Abstract
Crystallin genes are selectively expressed during lens development. Maf and Sox family proteins synergistically enhanced γF-crystallin promoter activity in a lens cell line. Mutational analysis of the γF-crystallin promoter identified a composite regulatory element containing nonconsensus Maf and Sox recognition sequences. Mutations in these recognition sequences or changes in their spacing eliminated synergistic transcription activation. The transcriptional synergy was also affected by changes in the orientation of the Maf recognition sequence that had no detectable effect on binding affinity. The interaction between Maf and Sox proteins was visualized in living cells by bimolecular fluorescence complementation analysis. The N-terminal region of Maf mediated the interaction with Sox proteins in cells. Synergistic transcription activation required the N-terminal region of Maf as well as the ancillary DNA binding domain and the unique portion of the basic region that mediate specific recognition of the γF-crystallin promoter element. A mutation in the ancillary DNA binding domain of Maf (R288P) that has been shown to cause cataract eliminated the transcriptional activity of Maf but had no detectable effect on DNA binding in vitro. Whereas wild-type Maf was uniformly distributed in the nucleoplasm, R288P Maf was enriched in nuclear foci. Cajal bodies and gemini of coiled bodies were closely associated with the foci occupied by R288P Maf. Wild-type Maf formed complexes with Sox proteins in the nucleoplasm, whereas R288P Maf recruited Sox proteins as well as other interaction partners to the nuclear foci. The mislocalization of normal cellular proteins to these foci provides a potential explanation for the dominant disease phenotype of the R288P mutation in Maf.
Combinatorial interactions among transcription-regulatory proteins control tissue-specific patterns of gene expression (6). Most transcription factors are expressed in many different cell types and regulate the expression of different genes in different cells and in response to different extracellular stimuli. Multiple transcription factors cooperate to regulate the expression of individual genes in specific cell types and in response to unique stimuli.
The lens is a powerful model system for the investigation of transcription regulation. Different classes of crystallins constitute 90% of the total protein in the lens (14). The various crystallin genes differ in their spatial and temporal patterns of expression during lens development (50). Many transcription factors have been implicated in crystallin gene regulation and in lens development (28, 38). Targeted disruption of the gene encoding Maf, Sox1, Prox1, or ATF4 in mice causes changes in crystallin gene expression and defects in the differentiation of lens cells (24, 27, 36, 42, 48, 49). The expression of mutated variants of some of these proteins can interfere with crystallin gene expression and normal lens development (9, 17, 18, 41). Due to the close relationship between crystallin expression and lens development, it is often difficult to distinguish between direct effects of transcription factors on crystallin expression and indirect effects caused by changes in lens development.
Maf is a member of the basic region-leucine zipper (bZIP) family of transcription factors. Maf is the founding member of a unique subfamily of bZIP proteins that recognize a 13- to 14-bp binding site (MARE) (23, 25). The core of the MARE is similar to the recognition sequences for other bZIP family proteins, but mutations in the core have only modest effects on Maf binding (8, 25). The recognition sequences flanking the core are unique to Maf family proteins and contribute the majority of the recognition specificity (8, 25). Members of the Maf family contain an ancillary DNA binding region on the N-terminal side of the bZIP domain that undergoes a conformational change upon DNA binding (8, 44). Maf therefore differs from canonical bZIP proteins both in the length of the DNA recognition sequence and in the requirement for a region outside the bZIP domain for specific DNA binding.
The differential regulation of various crystallin genes during lens development is likely to be determined by combinatorial interactions between different transcription regulatory proteins. Maf family proteins can synergize with Sox2 to regulate the δ-crystallin gene, which is expressed in the lens of avian species (35, 41, 47). Maf can also synergize with Sox1 and the CBP coactivator to stimulate the γF-crystallin promoter in COS-1 cells (5).
The Sox family are high-mobility group (HMG) proteins related to the testis-determining factor SRY (21, 39). The expression of Sox proteins is both spatially and temporally modulated during lens development (20). Sox1, Sox2, and Sox3 contain closely related DNA binding domains and participate in different stages of lens differentiation. Sox 2 and Sox3 contribute to lens induction (20, 52), whereas Sox 1 as well as Sox2 are involved in lens fiber cell differentiation (20, 36, 41). Sox proteins often function in concert with other transcription regulatory proteins, in part because of their limited DNA recognition specificities. Sox2 can cooperate with the Oct3 and -4 proteins to regulate the fgf-4, UTF1, and osteopontin genes in the pregastrulation embryo (21, 37, 51). Sox2 also allows Pax-6 binding to a nonconsensus recognition sequence that is required for activation of the δ-crystallin gene (22).
A translocation and a point mutation in the gene encoding human Maf are responsible for dominant ocular abnormalities in two kindreds (18). The point mutation replaces an arginine in an α helix of the ancillary DNA binding region with a proline (R288P), which is likely to alter the secondary structure of this region. A point mutation in the DNA binding domain of murine Maf causes the dominant opaque flecks in lens (Ofl) phenotype (17). Mutations in the crystallin genes can also cause cataract (3, 4, 14). The aberrant regulation of crystallin gene expression is therefore a potential factor contributing to the pathological lens phenotypes caused by mutations in genes encoding transcription regulatory proteins.
We investigated the synergistic effects of Maf and Sox proteins on the expression of crystallin genes, and we visualized the interactions between these proteins in living cells. We also examined the effects of the R288P mutation on the properties of Maf in vitro and in cells. The results of these studies provide insight into the molecular mechanisms of combinatorial regulation of crystallin gene expression as well as a potential explanation for the dominant disease phenotype of the R288P mutation in Maf.
MATERIALS AND METHODS
Plasmid constructs.
The bacterial expression vector encoding Maf residues 241 to 344 [Maf (241-344)] has been described (26). The R288P Maf expression vector was constructed by site-directed mutagenesis of Maf (241-344). Plasmids for the expression of Sox1, Sox2, and Sox3 in mammalian cells as well as the pGEX-Sox1, -Sox2, and -Sox3 bacterial expression constructs containing the respective HMG domains were kindly provided by Robin Lovell-Badge (20).
The chicken γF-crystallin(−395/+44)-luciferase and chicken αA-crystallin(−240/+60)-luciferase constructs were a kind gift from Tom Glaser. The luciferase reporter genes were replaced by that for chloramphenicol acetyltransferase (CAT). The base substitutions, deletions, and insertions in the promoter are described in Table 1.
TABLE 1.
Effects of base substitutions in the γF-crystallin promoter on transcription activation by Maf and Sox proteins
| Name | Sequencea | Avg activationb (SD)
|
|||
|---|---|---|---|---|---|
| Maf | Maf + Sox1 | Maf + Sox2 | Maf + Sox3 | ||
| WT | GGCCCCTTTTGTGCTGTTCCTGCCAACACAGCAGACCTC | 3.4 (0.1) | 8.8 (0.6) | 11.6 (0.02) | 16.7 (0.3) |
| M1M2 | GGCCCCTTTTGTGCTGTTCCgcgCAACACAcgcGACCTC> | 1.3 (0.5) | 1.4 (0.1) | 1.9 (0.2) | 2.6 (0.8) |
| M1 | GGCCCCTTTTGTGCTGTTCCgcgCAACACAGCAGACCTC | 1.0 (0.05) | 1.9 (0.05) | 1.7 (0.02) | 4.6 (0.2) |
| M2 | GGCCCCTTTTGTGCTGTTCCTGCCAACACAcgcGACCTC | 1.2 (0.1) | 1.5 (0.02) | 1.9 (0.05) | 3.3 (0.3) |
| M3 | GGCCCCTTTTGTGCTGTTCCTtCCAACACAGCAGACCTC | 1.1 (0.1) | 0.8 (0.06) | 1.7 (0.03) | 1.9 (0.2) |
| M4 | GGCCCCTTTTGTGCTGTTCCTGCCAACACAGaAGACCTC | 1.1 (0.1) | 1.3 (0.01) | 1.6 (0.03) | 1.8 (0.01) |
| M5 | GGCCCCTTTTGgcgTGTTCCTGCCAACACAGCAGACCTC | 4.9 (0.2) | 6.7 (0.4) | 6.7 (0.4) | 15.7 (0.5) |
| S1 | GGCCCCTcagaTGCTGTTCCTGCCAACACAGCAGACCTC | 3.1 (0.3) | 1.5 (0.5) | 1.5 (0.6) | 2.2 (0.4) |
| 1SM | GGCCCCTTTTGTGCgcaTCCTGCCAACACAGCAGACCTC | 3.1 (0.02) | 4.6 (0.4) | 10.8 (1) | 12.4 (0.4) |
| Sp-5 | GGCCCCTTTTGTG GT TGCCAACACAGCAGACCTC | 2.9 (0.3) | 2.1 (0.09) | 2.3 (0.5) | 2.4 (0.02) |
| Sp+2 | GGCCCCTTTTGTGCTGTTCCtgTGCCAACACAGCAGACCTC | 3.3 (0.6) | 2.4 (0.2) | 2.3 (0.1) | 2.6 (0.04) |
| Sp+7 | GGCCCCTTTTGTGCTGTTCCtgccaacTGCCAACACAGCAGACCTC | 2.9 (0.6) | 3.1 (0.2) | 2.7 (0.5) | 3.1 (0.2) |
| Inv | GGCCCCTTTTGTGCTGTTCCTGCtgtgttgGCAGACCTC | 1.3 (0.5) | 1.3 (0.4) | 1.6 (0.3) | 1.7 (0.6) |
| ML | GGCCCCTTTTGTGCTGTgcaTGCCAACACAGCAGACCTC | 1.3 (0.03) | 1.3 (0.01) | 2.3 (0.04) | 1.9 (0.14) |
| MR | GGCCCCTTTTGTGCTGTTCCTGCCAACACAGCAtgcCTC | 3.3 (0.01) | 7.2 (0.01) | 7.1 (0.01) | 11.4 (0.02) |
| MARE | GGCCCCTTTTGTGCTGTTCCTGCtgaCtcaGCAGACCTC | 2.6 (0.9) | 6.4 (0.05) | 8.0 (0.7) | 14.3 (0.14) |
| M-6t | GGCCCCTTTTGTGCTGTTCCTtCtgaCtcaGCAGACCTC | 2.1 (0.07) | 3.2 (0.05) | 4.1 (0.2) | 8.9 (0.01) |
| M+6a | GGCCCCTTTTGTGCTGTTCCTGCtgaCtcaGaAGACCTC | 2.9 (0.01) | 7.5 (0.3) | 8.1 (1.4) | 15.7 (0.3) |
Oligonucleotide sequence with the Sox element (double underlined), distal Maf element (single underlined, with dots indicating the nonconsensus core of the Maf recognition sequence), and mutated and inserted bases (lowercase letters) indicated.
