Abstract
Objective
To characterize the rate and pattern of age-related and glaucomatous neuroretinal rim area changes in subjects of African descent (AD) and European descent (ED).
Design
Prospective longitudinal study.
Subjects
296 eyes of 157 healthy subjects (88 AD and 69 ED) and 73 progressing glaucoma eyes of 67 subjects (24 AD and 43 ED) from the Diagnostic Innovations in Glaucoma Study (DIGS) and the African Descent and Glaucoma Evaluation Study (ADAGES) were included.
Methods
Global and sectoral rim area was measured using confocal laser scanning ophthalmoscopy (CSLO). Progression of glaucomatous optic disc damage was determined by masked stereophoto review. The rates of absolute rim area loss and percent rim area loss in healthy and progressing glaucomatous eyes were compared using multivariable nested mixed-effects models.
Main Outcome Measures
Rate of rim area loss over time.
Results
The median (inter-quartile range) follow-up time was 5.0 years (2.0–7.4) for healthy eyes and 8.3 years (7.5–9.9) for progressing glaucoma eyes. The mean rate of global rim area loss was significantly faster in progressing glaucoma eyes compared with healthy eyes for both rim area loss (−10.2 ×10−3 mm2/year vs. −2.8 ×10−3 mm2/year, respectively, P<.001) and percent rim area loss (−1.1 %/year vs. −0.2 %/year, respectively, P<.001), but there was considerable overlap between the two groups. 63% of progressing glaucoma eyes had a rate of change faster than the 5th quantile of healthy eyes. For both healthy and progressing eyes, the pattern of rim area loss and percent rim area loss was similar; it tended to be fastest in the superior temporal and inferior temporal sectors. The rate of change was similar in AD and ED progressing eyes.
Conclusions
Compared with healthy eyes, the mean rate of global rim area loss was 3.7 times faster and the mean rate of global percent rim area loss was 5.4 times faster in progressing glaucoma eyes. A reference database of healthy eyes can be used to help clinicians distinguish age-related rim area loss from rim area loss due to glaucoma.
Keywords: glaucoma, neuroretinal rim area, aging, rate of rim area change
Introduction
Differentiating glaucomatous progression from age-related changes of the optic nerve is one of the most challenging aspects in the management of glaucoma patients and suspects. Age-related loss of retinal ganglion cells (RGCs) has been demonstrated in human non-glaucomatous eyes both by histological methods1–4 and by in vivo measurements of retinal nerve fiber layer (RNFL) thickness and neuroretinal rim area (RA), using digital technologies such as optical coherence tomography (OCT) and confocal scanning laser ophthalmoscopy (CSLO).5–13 In a longitudinal study using CSLO, See et al.14 found higher rates of neuroretinal rim area changes in glaucoma patients compared to healthy subjects, but with a remarkably similar pattern of fastest rim area loss in the inferior region. Other studies do not support this finding of age-related structural change.15–18
Epidemiological studies have shown not only that the prevalence of primary open angle glaucoma (POAG) increases exponentially with age,19–22 but that populations of African descent (AD) have a higher prevalence of POAG than those or European descent (ED), and also higher rates of blindness.23–25 In addition, studies have demonstrated racial variations in optic nerve head topography, measured by CSLO, with larger optic discs,26 optic cups, neuroretinal rims, and cup-disc ratios reported in individuals of AD.27,28
Advances in optical imaging instruments have brought a significant improvement in the ability in clinical practice to visualize and measure the optic disc in vivo, thereby improving measurements of changes that occur over time, either as part of a pathological process or as part of normal aging.29–31 Estimating the differences between age-related and glaucomatous changes in the optic disc rim area and the rate at which they occur, taking into account the racial variations, may help clinicians differentiate normal aging from glaucoma progression.
The objective of this study was to characterize and compare the rate and pattern of age-related and glaucomatous neuroretinal rim area changes in subjects of African Descent (AD) and European Descent (ED).
Methods
Participants
Participants were enrolled in the longitudinal Diagnostic Innovations in Glaucoma Study (DIGS) and/or the African Descent and Glaucoma Evaluation Study (ADAGES).28,32,33 As described previously,32,33 DIGS is conducted at the Hamilton Glaucoma Center at the University of California, San Diego (UCSD), whereas ADAGES is a multicenter study conducted at UCSD, the University of Alabama at Birmingham (UAB), and the New York Eye and Ear Infirmary (NYEE). Protocols were developed to ensure that testing procedures were comparable at all sites. These studies were undertaken to develop improved methods to detect and measure glaucoma, characterize relationships between structural and functional changes, and determine whether there are race-related differences in the rates of structural and functional change. Enrollment of participants is based on the inclusion/exclusion criteria specified below. Informed consent was obtained from each participant and the institutional review boards at all 3 sites approved all methodology. All methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects and to the Health Insurance Portability and Accountability Act. DIGS and ADAGES were registered at http://cilincaltrials.gov (NCT00221897 and NCT00221923, respectively) on September 14, 2005.
Eligible participants had best corrected visual acuity of 20/40 or better, spherical refraction within ±5.0 diopter (D), cylinder correction within ±3.0 D, and open angles on gonioscopy. All participants were older than 18 years. Participants were excluded if they had a history of intraocular surgery (except for uncomplicated cataract or glaucoma surgery). Eyes with coexisting retinal disease, uveitis, or non-glaucomatous optic neuropathy were excluded from the investigation. Diabetic participants with no evidence of retinal involvement were included. Self-reported information regarding systemic conditions, medications, and risk factors associated with glaucoma was recorded. Each participant underwent a comprehensive ophthalmologic examination including review of medical and family history, best-corrected visual acuity testing, central corneal thickness (CCT) measurement, slit lamp biomicroscopy including gonioscopy, intraocular pressure (IOP) measurement using Goldmann applanation tonometry, and dilated funduscopic examination. Stereoscopic optic disc photography (Kowa WX3D, Kowa Optimed, Inc, Torrance, CA or Nidek 3Dx, Nidek Inc, Fremont, CA) and standard automated perimetry with the 24-2 Swedish Interactive Threshold Algorithm (SAP-SITA, Humphrey Field Analyzer, Carl Zeiss Meditec, Dublin, CA) were obtained. The quality of visual fields was reviewed by the Visual Field Assessment Center (VisFACT) staff according to a standard protocol.34,35 Axial length was acquired with IOLMaster (Carl Zeiss Meditec, Dublin, CA). Healthy eyes were defined as having a healthy appearance on stereoscopic photographs, IOP of less than 22 mmHg, no history of elevated IOP, and at least two reliable normal visual fields, defined as a pattern standard deviation (PSD) within 95% confidence limits and a glaucoma hemifield test (GHT) result within normal limits. Progressing glaucoma eyes were defined as eyes having glaucomatous appearing optic discs (neuroretinal rim thinning, excavation or RNFL defect) and repeatable visual field damage (PSD outside 95% confidence limits or GHT outside normal limits) at baseline, with evidence of progression during follow-up based on stereophoto review by two masked graders. Discrepancies between graders were resolved by consensus or adjudication by a third experienced grader. In addition to the regular ADAGES follow-up visits described above, a cohort of stable glaucoma eyes at UCSD underwent IOP measurements, SAP-SITA, SD-OCT and CSLO testing once a week for 5 consecutive weeks. Our assumption is that detectable progression is unlikely to occur during this short period of time. Stable glaucoma eyes were defined as eyes having glaucomatous appearing optic discs (neuroretinal rim thinning, excavation or RNFL defect) and repeatable visual field damage (PSD outside 95% confidence limits or GHT outside normal limits) at baseline, with no evidence of progression by visual field or stereo photographs within 3 years prior to stable glaucoma testing.
Instrumentation
Measurements of rim area were obtained using CSLO with the Heidelberg Retina Tomograph (HRT II, Heidelberg Engineering, Heidelberg, Germany, software version 3.0). Rim area measurements were calculated using the “standard” reference plane automatically set at 50 µm posterior to the mean height contour along a small temporal section of the contour line outlining the disc margin.
