Abstract
In this study, we evaluated the protective effects of dietary walnuts on high fat diet (HFD)-induced fatty liver and studied the underlying mechanisms. Male C57BL/6J mice were fed either a regular rodent chow or HFD (45% energy-derived) with or without walnuts (21.5% energy-derived) for 20 weeks. Walnut supplementation did not change HFD-induced increase in body weight or visceral fat mass. However, dietary walnuts significantly decreased the amounts of hepatic triglyceride (TG) observed in HFD-fed mice. The addition of walnuts significantly altered the levels of proteins, involved in the hepatic lipid homeostasis, including AMP-activated protein kinase, fatty acid synthase and peroxisome proliferator-activated receptor-α. Since adipocyte inflammation and apoptosis are reportedly important in regulating hepatic fat accumulation, we also evaluated the protective effects of walnuts on adipose tissue injury. RT-PCR results revealed that adipose tissues isolated from mice fed the HFD+walnuts showed significantly decreased levels of macrophage infiltration with suppressed expression of pro-inflammatory genes compared to those significantly elevated in mice fed HFD alone. These improvements also coincided with reduction of HFD-induced apoptosis of adipocytes by dietary walnuts. However, the supplemented walnuts did not significantly alter HFD-induced peripheral glucose intolerance or insulin resistance despite a trend of improvement. Collectively, these results demonstrate that the protective effects of walnuts against HFD-induced hepatic TG accumulation in mice are mediated, at least partially, by modulating the key proteins in hepatic lipid homeostasis and suppression of the genes related to adipose tissue inflammation and macrophage infiltration as well as prevention of adipocyte apoptosis.
Keywords: Walnut, High-fat diet, Liver, Steatosis, Triglyceride, Adipose tissue, Inflammation
Graphical Abstract
1. Introduction
The incidence of non-alcoholic fatty liver disease (NAFLD), which refers to a wide pathophysiological spectrum of nonalcoholic liver disease ranging from hepatic steatosis to inflammatory steatohepatitis and fibrotic complications, is increasing rapidly in the developed countries due to rising rates of obesity and related metabolic complications [1]. NAFLD is recognized to be the consequence of a complex interplay between diet, environment, host genes, liver, and adipose tissues [2]. Many proteins are known to be involved in the development and progression of NAFLD. For instance, AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-α (PPAR-α) play key roles in the regulation of hepatic lipid synthesis and degradation as well as energy metabolism. Activation of liver AMPK suppresses de novo fatty acid and cholesterol biosynthesis by inhibiting and/or downregulating fatty acid synthase (FAS), leading to the decreased hepatic lipogenesis [3], while PPAR-α activation mainly influences the fatty acid oxidation in the peroxisomes and mitochondria [4].
Even though the pathogenetic mechanism of NAFLD is not fully understood yet, it has been suggested that intra-abdominal fat mass and adipose tissue dysfunction are associated with the development of hepatic steatosis [5]. White adipose tissues, once considered a mere storage depot of energy in the form of fat, are today recognized as an important endocrine organ that produces and secretes various bioactive molecules known as adipokines or adipocytokines, including tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) [6]. In addition, adipose tissue expansion and adipocyte hypertrophy lead to inflammation, as manifested by the infiltration of macrophages [5, 7]. Adipose tissue inflammation is now recognized as an important mediator link that may help explain the relationship between obesity and several metabolic abnormalities, including hepatic steatosis, insulin resistance, and vascular dysfunction [8–10]. Previous studies proved that the unbalanced production of fat-derived pro-inflammatory adipokines, including TNF-α, promote the early stages of NAFLD [11, 12]. Additionally, recent studies uncover a key pathogenic role of adipocyte apoptosis in the recruitment of macrophages to adipose tissues, subsequent inflammation and metabolic disruption, contributing to hepatic lipid accumulation [13–15].
In recent years, the health benefits of dietary supplements for preventing the development and progression of NAFLD have received increasing attention, since these substances have several advantages such as being widely available with low toxicities or minimal side effects [16]. Walnut is one of these dietary supplements which have several beneficial effects including reduction of inflammation, improvement of blood circulation, suppression of the risk of heart disease, anti-aging properties, eczema prevention, and stabilization of body hormones due to the presence of multiple phytonutrients [17–19]. In addition, a recent report showed that dietary walnut oil improved hepatic steatosis in Zucker rats through modulating lipoprotein assembly and hepatic fatty acid influx [20]. However, there was little information about the beneficial effects of walnuts on adipocyte inflammation and apoptosis, leading to hepatic fat accumulation. Based on the aforementioned information, the present study was aimed to investigate the effects of dietary walnuts on hepatic fat accumulation via restoring the levels of the key genes and/or proteins involved in the hepatic lipid homeostasis and modulating adipose tissue inflammation and apoptosis, which can promote hepatic fat accumulation, in C57BL/6J mice fed a high fat diet (HFD).
2. Materials and methods
2.1. Animals and diet feedings
Age-matched male C57BL/6 mice were obtained from Jackson Laboratories at 5 weeks of age. After one week of acclimation, mice were randomly assigned to three groups (n≥6/group) fed either a regular chow diet (14% fat by calories; 7017 NIH-31 open formula mouse/rat sterilizable diet) (http://www.envigo.com/products-services/teklad/laboratory-animal-diets/natural-ingredient/traditional/rodent/7017-nih-31-open-formula-mouse-rat-sterilizable-diet.aspx) or HFD (45% fat-derived calories; D12451) (Research Diets, New Brunswick NJ, USA) with or without physiologically relevant amounts of walnuts (21.5% total energy with 18.9% fat-derived calories) for 20 weeks [21, 22]. The HFD and HFD+walnut diets were designed to be isocaloric and the composition of regular chow diet, HFD or HFD+walnuts is shown in Table 1. The mice were housed in groups of three per cage at 22°C with a 12 h light/dark cycle and given free access to diet and water. The weight of each mouse was determined once a week and their food intake recorded daily during the feeding period. The food intake and food efficiency ratio (FER) were measured on a per-cage basis and divided by three. After 20 weeks, the livers and adipose tissues were excised from the mice fasted overnight (12 h) and snap frozen while trunk blood samples were collected from the unconscious animals into heparinized blood collection tubes. All samples were stored at −80°C until analysis. Animal experiments were performed in accordance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee.
Table 1.
Composition of standard chow diet, HFD and HFD+walnuts.
| Chow (3.0 kcal/g) |
HFD (4.7 kacl/g) |
HFD+walnuts (4.7 kcal/g) |
|||
|---|---|---|---|---|---|
| % kcal from | % kcal from | % kcal from | |||
| Protein | 24 | 20 | 20 | ||
| Carbohydrate | 62 | 35 | 35 | ||
| Fat | 14 | 45 | 45 | ||
| Ingredient | g | kcal | g | kcal | |
| Casein | 200 | 800 | 181 | 742 | |
| L-Cystine | 3 | 12 | 3 | 12 | |
| Corn Starch | 246.3 | 985 | 246.3 | 985 | |
| Maltodextrin | 100 | 400 | 100 | 400 | |
| Cellulose | 50 | 0 | 50 | 0 | |
| Soybean Oil | 25 | 225 | 25 | 225 | |
| Lard | 177.5 | 1598 | 96 | 864 | |
| Mineral mix | 10 | 0 | 10 | 0 | |
| DiCalcium Phosphate | 13 | 0 | 13 | 0 | |
| Calcium Carbonate | 5.5 | 0 | 5.5 | 0 | |
| Potassium Citrate | 16.5 | 0 | 16.5 | 0 | |
| Vitamin Mix | 10 | 40 | 10 | 40 | |
| Choline Bitartrate | 2 | 0 | 2 | 0 | |
| Walnuts | 0 | 0 | 125 | 897.5 | |
| Total | 858.8 | 4060 | 883.3 | 4165.5 | |
2.2. Histopathology analysis
Liver and adipose tissue sections were stained with hematoxylin and eosin (H&E). Following staining, hepatic histological examination was performed with the histological scoring system for NAFLD, as described by Kleiner et al. [23], with separate scores for steatosis (0–3), hepatocellular ballooning (0–2), and lobular inflammation (0– 3). NAFLD activity score is the sum of these individual scores, and values of >5 are correlated with a diagnosis of NASH. All liver specimens were assessed blindly by an independent pathologist who does not know the identities of the study groups. To determine adipocyte size, the cross-sectional area of adipocytes from epididymal fat was measured at a magnification of X200 under light microscopy and image processing performed using Image J software (National Institutes of Health, Bethesda, MD).