Activation of the γF-crystallin promoter (CAT activity expressed relative to the activity of the reporter gene cotransfected with a plasmid lacking a transcription factor coding region) by Maf alone or together with Sox1, Sox2, or Sox3. The data represent averages and standard deviations from three or more independent experiments.
Plasmids for mammalian expression of c-Maf, c-Fos, and c-Jun were constructed by insertion of the respective cDNA sequences into pcDNA 3.1+ (Invitrogen). Site-directed mutagenesis of c-Maf was done to construct the mammalian R288P Maf expression vector.
Plasmids for the analysis of protein localization were constructed by fusing the coding regions of Maf, R288P Maf, and Sox proteins to yellow fluorescent protein (YFP) in plasmids pEYFP-N3 and pECFP-N3 (Clontech). Plasmids for bimolecular fluorescence complementation (BiFC) analysis were constructed by fusing the same coding regions to sequences encoding YFP1-154 (YN) or YFP155-238 (YC) with the linkers described previously (16). The plasmids for BiFC analysis of Fos, Jun, and ATF2 have been described (16). Chimeric proteins containing sequences from Maf and ATF2 were constructed by PCR amplification of the respective coding sequences. The chimeric protein MA1 contains Maf sequences on the N-terminal side of the leucine zipper (1 to 312) fused to the leucine zipper and C-terminal sequences of ATF2 (381 to 505). MA2 contains Maf sequences on the N-terminal side of the conserved basic region (1 to 298), including the unique GY dipeptide required for the extended DNA recognition specificity (15), fused to the conserved bZIP domain and C-terminal sequences of ATF2 (366 to 505). MA3 contains Maf sequences on the N-terminal side of the ancillary DNA binding region (1 to 265) fused to the bZIP domain and C-terminal sequences of of ATF2 (350 to 505). The chimeric proteins were fused to full-length YFP in plasmid pEYFP-N3 as well as YN and YC.
Cell culture and transfection.
αTN4, COS-1, and HeLa cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal calf serum (Invitrogen), glutamine, and antibiotics at 37°C with 5% CO2. For transient transfections, 7 × 104 cells were plated in six-well dishes 24 h before transfection. In transactivation assays, the cells were transfected with 0.2 μg of Maf and 0.6 μg of Sox expression vectors (unless indicated otherwise), 2.5 μg of reporter plasmid, and 0.1 μg of pCMV-β-galactosidase with Fugene6 (Roche Diagnostics) as the transfection agent. The cells were harvested 48 h after transfection, and the CAT and β-galactosidase activities were measured.
Protein purification.
The DNA binding domains of Maf (241-344) and R288P Maf (241-344) were expressed in Escherichia coli DH5α as hexahistidine fusion proteins and purified by nickel chelate affinity chromatography (15). The glutathione S-transferase fusions of Sox1, Sox2, and Sox3 HMG domains were purified by glutathione-Sepharose affinity chromatography.
Electrophoretic mobility shift assay.
Nucleoprotein complex formation was examined by electrophoretic mobility shift analysis (15) in the presence of 25 μg of dG:dC (Sigma) competitor per ml. Oligonucleotide competitors were added to the binding reactions to determine the specificity of binding and the dissociation rates of the complexes.
Fluorescence imaging of living cells.
Cells were transfected with plasmids (0.25 to 0.5 μg) encoding the full-length proteins indicated with Fugene6. After 12 to 48 h, images were acquired with filters optimized for YFP (excitation 500/20, emission 535/30) and cyan fluorescent protein (CFP; excitation 436/10, emission 470/30) fluorescence. Western blot analysis was used to confirm the expression of the fusion proteins.
Immunostaining.
αTN4 and HeLa cells were transfected with plasmids (0.25 to 0.5 μg) encoding the proteins indicated with Fugene6. After 18 to 24 h, the cells were fixed, and the proteins were visualized with antibodies directed against Maf (Santa Cruz Biotechnology), PML (Santa Cruz Biotechnology), SC-35 (Sigma), coilin (R124; a kind gift from Greg Matera), and survival of motor neurons (SMN) protein (2B1; a kind gift from Gideon Dreyfuss). Images were acquired with an inverted fluorescence microscope (Nikon TN300) with filters designed for the detection of YFP (excitation 500/20, emission 535/30), Alexa 594 (excitation 560/20, emission 690/40) and Cascade Blue (excitation 397/8, emission 470/15) fluorescence.
RESULTS
Synergistic activation of the γF-crystallin promoter by Maf and Sox proteins.
To investigate the regulation of crystallin gene expression by Maf, we transfected a Maf expression vector into αTN4 lens epithelial cells together with a reporter gene controlled by either the αA- or γF-crystallin promoter. Maf expression produced comparable levels of activation of both reporter genes (Fig. 1A and B). The two promoters exhibited similar responses to different levels of Maf expression. Deletion of the region on the N-terminal side of the bZIP and ancillary DNA binding domains eliminated transcription activation (data not shown).
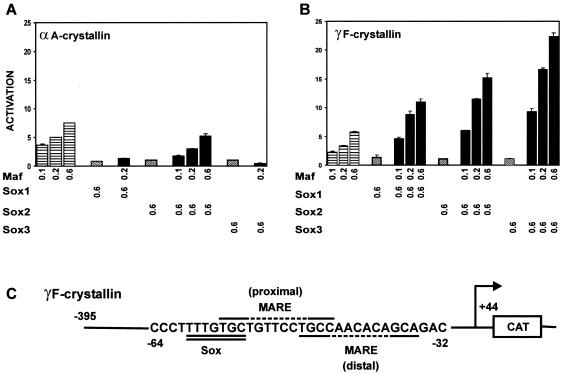
FIG. 1.
Maf and Sox proteins synergistically stimulate γF- but not αA-crystallin promoter activity. (A) Maf activation of αA-crystallin promoter activity is not stimulated by Sox proteins. The indicated amounts (in micrograms) of plasmids encoding the proteins indicated below the bars were transfected into mouse αTN4 lens epithelial cells together with a CAT reporter gene controlled by the αA crystallin promoter. The reporter gene activities were measured, and the efficiencies of transcription activation were calculated relative to cells transfected with the αA-crystallin reporter gene alone. The data in this and subsequent panels represent averages and standard deviations for at least three independent experiments, each with triplicate samples, performed on different days. (B) Maf and Sox proteins synergistically activate the γF-crystallin promoter. The efficiencies of transcription activation by the proteins indicated below the bars were measured at the γF-crystallin promoter as described for panel A. (C) Sequence of the region of the γF-crystallin promoter that contains Sox and Maf recognition elements. The Sox recognition element is double underlined, and two potential Maf recognition elements are overlined and underlined, respectively. The dashed line indicates the nonconsensus core of the Maf recognition sequences.
αA- and γF-crystallin gene expression is differentially regulated during development (50). To examine the molecular basis for the differential regulation of αA- and γF-crystallin gene expression, we compared the effects of other transcription factors that participate in lens-specific gene expression on the transcriptional activities of reporter genes regulated by these promoters. We found that coexpression of Sox1, Sox2, or Sox3 potentiated the transcriptional activity of Maf at the γF-crystallin gene promoter (Fig. 1B) but slightly inhibited Maf activation of the αA-crystallin promoter (Fig. 1A). The Sox proteins had no detectable effect on the transcriptional activities of these promoters when expressed alone, even at levels 10-fold higher than those required for synergistic activation with Maf. Sox proteins were also unable to activate the γF-crystallin gene when expressed together with a truncated Maf protein lacking the transcription activation domain.
The effects of Maf and Sox proteins on γF-crystallin promoter activity were synergistic at all concentrations tested. The coexpression of Sox proteins had no detectable effect on the level of Maf in the cell. Pax6 had no effect on γF-crystallin promoter activity when expressed alone or in combination with Maf and/or Sox proteins (data not shown). ATF4 likewise had no effect on γF-crystallin promoter activity when expressed alone or in combination with Maf or Sox proteins separately (data not shown). However, when expressed together with Maf and Sox proteins, ATF4 had differential effects on the synergy between Maf and different Sox proteins (data not shown).
Regions of Sox2 required for synergistic activation of the γF-crystallin promoter with Maf.
