Abstract
Human cytomegalovirus (CMV), the prototypical β-herpervirus, is a widespread pathogen that establishes a lifelong latent infection in myeloid progenitor, and possibly other cells as well. Although immunocompetent individuals show mild or no symptoms despite periodic reactivation during myeloid cell differentiation, CMV is responsible for considerable morbidity and mortality in older adults and in persons chronically infected with HIV. Indeed, in these individuals, reactivation of CMV can cause serious complications. This review will focus of the effects of CMV during aging and HIV/AIDS, with particular attention to the cellular immunity and age-related pathology outcomes from this persistent infection. The impact of the long-term chronic exposure to CMV antigens on the expansion of CD8 T cells with features of replicative senescence will be highlighted.
Keywords: CMV, aging, HIV, CD8 T cell, replicative senescence
1. Introduction
Extensive research on aging and HIV/AIDS—a disease associated with accelerated aging—indicates that chronic CMV infection has profound effects on the immune system. Even in the absence of overt reactivation, in most cases, CMV seropositivity is associated with multiple negative health and longevity outcomes. Interestingly, in persons with chronic infection with HIV-1, long-term administration of combination antiretroviral therapy, which controls the levels of HIV, paradoxically, accentuates the effect of CMV. Given the increased long-term survival of persons infected with HIV, it is predicted that the CMV immune effects associated with chronological aging may synergizewith those due to the HIV to further accelerate immunosenescence.
This review will focus on the deleterious effects of CMV in the context of both aging and HIV/AIDS. However, it should be noted that CMV also negatively impacts other clinical situations. For example, in the case of inflammatory bowel disease, CMV is associated with steroid resistance (Wu et al., 2015); in kidney transplant patients, CMV induces activation of several human endogenous retroviruses (Bergallo et al., 2015), and in stem cell transplant recipients, CMV is an important cause of mortality (Soland et al.., 2014). A recent, highly relevant finding is the consistent association between unfavorable glucose and lipid profiles with the accumulation of late stage CD8 T cells in a large cohort (n=400) of CMV+ individuals, as compared to uninfected controls that were matched for age, sex, sociodemographic variables and lifestyle factors. This novel observation links metabolic risk factors on immunity and health with an infectious pathogen that has been repeatedly implicated in immunosenescence(Rector et al., 2015).
2. The Herpesviruses
The role of the gastrointestinal bacterial population on human health and immunity has become a key focus of research over the last decade. In contrast to the intense interest in this so-called human microbiome, far less is known about the effects of viral symbionts, the most prevalent of which are the herpes viruses. The herpesviridae family consists of > 100 viruses that have been infecting vertebrate species, including humans, for hundreds of millions of years. The main feature of these viruses is their ability to develop lifelong, persistent infections, due to complex strategies to escape immune responses of the host. Most of these viruses are acquired early in life, and they infect the majority of the human population. (Gianella et al., 2015). Estimates of seroprevalence in the adult population range from 45–100%, depending on geographic locale.
Cytomegalovirus (CMV), the focus of this review, has a genome that is the largest of all viruses that infect humans. Experiments using over 200 protein-spanning peptide pools have revealed that human T cells from a donor population of 33 individuals of mixed HLA backgrounds can recognize at least 150 proteins. New evidence suggests that the human CMV proteome may be underestimated, since the genome actually provides more than 700 protein open reading frames (Terrazini& Kern, 2014; Stern-Ginossar et al. 2012; Holtappels et al., 2009). Overall, most researchers agree that the success of CMV to persist in the human population is based, in large part, on complex strategies of immune evasion, rather than rapid mutation of target proteins. Nonetheless, there is some genetic and antigenic heterogeneity among CMV isolates, which most likely affect pathogenic potential (Sijmons et al. 2015).
3. CMV and aging
As noted above, CMV has had millions of years to optimize its interaction with homo sapiens. Given the ubiquity of CMV infections in the human population worldwide, it is possible that certain beneficial effects during youth, a period associated with the need for reproductive success, might have favored its persistence over millions of years of evolution. Indeed, studies comparing young and old CMV-infected adults showed several beneficial immunological features associated with this virus. These include increased antibody response to influenza vaccination, elevated circulating levels of IFN gamma, and increased CD8 T cell sensitivity (Furman et al, 2015). Consistent with the observations in humans, studies in mice showed that murine CMV infection conferred cross-protection against a bacterial infection in young, but not old, animals (Barton et al., 2007). Indeed, it has been proposed that lifelong interaction between CMV and the host immune response not only controls viral reactivation, but also modulates the immune system. Given that the majority of humans harbor CMV and other latent herpervirus infections, one might even consider redefining the term “normal” immune system to take these persistent infection modulatory effects into account.(White et al., 2011).
The potential beneficial effects of CMV during youth are in stark contrast to the various deleterious outcomes associated with this persistent infection in older adults. It is possible that this age differential may relate to the length of time of the infection. But the phenomenon is also consistent with the notion of antagonistic pleiotropy. This theory posits that certain biological features that have been selected for as favorable during youth turn out to be harmful in old age, i.e., during a stage of life that is neutral in terms of evolutionary natural selection. Examples of antagonistic pleiotropy include high levels of estrogen, which favor reproductive success early in life, but which may enhance the growth of breast tumors in older women. The process of replicative (cellular) senescence, which suppresses tumor formation during youth, but may ultimately contribute to many-age-related pathologies, is a second example of antagonistic pleiotropy, as will be discussed below.
