Abstract
To investigate the toxicity, protective effects, and action mechanism of gallotannin-enriched extracts isolated from Galla Rhois (GEGR) against carbon tetrachloride (CCl4)-induced hepatotoxicity in Institute for Cancer Research (ICR) mice, alterations in serum biochemical indicators, histopathological structure, antioxidative status, hepatic apoptosis-related proteins, and liver fibrosis regulating factors were measured in mice pretreated with GEGR for five days before CCl4 injection. The GEGR/CCl4 treated group showed decreased levels of three serum marker enzymes (ALP, AST, and ALT) representing liver toxicity, although LDH levels remained constant. Necrotic area indicating hepatic cell death significantly inhibited, while malondialdehyde (MDA) concentration and superoxide dismutase (SOD) expression were dramatically recovered in the GEGR preadministrated group. In mechanism analyses of GEGR, the formation of active caspase-3 and enhancement of Bax/Bcl-2 expression was effectively inhibited in the GEGR/CCl4 treated group. The level of pro-inflammatory cytokines, TNF-α and IL-6, as well as the phosphorylation of p38 and JNK in the TNF-α downstream signaling pathway was rapidly recovered in the GEGR/CCl4 treated group, while anti-inflammatory cytokine (IL-10) increased slightly in the same group. Furthermore, the GEGR/CCl4 treated group showed a significant decrease in collagen accumulation results from alleviation of MMP-2 expression, TGF-β1 secretion and the phosphorylation of Smad2/3. Taken together, these results suggest that GEGR may induce remarkable protective effects against hepatic injury induced by CCl4 treatment through upregulation of the anti-inflammatory and antioxidant system.
Keywords: hepatotoxicity, hepatic fibrosis, Galla Rhois, inflammation, oxidative stress
1. Introduction
The liver is the primary organ responsible for metabolism of drugs and chemicals including paracetamol, also known as acetaminophen, diclofenac, and N-nitrosocompounds, as well as the primary target of many toxic chemicals such as carbon-tetrachloride (CCl4) and aflatoxin [1]. CCl4 is a well-known hepatotoxin that induces hepatic damage, necrosis [2], and apoptosis [3]. The administration of CCl4 for long periods leads to fibrosis, cirrhosis, and hepatic carcinoma [4]. Furthermore, CCl4-induced liver injuries are mediated through the activation of cytochrome P450 to produce reactive intermediates such as trichloromethyl free radical (CCl3•) and trichloromethyl peroxy radical (CCl3OO•). These radicals can bind to cellular molecules (nucleic acids, proteins, and lipids), impairing lipid metabolism and initiating hepatic cancer [5]. Therefore, the inhibition of production and activation of free radicals is considered a very important factor for the prevention and treatment of CCl4-induced liver injury.
Many natural products have recently received attention as key sources of antioxidants because they are highly active in the prevention and treatment of diseases induced by oxidative stress. However, a wide range of drugs has not been applied to treat or prevent hepatic disorders or investigate their molecular mechanisms until now [6,7,8]. Galla Rhois (GR), which is the excrescence formed by parasitic aphids, primarily Schlechtendalia chinensis Bell, on the leaf of sumac, Rhus javanica L. (Anacardiaceae) has been investigated as a natural product with favorable ethno pharmacological properties [9,10]. In Korea, GR has long been used as a traditional medicine for treatment of diarrhea, seminal emissions, excessive sweating, bleeding, and chronic cough, although there is little scientific evidence supporting these pharmacological effects [11,12,13]. Recent studies have revealed the therapeutic effects of GR against various human diseases and their mechanisms. Several compounds and extracts purified from GR exhibited good antibacterial activity against many pathogenic bacteria strains, including Salmonella spp., Escherichia coli, Eimeria tenella, Brucella abortus, Staphylococcus aurous, and Clostridium perfringens [14,15,16,17,18,19]. Methyl gallate and ethyl gallate isolated from GR were also found to exert significant anti-inflammatory activity in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages via the induction of heme oxygenase 1 and suppression of iNOS/COX-2 [20,21]. Moreover, galloylglucose (GG6-10) isolated from GR inhibited the invasion of metastatic HT-1080 cells into a reconstituted basement membrane via inhibition of gelationolysis mediated by MMP-2 and -9, while the ellagic acid extracted from GR showed anticancer activity against nasopharyngeal carcinoma cells through downregulated expression of COX-2 and stathmin [22,23]. Furthermore, oral administration of 85% methanol extract of GR reduced brain infarct volume by 37.5% and lipid-peroxidation in middle cerebral artery occlusion, while also improving sensory motor function in a transient focal cerebral ischemia rat model [24]. Moreover, tacrine, nitrofurantoinm, and tert-butyl hydroperoxide-induced hepatotoxicity in HepG2 cells was greatly retained by two hepatoprotective constituents of GR, an equilibrium mixture of 3-galloyl-gallic acid, 4-galloyl-gallic acid isomers, and 1,2,3,4,6-penta-O-galloyl-β-d-glucose [25,26]. However, the protective effects and their mechanisms against hepatotoxicity induced by oxidative stress after toxicant treatment have not yet been investigated in animal models.
Therefore, this study was conducted to investigate the protective effects of Galla Rhois against liver injury induced by CCl4 treatment. Gallotannin-enriched extracts isolated from Galla Rhois (GEGR) significantly inhibited the increase in serum biochemical markers of liver toxicity, histopathological damage, apoptosis induction, and hepatic fibrosis through suppression of oxidative stress and upregulation of antioxidant enzymes.
2. Experimental Section
2.1. Preparation of GEGR
Briefly, samples of GR were collected from plantations in the Hongcheon area of Korea during October of 2013 and dried in a hot-air drying machine (JSR, Seoul, Korea) at 60 °C. Voucher specimens of GR (WPC-14-001) were deposited in the functional materials bank of the PNU-Wellbeing RIS Center at Pusan National University. GEGR was prepared using a modified version of previously described methods [27,28,29,30]. Briefly, dry samples of GR were reduced to powder using an electric blender, after which water extract then obtained at 90 °C for 9 h in a fixed liquor ratio (solid GR powder/water ratio, 1:10) using circulating extraction equipment (IKA Labortechnik, Staufen, Germany). The extracts were subsequently filtered through a 0.4 μm filter, after which they were concentrated by vacuum evaporation and lyophilization using circulating extraction equipment (IKA Labortechnik, Staufen, Germany). Finally, the powder was dissolved in distilled water (dH2O) to 1 mg/mL, then further diluted with phosphate buffered saline (PBS) to the required concentration.
2.2. Analysis of Gallic Acid, Methyl Gallate, and Gallotanin Concentration
During analysis of the main components of GEGR, gallic acid monohydrate (IUPAC name; 3,4,5-trihydroxybenzoic acid, MW: 170.12 g/mol, Sigma-Aldrich Co., St. Louis, MO, USA), methyl gallate (IUPAC name; methyl 3,4,5-trihydroxybenzoate, MW: 184.15 g/mol, Sigma-Aldrich Co.) and gallotanin (IUPAC name; (3,5-dihydroxy-2-(3,4,5-trihydroxybenzoyl)oxy-6-[(3,4,5-trihydroxybenzoyl)oxymethyl]oxan-4-yl) 3,4,5-trihydroxybenzoate, MW: 1701.20 g/mol, Sigma-Aldrich Co.) were used as standard compounds. The wavelengths of the maximum absorption of pure gallic acid, pure methyl gallate, commercial gallotannin, and gallnut extract were 212/257, 214/268, 213/278, and 212/275 nm, respectively. The UV-VIS spectra of pure gallic acid, pure methyl gallate, pure gallotannin, and the gallnut extract showed two bands at 212–214 nm and 257–278 nm, which were both assigned to the π→π* transitions of the given aromatic units and C=O groups in the UV-VIS region [31]. Finally, the UV-Vis spectra were analyzed using a curve-resolving technique based on linear least-squares analysis to fit the combination of the Lorentzian and Gaussian curve shapes.
The HPLC analysis was conducted as previously described [32]. The HPLC system used for these experiments consisted of a Summit Dual-Gradient HPLC System (Dionex, Sunnyvale, CA, USA) with a PDA UV-vis detector at the Korea Bio-IT Foundry Busan Center. Separation was carried out on an YMC-Triart C18 column (S-5 mm/12 nm, 150 mm × 4.6 mm I.D.) maintained at 40 °C. The mobile phase consisted of solvent A (0.4% formic acid in water) and solvent B (acetonitrile). The gradient condition of the mobile phase was as follows: 0–5 min, 10% B; 5–6 min, 10%–15% B; 6–40 min, 15% B; 40–41 min, 15%–30% B; 41–50 min, 30% B; 50–55 min, 30%–10% B; 55–60 min, 10% B. The injection volume was 5 mL in full loop injection. The flow rate was 0.8 mL/min, and detection was performed at 280 nm.