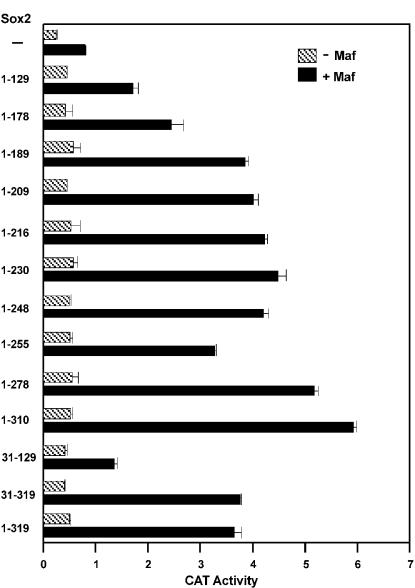
We used deletion derivatives of Sox2 (1) to identify the regions required for transcriptional synergy with Maf at the γF-crystallin promoter (Fig. 2). Deletions within the C-terminal half of Sox2 had only minor effects on synergistic activation with Maf. However, the deletion of sequences adjacent to the HMG domain (residues 129 to 189) progressively reduced synergy. Deletion of the short N-terminal extension before the HMG domain had little effect. Previous studies of these deletion derivatives have shown that the proteins are expressed at comparable levels (1). Thus, a region adjacent to the HMG domain of Sox2 was required for transcriptional synergy with Maf at the γF-crystallin promoter.
FIG. 2.
Regions of Sox2 required for synergistic transcription activation with Maf. The amino acid residues encoded by each Sox2 deletion derivative are indicated to the left of the bars that show the transcriptional activity of the γF-crystallin promoter in the presence of the Sox2 derivative alone (−Maf) and in combination with Maf (+Maf). The data represent averages and standard deviations from three independent experiments, each with triplicate samples.
Effects of a Maf mutation associated with cataract on transcription activation.
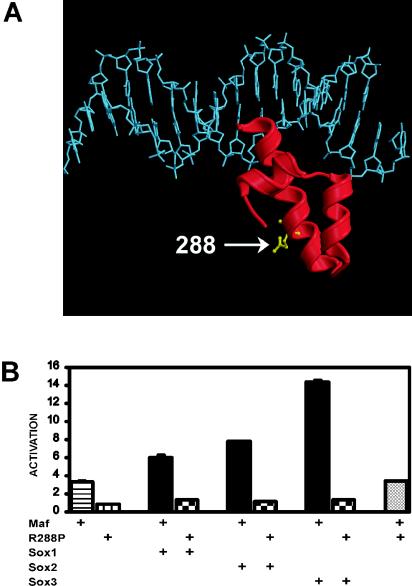
A dominant mutation that causes cataract and developmental abnormalities of the lens in humans was mapped to a single amino acid substitution (R288P) in Maf (18). This mutation is located within the ancillary DNA binding region of Maf (Fig. 3A). We examined the effects of this substitution on transcription activation by Maf (Fig. 3B). Expression of R288P Maf did not activate transcription from the γF-crystallin promoter either alone or in combination with Sox family proteins (Fig. 3B). R288P Maf also did not activate the αA-crystallin promoter (data not shown). The R288P mutation virtually eliminated the transcriptional activity of Maf at all promoter variants tested, including promoters that contained a consensus MARE sequence (see Table 1 below). R288P Maf was expressed at the same level as wild-type Maf, as determined by Western blot analysis (data not shown).
FIG. 3.
R288P mutation in Maf that causes cataracts in humans eliminates transcription activation by Maf alone and together with Sox proteins. (A) Position of the R288P mutation within the ancillary DNA binding domain of Maf. The model shows the structure of the ancillary DNA binding domain of MafG (29) superimposed on the structure of DNA from the Skn-1-DNA complex (44). The residue corresponding to R288 is shown in ball-and-stick representation. The alignment of the ancillary DNA binding domain of Maf is likely to be different from that observed for Skn-1 (8). (B) The R288P mutation in Maf eliminates transcription activation. The efficiencies of transcription activation by the proteins indicated below the bars were measured as described for Fig. 1A. The data represent averages from two independent experiments, each with triplicate samples.
Since the R288P mutation is dominant and Maf is not haploinsufficient in mice, it is likely that R288P Maf interferes with the functions of normal cellular proteins (17, 18). To examine the effect of R288P Maf expression on the activity of wild-type Maf, we coexpressed the proteins and determined their effects on transcription of the γF-crystallin reporter. Coexpression of R288P Maf had no detectable effect on the transcriptional activity of wild-type Maf either alone (Fig. 3B) or in combination with Sox proteins at the γF-crystallin promoter (data not shown). Likewise, R288P Maf did not interfere with activation of the αA-crystallin promoter by wild-type Maf (data not shown). R288P Maf therefore did not have a dominant negative effect on Maf activation of αA- or γF-crystallin transcription under the conditions used in these experiments.
DNA sequences required for synergistic transcription activation by Maf and Sox proteins.
The promoter-proximal region of the γF-crystallin promoter determines the lens-specific expression of the gene (33). Several binding sites for nuclear proteins have been identified in this region (13, 32). The element that is required for activation by Sox family proteins has been identified (Fig. 1C, double underline) (19). Inspection of the promoter sequence revealed two close matches to the Maf recognition element (TGCN7-8GCA) (23, 25) in close proximity to the element required for activation by Sox proteins (Fig. 1C, distal site underlined and proximal site overlined). Neither of these putative elements contained a close match to the consensus AP-1 site within the core of the Maf recognition sequence. We examined the roles of these sequence elements in the synergistic activation of the γF-crystallin promoter by Maf and Sox family proteins.
Mutational analysis of the sequences required for transcription activation by Maf and Sox proteins was carried out in the context of the native γF-crystallin promoter. Single and clustered nucleotide substitutions were made to test the roles of specific base pairs in transcription activation by Maf, Sox1, Sox2, and Sox3 (Fig. 4, Table 1). Clustered base pair substitutions in both half-sites of the distal MARE (overlapping one half-site of the proximal MARE) virtually eliminated activation by Maf alone or together with Sox (Table 1, M1M2). Similarly, clustered and single base pair substitutions in either half-site of the distal MARE eliminated activation by Maf alone and markedly reduced synergistic activation by Maf together with Sox proteins (Fig. 4, M2 and M3; Table 1, M1 and M4). The single base pair substitutions had slightly larger effects, consistent with their dramatic effects on the binding affinity and conformation of Maf (8). In contrast, clustered base substitutions in the proximal MARE that do not overlap the distal MARE did not reduce but slightly enhanced transcription activation by Maf (Fig. 4 and Table 1, M5). The distal MARE was therefore specifically required for activation of the γF-crystallin promoter both by Maf alone and by Maf together with Sox proteins.
FIG. 4.
Effects of base substitutions within the γF-crystallin promoter on synergistic transcription activation by Maf and Sox proteins. The mutations indicated in each panel and shown in Table 1 were introduced into the γF-crystallin promoter, and reporter gene (CAT) activity was measured in cells expressing the proteins indicated at the bottom of the figure. The diagrams in each panel indicate the positions of the base substitutions (solid boxes). The data in each panel represent averages and standard deviations from three or more independent experiments, each with triplicate samples.
The γF-crystallin promoter contains a divergent Sox recognition element, and this divergent sequence is conserved in other γ-crystallin promoters. Base substitutions within the Sox recognition element eliminated synergistic activation by all of the Sox proteins with Maf but had no significant effect on transcription activation by Maf alone (Fig. 4, S1). The coexpression of Sox family proteins slightly inhibited Maf activation of promoters lacking the Sox recognition element, reminiscent of their effect on Maf activation of the αA-crystallin promoter (Fig. 1A). Substitution of base pairs between the Maf and Sox recognition elements had no significant effect on transcription activation by Maf, but slightly altered the relative efficiencies of transcription activation by different Sox proteins in combination with Maf (Fig. 4, M5 and 1SM). Synergistic transcription activation by Maf and Sox therefore required specific DNA recognition sequences for both proteins.
We examined the influence of the spacing between the Maf and Sox recognition elements on the transcriptional activity of the γF-crystallin promoter. Deletion of five bp between the recognition sequences eliminated synergistic transcription activation by Sox and Maf, but did not significantly alter activation by Maf alone (Fig. 4, Sp-5). To confirm that the loss of synergistic activation did not result from deletion of essential promoter sequences, we duplicated either two or seven bp between the binding sites. Transcription activation by Maf alone was virtually unaffected by these duplications, indicating that the changes in spacing did not affect the transcriptional activity of Maf per se. However, the synergistic activation of transcription by Maf and each of the Sox proteins was eliminated by both of the duplications (Fig. 4, Sp+7; Table-1, Sp+2). Thus, synergistic transcription activation by Maf and Sox proteins required a fixed spacing between the binding sites, indicating that the sites constitute a composite regulatory element.
The sequence in the γF-crystallin promoter that mediated transcription activation by Maf has symmetrical extended recognition elements (TGCN7GCA),but an asymmetric core (CAAACACA) and asymmetric sequences flanking the extended elements (TCC… ..GAC). We examined the influence of this asymmetry on transcription activation by Maf and Sox family proteins. Surprisingly, inversion of the core sequence virtually eliminated transcription activation by Maf alone and in combination with Sox proteins (Table 1, Inv). Thus, the orientation of the asymmetric Maf recognition element was critical for transcription activation by Maf. Substitution of the base pairs on the left side of the Maf recognition element virtually eliminated transcription activation by Maf alone, and markedly reduced synergistic activation with Sox family proteins (Table 1, ML). In contrast, the same base substitutions on the right side had no detectable effect on transcription activation by Maf alone, and small effects on the synergy with Sox family proteins (Table 1, MR). Consequently, the activity of Maf at the γF-crystallin promoter was affected by the orientation of the Maf recognition element.
The effect of the orientation of the Maf recognition element on transcription activation was surprising because Maf is likely to bind this sequence as a homodimer. The conformation of Maf depends on the sequence of its binding site (8). We therefore compared transcription activation by Maf at a symmetrical consensus MARE and at binding sites containing single base pair substitutions that affect Maf conformation in opposite half-sites (Fig. 4, M−6t and M+6a) (8). Symmetry-related base substitutions in opposite half-sites had converse effects on transcription activation (Table 1). A single base pair substitution in the left half-site reduced transcription activation by Maf alone and by Maf in combination with Sox family proteins (Fig. 4, M−6t). In contrast, the same base pair substitution in the right half-site slightly enhanced transcription activation by Maf alone and in combination with Sox proteins (Fig. 4, M+6a). These results are consistent with the hypothesis that differences between the conformations of the two Maf subunits bound to the asymmetric regulatory element cause the orientation dependence of transcription activation at the γF-crystallin promoter.
