Stuendl et al. show that CSF exosomes of patients with Parkinson’s disease or dementia with Lewy bodies contain α-synuclein and induce α-synuclein aggregation in a reporter cell line. Thus, exosomes may support inter-neuronal transmission of α-synuclein pathology. CSF exosomal α-synuclein may serve as a biomarker in α-synuclein-related neurodegeneration.
Keywords: exosomes, α-synuclein, extracellular vesicles, Parkinson’s disease, cerebrospinal fluid
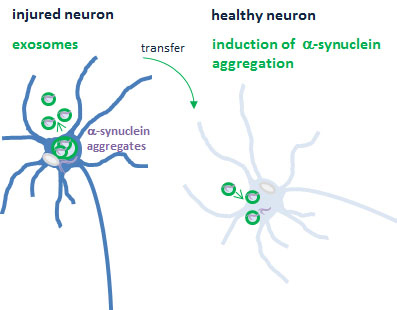
Stuendl et al. show that CSF exosomes of patients with Parkinson’s disease or dementia with Lewy bodies contain α-synuclein and induce α-synuclein aggregation in a reporter cell line. Thus, exosomes may support inter-neuronal transmission of α-synuclein pathology. CSF exosomal α-synuclein may serve as a biomarker in α-synuclein-related neurodegeneration.
Abstract
Extracellular α-synuclein has been proposed as a crucial mechanism for induction of pathological aggregate formation in previously healthy cells. In vitro, extracellular α-synuclein is partially associated with exosomal vesicles. Recently, we have provided evidence that exosomal α-synuclein is present in the central nervous system in vivo. We hypothesized that exosomal α-synuclein species from patients with α-synuclein related neurodegeneration serve as carriers for interneuronal disease transmission. We isolated exosomes from cerebrospinal fluid from patients with Parkinson’s disease, dementia with Lewy bodies, progressive supranuclear palsy as a non-α-synuclein related disorder that clinically overlaps with Parkinson’s disease, and neurological controls. Cerebrospinal fluid exosome numbers, α-synuclein protein content of cerebrospinal fluid exosomes and their potential to induce oligomerization of α-synuclein were analysed. The quantification of cerebrospinal fluid exosomal α-synuclein showed distinct differences between patients with Parkinson’s disease and dementia with Lewy bodies. In addition, exosomal α-synuclein levels correlated with the severity of cognitive impairment in cross-sectional samples from patients with dementia with Lewy bodies. Importantly, cerebrospinal fluid exosomes derived from Parkinson’s disease and dementia with Lewy bodies induce oligomerization of α-synuclein in a reporter cell line in a dose-dependent manner. Our data suggest that cerebrospinal fluid exosomes from patients with Parkinson’s disease and dementia with Lewy bodies contain a pathogenic species of α-synuclein, which could initiate oligomerization of soluble α-synuclein in target cells and confer disease pathology.
Introduction
Parkinson’s disease is a neurodegenerative disorder with motor symptoms that affects ∼1% of the population over 65 years of age (Pringsheim et al., 2014). It is characterized by the progressive loss of dopaminergic neurons in the substantia nigra and pathological aggregation of the intrinsically disordered protein α-synuclein into Lewy bodies (Spillantini et al., 1998; Braak et al., 2003a). Parkinson’s disease and other neurodegenerative diseases with Lewy body pathology, such as Parkinson’s disease dementia and dementia with Lewy bodies, are considered as a continuum of a disease spectrum termed Lewy body disorders (Lippa et al., 2007). Clinically, dementia with Lewy bodies is defined by dementia, visual hallucinations and fluctuating attention within 12 months of Parkinson syndrome onset. In contrast, the onset of dementia later than 12 months after initial motor symptoms specifies Parkinson’s disease dementia (McKeith et al., 2005b).
The accurate distinction between Parkinson’s disease, dementia with Lewy bodies and other non-α-synuclein variants with Parkinson syndrome is challenging due to an overlap of clinical symptoms and neuropathological changes. Several comprehensive studies have identified imaging and fluid biomarkers, including dopamine transporter scans, serum peptide markers, and CSF α-synuclein (Mollenhauer and Schlossmacher, 2010; Suzuki et al., 2015).
Although α-synuclein does not contain a sorting signal for extracellular release, soluble and aggregated α-synuclein was detected in tissue culture medium and body fluids, such as brain interstitial fluid, plasma and CSF (El-Agnaf et al., 2003, 2006; Lee et al., 2005, 2014; Tokuda et al., 2010; Emmanouilidou et al., 2011; Hansson et al., 2014). Extracellular α-synuclein was subsequently studied as a potential diagnostic biomarker, especially in the CSF, where the majority of α-synuclein is derived from the CNS rather than from peripheral blood (Mollenhauer et al., 2012). Most studies have shown a reduction of CSF α-synuclein levels in Parkinson’s disease and dementia with Lewy bodies (Tokuda et al., 2006; Hong et al., 2010; Mollenhauer et al., 2011); however, conflicting results with either no differences compared to controls or even increased levels of extracellular α-synuclein were reported (Noguchi-Shinohara et al., 2009; Ohrfelt et al., 2009; Reesink et al., 2010). In addition, the sensitivity and specificity of CSF α-synuclein to distinguish Parkinson’s disease or dementia with Lewy bodies from non-α-synuclein related Parkinson syndrome and other neurological controls are low and to date, α-synuclein has not been approved as a biomarker for clinical applications (Gao et al., 2015).
Extracellular α-synuclein was recently implied in the prion-like transmission of pathological α-synuclein from diseased to healthy neurons where misfolded α-synuclein might serve as a seed to induce the aggregation of soluble α-synuclein. This concept of interneuronal spreading is based on Braak’s observation that α-synuclein pathology in the brain propagates caudo-rostrally along axonal projections (Braak et al., 2003b). This hypothesis was further supported by the detection of α-synuclein aggregates in transplanted embryonic neurons in Parkinson’s disease patients’ brains (Li et al., 2008; Kordower and Brundin, 2009b), the observation of neuron-to-neuron transfer of α-synuclein in mouse brain (Desplats et al., 2009), internalization of exogenous α-synuclein fibrils and induction of neuronal α-synuclein aggregation in vitro and in vivo (Emmanouilidou et al., 2010; Nonaka et al., 2010; Volpicelli-Daley et al., 2011; Danzer et al., 2012a; Luk et al., 2012a, b; Mougenot et al., 2012). Recently, exosomes have been implicated in the dissemination of misfolded proteins in a variety of neurodegenerative disorders, including Parkinson’s disease (Bellingham et al., 2012; Schneider and Simons, 2013). Exosomes are extracellular vesicles of 40–120 nm diameters that are released from various cells including neurons (Faure et al., 2006). Exosomal release and transport of α-synuclein was reported by us and several other groups in vitro and in vivo (Emmanouilidou et al., 2010; Danzer et al., 2012b), followed by intracellular uptake and the induction of toxicity and cell death.
The aim of our study was to characterize exosomal α-synuclein in CSF from patients with α-synuclein-related neurodegeneration (Parkinson’s disease, dementia with Lewy bodies), patients with Parkinson syndrome without α-synuclein related degeneration (progressive supranuclear palsy) and neurological controls.
Materials and methods
Reagents
Primary antibodies were anti-Flotillin-2 (BD Biosciences), anti-Calnexin (Sigma-Aldrich), anti-α-synuclein (Invitrogen), anti-α-synuclein clone 42/α-synuclein (BD Transduction Laboratories). MJF-1 clone 12.1 was kindly provided by Dr Liyu Wu, Epitomics, Burlingame, USA. Secondary antibodies against mouse, rabbit and human IgG were obtained from Dako, Dianova and Invitrogen.
Plasmids
α-Synuclein hGLuc1 (S1) and α-synuclein hGLuc2 (S2) constructs were described previously (Outeiro et al., 2008).
