Abstract
Objective
This study aimed to increase our understanding of general self-management (SM) abilities in COPD by determining if SM can predict disease specific quality of life (QoL), by investigating whether specific SM domains are significant in COPD and by exploring the mediating effect of the positive/negative affect in the association between SM and QoL.
Methods
Cross-sectional study based on 292 patients with COPD. Measures included demographics, lung function, gait speed, health care utilization, positive/negative affect, SM abilities, breathlessness and disease specific QoL. We performed, correlation, multiple regression models and mediation analysis (positive/negative affect being mediator between SM and QoL association).
Results
After controlling for breathlessness, living alone, marital status, hospitalization history, age and lung function, SM related to QoL (p< 0.0001). Investment in behaviors (hobbies and social relationships) and self-efficacy are SM domains independently related to QoL in COPD. Positivity measured by the positive/negative affect ratio completely mediates the relationship of SM with QoL.
Conclusion
SM is independently associated with disease specific QoL in COPD after adjustment significant covariates but positive/negative affect ratio completely mediates the relationship of SM with QoL.
Practice implications
Measuring positive/negative affect and addressing investment behavior and self-efficacy are important in implementing COPD-SM programs.
Keywords: COPD, self-management, quality of life, positive affect
1. Introduction
Chronic obstructive pulmonary disease (COPD) is one of the main causes of morbidity and mortality worldwide and is expected to become the third cause of death and the fifth cause of disability-adjusted life years in 2020 (1). International guidelines for managing COPD posit self-management (SM) programs as a critical part of COPD treatment (2). Patients’ SM support, is a recognized component of the Chronic Care Model (3), and has received increasing attention in recent years in the field of COPD due to its impact on critically important outcomes such as disease specific quality of life (QoL) and hospitalizations (4). SM is particularly important in patients with COPD as they experience greater physical health losses and are dealing with greater depressive symptoms compared to patients with diabetes and cardiovascular disease (5). COPD-SM is most commonly associated with the use of written action plans for early signs of COPD exacerbations with differing results (6, 7). However, SM goes far beyond prescription of written plans for exacerbations. Recent data suggest that the effectiveness of SM programs may be limited if they are not incorporated in a more extensive individualized assessment of the whole patient that goes beyond the specificities of disease, COPD in this case (4).
There is a knowledge gap relating to SM abilities in COPD patients beyond the use of a written action plan for exacerbations and the association of those abilities with QoL, a critical outcome in COPD (1). In particular, little is known about the specific aspects of SM that are independently associated with improved QoL in COPD, with the goal of incorporating them into COPD SM or care programs.
Validated tools to comprehensively measure SM are not commonly used in COPD SM research. The Self-Management Ability Scale 30 (SMAS-30) is a tool to measure general SM in chronic disease that has been reported as a valid and reliable tool to comprehensively measure SM (5, 8–11). The tool measures abilities needed to achieve maintain or restore aspects of physical and social well-being. While there are reports on the association of this SMAS-30 with well-being in COPD patients, there is no report to-date in the literature about the association of this measure with the most critical outcomes in COPD, such as QoL, frailty measures (gait speed), hospitalizations, lung function, breathlessness level that can further inform the relevance of using SMAS-30 in COPD SM research.
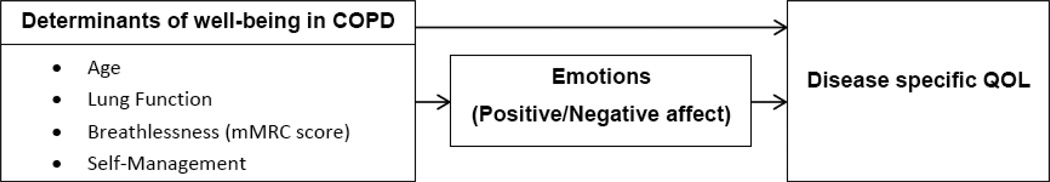
Another important aspect to consider at the time of implementing SM support programs is to know which factors moderate or mediate the effect of SM abilities on wellbeing (QoL). Given the importance of positive and negative affect in COPD, it is plausible to believe that they can at least moderate or even mediate the effect of SM support (12, 13). Positive affect has been shown to influence health in multiple ways (14). Positive affect interventions have had marked effects on people’s cognitive processes, social behavior, motivation, self-efficacy (which is at the very core of SM), and ability to cope with stressful life events. Individuals with positive affect have felt more intrinsically motivated and were more able to try new things (15, 16), both conditions that may be associated with the effect of SM support interventions. We hypothesize that positive affect may moderate or at most mediate the associations of SM with other meaningful outcomes in COPD (Figure 1).
Figure 1. Correlation coefficients between self-management (SMAS-30) and COPD outcomes.
The purpose of this cross-sectional cohort study was to test the following aims:
First, determine the association of SMAS-30 with the most important outcomes in COPD. Second, to establish if there is a significant and independent association between SM abilities and disease specific QoL, measured by the Chronic Respiratory Questionnaire, a cardinal outcome in COPD. Third, identify which aspects of SM, as defined by the SMAS-30 subdomains, are independently associated with disease specific QoL. Finally, if a significant association is found between SM and QoL, investigate if the association is mediated by positive affect, as proposed in previous research in other chronic diseases (17). We envisioned that the results of this study could be seminal to craft comprehensive SM interventions in COPD patients.
2. Methods
292 patients with COPD were prospectively recruited from the Pulmonary Clinic at Mayo Clinic, Rochester, MN (USA). The following inclusion criteria were used: diagnosis of COPD based on the Global Initiative for COPD 2011 guidelines (1) age > 40 years old, history of smoking more than 10 packs-year and able to complete questionnaires. This study received the Mayo Clinic Institutional Review Board approval (IRB number: 13-004603) and all patients signed research consent. Investigators recorded, at the moment of the recruitment, demographics, health care utilization and pulmonary function information.
