Abstract
Objective
Previous imaging studies with positron emission tomography (PET) have reliably demonstrated an age-associated decline in the dopamine system. Most of these studies have focused on the densities of dopamine receptor subtypes D2/3R (D2R family) in the striatum using antagonist radiotracers that are largely nonselective for D2R vs. D3R subtypes. Therefore, less is known about any possible age effects in D3-rich extrastriatal areas such as the substantia nigra/ventral tegmental area (SN/VTA) and hypothalamus. This study sought to investigate whether the receptor availability measured with [11C](+)PHNO, a D3R-preferring agonist radiotracer, also declines with age.
Methods
Forty-two healthy control subjects (9 females, 33 males; age range 19-55 years) were scanned with [11C](+)PHNO using a High Resolution Research Tomograph (HRRT). Parametric images were computed using the simplified reference tissue model (SRTM2) with cerebellum as the reference region. Binding potentials (BPND) were calculated for the amygdala, caudate, hypothalamus, pallidum, putamen, SN/VTA, thalamus, and ventral striatum and then confirmed at the voxel level with whole-brain parametric images.
Results
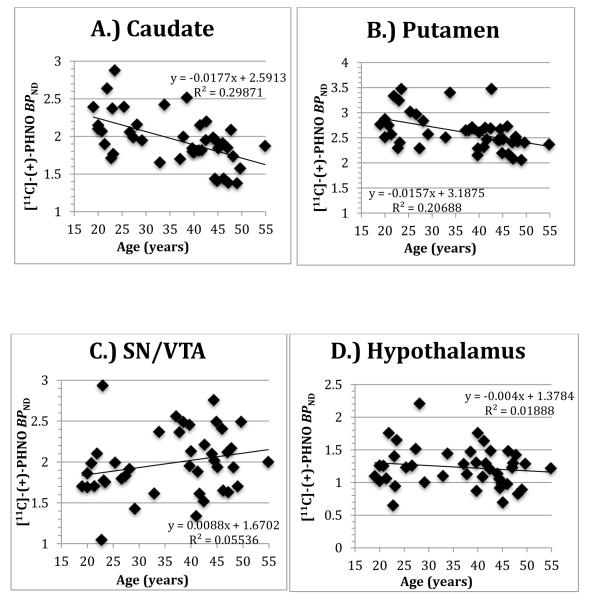
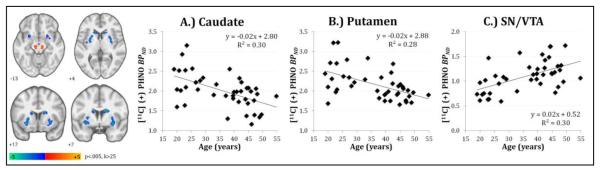
Regional [11C](+)PHNO BPND displayed a negative correlation between receptor availability and age in the caudate (r=−0.56, corrected p = 0.0008) and putamen (r=−0.45, corrected p = 0.02) in healthy subjects (respectively 8% and 5% lower per decade). No significant correlations with age were found between age and other regions (including the hypothalamus and SN/VTA). Secondary whole-brain voxel-wise analysis confirmed these ROI findings of negative associations and further identified a positive correlation in midbrain (SN/VTA) regions.
Conclusion
In accordance with previous studies, the striatum (an area rich in D2R) is associated with age-related declines of the dopamine system. We did not initially find evidence of changes with age in the SN/VTA and hypothalamus, areas previously found to have a predominantly D3R signal as measured with [11C](+)PHNO. A secondary analysis did find a significant positive correlation in midbrain (SN/VTA) regions, indicating that there may be differential effects of aging, whereby D2R receptor availability decreases with age while D3R availability stays unchanged or is increased.
Keywords: dopamine, aging, D2R, D3R, PET imaging, [11C](+)PHNO
1. Introduction
Previous imaging studies with positron emission tomography (PET) have reliably demonstrated an age-associated decline in the dopamine system of the brain (Ishibashi et al., 2009; Kim et al., 2011; Volkow et al., 2000; Volkow et al., 1998b). Dopamine D2/3 receptors (D2/3 R) in the striatum have been the most extensively studied with reductions of 6%–10% per decade typically reported (Alakurtti et al., 2013; Antonini et al., 1993; Rinne et al., 1993; Seeman et al., 1987; Volkow et al., 1998b). These decreases have also been found to have clinical significance as deficits in cognitive and motor function correlate with striatal D2/3 R in normal aging (Volkow et al., 1998a).
Age-related D2/3R findings have also been observed in extrastriatal areas in studies using high-affinity tracers [18F]fallypride and [11C]FLB 457 (Inoue et al., 2001; Kaasinen et al., 2000; Kegeles et al., 2010; Mukherjee et al., 2002). Specifically, D2/3R decreased 9–14% per decade of life in cortical areas (Inoue et al., 2001; Kaasinen et al., 2000) with [11C]FLB 457 and 5-10% in extrastriatal brain regions including the hippocampus, thalamus, amygdala and substantia nigra with [18F]fallypride and [11C]FLB 457 (Inoue et al., 2001; Kaasinen et al., 2000; Kegeles et al., 2010; Mukherjee et al., 2002).
To date, studies have largely employed antagonist radiotracers (e.g., raclopride) that cannot differentiate D2 vs. D3 subtypes in either striatal or extrastriatal areas, with the exception of a preliminary study with [18F]N-methylbenperidol ([18F]NMB), a tracer with higher specificity for D2R. This work found decreases in striatal but not extrastriatal areas, although the latter regions had low BPND that made accurate comparisons difficult (Eisenstein et al., 2012). Nonetheless, this study was the first to suggest age-related differences might be more specific in regards to D2R.
