Abstract
Several different human vaccines are available to protect against anthrax. We compared the human adaptive immune responses generated by three different anthrax vaccines or by previous exposure to cutaneous anthrax. Adaptive immunity was measured by ELISPOT to count cells that produce interferon (IFN)-γ in response to restimulation ex vivo with the anthrax toxin components PA, LF and EF and by measuring circulating IgG specific to these antigens. Neutralising activity of antisera against anthrax toxin was also assayed. We found that the different exposures to anthrax antigens promoted varying immune responses. Cutaneous anthrax promoted strong IFN-γ responses to all three antigens and antibody responses to PA and LF. The American AVA and Russian LAAV vaccines induced antibody responses to PA only. The British AVP vaccine produced IFN-γ responses to EF and antibody responses to all three antigens. Anti-PA (in AVA and LAAV vaccinees) or anti-LF (in AVP vaccinees) antibody titres correlated with toxin neutralisation activities. Our study is the first to compare all three vaccines in humans and show the diversity of responses against anthrax antigens.
Introduction
The disease anthrax has been long known to humankind. The causative agent, Bacillus anthracis, principally infects both wild and domestic herbivores. Human infection by B. anthracis is, however, possible and can be deadly. The pathogenesis of anthrax can be directly attributed to its virulence factors. The capacity for B. anthracis to form spores, which can last many years [1], means that the bacterium has an extended period where it can infect a suitable host. The anthrax bacilli are likely to gain entry into the natural host through contaminated food [2] and there is growing evidence that insect vectors may also play a role [3]. Once a host has been invaded, B. anthracis bacteria grow to large number, kill the host and form spores that survive in the expelled fluids of the decaying host.
Humans are not the primary niche for B. anthracis. This bacterium utilises potential routes of entry to the human body. Person to person spread is rare [4]. Intestinal anthrax is usually the result of the consumption of contaminated food or drink and is often fatal [5]. Spores can enter the body via cuts and abrasions causing cutaneous anthrax in which case the infection begins as, and may be limited to a characteristic, black eschar [6]. When spores enter the body by the respiratory route, they cause a serious pulmonary disease that can result in several different pathologies including ‘cardinal’s cap’ (a particularly unpleasant form of meningitis) and circulatory shock [7]. Airborne B. anthracis infection may occur in the handling of infected animal hides and fibres or as a consequence of a deliberate or accidental release. Recently a new and particularly invasive form of anthrax has emerged. Injectional anthrax occurred where heroin users injected themselves with narcotic preparations contaminated by spores of B. anthracis [8]. Following entry into the host, B. anthracis spores germinate to the vegetative state, where it evades host immune response via a tripartite toxin set and a capsule. The toxin proteins consist of Protective Antigen (PA), which is a pore-forming protein that associates into heptamers, Lethal Factor (LF) which is a zinc metalloprotease that cleaves the N-terminus of several mitogen-activated protein kinases kinases (MAPKKs), and Edema Factor (EF), which is an adenylate cyclase. These toxins are encoded on the plasmid pX01. The capsule (encoded on the plasmid pX02) provides the vegetative cell with protection from the physical stresses of phagocytosis. In pulmonary infection, these characteristics allow bacteria to escape air exchange surfaces and enter the mediastinal lymph node and pleural space, significantly impairing lung function [9]. During cutaneous infection the combination of dead leukocytes and oedema factor can cause dramatic pathologies (including limb oedema) but the disease will resolve in time through a combination of immune response and antibiotic intervention.
The long-term immune response after anthrax infection is poorly understood. In mice a CD4+ interferon-γ response to inactivated spores is protective, whereas antibody to spores is not [10]. Most adaptive immune response data has been gathered with the antigens PA and LF. T cell memory against PA and LF is generated after cutaneous anthrax [11–13] and injectional anthrax [14]. It is also known that the toxins of B. anthracis can directly influence the function of cells critical to the formation of the adaptive response [15, 16]. Investigations of individuals infected during the American postal attacks showed that individuals who survived airborne challenge have been found to generate antibody responses against PA that are able to neutralise the toxin [17]. Moreover it was demonstrated that high levels of anti-PA antibodies did not persist, particularly at one year after infection. In another study, the anthrax skin test appeared to indicate T cell activity against anthrax antigens [18], and this activity remained for several years after infection [19] indicating a possible T cell memory response. A recent study by Ingram and colleagues compared the cytokines released after LF stimulation by CD4+ T cells isolated from individuals who had previously contracted cutaneous anthrax and compared them to vaccinees [20]. The study found that the CD4+, LF specific T cells from recovered anthrax patients produced a wider spectrum of cytokines than those from vaccine recipients suggesting that disease produces a more complex and certainly different response. Whether this response is more protective or longer lasting, it is difficult to establish.
Several vaccines exist for clinical use to treat anthrax. The United Kingdom’s Anthrax Vaccine Precipitated (AVP) is an alum-precipitated cell-free filtrate of culture supernatant from a non-encapsulated strain of B. anthracis (Sterne), from which bacterial cells, and some LF and EF are removed by downstream filtering (http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1442552372697.pdf). The material is mostly PA, but also has EF, LF and other proteins, all of which are precipitated [21]. The American product, Anthrax Vaccine Adsorbed (AVA, also known as Biothrax™) is similar in formulation to AVP [22] (http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/UCM074923.pdf). The primary schedule for AVP consists of four doses administered intramuscularly at 0, 3, 6 and 32 weeks. The primary schedule for AVA consists of three doses administered intramuscularly at 0, 1 and 6 months. Annual booster doses are currently recommended for AVA. The Russian vaccine uses live attenuated STI strain B. anthracis spores and is often referred to as the live anthrax vaccine (LAV) or live attenuated anthrax vaccine (LAAV). The STI strain is stored in a lyophilized form and reconstituted for use. This vaccine is attenuated in that it lacks the plasmid that encodes the capsule, which is essential for virulence [23, 24]. The LAAV vaccine is delivered by 2 scarifications containing approximately 4 x 108 CFU of bacteria. The antibody responses generated by AVA and AVP have previously been compared to each other and to that of anthrax patients. It was observed that the antibody response in anthrax patients was more directed to LF, whereas the antibody responses generated by the two vaccines were more directed towards PA [25]. The relative merits of the different vaccines have been discussed by Splino and colleagues [26]. There is also considerable interest in the development of new vaccines, such as purified recombinant PA [27] or preparations containing novel adjuvants such as CPG [28], principally due to the potential for anthrax to be used as a deliberate release agent. Study of the immune response to anthrax antigens will help to inform the clinical development of such vaccines.
Our study had two goals. The primary goal was to compare the immunological effects of all three vaccines. The secondary objective was to look at what factors might influence the development of long-lasting immune responses to B. anthracis antigens following human infection.
