Abstract
The effects of berries consumption on cardiovascular disease (CVD) risk factors have not been systematically examined. Here, we aimed to conduct a meta-analysis with trial sequential analysis to estimate the effect of berries consumption on CVD risk factors. PubMed, Embase, and CENTRAL were searched for randomized controlled trials (RCTs) that regarding the effects of berries consumption in either healthy participants or patients with CVD. Twenty-two eligible RCTs representing 1,251 subjects were enrolled. The pooled result showed that berries consumption significantly lowered the low density lipoprotein (LDL)-cholesterol [weighted mean difference (WMD), −0.21 mmol/L; 95% confidence interval (CI), −0.34 to −0.07; P = 0.003], systolic blood pressure (SBP) (WMD, −2.72 mmHg; 95% CI, −5.32 to −0.12; P = 0.04), fasting glucose (WMD, −0.10 mmol/L; 95% CI, −0.17 to −0.03; P = 0.004), body mass index (BMI) (WMD, −0.36 kg/m2; 95% CI, −0.54 to −0.18, P < 0.00001), Hemoglobin A1c (HbA1c) (WMD, −0.20%; 95% CI, −0.39 to −0.01; P = 0.04) and tumor necrosis factor-α (TNF-α) (WMD, −0.99 ρg/mL; 95% CI, −1.96 to −0.02; P = 0.04). However, no significant changes were seen in other markers. The current evidence suggests that berries consumption might be utilized as a possible new effective and safe supplementary option to better prevent and control CVD in humans.
Cardiovascular disease (CVD) is one of the leading causes of morbidity and mortality globally, accounting for 17.5 million CVD-related deaths annually in the United States and other countries and is projected to rise to almost 23.6 million deaths annually by the year 20301,2. Although multiple risk factors for progression of CVD, dyslipidemia, which results from one or more abnormalities of blood lipids metabolism, remains a major key factor for this pathology and leads to the development of atherosclerotic plaques3,4. Observational epidemiologic evidence suggests that risk of heart attack in subjects with hyperlipidemia is 3 times higher than those in general population with normal lipid status, whereas a 1% decrease in serum cholesterol is strongly associated with 3% reduction in CVD risk3,5. Moreover, hypertension is also considered to be another important risk factor for CVD, since 60% of strokes and half of ischemic heart disease cases are attributable to elevated blood pressure (BP)6,7. From a public health perspective, nutraceuticals or functional foods composition intervention is considered a first approach in treating and controlling CVD8. In recent decades, both dietary therapy and pharmacologic interventions were used in CVD patients to improve their lipid profiles or BP. Multiple approaches to diet therapy have appreciable beneficial roles in preventing CVD, such as consumption of sour tea9, spirulina10 and barley-derived soluble fiber11.
Berries are rich in a number of polyphenols, including procyanidins, quercetin, phenolic acids, and particularly anthocyanins12,13. Accumulating evidence has shown that ingestion of berries display a wide range of biological activities in lowering the risk of CVD, including anti-inflammatory, antihypertensive, hypoglycaemic, anticoagulant and the improvement of lipid metabolism disorders13,14. However, randomized controlled trials (RCTs) have yielded conflicting results regarding this topic, and to our knowledge, there has not been any quantitative attempt to summarize the precise effect of berries consumption on cardiovascular risk factors. With accumulating evidence, we therefore performed a comprehensive assessment of the literature and carry out a meta-analysis by examining the change in lipid concentrations and BP induced by berries consumption. Additionally, we qualitatively reviewed other CVD risk factors that have been investigated in relation to berries consumption. Finally, we further applied trial sequential analysis (TSA) to determine whether the currently available evidence was sufficient and conclusive.
Results
Trial selection and trials characteristics
Based on the search strategy, the initial screening yielded 1,322 potentially eligible articles of which 45 were retrieved for complete review. The work of VALENTOVAÄ et al. was separated into 2 trials (since the intervention groups used different doses of cranberry juice on plasma lipid profiles)15. It should be noted that two trials conducted by Zhu et al. were reported in the same population16,17; therefore, we combined the informative data and retained only the latest article to avoid duplication of information16. Finally, 22 RCTs met our inclusion criteria and were included in the analysis of which one study was determined through checking reference lists of retrieved articles15,16,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36. The flow diagram shows the process of literature screening, study selection, and reasons for exclusion, can be found as Supplemental Fig. S1.
The characteristics of treatment groups selected for analysis in the 22 RCTs are outlined in Table 1. Main characteristics are as follow: (1) Publishing year; these included studies were published from 2004 to 2015. (2) Intervention duration; 15 RCTs have treatment durations of 8 weeks or shorter15,18,19,22,25,26,27,28,29,30,31,32,33,34, and the remaining 7 RCTs have treatment durations more than 8 weeks16,20,21,23,35,36. (3) Number of patients; the trials varied in size from 18 to 146 subjects. A total of 1,251 subjects were included in these 22 RCTs. (4) Age of patients; the mean age of participants in each trial ranged from 21.5 to 65.5 years, with healthy status or risk of cardiovascular. (5) Types of berry; Of the 22 trials, 9 trials were used cranberry15,18,20,21,27,28,29,32, 5 trials were used bilberry16,19,22,23,35, 3 trials were used blueberry25,26,30, 2 trials were used whortleberry33,34, and 2 trials were used elderberry24,31, and 1 trial was used raspberry36. (6) Study design; most of the trials (20 trials) adopted parallel study designs15,16,18,19,20,21,22,23,24,25,26,27,29,31,32,33,34,35,36, and 2 trials used crossover designs28,30.
Table 1. Characteristics of the 22 included randomized controlled trials1.