2.3. Design of Animal Experiment
The animal protocol used in this study was reviewed and approved by the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC; Approval Number PNU-2014-0662). All mice were handled in the Pusan National University-Laboratory Animal Resources Center, which is accredited by the Korea Food and Drug Administration (FDA), in accordance with the Laboratory Animal Act (Accredited Unit Number-000231) and AAALAC International according to the National Institutes of Health guidelines (Accredited Unit Number; 001525). Ten-week-old male ICR mice were purchased from Samtako (Osan, Korea) and provided with ad libitum access to a standard irradiated chow diet (Samtako) consisting of moisture (12.5%), crude protein (25.43%), crude fat (6.06%), crude fiber (3.9%), crude ash (5.31%), calcium (1.14%) and phosphorus (0.99%) and water throughout the feeding study. During the experiment, mice were maintained in a specific pathogen-free state under a strict light cycle (lights on at 08:00 h and off at 20:00 h) at 23 ± 2 °C and 50% ± 10% relative humidity.
ICR mice were divided into the following five groups (n = 8 per group): no treated group, vehicle/CCl4 treated group, low concentration GEGR (LGEGR)/CCl4 treated group, medium concentration GEGR (MGEGR)/CCl4 treated group and high concentration GEGR (HGEGR)/CCl4 treated group. The first group did not receive any extracts throughout the study period, while the second group repeatedly received a constant volume of desterilized water. The other three groups were treated with 250 mg (LGEGR/CCl4 treated group), 500 mg (MGEGR/CCl4 treated group), and 1000 mg (HGEGR/CCl4 treated group) doses of GEGR per kg of body weight/day. The GEGR was diluted in distilled water and administered via oral gavage for five days. After final administration of GEGR, the No treated group was administered a constant volume of olive oil by intraperitoneal (IP) injection as a control, while the remaining groups were injected IP with a single dose of CCl4 (0.7 mL/kg body weight diluted 1:9 in olive oil). At 24 h after CCl4 injection, mice in each group were euthanized using a chamber filled with CO2 gas, after which blood and organ samples were collected for further analysis.
2.4. Serum Biochemistry
After the final administration, all ICR mice in each group were fasted for 8 h, after which blood was collected from the abdominal veins and incubated for 30 min at room temperature. Whole blood was then centrifuged at 1500× g for 15 min to obtain the serum, after which serum biochemical components including alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine (CRE) were assayed using an automatic serum analyzer (Hitachi 747, Tokyo, Japan). All assays employed fresh serum and were conducted in duplicate.
2.5. Histological Analysis
Liver tissue was dissected from mice and fixed in 4% neutral buffered formaldehyde (pH 6.8) overnight, after which each liver was dehydrated and embedded in paraffin. Next, a series of liver sections (4 μm) was cut from paraffin-embedded tissue using a Leica microtome (Leica Microsystems, Bannockburn, IL, USA). These sections were then deparaffinized with xylene, rehydrated with ethanol at a graded decreasing concentration of 100%–70%, and finally washed with distilled water. The slides with liver sections were stained with hematoxylin (Sigma-Aldrich Co.) and eosin (Sigma-Aldrich Co.), then washed with dH2O, after which the necrotic area was measured using the Leica Application Suite (Leica Microsystems, Wetzlar, Germany).
2.6. Immunohistochemical Analysis
Immunohistochemical analysis was performed as previously described [33]. Briefly, liver tissue samples for the detection of collagen proteins using light microscopy were fixed in 4% formaldehyde for 12 h, embedded in paraffin, and sliced into 4 μm thick sections. These sections were then de-paraffinized with xylene, rehydrated, and pretreated for 30 min at room temperature with PBS-blocking buffer containing 10% goat serum. Next, the sections were incubated with anti-collagen antibody diluted 1:1000 in PBS-blocking buffer. The antigen-antibody complexes were then visualized with biotinylated secondary antibody (goat anti-rabbit)-conjugated HRP streptavidin (Histostain-Plus Kit) (Zymed, South San Francisco, CA, USA) at a dilution of 1:1500 in PBS blocking buffer. Finally, collagen proteins were detected using a stable DAB (Invitrogen Corp., Carlsbad, CA, USA) and Imaging Densitometer (Model GS-690, BioRad, Richmond, CA, USA).
2.7. Determination of Malondialdehyde (MDA) Levels
The MDA levels were assayed using a Lipid Peroxidation (MDA) Assay Kit (Sigma-Aldrich Co.) according to the manufacturer’s protocols. Briefly, the liver tissue was homogenized in MDA Lysis Buffer containing butylhydroxytoluene (BHT), after which the homogenates were stored at −20 °C until analysis. The sample or standards and TBA solution (70 mM thiobarbituric acid and 5 M glacial acetic acid) were incubated in microcentrifuge tubes at 95 °C for 60 min, then cooled to room temperature in an ice bath for 10 min, after which the reaction absorbance at 532 nm was read using a Vmax plate reader (Molecular Devices, Sunnyvale, CA, USA).
2.8. Western Blot
Proteins prepared from the liver tissue of mice were separated by 4%–20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for 2 h, after which the resolved proteins were transferred to nitrocellulose membranes for 2 h at 40 V. Each membrane was then incubated separately at 4 °C with the following primary antibodies overnight: anti-Bcl-2 (Abcam, Cambridge, UK), anti-Bax (Abcam), anti-caspase-3 (Cell Signaling, Danvers, MA, USA), anti-MMP-1 (Santa Cruz), anti-MMP-2 (Santa Cruz), anti-MMP-9 (Santa Cruz), anti-Smad 2/3 (Cell Signaling), anti-p-Smad2/3 (Cell Signaling), anti-p38 (Cell Signaling), anti-p-p38 (Cell Signaling), anti-SAPK/JNK (Cell Signaling), anti-p-SAPK/JNK (Cell Signaling), SOD (Abcam), and anti-actin antibody (Sigma-Aldrich Co.). The membranes were subsequently washed with washing buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 0.05% Tween 20) and incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Invitrogen Corp.) at a 1:1000 dilution and room temperature for 1 h. Membrane blots were developed using Amersham ECL Select Western Blotting detection reagent (GE Healthcare, Little Chalfont, UK).
2.9. Enzyme-Linked Immunosorbent Assay (ELISA) for Tumor Growth Factor (TGF)-β1
The concentration of TGF-β1 in blood serum was measured using a TGF-β1 ELISA kit (Biolegend, San Diego, CA, USA) according to the manufacturer’s protocols. Briefly, the sample for analysis was prepared by adding acidification solution and neutralization solution to liver tissue homogenate progressively. The liver samples or standards and buffer C were incubated in a 96-well plate at room temperature for 2 h while shaking at 200 rpm, after which 100 μL of TGF-β1 detection antibody solution was added to each well and samples were incubated at room temperature for 1 h with shaking. After washing, 100 μL of avidin-HRP D solution was added to each well and the plate was incubated at room temperature for 30 min with shaking. Next, 100 μL of substrate solution was added to each well and the plate was incubated for 10 min in the dark. The reaction was then quenched by the addition of 100 μL of stop solution, after which the plates were analyzed by evaluation of the absorbance at 450 nm using a Vmax plate reader (Molecular Devices, Sunnyvale, CA, USA).
2.10. RT-PCR
RT-PCR was conducted to measure the relative quantities of mRNA for inflammatory cytokines including TNF-α, IL-6 and IL-10. Briefly, the liver tissues were chopped with scissors and homogenized in RNAzol solution (Leedo Medical, Houston, TX, USA). The isolated RNA was then measured by UV spectroscopy (BioSpec-Nano) (Shimadzu Scientific Instruments, Columbia, MD, USA). Expression of inflammatory cytokines was assessed by RT-PCR with 3 μg of total RNA from the liver tissue of each group. Next, 500 ng of the oligo-dT primer (Invitrogen Corp.) was annealed at 70 °C for 10 min. Complementary DNA, which was used as the template for further amplification, was synthesized by the addition of dATP, dCTP, dGTP, and dTTP with 200 units of reverse transcriptase. Next, 10 pM each of the sense and antisense primers were added, and the reaction mixture was subjected to 25–30 cycles of amplification in a Perkin-Elmer Thermal Cycler as follows: 30 s at 94 °C, 30 s at 62 °C, and 45 s at 72 °C. RT-PCR was also conducted using GAPDH-specific primers to ensure RNA integrity. The primer sequences for TNF-α expression were as follows: sense, 5′-CCT GTA GCC CAC GTC GTA GC-3′ and antisense, 5′-TTG ACC TCA GCG CTG AGT TG-3′, IL-6: sense, 5′-TTG GGA CTG ATG TTG TTG ACA-3′ and antisense, 5′-TCA TCG CTG TTC ATA CAA TCA GA-3′, IL-10: sense, 5′-CCA AGC CTT ATC GGA AAT GA-3′, and antisense, 5′-TTT TCA CAG GGG AGA AAT CG-3′, GAPDH: sense, 5′-CTC ATG ACC ACA GTC CAT GC-3′, and antisense, 5′-TTC AGC TCT GGG ATG ACC TT-3′. The experiment was repeated three times, and all samples were analyzed in triplicate. The final PCR products were separated by 1.2% agarose gel electrophoresis and visualized by ethidium bromide staining.