Results
Statistical identification of potentially confounding factors
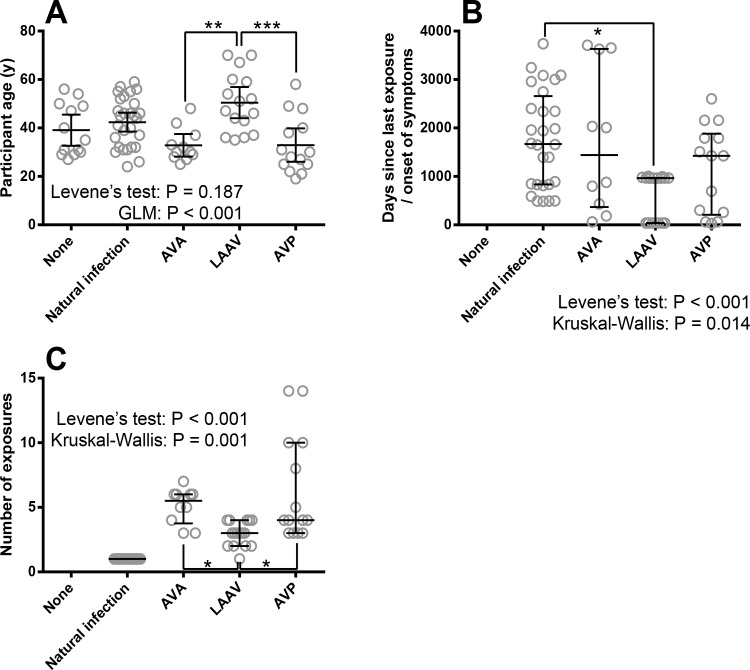
To understand whether we need to apply statistical controls for these factors, we performed analysis to establish whether these ‘secondary factors’ were evenly distributed across the different groups. The proportion of males and females was not constant between groups (Table 1). The AVP and AVA vaccinees and anthrax patients were mostly male (in fact no female AVP vaccinees were available for the study), whereas both genders were better represented in the unexposed control individuals and LAAV vaccinee groups. In addition, the distribution of age was not consistent between treatment groups (Fig 1A). Distribution of time since exposure to anthrax antigens across groups in study (excluding unexposed controls) and the total number of vaccination doses was investigated (excluding unvaccinated individuals) and found to be different between groups (Fig 1B and Fig 1C). In summary, ‘gender’, ‘age’, ‘time elapsed since exposure’ and ‘number of exposures’ were not equally distributed across exposure groups and were controlled for statistically where possible.
Table 1. Frequency of gender between groups in study including individuals with no history of Anthrax, recent Anthrax infection, or one of three different vaccines.
| Gender | Total | |||
|---|---|---|---|---|
| Male | Female | |||
| Immunological stimuli | None | 7 | 6 | 13 |
| Natural infection | 24 | 4 | 28 | |
| AVA | 9 | 2 | 11 | |
| LAAV | 5 | 11 | 16 | |
| AVP | 14 | 0 | 14 | |
| Total | 59 | 23 | 82 | |
Statistical investigation demonstrated by Pearson’s Chi squared test that the distribution of males and females was not consistent across participant groups (P < 0.001).
Fig 1. The characteristics of individuals enrolled into this study including: age, number of exposures to B. anthracis antigens and time since exposure in relation to the type of exposure to B. anthracis antigens.
Panel A shows the distribution of age across groups in study including individuals with no history of Anthrax, recent Anthrax infection, or one of three different vaccines. Each symbol denotes a single participant, the line is the mean and the error bars 95% Confidence Intervals. Statistical analysis is shown where Levene’s test validated GLM has been performed with Bonferroni’s post-tests. Panel B shows the distribution of time since exposure to vaccine or onset of anthrax symptoms across groups in the study. Each symbol denotes a single participant, the line is the median and the error bars the quartile range. The data were analysed by Kruskal-Wallis test (excluding naïve controls), which indicated differences between groups with Dunn’s individual comparisons. Panel C shows the distribution of number of anthrax-based immunological stimuli (doses) across groups in the study including percent people who had had Anthrax infection, or one of three different vaccines. Each symbol denotes a single participant, the line is the median and the error bars the quartile range. The data (excluding naïve and naturally infected controls) were analysed by Kruskal-Wallis test, which indicated differences between groups with Dunn’s individual comparisons. Significance markers indicate either P-values associated with either Bonferroni’s (For GLM) or Dunn’s (for Kruskal-Wallis) multiple comparisons where * indicates P < 0.05, ** indicates P < 0.01 and *** indicates P < 0.001).
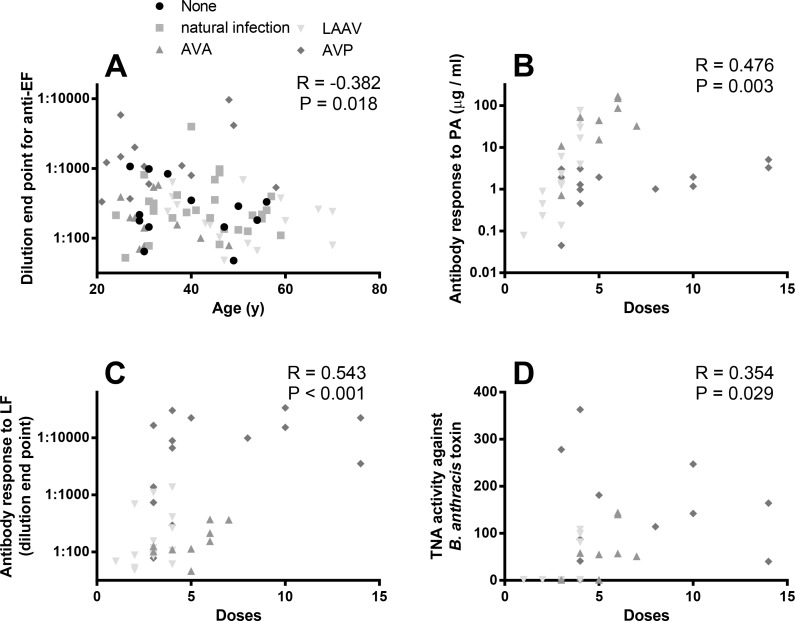
In order to better understand whether the potentially confounding factors noted above might influence the immune response, partial correlations were performed. In these correlations we statistically controlled for the different immunological stimuli/treatment groups. We found no correlation between any immune parameter and time elapsed from immune stimuli (omitting the naïve participants), however we concede that, when investigating the data, a larger dataset one might well give a significant interaction. The only correlation between immune parameter and age was a significant negative correlation with the antibody response to EF (Fig 2A). The number of received doses had significant positive correlations with the antibody response to PA (Fig 2B), antibody response to LF (Fig 2C) and toxin neutralisation activity (Fig 2D). We found that gender was too inconsistently represented across the groups to consider as a covariate across all groups. However Spearman’s Correlations were performed on immunological readouts and gender after the data was split into uninfected, natural infection and LAAV (which have representative number of males and females). We found no evidence for a gender-based effect for any responses except the antibody response to EF in naïve controls (P = 0.008).
