Abstract
The objectives of this study were 1) to describe maternal glucose and lipid kinetics and 2) to examine the relationships with infant birth weight in obese women with pregestational type 2 diabetes during late pregnancy. Using stable isotope tracer methodology and mass spectrometry, maternal glucose and lipid kinetic rates during the basal condition were compared in three groups: lean women without diabetes (Lean, n = 25), obese women without diabetes (OB, n = 26), and obese women with pregestational type 2 diabetes (OB+DM, n = 28; total n = 79). Glucose and lipid kinetics during hyperinsulinemia were also measured in a subset of participants (n = 56). Relationships between maternal glucose and lipid kinetics during both conditions and infant birth weight were examined. Maternal endogenous glucose production (EGP) rate was higher in OB+DM than OB and Lean during hyperinsulinemia. Maternal insulin value at 50% palmitate Ra suppression (IC50) for palmitate suppression with insulinemia was higher in OB+DM than OB and Lean. Maternal EGP per unit insulin and plasma free fatty acid concentration during hyperinsulinemia most strongly predicted infant birth weight. Our findings suggest maternal fatty acid and glucose kinetics are altered during late pregnancy and might suggest a mechanism for higher birth weight in obese women with pregestational diabetes.
Introduction
Pregnancy complicated by pregestational diabetes is associated with a host of adverse neonatal outcomes, including preterm birth, congenital malformations, increased birth weight, and neonatal hypoglycemia (1,2). Higher birth weight in infants born to women with diabetes contributes to elevated rates of birth injury and cesarean delivery, as well as higher risk for future obesity, diabetes, and cardiovascular disease (3–6). Maternal substrate metabolism during pregnancy plays a key role in fetal growth (7) and thus may be an important target for interventions aimed to modulate the deleterious effect of diabetes on infant birth weight.
Traditionally, higher birth weight in infants born to women with diabetes has been thought to be caused primarily by maternal hyperglycemia. Thus, current treatment in diabetic pregnancy focuses on achieving as close to maternal normoglycemia as possible (8). However, some past and more recent data suggest that maternal glycemia is a poor predictor of infant birth weight (9,10) and that markers of maternal lipid metabolism during pregnancy might contribute to and possibly be a better predictor of infant birth weight and adiposity (11–19). Previous studies demonstrating associations between indices of maternal glucose and lipid metabolism and infant birth weight have focused only on clinical measures of maternal metabolism, including fasting plasma glucose (9,16,17,20), HbA1c (15), oral glucose tolerance testing (9,11,16,18,19), and plasma concentrations of free fatty acid (FFA) (15), triglyceride (11–15,20), and cholesterol (20). Although clinical measures are easily collected and some are part of routine clinical care, these measures have not elucidated the pathologic abnormalities that lead to pregnancy-associated risks. Therefore, identification of the pathophysiologic mechanisms during both the fasting and hyperinsulinemic states that drive alterations in glucose and lipid metabolism in women with type 2 diabetes during pregnancy is needed.
Thus, the primary objective of the study was to compare maternal glucose and fatty acid kinetics during late pregnancy in obese women with diabetes to obese women without diabetes and lean, healthy control subjects. The secondary objective of the study was to examine the relationships between maternal glucose and fatty acid kinetics and infant birth weight. We hypothesized that glucose and fatty acid kinetics during basal and hyperinsulinemic conditions are abnormal in obese women with diabetes and would be better predictors of infant birth weight than clinical markers of glucose and lipid metabolism.
Research Design and Methods
Participants
Six hundred and twenty six women were screened at 24–26 weeks' gestation and 79 women were enrolled and studied at 32–36 weeks' gestation. Women were stratified into three separate groups: 1) obese women with pregestational type 2 diabetes (OB+DM, n = 28), 2) obese women without diabetes (pregestational or gestational) (OB, n = 26), and 3) lean control subjects without diabetes (Lean, n = 25). Participants were all receiving prenatal care at the Women’s Health Center at Barnes-Jewish Hospital/Washington University School of Medicine in St. Louis between May 2011 and December 2013. Inclusion criteria included women 18–44 years of age, confirmed singleton viable pregnancy with no identified fetal abnormalities (as determined by routine anatomy ultrasound at 18–22 weeks), and prepregnancy BMI between 18.0 and 24.9 kg/m2 for Lean or prepregnancy BMI between 30 and 45 kg/m2 for OB and OB+DM. Women with pregestational diabetes were diagnosed as White class B (preexisting diabetes with onset at 20 years or later with duration of ≤10 years, n = 20) or C (preexisting diabetes with onset between 10 and 19 years and with duration of 10–19 years, n = 8), were on insulin therapy, and had HbA1c ≤8% (183 mg/dL, 64 mmol/mol) for >3 months prior to pregnancy. All participants with diabetes were taking intermediate-acting insulin (100%, NPH, b.i.d.: morning/evening) and either rapid- (73%, aspart/Humalog, t.i.d.) or short-acting insulin (13%, regular, t.i.d.) at meals. Exclusion criteria for all women included 1) multiple gestation pregnancy, 2) inability to provide voluntary informed consent, 3) current self-reported use of illegal drugs (cocaine, methamphetamine, or opiates), 4) current smoker who did not consent to cessation, and 5) current usage of daily medications by class: corticosteroids, β-blockers (known to affect lipid metabolism), and antipsychotics (known to alter insulin resistance and metabolic profiles). For women without diabetes, exclusion criteria included 1) diagnosis or history of gestational diabetes mellitus, 2) prepregnancy diabetes, or 3) prior macrosomic (>4,500 g) infant. This study was approved by the Human Research Protection Office at Washington University in St. Louis (institutional review board no. 201012828, NCT01346527).
