Abstract
The potential indirect effect of observed maternal sensitivity at 5 months on the development of infants’ behavioral regulation of emotion from 5 to 10 months (i.e., distraction and maternal-orientation behaviors) via infant’s vagal withdrawal was investigated (N = 230). Results indicated that maternal sensitivity at 5 months was not directly associated with behavioral regulation at 10 months. However, greater maternal sensitivity at 5 months was associated with greater vagal withdrawal at 10 months, after controlling for vagal withdrawal at 5 months. Further, vagal withdrawal at 10 months was associated with greater orientation toward the mother at 10 months, after controlling for 5-month orientation behaviors. The indirect effect of maternal sensitivity on maternal-orientation behaviors was significant, suggesting that infant’s vagal withdrawal may be one potential mechanism through which maternal sensitivity is related to behavioral regulation.
Emotion regulation processes, which are observable at the biological and the behavioral level, refer to the behaviors, skills, and strategies that allow individuals to manage emotional arousal (Calkins & Hill, 2007; Fox & Calkins, 2003). The ability to regulate oneself during emotional situations emerges in infancy and has implications for multiple areas of development, including later social competence (Spinrad et al., 2006), school readiness (Denham, 2006), and psychological adjustment (Calkins, 1994; Hill, Degnan, Calkins, & Keane, 2006). Thus, one of the most fundamental skills for infants to acquire is the ability to modulate (i.e., inhibit or enhance) emotional expressions and experiences (Calkins & Hill, 2007; Stifter, 2002).
Maturation of prefrontal cortical and limbic system processes plays a role in the development and deployment of attention and the regulation of emotion (Posner & Rothbart, 2000). Because emotional responding is at least partially dependent on the biological maturation of these physiological systems, emotion regulation processes are thought to stem from early physiological regulation to more effortful regulation of behavior (Beauregard, Lévesque, & Paquette, 2004; Calkins, 2011; Ochsner & Gross, 2004). Although there has been much theorizing about these processes, more work is needed to better understand how the development of physiological systems is related to the development of behavioral regulation.
Biological and behavioral regulatory processes are also influenced by social interactions between infants and their caregivers (Calkins, 1994; Calkins & Hill, 2007), and a number of empirical studies have found caregiver behaviors to be associated with individual differences in infants’ regulatory strategies and physiological functioning (e.g., Calkins & Johnson, 1998; Kennedy, Rubin, Hastings, & Maisel, 2004; Moore et al., 2009). Far fewer studies, however, have assessed the role of specific caregiver behaviors on the development of individual biological systems and specific behavioral regulatory strategies during early infancy, a time when behaviors are becoming more purposeful and physiological systems are rapidly maturing (Porges & Furman, 2011). Given that the development of behavioral regulation is thought to stem from the maturation of physiological systems, and maternal sensitivity is found to be associated with individual differences in infants’ physiological functioning and behavioral regulatory strategies (Calkins & Johnson, 1998; Moore & Calkins, 2004; Moore et al., 2009), infant physiological regulation may be one way through which maternal sensitivity influences infants’ regulatory behaviors. Thus, the current study aimed to assess whether maternal sensitivity indirectly predicted greater behavioral regulatory abilities during early infancy through infants’ cardiac vagal withdrawal.
Emotion regulation behavior
Adaptive emotion regulation involves initiation and maintenance of emotion states, as well as the ability to reduce heightened levels of arousal in ways that allow individuals to meet regulatory goals and maintain positive social interactions with the environment (Bridges, Denham, & Ganiban, 2004). Although it is necessary to regulate both positive and negative emotions, the investigation of the regulation of negative emotions is particularly important because the task of coping with negative affect is thought to be more developmentally difficult than coping with positive affect (Ramsden & Hubbard, 2002). At birth, infants show individual temperamental differences in negative reactivity to emotionally charged situations (Calkins, Fox, & Marshall, 1996; Rothbart, Derryberry, & Hershey, 2000) and have little control over their own emotional arousal. By 3 months of age, however, the development of motor skills and attentional control mechanisms allows infants to begin to control their behaviors and arousal in contexts that evoke negative affect (Eisenberg, Smith, Sadovsky, & Spinrad, 2004; Rothbart, Ziaie, & O’Boyle, 1992; Ruff & Rothbart, 1996).
Specific regulatory strategies that have been the primary focus of theoretical and empirical work include disengagement of attention and mother-orientation behaviors (Rothbart et al., 1992). Rothbart, Posner, and Boylan (1990) demonstrated that by 6 months of age, infants are able to use voluntary control of visual attention to distract or avert attention away from the source of negative arousal to other less emotionally arousing objects in their environment (Johnson, Posner, & Rothbart, 1991). And indeed, much research, including contingency studies, has demonstrated that infants who avert their gaze or distract away from a distressing stimulus (i.e., frustrating or fearful) to a less arousing object show reduced negative affect in the moment and less anxious behavior over time (Crockenberg & Leerkes, 2004, 2006; Mangelsdorf, Shapiro, & Marzolf, 1995; Stifter & Spinrad, 2002). In addition, because young infants are just developing independent regulatory strategies and are often engaged in coregulatory processes to calm distress, averting attention away from the source of arousal and orienting toward the caregiver may serve as an adaptive regulatory strategy in that it facilitates coregulation between the infant and the mother in an active attempt to reduce arousal (Stifter & Braungart, 1995). Thus, both distraction and maternal-orientation behaviors involve an infant averting their attention away from the source of arousal. However, distraction behaviors during which infants direct their attention to other objects in the room, rather than a caregiver, may be considered more self-regulatory than maternal-orientation behaviors that aim to elicit a coregulatory dynamic.
Physiological underpinnings of behavioral emotion regulation
Emotion regulation theories that integrate biological and physiological aspects of regulation assume that adaptive emotion regulation behaviors result from the maturation of different biological systems across childhood (Calkins & Hill, 2007; Thompson, Lewis, & Calkins, 2008), and empirical work investigating biological markers associated with emotion regulation has underscored the importance of the parasympathetic nervous system (PNS) in the development of biobehavioral regulatory processes (Calkins, 2011; Graziano & Derefinko, 2013; Porges, 2007). Porges (1995) identified an index of the functional status of the PNS (i.e., vagal tone), which reflects vagal control of the heart, as a measurable variable that accounts for differences in the development of emotional expression and regulation. To assess vagal influence, Porges (1995) developed a method that measures the amplitude and period of the oscillations associated with inhalation and exhalation; this measure refers to the variability in heart rate which occurs at the frequency of breathing (respiratory sinus arrhythmia, RSA) and is thought to reflect parasympathetic influence on heart rate by way of the vagus nerve.
Research on the study of the physiological regulation of emotion has been particularly focused on vagal withdrawal during emotionally charged contexts. Vagal withdrawal is indexed by a decrease in RSA during situations where coping or emotional regulation is necessary. Specifically, the vagus nerve sends input to the heart and causes changes in cardiac activity that allows the body to transition between sustaining metabolic processes and generating responses to the environment (Porges, 2007). During situations that do not present environmental challenge, the vagus nerve inhibits the sympathetic nervous system’s influence on cardiac activity through increased parasympathetic influence, thus producing a relaxed and restorative state (Porges, 1995). However, during an environmentally challenging situation that calls for active coping, vagal influence is withdrawn to support an increase in heart rate and increased attention to the environment, which allow individuals to employ behaviors necessary to deal with challenging situations. Increased alertness and attentiveness, as a result of vagal withdrawal, may therefore facilitate the use of regulatory behaviors that function to reduce distress. In this way, vagal withdrawal (i.e., RSA during a baseline procedure—RSA during an emotion eliciting task) during challenge may be used as an indicator of infants’ physiological regulation.