Differentiation of D2R vs. D3R has become more important amid evidence suggesting that, despite similar homology, D2R and D3R have discrete anatomic and physiologic roles (Le Foll et al., 2005). This is true even though D2R and D3R are both in the D2-like subfamily and have a net inhibitory effect upon surrounding neurons by inhibiting adenylyl cyclase, production of cAMP and activation of protein kinase A (Bonci and Hopf, 2005). D2R (which has been more extensively studied) is a pre- and post-synaptic receptor with high concentrations in the striatum, among other areas, and has been implicated in a wide-range of clinical neuropsychiatric disorders (Bonci and Hopf, 2005). D3R has some unique properties that make it particularly valuable for investigating. First, D3R has a more limited anatomic distribution then D2R with dense concentrations in integral reward processing areas such as the ventral striatum and substantia nigra/ventral tegmental area (SN/VTA). Second, while D3R can exist as presynaptic or postsynaptic, its presynaptic autoreceptor has gathered the most attention as it is a controller of dopamine release (Fiorentini et al., 2010; Sokoloff et al., 2006). This quality may allow it work in opposing ways to D2R, and the D3-preferring agonist radiotracer [11C](+)PHNO has in fact found such opposing relationships in addiction and the reward system (Boileau et al., 2012; Erritzoe et al., 2014; Matuskey et al., 2014b; Matuskey et al., 2015;Payer et al., 2014) For recent reviews of PET imaging and D3 (including extrapyramidal motor disorders, psychoses and cognitive function) please see (Le Foll et al., 2014; Slifstein et al., 2013).
Given characteristic roles that are emerging it is important to know if D2R and D3R also have differences with regards to the aging process. A recent paper focused on the relationship of [11C](+)PHNO and [11C]raclopride (Nakajima et al., 2015) with aging, finding decreases in D2R, but not D3R rich regions such as the SN/VTA. The current work extends upon those findings by investigating whether receptor availability measured with [11C](+)PHNO is associated with age-related decreases in subcortical brain regions of interest (ROI) and whole-brain voxel-wise analyses in a completely independent cohort.
2. Material and methods
Forty-two healthy controls (9 females, 33 males; age range 19-55 years) were studied. All subjects had a comprehensive screening assessment that included a clinical interview, complete physical examination with medical history, routine blood tests, electrocardiogram, urine toxicology and pregnancy test (if female). Individuals were excluded if they self-reported or evaluation revealed a diagnosis of a current and/or lifetime psychiatric disorder; current or past serious medical or neurological illness (including a history of head injury with loss of consciousness); pregnancy (tested at screening and day of study) and/or breast feeding; metal in their body which would result in MRI exclusion; a history of substance abuse or dependence; and illicit drug use in the past 3 months. All subjects except one (with an average daily use of 5 cigarettes) were nonsmokers.
The study was performed under protocols approved by the Yale Human Investigation Committee, the Yale University Radiation Safety Committee, the Yale-New Haven Hospital Radioactive Drug Research Committee, and the Yale MRI Safety Committee. Subjects were recruited from Connecticut and surrounding states by paper and web advertisements, as well as personal referrals. Written informed consent was obtained from all participants at the beginning of screening after a full explanation of study procedures was performed.
Structural MR imaging was performed on a Siemens 3-T Trio system (Siemens Medical Solutions, Malvern, Pennsylvania) with a circularly polarized head coil for purposes of excluding individuals with anatomical abnormalities and anatomically coregistering with PET scans. The dimension and voxel size of MR images were 256 × 256 × 176 voxels and 0.98 × 0.98 × 1.0 mm3, respectively.
[11C](+)PHNO was prepared as previously reported (Gallezot et al., 2014; Wilson et al., 2005). All scans used a high-resolution research tomograph (HRRT) (Siemens/CTI, Knoxville, TN, USA), which acquires 207 slices (1.2mm slice separation) with a reconstructed image resolution of ~3mm. A transmission scan with a 137Cs point source was obtained before the emission scan. PET scans were acquired for 120min at rest with an average radioactivity dose of 417 ± 174 MBq and injected mass of 2.3±0.76 μg.
Dynamic PET scan data were reconstructed with all corrections (attenuation; normalization; scatter; randoms; deadtime and motion), using the MOLAR algorithm (Carson et al., 2003) with the following frame timing: 6 × 30 sec; 3 × 1 min; 2 × 2 min; 22 × 5 min. Motion correction was based on an optical detector (Vicra, NDI Systems, Waterloo, Ontario, Canada).
A summed image (0–10 min after injection) was created from the motion-corrected PET data and registered to the subject’s MR image, which in turn was nonlinearly registered to a MR template (MNI; Montreal Neurological Institute space) with Bioimagesuite (version 2.5; http://www.bioimagesuite.com). The cerebellum was defined by a Anatomical Automatic Labeling (AAL) template (Tzourio-Mazoyer et al., 2002) delineated on MR. This region has minimal D2/3R binding and was used as the reference region, as in previous studies (Boileau et al., 2012; Ginovart et al., 2007; Matuskey et al., 2014b; Mizrahi et al., 2011; Searle et al., 2010). Parametric images of BPND, which is linearly proportional to the density of available D2/3R, were computed using a simplified reference tissue model (2-parameter version: SRTM2). This method has been previously validated for [11C](+)PHNO (Gallezot et al., 2012; Gallezot et al., 2014; Wu and Carson, 2002) and reduces noise in the functional images (Matuskey et al., 2014b).