Glucose and Fatty Acid Kinetics
The evening prior to the study, participants were provided a standardized meal containing 12 kcal/kg body weight and 55% carbohydrate, 30% fat, and 15% protein at 1800 h provided by the Washington University in St. Louis Institute of Clinical and Translational Sciences Bionutrition Service. Participants then fasted overnight and until completion of the study the next day. OB+DM women were instructed to take their insulin the day prior as normal; however, they did not take insulin therapy the morning of the study until study completion. The following morning, at 0700 h, a catheter was inserted into an antecubital vein and used to administer stable isotope-labeled tracers where constant intravenous infusions of [6,6-2H2]glucose (0.25 μmol ⋅ kg−1 ⋅ min−1 with a 22.5 μmol/kg priming dose) and [U-13C]palmitate (12 nmol ⋅ kg-1 ⋅ min−1, unprimed) were initiated and maintained for 300 min. A second catheter was inserted into a hand vein on the contralateral arm; the hand was heated (55°C) using a thermostatically controlled box to obtain arterialized venous blood samples (21). We used palmitate as the choice for our tracer study as palmitate is one of the most abundant FFAs in the plasma, is thought to be representative of long-chain fatty acid metabolism, is relatively cheap, and is easily examined by gas chromatography–mass spectrometry (GC-MS) (22), and this tracer has been successfully used in our and other investigators’ studies examining fatty acid metabolism in a variety of disorders (23–25). All tracers came from Cambridge Isotope Laboratories (Andover, MA). All participants completed a basal period (0–120 min, n = 79). A subset of participants (n = 56) completed a one-stage hyperinsulinemic-euglycemic clamp (120–300 min) in order to evaluate glucose and fatty acid metabolism under hyperinsulinemic conditions. The Washington University in St. Louis Human Studies Committee determined partway through the study that the hypersinsulinemic clamp was more than minimal risk (i.e., necessary when a fetus is involved); therefore not all participants completed the clamp part of the study. During the hyperinsulinemic stage of the clamp, a primed (160 mU ⋅ m2 ⋅ min−1 × 5 min; 80 mU ⋅ m2 ⋅ min−1 × 5 min), constant (40 mU ⋅ m2 ⋅ min−1) infusion of regular human insulin was administered intravenously and continued for 180 min (total study time 300 min). This insulin dose range has been shown to increase glucose disposal in pregnant women (26) and moderately suppress hepatic glucose production and lipolysis in nongravid insulin-resistant adults in our previous study (27). Plasma glucose concentration was maintained at 5 mmol/L (90 mg/dL) by a variable-rate infusion of 20% dextrose containing 1.5% [6,6-2H2]glucose. Blood samples were obtained every 10 min during the clamp to quantify plasma glucose concentrations and used to adjust the 20% dextrose infusion rate. Blood and breath samples were collected in vacutainers before starting the tracer infusions to quantify background 2H, 13C, and 13CO2 enrichments, and every 10 min during the last 30 min of the basal and hyperinsulinemic periods to quantify hormone levels, substrate levels, and glucose and fatty acid kinetics. Whole-body oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured continuously for 15 min using indirect calorimetry (Parvo Medics, Sandy, UT) at 75 and 255 min of the study, as previously described (24).
Palmitate oxidation rate was corrected for incomplete labeled CO2 recovery by an acetate correction factor via an infusion of [1,2-13C]acetate for 120 or 300 min (depending on clamp participation) 1 week prior to or after the glucose and fatty acid metabolism study, as previously described (24).
Sample Analyses
Plasma glucose concentration was measured using an automated glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin levels were quantified using a chemiluminescent immunometric method (Immulite; Siemens, Los Angeles, CA). The plasma insulin assay range is 2–300 µU/mL and the interassay coefficient of variation is 4% in the low (10.5 µU/mL) and high insulin concentration range (55.1 µU/mL).
The tracer-to-tracee ratios (TTRs) for plasma [2H2]glucose and [U-13C]palmitate were quantified using capillary GC-MS (Agilent 6890N gas chromatograph and Agilent 5973N mass selective detector; Agilent, Palo Alto, CA), as previously described (24). For [2H2]glucose enrichment quantification, plasma proteins were precipitated with cold acetone, lipids were extracted into hexane, and the aqueous phase was dried (Labconco, Kansas City, MO). The heptafluorobutyric derivative of glucose was formed, and [2H2]glucose enrichment was quantified using GC-electron ionization-MS and selective ion monitoring (mass/charge ratio [m/z] 519 and 521). Plasma [U-13C]palmitate enrichment was quantified after plasma proteins were precipitated with cold acetone, lipids were extracted into hexane, and the fatty acid methyl esters were produced via iodomethane and dichloromethane. Plasma [U-13C]palmitate enrichment was quantified using GC-electron ionization-MS with selective ion monitoring (m/z 270 and 286). The GC-MS instrument response was calibrated using isotopic enrichment standards of known TTR for [2H2]glucose and [U-13C]palmitate. Breath 13CO2 enrichment was measured by isotope ratio mass spectrometry (Finnigan DELTAplus XL, Bremen, Germany).
Calculations
Plasma glucose and palmitate rates of appearance (Ra) were calculated by dividing each tracer infusion rate by the average TTR obtained during the last 30 min of each stage (i.e., basal, hyperinsulinemia) of the clamp, as previously described (24). Glucose rate of disappearance (Rd) was calculated as the sum of endogenous glucose Ra plus infused dextrose. Palmitate oxidation rate was determined by dividing breath 13CO2 production (13CO2 TTR × VCO2 production rate) by the plasma palmitate TTR and corrected for 13CO2 recovery as determined during the acetate infusion study. The insulin value at 50% palmitate Ra suppression (IC50) was calculated by log transforming insulin and palmitate Ra values and calculating the slope and intercept of 50% suppression of palmitate Ra by insulin, as previously described (28). Kinetic rates were expressed per kilogram body weight where appropriate.
Infant Birth Weight and Cord Blood Collection
Infant birth weight and length was measured per institutional protocol in the delivery room after initial infant assessment and drying by either the pediatric or labor and delivery nurse in attendance. Measurements were performed on infant naked, and weight was obtained by a digital scale. Cord blood was collected immediately after the delivery of the infants and before delivery of the placenta. A 60-mL syringe was used to extract the blood from the clamped umbilical cord by the obstetric resident or attending obstetrician overseeing the delivery. Cord blood was centrifuged, aliquoted, and frozen at −80°C by formally trained obstetric research nurses who provide 24/7 coverage of labor and delivery to obtain research specimens.