Cerebrospinal fluid collection
CSF specimens were collected at the Paracelsus-Elena Klinik in Kassel and the Göttingen University Memory Clinic, Department of Psychiatry, Germany [Institutional review board (IRB) approval by the local board of Hessen, Germany, IRB 09/07/04 and 26/07/02 and by the Ethics committee of the University Medical Centre, Göttingen, IRB 02/05/09] between 2009 and 2012 by lumbar puncture between 9 and 12 am. Specimens were collected in polypropylene tubes and centrifuged at 2000g for 10 min at room temperature, aliquoted and frozen at −80°C within 30 min of completion of the procedure (Mollenhauer et al., 2011). Samples with erythrocyte counts >50/mm3 were excluded. All samples were obtained in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
The cross-sectional Kassel cohort
The cohort is described in detail in Mollenhauer et al. (2011). All patients with Parkinson’s disease fulfilled UK Brain Bank criteria. Patients with dementia with Lewy body were diagnosed according to McKeith consensus criteria (McKeith et al., 2005a). Patients with progressive supranuclear palsy fulfilled the National Institute of Neurological Disorders and Stroke-Society for Progressive Supranuclear Palsy (NINDS-SPSP) criteria for possible or probable disease (Litvan et al., 1996). None of the neurological control patients suffered from Parkinson’s disease or dementia.
The DeNoPa cohort
Recruitment and diagnostic assessments of the longitudinal de novo Parkinson (DeNoPa) cohort have been published (Mollenhauer et al., 2013a). In brief, 159 patients had been diagnosed with Parkinson’s disease according to the UK Brain Bank criteria and were drug naïve at enrolment. One hundred and ten neurologically healthy controls matched according to age, gender and educational level without family history of Parkinson’s disease were enrolled in parallel. Clinical diagnosis at baseline was confirmed after a 2-year follow-up. Ninety-four per cent of subjects returned for follow-up investigations after 24 months; in 84% the clinical diagnosis was confirmed by follow-up. CSF was gained by lumbar puncture in the morning (between 8 and 9 am with the patients fastened in a sitting position). CSF (14 ml) was drawn in polypropylene tubes and processed by centrifugation and freezing within 30 min after lumbar puncture according to the published standard operating procedure (Mollenhauer et al., 2010). Baseline CSF samples of 76 patients with Parkinson’s disease and 58 healthy controls who had finalized the first follow-up with normal routine CSF parameters (i.e. haemoglobin, protein content), were randomly selected from this cohort.
Purification of exosomes from cerebrospinal fluid
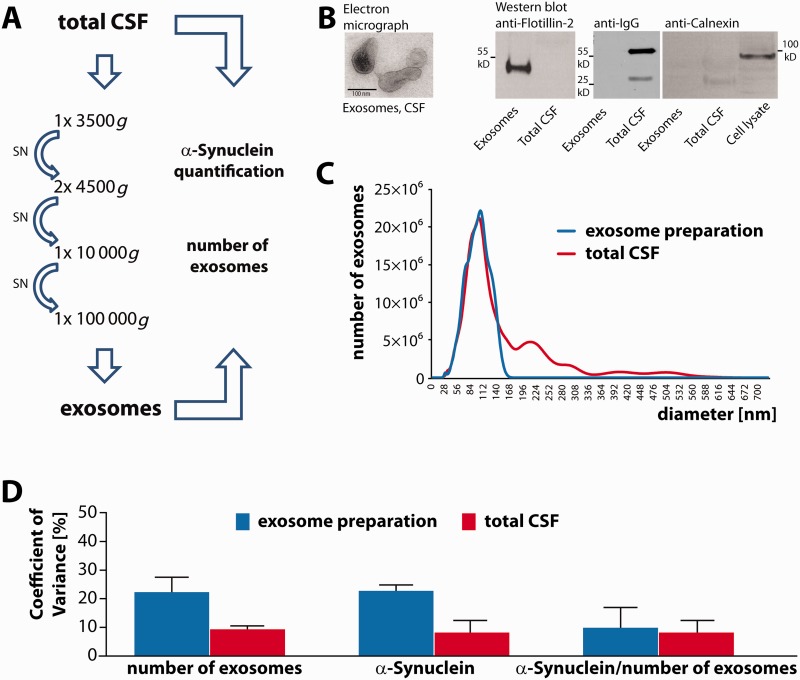
Exosomes were isolated as described previously (Strauss et al., 2010). CSF was thawed on ice and subjected to subsequent centrifugation steps at 4°C: 3500g for 10 min, two times 4500g for 10 min, 10 000g for 30 min and 100 000g for 60 min (Fig. 1A). The 100 000g pellet was washed once with phosphate-buffered saline (PBS) at 100 000g for 60 min before resuspension. Exosomes from human CSF for western blot analysis were prepared from 2.5 ml CSF and resuspended in Lämmli buffer. Exosomes for electrochemiluminescence measurements were dissolved in 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 5 mM EDTA, 50 mM Tris-HCl, pH 8.0 lysis buffer. Total CSF was diluted in 2× lysis buffer.
Figure 1.
Isolation and characterization of exosomes from CSF. (A) Exosomes were isolated by subsequent centrifugation rounds including a final 100 000g ultracentrifugation step from a CSF volume of 0.5 ml. Exosome numbers and α-synuclein content were quantified in total CSF and exosome fractions by nanoparticle tracking analysis and by electrochemoluminescence assay. (B) Left: Electron microscopy of the 100 000g exosome pellet derived from 4 ml of CSF. Scale bar = 100 nm. Right: For western blot analysis exosome pellets were prepared from 2.5 ml of CSF and resuspended in 20 µl of sample buffer. CSF was diluted 1:5 in sample buffer and 20 µl of the exosome preparation and 20 µl of total CSF were probed with an antibody against the exosomal marker protein flotillin-2 (left panel). As a negative control, we probed exosome preparations and CSF with a secondary antibody against human IgG (middle panel). To rule out microsomal contamination of the exosome preparation, 20 µl of the exosome pellet, total CSF and of a cell lysate of mouse neuroblastoma N2a cells were blotted and incubated with an antibody against the endoplasmatic reticulum protein calnexin (right panel). (C) Exosome numbers were determined by nanoparticle tracking analysis in both, total CSF and the 100 000g exosome pellet derived from 0.5 ml CSF after resuspension in PBS. A representative plot depicting vesicle size and number of vesicles is shown (total CSF: red curve; corresponding exosome pellet: blue curve; peak: 102 nm). The values were adjusted for the respective dilution factors and calculated to represent the absolute vesicle numbers in 1 ml of CSF and in exosomes derived from the same CSF volume. (D) The coefficient of variance was determined for the number of exosomes in the exosome preparation and in total CSF, for the amount of exosomal α-synuclein protein and total CSF α-synuclein protein, and for the ratio of exosomal α-synuclein protein levels to the number of exosomes. Blue bars: measurements in the exosome preparation; red bars: measurements in total CSF. CSF samples from two different patients were analysed in replicates of n = 3 and n = 4.
Nanoparticle tracking analysis
Exosomes in CSF or in the ultracentrifugation pellet were analysed by nanoparticle tracking analysis with a NanoSight LM10 instrument and a LM14 viewing unit equipped with a 532 nm laser (NanoSight Ltd). Total CSF samples were diluted 1:4 to 1:40 in PBS (Gibco) to a final volume of 400 µl prior to analysis. Pellets from 100 000g centrifugation derived from 0.5 ml total CSF were resuspended in 50 µl PBS and diluted 1:40 in PBS. Samples were recorded in triplicates (DeNoPa cohort) or six times (Kassel cohort) for 30 s. Particle numbers were then analysed with the Nanoparticle Tracking Analysis (NTA) 2.3 software.
Electrochemiluminescence assay for α-synuclein quantification
Quantification of α-synuclein protein in CSF and in exosomes prepared from CSF was performed as described (Kruse et al., 2012). Standard 96-well plates (Meso Scale Discovery) were coated overnight at 4°C with 3 µg/ml antibody MJF-1 clone 12.1 in PBS. After washing three times with 150 µl PBS + 0.05% Tween-20, plates were blocked with 150 µl 1% bovine serum albumin (Meso Scale Discovery) for 1 h under shaking at 300 rpm at room temperature. After washing three times with recombinant α-synuclein standards (kindly provided by Dr Omar El-Agnaf, Hamad Bin Khalifa University, Doha, Qatar), samples were applied in duplicate for 1 h at room temperature and under shaking at 700 rpm. After washing three times, Sulfo-TAG-labelled anti-α-synuclein clone 42 (1 µg/ml) was added for 1 h at room temperature and 700 rpm shaking. After washing three times 2× Read Buffer T (Meso Scale DiscoveryTM) was applied to each well and plates were measured in a Sector Imager 6000 (Meso Scale DiscoveryTM). Data analysis was performed with the MSD Discovery Workbench 3.0 Data Analysis Toolbox.