Co-occupancy of the γF-crystallin composite regulatory element by Maf and Sox.
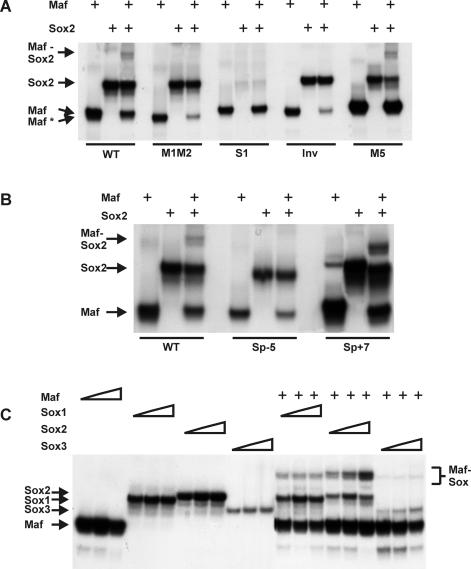
We investigated Maf and Sox binding to the composite regulatory element in the γF-crystallin promoter by electrophoretic mobility shift analysis (Fig. 5A). We used purified proteins encompassing the DNA binding domains of Maf, Sox1, Sox2, and Sox3 together with oligonucleotides containing the composite regulatory element with flanking sequences. Both Maf and each of the Sox proteins were able to bind independently to the element. Incubation of the oligonucleotide with Maf and Sox2 together produced a band that was not observed in the presence of either of the proteins alone (Fig. 5A, WT). Thus, Maf and Sox2 could co-occupy the composite regulatory element. The presence of Maf and Sox together did not increase the overall amount of complex formation, suggesting that the proteins did not bind cooperatively to this element.
FIG. 5.
Co-occupancy of the γF-crystallin promoter by Maf and Sox2. (A and B) The oligonucleotides indicated below the lanes (sequences shown in Table 1) were incubated with the DNA binding domain of Maf (10 nM) and/or the HMG domain of Sox2 (100 nM) as indicated above the lanes, and the complexes were analyzed by polyacrylamide gel electrophoresis. Complexes formed by the DNA binding domain of Maf at different sites migrated with different mobilities (Maf and Maf*). (C) Different concentrations of the DNA binding domain of Maf (10, 20, and 50 nM) as well as the HMG domains of Sox1 (0.5, 1, 1.5 and μM), Sox2 (50, 100, and 200 nM) or Sox3 (0.5, 1, and 1.5 μM) in the presence (+) or absence (−) of Maf (20 nM) were incubated with an oligonucleotide containing the consensus MARE (sequence in Table 1). The complexes were analyzed by polyacrylamide gel electrophoresis.
Mutation of both half-sites of the distal Maf recognition site (Fig. 5A, M1M2) prevented co-occupancy of the element by Maf and Sox2. This mutation reduced but did not eliminate binding by Maf alone, indicating that Maf was able to bind to the proximal site or to other sequences within the oligonucleotide. However, Maf was unable to co-occupy the element with Sox2 when bound to these sites. In contrast, mutation of the proximal site (M5) did not eliminate co-occupancy of the element by Maf and Sox (Fig. 5A, M5). Mutation of the Sox recognition element eliminated Sox2 binding and co-occupancy of the element with Maf (Fig. 5A, S1). Inversion of the distal Maf binding site also prevented co-occupancy of the element by Maf and Sox2 (Fig. 5A, Inv). Remarkably, co-occupancy of this element was not observed even at saturating Maf and Sox2 concentrations (data not shown). Co-occupancy of the γF-crystallin element by Maf and Sox2 therefore required the native orientation of the Maf recognition sequence.
The mobilities of complexes formed by Maf on oligonucleotides containing mutations in the distal site were slightly higher (Maf*) than those of complexes formed on oligonucleotides containing an intact distal site (Maf) (Fig. 5A). This mobility difference was reproducible and was observed for Maf complexes formed on oligonucleotides containing mutations or inversions in the distal MARE. There were no differences in the mobilities of the oligonucleotides alone or of complexes formed by Sox2 on these oligonucleotides. The altered mobility of Maf complexes at mutated or inverted binding sites correlated with the loss of transcription activation by Maf at promoters containing these sites. We have previously shown that Maf adopts different conformations when bound to different DNA sequences (8). The mobility differences may reflect differences between the structures of the complexes that Maf formed at the native and mutated binding sites.
We examined the effects of the changes in spacing that eliminated synergistic transcription activation on co-occupancy of the element by Maf and Sox2 (Fig. 5B). Deletion of 5 bp between the recognition sequences eliminated co-occupancy of the element by Maf and Sox2 (Fig. 5B, Sp-5). In contrast, insertion of either 2 or 7 bp between the sites did not prevent co-occupancy (Fig. 5B, Sp+7, data not shown). Thus, co-occupancy of the γF-crystallin promoter by Maf and Sox was not sufficient for transcriptional synergy, and changes in the spacing of the Maf and Sox recognition elements affected transcription activation through mechanisms other than changes in Maf and Sox binding.
We compared binding by the HMG domains of different Sox family proteins at a composite element containing a consensus MARE in the presence and absence of Maf (Fig. 5C). Sox2 exhibited the strongest binding, whereas Sox3 exhibited weak binding. All of the Sox family members tested were able to co-occupy the MARE element with Maf. However, there was little effect of Maf on the apparent binding affinities of the Sox proteins. Identical results were obtained when Sox2 derivatives containing the region adjacent to the HMG domain required for transcriptional synergy, Sox2(1-178) and Sox2(1-192), were used (data not shown). The DNA binding domains of Maf and Sox family proteins could therefore co-occupy the composite element, but did not facilitate DNA binding by each other.
Effects of the R288P mutation and the orientation of the recognition sequence on DNA binding by Maf.
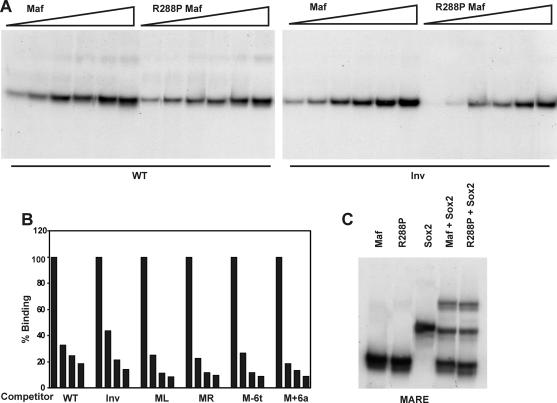
We examined if the effects of the R288P mutation in Maf and the orientation of the Maf recognition sequence on transcription activation were caused by differences in binding affinity. There were no significant differences in the apparent binding affinities of complexes formed by wild-type and R288P Maf or between complexes formed on oligonucleotides containing the native or the inverted binding site (Fig. 6A). There were also no significant differences in the efficiencies of competition by oligonucleotides containing the same base substitutions in opposite half-sites (Fig. 6B). Wild-type and R288P Maf were able to co-occupy the γF-crystallin regulatory element with Sox2 with comparable efficiencies (Fig. 6C). The dramatic differences between the transcriptional activities of R288P and wild-type Maf as well as between promoters containing symmetry-related base substitutions (Fig. 2 and 4 and Table 1) were therefore not caused by differences in nucleoprotein complex formation detectable in vitro.
FIG. 6.
Effects of the R288P mutation in Maf and of inversion of the core of the γF-crystallin element on complex formation. (A) Apparent binding affinities of the DNA binding domains of wild-type and R288P Maf at the native (WT) and inverted (Inv) γF-crystallin elements. Different concentrations (5 to 100 nM) of the proteins indicated above the lanes were incubated with the oligonucleotides indicated below the lanes, and complex formation was analyzed by polyacrylamide gel electrophoresis. (B) Relative efficiencies of competition by oligonucleotides containing symmetry-related base substitutions. The DNA binding domain of Maf was incubated with an oligonucleotide containing the γF-crystallin element. A molar excess (0-, 50-, 100-, and 250-fold) of the unlabeled oligonucleotides shown below the bars was added to the reaction, and the fraction of complexes remaining was quantified and plotted as a histogram. (C) Co-occupancy of the γF-crystallin MARE oligonucleotide by the DNA binding domain of wild-type and R288P Maf (10 nM) together with the HMG domain of Sox2 (100 nM). The proteins indicated above each lane were incubated with the γF-crystallin MARE oligonucleotide, and the complexes were analyzed by polyacrylamide gel electrophoresis.
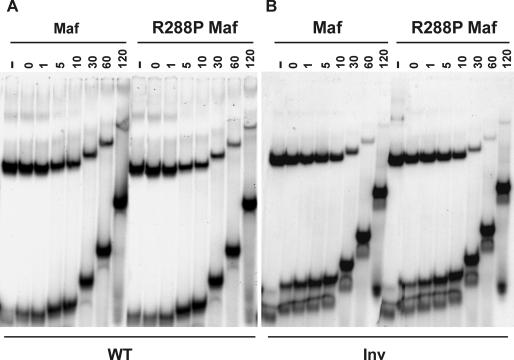
To compare the stabilities of complexes formed by wild-type and R288P Maf at different recognition sites, we measured the dissociation rates of the complexes in the presence of oligonucleotide competitors. Wild-type and R288P Maf formed complexes of indistinguishable stabilities at the native γF-crystallin promoter (Fig. 7A, t[1/2] = 5 min). The dissociation rates of both complexes were also identical at the consensus MARE element (t[1/2] = 24 h, data not shown). At the inverted binding site (Inv), a small difference in dissociation rates was measured (Fig. 7B, Maf t[1/2] = 15 min and R288P t[1/2] = 10 min), but this difference was smaller than the variation in dissociation rates between experiments. Likewise, symmetry-related base substitutions in the extended recognition elements (M−6t and M+6a) had equivalent effects on the dissociation rates of Maf complexes (data not shown). Thus, both the R288P mutation in Maf and the symmetry-related base substitutions in the Maf recognition sequence affected transcription activation through mechanisms other than changes in nucleoprotein complex stability.