Statistics
Normally distributed demographic, plasma metabolite, and hormone variables and glucose and fatty acid kinetics between groups were examined by one-way ANOVA and group differences were compared through post hoc testing using Tukey honestly significant difference testing. Nonnormally distributed variables determined by the Shapiro-Wilk test were examined by χ2 and independent samples Kruskal-Wallis one-way ANOVA. Due to the relationship between fatty acid kinetics and resting energy expenditure, fatty acid kinetic variables were analyzed by ANCOVA controlling for resting energy expenditure at baseline or during hyperinsulinemia. Relationships between maternal and infant demographic and metabolic outcomes were examined using univariate (Pearson product moment correlation). Stepwise linear regression analysis was used to refine the ability to predict birth weight combining multiple metabolic measures. Univariate analysis was used to determine the best predictors to enter in the model and then backward stepwise regression was performed. Statistical significance was considered at P < 0.05. Due to the lack of data regarding fatty acid kinetics during pregnancy in women with diabetes and the exploratory nature of the study, we used data from our previous work in nongravid insulin-resistant and control participants to estimate sample size (29), assuming an α of 0.05, where 25 subjects per group would provide 99% power to detect differences in baseline fatty acid oxidation rate between groups. All statistical analyses were performed using SPSS (IBM, Armonk, NY).
Results
Maternal Demographics
OB+DM was significantly older and heavier than OB and Lean groups and OB was significantly heavier than Lean. Rate of cesarean section tended to be higher in OB and OB+DM. Plasma interleukin-6 was significantly higher and total plasma cholesterol and LDL cholesterol concentrations were significantly lower in OB+DM than other groups. Plasma leptin concentration was significantly higher in both OB+DM and OB compared with Lean. Means ± SD for maternal demographics and plasma hormone and metabolite concentrations are reported in Table 1.
Table 1.
Maternal and infant demographics and plasma metabolites
| Lean (n = 25) | OB (n = 26) | OB+DM (n = 28) | F ratio P value | |
|---|---|---|---|---|
| Maternal variables | ||||
| Age (years) | 23 ± 3 | 25 ± 5 | 31 ± 6† | <0.001 |
| Height (cm) | 161.8 ± 5.3 | 168.0 ± 8.9 | 164.8 ± 5.5 | <0.001 |
| Weight (kg) | 70.8 ± 8.2 | 113.6 ± 21.8 | 127.6 ± 22.1*† | <0.001 |
| Gravida (n) | 2.3 ± 1.3 | 3.0 ± 1.7 | 3.6 ± 2.8 | 0.08 |
| Prenatal visits (n, %) | ||||
| 1–5 visits | 2, 8 | 2, 8 | 1, 4 | |
| 6–10 visits | 14, 56 | 14, 54 | 9, 32 | |
| >10 visits | 9, 36 | 10, 38 | 18, 64 | |
| Triglycerides (mg/dL) | 144.8 ± 44.1 | 159.8 ± 55.1 | 177.4 ± 91.2 | 0.47 |
| Total cholesterol (mg/dL) | 205.0 ± 33.3 | 197.7 ± 34.7 | 177.3 ± 37.9† | <0.001 |
| HDL cholesterol (mg/dL) | 71.2 ± 17.4 | 58.0 ± 13.1 | 56.8 ± 15.0 | 0.91 |
| LDL cholesterol (mg/dL) | 104.8 ± 29.3 | 107.8 ± 29.9 | 85.8 ± 32.4 | <0.001 |
| Leptin (μg/L) | 21.0 ± 6.4 | 49.3 ± 20.1† | 52.8 ± 37.1† | <0.001 |
| IL-6 (pg/mL) | 2.8 ± 1.1 | 3.5 ± 1.8 | 4.1 ± 2.0† | <0.02 |
| IGF-1 (ng/mL) | 313.2 ± 89.0 | 277.7 ± 100.0 | 382.0 ± 184.1 | 0.05 |
| Infant variables | ||||
| Gestational age (weeks) | 38.3 ± 1.3 | 38.8 ± 1.5 | 37.0 ± 1.9*† | <0.001 |
| Delivery mode | ||||
| Vaginal/cesarean | 68/32 | 42/58† | 32/68† | 0.05 |
| Sex | ||||
| Male/female (%) | 52/48 | 50/50 | 46/48 | 0.89 |
| Nursery admission | ||||
| Newborn/special care/NICU (%) | 80/20/0 | 81/15/4 | 50/42/8† | 0.01 |
| Resuscitative breathing | ||||
| Yes/no (%) | 12/88 | 23/77† | 43/57*† | 0.01 |
| Birth weight (g) | 3,070 ± 459 | 3,214 ± 756 | 3,604 ± 654*† | 0.01 |
| Birth length (cm) | 50.3 ± 2.6 | 50.8 ± 2.2 | 50.6 ± 2.9 | 0.91 |
| Ponderal index | 24.1 ± 2.3 | 25.7 ± 2.2 | 27.5 ± 3.5*† | 0.001 |
| Apgar, 1 min | 7.8 ± 1.4 | 7.1 ± 2.3 | 6.0 ± 2.9 | 0.01 |
| Apgar, 5 min | 8.8 ± 0.6 | 8.7 ± 0.9 | 7.8 ± 2.0 | 0.003 |
| Cord blood | ||||
| Glucose (mg/dL) | 93.1 ± 27.0 | 78.9 ± 14.4 | 86.9 ± 28.3 | 0.14 |
| Insulin (μU/mL) | 11.8 ± 7.4 | 10.9 ± 6.9 | 27.8 ± 24.1*† | <0.001 |
| C-peptide (ng/mL) | 0.87 ± 0.54 | 1.18 ± 0.81 | 1.83 ± 1.13*† | 0.001 |
| HOMA-insulin resistance | 2.8 ± 2.0 | 2.2 ± 1.7 | 6.0 ± 6.2*† | 0.001 |
| IGF-1 (ng/mL) | 61.2 ± 30.2 | 52.4 ± 19.7 | 70.5 ± 43.5 | 0.44 |
| FFA (mEq/L) | 0.14 ± 0.10 | 0.15 ± 0.10 | 0.16 ± 0.06 | 0.35 |
| Leptin (μg/L) | 11.2 ± 6.9 | 13.3 ± 9.1† | 30.9 ± 25.1*† | <0.001 |
| IL-6 (pg/mL) | 9.1 ± 10.4 | 10.0 ± 12.1 | 10.5 ± 13.2 | 0.93 |
Values are means ± SD. IL-6, interleukin 6; NICU, neonatal intensive care unit. Post hoc analysis:
*P < 0.05 vs. OB,
†P < 0.05 vs. Lean.