For this investigation, ROI included the amygdala, caudate, hypothalamus, pallidum (consisting of the globus pallidus and ventral pallidum), putamen, SN/VTA, thalamus, and ventral striatum (VST), and were based on the AAL template delineated on MR (Tzourio-Mazoyer et al., 2002) with the exception of hand-drawn SN and VST templates (that are not available in AAL). The SN/VTA was manually delineated as previously described on BPND images in template space because of greater anatomic visibility (Gallezot et al., 2014) and the VST template was based on guidelines from Mawlawi and colleagues (Mawlawi et al., 2001). The volume of the ROIs were computed after transfer from the template MRI to each individual PET image by computing the volume of the intersection of each PET voxel with the transferred ROI, and then summing the contribution of all PET voxels.
To confirm differences in BPND at the voxel level, whole-brain parametric BPND maps were registered to MNI space, smoothed with a 2mm FWHM Gaussian kernel, and statistically investigated using a simple regression model to assess correlations with age at every voxel (SPM12;Wellcome Trust Centre for Neuroimaging, London, United Kingdom). An initial whole-brain voxel-level threshold of p<.001 identified multiple peak correlations within ROIs, and a less stringent threshold of p<0.005 with an extent threshold (k) of 25 voxels was applied to examine spatial extent and contiguity of regional associations with age.
All outcomes were summarized descriptively and assessed for normality prior to analysis using normal probability plots and Kolmogorov test statistics. All outcomes were approximately normal. A linear mixed model was used to model the independent and joint effects of age (continuous) and brain region (within-subject factor) on BPND values. Slopes were estimated within each region post-hoc and decline per decade was calculated as a percent of the fitted value at age 20. Within-subject correlations were accounted for by fitting three variance-covariance structures to the data (unstructured, compound symmetry, and heterogeneous compound symmetry) and then selecting the best-fitting structure according to the Bayesian Information Criterion (BIC) (Matuskey et al., 2014a; Matuskey et al., 2012). All tests were two-tailed and adjusted for multiple comparisons using the Bonferroni adjustment. Adjusted p-values, presented here, were calculated by multiplying the raw p-value by the number of regional tests, in this case, eight. Analyses were conducted using SAS, version 9.3 (Cary, NC).
3. Results
The results of the primary mixed model showed an overall significant effect of ROIs (F7,280 78.2, p < 0.001) and an age-ROI interaction (F7, 280 7.31, p < 0.001). Table 1 presents average [11C](+)PHNO BPND levels (with standard deviations) for all ROIs along with the correlations and slope with age and the unadjusted p value.
Table 1.
| Region of Interest (ROI) |
BPND Mean (SD) |
Slope | Pearson R |
p value (unadjusted) |
% change per decade |
|---|---|---|---|---|---|
| Amygdala | 0.29 (0.09) | 0.0008 | 0.10 | 0.54 | 3% |
| Caudate | 1.95 (0.34) | −0.0177 | −0.56 | <0.0001 | −8% |
| Hypothalamus | 1.23 (0.31) | −0.0041 | −0.14 | 0.37 | −3% |
| Pallidum | 3.56 (0.49) | −0.0027 | −0.06 | 0.72 | −1% |
| Putamen | 2.62 (0.36) | −0.0157 | −0.45 | 0.003 | −5% |
| SN/VTA | 1.99 (0.39) | 0.0088 | 0.24 | 0.13 | 5% |
| Thalamus | 0.36 (0.09) | 0.0012 | 0.15 | 0.35 | 4% |
| VST | 3.71 (0.53) | −0.0067 | −0.14 | 0.39 | −2% |
Slope has units of change in BPND per year
p values were unadjusted for multiple comparisons
Significant negative associations between age and [11C](+)PHNO BPND values were observed in the caudate and putamen exclusively and averaged an 7.9±1.8% and 5.5±1.6% decline per decade, respectively. Both regions remained significant after a Bonferroni correction, with corrected p values of p = 0.0008 in the caudate and p = 0.02 in the putamen.
Individual data points, slope, intercept and R2 values are shown in Figure 1 for the A.) caudate and B.) putamen as well as the extrastriatial areas of the C.) SN/VTA and D.) hypothalamus for comparison. The magnitude of change with age in SN/VTA (+5%) was comparable to that in the caudate (−8%), however the SN/VTA correlation was not significant, in part due to higher variability in this small region. The volume of the ROIs did not correlate with age in any region studied (supplementary table and figure 1). Covarying for the effects of injection parameters, body mass index (BMI), and race in the above models did not affect the results (supplementary table 2 presents regional [11C](+)PHNO BPND correlations with individual injection parameters and the unadjusted p value).
Figure 1.
Secondary whole-brain voxel-wise analysis confirmed ROI findings of negative associations between age and [11C](+)PHNO BPND values in the caudate and putamen, and further identified a positive correlation in midbrain regions (Table 2; Figure 2). The bilateral midbrain clusters encompassed portions of the hand-drawn SN/VTA ROI (approximately 30% of cluster mass) and extended into posterior regions of the midbrain. The four striatal clusters survived cluster-level family-wise error (FWE) correction at pFWE<0.05 (k>83), and peak voxels in all reported regions survived initial voxel-wise threshold of p<.001.
Table 2.
| Region | k | x | y | z | t40 |
|---|---|---|---|---|---|
| R Caudate | 218 | 18 | 8 | 18 | −4.45 |
| L Caudate | 141 | −14 | 20 | 4 | −3.77 |
| R Putamen | 241 | 24 | 8 | −10 | −3.71 |
| L Putamen | 294 | −26 | 10 | −8 | −4.38 |
| R Midbrain (SN/VTA) | 36 | 12 | −24 | −12 | 4.04 |
| L Midbrain (SN/VTA) | 35 | −6 | −20 | −14 | 3.66 |
Details of age-related clusters identified by whole-brain voxel-wise analysis: spatial extent (k) in voxels, MNI coordinates (x, y, z) and t-score (t) of peak value.
