Abstract
With the prevalence of obesity increasing dramatically worldwide over the past several decades, an increasing body of literature has examined the impact of obesity in the context of rheumatoid arthritis (RA). Epidemiologic studies suggest that obesity may be associated with a modestly increased risk for the development of RA, although these studies have shown conflicting results. Among patients with established RA, obesity has been observed to be associated with greater subjective measures of disease activity and poor treatment response, but also with a decreased risk of joint damage and lower mortality. A comprehensive evaluation of the influence of obesity on the measurement of disease, response to therapies, and long-term prognosis is critical in order to understand these observations. This review therefore focuses on recent observations, potential explanations for these findings, and implications for clinicians and investigators caring for and studying patients with RA.
Keywords: Rheumatoid arthritis, Obesity, Adiposity
Introduction
The obesity epidemic has impacted practically every area of health, including the care of patients with rheumatoid arthritis (RA). Management of patients with RA therefore requires a comprehensive understanding of the complex interaction between the disease and obesity. The aim of this narrative review is to critically examine what has been learned from recent research and illustrate how this evidence should impact how rheumatologists consider obesity and adiposity in the care of patients with RA.
Obesity and the Risk of Developing RA
Numerous studies have attempted to identify environmental and lifestyle factors associated with the risk of developing RA. For example, smoking has consistently been found to be associated with an increased risk of RA, with an especially strong association in seropositive men and individuals with the shared epitope [1–5]. Alcohol use and higher socioeconomic status may be inversely associated with RA risk [6, 7]. In this context, obesity as a potential risk factor for the development of RA has been an area of interest for many years. It has been proposed that recent trends of increasing rates of RA found in some studies could potentially be related, at least in part, to the dramatic increase in rates of obesity [8–11, 12•]. Previous study results, however, have been mixed, with some showing an increased risk of RA among obese individuals [13–15] and others showing no association [16–18].
Several recent large case-control and cohort studies have contributed to evidence of an association between obesity and the development of RA, although definitive evidence of an association remains elusive. Among these large studies, obesity was not consistently associated with a significantly increased risk of incident RA in the population overall, and when present, the association was relatively modest, with HR ranging between 1.0 and 1.49 (Table 1) [12•, 19••, 20••, 21, 22•]. A recent meta-analysis of 11 studies, including 5 recent studies, did find a significant association between obesity and RA with a relative risk 1.31 (95 % CI 1.12 to 1.53), although because there was significant heterogeneity between studies, calculating a pooled relative risk is potentially problematic [23].
Table 1.
Recent studies examining the association between obesity ands the development of RA
| Study | Design | Country/population | # with RA | Agea | Confounders assessed in addition to age, sex, smoking | Overall association (95 % CI) | Significant association in sero-negative subgroup |
|---|---|---|---|---|---|---|---|
| Wesley et al. 2013 [19••] | Case-control | Sweden/EIRA | 2748 | 55 [range 18–70] | Alcohol, education | OR 1.0 (0.9 to 1.2) | Yesb |
| Crowson et al. 2013 [12•] | Case-control | USA/Olmsted county | 813 | 55.9 (15.7) | Calendar year | ORc 1.24 (1.01 to 1.53) | No |
| Lu et al. 2014 [20••] | Cohort | USA/NHS and NHSII | 1181 | 42.9 (7.2) NHS, 34.4 (4.7) NHSII | Alcohol, income, parity, physical activity | HR 1.37 (0.91 to 2.09) | Yes |
| Harpsoe et al. 2014 [21] | Cohort | Denmark/DNBC | 176 | 30.2 [IQR 27.4, 33.3] | Alcohol, socio-economic status, parity | HR 1.09 (0.63 to 1.88) | Not reported |
| Lahiri et al. 2014 [22•] | Cohort | UK/EPIC-Norfolk | 138 | 58.9 [IQR 50.9, 66.9] | Alcohol, education, socio-economic status, parity, diabetes | HR 1.49 (0.91 to 2.52) | Yes |
| Turesson et al. 2015 [24] | Case-control | Sweden/MDCS | 172 | 63.4 (8.0) | Alcohol, education, socio-economic status | Overall OR not reported | No |
| MPMP | 290 | 59.9 (8.8) |
EIRA Epidemiological Investigation of Rheumatoid Arthritis, NHS Nurses Health Study, DNBC Danish National Birth Cohort, EPIC-Norfolk European Prospective Investigation of Cancer, MDCS Malmo Diet Cancer Study, MPMP Malmo Preventive Medicine Program
Mean (SD) or median [IQR or range] age at cohort entry for cohort studies or at diagnosis for case-control studies
Significant association in anti-CCP negative women but not men
OR shown for history of obesity, obesity at diagnosis not significant
Some studies have showed an association between obesity and RA only for seronegative disease [15, 19••, 22•]. It is possible that the mechanism through which obesity contributes to RA development is specific to a distinct pathophysiology in seronegative inflammatory arthritis, much as smoking is specifically associated with CCP-positive RA and is influenced by presence of the shared epitope. In contrast to the smoking example, however, a mechanism has not been identified to explain why obesity would specifically contribute to the development of seronegative RA. Another potential explanation of this phenomenon is differential misclassification of RA. In other words, it is likely that some patients with seronegative RA in these studies have been misclassified and do not actually have inflammatory arthritis. Because obesity is associated with osteoarthritis, disability, and chronic pain, patients with obesity may be more likely to be misclassified—creating an apparent association between obesity and seronegative RA.