2.1. General survey
The general survey contains four items addressing the following: (1) the patient’s smoking status, (2) frequency of lung disease exacerbations, (3) frequency of hospitalizations for respiratory and non-respiratory issues and (4) frequency of ER/Urgent Care visits for respiratory and non-respiratory causes. Data regarding living situation (living alone or not), marital status, use of permanent oxygen, co-morbidities, and activities of daily living were also obtained from the medical record as it is part of the current visit questionnaire used for all patients at Mayo Clinic.
2.2. Pulmonary function testing
All patients included in the study had Pulmonary Function Tests completed in the outpatient Pulmonary Function Laboratory at Mayo Clinic, Rochester, MN (USA). The severity of the airflow limitation was classified based on the GOLD criteria (1). The Global Obstructive Lung Disease Initiative (GOLD) categorize COPD based on the degree lung obstruction in stages: Stage 1 COPD is considered mild and has few symptoms. Coughing is infrequent. Lung function tests reveal forced expiratory volume (FEV1), the measure of brionchial obstruction numbers at less than 80 percent of normal. Stage 2 COPD is considered moderate. Shortness of breath while exercising is common. Patients with moderate COPD benefit from self-management, health coaching and exercise. Stage 2 FEV1 numbers reflect between 50 and 79 percent of normal lung function. Stage 3 COPD patients have increased shortness of breath. Lung function tests show between 30 and 49 percent of normal function. Stage 4 COPD reflects severe lung damage. Symptoms worsen, and coughing and mucus production increase. Any activity is a challenge. People with stage 4 COPD rely on oxygen therapy. Flare-ups are increasingly serious, even possibly deadly.
2.3. Gait Speed
Four-meter Gait Speed was assessed as a measure of exercise capacity and frailty. A gait speed <0.8 m/s has been associated with frailty (18, 19). The rolling-start four-meter gait speed was measured at the patient’s usual pace in an unobstructed clinic hallway. Each walk was performed with a 2 meter rolling start, meaning each participant was already walking as they entered the measuring area. Canes, walkers and supplemental oxygen were used if the patient normally utilized the equipment in daily activities. Two cones were placed 8 meters apart, with the automated Dual Beam Wireless Infrared Timing System (TracTronix, Lenexa, KS) centered two meters after the first cone and two meters before the second cone. Patients were instructed to “walk at their usual pace” from one cone to the other.
2.4. Questionnaires
SM was measured using the SMAS-30 (20). In this validated questionnaire, already used in COPD, six core SM abilities are studied: taking initiatives (to be instrumental or self-motivating with regard to the realization of dimensions of well-being); investment behavior (to invest in resources for longer-term benefits); variety (to achieve and maintain various resources for each dimension of well-being); multi-functionality (to gain and maintain activities which serve multiple dimensions of well-being at the same time in a mutually reinforcing way); self-efficacy (to gain and maintain a belief in personal competence to achieve well-being), and positive frame of mind (to maintain a positive perspective regarding the future, rather than to focus on loss) (20). The total score of the entire scale on a 100-point scale is calculated from the average of the total scores of the six subscales (20).
QoL was assessed using the Chronic Respiratory Questionnaire Self-Administered Standardized Format (CRQ-SAS). The CRQ-SAS is a validated questionnaire to measure the health status of patients with COPD. It has a total score (main outcome in this study) and four sub-domains dyspnea, fatigue, emotional and mastery (21). Scores are on a 1–7-point scale; the lower the score, the greater the dysfunction (22). A difference of 0.5 in any dimension is considered clinically significant.
Emotions were measured using the Positive and Negative Affect Schedule (PANAS), a 20-item scale measuring the positive and negative affective components of well-being (23). The scale consists of 10 positive words and 10 negative words that describe different feelings and emotions. Higher sum scores on each sub-scale indicates more positive and more negative affect, respectively on a 50-point scale (23). We calculated the ratio of positive to negative affect, known as the critical positivity ratio (24), which claims to distinguish individuals that are emotionally balanced from those who are not. Individuals with higher ratios were found to have greater flexibility and resilience to adversity, more social resources, and better optimal functioning in many areas of their life (24). Researchers have long suggested that the balance of positive to negative affect is critically relevant to well-being and adjustment (25, 26). Recently, Larsen and Prizmic argued that the balance of positive to negative affect (hereafter referred to as the critical positivity ratio) is a key factor in subjective well-being and in defining whether a person flourishes (27). Larsen and Prizmic discussed work by several authors (24, 28–30) which suggests that to maintain an optimal level of emotional well-being and positive mental health, individuals need to experience approximately three times more positive than negative affect.
Breathlessness (Dyspnea mMRC)
The modified Medical Research Council (mMRC) Dyspnea scale is a 5-item tool used to assess dyspnea in patients with COPD (31). The MMRC is universally accepted tool to measure breathless ness and used to classify COPD based disease burden in the most recent Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management, and Prevention of COPD. (32)
2.5. Statistical Analysis
JMP statistical software (version 9.01, SAS, Cary, NC) was used for all analyses. Correlation analyses were tested between the measure variables. Univariate and multivariate models were built to predict QoL (dependent variable) including all significant variables from the univariate analysis as independent variables. We used bootstrapping methods (2000 iterations) to confirm the robustness of the models and to build a confidence interval for the Adjusted R square of the models. All tests were 2-sided and p-values less than 0.05 were considered statistically significant.