| Study | Year | No. of patients | Type of study | Type of Patient | Initial BMI | Initial |TC/LDL-c2 | Mean age2 | Gender (M/F) | Treatment group | Control group | Design duration | Initial SBP/DBP2 | Location | Outcomes of interest |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Murkovic et al.31 | 2004 | 34 | R, DB, PC, P | Healthy | 23.6 | 5.0/2.8 | 29.0 | 20/14 | Elderberry juice | Placebo | 2 week | NA/NA | Austria | TC, LDL, HDL, TG |
| Duthie et al.18 | 2006 | 20 | R, PC, P | Healthy | NA | 4.7/2.9 | 27.8 | 0/20 | Cranberry juice | Placebo | 2 week | NA/NA | UK | TC, LDL, HDL, TG |
| Valentova et al.15 | 2007a | 27 | R, DB, PC, P | Healthy | 20.8 | 4.7/2.7 | 21.5 | 0/27 | Cranberry juice | Placebo | 8 week | 114/75 | Czech | TC, LDL, HDL, TG, BP |
| Valentova et al.15 | 2007b | 30 | R, DB, PC, P | Healthy | 21.2 | 4.7/2.6 | 21.5 | 0/57 | Cranberry juice | Placebo | 8 week | 112/73 | Czech | TC, LDL, HDL, TG, BP |
| Karlsen et al.19 | 2007 | 118 | P, PC, P | Healthy | 24.5 | NA/NA | 61 | NA | Bilberry, black currants | Placebo | 3 weeks | NA/NA | Norway | IL-6, CRP, TNF-α, |
| Wang et al.20 | 2007 | 40 | R, PC, P | Healthy | 21.0 | 4.5/2.9 | NA | 20/20 | Cranberry vinegar | Placebo | 10 week | NA/NA | China | TC, LDL, TG |
| Lee et al.21 | 2008 | 30 | R, DB, PC, P | Type 2 diabetes | 26 | 5.2/3.1 | 65.5 | 16/14 | Cranberry capsule | Placebo | 12 week | 130/69 | China | TC, LDL, HDL, TG, BP, BMI, CRP, ox-LDL, glucose, HbA1c |
| Erlund et al.22 | 2008 | 71 | R,SB, PC, P | Cardiovascular risk factors | 26.2 | 6.3/NA | 57.9 | 25/46 | Bilberry, lingonberry | Placebo | 8 week | 129/81 | Finland | TC, HDL, TG, BP, sICAM-1 |
| Qin et al.23 | 2009 | 120 | R, DB, PC, P | Dyslipidemic | 26.1 | 5.8/4.1 | 40–65 | 42/78 | Bilberry, blackcurrant | Placebo | 12 week | 128/83 | China | TC, LDL, HDL, TG, BP, BMI, apo A-I, apo B, glucose |
| Curtis et al.24 | 2009 | 52 | R, DB, PC, P | Healthy postmenopausal | 24.7 | 5.5/3.5 | 58.2 | 0/52 | Elderberry | Placebo | 12 week | 126/80 | UK | TC, LDL, HDL, TG, BP, IL-6, BMI, CRP, TNF-α, glucose |
| Stull et al.25 | 2010 | 32 | R, DB, PC, P | Obese, insulin-resistant | 37.4 | 5.3/3.2 | 51.5 | 5/27 | Blueberry smoothie | Placebo | 6 week | 120/75 | USA | TC, LDL, HDL, TG, BP, BMI, CRP, TNF-α, glucose |
| Basu et al.26 | 2010 | 48 | R, SB, PC, P | Obese, metabolic, syndrome | 37.8 | NA/NA | 50 | 4/44 | Blueberry beverage | Placebo | 8 week | NA/NA | USA | TC, LDL, HDL, TG, BP, IL-6, CRP, sVCAM-1, sICAM-1, ox-LDL, glucose, HbA1C |
| Basu et al.27 | 2011 | 31 | R, DB, PC, P | Metabolic syndrome | 40.0 | 3.4/3.1 | 52.0 | Na | Cranberry juice | Placebo | 8 week | 132/83 | USA | TC, LDL, HDL, TG, BP, IL-6, CRP, ox-LDL, glucose |
| Dohadwala et al.28 | 2011 | 44 | R, DB, PC, C | Coronary artery disease | 29.5 | 4.1/2.3 | 62.0 | 30/14 | Cranberry juice | Placebo | 4 week | 132/73 | USA | TC, LDL, HDL, TG, BP, CRP, sICAM-1, glucose, HbA1c |
| Flammer et al.29 | 2013 | 69 | R, DB, PC, P | Cardiovascular risk factors | 27.4 | 4.7/NA | 48.1 | 31/38 | Cranberry juice | Placebo | 8 week | 116/71 | USA | TC, HDL, TG, BP, IL-6, CRP, TNF-α, sVCAM-1, sICAM-1, ox-LDL |
| Riso et al.30 | 2013 | 18 | R, PC, C | Cardiovascular risk factors | 24.8 | 5.8/3.8 | 47.8 | NA | Blueberry drink | Placebo | 6 week | 122/80 | Italy | TC, LDL, HDL, TG, BP, IL-6, BMI, CRP, TNF-α, sVCAM-1, glucose |
| Zhu et al. 16 | 2013 | 146 | R, DB, PC, P | Hypercholesterole-mia | 26.6 | 6.5/3.3 | 40-65 | NA | Bilberry, blackcurrant | Placebo | 24 week | 125/84 | China | TC, LDL, HDL, TG, BP, apo A-I, apo B, CRP, TNF-α, sVCAM-1 |
| Novotny et al.32 | 2014 | 56 | R, DB, PC, P | Healthy | 28.0 | 5.1/3.2 | 50.0 | 26/30 | Cranberry Juice | Placebo | 8 week | 117/71 | USA | TC, LDL, HDL, TG, BP, BMI, apo A-I, apo B, CRP, sVCAM-1, sICAM-1, glucose |
| Kianbakht et al.33 | 2014 | 80 | R, DB, PC, P | Hyperlipidemia | 29.9 | 7.6/4.3 | 53.5 | 38/42 | Whortleberry | Placebo | 8 week | NA/NA | Iran | TC, LDL, HDL, TG |
| Soltani et al.34 | 2014 | 50 | R, DB, PC, P | Hyperlipidemia | 25.3 | 5.8/3.3 | 47.2 | 20/30 | Whortleberry | Placebo | 4 week | NA/NA | Iran | TC, LDL, HDL, TG, BMI, CRP |
| Li et al.35 | 2015 | 58 | R, DB, PC, P | Diabetic | 24.0 | 5.0/3.2 | 57.8 | 34/24 | Bilberry | Placebo | 24 week | 129/81 | China | TC, LDL, HDL, TG, BP, IL-6, BMI, apo A-I, apo B, TNF-α, glucose, HbA1c |
| Jeong et al.36 | 2014 | 77 | R, DB, PC, P | Metabolic syndrome | 25.7 | 5.1/2.5 | 59.0 | 36/41 | Black Raspberry | Placebo | 12 week | NA/NA | Korea | TC, LDL, HDL, TG, apo A-I, apo B, IL-6, CRP, TNF-α,, sVCAM-1, sICAM-1 |
1R, randomized; DB, double-blind; SB, single-blinded; PC, placebo controlled; P, parallel; C, crossover; NA, not available; M, male; F, female; TC, total cholesterol; LDL, low density lipoprotein cholesterol; HDL, high density lipoprotein cholesterol; HbA1c, Hemoglobin A1c; TG, triglycerides; SBP, systolic blood pressure; DBP, diastolic blood pressure; sICAM, soluble intercellular adhesion molecule; sVCAM, soluble vascular cell adhesion molecule; CRP, C-reactive protein; TNF-α, Tumor Necrosis Factor-α; apo, apolipoprotein; ox-LDL, oxidized LDL; IL-6, interleukin-6.
2Values for age, BMI, baseline TC, LDL-c, SBP and DBP are means unless otherwise stated. For baseline TC and LDL-c, mmol/L; for baseline SBP and DBP, mm Hg; for BMI, kg/m2.
Risk of Bias Assessment
Details of risk-of-bias analysis can be found as Supplemental Fig. S2. Overall, all satisfied the criteria of complete outcome data, selective reporting and other bias. All included trials were described as “random”. Adequate randomized sequence was generated in eight trials, but 12 studies lacked appropriately described randomization procedures15,18,19,20,22,25,26,27,28,32,35,36. Three trials did not mention whether the blind method was adopted or not, and were considered as at high risk of bias18,19,20.