Maternal Glucose Kinetics
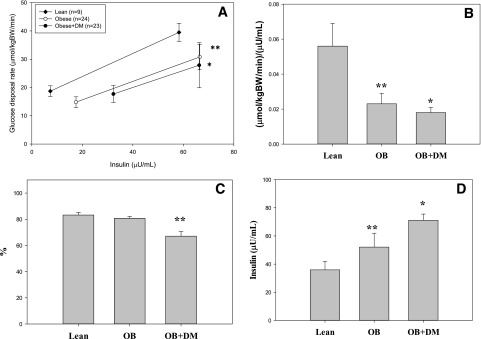
Basal plasma glucose and insulin concentrations were significantly higher in OB+DM compared with OB and Lean groups. Endogenous glucose production (EGP; glucose Ra) per kilogram body weight and per unit of plasma insulin during the basal condition was higher in OB+DM and OB versus Lean. Plasma glucose and insulin concentrations during hyperinsulinemia were similar between groups. EGP expressed absolutely and per kilogram of body weight during hyperinsulinemia was higher in OB+DM compared with other groups and significantly higher when expressed per unit plasma insulin in OB+DM compared with OB. EGP was suppressed less by hyperinsulinemia in OB+DM and OB compared with Lean and less in OB+DM versus OB (Fig. 1C). Glucose Rd expressed per kilogram of body weight was significantly lower in OB+DM compared with OB and Lean and was significantly lower in OB compared with Lean (Fig. 1A). Change in glucose Rd per change in plasma insulin from baseline to hyperinsulinemia was significantly lower in OB+DM versus OB (P = 0.08) and Lean and significantly lower in OB versus Lean (Fig. 1B). Means ± SD are reported in Table 2.
Figure 1.
A: Glucose Rd at baseline and hyperinsulinemia. B: Change in glucose disposal rate per change in plasma insulin. C: Endogenous glucose production suppression with insulin. D: Predicted IC50. *P < 0.05, different than OB and Lean; **P < 0.05, different than Lean. BW, body weight.
Table 2.
Maternal and infant metabolism
| Maternal variables | Lean (n = 25) | OB (n = 26) | OB+DM (n = 28) | F ratio P value |
|---|---|---|---|---|
| Basal | ||||
| Glucose (mg/dL) | 70.2 ± 11.1 | 81.2 ± 10.5† | 109.9 ± 30.3*† | <0.001 |
| Insulin (μU/mL) | 7.3 ± 5.0 | 17.5 ± 10.3† | 32.3 ± 30.5*† | <0.001 |
| REE (kcal/day) | 1,498 ± 270 | 2,125 ± 416† | 2,304 ± 484† | <0.001 |
| EGP (μmol/min) | 1,322 ± 157 | 1,660 ± 285† | 2,238 ± 443*† | <0.001 |
| EGP (μmol/kg BW/min) | 18.8 ± 1.9 | 14.8 ± 1.9† | 17.7 ± 3.0† | <0.001 |
| EGP (μmol/kg BW/min/μU/mL) | 4.0 ± 2.9 | 1.3 ± 1.2† | 0.9 ± 0.7† | <0.001 |
| FFA (mEq/L) | 0.45 ± 0.12 | 0.46 ± 0.13 | 0.57 ± 0.15*† | 0.001 |
| Palmitate Ra (μmol/min) | 105.0 ± 24.5 | 172.8 ± 47.7† | 195.7 ± 52.7† | <0.01 |
| Palmitate Ra (μmol/kg BW/min) | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.5 ± 0.3 | 0.99 |
| Palmitate Ox (μmol/min) | 9.7 ± 4.5 | 42.7 ± 15.7† | 45.1 ± 15.4† | <0.001 |
| Palmitate Ox (μmol/kg BW/min) | 0.34 ± 0.11 | 0.38 ± 0.13 | 0.35 ± 0.10 | 0.46 |
| % Uptake oxidized (%) | 23 ± 6 | 23 ± 5 | 25 ± 5 | 0.23 |
| Non-Ox Palm Disp (μmol/min) | 80.6 ± 20.8 | 129.7 ± 37.2† | 150.6 ± 40.4† | 0.004 |
| Non-Ox Palm Disp (μmol/kg BW/min) | 1.1 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.88 |
| Acetate recovery (%) | 38 ± 7 | 37 ± 6 | 39 ± 5 | 0.27 |
| Hyperinsulinemia# | ||||
| Glucose (mg/dL) | 89.3 ± 2.3 | 90.0 ± 2.0 | 90.2 ± 3.8 | 0.63 |
| Insulin (μU/mL) | 58.3 ± 13.5 | 66.3 ± 18.5 | 61.4 ± 26.3 | 0.53 |
| REE (kcal/day) | 1,559 ± 217 | 2,017 ± 355† | 2,124 ± 484† | 0.003 |
| EGP (μmol/min) | 229 ± 104 | 321 ± 145 | 791 ± 536*† | <0.001 |
| EGP (μmol/kg BW/min) | 3.1 ± 1.4 | 2.9 ± 1.2 | 6.2 ± 4.1*† | <0.001 |
| EGP (μmol/kg BW/min/μU/mL) | 0.06 ± 0.03 | 0.05 ± 0.02 | 0.09 ± 0.05* | <0.001 |
| EGP suppression (%) | 83 ± 7 | 81 ± 7 | 67 ± 18*† | 0.001 |
| Glucose Rd (μmol/kg BW/min) | 31.1 ± 3.0 | 22.7 ± 4.5† | 17.1 ± 4.5*† | <0.001 |
| Glucose Rd (μmol/kg BW/min/μU/mL) | 0.56 ± 0.14 | 0.40 ± 0.20† | 0.28 ± 0.13† | <0.001 |
| ΔRd/INS (μmol/kg BW/min/μU/mL) | 0.056 ± 0.039 | 0.023 ± 0.032 | 0.018 ± 0.016*† | <0.01 |
| FFA (mEq/L) | 0.06 ± 0.02 | 0.08 ± 0.06 | 0.22 ± 0.12*† | <0.001 |
| Palmitate Ra (μmol/min) | 42.0 ± 13.2 | 60.7 ± 38.5 | 97.4 ± 41.1*† | 0.004 |
| Palmitate Ra (μmol/kg BW/min) | 0.56 ± 0.41 | 0.54 ± 0.32 | 0.75 ± 0.24* | 0.04 |
| Palmitate Ra suppression (%) | 62 ± 14 | 66 ± 15 | 51 ± 13 | 0.002 |
| IC50 (μU/mL) | 35.9 ± 18.3 | 52.1 ± 50.6† | 71.1 ± 41.7*† | 0.01 |
| Palmitate Ox (μmol/min) | 17.1 ± 5.9 | 27.6 ± 20.6 | 38.8 ± 15.3 | 0.09 |
| Palmitate Ox (μmol/kg BW/min) | 0.23 ± 0.08 | 0.24 ± 0.17 | 0.30 ± 0.10 | 0.39 |
| % Uptake oxidized (%) | 41 ± 10 | 44 ± 11 | 41 ± 11 | 0.33 |
| Non-Ox Palm Disp (μmol/min) | 24.9 ± 10.6 | 33.1 ± 21.6 | 58.7 ± 31.6*† | 0.003 |
| Non-Ox Palm Disp (μmol/kg BW/min) | 0.17 ± 0.06 | 0.18 ± 0.12 | 0.23 ± 0.08 | 0.31 |
| Acetate recovery (%) | 76 ± 12 | 74 ± 11 | 75 ± 9 | 0.76 |
Values are means ± SD. Lipid variables analyzed via ANCOVA (REE). BW, body weight; Non-Ox Palm Disp, nonoxidative palmitate disposal; Ox, oxidation.