4. Replicative senescence in vitro
In the 1990’s, oligoclonal expansions of T cells were documented in older humans, and characterized as the T cell analogues of the well-known benign B cell gammapathies seen in many elderly persons (Posnett et al., 1994). Subsequent research provided evidence that these expanded populations of CMV-specific CD8 T cells showed many features that resembled T cells that arose in long-term cultures following multiple rounds of antigen-driven proliferation. These end stage cells show consistent changes in function and gene expression (Effros, 2007). In vivo, the presence of oligoclonally expanded CD8 T cell populations of similar late-differentiated phenotype cells is increasingly being implicated in advanced immunosenescence and predictive of poor prognosis (Hadrup et al. 2006). Although it cannot be definitively proven that cells have reached irreversible cell-cycle arrest in vivo, and in some cases have been shown to retain limited proliferative capacity (Weng et al. 2009) for the purpose of this review, these cells will be described as senescent, due to their overall similarity in function and phenotype to their in vitro counterparts.
Extensive in vitro studies have shown that, with proper activation via the T-cell receptor and constant exposure to IL-2, cultures of normal human T cells can undergo between 25 and 40 population doublings before reaching senescence and ceasing to proliferate (Effros, 1998). with an average lifespan of approximately 33 population doublings (Effros & Pawelec, 1997). Although aging is correlated with the accumulation of cell types with senescence markers in vivo, there is no clear correlation between in vitro lifespan of T cell cultures and chronological age of the donor (Perillo et al., 1993). The major changes associated with CD8 T cell senescence in cell culture are irreversible loss of gene and protein expression of CD28, apoptosis resistance, increased production of IL-6 and TNFα, loss of telomerase activity, shortened telomeres, and upregulation of the p16 and p21 cell cycle inhibitors (Effros et al., 2003, 2005). Other changes in cytokine production associated with T cell senescence include loss of IL-2, reduced Interferon-gamma, and increased secretion of RANKL (Effros et al., Graham et al., 2009). This short list of changes documented thus far for human T cells contrasts with the extensive number of secretory changes associated with fibroblast senescence, which include both cytokines, chemokines, and met various growth factors (Coppé et al. 2010).
5. Unique features of senescent human CD8 T cells
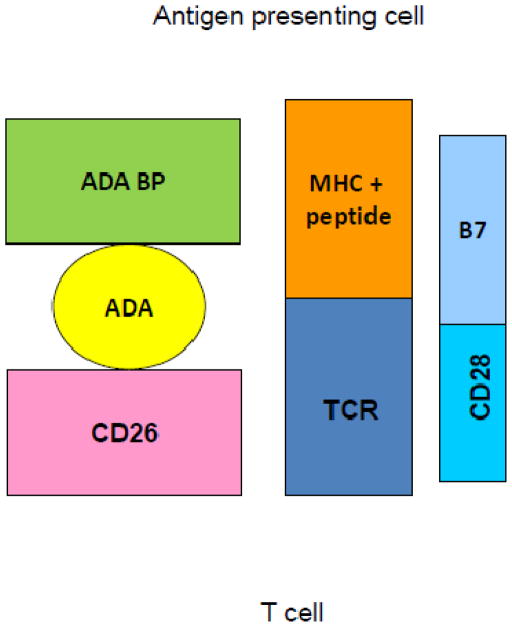
Recent studies have documented an unexpected role of adenosine deaminase (ADA) in modulating the process of T cell replicative senescence. ADA is the enzyme that converts immunosuppressive adenosine to inosine, thereby playing a key role in normal immune function (Gessi et al., 2007; Hovi et al., 1976). ADA is best known for its critical function in lymphoid development, where its absence results in severe combined immunodeficiency (Hershfield, 2005). However, ADA, present both intracellularly and on the surface, as ecto-ADA that is bound to CD26 (Kameoka et al., 1993), is also essential for optimal function of mature T lymphocytes. Indeed, the ADA/CD26 complex, which, like CD28, is a component of the immunological synapse, delivers a costimulatory signal upon T cell stimulation (Aran et al., 1991; Hovi et al., 1976; Kameoka et al., 1993; Martin et al., 1995; Pacheco et al., 2005). Our own studies documented for the first time that ADA is key to upregulation of telomerase activity, and that both intracellular and ecto-ADA decrease with increasing population doublings. Exposure to exogenous adenosine, to mimic the in vivo environment, was shown to accelerate the progression of CD8 T cells to replicative senescence, causing more rapid loss of CD28 expression and telomerase activity, and reduced proliferative potential(Parish et al., 2010).
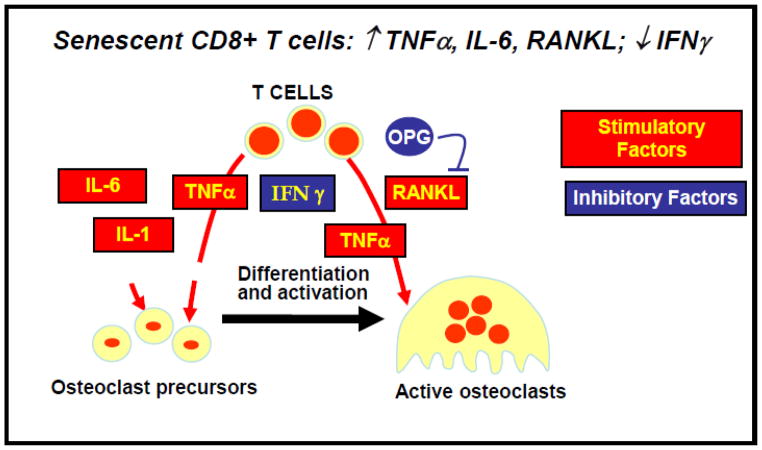
A second important change recently documented for T cells relates to the clinical association between chronic immune activation and bone loss (Crotti et al., 2015). Osteoporosis is a systemic disease that is associated with increased morbidity, mortality and health care costs (Pike et al., 2010). Whereas osteoclasts and osteoblasts are the main regulators of bone homeostasis, it is becoming increasingly clear that the immune system, particularly T cells, can produce both stimulatory and inhibitory factors that modulate the maturation and activation of bone-resorbing osteoclasts Interestingly, a small study on post-menopausal women showed a correlation between bone loss and the presence of increased proportions of CD8 T cells that lack CD28 expression (Peitschmann et al., 2001). This report is consistent with the increased production by senescent T cells of several soluble factors that enhance the activation of bone-resorbing osteoclasts, including IL-6, TNFα, and RANKL, which will be discussed below.