2.11. Statistical Analysis
One-way ANOVA was used to identify significant differences between the No and CCl4 treated groups (SPSS for Windows, Release 10.10, Standard Version, Chicago, IL, USA). In addition, differences in the responses of the vehicle/CCl4 treated group and GEGR/CCl4 treated groups were evaluated using a post hoc test (SPSS for Windows, Release 10.10, Standard Version). All values are reported as the mean ± standard deviation (SD), and a p-value of < 0.05 was considered significant.
3. Results
3.1. Detection of Gallotannin in GEGR
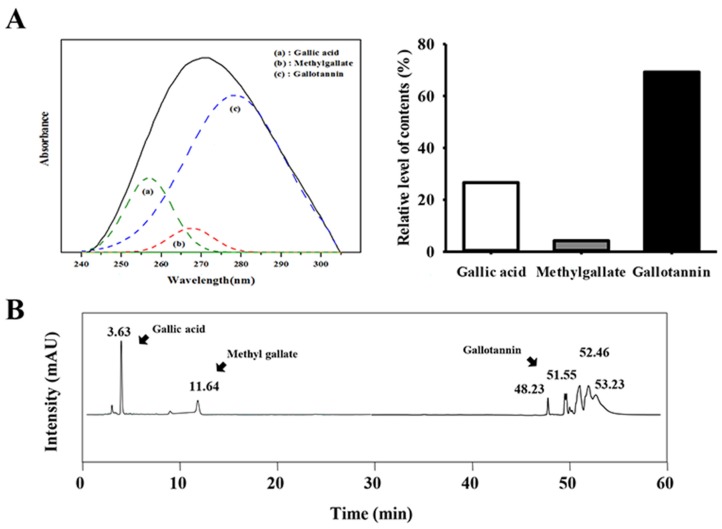
Three major compounds, gallic acid, methyl gallate, and gallotannin, were detected in GEGR at different wavelengths (257 nm, 268 nm and 278 nm) by UV-vis spectra. As shown in Figure 1A, gallotannin was present at the highest levels in GEGR (69.2%), followed by gallic acid (26.6%) and methyl gallate (4.2%). GEGR was composed only these three compounds without any significant contamination of other compounds. The chemical formula of gallic acid was very similar to that of methyl gallate, whereas gallotannin formed from the reaction of glucose with dimers or higher oligomers of gallic acid. Also, HPLC curves of GEGR were revealed that there characteristic peaks for gallic acid (3.58 min), methyl gallate (11.4 min), and gallotannin (48.26, 51.55, 52.46, and 53.23 min). These results demonstrate that GEGR contained a high concentration of only three bioactive components that were likely related to its hepatoprotective effects.
Figure 1.
Analysis of major components in GEGR. The contents of gallotannin, gallic acid, and methyl gallate in GEGR were determined based on the (A) UV-Vis spectra and (B) high-performance liquid chromatography.
3.2. Protective Effects of GEGR against Hepatic Toxicity
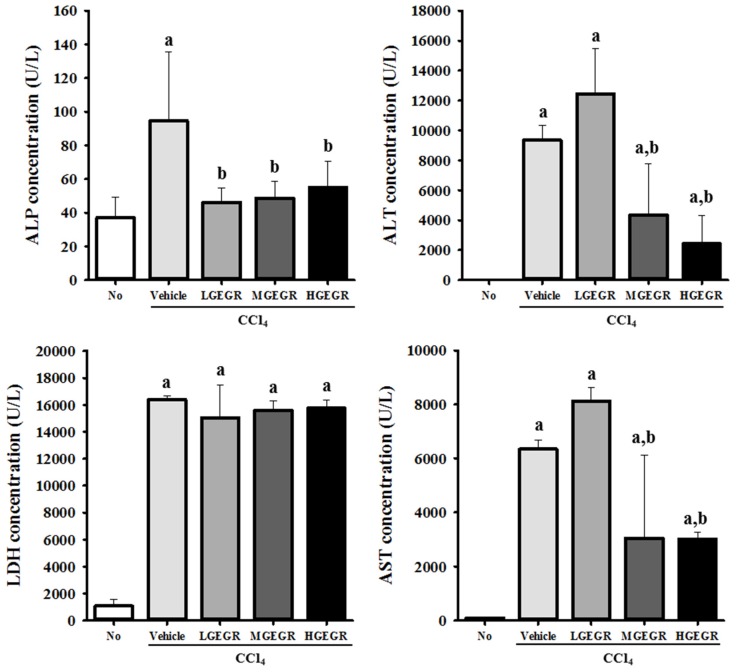
To investigate the protective effects of GEGR against CCl4-induced toxicity in terms of serum biochemical indicators, the levels of four indicators, ALP, AST, ALT, and LDH, were measured in vehicle/CCl4 and GEGR/CCl4 treated ICR mice. The levels of ALP, AST, ALT, and LDH were higher in vehicle/CCl4 treated rats than no treatment rats. The levels of all factors except LDH were significantly lower with average 54.5% in GEGR/CCl4 treated rats than vehicle/CCl4 treated rats, although the rate of decrease varied for each factor (Figure 2).
Figure 2.
Biochemical markers of hepatic toxicity. Blood was collected from the abdominal veins of vehicle/CCl4 and three GEGR/CCl4 treated ICR mice (no treatment and HGEGR group (n = 7), LGEGR group (n = 6), vehicle and MGEGR group (n = 8)). Serum concentrations of ALP, AST, ALT, and LDH were analyzed in duplicate using a serum biochemical analyzer as described in the Materials and Methods. Data represent the means ± SD of three replicates. a, p < 0.05 relative to the no treatment group; b, p < 0.05 relative to the vehicle/CCl4 treated group.
For ALP, the constant ratio of decrease level was detected in three different concentrations of GEGR treated groups. The levels of ALT and AST decreased significantly in the MGEGR and HGEGR treated group, while no significant change was observed in the LGEGR treated group. The LDH level was maintained at a constant value in all GEGR/CCl4 treated groups relative to the vehicle/CCl4 treated group (Figure 2). Taken together, these results show that pretreatment with GEGR may induce protective effects against CCl4-induced liver damage, although the level of protective effects varied according to each factor.
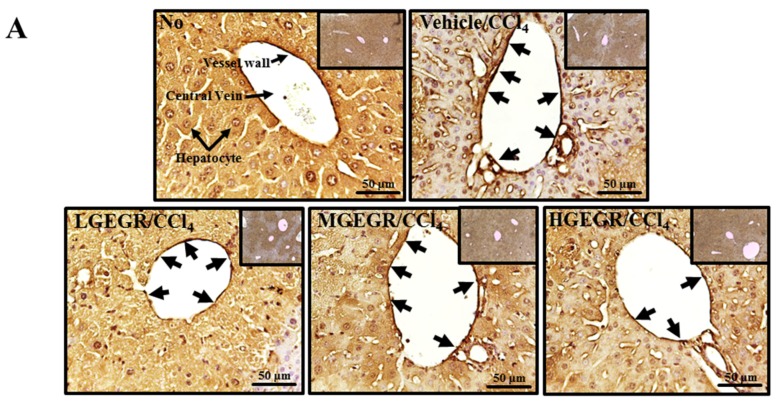
3.3. Protective Effects of GEGR against Alteration of Histopathological Structure
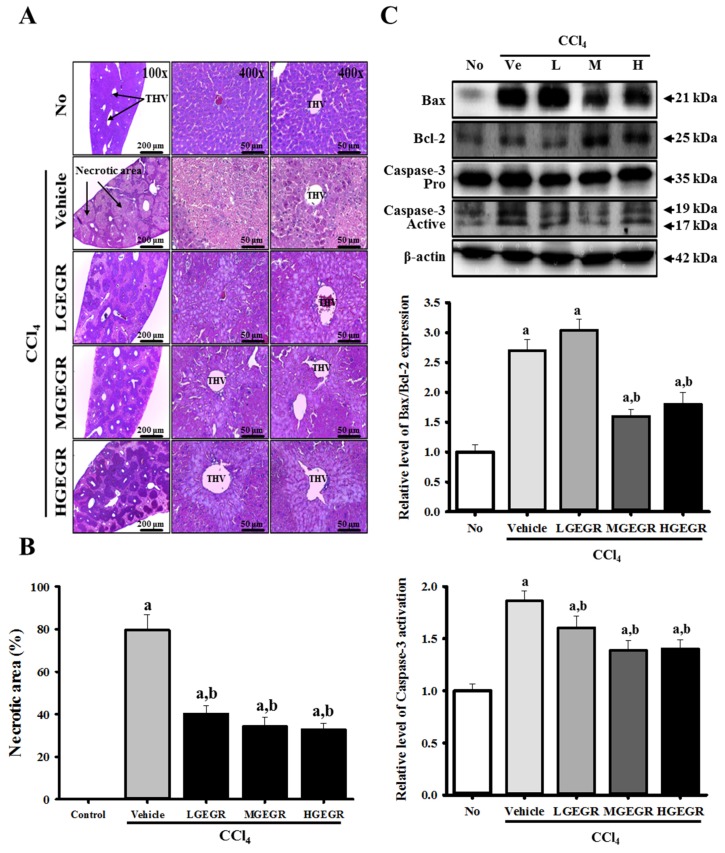
The differences between tissue components under normal and pathological conditions were visualized by histological staining using hematoxylin and eosin. Histopathological alterations during hepatocellular damage along with the protective effects of GEGR were first identified by histopathological analysis of the liver sections. In the no treatment group, the histopathology of the liver displayed a normal distribution of hepatocytes with clear visible nuclei, a portal triad, and central vein. However, extensive centrolobular necrosis was observed in and around the terminal hepatic venule (THV) of the liver following CCl4 treatment. Furthermore, the central vein was significantly dilated in the vehicle/CCl4 treated group relative to the no treatment group. However, the liver section of the group pretreated with GEGR for five days displayed low hepatocellular necrosis, a poorly dilated central vein, and regular arrangement of hepatocytes (Figure 3A). Additionally, the necrotic area was dramatically decreased by 51.3%–62.5% in the three GEGR/CCl4 treated groups (Figure 3A,B). Thus, our findings indicate that GEGR pretreatment may effectively inhibit the change in histopathological structure of the liver tissue induced by CCl4 treatment.