Primary outcome: Effect of berries consumption on lipid concentrations
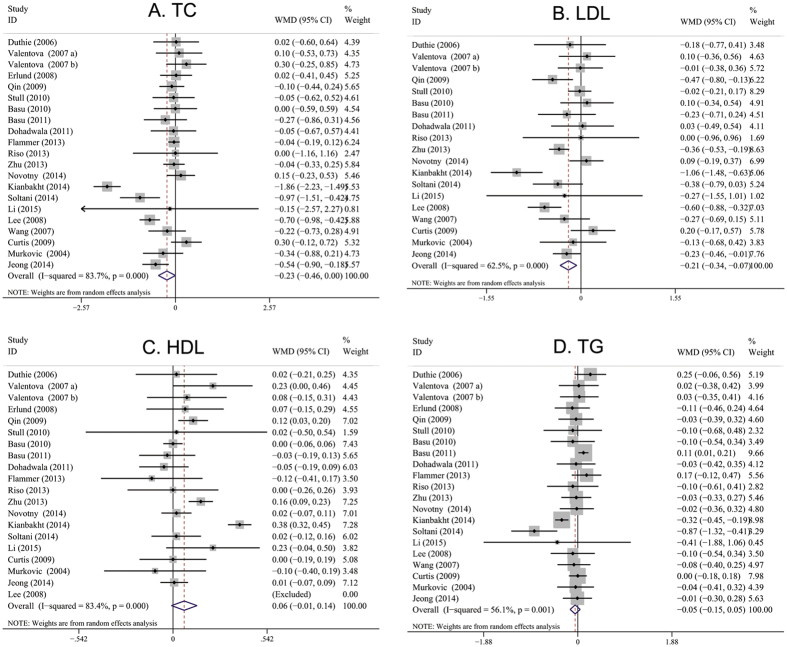
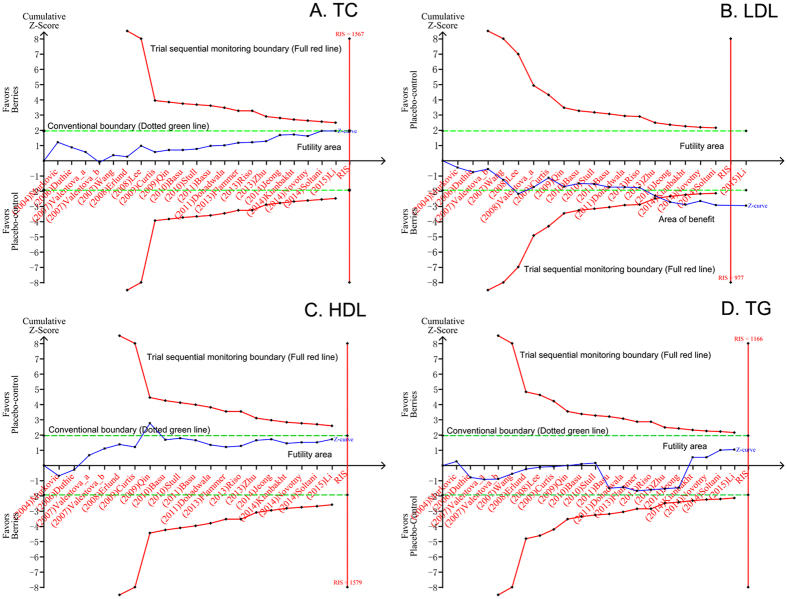
The results for total cholesterol (TC) were reported in 21 studies that represented 1,133 participants15,16,18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36. Compared with placebo-control group, berries consumption did not significantly change the serum TC level [weighted mean difference (WMD), −0.24 mmol/L; 95% confidence interval (CI), −0.49 to 0.01; P = 0.06; Fig. 1A]. Heterogeneity was observed for this outcome (I2 = 83.7%). We undertook a TSA at the level of α of 0.05, β of 0.2, and then the required information size (RIS) of 1,567 was calculated. Z-curve does not cross the trial sequential monitoring boundary and the RIS has not been reached, which demonstrate that evidence to reach a conclusion is insufficient and further trials are warranted (Fig. 2A).
Figure 1. Meta-analysis of effects of berries products consumption on lipid parameters (A, TC; B, LDL; C, HDL; D, TG) compared with control arms.
Sizes of data markers indicate the weight of each study in the analysis. WMD, weighted mean difference (the results were obtained from a random-effects model).
Figure 2. TSA on pooled result of effects of berries consumption on lipid profiles.
(A) TSA on pooled result of TC: the cumulative sample size over the RIS of 1,606 and the cumulative Z-curve did not cross both the conventional boundary and the trial sequential monitoring boundary. (B) TSA on pooled result of LDL cholesterol: the cumulative sample size over the RIS of 1,082 and the cumulative Z-curve crossed both the conventional boundary and the trial sequential monitoring boundary for benefit. (C) TSA on pooled result of HDL cholesterol: the cumulative sample size over the RIS of 1,792 and the cumulative Z-curve did not cross both the conventional boundary and the trial sequential monitoring boundary. (D) TSA on pooled result of TG: the cumulative sample size over the RIS of 1,192 and the cumulative Z-curve did not cross both the conventional boundary and the trial sequential monitoring boundary. RIS, required information size.
The mean change in low density lipoprotein (LDL)-cholesterol concentrations was reported in 19 studies representing 993 participants15,16,18,20,21,23,24,25,26,27,28,30,31,32,33,34,35,36. LDL-cholesterol was significantly lower in the berries-consumed subjects than in the placebo-treated subjects. The WMD in mean LDL cholesterol decreased by 0.21 mmol/L (95% CI: −0.34 to −0.07; P = 0.003; Fig. 1B), with significant heterogeneity (I2 = 62.5%). TSA was taken in the condition of α of 0.05, β of 0.2, and figured out RIS of 977. The accrued number of patients reached RIS, and the cumulative Z-curve cross conventional significance test boundary and RIS-adjusted boundary value, which established sufficient and conclusive evidence (Fig. 2B). Thus, further trials were not required and were unlikely to alter this conclusion.
The effect of berries on high density lipoprotein (HDL)-cholesterol was assessed in 20 trials based on the results of the meta-analysis15,16,18,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36. The pooled result show that the berries intervention group may not significantly change in serum HDL-cholesterol level compared with that of controls (WMD, 0.06 mmol/L; 95% CI, −0.01 to 0.14; P = 0.08; Fig. 1C), with significant heterogeneity (I2 = 83%). TSA was performed and demonstrated RIS of 1,579. Z-curve does not cross any of the boundaries and the RIS has not been reached, which demonstrate that evidence to reach a conclusion is insufficient and further trials are warranted (Fig. 2C).
Twenty-one trials totaling 1,133 patients provided data on triglycerides (TG) level15,16,18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36. Figure 1D shows the pooled results from the random-effects model combing the WMD for the effect of berries consumption on TG level in the study population, which demonstrated that the level of TG was not significantly changed in the berries treatment group compared with the control group (WMD, −0.05; 95%CI, −0.15 to 0.05; P = 0.30), with significant heterogeneity among the studies (I2 = 56%). TSA was performed and RIS of 1,166 was counted. Z-curve does not cross any of the boundaries and the RIS has not been reached, which demonstrate that evidence to reach a conclusion is insufficient and more trials are needed (Fig. 2D).
Secondary outcome: Effect of berries consumption on BP
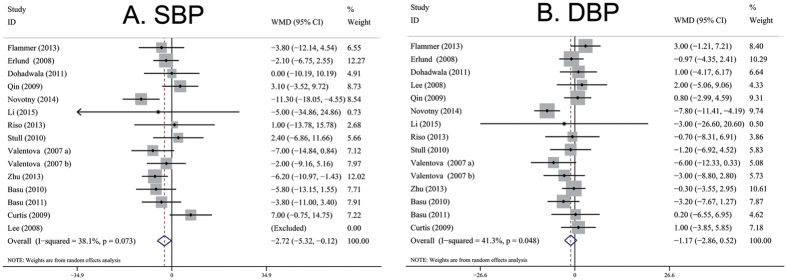
Pooled results from the random-effects model showed that berries consumption significantly reduced the systolic blood pressure (SBP) level (15 trials with 832 individuals; WMD, −2.72 mmHg; 95% CI, −5.32 to −0.12; P = 0.04; I2 = 38%; Fig. 3A). No effect was found for diastolic blood pressure (DBP) (15 trials with 832 individuals; WMD, −1.17 mmHg; 95% CI, −2.86 to 0.52; P = 0.18; I2 = 41%; Fig. 3B). TSA was performed and the results can be found as Supplemental Fig. S3. TSA demonstrate that evidence to reach a conclusion is insufficient and more trials are needed, because of both of the Z-curves does not cross any of the boundaries and the RIS has not been reached.
Figure 3. Meta-analysis of effects of berries consumption on BP (A, SBP; B, DBP) compared with control arms.
Sizes of data markers indicate the weight of each study in the analysis. WMD, weighted mean difference (the results were obtained from a random-effects model).