Quantification of CSF tau levels
The concentrations of total CSF tau were determined by a commercially available validated enzyme-linked immunosorbent assay (ELISA) (Innogenetics) according to the manufacturer’s instructions.
Cell culture and transfections
For oligomerization induction experiments, human H4 neuroglioma cells (Cell Lines Service) were maintained in Opti-MEM™ medium supplemented with 10% foetal bovine serum (both from Life Technologies) and incubated at 37°C and 5% CO2. Cells were plated 24 h prior to transfection. Transfection was performed at 80–90% confluency using Superfect (Qiagen) with equimolar ratios of plasmids according to the manufacturer’s instructions. After 2 h transfection mix was removed from cells and replaced by fresh Opti-MEM™ medium supplemented with 10% foetal bovine serum containing purified exosomes derived from CSF from patients with dementia with Lewy body, patients with Parkinson’s disease or neurological controls. After 12 h, the oligomerization of α-synuclein was measured using Gaussia luciferase protein-fragment complementation assay as described in Danzer et al. (2012a) and below.
Gaussia luciferase protein-fragment complementation assay
Complementation pairs of α-synuclein S1 and S2 wild-type were transfected into H4 cells in a 96-well plate format as described above. Twelve hours after transfection cells were washed with PBS and replaced with serum- and phenol-red free media. Luciferase activity from protein complementation was measured in live cells in an automated plate reader at 480 nm following the injection of the cell permeable substrate, Coelenterazine (20 µM) (p.j.k) with a signal integration time of 2 s.
Electron microscopy
Exosomes were prepared from CSF as described above, pelleted to glow-discharged Formvar-carbon-coated copper grids and fixed with 4% paraformaldehyde. The grids were negatively stained with 2% uranyl acetate containing 0.7 M oxalate, pH 7.0, and imaged with a LEO EM912 Omega electron microscope (Zeiss). Digital micrographs were obtained with an on-axis 2048 CCD camera (Proscan).
Data and statistical analysis
Data were analysed blinded to the diagnosis. Statistical analysis was performed using SPSS statistics software (SPSS-Statistics 17.0,) and Microsoft Excel software. Correlation analysis was performed by using Pearson’s correlation. Receiver operating characteristic (ROC) curves were used to evaluate sensitivity and specificity relationships to determine the diagnostic performance of exosomal α-synuclein as a diagnostic test.
Results
Our analysis included the quantification of CSF exosome numbers by nanoparticle tracking, measurement of CSF exosomal α-synuclein by a well-established electrochemiluminescence assay (Kruse et al., 2012) and determination of the potential of CSF exosomes to induce the oligomerization of α-synuclein in a split luciferase reporter cell assay (Danzer et al., 2009).
Isolation of exosomes from cerebrospinal fluid
We have previously shown that exosomes can be prepared from CSF by subsequent centrifugation rounds followed by a final 100 000g ultracentrifugation step (Kunadt et al., 2015) (Fig. 1A and B). The ultracentrifugation pellet contained vesicles of 40 to 120 nm diameters with the typical cup-shaped morphology of exosomes (Fig. 1B). The CSF exosome fraction was immunoreactive for the exosomal marker protein flotillin-2 (Fig. 1B) whereas in total CSF, flotillin-2 was only detectable at a much higher exposure time (data not shown). In contrast, the heavy and light chains of immunoglobulin G (IgG) were abundant in total CSF but absent from the CSF exosome fraction (Fig. 1B). In addition, the endoplasmatic reticulum protein calnexin was not detected in the CSF exosome preparation, indicating the absence of microsomal contamination (Fig. 1B). We have already demonstrated by western blot that CSF exosome preparations from 5 ml starting volume contain α-synuclein (Kunadt et al., 2015). However, for quantification of exosomal α-synuclein from smaller CSF volumes as low as 0.5 ml a more sensitive detection method was needed. We have previously described an optimized ELISA that is based on an electrochemiluminescence (ECL) platform and allows for CSF α-synuclein quantification with a large dynamic range and high sensitivity (Kruse et al., 2012). Exosomes were prepared from 0.5 ml CSF and the number and size distribution of vesicles in total CSF and in the CSF exosome preparations were measured by nanoparticle tracking analysis (NTA) (Sokolova et al., 2011; Oosthuyzen et al., 2013; van der Pol et al., 2014). A representative graph is depicted in Fig. 1C and shows vesicles in the range from 0 to 550 nm in total CSF (red line). In contrast, the exosome preparation predominantly consists of vesicles in the size range of exosomes (blue line). In addition, we quantified α-synuclein protein levels in CSF and CSF exosome preparations by an electrochemiluminescence assay using MJF-1 clone 12.1 as a capture and SULFO-TAG-labelled anti-α-synuclein clone 42 as a detection antibody (Kruse et al., 2012). This assay has an average lower limit of detection of 5 pg/ml α-synuclein, which allows the quantification of exosomal α-synuclein levels from a CSF starting volume as low as 0.5 ml. We next verified the reproducibility of these quantification methods (Fig. 1D). Exosomes were prepared from several aliquots of the same sample and exosome numbers as well as α-synuclein protein levels were measured in total CSF and in the exosome preparation. This was repeated with a second series of aliquots from an independent CSF sample. Coefficient of variance (CV) was <10% for the number of exosomes (9.4%) and for α-synuclein protein (8.2%) in total CSF. Coefficient of variance values for the same measurements carried out in the exosome preparations were 22.3% for both the number of exosomes and for exosomal α-synuclein (Fig. 1D). Coefficient of variance values were also calculated for the ratio of exosomal α-synuclein protein levels to the number of exosomes (9.9% for the determination of exosome numbers in the exosome pellet and 8.3% for the quantification of exosome numbers in the starting volume of total CSF). These data show that our exosome preparation is robust and suitable for quantitative analysis in clinical samples.
Quantification of exosomal α-synuclein in cerebrospinal fluid samples from early stage Parkinson’s disease
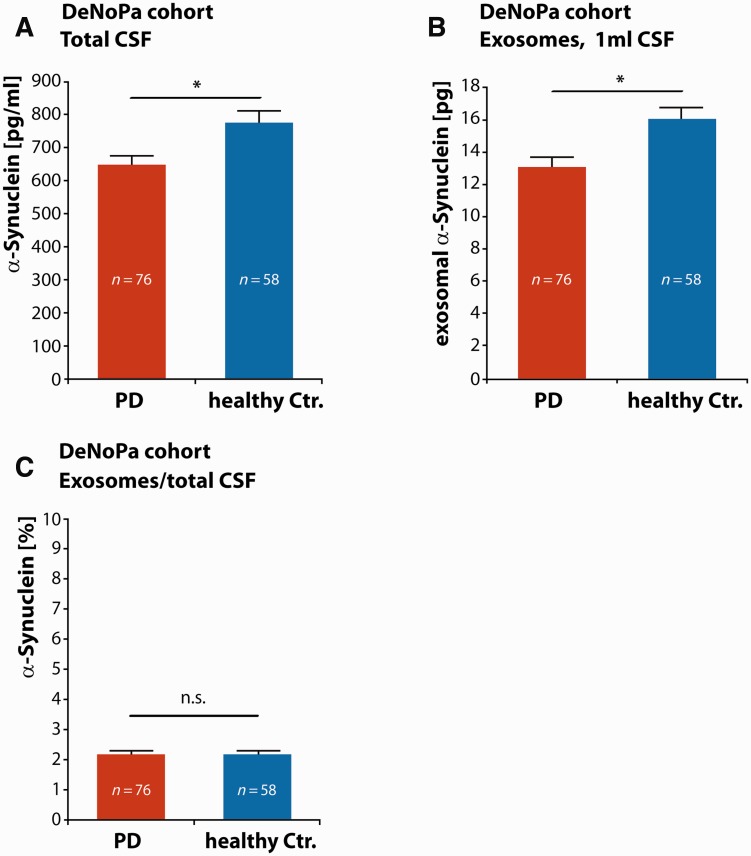
We first characterized exosomal α-synuclein levels in early stage Parkinson’s disease. Our single centre, prospective and longitudinal study cohort ‘DeNoPa’ comprises 159 early, at enrolment drug-naïve Parkinson’s disease subjects and 110 healthy controls (Mollenhauer et al., 2013b). From this cohort we selected 76 patients with Parkinson’s disease and 58 age- and gender-matched healthy control subjects (Supplementary Table 1). We prepared exosomes from 0.5 ml baseline CSF samples and quantified α-synuclein levels in the total CSF (Fig. 2A) as well as in the exosome preparation (Fig. 2B). As previously described, total α-synuclein levels in CSF were significantly lower in the Parkinson’s disease group compared to healthy controls (Parkinson’s disease: mean α-synuclein = 647.88 pg/ml, SEM = 26.95 pg/ml, n = 76; healthy controls: mean α-synuclein = 774.64 pg/ml, SEM = 36.13 pg/ml, n = 58; *P < 0.05, Student’s two-tailed t-test) (Fig. 2A). When we compared the absolute amounts of CSF exosomal α-synuclein between Parkinson’s disease and healthy control samples (Fig. 2B), we found a small but significant difference, with lower levels in the Parkinson’s disease group (Parkinson’s disease mean = 13.04 pg in exosomes derived from 1 ml CSF, SEM = 0.61 pg, n = 76; healthy controls mean = 16.01 pg in exosomes prepared from 1 ml CSF, SEM = 0.98 pg, n = 58, *P < 0.05, Student’s two-tailed t-test) (note that exosome preparations were performed from 0.5 ml CSF in this and all subsequent experiments; all numbers were normalized to 1 ml CSF starting volume after quantification). Next, we calculated the ratio of exosomal α-synuclein to total α-synuclein present in the starting volume of CSF (i.e. α-synuclein encapsulated within exosomes plus vesicle-free CSF α-synuclein). Interestingly, we found that only 2.17% (SEM = 0.12%) of CSF α-synuclein is present in exosomes in CSF from Parkinson’s disease patients and healthy controls (Parkinson’s disease mean = 2.17%, SEM = 0.12%, n = 76; healthy controls mean = 2.17%, SEM = 0.12%, n = 58, Student’s two-tailed t-test) (Fig. 2C).
