Abstract
Neurodegeneration is a phenomenon that occurs in the central nervous system through the hallmarks associating the loss of neuronal structure and function. Neurodegeneration is observed after viral insult and mostly in various so-called 'neurodegenerative diseases', generally observed in the elderly, such as Alzheimer's disease, multiple sclerosis, Parkinson's disease and amyotrophic lateral sclerosis that negatively affect mental and physical functioning. Causative agents of neurodegeneration have yet to be identified. However, recent data have identified the inflammatory process as being closely linked with multiple neurodegenerative pathways, which are associated with depression, a consequence of neurodegenerative disease. Accordingly, pro-inflammatory cytokines are important in the pathophysiology of depression and dementia. These data suggest that the role of neuroinflammation in neurodegeneration must be fully elucidated, since pro-inflammatory agents, which are the causative effects of neuroinflammation, occur widely, particularly in the elderly in whom inflammatory mechanisms are linked to the pathogenesis of functional and mental impairments. In this review, we investigated the role played by the inflammatory process in neurodegenerative diseases.
Keywords: cytokines, astrocytes, astroglia, neuroinflammation, neurodegeneration
1. Introduction
The degeneration of the central nervous system (CNS) is characterized by chronic progressive loss of the structure and functions of neuronal materials, resulting in functional and mental impairments (1). While the causes associated with neuronal degeneration remain poorly understood, the incidence of neurodegeneration increases with age, in mid-to-late adult life (2). This phenomenon, which mainly affects elder individuals (3,4), occurs in neurodegenerative diseases such as Alzheimer's disease (AD), multiple sclerosis (MS), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS) following viral infections. Viruses are able to directly injure neurons by direct killing or induction of apoptosis (5) to leading to neuro-degeneration (6,7). Similarly, in MS, the pathological features involve the permeability of the blood brain barrier (BBB), the destruction of myelin sheath, damage of the axon, the formation of glial scar and the presence of inflammatory cells, mostly lymphocytes infiltrated into the CNS (8). The loss of myelin is manifested in clinical symptoms together with neuropathic pain, paralysis, muscle spasms and optic neuritis (9).
Neurodegeneration induced by viruses, is noteworthy since it refers to the interaction between the CNS and environmental and viral factors, and suggests an important role of immune response in neurodegeneration (10). Immune activation in the CNS, always present in viral infections, immune-mediated disorders, and neurodegenerative diseases (11), involves microglia and astrocytes (12) which constitute the resident immune cells of the CNS and play an important role in the regulation of homeostasis of the brain during development, adulthood and aging (13). In the CNS, microglia constantly survey the microenvironment by producing factors that influence surrounding astrocytes and neurons (14), particularly in response to pathogen invasion or tissue damage thereby promoting an inflammatory response that further engages a self-limiting response through the immune system and initiates tissue repair (15). However, inflammation in tissue pathology that may result in the production of neurotoxic factors amplifying the disease states, indicates the persistence of inflammatory stimuli or failure in normal resolution mechanisms (16,17). Accordingly, specific inducers of inflammation associated with neurodegenerative diseases converge in mechanisms responsible in the sensing, transduction and amplification of the inflammatory processes that result in the production of neurotoxic mediators, such as cytokines and interleukins (18,19). These neurotoxic mediators are, in general, associated with several neurodegenerative diseases including AD, MS, PD and ALS, which are commonly linked to intracellular mechanisms such as the degradation of protein, the dysfunction of mitochondria, the defects of axonal transport and apoptosis (20–22). Inflammation associated with AD, MS, PD and ALS is not typically the initiating factor of neurodegenerative disease. However, the emerging evidence on the sustained inflammatory response associated with the contribution of microglia and astrocytes in disease progression, suggest contributory important roles of effectors of neuroinflammation in neuronal dysfunction and death. In this review, we assessed the role played by these inflammatory processes in neurodegenerative diseases.