Figure 3.
Histology of liver and expression of apoptosis-related proteins. (A) Liver tissues collected from ICR mice were fixed in 4% formalin and stained with H&E solution. Liver tissue was mainly observed around the terminal hepatic venule (THV) at a magnification 200× and 400×; (B) The necrotic area in each section was observed using Leica Application Suite (Leica Microsystems, Switzerland); (C) Expression levels of caspase-3, Bcl-2 and Bax proteins in the liver tissue were analyzed by western blot analysis. Membranes were incubated with antibodies for caspase-3, Bcl-2, and Bax, as well as β-actin protein from the liver. Expression levels were quantified by an imaging densitometer, and the sizes of the products indicated. Data represent the means ± SD of three replicates. a, p < 0.05 compared to the no treatment group; b, p < 0.05 compared to the vehicle/CCl4 treated group.
3.4. Protective Effects of GEGR on the Regulation of Oxidative Stress
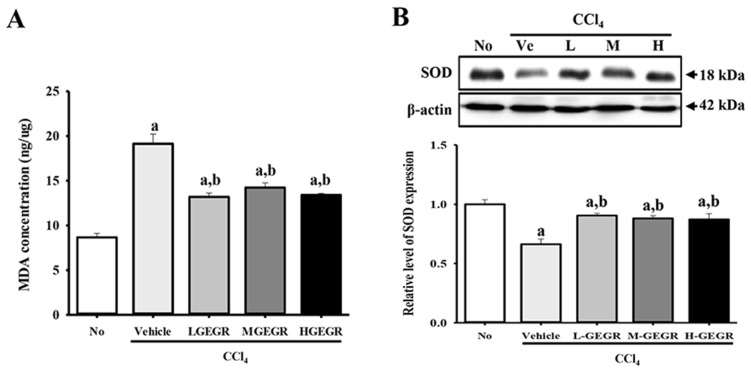
We investigated the protective effects of GEGR on the oxidative stress induced by CCl4 exposure. To accomplish this, the MDA concentration and SOD expression were measured in the liver tissue. The concentration of MDA was 221% higher in the vehicle/CCl4 treated group than the no treatment group, while the level was significantly lower in the GEGR/CCl4 treated groups; however, these levels were not correlated with GEGR dose (Figure 4A). Conversely, an opposite pattern of MDA concentrations was observed upon analysis of SOD expression. The vehicle/CCl4 treated group showed a significantly lower level of SOD expression than that of the no treatment group. However, this level was recovered to the level of the no treatment group after GEGR pretreatment (Figure 4B). Taken together, these results indicate that GEGR pretreatment may inhibit the oxidative stress induced by CCl4 exposure through the suppression of lipid peroxidation as well as increased SOD repression.
Figure 4.
Alteration of oxidant stress related factors. (A) The level of MDA was determined in serum collected from mice using a lipid peroxidation assay kit that could detect MDA at 0.1 nmole/mg to 20 nmole/mg; (B) The expression level of SOD was measured in the homogenate of liver tissue collected from subset groups. The intensity of each band was determined using an imaging densitometer and the relative level of each protein was calculated based on the intensity of actin protein as an endogenous control. Data represent the means ± SD of three replicates. a, p < 0.05 compared to the no treatment group; b, p < 0.05 compared to the vehicle/CCl4 treated group.
3.5. Anti-Apoptosis Effects of GEGR Treatment
To determine if GEGR treatment can prevent the activation of apoptosis induced by CCl4 exposure, alterations in the expression of apoptosis-related proteins were examined in the liver tissues of mice that had been pretreated with vehicle or GEGR for five days. Bcl-2 belongs to a family of proteins that includes both pro- and anti-apoptotic members. Among these members, Bcl-2 proteins stimulate anti-apoptosis, while Bax protein significantly inhibits the anti-apoptotic actions of Bcl-2 protein [34,35]. The level of Bax/Bcl-2 expression was higher in the vehicle/CCl4 treated group than the no treatment group. However, this level was significantly lower in the MGEGR and HGEGR treated groups than vehicle/CCl4-treated group, whereas it was maintained at a constant level in the LGEGR treated group, regardless of GEGR pretreatment (Figure 3C). Conversely, the change in the relative level of active caspase-3 was very similar to that in Bax/Bcl-2. After CCl4 injection, the pro-caspase-3 level was reduced, whereas the level of active-caspase-3 increased. In the GEGR/CCl4 treated group, the levels of pro-caspase 3 and active-caspase 3 decreased significantly by 15.1%–27.0% (Figure 3C). Therefore, western blot analysis of apoptotic protein indicated that GEGR pretreatment may protect against hepatocyte apoptosis induced by CCl4 injection via regulation of Bax/Bcl-2 expression and caspase-3 activation.
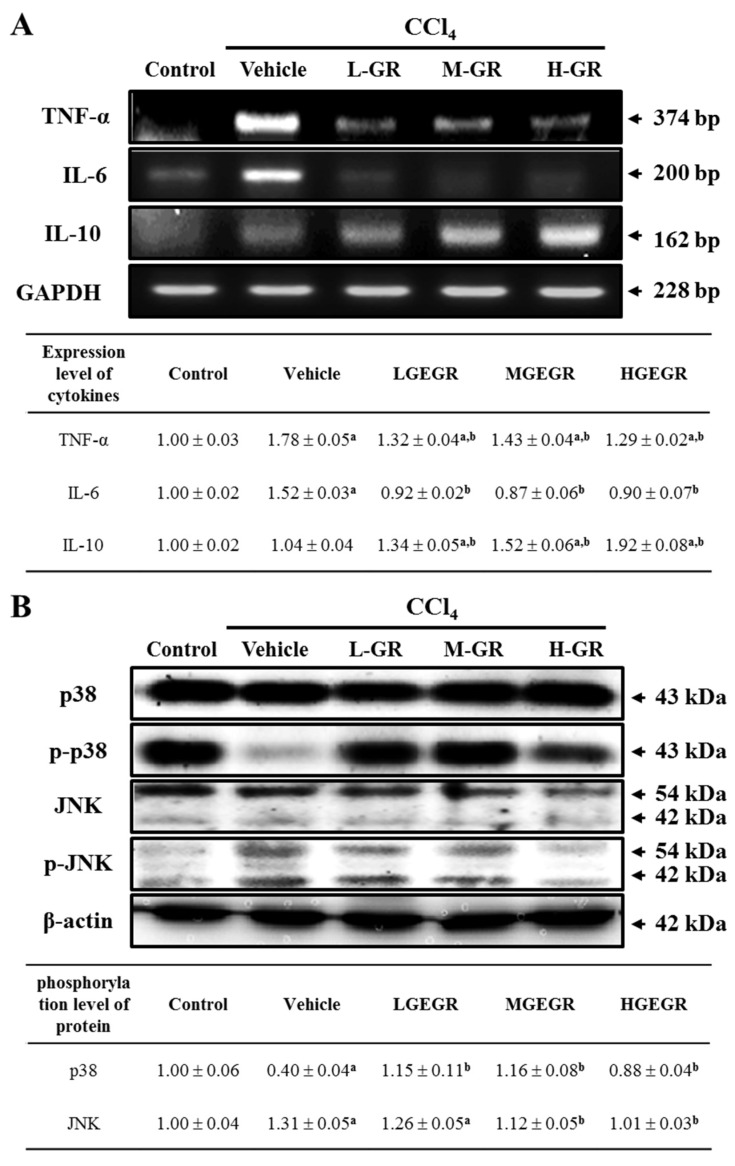
3.6. Anti-Inflammation Effects of GEGR Treatment
To investigate the effects of GEGR on inflammation of liver tissue, alterations in the transcript levels of pro- and anti-inflammatory cytokines were confirmed by RT-PCR analysis. Upon analysis of pro-inflammatory cytokines, the level of TNF-α and IL-6 mRNA was higher in the vehicle/CCl4 treated group than the no treatment group. However, these levels decreased in the three GEGR treated groups, although the rate of decrease was not dependent on GEGR dose. A complete recovery to that of the no treatment group was only observed for the IL-6 mRNA (Figure 5A). To determine if downregulation of the TNF-α mRNA was accompanied by alterations of the downstream signaling pathway, the phosphorylation levels of the key proteins were measured in the liver tissue. Interestingly, the phosphorylation level of p38 rapidly recovered to that of the no treatment group in the three GEGR treated groups, even though the vehicle/CCl4 treated group showed very low levels (Figure 5B). However, the phosphorylation level of JNK was increased in the vehicle/CCl4 treated group relative to the no treatment group, and this level decreased in a dose dependent manner in the GEGR treated groups, with those of the MGEGR/CCl4 and HGEGR/CCl4 treated groups showing complete recovery to that of the no treatment group (Figure 5B). Therefore, these results suggest that the TNF-α signaling pathway activated by GEGR pretreatment was closely related to the regulation of p38 and JNK phosphorylation.
