Abstract
Prairie voles (Microtus ochrogaster) are socially monogamous rodents that form pair bonds—a behavior composed of several social interactions including attachment with a familiar mate and aggression toward conspecific strangers. Therefore, this species has provided an excellent opportunity for the study of pair bonding behavior and its underlying neural mechanisms. In this chapter, we discuss the utility of this unique animal model in the study of aggression and review recent findings illustrating the neurochemical mechanisms underlying pair bonding-induced aggression. Implications of this research for our understanding of the neurobiology of human violence are also discussed.
I. INTRODUCTION
Mating induces aggression in several organisms throughout the animal kingdom. Within species, patterns of inter- and intrasexual aggression vary as a function of monogamy, parental investment, and group structure. In the wild, the appropriate coordination of social behavior is necessary for survival and reproductive success. How organisms make decisions about which behavior to display in the natural environment remains an important area of biological investigation. To address these questions, previous work has relied on using traditional laboratory rodents. However, these animals do not readily display certain types of social behaviors and thus are not appropriate for some investigations. For example, laboratory rats and mice do not exhibit strong social bonds between mates, and males typically do not display paternal behavior or female-directed aggression. Because mating naturally induces pair bonding, aggression, and biparental behavior in the socially monogamous prairie vole (Microtus ochrogaster), this species represents a unique animal model to study the underlying neural mechanisms regulating social behavior associated with a monogamous life strategy.
In this chapter, we begin by describing the prairie vole model and reviewing the neural correlates of pair bonding behavior. We focus on the neuropeptides arginine vasopressin (AVP) and oxytocin; neurotransmitters dopamine (DA), gamma-aminobutyric acid (GABA), and glutamate; and steroid hormones testosterone and estrogen in the regulation of aggression. We highlight the molecular genetics underlying courtship and aggression associated with monogamous pair bonding in voles and humans. Finally, we speculate on the potential for translation, of aggression studies in prairie voles, for research examining the etiology of violence in human populations—with a particular emphasis on the interactions between drug abuse and social behavior.
II. THE PRAIRIE VOLE MODEL
Voles are microtine (Microtus) rodents that are taxonomically and genetically similar, yet show remarkable differences in their social behavior (Young and Wang, 2004; Young et al., 2008, 2011a). These animals have provided an excellent opportunity for comparative studies examining social behaviors associated with different life strategies. For example, prairie (M. ochrogaster) and pine (M. pinetorum) voles are highly social and monogamous, whereas meadow (M. pennsylvanicus) and montane (M. montanus) voles are asocial and promiscuous (Dewsbury, 1987; Insel and Hulihan, 1995; Jannett, 1982). In the laboratory, prairie and pine voles are biparental, with both parents equally caring for their young, while meadow and montane voles are primarily maternal and males do not stay in the natal nest after female parturition (McGuire and Novak, 1984, 1986; Oliveras and Novak, 1986). Following mating, prairie voles develop pair bonds between mates (Fig. 6.1A; Young and Wang, 2004) and males even display aggression selectively toward conspecific strangers but not toward their familiar partner (Fig. 6.1B; Aragona et al., 2006; Gobrogge et al., 2007, 2009; Winslow et al., 1993)—behaviors that are not exhibited by promiscuous meadow or montane voles (Insel et al., 1995; Lim et al., 2004). Interestingly, these vole species do not differ in their nonsocial behavior (Tamarin, 1985), further indicating associations between species-specific social behavior and life strategy (Carter et al., 1995; Insel et al., 1998; Wang and Aragona, 2004; Young and Wang, 2004; Young et al., 1998). Therefore, prairie voles represent a unique model system to dissect the neural mechanisms underlying ethologically relevant social behavior.
Figure 6.1.
Neural correlates of selective aggression. (A) After 24 h, but not 6 h, of mating, male and female prairie voles display significantly more time in side-by-side physical contact with an opposite sex familiar partner than with a stranger. (B) Sexually naïve male prairie voles (Naïve) do not display aggression toward a stranger female, whereas 2 weeks of sexual and social experience (Paired) induces selective aggression toward both male and female strangers but not toward familiar female partners. (C) Photo depicts a pair bonded male prairie vole (left) preparing to attack a—sexually receptive—stranger female prairie vole (right). (D) Stereological estimates reveal a significantly higher density of Fos-ir cells in the anterior hypothalamus (AH) and medial amygdala (MeA) of pair bonded males displaying aggression toward a stranger female (Stranger) compared to males displaying affiliation toward their familiar female partner (Partner). (E) Photomicrographs showing Fos-ir (dark nuclear staining) in the AH and MeA from pair bonded males re-exposed to their familiar female partner (Partner) or to a stranger female (Stranger). F: fornix; OT: optic tract. Bars indicate means±standard error of the mean. Bars with different Greek letters differ significantly from each other. *p<0.05. Scale bar=100 μm. Adapted from Aragona and Wang (2009), Gobrogge and Wang (2009), Gobrogge et al. (2007), Wang et al. (1997a), Winslow et al. (1993), and Young et al. (2011a).
One behavioral index of pair bonding is selective aggression, which is more prominent in male than in female prairie voles. It has been suggested that selective aggression is a behavioral trait associated with mate guarding that is important for pair bonding (Carter et al., 1995). Selective aggression is studied using a resident–intruder test (RIT) (Fig. 6.1C; Gobrogge et al., 2007, 2009; Winslow et al., 1993; Wang et al., 1997a). A conspecific intruder is introduced into the male resident cage and their behavioral interactions are videotaped for 5–10 min (Aragona et al., 2006; Gobrogge et al., 2007, 2009; Wang et al., 1997a; Winslow et al., 1993). Subject’s behavioral interactions with the intruder are recorded and the frequency of aggressive behaviors including attacks, bites, chases, defensive/offensive upright postures, offensive sniffs, threats, and retaliatory attacks are calculated as a composite score (Gobrogge et al., 2007, 2009) as well as the duration of affiliative side-by-side physical contact (Gobrogge et al., 2007, 2009; Winslow et al., 1993). It is important to note that both offensive and defensive types of aggression are critical components of selective aggression in male prairie voles (Wang et al., 1997a; Winslow et al., 1993).
Selective aggression is associated with mating, as cohabitation in the absence of mating does not induce this behavior in male prairie voles (Insel et al., 1995; Wang et al., 1997a; Winslow et al., 1993). Selective aggression is also enduring (Aragona et al., 2006; Gobrogge et al., 2007, 2009; Insel et al., 1995) and lasts for at least 2 weeks after partner preference formation (Aragona et al., 2006; Gobrogge et al., 2007, 2009), even in the absence of continuous exposure to a partner (Insel et al., 1995). Importantly, males display aggression not only toward conspecific males (Fig. 6.1B; Aragona et al., 2006; Gobrogge et al., 2007, 2009; Insel et al., 1995; Wang et al., 1997a; Winslow et al., 1993) but also toward sexually receptive females (Fig. 6.1B and C; Gobrogge et al., 2007, 2009). This selective aggression functions to maintain monogamous pair bonds as males reject potential female mates (Fig. 6.1B and C; Aragona et al., 2006; Gobrogge et al., 2007, 2009; Wang et al., 1997a). Together, these reliably expressed and measurable agonistic behaviors make the prairie vole an excellent model for investigation of the neural mechanisms underlying naturally occurring aggression associated with monogamy. It should be mentioned that although female aggression has been less studied in voles, female prairie voles exhibit similar aggressive behavior as males and this behavior is influenced by a female’s social and sexual experience (Bowler et al., 2002).
III. NEURAL CORRELATES
With considerable overlap of brain areas involved in several forms of social (Newman, 1999) and agonistic (Table 6.1) behaviors, there is a significant amount of ambiguity regarding which brain areas may be involved in the regulation of selective aggression. Using a neuronal activation marker of an immediate early gene, c-fos, previous studies in voles have examined neuronal activity associated with aggression (Wang et al., 1997a), maternal (Katz et al., 1999) and paternal behavior (Kirkpatrick et al., 1994), mating (Curtis and Wang, 2003; Lim and Young, 2004), anxiety (Stowe et al., 2005), spatial learning (Kuptsov et al., 2005), chemosensory processing (Hairston et al., 2003; Tubbiola and Wysocki, 1997), social experience (Cushing et al., 2003a; Kramer et al., 2006), or pharmacological challenges (Curtis and Wang, 2005b; Cushing et al., 2003b; Gingrich et al., 1997). Within these studies, however, typically only one type of behavior was investigated. Little focus was aimed at examining other forms of social behavior, including affiliation or general social olfactory processing. Consequently, there is considerable overlap in brain–behavior relationships among these studies leading to ambiguity as to which brain areas regulate selective aggression. Nevertheless, in an early study, male prairie voles displayed aggression toward a male intruder following 24 h of mating, but not following 24 h of cohabitation with a female without mating (Wang et al., 1997a). However, despite their differences in sociosexual experience and in aggressive behavior, both types of male exposure led to equal levels of Fos-ir (immunoreactivity) expression in some brain areas, such as the bed nucleus of the stria terminalis (BNST). Males that mated for 24 h and displayed high levels of aggression toward either a male or a female intruder showed increased levels of Fos-ir expression in the medial amygdala (MeA; Wang et al., 1997a), compared to males that cohabitated with a female without mating, implicating the MeA as a brain area associated with the display of mating-induced selective aggression (Fig. 6.1D and E).
Table 6.1.