2.3. Determination of the hepatic triglyceride (TG) and plasma alanine aminotransferase (ALT) activity
Liver tissues (50 mg wet weight) were homogenized in 5% Triton X-100 solution and heated in 80–100°C water bath for 2–5 min to solubilize the TG. The samples were then centrifuged at 10,000 × g for 10 min, and the resulting supernatant was used to determine the TG level following the manufacturer’s protocol (BioVision Research products, Mountain View, CA). The level of plasma ALT was measured for each animal using a clinical IDEXX Vet Test Chemistry Analyzer system from IDEXX Laboratories (West brook, ME).
2.4. Immunoblot analysis
Hepatic total cell lysates (50 µg/sample) were separated by 10 or 12 % SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to nitrocellulose membranes. Upon completion of electrophoretic transfer of the proteins, membranes were blocked for 1 h in 5% milk powder in Tris-HCl buffered saline containing 0.01% Tween 20 (TBS-T). Nitrocellulose membranes were then probed with specific primary antibodies (1:1,000 dilution) for P-AMPK (Cell Signaling, Danvers, MA), AMPK (Cell Signaling, Danvers, MA), PPAR-α (Abcam, Cambridge, MA), FAS (Cell Signaling, Danvers, MA), or GAPDH (Cell Signaling, Danvers, MA) (1:20,000 dilution) in 5% milk powder in TBS-T, overnight at 4°C. After three cycles of separate washing steps to remove the primary antibodies, the membranes were either incubated with the goat anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution in 5% milk powder in TBS-T). Protein bands were detected by enhanced chemiluminescence and their densities quantified using UN-SCAN-IT gel version 6.1 from Silk Scientific, as previously described [4, 24].
2.5. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
The ApopTag peroxidase in situ apoptosis detection kit from Millipore Corporation (Billerica, MA) was used to identify apoptotic adipocytes by detecting DNA strand breaks with the TUNEL method following the manufacturer’s protocol. Numbers of TUNEL-positive adipocytes were counted in 10 high-power (200×) microscope fields (HPF).
2.6. Insulin resistance (IR) and glucose tolerance (GT) tests
After feeding for 18 and 19 weeks with the different diets without or with walnuts, GT and IR tests were conducted in mice fasted for 6 h or overnight, respectively, with subsequently intraperitoneal (i.p.) injection of glucose (2 g/kg) (18 weeks) or insulin (0.75 U/kg; Eli Lilly) (19 weeks). Glucose levels were then measured with tail blood from each mouse right before the injection (0 time point) as well as at 30, 60, 90, and 120 minutes following the intraperitoneal injection of insulin or glucose. Blood glucose levels were determined using the Elite glucometer (Bayer, Leverkusen, Germany). The kinetic curve as well as area under the curve were generated to compare the glucose levels between the different treatment groups.
2.7. Gene expression analysis
Total RNA was isolated from frozen liver and fat tissues from each mouse using a Trizol® from Life Technologies (Grand Island, NY), according to the manufacturer’s recommendations. The concentration of RNA samples was measured by Nanodrop® ND-1000 (Thermo Scientific, Wilmington, DE). Real-time quantitative PCR analyses were carried out in 7900HT Sequence Detection System from Applied Biosystems (Foster City, CA) and Eco Real-Time PCR system from Illumina (San Diego, CA) in 20 µl volume. The reaction was conducted using Power SYBR® Green RNA-to-CT™ 1-Step Kit from Life Technologies (Grand Island, NY) by following the manufacturer’s suggestions. Two hundred nM each of forward and reverse primers, and 40 ng of template RNA were used. All reactions were carried out in four biological replicates. The PCR analyses were conducted as the manufacturer’s recommendations. To distinguish the specific amplicons from the non-specific amplifications, a dissociation curve was generated and examined. The Ct-values were calculated with Sequence Detection System 2.3, RQ Manager 1.2 (Applied Biosystems), and Eco® software V4.0 (Illumina) with an automatic adjustment of base line and determination of Ct. The resulting Ct-values were imported to Microsoft Excel worksheet for further analysis. The primers used for the analysis were designed using Primer-BLAST software (Supplementary Table 1). The primer sequences were designed to span an intron region of the target gene of interest to avoid amplification of trace amounts of genomic DNA in the samples.
2.8. Statistical analysis
Data represent results from at least two separate measurements, unless otherwise stated. Each point represents the mean ± SEM (n≥6/group). The significance of differences between groups was determined by ANOVA followed by two-tailed multiple t-tests with the Bonferroni correction. Statistical analysis was conducted using Graphpad Prism software (GraphPad Software Inc.) and values with p < 0.05 were considered significant.
3. Results
3.1. Different effects of walnuts on HFD-induced body weight gain and hepatic TG accumulation
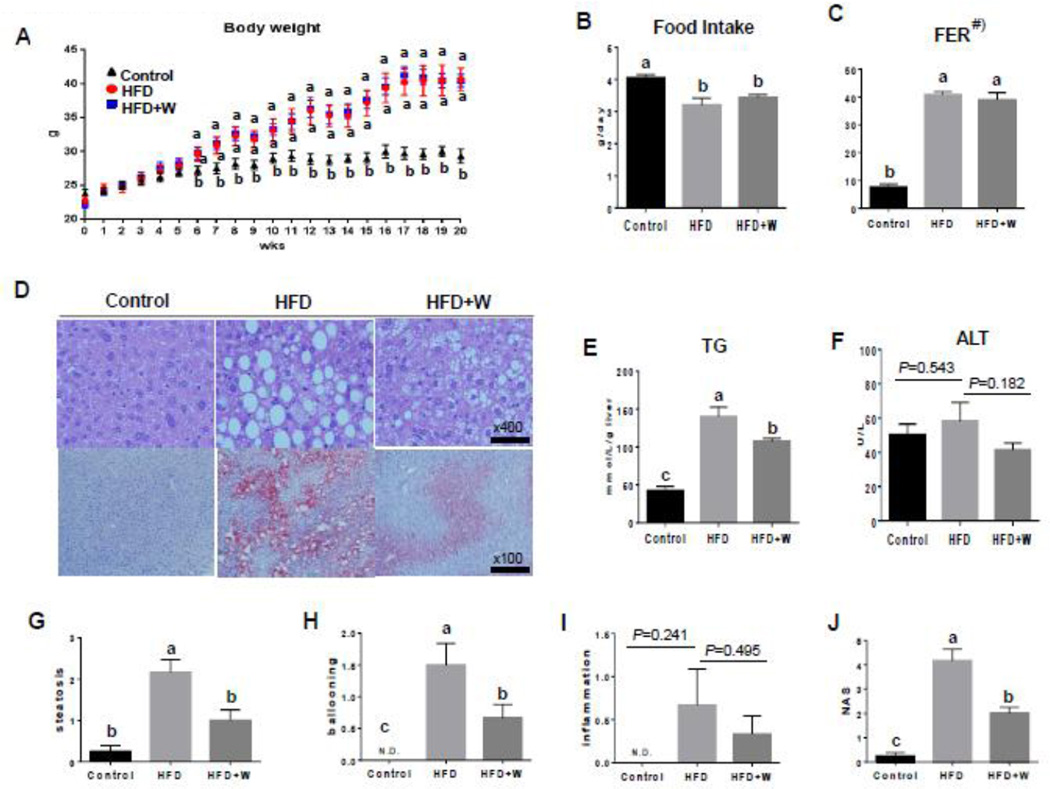
As expected, mice fed the HFD for 20 wk gained significantly more weight than mice fed the regular rodent chow (control) (P < 0.05, Fig. 1A). The addition of dietary walnuts to the HFD did not protect against HFD-induced weight gain, as the body weights of mice fed the HFD (40.6 ± 1.7 g) and the HFD+Walnuts (HFD+W) (40.5 ± 0.9 g) did not differ (Fig. 1A). In addition, there was no difference in relative liver weight (liver weight/body weight) among the groups (Control, 3.41 ± 0.05 vs. HFD, 3.65 ± 0.51 vs. HFD+W, 3.43 ± 0.23 g/100g BW). Furthermore, walnut supplementation did not change the amount of food consumed each day (Fig. 1B). However, HFD- and HFD+W-fed mice had greater food efficiency ratios (FER) compared with mice fed the control rodent chow (control mice) (Fig. 1C).