2. Sources of neuroinflammation
Vascular dementia and neuroinflammation
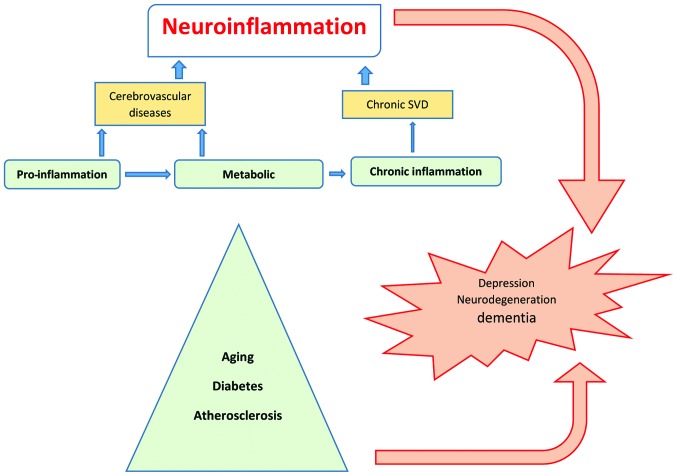
The cellular and molecular mechanisms of neuroinflammation are likely the same in aging and metabolic diseases such as hypertension, diabetes, depression, dementia or after cerebral insult such as stroke (23), and are considered as silent contributors of neuroinflammation (Fig. 1). In the elderly, inflammatory mechanisms have been associated with the pathogenesis of dementia and functional impairment. Systemic and local CNS inflammation significantly contributes to cerebral small vessel disease (SVD)-vascular dementia (24,25), hypothesized as microvascular changes that result in a state of chronic hypoperfusion, leading to continuous oligodendrocyte death and the consecutive degeneration of myelinated fibers that increase low-grade inflammation amplification of the risk of stroke (26). Another major risk factor for stroke and CNS tissue destruction is atherosclerosis, the disease of arteries that is characterized by vascular inflammation occasioned by the infiltration of monocytes into the injured vascular wall and an increase of interleukin (IL)-6 associated with future intracranial large artery stenosis progression after a stroke episode (27). Additional markers of inflammation such as C-reactive protein (CRP), which are well established in cardiovascular disease as strong predictors of subclinical and clinical atherosclerosis and progression of hemorrhagic stroke, were identified in SVD (28–31). Furthermore, adipose tissue dysfunction identified in obesity and hypertension, contributes to chronic and low-grade inflammation, predisposing to type 2 diabetes mellitus (DM) and cardiovascular disease (32,33) and could determine a worse outcome in stroke patients (34). Mortality in DM is primarily attributed to micro- and macro-vascular complication as well as sensory neuropathic complications, exacerbating the consequences of vascular disease. Sensory neuropathy promotes foot ulcers and abrogates warning symptoms during a heart attack. However, metabolic inflammatory disease (mataflammation) (35) occurring in unhealthy nutritional habits, can lead to a series of disorders and diseases such as CVD, stroke, hypertension, insulin resistance, metabolic syndrome and DM. Lipid hormone (sphingolipids and eicosanoids), cytokines and adipokines play an important role in mataflammation through the induction of adverse regulatory responses in target cells such as macrophages.
Figure 1.
Sources of neuroinflammation. Aging, metabolic diseases and viral infections are sources of inflammation that can affect vessels and neurons, leading to neurodegeneration. SVD, small vessel disease.
Depression and neuroinflammation
Normal aging is associated with an increase in the expression level of systemic inflammatory factors (36) such as pro-inflammatory cytokines (37–39). In the brain, this age-associated inflammation manifests initially as the chronic activation of perivascular and parenchymal macrophage/microglia expressing pro-inflammatory cytokines together with an increased number of astrocytes (40). Accordingly, the chronic activation of pro-inflammatory signals in aging may contribute to an increase in vulnerability to neuropsychiatric disorders (41). In obese women, the inflammation state was associated with a higher concentration of pro-inflammatory markers including IL-6, CRP and adipokines (42). These pro-inflammatory markers correlated positively with symptoms of depression and anxiety (43). Anxiety was alleviated with the reduction of inflammation following the surgical removal of fat tissue (44). In agreement with those findings, metabolic diseases such as obesity, hypertension, and being elderly are prevalent risk factors of depression, cognitive dysfunction and dementia (45) and there is an increase onset risk of aging-related diseases affecting the cardiovascular, cerebrovascular, neuroendocrine, metabolic, and immune systems in patients suffering major depression (46,47).