FIG. 7.
Comparison of the rates of dissociation of the DNA binding domains of wild-type and R288P Maf from the γF-crystallin element (WT) and the element containing an inverted core (Inv). The proteins indicated above the lanes (10 nM) were incubated with the oligonucleotides indicated below the lanes. A 1,000-fold excess of unlabeled competitor oligonucleotide was added, and aliquots were loaded onto a running polyacrylamide gel at the times (in minutes) indicated above the lanes. −, no competitor added.
Subcellular localization of wild-type and R288P Maf.
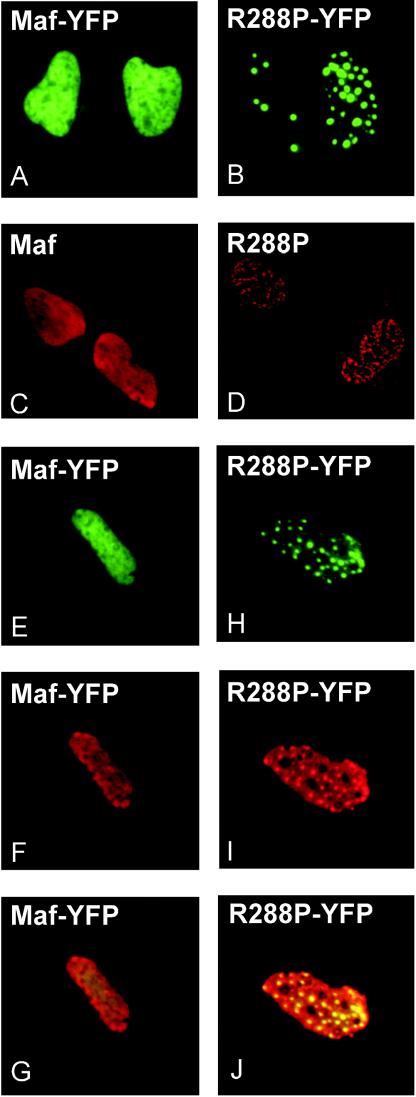
To investigate if R288P Maf had properties distinct from those of wild-type Maf in cells, we compared their localization in αTN4, HeLa and COS-1 cells (Fig. 8A and B). Wild-type Maf fused to YFP was distributed throughout the nucleoplasm in all cells (Fig. 8A). There was little variation in fluorescence intensity between different regions of the nucleoplasm (up to a 1.7-fold ratio between the highest and lowest fluorescence intensities in the nucleoplasm). In contrast, R288P Maf fused to YFP was localized to distinct foci in a subset of cells (20% in αTN4, 50% in HeLa, and 30% in COS-1) (Fig. 8B) and exhibited a larger variation between the fluorescence intensities of different regions of the nucleoplasm (up to a 3.2-fold ratio). The number of foci ranged from 1 to greater than 30 per nucleus. The foci were observed at the earliest times when fluorescence could be detected (6 h after transfection), and the number and size of the foci did not change with the level of protein expression or with the time after transfection.
FIG. 8.
Effect of the R288P mutation on Maf localization. Plasmids encoding the proteins indicated in each panel were transfected into αTN4 cells. The distributions of the proteins were visualized by YFP fluorescence (A, B, E, and H) or immunolabeling with Maf-specific antibody (C, D, F, and I). Panels G and J show the overlay of the YFP fluorescence and the immunoreactivity, confirming that the immunoreactivity reflected the localization of the proteins.
To examine if the YFP fusion affected the localization of R288P Maf, we compared the distributions of the unmodified proteins by indirect immunofluorescence. Nontransfected cells exhibited uniform nucleoplasmic immunoreactivity (data not shown). Cells transfected with a plasmid encoding wild-type Maf exhibited stronger immunoreactivity, which was uniformly distributed in the nucleoplasm (Fig. 8C). Expression of R288P Maf produced immunoreactivity in distinct nuclear foci in a subset (12%) of cells and more diffuse foci in a larger subpopulation (Fig. 8D). Similar results were obtained with four different antisera raised against two unrelated peptides from different regions of Maf. A subset of the cells (10%) expressing R288P Maf also exhibited an aberrant nuclear morphology, with multilobed nuclei.
To confirm that the immunoreactivity reflected the localization of wild-type and R288P Maf, we compared the distributions of YFP fluorescence and anti-Maf immunoreactivity in cells expressing either wild-type or R288P Maf. Cell transfected with a plasmid encoding wild-type Maf-YFP exhibited uniform nucleoplasmic YFP fluorescence and anti-Maf immunoreactivity (Fig. 8E and F). In contrast, expression of R288P Maf-YFP produced distinct nuclear foci of both YFP fluorescence and anti-Maf immunoreactivity (Fig. 8H and I). In both cases, the immunoreactivity closely matched the distribution of YFP fluorescence (Fig. 8G and J).
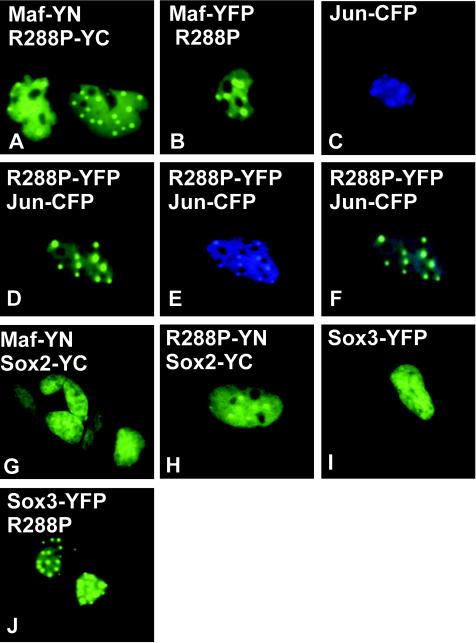
The dominant nature of the R288P mutation likely results from interactions between R288P Maf and normal cellular proteins. To determine if R288P Maf could form dimers with wild-type Maf, we used bimolecular fluorescence complementation (BiFC) analysis (16) to visualize dimerization in living cells (Fig. 9). The BiFC approach is based on the formation of a bimolecular fluorescent complex when two nonfluorescent fragments of YFP (YN and YC) are brought together by an interaction between proteins fused to the fragments (16). We expressed Maf-YN and R288P-YC in both αTN4 and COS-1 cells. The cells exhibited bright fluorescence that was localized to nuclear foci in a subset of cells (20%) (Fig. 9A). The same localization was observed regardless of which fragment of YFP was fused to R288P and which was fused to wild-type Maf. The dimerization between wild-type and R288P Maf therefore recruited wild-type Maf to the nuclear foci.
FIG. 9.
R288P Maf relocalizes interaction partners in cells. Plasmids encoding the proteins indicated in each panel were transfected into αTN4 cells. The distributions of protein complexes (A, G, H, and I) or individual proteins (B to F and J) were determined by imaging YFP (A, B, D, and G to J) or CFP (C and E) fluorescence. Panel F shows an overlay of the YFP and CFP emissions.
To determine if bimolecular complex formation was required for the recruitment of wild-type Maf to the foci, we coexpressed wild-type Maf fused to YFP with R288P Maf lacking a fusion. Wild-type Maf was localized to the nuclear foci in a subset of the cells cotransfected with a plasmid encoding R288P Maf (Fig. 9B). To examine if R288P Maf expression affected the localization of other interaction partners, we coexpressed R288P Maf fused to YFP with other nuclear proteins fused to CFP. Jun-CFP was localized to the nucleoplasm and enriched in the nucleoli when expressed alone (Fig. 9C). However, coexpression of R288P Maf-YFP resulted in relocalization of Jun-CFP to the nuclear foci occupied by R288P Maf and exclusion from nucleoli (Fig. 9D-F). JunB exhibited a similar shift in localization (data not shown). In contrast, R288P Maf-YFP expression had no detectable effect on the subcellular distributions of JunD-CFP, Fos-CFP, or CFP alone (data not shown). R288P Maf therefore selectively relocalized a subset of bZIP proteins to nuclear foci.
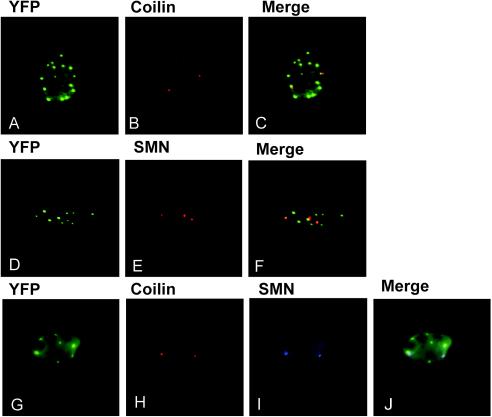
Several subnuclear compartments that are enriched in specific proteins have been identified in eukaryotic cells (34). To determine if R288P Maf was localized to a subnuclear compartment that had been characterized previously, we compared its localization with those of proteins that are localized to known compartments in HeLa cells (34). The distribution of R288P Maf-YFP was distinct from those of the promyelocytic leukemia protein, which is localized to PML bodies (2, 10), and the non-snRNP splicing factor SC35, which is localized to nuclear speckles (12) (data not shown). In contrast, coilin, which is localized to Cajal bodies (34), and the survival of motor neurons (SMN) protein, which is localized to gemini of coiled bodies (Gems) (31), were closely associated with the R288P Maf foci (Fig. 10). The R288P Maf foci did not coincide with either Cajal bodies or Gems, but a large majority of both Cajal bodies and Gems were in close proximity to these foci.