Figure 1.
Differential effects of walnut supplementation on HFD-induced body weight and hepatic injury. The changes in (A) body weight, (B) food intake, (C) food efficiency ratio (FER), (D) representative liver histology with H&E staining (top panels) and oil red-O staining (bottom panels), as indicated, (E) concentration of hepatic triglyceride, (F) serum ALT, (G) hepatic steatosis score, (H) ballooning score, (I) inflammation score, and (J) NASH score in the liver tissues of experimental mice are shown. All results are presented as mean ± SEM (n=6/group). Significance was determined by one-way ANOVA with the Bonferroni correction (P<0.05). Labeled characters without a common letter represent significant differences from the other group(s). #) FER (food efficiency ratio) = Body weight gain (g)/Food intake (g) for the experimental period.
The histopathological analyses and oil red-O staining results showed a moderate increase in the hepatic levels of lipid deposition in HFD-fed mice, as compared to those of control mice (Fig. 1D). Livers of HFD+W mice showed a marked decrease in lipid accumulation compared to HFD-fed mice (Fig. 1D). In addition, the elevated hepatic TG levels in the HFD group were also significantly alleviated by dietary walnut supplementation (Fig. 1E), further confirming the histopathology results. Serum ALT levels were slightly lower in the HFD+W group, although this difference was not statistically significant (Fig. 1F). As shown in Figures 1G–J, using the scoring system for NAFLD, as described by Kleiner et al. [23], the HFD group developed hepatocyte steatosis, ballooning, and scattered inflammatory cell infiltration at 20 weeks. Hepatocyte fat accumulation and ballooning were clearly reduced in the HFD+W mice. Although the inflammation score showed a similar pattern for the steatosis and ballooning scores of liver tissues, the differences among the three groups were not statistically significant (Fig. 1I). Collectively, the strikingly elevated NAFLD activity score in HFD group compared to control mice was significantly reduced in the HFD+W group (Fig.1J). These results indicate that the dietary supplementation of walnuts in HFD had marked effects on the improvement of hepatic TG accumulation in the experimental mice.
3.2. Effects of walnuts on the levels of hepatic proteins involved in lipid homeostasis
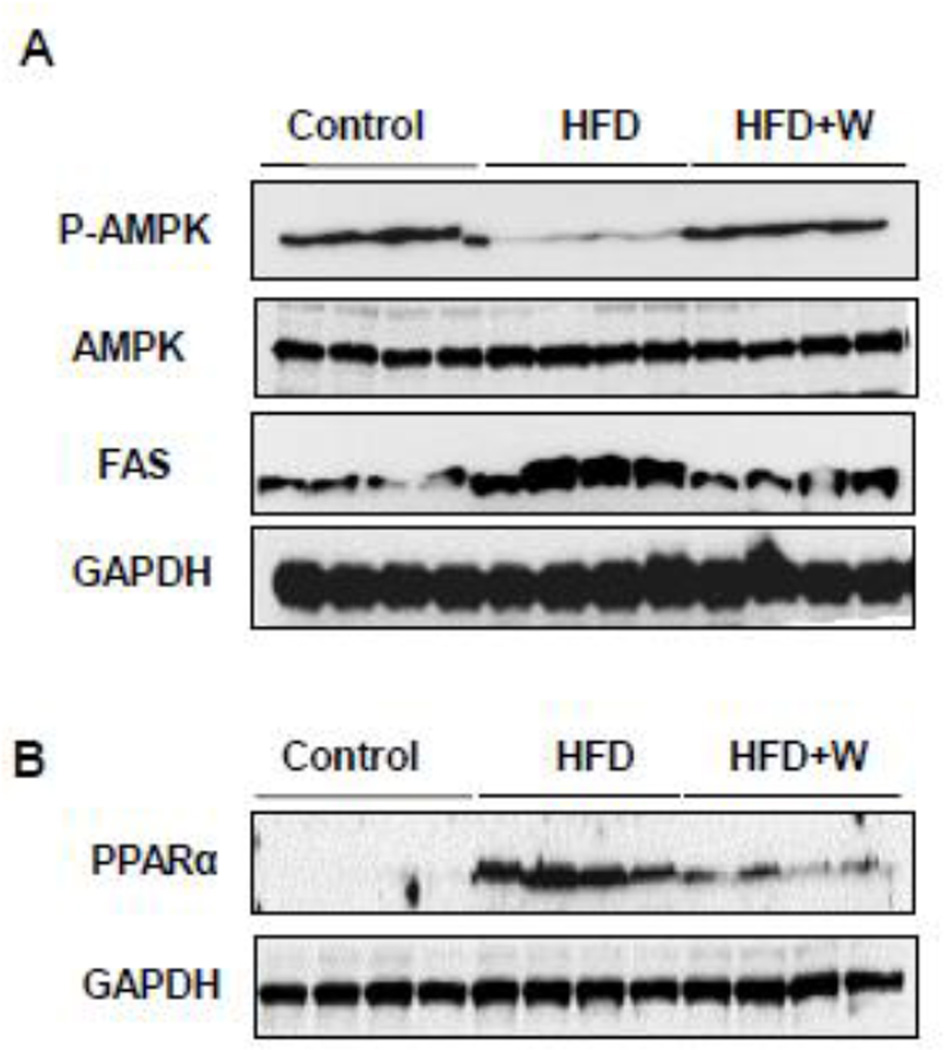
We next examined the effects of walnuts on key hepatic proteins involved in lipogenesis and FFA oxidation. The Western blot analyses showed that the phosphorylation (activation) of AMPK was markedly decreased in the livers of HFD group compared to control and that the walnut supplementation to HFD completely restored the activated p-AMPK to the levels of the control group (Fig. 2A, top panel), where AMPK was used as a loading control (Fig. 2A, second panel). In contrast, mice fed HFD exhibited a prominent elevation in FAS protein levels compared to control group and this increase was completely reversed in the livers of HFD+walnut mice, as similar to the levels of the control mice (Fig. 2A, third panel). GAPDH was used as a loading control for FAS (Fig. 2A, bottom panel). Lastly, the hepatic expression of PPAR-α was dramatically elevated in HFD-fed mice compared with control mice. However, its elevated levels were reduced by walnut supplementation (Fig. 2B, upper panel), where GAPDH was used as a loading control (Fig. 2B, lower panel).
Figure 2.
Walnut supplementation on the levels of key proteins in the signaling pathways and lipid homeostasis. Representative images of the immunoblot analysis for (A) P-AMPK, AMPK, FAS or GAPDH and (B) PPARα or GAPDH are shown.