The HRT device uses a diode laser and confocal imaging to produce a 3-dimensional topographic image of the optic nerve. The principles and operation of the HRT have been described in detail in previous publications.36–39 For each participating eye, 3 images were obtained, combined and automatically aligned to obtain a single mean topography that was used for the analysis. Image quality was evaluated by an experienced examiner from the UCSD Imaging and Data Evaluation and Assessment (IDEA) Reading Center that outlined the disc margin on the mean topographic image with the aid of stereoscopic photographs of the optic disc. To be included in the analysis images were designated as good quality, defined as a focused reflectance image with a standard deviation not greater than 50 µm. The parameter “rim area” was used in this analysis to evaluate change over time, because it is clinically relevant and has good reproducibility for assessment of longitudinal changes with CSLO.40
Statistical Analysis
Descriptive statistics were used to compare demographic characteristics by group (healthy and glaucoma subjects) and race (AD and ED). Fisher’s exact test was used to compare categorical variables and Wilcoxon rank-sum test was used to compare continuous variables.
Mixed effects models were used to calculate the rates of change (slopes) for rim area loss and percent rim area loss (calculated as percentage of baseline rim area) in univariate and multivariate models. These univariate, or single-covariate models, included time, group (healthy vs. progressing glaucoma) and an interaction term (time x group). Subsequently, we built 7 multivariate mixed-effects model that included the following covariates: baseline age, race, CCT, disc area, baseline visual field MD, IOP and standard deviation of the HRT topography image as time dependent covariates for healthy and progressing eyes separately. These covariates were chosen for analysis based on their importance in a previous publication41 and their statistical significance in the univariate models. In the multivariate models, appropriate 2-way interactions (e.g., covariate x time) were studied to evaluate change over time. Based on past ADAGES results in glaucoma suspects,42 an interaction term was included to account for the possibility of a non-linear relationship between IOP and rate of rim area change (IOP x IOP). To evaluate the possibility that racial differences in the rate of rim area loss varied by disease status, a 3-way interaction term (race x group x time) was also included in the multivariable model.
Mixed effects models with random intercepts and random slopes have been previously used in this setting to adjust for within-patient correlation in measurements between eyes from the same participant and to account for the repeated measurements over time.43–46 A P value less than 0.05 was considered statistically significant. Statistical analyses were performed using SAS software version 9.3 (SAS Institute, Cary, North Carolina, USA).
Results
Two-hundred and ninety-six eyes of 157 healthy subjects and 73 eyes of 67 progressing glaucoma patients were included in this study. In the healthy group, 88 (56%) subjects (169 eyes) were of AD and 69 (44%) subjects (127 eyes) of ED; in the progressing group 24 (36%) subjects (25 eyes) were of AD and 43 (64%) subjects (48 eyes) of ED. Demographic and baseline ocular characteristics are presented in Table 1. Progressing glaucoma subjects were older than healthy subjects (59.5 years [range, 25.9 to 80.6 years] and 48.9 years [range, 19.6 to 85.4 years], respectively, P<.001), had higher baseline IOP (17.9 ± 7.0 mmHg and 15.3 ± 2.7 mmHg, respectively, P=0.004), worse mean baseline SAP-SITA MD (−3.85 ± 4.7 dB and −0.38 ± 1.2 dB, respectively, P<.001), longer follow-up (median 8.3 years [inter quartile range (IQR), 7.5 to 9.9 years] and 5.0 years [IQR, 2.0 to 7.4 years], respectively, P<.001) and a larger number of HRT visits (median 11 [IQR, 9 to 12] and 4 [IQR 3 to 6], respectively, P<.001). Progressing eyes also had a significantly faster rate of MD and VFI change than the healthy eyes (P< 0.001) (Table 1). No statistically significant differences were found between healthy and progressing glaucoma eyes with regard to CCT or disc area.
Table 1.
Baseline demographics and ocular characteristics by group and race*
| Healthy | Progressing glaucoma | P** value |
Stable Glaucoma |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| By Subject | African Descent n=88 (53%) |
European Descent n=69 (47%) |
Total n=157 |
P | African Descent n=24 (35%) |
European Descent n=43 (65%) |
Total n=67 | P | 0.008 | n = 35 |
| Mean Age at baseline (years) |
48.4 ± 13.3 | 49.5 ± 15.3 | 48.9 ± 14.2 | 0.474 | 54.5 ± 13.2 | 62.3 ± 10.4 | 59.5 ± 12.0 | 0.014 | <.001 | 71.8 ± 9.2 |
| Gender, Female (%) | 53 (60%) | 43 (62%) | 96 (61%) | 0.869 | 15 (63%) | 22 (51%) | 37 (55%) | 0.447 | 0.458 | 14 (40%) |
| By Eye |
n=169 (57%) |
n=127 (43%) |
n=296 |
n=25 (34%) |
n=48 (66%) |
n=73 | 0.002 | n=63 | ||
| Baseline IOP (mmHg) | 15.1 ±2.7 | 15.5±2.7 | 15.3 ± 2.7 | 0.172 | 16.7 ± 4.3 | 18.5 ± 8.0 | 17.9 ± 7.0 | 0.510 | 0.004 | NA |
| Mean IOP during follow- up (mmHg) |
15.2 ± 2.5 | 15.3 ±2.3 | 15.2 ± 2.4 | 0.825 | 15.8 ±3.7 | 15.2 ± 4.2 | 15.5 ± 3.8 | 0.601 | 0.738 | 14.2 ± 4.6 |
| CCT (µm) | 530 ± 33 | 555 ± 37 | 541 ± 37 | <.001 | 525 ± 45 | 544 ±42 | 538 ± 44 | 0.101 | 0.819 | 550 ± 41 |
| Disc area (mm2) | 2.09 ± 0.5 | 1.9 ± 0.4 | 2.01 ± 0.4 | <.001 | 2.19 ± 0.4 | 1.97 ±0.4 | 2.04 ± 0.4 | 0.039 | 0.480 | 2.05 ± 0.5 |
| Baseline MD (dB) | −0.59 ±1.2 | −0.11 ± 1.0 | −0.38 ± 1.2 | <.001 | −4.49 ± 5.3 | −3.5 ± 4.4 | −3.85 ± 4.7 | 0.736 | <.001 | −7.3 ± 8.6 |
| Median No. of HRT visits, (Interquartile range) |
4 (3–7) |
4 (3–5) |
4 (3–6) |
0.006 | 11 (9–12) |
10.5 (9–12) |
11 (9–12) |
0.898 | <.001 | 5 (5–5) |
| Median Follow up (years) (Interquartile range) |
5.5 (3.1–7.3) |
4.9 (3.0–7.4) |
5.0 (2.0–7.4) |
0.470 | 7.8 (7–8.1) |
9.1 (7.8–11.4) |
8.3 (7.5–9.9) |
<.001 | <.001 | 4 weeks (4–4) |
| Rate VFI of change over time (%/year) |
−0.02±0.02 P=0.334 |
−0.001±0.03 P=0.960 |
−0.01±0.02 P=0.445 |
0.321 | −0.61±0.21 P=0.003 |
−0.64±0.13 P<0.001 |
−0.63±0.11 P<0.001 |
0.478 | <.001 | 0.09 ±8.1 P=0.740 |
| Rate of MD change over time (dB/year) |
0.03±0.02 P=0.614 |
0.04±0.01 P=0.400 |
−0.04±0.02 P=0.120 |
0.512 | −0.17±0.08 P=0.042 |
−0.22±0.05 P<0.001 |
−0.20±0.04 P<0.001 |
0.036 | <.001 | −0.03±3.3 P=0.960 |
Data are presented as mean±SD unless otherwise indicated
P-value representing the difference between the healthy and progressing glaucoma groups
Abbreviations: AD, African Descent; ED, European Descent; IOP, Intraocular Pressure; CCT, Central Corneal Thickness; MD, Mean Deviation; HRT, Heidelberg Retinal Tomograph.
In the progressing glaucoma group, AD subjects were younger than ED subjects (54.5±13.2 years and 62.3±10.4 years, respectively, P=0.014), whereas no age difference was found between AD and ED healthy subjects (48.4±13.3 years and 49.5±15.3 years, respectively, P=0.474). In both the healthy group and progressing group, AD eyes had thinner corneas than ED eyes and larger disc areas (Table 1). Healthy eyes of AD had a worse baseline visual field MD compared to ED eyes P<.001). Baseline MD did not vary by race in progressing eyes. Progressing glaucoma eyes of AD had a worse baseline MD compared to ED eyes, but the difference did not reach statistical significance (−4.49±0.53 dB and −3.5±4.4 dB, respectively, P=0.763). No statistically significant differences were found between healthy AD and ED eyes, and progressing glaucoma AD and ED eyes, with regard to baseline IOP and mean IOP during follow-up. Baseline global and sectoral rim area measurements (adjusted for disc area) by group and race are presented in Table 2. Healthy eyes had larger baseline rim area for all sectors when compared to glaucoma eyes. No racial differences were found in rim area in either healthy or progressing glaucoma eyes.