Although limited, research has supported the association between infant vagal withdrawal and infant regulatory behaviors. Most studies assessing this link examine vagal withdrawal within the still-face paradigm, a task designed to elicit distress by removing social and emotional communication with the mother through maternal depressed affect (Weinberg & Tronick, 1996). Infants are expected to show vagal withdrawal during the stressful still-face procedure, and many researchers have demonstrated this pattern (Bazhenova, Plonskaia, & Porges, 2001; Moore & Calkins, 2004; Weinberg & Tronick, 1996). Consistent with these findings, researchers have also found infants and children with greater vagal withdrawal show greater soothability, attentional control (Huffman et al., 1998), behavioral distraction (Calkins, 1997), and fewer socially withdrawn, depressed, and aggressive behaviors (Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996).
Effect of maternal sensitivity on behavioral and physiological emotion regulation
Both biological and behavioral processes associated with the regulation of emotion are influenced by and embedded within environmental contexts (Fox & Calkins, 2003), and the most influential context for young children is the caregiving environment. Over the first few years of life, there is a fundamental shift from dyadic to self-regulation that is characterized by a gradual transition from almost sole reliance on a caregiver to the ability to independently regulate emotional arousal in varying situations (Sameroff, 2010). Bowlby (1973) asserted that in the presence of sensitive and responsive caregiving (i.e., consistent and appropriate responses to infant signals that are considerate of the context and the infant’s developmental level), infants form strong bonds of emotional communication with their mothers in which they gain socioemotional competencies that get internalized into the capacity to appropriately regulate, generate, and maintain states of emotional security. More recent biobehavioral synchrony models have extended upon Bowlby’s work and posit that affiliative bonds, formed from repeated exposure to the coordination between physiological states and behaviors between infants and their caregivers, set the framework for infants’ emotional development and influence children’s lifelong capacity to modulate arousal and engage in regulatory behaviors, both of which are central components to children’s adaptive social and emotional functioning (Feldman, 2007, 2012). Thus, caregiving is thought to play a critical role in the development of infant’s behavioral and physiological regulatory capacities.
Maternal sensitivity and behavioral regulation of emotion
One way in which caregiving may influence infants’ experience and behavioral expressions of negative emotions is through coregulation processes (Fogel, 1993; Tronick, 1989). A history of shared experiences that are effective in reducing infants’ arousal may lead infants to independently orient toward their caregiver during emotionally charged situations (e.g., social referencing), thereby facilitating infant–caregiver coregulation. During these interactions, parents socialize infants’ emotions and emotion signals through modeling specific expressive styles in an effort to teach infants how to modulate their feeling states and express behavior in appropriate ways (Malatesta, Grigoryev, Lamb, Albin, & Culver, 1986; Malatesta & Haviland, 1982). In addition, by engaging in synchronous and well-regulated communicative patterns, caregivers can create a context in which infants associate their behaviors and the caregiver’s behaviors with their accompanying changes in emotional state (Gianino & Tronick, 1988; Kopp, 1989). For example, caregivers may engage in facial and vocal cues that distract infants when they are distressed therefore introducing the redirection of infant attention when distressed as a strategy to be employed when wishing to reduce negative arousal. By utilizing distraction techniques, caregivers may give infants opportunities to learn that shifts in attention can coincide with decreases in negative affect when presented with emotional challenge or threat (Spinrad & Stifter, 2002). Infants who repeatedly experience reduced negative affect through these interactions may then develop and repeat similar behaviors when confronting emotionally charged situations independently.
Cross-sectional and longitudinal studies have supported this association. Calkins and Johnson (1998), for example, found that when mothers used more positive guidance their 18-month-old infants engaged in more distraction and gaze aversion strategies during frustrating events, and researchers have demonstrated that mothers and fathers who were more sensitive had 4-month-old infants who displayed increased parent-orientation behaviors during the still-face paradigm (Braungart-Rieker, Garwood, Powers, & Notaro, 1998). Further, infants who spend more time engaged in collaborative joint attention during a parent-involved delay task have been found to avert their attention away from a delay object, providing support for the suggestion that parents who establish shared attention on objects during interaction may facilitate the development of children’s ability to use their own attention to reduce distress (Morales, Mundy, Crowson, Neal, & Delgado, 2005).
In a longitudinal controlled intervention study designed to improve mother’s ability to monitor infant signals attentively and respond contingently from 6 to 9 months of age, van den Boom (1994) reported that mothers in the intervention group were significantly more responsive, stimulating, and visually attentive to their 9-month-old infant’s behavior than mothers in the control group. In addition, it was found that mothers in the intervention group had infants who scored higher than control infants on sociability, behavioral regulation, and exploration at 9 months. Finally, Glöggler and Pauli-Pott (2008) reported that low maternal depression and high sensitivity at 4, 8, and 12 months predicted subsequent adaptive regulatory behaviors in 30-month-old children during a fear task. Taken together, these studies highlight the importance of sensitive caregiving in the development and deployment of behavioral regulation across infancy.
Maternal sensitivity and physiological regulation of emotion
In addition to behavioral regulation, caregiving has been found to be associated with infants’ ability to regulate physiologically. Early in development, children have rudimentary physiological abilities in place and are therefore reliant on external sources to achieve and maintain a regulated physiological state (Spangler & Grossmann, 1993). Warm, supportive, responsive, and sensitive parenting may aid in the organization and development of physiological systems to achieve regulation and reduce negative affect (e.g., Moore & Calkins, 2004; Moore et al., 2009). Although the exact process through which sensitive caregiving influences infants’ physiology is not known, several hypotheses have been posited. Sensitive caregiver responses may influence young children’s ability to regulate physiological stress by facilitating physiological homeostasis as caregivers help infants find a balance between meeting their individual needs and coping with environmental stimuli during emotionally challenging contexts (Hofer, 1987; Spangler & Grossmann, 1993). Recently, Porges and Furman (2011) have posited that social interactions with caregivers can calm and soothe an infant’s physiological state and that caregiving may facilitate greater myelination of vagal fibers and development of the vagal system. An increase in the myelination of vagal fibers may improve the modulation of physiological arousal and enable infants to engage in greater behavioral and attentional regulation, as well as positive social interactions (Porges & Furman, 2011).
Empirical work demonstrates associations between early caregiving behavior and vagal withdrawal. Maternal–child relationship quality at age 2, for example, was found to predict the degree of children’s vagal withdrawal at 5 years old even after controlling for behavior problems and vagal withdrawal at age 2, such that children with poorer early maternal–child relationships displayed significantly less vagal withdrawal at a later age (Calkins, Graziano, Berdan, Keane, & Degnan, 2008). In a similar study assessing the effects of maternal emotional support on the trajectory of vagal withdrawal, children of mothers who provided greater emotional support at age 3 had greater levels of vagal withdrawal at age 3 and age 4 when compared to children of mothers displaying lower emotional support (Perry et al., 2013). Further, infants who engaged in less synchronous interactions with caregivers displayed a less adaptive pattern of vagal withdrawal as evidenced by higher vagal withdrawal during a normal play episode, less vagal withdrawal during a situation meant to elicit distress, and more difficulty returning to previous levels of baseline vagal tone following distress (Moore & Calkins, 2004). Negative and controlling maternal behavior has also been associated with less vagal withdrawal (Calkins, Smith, Gill, & Johnson, 1998), and maternal positive touch has been found to reduce infant’s physiological reactivity to stress (Feldman, Singer, & Zagoory, 2010). Finally, Propper et al. (2008) found that infants who were at genetic risk for poor physiological regulation had vagal withdrawal similar to those not at genetic risk by 12 months of age when they were exposed to sensitive parenting during early infancy. In sum, these findings suggest that sensitive caregiving may promote the development of effective physiological regulation in young children.
