Abstract
The growth hormone/insulin-like growth factor (GH/IGF) axis is critically important for the regulation of bone formation, and deficiencies in this system have been shown to contribute to the development of osteoporosis and other diseases of low bone mass. The GH/IGF axis is regulated by a complex set of hormonal and local factors which can act to regulate this system at the level of the ligands, receptors, IGF binding proteins (IGFBPs), or IGFBP proteases. A combination of in vitro studies, transgenic animal models, and clinical human investigations has provided ample evidence of the importance of the endocrine and local actions of both GH and IGF-I, the two major components of the GH/IGF axis, in skeletal growth and maintenance. GH- and IGF-based therapies provide a useful avenue of approach for the prevention and treatment of diseases such as osteoporosis.
Keywords: Osteoporosis, Skeletal development, Growth hormone (GH), Insulin-like growth factors (IGFs), IGF binding proteins (IGFBPs)
1. Introduction
The dynamic process of bone remodeling involves the complex and coordinated interaction of osteoblast lineage cells, which form bone, and osteoclast lineage cells, which resorb bone. This process occurs at all stages of growth and development throughout the lifespan, and myriad systemic and local factors participate in the regulation of osteoblast and osteoclast function. Of particular note, the growth hormone/insulin-like growth factor (GH/IGF) axis plays an important role in the regulation of bone remodeling.
The first major component of the GH/IGF axis is GH, a single-chain polypeptide hormone which is secreted in a pulsatile manner by the anterior pituitary gland in response to hypothalamic stimulation by growth hormone releasing hormone (GHRH) and inhibition by somatostatin (SS) (Veldhuis and Bowers, 2003). Although GH can directly affect cells of various tissues, including osteoblasts and epiphyseal growth-plate chondrocytes (Wang et al., 2004), GH stimulates longitudinal bone growth primarily by activation of hepatic IGF-I production.
IGF-I and –II are small peptide hormones with significant structural similarity to insulin. While IGF-II is important for intrauterine growth, IGF-I regulates skeletal growth and maintenance during postnatal life. IGFs have endocrine actions, exerted systemically through the circulation, and local actions, exerted in an autocrine/paracrine manner (Mohan and Kesavan, 2012). GH-induced hepatic IGF-I secretion is responsible for systemic, circulating IGF-I while IGF-I production in peripheral tissues is controlled by a multitude of interacting endocrine and local signals, some of which are GH-independent (Mohan et al., 2003).
The GH/IGF axis significantly regulates both longitudinal bone growth, which is important during childhood, and appositional bone growth, which is important for bone maintenance in adulthood. This chapter will examine the role of the GH/IGF axis in the regulation of bone remodeling and the potential of GH/IGF-based therapies to treat and prevent osteoporosis and other diseases of low bone mass.
2. The GH/IGF axis
2.1 GH secretion and signaling
Many factors work together to produce distinct profiles of pulsatile GH secretion which vary depending on age, sex, and species (Clark et al., 1987; Edén, 1979; Saunders et al., 1976). Hypothalamic GHRH and SS respectively simulate and inhibit pituitary somatotroph secretion of GH (Brazeau et al., 1973; Ling et al., 1984; Spiess et al., 1983). Additionally, several factors interact with the hypothalamus and pituitary to stimulate GH release in response to an organism’s metabolic state, consistent with GH’s dual role in regulating both metabolism and growth. Along these lines, free fatty acids act at the level of the pituitary to inhibit GH secretion, providing negative feedback to counteract GH’s stimulation of lipid release (Imaki et al., 1985; Muggeo et al., 1975). Leptin, a so-called energy-sensing hormone released by adipocytes, modulates GHRH and SS action to trigger hypothalamic GH release (Carro et al., 1997; Tannenbaum et al., 1998; Vuagnat et al., 1998). Furthermore, a class of several peptide and non-peptide compounds which stimulate GH release have been identified and synthesized. These compounds have been termed GH-releasing peptides (GHRPs) or GH secretagogues (GHSs) (Smith et al., 1997). Compounds of note in this class include the hexapeptides GHRP-1, GHRP-2, GHRP-6, and hexarelin (Deghenghi et al., 1994) as well as the non-peptide GHRP mimetic MK-0677 (Patchett et al., 1995), some of which exhibit considerable oral bioavailability and activity (Ghigo et al., 1994). These compounds stimulate GH release via the GHS receptor (GHS-R) (Howard et al., 1996; Smith et al., 1996), a G protein-coupled receptor whose endogenous ligand, ghrelin, was later discovered and identified as a 28 amino acid peptide produced mainly in the stomach (Kojima et al., 1999). GH production in response to ghrelin has been demonstrated in both rats and humans (Takaya et al., 2000; Wren et al., 2000). In addition, sex steroids have been shown to regulate the secretion and action of GH (Ho et al., 1996). A complex interaction of many factors regulates the secretion of GH, which exerts its effects on tissues throughout the body.
The binding of GH to the GH-receptor (GHR) initiates a signal transduction cascade responsible for the actions of GH on peripheral tissues. The GHR, a transmembrane protein which is almost ubiquitously expressed on cells of all tissues, undergoes several posttranscriptional and posttranslational modifications, the most notable of which is the formation of soluble GH binding protein (GHBP) from the extracellular ligand-binding domain of the GHR (Baumann et al., 1986; Leung et al., 1987). Activation of the GHR induces the Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling pathway. GH binding to the GHR causes the GHR to associate with and activate JAK2, a receptor-associated tyrosine kinase. Activated JAK2 phosphorylates tyrosine residues on STAT-1, -3, -5a, and -5b (Brooks et al., 2014; Carter-Su and Smit, 1998), which, in turn, undergo nuclear translocation and act as transcription factors, binding directly to specific DNA sequences and modulating gene expression (Leonard and O’Shea, 1998; Takeda and Akira, 2000), leading especially to the upregulation of IGF-I expression. GHR is also known to interact with IGF and insulin receptor signaling pathways via phosphorylation of insulin receptor substrates IRS-1, -2, and -3, leading to activation of phosphatidylinositol (PI) 3′-kinase (Argetsinger et al., 1996; Herrington and Carter-Su, 2001; Souza et al., 1994). In addition, GHR can interact directly with IGF-IR, even in the absence of IGF binding, to modulate GH signaling (Gan et al., 2014a, 2014b). GH signaling may also occur through influx of extracellular calcium ions through voltage-dependent L-type calcium channels (Gaur et al., 1996) and increased intracellular diacylglycerol (DAG), leading to protein kinase C (PKC)-mediated activation of MAP kinases, p90rsk, and c-fos induction (Anderson, 1992; Campbell et al., 1992; Smal and De Meyts, 1987). Suppressor of cytokine signaling (SOCS) proteins accomplish further regulation of GH signaling through inhibition of the JAK-STAT pathway, leading to either suppression or augmentation of GH signaling (Corva et al., 2004; Greenhalgh et al., 2002; Krebs and Hilton, 2001; Ram and Waxman, 1999). GH signaling occurs through the interaction of several signaling pathways triggered by GHR activation.
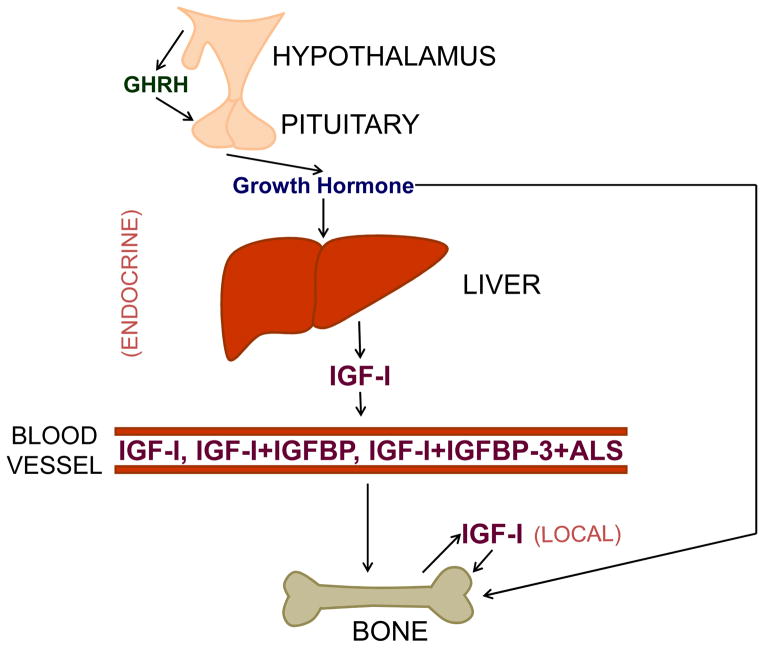
2.2 GH effects: with and without IGF
Many of the actions of GH are mediated through production of IGF-I, widely accepted as a key regulator of skeletal growth, development, and metabolism (Locatelli and Bianchi, 2014; Mohan and Kesavan, 2012; Yakar et al., 2010). The preponderance of circulating IGF-I is produced by the liver in response to GH stimulation; thus, the original somatomedin hypothesis proposed that GH induces skeletal growth only indirectly via stimulation of hepatic IGF-I production (Salmon and Daughaday, 1957) (Fig. 1). However, additional studies suggest that GH can have direct effects on chondrocytes (Isaksson et al., 1982; Schlechter et al., 1986); the more recently proposed dual effector theory of GH action accounts for the inability of IGF-I to reproduce all of GH’s effects (Green et al., 1985). Besides the effects of increased IGF-I expression, GH can directly stimulate the differentiation of prechondrocytes (Lindahl et al., 1987, 1986; Ohlsson et al., 1992), leading to longitudinal growth at the epiphyseal growth plate, data which have been recently confirmed by growth plate-specific postnatal knockout of the IGF-IR in mice (Wu et al., 2015). Additionally, targeted disruption of the GHR in osteoblasts suggests that, besides its stimulation of local IGF-I action, GH may play a role in directly regulating the development of skeletal sexual dimorphism in mice (Singhal et al., 2013). The intracellular molecular mechanisms that contribute to IGF-independent, growth-promoting effects of GH are yet to be identified.
Fig. 1.
Model overview of GH/IGF-I regulation of skeletal growth, including both endocrine and local actions of IGF-I. GH acts by increasing hepatic IGF-I production, by increasing local skeletal IGF-I production, and by influencing the bone directly, independent of IGF-I. Hepatic IGF-I acts in an endocrine manner, circulating in the blood primarily as a ternary complex with acid-labile subunit (ALS) and IGF binding protein (IGFBP)-3, although IGF-I can also complex with other IGFBPs. Only a small amount circulates as free IGF-I. Locally produced IGF-I acts on the bone in an autocrine/paracrine manner.
2.3 IGF expression and signaling
Systemic regulation of IGF-I expression occurs via the effects of several hormones in addition to GH. Notably, parathyroid hormone (PTH), 17β-estradiol, and thyroid hormone (TH) are known to upregulate IGF-I expression in osteoblasts (Bikle and Wang, 2012; Ernst and Rodan, 1991; Gray et al., 1989; McCarthy et al., 1997; Xing et al., 2012) while glucocorticoids and 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3] can downregulate IGF-I expression (Canalis, 2005; Chen et al., 1991; Scharla et al., 1991). The ability of PTH to stimulate osteoblast proliferation, differentiation, and survival as well as collagen synthesis, alkaline phosphatase (ALP) activity, and osteocalcin expression appears to be dependent upon local IGF-I production (Linkhart and Mohan, 1989; McCarthy et al., 1989), data which have been confirmed in vivo by genetic disruption of IGF-I (Bikle et al., 2002; Miyakoshi et al., 2001a) or IRS-1 (Yamaguchi et al., 2005) in mice. Sex steroids stimulate a rise in IGF-I expression during pubertal growth (Christoforidis et al., 2005; Styne, 2003; Veldhuis et al., 1997); the effect of androgens is dependent on aromatization (Weissberger and Ho, 1993), and the effect of estrogens is dependent on dose with high doses decreasing circulating free IGF-I (Veldhuis et al., 2005; Weissberger et al., 1991). In prepubertal mice, TH has been shown to have a greater effect on skeletal growth than GH, and this effect is mediated by an increase of IGF-I expression in both the liver and bone (Xing et al., 2012). Similarly, clinical studies in humans have shown that correction of serum TH levels with methimazole treatment in hyperthyroid patients is sufficient to lower elevated serum IGF-I levels to within normal limits (Lakatos et al., 2000). IGF-I expression levels increase with TH treatment in osteoblasts and chondrocytes (Lakatos et al., 1993; Xing et al., 2012) as there is a TH response element in the promoter of the IGF-I gene which stimulates transcription when bound by a complex of TH and TH receptor-α (Xing et al., 2012). The TH effect on IGF-I may also be modulated by interaction with the sympathetic nervous system (Fonseca et al., 2014). Thus, IGF-I has been predicted to be a critical mediator of TH effects on the skeleton.
Glucocorticoids have been shown to decrease osteoblast proliferation and function, resulting in reduced trabecular bone formation (Canalis, 2005, 1993; van der Eerden et al., 2003). These changes are associated with a fall in production of both IGF-I and stimulatory IGF binding proteins (Canalis, 2005; Cheng et al., 1998; Chevalley et al., 1996) as well as decreased basal and IGF-induced chondrocyte proliferation (Jux et al., 1998). Another stress hormone, prostaglandin E2 (PGE2), can increase IGF-I production via increased cAMP synthesis (Thomas et al., 1996), while hypoxic stress can interact with stress hormones to ultimately reduce IGF-I expression (McCarthy et al., 2014).