Descriptive analysis included calculating means and standard deviations (SDs). The mediation effect of the critical positivity ratio on the association between SM and QoL was evaluated based on conditions put forth by Baron and Kenny (33, 34) and Judd and Kenny (35). Condition 1: The theoretically specified independent variables must emerge as significant predictors of the outcome variable (QoL) in correlation analyses. Condition 2: The theoretically specified independent variables must emerge as significant predictors of the mediator variable critical positivity ratio in correlation analyses. Condition 3: The mediator variable must be significantly associated with the outcome variable after controlling for the independent variables. Condition 4: The relationship between the significant independent variables and the outcome variable (QoL) must be significantly reduced when the effects of the mediator variable (critical positivity ratio) is included in the model.
Sample Size
280 subjects with complete records was calculated as the minimum required sample size for the proposed study, a cross sectional study using a multiple regression model to predict the effect of the desired variable to be studied – self management and clinical positivity ratio- on the desired outcome (disease specific quality of life), given a probability level of 0.05 (α=0.05), anticipating 5 predictors in the model (initially expected to be at least age, degree of breathlessness (most common symptom in COPD and degree of bronchial obstruction (fev1%), -the latter are the most significant and universally accepted outcomes associated with COPD progress being most significant in COPD) with an the anticipated effect size (a change in R square in the model) for the variable studied >5%, and a statistical power level of 0.9. We anticipated a level of missingness of 5 %, so we aimed for at least 294 individuals to be recruited.
3. Results
Baseline characteristics of the cohort are shown in Table 1. Patients with COPD (n=292) were included (61% male, mean age 70 years). The spirometric impairment was predominantly severe (65% of the patients presented FEV1<50%). 40% of the patients suffered an exacerbation of the disease the previous year and 33% required a hospitalization for breathing problems. Patient reported outcomes are shown in Table 2.
Table 1.
Cohort characteristics.
| Mean (SD) or N (%) | |
|---|---|
| n | 292 |
| Age (yrs) | 70 (9.46) |
| Gender | |
| Male | 177 (61%) |
| BMI | 28.2 (6.8) |
| Live alone (yes) | 49 (23%) |
| Married | 156 (75%) |
| Use of permanent oxygen | 76 (37%) |
| Gait Speed (meters/second) | 1.07 (0.22) |
| Breathlessness mMRC (scale 0–4) | 2.00 (1.1) |
| PFT Data | |
| FVC % | 70.24 (17.4) |
| FEV1 % predicted | 40.8 (17.9) |
| FEV1/FVC | 47.8 (16.3) |
| DLCO % | 52.5 (18.3) |
| Exacerbations ≥ 2 | 114 (40) |
| Hospital admission previous year | 94 (33) |
| ADO index (range 0–14) | 8.8 (2) |
| GOLD stages | |
| A= mild | 59 (20%) |
| B = moderate | 46 (16%) |
| C-= severe | 53 (18%) |
| D= very severe | 134 (46%) |
Note: SD = Standard Deviation; BMI = Body Mass Index; PFT = Pulmonary Function Test; ADO = Age-Dyspnea-Obstruction Index; GOLD = Global initiative for Obstructive Lung Disease; mMRC= Modified Medical Research Council Dyspnea score
Table 2.
Patient reported outcomes.
| Mean (SD) | Min-Max Score | |
|---|---|---|
| CRQ Total | 4.85 (1.13) | 1–7 |
| Dyspnea | 5.03 (1.42) | 1–7 |
| Emotion | 5.03 (1.17) | 1–7 |
| Mastery | 5.23 (1.41) | 1–7 |
| Fatigue | 4.09 (1.22) | 1–7 |
| SMAS-30 | 67.83 (11.55) | 0–100 |
| Taking Initiatives | 69.06 (16.90) | 0–100 |
| Investment Behavior | 63.14 (17.83) | 0–100 |
| Self-Efficacy | 87.00 (11.38) | 0–100 |
| Variety | 46.65 (17.20) | 0–100 |
| Positive Frame of Mind | 69.63 (16.80) | 0–100 |
| Multifunctionality | 68.52 (13.49) | 0–100 |
| PANAS | ||
| Positive | 33.04 (7.62) | 10–50 |
| Negative | 16.06 (6.42) | 10–50 |
| Positive/Negative Ratio | 2.31 (1.02) | 0.2–5 |
Notes: SD = Standard Deviation; CRQ = Chronic Respiratory Questionnaire; SMAS 30 = Self-Management Ability Scale 30; PANAS = Positive and Negative Affect Schedule
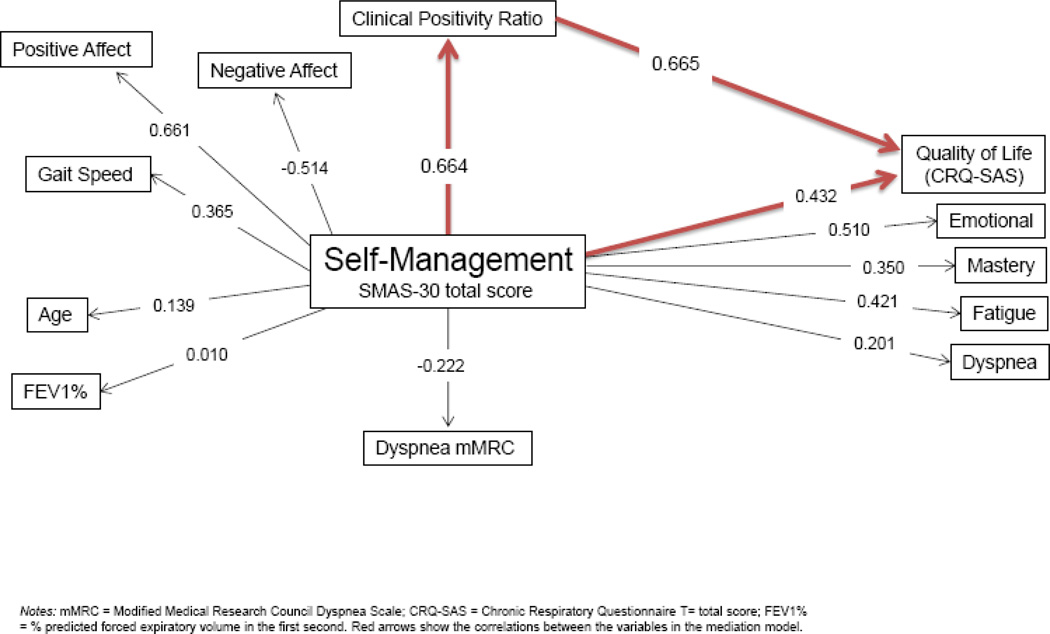
To study the construct of SMAS-30 in COPD, we included a correlation analysis as shown in Figure 2. The correlation analysis showed that SM is not significantly correlated with age or lung function but significantly correlated with affect (directly with positive affect and inversely with negative affect), gait speed and QoL.