Fig 2. Correlations between immune response to B. anthracis antigens and individual’s age and the number of vaccine doses.
Panel A shows the correlation between age and antibody response to EF, in participants of a study which included individuals who had suffered from cutaneous anthrax previously, been vaccinated with either AVA, LAAV or AVP, or have no history of exposure to anthrax antigens. Antibody responses were measured by ELISA estimating antigen specific antibody in μg / ml. Panels B-D show the correlation between the numbers of doses of vaccine received and antibody response. Antibody responses were measured by ELISA estimating antigen specific antibody against PA (panel B), LF (panel C) and toxin neutralisation activity (Panel D). Partial correlation analyses indicated a significant negative correlation between the parameters (marked on the graphs).
Comparison of adaptive immune responses
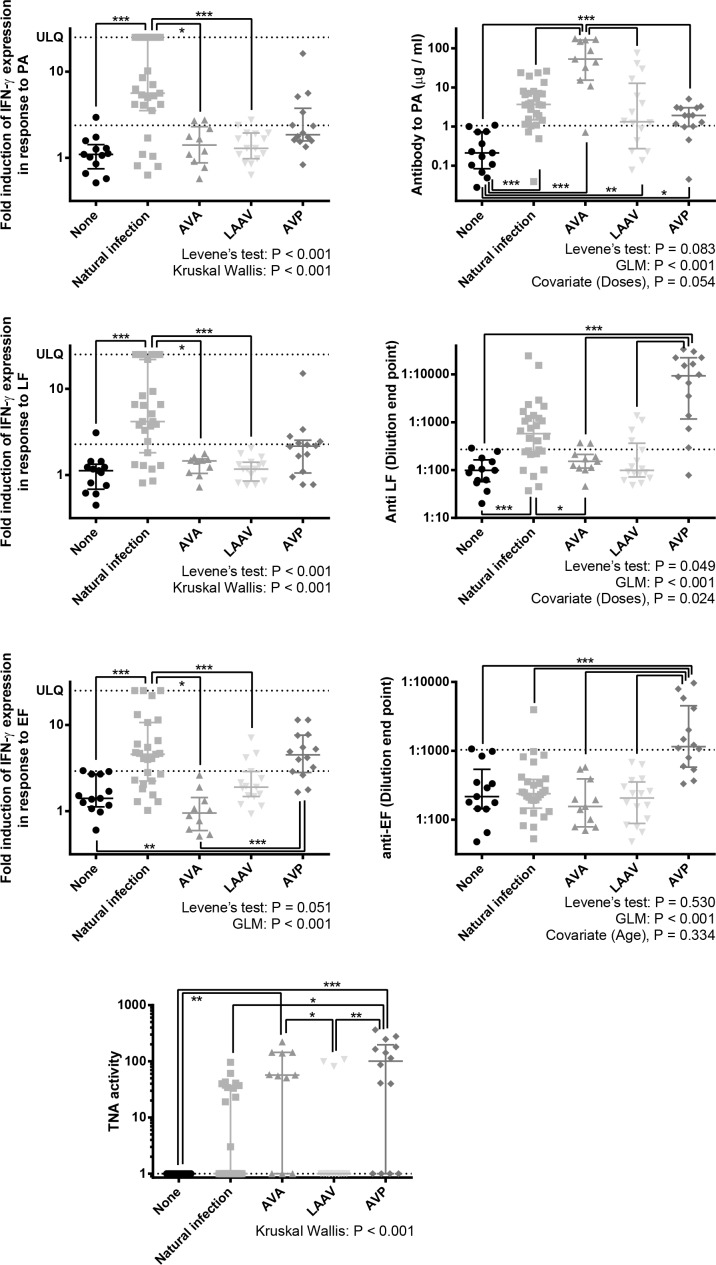
A variety of immune measurements were made including IgG antibody response to PA, IFN-γ ELISPOT responses to PA, LF and EF, and the toxin neutralisation activity (Fig 3).
Fig 3. The memory immune responses towards parts of the Anthrax toxin systems in individuals who had suffered from cutaneous anthrax previously, been vaccinated against anthrax with either AVA, LAAV or AVP, or have no history of exposure to anthrax antigens.
Antibody responses were measured by ELISA, estimating antigen specific antibody in μg / ml or a dilution end point. IFN-γ T cell responses were estimated by ELISPOT and numerated as fold induction in IFN-γ producing cells by exposure to anthrax antigen, when compared to unstimulated cells. Toxin neutralisation was measured in the ED50 of cells exposed to anthrax toxin in the presence of the serum. ULQ signifies the upper limit of quantification, due to confluence of spots on the ELISPOT plate, which was regarded as 25 fold. An additional line has been included which is indicative of the 90 percentile of the naïve group. The purpose of this is to give an approximate indication of the number of individuals with greater than background antigen recognition. Statistical analysis is shown. First, Levene’s tests of unequal variances were performed to establish suitability for parametric analysis. Unsuitability necessitated Kruskal-Wallis tests with Dunn’s post-tests. Suitability allowed for GLM analysis which included any potential covariates suggested previously, with Bonferroni’s multiple comparisons. The significance markers show results post-test comparisons: *** = P < 0.001, ** = P <0.01 * = P <0.05.
We found differences in the IFN-γ response to PA, LF and EF, between participant groups. Regarding the IFN-γ response to PA and LF, this response was statistically higher in naturally infected individuals than in those without exposure to anthrax antigens, AVA vaccine and LAAV. With regards to the IFN-γ response to EF, the response in naturally infected individuals was statistically higher than those in the unexposed individuals, AVA vaccine and LAAV. Moreover, in individuals who had been vaccinated with AVP, the IFN-γ response to EF was significantly greater than those who were unexposed and vaccinated with AVA.
We found differences in the antibody titres specific to PA, LF and EF, between participant groups. With regards to the antibody response to PA, titres were significantly raised in all vaccination / exposed groups when compared to the naïve control individuals. Anti-PA titres were greatest in the AVA vaccinated group, and this was significantly higher than in any other group. With regards to the antibody response to LF, both natural infection and AVP vaccination produced anti-LF antibody responses greater than background, whereas AVA and LAAV vaccination did not. The anti-LF response was significantly higher in AVP vaccinees when compared to the control group and the, AVA and LAAV vaccinees. The anti-LF response was significantly higher in anthrax patients when compared to AVA vaccinees. With regards to the antibody response to EF, the only difference between groups observed was that AVP vaccinees had a higher response to EF than all other groups.