Figure 5.
Analysis of inflammation related cytokines and TNF-α downstream signaling pathway. (A) Changes in the mRNA level of pro-inflammatory and anti-inflammatory cytokines in the no, vehicle/CCl4, and GEGR/CCl4 treated group. The mRNA levels of the TNF-α, IL-6 and IL-10 genes were examined by RT-PCR using specific primers; (B) Phosphorylation levels of p38 and JNK in liver tissue. To measure the expression levels of each protein, the membranes were incubated with antibodies specific for each protein, as well as β-actin protein from liver lysates. Three mice per group were assayed by western blotting. Values are reported as the means ± SD. a, p < 0.05 compared to the No treatment group; b, p < 0.05 compared to the vehicle/CCl4 treated group.
The anti-inflammatory cytokine, IL-10, showed a different pattern from that of the pro-inflammatory cytokines. The level of IL-10 mRNA was not changed in the vehicle/CCl4 treated group relative to the no treatment group; however, this level rapidly increased in the GEGR treated groups (Figure 5A). These results suggest that GEGR pretreatment can effectively prevent the inflammatory response induced by CCl4 treatment through suppression of pro-inflammatory cytokines and upregulation of anti-inflammatory cytokines.
3.7. Protective Effects of GEGR against Liver Fibrosis
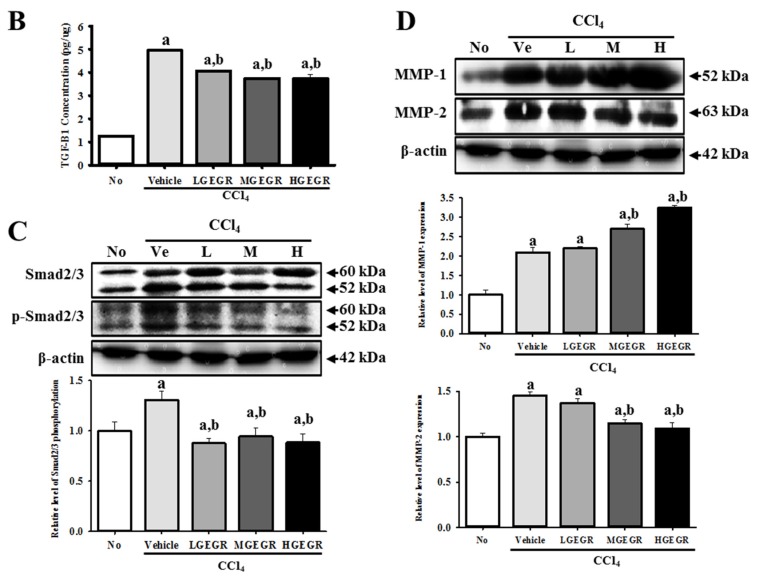
Hepatic fibrosis, which is a histologic hallmark of chronic liver disease, is characterized by the excessive accumulation of connective tissue in the liver and considered an indicator of persistent or progressive hepatic injury [36]. To examine whether GEGR pretreatment can affect collagen accumulation and MMP expression during liver fibrosis induced by CCl4 treatment, the levels of collagen and MMP expression were measured in the liver tissue of the subset group. In the case of immunostaining analysis for collagen, the excessive accumulation of collagen became densely stained and expanded in the portal tracts of the vehicle/CCl4 treated group when compared with the No treatment group. However, this accumulation was significantly decreased in the three GEGR treated group, even though the highest level of decrease was observed in the HGEGR treated group (Figure 6A). To investigate whether the collagen accumulation can accompany alteration of the collagen regulation enzymes, the MMP 1 and 2 expression was measured in the GEGR/CCl4 treated group. The expression level of both proteins was higher in the vehicle/CCl4 treated group than the no treatment group; however, the expression level of MMP-1 was significantly higher in the MGEGR/CCl4 and HGEGR/CCl4 treated group than the vehicle/CCl4 treated group, while the LGEGR/CCl4 treated group was maintained at a constant level. Upon analysis of MMP-2 expression, a significant decrease was observed in the MGEGR/CCl4 and HGEGR/CCl4 treated group (Figure 6D). Taken together, these results suggest that GEGR pretreatment may suppress collagen accumulation through downregulation of MMP-2 expression.
Figure 6.
Analysis of the regulatory factors of liver fibrosis. (A) Immunohistochemistry of collagen protein. After GEGR/CCl4 pretreatment for 5 days, the liver tissues were collected from all groups and slide sections of liver were prepared as described in the Materials and Methods. The distribution of collagen protein in the slide sections of liver tissue was determined by staining with collagen specific antibody followed by observation at 400×. Black arrows indicate the stained area in THV; (B) ELISA for TGF-β1. After collection of blood serum, the concentration of TGF-β1 was determined in the serum collected from the brains of mice using a TGF-β1 ELISA kit that could detect TGF-β1 at 3.5 pg/mL; (C) Western blot analysis for Smad2/3 expression. To measure the expression levels of Smad2/3 and p-Smad2/3 protein, the membranes were incubated with specific antibodies for each protein, as well as β-actin protein from liver lysates. Three mice per group were assayed by western blotting; (D) Western blot analysis of MMP expression. To measure the expression levels of MMP-1 and MMP-2 protein, the membranes were incubated with specific antibodies for each protein, as well as β-actin protein from liver lysates. Three mice per group were assayed by western blotting. Values are reported as the means ± SD. a, p < 0.05 compared to the no treatment group; b, p < 0.05 compared to the vehicle/CCl4 treated group.
The pathogenesis of liver fibrosis is also stimulated by TGF-β1 through promotion of ECM production, which inhibits production of MMPs for effective extracellular matrix deposition in the liver [37]. To investigate the molecular mechanism of the TGF-β1 signaling pathway after GEGR treatment, the concentration of TGF-β1 and the expression level of key proteins in the TGF-β1 signaling pathway were measured in the GEGR/CCl4 treated group. The concentration of TGF-β1 in the serum was 392% higher in the vehicle/CCl4 treated group than the no treatment group. However, this level decreased dramatically by 18.8%–25.0% in the three GEGRs treated group, even though the concentration of GEGR did not affect this concentration (Figure 6B). Moreover, the treatment of CCl4 induced an increase in Samd2/3 phosphorylation level in the TGF-β1 signaling pathway when compared with that of the no treatment group. However, these levels in the three GEGR/CCl4 treated group were effectively recovered to that of the no treatment group, even if there was no difference within the three GEGR/CCl4 treated groups (Figure 6C). Overall, these results suggest that GEGR pretreatment can reduce the secretion of TGF-β1 and therefore the phosphorylation levels of Smad2/3.
4. Discussion
Reactive oxygen species (ROS) are considered an important factor in the pathogenesis of various diseases including atherosclerosis, liver disorders, lung and kidney injury, aging, and diabetes [5]. ROS induced liver disease can be protected and treated by many antioxidants of natural origin, even though their origin varied [38,39]. GEGR has received a great deal of attention as a novel therapeutic drug candidate owing to its strong antioxidant activity. A number of related studies are currently being conducted to identify its novel functions and mechanism of action. In an effort to develop drugs for the treatment of oxidative stress induced diseases, we investigated the protective effects of GEGR in hepatic injury of ICR mice after CCl4 exposure. Our results demonstrated that GEGR pretreatment is associated with the prevention of the hepatocytes apoptosis, liver inflammation, and fibrosis via suppression of oxidative stress induced by CCl4 treatment.
Until now, composition of GR and their functions were investigated with various studies. Component analysis showed that GR consist of gallic acid, digallic acid, and galloylglucopyranoses (GGs) in ethyl acetate extract [39] and methyl gallate, galloyl-gallic acid isomer, and 1,2,3,4,6-penta-O-galloyl-β-d-glucose (PGG) in MeOH extract [25]. Especially, analysis of gallotannin of GR revealed gallotannin were mainly a mixture of mono-tetradeca-GGs, which have antioxidant and antibacterial activity [40,41,42]. Furthermore, PGG, precursor of gallotannin extract from GR showed remarkable hepatoprotective effects than gallic acid, methyl gallate against primary rat hepatocyte necrosis induced by tert-butyl hydroperoxide [26]. PGG also showed significant hepatoprotective effects against tacrine- and nitrofurantoin-induced cytotoxicity in HepG2 cells [25]. Therefore, hepatoprotective effects observed in the present study were considered due to mostly gallotannin rather than gallic acid and methyl gallate.