Figure 2. Theoretical framework of self-management in disease specific quality of life.
Notes: mMRC = Modified Medical Research Council Dyspnea Scale; CRQ-SAS = Chronic Respiratory Questionnaire T = total score; FEV1% = % predicted forced expiratory volume in the fast second. Red arrows show the correlations between the variables in the mediation model.
Univariate models to predict QoL (CRQ total) -not shown- indicated that CRQ SAS total score was significantly associated with age, COPD related hospitalization in the past year, being married, BMI, living alone, breathlessness (mMRC dyspnea scale), lung function, SM abilities, and the critical positivity ratio. Those variables were then included into the multivariate models. Non-significant variables were then not included neither in the correlation matrix nor in the multivariate models. Table 3 (correlation matrix) included continuos variables significant in the univariate model.
Table 3.
Correlation Matrix
| 1. | 2. | 3. | 4. | 5. | 6. | |
|---|---|---|---|---|---|---|
| 1. Age | ||||||
| 2. mMRC | −0.020 | |||||
| 3. CRQ Total | 0.209 | −0.502** | ||||
| 4. SMAS Total | 0.150 | −0.210* | 0.459** | |||
| 5. Positivity Ratio | 0.219 | −0.183 | 0.654*** | 0.670*** | ||
| 6. BMI | −0.008 | 0.214 | −0.236* | −0.212 | −0.234 | |
| 7. FEV1% Predicted | 0.105 | −0.289* | 0.177 | −0.078 | −0.029 | 0.040 |
Notes: mMRC = Modified Medical Research Council Dyspnea Scale; CRQ = Chronic Respiratory Questionnaire; SMAS = Self-Management Abilities Scale; Positivity Ratio = PANAS Positive/Negative affect ratio; BMI = body mass index; FEV1% Predicted = Forced expiratory volume/Forced vital capacity
p<0.05,
p<0.001,
p<0.0001
Our results met conditions 1 and 2 for the mediation analysis as shown by the correlations of independent variables with disease specific quality of life (CRQ total) as displayed in Table 3. The results indicated that CRQ total was significantly and directly associated with self-management abilities (SMAS), and the clinical positivity ratio and inversely and significantly associated with breathlessness and BMI. In multiple regression analyses using the mediating variable (clinical positivity ratio) as the dependent variable and after controlling for univariate significant variables, the results indicated that CRQ (outcome variable) was significantly related to clinical positivity ratio.
Multiple regression analyses were performed to test conditions 3 and 4 for mediation as shown in table 4 that present the direct effects of Breathlessness (dyspnea m MRC) self-management ability SMAS-30, marital status, age, living alone, bronchial obstruction (FEV1%, the lower the number the worst) and history of hospitalization on that outcome variable CRQ SAS total score (disease specific well-being). After controlling for all independent variables, clinical positivity ratio significantly affected well-being (β= 0.61) thus meeting condition 3 for mediation.
Table 4.
Multiple regression analysis of background characteristics and the mediating effect of Critical Positivity Ratio on QoL.
| Step 1 Rsq=0.41 (CI 0.37–0.48#) |
Step 2 Rsq=0.60 (CI 0.56–0.71#) |
|
|---|---|---|
| Independent Variable | β | β |
| Dyspnea mMRC | −0.35** | −0.31** |
| SMAS-30 | 0.03** | 0.002 |
| Married | 0.17 | 0.19 |
| Age | 0.01* | 0.01 |
| Live alone | 0.13 | 0.04 |
| FEV1% | 0.004 | 0.005 |
| Hospitalized for COPD in past year | 0.18* | 0.14* |
| Clinical Positivity Ratio | Not in model | 0.61** |
Notes: mMRC = Modified Medical Research Council Dyspnea Scale; Critical Positivity Ratio = PANAS Positive/Negative affect. QoL = Quality of life; relationship examined through Chronic Respiratory Questionnaire total score.(#: confidence interval of the adjusted R square after 2000 bootstrapped models)
In step 1 of the regression dyspnea (β= 0.35); self-management abilities (β = 0.03); p B 0.001), age (β=0.01) and a history of a COPD hospitalization in the last year (β=0.18) significantly affected the well-being of COPD patients. To meet condition 4, the relationship between the SMAS and the outcome variable CRQ (well-being) must be significantly reduced when the effects of the mediator (clinical positivity ration) are included in the model. Thus, clinical positivity ratio was included in step 2 of the regression analysis. Self-management abilities lost significance related to well-being (β = 0.002) indicating the complete mediating effect of the clinical positivity ratio between self-management abilities and quality of life in COPD. Furthermore, the age effect on wellbeing was also lost in step 2 indicating also a complete mediating effect by the clinical positivity ratio.