The current study
Vagal withdrawal has consistently been associated with behavioral outcomes across development in a variety of contexts (e.g., Hastings et al., 2008; Marcovitch et al., 2010). However, little is known regarding how the development of physiological systems relates to the development of behavioral regulation. Thus, the first goal of the current study was to assess whether developmental change in vagal withdrawal from 5 to 10 months was associated with developmental change in infant regulatory behaviors from 5 to 10 months. We expected that infants’ vagal withdrawal at 10 months, after controlling for vagal withdrawal at 5 months, would be associated with observed regulatory behavior at 10 months, after controlling for regulatory behavior at 5 months. Further, sensitive maternal behaviors have been found to be associated with both behavioral and physiological regulation across childhood (e.g., Calkins & Johnson, 1998; Moore & Calkins, 2004), but little longitudinal work has examined the way in which maternal sensitivity may be related to the development of these specific regulatory processes during early infancy. Therefore, the second goal of the current study was to examine whether maternal sensitivity predicts developmental change in infants’ vagal withdrawal and emotion regulation behaviors from 5 to 10 months. We expected maternal sensitivity at 5 months to be positively associated with greater vagal withdrawal at 10 months, after controlling for vagal withdrawal at 5 months, and greater behavioral regulation at 10 months, after controlling for 5-month behavioral regulation. Finally, given the theoretical and empirical links between physiology and behavior, and the associations between maternal sensitivity and physiological and behavioral regulatory process, the third goal of the study was to examine a potential indirect effect of maternal sensitivity on infant’s behavioral regulation. Maternal sensitivity was expected to be indirectly associated with infants’ regulatory behaviors through infants’ vagal withdrawal.
METHODS
Participants
The current study utilized data from infants and their mothers who are part of a larger, ongoing longitudinal study examining psychobiological processes in cognitive and emotional development (N = 410). Study participants were recruited by two research locations (Greensboro, North Carolina, and Blacksburg, Virginia), with each location recruiting half of the total sample. Participants at each site did not differ in terms of sex, χ2 (1, N = 388) = 2.26, p = ns. However, the Blacksburg site had mothers with higher levels of education on average, t(378) = −3.26, p < .001, and the Greensboro site had a greater number of ethnic minority participants, χ2 (1, N = 384) = 26.65, p < .001. Infants were recruited via commercial mailing lists, flyers and newspaper advertisements, and word of mouth. Of the original 410 study participants, 22 were flagged as having a developmental delay (i.e., weighing <5lbs 8 oz at birth, being born more than 28 days early, or being diagnosed with a particular developmental delay) and were therefore removed, resulting in a total of 388 healthy, full-term infants.
The sample utilized in the current study included 230 infants (124 female, 106 male) who participated in a frustrating arm-restraint task and who had available RSA data (i.e., both baseline RSA and task RSA) at the 5- or 10-month visit. Of the 230 infants, 179 were Caucasian, 30 were African American, 20 identified as multiracial or other, and 1 reported as Asian; 61% of mothers had a college education or higher. All infants were born within 15 days of their calculated due dates and were healthy at the time of testing. Infants’ mean age (in days) was 162 (SD = 8) at the 5-month visit and 314 (SD = 13) at the 10-month visit.
As part of laboratory visit protocol, if infants got highly upset prior to the administration of the arm-restraint task, the arm-restraint task was not administered. Thus, the primary reason for the decrease in sample size from the overall sample to the sample utilized in the current study was nonparticipation in the arm-restraint procedure. This was the case for 123 infants at the 5-month visit and 103 infants at the 10-month visit. Data were also lost due to RSA artifact (N = 7) and equipment failure (N = 10). Tests of mean comparisons revealed that the current sample did not differ from the overall sample in terms of gender, χ2 (1, N = 388) = 1.55, p = ns, race, χ2 (1, N = 384) = 0.63, p = ns, maternal sensitivity, t(357) = −1.45, p = ns, baseline RSA at 5 months, t(357) = 1.88, p = ns, or baseline RSA at 10 months, t(336) = .68, p = ns. Because infants did not participate in the arm-restraint task if they became upset prior to its administration, and this may be a consequence of the fact that these infants may be more negatively reactive in general, we also tested whether there were significant differences between the overall sample and the current sample in mother-reported infant negative affectivity as measured by the Infant Behavior Questionnaire (Rothbart & Derryberry, 1981). However, no significant differences were found at 5 months, t(374) = 1.22, p = ns, or 10 months, t(344) = 1.55, p = ns.
Procedures
The experimental protocol was approved by the University Institutional Review Boards at each research location (Greensboro; Blacksburg). Data were collected in both research locations using identical protocols. Research assistants from both locations were trained together on protocol administration and behavioral and psychophysiological coding and scoring by the last author. To ensure that identical protocol administration was maintained between the laboratories, the Blacksburg site periodically viewed DVD recordings and psychophysiology files collected by the Greensboro site. To ensure that identical coding criteria were maintained between laboratories, the Blacksburg site coded all behavioral data collected by both laboratories and provided reliability coding of artifact screening for psychophysiology data collected by the Greensboro site.
Upon arrival at the research laboratory, infants and mothers were greeted by a research assistant who explained the study procedures and obtained signed consent from the mother. After a brief 5-min warm-up period, infants sat on their mothers’ laps, while a researcher applied heart rate electrodes. Infants participated in laboratory tasks assessing cognitive, emotional, and maternal–child interaction processes. The 5- and 10-month laboratory visits were identical, and each session was digitally recorded for later behavioral coding. Parents were paid $50 for each laboratory visit.
Measures
Observed maternal sensitivity
Maternal sensitivity was observed during two mother–child interaction tasks. For each task, mothers were asked to interact with their infants as they normally would. During the first interaction task, mothers were given two age appropriate infant toys and asked to play with her infant for 2 min. This was followed immediately by a 2-min peekaboo task in which mothers were instructed to play peekaboo with their infants using their hands or a washcloth provided.
Maternal sensitivity was coded in 30 sec epochs during the mother–child interaction tasks using a coding scheme adapted from previous work (Calkins, Hungerford, & Dedmon, 2004; Fish, Stifter, & Belsky, 1991; Shapiro & Mangelsdorf, 1994). Mother sensitivity was rated on a 1–4 scale for each epoch according to how skillfully and appropriately the mother facilitated the infant’s response to an object or the peekaboo game and/or how well she followed and contingently responded to the infant’s attention and behavior. A score of 1 on an epoch indicated that the mother showed very low or no facilitation of the infant’s attention to the task or objects in the task and did not respond to the infant’s attention and spontaneous behaviors. A score of 4 indicated that the mother showed repeated instances of appropriate and well-timed facilitative behaviors, sensitive and contingent responding to the infant, and adapted her own behavior according to the infant’s spontaneous behavior. Final scores for maternal sensitivity on each task were created by summing scores across epochs and dividing by the number of epochs to create an average score for the task. As maternal sensitivity on the two interaction tasks was significantly positively correlated (r = .37, p < .001), an average maternal sensitivity score was created by averaging the scores for the two tasks.
Reliability coding for maternal sensitivity was accomplished on 36% of the sample. The interclass correlations (ICCs) between each pair of coders were examined and determined to be acceptable for each task (.74 for both).
Observed infant regulation
Following both mother–child interaction tasks, infant regulatory behaviors were observed and coded during a 2-min arm-restraint procedure, which has been shown to reliably produce short-term distress and frustration in infants and has been widely used as a challenge task within the developmental literature (Calkins, Dedmon, Gill, Lomax, & Johnson, 2002). Mothers were instructed to stand to the right or left of the highchair and hold their infant’s arms down at their sides, so that their arm movements would be restricted. Mothers were also told to maintain a neutral facial expression and use no vocalizations. If infants became extremely distressed (i.e., hard crying), experimenters were instructed to terminate the procedure.
Two regulatory behaviors, mother-orientation and distraction, were coded in 10 sec epochs. Mother orienting was coded when the infant was looking, vocalizing, or otherwise attempting to engage with the mother. Distraction was coded when the infant was visually attending to or attempting to manipulate an object other than the mother. Final scores for mother orienting and distraction behaviors were calculated as the number of epochs on which the behavior occurred out of the total number of epochs for the task, which yielded a proportion score for each measure. Reliability coding was accomplished on 30% of the sample. The ICCs between each pair of coders were examined and determined to be acceptable: mother orienting (.93), distraction (.95).
Vagal withdrawal
To measure vagal withdrawal, continuous electrocardiogram (ECG) data were recorded during a baseline task and during the arm-restraint procedure (described above). During the baseline task, infants’ ECG was recorded for 1 min, while they were seated on their mother’s laps and watched a research assistant manipulate a toy containing brightly colored balls on a testing table 1.1 m in front of them. This procedure quieted the infant and yielded minimal gross motor movements. Mothers were instructed not to talk or interact with their infant during the task.