Summary of Brain Areas Implicated in Aggression
| Brain area | Species | References |
|---|---|---|
| Anterior hypothalamus (AH) | Human | Sano et al. (1966), Ramamurthi (1988) |
| Prairie vole | Gobrogge et al. (2007, 2009), Gobrogge and Wang (2009) | |
| Rat | Veening et al. (2005), Kruk (1991), Bermond et al. (1982), Kruk et al. (1984), Adams et al. (1993), Roeling et al. (1993), Haller et al. (1998), Veenema et al. (2006), Motta et al. (2009) | |
| Syrian hamster | Delville et al. (2000), Ferris and Potegal (1988), Caldwell and Albers (2004), Ferris et al. (1997, 1989), Albers et al. (2006), Harrison et al. (2000b), Jackson et al. (2005), Grimes et al. (2007), Ricci et al. (2009), Schwartzer et al. (2009), Schwartzer and Melloni (2010a,2010b) | |
| Lateral septum (LS) | Rat | Veenema et al. (2010) |
| Medial amygdala (MeA) | Human | Ramamurthi (1988) |
| Prairie vole | Wang et al. (1997a), Gobrogge and Wang (2009) | |
| Rat | Koolhaas et al. (1990) | |
| Nucleus accumbens (NAcc) | Prairie vole | Aragona et al. (2006) |
| Ventromedial hypothalamus (VMH) | Mouse | Choi et al. (2005), Lin et al. (2011) |
Brain structures involved in aggression, across species, with corresponding references to ground brain area acronyms used throughout the chapter.
In a more recent study, several brain areas including the BNST, medial preoptic area (MPOA), paraventricular nucleus (PVN), and lateral septum (LS) showed higher levels of Fos expression in pair bonded males that had experienced an RIT compared to pair bonded males not exposed to a social intruder (Gobrogge et al., 2007). However, no group differences in Fos expression across these brain areas were found among males that were exposed to different social stimuli or displaying different patterns of social behavior, including aggression or affiliation toward intruders. These data suggest that the increased neuronal activation in these brain regions is probably due to olfactory stimulation or general arousal associated with exposure to a conspecific, but such a response is nonselective. A unique pattern of Fos expression was found in the anterior hypothalamus (AH), in which exposure to a conspecific stranger, either male or female, induced a significant increase in AH-Fos over those reexposed to their familiar partner (Fig. 6.1D and E; Gobrogge et al., 2007). This increase in Fos staining may indicate a stimulus-specific response. The AH appears to be more responsive to chemosensory, tactile, and/or visual cues associated with conspecific strangers, but not familiar partners (Gobrogge et al., 2007). These data indicate that the increased neuronal activation in the AH may be involved in aggressive behavior displayed by pair bonded male prairie voles (Gobrogge et al., 2007). This notion is corroborated by previous research documenting a critical role of the hypothalamus in regulating aggression across several mammalian species. For example, the ventromedial hypothalamus (VMH) in mice (Choi et al., 2005; Lin et al., 2011) and AH in rats (Veening et al., 2005) is responsive to conspecific chemosensory cues, which elicit aggressive behavior. Electrical stimulation applied directly to the AH induces attack toward conspecifics in rats (Kruk, 1991) and other animals (Albert and Walsh, 1984; Siegel et al., 1999). Interestingly, in humans, surgical lesioning of the AH reduces physical violence (Ramamurthi, 1988; Sano et al., 1966). In summary, data from vole studies demonstrate that activation of the MeA and AH is associated with the display of selective aggression (Gobrogge et al., 2007; Wang et al., 1997a).
IV. NEURAL CIRCUITRY
To directly evaluate the neural circuitry programming selective aggression, we performed a series of tract tracing experiments focusing on the AH and MeA. Intra-AH injections of an anterograde tracer, biotinylated dextran amine (BDA), resulted in BDA-ir staining in several brain regions. The AH projected to areas involved in processing chemosensory cues including the MeA; areas important for regulating social behavior including the LS, BNST, MPOA, VMH, and dorsal raphe (DR); and areas coordinating motor output, such as the periaqueductal gray (Gobrogge and Wang, 2009). The AH also projected to brain areas implicated in evaluating incentive salience including the medial prefrontal cortex (mPFC), nucleus accumbens (NAcc), and ventral pallidum (VP), as well as areas involved in memory formation and consolidation including hippocampal regions CA3 and the dentate gyrus (Gobrogge and Wang, 2009). Further, site-specific injections of a retrograde tracer, fluorogold (FG), into the LS, NAcc, or MeA resulted in FG-ir staining in the AH, indicating reciprocal connections between the AH and regions involved in motivation and chemosensory communication (Gobrogge and Wang, 2009).
Fos-ir staining was also used to assess neuronal activation, in this circuit, associated with the display of pair bonding behavior. Males displaying aggression toward an unfamiliar female showed a significantly higher density of Fos-ir in the AH and MeA relative to males displaying affiliation toward their female partner (Fig. 6.1D and E), replicating our previous findings (Gobrogge et al., 2007; Wang et al., 1997a). Interestingly, we identified a MeA-AH-LS circuit that was activated when males were displaying aggression and a DR-AH circuit that was recruited when males were displaying affiliation (Gobrogge and Wang, 2009). The identification of these two neural circuits indicates a specific neuronal framework associated with the choice between affiliation (DR-AH circuit) and aggression (MeA-AH-LS circuit) in pair bonded male prairie voles.
V. NEUROCHEMICAL REGULATION OF SELECTIVE AGGRESSION
Previous work has primarily focused on partner preference formation and documented a growing list of neurochemicals, including oxytocin (OT), AVP, corticotropin releasing hormone, DA, GABA, and glutamate, as well as their interactions in the regulation of pair bonding behavior (Aragona et al., 2003; Curtis and Wang, 2005a,b; Carter et al., 1995; DeVries et al., 1995; Gingrich et al., 2000; Lim and Young, 2004; Liu and Wang, 2003; Lim et al., 2004; Liu et al., 2001; Smeltzer et al., 2006; Wang et al., 1998, 1999; Williams et al., 1992, 1994; Winslow et al., 1993). Importantly, data from several studies indicate a select subset of neurochemicals in the regulation of selective aggression.
A. Neuropeptides
Comparative approaches have been utilized in studies examining neuroendocrine mechanisms regulating social behavior in voles (Insel, 2010). Studies have focused on examining the central AVP system, a nine amino acid neuropeptide with diverse forebrain projections, across monogamous and promiscuous vole species. AVP is an antidiuretic hormone and has been shown to stimulate three structurally distinct receptors V1a, V1b, and V2, each activating very specific second messenger systems (Michell et al., 1979). Classically, AVP was first described as a primary homeostatic factor controlling kidney water reabsorption, blood volume/pressure, and vasodilatation in the peripheral nervous system. AVP and its receptors have been shown to be widely expressed in the central nervous system (Thibonnier, 1992), within specific brain regions (Johnson et al., 1993).
The V1a AVP receptor subtype (V1aR), in particular, has been extensively studied in the regulation of social behavior (Insel et al., 1994) including aggression (Albers et al., 2006; Cooper et al., 2005; Ferris et al., 2006; Winslow et al., 1993). V1aRs are directly coupled to stimulatory (s) Gq-11 proteins (Thibonnier et al., 1993). Stimulation of these G-proteins leads to activation of adenylate cyclase, cAMP, protein kinase C, and phospholipases C, A2, and D (Raggenbass et al., 1991; Thibonnier, 1992; Thibonnier et al., 1992, 1994) enhancing calcium influx through L-type calcium channels (Son and Brinton, 2001). Such activation enhances learning and memory in the aging brain (Deyo et al., 1989; Yamada et al., 1996) via direct effects on gene expression (Murphy et al., 1991).
Because AVP regulates species-specific social behaviors such as aggression (Ferris et al., 1984; Ryding et al., 2008), it was hypothesized that the organization of central AVP systems may differ between monogamous and promiscuous vole species (Bamshad et al., 1993b; Insel and Shapiro, 1992). To test this hypothesis, the distribution pattern of AVP cells, fibers, and receptors were mapped in the vole brain. In all vole species examined, AVP-ir neurons were found in several brain regions, including the PVN and SON (supraoptic nucleus) of the hypothalamus, the BNST, MeA, AH, and MPOA (Bamshad et al., 1993b; Gobrogge et al., 2007; Wang, 1995; Wang et al., 1996). AVP-ir fibers were localized in the LS, lateral habenular nucleus, diagonal band, BNST, MPOA, and MeA (Bamshad et al., 1993b; Wang et al., 1996). Overall, AVP distribution patterns were highly conserved between monogamous and promiscuous vole species (Wang, 1995; Wang et al., 1996). Dramatic species differences in the distribution patterns and regional densities of V1aRs, however, were observed between vole species exhibiting different life strategies (Hammock and Young, 2002). For example, prairie voles have higher densities of V1aRs in the BNST, VP, central and basolateral nuclei of the amygdala, and accessory olfactory bulb, whereas montane voles exhibit a higher density of V1aRs in the LS and mPFC (Insel et al., 1994; Lim et al., 2004; Smeltzer et al., 2006; Wang et al., 1997c; Young et al., 1997). Further, prairie and pine voles exhibit similar patterns of V1aR binding, which differ from that of promiscuous meadow and montane voles (Insel et al., 1994; Lim et al., 2004). Species differences in V1aR distribution are stable across the lifespan (Wang et al., 1997b,c) and are receptor-specific, as no species differences are found in either the benzodiazepine or opiate receptor systems (Insel and Shapiro, 1992). In addition, monogamous prairie and promiscuous meadow voles differ in central AVP activity during mating and reproduction (Bamshad et al., 1993a; Wang et al., 1994). Because of these anatomical and functional differences, central AVP was thought to underlie selective aggression in male prairie voles (Winslow et al., 1993).
Among the neuropeptides underlying aggression (Miczek et al., 2007; Siever, 2008), AVP, and its homolog vasotocin, have been found to regulate several forms of aggression across species (Caldwell et al., 2008; Riters and Panksepp, 1997) and diverse taxa (Backstrom and Winberg, 2009; Goodson, 2008). In humans, central AVP correlates with aggressive behavior (Coccaro et al., 1998) and mediates anger (Thompson et al., 2004). Thus, the central AVP system may have evolved to be primed by a wide variety of experiences to induce aggression, when appropriate, in social animals (Donaldson and Young, 2008). Because central AVP underlies territorial aggression in other rodents (Ferris et al., 1984), AVP was proposed to regulate mating-induced aggression in prairie voles. In a pharmacological study, injections of a V1aR antagonist (V1aR Ant) into the lateral ventricle of male prairie voles blocked selective aggression induced by mating whereas injections of AVP-induced aggression toward an intruder in the absence of mating (Winslow et al., 1993). These effects were neuropeptide specific, as intracerebroventricular (ICV) infusion of an OT receptor antagonist had no effect on mating-induced aggression (Winslow et al., 1993). Importantly, developmental exposure to either AVP in male prairie voles (Stribley and Carter, 1999) or OT in female prairie voles (Bales and Carter, 2003) facilitates aggression in adulthood. Together, these data highlight a critical role of neuropeptide regulation of prairie vole aggression. However, the action site and release dynamics of neuropeptides in the regulation of selective aggression were unclear.