Although biological mechanisms of depression are poorly understood, conventional antidepressant treatments procuring beneficial effects were unsuccessful on one-third of depressed patients due to the inflammation that contributed to treatment resistance (48). The putative mechanism linking inflammation and depression involved oxidative stress, elevated pro-inflammatory cytokines IL-6 and IL-8 (49), endothelial nitric oxide synthase uncoupling and hyperglutamatergia. Accordingly, indirect evidence of neurovascular dysfunction have been found in major depressive disorder (MDD) (50,51), a severe psychiatric illness that is associated with increased levels of inflammatory markers in periphery, depression and mortality from suicide (52). Therefore, inflammatory markers identified in neurodegenerative diseases including MDD cover chemokines, adhesion molecules, cytokines and acute phase proteins (53).
Infections and neuroinflammation
Dynamic immune and inflammatory responses result from several offences in the CNS, of which infection is one (54). A virus can enter the CNS through two distinct hypothetical mechanisms, including hematogenous dissemination by which the virus gains access to the brain by BBB (55), and neuronal retrograde dissemination (56). However, it has been suggested that a virus can replicate in macrophage and CCR5+ T cells inside of the CNS in relation to the development and progression of dementia (57), as is the case for HIV proteins gp120 (58) and Tat (59) which are respectively able to induce the apoptosis of neurons through the enhancement of CXCR4-PKC (58), and to cause neuronal dysfunction through the disruption of miRNA expression (59). Most importantly, as in the case of HIV infection, other viral insults are associated with highly secreted cytokines, cholesterol increase, elevations of lipopolysaccharide (LPS) concentration, insulin resistance, testosterone deficiency and APOE4 (60), which are all involved in inflammation of the CNS.
Thus, inflammatory responses appear as the prevalent triggering mechanism driving tissue damage that is likely associated with different age-related diseases, as age-dependent upregulation of the inflammatory response is a consequence of chronic stress.
3. Neurodegeneration-induced neuroinflammation
The CNS is an immune-privileged organ with the innate and acquired immune response being closely controlled in relation with the periphery. Evidence suggests that a strong inflammatory response in the periphery from systemic LPS (61) or viral infections (62) results in the subsequent infiltration of leukocytes from the periphery to the CNS with consequent neuroinflammation and neurodegeneration. An offense is followed by the initial activation of microglia, which induce the release of pro-inflammatory mediators that favour the permeabilisation of the BBB. The subsequent infiltration of peripheral leukocytes occurs inside of the CNS, including T cells and macrophages, which share several functional features with microglia (61) including, the expression of toll-like receptors (TLRs), and consequently the ability to be activated by aggregated proteins or pathogen-associated molecular patterns (61,63); the expression of class II major histocompatibility complex, and the ability to present antigens to CD4+ T cells to exert an influence on the functional phenotype of T cells (64); as well as the ability to polarise their functional phenotype towards inflammatory M1 and anti-inflammatory M2 phenotypes, which can be influenced by inflammatory T cells and the lymphocyte regulatory T cells (65). Therefore, subsequent permeability of BBB leads to the possibility that peripheral macrophages can acquire a relevant role in the outcome of neuroinflammation. Accordingly, the alteration of CD4+ and CD8+ T cells has been observed in the periphery of neurodegenerative disease patients, suggesting a persisting antigenic challenge and that T cells may play a role in neurode-generative diseases. Of note, the ratio of CD8+ to CD4+ T cells or the shift to a Tc1/Th1-type immune response may contribute to a harmful brain inflammatory reaction, and the presence of antibodies against neuronal antigen observed in neurodegenerative diseases (some of them being pathogenic), solidify the involvement of the immune system in neurodegenerative diseases (5). Consequently, an acute neuro-inflammatory response is beneficial to the CNS, minimizing the injury by activating the innate immune system (66,67). By contrast, chronic inflammation is characterized by the long-standing activation of microglia that sustained release of inflammatory mediators, leading to an increase of oxidative and nitrosative stress which perpetuate the inflammatory cycle (68), further prolonging inflammation (54,69), which is detrimental for several neurodegenerative diseases (70).