Other outcomes: Effect of berries consumption on other markers of cardiovascular disease
Other identified risk factors, such as markers of oxidative stress, markers of inflammation and endothelial function, were mentioned in our systematic literature search of clinical trials of berries consumption. Table 2 outline secondary outcomes. Compared with the control arms, berries consumption were associated with decreases in fasting glucose (WMD, −0.10 mmol/L; 95% CI, −0.17 to −0.03; P = 0.004), Hemoglobin A1c (HbA1c) (WMD, −0.20%; 95% CI, −0.39 to −0.01; P = 0.04), body mass index (BMI) (WMD, −0.36 kg/m2; 95% CI, −0.54 to −0.18, P < 0.00001) and tumor necrosis factor-α (TNF-α) (WMD, −0.99 ρg/mL; 95% CI, −1.96 to −0.02; P = 0.04), whereas no meaningful differences were observed for all other markers considered.
Table 2. Effect of berries consumption on other markers of cardiovascular disease1.
| Markers outcomes | No. Trials | No. Patients | WMD (95% CI)1 | P Value | I2, % | P Value of Heterogeneity | Model used |
|---|---|---|---|---|---|---|---|
| Glucose (mmol/L) | 10 | 489 | −0.10 (−0.17 to −0.03)* | 0.004 | 0 | 0.62 | F |
| HbA1c (%) | 3 | 136 | −0.20 (−0.39 to −0.01)* | 0.04 | 80 | 0.006 | R |
| BMI (kg/m2) | 8 | 416 | −0.36 (−0.54 to −0.18)* | 0.0001 | 0 | 1.00 | F |
| Apo A-I (mg/dL) | 5 | 457 | 0.85 (−3.53 to 5.23) | 0.70 | 50 | 0.09 | R |
| Apo B (mg/dL) | 5 | 457 | −3.68 (−10.48 to 3.13) | 0.29 | 75 | 0.003 | R |
| Ox-LDL (μmol/l) | 4 | 178 | −3.45 (−10.01 to 3.11) | 0.30 | 40 | 0.17 | R |
| IL-6 (ρg/mL) | 8 | 471 | −0.14 (−0.45 to 0.16) | 0.35 | 40 | 0.11 | R |
| TNF-alpha (ρg/mL) | 8 | 570 | −0.99 (−1.96 to −0.02)* | 0.04 | 21 | 0.26 | R |
| CRP (mg/L) | 13 | 771 | −0.08 (−0.30 to 0.15) | 0.52 | 0 | 0.98 | F |
| sICAM-1 (ηg/mL) | 6 | 365 | 3.61 (−9.85 to 17.08) | 0.60 | 53 | 0.06 | R |
| sVCAM-1 (ηg/mL) | 6 | 414 | −13.55 (−65.95 to 38.84) | 0.61 | 75 | 0.001 | R |
1R, Random-effects model; F, Fixed-effects model; WMD, weighted mean difference; CI, confidence interval; *Indicates a significant result.
Subgroup analyses and Sensitivity Analyses
Subgroup analyses were planned a priori to determine whether the mean age, intervention duration, types of berry, study design and type of patients modified the effects of berries on lipid profile. Briefly, based on the current subgroup analyses, significant reductions in the level of serum LDL cholesterol and significant increases the HDL-cholesterol level were observed in subjects with longer-term intervention duration (≥8 weeks), and bilberry consumption group. Statistical differences were also found in comparisons of whortleberry versus placebo-control in TC, LDL, and TG levels. A significant reduction was also observed in the level of serum TC and LDL cholesterol in subjects with black raspberry intervention. In a stratified analysis by type of patient, a significant reduction in TC and LDL concentration were observed in subjects with cardiovascular risk factors. Detailed subgroup results of lipids changes are summarized in Table 3.
Table 3. Subgroup estimation of the effects of berries consumption on lipid concentrations according to predefined study characteristics1.
| Variables | No. trials | Total cholesterol |
No. trials | LDL cholesterol |
No. trials | HDL cholesterol |
No. trials | Triglycerides |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WMD (95%CI) | P | I2(%) | WMD (95%CI) | P | I2(%) | WMD (95%CI) | P | I2(%) | WMD (95%CI) | P | I2(%) | |||||
| Total | 21 | −0.23 (−0.46 to 0.00) | 0.05 | 84 | 19 | −0.21 (−0.34 to −0.07)* | 0.003 | 62 | 20 | 0.06 (−0.01 to 0.14) | 0.08 | 83 | 21 | −0.05 (−0.15 to 0.05) | 0.30 | 56 |
| Subgroup analysis | ||||||||||||||||
| Mean age | ||||||||||||||||
| <50 y | 8 | −0.12 (−0.38 to 0.13) | 0.34 | 49 | 7 | −0.08 (−0.26 to 0.11) | 0.41 | 0 | 8 | 0.01 (−0.04 to 0.06) | 0.68 | 0 | 8 | −0.05 (−0.27 to 0.17) | 0.64 | 61 |
| ≥50 y | 10 | −0.34 (−0.80 to 0.12) | 0.14 | 90 | 9 | −0.22 (−0.47 to 0.02) | 0.07 | 78 | 10 | 0.08 (−0.07 to 0.22) | 0.30 | 90 | 10 | −0.06 (−0.21 to 0.08) | 0.38 | 67 |
| Intervention duration | ||||||||||||||||
| shorter-term (≤8 weeks) | 14 | −0.23 (−0.57 to 0.11) | 0.19 | 87 | 12 | −0.14 (−0.32 to 0.05) | 0.14 | 58 | 14 | 0.05 (−0.06 to 0.15) | 0.38 | 87 | 14 | −0.06 (−0.21 to 0.08) | 0.40 | 71 |
| longer-term (>8 weeks) | 7 | −0.23 (−0.52 to 0.07) | 0.13 | 73 | 7 | −0.31 (−0.48 to −0.13)* | 0.0006 | 54 | 6 | 0.09 (0.02 to 0.17)* | 0.02 | 61 | 7 | −0.03 (−0.14 to 0.09) | 0.63 | 0 |
| Types of berry | ||||||||||||||||
| Cranberry juice | 9 | −0.11 (−0.34 to 0.13) | 0.36 | 65 | 8 | −0.15 (−0.36 to 0.06) | 0.17 | 55 | 8 | 0.01 (−0.05 to 0.07) | 0.67 | 0 | 9 | 0.09 (−0.00 to 0.17) | 0.05 | 0 |
| Bilberry | 4 | −0.05 (−0.24 to 0.15) | 0.63 | 0 | 3 | −0.38 (−0.53 to −0.23)* | 0.0001 | 0 | 4 | 0.14 (0.09 to 0.19)* | 0.0001 | 0 | 4 | −0.06 (−0.25 to 0.13) | 0.53 | 0 |
| Blueberry | 3 | −0.02 (−0.41 to 0.36) | 0.91 | 0 | 3 | −0.00 (−0.17 to 0.17) | 0.99 | 0 | 3 | 0.00 (−0.05 to 0.05) | 0.99 | 0 | 3 | −0.10 (−0.39 to 0.19) | 0.50 | 0 |
| Whortleberry | 2 | −1.44 (−2.32 to −0.56)* | 0.001 | 86 | 2 | −0.72 (−1.38 to −0.05)* | 0.03 | 80 | 2 | 0.21 (−0.15 to 0.56) | 0.26 | 95 | 2 | −0.55 (−1.08 to −0.02)* | 0.04 | 80 |
| Black Raspberry | 1 | −0.54 (−0.90 to −0.18)* | 0.003 | NA | 1 | −0.24 (−0.46 to −0.01)* | 0.04 | NA | 1 | 0.01 (−0.07 to 0.09) | 0.86 | NA | 1 | −0.01 (−0.30 to 0.28) | 0.96 | NA |