Table 2.
Baseline global and sectoral HRT rim area measurements (adjusted for disc area) by group and race*
| Healthy | Progressing glaucoma | Total | Stable glaucoma |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rim Area (mm2) by Sector |
African Descent n=169 |
European Descent n=127 |
Difference P value |
African Descent n=25 |
European Descent n=48 |
Difference P value |
Healthy n=296 |
Progressing glaucoma n=73 |
Difference P value |
n=63 |
| Global | 1.44±0.016 | 1.44±0.019 | 0.987 | 1.05±0.07 | 1.08±0.05 | 0.684 | 1.44±0.01 | 1.06±0.03 | <.001 | 1.26±0.00 |
| Superior | 0.20±0.003 | 0.20±0.003 | 0.588 | 0.15±0.01 | 0.15±0.01 | 0.842 | 0.20±0.00 | 0.15±0.00 | <.001 | 0.17±0.00 |
| Inferior | 0.20±0.002 | 0.20±0.003 | 0.976 | 0.14±0.01 | 0.14±0.01 | 0.764 | 0.20±0.00 | 0.14±0.00 | <.001 | 0.17±0.00 |
| Nasal | 0.41±0.005 | 0.41±0.006 | 0.582 | 0.32±0.02 | 0.34±0.02 | 0.666 | 0.41±0.00 | 0.32±0.01 | <.001 | 0.38±0.01 |
| Temporal | 0.22±0.005 | 0.23±0.006 | 0.391 | 0.15±0.01 | 0.17±0.01 | 0.231 | 0.23±0.00 | 0.16±0.01 | <.001 | 0.21±0.01 |
Data are presented as mean±SD unless otherwise indicated
Abbreviation: HRT, Heidelberg Retinal Tomograph
The covariates included in the multivariable model for the rate of global rim area loss are presented in Table 3. Baseline age, CCT, IOP as a time dependent covariate, disc area, baseline visual field MD, standard deviation of the topography image as a time dependent covariate, and the interaction term group x time were significantly associated with the rate of rim area loss. The interaction terms race x group, group x time and race x group x time were not statistically significant, indicating no racial difference in rate of rim area change within each group. The interaction term IOP x IOP was not significantly associated with the rate of rim area change in the multivariable model despite showing significance in the univariate model for global and superior sectors.
Table 3.
Results of multivariable model investigating the relationship between predictive factors and the rate of global rim area loss*
| Predictive Factor | Estimate | 95% Confidence Interval | P value |
|---|---|---|---|
| Race | 70.6 | −46.5 to 187.7 | 0.237 |
| Baseline age | 3.1 | −0.9 to 5.2 | 0.005 |
| Central corneal thickness | 1.3 | 0.6 to 2.0 | 0.001 |
| IOP as a time dependent covariate | −-4.3 | −7.6 to −1.0 | 0.011 |
| Disc area | 382.5 | 323.1 to 442 | <.001 |
| Baseline VF mean deviation | 30.4 | 20.1 to 40.6 | <.001 |
| Standard deviation of the topography image as a time dependent covariate |
−0.7 | −1.3 to 0.0 | 0.040 |
| Time | 0.0 | −5.6 to 1.9 | 0.996 |
| IOP X IOP interaction term | 0.1 | −0.0 to 0.1 | 0.169 |
| Race X time interaction term | 2.2 | −5.2 to 9.6 | 0.557 |
| Race X group interaction term | −23.7 | −89.9 to 42.5 | 0.482 |
| Group X time interaction term | −2.8 | −5.3 to −0.3 | 0.029 |
| Race X time X group interaction term | −1.7 | −8.4 to 5.0 | 0.623 |
- Results are presented as X10−3 mm2/year
- Abbreviations: IOP, Intra Ocular Pressure
- Group: Progressing glaucoma vs healthy group
The multivariable rates of global and sectoral rim area and percent rim area loss in healthy and progressing glaucoma eyes are presented in Table 4. Mean rates of rim area loss were significantly faster in progressing glaucoma eyes compared with healthy eyes in all but the temporal sector. Rates of percent rim area loss were significantly faster in progressing glaucoma eyes compared with healthy eyes in all sectors. The mean rate of global rim area loss in progressing glaucoma eyes was 3.7 times faster than healthy eyes (−10.2 ×10−3 mm2/year and −2.8 ×10−3 mm2/year, respectively, P<.001). The mean rate of global percent rim area loss was 5.4 times faster in progressing glaucoma eyes compared with healthy eyes (−1.1 %/year and −0.2 %/year, respectively, P<.001). Specifically, progressing glaucoma eyes had a mean baseline rim area of 1.06 mm2 and the mean percentage decrease from baseline rim area was 1.1 %/year (95% CI, −1.5% to −0.8%/year); in healthy eyes mean baseline rim area was 1.44 mm2 and the mean percentage decrease from baseline rim area was 0.2%/year (95% CI, −0.5% to 0.1%/year). Mean rates of rim area loss tended to be faster in progressing glaucoma eyes of AD compared to ED participants but these differences did not reach statistical significance (global rim area change AD −12.1 (95% CI, −18.3 to −5.8) X10−3 mm2/year, ED −9.1 (−15.7 to −2.5) X10−3 mm2/year, P=0.452; percent rim area change AD −1.2 (−1.9 to −0.6) %/year, ED −1.1 (−1.8 to −0.4)%/year, P=0.697). No racial differences were found in the healthy group (global rim area change AD −2.1 (95% CI, −4.2 to −0.02) X10−3 mm2/year, ED −2.3 (−4.9 to 0.3) X10−3 mm2/year, P=0.918; percent rim area change AD −0.2 (−0.3 to 0.1) %/year, ED −0.2 (−0.4 to 0.03) %/year, P=0.678).
Table 4.