Electrocardiogram data were recorded from two neonatal disposable electrodes using modified lead II alignment (right collarbone and lower left rib; Stern, Ray, & Quigley, 2001). The cardiac electrical activity was amplified using a SA Instrumentation Bioamp (San Diego, CA, USA) and bandpassed from 0.1 to 100 Hz. The QRS complex was displayed on the acquisition computer monitor and digitized at 512 samples per second. The acquisition software was Snapshot-Snapstream (HEM Corporation, Southfield, MI, USA), and the raw data were stored for later R-wave detection and analyses.
Electrocardiogram data were examined and analyzed using IBI Analysis System software developed by James Long Company (Caroga Lake, NY, USA). First, R-waves were detected offline with a four-pass peak detection algorithm, resulting in a data file with onset times for each detected R-wave. To edit ECG artifact, the ECG signal was viewed alongside tick marks representing the times of software-detected R-waves. If an R-wave was not detected by the software, a tick mark was inserted into the graphical ECG record. If the undetected R-wave was visible in the ECG, it was marked manually. If the R-wave was not visible, the tick mark was placed based on the specific editing rules of Byrne and Porges (1993). Movement artifact was designated by the absence of at least three consecutive Rwaves. These artifact-scored epochs were eliminated from all calculations. The edited R-wave was converted to heart period (i.e., time between heart beats).
Because vagal influence is measured by RSA (i.e., the variability in heart rate which occurs at the frequency of breathing), spectral analysis was used to calculate high-frequency variability (i.e., RSA) in the heart period data, using a discrete Fourier transform with a 16-sec Hanning window and 50% overlap. The frequency band for quantification of RSA at each age was 0.24–1.04 Hz. This frequency band is appropriate for all age groups between infancy and early childhood (Bar-Haim, Marshall, & Fox, 2000). The RSA data were transformed using natural log to normalize the distribution. To calculate vagal withdrawal, RSA during the arm-restraint task was subtracted from RSA during the baseline task, so that positive values indicate greater withdrawal and increased vagal withdrawal.
RESULTS
Descriptive statistics for all study variables are presented in Table 1. Given the number of correlations presented in Table 1, Bonferroni corrections were made to reduce type 1 error. Using the stricter Bonferroni correction, only estimates with p-values < .001 were significant. A path analysis was conducted to examine the associations between maternal sensitivity and infant emotion regulation behaviors through vagal withdrawal utilizing Mplus (Version 7; Muthén & Muthén, 2012). Full information maximum likelihood (FIML) was used to handle missing data, and infant gender, maternal education, and race were examined as potential covariates. These variables were not correlated with primary study variables and their inclusion in the model weakened model fit and left structural paths unchanged. Therefore, gender, race, and maternal education were not included in the current analyses. However, because baseline RSA is thought to impact the magnitude of vagal withdrawal during challenge such that higher baseline levels allow for greater decreases in RSA (Graziano & Derefinko, 2013), baseline RSA at 5 and 10 months was entered into the model as control variables. Finally, given our interest in examining whether maternal sensitivity predicted developmental change in infants’ vagal withdrawal from 5 to 10 months, and whether this was related to developmental changes in infant’s regulatory behaviors from 5 to 10 months, 5-month vagal withdrawal, distraction behaviors, and maternal-orientation behaviors were also controlled for in the model.
TABLE 1.
Descriptive Statistics and Correlations Among Model Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Baseline RSA at 5 montds | – | ||||||||
| 2. Baseline RSA at 10 months | .35** | – | |||||||
| 3. Vagal withdrawal at 5 months | .38** | .14 | – | ||||||
| 4. Vagal withdrawal at 10 months | .11 | .45** | .20 | – | |||||
| 5. Distraction at 5 months | .10 | .04 | −.14 | −.05 | – | ||||
| 6. Distraction at 10 months | .12 | .02 | −.01 | −.14 | −.02 | – | |||
| 7. Maternal orientation at 5 months | −.02 | .10 | .11 | .31** | −.21** | .02 | – | ||
| 8. Maternal orientation at 10 months | −.03 | .10 | −.02 | .22* | −.00 | −.17* | .18* | – | |
| 9. Maternal sensitivity at 5 months | .08 | .06 | .15* | .15 | .08 | −.02 | .20** | −.01 | – |
| Mean | 3.88 | 4.61 | −.54 | −.10 | .52 | .42 | .40 | .55 | 3.49 |
| Standard Deviation | 1.18 | 1.10 | 1.45 | 1.40 | .35 | .31 | .31 | .32 | .43 |
| Skew (SE) | 1.78 (.16) | .50 (17) | 1.13 (.19) | .18 (.22) | −.17 (.18) | .18 (.19) | .50 (.18) | −.06 (.19) | −.94 (.16) |
| N | 220 | 207 | 171 | 122 | 183 | 172 | 183 | 172 | 222 |
Note. RSA = respiratory sinus arrhythmia.
Based on Bonferroni corrections to reduce type 1 error, only estimates with p-values < .001 are significant.
p < .05,
p < .001.
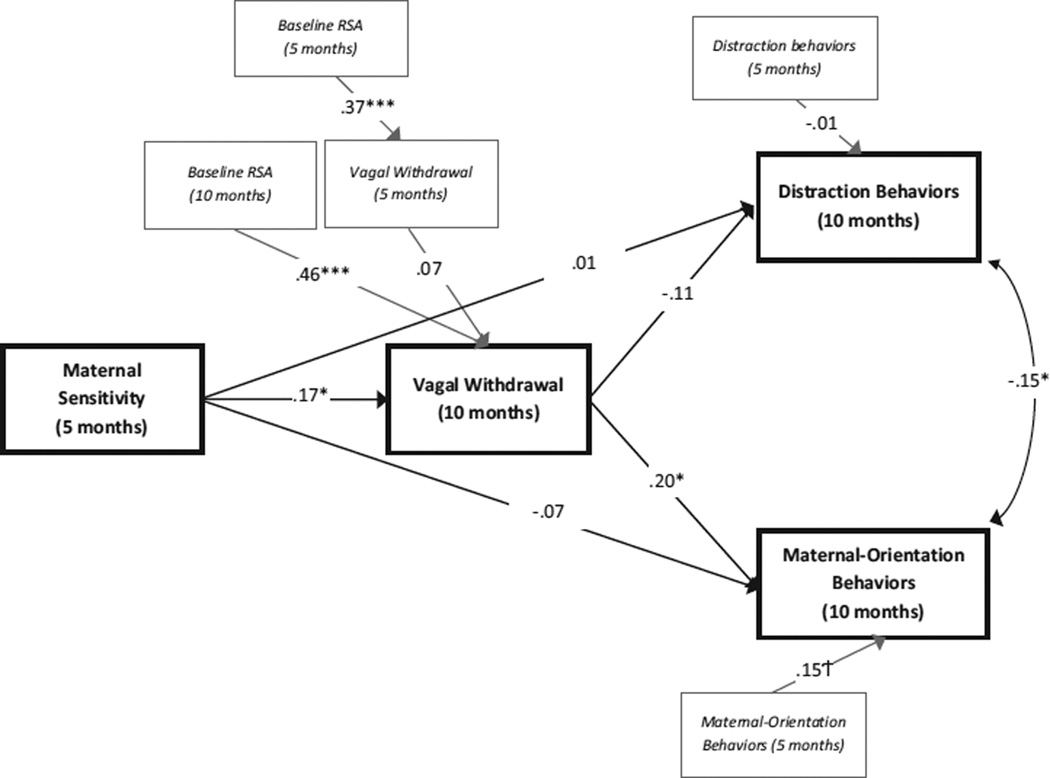
The hypothesized model fit well, χ2 (18, N = 230) = 23.01, p = .19, CFI = .95, RMSEA = .03 [CI = .00, .07] (standardized coefficients are presented in Figure 1). The first aim of the study was to assess whether developmental change in vagal withdrawal was associated with developmental change in infant regulatory behaviors. After controlling for 5-month vagal withdrawal and 5-month maternal-orientation behaviors, vagal withdrawal at 10 months was associated positively with infant maternal-orientation behaviors at 10 months. In contrast, after controlling for 5-month vagal withdrawal and 5-month distraction behaviors, vagal withdrawal at 10 months was not significantly associated with infant distraction behaviors at 10 months.