In addition to systemic regulation of IGF expression, local regulators also play an important role. Fibroblast growth factor 2, transforming growth factor-β1, bone morphogenetic protein 7, and interleukin-1 are locally produced growth factors which influence IGF-I production (Knutsen et al., 1995; McCarthy and Centrella, 2001; Tremollieres et al., 1991; X. Zhang et al., 2002). Moreover, the introduction of mechanical strain to bones rapidly increases IGF-I expression (Lean et al., 1996, 1995; Triplett et al., 2007; Xing et al., 2005); in fact, in one study, 10 minutes of mechanical loading was enough to increase IGF-I levels in osteocytes of the loaded bone in rats (Lean et al., 1996). Studies of mechanical strain using TE85 cells and primary human osteoblasts in vitro have shown that the proliferative response of osteoblasts to the combination of mechanical strain and estrogen is dependent on the estrogen receptor-α and the IGF-I receptor (IGF-IR) (Cheng et al., 2002, 1999; Rawlinson et al., 1993). In vivo, mice overexpressing IGF-I in osteoblasts have a several-fold greater increase in periosteal bone formation in response to low-magnitude, noninvasive loading compared to wild-type mice, which had no response to low-magnitude loading (Gross et al., 2002). Furthermore, the removal of normal loading in rats deficient in GH blocked the ability of IGF-I treatment to induce bone formation (Sakata et al., 2004, 2003). The mechanical effect on IGF-I expression is known to be mediated via an integrin-dependent process in which IGF-IR is phosphorylated and the phosphatases SHP-1 and -2 are recruited away from IGF-IR (Kapur et al., 2005; Lau et al., 2006). Additionally, the integrin-requiring resistance to IGF-I induced by skeletal unloading has been shown to be a unique feature of IGF-I signaling, over and against platelet-derived growth factor (PDGF) signaling (Long et al., 2011). More recently, the link between IGF-I upregulation and bone formation in response to mechanical loading has been more definitively demonstrated through the use of conditional IGF-I gene disruption in mouse osteoblasts expressing type 1α collagen (Kesavan et al., 2011). Osteocyte-derived IGF-I has also been shown to be a key determinant in bone mechanosensitivity, as well as bone growth, remodeling, and regeneration (Lau et al., 2013; Sheng et al., 2014), although it is not required for bone repletion after a low-calcium challenge (Lau et al., 2015). Regulation of IGF-I expression is critically important and is accomplished a multitude of diverse systemic and local factors and their interactions.
The effects of IGF-I are mediated through binding to the IGF-IR, a transmembrane receptor tyrosine kinase arranged in an α2β2-configuration in which the extracellular α-subunits form a binding pocket which, upon IGF binding, causes autophosphorylation and activation of the intracellular tyrosine kinase domains of the β-subunits (LeRoith et al., 1995; Sasaki et al., 1985; Steele-Perkins et al., 1988). IGF-I and -II act physiologically in osteoblasts by binding the IGF-IR (Centrella et al., 1990; Slootweg et al., 1990), which shares significant structural similarity with the related insulin receptor (IR). Both the IR and IGF-IR signal primarily through phosphorylation of IRS-I and -II (Kadowaki et al., 1996), both of which are necessary for proper bone metabolism; mice deficient in IRS-I display significant osteopenia (Ogata et al., 2000) and impaired bone healing (Shimoaka et al., 2004) while osteoblasts lacking IRS-2 show impaired anabolic activity and increased support of osteoclastogenesis (Akune et al., 2002). Indeed, dysregulation of some of the metabolic actions of IGF signaling in conditions such as type 2 diabetes mellitus or sarcopenia in the elderly could provide a connection between these conditions and the osteoporosis often observed in states of chronic disease. In addition, the extent to which the hepatic effects of the GH/IGF axis on bone are influenced by changes in hepatic glucose or lipid metabolism as would be seen in diabetes remains yet to be determined. However, it is clear that IGF signaling is critical for bone development and maintenance.
2.4 IGF binding proteins
IGF-I is regulated via interaction with several binding proteins, known as IGFBP-1 through -6. Although there is a relatively large amount of IGF-I in circulation, up to 75% of IGF-I in plasma exists in a ternary complex with IGFBP-3 and an acid-labile subunit (ALS); IGFBP-3 regulates the active concentration of free IGF-I (Bagi et al., 1994; Jones and Clemmons, 1995; Rajaram et al., 1997; Rosen et al., 1994). IGFBPs can have opposing effects on IGF actions. IGFBPs-3 and -5 are generally regarded as stimulating IGF-I’s effects on osteoblasts; they bind IGF-I in ternary complexes and prolong its half-life in circulation. IGFBP-5 in particular will associate with cell surface proteins, locally increasing IGF concentrations in the areas most likely to encourage binding with IGF-IRs. In addition, IGFBP-5 has been shown to act independent of IGFs (Govoni et al., 2005). By contrast, IGFBPs-1, -2, -4, and -6 are considered inhibitory; they bind IGF-I and prevent interaction with the IGF-IR (Govoni et al., 2005). For example, overexpression of IGFBP-4 in mouse osteoblasts leads to decreased bone size parameters, consistent with an increased inhibition of IGF-I activity (Zhang et al., 2003). A number of systemic agents have been shown to differentially regulate IGFBP production in varying cell types. PTH can increase expression of IGFBP-4 (Honda et al., 1996), and 1,25-(OH)2D3 has been shown to increase production of IGFPBs-2, -3, and -4 (Kveiborg et al., 2001; Scharla et al., 1993). Glucocorticoids increase expression of inhibitory IGFBPs-2, -4, and -6 while decreasing expression of IGFBPs-3 and -5 (Chevalley et al., 1996; Gabbitas and Canalis, 1996a; Okazaki et al., 1994), and a portion of estrogen’s suppression of bone formation may be due to upregulation of IGFBP-4 expression (Denger et al., 2008; Kassem et al., 1996). Indeed, a strong interaction between IGFBP-2 and estrogen has been suggested, as Igfbp2−/− mice show accelerated bone loss in the context of ovariectomy (DeMambro et al., 2015). Retinoic acid similarly causes an increase in inhibitory IGFBPs-4 and -6 along with a corresponding decrease in stimulatory IGFBP-5 (Gabbitas and Canalis, 1996b; Zhou et al., 1996). Local factors, including IGFs, TGF-β, BMPs, FGF, PDGF, interleukins, and IGFBP proteases, are additional regulators of IGFBP expression and activity in bone (Conover, 1995; Hayden et al., 1997; Kanzaki et al., 1994; Knutsen et al., 1995; Malpe et al., 1997; Qin et al., 2006). IGFBP actions are acutely regulated by IGFBP proteases in response to systemic and local cues. For example, studies involving transgenic overexpression and targeted knockout of pregnancy-associated plasma protein (PAPP)-A, a specific IGFBP-4 protease, have shown a key role for this protease in regulating skeletal metabolism via modulation of IGF bioavailability (Qin et al., 2006; Tanner et al., 2008). Another study showed that PAPP-A2, an IGFBP-5 protease, regulates bone size and shape (Christians et al., 2013). Thus, modulation of IGFBP protease activity may represent a therapeutic strategy to increase IGF bioavailability and to promote IGF actions in target tissues.
2.5 IGF-I action: endocrine vs. local
Although circulating IGF-I is produced largely by the liver, IGF-I is expressed by cells of all tissue types, including osteoblasts and chondrocytes. Conditional disruption of hepatic IGF-I (Sjögren et al., 1999; Yakar et al., 1999) or GHR (Fan et al., 2009) in transgenic mouse models has shown that longitudinal bone growth was largely unaffected by up to 90% reduction in circulating IGF-I levels, although trabecular bone volume was significantly decreased in hepatic GHR-deficient mice. To further confirm the relative lack of a skeletal phenotype in mice lacking hepatic IGF-I, double and triple knockout mice were generated, which lack either total ALS or total ALS and total IGFBP-3 in addition to lacking hepatic IGF-I (Yakar et al., 2009, 2002). In these mice, circulating serum IGF-I levels were reduced by 90% and 97.5%, respectively, and although the triple knockout mice showed a significant decrease in body length, the effect was relatively small (Table 1). By contrast, the triple knockout mice exhibit a 50% reduction in cortical bone which is similar in magnitude to the reduction in cortical bone observed in total IGF-I knockout mice (Mohan et al., 2003), indicating that endocrine IGF-I plays a more significant role in regulating bone size and may act by a different mechanism in regulating bone length versus bone width/size. Indeed, cortical bone size and periosteal expansion are primary determinants of bone strength; the ability of IGF-I to increase periosteal bone formation provides an important basis for the potential use of IGF-I to treat and prevent osteoporosis and osteoporotic fractures. Furthermore, a mouse model with hepatic IGF-I knock-in in an IGF-I null background revealed a roughly 30% rescue of body size by endocrine IGF-I action (Stratikopoulos et al., 2008), and further studies in mice with hepatic disruption of the GHR (List et al., 2014) and JAK2-mediated GH signaling (Nordstrom et al., 2011) suggest that endocrine IGF-I action does indeed contribute to overall skeletal growth.
Table 1.
Skeletal changes in transgenic mouse models of disrupted endocrine and local IGF-I actions.
| Mode of action | Gene(s) altered | Bone length | Periosteal circumference | BMD |
|---|---|---|---|---|
| Endocrine + Local | Total IGF-I KO 1 | 40% decrease | 38% decrease | 32% decrease |
|
| ||||
| Endocrine | Hepatic IGF-I KO 2 | 6% decrease | 11% decrease | 9% decrease |
| Endocrine | Total ALS KO 2 | 7.5% decrease | 18% decrease | 9% decrease |
| Endocrine | Hepatic IGF-I + Total ALS KO 2 | 20% decrease | 33% decrease | 6% decrease |
|
| ||||
| Local | Osteoblast IGF-I KO 3 | 15% decrease | 24% decrease | 25% decrease (DXA) 7 5% decrease (pQCT) |
| Local | Osteocyte IGF-I KO 4 | 5% decrease | 12% decrease | No change |
| Local | Chondrocyte IGF-I KO 5 | 6% decrease | 4% decrease | 5% decrease (DXA) 7 |
| Local | Osteoblast IGFBP-4 OE 6 | 14% decrease | 29% decrease | No change |
Abbreviations: BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; KO, knock-out; OE, overexpression; pQCT, peripheral quantitative computed tomography.
(Sheng et al., 2013)
BMD measured by DXA may be affected by bone size.
The role of local IGF-I acting in an autocrine and/or paracrine manner is also important to bone function. IGF-I is both produced by skeletal tissues and stored in mineralized matrix from which it can be released during matrix degradation and bone remodeling. Without locally produced IGFs in serum-free cultures, osteoblast proliferation was reduced by almost 50%, indicating a role for local IGF production in basal osteoblast proliferation (Mohan et al., 1989). Furthermore, mice overexpressing IGF-I only in mature osteoblasts which express osteocalcin showed augmented volume and rate of formation of trabecular bone by increasing osteoblast activity rather than number (Zhao et al., 2000). Similarly, IGF-I overexpression in osteoblasts using rat type I collagen αI regulatory elements showed increases in measures of bone formation and bone resorption (Jiang et al., 2006). Moreover, overexpressing other components of the IGF system which modulate local IGF bioavailability, such as IGFBPs and their corresponding proteases, also indicates that local production of IGF-I is important for bone formation (Devlin et al., 2002; Miyakoshi et al., 2001b, 1999; Qin et al., 2006; Richman et al., 1999; Zhao et al., 2000). These findings have been further confirmed both by using the osteocalcin promoter to disrupt the IGF-IR (M. Zhang et al., 2002) and by using the type I collagen α2 promoter to disrupt IGF-I (Govoni et al., 2007b) specifically in osteoblasts; in these studies, serum IGF-I levels were unchanged while significant reductions in bone mineral density (BMD), bone size, and bone formation indices were observed (Table 1). In addition, the GH effect on osteoblast number depends on local IGF-I production (DiGirolamo et al., 2007).
Furthermore, IGF-I expression in cartilage plays a crucial role in bone development. Targeted disruption of IGF-I in chondrocytes results in decreased bone length, total body areal BMD, and bone width (Govoni et al., 2007a); the corresponding decrease in parathyroid hormone-related protein (PTHrP), Dlx-5, and Sox-9 expression in the bones of these mice indicates that locally produced IGF-I may regulate bone growth in part by modulating chondrocyte differentiation and proliferation. In addition, chondrocyte-specific IGF-I is needed for proper growth plate organization and function (Wang et al., 2015, 2011). The importance of both endocrine and locally produced IGF-I for skeletal growth is clear.
3. GH/IGF effects on bone
3.1 Bone acquisition
Though GH is largely not required for intrauterine growth (Gluckman et al., 1981; Le Roith et al., 2001), GH contributes to longitudinal bone growth and peak BMD acquisition during postnatal and pubertal growth. This effect can be seen both in mice, in which GH overexpression increases BMD (Saban et al., 1996) and lit/lit GH-deficient or GHR knockout mice have poor peak BMD (Mohan et al., 2003; Sims et al., 2000; Sjögren et al., 2000), and in humans, in which childhood onset GH deficiency leads to decreased BMD and increased fracture risk in adulthood (de Boer et al., 1994; Holmes et al., 1994; Rosén et al., 1997; Simpson et al., 2002). GH supplementation in normal human subjects increases levels of serum bone formation markers (Holloway et al., 1994), and treatment of both animals and patients deficient in GH significantly increases their reduced BMD (Bouillon and Prodonova, 2000; Cowell et al., 2000; Simpson et al., 2002). Many of these effects of GH, however, are dependent upon age and growth period; GH deficiency leads to a 4-fold greater reduction in BMD during postpubertal growth than during prepubertal growth (Mohan et al., 2003), an effect which may be explained in part by the failure of GH to appropriately activate hepatic gene transcription in prepubertal rats (Choi and Waxman, 2000). In addition, the magnitude of GH’s ability to rescue BMD in GH-deficient mice depends on the age of GH administration (Kasukawa et al., 2003).
Many of the effects of GH are mediated via IGF-I. Patients with GH insensitivity, particularly Laron syndrome caused by to mutations in the GHR, produce little to no IGF-I or IGFBP-3, resulting in decreased longitudinal bone growth; short body length at birth is followed by short stature during and after growth (Laron and Kauli, 2015). Mouse models of GH insensitivity indicate significant reductions in cortical bone parameters as well as measures of endosteal and periosteal bone formation (Wu et al., 2013). In GH-insensitive patients, IGF-I administration can stimulate some longitudinal bone growth, although it cannot fully compensate for the absent GH effects (Collett-Solberg et al., 2008; Laron and Kauli, 2015; Laron and Klinger, 1994; Pfäffle, 2015; Walker et al., 1992; Wu et al., 2013). Furthermore, GH and IGF synergize to produce greater effects than either would independently (Fielder et al., 1996). IGF-I has an augmented effect on osteoblasts when GH and IGFBP-3 are present (Ernst and Rodan, 1990); similarly, GH administration to mice which have been given saturating doses of IGF-I causes a further increase in growth (Hazel et al., 1994).
