Abstract
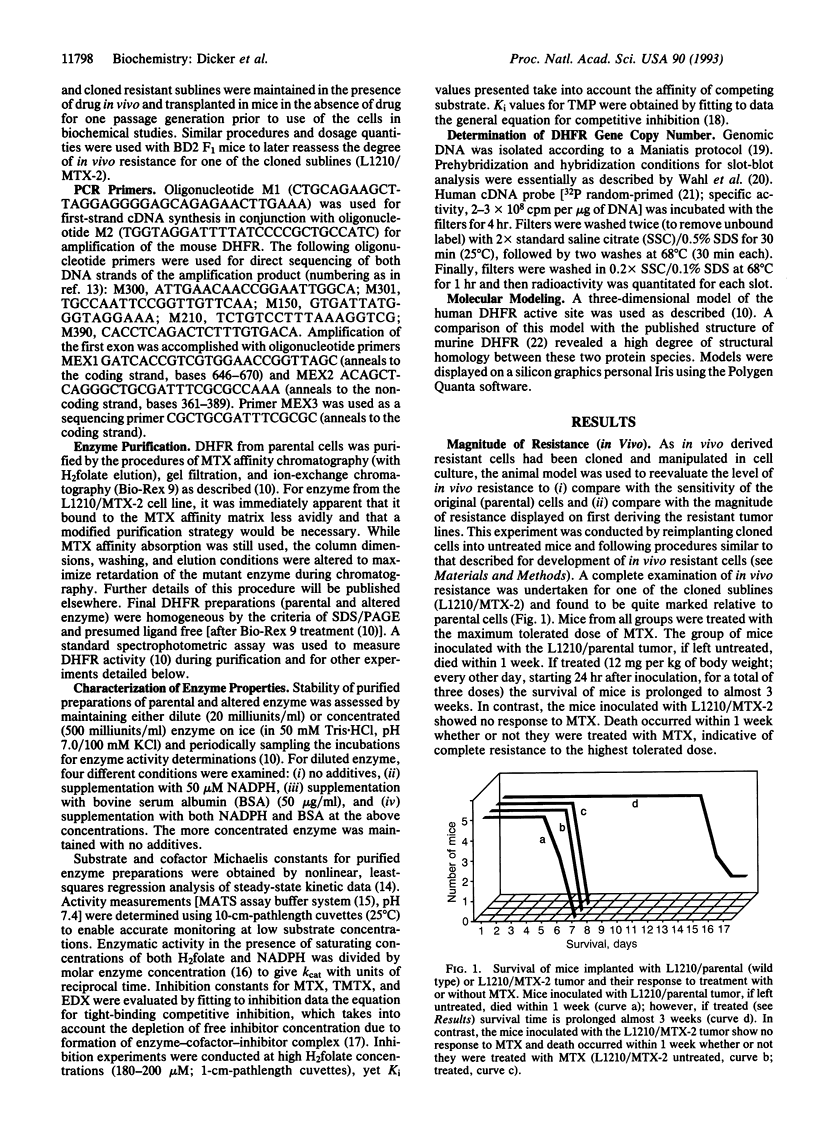
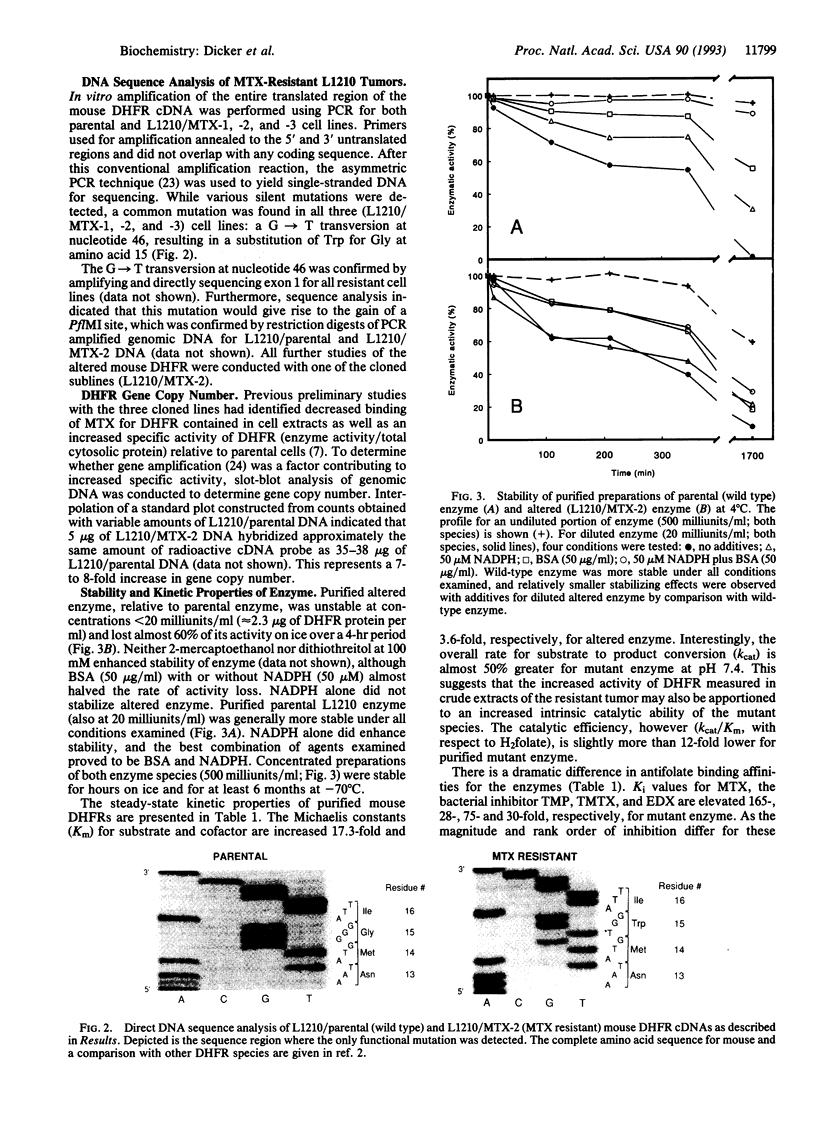
A series of methotrexate (MTX)-resistant L1210 leukemia murine ascites tumors were developed in vivo and analyzed for drug resistance. Three of 20 tumors studied expressed an altered dihydrofolate reductase (DHFR) and each was identical, having a C to T base transition at nucleotide 46 in the DHFR gene as demonstrated by PCR and direct sequencing. This transition results in a Gly to Trp substitution at amino acid 15 of the enzyme. Purified altered enzyme displays significantly lower binding affinity for the antifolates MTX, trimetrexate, edatrexate, and trimethoprim with respective Ki values 165-, 76-, 30-, and 28-fold higher than values obtained for enzyme isolated from parental tumor (wild-type enzyme). Substrate (dihydrofolate) and cofactor (NADPH) binding is also diminished for the mutant enzyme, although to a lesser extent (17.3- and 3.6-fold higher Km, respectively). Gly-15 is highly conserved for all vertebrate species of DHFR but has no known interaction(s), either directly or indirectly, with bound cofactor, substrate, or inhibitor. Protein molecular modeling reveals that the affected residue is 9-12 A away from the enzyme active site and located in a region analogous to the mobile Met-20 loop domain characterized for Escherichia coli DHFR.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleman J. R., Prendergast N., Delcamp T. J., Freisheim J. H., Blakley R. L. Kinetics of the formation and isomerization of methotrexate complexes of recombinant human dihydrofolate reductase. J Biol Chem. 1988 Jul 25;263(21):10304–10313. [PubMed] [Google Scholar]
- Bystroff C., Kraut J. Crystal structure of unliganded Escherichia coli dihydrofolate reductase. Ligand-induced conformational changes and cooperativity in binding. Biochemistry. 1991 Feb 26;30(8):2227–2239. doi: 10.1021/bi00222a028. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., Simonsen C. C., McEwan R. N., Schimke R. T. Structure of amplified normal and variant dihydrofolate reductase genes in mouse sarcoma S180 cells. J Biol Chem. 1982 Jul 10;257(13):7887–7897. [PubMed] [Google Scholar]
- Davies J. F., 2nd, Delcamp T. J., Prendergast N. J., Ashford V. A., Freisheim J. H., Kraut J. Crystal structures of recombinant human dihydrofolate reductase complexed with folate and 5-deazafolate. Biochemistry. 1990 Oct 9;29(40):9467–9479. doi: 10.1021/bi00492a021. [DOI] [PubMed] [Google Scholar]
- Dicker A. P., Volkenandt M., Schweitzer B. I., Banerjee D., Bertino J. R. Identification and characterization of a mutation in the dihydrofolate reductase gene from the methotrexate-resistant Chinese hamster ovary cell line Pro-3 MtxRIII. J Biol Chem. 1990 May 15;265(14):8317–8321. [PubMed] [Google Scholar]