Figure 2.
Whole-brain voxel-wise analysis of age-related [11C](+)PHNO BPND displayed on MNI template at p<.005, k>25. Scatter plots of smoothed parametric BPND values averaged across bilateral clusters identified in the A) caudate, B) putamen, and C) midbrain region that includes the SN/VTA ROI.
In an exploratory analysis, gender was examined (out of 42 subjects, 9 were females) and an age-ROI-gender interaction was observed (F7,266 3.24, p < 0.003). While negative associations with age were observed in the caudate (male and female) and putamen (male only), a significant age-related reduction was observed in VST BPND for females (Slope= −0.037±0.015, r = −0.78, p=0.012), but not males (Slope = 0.002±0.009, r = 0.03, p=0.86) (supplementary table 3 and figure 2). These exploratory findings will require validation in a sample with more female subjects.
4. Discussion
This study investigated the relationship between dopamine receptor availability and age with [11C](+)PHNO and demonstrated an age-related reduction specific to the caudate and putamen. These findings were confirmed in a whole-brain voxel-wise analysis and are largely consistent with previous studies finding age-related differences in the striatum (Nakajima et al., 2015). ROI analysis did not find age-related changes in in the high-binding extrastriatial areas of the SN/VTA and hypothalamus; however, whole-brain analysis identified regions of the midbrain where BPND was positively associated with age. Overall, our current results are likely due to tracer and receptor differences, as described below.
[11C](+)PHNO binding potentials have previously been interpreted to be relatively specific for D2R or D3R depending upon the brain region (Graff-Guerrero et al., 2008; Searle et al., 2010; Tziortzi et al., 2011). This, along with excellent signal-to-noise properties, allow the delineation of region-specific D2R and D3R that was not possible with other dopamine radiotracers. Specifically, when parsed by a D3R antagonist and/or modeling techniques, upwards of 90% of the [11C](+)PHNO signal in the dorsal striatum (caudate and putamen) has been attributed to D2R, in contrast with 100% of the SN/VTA and hypothalamus signal credited to D3R binding (Gallezot et al., 2012; Searle et al., 2010; Tziortzi et al., 2011).
Our findings in the D2-rich areas of the caudate and putamen were not surprising considering the wealth of existing literature on age-related declines in these regions with nonselective D2/3 ligands (such as [11C]raclopride )(Antonini et al., 1993; Ichise et al., 1998; Ishibashi et al., 2009; Kim et al., 2011; Rinne et al., 1993; Severson et al., 1982; Volkow et al., 1998a; Volkow et al., 2000; Volkow et al., 1998b; Wong et al., 1997). It is interesting to note, however, that dramatic reductions with the D2/3 antagonist [11C]raclopride have not been found with the D2/3 agonist [11C](+)PHNO in addictive disorders such as cocaine, methamphetamine and alcohol dependence (Boileau et al., 2012; Erritzoe et al., 2014; Matuskey et al., 2014b; Payer et al., 2014). In addition, prior studies in healthy humans suggest differences in striatal D2/3R availability as imaged by antagonist vs. agonist tracers (Graff-Guerrero et al., 2008; Narendran et al., 2011; Payer et al., 2014). While it is currently unknown why this may exist, possibilities include a preference for high-affinity state receptors that are coupled to G-proteins (D2High) or a greater sensitivity for endogenous dopamine with [11C](+)PHNO (Seeman, 2012; Shotbolt et al., 2012; Skinbjerg et al., 2012). Despite these tracer dissimilarities, the current results of receptor availability decline with age show no differences in the net measurement of age effects when compared to previously studied ligands. This suggests a D2R specific mechanism in the dorsal striatum, similar to previous findings with [18F]NMB and [11C](+)PHNO (Eisenstein et al., 2012; Nakajima et al., 2015).
The VST, an area important in reward processing, showed decreased binding with age only in the female population. This was an exploratory analysis and because of the smaller sample (N=9) in females, it is not clear if this due to a sampling bias. Recent work with high resolution [11C](+)PHNO and [11C]raclopride has found age differences in the VST (Nakajima et al., 2015), complementing earlier work with [11C]raclopride (Kim et al., 2011) . However, in the previous study the posterior putamen had greater decreases in D2/3R binding with age then the VST, leading some speculation that aging may differentially affect dopamine receptors in subregions of the striatum, with the sensorimotor areas of the dorsal striatum particularly vulnerable to the effects of age (Alakurtti et al., 2013; Kim et al., 2011).
While ROI analysis found no relationship between age and BPND in the D3R rich brain regions of the SN/VTA and hypothalamus, whole-brain voxel-wise analysis identified a positive relationship in midbrain regions, with no significant results in the hypothalamus. This differential finding in the SN/VTA was somewhat surprising, given the consistency in ROI and voxel-based results in other regions. One possible factor in these results could be the higher variability in BPND, differentially affecting this small ROI. This influence could be partially mitigated by the whole-brain analysis, as identified regions in the midbrain extended beyond the SN/VTA ROI, bringing significance (albeit non-corrected) to where ROI analysis indicated a non-significant increase (Table 1 and Figure 1C). The whole-brain analysis results also differ from the previous study with [11C](+)PHNO (Nakajima et al., 2015) where no correlations were seen with age in the SN/VTA. BPND means and volumes were similar in that study to our ROI values, suggesting that SN/VTA placement was comparable. In addition, Other groups have found that [11C](+)PHNO studies may not meet tracer conditions (Lee et al., 2013; Rabiner and Laruelle, 2010), particularly at D3R sites, possibly obscuring relationships in the SN/VTA due to its higher binding affinity to the tracer. In fact, we had a total of six subjects that had a physiological response to [11C](+)PHNO with mild-to-moderate nausea. Removing those subjects from the analysis had no significant effect upon our primary results in the caudate (R= −0.512, p value 0.001, N=36) or putamen (R=−0.391, p value 0.018, N=36), but in the SN/VTA, correlations became significant (R=0.336, p value 0.045, N=36).