3.3. Little effects of walnuts on HFD-induced glucose tolerance and insulin resistance
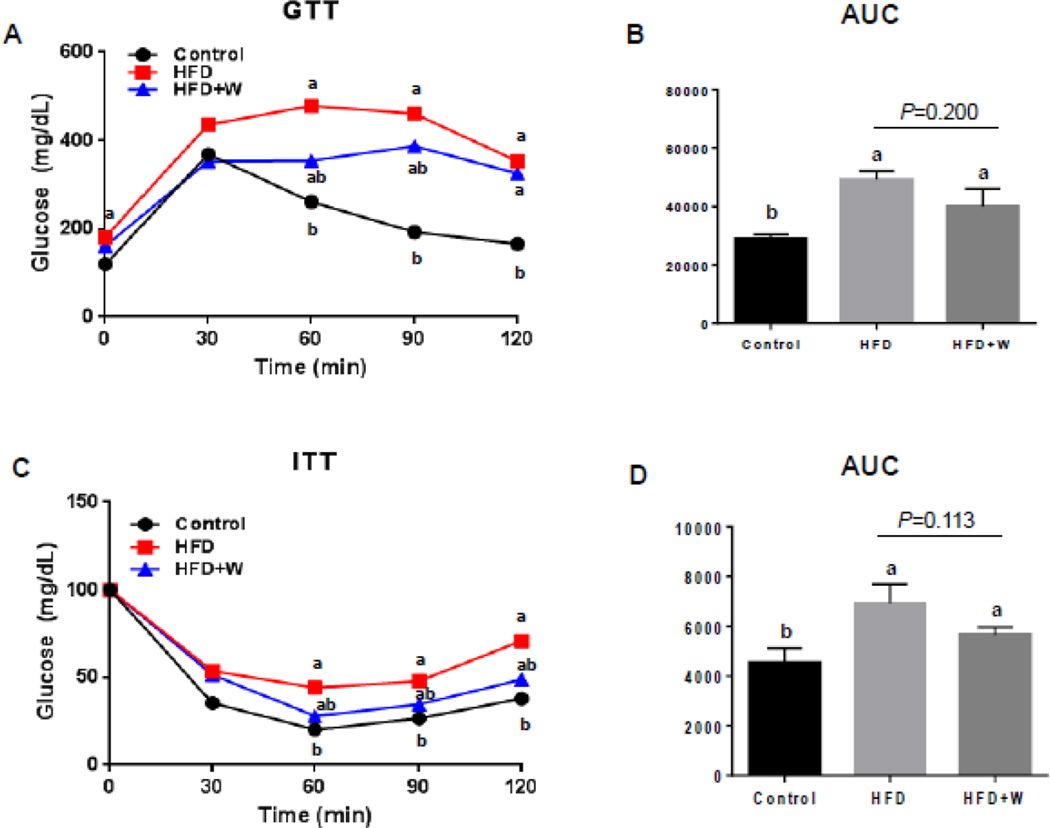
As shown in Figure 3, glucose tolerance (GT) and insulin resistance (IR) on all mouse groups were determined at 18 and 19 weeks, respectively. In the present study, HFD-fed mice exhibited both glucose intolerance after 18 weeks (Figs. 3A and B) and insulin resistance after 19 weeks (Figs. 3C and D), respectively. In addition, both parameters were not significantly changed by dietary walnuts when compared to HFD, despite the tendency of improvements in the HFD+walnut group (Figs. 3A–D). Taken together, dietary walnuts showed only a modest but not significant effects on HFD-induced glucose intolerance and insulin resistance.
Figure 3.
Little effects of walnut supplementation on HFD-induced glucose tolerance and insulin resistance. The results of (A) glucose tolerance test (GTT), (B) glucose area under the curve (AUC) during GTT, (C) insulin tolerance test (ITT), and (D) glucose AUC during ITT are shown. All results are presented as mean ± SEM (n=6/group). Significance was determined by one-way ANOVA with the Bonferroni correction (P<0.05). Labeled characters without a common letter represent significant differences from the other group(s).
3.4. Significant suppression of HFD-induced adipose tissue inflammation and apoptosis by walnuts
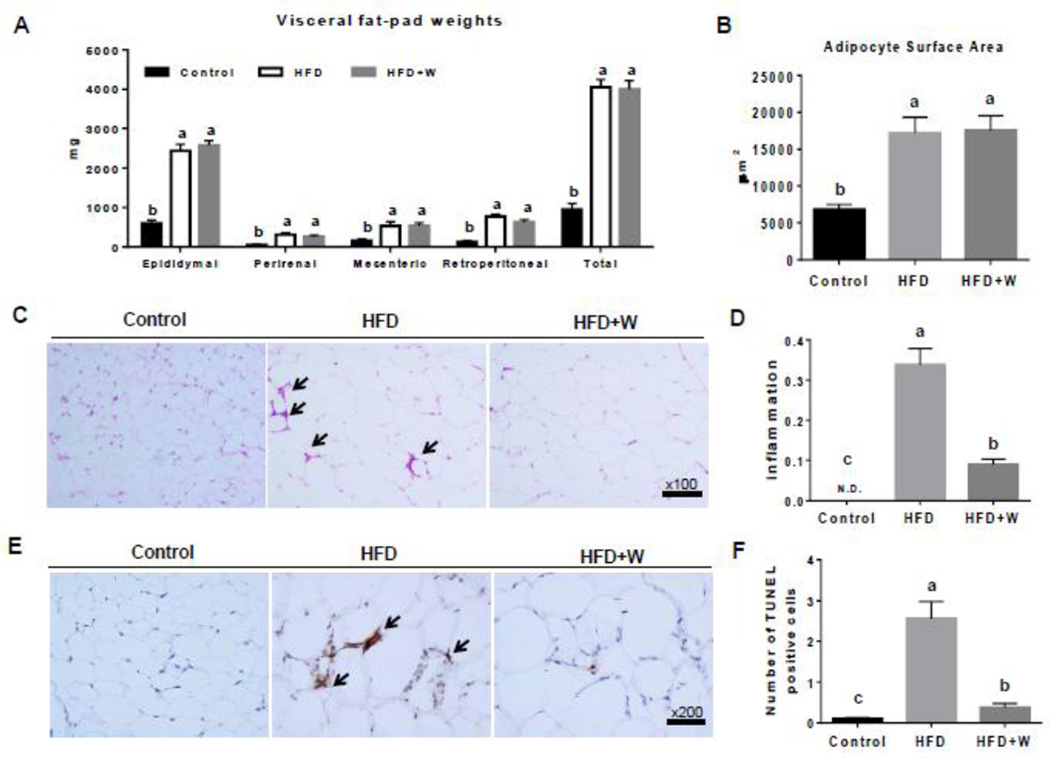
Similar to the results in body weight, the visceral fat mass as well as adipocyte size in epididymal adipose tissues of mice fed the HFD or HFD+W were greater than those of control mice, but walnut supplementation did not change these parameters from HFD group (Figs. 4A and B). These results indicate that the addition of walnuts to the HFD did not prevent adiposity and adipocyte hypertrophy induced by HFD.
Figure 4.
Preventive effects of walnut supplementation on HFD-induced adiposity and adipose tissue inflammation and apoptosis. The results of (A) visceral fat-pad weights, (B) adipocyte surface area, (C) representative H&E staining showing inflammation as indicated with black arrows, (D) inflammation score for C, (E) TUNEL positive adipocytes were identified by black arrows and (E) quantification in 20 high-power fields are shown. Data are presented as mean ± SEM (n=6/group). Significance was determined by one-way ANOVA with the Bonferroni correction (P<0.05). Labeled characters without a common letter represent significant differences from the other group(s).
Adipose tissue inflammation in obesity is characterized by macrophage infiltration. The infiltrated macrophages are responsible for the production of proinflammatory cytokines and the modulation of adipocyte-derived adipokines. There is increasing evidence that visceral adipose tissue is a causative risk factor in promoting fatty liver [8–10]. Therefore, we next determined the development of inflammation and macrophage infiltration in adipose tissues of experimental mice via H&E stain. Indeed, the HFD-fed mice showed accumulation of macrophages and increased inflammation in the adipose tissues (Figs. 4C and D), compared with mice fed a control diet. Walnut supplementation to the HFD significantly decreased the degree of inflammation in adipose tissues, compared with the HFD alone (Fig. 4C and 4D). In addition, in order to evaluate the protective effects of walnuts on HFD-induced adipocyte death, we performed TUNEL assay in mouse epididymal adipose tissues from different experimental groups. The number of dead adipocytes was markedly higher per HPF in HFD-fed mice than in mice fed the control diet (Figs. 4E and F). Walnut supplementation significantly suppressed the death of adipocytes (by more than 85%) compared with the HFD alone (Figs. 4E and F).