In summary Table 4 Step 1 (R square= 0.41) SM is independently associated to QoL, after adjusting for FEV1% predicted, age, hospitalizations. In Step 2, when we added the ratio of critical positivity ratio to the model, not only the adjusted R square significantly improved to 0.60 but also the association between SM and QoL lost significance, completely meditated by the critical positivity ratio
We further investigated the differences QoL and SM values using the proposed cutoff of 2.9 for the positive/negative affect ratio (the values of QoL and SMAS in subjects with a clinical positivity ration below 2.9 and above 2,9) and found a statistically and clinically significant difference: 0.9 points (p=0.0001) for CRQ total (minimally clinically important difference is 0.5 points) and for SMAS total score 12-point difference (p=0.0001) which is higher than one standard deviation difference which can be considered a clinically significant difference.
Lastly we explore which domains of SMAS were independently associated with QoL (CRQ SAS) after adjusting for the univariate significant variables and found that only the domains Investment Behaviors (β=0.01, p<0.05) and Self-efficacy (β=0.02, p<0.05) were the only ones significant in this large cohort of COPD patients explaining 14 and 13 % respectively of the overall variance of the model and confirmed by bootstrap analysis.
4. Discussion and Conclusion
4.1. Discussion
We found that SM measured by the SMAS-30 was significantly and independently associated with disease specific QoL in COPD, confirming previous reports (4, 5). The novelty of our results relates to the fact that that association of SM abilities with QoL is totally mediated by the critical positivity ratio or the degree of positivity of the individual. The SMAS-30 domains of investment behavior and self-efficacy were the only two domains that were independently associated with COPD disease specific QoL. Our work extends previous reports by Cramm et al. (10) by confirming the association between SMAS-30 and well-being in a much more characterized cohort of COPD patients. We measured lung function, gait speed, disease specific QoL, and positive and negative affect in addition to demographics and background measures.
The association of SM and QoL (CRQ SAS total) is not affected by lung function or living condition, and is minimally affected by age suggesting that increasing age and more limited functional lung capacity does not necessarily affect SM abilities. While SMAS-30 measures a wide range of SM abilities; we found that the domains of self-efficacy and investment behavior are the ones that independently predict QoL in this cohort of well characterized COPD patients. Lower levels of reported self-efficacy in managing breathing issues have been related to increases in anxiety and depression and QoL impairment (36). More specifically, the knowledge and ability to continue to perform tasks and activities that are valued by the individual was a strong indicator of self-reported health status in COPD, even when physical functioning and COPD specific factors were accounted for (37). The domain of investment behavior from SMAS-30 includes questions about the individual’s interests on a regular basis (such as a hobbies), physical exercise engagement to maintain fitness, efforts made to keep in good contact with acquaintances, as well as time and attention devoted to significant others. The latter can be very informative in generating dialogue and formulating goals in self-management like health coaching.
Previous research in COPD supports our findings regarding the relevancy of these two domains: In a study by Katz et al., it was found that the declining in doing valued life activities (like hobbies) in COPD patients were associated with onset of depression symptoms (38), a known contributor to poorer treatment adherence and self-care (5). Social exposure was associated with lower levels of anxiety and depression (36), and with improved coping strategies in disease management (39) and when social support was lacking, individuals identified it as a barrier to active SM of disease conditions (40).
The most significant and novel finding of this report is the direct mediating effect of the critical positivity ratio in the association between SM and QoL. Positive affect demonstrated the highest correlation and independent association with QoL, after adjusting for age, breathlessness measures and degree of bronchial obstruction (FEV1% predicted), and the addition in the prediction model increased the explanation of variance of QoL to 60% (from 40% in the step 1 model without the critical positivity ratio Table 4). The robustness of the model is suggested by the tight confidence interval of the R square after the 2000 iteration of the model in the bootstrap analysis (table 4). The mediation model, included very meaningful outcome variables for COPD make our results very relevant to understanding SM in COPD. To our knowledge, these are novel findings and may translate directly into initiatives for the care of patients.
Positive affect has been shown to have an impact on SM abilities and QoL in individuals living with chronic disease (41) Positivity and its implications have been studied broadly (42–44), particularly its importance in mental health (45) and well-being (46). Decreased positivity has shown to be related to higher incidents of catastrophizing (47). Positive affect reflects the extent a person feels enthusiastic and active, so high positive affect is a state of high energy, full concentration and pleasure engagement, whereas low positive affect is characterized by sadness and lethargy (48). A previous study showed that positive attitudes could improve mood, emotional and physical well-being in patients with a chronic disease, such a depression a very prevalent problem in COPD (49).
The clinical implications of our findings are rooted in the fact that positive affect can be fostered through training that may be incorporated into SM programs when people are found to have a low critical positivity ratio. The previously proposed threshold of 2.9 points in the clinical positivity ratio (24) may serve at least as a reference in clinical daily care of patients with COPD to identify people with low positivity (<2.9). We acknowledge the existing controversies about the rigid validity of the cutpoint but having a simple reference will help implementation. The value of measuring and quickly understanding the measure may derive in salutary consequences: increasing the awareness of clinicians and patients and creating condition for dialogue on that matter and instituting interventions that may increase positivity.