The complex regulation of activation-induced T lymphocyte production of Receptor Activator of NFκB Ligand (RANKL) in chronically stimulated human T cellshas recently been reviewed (Crotti et al., 2015). Given that lipid oxidation products mediate inflammatory and metabolic disorders such as osteoporosis and atherosclerosis, and since oxidized lipids affect several T lymphocyte functions, we hypothesized that RANKL production might also be subject to modulation by oxidized lipids. Our analysis demonstrated that short term exposure of both unstimulated and activated human T lymphocytes to minimally oxidized low density lipoprotein (LDL), but not native LDL, significantly enhances RANKL message and protein, and promotes the surface expression of the lectin-like oxidized LDL (LOX-1) receptor (Graham et al., 2009). The link between oxidized lipids and T lymphocytes was further reinforced by analysis of hyperlipidemic mice, in which the loss of bone mass is associated with both increased RANKL mRNA in T lymphocytes and elevated RANKL serum levels (Graham et al., 2009). Our results suggest a novel pathway by which T lymphocytes contribute to bone loss, namely, via oxidized lipid enhancement of RANKL production. These findings may help elucidate clinical associations between cardiovascular disease and decreased bone mass, and may also lead to new immune-based approaches to osteoporosis.
6. CMV and Immunosenescence
The most compelling evidence for a role of CMV as a driver of immune system aging comes from the Swedish OCTO/NONA studies, which identified an immune risk profile (IRP) present in 15–20% of 85 year olds that was associated with 2-, 4-, and 6-year mortality at follow-up. The major risk factors included an inverted CD4:CD8 ratio, apparently caused by accumulation of CD8 cells lacking CD28 expression, poor T cell proliferative response to mitogen, and low B cell numbers(Pawelec et al., 2010). It was subsequently shown that seropositivity for human CMV (but not for other persistent viruses, such as EBV, HSV or VZV) was also predictive of the IRP. Indeed, it turned out that 100% of 85 year olds with the IRP group were seropositive for CMV, versus only 85% who were not in the IRP (Pawelec et al. 2010). It was demonstrated the accumulation of CD8 T cells responsible for the inverted CD4:CD8 ratio tended to be late-differentiated oligoclonal expansions specific for CMV antigens (Hadrup et al., 2006; Khan et al., 2004 a & b), comprising up to 45% of the total CD8 T cell pool (Khan et al., 2004b). These late-differentiated CMV-specific clones in the elderly were subsequently shown to have reduced functionality in vitro compared to CMV clones from younger subjects (Hadrup et al., 2006; Ouyang et al., 2004). Whatever the underlying cause for the divergence of the CMV effects at different life stages, it is clear that this persistent CMV infection is associated with multiple negative effects in older adults.
Most of the above studies focus on correlations between CMV and immunological aging, but there is some evidence that CMV may actually play a causal role inimmunosenescence, independent of age. For example, reduced CD4/8 T cell ratio, higher levels of serum IL-6, and reduced influenza vaccine responses, have been observed even in young CMV+ adults (Turner et al. 2014). Another study showed a strong association between CMV seropositivity and increased number of CD4 and CD8 and T cells lacking CD28 expression irrespective of age (Looney et al., 1999). Moreover, across all age groups, individuals who are CMV seropositive show a 60% increase in the size of CD8 T cell memory pool and with a reduction in the proportion of naïve CD8 T cells (Chidrawar et al., 2009). CMV seropositivity is associated with a Th1 polarization of the immune system, possibly resulting in increased inflammation (Almazar et al., 2005). Supporting this notion, two epidemiological studies have noted a correlation between CMV seropositivity, increased inflammatory biomarkers, and morbidity in the elderly (Schmaltz et al., 2005; Aiello et al., 2008). Finally, it should be noted that the rare CMV-negative elderly persons do, in fact, show some characteristics of immunosenescence (Pawelec et al., 2009), changes that may, in some cases, be driven by other persistent viruses, such as EBV (Ouyang et al., 2003).
7. Accelerated immune system aging in chronic HIV disease
There is increasing evidence that chronic infection with HIV-1 accelerates many features associated with normal aging (Appay et al., 2007; Effros et al., 2008). The most dramatic evidence in this regard is the increased susceptibility of successfully treated HIV-infected persons to morbidities more commonly observed in older uninfected adults. These include frailty (Desquilbet et al., 2007), non-Hodgkins lymphoma (Engels et al., 2010), cervical carcinomas (D’Souza et al., 2001; Dorrucci et al., 2001), Kaposi’s sarcoma (Unemori et al. 2013), osteoporosis (Fausto et al., 2006; Thomas & Doherty,2003), liver (Weber et al., 2006; Hooshyar et al., 2007) and renal impairment (Arnold et al., 2006), cardiovascular disease (Baker et al., 2008; Smit et al., 2006) diabetes (Triant et al., 2007), and hypertension (Hsue et al., 2008). Moreover, older HIV-infected adults show more rapid disease progression (Blanco et al., 2010). It has also been recently been shown that untreated HIV infection has epigenetic effects that are additive with aging and accelerate, by ~14 years, the formation of an aging-related methylation pattern (Rickabaugh et al., 2015). Thus, multiple facets of normal aging have been documented for HIV infection.