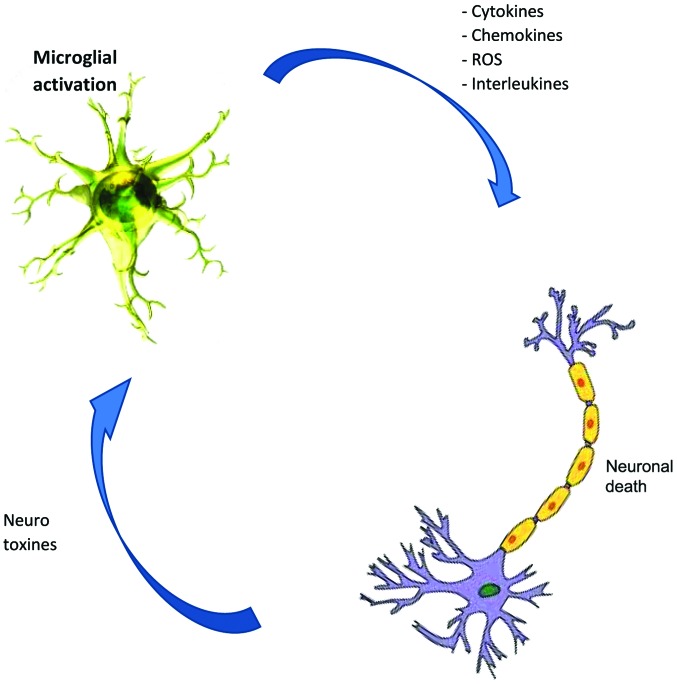
However, cell factors that influence microglial fate invade the epithelial cells of the BBB while T cells infiltrate the CNS, astrocytes and neurons (71), the most abundant glial cell population of the CNS which also participates in the innate immune response, triggered as a consequence of constant insult during inflammation or infection. Astrocytes are reservoirs of HIV-1, playing significant role in virus-mediated neurodegeneration (17,57). Accordingly, chronic neuroinflammation and microglia activation play central roles in the pathophysiology of neurodegenerative disease. For example, IL-1-positive activated microglia, colocalized with amyloid β plaques and neurofibrillary tangles in AD or present in degenerative motor neuron regions in patients suffering ALS (72) lead to abnormal phosphorylation of τ (73). Similarly, neurotropic viruses trigger long-term neuroimmune activation to underlying mechanisms of viral neurodegenerative diseases (74). Furthermore, neuroinflammation has been associated with either the cause or consequence of chronic oxidative stress, a key feature of all the neurodegenerative diseases that causes genetic structural alteration, lipid and protein, resulting in neurodegeneration. Microglial cells are the main source of reactive oxygen species and nitrogen species, tumor necrosis-α and glutamate, all of which are neurotoxic when released at a high dose after the activation of microglia (71,75,76) (Fig. 2), likely due to the stimulus from TLRs through the aggregated proteins (77,78) as is the case of AD patients (79), (MS) (9), PD (80), and ALS (81).
Figure 2.
Relationship between microglial activation and neuronal death. Microglial activation may result in the release of neurotoxicity or neuroprotection. The increase of neurotoxic molecules favours neuroinflammation or neuronal death leading to neurodegeneration. ROS, reactive oxygen species.