FIG. 10.
Comparison of the distributions of R288P Maf foci with Cajal bodies and Gems. R288P Maf-YFP was expressed in HeLa cells and visualized by YFP fluorescence (A, D, and G). The cells were stained with antibodies directed against coilin (B and H) and SMN protein (E and I). The fluorescence images are superimposed in panels C, F, and J.
To compare the positions of Cajal bodies and Gems relative to the R288P Maf foci, we visualized all three structures in the same cells. Cajal bodies and Gems colocalized in our HeLa cell line at the resolution of our microscope. Most cells had a larger number of R288P Maf foci than Gems or Cajal bodies, but even in cells with a small number of foci, the Cajal bodies and Gems were juxtaposed against these foci. Thus, the juxtaposition of Cajal bodies and Gems with R288P Maf foci did not result from a random distribution of these structures.
Interactions between Maf and Sox proteins in living cells.
To investigate Maf interactions with Sox proteins in living cells, we used BiFC analysis in αTN4 and COS-1 cells. We examined complementation between YN fused to the C-terminal end of Maf and YC fused to the C-terminal end of Sox2 and vice versa. Expression of either combination of proteins produced uniform nucleoplasmic fluorescence (Fig. 9G). Equivalent results were obtained when Sox3 was used in place of Sox2 (data not shown). In contrast, expression of R288P Maf fused to YN with either Sox2 or Sox3 fused to YC or vice versa produced nuclear foci similar to those observed for R288P Maf-YFP alone (Fig. 9H and data not shown). The R288P mutation therefore did not eliminate the interactions between Maf and Sox proteins in cells, but it altered the subnuclear localization of the complexes.
To determine if the relocalization of Sox proteins by R288P Maf required bimolecular complex formation, we cotransfected a plasmid encoding R288P Maf with a plasmid encoding either Sox1, Sox2, or Sox3 fused to full-length YFP. Sox1, Sox2, and Sox3 exhibited uniform nucleoplasmic distributions in the absence of R288P Maf (Fig. 9I). Coexpression of R288P Maf resulted in relocalization of these proteins to nuclear foci in a subset of cells (8% on average; Fig. 9J). These results confirm that R288P Maf and Sox proteins could interact in living cells and demonstrate that bimolecular complex formation was not required for relocalization of Sox proteins by R288P Maf.
Maf sequences required for interactions with Sox proteins in living cells.
To investigate the specificity of Sox2 interactions with bZIP proteins, we examined complementation by Sox2 with Jun, JunB, JunD, Fos, Fra1, and ATF2 with the BiFC assay. Jun, JunB, and JunD exhibited complementation with Sox2, whereas Fos, Fra1, and ATF2 exhibited no detectable complementation with Sox2 when fused to YN and YC or vice versa (Fig. 11A and B and data not shown). Identical results were obtained in experiments with Sox3 fusions (data not shown). Fos, Fra1, and ATF2 exhibited complementation with other proteins (i.e., Jun) when fused to YN and YC (16). To confirm that the lack of complementation by Sox2 with Fos and Fra1 reflected the absence of interactions between these proteins, we coexpressed unmodified Jun in the same cells. Coexpression of Jun with Fos-YN and Sox2-YC (or Sox3-YC) or Fra1-YN and Sox2-YC resulted in fluorescence complementation, demonstrating that Fos-Jun and Fra1-Jun heterodimers can interact with Sox2. Jun had no detectable effect on complementation by Maf-YN and Sox-YC. Thus, Sox2 exhibited selective fluorescence complementation with a subset of bZIP dimers in cells.
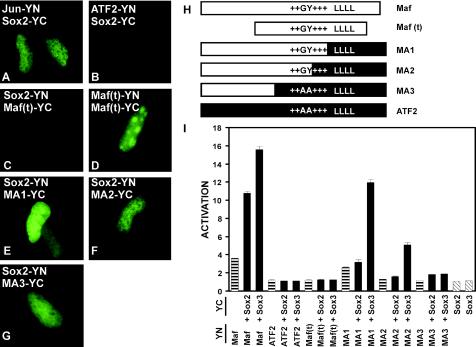
FIG. 11.
Regions of Maf required for interactions with Sox proteins in cells and for synergistic activation of the γF-crystallin promoter. (A to G) BiFC analysis of interactions between the proteins indicated in each panel. The proteins indicated were expressed in αTN4 cells, and the bimolecular fluorescent complexes were visualized by YFP fluorescence. The images are representative of greater than 95% of the fluorescent cells in each population. (H) Diagrams of the regions of Maf and ATF2 in each of the truncated and chimeric proteins. Maf(t) corresponds to the truncated Maf(241-344) protein, encompassing the bZIP and ancillary DNA binding domains. The unique GY dipeptide (15), the basic region (+++), and the leucine zipper (LLLL) are indicated. (I) The transcriptional activities of the protein combinations indicated below each bar at the γF-crystallin promoter were measured in αTN4 cells. The proteins were fused to the fluorescent protein fragments indicated on the left. The data represent averages from two or three independent experiments, each with duplicate samples.
To identify the region of Maf required for interactions with Sox proteins, we examined complementation by truncated and chimeric Maf proteins with Sox2 when fused to YN and YC or vice versa. Truncated Maf encompassing the bZIP and ancillary DNA binding regions exhibited no detectable fluorescence complementation with Sox2, but produced bright fluorescence enriched in nucleoli when expressed as a homodimer of subunits fused to YN and YC (Fig. 11C and D). To examine if regions outside the bZIP and ancillary DNA binding regions could mediate interactions with Sox2, we prepared chimeric proteins in which Maf sequences on the N-terminal side of the leucine zipper (MA1), the conserved basic region (MA2), or the ancillary DNA binding domain (MA3) were fused to the corresponding C-terminal sequences from ATF-2 (Fig. 11H). Each of the chimeric proteins exhibited fluorescence complementation with Sox2 when fused to YN and YC or vice versa (Fig. 11E, F, and G). Equivalent results were obtained when complementation with Sox3 was examined (data not shown). The chimeric proteins were expressed at levels comparable to that of Maf, and the localizations of both the chimeras alone as well as the complexes they formed with Sox proteins were similar to those of Maf. No complementation was observed between Sox2 or Sox3 and the bZIP domain of ATF2 fused to YN and YC (data not shown). Bimolecular fluorescence complementation with Sox proteins was therefore mediated by Maf sequences located on the N-terminal side of the ancillary DNA binding domain.
Regions of Maf required for synergistic transcription activation with Sox proteins at the γF-crystallin promoter.
We examined if fusion of the fluorescent protein fragments to Maf and Sox affected their transcriptional activities or synergy at the γF-crystallin promoter (Fig. 11I). Maf-YN and Maf-YC had transcriptional activities that were virtually indistinguishable from that of unmodified Maf (compare Fig. 1 and 11I). Maf-YN and Maf-YC also exhibited the same levels of transcriptional synergy with Sox2-YN, Sox2-YC, Sox3-YN, and Sox3-YC as the unmodified proteins. Thus, the fluorescent protein fusions and bimolecular fluorescent complex formation did not alter the transcriptional activity of Maf or its transcriptional synergy with Sox proteins.
Since Jun was able to interact with both Maf and Sox proteins in cells, we examined the effects of different bZIP family proteins on the transcriptional activity of the γF-crystallin promoter. Fos, Jun, and ATF2 did not have any effect on transcriptional activity either alone or in combination with Sox proteins (Fig. 11I and data not shown). Fos and Jun also had no effect on transcription activation by Maf or on its synergy with Sox proteins (data not shown). Thus, the interaction of Jun with Sox proteins was not sufficient to mediate synergistic transcription activation at the γF-crystallin promoter.
To identify the regions of Maf required for synergistic transcription activation with Sox proteins, we examined the activities of truncated and chimeric Maf proteins. Truncated Maf encompassing the bZIP and ancillary DNA binding regions had no detectable transcriptional activity either alone or in combination with Sox proteins (Fig. 11I). The MA1 chimera containing the sequences on the N-terminal side of the leucine zipper of Maf fused to the leucine zipper and C-terminal sequences of ATF2 had approximately 70% of the transcriptional activity of intact Maf alone. MA1 also exhibited transcriptional synergy with Sox3 that resulted in 75% of the transcriptional activity observed with intact Maf, but little or no transcriptional synergy with Sox2 was observed. The MA2 chimera containing Maf sequences on the N-terminal side of the conserved portion of the basic region fused to the conserved bZIP domain and C-terminal sequences from ATF2 had minimal transcriptional activity alone but induced 30% of the transcriptional activity of wild-type Maf in combination with Sox3. Little or no synergy with Sox2 was observed. The MA3 chimera containing sequences on the N-terminal side of the ancillary DNA binding domain of Maf fused to the bZIP domain and C-terminal sequences of ATF2 had no significant transcriptional activity alone and exhibited little synergistic activation together with either Sox2 or Sox3. Thus, the N-terminal region of Maf together with the ancillary DNA binding region and the portion of the basic region unique to Maf family proteins were sufficient to mediate synergistic transcription activation with Sox3 when fused to the bZIP domain of ATF2.
DISCUSSION
The selective regulation of individual genes in mammalian genomes requires combinatorial interactions among transcription regulatory proteins. One mechanism for combinatorial transcription regulation is the synergistic action of multiple transcription factors at composite regulatory elements. The synergistic transcriptional activity of Maf and Sox proteins at the γF-crystallin promoter required a composite regulatory element consisting of nonconsensus recognition sequences for both Maf and Sox proteins.