The present study showed that GEGR pretreatment inhibited serum marker enzymes representing hepatic toxicity in ICR mice with liver injury. Strong decreases in ALT, ALP, and AST in serum were detected in the GEGR/CCl4 treated group, although the LDH level was maintained at a constant level (Figure 3). These findings were consistent with those of many previous studies that showed administration of several antioxidant mixtures significantly decreased the levels of serum marker enzymes relative to vehicle groups in CCl4 treated animals. For example, the level of ALT, ALP, and AST was significantly decreased by methanol extract of Carissa opaca leaves (MCL) [43], aqueous leaf extract of Persea Americana (AEPA) [44], Berchemia lineate ethanol extract (BELE) [45], methanol extract of Melaleuca styphelioides (MSE) [46], and polyphenols-enriched extract from Huangshan Maofeng green tea (HMTP) [47]. However, MCL treatment induced a decrease in LDH level [43], which may have been caused by differences in the composition or concentration of bioactive compounds among studies.
Another conclusion of this study is that GEGR pretreatment applied to the CCl4-induced liver injury of ICR mice stimulated a protective effect of hepatocytes against apoptosis and necrosis. Similar results have been reported for the CCl4-induced liver injury of ICR mice treated with several bioactive mixtures or compounds. A marked change in liver histopathology and increase in the expression level of apoptotic proteins including caspase-3, Bax, and Bcl-2 were induced by CCl4 injection. However, protective effects against CCl4-induced acute liver damage were observed in animals treated with water-soluble extract of Salvia miltiorrhiza [24], dibenzoyl glycoside from Salvinia natans [48], dioscin [49], glycyrrhizic acid [50], and the flavonoid fraction from Rosa laevigata Michx fruit [51]. Therefore, the results of present study provide additional evidence that GEGR treatment may induce the attenuation of CCl4-induced liver damage.
Oxidative stress induced by CCl4 exposure can regulate numerous redox-sensitive transcription factors such as NF-κB, activator protein 1 (AP-1) and early growth response 1 (EGR1). NF-κB activation was tightly associated with the release of pro-inflammatory cytokines including TNF-α, IL-6, and acute phase reactants (C-reactive protein, CRP) [52,53]. CCl4 treated animals showed a significant increase in TNF-α and IL-6 concentration; however, these levels were inhibited by several compounds and mixtures including curcumin [54], polysaccharide from Tarphochlamys affinis (PTA) [55], and ursolic acid [56]. The results of the present study are in agreement with the above reports, although the rate of decrease varied. However, there are conflicting results regarding the expression of anti-inflammatory cytokines (IL-10) in CCl4 injected animals. The IL-10 level in the hepatic supernatant of SD rats with CCl4-induced liver injury did not change in response to treatment with ginseng extract and ginsenoside Rb1 for two weeks, although CCl4 injection alone induced a slight decrease in their level [57]. However, the IL-10 level in the serum of Balb/c mice with CCl4-induced liver injury was significantly reduced by pretreatment with ruxolitinib (15 mg/kg) for 2 h, whereas the levels in the CCl4 injected group were higher than in the control group [58]. In this study, the expression of IL-10 was dramatically enhanced by GEGR pretreatment in a dose dependent manner, which differed from the results of previous studies. We thought that this difference in IL-10 expression could be caused by distinction of the properties of bioactive compounds and the genetic background of the experimental animal.
Liver fibrosis is known to be a common response of the liver to a variety of damage including toxic, infectious, or metabolic agents. Fibrosis is also characterized by excessive accumulation of extracellular matrix (ECM) through the abnormal regulation of connective tissue synthesis and ECM homeostasis [59,60]. Many herbs or their bioactive compounds, such as Fufang-Liu-Yue-Qing [61], epigallocatechin-3-gallate (EGCG) [62], silymarin [63] and curcumin [64], have been shown to reduce the severity of hepatic fibrosis in mice and rats. Indeed, the level of TGF-β1, MMP-2 expression and collagen was significantly reduced in response to treatment with most of the above herbs or compounds. Conversely, our finding that GEGR pretreatment induced the effective attenuation of hepatic injury is very similar to the results of previous studies, although the rate of decrease differed.
Lipid peroxidation is considered a critical factor in the pathogenesis of CCl4 induced hepatic injury [43]. Therefore, many approaches for the detection of hepatic injury include measurement of lipid peroxides [43]. A significant decrease in lipid peroxides in liver tissues following cotreatment with CCl4 and antioxidants indicated the protective effects of many antioxidant compounds and mixtures such as MCL [43], polysaccharide from Angelica and Astragalus (AAP) [65], ellagitannin-enriched Melaleuca styphelioides Sm. (Myrtaceae) [46], and Fagonia schweinfurthii (Hadidi) Hadidi (Family: Zygophyllaceae) [66] by scavenging of free radicals produced by CCl4 injection. In the present study, GEGR pretreatment caused a significant decrease in MDA level, as shown in Figure 4A. These findings are in complete agreement with the results of previous studies and provide novel evidence of the effects of GEGR on lipid metabolism.
SOD decrease the concentration of highly reactive superoxide radicals by transforming them to H2O2, while catalase (CAT), glutathione (GSH), and glutathione peroxidase (GPx) degrade H2O2 to protect the liver tissue from ROS produced by CCl4 exposure [43,46]. After decrease of the activity of SOD by CCl4 injection, the activity and level of antioxidant enzymes was dramatically recovered in response to treatment with MSE [46], MCL [43], HMTP [47], AAP [65] and Fangonia schweinfurthii (Hadidi) Hadidi extract [66]. The present study is the first to demonstrate the alteration of SOD expression in the liver of GEGR/CCl4 treated mice. Fundamentally, GEGR pretreatment attenuates the decrease of SOD expression relative to the vehicle/CCl4 treated group. These results are concordant with those of previous studies showing the correlation between the level of antioxidant enzymes and the administration of antioxidant mixture.
We investigated the protective effects of GEGR against CCl4-induced hepatic injury of ICR mice. GEGR pretreatment prevented the increase of serum marker enzymes, enhancing hepatic apoptosis and necrosis, liver fibrosis, and inflammation through the suppression of lipid peroxidation and induction of antioxidant enzyme expression. Therefore, the results of this study indicate that GEGR should be considered a candidate for the treatment and prevention of hepatotoxicity in the liver.
Acknowledgments
We thank Jin Hyang Hwang, the animal technician, for directing the Animal Facility and Care at the Laboratory Animal Resources Center.