Figure 1.
Standardized estimates for the indirect effects model predicting infant regulatory behaviors. Italicized wording delineates variables included for the purposes of controlling for previous levels. †p < .10, *p < .05, ***p < .001.
We also aimed to examine whether maternal sensitivity is associated with infants’ vagal withdrawal and emotion regulation behaviors across early infancy. Contrary to hypotheses, direct paths from maternal sensitivity at 5 months to infant distraction behaviors and maternal orientation behaviors at 10 months were not significant. However, maternal sensitivity was associated positively with infant’s vagal withdrawal at 10 months after controlling for vagal withdrawal at 5 months, suggesting that greater sensitivity at 5 months is associated with increases in vagal withdrawal from 5 to 10 months.
Finally, we aimed to assess whether maternal sensitivity was an indirect predictor of infant regulation behaviors via infants’ vagal withdrawal. Although it was hypothesized that early maternal sensitivity may be indirectly related to later distraction and maternal-orientation behaviors through infants’ vagal withdrawal, vagal withdrawal at 10 months was not significantly associated with infant distraction behaviors at 10 months. Thus, the indirect effect could not be considered. However, the indirect effect from maternal sensitivity at 5 months to maternal-orientation behaviors at 10 months was tested using a bias-corrected bootstrapping procedure (10,000 draws). This approach has been shown to generate the most accurate confidence intervals for indirect effects, reducing type 1 error rates and increasing power over other similar tests (MacKinnon, Lockwood, & Williams, 2004). The indirect path was significant (unstandardized estimate = .03, 95% BC bootstrap CI [.01, .08]), indicating that greater maternal sensitivity when infants were 5 months was associated with greater developmental change in maternal-orientation behaviors from 5 to 10 months through its influence on developmental changes in infants’ vagal withdrawal from 5 to 10 months.
DISCUSSION
Emotion regulation is a fundamental skill that operates at various levels of child functioning and has implications for multiple developmental outcomes (Vohs & Baumeister, 2010). The ability to regulate is thought to be rooted in the biological maturation of physiological systems over time, and regulation continues to function at both a biological and behavioral level across development (Calkins & Fox, 2002). Although often theoretically speculated, a limited amount of empirical work demonstrates associations between the development of physiological systems and changes in behavior. Even fewer studies assess this association during early infancy, a time when rapid maturation in regulatory processes occurs (Kopp, 1982). Therefore, the current study aimed to assess the way in which infants’ cardiac vagal withdrawal is associated with infant’s observed regulation behaviors from 5 to 10 months. As expected, greater vagal withdrawal was associated with more time spent engaging in maternal-orientation behaviors during frustration. Contrary to expectations, however, vagal withdrawal was not associated with increases in distraction behaviors.
A core component of vagal withdrawal is control of attentional processes (Porges, 1992). During a task designed to elicit emotional arousal, increased vagal withdrawal is thought to heighten awareness and allow individuals to adaptively redirect their attention to a less stimulating object or person that serves to effectively reduce arousal. And indeed, prior research employing frustration or stress paradigms (e.g., still-face paradigm; Tronick, Als, Adamson, Wise, & Brazelton, 1978) has shown young infants to distract away from a source of distress as a means of modulating arousal (e.g., Planalp & Braungart-Rieker, 2015). Therefore, it is somewhat surprising that developmental changes in infants’ vagal withdrawal from 5 to 10 months were not associated with greater increases in the use of distraction behaviors during this time. However, during early infancy, it may be more appropriate and adaptive for infants to engage in more maternal-orientation behaviors and attempt to facilitate coregulation when the opportunity permits, rather than employing a more independent strategy such as distraction. Infants’ regulatory capabilities are extremely limited during the first year of life and are built from within the caregiver–infant dyad (Propper & Moore, 2006). Over time, infants begin to trust that their caregiver will assist them if they fail to reduce their own distress; in turn, this trust serves as a safety net that eventually allows infants to explore new situations, and their own abilities, more freely (Sroufe, 1996). Thus, directing attention to caregivers and making bids for assistance in reducing heightened negative arousal during unfamiliar contexts may promote stronger emotional bonds that lead to the development of more independent regulatory strategies such as distraction in toddlerhood and preschool.
In addition to gaining a better understanding regarding the physiological underpinnings of behavioral regulation, we aimed to examine the way in which maternal sensitivity is related to infants’ physiological and behavioral regulation of emotion. The importance of sensitive caregiving in teaching and facilitating the use of appropriate behavioral strategies and helping children develop and manage physiological states has been both theorized and empirically supported. Only a small body of research, however, has examined the relations between parenting and infants’ physiological and behavioral regulation simultaneously. As expected, maternal sensitivity when infants were 5 months was associated with vagal withdrawal at 10 months after controlling for 5-month vagal withdrawal. Early infancy is a developmental time period during which there is a rapid biological maturation of the vagus system (Sachis, Armstrong, Becker, & Bryan, 1982). And, although the specific ways in which caregiving behavior influences changes in physiological systems remain unclear, these findings support theories positing that sensitive and stimulating emotional interactions, serving to upregulate and downregulate arousal, may facilitate the development of infants’ vagal functioning and aid infants in finding ways to process and cope with environmental stimuli (Fields, 2005; Porges & Furman, 2011). It should be noted, however, that empirical research has found transactional relations among these two constructs. For example, in a longitudinal crosslagged model, Perry, Mackler, Calkins, and Keane (2014) also found maternal sensitivity in infancy to predict vagal withdrawal in preschool, but vagal withdrawal in preschool subsequently predicted maternal sensitivity at the start of school. The authors suggested that children who are better able to physiologically regulate may be easier to parent and therefore elicit more sensitive caregiving. In addition, in a longitudinal approach similar to that of the current study, Kennedy et al. (2004) showed baseline cardiac vagal tone to predict restrictive and supportive parenting practices from age 2 to age 4. Thus, although a growing body of research supports the caregivers’ role in the development of early physiological systems, the developmental time period and bidirectional associations should be taken into consideration when interpreting empirical research examining these constructs.
Contrary to hypotheses, maternal sensitivity was not directly related to increases in infants’ distraction behaviors or maternal-orientation behaviors. It may be that direct associations appear after the first year as opportunities for parental assistance in regulating emotions increase and children are able to gauge the effectiveness of specific caregiver strategies. Cognitive capabilities that allow infants to link caregiver-assisted redirection of attention with the reduction in arousal in a way that facilitates independent use of distraction behaviors may not yet be fully developed by 10 months of age. Although voluntary shifts in attention do begin to occur between 3 and 6 months (Rothbart et al., 1990), additional shifts in voluntary attention indicative of increased cognitive control have been demonstrated by 12 months (Diamond, 1991). And indeed, purposeful redirection of attention away from a distressing stimulus and toward another object or event is most frequently used by older infants and toddlers (Grolnick, Kurowski, McMenamy, Rivkin, & Bridges, 1998; Grolnick, McMenamy, & Kurowski, 2006). Thus, mothers may be more able to directly impact infant’s physiological functioning during early infancy, a time of rapid biological maturation, and teach or model more sophisticated emotion regulation strategies in late infancy and early childhood.