Composite regulatory elements frequently contain nonconsensus recognition sequences (6), possibly to reduce activation by the individual proteins that function synergistically at the element. The nonconsensus recognition sequences may also contribute to the specificity of transcription activation by inducing specific conformational changes in the transcription factors that bind to the element (40). Inversion of the asymmetric Maf recognition element eliminated activation of the γF-crystallin promoter, and symmetry-related base substitutions in the two half-sites had distinct effects on transcriptional activity.
Maf undergoes a conformational change upon binding to DNA, and the nature of the conformational change is affected by the sequence of the binding site (8). The symmetry-related base substitutions in opposite half-sites (i.e., M−6t and M+ 6a) are likely to affect the conformations of different subunits of the homodimer (8). It is therefore likely that the orientation of the nonconsensus Maf recognition sequence influences transcription activation because the two subunits of the Maf homodimer adopt distinct conformations at this site.
Synergistic transcription activation can be mediated by cooperative DNA binding or by the concerted functions of transcription factors subsequent to DNA binding. The DNA binding domains of Maf and Sox proteins were able to co-occupy the γF-crystallin element but did not stabilize binding by each other. Regions outside the DNA binding domains of both Maf and Sox2 were required both for interactions between the proteins in cells and for transcriptional synergy. The region adjacent to the HMG domain of Sox2 that was required for transcriptional synergy did not affect the DNA binding affinity or specificity of Sox2, nor did it affect the co-occupancy of the γF-crystallin element with Maf. This region was distinct from the regions of Sox2 required for transcriptional synergy with Oct2 at the fgf-4 gene enhancer (1). Synergistic transcription activation by Sox2 with different transcription factors is therefore likely to involve interactions with different proteins.
The R288P mutation eliminated the transcriptional activity of Maf alone and in combination with Sox proteins at the γF-crystallin promoter. Surprisingly, this helix-breaking substitution within an α helix in the ancillary DNA binding region had no detectable effect on the DNA binding affinity or specificity of Maf. The mutation also had no effect on the co-occupancy of Maf and Sox proteins on the γF-crystallin promoter element in vitro. Thus, neither the lack of transcriptional activity nor the pathological phenotype of R288P Maf can be accounted for by changes in DNA binding detected with purified proteins.
R288P Maf had a tendency to form inactive oligomers in vitro that were resistant to disruption in the presence of detergent and reducing agents. When it was expressed in cells, R288P Maf exhibited a tendency to localize to subnuclear foci in all cell lines tested. Such foci were never observed in cells that expressed only wild-type Maf. The aberrant localization of R288P Maf is likely to represent an intrinsic property of the protein rather than an indirect consequence of its expression, since it was observed from the earliest time that fluorescence could be detected. This aberrant localization may contribute to the lack of transcription activation by R288P Maf. However, since only a subset of cells exhibited visually detectable foci, it is possible that other mechanisms contribute to the lack of transcription activation or that some foci are too small to be observed by microscopy.
Most Cajal bodies and Gems were associated with R288P Maf foci in HeLa cells. Several nuclear structures have been previously shown to be associated with Cajal bodies (34). Cleavage bodies, nuclear structures occupied by RNA 3′-processing factors, have been shown to contact or to overlap Cajal bodies (45). Cleavage bodies are also observed in only a subset of asynchronously growing cells, since they disappear during the G2 phase of the cell cycle (46). The association of Cdk2-cyclin E complexes with Cajal bodies is also regulated in a cell cycle-dependent manner (30). The cell cycle-regulated histone genes as well as the U1, U2, and U3 small nuclear RNA genes are closely associated with Cajal bodies (11, 46). The functional roles of the association of Cajal bodies and Gems with R288P Maf foci and the other nuclear structures remain unknown.
Since the R288P mutation is dominant in humans, its pathological effect is likely to be caused by a gain of function (18). The observation that R288P Maf can recruit other interaction partners to the subnuclear foci represents a gain of function with potential deleterious effects. The aberrant recruitment of Sox and other Maf interaction partners to the foci could alter the expression of crystallins and other lens-specific proteins. Prevention of Maf accumulation within these foci and/or inhibition of the recruitment of other proteins to the foci could provide a therapeutic strategy. The bimolecular fluorescence complementation assay provides a potential approach for the identification of lead compounds that can inhibit these interactions in the normal cellular environment.
Numerous interactions among transcription regulatory proteins have been identified with in vitro assays and genetic screens in Saccharomyces cerevisiae. Only a small number of these interactions have been visualized in living cells (7, 16, 43). We found that Maf could form complexes with Sox2 as well as Sox3 in cells using the BiFC assay (16). Jun family proteins could also interact with Sox proteins, but no interactions between Sox proteins and Fos, Fra1, or ATF2 alone were observed. Coexpression of unmodified Jun induced bimolecular fluorescent complex formation between Sox proteins and Fos as well as Fra1, likely through the formation of Fos-Jun and Fra1-Jun heterodimers. These results indicate that bimolecular fluorescence complementation does not require direct contact between the proteins that are fused to the fluorescent protein fragments but that it can occur when these proteins are present in the same macromolecular complex.
We compared the interactions between Sox and bZIP proteins that were observed using BiFC analysis in living cells (Fig. 9) with those that were observed using glutathione S-transferase affinity precipitation in vitro (data not shown). The selectivity of Sox protein interactions with the DNA binding domains of different bZIP proteins was apparently identical for proteins that were tested with both BiFC analysis and glutathione S-transferase affinity precipitation. However, the regions of Maf that mediated interactions with Sox proteins were distinct in the two assays. Whereas the bZIP and ancillary DNA binding domains of Maf were sufficient to mediate interactions with the HMG domains of Sox proteins in vitro (data not shown), the region on the N-terminal side of these domains mediated the interaction in cells (Fig. 11). Since the N-terminal region of Maf was also required for transcriptional synergy, it is likely to be the region that mediates the functionally relevant interaction in the normal cellular context. Thus, interactions between proteins in vitro do not necessarily correspond to functionally relevant interactions in the normal cellular environment.
Maf sequences outside the bZIP and ancillary DNA binding domains were required for the interaction with Sox proteins in cells, and the sequences on the N-terminal side of these regions were sufficient to mediate the interaction when fused to the bZIP domain of ATF2. Sequences outside the bZIP and ancillary DNA binding domains of Maf were also required for transcriptional synergy with Sox proteins. In addition to the N-terminal region that mediates the interaction with Sox proteins, synergistic transcription activation required the ancillary DNA binding region and the unique portion of the basic region of Maf that mediates the extended DNA recognition specificity (15). The chimeric proteins exhibited transcriptional synergy with Sox3 but not with Sox2 for reasons that are not clear at present. Thus, both Maf sequences that mediate interactions with Sox proteins in cells and sequences that allow specific recognition of the γF-crystallin promoter were required for transcriptional synergy with Sox3.
Acknowledgments
We thank Lisa Dailey, Tom Glaser, Tsonwin Hai, Jeffrey Leiden, Robin Lovell-Badge, Gregory Matera, and G. Dreyfuss for generously sharing materials. We are grateful to Changdeng Hu for development of the BiFC assay and advice on its application, to Mensur Dlakic for providing the model shown in Fig. 2A, and to Yurii Chinenov for sharing his BiFC constructs and for assistance with microscopy and imaging. We also thank all the members of the Kerppola laboratory for stimulating discussions and critical comments on the manuscript.