Although IGF-II is crucial for growth during the embryonic period, IGF-I is required for growth throughout development (Baker et al., 1993; Liu et al., 1993). Targeted IGF-I gene disruption in mice leads to significantly reduced femoral length, size, and BMD (Mohan et al., 2003); histomorphometric measures also show reduced bone formation and mineral apposition rates in IGF-I knockout mice (Bikle et al., 2001). These effects of IGF-I are the result of a combination of GH-dependent and GH-independent mechanisms (Mohan et al., 2003; Mohan and Baylink, 2005; Xing et al., 2012). However, IGF-I knockout led to increased trabecular bone volume in the proximal tibia, resulting from increased trabecular connectivity, number, and spacing, and this effect was lessened in the lumbar vertebrae (Bikle et al., 2001). These conflicting data suggest a complex role for IGF-I in regulating bone structure, which may vary in cortical vs. trabecular bone compartments and in appendicular vs. axial skeletal regions. The effect of IGF-I deficiency has been confirmed in humans, in which patients with a mutant IGF-IR show growth retardation both before and after birth, and a mutant IGF-I gene can lead to significantly decreased BMD (Camacho-Hübner et al., 2002; Walenkamp et al., 2005; Walenkamp and Wit, 2007). Furthermore, a recent study showed a significant association between polymorphisms in the IGF-I gene and risk of osteoporosis in a population of Chinese women (Zhang et al., 2015). Thus, both GH and IGF-I are key regulators of peak bone mass acquisition in animals and in humans.
3.2 Bone loss
An additional IGF-independent ability of GH is to increase bone resorption and turnover (Colao et al., 1999; Thomas and Monson, 2009), as seen in the increased risk of fracture in patients with excess GH (Claessen et al., 2013; Mazziotti et al., 2013, 2008; Mormando et al., 2014; Padova et al., 2011; Wassenaar et al., 2011) and the deterioration of bone architecture and strength observed in GH-overexpressing mice (Lim et al., 2015). This increase in bone turnover may be mediated at least in part by increased expression of inflammatory cytokines such as interleukin-6 (Swolin and Ohlsson, 1996).
However, the more significant manner in which the GH/IGF system contributes to bone loss is through its overall decline with age. GH secretion is known to decrease as an individual ages (Corpas et al., 1993; Müller et al., 1999), a result of the confluence of any of a number of multiple factors including decreased GHRH stimulation, increased somatostatin inhibition, decreased sex steroid levels, lower levels of physical activity, poor sleeping patterns, inadequate nutrition, and increased negative feedback from GH and IGF-I (Bartke, 1998; Misra et al., 2003; Veldhuis and Bowers, 2003; Veldhuis and Iranmanesh, 1996). In addition, serum and bone IGF-I decrease in both concentration (Benbassat et al., 1997; Seck et al., 1999) and effectiveness at stimulating osteoprogenitor cells (Tanaka and Liang, 1996) with age, and IGF-I levels correlate well with BMD in postmenopausal women and men with or without osteoporosis (P. Gillberg et al., 2002; Muñoz-Torres et al., 2001). IGFBPs also change with age (Karasik et al., 2002; Mohan et al., 1995; Nicolas et al., 1995). In fact, a cross-sectional study of elderly women with femoral neck fractures revealed significantly decreased levels of IGF-I, IGF-II, IGFBP-3, and IGFBP-5, suggesting deficiencies in components of the IGF system may predispose elderly women to fracture (Boonen et al., 1999). However, there are also reports in the literature that show little to no effects of IGFs on fracture risk (Gillberg et al., 2001; Kassem et al., 1994b; Martini et al., 2001; Seck et al., 1999; Zofková et al., 2001). Thus, the extent to which IGF system components contribute to age-related bone loss is yet to be determined.
4. GH/IGF therapy
4.1 Therapy in children
Childhood deficiency in major components of the GH/IGF axis results in severely impaired longitudinal bone growth and failure to attain normal height in adulthood. Thus, treatment with GH or IGF-I is a logical therapy for children with such conditions. In fact, long-term administration of GH in children with GH-deficient conditions including isolated GH deficiency and multiple pituitary hormone deficiency significantly improved age-adjusted height in a manner consistent with growth approaching the children’s genetic height potential (Ross et al., 2015). GH has even been effectively used to promote growth in children without proven GH deficiencies, born small for gestational age or with idiopathic short stature. However, further studies are needed to determine the safety and magnitude of increase in final height resulting from long-term GH therapy in all patient groups (Pfäffle, 2015). Furthermore, children with GH insensitivity, most commonly caused by resistance to GH due to mutations in the GHR (called Laron syndrome, described in section 3.1), have been successfully treated with IGF-I (Laron and Kauli, 2015). The inability of IGF-I to fully compensate for the lack of GH action in these patients may be attributable to the lack of IGFBP-3 carrying, stabilizing, and enhancing IGF-I in circulation as well as the lack of direct GH stimulation in bone (Pfäffle, 2015).
4.2 Therapy in adults
Since GH and IGF-I appear to be involved in age-related and osteoporotic bone loss, multiple clinical trials have been run to evaluate the possible therapeutic effects of GH and IGF-I administration in patients with and without osteoporosis. The first trial of GH therapy in patients with primary and secondary osteoporosis found increased periosteal bone formation and osteoblast activity, along with increased bone turnover (Kruse and Kuhlencordt, 1975). Since then, the effects of GH therapy have been assessed in healthy subjects (Bianda et al., 1997; Brixen et al., 1990; Holloway et al., 1994; Rudman et al., 1990), women with postmenopausal osteoporosis (Brixen et al., 1995; Clemmesen et al., 1993; Erdtsieck et al., 1995; Holloway et al., 1997, 1994; Joseph et al., 2008; Kassem et al., 1998, 1994a, 1994b; Landin-Wilhelmsen et al., 2003; Sääf et al., 1999; Sugimoto et al., 2002, 1999), and men with idiopathic osteoporosis (Peter Gillberg et al., 2002; Johansson et al., 1996). Overall, GH treatment has been well-tolerated, with few side effects beyond fluid retention and carpal tunnel syndrome. GH administration has resulted in increased bone turnover with a net anabolic effect and increased BMD (Locatelli and Bianchi, 2014). However, a minority of studies showed no net improvement with GH treatment (Clemmesen et al., 1993; Erdtsieck et al., 1995; Holloway et al., 1994; Sääf et al., 1999); in these cases, the lack of a positive GH effect is likely due to a combination of confounding factors such as poor nutrition as well as the low dosage of GH administered. In addition, dysregulation of PTH secretion patterns and peripheral PTH sensitivity are rescued by GH treatment in women with postmenopausal osteoporosis, further contributing to an increased BMD (Joseph et al., 2008; White et al., 2005). By contrast, concomitant estrogen administration attenuated GH effects on bone (Holloway et al., 1994), lending confirmation to the previous observation that oral estrogen administration decreases hepatic IGF-I production (Ho and Weissberger, 1992). Since GH treatment has been shown to increase both bone formation and resorption, GH therapy has not been evaluated fully to treat osteoporotic patients. Promisingly, a recent study has reported that GH therapy in postmenopausal women with osteoporosis significantly improved BMD and fracture outcomes after ten years, although overall quality of life measures were unaffected (Krantz et al., 2015). What effect this report will have on the use of GH to treat osteoporosis remains to be seen.
Moreover, exogenous GH administration has been used to supplement GH-deficient adults, whose symptoms can include metabolic syndrome, osteoporosis, muscle wasting, and overall diminished quality of life (Tzanela, 2007). In general, GH replacement in GH-deficient adults has been shown to increase BMD in the context of augmented markers of bone formation and decreased markers of bone resorption (Kužma et al., 2014). However, a recent study suggests that GH only reduces the incidence of fractures in GH-deficient adults without preexisting osteoporosis; it appears that GH therapy cannot compensate for the combined effects of GH deficiency and osteoporosis (Mo et al., 2015). A possible interpretation of these data would suggest that beginning GH administration in these GH-deficient patients before the onset of osteoporosis might prevent future fractures.
Additionally, recombinant human IGF-I (rhIGF-I) has also been investigated in several clinical studies for the treatment of osteoporosis. On the whole, IGF-I therapy resulted in significantly increased bone turnover and improved bone formation, with no significant adverse effects reported (Locatelli and Bianchi, 2014). IGF-I administration has been studied in healthy subjects (Bianda et al., 1997; Ghiron et al., 1995; Mauras et al., 1996), fasting subjects (Grinspoon et al., 1995), anorexic/osteopenic subjects (Grinspoon et al., 2002, 1996; Misra et al., 2009), postmenopausal women with and without osteoporosis (Ebeling et al., 1993; Friedlander et al., 2001), osteopenic men (Johansson et al., 1996, 1992), subjects with osteoporosis secondary to Werner syndrome (Rubin et al., 1994), and subjects with glucocorticoid-induced osteoporosis (Berneis et al., 1999). The only study which did not find any differences between IGF-I treatment and placebo (Friedlander et al., 2001) was a relatively long-term study in which negative feedback from exogenous IGF-I could have suppressed GH secretion. By contrast, other long-term studies have shown a positive IGF-I effect (Locatelli and Bianchi, 2014). In addition, treatment of patients with osteoporotic femoral fractures with an IGF-I/IGFBP-3 complex led to recovery of lost bone mass (Boonen et al., 2002). Combined treatment with IGF-I and IGBP-3 seems to have allowed for safe and effective administration of higher doses of IGF-I than would have been possible with IGF-I alone; however, this theoretical benefit has not always been observed, and use of the combined treatment may not be indicated (Pfäffle, 2015). In summary, both short-term and long-term IGF-I treatment can increase bone turnover with a predominantly anabolic effect.
5. Conclusions
The GH/IGF axis is regulated by a host of interacting mechanisms and factors, particularly as it participates in the regulation of skeletal growth and maintenance. Age-related loss of bone mass occurs in part due to a decrease in components of the GH/IGF system with age. Therefore, supplemental anabolic GH and IGF-I therapies for the treatment of age-related bone loss and osteoporosis show great promise, especially as they demonstrate a relatively safe adverse effect profile. Ultimately, a better understanding of the GH/IGF axis will allow greater pharmacological manipulation of the pathophysiological changes which lead to diseases of low bone mass, helping to treat and, finally, to prevent debilitating conditions which plague so many worldwide.
Highlights.
GH and IGF-I are major regulators of skeletal metabolism.
GH affects the skeleton with and without IGF-I mediation.
IGF-I is regulated through its synthesis, receptors, IGFBPs, and IGFBP proteases.
Deficiency in GH/IGF-I actions contributes to the pathogenesis of osteoporosis.
GH and IGF-I therapies have increased bone turnover with mainly anabolic effects.
Acknowledgments
The authors acknowledge funding support from NIH (AR048139 and 2 R25 GM060507) and the US Department of Veterans Affairs.