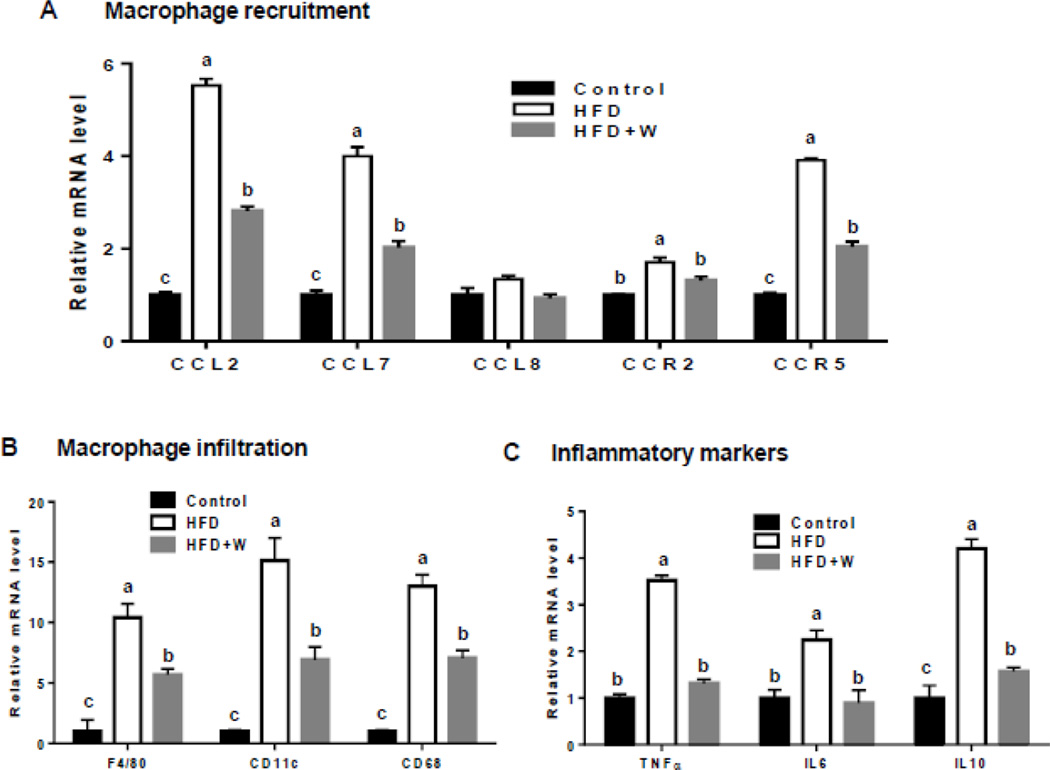
3.5. Differential effects of walnuts on the expression of genes related to macrophage infiltration and inflammatory markers in adipose and liver tissues from HFD-exposed mice
The mRNA levels of the genes involved in macrophage recruitment including the inflammatory chemokine ligands [e.g., chemokine ligand 2 (CCL2), CCL7, CCL8] and chemokine receptors [e.g., C-C chemokine receptor type 2 (CCR2), CCR5)] were upregulated in epididymal adipose tissues of HFD-fed mice compared to control group (Fig. 5A). Walnut supplementation markedly decreased the mRNA levels of these chemokine-related genes up-regulated by HFD (Fig. 5A). To further investigate macrophage infiltration and accumulation following exposure to different diets including the HFD with walnuts, gene expression profiles of several macrophage and inflammatory markers were evaluated. The mRNA levels of three macrophage markers F4/80, CD11c, and CD68 were highly up-regulated in adipose tissues of HFD-fed mice compared to control mice and the addition of walnuts significantly prevented the HFD-mediated up-regulation of these genes (Fig. 5B). Consistently, walnut supplementation suppressed the expression of three inflammatory markers (i.e., TNF-α, IL6, and IL10), all of which were highly elevated in HFD-exposed adipose tissues (Fig. 5C), as similar to those macrophage markers (Fig. 5B). In contrast to the results with the adipose tissues, there was little or no significant change in the expression of these genes in the liver (data not shown). Only the mRNA level of hepatic IL10 was significantly downregulated in walnut-fed mice compared to HFD-fed mice (data not shown). Taken together, the addition of walnuts ameliorated both HFD-induced adipose tissue inflammation and apoptosis.
Figure 5.
Preventive effects of walnut supplementation on the expression of the genes related to macrophage recruitment and infiltration and inflammatory markers in epididymal adipose tissues of mice fed different diets, as indicated. Relative mRNA levels of the genes involved in: (A) macrophage recruitment, (B) macrophage infiltration, and (C) inflammatory markers are shown. Relative expression of each target mRNA has been standardized to cyclophilin. Data are presented as mean ± SEM (n=6/group). Significance was determined by one-way ANOVA with the Bonferroni correction (P<0.05). Labeled characters without a common letter represent significant differences from the other group(s).
4. Discussion
The multiple factors are known to be involved in the molecular etiology of NAFLD. These factors include genetic and non-genetic environmental mechanisms, contributing to the development of the NAFLD [25, 26]. In addition to HFD [4, 21, 24], many other substances, such as fructose and sucrose [27–30], are known to promote NAFLD with or without inflammation, depending on the outcomes of gene and environmental interactions. It is well-established that intrahepatic accumulation of TG in NAFLD is primarily caused by imbalance between de novo lipogenesis and lipid oxidation exclusively in the liver.
Indeed, the HFD used in this study moderately increased the hepatic levels of lipid accumulation, although the hepatic fat content does not seem to reach 10% of the liver weight. The addition of walnut clearly ameliorated hepatic TG accumulation despite the fact that it did neither improve the body weight nor the relative liver weight. The lack of the effect of walnut on the body weight could be due to the high caloric intake when combining both HFD (45% fat-derived calories) and walnut (21.5% total energy with 18.9% fat-derived calories). Even though the quality of fat was improved by the addition of walnut with replacement of saturated fat in lard, nonetheless the amount of high caloric intake is still similar to the HFD-fed mice. This might explain the similar weight gains in both HFD and HFD+walnut groups, and obesity might be explained as a product of the total energy intake in our model. Since the HFD-induced hepatic steatosis was moderate in our model, and the body weight gains were comparable in both HFD and HFD+walnut groups, it is conceivable that the relative liver weight was also similar between the two groups, despite the lower tendency in the HFD+walnut compared to HFD alone (Fig. 1).
To characterize the potential mechanism(s) by which walnut decreased the liver fat accumulation, we therefore first studied hepatic lipid homeostasis in C57BL/6J mice. We specifically targeted three very well-established proteins or genes that play a prominent role in regulating the hepatic fat metabolism namely, AMPK, FAS, and PPAR-α. The signaling molecule activated p-AMPK has been implicated in the regulation of hepatic lipid homeostasis by modulating hepatic lipogenesis through phosphorylating its down-stream targets including FAS [3], since FAS is a key enzyme involved in de novo fatty acid and TG synthesis in mammals and can be down-regulated and/or inhibited by activated p-AMPK, leading to decreased hepatic fat accumulation [31]. In contrast, PPAR-α is essential in the modulation of lipid transport and metabolism via increased fatty acid β-oxidation, particularly when the organ is subjected to high fat overload as in the case of HFD exposure [4]. Indeed, PPAR-α was reported to protect the liver from the development and/or advancement of NAFLD in different HFD models using Ppar-α-null mice [4, 32]. Our current results revealed that the addition of walnuts with replacement of saturated fat in lard with unsaturated fatty acids in walnuts prevented the HFD-induced inhibition of activated p-AMPK and up-regulation of FAS, leading to restoration of their levels to approximately control levels. These results suggest that the observed reduction of hepatic TG accumulation by dietary walnuts seems to be partly regulated by the inhibition of fatty acid synthesis through the restoration of p-AMPK activity. Indeed, recent findings showing that hepatic AMPK activation has been validated as a therapeutic target for metabolic disorders including hepatic steatosis [33]. For instance, metformin and thiazolidinediones markedly reduced hepatic steatosis both in rodents and humans, presumably acting through hepatic AMPK activation [34, 35]. On the other hand, it is also known that PPAR-α activation by various ligands up-regulates the expression of the genes involved in the oxidation of fatty acids [31, 36, 37]. However, to our surprise, PPAR-α was markedly up-regulated by HFD alone and that the addition of walnuts restored PPAR-α to the levels comparable in control mice. This unexpected increase of PPAR-α can be explained by a cellular defense mechanism to compensate against the increased FFA load either through the HFD or the upregulation of FAS. Our result is in agreement with another report by Patsouris et al. [38] who explained that fatty liver was still observed, despite up-regulation of PPAR-α and numerous PPAR-α target genes involved in the fatty acid oxidation in response to chronic feeding of HFD. These results indicate that the up-regulation of PPAR-α and its down-stream targets was not sufficient to efficiently catabolize the extra load of fatty acids.