In a more recent study, it was found that the display of selective aggression was associated with increased neuronal activation in the AH, specifically in neurons expressing AVP (Fig. 6.2A and B; Gobrogge et al., 2007). In a previous study in Syrian hamsters (Mesocricetus auratus), aggression has also been shown to be associated with an increase in AVP-ir/Fos-ir double-labeled cells in the nucleus circularis (NC), medial SON (mSON), and surrounding areas ventral to the fornix in the AH (Delville et al., 2000). Our data, combined with previous results from other species, suggest that the AH may be a brain area in which AVP regulates selective aggression. Indeed, AH-AVP has been implicated in various forms of aggressive behavior. In Syrian hamsters, for example, blockade of V1aRs in the AH diminished offensive aggression (Caldwell and Albers, 2004; Ferris and Potegal, 1988) whereas intra-AH administration of a V1aR agonist enhanced aggressive behavior (Caldwell and Albers, 2004; Ferris et al., 1997). More recently, the density of V1aRs in the AH has been found to increase significantly in Syrian hamsters after their display of offensive aggression (Albers et al., 2006).
Figure 6.2.
Vasopressin (AVP) regulation of selective aggression. (A) Pair bonded male prairie voles that display aggression toward female or male strangers exhibit a significantly higher density of AVP-ir/Fos-ir double-labeled cells in the AH relative to pair bonded males re-exposed to their female partner or to males not exposed to any social stimulus (Control). (B) Photomicrograph of AVP-ir cell bodies and fibers (brown cytoplasmic staining), Fos-ir (dark nuclear staining), or AVP-ir/Fos-ir double labeled cells (insert) in the anterior hypothalamus (AH). (C) In vivo brain microdialysis reveals a significant increase in AH-AVP release in pair bonded male prairie voles displaying aggression toward a stranger female compared to males reexposed to their female partner. (D) Intra-AH AVP microinfusion, in sexually naïve males (Naïve), is sufficient to induce aggression toward a stranger female compared to control males infused with cerebral spinal fluid (CSF). AH-AVP-induced aggression is blocked with concurrent administration of AVP with a V1aR Ant. Pair bonded males (Paired) display aggression toward stranger females, which is blocked by intra-AH infusion of a V1aR Ant. (E) Pair bonded males (Paired) exhibit a significantly higher density of AVP-V1a receptor (V1aR) binding in the AH relative to sexually naïve males (Naïve). (F) Sexually naïve males infused with an adeno-associated virus expressing the V1aR gene (AAV-V1aR) in the AH exhibit enhanced aggression toward stranger females relative to males infused with the LacZ-gene (Control). F, fornix; OT, optic tract. Bars indicate means ± standard error of the mean. Bars with different Greek letters differ significantly from each other. *p<0.05. Scale bars=100 μm, insert scale bars=10 μm. Adapted from Aragona et al. (2006), Gobrogge et al. (2007, 2009), and Young et al. (2011a).
Because selective aggression was associated with neuronal activation in the AH, specifically in AVP-containing neurons (Fig. 6.2A and B; Gobrogge et al., 2007), we tested the hypothesis that selective aggression is associated with AH-AVP release. In vivo brain microdialysis, with ELISA, was performed on male prairie voles that were pair bonded for 2 weeks. Pair bonded males displayed significantly higher levels of aggression toward novel females but more side-by-side affiliation with their familiar female partner (Gobrogge et al., 2009). ELISA analysis indicated that exposure to a stranger female, compared to a familiar partner, increased AH-AVP release (Fig. 6.2C), which is further confirmed by correlation analyses indicating that AH-AVP release was coupled positively with aggression and negatively with affiliation (Gobrogge et al., 2009). Moreover, intra-AH administration of AVP at a high (500 ng/side), but not a low (5 ng/side), dose in sexually naïve males induced aggression toward a novel female, and this effect was mediated by V1aR as concurrent administration of a 10-fold higher dose of a V1aR Ant blocked AVP-induced aggression (Fig. 6.2D; Gobrogge et al., 2009). Further, intra-AH infusions of a V1aR Ant (5 μg/side), in pair bonded males, blocked aggression and facilitated social affiliation toward novel females (Fig. 6.2D; Gobrogge et al., 2009). Thus, AH-AVP is both necessary and sufficient to regulate mating-induced selective aggression in male prairie voles.
Prior research has shown that the social environment has a significant impact on signaling and structural components of AVP systems in the brain. In marmoset monkeys, for example, prefrontal V1aR increases during fatherhood (Kozorovitskiy et al., 2006). In hamsters, several social and drug paradigms have been shown to directly alter the AH-AVP system to regulate offensive aggression (Ferris et al., 1989; Grimes et al., 2007; Harrison et al., 2000b; Jackson et al., 2005). In a previous study in male prairie voles, cohabitation with a female significantly increased the number of AVP mRNA labeled cells in the BNST (Wang et al., 1994). Because social isolation increases the density of V1aRs in the AH to regulate offensive aggression in golden hamsters (Albers et al., 2006), we tested the hypothesis that the density of AH-V1aR changes with pair bonding experience to engage selective aggression. Pair bonded males showed higher densities of V1aR binding, site specifically, in the AH (Fig. 6.2E), with no change in OT receptor binding, demonstrating that pair bonding experience induces a neural plastic reorganization of V1aRs in a region- and receptor-specific manner (Gobrogge et al., 2009). To determine whether this increase in V1aR density in the AH, following pair bonding, was directly related to the emergence of aggression toward novel females, we used viral vector mediated gene transfer to artificially elevate V1aR density in the AH. Males that received intra-AH infusions of the AAV-V1aR displayed higher levels of aggression toward a novel female compared to control males that received infusions of the LacZ-gene (Fig. 6.2F; Gobrogge et al., 2009). Similar viral vector mediated increases in V1aR expression in the VP in voles (Lim et al., 2004; Pitkow et al., 2001) and LS in mice (Bielsky et al., 2005) enhanced affiliation. In male rats, intermale aggression correlates with AVP release in the LS while AVP release in the BNST is inversely related to aggression levels (Veenema et al., 2010). Together, these data support the notion that region-specific AVP functioning regulates specific types of social behaviors, and multiple brain regions serve as a circuit in which AVP coordinates a range of adaptive behaviors important for reproductive success.
B. Dopamine
Anatomically, central DA is divided into three distinct pathways: nigrostriatal, incertohypothalamic, and mesocorticolimbic. DA cell bodies projecting from the substantia nigra synapse in the dorsal striatum and comprise the nigrostriatal path (Swanson, 1982). Incertohypothalamic paths extend from DA cell bodies of the A12–14 cell groups and project to the MPOA and PVN (Cheung et al., 1998). The mesocorticolimbic path represents DA cell bodies originating in the ventral tegmental area (VTA; Fig. 6.3C) projecting to the mPFC and NAcc (Fig. 6.3C; Carr and Sesack, 2000; Swanson, 1982). In addition, DA cells in the AH (Fig. 6.3B) project to forebrain areas including the striatum, LS, NAcc, and mPFC (Alcaro et al., 2007; Lindvall and Stenevi, 1978; Maeda and Mogenson, 1980).
Figure 6.3.
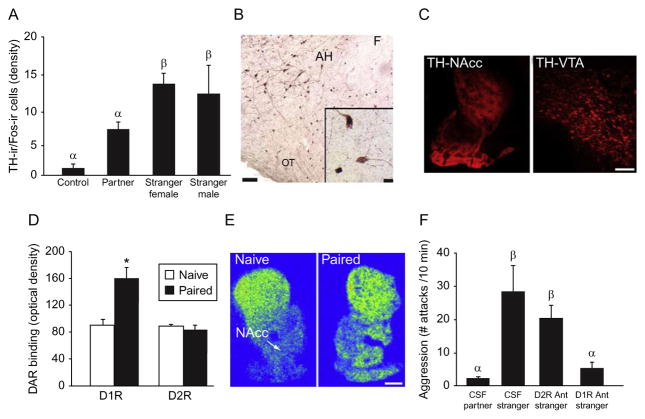
Dopamine (DA) regulation of selective aggression. (A) Pair bonded male prairie voles that display aggression toward female or male strangers exhibit a significantly higher density of TH-ir/Fos-ir double-labeled cells in the AH relative to pair bonded males re-exposed to their female partner or to males not exposed to any social stimulus (Control). (B) Photomicrograph of TH-ir cell bodies and fibers (brown cytoplasmic staining), Fos-ir (dark nuclear staining), or TH-ir/Fos-ir double labeled cells (insert) in the anterior hypothalamus (AH). (C) Photomicrograph of TH-ir fibers in the nucleus accumbens (NAcc) and neurons in the ventral tegmental area (VTA). (D, E) Pair bonded males (Paired) exhibit a significantly higher density of DA D1-like (D1R), but not D2-like (D2R), receptor binding in the NAcc compared to sexually naïve males (Naïve). (F) Pair bonded males, infused with CSF in the NAcc, display aggression toward a stranger female but not toward their female partner. Concurrent infusion of CSF with a DA D1R antagonist (D1R Ant), but not D2R antagonist (D2R Ant), in the NAcc is sufficient to attenuate pair bonding-induced aggression toward a stranger female. F, fornix; OT, optic tract. Bars indicate means±standard error of the mean. Bars with different Greek letters differ significantly from each other. *p<0.05. Scale bars=100 μm, insert scale bars=10 μm. Adapted from Aragona et al. (2006), Gobrogge et al. (2007, 2009), and Young et al. (2011a).