Figure 2.
Quantification of exosomal α-synuclein in CSF samples from the DeNoPa cohort. (A) CSF concentrations of α-synuclein in total CSF were determined [red bar: Parkinson’s disease (PD) patients, blue bar: healthy control patients], n = 76 Parkinson’s disease patients, n = 58 healthy control patients, *P < 0.05, two-sided Student’s t-test. (B) Exosomal α-synuclein protein levels were quantified in exosomes prepared from 0.5 ml CSF starting material and levels were normalized to 1 ml (red bar: Parkinson’s disease patients, blue bar: healthy control patients), n = 76 Parkinson’s disease patients, n = 58 healthy control patients, *P < 0.05, two-sided Student’s t-test. (C) The ratio of exosomal α-synuclein (prepared from 0.5 ml CSF and normalized to 1 ml) to α-synuclein in total CSF (normalized to 1 ml) was determined in samples from patients with Parkinson’s disease (red bar) and healthy controls (blue bar) and is given in %, n = 76 Parkinson’s disease patients, n = 58 healthy control patients, n.s. = not significant, two-sided Student’s t-test.
CSF exosomal α-synuclein distinguishes between Parkinson’s disease, dementia with Lewy bodies and neurological controls and correlates with cognitive impairment
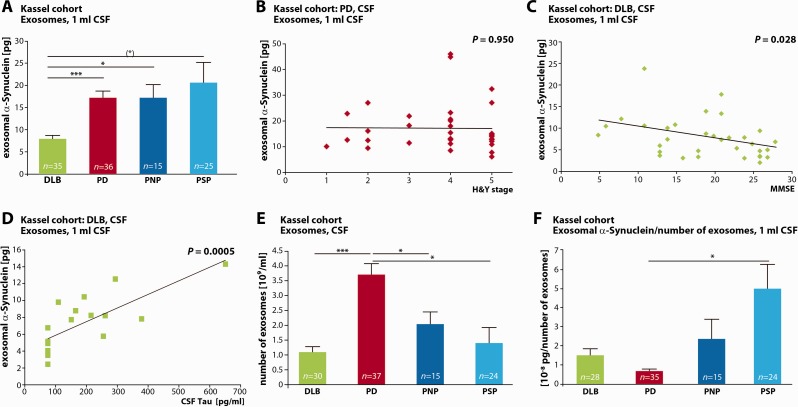
We next examined CSF exosomal α-synuclein in more progressed disease stages. To this end we prepared CSF exosomes from 37 clinically diagnosed patients with Parkinson’s disease with different grades of motor symptoms as assessed by Hoehn and Yahr staging (mean Hoehn and Yahr stage = 3.9, SEM = 0.20). All samples were randomly selected from the ‘Kassel cohort’ and complemented with two different neurological control groups (Supplementary Table 2 andSupplementary Fig. 1) (Mollenhauer et al., 2011). One group consisted of 15 patients with gait problems due to polyneuropathy but without Parkinson’s disease. A second group contained 25 patients with progressive supranuclear palsy, which is characterized by a Parkinson syndrome without underlying α-synuclein but known tau pathology. When we quantified absolute amounts of α-synuclein protein in the exosome fraction of equalized CSF starting volumes we found no significant differences between this more advanced Parkinson’s disease, polyneuropathy and progressive supranuclear palsy groups (one-way ANOVA, P = 0.000691. Parkinson’s disease mean = 17.20 pg in exosomes derived from 1 ml CSF, SEM = 1.50 pg, n = 36; polyneuropathy control mean = 17.20 pg in exosomes derived from 1 ml CSF, SEM = 2.97 pg, n = 15, progressive supranuclear palsy control mean = 20.60 pg in exosomes derived from 1 ml CSF, SEM = 4.56 pg, n = 25, no significant differences, Games-Howell post hoc test) (Fig. 3A). This finding is in contrast to the small difference in exosomal α-synuclein observed between very early Parkinson’s disease and healthy controls in the DeNoPa cohort. It is possible that CSF exosomal α-synuclein levels could increase during disease progression. However, in the Kassel cohort with more advanced disease stages, no correlation was observed between exosomal α-synuclein and the severity of motor symptoms as assessed by Hoehn and Yahr staging (Pearson’s coefficient, r = −0.011, P = 0.950) (Fig. 3B). Another possible reason for the discrepancy between the two cohorts could be the differences in the control groups which consisted of neurologically healthy controls in one (Fig. 2) and patients with polyneuropathy in the second group (Fig. 3), who were referred to the movement disorder clinic in Kassel due to gait disturbances and symmetric positional tremor mimicking Parkinson’s disease. Although a Parkinson’s disease was ruled out by standardized levodopa testing, smell test and brainstem ultrasound, it is still possible that some of these patients had an early stage of Parkinson’s disease. Furthermore, we cannot rule out that the lack of a difference in exosomal α-synuclein between Parkinson’s disease and neurological controls is caused by the relatively smaller number of samples tested.
Figure 3.
Characterization of exosomal α-synuclein in dementia with Lewy bodies, Parkinson’s disease and non α-synuclein-related disease controls in the Kassel cohort. (A) Quantification of exosomal α-synuclein protein content. Exosomes were prepared from CSF samples from patients with dementia with Lewy bodies (DLB, green bar), Parkinson’s disease (PD, red bar), polyneuropathy (PNP, dark blue bar) and progressive supranuclear palsy (PSP, light blue bar), n = 35 DLB, n = 36 PD, n = 15 PNP control, n = 25 PSP control. (*) P = 0.05, *P < 0.05, ***P < 0.0005, Games-Howell post hoc test. One-way ANOVA P = 0.000691. For ROC curve analysis, see Supplementary Fig. 2 andSupplementary Table 3A. (B) Exosomal α-synuclein amounts do not correlate with the severity of motor symptoms determined by Hoehn and Yahr staging (H&Y) in Parkinson’s disease (n = 36, Pearson’s correlation coefficient r = −0.011, P = 0.950, two-tailed probability). (C) Correlation of CSF exosomal α-synuclein with impaired cognitive function in patients with dementia with Lewy bodies. Cognitive state was measured by MMSE (lower scores indicate a decrease in cognitive function) (n = 32, Pearson’s correlation coefficient r = −0.382, P = 0.028, two-tailed probability). (D) Correlation of CSF exosomal α-synuclein with increasing levels of CSF total tau indicating more progressed neuronal degeneration in patients with dementia with Lewy bodies (n = 17, Pearson’s correlation coefficient r = 0.751, P = 0.0005, two-tailed probability). (E) The number of exosomes per ml CSF was quantified by nanoparticle tracking analysis in CSF from patients with dementia with Lewy bodies (green bar), Parkinson’s disease (red bar), polyneuropathy (dark blue bar) and progressive supranuclear palsy (light blue bar) (n = 30 DLB, n = 37 PD, n = 15 PNP, n = 24 PSP, *P < 0.05, ***P < 0.0005, Games-Howell post hoc test. One-way ANOVA P < 0.0001). (F) Ratio of exosomal α-synuclein to number of exosomes in CSF from patients with dementia with Lewy bodies (green bar), Parkinson’s disease (red bar), polyneuropathy (dark blue bar) and progressive supranuclear palsy (light blue bar) (n = 28 DLB, n = 35 PD, n = 15 PNP, n = 24 PSP, *P < 0.05, Games-Howell post hoc test. One-way ANOVA P = 0.000377). For ROC curve analysis, see Supplementary Fig. 2 andSupplementary Table 3B.