Abbreviations
- 1,25-(OH)2D3
1,25-dihydroxyvitamin D3
- ALS
acid-labile subunit
- GH
growth hormone
- GHR
growth hormone receptor
- GHRH
growth hormone releasing hormone
- GHRP
GH-releasing peptide
- IGF
insulin-like growth factor
- IGFBP
insulin-like growth factor binding protein
- IGF-IR
insulin-like growth factor-I receptor
- IR
insulin receptor
- IRS
insulin receptor substrate
- PAPP-A
pregnancy-associated plasma protein-A
- PAPP-A2
pregnancy-associated plasma protein-A2
- PTH
parathyroid hormone
- SS
somatostatin
- TH
thyroid hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akune T, Ogata N, Hoshi K, Kubota N, Terauchi Y, Tobe K, Takagi H, Azuma Y, Kadowaki T, Nakamura K, Kawaguchi H. Insulin receptor substrate-2 maintains predominance of anabolic function over catabolic function of osteoblasts. J Cell Biol. 2002;159:147–156. doi: 10.1083/jcb.200204046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NG. Growth hormone activates mitogen-activated protein kinase and S6 kinase and promotes intracellular tyrosine phosphorylation in 3T3-F442A preadipocytes. Biochem J. 1992;284 (Pt 3):649–652. doi: 10.1042/bj2840649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argetsinger LS, Norstedt G, Billestrup N, White MF, Carter-Su C. Growth hormone, interferon-gamma, and leukemia inhibitory factor utilize insulin receptor substrate-2 in intracellular signaling. J Biol Chem. 1996;271:29415–29421. doi: 10.1074/jbc.271.46.29415. [DOI] [PubMed] [Google Scholar]
- Bagi CM, Brommage R, Deleon L, Adams S, Rosen D, Sommer A. Benefit of systemically administered rhIGF-I and rhIGF-I/IGFBP-3 on cancellous bone in ovariectomized rats. J Bone Miner Res Off J Am Soc Bone Miner Res. 1994;9:1301–1312. doi: 10.1002/jbmr.5650090820. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Bartke A. Growth hormone and aging. Endocrine. 1998;8:103–108. doi: 10.1385/ENDO:8:2:103. [DOI] [PubMed] [Google Scholar]
- Baumann G, Stolar MW, Amburn K, Barsano CP, DeVries BC. A specific growth hormone-binding protein in human plasma: initial characterization. J Clin Endocrinol Metab. 1986;62:134–141. doi: 10.1210/jcem-62-1-134. [DOI] [PubMed] [Google Scholar]
- Benbassat CA, Maki KC, Unterman TG. Circulating levels of insulin-like growth factor (IGF) binding protein-1 and -3 in aging men: relationships to insulin, glucose, IGF, and dehydroepiandrosterone sulfate levels and anthropometric measures. J Clin Endocrinol Metab. 1997;82:1484–1491. doi: 10.1210/jcem.82.5.3930. [DOI] [PubMed] [Google Scholar]
- Berneis K, Oehri M, Kraenzlin M, Keller U. Effects of IGF-I combined with GH on glucocorticoid-induced changes of bone and connective tissue turnover in man. J Endocrinol. 1999;162:259–264. doi: 10.1677/joe.0.1620259. [DOI] [PubMed] [Google Scholar]
- Bianda T, Hussain MA, Glatz Y, Bouillon R, Froesch ER, Schmid C. Effects of short-term insulin-like growth factor-I or growth hormone treatment on bone turnover, renal phosphate reabsorption and 1,25 dihydroxyvitamin D3 production in healthy man. J Intern Med. 1997;241:143–150. doi: 10.1046/j.1365-2796.1997.94101000.x. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res Off J Am Soc Bone Miner Res. 2002;17:1570–1578. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Wang Y. Insulin like growth factor-I: a critical mediator of the skeletal response to parathyroid hormone. Curr Mol Pharmacol. 2012;5:135–142. [PMC free article] [PubMed] [Google Scholar]
- Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res Off J Am Soc Bone Miner Res. 2001;16:2320–2329. doi: 10.1359/jbmr.2001.16.12.2320. [DOI] [PubMed] [Google Scholar]
- Boonen S, Mohan S, Dequeker J, Aerssens J, Vanderschueren D, Verbeke G, Broos P, Bouillon R, Baylink DJ. Down-regulation of the serum stimulatory components of the insulin-like growth factor (IGF) system (IGF-I, IGF-II, IGF binding protein [BP]-3, and IGFBP-5) in age-related (type II) femoral neck osteoporosis. J Bone Miner Res Off J Am Soc Bone Miner Res. 1999;14:2150–2158. doi: 10.1359/jbmr.1999.14.12.2150. [DOI] [PubMed] [Google Scholar]
- Boonen S, Rosen C, Bouillon R, Sommer A, McKay M, Rosen D, Adams S, Broos P, Lenaerts J, Raus J, Vanderschueren D, Geusens P. Musculoskeletal effects of the recombinant human IGF-I/IGF binding protein-3 complex in osteoporotic patients with proximal femoral fracture: a double-blind, placebo-controlled pilot study. J Clin Endocrinol Metab. 2002;87:1593–1599. doi: 10.1210/jcem.87.4.8426. [DOI] [PubMed] [Google Scholar]
- Bouillon R, Prodonova A. Growth and hormone deficiency and peak bone mass. J Pediatr Endocrinol Metab JPEM. 2000;13(Suppl 6):1327–1336. doi: 10.1515/jpem-2000-s604. [DOI] [PubMed] [Google Scholar]
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Brixen K, Kassem M, Nielsen HK, Loft AG, Flyvbjerg A, Mosekilde L. Short-term treatment with growth hormone stimulates osteoblastic and osteoclastic activity in osteopenic postmenopausal women: a dose response study. J Bone Miner Res Off J Am Soc Bone Miner Res. 1995;10:1865–1874. doi: 10.1002/jbmr.5650101205. [DOI] [PubMed] [Google Scholar]
- Brixen K, Nielsen HK, Mosekilde L, Flyvbjerg A. A short course of recombinant human growth hormone treatment stimulates osteoblasts and activates bone remodeling in normal human volunteers. J Bone Miner Res Off J Am Soc Bone Miner Res. 1990;5:609–618. doi: 10.1002/jbmr.5650050610. [DOI] [PubMed] [Google Scholar]
- Brooks AJ, Dai W, O’Mara ML, Abankwa D, Chhabra Y, Pelekanos RA, Gardon O, Tunny KA, Blucher KM, Morton CJ, Parker MW, Sierecki E, Gambin Y, Gomez GA, Alexandrov K, Wilson IA, Doxastakis M, Mark AE, Waters MJ. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science. 2014;344:1249783. doi: 10.1126/science.1249783. [DOI] [PubMed] [Google Scholar]
- Camacho-Hübner C, Woods KA, Clark AJL, Savage MO. Insulin-like growth factor (IGF)-I gene deletion. Rev Endocr Metab Disord. 2002;3:357–361. doi: 10.1023/a:1020957809082. [DOI] [PubMed] [Google Scholar]
- Campbell GS, Pang L, Miyasaka T, Saltiel AR, Carter-Su C. Stimulation by growth hormone of MAP kinase activity in 3T3-F442A fibroblasts. J Biol Chem. 1992;267:6074–6080. [PubMed] [Google Scholar]
- Canalis E. Mechanisms of glucocorticoid action in bone. Curr Osteoporos Rep. 2005;3:98–102. doi: 10.1007/s11914-005-0017-7. [DOI] [PubMed] [Google Scholar]
- Canalis E. Insulin like growth factors and the local regulation of bone formation. Bone. 1993;14:273–276. doi: 10.1016/8756-3282(93)90151-y. [DOI] [PubMed] [Google Scholar]
- Carro E, Señaris R, Considine RV, Casanueva FF, Dieguez C. Regulation of in vivo growth hormone secretion by leptin. Endocrinology. 1997;138:2203–2206. doi: 10.1210/endo.138.5.5238. [DOI] [PubMed] [Google Scholar]
- Carter-Su C, Smit LS. Signaling via JAK tyrosine kinases: growth hormone receptor as a model system. Recent Prog Horm Res. 1998;53:61–82. discussion 82–83. [PubMed] [Google Scholar]
- Centrella M, McCarthy TL, Canalis E. Receptors for insulin-like growth factors-I and -II in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1990;126:39–44. doi: 10.1210/endo-126-1-39. [DOI] [PubMed] [Google Scholar]
- Cheng MZ, Rawlinson SCF, Pitsillides AA, Zaman G, Mohan S, Baylink DJ, Lanyon LE. Human osteoblasts’ proliferative responses to strain and 17beta-estradiol are mediated by the estrogen receptor and the receptor for insulin-like growth factor I. J Bone Miner Res Off J Am Soc Bone Miner Res. 2002;17:593–602. doi: 10.1359/jbmr.2002.17.4.593. [DOI] [PubMed] [Google Scholar]
- Cheng SL, Zhang SF, Mohan S, Lecanda F, Fausto A, Hunt AH, Canalis E, Avioli LV. Regulation of insulin-like growth factors I and II and their binding proteins in human bone marrow stromal cells by dexamethasone. J Cell Biochem. 1998;71:449–458. doi: 10.1002/(sici)1097-4644(19981201)71:3<449::aid-jcb13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Cheng Mz, Zaman G, Rawlinson SC, Mohan S, Baylink DJ, Lanyon LE. Mechanical strain stimulates ROS cell proliferation through IGF-II and estrogen through IGF-I. J Bone Miner Res Off J Am Soc Bone Miner Res. 1999;14:1742–1750. doi: 10.1359/jbmr.1999.14.10.1742. [DOI] [PubMed] [Google Scholar]
- Chen TL, Chang LY, Bates RL, Perlman AJ. Dexamethasone and 1,25-dihydroxyvitamin D3 modulation of insulin-like growth factor-binding proteins in rat osteoblast-like cell cultures. Endocrinology. 1991;128:73–80. doi: 10.1210/endo-128-1-73. [DOI] [PubMed] [Google Scholar]
- Chevalley T, Strong DD, Mohan S, Baylink D, Linkhart TA. Evidence for a role for insulin-like growth factor binding proteins in glucocorticoid inhibition of normal human osteoblast-like cell proliferation. Eur J Endocrinol Eur Fed Endocr Soc. 1996;134:591–601. doi: 10.1530/eje.0.1340591. [DOI] [PubMed] [Google Scholar]
- Choi HK, Waxman DJ. Plasma growth hormone pulse activation of hepatic JAK-STAT5 signaling: developmental regulation and role in male-specific liver gene expression. Endocrinology. 2000;141:3245–3255. doi: 10.1210/endo.141.9.7638. [DOI] [PubMed] [Google Scholar]
- Christians JK, de Zwaan DR, Fung SHY. Pregnancy associated plasma protein A2 (PAPP-A2) affects bone size and shape and contributes to natural variation in postnatal growth in mice. PloS One. 2013;8:e56260. doi: 10.1371/journal.pone.0056260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis A, Maniadaki I, Stanhope R. Growth hormone/insulin-like growth factor-1 axis during puberty. Pediatr Endocrinol Rev PER. 2005;3:5–10. [PubMed] [Google Scholar]
- Claessen KMJA, Kroon HM, Pereira AM, Appelman-Dijkstra NM, Verstegen MJ, Kloppenburg M, Hamdy NaT, Biermasz NR. Progression of vertebral fractures despite long-term biochemical control of acromegaly: a prospective follow-up study. J Clin Endocrinol Metab. 2013;98:4808–4815. doi: 10.1210/jc.2013-2695. [DOI] [PubMed] [Google Scholar]
- Clark RG, Carlsson LM, Robinson IC. Growth hormone secretory profiles in conscious female rats. J Endocrinol. 1987;114:399–407. doi: 10.1677/joe.0.1140399. [DOI] [PubMed] [Google Scholar]
- Clemmesen B, Overgaard K, Riis B, Christiansen C. Human growth hormone and growth hormone releasing hormone: a double-masked, placebo-controlled study of their effects on bone metabolism in elderly women. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 1993;3:330–336. doi: 10.1007/BF01637319. [DOI] [PubMed] [Google Scholar]
- Colao A, Di Somma C, Pivonello R, Loche S, Aimaretti G, Cerbone G, Faggiano A, Corneli G, Ghigo E, Lombardi G. Bone loss is correlated to the severity of growth hormone deficiency in adult patients with hypopituitarism. J Clin Endocrinol Metab. 1999;84:1919–1924. doi: 10.1210/jcem.84.6.5742. [DOI] [PubMed] [Google Scholar]
- Collett-Solberg PF, Misra M Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. The role of recombinant human insulin-like growth factor-I in treating children with short stature. J Clin Endocrinol Metab. 2008;93:10–18. doi: 10.1210/jc.2007-1534. [DOI] [PubMed] [Google Scholar]
- Conover CA. Insulin-like growth factor binding protein proteolysis in bone cell models. Prog Growth Factor Res. 1995;6:301–309. doi: 10.1016/0955-2235(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev. 1993;14:20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- Corva PM, Mucci NC, Evans K, Medrano JF. Diet effects on female reproduction in high growth (hg/hg) mice that are deficient in the Socs-2 gene. Reprod Nutr Dev. 2004;44:303–312. doi: 10.1051/rnd:2004035. [DOI] [PubMed] [Google Scholar]
- Cowell CT, Woodhead HJ, Brody J. Bone markers and bone mineral density during growth hormone treatment in children with growth hormone deficiency. Horm Res. 2000;54(Suppl 1):44–51. doi: 10.1159/000063447. 63447. [DOI] [PubMed] [Google Scholar]
- de Boer H, Blok GJ, van Lingen A, Teule GJ, Lips P, van der Veen EA. Consequences of childhood-onset growth hormone deficiency for adult bone mass. J Bone Miner Res Off J Am Soc Bone Miner Res. 1994;9:1319–1326. doi: 10.1002/jbmr.5650090822. [DOI] [PubMed] [Google Scholar]
- Deghenghi R, Cananzi MM, Torsello A, Battisti C, Muller EE, Locatelli V. GH-releasing activity of Hexarelin, a new growth hormone releasing peptide, in infant and adult rats. Life Sci. 1994;54:1321–1328. doi: 10.1016/0024-3205(94)00510-9. [DOI] [PubMed] [Google Scholar]
- DeMambro VE, Le PT, Guntur AR, Maridas DE, Canalis E, Nagano K, Baron R, Clemmons DR, Rosen CJ. Igfbp2 deletion in ovariectomized mice enhances energy expenditure but accelerates bone loss. Endocrinology. 2015:en20141452. doi: 10.1210/en.2014-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denger S, Bähr-Ivacevic T, Brand H, Reid G, Blake J, Seifert M, Lin CY, May K, Benes V, Liu ET, Gannon F. Transcriptome profiling of estrogen-regulated genes in human primary osteoblasts reveals an osteoblast-specific regulation of the insulin-like growth factor binding protein 4 gene. Mol Endocrinol Baltim Md. 2008;22:361–379. doi: 10.1210/me.2007-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RD, Du Z, Buccilli V, Jorgetti V, Canalis E. Transgenic mice overexpressing insulin-like growth factor binding protein-5 display transiently decreased osteoblastic function and osteopenia. Endocrinology. 2002;143:3955–3962. doi: 10.1210/en.2002-220129. [DOI] [PubMed] [Google Scholar]