| Elderberry | 2 | −0.01 (−0.61 to 0.63) | 0.98 | 70 | 2 | 0.10 (−0.21 to 0.40) | 0.53 | 0 | 2 | −0.03 (−0.19 to 0.13) | 0.71 | 0 | 5 | −0.01 (−0.17 to 0.15) | 0.92 | 0 |
| Type of Patient | ||||||||||||||||
| Healthy | 7 | 0.08 (−0.11 to 0.26) | 0.43 | 0 | 7 | 0.01 (−0.14 to 0.16) | 0.88 | 0 | 6 | 0.03 (−0.03 to 0.10) | 0.31 | 0 | 7 | 0.02 (−0.09 to 0.13) | 0.72 | 0 |
| Cardiovascular risk factors | 14 | −0.37 (−0.68 to −0.06)* | 0.02 | 88 | 12 | −0.31 (−0.49 to −0.14)* | 0.0003 | 66 | 14 | 0.07 (−0.02 to 0.17) | 0.13 | 88 | 14 | −0.10 (−0.25 to 0.04) | 0.18 | 69 |
| Study design | ||||||||||||||||
| Parallel | 19 | −0.24 (−0.49 to 0.00) | 0.05 | 85 | 17 | −0.22 (−0.36 to −0.08)* | 0.003 | 66 | 18 | 0.08 (−0.00 to 0.15) | 0.06 | 85 | 19 | −0.05 (−0.16 to 0.05) | 0.33 | 60 |
| Crossover | 2 | −0.04 (−0.59 to 0.51) | 0.88 | 0 | 2 | 0.02 (−0.43 to 0.47) | 0.93 | 0 | 2 | −0.04 (−0.16 to 0.08) | 0.53 | 0 | 2 | −0.06 (−0.37 to 0.25) | 0.71 | 0 |
| Types of interventions | ||||||||||||||||
| whole berries | 17 | −0.29 (−0.56 to −0.01)* | 0.04 | 86 | 15 | −0.20 (−0.36 to −0.04)* | 0.02 | 63 | 16 | 0.05 (−0.05 to 0.14) | 0.35 | 86 | 17 | −0.06 (−0.18 to 0.06) | 0.33 | 65 |
| Purified berries-derived anthocyanins | 4 | 0.02 (−0.18 to 0.21) | 0.86 | 0 | 4 | −0.24 (−0.54 to 0.07) | 0.13 | 65 | 4 | 0.14 (0.08 to 0.19)* | 0.0001 | 2 | 4 | −0.02 (−0.15 to 0.12) | 0.20 | 0 |
| Sensitivity analysis | ||||||||||||||||
| Exclude high-risk research | 19 | −0.24 (−0.49 to 0.01) | 0.06 | 85 | 17 | −0.20 (−0.35 to −0.05)* | 0.008 | 67 | 19 | 0.07 (−0.01 to 0.14) | 0.09 | 84 | 19 | −0.07 (−0.18 to 0.04) | 0.20 | 57 |
1NA, Not applicable; *Indicates a significant result.
In sensitivity analyses, the pooled effects of berries on lipid profile did not change after systematically dropping each trial. Furthermore, we also omitted the studies with high risk of bias. The aggregated results were similar when compared with the overall analysis (Table 3). All results of the sensitivity analysis suggest that the data in this meta-analysis are relatively stable and credible.
Publication Bias
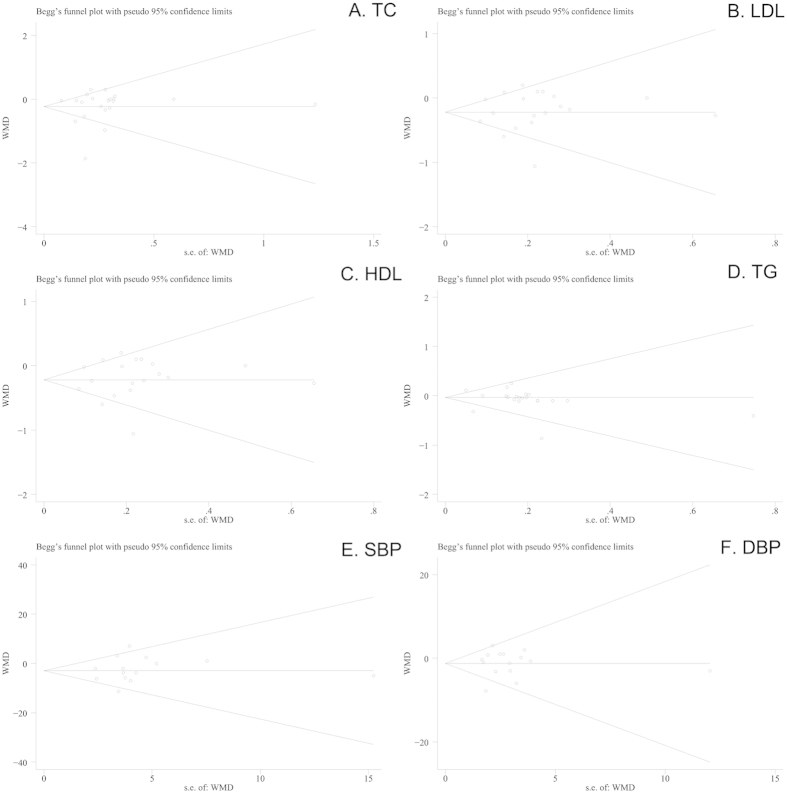
For the meta-analysis of the effect of berries consumption on lipid concentrations or BP, there was no evidence of publication bias by inspection of the funnel plot and formal statistical (for TC, Egger’s test P = 0.740; for LDL, Egger’s test P = 0.529; for HDL, Egger’s test P = 0.834; for TG, Egger’s test P = 0.815; for SBP, Egger’s test P = 0.511; for DBP, Egger’s test P = 0.488; respectively). No evidence of publication bias was indicated by the funnel plots (Fig. 4).
Figure 4. Tests for publication bias of effects of berries consumption on lipid profiles (A, TC; B, LDL; C, HDL, D, TG) and BP (E, SBP; F, DBP).
Discussion
To our knowledge, this is the first meta-analysis to estimate of the relationship between berries consumption and changes of cardiovascular risk factors in subjects. Our analyses, with a total of 1,251 participants, showed a statistically significant reduction in LDL, SBP, fasting glucose, HbA1c, BMI and TNF-α levels after berries consumption. However, no statistically significant effects were observed in serum TC, TG, apolipoprotein (apo) A-I, apo B, HDL, DBP, oxidized LDL (ox-LDL), interleukin-6 (IL-6), C-reactive protein (CRP), soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular cell adhesion molecule-1 (sVCAM-1) with berries ingestion when compared with placebo. The effects of berries on LDL-cholesterol reduction were found in several subgroups of individuals (i.e., longer-term intervention duration, and parallel study design). Similarly, subgroup analyses showed that significant reductions in the level of SBP were observed in subjects with shorter-term intervention duration (≤8 weeks), age ≥50 y, cranberry consumption group and parallel study design group. In addition, finding from subgroup analyzes also clearly show that berries products consumption significantly decreased the levels of serum TC, LDL-cholesterol and SBP in subjects with cardiovascular risk factors.
The precise mechanisms responsible for the presumed lipids-lowering properties of berries are not fully explored. However, an inhibition of cholesteryl ester transfer protein and the suppression of LDL oxidation, as well as improvement in HDL-associated paraoxonase 1 activity are considered the likely pathway17,23,37. Other mechanisms such as the inhibition of release reactive oxygen species from activated human granulocytes or suppress free-radical mediated lipid peroxidation and cell death in cultured aortic endothelial cells may also contribute to the antihyperlipidemic effect38. Furthermore, berries derived anthocyanins also showed similar beneficial properties in vitro by the promotion of cholesterol efflux from macrophages, which may also contribute to their beneficial effects on lipid profiles39. Similarly, the observed effects of berries intake on SBP is also supported by several in vitro mechanistic study findings, in which purified berries-derived anthocyanins have been shown to affect signaling pathways involved in inflammation and exert antiangiogenic effects including the inhibition of platelet-derived growth-factor signaling40. In an apolipoprotein E–deficient mice model, purified berries-derived anthocyanin ingestion directly inhibited atherosclerosis development and suppressed the development of atherosclerotic lesions41,42.