Specific tools that have been utilized to increase positive experiences that could be part of support SM including the use of affirmations (48), journaling, gratefulness practice (50), mindfulness of emotions and thoughts and utilizing daily data logs (51). Mindfulness has been shown to decrease catastrophizing (52, 53) (that is well reported in COPD (54, 55) in pain-related symptoms (56, 57). Decreasing the rigidity of catastrophizing and working towards more flexible, positive thinking through the use of concrete tools has proved effective. Not only have these techniques been shown decrease negative thinking, but they also contribute to increased self-efficacy, regardless of disease characteristics.
Our results of the mediating effect of positive affect have been suggested in other chronic states, such as chronic pain syndromes, in which individuals need to cope with persistent and many times “unfixable” symptoms, such as breathlessness in many patients with COPD. Positive affect offers an "upward spiral" model that can translate to resilience and promote active engagement in valued goals that enhance SM (17). In clinical studies, the association of positive affect and chronic symptoms is dynamic, time variant, and may be best considered in the context of its interacting role with negative affect, like we present in this study (the critical positivity ratio) (48).
4.1.1 Limitations
Our study has some limitations. First, causal relationships could not be inferred, as the data collected were cross-sectional. While we showed that SM is a predictor of disease specific QoL, we did not investigate whether interventions aimed to enhance SM abilities lead to improved QoL or if positive affect interventions have an effect on QoL. Further research is necessary to establish those causal relationships. Second, because of the cross-sectional data, further validation in other cohorts is desirable, even though the results were rooted in robust models confirmed by the bootstrapping analysis. The specific SM abilities found significantly related to QoL (investment behaviors and self-efficacy) are not universal to all outcomes in COPD may not translate to other outcomes beyond QoL, such as decreasing hospitalizations.
4.2. Conclusions
SM ability, measured by the total score of SMAS-30 is independently associated with disease specific quality of life after adjusting with the most meaningful co-variates for COPD. However that association is totally mediated by the critical positivity ratio or degree of positivity of the individual. Our results are of relevance in crafting and implementing SM programs for patients with COPD. The SMAS-30 is related to meaningful outcomes in COPD and represents a good tool to measure SM abilities in this condition.
4.3. Practice Implications
This study suggests that measuring the critical positivity ratio, in patients with COPD is not only significant because of increasing awareness of the degree of positivity of the individual that impact disease specific quality of life in COPD but also because may have significant implications on the effect of COPD SM programs. The 2.9 cutoff point of the critical positivity ratio may be a useful in clinical practice to quickly assess positivity, to generate dialogue about positivity and its benefits and to propose interventions that may improve positive affect. Mindfulness, Emotional Intelligence and Gratitude training are potential interventions to increase the clinical positivity ratio, promoting flourishing in COPD patients.
Investment time in meaningful behaviors (for the individual) and self-efficacy are likely key ingredients in COPD SM programs.
Highlights.
Self management is independently associated with disease specific quality of life in COPD
Positive/Negative Affect Ratio mediates the association of self-management-quality of life
Significant self management domains in COPD: Investment in behaviors and self-efficacy
Age and lung function are not significantly associated to self-management abilities in COPD
Acknowledgments
The authors want to thank Janae L. Kirsch for assisting in the preparation and editing of the manuscript.
This study was funded by Grant 1R01 HL 94680-01A2from the National Institutes of Health, National Heart, Lung and Blood Institute. R Benzo (PI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
Authors’ contributions
RB: Project conception and hypothesis, designed data analysis, wrote first draft of the manuscript, revised manuscript and approved final version to be published.
BAB: Conducted the trial, revised manuscript and approved final version to be published.
RB and BAB are co-primary authors
MD: Conducted the trial, revised manuscript and approved final version to be published.
References
- 1.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: Gold executive summary. American journal of respiratory and critical care medicine. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Spruit MA, Singh SJ, Garvey C, Zuwallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, Pitta F, Sewell L, Raskin J, Bourbeau J, Crouch R, Franssen FM, Casaburi R, Vercoulen JH, Vogiatzis I, Gosselink R, Clini EM, Effing TW, Maltais F, van der Palen J, Troosters T, Janssen DJ, Collins E, Garcia-Aymerich J, Brooks D, Fahy BF, Puhan MA, Hoogendoorn M, Garrod R, Schols AM, Carlin B, Benzo R, Meek P, Morgan M, Rutten-van Molken MP, Ries AL, Make B, Goldstein RS, Dowson CA, Brozek JL, Donner CF, Wouters EF. An official american thoracic society/european respiratory society statement: Key concepts and advances in pulmonary rehabilitation. American journal of respiratory and critical care medicine. 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 3.Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the chronic care model in the new millennium. Health Aff. 2009;28:75–85. doi: 10.1377/hlthaff.28.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zwerink M, Brusse-Keizer M, van der Valk PD, Zielhuis GA, Monninkhof EM, van der Palen J, Frith PA, Effing T. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;19 doi: 10.1002/14651858.CD002990.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramm JM, Nieboer AP. Self-management abilities, physical health and depressive symptoms among patients with cardiovascular diseases, chronic obstructive pulmonary disease, and diabetes. Patient Educ Couns. 2012;87:411–415. doi: 10.1016/j.pec.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, Renzi P, Nault D, Borycki E, Schwartzman K, Singh R, Collet JP. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: A disease-specific self-management intervention. Archives of internal medicine. 2003;163:585–591. doi: 10.1001/archinte.163.5.585. [DOI] [PubMed] [Google Scholar]