Multivariable Rates of Global and Sectoral Rim Area Loss by group and Race
| Healthy | Progressing glaucoma | TOTAL | Stable glaucoma |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| African Descent (n=169) |
European Descent (n=127) |
Difference | African Descent (n=25) |
European Descent (n=48) |
Difference | Healthy (n=296) |
Progressing glaucoma (n=73) |
Difference | n=63 | ||
|
HRT Sector |
Mean (95% CI) P-value |
Mean (95% CI) P-value |
P value | Mean (95% CI) P-value |
Mean (95% CI) P-value |
P value | Mean (95% CI) P-value |
Mean (95% CI) P-value |
P value | Mean (95% CI) P-value |
|
|
Rim Area change X10−3 (mm2/year) |
Global |
−2.1 (−4.2, −0.02) 0.048 |
−2.3 (−4.9, 0.3) 0.081 |
0.918 |
−12.1 (−18.3, −5.8) <.001 |
−9.1 (−15.7, −2.5) 0.019 |
0.452 | −2.8 (−6.3, 0.7) 0.125 |
−10.2 (−13.9, −6.4) <.001 |
<.001 | −0.25 (−2.7, 4.0) 0.410 |
| Superior | −0.3 (−0.6, 0.1) 0.184 |
−0.6 (−1.1, −0.1) 0.011 |
0.248 |
−1.9 (−2.9, −0.8) <.001 |
−1.5 (−2.6, −0.4) 0.019 |
0.580 |
−0.7 (−1.3, −0.1) 0.024 |
−1.8 (−2.5, −1.2) <.001 |
<.001 | −0.24 (−0.5, 0.4) 0.958 |
|
| Inferior |
−0.5 (−0.7, −0.2) 0.001 |
−0.3 (−0.6, 0.03) 0.076 |
0.501 |
−2.1 (−3.0, −1.2) <.001 |
−1.6 (−2.6, −0.7) 0.010 |
0.392 | −0.3 (−0.8, 0.2) 0.196 |
−1.8 (−2.3, −1.2) <.001 |
<.001 | −0.38 (−0.6, 0.2) 0.170 |
|
| Nasal | −0.06 (−0.7, 0.2) 0.862 |
−0.5 (−1.3, 0.3) 0.200 |
0.379 | −1.5 (−3.3, 0.4) 0.118 |
−1.3 (−3.2, 0.6) 0.138 |
0.882 | −0.4 (−1.5, 0.6) 0.399 |
−1.7 (−2.8, −0.6) 0.002 |
0.024 | −0.41 (−0.7, 0.2) 0.460 |
|
| Temporal |
−0.9 (−1.7, −0.2) 0.041 |
−0.1 (−1.2, 0.9) 0.806 |
0.271 |
−2.8 (−4.8, −0.8) 0.007 |
−1.4 (−3.5, −0.8) 0.149 |
0.261 | −0.3 (−1.5, 0.9) 0.654 |
−1.2 (−2.6, −0.1) 0.076 |
0.168 | −0.43 (−0.8, 0.8) 0.723 |
|
|
% Rim Area Change from baseline (%/year) |
Global | −0.2 (−0.3, 0.1) 0.174 |
−0.2 (−0.4, 0.03) 0.098 |
0.678 |
−1.2 (−1.9, −0.6) <.001 |
−1.1 (−1.8, −0.4) 0.013 |
0.697 | −0.2 (−0.5, 0.1) 0.196 |
−1.1 (−1.5, −0.8) <.001 |
<.001 | −0.08 (−0.2, 0.1) 0.290 |
| Superior | −0.1 (−0.3, 0.2) 0.554 |
−0.3 (−0.6, −0.02) 0.039 |
0.223 |
−1.5 (−2.3, −0.7) <.001 |
−1.2 (−2.0, −0.3) 0.018 |
0.509 | −0.4 (−0.7, 0.0) 0.050 |
−1.3 (−1.8, −0.9) <.001 |
<.001 | 0.03 (−0.2, 0.4) 0.573 |
|
| Inferior |
−0.2 (−0.3, −0.1) 0.009 |
−0.2 (−0.4, 0.0) 0.056 |
0.845 |
−1.7 (−2.5, −0.8) <.001 |
−1.5 (−2.3, −0.6) 0.009 |
0.723 | −0.2 (−0.5, 0.2) 0.268 |
−1.5 (−1.9, −1.1) <.001 |
<.001 | −0.11 (−0.3, 0.1) 0.170 |
|
| Nasal | 0.04 (−0.1, 0.2) 0.612 |
−0.1 (−0.3, 0.1) 0.237 |
0.215 | −0.5 (−1.4, 0.4) 0.245 |
−0.7 (−1.6, 0.2) 0.089 |
0.694 | −0.2 (−0.6, 0.3) 0.441 |
−0.7 (−1.2, −0.2) 0.003 |
0.023 | −0.03 (−0.1, 0.1) 0.183 |
|
| Temporal | −0.2 (−0.7, 0.3) 0.460 |
0.1 (−0.5, 0.7) 0.685 |
0.432 |
−2.0 (−3.3, −0.8) 0.001 |
−1.1 (−2.4, 0.2) 0.077 |
0.213 | −0.01 (−0.7, 0.7) 0.974 |
−1.16 (−1.2, −0.4) 0.005 |
0.004 | −0.1 (−0.2, 0.2) 0.390 |
|
Abbreviations: HRT, Heidelberg Retina Tomograph; CI, Confidence Interval
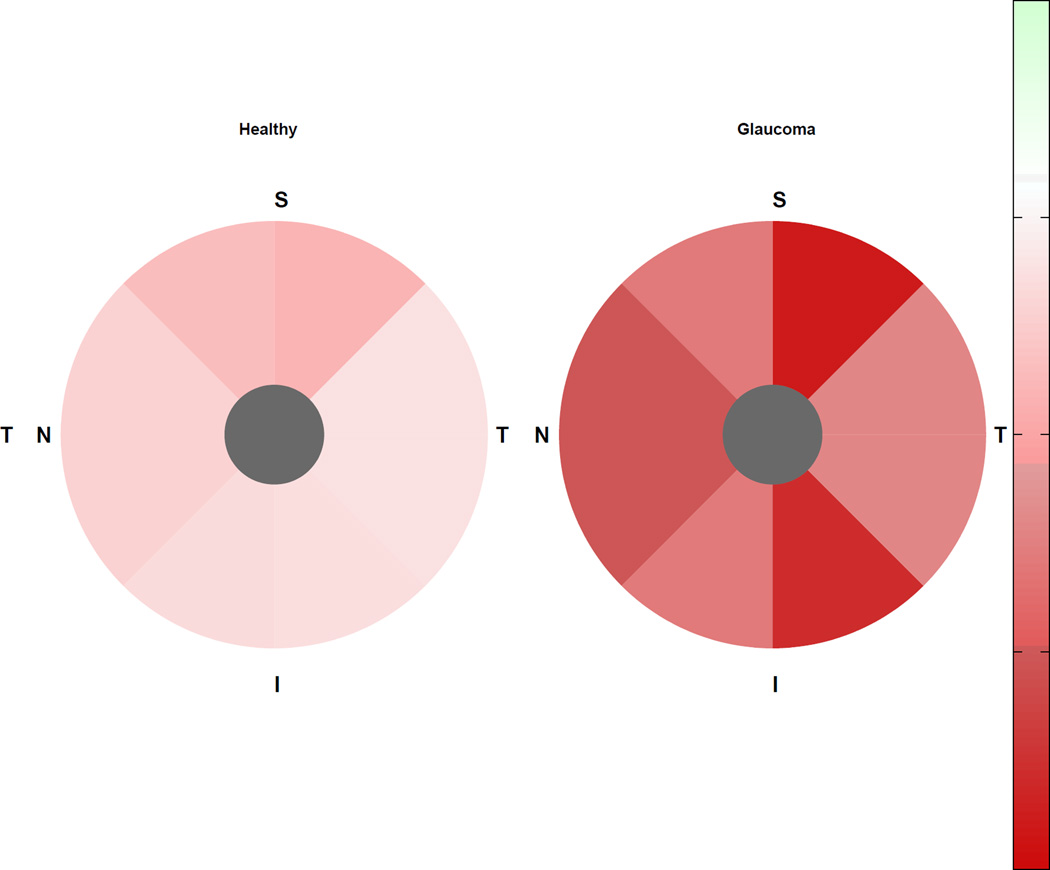
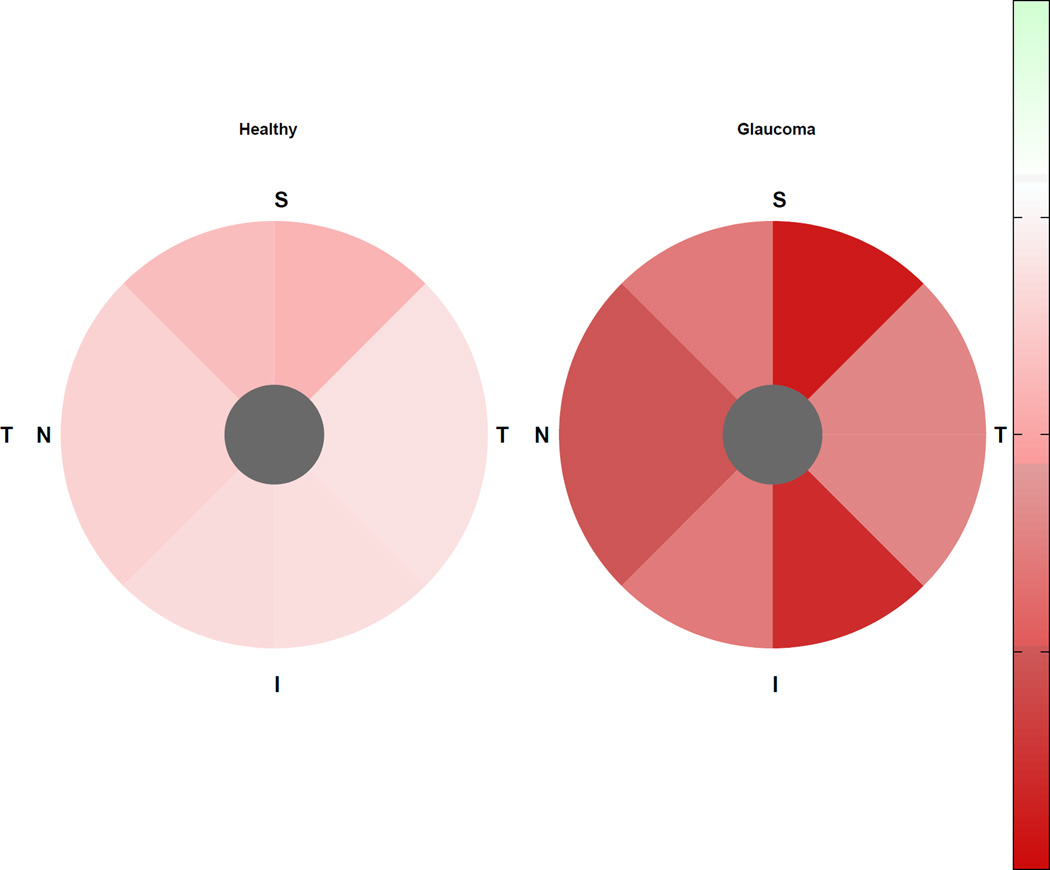
In healthy eyes, the rate of rim area loss was significantly different from zero only in the superior sector; whereas the rate of percent rim area loss was not significantly different from zero globally or in any sector. In progressing glaucoma eyes, rates of rim area loss were significantly different from zero globally and in all but the temporal sector; rates of percent rim area loss were significantly different from zero globally and in all sectors. In stable glaucoma eyes, rates of rim area and percent rim area loss were not significantly different from zero in any sector. The pattern of change was similar in healthy and progressing glaucoma eyes with fastest rates of rim area loss in the superior-temporal sector, and fastest percent rim area loss in the superior and inferior temporal sectors (see Figures 1 and 2).