We additionally analysed exosomal α-synuclein levels in CSF from 35 patients with dementia with Lewy bodies from both the Kassel and the Göttingen cohorts, as another disease with α-synuclein-related Parkinson syndrome. As shown in Fig. 3A, we found significantly lower CSF exosomal α-synuclein from patients with dementia with Lewy bodies as compared to Parkinson’s disease, polyneuropathy and progressive supranuclear palsy groups [dementia with Lewy bodies mean = 7.92 pg in exosomes derived from 1 ml, SEM = 0.78 pg, n = 35, (*)P = 0.05, *P < 0.05, ***P < 0.0005, Games-Howell post hoc test]. Importantly, exosomal α-synuclein levels correlated inversely with the dementia with Lewy bodies patients’ Mini-Mental State Examination (MMSE) scores (Pearson’s coefficient, r = −0.382, n = 32 for whom MMSE data were available, P = 0.028, two-tailed probability) (Fig. 3C). These data indicate that higher amounts of exosomal α-synuclein in CSF are associated with a lower MMSE performance and therefore with cognitive impairment. It is feasible to assume that during disease progression, an increasing number of neurons contain pathological α-synuclein aggregates, which could be released with exosomes. This notion is further supported by the strong positive correlation of exosomal α-synuclein levels with tau protein levels rising in CSF (Pearson’s coefficient, r = 0.751, n = 17 for whom CSF tau data were available, P = 0.0005, two-tailed probability) (Fig. 3D). Tau is an intraneuronal protein required to maintain the structural integrity of neurons by stabilizing the microtubule cytoskeleton. It is assumed that neuronal damage leads to the release of tau from disintegrating neurons to the CSF. Thus, an increase in CSF total tau levels is generally considered as an unspecific marker of neurodegeneration, e.g. in Alzheimer’s disease and dementia with Lewy bodies (Mollenhauer et al., 2005; Montine et al., 2010; Musiek and Holtzman, 2012; Kang et al., 2013). Therefore, higher levels of exosomal α-synuclein in progressed stages of dementia with Lewy bodies could likely reflect an increased disease activity, although it is not clear why such a correlation between disease progression and exosomal α-synuclein is not observed in Parkinson’s disease. The correlation between increasing exosomal α-synuclein with clinical disease progression and the rise in the neuronal injury marker tau contrasts with the overall lower exosomal α-synuclein levels in the group of patients with dementia with Lewy bodies compared to the other groups.
We next performed a preliminary evaluation of the performance of exosomal α-synuclein as a potential diagnostic biomarker by ROC curve analysis (Supplementary Fig. 2 andSupplementary Table 3A). The sensitivity and specificity of CSF exosomal α-synuclein to distinguish dementia with Lewy bodies from Parkinson’s disease were 85.7% and 80.6% with a positive and negative predictive value of 81.1% and 85.3%, respectively. The sensitivity and specificity for the distinction of dementia with Lewy bodies from polyneuropathy were 85.7% and 66.7%; that for dementia with Lewy bodies versus progressive supranuclear palsy 71.4% and 92.0% (Supplementary Fig. 2 andSupplementary Table 3A).
As depicted in Fig. 3E, the number of exosomes in CSF differs highly between the various diagnostic groups, with nearly 4-fold higher values in Parkinson’s disease as compared to dementia with Lewy bodies (one-way ANOVA, P < 0.0001. Parkinson’s disease mean = 3.69 × 109 exosomes/ml CSF, SEM = 1.83 × 108 exosomes/ml CSF, n = 37; dementia with Lewy bodies mean = 1.08 × 109 exosomes/ml CSF, SEM = 1.83 × 108 exosomes/ml CSF, n = 30, P < 0.000, Games-Howell post hoc test) and ∼2 times higher numbers as compared to polyneuropathy and progressive supranuclear palsy (polyneuropathy mean = 2.03 ×109 exosomes/ml CSF, SEM = 4.09 × 108 exosomes/ml CSF, n = 15; progressive supranuclear palsy mean = 1.39 × 109 exosomes/ml CSF, SEM = 5.23 × 109 exosomes/ml CSF, n = 24, *P < 0.05, ***P < 0.0005 Games-Howell post hoc test). In contrast, no significant differences were observed between the polyneuropathy and progressive supranuclear palsy groups (Fig. 3E).
We next calculated the ratio of exosomal α-synuclein per number of exosomes in the different diagnostic groups (Fig. 3F). Interestingly, an approximately two to three times lower ratio was detected in patients with Parkinson’s disease compared to all other groups (one-way ANOVA P = 0.000377; Parkinson’s disease mean = 6.74 × 10−9 pg α-synuclein/number of exosomes, SEM = 1.04 × 10−9 pg α-synuclein/number of exosomes, n = 35; dementia with Lewy bodies mean = 1.50 × 10−8 pg α-synuclein/number of exosomes, SEM = 3.37 × 10−9 pg α-synuclein/number of exosomes, n = 28; polyneuropathy mean = 2.63 × 10−8 pg α-synuclein/number of exosomes, SEM = 1.03 × 10−8 pg α-synuclein/number of exosomes, n = 15; progressive supranuclear palsy mean = 4.99 × 10−8 pg α-synuclein/number of exosomes, SEM = 1.27 × 10−8 pg α-synuclein/number of exosomes, n = 24, *P < 0.05, Games-Howell post hoc test). In contrast, no significant differences were found between polyneuropathy and progressive supranuclear palsy groups. ROC curve analysis of this marker for the distinction of Parkinson’s disease versus dementia with Lewy bodies, polyneuropathy and progressive supranuclear palsy is shown in Supplementary Fig. 2 and in Supplementary Table 3B. The ratio of CSF exosomal α-synuclein to CSF exosome numbers distinguishes Parkinson’s disease from dementia with Lewy bodies with a sensitivity of 74.3% and a specificity of 60.7% (Supplementary Table 3B).
Taken together, exosomal α-synuclein is significantly decreased in CSF from patients with dementia with Lewy bodies compared to neurological controls, mainly due to a lower absolute number of CSF exosomes. Disease progression in dementia with Lewy bodies, defined by the severity of cognitive dysfunction and increasing concentrations of the neuronal injury marker CSF tau, is paralleled by higher exosomal α-synuclein levels (Fig. 3C and D). In Parkinson’s disease, exosome numbers are significantly increased compared to dementia with Lewy bodies and neurological controls with comparable levels of total exosomal α-synuclein and a lower ratio of α-synuclein per exosomal particle (for a graphic summary and model see Fig. 4E).
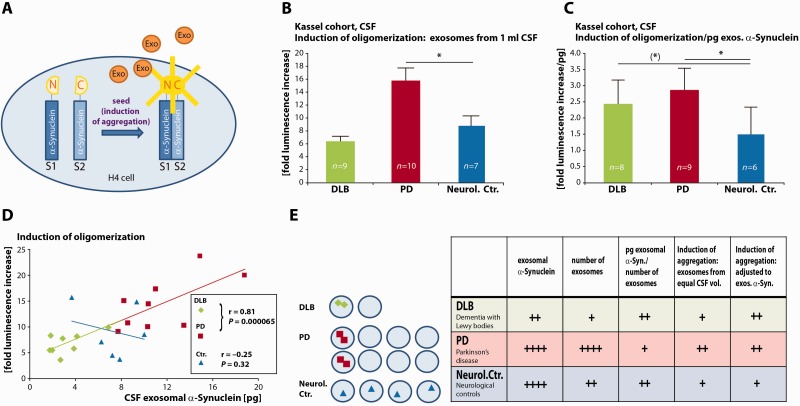
Figure 4.