#n under hyperinsulinemic conditions: Lean (n = 9), OB (n = 24), and OB+DM (n = 23). Between-group post hoc analysis:
*P < 0.05 vs. OB,
†P < 0.05 vs. Lean.
Maternal Fatty Acid Kinetics
Basal and hyperinsulinemic plasma FFA concentrations were significantly higher in OB+DM compared with other groups. Absolute palmitate Ra, palmitate oxidation rate, and nonoxidative disposal rate during the basal period were significantly higher in OB+DM and OB compared with Lean but not different between groups when expressed per kilogram body weight. Palmitate Ra expressed absolutely during hyperinsulinemia was significantly higher in OB+DM than other groups. Palmitate Ra suppression by hyperinsulinemia tended to be blunted in OB+DM compared with OB and Lean (P = 0.099). The predicted IC50 was significantly higher in OB+DM versus other groups and higher in OB versus Lean (Fig. 1D). Ninety-one percent of participants completed the acetate infusion study; the average recovery for each group was used for those participants who did not complete the study. Acetate recovery factors were not different between groups during the basal or hyperinsulinemic conditions. Means ± SD are reported in Table 2.
Infant Demographics and Metabolism
Infants of OB+DM were born significantly earlier than other groups. Infants born to OB+DM tended to need more advanced care after delivery, including resuscitative breathing and admission to the special care nursery or the neonatal intensive care unit. One-minute Apgar was significantly lower in OB+DM than other groups. Infant birth weight was significantly greater in OB+DM than Lean and tended (P = 0.07) to be greater than OB. Cord plasma insulin, HOMA-insulin resistance, C-peptide, and leptin were significantly higher in OB+DM than OB and Lean. There were no differences in labor duration (i.e., fasting before cord blood collection) between the groups, no relationships between labor duration and cord HOMA or FFA, and no differences in cord blood variables between those with natural delivery versus cesarean section (data not shown). Means ± SD are reported in Table 1.
Correlations Between Maternal Substrate Kinetics and Infant Birth Weight
Maternal fasting plasma glucose, baseline EGP per unit plasma insulin, change in glucose Rd per change in plasma insulin from baseline to hyperinsulinemia, IC50, and plasma FFA concentration during hyperinsulinemia were the greatest univariate predictors of infant birth weight (all participants included) (Table 3). These variables remained the strongest predictors when only OB+DM and OB were analyzed (maternal fasting glucose: r = 0.40; EGP/plasma insulin [INS]: r = −0.38; Δglucose Rd/INS: r = −0.39; IC50: r = 0.28; FFA concentration: r = 0.46 [all P < 0.01]). Using these variables in a backward stepwise regression analysis (multicollinearity: tolerance = 0.82, variance inflation factor = 1.2), a model including plasma FFA concentration during hyperinsulinemia and baseline EGP per unit plasma insulin most strongly predicted of birth weight (adjusted R2 = 0.33) (Table 3). Regression coefficients out of the model were β = −0.382 (EGP/INS) and β = 0.455 (FFA).
Table 3.
Correlation and regression coefficients
| Variable | Birth weight | Fasting glucose | FFA clamp | Standardized coefficient (β) |
|---|---|---|---|---|
| Basal | ||||
| Glucose (mg/dL) | 0.47 (0.001) | 0.71 (0.001) | ||
| EGP (μmol/min/kg BW/μU/mL) | −0.43 (0.001) | −0.44 (0.001) | −0.42 (0.001) | −0.355 |
| Hyperinsulinemia* | ||||
| IC50 (μU/mL) | 0.33 (0.02) | 0.28 (0.04) | 0.56 (0.001) | |
| ΔGluRd/INS (μmol/min/kg BW/μU/mL) | −0.47 (0.001) | −0.26 (0.06) | −0.35 (0.001) | |
| FFA (mEq/L) | 0.50 (0.001) | 0.71 (0.001) | 0.353 | |
| Model** | R | R2 | Adjusted R2 | Standard error |
| Baseline EGP/INS, FFA clamp | 0.597 | 0.357 | 0.332 | 457.9 |
Data are r (P value), unless otherwise stated. BW, body weight; glucose, maternal fasting plasma glucose concentration; ΔGluRd/INS, change in glucose rate of disappearance per unit change in plasma insulin.
*n under hyperinsulinemic conditions: Lean (n = 9), OB (n = 24), and OB+DM (n = 23).
**Backward regression model including variables identified as best predictors from univariate analyses. Initial variable included above, final variables shown with regression coefficients.
Discussion
To our knowledge, this is the first study to report maternal glucose and fatty acid kinetics during basal and hyperinsulinemic conditions in obese women with pregestational diabetes during late pregnancy. The principal findings of the study were that obese women with pregestational type 2 diabetes had 1) hepatic, adipose tissue, and skeletal muscle insulin resistance; 2) elevated adipose tissue lipolysis and plasma FFA concentration; and 3) higher infant birth weight compared with obese and lean women without diabetes during late pregnancy. Further, we found that a statistical model consisting of maternal baseline EGP rate per unit of plasma insulin and plasma FFA concentration during hyperinsulinemia best predicted infant birth weight in a cohort of obese and lean women with and without diabetes.