- 7.Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, Kumari S, Thomas M, Geist LJ, Beaner C, Caldwell M, Niewoehner DE. Disease management program for chronic obstructive pulmonary disease: A randomized controlled trial. American journal of respiratory and critical care medicine. 2010;182:890–896. doi: 10.1164/rccm.200910-1579OC. [DOI] [PubMed] [Google Scholar]
- 8.Cramm JM, Twisk J, Nieboer AP. Self-management abilities and frailty are important for healthy aging among community-dwelling older people; a cross-sectional study. BMC Geriatr. 2014;14:1471–2318. doi: 10.1186/1471-2318-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramm JM, Nieboer AP. The relationship between self-management abilities, quality of chronic care delivery, and wellbeing among patients with chronic obstructive pulmonary disease in the netherlands. Int J Chron Obstruct Pulmon Dis. 2013;8:209–214. doi: 10.2147/COPD.S42667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cramm JM, Hartgerink JM, Steyerberg EW, Bakker TJ, Mackenbach JP, Nieboer AP. Understanding older patients' self-management abilities: Functional loss, self-management, and well-being. Qual Life Res. 2013;22:85–92. doi: 10.1007/s11136-012-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramm JM, Strating MM, de Vreede PL, Steverink N, Nieboer AP. Validation of the self-management ability scale (smas) and development and validation of a shorter scale (smas-s) among older patients shortly after hospitalisation. Health Qual Life Outcomes. 2012;10:1477–7525. doi: 10.1186/1477-7525-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang MW, Ho RC, Cheung MW, Fu E, Mak A. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: A systematic review, meta-analysis and meta-regression. General hospital psychiatry. 2011;33:217–223. doi: 10.1016/j.genhosppsych.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Eisner MD, Blanc PD, Yelin EH, Katz PP, Sanchez G, Iribarren C, Omachi TA. Influence of anxiety on health outcomes in copd. Thorax. 2010;65:229–234. doi: 10.1136/thx.2009.126201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steele CM. The psychology of self-affirmation: Sustaining the integrity of the self. Advances in experimental social psychology. 1988;21:261–302. [Google Scholar]
- 15.Kahn BE, Isen AM. The influence of positive affect on variety seeking among safe, enjoyable products. Journal of Consumer Research. 1993;20:257–270. [Google Scholar]
- 16.Isen A, Reeve J. Positive affect promotes intrinsic motivation. Ithaca: Cornell University. 2002 [Google Scholar]
- 17.Finan PH, Garland EL. The role of positive affect in pain and its treatment. Clin J Pain. 2015;31:177–187. doi: 10.1097/AJP.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benzo R, Siemion W, Novotny P, Sternberg A, Kaplan RM, Ries A, Wise R, Martinez F, Utz J, Sciurba F. Factors to inform clinicians about the end of life in severe chronic obstructive pulmonary disease. J Pain Symptom Manage. 2013 doi: 10.1016/j.jpainsymman.2012.10.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karpman C, Depew ZS, Lebrasseur NK, Novotny PJ, Benzo RP. Determinants of gait speed in chronic obstructive lung disease. Chest. 2014 doi: 10.1378/chest.13-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuurmans H, Steverink N, Frieswijk N, Buunk BP, Slaets JP, Lindenberg S. How to measure self-management abilities in older people by self-report. The development of the smas-30. Qual Life Res. 2005;14:2215–2228. doi: 10.1007/s11136-005-8166-9. [DOI] [PubMed] [Google Scholar]
- 21.Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T, Ikeda A, Mishima M. Health status measured with the crq does not predict mortality in copd. Eur Respir J. 2002;20:1147–1151. doi: 10.1183/09031936.02.00303702. [DOI] [PubMed] [Google Scholar]
- 22.Williams JE, Singh SJ, Sewell L, Guyatt GH, Morgan MD. Development of a self-reported chronic respiratory questionnaire (crq-sr) Thorax. 2001;56:954–959. doi: 10.1136/thorax.56.12.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawford JR, Henry JD. The positive and negative affect schedule (panas): Construct validity, measurement properties and normative data in a large non-clinical sample. The British journal of clinical psychology / the British Psychological Society. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- 24.Fredrickson BL, Losada MF. Positive affect and the complex dynamics of human flourishing. Am Psychol. 2005;60:678–686. doi: 10.1037/0003-066X.60.7.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradburn NM. The structure of psychological well-being. 1969 [Google Scholar]
- 26.Kahneman D. 1 objective happiness. Well-being: Foundations of hedonic psychology. 1999:1. [Google Scholar]
- 27.Larsen RJ, Prizmic Z. Regulation of emotional well-being. The science of subjective well-being. 2008:258–289. [Google Scholar]
- 28.Schwartz RM. Consider the simple screw: Cognitive science, quality improvement, and psychotherapy. Journal of Consulting and Clinical Psychology. 1997;65:970. doi: 10.1037//0022-006x.65.6.970. [DOI] [PubMed] [Google Scholar]
- 29.Gottman JM. Spaff audiotape 2. Tonkassette 2. Erlbaum; 1996. What predicts divorce?: The measures. [Google Scholar]