The capacity for the participant’s antibodies to neutralise lethal toxin was compared between participant groups. The capacity for serum to neutralise toxin activity was greatest in subjects who had received the AVP vaccine and the response in these individuals was statistically greater than those in LAAV vaccinees, anthrax patients and naïve participants. The next highest response was found in AVA vaccinees who had significantly greater activity than the LAAV vaccinees and the naïve participants.
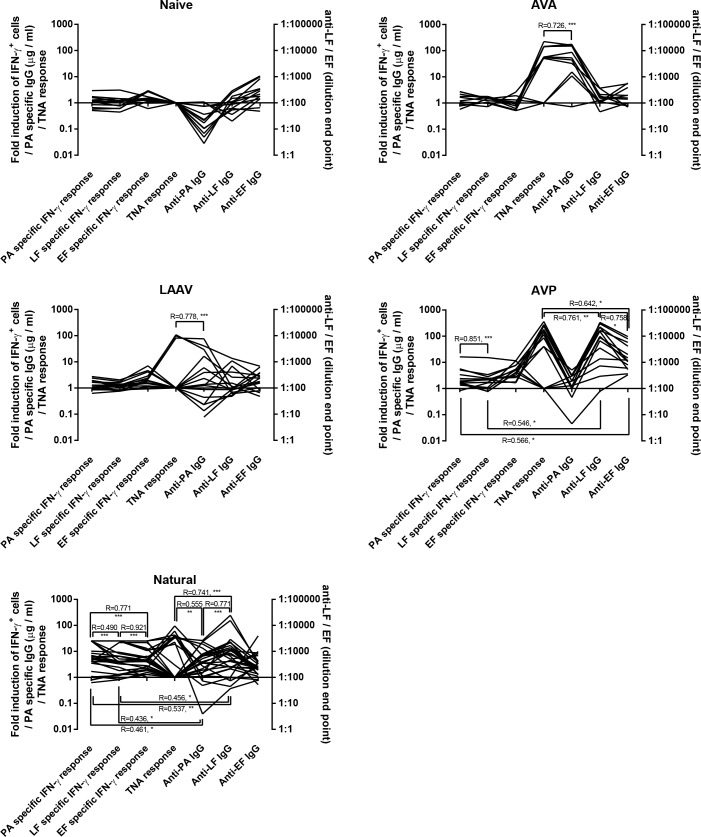
Our study provided an excellent opportunity to explore how the 7 different readings of adaptive immunity quantified in this study correlate (Fig 4). A comprehensive correlation matrix was constructed for each group. Of the immunological measurements taken we found several instances where two of more parameters correlated together. All correlations were found to be positive. In anthrax patients: the T cell response to all antigens correlated together, the antibody response to PA, LF and toxin neutralisation correlated together, and the antibody responses and T cell responses to PA and LF correlated together.
Fig 4. The relativity of different antigen specific responses in groups exposed to B. anthracis antigens in different ways.
The panels show the five different groups investigated in the course of this study. Participants included individuals who had suffered from cutaneous anthrax previously, been vaccinated with either AVA, LAAV or AVP, or had no history of exposure to anthrax antigens. Each line represents an individual with 7 different immune measurements take. Antibody responses were measured by ELISA estimating antigen specific antibody in μg / ml or a dilution end point. IFN-γ T cell responses were estimated by ELISPOT and numerated as fold induction in IFN-γ producing cells by exposure to anthrax antigen, when compared to unstimulated cells. Toxin neutralisation was measured in the ED50 of cells exposed to anthrax toxin in the presence of the serum. The analyses are Pearson Correlations and the R values are given with the significance markers of *** = P < 0.001, ** = P <0.01 * = P <0.05.
Concerning individuals who had revived AVP vaccination, the antibody response to LF and EF and toxin neutralisation all correlated together, the T cell response to PA and LF correlated together, the T cells response to PA correlated with the antibody response to EF and the T cells response to LF correlated with the antibody response to LF. The only observed correlation between immune parameters in individuals receiving the LAAV or AVA vaccines were correlation between the antibody response to PA and the toxin neutralisation activity of the serum.
In short, is seems that the different exposures to anthrax antigens promote different responses; however all of the responses that were generated to anthrax antigens did correlate well together. Natural infection promotes a response that includes PA, LF and EF specific IFN-γ production and antibody response to PA and LF and toxin neutralisation. The AVA and LAAV vaccines only instigate an anti-PA antibody response which can neutralise toxin when sufficient titres are reached. The AVP vaccine is more complex; it generates an IFN-γ response to EF and antibody responses to PA, LF and EF that can neutralise toxin when anti-LF titres are sufficiently high.
Discussion
Anthrax is a disease that can be prevented by a primed adaptive immune response. We know this because animals and humans can be protected by vaccination [26] and that reinfections are very rare events (Prof Mehmet Doganay, personal communication). Protection against anthrax can be elicited through the recognition of a variety of different antigens. For example, CD4+ IFN-γ+ T cells specific to spore proteins can protect whereas antibodies to the same targets do not [10] and, conversely, antibodies to elements of the B. anthracis toxin can be sufficient alone to prevent death [29]. There is a possibility that the spore vaccinated subjects were actually protected through anti-toxin activity; it has been suggested that the spores may express low levels of PA prior to germination [30]. The above studies suggest an importance in considering both T and B cell function in protection. In our study we have looked at the host recognition of toxin elements and compared the recipients of three different vaccines and individuals who were previously infected with B. anthracis.
Comparison between our groups is complex and we first considered the homogeneity of the groups. We gathered additional data to explore how these groups differ. ‘Gender’, ‘age’, ‘time elapsed since exposure’ and ‘number of exposures’ were not equally distributed across exposure groups. Additionally, ‘time elapsed since exposure’ and ‘number of exposures’ also correlated together, indicating that any significant effect that might be observed between either of these factors is likely to be intrinsically confounded. These differences between exposure groups need to be considered as limitations on any extrapolation of the data. For this reason, we explored whether these factors influenced the immunological readouts, independently to groups. The antibody response to PA was positively affected by number of doses (using only the vaccinee data) and this is entirely consistent with previous data [31]. Using statistical methodologies we were able to control for the effect of the different numbers of vaccine doses between groups. We also found that age affected the IFN-γ response to PA, declining slightly but significantly with age. However, we were not able to statistically control for this. The older groups were the anthrax patients and the LAAV vaccinees. The anthrax patients had statistically greater IFN-γ responses than all other groups; it is possible that these individuals would have an even more substantial response if the individuals were younger. The other older group in this study were the vaccinees receiving LAAV. The LAAV vaccinees did not have significantly different IFN-γ responses from the unexposed control group. It is possible that some affect might be masked by the age of the vaccinees; however this effect is not likely to be large and this is demonstrable from the correlation coefficient and lack statistical significance between age and IFN-γ responses. One further limitation of this investigation was that subjects originated from a variety of different ethnic and geographic backgrounds.