DA preferentially binds to two families of receptors: D1-like and D2-like. Both types of DA receptors are found in the mPFC, NAcc, LS, and MeA (Boyson et al., 1986). D1-like receptors are directly coupled to both stimulatory (s) Gα and Gαolf proteins (Neve et al., 2004). Stimulation of these G-proteins leads to activation of adenylate cyclase, cAMP, and protein phosphatase-1 inhibitor DARP-32 (Neve et al., 2004). Conversely, D2-like receptors couple to inhibitory (i) Gα proteins and, when activated, downregulate adenylate cyclase, cAMP, and protein phosphatase-1 inhibitor DARP-32 (Neve et al., 2004). D1-receptor stimulation plays a critical role in calcium influx, via L-type calcium channels, which is important for cellular long-term potentiation facilitating learning and memory in the aging brain (Deyo et al., 1989; Yamada et al., 1996) through direct influences on gene expression (Murphy et al., 1991).
Because mesocorticolimbic DA underlies partner preference formation (Aragona and Wang, 2009; Aragona et al., 2003; Gingrich et al., 2000; Gobrogge et al., 2008; Wang et al., 1999), studies focused on examining the potential role of central DA regulating selective aggression (Gobrogge et al., 2008). Pair bonded male prairie voles have significantly higher levels of DA D1-type receptors (D1Rs), but not D2-type receptors (D2Rs), in the NAcc (Fig. 6.3D and E; Aragona et al., 2006). Males that cohabitated with a female for 24 h, with or without mating, did not exhibit an increase in D1Rs in the NAcc, supporting the idea that upregulation of NAcc D1Rs, after pair bonding, may directly regulate selective aggression. To test this notion, pair bonded male prairie voles were injected with a D1R antagonist (D1R Ant) into the NAcc. NAcc-D1R, not D2R (D2R Ant), antagonism was sufficient to block selective aggression in pair bonded male prairie voles (Fig. 6.3F; Aragona et al., 2006). In other work, both brief and extended cohabitation with unfamiliar conspecifics in female prairie voles significantly increased the number of DA-ergic cells in the BNST and MeA (Cavanaugh and Lonstein, 2010) and blocking D2Rs, during development, decreased aggression-related behavior including infanticide in adult female, but not male, prairie voles (Hostetler et al., 2010). Further, pair bonded male prairie voles—displaying aggression toward either male or female intruders, had a significantly higher density of cells in the AH that were double-labeled for tyrosine hydroxylase-ir (TH—rate-limiting enzyme in DA biosynthesis) and Fos-ir than males not exposed to a social stimulus or males that were re-exposed to their familiar female partner (Fig. 6.3A and B), implicating AH-DA involvement in selective aggression (Gobrogge et al., 2007).
C. Steroid hormones
Physical aggression is significantly more common in males than females and these behavioral sex differences have been observed across many species (Gatewood et al., 2006). Research describing biological contributions underlying these sex differences has focused primarily on steroid hormones (Gatewood et al., 2006). Several studies have examined the role of androgens in the development of aggressive behavior, both organizationally (e.g., treatment with prenatal testosterone) and activationally (e.g., treatment with postnatal testosterone). Previous research has found that prenatal androgen exposure increases the behavioral expression of adult aggression (Michard-Vanhee, 1988; Vale et al., 1972). Although organizational and activational influences of androgen on aggression have been noted, some inconsistent results have been reported. For example, castration in male rats (Koolhaas et al., 1990) and male prairie voles (Demas et al., 1999) has no affect on aggression. Thus, circulating testosterone, alone, cannot solely contribute to the expression of aggressive behavior in all rodent species. However, these findings do not rule out the possible effects of testosterone having organizational influences on other neurochemical systems in the brain—which, together, may regulate aggression in adulthood. Thus, neurochemical–steroid hormone interactions—underlying aggression—have also been examined. For example, AVP administered directly into the MeA facilitates territorial aggression in male rats and is sufficient to block the effects of castration on reducing aggression (Koolhaas et al., 1990). Further, castration (Bermond et al., 1982), but not ovariectomy (Kruk et al., 1984), decreases the excitability of neurons in the AH—blocking electrically induced aggression, which in castrates can be reversed by testosterone treatment (Bermond et al., 1982). Therefore, circulating testosterone may be acting as a potent neuromodulator—interacting with neurochemicals, like AVP, to regulate aggression.
Additional evidence demonstrating steroid hormone–neurotransmitter interactions exists in research investigating central DA. For example, 75% of TH-ir expressing cells in the hamster posterior MeA contain androgen receptors, are DA-ergic (i.e., they do not co-label with DA beta hydroxylase), and are highly influenced by gonadal hormones compared to TH-ir cells found in the anterior MeA (Asmus and Newman, 1993; Asmus et al., 1992). Interestingly, this same group of TH-ir cells is found in the posterior MeA and BNST in male prairie voles, which appears to be influenced by testosterone (Northcutt et al., 2007) and activated after mating and social experience (Northcutt and Lonstein, 2009). Together, these data suggest interactions between steroid hormones and central DA, in areas such as the MeA, in processing chemosensory cues related to social communication.
D. Classical neurotransmitters
In addition to the effects of neurotransmitters and hormones on aggression, neuromodulators such as GABA and glutamate have also been shown to be involved in the display of agonistic behavior. For example, microinjection of a GABA antagonist (Adams et al., 1993; Roeling et al., 1993) with concurrent treatment of a glutamate agonist (Haller et al., 1998) in the AH facilitates attack behavior in rodents—with higher doses having greater behavioral effects. Further, reverse microdialysis infusion with a glutamate agonist and a GABA-A antagonist into the AH of rats, recently having experienced an agonistic encounter, also facilitates aggression (Haller et al., 1998). Finally, it is worth mentioning that neuromodulators in the VTA, which provides the major source of DA projections to the NAcc (Fig. 6.3C) and mPFC, are also involved in pair bonding behavior. Glutamate and GABA receptor blockade in the VTA, which alters DA activity in the NAcc, induces partner preference formation in the absence of mating in male prairie voles (Curtis and Wang, 2005b).
VI. MOLECULAR GENETICS OF SELECTIVE AGGRESSION
Monogamous male prairie voles carry several repetitive microsatellite DNA sequences in the promoter region of the V1aR gene that are not found in promiscuous male voles (Hammock and Young, 2002, 2004; Young, 1999). These genetic differences directly contribute to species differences in social organization (Landgraf et al., 2003; Lim et al., 2004; Pitkow et al., 2001). Further, mice carrying a transgene coding for the prairie vole V1aR exhibit central V1aR patterns similar to prairie voles and, when injected with AVP, display enhanced social affiliation (Young et al., 1999). Male voles injected in the VP, with a virus expressing V1aR, display enhanced partner preference in the absence of mating (Lim et al., 2004; Pitkow et al., 2001). Interestingly, individual variability in the genetic sequences coding V1aRs has revealed remarkable within species differences in the strength of monogamous pair bonds in prairie voles (Hammock and Young, 2005; Ophir et al., 2008).
Because prairie voles carry varying lengths of DNA to code V1aRs (Hammock and Young, 2005), this genomic predisposition enables their brain to dynamically express V1aRs after sociosexual experience. This genetic loading distinguishes prairie voles from traditional laboratory rodents that lack this genetic makeup. Future work would benefit from comparing aggression levels between male prairie voles carrying long versus short versions of the promoter region encoding the V1aR gene. In humans, polymorphisms in the promoter region encoding V1aR are associated with differences in sociosexual bonding behaviors (Prichard et al., 2007; Walum et al., 2008) including altruism (Israel et al., 2008) and deficits in social communication observed in individuals with autistic spectrum disorders (Israel et al., 2008; Kim et al., 2002; Meyer-Lindenberg et al., 2008). To date, no study has examined potential associations between the V1aR gene and patterns of aggression in humans. Thus, it would be interesting to examine polymorphisms in the V1aR gene in patients with a lifetime history of pathological violence, as the V1aR system may harbor susceptibility genes underlying extreme forms of aggression, increasing the prevalence of homicide and suicide in human populations.
VII. DRUG-INDUCED AGGRESSION
The prairie vole has been established as an animal model for depression related to social separation (Bosch et al., 2009; Grippo et al., 2007). Further, chronic metal ingestion—a potential model for autism—produces social avoidance in male, but not female, prairie voles exposed to unfamiliar same-sex conspecific strangers (Curtis et al., 2010), suggesting a developmental mechanism underlying the social deficits associated with autistic spectrum disorders in humans. Recently, prairie voles have also been utilized to examine the effects of drugs of abuse on pair bonding behavior (Gobrogge et al., 2009; Liu et al., 2010; Young et al., 2011b).
Drug addiction is a significant problem for many humans because drug abuse has such a powerful control over social behavior essential for survival (Kelley and Berridge, 2002; Nesse and Berridge, 1997; Panksepp et al., 2002). In humans, substance abuse has been associated with weapon-related violence and homicide (Hagelstam and Hakkanen, 2006; Madan et al., 2001; Spunt et al., 1998), intimate partner aggression, including partner-directed physical and psychological aggression (Chermack et al., 2008; O’Farrell and Fals-Stewart, 2000), sexual (El-Bassel et al., 2001), and child abuse (Haapasalo and Hamalainen, 1996; Mokuau, 2002; Walsh et al., 2003). Collectively, drug-related violence leads to family system dysfunction and incarceration (Krug et al., 2002), creating significant societal concerns. While aggression research in humans has provided valuable information regarding relationships between drug abuse and violence, animal models have been used to examine neural mechanisms underlying drug-induced aggression.