Figure 1.
Rates of sectoral rim area change (X10−3 mm2/year) in healthy eyes (left) and progressing glaucoma eyes (right). I = inferior; N = nasal; S = superior; T = temporal
Figure 2.
Rates of sectoral percent rim area change (%/year) in healthy eyes (left) and progressing glaucoma eyes (right). I = inferior; N = nasal; S = superior; T = temporal.
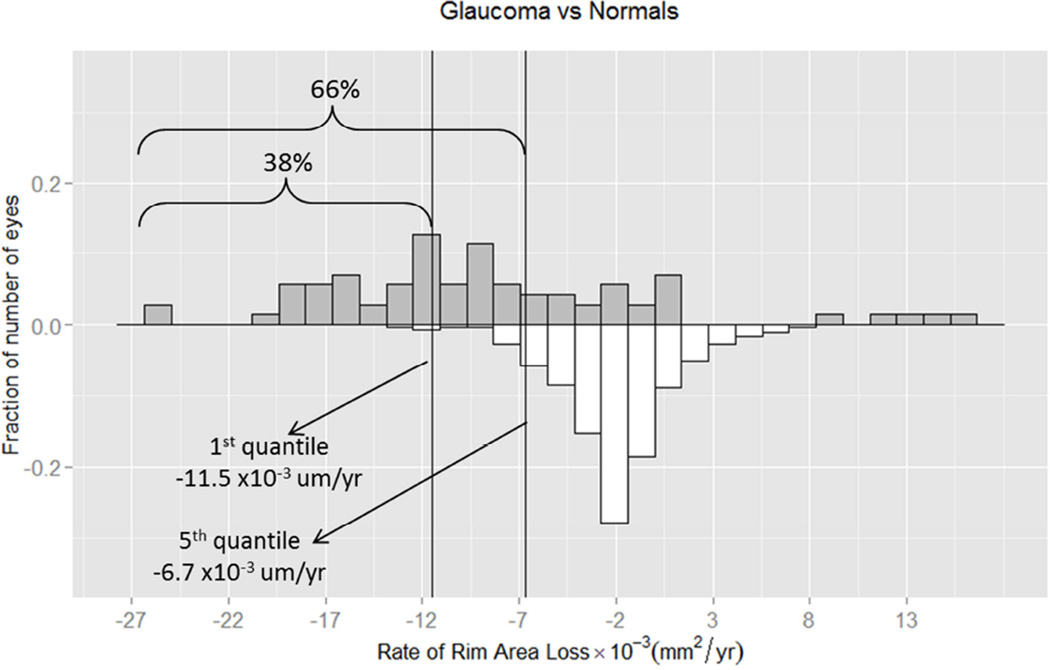
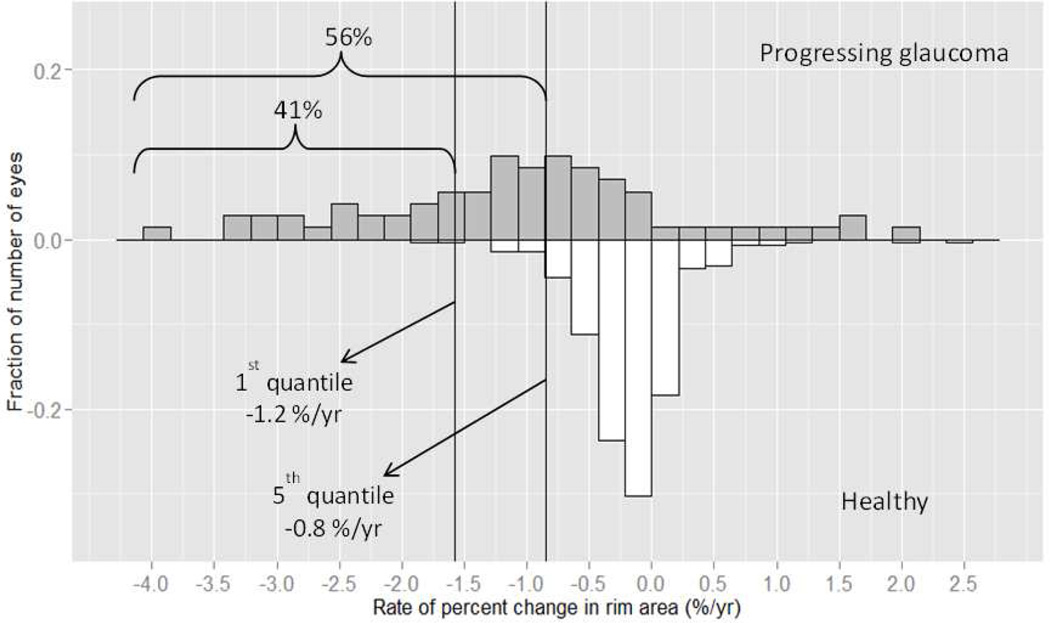
In addition, we created a reference database of rim area loss in the healthy group to calculate the 1st and 5th quantiles of fastest age-related rates of rim area and percent rim area loss. Figures 3 & 4 show the distribution of the rates of rim area and percent rim area change, respectively, for both groups. We found that for rim area loss, 66% of progressing glaucoma eyes had a rate of change that was faster than the 5th quantile and 38% had a rate of change that was faster than the 1st quantile. For percent rim area loss, 56% of progressing glaucoma eyes had a rate of change faster than 5th quantile, and 41% had a rate faster than the 1st quantile.
Figure 3.
Distribution of the rates of rim area change (X10−3 mm2/year) of progressing glaucoma eyes (upper panel) and healthy eyes (lower panel). Left line represents 1st quantile (1% fastest healthy eyes, −11.5 ×10−3 mm2/year) and middle line represents 5th quantile (−6.7 ×10−3 mm2/year) criteria calculated using healthy eyes data. 66% of progressing glaucoma eyes had a rate of change faster than the 5th quantile; 38% of progressing glaucoma eyes had a rate of change faster than the 1st quantile.
Figure 4.
Distribution of the rates of percent rim area change (%/year) of progressing glaucoma eyes (upper panel) and healthy eyes (lower panel). Left line represents 1st quantile (1% fastest healthy eyes, −1.2 %/year) and middle line represents 5th quantile (−0.8 %/year) criteria calculated using healthy eyes data. 56% of progressing glaucoma eyes had a rate of change faster than the 5th quantile; 41% of progressing glaucoma eyes had a rate of change faster than the 1st quantile.
Rates of change of rim area loss were calculated for 63 eyes of 35 stable glaucoma subjects, as a measure of the specificity. The rate of rim area change in the stable eyes was similar to that in healthy eyes; the rate of rim area loss and percent rim area loss in 100% of stable eyes was within the 95th percentile of rim area change in healthy eyes. Specifically, the mean (95%CI) rates of global rim area loss and percent rim area loss were −0.8 ×10−3 mm2/year (−2.7 to 4 ×10−3 mm2/year) and −0.08 %/year (−0.2 to 0.1%/year), respectively.
Discussion
In this study, the estimated rate of neuroretinal rim area loss was 3.7 times faster in progressing glaucoma eyes compared with healthy eyes. When measured as a percentage of baseline rim area, the estimated rate of loss was 5.4 times faster in the glaucomatous eyes. In addition, we found that the rate of rim area loss in healthy eyes was significantly different from zero only in the superior sector while the rate of percent rim area loss was not statistically significant from zero in any sector. We did not find differences in rates of rim area change between AD and ED eyes in either the healthy or the progressing glaucoma group.