REFERENCES
- 1.Ambrosetti, D. C., H. R. Scholer, L. Dailey, and C. Basilico. 2000. Modulation of the activity of multiple transcriptional activation domains by the DNA binding domains mediates the synergistic action of Sox2 and Oct-3 on the fibroblast growth factor-4 enhancer. J. Biol. Chem. 275:23387-23397. [DOI] [PubMed] [Google Scholar]
- 2.Borden, K. L. 2002. Pondering the promyelocytic leukemia protein (PML) puzzle: possible functions for PML nuclear bodies. Mol. Cell. Biol. 22:5259-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle, D. L., L. Takemoto, J. P. Brady, and E. F. Wawrousek. 2003. Morphological characterization of the AlphaA- and AlphaB-crystallin double knockout mouse lens. BMC Ophthalmol. 3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady, J. P., D. Garland, Y. Duglas-Tabor, W. G. Robison, Jr., A. Groome, and E. F. Wawrousek. 1997. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc. Natl. Acad. Sci. USA 94:884-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Q., D. H. Dowhan, D. Liang, D. D. Moore, and P. A. Overbeek. 2002. CREB-binding protein/p300 co-activation of crystallin gene expression. J. Biol. Chem. 277:24081-24089. [DOI] [PubMed] [Google Scholar]
- 6.Chinenov, Y., and T. K. Kerppola. 2001. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 20:2438-2452. [DOI] [PubMed] [Google Scholar]
- 7.De Angelis, D. A., G. Miesenbock, B. V. Zemelman, and J. E. Rothman. 1998. PRIM: proximity imaging of green fluorescent protein-tagged polypeptides. Proc. Natl. Acad. Sci. USA 95:12312-12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dlakic, M., A. V. Grinberg, D. A. Leonard, and T. K. Kerppola. 2001. DNA sequence-dependent folding determines the divergence in binding specificities between Maf and other bZIP proteins. EMBO J. 20:828-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan, M. K., A. Cvekl, X. Li, and J. Piatigorsky. 2000. Truncated forms of Pax-6 disrupt lens morphology in transgenic mice. Investig. Ophthalmol. Vis. Sci. 41:464-473. [PubMed] [Google Scholar]
- 10.Dyck, J. A., G. G. Maul, W. H. Miller, Jr., J. D. Chen, A. Kakizuka, and R. M. Evans. 1994. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell 76:333-343. [DOI] [PubMed] [Google Scholar]
- 11.Frey, M. R., and A. G. Matera. 1995. Coiled bodies contain U7 small nuclear RNA and associate with specific DNA sequences in interphase human cells. Proc. Natl. Acad. Sci. USA 92:5915-5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu, X. D., and T. Maniatis. 1990. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature 343:437-441. [DOI] [PubMed] [Google Scholar]
- 13.Goring, D. R., D. M. Bryce, L. C. Tsui, M. L. Breitman, and Q. Liu. 1993. Developmental regulation and cell type-specific expression of the murine gamma F-crystallin gene is mediated through a lens-specific element containing the gamma F-1 binding site. Dev. Dyn. 196:143-152. [DOI] [PubMed] [Google Scholar]
- 14.Graw, J. 1997. The crystallins: genes, proteins and diseases. Biol. Chem. 378:1331-1348. [PubMed] [Google Scholar]
- 15.Grinberg, A. V., and T. Kerppola. 2003. Both Max and TFE3 cooperate with Smad proteins to bind the plasminogen activator inhibitor-1 promoter, but they have opposite effects on transcriptional activity. J. Biol. Chem. 278:11227-11236. [DOI] [PubMed] [Google Scholar]
- 16.Hu, C. D., Y. Chinenov, and T. K. Kerppola. 2002. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell 9:789-798. [DOI] [PubMed] [Google Scholar]
- 17.Jamieson, R. V., F. Munier, A. Balmer, N. Farrar, R. Perveen, and G. C. Black. 2003. Pulverulent cataract with variably associated microcornea and iris coloboma in a MAF mutation family. Br. J. Ophthalmol. 87:411-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamieson, R. V., R. Perveen, B. Kerr, M. Carette, J. Yardley, E. Heon, M. G. Wirth, V. van Heyningen, D. Donnai, F. Munier, and G. C. Black. 2002. Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum. Mol. Genet. 11:33-42. [DOI] [PubMed] [Google Scholar]
- 19.Kamachi, Y., S. Sockanathan, Q. Liu, M. Breitman, R. Lovell-Badge, and H. Kondoh. 1995. Involvement of SOX proteins in lens-specific activation of crystallin genes. EMBO J. 14:3510-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamachi, Y., M. Uchikawa, J. Collignon, R. Lovell-Badge, and H. Kondoh. 1998. Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development 125:2521-2532. [DOI] [PubMed] [Google Scholar]
- 21.Kamachi, Y., M. Uchikawa, and H. Kondoh. 2000. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 16:182-187. [DOI] [PubMed] [Google Scholar]
- 22.Kamachi, Y., M. Uchikawa, A. Tanouchi, R. Sekido, and H. Kondoh. 2001. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 15:1272-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kataoka, K., M. Noda, and M. Nishizawa. 1994. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol. Cell. Biol. 14:700-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawauchi, S., S. Takahashi, O. Nakajima, H. Ogino, M. Morita, M. Nishizawa, K. Yasuda, and M. Yamamoto. 1999. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J. Biol. Chem. 274:19254-19260. [DOI] [PubMed] [Google Scholar]
- 25.Kerppola, T. K., and T. Curran. 1994. A conserved region adjacent to the basic domain is required for recognition of an extended DNA binding site by Maf/Nrl family proteins. Oncogene 9:3149-3158. [PubMed] [Google Scholar]
- 26.Kerppola, T. K., and T. Curran. 1994. Maf and Nrl can bind to AP-1 sites and form heterodimers with Fos and Jun. Oncogene 9:675-684. [PubMed] [Google Scholar]
- 27.Kim, J. I., T. Li, I. C. Ho, M. J. Grusby, and L. H. Glimcher. 1999. Requirement for the c-Maf transcription factor in crystallin gene regulation and lens development. Proc. Natl. Acad. Sci. USA 96:3781-3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondoh, H. 1999. Transcription factors for lens development assessed in vivo. Curr. Opin. Genet. Dev. 9:301-308. [DOI] [PubMed] [Google Scholar]
- 29.Kusunoki, H., H. Motohashi, F. Katsuoka, A. Morohashi, M. Yamamoto, and T. Tanaka. 2003. Solution structure of the DNA-binding domain of MafG. Nat. Struct. Biol. 9:252-256. [DOI] [PubMed] [Google Scholar]
- 30.Liu, J., M. D. Hebert, Y. Ye, D. J. Templeton, H. Kung, and A. G. Matera. 2000. Cell cycle-dependent localization of the CDK2-cyclin E complex in Cajal (coiled) bodies. J. Cell Sci. 113:1543-1552. [DOI] [PubMed] [Google Scholar]
- 31.Liu, Q., and G. Dreyfuss. 1996. A novel nuclear structure containing the survival of motor neurons protein. EMBO J. 15:3555-3565. [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, Q. R., M. Tini, L. C. Tsui, and M. L. Breitman. 1991. Interaction of a lens cell transcription factor with the proximal domain of the mouse gamma F-crystallin promoter. Mol. Cell. Biol. 11:1531-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lok, S., M. L. Breitman, A. B. Chepelinsky, J. Piatigorsky, R. J. Gold, and L. C. Tsui. 1985. Lens-specific promoter activity of a mouse gamma-crystallin gene. Mol. Cell. Biol. 5:2221-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matera, A. G. 1999. Nuclear bodies: multifaceted subdomains of the interchromatin space. Trends Cell Biol. 9:302-309. [DOI] [PubMed] [Google Scholar]
- 35.Muta, M., Y. Kamachi, A. Yoshimoto, Y. Higashi, and H. Kondoh. 2002. Distinct roles of SOX2, Pax6 and Maf transcription factors in the regulation of lens-specific delta1-crystallin enhancer. Genes Cells 7:791-805. [DOI] [PubMed] [Google Scholar]
- 36.Nishiguchi, S., H. Wood, H. Kondoh, R. Lovell-Badge, and V. Episkopou. 1998. Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 12:776-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimoto, M., A. Fukushima, A. Okuda, and M. Muramatsu. 1999. The gene for the embryonic stem cell coactivator UTF1 carries a regulatory element which selectively interacts with a complex composed of Oct-3/4 and Sox-2. Mol. Cell. Biol. 19:5453-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogino, H., and K. Yasuda. 2000. Sequential activation of transcription factors in lens induction. Dev. Growth Differ. 42:437-448. [DOI] [PubMed] [Google Scholar]
- 39.Pevny, L. H., and R. Lovell-Badge. 1997. Sox genes find their feet. Curr. Opin. Genet. Dev. 7:338-344. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez-Carrozzi, V., and T. Kerppola. 2003. Asymmetric recognition of nonconsensus AP-1 sites by Fos-Jun and Jun-Jun influences transcriptional cooperativity with NFAT1. Mol. Cell. Biol. 23:1737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reza, H. M., H. Ogino, and K. Yasuda. 2002. L-Maf, a downstream target of Pax6, is essential for chick lens development. Mech. Dev. 116:61-73. [DOI] [PubMed] [Google Scholar]
- 42.Ring, B. Z., S. P. Cordes, P. A. Overbeek, and G. S. Barsh. 2000. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development 127:307-317. [DOI] [PubMed] [Google Scholar]
- 43.Rossi, F., C. A. Charlton, and H. M. Blau. 1997. Monitoring protein-protein interactions in intact eukaryotic cells by beta-galactosidase complementation. Proc. Natl. Acad. Sci. USA 94:8405-8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rupert, P. B., G. W. Daughdrill, B. Bowerman, and B. W. Matthews. 1998. A new DNA-binding motif in the Skn-1 binding domain-DNA complex. Nat. Struct. Biol. 5:484-491. [DOI] [PubMed] [Google Scholar]
- 45.Schul, W., B. Groenhout, K. Koberna, Y. Takagaki, A. Jenny, E. M. Manders, I. Raska, R. van Driel, and L. de Jong. 1996. The RNA 3′ cleavage factors CstF 64 kDa and CPSF 100 kDa are concentrated in nuclear domains closely associated with coiled bodies and newly synthesized RNA. EMBO J. 15:2883-2892. [PMC free article] [PubMed] [Google Scholar]
- 46.Schul, W., I. van Der Kraan, A. G. Matera, R. van Driel, and L. de Jong. 1999. Nuclear domains enriched in RNA 3′-processing factors associate with coiled bodies and histone genes in a cell cycle-dependent manner. Mol. Biol. Cell 10:3815-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimada, N., T. Aya-Murata, H. M. Reza, and K. Yasuda. 2003. Cooperative action between L-Maf and Sox2 on delta-crystallin gene expression during chick lens development. Mech. Dev. 120:455-465. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka, T., T. Tsujimura, K. Takeda, A. Sugihara, A. Maekawa, N. Terada, N. Yoshida, and S. Akira. 1998. Targeted disruption of ATF4 discloses its essential role in the formation of eye lens fibres. Genes Cells 3:801-810. [DOI] [PubMed] [Google Scholar]
- 49.Wigle, J. T., K. Chowdhury, P. Gruss, and G. Oliver. 1999. Prox1 function is crucial for mouse lens-fibre elongation. Nat. Genet. 21:318-322. [DOI] [PubMed] [Google Scholar]
- 50.Wistow, G. J., and J. Piatigorsky. 1988. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu. Rev. Biochem. 57:479-504. [DOI] [PubMed] [Google Scholar]
- 51.Yuan, H., N. Corbi, C. Basilico, and L. Dailey. 1995. Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev. 9:2635-2645. [DOI] [PubMed] [Google Scholar]
- 52.Zygar, C. A., T. L. Cook, and R. M. Grainger, Jr. 1998. Gene activation during early stages of lens induction in Xenopus. Development 125:3509-3519. [DOI] [PubMed] [Google Scholar]