- Eisenhauer E. A., Wierzbicki R., Knowling M., Bramwell V. H., Quirt I. C. Phase II trials of trimetrexate in advanced adult soft tissue sarcoma. Studies of the Canadian Sarcoma Group and the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 1991 Oct;2(9):689–690. doi: 10.1093/oxfordjournals.annonc.a058051. [DOI] [PubMed] [Google Scholar]
- Ellis K. J., Morrison J. F. Buffers of constant ionic strength for studying pH-dependent processes. Methods Enzymol. 1982;87:405–426. doi: 10.1016/s0076-6879(82)87025-0. [DOI] [PubMed] [Google Scholar]
- Farnum M. F., Magde D., Howell E. E., Hirai J. T., Warren M. S., Grimsley J. K., Kraut J. Analysis of hydride transfer and cofactor fluorescence decay in mutants of dihydrofolate reductase: possible evidence for participation of enzyme molecular motions in catalysis. Biochemistry. 1991 Dec 10;30(49):11567–11579. doi: 10.1021/bi00113a012. [DOI] [PubMed] [Google Scholar]
- Filman D. J., Bolin J. T., Matthews D. A., Kraut J. Crystal structures of Escherichia coli and Lactobacillus casei dihydrofolate reductase refined at 1.7 A resolution. II. Environment of bound NADPH and implications for catalysis. J Biol Chem. 1982 Nov 25;257(22):13663–13672. [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Libshitz H. I., Murphy W. K., Jeffries D., Hong W. K. Phase II study of 10-ethyl-10-deaza-aminopterin (10-EdAM; CGP 30 694) for stage IIIB or IV non-small cell lung cancer. Invest New Drugs. 1990 Aug;8(3):299–304. doi: 10.1007/BF00171841. [DOI] [PubMed] [Google Scholar]
- Li L., Falzone C. J., Wright P. E., Benkovic S. J. Functional role of a mobile loop of Escherichia coli dihydrofolate reductase in transition-state stabilization. Biochemistry. 1992 Sep 1;31(34):7826–7833. doi: 10.1021/bi00149a012. [DOI] [PubMed] [Google Scholar]
- Masters J. N., Attardi G. The nucleotide sequence of the cDNA coding for the human dihydrofolic acid reductase. Gene. 1983 Jan-Feb;21(1-2):59–63. doi: 10.1016/0378-1119(83)90147-6. [DOI] [PubMed] [Google Scholar]
- Melera P. W., Davide J. P., Hession C. A., Scotto K. W. Phenotypic expression in Escherichia coli and nucleotide sequence of two Chinese hamster lung cell cDNAs encoding different dihydrofolate reductases. Mol Cell Biol. 1984 Jan;4(1):38–48. doi: 10.1128/mcb.4.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melera P. W., Davide J. P., Oen H. Antifolate-resistant Chinese hamster cells. Molecular basis for the biochemical and structural heterogeneity among dihydrofolate reductases produced by drug-sensitive and drug-resistant cell lines. J Biol Chem. 1988 Feb 5;263(4):1978–1990. [PubMed] [Google Scholar]