Glucose and Fatty Acid Kinetics
Glucose and lipid abnormalities are well described in nongravid adults with diabetes (30). Previous studies have also demonstrated hepatic insulin resistance (31) and impaired FFA response to insulin (32) in women with gestational diabetes mellitus during pregnancy. This study extends those observations into obese women with pregestational type 2 diabetes and further reports lower insulin-stimulated glucose disposal rate, elevated adipose tissue lipolytic rate during hyperinsulinemia, and higher IC50 for palmitate suppression compared with obese and lean women without diabetes. Higher maternal adipose tissue lipolytic rate during hyperinsulinemia and higher IC50 for palmitate suppression likely resulted in higher maternal FFA concentrations as seen in the current study. Indeed, we found strong relationships between maternal adipose tissue lipolytic rates, IC50, and maternal FFA concentration during both conditions (data not shown). In addition, palmitate oxidation rate during baseline and hyperinsulinemia expressed absolutely tended to be higher in obese women with and without diabetes compared with lean women without diabetes. However, these differences were not present when normalized per body mass, suggesting skeletal muscle fatty acid oxidation is not impaired. This tendency toward higher absolute palmitate oxidation rate in obese women with diabetes might be important as higher lipid oxidation is associated with greater reactive oxygen species generation and systemic inflammation (33). Indeed, we found higher maternal systemic inflammation (interleukin-6) in obese women with diabetes. Lastly, obese women with pregestational type 2 diabetes demonstrated hepatic and skeletal muscle insulin resistance during late pregnancy. This resistance was significantly greater than the physiologic insulin resistance of pregnancy (34) and appeared to contribute equally (through univariate analysis) to elevated maternal plasma glucose concentration. On the basis of our findings, interventions targeting hepatic, skeletal muscle, and adipose tissue insulin resistance during pregnancy might improve maternal metabolic health and modulate infant birth weight.
Infant Birth Weight
Higher infant birth weight is well documented in pregnancies independently complicated by diabetes (35) and obesity (36,37). However, we found higher infant birth weight only in women with both obesity and pregestational diabetes. Traditionally, maternal fasting plasma glucose was thought to be the most important predictor of infant birth weight in women with diabetes (8). However, more recent data suggest that maternal glucose only explains some of the variance in infant birth weight (10) and that markers of maternal lipid metabolism might better predict birth weight in pregnancies complicated by obesity and diabetes (11–20). Previous studies demonstrating these relationships only used clinically obtained measures (e.g., maternal plasma concentrations of triglyceride, FFA, and cholesterol) where the current study also examined the physiologic mechanisms (i.e., kinetics) that might contribute to increased infant birth weight. Although correlation analysis revealed that maternal glucose was strongly related to infant birth weight, a multiple regression model found that maternal glucose kinetics (baseline EGP per unit plasma insulin) and a clinical marker of fatty acid metabolism (plasma FFA concentration) during the hyperinsulinemic condition best predicted infant birth weight. This model predicted ∼16% more variance in infant birth weight than a model including clinically obtainable measures (fasting plasma glucose, insulin, FFA, and HDL and total cholesterol concentrations; data not shown). Taken together, these data indicate that performing hyperinsulinemic clamps during pregnancy in all obese women with pregestational diabetes is neither clinically nor scientifically warranted but might suggest that measurement of postprandial plasma FFA (and possibly glucose) concentration (i.e., hyperinsulinemic condition) might be the best, clinically obtainable predictor of infant birth weight in this population, but this needs further study. Surprisingly, we did not find a significant correlation between maternal plasma triglyceride concentration and infant birth weight as seen in other studies in gestational diabetes mellitus (12,38). These studies examined Caucasian and Asian women and our study had a large proportion of African American women who have been previously shown to have reduced VLDL-triglyceride secretion rates compared with Caucasians (39); these differences might have modulated this effect in our study. We also found lower total plasma cholesterol in women with diabetes. It is likely that total cholesterol was lower in the diabetes group because HDL levels were ∼15 mg/dL lower in the diabetes group. The most common lipid abnormalities in type 2 diabetes are low HDL cholesterol and hypertriglyceridemia, which is exacerbated by uncontrolled hyperglycemia; elevations in total LDL are not typically seen in type 2 diabetes alone (40). We selected patients with relatively well-controlled diabetes and therefore would anticipate that the patients would have near-normal triglyceride levels but would continue to have a low HDL cholesterol.
Umbilical Cord Blood Metabolites and Hormones
Cord plasma insulin, C-peptide, and leptin were higher in infants born to obese women with pregestational diabetes compared with obese and lean women without diabetes. However, based on the foreseen maternal fatty acid metabolic abnormalities in obese women with pregestational diabetes and the strong predictive value of maternal plasma FFA concentration of infant birth weight, we were surprised that umbilical cord plasma FFA concentration was not different among groups and that we did not find a correlation between maternal and umbilical cord FFA concentrations as has been previously reported (38). Our data are however consistent with a previous report finding no difference in umbilical cord plasma FFA concentration in women with gestational diabetes mellitus (41). Similar cord blood FFA concentrations in the current study might be due to the possibility that fetal delivery of FFAs is likely not static and fluctuates with changes in maternal FFA concentration (42). During fasting, maternal oxidation of fatty acids for maternal energy needs is at its highest (43). Umbilical cord blood collection for scientific and clinical purposes is typically obtained after an extended maternal fast (both natural labor and cesarean delivery) and likely does not reflect quantitative fetal FFA delivery. Although difficult to measure, it is reasonable to speculate that fetal delivery of fatty acids (via placental uptake of FFAs and triglycerides) is highest after a meal and that postprandial insulin resistance might further facilitate fatty acid delivery (and glucose) to the fetus and thus contribute to fetal growth and adiposity. This notion is supported by data that demonstrate placental triglyceride accumulation (44) and fatty acid transporter content (45) is higher in pregnancy complicated by diabetes and obesity. Thus, further research examining the role of postprandial plasma FFA and/or triglyceride metabolism on fetal growth is warranted.