We observed strong IFN-γ responses in recovered anthrax patients and no significant responses in the vaccinees, with the exception of a significant IFN-γ response to EF in AVP recipients. The response observed in recovered anthrax patients were consistent with published data [13]. It is possible and, indeed likely, that T cell responses to the vaccine antigens did exist at times prior to recruitment and testing but had subsided. IFN-γ responses in vaccinees had previously been measured at time closer to vaccination [11]. It is also the case that with the ELISPOT technique single cells circulating are measured whereas, with the ELISA we measure the product of cells and each cell will produce large numbers of antibodies. This difference in sensitivity may go somewhere to explain the why we observed antibody but no T cell immunity ion the vaccinees. The presence of a measureable IFN-γ response to EF in recipients of the AVP vaccine is noteworthy. AVP provides all three antigens, as does cutaneous infection, yet only EF specific IFN-γ responses endured to the time of sampling in the AVP vaccinees. This may mean that EF stimulates longer lasting IFN-γ+ T cell memory than PA and LF.
We found that the different exposures to anthrax antigens promote different responses; however all of the different responses correlated well together. Natural infection promoted a response that included PA, LF and EF specific IFN-γ production and an antibody response to PA and LF and toxin neutralisation in individuals with high enough antibody titres. The AVA and LAAV vaccines only instigated an anti-PA antibody response and this response can neutralise toxin when sufficient titres are reached. The AVP vaccine is more complex in that it generates an IFN-γ response to EF as well as antibody responses to PA, LF and EF that can neutralise toxin when titres are sufficiently high.
The LAAV vaccine did not promote a strong adaptive immune response in our study. This needs to be put into context with three considerations. Firstly, most individuals from this group had only received 2 or 3 doses, whereas those who had received the AVA or AVP vaccines had received more. Secondly, as a whole cell live attenuated vaccine, this vaccine would likely have other important antigens that we have not measured here. Thirdly, the mean LAAV vaccinee age, in this study was older, although we found no evidence in our data for a correlation between age and toxin specific antibody.
Both the LAAV and AVA vaccines only produced significant anti-PA antibody responses and these significantly correlated with toxin neutralisation. AVP produced significant antibody responses to all three antigens and an IFN-γ response to EF and we observed strong positive correlations between these different responses. LAAV also contains LF and EF yet only a response to PA was observed. Conversely, the anti-PA antibody response to PA in recipients of AVP, which also contains all three antigens, was substantially lower than in AVA and LAAV. It is possible that the preparation process of AVP removes much of the PA and this could be assayed using quantitative ELISA. LF is not likely to be immune-dominant to PA and this is shown in the subjects that had B. anthracis infection where the infection induces antisera to both antigens. All three products have been extensively used in humans and produce toxin neutralising effects.
In summary, we performed a study to compare the adaptive immune response generated by three different anthrax vaccines to each other and to individuals with a past history or cutaneous anthrax. PA, LF and EF specific IFN-γ and circulating IgG responses were measured, as was the ability of the individual’s serum to neutralise anthrax toxin. Cutaneous anthrax promoted strong IFN-γ response to all three antigens and antibody responses to PA and LF. Clear differences were demonstrated in responses to the three vaccines. The American AVA and Russian LAAV vaccines both produced antibody responses to PA only. The British AVP vaccine produced IFN-γ responses to EF and antibody responses to all three antigens. Where PA or LF titres were high enough, serum demonstrated toxin neutralising activity. These findings should help to inform the development of new anthrax vaccines.
Materials and Methods
Subjects
This work was carried out under the protocol (FY06-23) approved by the appropriate committees: Human Use Committee (HUC), US Army Medical Research Institute of Infectious Diseases (USAMRIID), US and Institutional Review Board (IRB), National Center for Disease Control and Public Health (NCDC) of the republic of Georgia. UK nationals gave blood samples under a protocol (CBD VP 141/06) approved by the CBD Independent Ethics Committee for the UK Ministry of Defence. In all cases, written consent was given.
A log of subjects with documented cutaneous anthrax infection during a period of 1 month to 10 years prior to study initiation was created after reviewing the medical archives of the two major referral hospitals in eastern Georgia. Informed consent was obtained from all volunteers. Past and current epidemiological/clinical data relevant to anthrax infection were collected from each subject. Additional epidemiological, clinical and laboratory data were also retrieved from the medical records of the hospital archives. LAAV Vaccinees were recruited from the laboratory personnel of NCDC. AVA/AVP vaccinated US and UK nationals visiting Georgia were also recruited. Additionally, 13 Georgian Nationals with no history of prior anthrax infection/vaccination were evaluated as controls for the immunologic assays include in the study.
Blood (43 mL) was obtained from all subjects enrolled in the study and tested for the presence of B. anthracis-specific cellular and humoral immune responses. For Georgian patients with a history of cutaneous anthrax, venous blood was collected at the volunteer’s home into sodium citrate-containing vacutainer cell preparation tubes (CPTs) and serum tubes (BD bioscience®). The blood samples were transported to a laboratory at the NCDC, Tbilisi, and processed within 4 h of collection. Immune responses (serological and cell mediated) to anthrax toxin components were measured. Blood samples from vaccine recipients and naïve controls were taken in appropriate clinical settings in Tbilisi and were also all processed within 4 h of collection. The number of previous vaccinations and time elapsed since last vaccination were recorded for each vaccine recipient as was the time since diagnosis of infection for patients with a history of cutaneous anthrax. Some of the recipients of AVP vaccinations, the precise date for their most recent vaccination was only known to the nearest year. In these instances the date was taken as the 1st of July of that year.