Drug use can override neurobiological programs to activate maladaptive forms of agonistic behavior, engaging inappropriate types of physical aggression (Swartz et al., 1998) such as domestic violence (Moore et al., 2008) and intimate partner homicide (Farooque et al., 2005). As a result, chronic drug abuse can cause permanent neural reorganization (Nestler and Aghajanian, 1997; White and Kalivas, 1998), impairing the adaptive—social brain (Panksepp et al., 2002), leading to the display of maladaptive social behavior (Wise, 2002). Multiple studies have demonstrated that aggression may be altered shortly after drug exposure and that the directionality of these effects depends on drug, dose, and individual differences between subjects.
Repeated exposure to several drugs of abuse, during adolescence or adulthood, persistently enhances agonistic behaviors, specifically those associated with offensive aggression. For example, Syrian hamsters treated during adolescence with cocaine (DeLeon et al., 2002a; Harrison et al., 2000a; Jackson et al., 2005; Knyshevski et al., 2005a,b; Melloni et al., 2001) or anabolic-androgenic steroids (AASs) (DeLeon et al., 2002b; Harrison et al., 2000b; Melloni and Ferris, 1996; Melloni et al., 1997) display enhanced offensive aggression in adulthood. Interestingly, these drug experiences reorganize AVP (Grimes et al., 2007; Harrison et al., 2000b; Jackson et al., 2005), DA (Ricci et al., 2009; Schwartzer et al., 2009), and GABA (Schwartzer et al., 2009) signaling in the AH.
For example, when compared with nonaggressive sesame oil-treated control males, aggressive AAS-treated males exhibit significant neuroplastic changes in the AH including increased AVP-ir fiber density and AVP content (Harrison et al., 2000b), an increase in TH-ir cell and fiber density (Ricci et al., 2009), enhanced DA-D2R expression (Schwartzer et al., 2009), a higher number of GAD67-ir cells (Schwartzer et al., 2009), and decreased GABAA receptor expression (Schwartzer et al., 2009). Further, pharmacological blockade of D2 (Schwartzer and Melloni, 2010a,b), but not D5 (Schwartzer and Melloni, 2010b), DA receptors in the AH abolishes these effects. Together, results from this work suggests that AVP and DA signaling facilitates aggression by GABA inhibition in the AH of AAS-treated male Syrian hamsters.
As noted above, previous work has shown that exposure to drugs of abuse, such as cocaine, enhances male–male aggression by reorganizing the AH-AVP system in hamsters (Jackson et al., 2005). Therefore, we tested the hypothesis that amphetamine (AMPH), another commonly abused psychostimulant, would act in a similar fashion in affecting male-to-female aggression in prairie voles. Because our recent data revealed that repeated AMPH treatment—(1 mg/ kg) for 3 consecutive days—induces a conditioned place preference (Aragona et al., 2007) and blocks mating-induced partner preference (Fig. 6.4A; Liu et al., 2010), this treatment regimen was used. To examine the selectivity of AMPH-induced aggression, males treated with saline or AMPH were tested for aggression toward an unfamiliar female or a familiar female (that cohabitated with a male across a wire mesh screen for 24 h without mating). Compared with saline-treated controls, AMPH-treated males displayed significantly higher levels of aggression toward either familiar or unfamiliar females (Fig. 6.4B), indicating that AMPH exposure induces generalized aggression, rather than being selective to novel females. This AMPH treatment also induced an increase in the density of AVP-V1aR binding in the AH, but not MPOA, relative to saline control males (Fig. 6.4C). Further, intra-AH infusions of CSF containing the V1aR Ant, but not CSF alone, diminished AMPH-induced aggression toward novel females (Fig. 6.4D). These data suggest that repeated exposure to AMPH can induce female-directed aggression and that this behavior is mediated by AH-AVP. Interestingly, these behavioral effects coincide with upregulation of D1Rs in the NAcc (Liu et al., 2010) and V1aRs in the AH (Gobrogge et al., 2009)—which both facilitate aggression toward novel females (Aragona et al., 2006; Gobrogge et al., 2009); indicating that drugs of abuse can hijack neuroplasticity evolved to maintain monogamous pair bonds.
Figure 6.4.
Drug experience impairs pair bonding behavior. (A) Pair bonded (Intact) and saline treated (0.0) male prairie voles, receiving 3-day—once daily; repeated injections, spend significantly more time in physical side-by-side contact with their familiar female partner than with an unfamiliar stranger female. Pair bonded males injected (i.p., intraperitoneal) with 1.0 or 5.0 mg/kg amphetamine (AMPH) spent equal amounts of time in physical side-by-side contact with their female partner as with a stranger female. (B, C) Repeated AMPH exposure, in sexually naïve males, increases aggression toward both familiar and unfamiliar females and enhances the density of vasopressin (AVP) receptor (V1aR) expression in the anterior hypothalamus (AH) but not in the medial preoptic area (MPOA). (D) Site-specific microinfusion of an AVP-V1aR antagonist (V1aR Ant) into the AH, of males receiving 3-day repeated AMPH exposure (i.p.), significantly decreases AMPH-induced aggression toward novel females relative to males receiving intra-AH infusions of cerebral spinal fluid (CSF). Bars indicate means±standard error of the mean. Bars with different Greek letters differ significantly from each other. *p<0.05; **p<0.01. Adapted from Gobrogge et al. (2009), Liu et al. (2010), and Young et al. (2011a).
VIII. CONCLUSIONS AND FUTURE DIRECTIONS
Prairie voles have provided an excellent model system to study the neurobiology of ethologically meaningful aggression associated with monogamous pair bonds. Aggression can be easily manipulated under laboratory conditions and reliably expressed following mating and social cohabitation. Several brain areas: MeA, AH, and NAcc, work in a neural circuit to regulate selective aggression via AVP and DA. Commonly abused drugs, like AMPH, can usurp these neurochemical circuits to engage maladaptive social behavior impairing monogamous pair bonds.
Although male-to-male aggression has been studied in a variety of mammals, we know surprisingly little about male-to-female aggression and its underlying neuromechanisms. Interestingly, pair bonded male prairie voles naturally display aggression toward conspecific females but not toward their female partner and, therefore, selective aggression allows for investigation of the neurobiology of male-to-female aggression. Data have demonstrated that this form of selective aggression is mediated by elevated AVP release and increased V1aR expression in the AH—priming male prairie voles to respond aggressively to novel females. In addition, data have also shown that the same AH-AVP system mediates generalized, female-directed aggression induced by AMPH. Together with previous research from other animals (Ferris et al., 1989, 1997; Grimes et al., 2007; Harrison et al., 2000b; Jackson et al., 2005; Veenema et al., 2006), these data demonstrate a unique point of convergence in the mammalian brain (Choi et al., 2005; Motta et al., 2009). The AH-AVP system is highly conserved and functions to control different forms of aggression to maintain a wide range of resources important for reproductive success. These highly evolved neuropeptide systems appear to be extremely vulnerable to drugs of abuse, as our data show that hypothalamic AVP controls both naturally occurring as well as drug-facilitated female-directed aggression, suggesting that psychostimulant drugs, like AMPH, are capable of switching adaptive (functional) forms of aggression (e.g., mate guarding) to aberrant (dysfunctional) forms of violent behavior (e.g., partner-directed aggression). Together, these data demonstrate the utility of the prairie vole model for evaluation of the effects of drug abuse on neural systems controlling adaptive forms of aggression—such as mate guarding.
Finally, because other neurochemicals, such as DA (Aragona et al., 2006) and serotonin (Villalba et al., 1997), also regulate selective aggression in prairie voles, offensive aggression related to drug experience (Tidey and Miczek, 1992) and AVP/5-HT interactions mediate aggression in other rodents (Ferris et al., 1997; Veenema et al., 2006), future studies should examine potential neurochemical interactions in the regulation of selective aggression. By understanding the basic neuroendocrinology of pair bonding in prairie voles, we may eventually be able to better clarify the neural chemistry of mental health deficits associated with aberrations in social behavior in patients suffering from drug addiction or pathological violence.
Acknowledgments
The authors would like to thank Benjamin William Tyson for critically reading this manuscript. The work reviewed in this chapter was supported by National Institutes of Health grants MHF31-79600 to K. L. G., MHR01-58616, MHR01-89852, DAR01-19627, and DAK02-23048 to Z. X. W., and NIH Program Training Grant T32 NS-07437.