Research on the immune system has provided substantial evidence that immunosenescence is accelerated in persons chronically infected with HIV. Within the CD8 T cell subset, there is a significant increase in cells that share features with senescent T cells that arise in vitro following multiple rounds of stimulation with either alloantigen, anti-CD2/3/28-coated microbeads, or HIV-peptide pulsed antigen presenting cells (Dagarag et al., 2003). Interestingly, the telomere length of CD8+CD28− T cells from HIV-infected persons is shorter than the corresponding subset from age-matched HIV-negative adults (Effros et al., 1996), possibly reflecting the dual impact of CMV and HIV in the former group. In addition, early in the infection, the proportion of CD8 T cells lacking CD28 expression is predictive of the rate of HIV disease progression (Cao et al., 2009). HIV-1-infection is also associated with a significant decrease in the number of CD31+ naïve CD4+ T-cells and shortening of telomeres in the overall naïve CD4+ T-cell subpopulation, rendering this cellular compartment more phenotypically similar to that of an uninfected adult 20–30 years older (Rickabaugh et al., 2011). Interestingly, those HIV-infected persons who are seronegative for CMV show greater resilience in terms of immune recovery following antiretroviral therapy (Barrett et al. 2014).
8. CMV vs. HIV
It is clear that CMV coinfection is very common in HIV-infected populations, reaching levels of 90%–100% (Lang et al. 1989). In fact, long-term successfully treated HIV-infected individuals have high levels of CMV-specific effector cells, similar to that observed in the elderly participants, but occurring about 40 years earlier (Naeger et al., 2010). There is also evidence the CMV may play a role in cardiovascular pathologies seen in HIV/AIDS. It has been reported that HIV-infected women with increased CMV immunoglobulin G levels were more likely to have carotid artery stiffness compared with uninfected controls (Parinello et al., 2012) and in both men and women who are HIV+, carotid intima-media thickness has been reported. Although HIV-infected persons show multiple features in common, such as higher T-cell activation, high-sensitivity C-reactive protein levels, and CMV-specific T-cell responses, only CMV-specific T-cell responses were independently associated with intima-media thickness (Hsue et al., 2006). Thus, CMV and HIV may influence immunological aging in an additive manner, but it appears that CMV may exert a more dramatic effect than HIV (Pathai et al., 2014).
Possible mechanisms for the very high frequencies of CMV-specific T cells in antiretroviral-treated HIV disease include subclinical CMV replication and/or a dysregulated and heightened immunologic response to a normal level of CMV replication(Aiello et al., 2012; Parinello et al, 2012). Given that a large proportion of the senescent CD8 T cells present in HIV-infected persons are CMV-specific, CMV may also be implicated in the accelerated bone loss associated with HIV disease (Fausto et al. 2006), due the pro-inflammatory secretory profile of the end-stage CD8 T cells.
As noted above, nearly all HIV-infected persons are also infected with CMV, and the proportion of CMV-specific CD8 T cells often exceeds that of the HIV-specific T cells. Large cohort studies have documented the impact of CMV at various stages of the HIV infection (Naeger et al., 2010). Well before the onset of immunodeficiency, the frequency of pp65-specific (a major CMV immunogen) CD8 T cells was higher than in HIV uninfected controls. Surprisingly, this pattern was exaggerated in anti-retrovirally- treated persons, even though only 2 of the 200 potential T cell CMV antigens were measured.
The question arises as to why HIV fails to establish the same mutually symbiotic relationship with humans as CMV. First and foremost, it would seem that the long evolutionary history between humans and CMV has selected for those hosts who have the ability to harbor latent viruses that confer the benefit of the protective effects against other infections early in life. In that sense, HIV, which only recently entered the human population, has not had sufficient time to evolve analogous mechanisms of mutually beneficial co-existence. Thus, one might view HIV as “CMV in–training”. Second, it is clear that the cell type in which each virus establishes latency has profound effects on the relationship with the host. Indeed, even among the herpesviruses, such as Epstein-Barr virus and Herpes simplex, CMV has the most profound effect on humans, which may relate to the different sites of latency among this family of viruses. Indeed, CMV can infect a host of different cell types, including fibroblasts, endothelial cells, muscle cells, and brain-derived pericytes. Recent studies have added perivascular mesenchymal stromal cells within multiple organs to this list (Soland et al., 2014). These cells are present not only within the hematopoietic microenvironmental niche, but also as cells that encircle capillaries and vessels throughout the body, allowing intimate contact with circulating lymphocytes as they extravasate across vessel walls (Soland et al., 2014). These observations may explain the fact that the magnitude of the CD8 T cell response during even clinical latency is extraordinarily high, ranging from 10–20% in otherwise healthy adults, and reaches levels of 50% in the HIV-infected population.
9. Translational implications
Immunosenescence and HIV/AIDS are both characterized by the accumulation of memory CD8 T lymphocytes with features of replicative senescence. The driving force seems to be chronic antigenic stimulation by persistent viruses, in particular, CMV. Whereas prevention of primary infection with these viruses would be the most efficient strategy to prevent replicative senescence, it is highly unlikely that prophylactic vaccines against CMV and HIV-1 will be developed in the foreseeable future. In fact, based on the proposed idea that CMV and the human immune system have a mutually symbiotic relationship (White et al., 2011), prevention of CMV infection requires careful cost/benefit analysis, and may ultimately be restricted to specific situations of high risk, such as organ transplant candidates. Alternatively, reduction of the antigenic burden by anti-CMV viral medication—usually reserved for situations of extreme immunosuppression, such as in organ transplant patients or the final stages of HIV disease—or using therapeutic vaccines, may lead to improved immune function during aging and HIV/AIDS.