CSF exosomal α-synuclein from patients with dementia with Lewy bodies and Parkinson’s disease induces the oligomerization of soluble α-synuclein. (A) Illustration of the assay: human neuroglioma H4 cells are co-transfected with α-synuclein fused to split luciferase constructs (S1: N-terminal part of luciferase, S2: C-terminal part of luciferase). A luminescence signal is measured only after complementation of both split luciferase fragments (e.g. during dimerization and oligomerization of α-synuclein) and indicates the induction of α-synuclein aggregation. (B) Luminescence increase in reporter cells upon treatment with exosomes prepared from equal volumes of CSF. CSF was derived from the ‘Kassel cohort’ [dementia with Lewy bodies (DLB) patients: green bar, n = 9; Parkinson’s disease (PD) patients: red bar, n = 10 and neurological controls (Neurol. Ctr.), blue bar, n = 7]. All measurements were performed in duplicates (*P < 0.05, two-sided Student’s t-test, one-way ANOVA P = 0.000513). (C) Ratio of luminescence increase in reporter cells to CSF exosomal α-synuclein protein levels (patients with dementia with Lewy bodies: green bar, n = 8; Parkinson’s disease patients: red bar n = 9; neurological controls: blue bar, n = 6). All measurements were performed in duplicates. [(*)P < 0.10, *P < 0.05, two-tailed Mann-Whitney U-test, one-way ANOVA P = 0.00698]. (D) Correlation of luminescence increase indicative of α-synuclein oligomerization in the reporter cells with exosomal α-synuclein levels (prepared from 1 ml CSF) (dementia with Lewy bodies: green diamonds, n = 8, Parkinson’s disease: red rectangles, n = 9; neurological controls: blue triangles, n = 6). Pearson correlation coefficient: dementia with Lewy bodies and Parkinson’s disease r = 0.85, P = 1.6 × 10−5; neurological controls r = −0.25, P = 0.64. (E) Model (left) and summary table (right) of exosomal α-synuclein in different diseases (left). Exosomal α-synuclein in dementia with Lewy bodies: green diamonds, in Parkinson’s disease: red rectangles, in neurological controls: blue triangles. Depicting α-synuclein symbols as dimers in dementia with Lewy bodies and Parkinson’s disease exosomes indicates their potential to act as a seed to induce aggregate formation.
CSF exosomal α-synuclein from patients with Parkinson’s disease and dementia with Lewy bodies induces the oligomerization of α-synuclein
Exosomes could contribute to spreading of α-synuclein disease pathology by the transfer of α-synuclein oligomers to healthy neurons where they could induce the oligomerization of soluble intracellular α-synuclein. We have previously shown that exosomal α-synuclein oligomers are efficiently internalized into target cells (Danzer et al., 2012b). We therefore quantified the seeding potential of CSF exosomes to induce oligomerization of α-synuclein in a cell line. Exosomes were prepared from CSF from patients with dementia with Lewy bodies, Parkinson’s disease and neurological controls. Controls included six patients with polyneuropathy, one patient with normal pressure hydrocephalus and one with corticobasal degeneration. All CSF samples were randomly selected from the ‘Kassel cohort’. Induction of oligomerization was measured by a split luciferase fragment complementation technique, which is one of the most sensitive and dynamic detection methods to study protein-protein interactions (Luker and Piwnica-Worms, 2004; Danzer et al., 2012b). In short, luciferase is split into two non-bioluminiscent fragments S1 and S2, and fused with a short linker to the C-terminus of α-synuclein (Fig. 4A). Interaction of α-synuclein molecules, e.g. during oligomerization and higher order aggregation, brings both luciferase fragments into close proximity. The resulting luciferase complementation can be measured as a luminescence signal in the presence of the substrate (Fig. 4A). Unspecific complementation of both luciferase fragments had previously been excluded by a series of experiments where no complementation was shown for the combination of α-synuclein S1 with amyloid-β fused to the C-terminal fragment of luciferase nor for α-synuclein S2 and amyloid-β fused to the N-terminal fragment of luciferase (Danzer et al., 2011). As a reporter cell line we used H4 neuroglioma cells co-transfected with the split luciferase α-synuclein constructs S1 and S2. The reporter assay was previously characterized by size exclusion chromatography and revealed the detection of α-synuclein S1/S2 multimers in the size range of dimers to 30mers (Danzer et al., 2011). The activity change of the bioluminescence signal induced by treatment with CSF-derived exosomes was quantified as ‘fold luminescence increase’ compared to mock treated control, e.g. cells that were exposed to exosomes but were treated with transfection reagent without the plasmids coding for S1/S2 α-synuclein. Incubation of the reporter cell line with exosomes prepared from equal amounts of either dementia with Lewy bodies, Parkinson’s disease or neurological control CSF resulted in ∼2-fold increase of the bioluminescence signal in cells treated with Parkinson’s disease-derived exosomes compared to neurological control or dementia with Lewy bodies exosomes (one-way ANOVA, P = 0.000513; dementia with Lewy bodies mean = 6.40-fold increase, SEM = 0.77-fold increase, n = 9; Parkinson’s disease mean = 15.79-fold increase, SEM = 1.73-fold increase, n = 10; control mean = 8.79-fold increase, SEM = 1.58-fold increase, n = 7, *P < 0.05, Student’s two-tailed t-test) (Fig. 4B). This indicates that exosomes in the CSF of Parkinson’s disease patients can serve as seeds to induce the oligomerization of α-synuclein in recipient cells.
As shown above, the overall amount of exosomal α-synuclein and the number of exosomes in CSF from patients with dementia with Lewy bodies is significantly lower compared to Parkinson’s disease and neurological controls. We therefore analysed the potential of CSF-derived exosomes to induce oligomerization after adjusting for CSF exosomal α-synuclein levels (Fig. 4C). The ratio of luminescence increase as a measure for oligomer inducing potential to CSF exosomal α-synuclein protein levels was calculated for each preparation. Interestingly, when adjusted to exosomal α-synuclein content, CSF exosomes derived from patients with dementia with Lewy bodies and Parkinson’s disease had a higher potential to induce α-synuclein oligomerization as CSF exosomes from neurological controls [one-way ANOVA P = 0.00698; dementia with Lewy bodies mean = 2.43-fold increase/pg exosomal α-synuclein, SEM = 1.05-fold increase/pg exosomal α-synuclein, n = 8; Parkinson’s disease mean = 2.87-fold increase/pg exosomal α-synuclein, SEM = 0.95-fold increase/pg exosomal α-synuclein, n = 9; neurological controls mean = 1.50-fold increase/pg exosomal α-synuclein, SEM = 1.19-fold increase/pg exosomal α-synuclein, n = 6, (*)P < 0.1 indicates strong statistical tendency due to small sample sizes, Mann-Whitney U-Test, *P < 0.05, Mann-Whitney U-Test]. When we plotted the oligomerization inducing activity versus exosomal α-synuclein content we found a strong correlation for CSF exosome preparations derived from dementia with Lewy bodies and Parkinson’s disease patients but not for preparations from the neurological control group (Fig. 4D) (dementia with Lewy bodies and Parkinson’s disease group r = 0.85, n = 17, P = 1.6 × 10−5, neurological control group r = −0.25, n = 6, P = 0.64, Pearson’s correlation).
Discussion
The findings from our work address two major aspects: (i) we provide the first comprehensive analysis and quantification of exosomal α-synuclein in CSF from patient cohorts; and (ii) we show that CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies contain a pathogenic α-synuclein species which serves as a seed to induce the oligomerization of soluble α-synuclein in recipient cells. These findings may shed light on several aspects of α-synuclein spreading and clearance in α-synuclein-related disorders. Moreover, replication provided, CSF exosomal α-synuclein may well be suited as a diagnostic marker in the future.