4. Conclusion
Neuroinflammatory disorders are conditions involving the immune response damage component of the nervous system. In the CNS, inflammatory effectors derived from innate and acquired immune systems as well as glial cells, particularly, microglia, act as sensors for disturbed brain tissue homeostasis and accumulate locally in response to neuronal cell injury or foreign entry in the brain; the differential activation of microglia cells being the central point that regulates neuroinflammation, which results in neurotoxicity or neuroprotection. The environmental exposure is therefore, the critical element for the fate of neurons with regard to degeneration or protection. Additional studies must be undertaken to benefit from the versatility of microglia, since activated microglia can also produce anti-inflammatory mediators and neurotrophic factors such as insulin-like growth factor-1, glial cell-derived neurotrophic factor, brain-derived neurotrophic factors and other factors (82–84), and procure beneficial effects.
References
- 1.Campbell IL, Krucker T, Steffensen S, Akwa Y, Powell HC, Lane T, Carr DJ, Gold LH, Henriksen SJ, Siggins GR. Structural and functional neuropathology in transgenic mice with CNS expression of IFN-α. Brain Res. 1999;835:46–61. doi: 10.1016/S0006-8993(99)01328-1. [DOI] [PubMed] [Google Scholar]
- 2.Hof PR, Mobbs CV, editors. Handbook of the neuroscience of aging. Elsevier/Academic Press; Amsterdam: 2010. pp. 1–53. [Google Scholar]
- 3.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 4.Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: What is it and where are we? J Clin Invest. 2003;111:3–10. doi: 10.1172/JCI200317522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinya K, Shimada A, Ito T, Otsuki K, Morita T, Tanaka H, Takada A, Kida H, Umemura T. Avian influenza virus intranasally inoculated infects the central nervous system of mice through the general visceral afferent nerve. Arch Virol. 2000;145:187–195. doi: 10.1007/s007050050016. [DOI] [PubMed] [Google Scholar]
- 7.Reinacher M, Bonin J, Narayan O, Scholtissek C. Pathogenesis of neurovirulent influenza A virus infection in mice. Route of entry of virus into brain determines infection of different populations of cells. Lab Invest. 1983;49:686–692. [PubMed] [Google Scholar]
- 8.Jadidi-Niaragh F, Mirshafiey A. Histamine and histamine receptors in pathogenesis and treatment of multiple sclerosis. Neuropharmacology. 2010;59:180–189. doi: 10.1016/j.neuropharm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Chastain EM, Duncan DS, Rodgers JM, Miller SD. The role of antigen presenting cells in multiple sclerosis. Biochim Biophys Acta. 2011;1812:265–274. doi: 10.1016/j.bbadis.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czirr E, Wyss-Coray T. The immunology of neurodegeneration. J Clin Invest. 2012;122:1156–1163. doi: 10.1172/JCI58656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ransohoff RM, Perry VH. Microglial physiology: Unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 12.Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Sem Immunopathol. 2013;35:601–612. doi: 10.1007/s00281-013-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz M, Kipnis J, Rivest S, Prat A. How do immune cells support and shape the brain in health, disease, and aging? J Neurosci. 2013;33:17587–17596. doi: 10.1523/JNEUROSCI.3241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sofroniew MV, Vinters HV. Astrocytes: Biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease - a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/S0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 16.Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7:354–365. doi: 10.1016/j.nurt.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das Sarma J. Microglia-mediated neuroinflammation is an amplifier of virus-induced neuropathology. J Neurovirol. 2014;20:122–136. doi: 10.1007/s13365-013-0188-4. [DOI] [PubMed] [Google Scholar]