- 30.Schwartz RM, Reynolds CF, III, Thase ME, Frank E, Fasiczka AL, Haaga DA. Optimal and normal affect balance in psychotherapy of major depression: Evaluation of the balanced states of mind model. Behavioural and Cognitive Psychotherapy. 2002;30:439–450. [Google Scholar]
- 31.Bestall JC, Paul EA, Garrod R, Garnham R, Jones RW, Wedzicha AJ. Longitudinal trends in exercise capacity and health status after pulmonary rehabilitation in patients with copd. Respir Med. 2003;97:173–180. doi: 10.1053/rmed.2003.1397. [DOI] [PubMed] [Google Scholar]
- 32.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Oga T, Izumi T. A comparison of the level of dyspnea vs disease severity in indicating the health-related quality of life of patients with copd. Chest. 1999;116:1632–1637. doi: 10.1378/chest.116.6.1632. [DOI] [PubMed] [Google Scholar]
- 33.Kenny DA. Meditation. Baron and kenny steps. 2010 Available from: http://davidakenny.net/cm/mediate.htm#BK. [Google Scholar]
- 34.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 35.Judd CM, Kenny DA. Process analysis: Estimating mediation in treatment evaluations. Evaluation Review. 1981;5:602–619. [Google Scholar]
- 36.McCathie HC, Spence SH, Tate RL. Adjustment to chronic obstructive pulmonary disease: The importance of psychological factors. Eur Respir J. 2002;19:47–53. doi: 10.1183/09031936.02.00240702. [DOI] [PubMed] [Google Scholar]
- 37.Katz P, Morris A, Gregorich S, Yazdany J, Eisner M, Yelin E, Blanc P. Valued life activity disability played a significant role in self-rated health among adults with chronic health conditions. J Clin Epidemiol. 2009;62:158–166. doi: 10.1016/j.jclinepi.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katz PP, Julian LJ, Omachi TA, Gregorich SE, Eisner MD, Yelin EH, Blanc PD. The impact of disability on depression among individuals with copd. Chest. 2010;137:838–845. doi: 10.1378/chest.09-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marques A, Jacome C, Cruz J, Gabriel R, Brooks D, Figueiredo D. Family-based psychosocial support and education as part of pulmonary rehabilitation in copd: A randomized controlled trial. Chest. 2015;147:662–672. doi: 10.1378/chest.14-1488. [DOI] [PubMed] [Google Scholar]
- 40.Jerant AF, Friederichs-Fitzwater MMv, Moore M. Patients’ perceived barriers to active self-management of chronic conditions. Patient Education and Counseling. 2005;57:300–307. doi: 10.1016/j.pec.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 42.Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological review. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 43.Fordyce MW. Development of a program to increase personal happiness. Journal of Counseling Psychology. 1977;24:511–521. [Google Scholar]
- 44.Fordyce MW. A program to increase happiness: Further studies. Journal of Counseling Psychology. 1983;30:483–498. [Google Scholar]
- 45.Macleod AK, Moore R. Positive thinking revisited: Positive cognitions, well-being and mental health. Clinical Psychology & Psychotherapy. 2000;7:1–10. [Google Scholar]
- 46.Otto LM, Howerter A, Bell IR, Jackson N. Exploring measures of whole person wellness: Integrative well-being and psychological flourishing. EXPLORE: The Journal of Science and Healing. 2010;6:364–370. doi: 10.1016/j.explore.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borsbo B, Gerdle B, Peolsson M. Impact of the interaction between self-efficacy, symptoms and catastrophising on disability, quality of life and health in with chronic pain patients. Disabil Rehabil. 2010;32:1387–1396. doi: 10.3109/09638280903419269. [DOI] [PubMed] [Google Scholar]
- 48.Charlson ME, Wells MT, Peterson JC, Boutin-Foster C, Ogedegbe GO, Mancuso CA, Hollenberg JP, Allegrante JP, Jobe J, Isen AM. Mediators and moderators of behavior change in patients with chronic cardiopulmonary disease: The impact of positive affect and self-affirmation. Transl Behav Med. 2014;4:7–17. doi: 10.1007/s13142-013-0241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan VS, Ramsey SD, Giardino ND, Make BJ, Emery CF, Diaz PT, Benditt JO, Mosenifar Z, McKenna R, Jr, Curtis JL, Fishman AP, Martinez FJ. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Archives of internal medicine. 2007;167:2345–2353. doi: 10.1001/archinte.167.21.2345. [DOI] [PubMed] [Google Scholar]
- 50.Layous K, Chancellor J, Lyubomirsky S. Positive activities as protective factors against mental health conditions. Journal of Abnormal Psychology. 2014;123:3–12. doi: 10.1037/a0034709. [DOI] [PubMed] [Google Scholar]
- 51.Padesky CA. Schema change processes in cognitive therapy. Clinical Psychology & Psychotherapy. 1994;1:267–278. [Google Scholar]
- 52.Cassidy EL, Atherton RJ, Robertson N, Walsh DA, Gillett R. Mindfulness, functioning and catastrophizing after multidisciplinary pain management for chronic low back pain. Pain. 2012;153:644–650. doi: 10.1016/j.pain.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 53.Day MA, Smitherman A, Ward LC, Thorn BE. An investigation of the associations between measures of mindfulness and pain catastrophizing. Clin J Pain. 2015;31:222–228. doi: 10.1097/AJP.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 54.Plaufcan MR, Wamboldt FS, Holm KE. Behavioral and characterological self-blame in chronic obstructive pulmonary disease. Journal of Psychosomatic Research. 2012;72:78–83. doi: 10.1016/j.jpsychores.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arne M, Emtner M, Janson S, Wilde-Larsson B. Copd patients perspectives at the time of diagnosis: A qualitative study. Primary Care Respiratory Journal. 2007;16:215–221. doi: 10.3132/pcrj.2007.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schutze R, Rees C, Preece M, Schutze M. Low mindfulness predicts pain catastrophizing in a fear-avoidance model of chronic pain. Pain. 2010;148:120–127. doi: 10.1016/j.pain.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 57.de Boer MJ, Steinhagen HE, Versteegen GJ, Struys MM, Sanderman R. Mindfulness, acceptance and catastrophizing in chronic pain. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087445. [DOI] [PMC free article] [PubMed] [Google Scholar]