An alternative to reducing the antigenic burden is to augment the function of the virus-specific CD8 T cells by retarding replicative senescence via CD28 or telomerase gene therapy. Indeed, the re-expression of an intact signaling CD28 molecule in CMV- or HIV-specific CD8 T cells that had lost CD28 expression led to the restoration of IL-2 production and autocrine-induced proliferation in response to antigen recognition (Topp et al., 2003). Also, transduction of HIV-specific CD8 T cells isolated from HIV-infected persons with the gene for hTERT (the catalytic telomerase component) results in increased proliferative potential, telomere length stabilization, and enhanced ability to control viral replication (Dagarag et al., 2003; Dagarag et al. 2004). Avoidance of the complications of telomerase gene therapy may be possible using small molecule telomerase activators, which, in cell culture studies, leads to increased proliferation and anti-viral function (Fauce et al., 2008).
Irrespective of the driving force leading to senescent T cell accumulation during aging and HIV disease, reduction in the proportion of these cells would be predicted to diminish many of the associated deleterious clinical effects. Blunting the chronic state of inflammation, which is due, at least in part, to the production of pro-inflammatory cytokines by senescent T cells, might reduce the severity of such age-related pathologies as atherosclerosis and bone loss. An additional benefit of immune-based approaches to therapy may be a reduced need for drugs that target HIV-1. Many of the current drug treatments are associated with metabolic changes normally associated with aging, including lipodystrophy, dyslipidemia and insulin resistance, all of which increase the risk of cardiovascular disease (Morse & Kovacs, 2006).
Conclusions
The question of whether HIV accelerates aging is most probably organ and disease-specific. The most dramatic example of premature aging is in the immune system, where T cell telomere length, accumulation of CD8+CD28− T cells, reduced naïve T cell generation and evidence of chronic immune activation implicate the contribution of replicative senescence (Pathai et al. 2014). Since nearly all HIV-infected persons are also infected with CMV, it seems likely that this persistent infection, probably acquired prior to HIV, plays a substantial role in the accelerated immunosenescence. Clinically, the development of such geriatric syndromes as frailty, and multimorbidity are hastened in chronic HIV infection, and many age-associated pathologies, such as cardiovascular disease and bone loss may be accentuated, suggesting that HIV and CMV may confer ‘double indemnity.
FIG. 1. T lymphocyte regulation of cytokines involved in bone loss.
T cells produce various cytokines that regulate the maturation and activation of bone-resorbing osteoclasts. Senescent cultures of human CD8 T cells secrete several factors that are predicted to favor bone loss. Stimulatory factors (red) and inhibitory factors (blue).
FIG. 2. Ecto-ADA and the immunological synapse.
the ADA/CD26 complex, which, like CD28, is a component of the immunological synapse, delivers a costimulatory signal upon T cell stimulation via the binding of the T cell receptor (TCR) to the antigen-MHC complex on the antigen-presenting cell. (Parish et al. 2010)
Highlights.
Importance of herperviruses to human health
Replicative senescence within the human immune system
Senescent T cells accumulate with age
Cytomegalovirus is a major driver of senescence
Cytomegalovirus impacts both normal aging and HIV/AIDS
Acknowledgments
The work described in this review was, in part, supported by grants from the NIH for over 25 years, and is currently supported by AG032422. The author acknowledges the contributions by members of her laboratory, including Drs. Nancy Perillo, Carolyn Spaulding, Hector Valenzuela, Steven Fauce, Lucy Graham, Stanley Parish, Jennifer Chou and Jeffrey Dock, whose research is summarized in this review..
Footnotes
Conflict of interest
The author declares no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello AE, Simanek AM. Cytomegalovirus and immunological aging: the real driver of HIV and heart disease? J Infect Dis. 2012;205:1772–1774. doi: 10.1093/infdis/jis288. [DOI] [PubMed] [Google Scholar]
- Aiello AE, et al. Persistent infection, inflammation, and functional impairment in older Latinos. The journals of gerontology. Series A, Biological sciences and medical sciences. 2008;63(6):610–8. doi: 10.1093/gerona/63.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almanzar G, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. Journal of virology. 2005;79(6):3675–83. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almanzar G, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. Journal of Virology. 2005;79(6):3675–83. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV-1 infection. Experimental Gerontology. 2007;42:432–437. doi: 10.1016/j.exger.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Aramă V, Mihăilescu R, Rădulescu M, Aramă SŞ, Streinu-Cercel A, Youle M CMV-HIV Study group. Clinical relevance of the plasma load of cytomegalovirus in patients infected with HIV--a survival analysis. J Med Virol. 2014 Nov;86(11):1821–7. doi: 10.1002/jmv.24027. [DOI] [PubMed] [Google Scholar]
- Aran JM, Colomer D, Matutes E, Vives-Corrons JL, Franco R. Presence of adenosine deaminase on the surface of mononuclear blood cells: immunochemical localization using light and electron microscopy. J Histochem Cytochem. 1991 Aug;39(8):1001–8. doi: 10.1177/39.8.1856451. [DOI] [PubMed] [Google Scholar]
- Arnold DM, Julian JA, Walker IR. Mortality rates and causes of death among all HIV-positive individuals with hemophilia in Canada over 21 years of follow-up. Blood. 2006;108: 460–464. doi: 10.1182/blood-2005-11-4407. [DOI] [PubMed] [Google Scholar]