- 18.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: Underlying mechanisms. Neuroscience. 2009;158:1062–1073. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–1995. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- 21.Chevalier-Larsen E, Holzbaur EL. Axonal transport and neurodegenerative disease. Biochim Biophys Acta. 2006;1762:1094–1108. doi: 10.1016/j.bbadis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Chan DC. Mitochondrial dynamics - fusion, fission, movement, and mitophagy - in neurodegenerative diseases. Hum Mol Genet. 2009;18(R2):R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allison DJ, Ditor DS. The common inflammatory etiology of depression and cognitive impairment: A therapeutic target. J Neuroinflammation. 2014;11:151. doi: 10.1186/s12974-014-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/125.4.765. [DOI] [PubMed] [Google Scholar]
- 25.Schiffrin EL. Inflammation, immunity and development of essential hypertension. J Hypertens. 2014;32:228–229. doi: 10.1097/HJH.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu M, Ishikawa J, Yano Y, Hoshide S, Shimada K, Kario K. The relationship between the morning blood pressure surge and low-grade inflammation on silent cerebral infarct and clinical stroke events. Atherosclerosis. 2011;219:316–321. doi: 10.1016/j.atherosclerosis.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Tousoulis D, Kampoli AM, Papageorgiou N, Androulakis E, Antoniades C, Toutouzas K, Stefanadis C. Pathophysiology of atherosclerosis: The role of inflammation. Curr Pharm Des. 2011;17:4089–4110. doi: 10.2174/138161211798764843. [DOI] [PubMed] [Google Scholar]
- 28.Di Napoli M, Godoy DA, Campi V, Masotti L, Smith CJ, Parry Jones AR, Hopkins SJ, Slevin M, Papa F, Mogoanta L, et al. C-reactive protein in intracerebral hemorrhage: Time course, tissue localization, and prognosis. Neurology. 2012;79:690–699. doi: 10.1212/WNL.0b013e318264e3be. [DOI] [PubMed] [Google Scholar]
- 29.Di Napoli M, Parry-Jones AR, Smith CJ, Hopkins SJ, Slevin M, Masotti L, Campi V, Singh P, Papa F, Popa-Wagner A, et al. C-reactive protein predicts hematoma growth in intracerebral hemorrhage. Stroke. 2014;45:59–65. doi: 10.1161/STROKEAHA.113.001721. [DOI] [PubMed] [Google Scholar]
- 30.Rizzo M, Corrado E, Coppola G, Muratori I, Mezzani A, Novo G, Novo S. The predictive role of C-reactive protein in patients with hypertension and subclinical atherosclerosis. Intern Med J. 2009;39:539–545. doi: 10.1111/j.1445-5994.2009.01955.x. [DOI] [PubMed] [Google Scholar]
- 31.Rizzo M, Corrado E, Coppola G, Muratori I, Novo G, Novo S. Markers of inflammation are strong predictors of subclinical and clinical atherosclerosis in women with hypertension. Coron Artery Dis. 2009;20:15–20. doi: 10.1097/MCA.0b013e3283109065. [DOI] [PubMed] [Google Scholar]
- 32.Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206–218. doi: 10.1016/j.physbeh.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Čajlaković M, Ribitsch V, Clément K, et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 34.Howcroft TK, Campisi J, Louis GB, Smith MT, Wise B, Wyss-Coray T, Augustine AD, McElhaney JE, Kohanski R, Sierra F. The role of inflammation in age-related disease. Aging (Albany NY) 2013;5:84–93. doi: 10.18632/aging.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 36.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Fagiolo U, Cossarizza A, Santacaterina S, Ortolani C, Monti D, Paganelli R, Franceschi C. Increased cytokine production by peripheral blood mononuclear cells from healthy elderly people. Ann N Y Acad Sci. 1992;663:490–493. doi: 10.1111/j.1749-6632.1992.tb38712.x. [DOI] [PubMed] [Google Scholar]
- 38.Fagiolo U, Amadori A, Cozzi E, Bendo R, Lama M, Douglas A, Palù G. Humoral and cellular immune response to influenza virus vaccination in aged humans. Aging (Milano) 1993;5:451–458. doi: 10.1007/BF03324202. [DOI] [PubMed] [Google Scholar]