Treatment particularly aimed at decreasing LDL-cholesterol is effective in reducing the risk of death from coronary heart disease and stroke. Epidemiological and prospective studies have established the benefit of reducing LDL-C, with a 1% reduction associated with a 1% decrease in CVD events43. According to the updated clinical guidelines for cholesterol testing and management of the National Cholesterol Education Program (NCEP), the first priority of drug therapy have recommended progressively lower LDL-C for cholesterol treatment and CVD prevention as the primary target of therapy43. For this reason, treatment with LDL-lowering agents should be started as early as possible. Despite dramatic reductions in cardiovascular risk with intensively LDL-cholesterol lowering therapy, a substantial residual cardiovascular risk (up to 70% of baseline) remains significant after LDL-C goals are achieved with lipid-lowering treatments in most patient populations44,45. Early epidemiological studies have identified low levels of HDL-cholesterol (<1.0 mmol/L or 40 mg/dl) to be an independent determinant of increased cardiovascular risk46. Therefore, raising HDL cholesterol represents an important strategy for reducing residual cardiovascular risk in patients already optimally treated with LDL-cholesterol lowering therapy, and should lead to further improvements in clinical outcomes in these patient groups47. Analysis of the epidemiological data available suggests that cardiovascular risk increases by 2–3% for every increase of 0.03 mmol/L or 1 mg/dl HDL-C48. Dietary or dietary nutraceuticals composition intervention is now considered as potential candidates to protect against CVD for many reasons, including the poor adherence to drugs, contraindications to drugs, drug-related adverse effects (AEs) or personal preference for natural or alternative therapies. In our subgroup meta-analysis, the pooled result revealed that daily bilberries products consumption may significantly reduce the LDL-cholesterol level (0.38 mmol/L) and significant increases the HDL cholesterol level (0.14 mmol/L) without obviously AEs, which suggests that berries may be considered as a possible new effective and safe supplementary option for treatment of dyslipidemia and reduction of residual vascular risk in public population. Furthermore, this beneficial effect can be also found in subjects with longer-term intervention duration (≥8 weeks).
We also assessed the effects of berries products consumption on the other markers of cardiovascular disease. A number of studies have also examined the role of various proinflammatory cytokines in the progression of atherosclerosis49,50,51. CRP, a plasma protein synthesized by the liver, binds to LDL and is present in atherosclerotic plaques, so it has been proposed that CRP may have a causal role in coronary heart disease. VCAM-1, ICAM-1, IL-6, and TNF-α may provide additional information for cardiovascular risk stratification and prediction and have been regarded as useful biomarkers in assessing cardiovascular events in populations with various disease settings. In our present study, changes in plasma levels of CRP, IL-6, sVCAM-1, sICAM-1 and TNF-α did not differ between the berries and control groups. Furthermore, previous studies have demonstrated that the development of atherosclerosis is also accompanied by an accumulation of oxLDL52. In the present study, berries consumption, compared with control arms, produced a slight, but significant reduction of 0.99 ρg/mL (P = 0.04) in TNF-α level, whereas no meaningful differences were observed for all other antioxidant and inflammatory markers considered.
A major strength of this meta-analysis was following the PRISMA guidelines and the recommendations of the Cochrane Collaboration. To increase the robustness of our study, we applied TSA to assess the impact of random error and repetitive testing. Furthermore, funnel plot did not reflect obvious asymmetry, and Egger’s test further indicated no considerable publication bias in our present meta-analysis. This made our results more reliable to some extent. However, some potential limitations in the current study should be worthy of comment. Firstly, because of the various confounders appear in our screening trials, the lipid-lowering effect of berries consumption in subjects could be attributed to other healthy habits, such as cocoa, red wine, and tea consumption. The synergistic effects of other coexisting substances in berries foods on the clinical outcomes need to be excluded. Secondly, although extensive searches and clear inclusion criteria were made, it cannot be entirely sure that all relevant articles were selected, for the reason of the measures for lipid profiles or BP control were not primary outcomes in most of the trials selected in this meta-analysis, and the null findings of secondary outcomes may not have always been published. Finally, it should be noted that lipid profiles and BP change soon after switching diets, berries products consumption would need to be maintained indefinitely to maintain lower concentrations. Long-term compliance with consumption is often a concern with dietary interventions.
In conclusion, our meta-analysis showed that berries consumption significantly reduced the levels of LDL cholesterol, SBP, fasting glucose, HbA1c, BMI and TNF-α. However, no significantly changes were found in TC, HDL-cholesterol, TG, DBP, apo A-I, apo B, ox-LDL, IL-6, CRP, sICAM-1 and sVCAM-1. Our subgroup analyses demonstrated that berries products might be utilized as a possible new effective and safe supplementary option to better prevent and control CVD in subjects with cardiovascular risk factors. Further studies evaluating the effects of berries food consumption on clinical endpoints, such as cardiovascular morbidity and all-cause mortality, are required.
Methods
Literature search strategy
For this meta-analysis, we performed a search of PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases from their inception to August 2015 to identify RCTs that examined the effects of berries consumption in either healthy participants or patients with cardiovascular diseases. A search strategy was performed with the use of exploded Medical Subject Heading (MeSH) terms and corresponding key words. Briefly, we used the following format of search terms: (“cranberry*” OR “raspberry*” OR “blueberr*” OR “whortleberry*” OR “bilberr*” OR “lingonberr*” OR “berr*” OR “Huckleberr*” OR “Vaccinium macrocarpon Ait” OR “Vaccinium corymbosum L.”). The search was limited to the criteria “clinical trials” and “human”, with no language restrictions. In order to identify RCTs, a highly sensitive filter was used. Two investigators independently screened the titles and abstracts resulting from the search strategies. Articles were excluded on initial screening if titles or abstracts were clearly irrelevant. Citation lists of retrieved articles were manually screened to ensure sensitivity of the search strategy. Bibliographies of accepted studies and recent reviews were also screened to ensure a complete study listing.
Study selection criteria
To be selected for analysis, full publications were retrieved for evaluation on the basis of criteria established a priori. Studies were selected for this analysis if they met the following criteria: 1) subjects needed to have specifically ingested the berries interventions; 2) study was an RCT in human with either a parallel or crossover design; 3) studies used placebo as comparison intervention; 4) food-intake control regimens of experimental groups were consistent with those of control groups; 5) studies had to assess at least one primary outcomes [i.e., lipids (TC, LDL, HDL, TG), BP (SBP, DBP) or other pre-determined CVD risk factors]; 6) the baseline and endpoint information (or their difference) with SDs, SEMs, or 95% CIs were available for each group in the study.
Risk of bias assessment
Risk-of-bias assessment was performed in accordance with guidelines outlined in the cochrane risk-of-bias tool53. For each study, two investigators subjectively assigned a value of ‘high’, ‘low’, or ‘unclear’ to the following 7 domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other bias.
Data extraction
Two investigators independently collected the data, crosschecked and reached a consensus on all items. The following variables were extracted from the including studies: first author’s name, publication year, number of subjects enrolled (intervention/control), types of study design, patients’ characteristics [including mean age, sex, BMI, health status, baseline LDL and TC levels, baseline SBP and DBP], medication strategies of treatment and control arms, duration of treatment, location, and outcome measures for the analysis. When the same patients were reported in several publications, we retained only the largest study to avoid duplication of information. If trials included in our analysis have multiple intervention groups, we grouped together all the experimental groups and compared them with the control group, respectively. Any disagreements in abstracted data between the reviewers were adjudicated by a third author.
