Abstract
The effect of Zn2+ on a voltage-dependent, transient potassium current (IA) in acutely dissociated neurons from the suprachiasmatic nucleus was studied with the whole-cell patch-clamp technique. At micromolar concentrations, Zn2+ markedly potentiated IA activated from a holding potential of -60 mV, which is the resting potential of these neurons. This potentiation occurred at a Zn2+ concentration as low as 2 microM and increased with higher Zn2+ concentrations. The Zn2+ action appears to arise from a shift in the steady-state inactivation of IA to more positive voltages. At 30 microM, Zn2+ shifted the half-inactivation voltage by +20 mV (from -80 mV to -60 mV), and 200 microM Zn2+ shifted this voltage by +45 mV (from -80 mV to -35 mV). Histochemically, we have also observed Zn2+ staining throughout the suprachiasmatic nucleus; the staining is particularly intense in the ventrolateral region of the nucleus, which receives the major fiber inputs. Our findings suggest that Zn2+, presumably synaptically released, may modulate the electrical activity of suprachiasmatic nucleus neurons through IA. Because vesicular Zn2+ is fairly widespread in the central nervous system, it is conceivable that this kind of Zn2+ modulation on IA, and possibly on other voltage-activated currents, exists elsewhere in the brain.
Full text
PDF
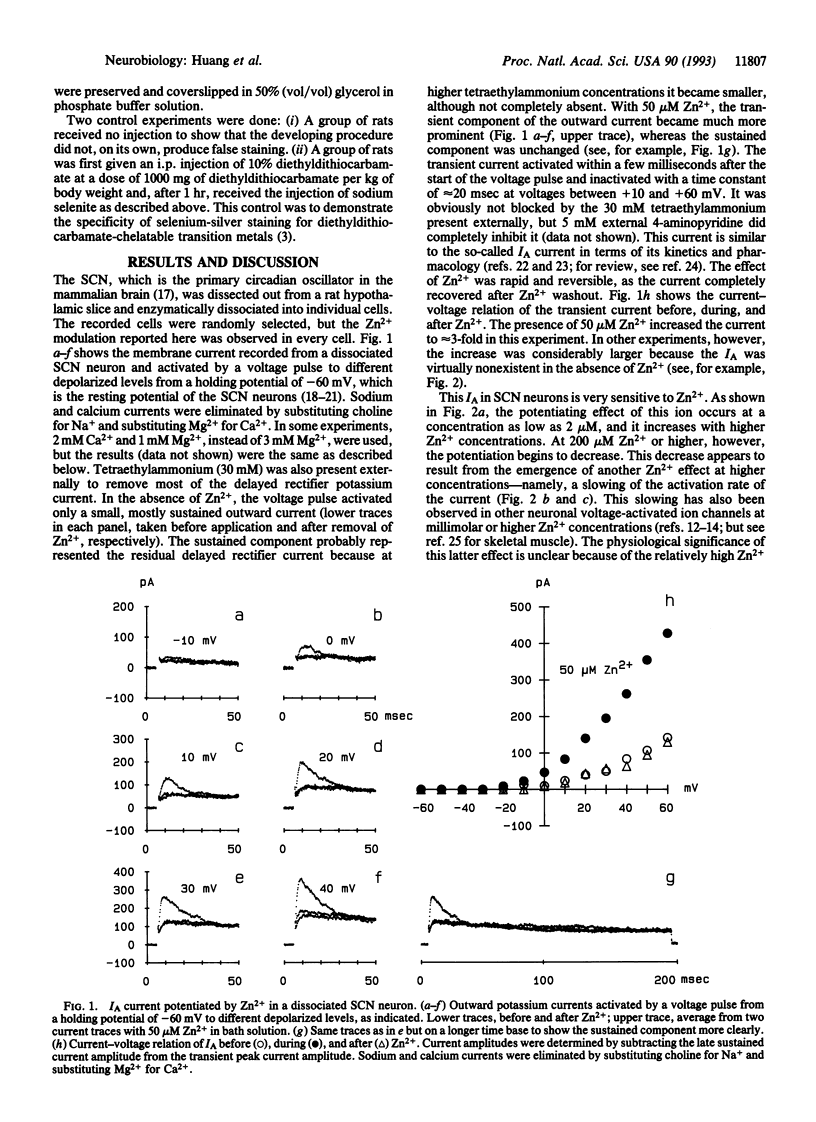
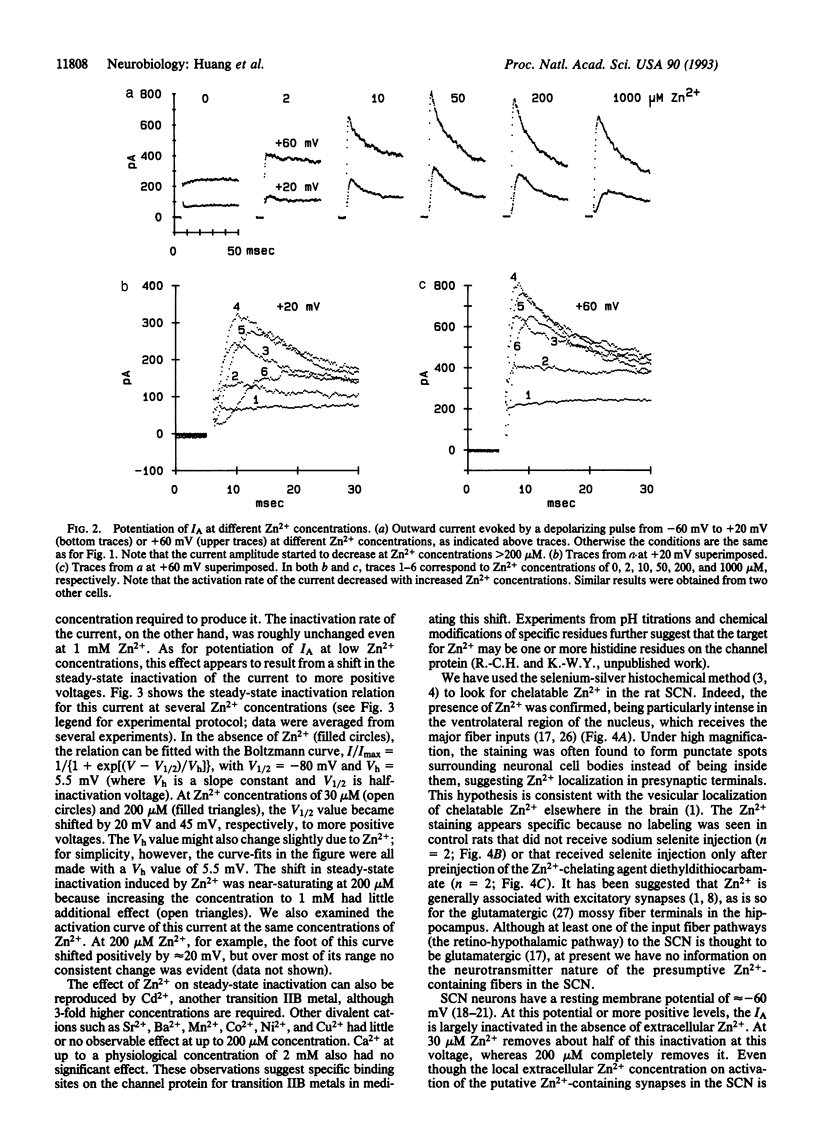
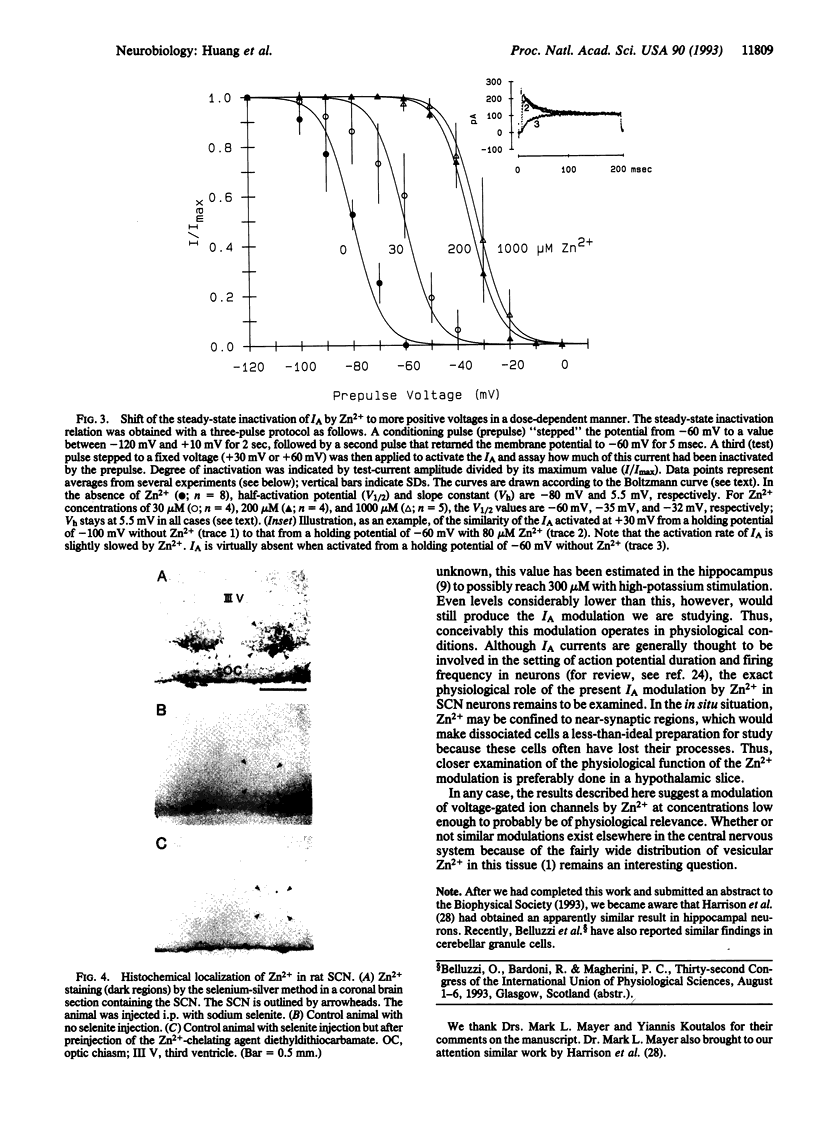

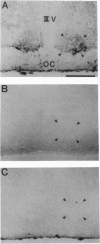
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assaf S. Y., Chung S. H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984 Apr 19;308(5961):734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. L., Connor J. D. Localization and release of glutamic acid in relation to the hippocampal mossy fibre pathway. Nature. 1973 Aug 17;244(5416):442–443. doi: 10.1038/244442a0. [DOI] [PubMed] [Google Scholar]