We do not know the reason why dietary walnuts did not prevent HFD-induced glucose intolerance and insulin resistance despite the walnut-mediated amelioration of HFD-induced hepatic steatosis, although hepatic fat accumulation is widely believed to result in insulin resistance. However, the causal relationship between hepatic steatosis and insulin resistance is uncertain. For instance, several recent studies have shown that it is possible to experimentally induce insulin resistance without NAFLD, or induce NAFLD without insulin resistance under certain conditions [39–41]. Our results indicate that dietary walnut supplementation is insufficient to improve the peripheral insulin sensitivity under current experimental conditions, despite the trend of improvement, possibly due to too much influence by high contents of saturated and n-6 fatty acids in the HFD. In line with our results, Brennan et al. reported that walnuts did not show any protective effect against insulin resistance compared to placebo diet [42]. Another randomized study reported that a diet containing high walnut content did not show beneficial effects on any metabolic parameters of the metabolic syndrome [43]. Therefore, our results showed that dietary walnuts significantly reduced HFD-induced TG content through modulating hepatic proteins involved in fat homeostasis and adipose tissue apoptosis and inflammation, independent from alleviating HFD-induced insulin resistance.
Several studies have shown that n-3 α-linolenic acid (ALA), an important component of walnuts, appears to elicit anti-inflammatory effects in clinical studies. For instance, Zhao et al. [44] found that the diet high in ALA inhibited the production of IL-6, IL-1β and TNF-α in cultured peripheral blood mononuclear cells (PBMCs) and decreased serum TNF-α concentrations compared to an average American diet. In addition, a walnut-containing breakfast (4% ALA) decreased mRNA expression of IL-6 in PBMCs in healthy subjects compared to that following a butter breakfast [45]. On the other hand, walnuts are fat-rich and energy-dense foods but most of the fats are unsaturated, according to the U.S. Department of Agriculture. The unsaturated fats, including ω-3 ALA contained in walnuts, could partly explain their beneficial effects. In the present study, the isocaloric replacement of HFD with walnuts prevents hepatic fat accumulation and adipocyte apoptosis as well as macrophage infiltration into the adipose tissues of HFD-fed mice, although there was no significant difference in total calorie intake between HFD and HFD+W groups. The high caloric intakes might explain the comparable weight gains in both the HFD and HFD+walnut groups and obesity might reflect a by-product of high energy intake. Thus, the beneficial effect of walnuts on adipose tissue inflammation observed in our study might have been due to partial replacement of saturated fatty acids present in the HFD with beneficial fats and antioxidants in walnuts, when mice were fed HFD+walnuts. Accumulation of immune cells, particularly macrophages, in adipose tissues is an important component of obesity-associated inflammation and contributes to the development of fatty liver [46]. The important mechanisms related to invasion of adipose tissues by macrophages occur through the release of soluble mediators from adipocytes [46] as well as up-regulated expression of pro-inflammatory chemokines [47]. In the present study, the adipose tissues of walnut-fed mice showed markedly decreased mRNA levels of the genes involved in macrophage recruitment including inflammatory chemokine ligands (e.g., CCL2, CCL7, CCL8) and their receptors (e.g., CCR2 and CCR5) and macrophage markers F4/80, CD11c, and CD68 compared to those in HFD-fed mice. Kanda et al. [12] showed that mice overexpressing CCL2 specifically in the adipose tissue develop adipose tissue inflammation and hepatic steatosis. Moreover, alterations induced by a HFD were blunted in the presence of a dominant-negative mutant CCL2. On the other hand, genetic deletion of the CCR2 receptor, known to bind the ligands CCL2, CCL7, or CCL8, was found to attenuate obesity and recruitment of macrophages to the adipose tissues [48] in agreement with the results with antagonists of CCR2 [48, 49]. In addition, CCR5-deficient mice were protected from insulin resistance and hepatic steatosis with a shift toward M2-type macrophages (alternatively activation of non-inflammatory macrophages) in the adipose tissues [50]. These results collectively suggest that inflammatory chemokines may directly affect hepatic lipid accumulation. This concept was also shown by in vitro experiments in which conditioned medium of human adipose tissue explants induced significant fat accumulation in hepatocyte cell lines. In this case, CCL2 secreted by adipose tissues may induce steatosis not only recruiting macrophages but also acting directly on hepatocytes [51]. Furthermore, recent findings revealed that adipocyte death and adipose tissue macrophage infiltration are mechanistically associated with the pathogenesis of adipose tissue inflammation. For instance, Alkhouri et al. [52] reported that the adipocyte apoptosis is a key factor that initiates macrophage infiltration into adipose tissues accompanied with hepatic steatosis and obesity in both mice and humans. Although a recent report showed that adipose tissue expansion is an important factor triggering the inflammatory processes during the development of obesity, other studies showed that the amount and composition of dietary fat are also important factors in determining the activation of pro-inflammatory process [53]. Indeed, some studies described that adipose tissue inflammation can be decreased independently of adipose mass [54, 55]. These results are also consistent with our current findings that dietary walnuts significantly prevent HFD-induced adipose macrophage infiltration and inflammation without changing fat mass and adipocyte size in C57BL/6J mice.
Many lines of evidence have shown that the metabolic link between the liver and adipose tissues may play an important role in causing intrahepatic TG accumulation associated with obesity. Indeed, adipose tissue inflammation, caused by various pro-inflammatory chemokines and cytokines, greatly accelerated hepatic fat accumulation in obesity. Thus, blocking the development of inflammation in adipose tissue can prevent HFD-induced hepatic steatosis [56–59]. Crespo et al. [60] showed that the TNF-α mRNA expression was significantly increased both in the liver and in adipose tissues of obese patients with steatohepatitis. It has been also established that certain TNF-α polymorphisms can be associated with the susceptibility towards NAFLD [61]. Furthermore, IL6 can induce excessive inflammation, which is considered a potential mediator of NAFLD [62]. However, the mRNA level of the anti-inflammatory cytokine IL10 was also elevated in our HFD-fed mice. Our results are consistent with those of previous studies in mice [63] and humans [64] and this phenomenon likely represents a protective response of the body to counteract pro-inflammatory events. None-the-less, these results suggest that the protective effect of dietary walnuts on hepatic TG content observed in the present study is likely due to, at least partially, decreased inflammatory cytokines secreted from adipose tissues in HFD-fed obese mice. Furthermore, our results showed that walnuts selectively suppressed the expression of various chemokines and adipokines in adipose tissues without affecting those of hepatocytes in HFD-fed mice. These results suggest an important role of adipose tissues in promoting hepatic fat accumulation and the primary source of serum cytokines and chemokines could be adipose tissues rather than hepatocytes. However, the specific mechanisms of the hepatic responses to the circulating cytokines and chemokines remain to be elucidated.