As hypothesized, maternal sensitivity was indirectly associated with increases in infant’s maternal-orientation behaviors through increases in vagal withdrawal, thus providing preliminary empirical evidence that vagal withdrawal may be one early mechanism through which the caregiving context is internalized into later behavioral social and emotional competencies across childhood. In line with current biobehavioral theories, findings suggest that maternal sensitivity may promote increased maturation of the vagal system and greater vagal withdrawal. Infants with greater vagal withdrawal and increased attentional abilities may be more likely to recognize when they need caregiver assistance and be more likely to give caregivers direct signals (i.e., orientating to them) when help is needed. In turn, an increased number of infant–caregiver interactions may provide caregivers with more opportunities for teaching self-regulatory strategies and may allow for the transition from sole reliance on a caregiver to greater self-regulation to occur at a faster rate. Although the current findings provide important insight into biobehavioral processes of emotion regulation and support vagal withdrawal as one mechanism through which maternal behavior influences infants’ behavior, it is important to keep in mind that autonomic regulation is likely not the only mechanism. For example, mothers’ behavior may influence other physiological systems (i.e., neural activation or HPA functioning), or infants’ executive functioning, in turn making them more cognitively skilled at choosing appropriate and effective regulatory behaviors for specific contexts. Thus, future work examining emotion regulation from a biobehavioral perspective is needed to disentangle the multiple processes through which social interactions between caregivers and children impact behavioral adjustment and maladjustment. We do, however, believe this study to be an important first step in beginning to identify the specific process mechanisms associated with the development of adaptive emotion regulation skills, especially given the early developmental time period in which we are examining.
Additional research is needed to better understand the longitudinal relations between parenting and the development of both behavioral and biological regulatory processes. However, the current study has multiple implications for developmental theory and research. First, findings highlight the importance of moving beyond studying single early time points as predictors of later outcomes and underscore the need for researchers to better understand how development in constructs over time is associated with one another. In addition, because a significant indirect effect emerged linking maternal sensitivity to infant maternal-orientation behaviors through vagal withdrawal, findings suggest that a regulated physiological state that allows infants to purposefully initiate caregiver assistance during heightened arousal may be an important skill that increases opportunities for parents to build infant trust and teach appropriate behavioral regulation strategies. Also noteworthy is the use of a biobehavioral frame-work that aims to better understand the link between emotion regulation processes at multiple levels. For over a decade, researchers have been theorizing biological underpinnings of observed behaviors. Relatively, little empirical work, however, has been able to longitudinally address their associations in combination with extrinsic predictors of this association. By examining the relation between early caregiver behavior and its impact on developmental changes in biological and behavioral emotion regulation processes across early infancy, the current study provides preliminary insight into these complex biobehavioral processes.
Although the current study had several strengths, there are some noteworthy limitations. First, we focused on the regulation of frustration. Infants may show different behavioral and physiological responses to other emotionally charged contexts such as fear or excitement. In addition, some recent research suggests varying levels of vagal influence are adaptive in some social and cognitive contexts (Calkins, Graziano, & Keane, 2007; Gazelle & Druhen, 2009; Hastings et al., 2008; Marcovitch et al., 2010). Thus, it will be important for future research to gain a better understanding of the relations addressed in the current study during other social, cognitive, and emotion eliciting tasks.
Second, the measure of maternal sensitivity used in the current study was a composite score of general sensitivity and mothers in the current sample scored somewhat high. Therefore, the study is limited in that it cannot speak to the influence of specific sensitive behaviors that may be related to infant’s behavioral and physiological regulatory capabilities, nor can it address the effects of extremely insensitive behaviors such as maltreatment. Future research should examine these associations in more atrisk samples with greater variation in caregiving behavior. In addition, examining maternal sensitivity for longer periods than was done in the current study may allow researchers to better assess the direct impact of maternal sensitive behavior and disentangle the measure of maternal sensitivity into a continuum of specific caregiving behaviors, which would provide valuable insight into the specific process mechanisms that underlie the relation between sensitive caregiving and infant regulation.
Finally, although the current sample was a relatively diverse sample, the majority of mothers in our sample had a college education or higher (61%). Therefore, an important goal for future work will be to recruit mothers with more variability in education level and/or income, as both of these have consistently been associated with maternal sensitivity in multiple empirical studies (e.g., Mills-Koonce et al., 2009; NICHD Early Child Care Research Network, 2004). Moreover, the sample utilized in the current study was a smaller subset of the overall project sample that only included infants who had available data for the arm-restraint task. Although tests of mean comparisons suggest that the current study sample did not systematically differ from the overall study sample with regard to any study variables or mother-reported negative affect, infants with available arm-restraint data did in fact express less negativity prior to the administration of the frustration task. The time of day in which infants visited the laboratory, their sleep schedules, and their daily routine were not considered in the current analyses and may have contributed to infants’ irritability and negative affect prior to the administration of the task, thus increasing the number of infants excluded. Additional work considering these factors is needed to replicate the current findings.
Valuable information regarding the associations between caregiving and vagal withdrawal was provided by the current study, and vagal withdrawal was shown to play a role in the link between maternal sensitivity and the development of infant’s regulatory behaviors. Thus, our findings highlight the need to assess both intrinsic and extrinsic child factors when attempting to understand individual differences in the development of emotion regulation across childhood (Fox & Calkins, 2003). In addition, findings underscore the importance of assessing the longitudinal relation between both contextual and individual characteristics, and potential mediating mechanisms, when gaining insight into developmental processes involved in the regulation of emotion.
ACKNOWLEDGEMENTS
This research was supported by Grants HD049878 and HD043057 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to the last author. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We are grateful to the families for their participation in our research and to our research teams at Blacksburg and Greensboro for their assistance with data collection and coding. [Corrections added on 8 September 2015, after first online publication: Acknowledgements section has been added.]
Contributor Information
Nicole B. Perry, Department of Human Development and Family Studies, University of North Carolina at Greensboro
Susan D. Calkins, Department of Human Development and Family Studies, University of North Carolina at Greensboro and Department of Psychology, University of North Carolina at Greensboro
Martha Ann Bell, Department of Psychology, Virginia Tech.