ELISPOT
Peripheral blood mononuclear cells (PBMCs) were prepared and washed in RPMI medium supplemented with 10% fetal calf serum, penicillin, streptomycin, and L-glutamine. ELISPOTs were performed as described in the manufacturer’s instructions (BD biosciences®). Briefly, 5 × 105 cells were added to each well of the ELISPOT plates, which were pre-coated with antibodies to interferon-γ (IFN-γ). The antigen, recombinant Protective Antigen (rPA), LF or EF (List Biological Laboratories Campbell, Calif.) was added at a final concentration of 12.5 μg/ml for PA or 25 μg/ml for either LF or EF. The plates were incubated overnight at 37°C in a 5% CO2, humidified atmosphere. Subsequently, the cells were washed away, and the plates were developed by the addition of an appropriate biotinylated cytokine-detection antibody, followed by incubation at room temperature for 2 h. Any unbound antibodies were removed by washing three times with wash buffer, and then streptavidin horseradish peroxidase conjugate was added. After incubation, the enzyme conjugate was removed by washing three times, and the final development of the spots was performed with 3-amino-9-ethylcarbazole (AEC Chromogen kit, Sigma). The spots were enumerated (intensity 3, size 8, gradient 1 and well saturation 83%) using an ELISPOT Reader system ELR04 (Advanced Imaging Devices, GmbH) and the ELISPOT Reader 4.0 software (Autoimmune Diagnostika, GmbH). Concanavalin A (ConA) (2 μg/ml, Sigma Aldrich) was used as a positive control; when cells failed to react to ConA, the samples were not considered for further analysis. Unstimulated (blank) controls were also included for each sample that consisted of cells cultured in medium alone. All of the conditions were assayed in triplicate for each blood sample. The results were considered to be positive when greater than a threefold induction of cytokine excretion was observed over the background.
Quantitative anti-PA IgG ELISA
Wells of 96-well microtiter plates (Immulon 2HB; Dynex Technologies, Chantilly, Va.) were coated by adding of 100 μl rPA (List Biological Laboratories Campbell, Calif.) diluted to 1 μg/ml in PBS. Plates were incubated overnight at 4°C. Human anti-AVA Biothrax serum AVR 801 [32] (109.4 μg/ml; CDC, Atlanta, Ga.) was used as the reference antiserum for the quantitative standard curve. The plates were washed for 1 cycle (three washes) with PBS containing 0.1% Tween 20 (PBST) before adding reference antiserum, controls, and test samples. Each plate contained triplicate wells of high and low positive controls, normal human serum negative controls, blank, and three-fold dilutions of the reference antiserum. Samples were diluted in PBS containing 0.5% Tween 20 and 5% milk (MASP). The plates were incubated for 1 h at 37°C then washed with PBST for 1 cycle. Horseradish peroxidase (HRP)-conjugated goat anti-human IgG(H+L) antibody (Kirkegaard and Perry Laboratory, Gaithersberg, Md.) diluted to 1:1000 in MASP was added to the wells and the plates were incubated for 1 h at 37°C. The plates were washed for 1 cycle with PBST, rotated 180°, and washed again for 1 cycle before adding two-component substrate (2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt; ABTS; Kirkegaard and Perry Laboratory) to the wells. The plates were incubated at 37°C for 30 min. Stop solution (Kirkegaard and Perry Laboratory) was added and absorbance readings at 405 nm were obtained using a BioTek 312e microplate reader (BioTek Instruments, Winoski, Vt.). The mean absorbance values at 405 nm and coefficient of variation (%CV) for each triplicate dilution of all reference standards and controls were obtained using the KC4 software program (BioTek Instruments). A four-parameter regression equation of the KC4 program was used to evaluate the standard curve. Titres for the quantitative anti-PA IgG ELISA were presented as μg of anti-PA IgG per ml. Acceptance criteria included (i) the coefficient of determination (r2) for the standard curve (eight separate dilutions) must be ≥ 0.9970, (ii) %CV for the triplicate absorbance readings of the standards S1-S7 and samples must be ≤ 20%, (iii) no more than 1 outlier, as identified by the Dixon Gap Test [33] could be removed from the standard curve, and (iv) if the %CV the first sample dilution that could be read directly from the linear portion of the standard curve was >20%, the next sample dilution that could be read directly from the linear portion of the standard curve was used. If the sample was negative (below the limit of quantitation), the acceptance criteria for the standard were disregarded and the concentration of the sample was extrapolated from the standard curve or assigned a value of 0.025 μg IgG per ml, chosen by multiplying the lowest standard concentration (0.50 ng/ml) by the highest starting dilution tested (1:50).
Lethal toxin neutralization assay (TNA)
For the TNA assay, cell culture-treated, 96-well plates were seeded with approximately 1 x 105 J774A.1 cells per well in 200 μl volumes 18 to 22 h before testing. Cells were maintained in Dulbecco’s Minimal Essential Medium high glucose (D-MEM) containing 5% heat-inactivated fetal bovine serum, 4 mM glutamine, and 100 units of Penicillin G and 100 μg of streptomycin per ml. Serial 2-fold dilutions of samples were prepared using D-MEM containing 5% heat-inactivated fetal bovine serum, 4 mM glutamax, 25 mM HEPES, and 100 units of Penicillin G and 100 μg of streptomycin per ml. Lethal toxin (LeTx; 100 ng of rPA/ml and 50 ng of LF/ml, final concentrations) was prepared in the same medium and pre-incubated with the sample dilutions in a humidified incubator set at 37°C, 5% CO2 for 1 h. LeTx was prepared using PA obtained from List Biological Laboratories (see above) and LF was purified from BH450, a recombinant Escherichia coli strain expressing LF [34]. Medium was removed from wells of plates containing the J774A.1 cells and replaced with 100 μl per well of the samples pre-incubated with LeTx. After incubating for 4 hrs at 37°C, 5% CO2, 25 μl of 3-[4,5-dimethylthiazol-2-yl]2,5-diphenyltetrazolium bromide (MTT, Sigma Chemical Company, St. Louis, Mo.) at 5 mg/ml of PBS was added to each well. After incubation at 37°C, 5% CO2 for 2 h, the cells were lysed and the precipitate was dissolved by adding 100 μl per well of solubilisation buffer which consisted of 10% SDS in 50% dimethylformamide, pH 4.8 [35]. Absorbance readings were obtained after an overnight incubation at 37°C at a reading wavelength of 570nm less the reference wavelength of 690nm using a BioTek 312e (BioTek Instruments) microplate reader. Each dilution of sample was tested in triplicate. Nine wells contained only medium and served as medium controls. Three wells contained only LeTx and served as toxin controls. The percent viability was plotted against each respective test dilution using a 4-parameter logistic equation algorithm and using XLfit software (IDBS, Inc., Emeryville, Calif.). TNA assay titers were expressed as the reciprocal of the dilution of antibody in serum that neutralized the cytotoxic activity of LeTx on J774A.1 cells at 50% of control values (midpoint for each curve; ED50) using XLfit software (IDBS). The starting sample dilution for the TNA assay was 1:50 and negative samples that could not be measured from their 4-parameter curve were assigned an ED50 value of ‘10’. Acceptance criteria included (i) the r2 for the sample curves must be ≥ 0.9700 and (ii) the %CV for the triplicate absorbance readings for the samples must be ≤ 25%. The acceptance criteria for the standards were ignored if the sample was negative. Titres <50 were extrapolated by XLfit but the 4-parameter plots were visually-inspected to evaluate titres. Human anti-AVA Biothrax serum AVR801 was titered on approximately 25% of the plates as an internal control. Relative reactivity of the test samples were not measured against AVR801.