- DiGirolamo DJ, Mukherjee A, Fulzele K, Gan Y, Cao X, Frank SJ, Clemens TL. Mode of growth hormone action in osteoblasts. J Biol Chem. 2007;282:31666–31674. doi: 10.1074/jbc.M705219200. [DOI] [PubMed] [Google Scholar]
- Ebeling PR, Jones JD, O’Fallon WM, Janes CH, Riggs BL. Short-term effects of recombinant human insulin-like growth factor I on bone turnover in normal women. J Clin Endocrinol Metab. 1993;77:1384–1387. doi: 10.1210/jcem.77.5.8077337. [DOI] [PubMed] [Google Scholar]
- Edén S. Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology. 1979;105:555–560. doi: 10.1210/endo-105-2-555. [DOI] [PubMed] [Google Scholar]
- Erdtsieck RJ, Pols HA, Valk NK, van Ouwerkerk BM, Lamberts SW, Mulder P, Birkenhäger JC. Treatment of post-menopausal osteoporosis with a combination of growth hormone and pamidronate: a placebo controlled trial. Clin Endocrinol (Oxf) 1995;43:557–565. doi: 10.1111/j.1365-2265.1995.tb02920.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Rodan GA. Estradiol regulation of insulin-like growth factor-I expression in osteoblastic cells: evidence for transcriptional control. Mol Endocrinol Baltim Md. 1991;5:1081–1089. doi: 10.1210/mend-5-8-1081. [DOI] [PubMed] [Google Scholar]
- Ernst M, Rodan GA. Increased activity of insulin-like growth factor (IGF) in osteoblastic cells in the presence of growth hormone (GH): positive correlation with the presence of the GH-induced IGF-binding protein BP-3. Endocrinology. 1990;127:807–814. doi: 10.1210/endo-127-2-807. [DOI] [PubMed] [Google Scholar]
- Fan Y, Menon RK, Cohen P, Hwang D, Clemens T, DiGirolamo DJ, Kopchick JJ, Le Roith D, Trucco M, Sperling MA. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284:19937–19944. doi: 10.1074/jbc.M109.014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielder PJ, Mortensen DL, Mallet P, Carlsson B, Baxter RC, Clark RG. Differential long-term effects of insulin-like growth factor-I (IGF-I) growth hormone (GH), and IGF-I plus GH on body growth and IGF binding proteins in hypophysectomized rats. Endocrinology. 1996;137:1913–1920. doi: 10.1210/endo.137.5.8612531. [DOI] [PubMed] [Google Scholar]
- Fonseca TL, Teixeira MBCG, Miranda-Rodrigues M, Rodrigues-Miranda M, Silva MV, Martins GM, Costa CC, Arita DY, Perez JD, Casarini DE, Brum PC, Gouveia CHA. Thyroid hormone interacts with the sympathetic nervous system to modulate bone mass and structure in young adult mice. Am J Physiol Endocrinol Metab. 2014;307:E408–418. doi: 10.1152/ajpendo.00643.2013. [DOI] [PubMed] [Google Scholar]
- Friedlander AL, Butterfield GE, Moynihan S, Grillo J, Pollack M, Holloway L, Friedman L, Yesavage J, Matthias D, Lee S, Marcus R, Hoffman AR. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J Clin Endocrinol Metab. 2001;86:1496–1503. doi: 10.1210/jcem.86.4.7377. [DOI] [PubMed] [Google Scholar]
- Gabbitas B, Canalis E. Cortisol enhances the transcription of insulin-like growth factor-binding protein-6 in cultured osteoblasts. Endocrinology. 1996a;137:1687–1692. doi: 10.1210/endo.137.5.8612502. [DOI] [PubMed] [Google Scholar]
- Gabbitas B, Canalis E. Retinoic acid stimulates the transcription of insulin-like growth factor binding protein-6 in skeletal cells. J Cell Physiol. 1996b;169:15–22. doi: 10.1002/(SICI)1097-4652(199610)169:1<15::AID-JCP2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Gan Y, Buckels A, Liu Y, Zhang Y, Paterson AJ, Jiang J, Zinn KR, Frank SJ. Human GH receptor-IGF-1 receptor interaction: implications for GH signaling. Mol Endocrinol Baltim Md. 2014a;28:1841–1854. doi: 10.1210/me.2014-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, Paterson AJ, Zhang Y, Jiang J, Frank SJ. Functional collaboration of insulin-like growth factor-1 receptor (IGF-1R), but not insulin receptor (IR), with acute GH signaling in mouse calvarial cells. Endocrinology. 2014b;155:1000–1009. doi: 10.1210/en.2013-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur S, Yamaguchi H, Goodman HM. Growth hormone regulates cytosolic free calcium in rat fat cells by maintaining L-type calcium channels. Am J Physiol. 1996;270:C1478–1484. doi: 10.1152/ajpcell.1996.270.5.C1478. [DOI] [PubMed] [Google Scholar]
- Ghigo E, Arvat E, Gianotti L, Imbimbo BP, Lenaerts V, Deghenghi R, Camanni F. Growth hormone-releasing activity of hexarelin, a new synthetic hexapeptide, after intravenous, subcutaneous, intranasal, and oral administration in man. J Clin Endocrinol Metab. 1994;78:693–698. doi: 10.1210/jcem.78.3.8126144. [DOI] [PubMed] [Google Scholar]
- Ghiron LJ, Thompson JL, Holloway L, Hintz RL, Butterfield GE, Hoffman AR, Marcus R. Effects of recombinant insulin-like growth factor-I and growth hormone on bone turnover in elderly women. J Bone Miner Res Off J Am Soc Bone Miner Res. 1995;10:1844–1852. doi: 10.1002/jbmr.5650101203. [DOI] [PubMed] [Google Scholar]
- Gillberg P, Johansson AG, Blum WF, Groth T, Ljunghall S. Growth hormone secretion and sensitivity in men with idiopathic osteoporosis. Calcif Tissue Int. 2001;68:67–73. doi: 10.1007/BF02678143. [DOI] [PubMed] [Google Scholar]
- Gillberg P, Mallmin H, Petrén-Mallmin M, Ljunghall S, Nilsson AG. Two years of treatment with recombinant human growth hormone increases bone mineral density in men with idiopathic osteoporosis. J Clin Endocrinol Metab. 2002;87:4900–4906. doi: 10.1210/jc.2002-020231. [DOI] [PubMed] [Google Scholar]
- Gillberg P, Olofsson H, Mallmin H, Blum WF, Ljunghall S, Nilsson AG. Bone mineral density in femoral neck is positively correlated to circulating insulin-like growth factor (IGF)-I and IGF-binding protein (IGFBP)-3 in Swedish men. Calcif Tissue Int. 2002;70:22–29. doi: 10.1007/s002230020048. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Grumbach MM, Kaplan SL. The neuroendocrine regulation and function of growth hormone and prolactin in the mammalian fetus. Endocr Rev. 1981;2:363–395. doi: 10.1210/edrv-2-4-363. [DOI] [PubMed] [Google Scholar]
- Govoni KE, Baylink DJ, Mohan S. The multi-functional role of insulin-like growth factor binding proteins in bone. Pediatr Nephrol. 2005;20:261–8. doi: 10.1007/s00467-004-1658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni KE, Lee SK, Chung YS, Behringer RR, Wergedal JE, Baylink DJ, Mohan S. Disruption of insulin-like growth factor-I expression in type IIalphaI collagen-expressing cells reduces bone length and width in mice. Physiol Genomics. 2007a;30:354–62. doi: 10.1152/physiolgenomics.00022.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1alpha2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology. 2007b;148:5706–15. doi: 10.1210/en.2007-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TK, Mohan S, Linkhart TA, Baylink DJ. Estradiol stimulates in vitro the secretion of insulin-like growth factors by the clonal osteoblastic cell line, UMR106. Biochem Biophys Res Commun. 1989;158:407–412. doi: 10.1016/s0006-291x(89)80062-2. [DOI] [PubMed] [Google Scholar]
- Greenhalgh CJ, Metcalf D, Thaus AL, Corbin JE, Uren R, Morgan PO, Fabri LJ, Zhang JG, Martin HM, Willson TA, Billestrup N, Nicola NA, Baca M, Alexander WS, Hilton DJ. Biological evidence that SOCS-2 can act either as an enhancer or suppressor of growth hormone signaling. J Biol Chem. 2002;277:40181–40184. doi: 10.1074/jbc.C200450200. [DOI] [PubMed] [Google Scholar]
- Green H, Morikawa M, Nixon T. A dual effector theory of growth-hormone action. Differ Res Biol Divers. 1985;29:195–198. doi: 10.1111/j.1432-0436.1985.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Baum H, Lee K, Anderson E, Herzog D, Klibanski A. Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81:3864–3870. doi: 10.1210/jcem.81.11.8923830. [DOI] [PubMed] [Google Scholar]
- Grinspoon SK, Baum HB, Peterson S, Klibanski A. Effects of rhIGF-I administration on bone turnover during short-term fasting. J Clin Invest. 1995;96:900–906. doi: 10.1172/JCI118137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2002;87:2883–2891. doi: 10.1210/jcem.87.6.8574. [DOI] [PubMed] [Google Scholar]
- Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res Off J Am Soc Bone Miner Res. 2002;17:493–501. doi: 10.1359/jbmr.2002.17.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden JM, Strong DD, Baylink DJ, Powell DR, Sampath TK, Mohan S. Osteogenic protein-1 stimulates production of insulin-like growth factor binding protein-3 nuclear transcripts in human osteosarcoma cells. Endocrinology. 1997;138:4240–4247. doi: 10.1210/endo.138.10.5457. [DOI] [PubMed] [Google Scholar]
- Hazel SJ, Gillespie CM, Moore RJ, Clark RG, Jureidini KF, Martin AA. Enhanced body growth in uremic rats treated with IGF-I and growth hormone in combination. Kidney Int. 1994;46:58–68. doi: 10.1038/ki.1994.244. [DOI] [PubMed] [Google Scholar]
- Herrington J, Carter-Su C. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol Metab TEM. 2001;12:252–257. doi: 10.1016/s1043-2760(01)00423-4. [DOI] [PubMed] [Google Scholar]
- Ho KK, O’Sullivan AJ, Weissberger AJ, Kelly JJ. Sex steroid regulation of growth hormone secretion and action. Horm Res. 1996;45:67–73. doi: 10.1159/000184762. [DOI] [PubMed] [Google Scholar]
- Ho KK, Weissberger AJ. Impact of short-term estrogen administration on growth hormone secretion and action: distinct route-dependent effects on connective and bone tissue metabolism. J Bone Miner Res Off J Am Soc Bone Miner Res. 1992;7:821–827. doi: 10.1002/jbmr.5650070711. [DOI] [PubMed] [Google Scholar]
- Holloway L, Butterfield G, Hintz RL, Gesundheit N, Marcus R. Effects of recombinant human growth hormone on metabolic indices, body composition, and bone turnover in healthy elderly women. J Clin Endocrinol Metab. 1994;79:470–479. doi: 10.1210/jcem.79.2.7519191. [DOI] [PubMed] [Google Scholar]
- Holloway L, Kohlmeier L, Kent K, Marcus R. Skeletal effects of cyclic recombinant human growth hormone and salmon calcitonin in osteopenic postmenopausal women. J Clin Endocrinol Metab. 1997;82:1111–1117. doi: 10.1210/jcem.82.4.3901. [DOI] [PubMed] [Google Scholar]
- Holmes SJ, Economou G, Whitehouse RW, Adams JE, Shalet SM. Reduced bone mineral density in patients with adult onset growth hormone deficiency. J Clin Endocrinol Metab. 1994;78:669–674. doi: 10.1210/jcem.78.3.8126140. [DOI] [PubMed] [Google Scholar]
- Honda Y, Landale EC, Strong DD, Baylink DJ, Mohan S. Recombinant synthesis of insulin-like growth factor-binding protein-4 (IGFBP-4): Development, validation, and application of a radioimmunoassay for IGFBP-4 in human serum and other biological fluids. J Clin Endocrinol Metab. 1996;81:1389–1396. doi: 10.1210/jcem.81.4.8636339. [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Shizume K, Masuda A, Hotta M, Kiyosawa Y, Jibiki K, Demura H, Tsushima T, Ling N. The effect of free fatty acids on growth hormone (GH)-releasing hormone-mediated GH secretion in man. J Clin Endocrinol Metab. 1985;60:290–293. doi: 10.1210/jcem-60-2-290. [DOI] [PubMed] [Google Scholar]
- Isaksson OG, Jansson JO, Gause IA. Growth hormone stimulates longitudinal bone growth directly. Science. 1982;216:1237–1239. doi: 10.1126/science.7079756. [DOI] [PubMed] [Google Scholar]
- Jiang J, Lichtler AC, Gronowicz GA, Adams DJ, Clark SH, Rosen CJ, Kream BE. Transgenic mice with osteoblast-targeted insulin-like growth factor-I show increased bone remodeling. Bone. 2006;39:494–504. doi: 10.1016/j.bone.2006.02.068. [DOI] [PubMed] [Google Scholar]
- Johansson AG, Lindh E, Blum WF, Kollerup G, Sørensen OH, Ljunghall S. Effects of growth hormone and insulin-like growth factor I in men with idiopathic osteoporosis. J Clin Endocrinol Metab. 1996;81:44–48. doi: 10.1210/jcem.81.1.8550792. [DOI] [PubMed] [Google Scholar]
- Johansson AG, Lindh E, Ljunghall S. Insulin-like growth factor I stimulates bone turnover in osteoporosis. Lancet Lond Engl. 1992;339:1619. doi: 10.1016/0140-6736(92)91889-g. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Joseph F, Ahmad AM, Ul-Haq M, Durham BH, Whittingham P, Fraser WD, Vora JP. Effects of growth hormone administration on bone mineral metabolism, PTH sensitivity and PTH secretory rhythm in postmenopausal women with established osteoporosis. J Bone Miner Res Off J Am Soc Bone Miner Res. 2008;23:721–729. doi: 10.1359/jbmr.071117. [DOI] [PubMed] [Google Scholar]
- Jux C, Leiber K, Hügel U, Blum W, Ohlsson C, Klaus G, Mehls O. Dexamethasone impairs growth hormone (GH)-stimulated growth by suppression of local insulin-like growth factor (IGF)-I production and expression of GH- and IGF-I-receptor in cultured rat chondrocytes. Endocrinology. 1998;139:3296–3305. doi: 10.1210/endo.139.7.6099. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Tobe K, Honda-Yamamoto R, Tamemoto H, Kaburagi Y, Momomura K, Ueki K, Takahashi Y, Yamauchi T, Akanuma Y, Yazaki Y. Signal transduction mechanism of insulin and insulin-like growth factor-1. Endocr J. 1996;43(Suppl):S33–41. doi: 10.1507/endocrj.43.suppl_s33. [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Hilliker S, Baylink DJ, Mohan S. Evidence that human bone cells in culture produce insulin-like growth factor-binding protein-4 and -5 proteases. Endocrinology. 1994;134:383–392. doi: 10.1210/endo.134.1.7506211. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mohan S, Baylink DJ, Lau KHW. Fluid shear stress synergizes with insulin-like growth factor-I (IGF-I) on osteoblast proliferation through integrin-dependent activation of IGF-I mitogenic signaling pathway. J Biol Chem. 2005;280:20163–20170. doi: 10.1074/jbc.M501460200. [DOI] [PubMed] [Google Scholar]