Author Contributions
J.G., J.E.K., E.K.K., S.H.S., J.E.S., H.A.L., and D.Y.H. participated in the design of the study, sample preparation, animal experiments and data analyses, where Y.L. performed the serum biochemical analysis of GEGR-treated animal. Y.H.L. helped with preparation and HPLC analysis of GEGR. J.T.H. helped with data analysis and manuscript preparation. Also, all authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Besednova N.N., Zaporozhets T.S., Kuznetsova T.A., Kryzhanovskiĭ S.P., Kovalev N.N., Zviagintseva T.N. Hepatoprotective effects of extracts and polysaccharides from seaweed. Antibiot. Khimioterapiia. 2014;59:30–37. [PubMed] [Google Scholar]
- 2.Siviková K., Piesová E., Dianovský J. The protection of Vitamin E and selenium against carbon tetrachloride-induced genotoxicity in ovine peripheral blood lymphocytes. Mutat. Res. 2001;494:135–142. doi: 10.1016/S1383-5718(01)00190-5. [DOI] [PubMed] [Google Scholar]
- 3.Shi J., Aisaki K., Ikawa Y., Wake K. Evidence of hepatocyte apoptosis in rat liver after the administration of carbon tetrachloride. Am. J. Pathol. 1998;153:515–525. doi: 10.1016/S0002-9440(10)65594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wernke M.J., Schell J.D. Solvents and malignancy. Clin. Occup. Environ. Med. 2004;4:513–527. doi: 10.1016/j.coem.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Singh N., Kamath V., Narasimhamurthy K., Rajini P.S. Protective effect of potato peel extract against carbon tetrachloride-induced liver injury in rats. Environ. Toxicol. Pharmacol. 2008;26:241–246. doi: 10.1016/j.etap.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Sreelatha S., Padma P.R., Umadevi M. Protective effects of Coriandrum sativum extracts on carbon tetrachloride-induced hepatotoxicity in rats. Food Chem. Toxicol. 2009;47:702–708. doi: 10.1016/j.fct.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava A., Shivanandappa T. Hepatoprotective effect of the aqueous extract of the roots of Decalepis hamiltonii against carbon tetrachloride-induced oxidative stress in rats. Food Chem. 2010;118:411–417. doi: 10.1016/j.foodchem.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Sahreen S., Khan M.R., Khan R.A. Evaluation of antioxidant activities of various solvent extracts of Carissa opaca fruits. Food Chem. 2010;122:1205–1211. doi: 10.1016/j.foodchem.2010.03.120. [DOI] [Google Scholar]
- 9.Lee S.M., Lee D.W., Park J.D., Kim J.I. Study on Formation and Development of Schlechtendalis chinensis Gall in Rhus javanica. Korean J. Appl. Entomol. 1997;36:83–87. [Google Scholar]
- 10.Ren Z., Zhu B., Wang D., Ma E., Su D., Zhong Y. Comparative population structure of Chinese sumac aphid Schlechtendalia chinensis and its primary host-plant Rhus chinensis. Genetica. 2008;132:103–112. doi: 10.1007/s10709-007-9153-6. [DOI] [PubMed] [Google Scholar]
- 11.Song G.Y., Park B.J., Kim S.H. Antithrombotic effect of galla rhois. Korean J. Pharmacogn. 2002;33:120–123. [Google Scholar]
- 12.Shim Y.J., Doo H.K., Ahn S.Y., Kim Y.S., Seong J.K., Park I.S., Min B.H. Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J. Ethnopharmacol. 2003;85:283–287. doi: 10.1016/S0378-8741(02)00370-7. [DOI] [PubMed] [Google Scholar]
- 13.Kim S.H., Park H.H., Lee S., Jun C.D., Choi B.J., Kim S.Y., Kim S.H., Kim D.K., Park J.S., Chae B.S., et al. The anti-anaphylactic effect of the gall of Rhus javanica is mediated through inhibition of histamine release and inflammatory cytokine secretion. Int. Immunopharmacol. 2005;5:1820–1829. doi: 10.1016/j.intimp.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Ahn Y.J., Lee C.O., Kweon J.H., Ahn J.W., Park J.H. Growth-inhibitory effects of Galla Rhois-derived tannins on intestinal bacteria. J. Appl. Microbiol. 1998;84:439–443. doi: 10.1046/j.1365-2672.1998.00363.x. [DOI] [PubMed] [Google Scholar]
- 15.Choi J.G., Kang O.H., Lee Y.S., Oh Y.C., Chae H.S., Jang H.J., Kim J.H., Sohn D.H., Shin D.W., Park H., et al. In vitro activity of methyl gallate isolated from galla rhois alone and in combination with ciprofloxacin against clinical isolates of salmonella. J. Microbiol. Biotechnol. 2008;18:1848–1852. doi: 10.4014/jmb.0800.025. [DOI] [PubMed] [Google Scholar]
- 16.Choi J.G., Kang O.H., Lee Y.S., Oh Y.C., Chae H.S., Jang H.J., Shin D.W., Kwon D.Y. Antibacterial activity of methyl gallate isolated from Galla Rhois or carvacrol combined with nalidixic acid against nalidixic acid resistant bacteria. Molecules. 2009;14:1773–1780. doi: 10.3390/molecules14051773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi J.G., Mun S.H., Chahar H.S., Bharaj P., Kang O.H., Kim S.G., Shin D.W., Kwon D.Y. Methyl gallate from Galla rhois successfully controls clinical isolates of Salmonella infection in both in vitro and in vivo systems. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0102697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J.J., Bae J.H., Kim D.H., Lim J.J., Kim D.G., Lee H.J., Min W., Rhee M.H., Chang H.H., Park H., et al. Intracellular replication inhibitory effects of Galla Rhois ethanol extract for Brucella abortus infection. J. Ethnopharmacol. 2011;138:602–609. doi: 10.1016/j.jep.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Lee J.J., Kim D.H., Lim J.J., Kim D.G., Min W., Kim G.S., Lee H.J., Rhee M.H., Park H., Kim S.C., et al. Anticoccidial effect of supplemental dietary Galla Rhois against infection with Eimeria tenella in chickens. Avian Pathol. 2012;41:403–407. doi: 10.1080/03079457.2012.702888. [DOI] [PubMed] [Google Scholar]
- 20.Chae H.S., Kang O.H., Choi J.G., Oh Y.C., Lee Y.S., Brice O.O., Chong M.S., Lee K.N., Shin D.W., Kwon D.Y. Methyl gallate inhibits the production of interleukin-6 and nitric oxide via down-regulation of extracellular-signal regulated protein kinase in RAW 264.7 cells. Am. J. Chin. Med. 2010;38:973–983. doi: 10.1142/S0192415X10008391. [DOI] [PubMed] [Google Scholar]
- 21.Park P.H., Hur J., Kim Y.C., An R.B., Sohn D.H. Involvement of heme oxygenase-1 induction in inhibitory effect of ethyl gallate isolated from Galla Rhois on nitric oxide production in RAW 264.7 macrophages. Arch. Pharm. Res. 2011;34:1545–1552. doi: 10.1007/s12272-011-0917-2. [DOI] [PubMed] [Google Scholar]
- 22.Ata N., Oku T., Hattori M., Fujii H., Nakajima M., Saiki I. Inhibition by galloylglucose (GG6-10) of tumor invasion through extracellular matrix and gelatinase-mediated degradation of type IV collagens by metastatic tumor cells. Oncol. Res. 1996;8:503–511. [PubMed] [Google Scholar]
- 23.Xiang Q., Fan C., Xiao S., Pan H., Wang J., Zhao N., Tian J. Effect of gallnut extract on nasopharyngeal carcinoma 5–8F cells and its mechanism. Zhong Nan da Xue Xue Bao Yi Xue Ban. 2012;37:871–875. doi: 10.3969/j.issn.1672-7347.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Lee T.Y., Chang H.H., Wang G.J., Chiu J.H., Yang Y.Y., Lin H.C. Water-soluble extract of Salvia miltiorrhiza ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. J. Pharm. Pharmacol. 2006;58:659–665. doi: 10.1211/jpp.58.5.0011. [DOI] [PubMed] [Google Scholar]
- 25.An R.B., Oh H.C., Kim Y.C. Phenolic constituents of galla Rhois with hepatoprotective effects on tacrine- and nitrofurantoin-induced cytotoxicity in Hep G2 cells. Biol. Pharm. Bull. 2005;28:2155–2157. doi: 10.1248/bpb.28.2155. [DOI] [PubMed] [Google Scholar]
- 26.Park E.J., Zhao Y.Z., An R.B., Kim Y.C., Sohn D.H. 1,2,3,4,6-penta-O-galloyl-beta-d-glucose from Galla Rhois protects primary rat hepatocytes from necrosis and apoptosis. Planta Med. 2008;11:1380–1383. doi: 10.1055/s-2008-1081300. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y.H. Dyeing, fastness, and deodorizing properties of cotton, silk, and wool fabrics dyed with coffee sludge (Coffea arabica L.) extract. J. Appl. Polym. Sci. 2007;103:251–257. doi: 10.1002/app.25221. [DOI] [Google Scholar]
- 28.Hwang E.K., Lee Y.H., Kim H.D. Dyeing and deodorizing properties of cotton, silk, and wool fabrics dyed with various natural colorants. J. Korean Soc. Dyers Finish. 2007;19:12–20. [Google Scholar]
- 29.Lee Y.H., Hwang E.K., Jung Y.J., Do S.K., Kim H.D. Dyeing and deodorizing properties of cotton, silk, wool fabrics dyed with Amur Cork tree, Dryopteris crassirhizoma, Chrysanthemum boreale, Artemisia Extracts. J. Appl. Polym. Sci. 2010;115:2246–2253. doi: 10.1002/app.31357. [DOI] [Google Scholar]
- 30.Lee Y.H., Hwang E.K., Baek Y.M., Lee M.S., Lee D.J., Jung Y.J., Kim H.D. Deodorizing and antibacterial performance of cotton, silk and wool fabrics dyed with Punica granatum L. extracts. Fiber Polym. 2013;14:1445–1453. doi: 10.1007/s12221-013-1445-0. [DOI] [Google Scholar]
- 31.Albu M.G., Ghica M.V., Giurginca M., Trandafir V., Popa L., Cotrut C. Spectral characteristics and antioxidant properties of tannic acid immobilized on collagen drug-delivery systems. Rev. Chim. 2009;60:666–672. [Google Scholar]