Additional subgroup analyses have suggested that the association between obesity and the development of RA may depend on age and gender, with stronger associations identified among younger women. Again, these results vary in recent studies. While some studies have shown similar results in men and women [12•, 22•], others have suggested that obesity may be associated with a decreased risk of RA among men [19••, 24]. Some studies have also shown an association between obesity and RA only in younger subgroups [12•, 20••], and it has been proposed that an effect specific to younger individuals might explain why no association between obesity and RA has been identified in some older cohorts [22•]. With varied results across studies and small subgroups limiting analysis in some cases, it remains unclear whether age and gender are important, perhaps suggesting a hormonal mechanism between the association of obesity and RA.
The observed associations between obesity and RA may also be affected by residual confounding. Detecting a modest association requires large sample sizes, most easily obtained though large longitudinal cohort and case-control studies. Yet these studies, in which data is typically not collected with the study of obesity specifically in mind, are limited in their ability to adjust for all potential confounders. While age, sex, smoking, parity, and socioeconomic status have been appropriately considered in many of the existing studies, other potential confounders such as diet, physical activity, chronic pain, differences in the gut microbiome, and genetic predispositions to both obesity and RA have not been adequately explored.
Furthermore, a potential biologic mechanism to explain an association between obesity and RA has yet to be determined. Adipocytokines, because of their association with adiposity and their pro-inflammatory effects, have been proposed as potential mediators [25, 26]. Studies to date, however, have not established adipocytokines as a direct link between obesity and the development of RA. Vitamin D deficiency, sex hormone differences, and insulin resistance leading to increased inflammation have been proposed as other potential mechanisms although are currently hypothetical [12•, 20••, 27].
In summary, there is some evidence of modest association between obesity and the development of RA, perhaps especially in seronegative patients and younger women. It is possible, however, that these observations are influenced by differential misclassification and residual confounding. Because of the high prevalence of obesity, even a weak association could have a substantial impact on the burden of RA in the population as a whole. If more justification were needed for public health interventions on obesity, RA perhaps could be added to the already substantial list of associated conditions contributing to morbidity and mortality. This relatively modest association, however, is unlikely to have substantial implications for clinicians diagnosing RA at the individual patient level in clinical practice.
Obesity, Disease Activity, and Treatment Response
Recently, there has been substantial interest in obesity as a potential predictor of refractory disease and poor treatment response among patients with RA. In 2011, Klaasen et al. reported that obesity was associated with poorer response to TNF-α inhibition. Their study evaluated 89 patients in a prospective cohort treated with 3 mg/kg of infliximab [28]. Higher BMI was associated with less improvement in the DAS28 at 16 weeks. Only 50 % of patients with BMI >30 kg/m2 had a response at 16 weeks as defined by improvement of DAS28 of 1.2 compared to 75 % with BMI 20–30 (p = 0.04). A large Italian study of 641 patients starting TNF-α antagonists found that obese patients were less than half as likely to achieve DAS28 remission at 1 year [29•].
While these studies raised concern that TNF-α inhibition in particular could be less effective in obese patients, subsequent studies have shown lower response rates in obese patients treated with both biologic and non-biologic therapy, even after adjustment for age, sex, smoking, and seropositivity [30•, 31••, 32–34]. Notable among these studies is an analysis of the BeST trial, showing lower response rates to initial treatment in patients with BMI ≥25 kg/m2[30•], and a large Swedish study of DMARD-naïve patients, most treated with methotrexate, in which patients with obesity were significantly less likely to achieve low disease activity at 3 and 6 months [31••]. While small studies have shown no differences in response rates to rituximab or tocilizumab, larger studies are needed to verify these results [35, 36]. At this time, there is no sufficient evidence to suggest that obesity is associated with poor response to a specific class of medications or to support individualizing treatment decisions based on BMI. Future studies aimed at determining whether obesity is an important predictor of treatment response to a specific class of medication would ideally focus on comparative effectiveness of two similarly effective medication classes in order to evaluate for an interaction between obesity and class of drug.