- 39.Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, Monti D, Franceschi C, Paganelli R. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- 40.Johnson FA, Dawson AJ, Meyer RL. Activity-dependent refinement in the goldfish retinotectal system is mediated by the dynamic regulation of processes withdrawal: An in vivo imaging study. J Comp Neurol. 1999;406:548–562. doi: 10.1002/(SICI)1096-9861(19990419)406:4<548::AID-CNE8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ, Maisano C, Jones L, Murrah NV, Vaccarino V. Depressive symptoms and metabolic syndrome: Is inflammation the underlying link? Biol Psychiatry. 2008;64:896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capuron L, Poitou C, Machaux-Tholliez D, Frochot V, Bouillot JL, Basdevant A, Layé S, Clément K. Relationship between adiposity, emotional status and eating behaviour in obese women: Role of inflammation. Psychol Med. 2011;41:1517–1528. doi: 10.1017/S0033291710001984. [DOI] [PubMed] [Google Scholar]
- 44.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 45.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 46.McIntyre RS, Soczynska JK, Konarski JZ, Woldeyohannes HO, Law CW, Miranda A, Fulgosi D, Kennedy SH. Should depressive syndromes be reclassified as 'metabolic syndrome type II'? Ann Clin Psychiatry. 2007;19:257–264. doi: 10.1080/10401230701653377. [DOI] [PubMed] [Google Scholar]
- 47.Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: Do stress and depression accelerate cell aging? Depress Anxiety. 2010;27:327–338. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- 48.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 49.Baune BT, Smith E, Reppermund S, Air T, Samaras K, Lux O, Brodaty H, Sachdev P, Trollor JN. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: The prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012;37:1521–1530. doi: 10.1016/j.psyneuen.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Najjar S, Pearlman DM, Devinsky O, Najjar A, Zagzag D. Neurovascular unit dysfunction with blood-brain barrier hyper-permeability contributes to major depressive disorder: A review of clinical and experimental evidence. J Neuroinflammation. 2013;10:142. doi: 10.1186/1742-2094-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135–151. doi: 10.1007/7854_2012_211. [DOI] [PubMed] [Google Scholar]
- 52.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 53.Papakostas GI, Shelton RC, Kinrys G, Henry ME, Bakow BR, Lipkin SH, Pi B, Thurmond L, Bilello JA. Assessment of a multi-assay, serum-based biological diagnostic test for major depressive disorder: A pilot and replication study. Mol Psychiatry. 2013;18:332–339. doi: 10.1038/mp.2011.166. [DOI] [PubMed] [Google Scholar]
- 54.Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 55.Yang WX, Terasaki T, Shiroki K, Ohka S, Aoki J, Tanabe S, Nomura T, Terada E, Sugiyama Y, Nomoto A. Efficient delivery of circulating poliovirus to the central nervous system independently of poliovirus receptor. Virology. 1997;229:421–428. doi: 10.1006/viro.1997.8450. [DOI] [PubMed] [Google Scholar]
- 56.Aronsson F, Robertson B, Ljunggren HG, Kristensson K. Invasion and persistence of the neuroadapted influenza virus A/WSN/33 in the mouse olfactory system. Viral Immunol. 2003;16:415–423. doi: 10.1089/088282403322396208. [DOI] [PubMed] [Google Scholar]
- 57.Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog. 2011;7:e1002286. doi: 10.1371/journal.ppat.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L, Liu J, Xu C, Keblesh J, Zang W, Xiong H. HIV-1gp120 induces neuronal apoptosis through enhancement of 4-aminopyridine-senstive outward K+ currents. PLoS One. 2011;6:e25994. doi: 10.1371/journal.pone.0025994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang JR, Mukerjee R, Bagashev A, Del Valle L, Chabrashvili T, Hawkins BJ, He JJ, Sawaya BE. HIV-1 Tat protein promotes neuronal dysfunction through disruption of microRNAs. J Biol Chem. 2013;288:8564. doi: 10.1074/jbc.A111.268466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G. Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol. 2009;4:163–174. doi: 10.1007/s11481-008-9143-1. [DOI] [PubMed] [Google Scholar]