REFERENCE
- Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology. 2000;37(1):44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Bazhenova OV, Plonskaia O, Porges SW. Vagal reactivity and affective adjustment in infants during interaction challenges. Child Development. 2001;72:1314–1326. doi: 10.1111/1467-8624.00350. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Lévesque J, Paquette V. Neural basis of conscious and voluntary self-regulation of emotion. In: Beauregard M, editor. Consciousness, emotional self-regulation and the brain. Amsterdam, the Netherlands: John Benjamins Publishing Company; 2004. pp. 163–194. [Google Scholar]
- van den Boom DC. The influence of temperament and mothering on attachment and exploration: An experimental manipulation of sensitive responsiveness among lower-class mothers with irritable infants. Child Development. 1994;65:1457–1477. doi: 10.1111/j.1467-8624.1994.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss: Vol. 2 Separation: Anxiety and anger. New York, NY: Basic Books; 1973. [Google Scholar]
- Braungart-Rieker J, Garwood M, Powers BP, Notaro PC. Infant affect and affect regulation during the still-face paradigm with mothers and fathers: The role of infant characteristics and parental sensitivity. Developmental Psychology. 1998;34:1428–1437. doi: 10.1037//0012-1649.34.6.1428. [DOI] [PubMed] [Google Scholar]
- Bridges LJ, Denham SA, Ganiban JM. Definitional issues in emotion regulation research. Child Development. 2004;75:340–345. doi: 10.1111/j.1467-8624.2004.00675.x. [DOI] [PubMed] [Google Scholar]
- Byrne EA, Porges SW. Data-dependent filter characteristics of peak-valley respiratory sinus arrhythmia estimation: A cautionary note. Psychophysiology. 1993;30:397–404. doi: 10.1111/j.1469-8986.1993.tb02061.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Individual differences in the biological aspects of temperament. In: Bates JE, Wachs TD, editors. Temperament: Individual differences at the interface of biology and behavior. Washington, DC: American Psychological Association; 1994. pp. 199–217. [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31:125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Caregiving as coregulation: Psychobiological processes and child functioning. In: Booth A, McHale SM, Landale NS, editors. Biosocial foundations of family processes. New York, NY: Springer Science + Business Media; 2011. pp. 49–59. [Google Scholar]
- Calkins SD, Dedmon S, Gill K, Lomax L, Johnson L. Frustration in infancy: Implications for emotion regulation, physiological processes, and temperament. Infancy. 2002;3:175–198. doi: 10.1207/S15327078IN0302_4. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA. Self-regulatory processes in early personality development: A multilevel approach to the study of childhood social withdrawal and aggression. Development and Psychopathology. 2002;14:477–498. doi: 10.1017/s095457940200305x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Berdan LE, Keane SP, Degnan KA. Predicting cardiac vagal withdrawal in early childhood from maternal–child relationship quality during toddlerhood. Developmental Psychobiology. 2008;50:751–766. doi: 10.1002/dev.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal withdrawal differentiates among children at risk for behavior problems. Biological Psychology. 2007;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins S, Hill A. Caregiver influences on emerging emotion regulation. In: Gross JJ, editor. Handbook of emotion regulation. New York, NY: Guilford Press; 2007. pp. 229–248. [Google Scholar]
- Calkins SD, Hungerford A, Dedmon SE. Mothers’ interactions with temperamentally frustrated infants. Infant Mental Health Journal. 2004;25:219–239. [Google Scholar]
- Calkins SD, Johnson MC. Toddler regulation of distress to frustrating events: Temperamental and maternal correlates. Infant Behavior & Development. 1998;21:379–395. [Google Scholar]
- Calkins SD, Smith CL, Gill KL, Johnson MC. Maternal interactive style across contexts: Relations to emotional, behavioral, and physiological regulation during toddlerhood. Social Development. 1998;7:350–369. [Google Scholar]
- Crockenberg SC, Leerkes EM. Infant and maternal behaviors regulate infant reactivity to novelty at six months. Developmental Psychology. 2004;40:1123–1132. doi: 10.1037/0012-1649.40.6.1123. [DOI] [PubMed] [Google Scholar]
- Crockenberg SC, Leerkes EM. Infant and maternal behavior moderate reactivity to novelty to predict anxious behavior at 2.5 years. Development and Psychopathology. 2006;18(1):17–34. doi: 10.1017/S0954579406060020. [DOI] [PubMed] [Google Scholar]
- Denham S. Social emotional competence as a support for school readiness: What is it and how do we assess it? Early Education and Development. 2006;17(1):57–89. [Google Scholar]
- Diamond A. Frontal lobe involvement in cognitive changes during the first year of life. In: Gibson KR, Petersen AC, editors. Brain maturation and cognitive development: Comparative and cross-cultural perspectives. Hawthorne, NY: Aldine de Gruyter; 1991. pp. 127–180. [Google Scholar]
- Eisenberg N, Smith CL, Sadovsky A, Spinrad TL. Effortful control: Relations with emotion regulation, adjustment, and socialization in childhood. In: Baumeister RR, Vohs KD, editors. Handbook of self-regulation: Research, theory & applications. New York, NY: The Guilford Press; 2004. pp. 259–282. [Google Scholar]
- Feldman R. Parent-infant synchrony: Biological foundations and developmental outcomes. Current Directions in Psychological Science. 2007;16:340–345. [Google Scholar]
- Feldman R. Physiological measures of emotion from a developmental perspective: State of the science: Parent–infant synchrony: A biobehavioral model of mutual influences in the formation of affiliative bonds. Monographs of the Society for Research in Child Development. 2012;77(2):42–51. [Google Scholar]
- Feldman R, Singer M, Zagoory O. Touch attenuates infants physiological reactivity to stress. Developmental Science. 2010;13:271–278. doi: 10.1111/j.1467-7687.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- Fields RD. Myelination: An overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11:528–531. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish M, Stifter CA, Belsky J. Conditions of continuity and discontinuity in infant negative emotionality: Newborn to five months. Child Development. 1991;62:1525–1537. [PubMed] [Google Scholar]
- Fogel A. Developing through relationships: Origins of communication, self, and culture. Chicago, IL: University of Chicago Press; 1993. [Google Scholar]
- Fox NA, Calkins SD. The development of self-control of emotion: Intrinsic and extrinsic influences. Motivation and Emotion. 2003;27(1):7–26. [Google Scholar]
- Gazelle H, Druhen MJ. Anxious solitude and peer exclusion predict social helplessness, upset affect, and vagal withdrawal in response to behavioral rejection by a friend. Developmental Psychology. 2009;45:1077–1096. doi: 10.1037/a0016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianino AA, Tronick EZ. The mutual regulation model: The infant’s self and interactive regulation and coping and defensive capacities. In: Field TM, McCabe PM, Schneiderman N, editors. Stress and coping across development. Hillsdale, NJ: Lawrence Erlbaum Associates Inc; 1988. pp. 47–68. [Google Scholar]
- Glöggler B, Pauli-Pott U. Different fear-regulation behaviors in toddlerhood: Relations to preceding infant negative emotionality, maternal depression, and sensitivity. Merrill-Palmer Quarterly: Journal of Developmental Psychology. 2008;54(1):86–101. [Google Scholar]
- Graziano P, Derefinko K. Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology. 2013;94(1):22–23. doi: 10.1016/j.biopsycho.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolnick WS, Kurowski CO, McMenamy JM, Rivkin I, Bridges LJ. Mothers’ strategies for regulating their toddlers’ distress. Infant Behavior & Development. 1998;21:437–450. [Google Scholar]
- Grolnick WS, McMenamy JM, Kurowski CO. Emotional self-regulation in infancy and toddlerhood. In: Balter L, Tamis-LeMonda CS, editors. Child psychology: A handbook of contemporary issues. 2nd ed. New York, NY: Psychology Press; 2006. pp. 3–25. [Google Scholar]
- Hastings PD, Nuselovici JN, Utendale WT, Coutya J, McShane KE, Sullivan C. Applying the polyvagal theory to children’s emotion regulation: Social context, socialization, and adjustment. Biological Psychology. 2008;79:299–306. doi: 10.1016/j.biopsycho.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Hill AL, Degnan KA, Calkins SD, Keane SP. Profiles of externalizing behavior problems for boys and girls across preschool: The roles of emotion regulation and inattention. Developmental Psychology. 2006;42:913–928. doi: 10.1037/0012-1649.42.5.913. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Shaping forces within early social relationships. In: Krasnegor NA, Blass EM, Hofer MA, editors. Perinatal development: A psychobiological perspective. San Diego, CA: Academic Press; 1987. pp. 251–274. [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: Assessments at twelve weeks of age. Child Development. 1998;69:624–635. [PubMed] [Google Scholar]
- Johnson MH, Posner MI, Rothbart MK. Components of visual orienting in early infancy: Contingency learning, anticipatory looking, and disengaging. Journal of Cognitive Neuroscience. 1991;3:335–344. doi: 10.1162/jocn.1991.3.4.335. [DOI] [PubMed] [Google Scholar]