Evidence from preclinical work in the SN/VTA highlights that D2R and D3R might be differentially regulated with aging. In contrast to the well documented D2R age-related decreases in the striatum (Kaasinen et al., 2000; Rinne et al., 1993; Seeman et al., 1987), aged rats have shown no alteration of D3R mRNA in the SN and D3R binding has been found to be upregulated in the striatum when compared to younger rats (Valerio et al., 1994; Wallace and Booze, 1996). Thus, the current work provides some evidence that a similar process might be present in humans. Another possible rationale focuses on endogenous dopamine, which has a higher affinity for D3R (compared to D2R) (Narendran et al., 2006; Shotbolt et al., 2012). This preference, along with endogenous dopamine possibly decreasing with age (Nakano and Mizuno, 1996), has led to the suggestion that D3R availability may be disproportionately affected with aging (Nakajima et al., 2015). This could create a scenario where normal aging is decreasing both receptor density and endogenous dopamine. This would work in opposing directions on receptor availability (BPND), essentially creating no net differences (or even increases) in BPND in D3R rich sites such as the SN/VTA. Available evidence for this is lacking however, as the only published study with [11C](+)PHNO (Caravaggio et al., 2014) using a dopamine depletion paradigm to determine the influence of endogenous dopamine found no significant change in BPND in the SN, suggesting extrasynaptic dopamine levels may not have an influential role with this tracer (please see the (Caravaggio et al., 2014) supplementary text for a full discussion of intra vs. extrasynaptic dopamine detection with [11C](+)PHNO).
Clinical and functional implications of these findings are beyond the scope of this study and largely speculative. However, wide-spread motor deficits are involved in aging and have been linked to the dopamine system, including striatal D2/3R (Seidler et al., 2010). Cognitive difficulties in older adults can also show decreases in reward-based acquisition of behavior, flexible adaptation and punishment sensitivity (Eppinger et al., 2011), and reward system differences have recently been found in aging and midbrain dopamine function and the dorsolateral prefrontal cortex (Dreher et al., 2008). Thus, both motor and cognitive age-related decreases could be at least partially mediated by D2R specific decreases in the striatum and SN/VTA (the latter not detectible in the current study because of [11C](+)PHNO’s D3R preference in that region).
One of the current study’s advantage over previous work (Nakajima et al., 2015) was the 120 minute acquisition time, which provides lower variance quantification in regions with slow kinetics, such as D3R-rich regions. (Girgis et al., 2011) Limitations of the current work include a gender imbalance (79% male) and a relatively narrow age distribution (36 years with a maximum age of 55) for an age-specific study. Further studies could expand upon these findings and include a more balanced distribution of gender, a wider age range of older adults and potentially investigate clinical measures. In addition, this study did not correct for partial-volume effects (PVE). While PVE could affect the current results, the lack of a ROI volume-age correlation argues against any significant PVE effects. In addition, all scans were performed with high-resolution scanning (HRRT) and others have found minimal PVE in subcortical areas (Alakurtti et al., 2013; Kim et al., 2011; Leroy et al., 2007; Varrone et al., 2009) or that PVE corrections were not necessary when aging in a healthy population is examined with similar methods (Uchida et al., 2011). It should also be mentioned that extrastriatal areas (including D3R specific areas) have poorer test-retest variation which could contribute to our lack of findings in those regions (Gallezot et al., 2014; Ginovart et al., 2007; Lee et al., 2013; Willeit et al., 2008). In addition, BPND was calculated with the cerebellum as a reference region. There is some evidence of [11C](+)PHNO specific binding in this region (Searle et al., 2010; Searle et al., 2013; Tziortzi et al., 2011) and this could potentially lead to underestimation of D3R specific binding (Salinas et al., 2015; Searle et al., 2013) as well as confound results if there are age-dependent changes in cerebellum. It cannot be ruled out that this may have affected our D3R and age results. Lastly, BPND measures available receptors and as such does not take into account receptors currently occupied by endogenous dopamine or the injected mass from the tracer. However, removing those subjects with nausea or controlling for injection parameters (supplementary section) had no significant effect upon our primary results. It is not currently clear, though, if endogenous dopamine would differentially affect our results through age-dependent changes in dopamine. For example, PET studies of [18F]FDOPA have not consistently shown declines in the capacity for dopamine synthesis with aging (Kumakura et al., 2010). Further studies (such as dopamine depletion) are necessary to fully understand any possible influence of endogenous dopamine in age-related effects.
5. Conclusion
Age-related declines of the striatum (an area rich in D2R), but not in the SN/VTA and hypothalamus (areas rich in D3R) were found with the agonist radiotracer [11C](+)PHNO. This indicates that there may be differential effects of aging, whereby D2R binding decreases with age while D3R binding remains relatively unchanged or increased.