References
- Adams DB, Boudreau W, Cowan CW, Kokonowski C, Oberteuffer K, Yohay K. Offense produced by chemical stimulation of the anterior hypothalamus of the rat. Physiol Behav. 1993;53:1127–1132. doi: 10.1016/0031-9384(93)90369-q. [DOI] [PubMed] [Google Scholar]
- Albers EH, Dean A, Karom MC, Smith D, Huhman KL. Role of V1a vasopressin receptors in the control of aggression in Syrian hamsters. Brain Res. 2006;1073–1074:425–430. doi: 10.1016/j.brainres.2005.12.081. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Walsh ML. Neural systems and the inhibitory modulation of agonistic behavior: A comparison of mammalian species. Neurosci Biobehav Rev. 1984;8:5–24. doi: 10.1016/0149-7634(84)90017-4. [DOI] [PubMed] [Google Scholar]
- Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: An affective neuroethological perspective. Brain Res Rev. 2007;56:283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Front Behav Neurosci. 2009;3:15. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Detwiler JM, Wang Z. Amphetamine reward in the monogamous prairie vole. Neurosci Lett. 2007;418:190–194. doi: 10.1016/j.neulet.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmus SE, Newman SW. Tyrosine hydroxylase neurons in the male hamster chemosensory pathway contain androgen receptors and are influenced by gonadal hormones. J Comp Neurol. 1993;331:445–457. doi: 10.1002/cne.903310402. [DOI] [PubMed] [Google Scholar]
- Asmus SE, Kincaid AE, Newman SW. A species-specific population of tyrosine hydroxylase-immunoreactive neurons in the medial amygdaloid nucleus of the Syrian hamster. Brain Res. 1992;575:199–207. doi: 10.1016/0006-8993(92)90080-s. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Winberg S. Arginine-vasotocin influence on aggressive behavior and dominance in rainbow trout. Physiol Behav. 2009;96:470–475. doi: 10.1016/j.physbeh.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm Behav. 2003;44:178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster and meadow voles, Microtus pennsylvanicus. J Neuroendocrinol. 1993a;5:247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, deVries AC. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) J Neuroendocrinol. 1993b;5:247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Bermond B, Mos J, Meelis W, van der Poel AM, Kruk MR. Aggression induced by stimulation of the hypothalamus: Effects of androgens. Pharmacol Biochem Behav. 1982;16:41–45. doi: 10.1016/0091-3057(82)90010-7. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: A gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler CM, Cushing BS, Carter CS. Social factors regulate female-female aggression and affiliation in prairie voles. Physiol Behav. 2002;76:559–566. doi: 10.1016/s0031-9384(02)00755-2. [DOI] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE. Effect of photoperiod on vasopressin-induced aggression in Syrian hamsters. Horm Behav. 2004;46:444–449. doi: 10.1016/j.yhbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS., 3rd Vasopressin: Behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: Target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: The prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Cavanaugh BL, Lonstein JS. Social novelty increases tyrosine hydroxylase immunoreactivity in the extended olfactory amygdala of female prairie voles. Physiol Behav. 2010;100:381–386. doi: 10.1016/j.physbeh.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chermack ST, Murray RL, Walton MA, Booth BA, Wryobeck J, Blow FC. Partner aggression among men and women in substance use disorder treatment: Correlates of psychological and physical aggression and injury. Drug Alcohol Depend. 2008;98:35–44. doi: 10.1016/j.drugalcdep.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S, Ballew JR, Moore KE, Lookingland KJ. Contribution of dopamine neurons in the medial zona incerta to the innervation of the central nucleus of the amygdala, horizontal diagonal band of Broca and hypothalamic paraventricular nucleus. Brain Res. 1998;808:174–181. doi: 10.1016/s0006-8993(98)00809-9. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF. Cerebrospinal fluid vasopressin levels: Correlates with aggression and serotonin function in personality-disordered subjects. Arch Gen Psychiatry. 1998;55:708–714. doi: 10.1001/archpsyc.55.8.708. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Karom M, Huhman KL, Albers HE. Repeated agonistic encounters in hamsters modulate AVP V1a receptor binding. Horm Behav. 2005;48:545–551. doi: 10.1016/j.yhbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Wang Z. Forebrain c-fos expression under conditions conducive to pair bonding in female prairie voles (Microtus ochrogaster) Physiol Behav. 2003;80:95–101. doi: 10.1016/s0031-9384(03)00226-9. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Wang Z. Glucocorticoid receptor involvement in pair bonding in female prairie voles: The effects of acute blockade and interactions with central dopamine reward systems. Neuroscience. 2005a;134:369–376. doi: 10.1016/j.neuroscience.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Wang Z. Ventral tegmental area involvement in pair bonding in male prairie voles. Physiol Behav. 2005b;86:338–346. doi: 10.1016/j.physbeh.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Hood AN, Chen Y, Cobb GP, Wallace DR. Chronic metals ingestion by prairie voles produces sex-specific deficits in social behavior: An animal model of autism. Behav Brain Res. 2010;213:42–49. doi: 10.1016/j.bbr.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing BS, Mogekwu N, Le WW, Hoffman GE, Carter CS. Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Res. 2003a;965:203–211. doi: 10.1016/s0006-8993(02)04199-9. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Yamamoto Y, Hoffman GE, Carter CS. Central expression of c-Fos in neonatal male and female prairie voles in response to treatment with oxytocin. Brain Res Dev Brain Res. 2003b;143:129–136. doi: 10.1016/s0165-3806(03)00105-6. [DOI] [PubMed] [Google Scholar]
- DeLeon KR, Grimes JM, Connor DF, Melloni RH., Jr Adolescent cocaine exposure and offensive aggression: Involvement of serotonin neural signaling and innervation in male Syrian hamsters. Behav Brain Res. 2002a;133:211–220. doi: 10.1016/s0166-4328(02)00004-9. [DOI] [PubMed] [Google Scholar]
- DeLeon KR, Grimes JM, Melloni RH., Jr Repeated anabolic-androgenic steroid treatment during adolescence increases vasopressin V(1A) receptor binding in Syrian hamsters: Correlation with offensive aggression. Horm Behav. 2002b;42:182–191. doi: 10.1006/hbeh.2002.1802. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Demas GE, Moffatt CA, Drazen DL, Nelson RJ. Castration does not inhibit aggressive behavior in adult male prairie voles (Microtus ochrogaster) Physiol Behav. 1999;66:59–62. doi: 10.1016/s0031-9384(98)00268-6. [DOI] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans S, Carter CS. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proc Natl Acad Sci USA. 1995;92:7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewsbury DA. The comparative psychology of monogamy. Nebr Symp Motiv. 1987;35:1–50. [PubMed] [Google Scholar]
- Deyo RA, Straube KT, Disterhoft JF. Nimodipine facilitates associative learning in aging rabbits. Science. 1989;243:809–811. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- El-Bassel N, Witte SS, Wada T, Gilbert L, Wallace J. Correlates of partner violence among female street-based sex workers: Substance abuse, history of childhood abuse, and HIV risks. AIDS Patient Care STDS. 2001;15:41–51. doi: 10.1089/108729101460092. [DOI] [PubMed] [Google Scholar]
- Farooque RS, Stout RG, Ernst FA. Heterosexual intimate partner homicide: Review of ten years of clinical experience. J Forensic Sci. 2005;50:648–651. [PubMed] [Google Scholar]
- Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol Behav. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Albers HE, Wesolowski SM, Goldman BD, Luman SE. Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters. Science. 1984;224:521–523. doi: 10.1126/science.6538700. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Axelson JF, Martin AM, Roberge LF. Vasopressin immunoreactivity in the anterior hypothalamus is altered during the establishment of dominant/subordinate relationships between hamsters. Neuroscience. 1989;29:675–683. doi: 10.1016/0306-4522(89)90140-1. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Lu SF, Messenger T, Guillon CD, Heindel N, Miller M, Koppel G, Robert Bruns F, Simon NG. Orally active vasopressin V1a receptor antagonist, SRX251, selectively blocks aggressive behavior. Pharmacol Biochem Behav. 2006;83:169–174. doi: 10.1016/j.pbb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich BS, Huot RL, Wang Z, Insel TR. Differential Fos expression following microinjection of oxytocin or vasopressin in the prairie vole brain. Ann N Y Acad Sci. 1997;807:504–505. doi: 10.1111/j.1749-6632.1997.tb51952.x. [DOI] [PubMed] [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behav Neurosci. 2000;114:173–183. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Wang Z. Neural Circuitry Underlying Pair Bonding-induced Aggression in Monogamous Male Prairie Voles. Society for Neuroscience Abstracts #377.4; Chicago, IL: 2009. [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Wang Z. Dopamine regulation of pair bonding in monogamous prairie voles. In: Bridges R, editor. The Neurobiology of the Parental Brain. Elsevier; San Diego, CA: 2008. pp. 345–358. [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci USA. 2009;106:19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH., Jr Alterations in anterior hypothalamic vasopressin, but not serotonin, correlate with the temporal onset of aggressive behavior during adolescent anabolic-androgenic steroid exposure in hamsters (Mesocricetus auratus) Behav Neurosci. 2007;121:941–948. doi: 10.1037/0735-7044.121.5.941. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo J, Hamalainen T. Childhood family problems and current psychiatric problems among young violent and property offenders. J Am Acad Child Adolesc Psychiatry. 1996;35:1394–1401. doi: 10.1097/00004583-199610000-00027. [DOI] [PubMed] [Google Scholar]