CSF exosomes from patients with dementia with Lewy bodies and Parkinson’s disease induce α-synuclein oligomerization
We and others have demonstrated previously in vivo uptake and functional recovery of exosomal proteins and RNA in neurons (Fruhbeis et al., 2012; El Andaloussi et al., 2013). Exosomal release and transfer of neuron-derived oligomeric α-synuclein was reported in vitro (Danzer et al., 2012a) and we have recently shown first in vivo evidence of exosomal α-synuclein in the CNS (Kunadt et al., 2015). Our study revealed that exosomal α-synuclein can be isolated from human CSF. Interestingly, here we find that CSF exosomes from patients with Parkinson’s disease induce α-synuclein oligomerization in a reporter cell line. CSF from patients with dementia with Lewy bodies contains lower numbers of exosomes compared to Parkinson’s disease and neurological controls. As a result, exosomes isolated from equal CSF volumes from dementia with Lewy bodies and neurological controls do not differ in their activity to induce α-synuclein oligomerization. However, after adjusting to exosomal α-synuclein protein levels, the oligomerization-inducing potential of dementia with Lewy bodies and Parkinson’s disease CSF exosomes was approximately two times higher compared to neurological controls. Furthermore, a linear correlation between exosomal α-synuclein levels and their activity to induce α-synuclein oligomerization was observed for dementia with Lewy bodies and Parkinson’s disease but not for control CSF exosomes. Our results thus suggest that exosomes derived from Parkinson’s disease and dementia with Lewy bodies CSF may contain a pathogenic species of α-synuclein that is distinct from neurological controls (Fig. 4E). It is tempting to speculate that exosomes are enriched for misfolded oligomers of α-synuclein since oligomerization was described to target proteins for exosomal release (Fang et al., 2007). In Parkinson’s disease and dementia with Lewy bodies, pathogenic α-synuclein oligomers may be preferentially sorted into exosomes and act as a seed for α-synuclein fibril growth in recipient cells. Interneuronal transfer of exosomal α-synuclein may thus explain the propagation of pathology along interconnected neuronal tracts, which is observed in α-synuclein-related neurodegeneration (Muller et al., 2005; Kordower and Brundin, 2009a; Li et al., 2010; Lee et al., 2012; Luk et al., 2012a; Ulusoy et al., 2013).
Exosomal secretion of superfluous or toxic cellular content has been described under various physiological and pathophysiological conditions previously (Vidal et al., 1997; Strauss et al., 2010). Therefore, exosomal release of pathological α-synuclein intermediates may represent a cellular response to remove neurotoxic substances, especially in cells with low degradative capacity. Supporting this notion, increased exosomal secretion of α-synuclein was observed after lysosomal inhibition in vitro (Alvarez-Erviti et al., 2011; Danzer et al., 2012b). Interestingly, exosomes are most efficiently internalized into microglia whereas neuronal uptake rates are much lower (Fitzner et al., 2011; Zhuang et al., 2011; Fruhbeis et al., 2013). This suggests that pathogenic α-synuclein may be released by exosomes and subsequently degraded by microglia. However, insufficient microglial clearance function, as observed in many neurodegenerative diseases, may pave the way for interneuronal propagation of pathogenic exosomal α-synuclein species (Luo et al., 2010). Interestingly, the levels of exosomal α-synuclein rise in later disease stages of dementia with Lewy bodies as reflected by deteriorating cognitive function and increasing levels of the neuronal injury marker tau. On a more speculative basis we suggest that the initially decreased levels of exosome numbers and hence exosomal α-synuclein in dementia with Lewy bodies CSF might mirror a clearance deficit for α-synuclein, which might explain the pan-cortical presence of α-synuclein aggregates. It is feasible to assume that during disease progression, more neurons contain pathological α-synuclein aggregates, which could be released with exosomes. Therefore, higher levels of exosomal α-synuclein in progressed stages of dementia with Lewy bodies could likely reflect an increased disease activity and the higher number of affected neurons could contribute to the rise of exosomal α-synuclein levels in CSF. Clearly, this assumption needs to be corroborated by further experimentation, including a comparison of exosomal release rates from cortical neurons in dementia with Lewy bodies and controls, e.g. by using induced pluripotent stem cell-derived cortical neurons from the respective patient groups or by using neuronal subtype-specific exosomal marker proteins, which would allow us to quantify exosomes in CSF, dependent on their cellular origin.
Preliminary evaluation of CSF exosomal α-synuclein as a potential biomarker in α-synuclein-related disorders
We demonstrate that exosomal α-synuclein is present in CSF where it is accessible for quantification as a potential biomarker to detect α-synuclein-related pathology (Fig. 4E). Preliminary ROC curve analysis revealed a high sensitivity and specificity for the discrimination of dementia with Lewy bodies, Parkinson’s disease and neurological controls. Moreover, we detected a highly significant correlation of CSF exosomal α-synuclein with cognition and the neuronal injury marker tau in dementia with Lewy bodies. Thus, CSF exosomal α-synuclein could additionally serve as a surrogate marker to monitor disease progression. Such markers have not been available in the past and could aid future interventional studies in dementia with Lewy bodies. Clearly, further experiments including larger cohorts and independent validation samples are needed to verify the potential of CSF exosomal α-synuclein as a biomarker in Parkinson’s disease and dementia with Lewy bodies.
Interestingly, exosomal α-synuclein was recently characterized in plasma samples from a large cohort of patients with Parkinson’s disease and healthy controls. There, exosomes derived from the CNS were immune-captured by an antibody directed against the neural L1 cell adhesion molecule L1CAM (Shi et al., 2014). Using this approach and in contrast to our findings, Shi et al. (2014) report increased amounts of exosomal α-synuclein in Parkinson’s disease compared to healthy controls. However, this may be caused by increased rates of transport from the CNS to the blood that are described in Parkinson’s disease (Popescu et al., 2009).
In summary, our study is the first detailed analysis of α-synuclein in CSF exosomes from different diseases with α-synuclein-related neurodegeneration. CSF exosomes from patients with α-synuclein-related neurodegeneration possess the capacity to induce oligomerization of soluble α-synuclein in an in vitro model. These data are therefore highly relevant for disease pathogenesis. In addition, upon independent validation in larger cohorts, we propose exosomal α-synuclein as a potential future biomarker in α-synuclein-related neurodegenerative disorders.
Funding
A.S. and M.K. were supported by grants from the German Research foundation Cluster of Excellence ‘Nanoscale Microscopy and Molecular Physiology of the Brain’ (CNMPB) and the Center for Molecular Physiology of the Brain (CMPB). A.S. received funding by the German Research Foundation (Deutsche Forschungsgemeinschaft) grants SCHN1265 2-1 and 1-1. A.S. received funding from ERA-NET EuroNanoMed II, GlioEx consortium. A.S. and B.M. received funds from the Michael J. Fox Foundation of Parkinson’s Research. The DeNoPa study was supported by unrestricted grants from the Paracelsus-Elena-Klinik, Kassel, Germany and from TEVA Pharma.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
Abbreviation
- ROC
receiver operating curve
References
- Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis 2011; 42: 360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham SA, Guo BB, Coleman BM, Hill AF. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol 2012; 3: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003a; 24: 197–211. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 2003b; 110: 517–36. [DOI] [PubMed] [Google Scholar]
- Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 2012a; 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 2012b; 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer KM, Krebs SK, Wolff M, Birk G, Hengerer B. Seeding induced by alpha-synuclein oligomers provides evidence for spreading of alpha-synuclein pathology. J Neurochem 2009; 111: 192–203. [DOI] [PubMed] [Google Scholar]
- Danzer KM, Ruf WP, Putcha P, Joyner D, Hashimoto T, Glabe C, et al. Heat-shock protein 70 modulates toxic extracellular alpha-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J 2011; 25: 326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA 2009; 106: 13010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, et al. Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J 2003; 17: 1945–7. [DOI] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, et al. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J 2006; 20: 419–25. [DOI] [PubMed] [Google Scholar]
- El Andaloussi S, Lakhal S, Mager I, Wood MJ. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev 2013; 65: 391–7. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Elenis D, Papasilekas T, Stranjalis G, Gerozissis K, Ioannou PC, et al. Assessment of alpha-synuclein secretion in mouse and human brain parenchyma. PLoS One 2011; 6: e22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 2010; 30: 6838–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol 2007; 5: e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 2006; 31: 642–8. [DOI] [PubMed] [Google Scholar]
- Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci 2011; 124(Pt 3): 447–58. [DOI] [PubMed] [Google Scholar]