- Rumberger B. G., Schmid F. A., Otter G. M., Sirotnak F. M. Preferential selection during therapy in vivo by edatrexate compared to methotrexate of resistant L1210 cell variants with decreased folylpolyglutamate synthetase activity. Cancer Commun. 1990;2(9):305–310. [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured cells. J Biol Chem. 1988 May 5;263(13):5989–5992. [PubMed] [Google Scholar]
- Schweitzer B. I., Dicker A. P., Bertino J. R. Dihydrofolate reductase as a therapeutic target. FASEB J. 1990 May;4(8):2441–2452. doi: 10.1096/fasebj.4.8.2185970. [DOI] [PubMed] [Google Scholar]
- Schweitzer B. I., Srimatkandada S., Gritsman H., Sheridan R., Venkataraghavan R., Bertino J. R. Probing the role of two hydrophobic active site residues in the human dihydrofolate reductase by site-directed mutagenesis. J Biol Chem. 1989 Dec 5;264(34):20786–20795. [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotnak F. M., Moccio D. M., Kelleher L. E., Goutas L. J. Relative frequency and kinetic properties of transport-defective phenotypes among methotrexate-resistant L1210 clonal cell lines derived in vivo. Cancer Res. 1981 Nov;41(11 Pt 1):4447–4452. [PubMed] [Google Scholar]
- Srimatkandada S., Schweitzer B. I., Moroson B. A., Dube S., Bertino J. R. Amplification of a polymorphic dihydrofolate reductase gene expressing an enzyme with decreased binding to methotrexate in a human colon carcinoma cell line, HCT-8R4, resistant to this drug. J Biol Chem. 1989 Feb 25;264(6):3524–3528. [PubMed] [Google Scholar]
- Stammers D. K., Champness J. N., Beddell C. R., Dann J. G., Eliopoulos E., Geddes A. J., Ogg D., North A. C. The structure of mouse L1210 dihydrofolate reductase-drug complexes and the construction of a model of human enzyme. FEBS Lett. 1987 Jun 22;218(1):178–184. doi: 10.1016/0014-5793(87)81042-6. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Morrison J. F. Mechanism of inhibition of dihydrofolate reductases from bacterial and vertebrate sources by various classes of folate analogues. Biochim Biophys Acta. 1986 Feb 14;869(3):275–285. doi: 10.1016/0167-4838(86)90067-1. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. W., Morrison J. F., Duggleby R. G. Methotrexate, a high-affinity pseudosubstrate of dihydrofolate reductase. Biochemistry. 1979 Jun 12;18(12):2567–2573. doi: 10.1021/bi00579a021. [DOI] [PubMed] [Google Scholar]
- Williams J. W., Morrison J. F. The kinetics of reversible tight-binding inhibition. Methods Enzymol. 1979;63:437–467. doi: 10.1016/0076-6879(79)63019-7. [DOI] [PubMed] [Google Scholar]