Furthermore, we found that despite considerable overlap in the distribution of the rate of rim area change, 66% of progressing glaucoma eyes had a rate of rim area change faster than the fastest 5% of healthy eyes, and 38% had a rate of change faster than the fastest 1% of healthy eyes (Figure 3). When evaluated as percent of baseline rim area these differences were similar (56% of progressing glaucoma eyes faster than the fastest 5% of healthy eyes; 41% faster than the fastest 1% of healthy eyes, Figure 4). These results suggest that a reference database of the rate of rim area change can provide important information for distinguishing between glaucomatous and age-related rim loss. At the same time, although all progressing glaucoma eyes had evidence of progression detected by stereophoto review, 37% had a rate of rim area change measured by HRT that was not faster than 95% of healthy eyes. This may be explained, at least in part, by the fact that progression on stereophotos was defined as either rim thinning or new RNFL defect, while HRT measured rim loss only. Disc size, glaucoma severity, corneal thickness, gender were not associated with whether the rate of rim area change of the progressing glaucoma eyes was within or outside the 5% and 1% limits of healthy eyes (data not shown). For this reason, information on structural change from imaging instruments, even when compared to reference databases, should be evaluated in conjunction with the clinical examination and visual field testing.
Moreover, several studies have reported poor agreement between stereophotos, visual field testing, and optic nerve head imaging modalities (such as HRT) in detection of glaucoma progression.47–52 In our study, there was a wide variation of disease severity among progressing glaucoma eyes (mean baseline MD −3.85±4.7, range −23.3 to 1.44). Several studies suggest that the ability of tests to detect glaucoma progression varies in different stages of the disease. Subtle progression of early glaucoma may remain undetected by stereophotos or visual field, but may be detected by OCT. In contrast, changes in advanced stages may be better detected by perimetry.53–55 Further studies are needed to evaluate differences in the rate of rim area loss at different stages of the disease.
Previous studies have reported rates of rim area loss over time in glaucoma and healthy subjects. Zeyen and Caprioli56 reported a rate of −2.1%/year in eyes with glaucomatous visual field loss and Airkasinen et al57 reported a faster rate of rim area change in progressing glaucoma eyes compared to healthy eyes (−3.47%/year and −0.23%/year, respectively) using computer-assisted planimetry. Leung and associates52 reported a mean rate of rim area change of −1.06 %/year in a cohort of glaucoma patients using HRT3. See and associates14 have also reported that the rate of rim area change is faster in glaucoma eyes than healthy eyes. In their report, the rate of global HRT1 neuroretinal rim area change was more than 4 times faster in glaucoma eyes than healthy eyes when measured in absolute units (median, −5.33 × 10−3 mm2/year and −1.25 × 10−3 mm2/year, respectively, P=.006) and 6 times faster when measured as a percentage from baseline rim area (median, −0.42 %/year and −0.07 %/year, respectively, P<.001). Compared to See et al, our rates of rim area loss and percent rim area loss in both the healthy and progressing glaucoma group are twice as fast (median, −9.6 × 10−3 mm2/year and −2.5 × 10−3 mm2/year, respectively, P<.001; −0.91 %/year and −0.16 %/year, respectively, P<.001), but the ratios of glaucoma to healthy rates of rim area loss are similar (median rim area loss 3.8 times faster, percent rim area loss 5.7 times faster), even though we restricted our glaucoma group to progressing eyes and See et al did not. While all of these studies confirm loss of rim area over time in healthy eyes, they differ in the magnitude of the rate of change. These differences may be explained by the differences in instruments, software version, populations, sample size and length of follow up among the different studies.
In the Ocular Hypertension Treatment Study (OHTS),41 mean rates of rim area loss using the HRT in eyes that developed POAG were approximately 5 times faster compared to eyes that did not (−13.1 ×10−3 mm2/year and −2.6 mm2/year, respectively, P<.001; and −0.89 %/year and −0.17 %/year, respectively, P<.001). A recent report of rim area measurements over time in an ADAGES glaucoma suspects cohort,58 shows similar results among participants who developed visual field loss compared to those who did not (mean global rim area change −11.0 ×10−3 mm2/year and −3.0 mm2/year, respectively, P<.001). The mean rate of rim area loss in ocular hypertensive eyes that developed POAG and among glaucoma suspects, who developed visual field loss, is very close to the mean rate we found in progressing glaucoma eyes (−10.2 ×10−3 mm2/year). Similarly, the rate of rim area loss in OHTS eyes that did not develop POAG and glaucoma suspect eyes that did not develop visual field defect was very close to the rate we found in our healthy eyes group (−2.8 ×10−3 mm2/year).
When examining the pattern of sectoral rim area loss, See et al14 found that the fastest rates of loss for glaucoma eyes was in 2 of the 3 clock-hour sectors in the inferior-temporal quadrant, followed by 2 sectors in the superior-temporal quadrant. In the healthy eyes, the pattern was very similar. Our results are in agreement with See et al,14 in showing a consistent pattern of the fastest rate of change in the superior temporal and inferior temporal sectors.
We did not find racial differences in the rate of rim area loss in the healthy or progressing glaucoma eyes. Progressing AD eyes tended to have a faster rate of change when compared with ED eyes (global rim area measurements −12.1 ×10−3 mm2/year and −9.1 ×10−3 mm2/year, respectively, P=0.452; −1.2 %/year and −1.1 %/year, respectively, P=0.697). Rates of change of healthy AD and ED eyes were very similar (−2.1 ×10−3 mm2/year and −2.3 ×10−3 mm2/year, respectively, P=0.918; −0.2 %/year and −0.2 %/year, respectively, P=0.678). In the OHTS, the mean rate of rim area change over time among participants who developed POAG was significantly faster in African American participants compared to other participants (−18.2 ×10−3 mm2/year and −11.6 ×10−3 mm2/year, respectively, P=0.026). Among those who did not develop POAG the rate of rim area loss was very similar in African American and other participants (−2.4 ×10−3 mm2/year and −2.6 ×10−3 mm2/year, respectively).41 In another recent ADAGES report42 AD suspect eyes that developed visual field loss (average follow-up 7.5±2.0 years), had a significantly faster rate of rim area loss than ED eyes. Racial differences were not as pronounced in the current study, perhaps because both AD and ED eyes in the progressing glaucoma group had documented photography-based optic disc damage at baseline and agreement in the rate of HRT change and photograph-based progression are limited.59
Limitations to this study include the relatively short follow up time, the relatively small number of HRT exams in the healthy group and the limited age range, particularly of the healthy subjects. As clinicians would like to determine if there is glaucoma progression in the shortest time period possible, one can argue that the relatively short follow up time (median 5 years) and relatively small number of HRT exams (median 4) in the healthy eyes are clinically relevant. In addition, we estimated specificity in a group of confirmed stable eyes followed frequently for 2 months. Longer follow-up in a stable group is needed to confirm the specificity estimated in this cohort.
Another limitation to this study is the relatively small number of progressing glaucoma eyes (24 AD and 54 ED eyes). In addition, we used self-reported race in our investigation, which involves geographic, cultural and socio-economic factors, and is not a pure biological measure.60,61 However, as recently reported,62 blood samples from 224 AD and 245 ED ADAGES participants were analyzed for a total of 20–31 ancestry informative markers (AIMs) for analysis of biogeographic ancestry. The median estimated African proportion among AD participants was 92.0%, while the median estimated African proportion among ED participants was 0.54%. Therefore, in the ADAGES cohort, self-reported race is in close agreement with genetically defined biogeographic ancestry.
Finally, given that our study estimated the rate of rim area loss in progressing glaucoma patients with evidence of progression based on optic disc stereophotographs, one can argue that it is not surprising that we found faster rim area loss in these progressing patients compared to healthy subjects. However, the objective of this study was not to show that HRT-based rim area loss is faster in progressing eyes. Rather, the objective was to characterize the rate and pattern of rim area loss in AD and ED healthy eyes and eyes with documented optic nerve head change and to determine whether reference databases aging loss can improve our ability to detect change.