- Baker JV, Peng G, Rapkin J, Abrams DI, Silverberg MJ, MacArthur RD, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22: 841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L, Fowke KR, Grant MD. Cytomegalovirus, aging, and HIV: a perfect storm. AIDS Rev. 2012 Jul-Sep;14(3):159–67. [PubMed] [Google Scholar]
- Barrett L, Stapleton SN, Fudge NJ, Grant MD. Immune resilience in HIV-infected individuals seronegative for cytomegalovirus. AIDS. 2014 Sep 10;28(14):2045–9. doi: 10.1097/QAD.0000000000000405. [DOI] [PubMed] [Google Scholar]
- Bergallo M, Galliano I, Montanari P, Gambarino S, Mareschi K, Ferro F, Fagioli F, Tovo PA, Ravanini P. CMV induces HERV-K and HERV-W expression in kidney transplant recipients. J Clin Virol. 2015 Jul;68:28–31. doi: 10.1016/j.jcv.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Blanco JR, Caro AM, Pérez-Cachafeiro S, Gutiérrez F, Iribarren JA, González-García J, Ferrando-Martínez S, Navarro G, Moreno S. HIV infection and aging. AIDS Rev. 2010 Oct-Dec;12(4):218–30. [PubMed] [Google Scholar]
- Bocklandt S, Lin W, Sehl ME, Sanchez FJ, Sinsheimer JS, Horvath S, et al. Epigenetic predictor of age. PLoS One. 2011;6 doi: 10.1371/journal.pone.0014821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Jamieson BD, Hultin LE, Hultin PM, Effros RB, Detels R. Premature aging of T cells is associated with faster HIV-1 disease progression. J Acquir Immune Defic Syndr. 2009 Feb 1;50(2):137–47. doi: 10.1097/QAI.0b013e3181926c28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky FA, Brach JS, Satterfield S, Bauer DC, Visser M, Rubin SM, Harris T, Pahor BM. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005 Oct;53(10):1675. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- Chidrawar S, et al. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clinical and experimental immunology. 2009;155(3):423–32. doi: 10.1111/j.1365-2249.2008.03785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotti TN, Dharmapatni AA, Alias E, Haynes DR. Osteoimmunology: Major and Costimulatory Pathway Expression Associated with Chronic Inflammatory Induced Bone Loss. J Immunol Res. 2015;2015:281287. doi: 10.1155/2015/281287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagarag MD, Evazyan T, Rao N, Effros RB. Genetic manipulation of telomerase in HIV-specific CD8+ T cells: enhanced anti-viral functions accompany the increased proliferative potential and telomere length stabilization. Journal of Immunology. 2004;173:6303–6311. doi: 10.4049/jimmunol.173.10.6303. [DOI] [PubMed] [Google Scholar]
- Dagarag MD, Ng H, Lubong R, Effros RB, Yang OO. Differential impairment of lytic and cytokine functions in senescent HIV-1-specific cytotoxic T lymphocytes. Journal of Virology. 2003;77:3077–3083. doi: 10.1128/JVI.77.5.3077-3083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62: 1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- Dorrucci M, Suligoi B, Serraino D, Tirelli U, Rezza G. Incidence of invasive cervical cancer in a cohort of HIV-seropositive women before and after the introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;26: 377–380. doi: 10.1097/00126334-200104010-00016. [DOI] [PubMed] [Google Scholar]
- D’Souza G, Wiley DJ, Li X, Chmiel JS, Margolick JB, Cranston RD, et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2008;48: 491–499. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB. Replicative senescence in the immune system: impact of the Hayflick limit on T-cell function in the elderly. Am J Hum Genet. 1998;62:1003–100. doi: 10.1086/301845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros R. Replicative senescence: the final stage of memory T cell differentiation? Current HIV Research. 2003;1(2):153–157. doi: 10.2174/1570162033485348. [DOI] [PubMed] [Google Scholar]
- Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007;25:599–604. doi: 10.1016/j.vaccine.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, et al. Shortened telomeres in the expanded CD28− CD8 + cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10: F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clinical Infectious Diseases. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB. Telomeres and HIV disease. Microbes and Infection. 2000;2: 69–76. doi: 10.1016/s1286-4579(00)00283-5. [DOI] [PubMed] [Google Scholar]
- Effros RB, Pawelec G. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol Today. 1997;18:450–454. doi: 10.1016/s0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]
- Effros RB, Allsopp R, Chiu CP, Wang L, Hirji K, Harley CB, et al. Shortened telomeres in the expanded CD28−CD8+ subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS/Fast Track. 1996;10:F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford RL, et al. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29:601–609. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Effros RB, Dagarag MD, Valenzuela HF. In vitro senescence of immune cells. Exp Gerontol. 2003;38(11–12):1243–9. doi: 10.1016/j.exger.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Effros RB, Dagarag M, Spaulding C, Man J. The Role of CD8 T cell replicative senescence in human aging. Immunological Revs. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Engels EA, Pfeiffer RM, Landgren O, Moore RD. Immunologic and virologic predictors of AIDS-related non-hodgkin lymphoma in the highly active antiretroviral therapy era. J Acquir Immune Defic Syndr. 2010;54: 78–84. doi: 10.1097/01.qai.0000371677.48743.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson KM, Allshouse AA, Rapaport E, Palmer BE, Wilson CC, Weinberg A, MaWhinney S, Campbell TB. Physical Function Impairment of Older, HIV-Infected Adults Is Associated with Cytomegalovirus Immunoglobulin Response. AIDS Res Hum Retroviruses. 2015 Jul 8;:905–12. doi: 10.1089/aid.2015.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauce SR, Jamieson BD, Chin AC, Mitsuyasu RT, Parish ST, Ng HL, Kitchen CM, Yang OO, Harley CB, Effros RB. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J Immunol. 