- Fruhbeis C, Frohlich D, Kramer-Albers EM. Emerging roles of exosomes in neuron-glia communication. Front Physiol 2012; 3: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol 2013; 11: e1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Tang H, Nie K, Wang L, Zhao J, Gan R, et al. Cerebrospinal fluid alpha-synuclein as a biomarker for Parkinson's disease diagnosis: a systematic review and meta-analysis. Int J Neurosci 2015; 125: 145–54. [DOI] [PubMed] [Google Scholar]
- Hansson O, Hall S, Ohrfelt A, Zetterberg H, Blennow K, Minthon L, et al. Levels of cerebrospinal fluid alpha-synuclein oligomers are increased in Parkinson's disease with dementia and dementia with Lewy bodies compared to Alzheimer's disease. Alzheimers Res Ther 2014; 6: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain 2010; 133(Pt 3): 713–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, Coffey CS, et al. Association of cerebrospinal fluid beta-amyloid 1-42, T-tau, P-tau181, and alpha-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol 2013; 70: 1277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Brundin P. Lewy body pathology in long-term fetal nigral transplants: is Parkinson's disease transmitted from one neural system to another? Neuropsychopharmacology 2009a; 34: 254. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Brundin P. Propagation of host disease to grafted neurons: accumulating evidence. Exp Neurol 2009b; 220: 224–5. [DOI] [PubMed] [Google Scholar]
- Kruse N, Schulz-Schaeffer WJ, Schlossmacher MG, Mollenhauer B. Development of electrochemiluminescence-based singleplex and multiplex assays for the quantification of alpha-synuclein and other proteins in cerebrospinal fluid. Methods 2012; 56: 514–8. [DOI] [PubMed] [Google Scholar]
- Kunadt M, Eckermann K, Stuendl A, Gong J, Russo B, Strauss K, et al. Extracellular vesicle sorting of alpha-Synuclein is regulated by sumoylation. Acta Neuropathol 2015; 129: 695–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Bae EJ, Lee SJ. Extracellular alpha—synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol 2014; 10: 92–8. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci 2005; 25: 6016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Desplats P, Lee HJ, Spencer B, Masliah E. Cell-to-cell transmission of alpha-synuclein aggregates. Methods Mol Biol 2012; 849: 347–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med 2008; 14: 501–3. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Widner H, Rehncrona S, Bjorklund A, Lindvall O, et al. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson's disease. Mov Disord 2010; 25: 1091–6. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Duda JE, Grossman M, Hurtig HI, Aarsland D, Boeve BF, et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology 2007; 68: 812–9. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996; 47: 1–9. [DOI] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012a; 338: 949–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med 2012b; 209: 975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luker KE, Piwnica-Worms D. Optimizing luciferase protein fragment complementation for bioluminescent imaging of protein-protein interactions in live cells and animals. Methods Enzymol 2004; 385: 349–60. [DOI] [PubMed] [Google Scholar]
- Luo XG, Ding JQ, Chen SD. Microglia in the aging brain: relevance to neurodegeneration. Mol Neurodegener 2010; 5: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005a; 65: 1863–72. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005b; 65: 1863–72. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Cepek L, Bibl M, Wiltfang J, Schulz-Schaeffer WJ, Ciesielczyk B, et al. Tau protein, Abeta42 and S-100B protein in cerebrospinal fluid of patients with dementia with Lewy bodies. Dement Geriatr Cogn Disord 2005; 19: 164–70. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, El-Agnaf OM, Marcus K, Trenkwalder C, Schlossmacher MG. Quantification of alpha-synuclein in cerebrospinal fluid as a biomarker candidate: review of the literature and considerations for future studies. Biomark Med 2010; 4: 683–99. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Doring F, Trenkwalder C, Schlossmacher MG. alpha-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol 2011; 10: 230–40. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Schlossmacher MG. CSF synuclein: adding to the biomarker footprint of dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2010; 81: 590–1. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Trautmann E, Otte B, Ng J, Spreer A, Lange P, et al. alpha-Synuclein in human cerebrospinal fluid is principally derived from neurons of the central nervous system. J Neural Transm 2012; 119: 739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer B, Trautmann E, Sixel-Doring F, Wicke T, Ebentheuer J, Schaumburg M, et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology 2013a; 81: 1226–34. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Trautmann E, Taylor P, Manninger P, Sixel-Doring F, Ebentheuer J, et al. Total CSF alpha-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci Lett 2013b; 532: 44–8. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Shi M, Quinn JF, Peskind ER, Craft S, Ginghina C, et al. CSF Abeta and tau in Parkinson's disease with cognitive impairment. Mov Disord 2010; 25: 2682–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougenot AL, Nicot S, Bencsik A, Morignat E, Verchere J, Lakhdar L, et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol Aging 2012; 33: 2225–8. [DOI] [PubMed] [Google Scholar]
- Muller CM, de Vos RA, Maurage CA, Thal DR, Tolnay M, Braak H. Staging of sporadic Parkinson disease-related alpha-synuclein pathology: inter- and intra-rater reliability. J Neuropathol Exp Neurol 2005; 64: 623–8. [DOI] [PubMed] [Google Scholar]
- Musiek ES, Holtzman DM. Origins of Alzheimer's disease: reconciling cerebrospinal fluid biomarker and neuropathology data regarding the temporal sequence of amyloid-beta and tau involvement. Curr Opin Neurol 2012; 25: 715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi-Shinohara M, Tokuda T, Yoshita M, Kasai T, Ono K, Nakagawa M, et al. CSF alpha-synuclein levels in dementia with Lewy bodies and Alzheimer's disease. Brain Res 2009; 1251: 1–6. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Watanabe ST, Iwatsubo T, Hasegawa M. Seeded aggregation and toxicity of {alpha}-synuclein and tau: cellular models of neurodegenerative diseases. J Biol Chem 2010; 285: 34885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrfelt A, Grognet P, Andreasen N, Wallin A, Vanmechelen E, Blennow K, et al. Cerebrospinal fluid alpha-synuclein in neurodegenerative disorders-a marker of synapse loss? Neurosci Lett 2009; 450: 332–5. [DOI] [PubMed] [Google Scholar]
- Oosthuyzen W, Sime NE, Ivy JR, Turtle EJ, Street JM, Pound J, et al. Quantification of human urinary exosomes by nanoparticle tracking analysis. J Physiol 2013; 591(Pt 23): 5833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Putcha P, Tetzlaff JE, Spoelgen R, Koker M, Carvalho F, et al. Formation of toxic oligomeric alpha-synuclein species in living cells. PLoS One 2008; 3: e1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu BO, Toescu EC, Popescu LM, Bajenaru O, Muresanu DF, Schultzberg M, et al. Blood-brain barrier alterations in ageing and dementia. J Neurol Sci 2009; 283: 99–106. [DOI] [PubMed] [Google Scholar]
- Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2014; 29: 1583–90. [DOI] [PubMed] [Google Scholar]
- Reesink FE, Lemstra AW, van Dijk KD, Berendse HW, van de Berg WD, Klein M, et al. CSF alpha-synuclein does not discriminate dementia with Lewy bodies from Alzheimer's disease. J Alzheimers Dis 2010; 22: 87–95. [DOI] [PubMed] [Google Scholar]
- Schneider A, Simons M. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res 2013; 352: 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, et al. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson's disease. Acta Neuropathol 2014; 128: 639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple M, et al. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B Biointerfaces 2011; 87: 146–50. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci USA 1998; 95: 6469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss K, Goebel C, Runz H, Mobius W, Weiss S, Feussner I, et al. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J Biol Chem 2010; 285: 26279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I, Noguchi M, Arito M, Sato T, Omoteyama K, Maedomari M, et al. Serum peptides as candidate biomarkers for dementia with Lewy bodies. Int J Geriatr Psychiatry 2015; 30: 1195–206. [DOI] [PubMed] [Google Scholar]
- Tokuda T, Qureshi MM, Ardah MT, Varghese S, Shehab SA, Kasai T, et al. Detection of elevated levels of alpha-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 2010; 75: 1766–72. [DOI] [PubMed] [Google Scholar]
- Tokuda T, Salem SA, Allsop D, Mizuno T, Nakagawa M, Qureshi MM, et al. Decreased alpha-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson's disease. Biochem Biophys Res Commun 2006; 349: 162–6. [DOI] [PubMed] [Google Scholar]
- Ulusoy A, Rusconi R, Perez-Revuelta BI, Musgrove RE, Helwig M, Winzen-Reichert B, et al. Caudo-rostral brain spreading of alpha-synuclein through vagal connections. EMBO Mol Med 2013; 5: 1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pol E, Coumans FA, Grootemaat AE, Gardiner C, Sargent IL, Harrison P, et al. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost 2014; 12: 1182–92. [DOI] [PubMed] [Google Scholar]
- Vidal M, Mangeat P, Hoekstra D. Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J Cell Sci 1997; 110 (Pt 16): 1867–77. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011; 72: 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 2011; 19: 1769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.