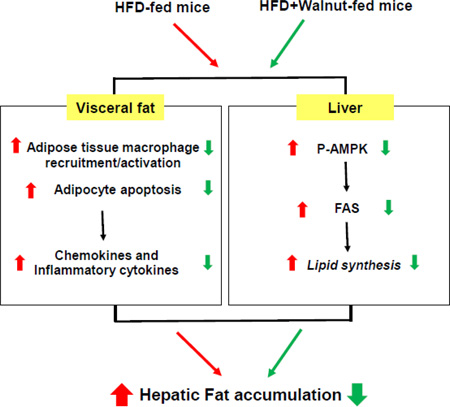
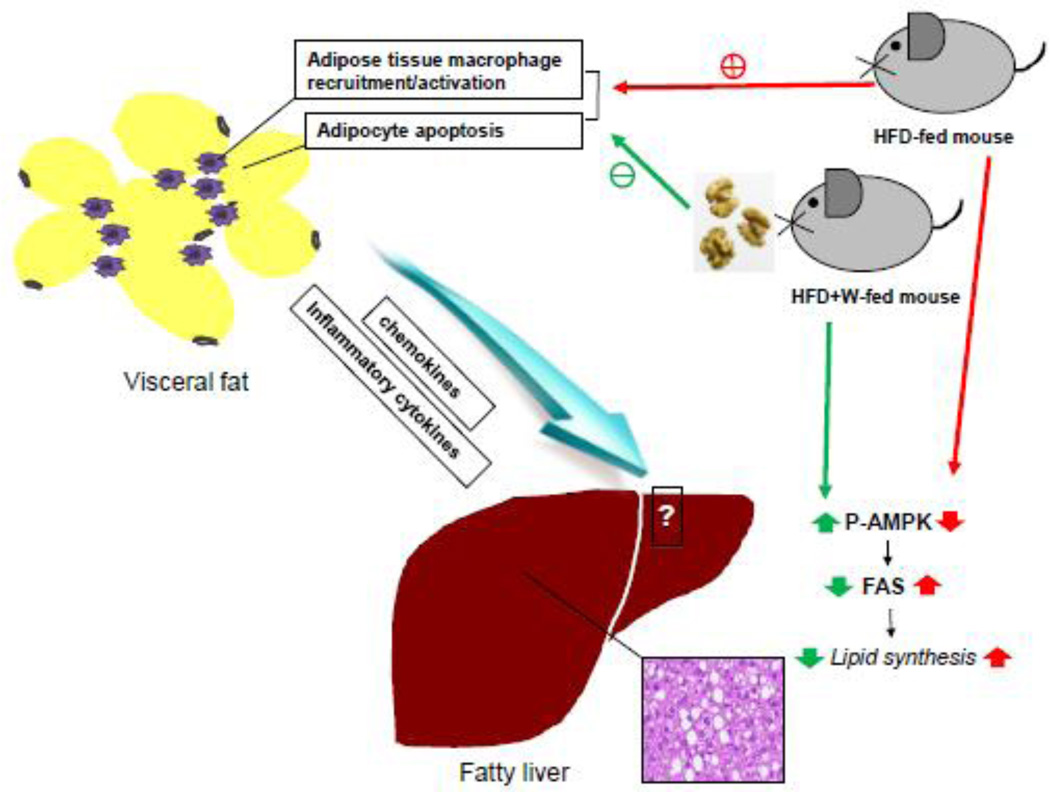
In conclusion, the present study demonstrate for the first time that walnut supplementation prevents inactivation and restoration of AMPK activity accompanied with reduction of the level of FAS, resulting in decreased fat synthesis. In addition, walnuts were particularly effective in lowering adipocyte apoptosis and macrophage infiltration into the adipose tissues of HFD-fed mice. We propose that these two crucial effects by walnuts play a central role in alleviating HFD-induced hepatic TG accumulation (Fig. 6). However, these mechanistic results warrant for future studies about the temporal changes in the expression of many other genes involved in hepatic fat accumulation and inflammation.
Figure 6.
Proposed mechanisms for the protective effects of dietary walnuts against hepatic fat accumulation in HFD-fed mice. Walnut supplementation activates AMPK with decreased FAS protein level in the livers of HFD-fed mice. In addition, dietary walnuts decrease macrophage infiltration and apoptosis in the adipose tissues of HFD-fed mice, contributing to decreased levels of circulating cytokines and thus preventing diet-induced hepatic fat accumulation.
Acknowledgments
This research was supported by the Intramural Program of National Institute on Alcohol Abuse and Alcoholism. This work was also supported by a grant to Youngshim Choi from the KRIBB Research Initiative Program (Korean Biomedical Scientist Fellowship Program), Korea Research Institute of Bioscience and Biotechnology, Republic of Korea. The authors are thankful to Dr. Klaus Gawrisch for supporting this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM : monthly journal of the Association of Physicians. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang E, Park CY, Park SW. Role of thiazolidinediones, insulin sensitizers, in non-alcoholic fatty liver disease. Journal of diabetes investigation. 2013;4:517–524. doi: 10.1111/jdi.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell metabolism. 2009;9:407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. The Journal of nutrition. 2011;141:603–610. doi: 10.3945/jn.110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duval C, Thissen U, Keshtkar S, Accart B, Stienstra R, Boekschoten MV, et al. Adipose tissue dysfunction signals progression of hepatic steatosis towards nonalcoholic steatohepatitis in C57BL/6 mice. Diabetes. 2010;59:3181–3191. doi: 10.2337/db10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nature reviews Immunology. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotronen A, Westerbacka J, Bergholm R, Pietilainen KH, Yki-Jarvinen H. Liver fat in the metabolic syndrome. The Journal of clinical endocrinology and metabolism. 2007;92:3490–3497. doi: 10.1210/jc.2007-0482. [DOI] [PubMed] [Google Scholar]
- 8.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annual review of physiology. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 9.Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 10.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le KA, Mahurkar S, Alderete TL, Hasson RE, Adam TC, Kim JS, et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-kappaB stress pathway. Diabetes. 2011;60:2802–2809. doi: 10.2337/db10-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of clinical investigation. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 14.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. Journal of lipid research. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. Journal of lipid research. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Firenzuoli F, Gori L. Herbal medicine today: clinical and research issues. Evidence-based complementary and alternative medicine : eCAM. 2007;4:37–40. doi: 10.1093/ecam/nem096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berryman CE, Grieger JA, West SG, Chen CY, Blumberg JB, Rothblat GH, et al. Acute consumption of walnuts and walnut components differentially affect postprandial lipemia, endothelial function, oxidative stress, and cholesterol efflux in humans with mild hypercholesterolemia. The Journal of nutrition. 2013;143:788–794. doi: 10.3945/jn.112.170993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullner E, Brath H, Pleifer S, Schiermayr C, Baierl A, Wallner M, et al. Vegetables and PUFA-rich plant oil reduce DNA strand breaks in individuals with type 2 diabetes. Molecular nutrition & food research. 2013;57:328–338. doi: 10.1002/mnfr.201200343. [DOI] [PubMed] [Google Scholar]
- 19.Anand R, Kaithwas G. Anti-inflammatory potential of alpha-linolenic acid mediated through selective COX inhibition: computational and experimental data. Inflammation. 2014;37:1297–1306. doi: 10.1007/s10753-014-9857-6. [DOI] [PubMed] [Google Scholar]
- 20.Fink A, Rufer CE, Le Grandois J, Roth A, Aoude-Werner D, Marchioni E, et al. Dietary walnut oil modulates liver steatosis in the obese Zucker rat. European journal of nutrition. 2014;53:645–660. doi: 10.1007/s00394-013-0573-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis PA, Vasu VT, Gohil K, Kim H, Khan IH, Cross CE, et al. A high-fat diet containing whole walnuts (Juglans regia) reduces tumour size and growth along with plasma insulin-like growth factor 1 in the transgenic adenocarcinoma of the mouse prostate model. The British journal of nutrition. 2012;108:1764–1772. doi: 10.1017/S0007114511007288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagel JM, Brinkoetter M, Magkos F, Liu X, Chamberland JP, Shah S, et al. Dietary walnuts inhibit colorectal cancer growth in mice by suppressing angiogenesis. Nutrition. 2012;28:67–75. doi: 10.1016/j.nut.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 24.Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. Journal of hepatology. 2012;57:860–866. doi: 10.1016/j.jhep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amarapurkar DN, Hashimoto E, Lesmana LA, Sollano JD, Chen PJ, Goh KL, et al. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? Journal of gastroenterology and hepatology. 2007;22:788–793. doi: 10.1111/j.1440-1746.2007.05042.x. [DOI] [PubMed] [Google Scholar]
- 26.Das K, Das K, Mukherjee PS, Ghosh A, Ghosh S, Mridha AR, et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51:1593–1602. doi: 10.1002/hep.23567. [DOI] [PubMed] [Google Scholar]
- 27.Kanuri G, Spruss A, Wagnerberger S, Bischoff SC, Bergheim I. Role of tumor necrosis factor alpha (TNFalpha) in the onset of fructose-induced nonalcoholic fatty liver disease in mice. The Journal of nutritional biochemistry. 2011;22:527–534. doi: 10.1016/j.jnutbio.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Spruss A, Kanuri G, Uebel K, Bischoff SC, Bergheim I. Role of the inducible nitric oxide synthase in the onset of fructose-induced steatosis in mice. Antioxidants & redox signaling. 2011;14:2121–2135. doi: 10.1089/ars.2010.3263. [DOI] [PubMed] [Google Scholar]
- 29.Schultz A, Barbosa-da-Silva S, Aguila MB, Mandarim-de-Lacerda CA. Differences and similarities in hepatic lipogenesis, gluconeogenesis and oxidative imbalance in mice fed diets rich in fructose or sucrose. Food & function. 2015;6:1684–1691. doi: 10.1039/c5fo00251f. [DOI] [PubMed] [Google Scholar]