- Karasik D, Rosen CJ, Hannan MT, Broe KE, Dawson-Hughes B, Gagnon DR, Wilson PWF, Visser M, Langlois JA, Mohan S, Kiel DP. Insulin-like growth factor binding proteins 4 and 5 and bone mineral density in elderly men and women. Calcif Tissue Int. 2002;71:323–328. doi: 10.1007/s00223-002-1002-0. [DOI] [PubMed] [Google Scholar]
- Kassem M, Brixen K, Blum WF, Mosekilde L, Eriksen EF. Normal osteoclastic and osteoblastic responses to exogenous growth hormone in patients with postmenopausal spinal osteoporosis. J Bone Miner Res Off J Am Soc Bone Miner Res. 1994a;9:1365–1370. doi: 10.1002/jbmr.5650090907. [DOI] [PubMed] [Google Scholar]
- Kassem M, Brixen K, Blum W, Mosekilde L, Eriksen EF. No evidence for reduced spontaneous or growth-hormone-stimulated serum levels of insulin-like growth factor (IGF)-I, IGF-II or IGF binding protein 3 in women with spinal osteoporosis. Eur J Endocrinol Eur Fed Endocr Soc. 1994b;131:150–155. doi: 10.1530/eje.0.1310150. [DOI] [PubMed] [Google Scholar]
- Kassem M, Brixen K, Mosekilde L, Blum WF, Flyvbjerg A. Effects of growth hormone treatment on serum levels of insulin-like growth factors (IGFs) and IGF binding proteins 1-4 in postmenopausal women. Clin Endocrinol (Oxf) 1998;49:747–756. doi: 10.1046/j.1365-2265.1998.00606.x. [DOI] [PubMed] [Google Scholar]
- Kassem M, Okazaki R, De León D, Harris SA, Robinson JA, Spelsberg TC, Conover CA, Riggs BL. Potential mechanism of estrogen-mediated decrease in bone formation: estrogen increases production of inhibitory insulin-like growth factor-binding protein-4. Proc Assoc Am Physicians. 1996;108:155–164. [PubMed] [Google Scholar]
- Kasukawa Y, Baylink DJ, Guo R, Mohan S. Evidence that sensitivity to growth hormone (GH) is growth period and tissue type dependent: studies in GH-deficient lit/lit mice. Endocrinology. 2003;144:3950–3957. doi: 10.1210/en.2002-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavan C, Wergedal JE, Lau KHW, Mohan S. Conditional disruption of IGF-I gene in type 1α collagen-expressing cells shows an essential role of IGF-I in skeletal anabolic response to loading. Am J Physiol Endocrinol Metab. 2011;301:E1191–1197. doi: 10.1152/ajpendo.00440.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen R, Honda Y, Strong DD, Sampath TK, Baylink DJ, Mohan S. Regulation of insulin-like growth factor system components by osteogenic protein-1 in human bone cells. Endocrinology. 1995;136:857–865. doi: 10.1210/endo.136.3.7532581. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Krantz E, Trimpou P, Landin-Wilhelmsen K. Effect of Growth Hormone Treatment on Fractures and Quality of Life in Postmenopausal Osteoporosis: A 10-Year Follow-Up Study. J Clin Endocrinol Metab. 2015;100:3251–3259. doi: 10.1210/jc.2015-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells Dayt Ohio. 2001;19:378–387. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- Kruse HP, Kuhlencordt F. On an attempt to treat primary and secondary osteoporosis with human growth hormone. Horm Metab Res Horm Stoffwechselforschung Horm Métabolisme. 1975;7:488–491. doi: 10.1055/s-0028-1093710. [DOI] [PubMed] [Google Scholar]
- Kužma M, Kužmová Z, Zelinková Z, Killinger Z, Vaňuga P, Lazurová I, Tomková S, Payer J. Impact of the growth hormone replacement on bone status in growth hormone deficient adults. Growth Horm IGF Res Off J Growth Horm Res Soc Int IGF Res Soc. 2014;24:22–28. doi: 10.1016/j.ghir.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Kveiborg M, Flyvbjerg A, Eriksen EF, Kassem M. 1,25-Dihydroxyvitamin D3 stimulates the production of insulin-like growth factor-binding proteins-2, -3 and -4 in human bone marrow stromal cells. Eur J Endocrinol Eur Fed Endocr Soc. 2001;144:549–557. doi: 10.1530/eje.0.1440549. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Caplice MD, Khanna V, Stern PH. Thyroid hormones increase insulin-like growth factor I content in the medium of rat bone tissue. J Bone Miner Res Off J Am Soc Bone Miner Res. 1993;8:1475–1481. doi: 10.1002/jbmr.5650081210. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Foldes J, Nagy Z, Takacs I, Speer G, Horvath C, Mohan S, Baylink DJ, Stern PH. Serum insulin-like growth factor-I, insulin-like growth factor binding proteins, and bone mineral content in hyperthyroidism. Thyroid Off J Am Thyroid Assoc. 2000;10:417–423. doi: 10.1089/thy.2000.10.417. [DOI] [PubMed] [Google Scholar]
- Landin-Wilhelmsen K, Nilsson A, Bosaeus I, Bengtsson BA. Growth hormone increases bone mineral content in postmenopausal osteoporosis: a randomized placebo-controlled trial. J Bone Miner Res Off J Am Soc Bone Miner Res. 2003;18:393–405. doi: 10.1359/jbmr.2003.18.3.393. [DOI] [PubMed] [Google Scholar]
- Laron Z, Kauli R. Fifty seven years of follow-up of the Israeli cohort of Laron Syndrome patients-From discovery to treatment. Growth Horm IGF Res Off J Growth Horm Res Soc Int IGF Res Soc. 2015 doi: 10.1016/j.ghir.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Laron Z, Klinger B. IGF-I treatment of adult patients with Laron syndrome: preliminary results. Clin Endocrinol (Oxf) 1994;41:631–638. doi: 10.1111/j.1365-2265.1994.tb01829.x. [DOI] [PubMed] [Google Scholar]
- Lau KHW, Baylink DJ, Sheng MHC. Osteocyte-derived insulin-like growth factor I is not essential for the bone repletion response in mice. PloS One. 2015;10:e0115897. doi: 10.1371/journal.pone.0115897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau KHW, Baylink DJ, Zhou XD, Rodriguez D, Bonewald LF, Li Z, Ruffoni D, Müller R, Kesavan C, Sheng MHC. Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am J Physiol Endocrinol Metab. 2013;305:E271–281. doi: 10.1152/ajpendo.00092.2013. [DOI] [PubMed] [Google Scholar]
- Lau KHW, Kapur S, Kesavan C, Baylink DJ. Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J Biol Chem. 2006;281:9576–9588. doi: 10.1074/jbc.M509205200. [DOI] [PubMed] [Google Scholar]
- Lean JM, Jagger CJ, Chambers TJ, Chow JW. Increased insulin-like growth factor I mRNA expression in rat osteocytes in response to mechanical stimulation. Am J Physiol. 1995;268:E318–327. doi: 10.1152/ajpendo.1995.268.2.E318. [DOI] [PubMed] [Google Scholar]
- Lean JM, Mackay AG, Chow JW, Chambers TJ. Osteocytic expression of mRNA for c-fos and IGF-I: an immediate early gene response to an osteogenic stimulus. Am J Physiol. 1996;270:E937–945. doi: 10.1152/ajpendo.1996.270.6.E937. [DOI] [PubMed] [Google Scholar]
- Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Werner H, Beitner-Johnson D, Roberts CT. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- Leung DW, Spencer SA, Cachianes G, Hammonds RG, Collins C, Henzel WJ, Barnard R, Waters MJ, Wood WI. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature. 1987;330:537–543. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- Lim SV, Marenzana M, Hopkinson M, List EO, Kopchick JJ, Pereira M, Javaheri B, Roux JP, Chavassieux P, Korbonits M, Chenu C. Excessive growth hormone expression in male GH transgenic mice adversely alters bone architecture and mechanical strength. Endocrinology. 2015;156:1362–1371. doi: 10.1210/en.2014-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl A, Isgaard J, Isaksson OG. Growth hormone in vivo potentiates the stimulatory effect of insulin-like growth factor-1 in vitro on colony formation of epiphyseal chondrocytes isolated from hypophysectomized rats. Endocrinology. 1987;121:1070–1075. doi: 10.1210/endo-121-3-1070. [DOI] [PubMed] [Google Scholar]
- Lindahl A, Isgaard J, Nilsson A, Isaksson OG. Growth hormone potentiates colony formation of epiphyseal chondrocytes in suspension culture. Endocrinology. 1986;118:1843–1848. doi: 10.1210/endo-118-5-1843. [DOI] [PubMed] [Google Scholar]
- Ling N, Esch F, Böhlen P, Brazeau P, Wehrenberg WB, Guillemin R. Isolation, primary structure, and synthesis of human hypothalamic somatocrinin: growth hormone-releasing factor. Proc Natl Acad Sci U S A. 1984;81:4302–4306. doi: 10.1073/pnas.81.14.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkhart TA, Mohan S. Parathyroid hormone stimulates release of insulin-like growth factor-I (IGF-I) and IGF-II from neonatal mouse calvaria in organ culture. Endocrinology. 1989;125:1484–1491. doi: 10.1210/endo-125-3-1484. [DOI] [PubMed] [Google Scholar]
- List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Masternak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155:1793–1805. doi: 10.1210/en.2013-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Locatelli V, Bianchi VE. Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis. Int J Endocrinol. 2014;2014 doi: 10.1155/2014/235060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long RK, Nishida S, Kubota T, Wang Y, Sakata T, Elalieh HZ, Halloran BP, Bikle DD. Skeletal unloading-induced insulin-like growth factor 1 (IGF-1) nonresponsiveness is not shared by platelet-derived growth factor: the selective role of integrins in IGF-1 signaling. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26:2948–2958. doi: 10.1002/jbmr.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpe R, Baylink DJ, Linkhart TA, Wergedal JE, Mohan S. Insulin-like growth factor (IGF)-I, -II, IGF binding proteins (IGFBP)-3, -4, and -5 levels in the conditioned media of normal human bone cells are skeletal site-dependent. J Bone Miner Res Off J Am Soc Bone Miner Res. 1997;12:423–430. doi: 10.1359/jbmr.1997.12.3.423. [DOI] [PubMed] [Google Scholar]
- Martini G, Valenti R, Giovani S, Franci B, Campagna S, Nuti R. Influence of insulin-like growth factor-1 and leptin on bone mass in healthy postmenopausal women. Bone. 2001;28:113–117. doi: 10.1016/s8756-3282(00)00408-7. [DOI] [PubMed] [Google Scholar]
- Mauras N, Doi SQ, Shapiro JR. Recombinant human insulin-like growth factor I, recombinant human growth hormone, and sex steroids: effects on markers of bone turnover in humans. J Clin Endocrinol Metab. 1996;81:2222–2226. doi: 10.1210/jcem.81.6.8964855. [DOI] [PubMed] [Google Scholar]
- Mazziotti G, Bianchi A, Bonadonna S, Cimino V, Patelli I, Fusco A, Pontecorvi A, De Marinis L, Giustina A. Prevalence of vertebral fractures in men with acromegaly. J Clin Endocrinol Metab. 2008;93:4649–4655. doi: 10.1210/jc.2008-0791. [DOI] [PubMed] [Google Scholar]
- Mazziotti G, Bianchi A, Porcelli T, Mormando M, Maffezzoni F, Cristiano A, Giampietro A, De Marinis L, Giustina A. Vertebral fractures in patients with acromegaly: a 3-year prospective study. J Clin Endocrinol Metab. 2013;98:3402–3410. doi: 10.1210/jc.2013-1460. [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M. Local IGF-I expression and bone formation. Growth Horm IGF Res Off J Growth Horm Res Soc Int IGF Res Soc. 2001;11:213–219. doi: 10.1054/ghir.2001.0236. [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M, Canalis E. Parathyroid hormone enhances the transcript and polypeptide levels of insulin-like growth factor I in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1989;124:1247–1253. doi: 10.1210/endo-124-3-1247. [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Ji C, Shu H, Casinghino S, Crothers K, Rotwein P, Centrella M. 17beta-estradiol potently suppresses cAMP-induced insulin-like growth factor-I gene activation in primary rat osteoblast cultures. J Biol Chem. 1997;272:18132–18139. doi: 10.1074/jbc.272.29.18132. [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Yun Z, Madri JA, Centrella M. Stratified control of IGF-I expression by hypoxia and stress hormones in osteoblasts. Gene. 2014;539:141–151. doi: 10.1016/j.gene.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, McGrane J, Miller KK, Goldstein MA, Ebrahimi S, Weigel T, Klibanski A. Effects of rhIGF-1 administration on surrogate markers of bone turnover in adolescents with anorexia nervosa. Bone. 2009;45:493–498. doi: 10.1016/j.bone.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, Ott M, Herzog DB, Johnson ML, Klibanski A. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003;88:5615–5623. doi: 10.1210/jc.2003-030532. [DOI] [PubMed] [Google Scholar]
- Miyakoshi N, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that anabolic effects of PTH on bone require IGF-I in growing mice. Endocrinology. 2001a;142:4349–4356. doi: 10.1210/endo.142.10.8436. [DOI] [PubMed] [Google Scholar]
- Miyakoshi N, Richman C, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that IGF-binding protein-5 functions as a growth factor. J Clin Invest. 2001b;107:73–81. doi: 10.1172/JCI10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakoshi N, Richman C, Qin X, Baylink DJ, Mohan S. Effects of recombinant insulin-like growth factor-binding protein-4 on bone formation parameters in mice. Endocrinology. 1999;140:5719–5728. doi: 10.1210/endo.140.12.7175. [DOI] [PubMed] [Google Scholar]