2008 Nov 15;181(10):7400–6. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto A, Bongiovanni M, Cicconi P, Menicagli L, Ligabo EV, Melzi S, et al. Potential predictive factors of osteoporosis in HIV-positive subjects. Bone. 2006;38: 893–897. doi: 10.1016/j.bone.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000 Jan;55(1):M43. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- Furman D, Jojic V, Sharma S, Shen-Orr SS, Angel CJ, Onengut-Gumuscu S, Kidd BA, Maecker HT, Concannon P, Dekker CL, Thomas PG, Davis MM. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med. 2015 Apr 1;7(281):281ra43. doi: 10.1126/scitranslmed.aaa2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianella S, Massanella M, Wertheim JO, Smith DM. 3. The Sordid Affair Between Human Herpesvirus and HIV. J Infect Dis. 2015 Mar 6; doi: 10.1093/infdis/jiv148. pii: jiv 148. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham LS, Parhami F, Tintut Y, Kitchen CM, Demer LL, Effros RB. Oxidized lipids enhance RANKL production by T lymphocytes: implications for lipid-induced bone loss. Clin Immunol. 2009 Nov;133(2):265–75. doi: 10.1016/j.clim.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrup SR, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoirwe hrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. Journal of immunology. 2006;176(4):2645–53. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- Holtappels R, Thomas D, Reddehase MJ. The efficacy of antigen processing is critical for protection against cytomegalovirus disease in the presence of viral immune evasion proteins. J Virol. 2009;83:9611–5. doi: 10.1128/JVI.00936-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooshyar D, Hanson DL, Wolfe M, Selik RM, Buskin SE, McNaghten AD. Trends in perimortal conditions and mortality rates among HIV-infected patients. AIDS. 2007;21: 2093–2100. doi: 10.1097/QAD.0b013e3282e9a664. [DOI] [PubMed] [Google Scholar]
- Hovi T, Smyth JF, Allison AC, Williams SC. Role of adenosine deaminase in lymphocyte proliferation. Clin Exp Immunol. 1976 Mar;23(3):395–403. [PMC free article] [PubMed] [Google Scholar]
- Hsue PY, Deeks SG, Farah HH, Palav S, Ahmed SY, Schnell A, et al. Role of HIV and human herpesvirus-8 infection in pulmonary arterial hypertension. AIDS. 2008;22: 825–833. doi: 10.1097/QAD.0b013e3282f7cd42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovi-rus-specific T-cell responses. AIDS. 2006;20:2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993 Jul 23;261(5120):466–9. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- Khan N, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. Journal of immunology. 2002;169(4):1984–92. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- Khan N, et al. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. Journal of immunology. 2004;173(12):7481–9. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008 Oct 21;5(10):e203. doi: 10.1371/journal.pmed.0050203. 2570418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Kovacs AA, Zaia A, et al. Seroepidemiologic studies of cytomegalovirus and Epstein-Barr virus infections in relation to human immunodeficiency virus type 1 infection in selected recipient populations. Transfusion Safety Study Group. J Acquir Immune Defic Syndr. 1989;2:540–549. [PubMed] [Google Scholar]
- Lichtner M, Cicconi P, Vita S, Cozzi-Lepri A, Galli M, Lo Caputo S, Saracino A, De Luca A, Moioli M, Maggiolo F, Marchetti G, Vullo V, d’Arminio Monforte A ICONA Foundation Study. Cytomegalovirus coinfection is associated with an increased risk of severe non-AIDS-defining events in a large cohort of HIV-infected patients. J Infect Dis. 2015 Jan 15;211(2):178–86. doi: 10.1093/infdis/jiu417. [DOI] [PubMed] [Google Scholar]
- Looney RJ, et al. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clinical immunology. 1999;90(2):213–9. doi: 10.1006/clim.1998.4638. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Mehta SH, Atta MG, Kirk GD, Galai N, Vlahov D, et al. End-stage renal disease and chronic kidney disease in a cohort of African-American HIV-infected and at-risk HIV-seronegative participants followed between 1988 and 2004. AIDS. 2007;21: 2435–2443. doi: 10.1097/QAD.0b013e32827038ad. [DOI] [PubMed] [Google Scholar]
- Morse CG, Kovacs JA. Metabolic and skeletal complications of HIV infection: the price of success. Jama. 2006;296:844–854. doi: 10.1001/jama.296.7.844. [DOI] [PubMed] [Google Scholar]
- Naeger DM, Martin N, Sinclair E, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One. 2010;5:e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Q, et al. An age-related increase in the number of CD8+ T cells carrying receptors for an immunodominant Epstein-Barr virus (EBV) epitope is counteracted by a decreased frequency of their antigen-specific responsiveness. Mechanisms of ageing and development. 2003;124(4):477–85. doi: 10.1016/s0047-6374(03)00026-5. [DOI] [PubMed] [Google Scholar]
- Ouyang Q, et al. Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Experimental gerontology. 2004;39(4):607–13. doi: 10.1016/j.exger.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Pacheco R, Martinez-Navio JM, Lejeune M, Climent N, Oliva H, Gatell JM, Gallart T, Mallol J, Lluis C, Franco R. CD26, adenosine deaminase, and adenosine receptors mediate costimulatory signals in the immunological synapse. Proc Natl Acad Sci U S A. 2005 Jul 5;102(27):9583–8. doi: 10.1073/pnas.0501050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish ST, Kim S, Sekhon RK, Wu JE, Kawakatsu Y, Effros RB. Adenosine deaminase modulation of telomerase activity and replicative senescence in human CD8 T lymphocytes. J Immunol. 2010;184:2847–54. doi: 10.4049/jimmunol.0903647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello CM, Sinclair E, Landay AL, et al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis. 2012;205:1788–1796. doi: 10.1093/infdis/jis276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathai S, Bajillan H, Alnday AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol Biol Sci Med Sci. 2014;7:833–42. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Larbi A, Derhovanessian E. Senescence of the human immune system. Journal of comparative pathology. 2010;142(Suppl 1):S39–44. doi: 10.1016/j.jcpa.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Pawelec G, et al. Cytomegalovirus and human immunosenescence. Reviews in medical virology. 2009;19(1):47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- Pawelec G, et al. Human immunosenescence: is it infectious? Immunological reviews. 2005;205:257–68. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Perillo NL, Naeim F, Walford RL, Effros RB. The in vitro senescence of human T lymphocytes: failure to divide is not associated with a loss of cytolytic activity or memory T cell phenotype. Mech Ageing Dev. 1993;67:173–185. doi: 10.1016/0047-6374(93)90121-7. [DOI] [PubMed] [Google Scholar]
- Pike C, Birnbaum HG, Schiller M, Sharma H, Burge R, Edgell ET. Direct and indirect costs of non-vertebral fracture patients with osteoporosis in the US. Pharmacoeconomics. 2010;28(5):395–409. doi: 10.2165/11531040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Pietschmann P, Grisar J, Thien R, Willheim M, Kerschan-Schindl K, Preisinger E, Peterlik M. Immune phenotype and intracellular cytokine production of peripheral blood mononuclear cells from postmenopausal patients with osteoporotic fractures. Exp Gerontol. 2001 Nov;36(10):1749–59. doi: 10.1016/s0531-5565(01)00125-5. [DOI] [PubMed] [Google Scholar]
- Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. Exp Med. 1994 Feb 1;179(2):609–18. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector JL, Thomas GN, Burns VE, Dowd JB, Herr RM, Moss PA, Jarczok MN, Hoffman K, Fischer JE, Bosch JA. Elevated HbA1c levels and the accumulation of differentiated T cells in CMV+ individuals. Diabetologia. 2015 Aug 20; doi: 10.1007/s00125-015-3731-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickabaugh TM, Kilpatrick RD, Hultin LE, Hultin PM, Hausner MA, Sugar CA, et al. The dual impact of HIV-1 infection and aging on naive CD4 T-cells: additive and distinct patterns of impairment. PLoS One. 2011;6:e16459. doi: 10.1371/journal.pone.0016459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickabaugh TM, Baxter RM, Sehl M, Sinsheimer JS, Hultin PM, Hultin LE, Quach A, Martinez-Maza O, Horvath S, Vilain E, Jamieson BD. Acceleration of Age-Associated Methylation Patterns in HIV-1-Infected Adults. PLoS One. 2015;10(3):e0119201. doi: 10.1371/journal.pone.0119201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaltz HN, et al. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. Journal of the American Geriatrics Society. 2005;53(5):747–54. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- Sijmons S, Thys K, Mbong Ngwese M, Van Damme E, Dvorak J, Van Loock M, Li G, Tachezy R, Busson L, Aerssens J, Van Ranst M, Maes P. High-throughput analysis of human cytomegalovirus genome diversity highlights the widespread occurrence of gene-disrupting mutations and pervasive recombination. J Virol. 2015 May 13; doi: 10.1128/JVI.00578-15. pii: JVI.00578-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit C, Geskus R, Walker S, Sabin C, Coutinho R, Porter K, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20: 741–749. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- Soland MA, Keyes LR, Bayne R, Moon J, Porada CD, St Jeor S, Almeida-Porada G. Perivascular stromal cells as a potential reservoir of human cytomegalovirus. Am J Transplant. 2014 Apr;14(4):820–30. doi: 10.1111/ajt.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern-Ginossar N, Weisburd B, Michalski A, Le V, Hein MY, Huang S, Ma M, Shen B, Qian S, Hengel H, Mann M, Ingolia NT, Weissman JS. Decoding human cytomegalovirus. Science. 2012;338:1088–93. doi: 10.1126/science.1227919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011 Jan 5;305(1):50.3080184. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrazzini N, Kern F. Cell-mediated immunity to human CMV infection: a brief overview. F 1000 Prime Rep. 2014 May 6;6:28. doi: 10.12703/P6-28.eCollection2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Doherty SM. HIV infection—a risk factor for osteoporosis. J Acquir Immune Defic Syndr. 2003;33: 281–291. doi: 10.1097/00126334-200307010-00001. [DOI] [PubMed] [Google Scholar]
- Topp MS, Riddell SR, Akatsuka Y, Jensen MC, Blattman JN, Greenberg PD. Restoration of CD28 expression in CD28− CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J Exp Med. 2003;198:947–955. doi: 10.1084/jem.20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92: 2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JE, Campbell JP, Edwards KM, Howarth LJ, Pawelec G, Aldred S, Moss P, Drayson MT, Burns VE, Bosch JA. Rudimentary signs of immunosenescence in Cytomegalovirus-seropositive healthy young adults. Age (Dordr) 2014 Feb;36(1):287–97. doi: 10.1007/s11357-013-9557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemori P, Leslie KS, Hunt PW, Sinclair E, Epling L, Mitsuyasu R, Effros RB, Dock J, Dollard SG, Deeks SG, Martin JN, Maurer TA. Immunosenescence is associated with presence of Kaposi’s sarcoma in antiretroviral treated HIV infection. AIDS. 2013 Jul 17;27(11):1735–42. doi: 10.1097/QAD.0b013e3283601144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Archives of Internal Medicine. 2006;166: 1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DW, Suzanne Beard R, Barton ES. Immune modulation during latent herpesvirusinfection. Immunol Rev. 2012 Jan;245(1):189–208. doi: 10.1111/j.1600-065X.2011.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XW, Wu L, Ji HZ, Wang FY. Relationship Between Cytomegalovirus Infection and Steroid Resistance in Inflammatory Bowel Disease: A Meta-Analysis. Dig Dis Sci. 2015 Jun 2; doi: 10.1007/s10620-015-3733-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]