- 32.Lee Y.H., Hwang E.K., Baek Y.M., Kim H.D. Deodorizing function and antibacterial activity of fabrics dyed with gallnut (Galla chinensis) extract. Text. Res. J. 2015;85:1045–1054. doi: 10.1177/0040517514559580. [DOI] [Google Scholar]
- 33.Hwang D.Y., Chae K.R., Kang T.S., Hwang J.H., Lim C.H., Kang H.K., Goo J.S., Lee M.R., Lim H.J., Min S.H., et al. Alterations in behavior, amyloid beta-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of Alzheimer’s disease. FASEB J. 2002;16:805–813. doi: 10.1096/fj.01-0732com. [DOI] [PubMed] [Google Scholar]
- 34.Joensuu H., Pylkkänen L., Toikkanen S. Bcl-2 protein expression and long-term survival in breast cancer. Am. J. Pathol. 1994;145:1191–1198. [PMC free article] [PubMed] [Google Scholar]
- 35.Apakama I., Robinson M.C., Walter N.M., Charlton R.G., Royds J.A., Fuller C.E., Neal D.E., Hamdy F.C. Bcl-2 overexpression combined with p53 protein accumulation correlates with hormone-refractory prostate cancer. Br. J. Urol. 1996;74:1258–1262. doi: 10.1038/bjc.1996.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bissell D.M. Cell-matrix interaction and hepatic fibrosis. Prog. Liver Dis. 1990;9:143–155. [PubMed] [Google Scholar]
- 37.Friedman S.L., Millward-Sadler G.H., Arthur M.J.P. Wright’s Liver and Biliary Disease. 3rd ed. W.B. Saunders; Philadelphia, PA, USA: 1992. pp. 821–881. [Google Scholar]
- 38.Liu G.T., Zhang T.M., Wang B.E., Wang Y.W. Protective action of seven natural phenolic compounds against peroxidative damage to biomembranes. Biochem Pharmacol. 1992;43:147–152. doi: 10.1016/0006-2952(92)90271-J. [DOI] [PubMed] [Google Scholar]
- 39.Domitrović R., Jakovac H., Blagojević G. Hepatoprotective activity of berberine is mediated by inhibition of TNF-α, COX-2, and iNOS expression in CCl4-intoxicated mice. Toxicology. 2001;280:33–43. doi: 10.1016/j.tox.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Tian F., Li B., Ji B., Zhang G., Luo Y. Identification and structure-activity relationship of gallotannins separated from Galla chinensis. Food Sci. Technol. 2009;42:1289–1295. doi: 10.1016/j.lwt.2009.03.004. [DOI] [Google Scholar]
- 41.Beasley T.H., Ziegler H.W., Bell A.D. Determination and characterization of gallotannin by high performance liquid chromatography. Anal. Chem. 1977;49:238–243. doi: 10.1021/ac50010a016. [DOI] [Google Scholar]
- 42.Xiang P., Lin Y.M., Lin P., Xiang C., Yang Z.W., Lu Z.M. Effect of cationization reagents on the matrix-assisted laser desorption/ionization time-of-flight mass spectrum of Chinese gallotannins. J. Appl. Polym. Sci. 2007;105:859–864. doi: 10.1002/app.26373. [DOI] [Google Scholar]
- 43.Sahreen S., Khan M.R., Khan R.A. Hepatoprotective effects of methanol extract of Carissa opaca leaves on CCl4-induced damage in rat. BMC Complement. Altern. Med. 2011;11:48–56. doi: 10.1186/1472-6882-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brai B.I., Adisa R.A., Odetola A.A. Hepatoprotective properties of aqueous leaf extract of Persea Americana, Mill (Lauraceae) “avocado” against CCl4-induced damage in rats. Afr. J. Tradit. Complement. Altern. Med. 2014;28:237–244. doi: 10.4314/ajtcam.v11i2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li C., Yi L.T., Geng D., Han Y.Y., Weng L.J. Hepatoprotective effect of ethanol extract from Berchemia lineate against CCl4-induced acute hepatotoxicity in mice. Pharm. Biol. 2015;53:767–772. doi: 10.3109/13880209.2014.941506. [DOI] [PubMed] [Google Scholar]
- 46.Al-Sayed E., El-Lakkany N.M., Seif El-Din S.H., Sabra A.N., Hammam O.A. Hepatoprotective and antioxidant activity of Melaleuca styphelioides on carbon tetrachloride-induced hepatotoxicity in mice. Pharm. Biol. 2014;52:1581–1590. doi: 10.3109/13880209.2014.908398. [DOI] [PubMed] [Google Scholar]
- 47.Cui Y., Yang X., Lu X., Chen J., Zhao Y. Protective effects of polyphenols-enriched extract from Huangshan Maofeng green tea against CCl4-induced liver injury in mice. Chem. Biol. Interact. 2014;220:75–83. doi: 10.1016/j.cbi.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Srilaxmi P., Sareddy G.R., Kavi Kishor P.B., Setty O.H., Babu P.P. Protective efficacy of natansnin, a dibenzoyl glycoside from Salvinia natans against CCl4 induced oxidative stress and cellular degeneration in rat liver. BMC Pharmacol. 2010;10:13–25. doi: 10.1186/1471-2210-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu B., Xu Y., Xu L., Cong X., Yin L., Li H., Peng J. Mechanism investigation of dioscin against CCl4-induced acute liver damage in mice. Environ. Toxicol. Pharmacol. 2012;34:127–135. doi: 10.1016/j.etap.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Guo X., Liang B., Wang X., Fan F., Jin J., Lan R., Yang J., Wang X., Jin L., Cao Q. Glycyrrhizic acid attenuates CCl4-induced hepatocyte apoptosis in rats via a p53-mediated pathway. World J. Gastroenterol. 2013;19:3781–3791. doi: 10.3748/wjg.v19.i24.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang S., Lu B., Han X., Xu L., Qi Y., Yin L., Xu Y., Zhao Y., Liu K., Peng J. Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2013;55:60–69. doi: 10.1016/j.fct.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 52.Iyer A., Fairlie D.P., Prins J.B., Hammock B.D., Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nat. Rev. Endocrinol. 2010;6:71–82. doi: 10.1038/nrendo.2009.264. [DOI] [PubMed] [Google Scholar]
- 53.Muñoz A., Costa M. Nutritionally mediated oxidative stress and inflammation. Oxid. Med. Cell Longev. 2013;2013 doi: 10.1155/2013/610950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reyes-Gordillo K., Segovia J., Shibayama M., Vergara P., Moreno M.G., Muriel P. Curcumin protects against acute liver damage in the rat by inhibiting NF-kappaB, proinflammatory cytokines production and oxidative stress. Biochim. Biophys. Acta. 2007;1770:989–996. doi: 10.1016/j.bbagen.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Lin X., Liu X., Huang Q., Zhang S., Zheng L., Wei L., He M., Jiao Y., Huang J., Fu S., et al. Hepatoprotective effects of the polysaccharide isolated from Tarphochlamys affinis (Acanthaceae) against CCl4-induced hepatic injury. Biol. Pharm. Bull. 2012;35:1574–1580. doi: 10.1248/bpb.b12-00494. [DOI] [PubMed] [Google Scholar]
- 56.Ma J.Q., Ding J., Zhang L., Liu C.M. Ursolic acid protects mouse liver against CCl4-induced oxidative stress and inflammation by the MAPK/NF-κB pathway. Environ. Toxicol. Pharmacol. 2014;37:975–983. doi: 10.1016/j.etap.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Hou Y.L., Tsai Y.H., Lin Y.H., Chao J.C. Ginseng extract and ginsenoside Rb1 attenuate carbon tetrachloride-induced liver fibrosis in rats. BMC Complement. Altern. Med. 2014;14:415–425. doi: 10.1186/1472-6882-14-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hazem S.H., Shaker M.E., Ashamallah S.A. The novel Janus kinase inhibitor ruxolitinib confers protection against carbon tetrachloride-induced hepatotoxicity via multiple mechanisms. Chem. Biol. Interact. 2014;220:116–127. doi: 10.1016/j.cbi.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 59.Friedman S.L., Maher J.J., Bissell D.M. Mechanisms and therapy of hepatic fibrosis: report of the AASLD Single Topic Basic Research Conference. Hepatology. 2000;32:1403–1408. doi: 10.1053/jhep.2000.20243. [DOI] [PubMed] [Google Scholar]
- 60.Wells R.G. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J. Clin. Gastroenterol. 2005;46:295–303. doi: 10.1097/01.mcg.0000155516.02468.0f. [DOI] [PubMed] [Google Scholar]
- 61.Lin X., Zhang S., Huang Q., Wei L., Zheng L., Chen Z., Jiao Y., Huang J., Fu S., Huang R. Protective effect of Fufang-Liu-Yue-Qing, a traditional Chinese herbal formula, on CCl4 induced liver fibrosis in rats. J. Ethnopharmacol. 2012;142:548–556. doi: 10.1016/j.jep.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 62.Tipoe G.L., Leung T.M., Liong E.C., Lau T.Y., Fung M.L., Nanji A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology. 2010;273:45–52. doi: 10.1016/j.tox.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 63.Mata-Santos H.A., Lino F.G., Rocha C.C., Paiva C.N., Castelo Branco M.T., Pyrrho Ados S. Silymarin treatment reduces granuloma and hepatic fibrosis in experimental schistosomiasis. Parasitol. Res. 2010;107:1429–1434. doi: 10.1007/s00436-010-2014-8. [DOI] [PubMed] [Google Scholar]
- 64.Fu Y., Zheng S., Lin J., Ryerse J., Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol. Pharmacol. 2008;73:339–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- 65.Pu X., Fan W., Yu S., Li Y., Ma X., Liu L., Ren J., Zhang W. Polysaccharides from Angelica and Astragalus exert hepatoprotective effects against carbon-tetrachloride-induced intoxication in mice. Can. J. Physiol. Pharmacol. 2015;93:39–43. doi: 10.1139/cjpp-2014-0331. [DOI] [PubMed] [Google Scholar]
- 66.Pareek A., Godavarthi A., Issarani R., Nagori B.P. Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. J. Ethnopharmacol. 2013;150:973–981. doi: 10.1016/j.jep.2013.09.048. [DOI] [PubMed] [Google Scholar]