Supplementary Material
Research Support and Acknowledgements
We would like to thank the staff of the Yale PET Center, Clinical Neuroscience Research Unit (CNRU) at the Connecticut Mental Health Center (CMHC) of the Connecticut Department of Mental Health and Addiction Services (DMHAS), the Hospital Research Unit (HRU) at Yale-New Haven Hospital (YNHH) and the Yale Magnetic Resonance Research Center (MRRC). This work was supported by the National Institute on Drug Abuse (NIDA) (K24 DA017899; 1R03DA027456-01; RTM; K12DA00167; JH; P20 DA027844; RTM, MNP, REC), the National Institute of Mental Health (NIMH; T32 MH019961; DM/RTM), NARSAD Young Investigator Award Grant (M132018; DM), Yale PET Center and Yale Center for Clinical Investigation (YCCI) Pilot Projects Utilizing Core Technologies. This work was also made possible by CTSA Grant Number UL1 TR000142 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alakurtti K, Johansson JJ, Tuokkola T, Nagren K, Rinne JO. Rostrocaudal gradients of dopamine D2/3 receptor binding in striatal subregions measured with [(11)C]raclopride and high-resolution positron emission tomography. Neuroimage. 2013;82:252–259. doi: 10.1016/j.neuroimage.2013.05.091. [DOI] [PubMed] [Google Scholar]
- Antonini A, Leenders KL, Reist H, Thomann R, Beer HF, Locher J. Effect of age on D2 dopamine receptors in normal human brain measured by positron emission tomography and 11C-raclopride. Arch Neurol. 1993;50:474–480. doi: 10.1001/archneur.1993.00540050026010. [DOI] [PubMed] [Google Scholar]
- Boileau I, Guttman M, Rusjan P, Adams JR, Houle S, Tong J, Hornykiewicz O, Furukawa Y, Wilson AA, Kapur S, Kish SJ. Decreased binding of the D3 dopamine receptor-preferring ligand [11C]-(+)-PHNO in drug-naive Parkinson's disease. Brain. 2009;132:1366–1375. doi: 10.1093/brain/awn337. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, Wilkins D, Selby P, George TP, Zack M, Furukawa Y, McCluskey T, Wilson AA, Kish SJ. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Hopf FW. The dopamine D2 receptor: new surprises from an old friend. Neuron. 2005;47:335–338. doi: 10.1016/j.neuron.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Caravaggio F, Nakajima S, Borlido C, Remington G, Gerretsen P, Wilson A, Houle S, Menon M, Mamo D, Graff-Guerrero A. Estimating endogenous dopamine levels at D2 and D3 receptors in humans using the agonist radiotracer [(11)C]-(+)-PHNO. Neuropsychopharmacology. 2014;39:2769–2776. doi: 10.1038/npp.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson R, Barker W, Liow J, Johnson C. Design of a motion-compensation OSEM List-mode Algorithm for Resolution-Recovery Reconstruction of the HRRT Conf Record. IEEE Nuclear Science Symposium and Medical Imaging Conference; Portland, OR. 2003. pp. 3281–3285. [Google Scholar]
- Dreher JC, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proc Natl Acad Sci U S A. 2008;105:15106–15111. doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein SA, Koller JM, Piccirillo M, Kim A, Antenor-Dorsey JA, Videen TO, Snyder AZ, Karimi M, Moerlein SM, Black KJ, Perlmutter JS, Hershey T. Characterization of extrastriatal D2 in vivo specific binding of [(1)(8)F](N-methyl)benperidol using PET. Synapse. 2012;66:770–780. doi: 10.1002/syn.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Hammerer D, Li SC. Neuromodulation of reward-based learning and decision making in human aging. Ann N Y Acad Sci. 2011;1235:1–17. doi: 10.1111/j.1749-6632.2011.06230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erritzoe D, Tziortzi A, Bargiela D, Colasanti A, Searle GE, Gunn RN, Beaver JD, Waldman A, Nutt DJ, Bani M, Merlo-Pich E, Rabiner EA, Lingford-Hughes A. In vivo imaging of cerebral dopamine D3 receptors in alcoholism. Neuropsychopharmacology. 2014;39:1703–1712. doi: 10.1038/npp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Spano P, Missale C. Dimerization of dopamine D1 and D3 receptors in the regulation of striatal function. Current opinion in pharmacology. 2010;10:87–92. doi: 10.1016/j.coph.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T, Slifstein M, Fowles K, Ding YS, Huang Y, Laruelle M, Carson RE, Rabiner EA. Affinity and selectivity of [(1)(1)C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse. 2012;66:489–500. doi: 10.1002/syn.21535. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Zheng MQ, Lim K, Lin SF, Labaree D, Matuskey D, Huang Y, Ding YS, Carson RE, Malison RT. Parametric Imaging and Test-Retest Variability of 11C-(+)-PHNO Binding to D2/D3 Dopamine Receptors in Humans on the High-Resolution Research Tomograph PET Scanner. J Nucl Med. 2014;55:960–966. doi: 10.2967/jnumed.113.132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S, Kapur S, Wilson AA. Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab. 2007;27:857–871. doi: 10.1038/sj.jcbfm.9600411. [DOI] [PubMed] [Google Scholar]
- Girgis RR, Xu X, Miyake N, Easwaramoorthy B, Gunn RN, Rabiner EA, Abi-Dargham A, Slifstein M. In Vivo Binding of Antipsychotics to D3 and D2 Receptors: A PET Study in Baboons with [ 11C]-(+)-PHNO. Neuropsychopharmacology. 2011;36:887–895. doi: 10.1038/npp.2010.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S. Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp. 2008;29:400–410. doi: 10.1002/hbm.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise M, Ballinger JR, Tanaka F, Moscovitch M, St George-Hyslop PH, Raphael D, Freedman M. Age-related changes in D2 receptor binding with iodine-123-iodobenzofuran SPECT. J Nucl Med. 1998;39:1511–1518. [PubMed] [Google Scholar]