Data synthesis and statistical analysis
The primary end points of this study were the mean difference in lipid profiles (mmol/L; TC, LDL, HDL and TG). Secondary outcome was the mean difference in BP (mmHg; SBP and DBP). Net changes in each of the study variables, which were calculated from baseline and follow-up means and SDs, were used to estimate the principal effect. Intention to treat (ITT) data from all eligible trials was used in this meta-analysis. Since ITT is intended to avoid various misleading artifacts that can arise in intervention research such as non-random attrition of participants from the study or crossover54. Studies that reported results in mg/dL were converted to mmol/L using the standard conversion factors. The conversion factor was 1 mg/dL = 0.02586 mmol/L for cholesterol, 1 mg/dL = 0.01129 mmol/L for TG and 1mg/dL = 0.056 mmol/L for glucose. These values were captured as mean ± SD. When SDs were not directly available, they were calculated from SE or 95% CI according to the Cochrane Handbook for Systematic Reviews of Interventions55. Other cardiovascular risk factors were also captured in this way, including apo A-I and apo B, BMI, ox-LDL, glucose, HbA1c, IL-6, TNF-α, VCAM-1, ICAM-1 and CRP.
Our meta-analysis and statistical analyses were performed with STATA (version 12; StataCorp, College Station, TX). For continuous outcomes, summary estimates of WMD with 95% CI were calculated for net changes for each intervention and control group. Clinical heterogeneity was assessed by considering the design of each study. The statistical heterogeneity across studies was tested by using the I2 statistic, which was a quantitative measure of inconsistency across studies. We considered an I2 value 50% to indicate significant heterogeneity between the trials56. Primary analyses were done with a fixed effects model; secondary confirmatory analyses were done with a random effects model if there was significant heterogeneity. To check the influence of various factors on cardiovascular risk factors of berries intervention, we further performed a priori subgroup analysis according to participant characteristics. The subgroup analyses were performed only for the effect of berries products on lipid profiles and BP because of small numbers of studies for other outcome. Furthermore, sensitivity analysis was also performed by removing each study at a time to evaluate the stability of the results. Furthermore, we evaluated potential publication bias of the studies included with Funnel plots and Egger’s regression asymmetry test (P < 0.05 was considered representative of statistically significant publication bias)57. All tests were two-tailed and a P value of less than 0.05 was deemed statistically significant.
Trial sequential analysis
The estimation of sample size is required by the repeatability principle of clinical trials aiming at the study power in statistics. In a meta-analysis, repeated significance test of sparse and accumulated data may increase the risk of random errors which cause false positive or negative results58,59. A method that aims to correct for the increased risk of random errors is called Trial sequential analyses, which were performed post hoc to assess the risk of random errors, false positive results, and to help clarify the need for additional trials. If the cumulative Z-curve crosses the trial sequential monitoring boundary or enters the futility area, then we can draw the conclusion that a sufficient level of evidence for the anticipated intervention effect may have been reached and no further trials are needed; whereas the Z-curve does not cross any of the boundaries and the required information size has not been reached, evidence to reach a conclusion is insufficient.
The primary outcomes in our meta-analysis were continuous data category, so we estimated the RIS based on the empirical data autogenerated from software according to the data input. The TSA was performed at the level of an overall 5% risk of a type I error (two sided) and 20% of the type II error (a statistical test power of 80%)60. TSA software (version 0.9 beta; http://www.ctu.dk/tsa) were used for these analyses.
Additional Information
How to cite this article: Huang, H. et al. Effects of Berries Consumption on Cardiovascular Risk Factors: A Meta-analysis with Trial Sequential Analysis of Randomized Controlled Trials. Sci. Rep. 6, 23625; doi: 10.1038/srep23625 (2016).
Supplementary Material
Footnotes
Author Contributions H.H. Conception and design of the study, acquisition of data, analysis and interpretation of data, discussed the idea of the meta-analysis, drafted the manuscript, submitted the paper. G.C. and D.L. Completed the database searches and selected, screened, and reviewed the articles. Y.Z. and X.X. reviewed and extracted the data, and performed the data analyses. All authors read and approved the final manuscript. H.H. had primary responsibility for final content.
References
- World Health Organization Statistics 2013. Cardiovascular diseases. Available at: http://www.who.int/mediacentre/factsheets/fs317/en/index. html. (Accessed: 27 February 2014).
- Smith S. C. Jr. et al. Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke). Circulation 126, 2769–2775, doi: 10.1161/CIR.0b013e318267e99f (2012). [DOI] [PubMed] [Google Scholar]
- Yusuf S. et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364, 937–952, doi: 10.1016/s0140-6736(04)17018-9 (2004). [DOI] [PubMed] [Google Scholar]
- Jain K. S., Kathiravan M. K., Somani R. S. & Shishoo C. J. The biology and chemistry of hyperlipidemia. Bioorg Med Chem 15, 4674–4699, doi: 10.1016/j.bmc.2007.04.031 (2007). [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D. et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121, e46–e215, doi: 10.1161/circulationaha.109.192667 (2010). [DOI] [PubMed] [Google Scholar]
- Lewington S., Clarke R., Qizilbash N., Peto R. & Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360, 1903–1913 (2002). [DOI] [PubMed] [Google Scholar]
- Banach M. & Aronow W. S. Hypertension therapy in the older adults-do we know the answers to all the questions? The status after publication of the ACCF/AHA 2011 expert consensus document on hypertension in the elderly. J Hum Hypertens 26, 641–643, doi: 10.1038/jhh.2012.3 (2012). [DOI] [PubMed] [Google Scholar]
- Chen Z. Y., Jiao R. & Ma K. Y. Cholesterol-lowering nutraceuticals and functional foods. J Agric Food Chem 56, 8761–8773, doi: 10.1021/jf801566r (2008). [DOI] [PubMed] [Google Scholar]
- Serban C., Sahebkar A., Ursoniu S., Andrica F. & Banach M. Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: a systematic review and meta-analysis of randomized controlled trials. J Hypertens 33, 1119–1127, doi: 10.1097/hjh.0000000000000585 (2015). [DOI] [PubMed] [Google Scholar]
- Serban M. C. et al. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin Nutr, doi: 10.1016/j.clnu.2015.09.007 (2015). [DOI] [PubMed] [Google Scholar]
- Talati R., Baker W. L., Pabilonia M. S., White C. M. & Coleman C. I. The effects of barley-derived soluble fiber on serum lipids. Ann Fam Med 7, 157–163, doi: 10.1370/afm.917 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatta-Riihinen K. R., Kamal-Eldin A., Mattila P. H., Gonzalez-Paramas A. M. & Torronen A. R. Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J Agric Food Chem 52, 4477–4486, doi: 10.1021/jf049595y (2004). [DOI] [PubMed] [Google Scholar]
- Milbury P. E., Vita J. A. & Blumberg J. B. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J Nutr 140, 1099–1104, doi: 10.3945/jn.109.117168 (2010). [DOI] [PubMed] [Google Scholar]
- Ruel G. & Couillard C. Evidences of the cardioprotective potential of fruits: the case of cranberries. Mol Nutr Food Res 51, 692–701, doi: 10.1002/mnfr.200600286 (2007). [DOI] [PubMed] [Google Scholar]
- Valentova K. et al. Biosafety, antioxidant status, and metabolites in urine after consumption of dried cranberry juice in healthy women: a pilot double-blind placebo-controlled trial. J Agric Food Chem 55, 3217–3224, doi: 10.1021/jf0636014 (2007). [DOI] [PubMed] [Google Scholar]
- Zhu Y. et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis 23, 843–849, doi: 10.1016/j.numecd.2012.06.005 (2013). [DOI] [PubMed] [Google Scholar]
- Zhu Y. et al. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin Chem 57, 1524–1533, doi: 10.1373/clinchem.2011.167361 (2011). [DOI] [PubMed] [Google Scholar]