- Mo D, Fleseriu M, Qi R, Jia N, Child CJ, Bouillon R, Hardin DS. Fracture risk in adult patients treated with growth hormone replacement therapy for growth hormone deficiency: a prospective observational cohort study. Lancet Diabetes Endocrinol. 2015;3:331–338. doi: 10.1016/S2213-8587(15)00098-4. [DOI] [PubMed] [Google Scholar]
- Mohan S, Bautista CM, Wergedal J, Baylink DJ. Isolation of an inhibitory insulin-like growth factor (IGF) binding protein from bone cell-conditioned medium: a potential local regulator of IGF action. Proc Natl Acad Sci U S A. 1989;86:8338–8342. doi: 10.1073/pnas.86.21.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S, Baylink DJ. Impaired skeletal growth in mice with haploinsufficiency of IGF-I: genetic evidence that differences in IGF-I expression could contribute to peak bone mineral density differences. J Endocrinol. 2005;185:415–420. doi: 10.1677/joe.1.06141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S, Farley JR, Baylink DJ. Age-related changes in IGFBP-4 and IGFBP-5 levels in human serum and bone: implications for bone loss with aging. Prog Growth Factor Res. 1995;6:465–473. doi: 10.1016/0955-2235(95)00027-5. [DOI] [PubMed] [Google Scholar]
- Mohan S, Kesavan C. Role of insulin-like growth factor-1 in the regulation of skeletal growth. Curr Osteoporos Rep. 2012;10:178–186. doi: 10.1007/s11914-012-0100-9. [DOI] [PubMed] [Google Scholar]
- Mohan S, Richman C, Guo R, Amaar Y, Donahue LR, Wergedal J, Baylink DJ. Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology. 2003;144:929–936. doi: 10.1210/en.2002-220948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormando M, Nasto LA, Bianchi A, Mazziotti G, Giampietro A, Pola E, Pontecorvi A, Giustina A, De Marinis L. GH receptor isoforms and skeletal fragility in acromegaly. Eur J Endocrinol Eur Fed Endocr Soc. 2014;171:237–245. doi: 10.1530/EJE-14-0205. [DOI] [PubMed] [Google Scholar]
- Muggeo M, Tiengo A, Fedele D, Crepaldi G. The influence of plasma triglycerides on human growth hormone response to arginine and insulin: a study in hyperlipemics and normal subjects. Horm Metab Res Horm Stoffwechselforschung Horm Métabolisme. 1975;7:367–374. doi: 10.1055/s-0028-1093729. [DOI] [PubMed] [Google Scholar]
- Müller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiol Rev. 1999;79:511–607. doi: 10.1152/physrev.1999.79.2.511. [DOI] [PubMed] [Google Scholar]
- Muñoz-Torres M, Mezquita-Raya P, Lopez-Rodriguez F, Torres-Vela E, de Dios Luna J, Escobar-Jimenez F. The contribution of IGF-I to skeletal integrity in postmenopausal women. Clin Endocrinol (Oxf) 2001;55:759–766. doi: 10.1046/j.1365-2265.2001.01390.x. [DOI] [PubMed] [Google Scholar]
- Nicolas V, Mohan S, Honda Y, Prewett A, Finkelman RD, Baylink DJ, Farley JR. An age-related decrease in the concentration of insulin-like growth factor binding protein-5 in human cortical bone. Calcif Tissue Int. 1995;57:206–212. doi: 10.1007/BF00310260. [DOI] [PubMed] [Google Scholar]
- Nordstrom SM, Tran JL, Sos BC, Wagner KU, Weiss EJ. Liver-derived IGF-I contributes to GH-dependent increases in lean mass and bone mineral density in mice with comparable levels of circulating GH. Mol Endocrinol Baltim Md. 2011;25:1223–1230. doi: 10.1210/me.2011-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N, Chikazu D, Kubota N, Terauchi Y, Tobe K, Azuma Y, Ohta T, Kadowaki T, Nakamura K, Kawaguchi H. Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J Clin Invest. 2000;105:935–943. doi: 10.1172/JCI9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Nilsson A, Isaksson O, Lindahl A. Growth hormone induces multiplication of the slowly cycling germinal cells of the rat tibial growth plate. Proc Natl Acad Sci U S A. 1992;89:9826–9830. doi: 10.1073/pnas.89.20.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R, Riggs BL, Conover CA. Glucocorticoid regulation of insulin-like growth factor-binding protein expression in normal human osteoblast-like cells. Endocrinology. 1994;134:126–132. doi: 10.1210/endo.134.1.7506203. [DOI] [PubMed] [Google Scholar]
- Padova G, Borzì G, Incorvaia L, Siciliano G, Migliorino V, Vetri M, Tita P. Prevalence of osteoporosis and vertebral fractures in acromegalic patients. Clin Cases Miner Bone Metab Off J Ital Soc Osteoporos Miner Metab Skelet Dis. 2011;8:37–43. [PMC free article] [PubMed] [Google Scholar]
- Patchett AA, Nargund RP, Tata JR, Chen MH, Barakat KJ, Johnston DB, Cheng K, Chan WW, Butler B, Hickey G. Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. Proc Natl Acad Sci U S A. 1995;92:7001–7005. doi: 10.1073/pnas.92.15.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfäffle R. Hormone replacement therapy in children: The use of growth hormone and IGF-I. Best Pract Res Clin Endocrinol Metab. 2015;29:339–352. doi: 10.1016/j.beem.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Qin X, Wergedal JE, Rehage M, Tran K, Newton J, Lam P, Baylink DJ, Mohan S. Pregnancy-associated plasma protein-A increases osteoblast proliferation in vitro and bone formation in vivo. Endocrinology. 2006;147:5653–5661. doi: 10.1210/en.2006-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- Ram PA, Waxman DJ. SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J Biol Chem. 1999;274:35553–35561. doi: 10.1074/jbc.274.50.35553. [DOI] [PubMed] [Google Scholar]
- Rawlinson SC, Mohan S, Baylink DJ, Lanyon LE. Exogenous prostacyclin, but not prostaglandin E2, produces similar responses in both G6PD activity and RNA production as mechanical loading, and increases IGF-II release, in adult cancellous bone in culture. Calcif Tissue Int. 1993;53:324–329. doi: 10.1007/BF01351837. [DOI] [PubMed] [Google Scholar]
- Richman C, Baylink DJ, Lang K, Dony C, Mohan S. Recombinant human insulin-like growth factor-binding protein-5 stimulates bone formation parameters in vitro and in vivo. Endocrinology. 1999;140:4699–4705. doi: 10.1210/endo.140.10.7081. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Donahue LR, Hunter SJ. Insulin-like growth factors and bone: the osteoporosis connection. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 1994;206:83–102. doi: 10.3181/00379727-206-43726. [DOI] [PubMed] [Google Scholar]
- Rosén T, Wilhelmsen L, Landin-Wilhelmsen K, Lappas G, Bengtsson BA. Increased fracture frequency in adult patients with hypopituitarism and GH deficiency. Eur J Endocrinol Eur Fed Endocr Soc. 1997;137:240–245. doi: 10.1530/eje.0.1370240. [DOI] [PubMed] [Google Scholar]
- Ross JL, Lee PA, Gut R, Germak J. Attaining genetic height potential: Analysis of height outcomes from the ANSWER Program in children treated with growth hormone over 5years. Growth Horm IGF Res Off J Growth Horm Res Soc Int IGF Res Soc. 2015 doi: 10.1016/j.ghir.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Rubin CD, Reed B, Sakhaee K, Pak CY. Treating a patient with the Werner syndrome and osteoporosis using recombinant human insulin-like growth factor. Ann Intern Med. 1994;121:665–668. doi: 10.7326/0003-4819-121-9-199411010-00007. [DOI] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323:1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- Sääf M, Hilding A, Thorën M, Troell S, Hall K. Growth hormone treatment of osteoporotic postmenopausal women - a one-year placebo-controlled study. Eur J Endocrinol Eur Fed Endocr Soc. 1999;140:390–399. doi: 10.1530/eje.0.1400390. [DOI] [PubMed] [Google Scholar]
- Saban J, Schneider GB, Bolt D, King D. Erythroid-specific expression of human growth hormone affects bone morphology in transgenic mice. Bone. 1996;18:47–52. doi: 10.1016/8756-3282(95)00419-x. [DOI] [PubMed] [Google Scholar]
- Sakata T, Halloran BP, Elalieh HZ, Munson SJ, Rudner L, Venton L, Ginzinger D, Rosen CJ, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor I on bone formation. Bone. 2003;32:669–680. doi: 10.1016/s8756-3282(03)00088-7. [DOI] [PubMed] [Google Scholar]
- Sakata T, Wang Y, Halloran BP, Elalieh HZ, Cao J, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. J Bone Miner Res Off J Am Soc Bone Miner Res. 2004;19:436–446. doi: 10.1359/JBMR.0301241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon WD, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957;49:825–836. [PubMed] [Google Scholar]
- Sasaki N, Rees-Jones RW, Zick Y, Nissley SP, Rechler MM. Characterization of insulin-like growth factor I-stimulated tyrosine kinase activity associated with the beta-subunit of type I insulin-like growth factor receptors of rat liver cells. J Biol Chem. 1985;260:9793–9804. [PubMed] [Google Scholar]
- Saunders A, Terry LC, Audet J, Brazeau P, Martin JB. Dynamic studies of growth hormone and prolactin secretion in the female rat. Neuroendocrinology. 1976;21:193–203. doi: 10.1159/000122525. [DOI] [PubMed] [Google Scholar]
- Scharla SH, Strong DD, Mohan S, Baylink DJ, Linkhart TA. 1,25-Dihydroxyvitamin D3 differentially regulates the production of insulin-like growth factor I (IGF-I) and IGF-binding protein-4 in mouse osteoblasts. Endocrinology. 1991;129:3139–3146. doi: 10.1210/endo-129-6-3139. [DOI] [PubMed] [Google Scholar]
- Scharla SH, Strong DD, Rosen C, Mohan S, Holick M, Baylink DJ, Linkhart TA. 1,25-Dihydroxyvitamin D3 increases secretion of insulin-like growth factor binding protein-4 (IGFBP-4) by human osteoblast-like cells in vitro and elevates IGFBP-4 serum levels in vivo. J Clin Endocrinol Metab. 1993;77:1190–1197. doi: 10.1210/jcem.77.5.7521341. [DOI] [PubMed] [Google Scholar]
- Schlechter NL, Russell SM, Greenberg S, Spencer EM, Nicoll CS. A direct growth effect of growth hormone in rat hindlimb shown by arterial infusion. Am J Physiol. 1986;250:E231–235. doi: 10.1152/ajpendo.1986.250.3.E231. [DOI] [PubMed] [Google Scholar]
- Seck T, Bretz A, Krempien R, Krempien B, Ziegler R, Pfeilschifter J. Age-related changes in insulin-like growth factor I and II in human femoral cortical bone: lack of correlation with bone mass. Bone. 1999;24:387–393. doi: 10.1016/s8756-3282(98)00186-0. [DOI] [PubMed] [Google Scholar]
- Sheng MHC, Lau KHW, Baylink DJ. Role of Osteocyte-derived Insulin-Like Growth Factor I in Developmental Growth, Modeling, Remodeling, and Regeneration of the Bone. J Bone Metab. 2014;21:41–54. doi: 10.11005/jbm.2014.21.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoaka T, Kamekura S, Chikuda H, Hoshi K, Chung UI, Akune T, Maruyama Z, Komori T, Matsumoto M, Ogawa W, Terauchi Y, Kadowaki T, Nakamura K, Kawaguchi H. Impairment of bone healing by insulin receptor substrate-1 deficiency. J Biol Chem. 2004;279:15314–15322. doi: 10.1074/jbc.M312525200. [DOI] [PubMed] [Google Scholar]
- Simpson H, Savine R, Sönksen P, Bengtsson BA, Carlsson L, Christiansen JS, Clemmons D, Cohen P, Hintz R, Ho K, Mullis P, Robinson I, Strasburger C, Tanaka T, Thorner M GRS Council. Growth hormone replacement therapy for adults: into the new millennium. Growth Horm IGF Res Off J Growth Horm Res Soc Int IGF Res Soc. 2002;12:1–33. doi: 10.1054/ghir.2001.0263. [DOI] [PubMed] [Google Scholar]
- Sims NA, Clément-Lacroix P, Da Ponte F, Bouali Y, Binart N, Moriggl R, Goffin V, Coschigano K, Gaillard-Kelly M, Kopchick J, Baron R, Kelly PA. Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest. 2000;106:1095–1103. doi: 10.1172/JCI10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal V, Goh BC, Bouxsein ML, Faugere MC, DiGirolamo DJ. Osteoblast-restricted Disruption of the Growth Hormone Receptor in Mice Results in Sexually Dimorphic Skeletal Phenotypes. Bone Res. 2013;1:85–97. doi: 10.4248/BR201301006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren K, Bohlooly-Y M, Bohlooly YM, Olsson B, Coschigano K, Törnell J, Mohan S, Isaksson OG, Baumann G, Kopchick J, Ohlsson C. Disproportional skeletal growth and markedly decreased bone mineral content in growth hormone receptor −/− mice. Biochem Biophys Res Commun. 2000;267:603–608. doi: 10.1006/bbrc.1999.1986. [DOI] [PubMed] [Google Scholar]
- Sjögren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Törnell J, Isaksson OG, Jansson JO, Ohlsson C. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slootweg MC, Hoogerbrugge CM, de Poorter TL, Duursma SA, van Buul-Offers SC. The presence of classical insulin-like growth factor (IGF) type-I and -II receptors on mouse osteoblasts: autocrine/paracrine growth effect of IGFs? J Endocrinol. 1990;125:271–277. doi: 10.1677/joe.0.1250271. [DOI] [PubMed] [Google Scholar]
- Smal J, De Meyts P. Role of kinase C in the insulin-like effects of human growth hormone in rat adipocytes. Biochem Biophys Res Commun. 1987;147:1232–1240. doi: 10.1016/s0006-291x(87)80202-4. [DOI] [PubMed] [Google Scholar]
- Smith RG, Pong SS, Hickey G, Jacks T, Cheng K, Leonard R, Cohen CJ, Arena JP, Chang CH, Drisko J, Wyvratt M, Fisher M, Nargund R, Patchett A. Modulation of pulsatile GH release through a novel receptor in hypothalamus and pituitary gland. Recent Prog Horm Res. 1996;51:261–285. discussion 285–286. [PubMed] [Google Scholar]