- Hagelstam C, Hakkanen H. Adolescent homicides in Finland: Offence and offender characteristics. Forensic Sci Int. 2006;164:110–115. doi: 10.1016/j.forsciint.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Hairston JE, Ball GF, Nelson RJ. Photoperiodic and temporal influences on chemosensory induction of brain fos expression in female prairie voles. J Neuroendocrinol. 2003;15:161–172. doi: 10.1046/j.1365-2826.2003.00963.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Abraham I, Zelena D, Juhasz G, Makara GB, Kruk MR. Aggressive experience affects the sensitivity of neurons towards pharmacological treatment in the hypothalamic attack area. Behav Pharmacol. 1998;9:469–475. doi: 10.1097/00008877-199809000-00010. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Variation in the vasopressin V1a receptor promoter and expression: Implications for inter- and intraspecific variation in social behaviour. Eur J Neurosci. 2002;16:399–402. doi: 10.1046/j.1460-9568.2002.02083.x. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Functional microsatellite polymorphism associated with divergent social structure in vole species. Mol Biol Evol. 2004;21:1057–1063. doi: 10.1093/molbev/msh104. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308:1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- Harrison RJ, Connor DF, Nowak C, Melloni RH., Jr Chronic low-dose cocaine treatment during adolescence facilitates aggression in hamsters. Physiol Behav. 2000a;69:555–562. doi: 10.1016/s0031-9384(00)00220-1. [DOI] [PubMed] [Google Scholar]
- Harrison RJ, Connor DF, Nowak C, Nash K, Melloni RH., Jr Chronic anabolic-androgenic steroid treatment during adolescence increases anterior hypothalamic vasopressin and aggression in intact hamsters. Psychoneuroendocrinology. 2000b;25:317–338. doi: 10.1016/s0306-4530(99)00057-8. [DOI] [PubMed] [Google Scholar]
- Hostetler CM, Harkey SL, Bales KL. D2 antagonist during development decreases anxiety and infanticidal behavior in adult female prairie voles (Microtus ochrogaster) Behav Brain Res. 2010;210:127–130. doi: 10.1016/j.bbr.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci USA. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang Z, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Preston S, Winslow JT. Mating in the monogamous male: Behavioral consequences. Physiol Behav. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Insel TR, Winslow JT, Wang Z, Young LJ. Oxytocin, vasopressin, and the neuroendocrine basis of pair bond formation. Adv Exp Med Biol. 1998;449:215–224. doi: 10.1007/978-1-4615-4871-3_28. [DOI] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, Uzefovsky F, Reibold M, Bachner-Melman R, Granot R, Bornstein G, Knafo A, Yirmiya N, Ebstein RP. Molecular genetic studies of the arginine vasopressin 1a receptor (AVPR1a) and the oxytocin receptor (OXTR) in human behaviour: From autism to altruism with some notes in between. Prog Brain Res. 2008;170:435–449. doi: 10.1016/S0079-6123(08)00434-2. [DOI] [PubMed] [Google Scholar]
- Jackson D, Burns R, Trksak G, Simeone B, DeLeon KR, Connor DF, Harrison RJ, Melloni RH., Jr Anterior hypothalamic vasopressin modulates the aggression-stimulating effects of adolescent cocaine exposure in Syrian hamsters. Neuroscience. 2005;133:635–646. doi: 10.1016/j.neuroscience.2005.02.047. [DOI] [PubMed] [Google Scholar]
- Jannett F. Nesting patterns of adult voles, Microtus montanus, in field populations. J Mammal. 1982;63:495–498. [Google Scholar]
- Johnson AE, Audigier S, Rossi F, Jard S, Tribollet E, Barberis C. Localization and characterization of vasopressin binding sites in the rat brain using an iodinated linear AVP antagonist. Brain Res. 1993;622:9–16. doi: 10.1016/0006-8993(93)90795-o. [DOI] [PubMed] [Google Scholar]
- Katz LF, Ball GF, Nelson RJ. Elevated Fos-like immunoreactivity in the brains of postpartum female prairie voles, Microtus ochrogaster. Cell Tissue Res. 1999;298:425–435. doi: 10.1007/s004419900123. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchesne R, Courchesne E, Lord C, Leventhal BL, Cook EH, Jr, Insel TR. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol Psychiatry. 2002;7:503–507. doi: 10.1038/sj.mp.4001125. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Kim JW, Insel TR. Limbic system fos expression associated with paternal behavior. Brain Res. 1994;658:112–118. doi: 10.1016/s0006-8993(09)90016-6. [DOI] [PubMed] [Google Scholar]
- Knyshevski I, Connor DF, Harrison RJ, Ricci LA, Melloni RH., Jr Persistent activation of select forebrain regions in aggressive, adolescent cocaine-treated hamsters. Behav Brain Res. 2005a;159:277–286. doi: 10.1016/j.bbr.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Knyshevski I, Ricci LA, McCann TE, Melloni RH., Jr Serotonin type-1A receptors modulate adolescent, cocaine-induced offensive aggression in hamsters. Physiol Behav. 2005b;85:167–176. doi: 10.1016/j.physbeh.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Van den Brink THC, Roozendaal B, Boorsma F. Medial amygdala and aggressive behavior: Interaction between testosterone and vasopressin. Aggress Behav. 1990;16:223–229. [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Choe C, Carter CS, Cushing BS. Developmental effects of oxytocin on neural activation and neuropeptide release in response to social stimuli. Horm Behav. 2006;49:206–214. doi: 10.1016/j.yhbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Krug EG, Dahlberg LL, Mercy JA, Zwi AB, Lozito R. World Report on Violence and Health. World Health Organization; Geneva: 2002. [Google Scholar]
- Kruk MR. Ethology and pharmacology of hypothalamic aggression in the rat. Neurosci Biobehav Rev. 1991;15:527–538. doi: 10.1016/s0149-7634(05)80144-7. [DOI] [PubMed] [Google Scholar]
- Kruk MR, Van der Laan CE, Mos J, Van der Poel AM, Meelis W, Olivier B. Comparison of aggressive behaviour induced by electrical stimulation in the hypothalamus of male and female rats. Prog Brain Res. 1984;61:303–314. doi: 10.1016/S0079-6123(08)64443-X. [DOI] [PubMed] [Google Scholar]
- Kuptsov PA, Pleskacheva MG, Voronkova DN, Lipp HP, Anokhin KV. Features of the c-Fos gene expression along the hippocampal rostro-caudal axis in common voles after rapid spatial learning. Zh Vyssh Nerv Deiat Im I P Pavlova. 2005;55:231–240. [PubMed] [Google Scholar]
- Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X, Terwilliger EF, Niwa M, Wigger A, Young LJ. Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: Improved social discrimination and active social behaviour. Eur J Neurosci. 2003;18:403–411. doi: 10.1046/j.1460-9568.2003.02750.x. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Stenevi U. Dopamine and noradrenaline neurons projecting to the septal area in the rat. Cell Tissue Res. 1978;190:383–407. doi: 10.1007/BF00219554. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Z. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Liu Y, Aragona BJ, Young KA, Dietz DM, Kabbaj M, Mazei-Robison M, Nestler EJ, Wang Z. Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proc Natl Acad Sci USA. 2010;107:1217–1222. doi: 10.1073/pnas.0911998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan A, Beech DJ, Flint L. Drugs, guns, and kids: The association between substance use and injury caused by interpersonal violence. J Pediatr Surg. 2001;36:440–442. doi: 10.1053/jpsu.2001.21599. [DOI] [PubMed] [Google Scholar]
- Maeda H, Mogenson GJ. An electrophysiological study of inputs to neurons of the ventral tegmental area from the nucleus accumbens and medial preoptic-anterior hypothalamic areas. Brain Res. 1980;197:365–377. doi: 10.1016/0006-8993(80)91122-1. [DOI] [PubMed] [Google Scholar]
- McGuire B, Novak M. A comparison of maternal behavior in the meadow vole (Microtus pennsylvanicus), prairie vole (M. ochrogaster) and pine vole (M. pinetorum) Anim Behav. 1984;32:1132–1141. [Google Scholar]
- McGuire B, Novak M. Parental care and its relation to social organization in the montane vole. J Mammal. 1986;67:305–311. [Google Scholar]
- Melloni RH, Jr, Ferris CF. Adolescent anabolic steroid use and aggressive behavior in golden hamsters. Ann N Y Acad Sci. 1996;794:372–375. doi: 10.1111/j.1749-6632.1996.tb32546.x. [DOI] [PubMed] [Google Scholar]
- Melloni RH, Jr, Connor DF, Hang PT, Harrison RJ, Ferris CF. Anabolic-androgenic steroid exposure during adolescence and aggressive behavior in golden hamsters. Physiol Behav. 1997;61:359–364. doi: 10.1016/s0031-9384(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Melloni RH, Jr, Connor DF, Todtenkopf MS, DeLeon KR, Sanyal P, Harrison RJ. Repeated cocaine treatment activates flank marking in adolescent female hamsters. Physiol Behav. 2001;73:561–570. doi: 10.1016/s0031-9384(01)00478-4. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, Dean M, Weinberger DR. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Mol Psychiatry. 2008;10:968–975. doi: 10.1038/mp.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard-Vanhee C. Aggressive behavior induced in female mice by an early single injection of testosterone is genotype dependent. Behav Genet. 1988;18:1–12. doi: 10.1007/BF01067071. [DOI] [PubMed] [Google Scholar]
- Michell RH, Kirk CJ, Billah MM. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979;7:861–865. doi: 10.1042/bst0070861. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Almeida RM, Kravitz EA, Rissman EF, de Boer SF, Raine A. Neurobiology of escalated aggression and violence. J Neurosci. 2007;27:11803–11806. doi: 10.1523/JNEUROSCI.3500-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokuau N. Culturally based interventions for substance use and child abuse among native Hawaiians. Public Health Rep. 2002;117(Suppl 1):S82–S87. [PMC free article] [PubMed] [Google Scholar]
- Moore TM, Stuart GL, Meehan JC, Rhatigan DL, Hellmuth JC, Keen SM. Drug abuse and aggression between intimate partners: A meta-analytic review. Clin Psychol Rev. 2008;28:247–274. doi: 10.1016/j.cpr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Motta SC, Goto M, Gouveia FV, Baldo MV, Canteras NS, Swanson LW. Dissecting the brain’s fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc Natl Acad Sci USA. 2009;106:4870–4875. doi: 10.1073/pnas.0900939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Worley PF, Baraban JM. L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron. 1991;7:625–635. doi: 10.1016/0896-6273(91)90375-a. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Berridge KC. Psychoactive drug use in evolutionary perspective. Science. 1997;278:63–66. doi: 10.1126/science.278.5335.63. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Northcutt KV, Lonstein JS. Social contact elicits immediate-early gene expression in dopaminergic cells of the male prairie vole extended olfactory amygdala. Neuroscience. 2009;163:9–22. doi: 10.1016/j.neuroscience.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt KV, Wang Z, Lonstein JS. Sex and species differences in tyrosine hydroxylase-synthesizing cells of the rodent olfactory extended amygdala. J Comp Neurol. 2007;500:103–115. doi: 10.1002/cne.21148. [DOI] [PubMed] [Google Scholar]