In summary, the overall rate of neuroretinal rim area loss is 3.7 times faster in progressing glaucoma eyes compared with healthy eyes, with 66% of progressing eyes demonstrating a rate of change faster than the fastest 5% of healthy eyes. No differences in the rates of rim area change were found between AD and ED healthy and progressing glaucoma eyes. These results suggest that measuring the rate of neuroretinal rim area loss can play an important role in the clinical management of glaucoma patients and that establishing reference limits for age-related change can improve the clinician’s ability to differentiate disease progression from normal aging.
Acknowledgments
Funding: P30EY022589 (the core grant); EY11008, U10EY14267, EY019869 (LMZ); EY021818 (FAM); EY022039 (CB); Eyesight Foundation of Alabama; Alcon Laboratories Inc.; Heidelberg Engineering GmbH; Allergan Inc.; Pfizer Inc.; Merck Inc.; Santen Inc.; and the Edith C. Blum Research Fund of the New York Glaucoma Research Institute, New York, NY; Unrestricted grant from Research to Prevent Blindness, New York, NY
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dolman CL, McCormick AQ, Drance SM. Aging of the optic nerve. Archives of ophthalmology. 1980 Nov;98(11):2053–2058. doi: 10.1001/archopht.1980.01020040905024. [DOI] [PubMed] [Google Scholar]
- 2.Jonas JB, Schmidt AM, Muller-Bergh JA, Schlotzer-Schrehardt UM, Naumann GO. Human optic nerve fiber count and optic disc size. Investigative ophthalmology & visual science. 1992 May;33(6):2012–2018. [PubMed] [Google Scholar]
- 3.Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Investigative ophthalmology & visual science. 1992 Jan;33(1):1–17. [PubMed] [Google Scholar]
- 4.Harman A, Abrahams B, Moore S, Hoskins R. Neuronal density in the human retinal ganglion cell layer from 16–77 years. The Anatomical record. 2000 Oct 1;260(2):124–131. doi: 10.1002/1097-0185(20001001)260:2<124::AID-AR20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.Kanamori A, Escano MF, Eno A, et al. Evaluation of the effect of aging on retinal nerve fiber layer thickness measured by optical coherence tomography. Ophthalmologica. Journal international d’ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2003 Jul-Aug;217(4):273–278. doi: 10.1159/000070634. [DOI] [PubMed] [Google Scholar]
- 6.Toprak AB, Yilmaz OF. Relation of optic disc topography and age to thickness of retinal nerve fibre layer as measured using scanning laser polarimetry, in normal subjects. The British journal of ophthalmology. 2000 May;84(5):473–478. doi: 10.1136/bjo.84.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007 Jun;114(6):1046–1052. doi: 10.1016/j.ophtha.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh RS, Parikh SR, Sekhar GC, Prabakaran S, Babu JG, Thomas R. Normal age-related decay of retinal nerve fiber layer thickness. Ophthalmology. 2007 May;114(5):921–926. doi: 10.1016/j.ophtha.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Sung KR, Wollstein G, Bilonick RA, et al. Effects of age on optical coherence tomography measurements of healthy retinal nerve fiber layer, macula, and optic nerve head. Ophthalmology. 2009 Jun;116(6):1119–1124. doi: 10.1016/j.ophtha.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girkin CA, McGwin G, Jr, Sinai MJ, et al. Variation in optic nerve and macular structure with age and race with spectral-domain optical coherence tomography. Ophthalmology. 2011 Dec;118(12):2403–2408. doi: 10.1016/j.ophtha.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology. 2012 Apr;119(4):731–737. doi: 10.1016/j.ophtha.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Leung CK, Ye C, Weinreb RN, Yu M, Lai G, Lam DS. Impact of age-related change of retinal nerve fiber layer and macular thicknesses on evaluation of glaucoma progression. Ophthalmology. 2013 Dec;120(12):2485–2492. doi: 10.1016/j.ophtha.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Garway-Heath DF, Wollstein G, Hitchings RA. Aging changes of the optic nerve head in relation to open angle glaucoma. The British journal of Ophthalmology. 1997 Oct;81(10):840–845. doi: 10.1136/bjo.81.10.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.See JL, Nicolela MT, Chauhan BC. Rates of neuroretinal rim and peripapillary atrophy area change: a comparative study of glaucoma patients and normal controls. Ophthalmology. 2009 May;116(5):840–847. doi: 10.1016/j.ophtha.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Repka MX, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989 Jan;96(1):26–32. doi: 10.1016/s0161-6420(89)32928-9. [DOI] [PubMed] [Google Scholar]
- 16.Funaki S, Shirakashi M, Abe H. Relation between size of optic disc and thickness of retinal nerve fibre layer in normal subjects. The British journal of Ophthalmology. 1998 Nov;82(11):1242–1245. doi: 10.1136/bjo.82.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramakrishnan R, Mittal S, Ambatkar S, Kader MA. Retinal nerve fibre layer thickness measurements in normal Indian population by optical coherence tomography. Indian journal of Ophthalmology. 2006 Mar;54(1):11–15. doi: 10.4103/0301-4738.21608. [DOI] [PubMed] [Google Scholar]
- 18.Moya FJ, Brigatti L, Caprioli J. Effect of aging on optic nerve appearance: a longitudinal study. The British journal of Ophthalmology. 1999 May;83(5):567–572. doi: 10.1136/bjo.83.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuck MW, Crick RP. The age distribution of primary open angle glaucoma. Ophthalmic epidemiology. 1998 Dec;5(4):173–183. doi: 10.1076/opep.5.4.173.4192. [DOI] [PubMed] [Google Scholar]
- 20.Leske MC, Connell AM, Wu SY, et al. Incidence of open-angle glaucoma: the Barbados Eye Studies. The Barbados Eye Studies Group. Archives of Ophthalmology. 2001 Jan;119(1):89–95. [PubMed] [Google Scholar]
- 21.Mukesh BN, McCarty CA, Rait JL, Taylor HR. Five-year incidence of open-angle glaucoma: the visual impairment project. Ophthalmology. 2002 Jun;109(6):1047–1051. doi: 10.1016/s0161-6420(02)01040-0. [DOI] [PubMed] [Google Scholar]
- 22.de Voogd S, Ikram MK, Wolfs RC, Jansonius NM, Hofman A, de Jong PT. Incidence of open-angle glaucoma in a general elderly population: the Rotterdam Study. Ophthalmology. 2005 Sep;112(9):1487–1493. doi: 10.1016/j.ophtha.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. The New England journal of medicine. 1991 Nov 14;325(20):1412–1417. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 24.Sommer A. Epidemiology, ethnicity, race, and risk. Archives of Ophthalmology. 2003 Aug;121(8):1194. doi: 10.1001/archopht.121.8.1194. [DOI] [PubMed] [Google Scholar]
- 25.Racette L, Wilson MR, Zangwill LM, Weinreb RN, Sample PA. Primary open-angle glaucoma in blacks: a review. Survey of Ophthalmology. 2003 May-Jun;48(3):295–313. doi: 10.1016/s0039-6257(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 26.Girkin CA, McGwin G, Jr, Xie A, Deleon-Ortega J. Differences in optic disc topography between black and white normal subjects. Ophthalmology. 2005 Jan;112(1):33–39. doi: 10.1016/j.ophtha.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 27.Zangwill LM, Weinreb RN, Berry CC, et al. Racial differences in optic disc topography: baseline results from the confocal scanning laser ophthalmoscopy ancillary study to the ocular hypertension treatment study. Archives of Ophthalmology. 2004 Jan;122(1):22–28. doi: 10.1001/archopht.122.1.22. [DOI] [PubMed] [Google Scholar]
- 28.Girkin CA, Sample PA, Liebmann JM, et al. African Descent Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Archives of Ophthalmology. 2010 May;128(5):541–550. doi: 10.1001/archophthalmol.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chauhan BC, LeBlanc RP, McCormick TA, Rogers JB. Test-retest variability of topographic measurements with confocal scanning laser tomography in patients with glaucoma and control subjects. American journal of Ophthalmology. 1994 Jul 15;118(1):9–15. doi: 10.1016/s0002-9394(14)72836-3. [DOI] [PubMed] [Google Scholar]
- 30.Iester M, Broadway DC, Mikelberg FS, Drance SM. A comparison of healthy, ocular hypertensive, and glaucomatous optic disc topographic parameters. Journal of glaucoma. 1997 Dec;6(6):363–370. [PubMed] [Google Scholar]