End-point ELISA for LF and EF
Wells of 96-well microtitre plates (Immulon 2HB) were coated with either LF or EF (List Biological Laboratories) at 1 μg/ mL in PBS (100 μL per well). After incubating overnight at 4°C, the plates were washed with PBST. Samples, at an appropriate starting dilution in MASP, were added to the wells in triplicate and titrated down the plate by appropriate serial dilutions in 100 μL volumes of MASP. After incubation for 1 h at 37°C, the plates were washed for 1 cycle with PBST and 100 μL of a 1:1000 dilution of HRP-conjugated anti-human IgG(H+L) was added to the wells. Plates were incubated for 1 h at 37°C and washed for 2 cycles in PBST rotating the plates 180° between cycles. The plates were incubated with ABTS substrate at 37°C for 30 min after which 100 μl stop solution was added to each well. The absorbance readings were obtained within 60 min. The end-point anti-LF and anti-EF ELISA titres were determined by fitting the absorbance values verse the corresponding reciprocal of the dilution in a four-parameter titration curve using XLfit software (IDBS). Results were presented as the reciprocal of the dilution of test serum at an absorbance value of 0.200 calculated from XLfit. Acceptance criteria included (i) blank absorbance values ≤ 0.100 and ≤ 20% CV, (ii) r2 of the sample titration curve ≥ 0.9700, (iii) ≤ 25% CV for sample dilution absorbance values ≥ 0.200, and (iv) the absorbance value of the lower asymptote of the sample titration curve ≤ 0.200. Outliers were removed from the titration.
Statistical Methods
ELISPOT data were condensed into fold increase in spot forming units when compared to unstimulated cells from the same source. ELISPOT data were disregarded when the Concanavalin A control had not enhanced IFN-γ production. Where ELISPOT readouts were so high that meaningful numbers (i.e. confluence of spots) were impossible to estimate, the figure 25 fold was assigned. The figure 25 fold was used because it was slightly higher than the maximum value in the complete data set that could be resolved (21.8 fold). Graphs were constructed using Graphpad PRISM V6.0 and analysis was performed using IBM SPSS V21.0. For analysis purposes and to deal with their exponential nature, serology (μg / ml of antibody) and T cell response (fold-change in spot forming units over background) data were subjected to transformation to the logarithm of 10. A further square root transformation was required for analysis of the LF data, for GLM analysis. In order to assess the suitability of these data sets for analysis QQ plots were constructed (data not shown).
Supporting Information
Data is in raw (untransformed) format.
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
Funding for this project was provided by the UK Ministry of Defence and US Department of Defense Cooperative Threat Reduction Program, implemented by the US Defense Threat Reduction Agency. The views of the authors do not purport to reflect the positions of the US Department of Defense. During this study, author T. Kuchuloria worked as a sub-contractor for the commercial entity Technology Management Company (TMC), on behalf of the US Defense Threat Reduction Agency via USAMRIID. TMC administered a salary for T. Kuchuloria but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Graham-Smith GS. The Longevity of Dry Spores of B. anthracis. The Journal of hygiene. 1930;30(2):213–5. Epub 1930/06/01. ; PubMed Central PMCID: PMCPmc2170553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner WC, Kausrud KL, Krishnappa YS, Cromsigt JP, Ganz HH, Mapaure I, et al. Fatal attraction: vegetation responses to nutrient inputs attract herbivores to infectious anthrax carcass sites. Proceedings Biological sciences / The Royal Society. 2014;281(1795). Epub 2014/10/03. 10.1098/rspb.2014.1785 ; PubMed Central PMCID: PMCPmc4213624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turell MJ, Knudson GB. Mechanical transmission of Bacillus anthracis by stable flies (Stomoxys calcitrans) and mosquitoes (Aedes aegypti and Aedes taeniorhynchus). Infection and immunity. 1987;55(8):1859–61. Epub 1987/08/01. ; PubMed Central PMCID: PMCPmc260614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doganay M, Metan G. Human anthrax in Turkey from 1990 to 2007. Vector borne and zoonotic diseases (Larchmont, NY). 2009;9(2):131–40. Epub 2008/10/24. 10.1089/vbz.2008.0032 . [DOI] [PubMed] [Google Scholar]
- 5.Owen JL, Yang T, Mohamadzadeh M. New insights into gastrointestinal anthrax infection. Trends in molecular medicine. 2014. Epub 2015/01/13. 10.1016/j.molmed.2014.12.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goel AK. Anthrax: A disease of biowarfare and public health importance. World journal of clinical cases. 2015;3(1):20–33. Epub 2015/01/23. 10.12998/wjcc.v3.i1.20 ; PubMed Central PMCID: PMCPmc4295216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brojatsch J, Casadevall A, Goldman DL. Molecular determinants for a cardiovascular collapse in anthrax. Frontiers in bioscience (Elite edition). 2014;6:139–47. Epub 2014/01/07. ; PubMed Central PMCID: PMCPmc4294697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger T, Kassirer M, Aran AA. Injectional anthrax—new presentation of an old disease. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2014;19(32). Epub 2014/08/21. . [DOI] [PubMed] [Google Scholar]
- 9.Guidi-Rontani C. The alveolar macrophage: the Trojan horse of Bacillus anthracis. Trends in microbiology. 2002;10(9):405–9. Epub 2002/09/10. . [DOI] [PubMed] [Google Scholar]
- 10.Glomski IJ, Corre JP, Mock M, Goossens PL. Cutting Edge: IFN-gamma-producing CD4 T lymphocytes mediate spore-induced immunity to capsulated Bacillus anthracis. Journal of immunology (Baltimore, Md: 1950). 2007;178(5):2646–50. Epub 2007/02/22. . [DOI] [PubMed] [Google Scholar]
- 11.Ascough S, Ingram RJ, Altmann DM. Anthrax lethal toxin and the induction of CD4 T cell immunity. Toxins. 2012;4(10):878–99. Epub 2012/11/20. 10.3390/toxins4100878 ; PubMed Central PMCID: PMCPmc3496994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingram RJ, Harris A, Ascough S, Metan G, Doganay M, Ballie L, et al. Exposure to anthrax toxin alters human leucocyte expression of anthrax toxin receptor 1. Clinical and experimental immunology. 2013;173(1):84–91. Epub 2013/04/24. 10.1111/cei.12090 ; PubMed Central PMCID: PMCPmc3694538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingram RJ, Metan G, Maillere B, Doganay M, Ozkul Y, Kim LU, et al. Natural exposure to cutaneous anthrax gives long-lasting T cell immunity encompassing infection-specific epitopes. Journal of immunology (Baltimore, Md: 1950). 2010;184(7):3814–21. Epub 2010/03/09. 10.4049/jimmunol.0901581 . [DOI] [PubMed] [Google Scholar]