- Inoue M, Suhara T, Sudo Y, Okubo Y, Yasuno F, Kishimoto T, Yoshikawa K, Tanada S. Age-related reduction of extrastriatal dopamine D2 receptor measured by PET. Life Sci. 2001;69:1079–1084. doi: 10.1016/s0024-3205(01)01205-x. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Ishii K, Oda K, Kawasaki K, Mizusawa H, Ishiwata K. Regional analysis of age-related decline in dopamine transporters and dopamine D2-like receptors in human striatum. Synapse. 2009;63:282–290. doi: 10.1002/syn.20603. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, Farde L, Rinne J. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Slifstein M, Xu X, Urban N, Thompson JL, Moadel T, Harkavy-Friedman JM, Gil R, Laruelle M, Abi-Dargham A. Striatal and extrastriatal dopamine D2/D3 receptors in schizophrenia evaluated with [18F]fallypride positron emission tomography. Biol Psychiatry. 2010;68:634–641. doi: 10.1016/j.biopsych.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Son YD, Kim HK, Lee SY, Cho SE, Kim YB, Cho ZH. Effects of age on dopamine D2 receptor availability in striatal subdivisions: a high-resolution positron emission tomography study. Eur Neuropsychopharmacol. 2011;21:885–891. doi: 10.1016/j.euroneuro.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Kumakura Y, Vernaleken I, Buchholz HG, Borghammer P, Danielsen E, Grunder G, Heinz A, Bartenstein P, Cumming P. Age-dependent decline of steady state dopamine storage capacity of human brain: an FDOPA PET study. Neurobiol Aging. 2010;31:447–463. doi: 10.1016/j.neurobiolaging.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wilson AA, Graff A, Boileau I, Di Ciano P. Recent methods for measuring dopamine D3 receptor occupancy in vivo: importance for drug development. Front Pharmacol. 2014;5:161. doi: 10.3389/fphar.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DE, Gallezot J-D, Zheng M-Q, Lim K, Ding Y-S, Huang Y, Carson RE, Morris ED, Cosgrove KP. Test-Retest Reproducibility of [11C]-(+)-PHNO PET Using the Bolus Plus Constant Infusion Paradigm. Molecular imaging. 2013;12:77. [PMC free article] [PubMed] [Google Scholar]
- Leroy C, Comtat C, Trebossen R, Syrota A, Martinot JL, Ribeiro MJ. Assessment of 11C-PE2I binding to the neuronal dopamine transporter in humans with the high-spatial-resolution PET scanner HRRT. J Nucl Med. 2007;48:538–546. doi: 10.2967/jnumed.106.037283. [DOI] [PubMed] [Google Scholar]
- Matuskey D, Bhagwagar Z, Planeta B, Pittman B, Gallezot JD, Chen J, Wanyiri J, Najafzadeh S, Ropchan J, Geha P, Huang Y, Potenza MN, Neumeister A, Carson RE, Malison RT. Reductions in brain 5-HT1B receptor availability in primarily cocaine-dependent humans. Biol Psychiatry. 2014a;76:816–822. doi: 10.1016/j.biopsych.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskey D, Gaiser EC, Gallezot J-D, Angarita GA, Pittman B, Nabulsi N, Ropchan J, MaCleod P, Cosgrove KP, Ding Y-S. A preliminary study of dopamine D 2/3 receptor availability and social status in healthy and cocaine dependent humans imaged with [11 C](+) PHNO. Drug and alcohol dependence. 2015;154:167–173. doi: 10.1016/j.drugalcdep.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskey D, Gallezot JD, Pittman B, Williams W, Wanyiri J, Gaiser E, Lee DE, Hannestad J, Lim K, Zheng MQ, Lin SF, Labaree D, Potenza MN, Carson RE, Malison RT, Ding YS. Dopamine D(3) receptor alterations in cocaine-dependent humans imaged with [(1)(1)C](+)PHNO. Drug Alcohol Depend. 2014b;139:100–105. doi: 10.1016/j.drugalcdep.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskey D, Pittman B, Planeta-Wilson B, Walderhaug E, Henry S, Gallezot JD, Nabulsi N, Ding YS, Bhagwagar Z, Malison R, Carson RE, Neumeister A. Age effects on serotonin receptor 1B as assessed by PET. J Nucl Med. 2012;53:1411–1414. doi: 10.2967/jnumed.112.103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Suzuki JS. Aging and extrapyramidal function. Arch Neurol. 1977;34:33–35. doi: 10.1001/archneur.1977.00500130053010. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Agid O, Borlido C, Suridjan I, Rusjan P, Houle S, Remington G, Wilson AA, Kapur S. Effects of antipsychotics on D3 receptors: a clinical PET study in first episode antipsychotic naive patients with schizophrenia using [11C]-(+)-PHNO. Schizophr Res. 2011;131:63–68. doi: 10.1016/j.schres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, Mantil J. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Caravaggio F, Boileau I, Chung J, Plitman E, Gerretsen P, Wilson A, Houle S, Mamo D, Graff-Guerrero A. Lack of age-dependent decrease in dopamine D3 receptor availability: a [(11) C]-(+)-PHNO and [(11) C]-raclopride positron emission tomography study. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35:1812–1818. doi: 10.1038/jcbfm.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Mizuno T. Age-related changes in the metabolism of neurotransmitters in rat striatum: a microdialysis study. Mechanisms of ageing and development. 1996;86:95–104. doi: 10.1016/0047-6374(95)01680-5. [DOI] [PubMed] [Google Scholar]