- O’Farrell TJ, Fals-Stewart W. Behavioral couples therapy for alcoholism and drug abuse. J Subst Abuse Treat. 2000;18:51–54. doi: 10.1016/s0740-5472(99)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveras D, Novak M. A comparison of paternal behavior in the meadow vole, Microtus pennsylvanicus, the pine vole, Microtus pinetorum, and prairie vole, Microtus ochrogaster. Anim Behav. 1986;34:519–526. [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proc Natl Acad Sci USA. 2008;105:1249–1254. doi: 10.1073/pnas.0709116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addictions: A neuro-evolutionary perspective and new ‘self-report’ animal model. Addiction. 2002;97:459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard ZM, Mackinnon AJ, Jorm AF, Easteal S. AVPR1A and OXTR polymorphisms are associated with sexual and reproductive behavioral phenotypes in humans. Hum Mutat. 2007;28:1150. doi: 10.1002/humu.9510. Mutation in brief no. 981. Online. [DOI] [PubMed] [Google Scholar]
- Raggenbass M, Goumaz M, Sermasi E, Tribollet E, Dreifuss JJ. Vasopressin generates a persistent voltage-dependent sodium current in a mammalian motoneuron. J Neurosci. 1991;11:1609–1616. doi: 10.1523/JNEUROSCI.11-06-01609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi B. Stereotactic operation in behaviour disorders. Amygdalotomy and hypothalamotomy. Acta Neurochir Suppl. 1988;44:152–157. doi: 10.1007/978-3-7091-9005-0_29. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Schwartzer JJ, Melloni RH., Jr Alterations in the anterior hypothalamic dopamine system in aggressive adolescent AAS-treated hamsters. Horm Behav. 2009;55:348–355. doi: 10.1016/j.yhbeh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Riters LV, Panksepp J. Effects of vasotocin on aggressive behavior in male Japanese quail. Ann N Y Acad Sci. 1997;807:478–480. doi: 10.1111/j.1749-6632.1997.tb51943.x. [DOI] [PubMed] [Google Scholar]
- Roeling TA, Kruk MR, Schuurmans R, Veening JG. Behavioural responses of bicucculline methiodide injections into the ventral hypothalamus of freely moving, socially interacting rats. Brain Res. 1993;615:121–127. doi: 10.1016/0006-8993(93)91122-9. [DOI] [PubMed] [Google Scholar]
- Ryding E, Lindstrom M, Traskman-Bendz L. The role of dopamine and serotonin in suicidal behaviour and aggression. Prog Brain Res. 2008;172:307–315. doi: 10.1016/S0079-6123(08)00915-1. [DOI] [PubMed] [Google Scholar]
- Sano K, Yoshioka M, Ogashiwa M, Ishijima B, Ohye C. Postero-medial hypothalamotomy in the treatment of aggressive behaviors. Confin Neurol. 1966;27:164–167. doi: 10.1159/000103949. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Melloni RH., Jr Anterior hypothalamic dopamine D2 receptors modulate adolescent anabolic/androgenic steroid-induced offensive aggression in the Syrian hamster. Behav Pharmacol. 2010a;21:314–322. doi: 10.1097/FBP.0b013e32833b10f1. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Melloni RH., Jr Dopamine activity in the lateral anterior hypothalamus modulates AAS-induced aggression through D2 but not D5 receptors. Behav Neurosci. 2010b;124:645–655. doi: 10.1037/a0020899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Ricci LA, Melloni RH., Jr Interactions between the dopaminergic and GABAergic neural systems in the lateral anterior hypothalamus of aggressive AAS-treated hamsters. Behav Brain Res. 2009;203:15–22. doi: 10.1016/j.bbr.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Siegel A, Roeling TA, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165:429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394:146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Son MC, Brinton RD. Regulation and mechanism of L-type calcium channel activation via V1a vasopressin receptor activation in cultured cortical neurons. Neurobiol Learn Mem. 2001;76:388–402. doi: 10.1006/nlme.2001.4020. [DOI] [PubMed] [Google Scholar]
- Spunt B, Brownstein HH, Crimmins SM, Langley S, Spanjol K. Alcohol-related homicides committed by women. J Psychoactive Drugs. 1998;30:33–43. doi: 10.1080/02791072.1998.10399669. [DOI] [PubMed] [Google Scholar]
- Stowe JR, Liu Y, Curtis JT, Freeman ME, Wang Z. Species differences in anxiety-related responses in male prairie and meadow voles: The effects of social isolation. Physiol Behav. 2005;86:369–378. doi: 10.1016/j.physbeh.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Stribley JM, Carter CS. Developmental exposure to vasopressin increases aggression in adult prairie voles. Proc Natl Acad Sci USA. 1999;96:12601–12604. doi: 10.1073/pnas.96.22.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swartz MS, Swanson JW, Hiday VA, Borum R, Wagner HR, Burns BJ. Violence and severe mental illness: The effects of substance abuse and nonadherence to medication. Am J Psychiatry. 1998;155:226–231. doi: 10.1176/ajp.155.2.226. [DOI] [PubMed] [Google Scholar]
- Tamarin R. Am Soc Mamm Spec Pub. Shippensburg, PA: 1985. Biology of new world Microtus; p. 8. [Google Scholar]
- Thibonnier M. Signal transduction of V1-vascular vasopressin receptors. Regul Pept. 1992;38:1–11. doi: 10.1016/0167-0115(92)90067-5. [DOI] [PubMed] [Google Scholar]
- Thibonnier M, Bayer AL, Simonson MS, Douglas JG. Effects of amiloride analogues on AVP binding and activation of V1-receptor-expressing cells. Am J Physiol. 1992;262:E76–E86. doi: 10.1152/ajpendo.1992.262.1.E76. [DOI] [PubMed] [Google Scholar]
- Thibonnier M, Goraya T, Berti-Mattera L. G protein coupling of human platelet V1 vascular vasopressin receptors. Am J Physiol. 1993;264:C1336–C1344. doi: 10.1152/ajpcell.1993.264.5.C1336. [DOI] [PubMed] [Google Scholar]
- Thibonnier M, Auzan C, Madhun Z, Wilkins P, Berti-Mattera L, Clauser E. Molecular cloning, sequencing, and functional expression of a cDNA encoding the human V1a vasopressin receptor. J Biol Chem. 1994;269:3304–3310. [PubMed] [Google Scholar]
- Thompson R, Gupta S, Miller K, Mills S, Orr S. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology. 2004;29:35–48. doi: 10.1016/s0306-4530(02)00133-6. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Morphine withdrawal aggression: Modification with D1 and D2 receptor agonists. Psychopharmacology (Berl) 1992;108:177–184. doi: 10.1007/BF02245304. [DOI] [PubMed] [Google Scholar]
- Tubbiola ML, Wysocki CJ. FOS immunoreactivity after exposure to conspecific or heterospecific urine: Where are chemosensory cues sorted? Physiol Behav. 1997;62:867–870. doi: 10.1016/s0031-9384(97)00254-0. [DOI] [PubMed] [Google Scholar]
- Vale JR, Ray D, Vale CA. The interaction of genotype and exogenous neonatal androgen: Agonistic behavior in female mice. Behav Biol. 1972;7:321–334. doi: 10.1016/s0091-6773(72)80104-4. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Blume A, Niederle D, Buwalda B, Neumann ID. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur J Neurosci. 2006;24:1711–1720. doi: 10.1111/j.1460-9568.2006.05045.x. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm Behav. 2010;58:273–281. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Veening JG, Coolen LM, de Jong TR, Joosten HW, de Boer SF, Koolhaas JM, Olivier B. Do similar neural systems subserve aggressive and sexual behaviour in male rats? Insights from c-Fos and pharmacological studies. Eur J Pharmacol. 2005;526:226–239. doi: 10.1016/j.ejphar.2005.09.041. [DOI] [PubMed] [Google Scholar]
- Villalba C, Boyle PA, Caliguri EJ, De Vries GJ. Effects of the selective serotonin reuptake inhibitor fluoxetine on social behaviors in male and female prairie voles (Microtus ochrogaster) Horm Behav. 1997;32:184–191. doi: 10.1006/hbeh.1997.1420. [DOI] [PubMed] [Google Scholar]
- Walsh C, MacMillan HL, Jamieson E. The relationship between parental substance abuse and child maltreatment: Findings from the Ontario Health Supplement. Child Abuse Negl. 2003;27:1409–1425. doi: 10.1016/j.chiabu.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, Lichtenstein P. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci USA. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Species differences in the vasopressin-immunoreactive pathways in the bed nucleus of the stria terminalis and medial amygdaloid nucleus in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Behav Neurosci. 1995;109:305–311. doi: 10.1037//0735-7044.109.2.305. [DOI] [PubMed] [Google Scholar]
- Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav. 2004;83:319–328. doi: 10.1016/j.physbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Wang Z, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: A quantitative comparative study. J Comp Neurol. 1996;366:726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997a;767:321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu Y, Young LJ, Insel TR. Developmental changes in forebrain vasopressin receptor binding in prairie voles (Microtus ochrogaster) and montane voles (Microtus montanus) Ann N Y Acad Sci. 1997b;807:510–513. doi: 10.1111/j.1749-6632.1997.tb51954.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Young LJ, Liu Y, Insel TR. Species differences in vasopressin receptor binding are evident early in development: Comparative anatomic studies in prairie and montane voles. J Comp Neurol. 1997c;378:535–546. [PubMed] [Google Scholar]
- Wang Z, Young LJ, De Vries GJ, Insel TR. Voles and vasopressin: A review of molecular, cellular, and behavioral studies of pair bonding and paternal behaviors. Prog Brain Res. 1998;119:483–499. doi: 10.1016/s0079-6123(08)61589-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, Insel TR. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): A mechanism for pair bonding? Behav Neurosci. 1999;113:602–611. doi: 10.1037//0735-7044.113.3.602. [DOI] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel T. Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Ann N Y Acad Sci. 1992;652:487–489. doi: 10.1111/j.1749-6632.1992.tb34393.x. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: Insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Yamada S, Uchida S, Ohkura T, Kimura R, Yamaguchi M, Suzuki M, Yamamoto M. Alterations in calcium antagonist receptors and calcium content in senescent brain and attenuation by nimodipine and nicardipine. J Pharmacol Exp Ther. 1996;277:721–727. [PubMed] [Google Scholar]
- Young LJ. Frank A. Beach Award. Oxytocin and vasopressin receptors and species-typical social behaviors. Horm Behav. 1999;36:212–221. doi: 10.1006/hbeh.1999.1548. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: Behavioral consequences. Behav Neurosci. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Insel TR. Neuroendocrine bases of monogamy. Trends Neurosci. 1998;21:71–75. doi: 10.1016/s0166-2236(97)01167-3. [DOI] [PubMed] [Google Scholar]
- Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400:766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- Young KA, Liu Y, Wang Z. The neurobiology of social attachment: A comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Physiol C Toxicol Pharmacol. 2008;148:401–410. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: Insights from a socially monogamous rodent. Front Neuroendocrinol. 2011a;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Wang Z. The role of mesocorticolimbic dopamine in regulating interactions between drugs of abuse and social behavior. Neurosci Biobehav Rev. 2011b;35:498–515. doi: 10.1016/j.neubiorev.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]