- 14.Ascough S, Ingram RJ, Abarra A, Holmes AJ, Maillere B, Altmann DM, et al. Injectional anthrax infection due to heroin use induces strong immunological memory. The Journal of infection. 2014;68(2):200–3. Epub 2014/02/12. 10.1016/j.jinf.2013.10.007 ; PubMed Central PMCID: PMCPmc4150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comer JE, Chopra AK, Peterson JW, Konig R. Direct inhibition of T-lymphocyte activation by anthrax toxins in vivo. Infection and immunity. 2005;73(12):8275–81. Epub 2005/11/22. 10.1128/iai.73.12.8275-8281.2005 ; PubMed Central PMCID: PMCPmc1307061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alileche A, Serfass ER, Muehlbauer SM, Porcelli SA, Brojatsch J. Anthrax lethal toxin-mediated killing of human and murine dendritic cells impairs the adaptive immune response. PLoS pathogens. 2005;1(2):e19 Epub 2005/10/29. 10.1371/journal.ppat.0010019 ; PubMed Central PMCID: PMCPmc1266308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn CP, Dull PM, Semenova V, Li H, Crotty S, Taylor TH, et al. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. The Journal of infectious diseases. 2004;190(7):1228–36. Epub 2004/09/04. 10.1086/423937 . [DOI] [PubMed] [Google Scholar]
- 18.Shlyakhov E, Rubinstein E, Novikov I. Anthrax post-vaccinal cell-mediated immunity in humans: kinetics pattern. Vaccine. 1997;15(6–7):631–6. Epub 1997/04/01. . [DOI] [PubMed] [Google Scholar]
- 19.Shlyakhov E, Rubinstein E. Evaluation of the anthraxin skin test for diagnosis of acute and past human anthrax. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 1996;15(3):242–5. Epub 1996/03/01. . [DOI] [PubMed] [Google Scholar]
- 20.Ingram RJ, Ascough S, Reynolds CJ, Metan G, Doganay M, Baillie L, et al. Natural cutaneous anthrax infection, but not vaccination, induces a CD4(+) T cell response involving diverse cytokines. Cell & bioscience. 2015;5:20 Epub 2015/06/16. 10.1186/s13578-015-0011-4 ; PubMed Central PMCID: PMCPmc4464127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baillie L, Hebdon R, Flick-Smith H, Williamson D. Characterisation of the immune response to the UK human anthrax vaccine. FEMS immunology and medical microbiology. 2003;36(1–2):83–6. Epub 2003/05/03. . [DOI] [PubMed] [Google Scholar]
- 22.Brachman PS, Gold H, Plotkin SA, Fekety FR, Werrin M, Ingraham NR. Field Evaluation of a Human Anthrax Vaccine. American journal of public health and the nation's health. 1962;52(4):632–45. Epub 1962/04/01. ; PubMed Central PMCID: PMCPmc1522900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feodorova VA, Sayapina LV, Corbel MJ, Motin VL. Russian vaccines against especially dangerous bacterial pathogens. Emerg Microbes Infect. 2014;3:e86 10.1038/emi.2014.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shlyakhov EN, Rubinstein E. Human live anthrax vaccine in the former USSR. Vaccine. 1994;12(8):727–30. Epub 1994/06/01. . [DOI] [PubMed] [Google Scholar]
- 25.Brenneman KE, Doganay M, Akmal A, Goldman S, Galloway DR, Mateczun AJ, et al. The early humoral immune response to Bacillus anthracis toxins in patients infected with cutaneous anthrax. FEMS immunology and medical microbiology. 2011;62(2):164–72. Epub 2011/03/16. 10.1111/j.1574-695X.2011.00800.x ; PubMed Central PMCID: PMCPmc3605738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Splino M, Patocka J, Prymula R, Chlibek R. Anthrax vaccines. Annals of Saudi medicine. 2005;25(2):143–9. Epub 2005/06/28. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller J, McBride BW, Manchee RJ, Moore P, Baillie LW. Production and purification of recombinant protective antigen and protective efficacy against Bacillus anthracis. Letters in applied microbiology. 1998;26(1):56–60. Epub 1998/03/07. . [DOI] [PubMed] [Google Scholar]
- 28.Williamson ED, Bennett AM, Perkins SD, Beedham RJ, Miller J, Baillie LW. Co-immunisation with a plasmid DNA cocktail primes mice against anthrax and plague. Vaccine. 2002;20(23–24):2933–41. Epub 2002/07/20. . [DOI] [PubMed] [Google Scholar]
- 29.Beedham RJ, Turnbull PC, Williamson ED. Passive transfer of protection against Bacillus anthracis infection in a murine model. Vaccine. 2001;19(31):4409–16. Epub 2001/08/03. . [DOI] [PubMed] [Google Scholar]
- 30.Cote CK, Rossi CA, Kang AS, Morrow PR, Lee JS, Welkos SL. The detection of protective antigen (PA) associated with spores of Bacillus anthracis and the effects of anti-PA antibodies on spore germination and macrophage interactions. Microbial pathogenesis. 2005;38(5–6):209–25. Epub 2005/06/01. 10.1016/j.micpath.2005.02.001 . [DOI] [PubMed] [Google Scholar]
- 31.Hepburn MJ, Dyson E, Simpson AJ, Brenneman KE, Bailey N, Wilkinson L, et al. Immune response to two different dosing schedules of the anthrax vaccine precipitated (AVP) vaccine. Vaccine. 2007;25(32):6089–97. Epub 2007/07/03. 10.1016/j.vaccine.2007.05.018 . [DOI] [PubMed] [Google Scholar]
- 32.Semenova VA, Steward-Clark E, Stamey KL, Taylor TH Jr., Schmidt DS, Martin SK, et al. Mass value assignment of total and subclass immunoglobulin G in a human standard anthrax reference serum. Clinical and diagnostic laboratory immunology. 2004;11(5):919–23. Epub 2004/09/11. 10.1128/cdli.11.5.919-923.2004 ; PubMed Central PMCID: PMCPmc515271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bliss CI. Linear regression Statistics in biology: statistical methods for research in the natural sciences. 1 New York: McGraw-Hill; 1967. p. 152–85. [Google Scholar]
- 34.Park S, Leppla SH. Optimized production and purification of Bacillus anthracis lethal factor. Protein expression and purification. 2000;18(3):293–302. Epub 2000/03/29. 10.1006/prep.2000.1208 . [DOI] [PubMed] [Google Scholar]
- 35.Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. Journal of immunological methods. 1989;119(2):203–10. Epub 1989/05/12. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data is in raw (untransformed) format.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.