- Narendran R, Martinez D, Mason NS, Lopresti BJ, Himes ML, Chen CM, May MA, Price JC, Mathis CA, Frankle WG. Imaging of dopamine D2/3 agonist binding in cocaine dependence: a [11C]NPA positron emission tomography study. Synapse. 2011;65:1344–1349. doi: 10.1002/syn.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, Reeder S, Rabiner E, Laruelle M. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- Payer DE, Behzadi A, Kish SJ, Houle S, Wilson AA, Rusjan PM, Tong J, Selby P, George TP, McCluskey T, Boileau I. Heightened D3 Dopamine Receptor Levels in Cocaine Dependence and Contributions to the Addiction Behavioral Phenotype: A Positron Emission Tomography Study with [(11)C]-(+)-PHNO. Neuropsychopharmacology. 2014;39:321–328. doi: 10.1038/npp.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiner EA, Laruelle M. Imaging the D3 receptor in humans in vivo using [11C](+)-PHNO positron emission tomography (PET) Int J Neuropsychopharmacol. 2010;13:289–290. doi: 10.1017/S1461145710000088. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Hietala J, Ruotsalainen U, Sako E, Laihinen A, Nagren K, Lehikoinen P, Oikonen V, Syvalahti E. Decrease in human striatal dopamine D2 receptor density with age: a PET study with [11C]raclopride. J Cereb Blood Flow Metab. 1993;13:310–314. doi: 10.1038/jcbfm.1993.39. [DOI] [PubMed] [Google Scholar]
- Salinas CA, Searle GE, Gunn RN. The simplified reference tissue model: model assumption violations and their impact on binding potential. JOURNAL OF CEREBRAL BLOOD FLOW AND METABOLISM. 2015;35:304–311. doi: 10.1038/jcbfm.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, Mugnaini M, Griffante C, Wilson AA, Merlo-Pich E, Houle S, Gunn R, Rabiner EA, Laruelle M. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol Psychiatry. 2010;68:392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Searle GE, Beaver JD, Tziortzi A, Comley RA, Bani M, Ghibellini G, Merlo-Pich E, Rabiner EA, Laruelle M, Gunn RN. Mathematical modelling of [11 C]-(+)-PHNO human competition studies. Neuroimage. 2013;68:119–132. doi: 10.1016/j.neuroimage.2012.11.033. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine agonist radioligand binds to both D2High and D2Low receptors, explaining why alterations in D2High are not detected in human brain scans. Synapse. 2012;66:88–93. doi: 10.1002/syn.20987. [DOI] [PubMed] [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, Bird ED, Riederer P, Jellinger K, Watanabe S, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson JA, Marcusson J, Winblad B, Finch CE. Age-correlated loss of dopaminergic binding sites in human basal ganglia. J Neurochem. 1982;39:1623–1631. doi: 10.1111/j.1471-4159.1982.tb07996.x. [DOI] [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, Plisson C, Miller SR, Huiban M, Beaver JD, Gunn RN, Laruelle M, Rabiner EA. Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab. 2012;32:127–136. doi: 10.1038/jcbfm.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinbjerg M, Sibley DR, Javitch JA, Abi-Dargham A. Imaging the high-affinity state of the dopamine D2 receptor in vivo: fact or fiction? Biochem Pharmacol. 2012;83:193–198. doi: 10.1016/j.bcp.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifstein M, Rabiner EA, Gunn RN. Imaging the Dopamine D3 Receptor In Vivo. Imaging of the Human Brain in Health and Disease. 2013:265. [Google Scholar]
- Sokoloff P, Diaz J, Foll BL, Guillin O, Leriche L, Bezard E, Gross C. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uchida H, Chow TW, Mamo DC, Kapur S, Mulsant BH, Houle S, Pollock BG, Graff-Guerrero A. Effects of aging on 5-HT(2A) R binding: a HRRT PET study with and without partial volume corrections. Int J Geriatr Psychiatry. 2011;26:1300–1308. doi: 10.1002/gps.2682. [DOI] [PubMed] [Google Scholar]
- Valerio A, Belloni M, Gorno ML, Tinti C, Memo M, Spano P. Dopamine D2, D3, and D4 receptor mRNA levels in rat brain and pituitary during aging. Neurobiol Aging. 1994;15:713–719. doi: 10.1016/0197-4580(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Varrone A, Sjoholm N, Eriksson L, Gulyas B, Halldin C, Farde L. Advancement in PET quantification using 3D-OP-OSEM point spread function reconstruction with the HRRT. Eur J Nucl Med Mol Imaging. 2009;36:1639–1650. doi: 10.1007/s00259-009-1156-3. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998a;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang GJ, Gur RC, Wong C, Felder C, Gatley SJ, Ding YS, Hitzemann R, Pappas N. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry. 2000;157:75–80. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Ding YS, Gur RC, Gatley J, Logan J, Moberg PJ, Hitzemann R, Smith G, Pappas N. Parallel loss of presynaptic and postsynaptic dopamine markers in normal aging. Ann Neurol. 1998b;44:143–147. doi: 10.1002/ana.410440125. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Booze RM. Dopamine D3 receptor density elevation in aged Fischer-344 × Brown-Norway (F1) rats. Eur J Pharmacol. 1996;308:283–285. doi: 10.1016/0014-2999(96)00354-8. [DOI] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S, Seeman P, Wilson AA, Kapur S. First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: A [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology. 2008;33:279–289. doi: 10.1038/sj.npp.1301400. [DOI] [PubMed] [Google Scholar]
- Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, Houle S, Seeman P, Ginovart N. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem. 2005;48:4153–4160. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]
- Wong DF, Young D, Wilson PD, Meltzer CC, Gjedde A. Quantification of neuroreceptors in the living human brain: III. D2-like dopamine receptors: theory, validation, and changes during normal aging. J Cereb Blood Flow Metab. 1997;17:316–330. doi: 10.1097/00004647-199703000-00009. [DOI] [PubMed] [Google Scholar]
- Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22:1440–1452. doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.