- Duthie S. J. et al. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur J Nutr 45, 113–122, doi: 10.1007/s00394-005-0572-9 (2006). [DOI] [PubMed] [Google Scholar]
- Karlsen A. et al. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr 137, 1951–1954 (2007). [DOI] [PubMed] [Google Scholar]
- Wang C. et al. Cardiovascular disease prevention of cranberry vinegar. Nutr Sci 32, No. 4, 129–132 (2007). [Google Scholar]
- Lee I. T., Chan Y. C., Lin C. W., Lee W. J. & Sheu W. H. Effect of cranberry extracts on lipid profiles in subjects with Type 2 diabetes. Diabet Med 25, 1473–1477, doi: 10.1111/j.1464-5491.2008.02588.x (2008). [DOI] [PubMed] [Google Scholar]
- Erlund I. et al. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr 87, 323–331 (2008). [DOI] [PubMed] [Google Scholar]
- Qin Y. et al. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr 90, 485–492, doi: 10.3945/ajcn.2009.27814 (2009). [DOI] [PubMed] [Google Scholar]
- Curtis P. J. et al. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J Nutr 139, 2266–2271, doi: 10.3945/jn.109.113126 (2009). [DOI] [PubMed] [Google Scholar]
- Stull A. J., Cash K. C., Johnson W. D., Champagne C. M. & Cefalu W. T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr 140, 1764–1768, doi: 10.3945/jn.110.125336 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A. et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr 140, 1582–1587, doi: 10.3945/jn.110.124701 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A. et al. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr Res 31, 190–196, doi: 10.1016/j.nutres.2011.02.003 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohadwala M. M. et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr 93, 934–940, doi: 10.3945/ajcn.110.004242 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammer A. J. et al. Polyphenol-rich cranberry juice has a neutral effect on endothelial function but decreases the fraction of osteocalcin-expressing endothelial progenitor cells. Eur J Nutr 52, 289–296, doi: 10.1007/s00394-012-0334-4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riso P. et al. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur J Nutr 52, 949–961, doi: 10.1007/s00394-012-0402-9 (2013). [DOI] [PubMed] [Google Scholar]
- Murkovic M. et al. Effects of elderberry juice on fasting and postprandial serum lipids and low-density lipoprotein oxidation in healthy volunteers: a randomized, double-blind, placebo-controlled study. Eur J Clin Nutr 58, 244–249, doi: 10.1038/sj.ejcn.1601773 (2004). [DOI] [PubMed] [Google Scholar]
- Novotny J. A., Baer D. J., Khoo C., Gebauer S. K. & Charron C. S. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J Nutr 145, 1185–1193, doi: 10.3945/jn.114.203190 (2015). [DOI] [PubMed] [Google Scholar]
- Kianbakht S., Abasi B. & Hashem Dabaghian F. Improved lipid profile in hyperlipidemic patients taking Vaccinium arctostaphylos fruit hydroalcoholic extract: a randomized double-blind placebo-controlled clinical trial. Phytother Res 28, 432–436, doi: 10.1002/ptr.5011 (2014). [DOI] [PubMed] [Google Scholar]
- Soltani R. et al. Evaluation of the Effects of Vaccinium arctostaphylos L. Fruit Extract on Serum Lipids and hs-CRP Levels and Oxidative Stress in Adult Patients with Hyperlipidemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Evid Based Complement Alternat Med 2014, 217451, doi: 10.1155/2014/217451 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Zhang Y., Liu Y., Sun R. & Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr 145, 742–748, doi: 10.3945/jn.114.205674 (2015). [DOI] [PubMed] [Google Scholar]
- Jeong H. S. et al. Effects of black raspberry on lipid profiles and vascular endothelial function in patients with metabolic syndrome. Phytother Res 28, 1492–1498, doi: 10.1002/ptr.5154 (2014). [DOI] [PubMed] [Google Scholar]
- Zhu Y. et al. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J Clin Endocrinol Metab 99, 561–569, doi: 10.1210/jc.2013-2845 (2014). [DOI] [PubMed] [Google Scholar]
- Youdim K. A., McDonald J., Kalt W. & Joseph J. A. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults (small star, filled). J Nutr Biochem 13, 282–288 (2002). [DOI] [PubMed] [Google Scholar]
- Xia M. et al. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proliferator-activated receptor {gamma}-liver X receptor {alpha}-ABCA1 pathway. J Biol Chem 280, 36792–36801, doi: 10.1074/jbc.M505047200 (2005). [DOI] [PubMed] [Google Scholar]
- Lamy S. et al. Delphinidin, a dietary anthocyanidin, inhibits platelet-derived growth factor ligand/receptor (PDGF/PDGFR) signaling. Carcinogenesis 29, 1033–1041, doi: 10.1093/carcin/bgn070 (2008). [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Makino K., Iwadate E., Deguchi Y. & Ishikawa F. Anthocyanins from purple sweet potato Ipomoea batatas cultivar Ayamurasaki suppress the development of atherosclerotic lesions and both enhancements of oxidative stress and soluble vascular cell adhesion molecule-1 in apolipoprotein E-deficient mice. J Agric Food Chem 56, 11485–11492, doi: 10.1021/jf801876n (2008). [DOI] [PubMed] [Google Scholar]
- Wang D., Wei X., Yan X., Jin T. & Ling W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J Agric Food Chem 58, 12722–12728, doi: 10.1021/jf103427j (2010). [DOI] [PubMed] [Google Scholar]
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Jama 285, 2486–2497 (2001). [DOI] [PubMed]
- Hobbs F. D., Banach M., Mikhailidis D. P., Malhotra A. & Capewell S. Is statin-modified reduction in lipids the most important preventive therapy for cardiovascular disease? A pro/con debate. BMC Med 14, 4, doi: 10.1186/s12916-016-0550-5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigent C. et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376, 1670–1681, doi: 10.1016/s0140-6736(10)61350-5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy D. J. & Yellon D. M. Targeting residual cardiovascular risk: raising high-density lipoprotein cholesterol levels. Heart 94, 706–714, doi: 10.1136/hrt.2007.125401 (2008). [DOI] [PubMed] [Google Scholar]
- Banach M. et al. Lipids, blood pressure and kidney update 2015. Lipids Health Dis 14, 167, doi: 10.1186/s12944-015-0169-0 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. J. et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 79, 8–15 (1989). [DOI] [PubMed] [Google Scholar]
- Herder C., Karakas M. & Koenig W. Biomarkers for the prediction of type 2 diabetes and cardiovascular disease. Clin Pharmacol Ther 90, 52–66, doi: 10.1038/clpt.2011.93 (2011). [DOI] [PubMed] [Google Scholar]
- Greenland P. et al. 2010. ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 122, e584–636, doi: 10.1161/CIR.0b013e3182051b4c (2010). [DOI] [PubMed] [Google Scholar]
- Ridker P. M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 107, 363–369 (2003). [DOI] [PubMed] [Google Scholar]
- Chen W. et al. Amelioration of atherosclerosis by tanshinone IIA in hyperlipidemic rabbits through attenuation of oxidative stress. Eur J Pharmacol 674, 359–364, doi: 10.1016/j.ejphar.2011.10.040 (2012). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928, doi: 10.1136/bmj.d5928 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montedori A. et al. Modified versus standard intention-to-treat reporting: are there differences in methodological quality, sponsorship, and findings in randomized trials? A cross-sectional study. Trials 12, 58, doi: 10.1186/1745-6215-12-58 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. & Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. (updated March 2011). Available at: http://handbook.cochrane.org/. (Accessed: 12 September 2011)
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560, doi: 10.1136/bmj.327.7414.557 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brok J., Thorlund K., Wetterslev J. & Gluud C. Apparently conclusive meta-analyses may be inconclusive–Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 38, 287–298, doi: 10.1093/ije/dyn188 (2009). [DOI] [PubMed] [Google Scholar]
- Wetterslev J., Thorlund K., Brok J. & Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Int J Epidemiol 61, 64–75, doi: 10.1016/j.jclinepi.2007.03.013 (2008). [DOI] [PubMed] [Google Scholar]
- Wetterslev J., Thorlund K., Brok J. & Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol 9, 86, doi: 10.1186/1471-2288-9-86 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.