- Danscher G. Exogenous selenium in the brain. A histochemical technique for light and electron microscopical localization of catalytic selenium bonds. Histochemistry. 1982;76(3):281–293. doi: 10.1007/BF00543951. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Divalent cations and the activation kinetics of potassium channels in squid giant axons. J Gen Physiol. 1982 Jun;79(6):965–996. doi: 10.1085/jgp.79.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Slowing of sodium channel opening kinetics in squid axon by extracellular zinc. J Gen Physiol. 1982 Jun;79(6):935–964. doi: 10.1085/jgp.79.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Radke H. K., Talukder G., Ffrench-Mullen J. M. Zinc modulates transient outward current gating in hippocampal neurons. Receptors Channels. 1993;1(2):153–163. [PubMed] [Google Scholar]
- Howell G. A., Welch M. G., Frederickson C. J. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984 Apr 19;308(5961):736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Huang R. C. Sodium and calcium currents in acutely dissociated neurons from rat suprachiasmatic nucleus. J Neurophysiol. 1993 Oct;70(4):1692–1703. doi: 10.1152/jn.1993.70.4.1692. [DOI] [PubMed] [Google Scholar]
- Ibata Y., Otsuka N. Electron microscopic demonstration of zinc in the hippocampal formation using Timm's sulfide silver technique. J Histochem Cytochem. 1969 Mar;17(3):171–175. doi: 10.1177/17.3.171. [DOI] [PubMed] [Google Scholar]
- Ito C., Wakamori M., Akaike N. Dual effect of glycine on isolated rat suprachiasmatic neurons. Am J Physiol. 1991 Feb;260(2 Pt 1):C213–C218. doi: 10.1152/ajpcell.1991.260.2.C213. [DOI] [PubMed] [Google Scholar]