- 30.Song Z, Deaciuc I, Zhou Z, Song M, Chen T, Hill D, et al. Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. American journal of physiology Gastrointestinal and liver physiology. 2007;293:G894–G902. doi: 10.1152/ajpgi.00133.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Do GM, Kwon EY, Ha TY, Park YB, Kim HJ, Jeon SM, et al. Tannic acid is more effective than clofibrate for the elevation of hepatic beta-oxidation and the inhibition of 3-hydroxy-3-methyl-glutaryl-CoA reductase and aortic lesion formation in apo E-deficient mice. The British journal of nutrition. 2011;106:1855–1863. doi: 10.1017/S000711451100256X. [DOI] [PubMed] [Google Scholar]
- 32.Stienstra R, Mandard S, Patsouris D, Maass C, Kersten S, Muller M. Peroxisome proliferator-activated receptor alpha protects against obesity-induced hepatic inflammation. Endocrinology. 2007;148:2753–2763. doi: 10.1210/en.2007-0014. [DOI] [PubMed] [Google Scholar]
- 33.Brooks SC, 3rd, Brooks JS, Lee WH, Lee MG, Kim SG. Therapeutic potential of dithiolethiones for hepatic diseases. Pharmacology & therapeutics. 2009;124:31–43. doi: 10.1016/j.pharmthera.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nature medicine. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 35.Bajaj M, Suraamornkul S, Pratipanawatr T, Hardies LJ, Pratipanawatr W, Glass L, et al. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes. 2003;52:1364–1370. doi: 10.2337/diabetes.52.6.1364. [DOI] [PubMed] [Google Scholar]
- 36.Schafer HL, Linz W, Falk E, Glien M, Glombik H, Korn M, et al. AVE8134, a novel potent PPARalpha agonist, improves lipid profile and glucose metabolism in dyslipidemic mice and type 2 diabetic rats. Acta pharmacologica Sinica. 2012;33:82–90. doi: 10.1038/aps.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peschel D, Koerting R, Nass N. Curcumin induces changes in expression of genes involved in cholesterol homeostasis. The Journal of nutritional biochemistry. 2007;18:113–119. doi: 10.1016/j.jnutbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Patsouris D, Reddy JK, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology. 2006;147:1508–1516. doi: 10.1210/en.2005-1132. [DOI] [PubMed] [Google Scholar]
- 39.Farese RV, Jr, Zechner R, Newgard CB, Walther TC. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell metabolism. 2012;15:570–573. doi: 10.1016/j.cmet.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Z, Lazar MA. Dissociating fatty liver and diabetes. Trends in endocrinology and metabolism: TEM. 2013;24:4–12. doi: 10.1016/j.tem.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brennan AM, Sweeney LL, Liu X, Mantzoros CS. Walnut consumption increases satiation but has no effect on insulin resistance or the metabolic profile over a 4-day period. Obesity. 2010;18:1176–1182. doi: 10.1038/oby.2009.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukuddem-Petersen J, Stonehouse Oosthuizen W, Jerling JC, Hanekom SM, White Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: a controlled feeding trial. The British journal of nutrition. 2007;97:1144–1153. doi: 10.1017/S0007114507682944. [DOI] [PubMed] [Google Scholar]
- 44.Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. The American journal of clinical nutrition. 2007;85:385–391. doi: 10.1093/ajcn/85.2.385. [DOI] [PubMed] [Google Scholar]
- 45.Jimenez-Gomez Y, Lopez-Miranda J, Blanco-Colio LM, Marin C, Perez-Martinez P, Ruano J, et al. Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis. 2009;204:e70–e76. doi: 10.1016/j.atherosclerosis.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Kanneganti TD, Dixit VD. Immunological complications of obesity. Nature immunology. 2012;13:707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- 47.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. The Journal of clinical investigation. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamura Y, Sugimoto M, Murayama T, Minami M, Nishikaze Y, Ariyasu H, et al. C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. Journal of atherosclerosis and thrombosis. 2010;17:219–228. doi: 10.5551/jat.3368. [DOI] [PubMed] [Google Scholar]
- 50.Kitade H, Sawamoto K, Nagashimada M, Inoue H, Yamamoto Y, Sai Y, et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012;61:1680–1690. doi: 10.2337/db11-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clement S, Juge-Aubry C, Sgroi A, Conzelmann S, Pazienza V, Pittet-Cuenod B, et al. Monocyte chemoattractant protein-1 secreted by adipose tissue induces direct lipid accumulation in hepatocytes. Hepatology. 2008;48:799–807. doi: 10.1002/hep.22404. [DOI] [PubMed] [Google Scholar]
- 52.Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, et al. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. The Journal of biological chemistry. 2010;285:3428–3438. doi: 10.1074/jbc.M109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvheim AR, Malde MK, Osei-Hyiaman D, Lin YH, Pawlosky RJ, Madsen L, et al. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity. 2012;20:1984–1994. doi: 10.1038/oby.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abe M, Matsuda M, Kobayashi H, Miyata Y, Nakayama Y, Komuro R, et al. Effects of statins on adipose tissue inflammation: their inhibitory effect on MyD88-independent IRF3/IFN-beta pathway in macrophages. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:871–877. doi: 10.1161/ATVBAHA.107.160663. [DOI] [PubMed] [Google Scholar]
- 55.DeFuria J, Bennett G, Strissel KJ, Perfield JW, 2nd, Milbury PE, Greenberg AS, et al. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. The Journal of nutrition. 2009;139:1510–1516. doi: 10.3945/jn.109.105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao M, Zhang C, Ma Y, Bu L, Yan L, Liu D. Hydrodynamic delivery of mIL10 gene protects mice from high-fat diet-induced obesity and glucose intolerance. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:1852–1861. doi: 10.1038/mt.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao M, Ma Y, Liu D. Rutin suppresses palmitic acids-triggered inflammation in macrophages and blocks high fat diet-induced obesity and fatty liver in mice. Pharmaceutical research. 2013;30:2940–2950. doi: 10.1007/s11095-013-1125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerner RR, Wieser V, Moschen AR, Tilg H. Metabolic inflammation: role of cytokines in the crosstalk between adipose tissue and liver. Canadian journal of physiology and pharmacology. 2013;91:867–872. doi: 10.1139/cjpp-2013-0050. [DOI] [PubMed] [Google Scholar]
- 59.Ma Y, Gao M, Liu D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharmaceutical research. 2015;32:1200–1209. doi: 10.1007/s11095-014-1526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crespo J, Cayon A, Fernandez-Gil P, Hernandez-Guerra M, Mayorga M, Dominguez-Diez A, et al. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 61.Zhou YJ, Li YY, Nie YQ, Yang H, Zhan Q, Huang J, et al. Influence of polygenetic polymorphisms on the susceptibility to non-alcoholic fatty liver disease of Chinese people. Journal of gastroenterology and hepatology. 2010;25:772–777. doi: 10.1111/j.1440-1746.2009.06144.x. [DOI] [PubMed] [Google Scholar]
- 62.Kishimoto T. IL-6: from its discovery to clinical applications. International immunology. 2010;22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 63.Oliveira MC, Menezes-Garcia Z, Henriques MC, Soriani FM, Pinho V, Faria AM, et al. Acute and sustained inflammation and metabolic dysfunction induced by high refined carbohydrate-containing diet in mice. Obesity. 2013;21:E396–E406. doi: 10.1002/oby.20230. [DOI] [PubMed] [Google Scholar]
- 64.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. Jama. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]