- Smith RG, Van der Ploeg LH, Howard AD, Feighner SD, Cheng K, Hickey GJ, Wyvratt MJ, Fisher MH, Nargund RP, Patchett AA. Peptidomimetic regulation of growth hormone secretion. Endocr Rev. 1997;18:621–645. doi: 10.1210/edrv.18.5.0316. [DOI] [PubMed] [Google Scholar]
- Souza SC, Frick GP, Yip R, Lobo RB, Tai LR, Goodman HM. Growth hormone stimulates tyrosine phosphorylation of insulin receptor substrate-1. J Biol Chem. 1994;269:30085–30088. [PubMed] [Google Scholar]
- Spiess J, Rivier J, Vale W. Characterization of rat hypothalamic growth hormone-releasing factor. Nature. 1983;303:532–535. doi: 10.1038/303532a0. [DOI] [PubMed] [Google Scholar]
- Steele-Perkins G, Turner J, Edman JC, Hari J, Pierce SB, Stover C, Rutter WJ, Roth RA. Expression and characterization of a functional human insulin-like growth factor I receptor. J Biol Chem. 1988;263:11486–11492. [PubMed] [Google Scholar]
- Stratikopoulos E, Szabolcs M, Dragatsis I, Klinakis A, Efstratiadis A. The hormonal action of IGF1 in postnatal mouse growth. Proc Natl Acad Sci U S A. 2008;105:19378–19383. doi: 10.1073/pnas.0809223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styne DM. The regulation of pubertal growth. Horm Res. 2003;60:22–26. doi: 10.1159/000071222. 71222. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Kaji H, Nakaoka D, Yamauchi M, Yano S, Sugishita T, Baylink DJ, Mohan S, Chihara K. Effect of low-dose of recombinant human growth hormone on bone metabolism in elderly women with osteoporosis. Eur J Endocrinol Eur Fed Endocr Soc. 2002;147:339–348. doi: 10.1530/eje.0.1470339. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Nakaoka D, Nasu M, Kanzawa M, Sugishita T, Chihara K. Effect of recombinant human growth hormone in elderly osteoporotic women. Clin Endocrinol (Oxf) 1999;51:715–724. doi: 10.1046/j.1365-2265.1999.00867.x. [DOI] [PubMed] [Google Scholar]
- Swolin D, Ohlsson C. Growth hormone increases interleukin-6 produced by human osteoblast-like cells. J Clin Endocrinol Metab. 1996;81:4329–4333. doi: 10.1210/jcem.81.12.8954036. [DOI] [PubMed] [Google Scholar]
- Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab. 2000;85:4908–4911. doi: 10.1210/jcem.85.12.7167. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. STAT family of transcription factors in cytokine-mediated biological responses. Cytokine Growth Factor Rev. 2000;11:199–207. doi: 10.1016/s1359-6101(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Liang CT. Mitogenic activity but not phenotype expression of rat osteoprogenitor cells in response to IGF-I is impaired in aged rats. Mech Ageing Dev. 1996;92:1–10. doi: 10.1016/s0047-6374(96)01793-9. [DOI] [PubMed] [Google Scholar]
- Tannenbaum GS, Gurd W, Lapointe M. Leptin is a potent stimulator of spontaneous pulsatile growth hormone (GH) secretion and the GH response to GH-releasing hormone. Endocrinology. 1998;139:3871–3875. doi: 10.1210/endo.139.9.6206. [DOI] [PubMed] [Google Scholar]
- Tanner SJ, Hefferan TE, Rosen CJ, Conover CA. Impact of pregnancy-associated plasma protein-a deletion on the adult murine skeleton. J Bone Miner Res Off J Am Soc Bone Miner Res. 2008;23:655–662. doi: 10.1359/jbmr.071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JDJ, Monson JP. Adult GH deficiency throughout lifetime. Eur J Endocrinol Eur Fed Endocr Soc. 2009;161(Suppl 1):S97–S106. doi: 10.1530/EJE-09-0258. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Umayahara Y, Shu H, Centrella M, Rotwein P, McCarthy TL. Identification of the cAMP response element that controls transcriptional activation of the insulin-like growth factor-I gene by prostaglandin E2 in osteoblasts. J Biol Chem. 1996;271:21835–21841. doi: 10.1074/jbc.271.36.21835. [DOI] [PubMed] [Google Scholar]
- Tremollieres FA, Strong DD, Baylink DJ, Mohan S. Insulin-like growth factor II and transforming growth factor beta 1 regulate insulin-like growth factor I secretion in mouse bone cells. Acta Endocrinol (Copenh) 1991;125:538–546. doi: 10.1530/acta.0.1250538. [DOI] [PubMed] [Google Scholar]
- Triplett JW, O’Riley R, Tekulve K, Norvell SM, Pavalko FM. Mechanical loading by fluid shear stress enhances IGF-1 receptor signaling in osteoblasts in a PKCzeta-dependent manner. Mol Cell Biomech MCB. 2007;4:13–25. [PubMed] [Google Scholar]
- Tzanela M. Adult growth hormone deficiency: to treat or not to treat. Expert Opin Pharmacother. 2007;8:787–795. doi: 10.1517/14656566.8.6.787. [DOI] [PubMed] [Google Scholar]
- van der Eerden BCJ, Karperien M, Wit JM. Systemic and local regulation of the growth plate. Endocr Rev. 2003;24:782–801. doi: 10.1210/er.2002-0033. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Bowers CY. Human GH pulsatility: an ensemble property regulated by age and gender. J Endocrinol Invest. 2003;26:799–813. doi: 10.1007/BF03345229. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Frystyk J, Iranmanesh A, Ørskov H. Testosterone and estradiol regulate free insulin-like growth factor I (IGF-I), IGF binding protein 1 (IGFBP-1), and dimeric IGF-I/IGFBP-1 concentrations. J Clin Endocrinol Metab. 2005;90:2941–2947. doi: 10.1210/jc.2004-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A. Physiological regulation of the human growth hormone (GH)-insulin-like growth factor type I (IGF-I) axis: predominant impact of age, obesity, gonadal function, and sleep. Sleep. 1996;19:S221–224. doi: 10.1093/sleep/19.suppl_10.s221. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Metzger DL, Martha PM, Mauras N, Kerrigan JR, Keenan B, Rogol AD, Pincus SM. Estrogen and testosterone, but not a nonaromatizable androgen, direct network integration of the hypothalamo-somatotrope (growth hormone)-insulin-like growth factor I axis in the human: evidence from pubertal pathophysiology and sex-steroid hormone replacement. J Clin Endocrinol Metab. 1997;82:3414–3420. doi: 10.1210/jcem.82.10.4317. [DOI] [PubMed] [Google Scholar]
- Vuagnat BA, Pierroz DD, Lalaoui M, Englaro P, Pralong FP, Blum WF, Aubert ML. Evidence for a leptin-neuropeptide Y axis for the regulation of growth hormone secretion in the rat. Neuroendocrinology. 1998;67:291–300. doi: 10.1159/000054326. [DOI] [PubMed] [Google Scholar]
- Walenkamp MJE, Karperien M, Pereira AM, Hilhorst-Hofstee Y, van Doorn J, Chen JW, Mohan S, Denley A, Forbes B, van Duyvenvoorde HA, van Thiel SW, Sluimers CA, Bax JJ, de Laat JaPM, Breuning MB, Romijn JA, Wit JM. Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J Clin Endocrinol Metab. 2005;90:2855–2864. doi: 10.1210/jc.2004-1254. [DOI] [PubMed] [Google Scholar]
- Walenkamp MJE, Wit JM. Genetic disorders in the GH IGF-I axis in mouse and man. Eur J Endocrinol Eur Fed Endocr Soc. 2007;157(Suppl 1):S15–26. doi: 10.1530/EJE-07-0148. [DOI] [PubMed] [Google Scholar]
- Walker JL, Van Wyk JJ, Underwood LE. Stimulation of statural growth by recombinant insulin-like growth factor I in a child with growth hormone insensitivity syndrome (Laron type) J Pediatr. 1992;121:641–646. doi: 10.1016/s0022-3476(05)81163-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhou J, Cheng CM, Kopchick JJ, Bondy CA. Evidence supporting dual, IGF-I-independent and IGF-I-dependent, roles for GH in promoting longitudinal bone growth. J Endocrinol. 2004;180:247–255. doi: 10.1677/joe.0.1800247. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cheng Z, Elalieh HZ, Nakamura E, Nguyen MT, Mackem S, Clemens TL, Bikle DD, Chang W. IGF-1R signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26:1437–1446. doi: 10.1002/jbmr.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Menendez A, Fong C, ElAlieh HZ, Kubota T, Long R, Bikle DD. IGF-I Signaling in Osterix-Expressing Cells Regulates Secondary Ossification Center Formation, Growth Plate Maturation, and Metaphyseal Formation during Postnatal Bone Development. J Bone Miner Res Off J Am Soc Bone Miner Res. 2015 doi: 10.1002/jbmr.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar MJE, Biermasz NR, Hamdy NaT, Zillikens MC, van Meurs JBJ, Rivadeneira F, Hofman A, Uitterlinden AG, Stokkel MPM, Roelfsema F, Kloppenburg M, Kroon HM, Romijn JA, Pereira AM. High prevalence of vertebral fractures despite normal bone mineral density in patients with long-term controlled acromegaly. Eur J Endocrinol Eur Fed Endocr Soc. 2011;164:475–483. doi: 10.1530/EJE-10-1005. [DOI] [PubMed] [Google Scholar]
- Weissberger AJ, Ho KK. Activation of the somatotropic axis by testosterone in adult males: evidence for the role of aromatization. J Clin Endocrinol Metab. 1993;76:1407–1412. doi: 10.1210/jcem.76.6.8501143. [DOI] [PubMed] [Google Scholar]
- Weissberger AJ, Ho KK, Lazarus L. Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women. J Clin Endocrinol Metab. 1991;72:374–381. doi: 10.1210/jcem-72-2-374. [DOI] [PubMed] [Google Scholar]
- White HD, Ahmad AM, Durham BH, Patwala A, Whittingham P, Fraser WD, Vora JP. Growth hormone replacement is important for the restoration of parathyroid hormone sensitivity and improvement in bone metabolism in older adult growth hormone-deficient patients. J Clin Endocrinol Metab. 2005;90:3371–3380. doi: 10.1210/jc.2004-1650. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Wu S, Yang W, De Luca F. Insulin-Like Growth Factor-Independent Effects of Growth Hormone on Growth Plate Chondrogenesis and Longitudinal Bone Growth. Endocrinology. 2015;156:2541–2551. doi: 10.1210/en.2014-1983. [DOI] [PubMed] [Google Scholar]
- Wu Y, Sun H, Basta-Pljakic J, Cardoso L, Kennedy OD, Jasper H, Domené H, Karabatas L, Guida C, Schaffler MB, Rosen CJ, Yakar S. Serum IGF-1 is insufficient to restore skeletal size in the total absence of the growth hormone receptor. J Bone Miner Res Off J Am Soc Bone Miner Res. 2013;28:1575–1586. doi: 10.1002/jbmr.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing W, Baylink D, Kesavan C, Hu Y, Kapoor S, Chadwick RB, Mohan S. Global gene expression analysis in the bones reveals involvement of several novel genes and pathways in mediating an anabolic response of mechanical loading in mice. J Cell Biochem. 2005;96:1049–1060. doi: 10.1002/jcb.20606. [DOI] [PubMed] [Google Scholar]
- Xing W, Govoni KE, Donahue LR, Kesavan C, Wergedal J, Long C, Bassett JHD, Gogakos A, Wojcicka A, Williams GR, Mohan S. Genetic evidence that thyroid hormone is indispensable for prepubertal insulin-like growth factor-I expression and bone acquisition in mice. J Bone Miner Res Off J Am Soc Bone Miner Res. 2012;27:1067–1079. doi: 10.1002/jbmr.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Courtland HW, Clemmons D. IGF-1 and bone: New discoveries from mouse models. J Bone Miner Res Off J Am Soc Bone Miner Res. 2010;25:2543–2552. doi: 10.1002/jbmr.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Bouxsein ML, Sun H, Mejia W, Kawashima Y, Wu Y, Emerton K, Williams V, Jepsen K, Schaffler MB, Majeska RJ, Gavrilova O, Gutierrez M, Hwang D, Pennisi P, Frystyk J, Boisclair Y, Pintar J, Jasper H, Domene H, Cohen P, Clemmons D, LeRoith D. Serum complexes of insulin-like growth factor-1 modulate skeletal integrity and carbohydrate metabolism. FASEB J Off Publ Fed Am Soc Exp Biol. 2009;23:709–719. doi: 10.1096/fj.08-118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Ogata N, Shinoda Y, Akune T, Kamekura S, Terauchi Y, Kadowaki T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H. Insulin receptor substrate-1 is required for bone anabolic function of parathyroid hormone in mice. Endocrinology. 2005;146:2620–2628. doi: 10.1210/en.2004-1511. [DOI] [PubMed] [Google Scholar]
- Zhang M, Faugere MC, Malluche H, Rosen CJ, Chernausek SD, Clemens TL. Paracrine overexpression of IGFBP-4 in osteoblasts of transgenic mice decreases bone turnover and causes global growth retardation. J Bone Miner Res Off J Am Soc Bone Miner Res. 2003;18:836–843. doi: 10.1359/jbmr.2003.18.5.836. [DOI] [PubMed] [Google Scholar]
- Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang LC, Chen H, Tang PF, Zhang LH. Association between polymorphisms in insulin-like growth factor-1 and risk of osteoporosis. Genet Mol Res GMR. 2015;14:7655–7660. doi: 10.4238/2015.July.13.10. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sobue T, Hurley MM. FGF-2 increases colony formation, PTH receptor, and IGF-1 mRNA in mouse marrow stromal cells. Biochem Biophys Res Commun. 2002;290:526–531. doi: 10.1006/bbrc.2001.6217. [DOI] [PubMed] [Google Scholar]
- Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141:2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Mohan S, Linkhart TA, Baylink DJ, Strong DD. Retinoic acid regulates insulin-like growth factor-binding protein expression in human osteoblast cells. Endocrinology. 1996;137:975–983. doi: 10.1210/endo.137.3.8603611. [DOI] [PubMed] [Google Scholar]
- Zofková I, Bahbouh R, Bendlová B. Systemic insulin-like growth factor-I, insulin and vitamin D status in relation to age-associated bone loss in women. Exp Clin Endocrinol Diabetes Off J Ger Soc Endocrinol Ger Diabetes Assoc. 2001;109:267–272. doi: 10.1055/s-2001-16346. [DOI] [PubMed] [Google Scholar]