Limitations and Strengths
Although infant birth weight among those born to women with diabetes is a reasonable surrogate for some adverse outcomes, it might be mediated by increases in fat mass and/or lean mass (46). However, our study did not measure infant adiposity and therefore is a limitation of the study. Another limitation is that not all women participated in the hyperinsulinemic-euglycemic clamp portion of the study; therefore, this might have reduced our power to detect differences in lean women from the obese groups during hyperinsulinemia. We also did not assess postprandial lipid and glucose metabolism, where in a previous study, it has been shown to correlate with infant adiposity (47). We also did not measure maternal body composition to which glucose and lipid kinetics could have been normalized. We did not collect maternal body composition due to the inherent inaccuracies (bioelectrical impedance, air displacement plethymosgraphy, and skin fold calipers) during pregnancy (48). Also, another limitation is that these data can only be applied to late pregnancy and not early or midgestation as we only measured glucose and lipid metabolism during late pregnancy. However, we believe our study also has some strengths to consider. Our relatively large sample size contained a mix of lean and obese women with and without diabetes. Also, we examined physiologic mechanisms (i.e., kinetics) of maternal glycemia and lipemia under both the fasting and hyperinsulinemic conditions using state-of-the-art techniques (i.e., metabolic tracers and mass spectrometry).
Conclusions
Maternal glucose and fatty acid kinetics during late pregnancy are abnormal in women with pregestational diabetes and reflect adipose tissue, hepatic, and skeletal muscle insulin resistance. Glucose kinetics and fatty acid concentration during hyperinsulinemia are the strongest predictors of infant birth weight in a mixed population of lean and obese women with and without diabetes. These findings suggest that in attempting to modulate infant birth weight (and likely other complications), maternal adipose tissue, hepatic, and skeletal muscle insulin resistance, rather than just maternal hyperglycemia, should also be considered during pregnancy in women with pregestational diabetes as clinical targets that deserve further investigation.
Article Information
Acknowledgments. The authors thank the nursing staff at the Washington University in St. Louis Institute of Clinical and Translational Sciences Clinical Research Unit for their hard work and altruism.
Funding. The authors acknowledge the following funding sources: Thrasher Research Fund, the American Diabetes Association, and the National Institutes of Health (NIH) (P30DK056341, P60DK020579, P41GM103422, and UL1RR024992 from the National Center for Research Resources and NIH Roadmap for Medical Research).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. W.T.C. and A.G.C. researched data and wrote and edited the manuscript. R.A.T., D.N.R., and B.W.P. researched data and reviewed and edited the manuscript. W.T.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. A portion of these data was presented in abstract form at the 35th Annual Meeting of the Society for Maternal-Fetal Medicine, San Diego, CA, 2–7 February 2015.
Footnotes
Clinical trial reg. no. NCT01346527, clinicaltrials.gov.
References
- 1.Hay WW, Jr. Care of the infant of the diabetic mother. Curr Diab Rep 2012;12:4–15 [DOI] [PubMed] [Google Scholar]
- 2.Casson IF, Clarke CA, Howard CV, et al. Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. BMJ 1997;315:275–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deierlein AL, Siega-Riz AM, Chantala K, Herring AH. The association between maternal glucose concentration and child BMI at age 3 years. Diabetes Care 2011;34:480–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leong NM, Mignone LI, Newcomb PA, et al. Early life risk factors in cancer: the relation of birth weight to adult obesity. Int J Cancer 2003;103:789–791 [DOI] [PubMed] [Google Scholar]
- 5.Murtaugh MA, Jacobs DR Jr, Moran A, Steinberger J, Sinaiko AR. Relation of birth weight to fasting insulin, insulin resistance, and body size in adolescence. Diabetes Care 2003;26:187–192 [DOI] [PubMed] [Google Scholar]
- 6.Rich-Edwards JW, Kleinman K, Michels KB, et al. Longitudinal study of birth weight and adult body mass index in predicting risk of coronary heart disease and stroke in women. BMJ 2005;330:1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoet JJ, Hanson MA. Intrauterine nutrition: its importance during critical periods for cardiovascular and endocrine development. J Physiol 1999;514:617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langer O. A spectrum of glucose thresholds may effectively prevent complications in the pregnant diabetic patient. Semin Perinatol 2002;26:196–205 [DOI] [PubMed] [Google Scholar]
- 9.Ouzilleau C, Roy MA, Leblanc L, Carpentier A, Maheux P. An observational study comparing 2-hour 75-g oral glucose tolerance with fasting plasma glucose in pregnant women: both poorly predictive of birth weight. CMAJ 2003;168:403–409 [PMC free article] [PubMed] [Google Scholar]
- 10.Sacks DA, Liu AI, Wolde-Tsadik G, Amini SB, Huston-Presley L, Catalano PM. What proportion of birth weight is attributable to maternal glucose among infants of diabetic women? Am J Obstet Gynecol 2006;194:501–507 [DOI] [PubMed] [Google Scholar]
- 11.Knopp RH, Magee MS, Walden CE, Bonet B, Benedetti TJ. Prediction of infant birth weight by GDM screening tests. Importance of plasma triglyceride. Diabetes Care 1992;15:1605–1613 [DOI] [PubMed] [Google Scholar]
- 12.Son GH, Kwon JY, Kim YH, Park YW. Maternal serum triglycerides as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Acta Obstet Gynecol Scand 2010;89:700–704 [DOI] [PubMed] [Google Scholar]
- 13.Di Cianni G, Miccoli R, Volpe L, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med 2005;22:21–25 [DOI] [PubMed] [Google Scholar]
- 14.Kitajima M, Oka S, Yasuhi I, Fukuda M, Rii Y, Ishimaru T. Maternal serum triglyceride at 24--32 weeks’ gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet Gynecol 2001;97:776–780 [DOI] [PubMed] [Google Scholar]