- Müller T. H., Misgeld U., Swandulla D. Ionic currents in cultured rat hypothalamic neurones. J Physiol. 1992 May;450:341–362. doi: 10.1113/jphysiol.1992.sp019130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., Koh J., Choi D. W. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987 May 1;236(4801):589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- Pérez-Clausell J., Danscher G. Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res. 1985 Jun 24;337(1):91–98. doi: 10.1016/0006-8993(85)91612-9. [DOI] [PubMed] [Google Scholar]
- Romans A. Y., Graichen M. E., Lochmüller C. H., Henkens R. W. Kinetics and mechanism of dissociation of zinc ion from carbonic anhydrase. Bioinorg Chem. 1978 Sep;9(3):217–229. doi: 10.1016/s0006-3061(78)80007-6. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Slomianka L., Danscher G., Frederickson C. J. Labeling of the neurons of origin of zinc-containing pathways by intraperitoneal injections of sodium selenite. Neuroscience. 1990;38(3):843–854. doi: 10.1016/0306-4522(90)90076-g. [DOI] [PubMed] [Google Scholar]
- Spires S., Begenisich T. Chemical properties of the divalent cation binding site on potassium channels. J Gen Physiol. 1992 Aug;100(2):181–193. doi: 10.1085/jgp.100.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of zinc ions on the gating of the delayed potassium conductance of frog sartorius muscle. J Physiol. 1975 Oct;251(3):711–735. doi: 10.1113/jphysiol.1975.sp011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Pol A. N. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980 Jun 15;191(4):661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987 Aug 13;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Wheal H. V., Thomson A. M. The electrical properties of neurones of the rat suprachiasmatic nucleus recorded intracellularly in vitro. Neuroscience. 1984 Sep;13(1):97–104. doi: 10.1016/0306-4522(84)90262-8. [DOI] [PubMed] [Google Scholar]
- Xie X. M., Smart T. G. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature. 1991 Feb 7;349(6309):521–524. doi: 10.1038/349521a0. [DOI] [PubMed] [Google Scholar]