- 31.Zangwill LM, van Horn S, de Souza Lima M, Sample PA, Weinreb RN. Optic nerve head topography in ocular hypertensive eyes using confocal scanning laser ophthalmoscopy. American journal of Ophthalmology. 1996 Oct;122(4):520–525. doi: 10.1016/s0002-9394(14)72112-9. [DOI] [PubMed] [Google Scholar]
- 32.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Archives of Ophthalmology. 2009 Sep;127(9):1136–1145. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Racette L, Liebmann JM, Girkin CA, et al. African Descent Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Archives of Ophthalmology. 2010 May;128(5):551–559. doi: 10.1001/archophthalmol.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sample PA, Johnson CA, Haegerstrom-Portnoy G, Adams AJ. Optimum parameters for short-wavelength automated perimetry. Journal of glaucoma. 1996 Dec;5(6):375–383. [PubMed] [Google Scholar]
- 35.Zeyen T, Miglior S, Pfeiffer N, Cunha-Vaz J, Adamsons I. Reproducibility of evaluation of optic disc change for glaucoma with stereo optic disc photographs. Ophthalmology. 2003 Feb;110(2):340–344. doi: 10.1016/s0161-6420(02)01754-2. [DOI] [PubMed] [Google Scholar]
- 36.Weinreb RN. Laser scanning tomography to diagnose and monitor glaucoma. Current opinion in Ophthalmology. 1993 Apr;4(2):3–6. [PubMed] [Google Scholar]
- 37.Zangwill LM, Bowd C, Berry CC, et al. Discriminating between normal and glaucomatous eyes using the Heidelberg Retina Tomograph, GDx Nerve Fiber Analyzer, and Optical Coherence Tomograph. Archives of Ophthalmology. 2001 Jul;119(7):985–993. doi: 10.1001/archopht.119.7.985. [DOI] [PubMed] [Google Scholar]
- 38.Chauhan BC, Blanchard JW, Hamilton DC, LeBlanc RP. Technique for detecting serial topographic changes in the optic disc and peripapillary retina using scanning laser tomography. Investigative ophthalmology & visual science. 2000 Mar;41(3):775–782. [PubMed] [Google Scholar]
- 39.Strouthidis NG, White ET, Owen VM, Ho TA, Hammond CJ, Garway-Heath DF. Factors affecting the test-retest variability of Heidelberg retina tomograph and Heidelberg retina tomograph II measurements. The British journal of Ophthalmology. 2005 Nov;89(11):1427–1432. doi: 10.1136/bjo.2005.067298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asaoka R, Strouthidis NG, Kappou V, Gardiner SK, Garway-Heath DF. HRT-3 Moorfields reference plane: effect on rim area repeatability and identification of progression. The British journal of Ophthalmology. 2009 Nov;93(11):1510–1513. doi: 10.1136/bjo.2008.148031. [DOI] [PubMed] [Google Scholar]
- 41.Zangwill LM, Jain S, Dirkes K, et al. The rate of structural change: the confocal scanning laser ophthalmoscopy ancillary study to the ocular hypertension treatment study. American journal of Ophthalmology. 2013 Jun;155(6):971–982. doi: 10.1016/j.ajo.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khachatryan N, Medeiros FA, Sharpsten L, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): Predictors of Visual Field Damage in Glaucoma Suspects. American journal of Ophthalmology. 2015 Jan 15; doi: 10.1016/j.ajo.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medeiros FA, Alencar LM, Zangwill LM, et al. Detection of progressive retinal nerve fiber layer loss in glaucoma using scanning laser polarimetry with variable corneal compensation. Investigative ophthalmology & visual science. 2009 Apr;50(4):1675–1681. doi: 10.1167/iovs.08-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medeiros FA, Alencar LM, Zangwill LM, Sample PA, Weinreb RN. The Relationship between intraocular pressure and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2009 Jun;116(6):1125–1133. doi: 10.1016/j.ophtha.2008.12.062. e1121–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medeiros FA, Alencar LM, Zangwill LM, Sample PA, Susanna R, Jr, Weinreb RN. Impact of atypical retardation patterns on detection of glaucoma progression using the GDx with variable corneal compensation. American journal of Ophthalmology. 2009 Jul;148(1):155–163. doi: 10.1016/j.ajo.2009.01.021. e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medeiros FA, Alencar LM, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Prediction of functional loss in glaucoma from progressive optic disc damage. Archives of Ophthalmology. 2009 Oct;127(10):1250–1256. doi: 10.1001/archophthalmol.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leung CK, Ye C, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography a study on diagnostic agreement with Heidelberg Retinal Tomograph. Ophthalmology. 2010 Feb;117(2):267–274. doi: 10.1016/j.ophtha.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 48.Alencar LM, Zangwill LM, Weinreb RN, et al. Agreement for detecting glaucoma progression with the GDx guided progression analysis, automated perimetry, and optic disc photography. Ophthalmology. 2010 Mar;117(3):462–470. doi: 10.1016/j.ophtha.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banegas SA, Anton A, Morilla-Grasa A, Bogado M, Ayala EM, Moreno-Montanes J. Agreement among spectral-domain optical coherence tomography, standard automated perimetry, and stereophotography in the detection of glaucoma progression. Investigative ophthalmology & visual science. 2015 Feb;56(2):1253–1260. doi: 10.1167/iovs.14-14994. [DOI] [PubMed] [Google Scholar]
- 50.Strouthidis NG, Scott A, Peter NM, Garway-Heath DF. Optic disc and visual field progression in ocular hypertensive subjects: detection rates, specificity, and agreement. Investigative ophthalmology & visual science. 2006 Jul;47(7):2904–2910. doi: 10.1167/iovs.05-1584. [DOI] [PubMed] [Google Scholar]
- 51.Bowd C, Balasubramanian M, Weinreb RN, et al. Performance of confocal scanning laser tomograph Topographic Change Analysis (TCA) for assessing glaucomatous progression. Investigative ophthalmology & visual science. 2009 Feb;50(2):691–701. doi: 10.1167/iovs.08-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leung CK, Liu S, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma a prospective analysis with neuroretinal rim and visual field progression. Ophthalmology. 2011 Aug;118(8):1551–1557. doi: 10.1016/j.ophtha.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 53.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Archives of Ophthalmology. 2005 Apr;123(4):464–470. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investigative ophthalmology & visual science. 2000 Mar;41(3):741–748. [PubMed] [Google Scholar]
- 55.Suh MH, Park KH, Kim H, et al. Glaucoma progression after the first-detected optic disc hemorrhage by optical coherence tomography. Journal of glaucoma. 2012 Aug;21(6):358–366. doi: 10.1097/IJG.0b013e3182120700. [DOI] [PubMed] [Google Scholar]
- 56.Zeyen TG, Caprioli J. Progression of disc and field damage in early glaucoma. Archives of Ophthalmology. 1993 Jan;111(1):62–65. doi: 10.1001/archopht.1993.01090010066028. [DOI] [PubMed] [Google Scholar]
- 57.Airaksinen PJ, Tuulonen A, Alanko HI. Rate and pattern of neuroretinal rim area decrease in ocular hypertension and glaucoma. Archives of Ophthalmology. 1992 Feb;110(2):206–210. doi: 10.1001/archopht.1992.01080140062028. [DOI] [PubMed] [Google Scholar]
- 58.Medeiros FA, Lisboa R, Zangwill LM, et al. Evaluation of progressive neuroretinal rim loss as a surrogate end point for development of visual field loss in glaucoma. Ophthalmology. 2014 Jan;121(1):100–109. doi: 10.1016/j.ophtha.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vizzeri G, Bowd C, Weinreb RN, et al. Determinants of agreement between the confocal scanning laser tomograph and standardized assessment of glaucomatous progression. Ophthalmology. 2010 Oct;117(10):1953–1959. doi: 10.1016/j.ophtha.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson MR. The use of “race” for classification in medicine: is it valid? Journal of glaucoma. 2003 Aug;12(4):293–294. doi: 10.1097/00061198-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Kosoko-Lasaki O, Gong G, Haynatzki G, Wilson MR. Race, ethnicity and prevalence of primary open-angle glaucoma. Journal of the National Medical Association. 2006 Oct;98(10):1626–1629. [PMC free article] [PubMed] [Google Scholar]
- 62.Girkin CA, Nievergelt CM, Kuo JZ, et al. Biogeographic Ancestry in the African Descent and Glaucoma Evaluation Study (ADAGES): Association With Corneal and Optic Nerve Structure. Investigative ophthalmology & visual science. 2015;56(3):2043–2049. doi: 10.1167/iovs.14-15719. [DOI] [PMC free article] [PubMed] [Google Scholar]