- Kennedy AE, Rubin KH, Hastings PD, Maisel B. Longitudinal relations between child vagal tone and parenting behavior: 2 to 4 years. Developmental Psychobiology. 2004;45(1):10–21. doi: 10.1002/dev.20013. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18:199–214. [Google Scholar]
- Kopp CB. Regulation of distress and negative emotions: A developmental view. Developmental Psychology. 1989;25:343–354. [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research. 2004;39(1):99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta CZ, Grigoryev P, Lamb C, Albin M, Culver C. Emotion socialization and expressive development in preterm and full-term infants. Child Development. 1986;57:316–330. doi: 10.1111/j.1467-8624.1986.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Malatesta CZ, Haviland JM. Learning display rules: The socialization of emotion expression in infancy. Child Development. 1982;53:991–1003. [PubMed] [Google Scholar]
- Mangelsdorf SC, Shapiro JR, Marzolf D. Developmental and temperamental differences in emotional regulation in infancy. Child Development. 1995;66:1817–1828. [PubMed] [Google Scholar]
- Marcovitch S, Leigh J, Calkins SD, O’Brien M, Leerkes EM, Blankson AN. Moderate vagal withdrawal in 3.5-year-old children is associated with optimal performance on executive function tasks. Developmental Psychobiology. 2010;52:603–608. doi: 10.1002/dev.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills-Koonce WR, Propper CB, Barnett M, Gariépy J-L, Moore G, Calkins S, Cox M. Psychophysiological correlates of parenting behavior in mothers of young children. Developmental Psychobiology. 2009;51:650–661. doi: 10.1002/dev.20400. [DOI] [PubMed] [Google Scholar]
- Moore GA, Calkins SD. Infants’ vagal withdrawal in the still-face paradigm is related to dyadic coordination of mother–infant interaction. Developmental Psychology. 2004;40:1068–1080. doi: 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- Moore GA, Hill-Soderlund AL, Propper CB, Calkins SD, Mills-Koonce W, Cox MJ. Mother-infant vagal withdrawal in the face-to-face still-face paradigm is moderated by maternal sensitivity. Child Development. 2009;80(1):209–223. doi: 10.1111/j.1467-8624.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- Morales M, Mundy P, Crowson MM, Neal A, Delgado CF. Individual differences in infant attention skills, joint attention, and emotion regulation behaviour. International Journal of Behavioral Development. 2005;29:259–263. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 7th ed. Los Angeles, CA: Muthén & Muthézn; 2012. [Google Scholar]
- NICHD Early Child Care Research Network. Affect dysregulation in the mother–child relationship in the toddler years: Antecedents and consequences. Development and Psychopathology. 2004;16(3):43–68. doi: 10.1017/s0954579404044402. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Thinking makes it so: A social cognitive neuroscience approach to emotion regulation. In: Baumeister RF, Vohs KD, editors. Handbook of self-regulation: Research, theory, and applications. New York, NY: Guilford Press; 2004. pp. 229–255. [Google Scholar]
- Perry NB, Mackler JS, Calkins SD, Keane SP. A transactional analysis of the relation between maternal sensitivity and child vagal regulation. Developmental Psychology. 2014;50:784–793. doi: 10.1037/a0033819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry NB, Nelson JA, Swingler MM, Leerkes EM, Calkins SD, Marcovitch S, O’Brien M. The relation between maternal emotional support and child physiological regulation across the preschool years. Developmental Psychobiology. 2013;55:382–394. doi: 10.1002/dev.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planalp EM, Braungart-Rieker JM. Trajectories of regulatory behaviors in early infancy: Determinants of infant self-distraction and self-comforting. Infancy. 2015;20:129–159. doi: 10.1111/infa.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. Autonomic regulation and attention. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Hillsdale, NJ: Lawrence Erlbaum Associates Inc.; 1992. pp. 201–223. [Google Scholar]
- Porges SW. Cardiac vagal tone: A physiological index of stress. Neuroscience & Biobehavioral Reviews. 1995;19:225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales A, Greenspan SI. Infant regulation of the vagal ‘brake’ predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Porges SW, Furman SA. The early development of the autonomic nervous system provides a neural platform for social behaviour: A polyvagal perspective. Infant and Child Development. 2011;20(1):106–118. doi: 10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M, Rothbart M. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Propper C, Moore GA. The influence of parenting on infant emotionality: A multi-level psychobiological perspective. Developmental Review. 2006;26:427–460. [Google Scholar]
- Propper C, Moore G, Mills-Koonce W, Halpern C, Hill-Soderlund AL, Calkins SD, Cox M. Gene-environment contributions to the development of infant vagal reactivity: The interaction of dopamine and maternal sensitivity. Child Development. 2008;79:1377–1394. doi: 10.1111/j.1467-8624.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- Ramsden S, Hubbard J. Family expressiveness and parental emotion coaching: Their role in children’s emotion regulation and aggression. Journal of Abnormal Child Psychology. 2002;30:657–667. doi: 10.1023/a:1020819915881. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Derryberry D. Development of individual differences in temperament. In: Lamb ME, Brown AL, editors. Advances in developmental psychology. Vol. 1. Hillsdale, NJ: Erlbaum; 1981. pp. 37–86. [Google Scholar]
- Rothbart MK, Derryberry D, Hershey K. Stability of temperament in childhood: Laboratory infant assessment to parent report at seven years. In: Molfese V, Molfese D, editors. Temperament and personality development across the lifespan. Mahwah, NJ: Erlbaum; 2000. pp. 85–119. [Google Scholar]
- Rothbart MK, Posner MI, Boylan A. Regulatory mechanisms in infant development. In: Enns JT, editor. The development of attention: Research and theory. Oxford, England and North-Holland: Elsevier Science Publishers; 1990. pp. 47–66. [Google Scholar]
- Rothbart MK, Ziaie H, O’Boyle CG. Self-regulation and emotion in infancy. In: Eisenberg N, Fabes RA, Eisenberg N, Fabes RA, editors. Emotion and its regulation in early development. San Francisco, CA: Jossey-Bass; 1992. pp. 7–23. [DOI] [PubMed] [Google Scholar]
- Ruff HA, Rothbart MK. Attention in early development: Themes and variations. New York, NY: Oxford University Press; 1996. [Google Scholar]
- Sachis PN, Armstrong DL, Becker LE, Bryan AC. Myelination of the human vagus nerve from 24 weeks postconceptional age to adolescence. Journal of Neuropathology and Experimental Neurology. 1982;41:466–472. doi: 10.1097/00005072-198207000-00009. [DOI] [PubMed] [Google Scholar]
- Sameroff A. A unified theory of development: A dialectic integration of nature and nurture. Child Development. 2010;81(1):6–22. doi: 10.1111/j.1467-8624.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- Shapiro JR, Mangelsdorf SC. The determinants of parenting competence in adolescent mothers. Journal of Youth and Adolescence. 1994;23:621–641. doi: 10.1007/BF01537633. [DOI] [PubMed] [Google Scholar]
- Spangler G, Grossmann KE. Biobehavioral organization in securely and insecurely attached infants. Child Development. 1993;64:1439–1450. doi: 10.1111/j.1467-8624.1993.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Spinrad TL, Eisenberg N, Cumberland A, Fabes RA, Valiente C, Shepard SA, Guthrie IK. Relation of emotion-related regulation to children’s social competence: A longitudinal study. Emotion. 2006;6:498–510. doi: 10.1037/1528-3542.6.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinrad TL, Stifter CA. Maternal sensitivity and infant emotional reactivity: Concurrent and longitudinal relations. Marriage & Family Review. 2002;34:243–263. [Google Scholar]
- Sroufe L. Emotional development: The organization of emotional life in the early years. New York, NY: Cambridge University Press; 1996. [Google Scholar]
- Stern RM, Ray WJ, Quigley KS. Psychophysiological recording. 2nd ed. New York, NY: Oxford University Press; 2001. [Google Scholar]
- Stifter CA. Individual differences in emotion regulation in infancy: A thematic collection. Infancy. 2002;3:129–132. doi: 10.1207/S15327078IN0302_1. [DOI] [PubMed] [Google Scholar]
- Stifter CA, Braungart JM. The regulation of negative reactivity in infancy: Function and development. Developmental Psychology. 1995;31:448–455. [Google Scholar]
- Stifter CA, Spinrad TL. The effect of excessive crying on the development of emotion regulation. Infancy. 2002;3:133–152. doi: 10.1207/S15327078IN0302_2. [DOI] [PubMed] [Google Scholar]
- Thompson RA, Lewis MD, Calkins SD. Reassessing emotion regulation. Child Development Perspectives. 2008;2:124–131. [Google Scholar]
- Tronick EZ. Emotions and emotional communication in infants. American Psychologist. 1989;44:112–119. doi: 10.1037//0003-066x.44.2.112. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, Als H, Adamson L, Wise S, Brazelton TB. The infant’s response to entrapment between contradictory messages in face-to-face interaction. Journal American Academy of Child Psychiatry. 1978;17(1):1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- Vohs KD, Baumeister RF. Handbook of self-regulation, second edition: Research, theory, and applications. New York, NY: Guilford Press; 2010. [Google Scholar]
- Weinberg KM, Tronick EZ. Infant affective reactions to the resumption of maternal interaction after the still-face. Child Development. 1996;67:905–914. [PubMed] [Google Scholar]