- 15.Crume TL, Shapiro AL, Brinton JT, et al. Maternal fuels and metabolic measures during pregnancy and neonatal body composition: the healthy start study. J Clin Endocrinol Metab 2015;100:1672–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerényi Z, Tamás G, Kivimäki M, et al. Maternal glycemia and risk of large-for-gestational-age babies in a population-based screening. Diabetes Care 2009;32:2200–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uvena-Celebrezze J, Fung C, Thomas AJ, et al. Relationship of neonatal body composition to maternal glucose control in women with gestational diabetes mellitus. J Matern Fetal Neonatal Med 2002;12:396–401 [DOI] [PubMed] [Google Scholar]
- 18.Figueroa D, Landon MB, Mele L, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Relationship between 1-hour glucose challenge test results and perinatal outcomes. Obstet Gynecol 2013;121:1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landon MB, Mele L, Spong CY, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal–Fetal Medicine Units (MFMU) Network . The relationship between maternal glycemia and perinatal outcome. Obstet Gynecol 2011;117:218–22421309194 [Google Scholar]
- 20.Kulkarni SR, Kumaran K, Rao SR, et al. Maternal lipids are as important as glucose for fetal growth: findings from the Pune Maternal Nutrition Study. Diabetes Care 2013;36:2706–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zello GA, Smith JM, Pencharz PB, Ball RO. Development of a heating device for sampling arterialized venous blood from a hand vein. Ann Clin Biochem 1990;27:366–372 [DOI] [PubMed] [Google Scholar]
- 22.Bougnères PF, Bier DM. Stable isotope dilution method for measurement of palmitate content and labeled palmitate tracer enrichment in microliter plasma samples. J Lipid Res 1982;23:502–507 [PubMed] [Google Scholar]
- 23.Ali AH, Mundi M, Koutsari C, Bernlohr DA, Jensen MD. Adipose tissue free fatty acid storage in vivo: effects of insulin versus niacin as a control for suppression of lipolysis. Diabetes 2015;64:2828–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cade WT, Spencer CT, Reeds DN, et al. Substrate metabolism during basal and hyperinsulinemic conditions in adolescents and young-adults with Barth syndrome. J Inherit Metab Dis 2013;36:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen SR, Sumner AE, Miller BV 3rd, Turkova H, Klein S, Jensen MD. Free fatty acid flux in African-American and Caucasian adults--effect of sex and race. Obesity (Silver Spring) 2013;21:1836–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catalano PM, Kirwan JP. Clinical utility and approaches for estimating insulin sensitivity in pregnancy. Semin Perinatol 2002;26:181–189 [DOI] [PubMed] [Google Scholar]
- 27.Reeds DN, Yarasheski KE, Fontana L, et al. Alterations in liver, muscle, and adipose tissue insulin sensitivity in men with HIV infection and dyslipidemia. Am J Physiol Endocrinol Metab 2006;290:E47–E53 [DOI] [PubMed] [Google Scholar]
- 28.Jensen MD, Nielsen S. Insulin dose response analysis of free fatty acid kinetics. Metabolism 2007;56:68–76 [DOI] [PubMed] [Google Scholar]
- 29.Cade WT, Reeds DN, Mittendorfer B, et al. Blunted lipolysis and fatty acid oxidation during moderate exercise in HIV-infected subjects taking HAART. Am J Physiol Endocrinol Metab 2007;292:E812–E819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 2004;88:787–835, ix [DOI] [PubMed] [Google Scholar]
- 31.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 1999;180:903–916 [DOI] [PubMed] [Google Scholar]
- 32.Catalano PM, Nizielski SE, Shao J, Preston L, Qiao L, Friedman JE. Downregulated IRS-1 and PPARgamma in obese women with gestational diabetes: relationship to FFA during pregnancy. Am J Physiol Endocrinol Metab 2002;282:E522–E533 [DOI] [PubMed] [Google Scholar]
- 33.McIntyre TM, Hazen SL. Lipid oxidation and cardiovascular disease: introduction to a review series. Circ Res 2010;107:1167–1169 [DOI] [PubMed] [Google Scholar]
- 34.Kalhan SC, D’Angelo LJ, Savin SM, Adam PA. Glucose production in pregnant women at term gestation. Sources of glucose for human fetus. J Clin Invest 1979;63:388–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clausen TD, Mathiesen E, Ekbom P, Hellmuth E, Mandrup-Poulsen T, Damm P. Poor pregnancy outcome in women with type 2 diabetes. Diabetes Care 2005;28:323–328 [DOI] [PubMed] [Google Scholar]
- 36.Ray JG, Vermeulen MJ, Shapiro JL, Kenshole AB; Diabetes Endocrine Pregnancy Outcome Study in Toronto . Maternal and neonatal outcomes in pregestational and gestational diabetes mellitus, and the influence of maternal obesity and weight gain: the DEPOSIT study. QJM 2001;94:347–356 [DOI] [PubMed] [Google Scholar]
- 37.Kumari AS. Pregnancy outcome in women with morbid obesity. Int J Gynaecol Obstet 2001;73:101–107 [DOI] [PubMed] [Google Scholar]
- 38.Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008;31:1858–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller BV 3rd, Patterson BW, Okunade A, Klein S. Fatty acid and very low density lipoprotein metabolism in obese African American and Caucasian women with type 2 diabetes. J Lipid Res 2012;53:2767–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diabetes Care 2002;25(Suppl. 1):S74–S77 [DOI] [PubMed] [Google Scholar]
- 41.Bomba-Opon D, Wielgos M, Szymanska M, Bablok L. Effects of free fatty acids on the course of gestational diabetes mellitus. Neuroendocrinol Lett 2006;27:277–280 [PubMed] [Google Scholar]
- 42.Herrera E, Ortega-Senovilla H. Lipid metabolism during pregnancy and its implications for fetal growth. Curr Pharm Biotechnol 2014;15:24–31 [DOI] [PubMed] [Google Scholar]
- 43.Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine 2002;19:43–55 [DOI] [PubMed] [Google Scholar]
- 44.Magnusson AL, Waterman IJ, Wennergren M, Jansson T, Powell TL. Triglyceride hydrolase activities and expression of fatty acid binding proteins in the human placenta in pregnancies complicated by intrauterine growth restriction and diabetes. J Clin Endocrinol Metab 2004;89:4607–4614 [DOI] [PubMed] [Google Scholar]
- 45.Dubé E, Gravel A, Martin C, et al. Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta. Biol Reprod 2012;87:14–, 1–11. [DOI] [PubMed] [Google Scholar]
- 46.Kehl RJ, Krew MA, Thomas A, Catalano PM. Fetal growth and body composition in infants of women with diabetes mellitus during pregnancy. J Matern Fetal Med 1996;5:273–280 [DOI] [PubMed] [Google Scholar]
- 47.Horan MK, McGowan CA, Gibney ER, Donnelly JM, McAuliffe FM. Maternal low glycaemic index diet, fat intake and postprandial glucose influences neonatal adiposity--secondary analysis from the ROLO study. Nutr J 2014;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Widen EM, Gallagher D. Body composition changes in pregnancy: measurement, predictors and outcomes. Eur J Clin Nutr 2014;68:643–652 [DOI] [PMC free article] [PubMed] [Google Scholar]