- 61.Noh H, Jeon J, Seo H. Systemic injection of LPS induces region-specific neuroinflammation and mitochondrial dysfunction in normal mouse brain. Neurochem Int. 2014;69:35–40. doi: 10.1016/j.neuint.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Zhou L, Miranda-Saksena M, Saksena NK. Viruses and neurodegeneration. Virol J. 2013;10:172. doi: 10.1186/1743-422X-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ostanin DV, Bao J, Koboziev I, Gray L, Robinson-Jackson SA, Kosloski-Davidson M, Price VH, Grisham MB. T cell transfer model of chronic colitis: Concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135–G146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, Protschka M, Galle PR, Neurath MF, Blessing M. Cutting edge: TGF-β signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173:6526–6531. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 65.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crutcher KA, Gendelman HE, Kipnis J, Perez-Polo JR, Perry VH, Popovich PG, Weaver LC. Debate: 'is increasing neuroinflammation beneficial for neural repair?'. J Neuroimmune Pharmacol. 2006;1:195–211. doi: 10.1007/s11481-006-9021-7. [DOI] [PubMed] [Google Scholar]
- 67.Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9:481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- 68.Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson's disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol. 2007;208:1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmid CD, Melchior B, Masek K, Puntambekar SS, Danielson PE, Lo DD, Sutcliffe JG, Carson MJ. Differential gene expression in LPS/IFNgamma activated microglia and macrophages: In vitro versus in vivo. J Neurochem. 2009;109(Suppl 1):117–125. doi: 10.1111/j.1471-4159.2009.05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 71.González H, Elgueta D, Montoya A, Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J Neuroimmunol. 2014;274:1–13. doi: 10.1016/j.jneuroim.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 72.Henkel JS, Engelhardt JI, Siklós L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55:221–235. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- 73.Mrak RE, Griffin WST. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 74.Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942–964. doi: 10.1128/CMR.17.4.942-964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qian L, Tan KS, Wei SJ, Wu HM, Xu Z, Wilson B, Lu RB, Hong JS, Flood PM. Microglia-mediated neurotoxicity is inhibited by morphine through an opioid receptor-independent reduction of NADPH oxidase activity. J Immunol. 2007;179:1198–1209. doi: 10.4049/jimmunol.179.2.1198. [DOI] [PubMed] [Google Scholar]
- 76.Gordon R, Anantharam V, Kanthasamy AG, Kanthasamy A. Proteolytic activation of proapoptotic kinase protein kinase Cδ by tumor necrosis factor α death receptor signaling in dopaminergic neurons during neuroinflammation. J Neuroinflammation. 2012;9:82. doi: 10.1186/1742-2094-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Magro F, Fraga S, Ribeiro T, Soares-da-Silva P. Decreased availability of intestinal dopamine in transmural colitis may relate to inhibitory effects of interferon-γ upon L-DOPA uptake. Acta Physiol Scand. 2004;180:379–386. doi: 10.1111/j.1365-201X.2004.01260.x. [DOI] [PubMed] [Google Scholar]
- 78.Panaro MA, Lofrumento DD, Saponaro C, De Nuccio F, Cianciulli A, Mitolo V, Nicolardi G. Expression of TLR4 and CD14 in the central nervous system (CNS) in a MPTP mouse model of Parkinson's-like disease. Immunopharmacol Immunotoxicol. 2008;30:729–740. doi: 10.1080/08923970802278557. [DOI] [PubMed] [Google Scholar]
- 79.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 80.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 81.Saccon RA, Bunton-Stasyshyn RK, Fisher EM, Fratta P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain. 2013;136:2342–2358. doi: 10.1093/brain/awt097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q, Wang J, Chen M, Liu Y, Shen Y, et al. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci USA. 2014;111:E3432–E3440. doi: 10.1073/pnas.1408780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Appel SH. CD4+ T cells mediate cytotoxicity in neurodegenerative diseases. J Clin Invest. 2009;119:13–15. doi: 10.1172/JCI38096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reynolds AD, Stone DK, Mosley RL, Gendelman HE. Proteomic studies of nitrated alpha-synuclein microglia regulation by CD4+CD25+ T cells. J Proteome Res. 2009;8:3497–3511. doi: 10.1021/pr9001614. [DOI] [PMC free article] [PubMed] [Google Scholar]