Again, a definitive mechanism through which obesity could lead to refractory disease and poor response to therapy has not been established. Pharmacokinetics of therapy may be different in obese patients, even for medications with weight-based dosing, although the impact of pharmacokinetic differences has not been comprehensively studied. Some have proposed that obesity may be associated with more severe or more refractory inflammation through increased levels of the inflammatory adipocytokines leptin, resistin, or visfatin or decreased levels of the anti-inflammatory adipocytokine adiponectin. These adipocytokines impact the innate immune system, activating monocytes and increasing levels of inflammatory cytokines such as IL-6, IL-12, and TNF-α, and also the adaptive immune system, inducing proliferation of Th1 cells and decreasing proliferation of regulatory T cells [25, 26]. No studies, however, have provided direct evidence that adipocytokines mediate an association between obesity and more severe or refractory disease in RA.
An alternative explanation is that the apparently poor response to treatment in patients with obesity is due to differences in the performance of subjective disease activity measures. In studies for which specific components of the DAS28 are reported, the poor response in disease activity is driven largely by subjective components of the DAS28. Obese patients tend to have poor response in the patient global, tender joint count, and pain visual analog scale (VAS), without significant differences in improvement in inflammatory markers or, often, of swollen joint count [28, 30•, 31••]. These observations suggest that the impact of obesity on response to treatment may be more related to the impact of obesity on subjective outcomes rather than a refractory disease phenotype.
Indeed, data from several large cohorts has consistently showed that obesity is generally associated with worse subjective measures of disease activity. Wolfe and Michaud evaluated a large longitudinal cohort of 24,535 patients with RA and showed that both low BMI <18.5 and high BMI >30 kg/m2 were associated with worse scores for the Health Assessment Questionnaire (HAQ), patient global, fatigue, sleep scale, physical component scale, mental component scale, and quality of life. Also both low and high BMI were associated with opioid use and higher medical costs [37••]. Ajeganova et al., evaluating the BARFOT prospective observational cohort of early RA in Sweden, evaluated 1596 patients who had BMI information available [38•]. At baseline and in follow-up, obese patients had worse HAQ, VAS pain, and patient global scores. In contrast, inflammatory markers were not significantly different by BMI group.
Obesity is known to be associated with increased pain and comorbidity in the population in general [39, 40], and is associated with a substantially increased risk of physical disability in many populations, including in RA [41–43]. It is likely that obesity contributes to elevated composite indices of disease activity through obesity-related symptoms that would not be expected to respond to RA treatment alone.
To further undermine the concept that obesity is associated with more severe and refractory RA, radiographic damage is not more likely among obese individuals. As discussed in greater detail below, radiographic studies have actually consistently demonstrated that obesity is associated with a significantly lower risk of radiographic damage over time [42, 44–46]. These findings do not support the hypothesis that obesity itself worsens inflammatory disease in RA.
In general, for the practicing rheumatologist, while there is insufficient evidence to support using BMI to inform choice of therapy, clinicians and investigators should consider the potential impact of obesity on the performance of composite disease activity scores and physical function measures in RA.
The Dynamic Nature of Weight in RA and Importance for Long-Term Outcomes
In order to understand the association between obesity and long-term outcomes in RA, it is important to first realize that weight is dynamic over time, particularly in chronic illness. It is not likely to be sufficient to look only at an individual’s current BMI. Understanding the relationship between BMI and long-term outcomes requires an evaluation of changes in BMI and body composition as well as the underlying causes of that change. In other words, with regard to BMI, it is not just where patients are, but how they got there.
Active RA can result in weight loss. Evolutionarily speaking, the body’s first priority is to make available sufficient energy to fight infection and heal wounds. Therefore, disease processes that demand greater energy expenditure will result in inhibition of growth, utilization of energy stores, and weight loss. Historically, rheumatoid cachexia has been described as severe weight loss in the setting of active and severe RA due to excessive resting energy expenditure [47–49]. Two recent studies observed that, even in current practice settings, systemic inflammation and greater disease activity are associated with greater weight loss in patients with RA [50•, 51•].
Conversely, successful control of inflammation in RA may subsequently result in weight gain. Jurgens et al. demonstrated that, among patients treated with methotrexate and prednisone, those who achieved low disease activity gained significantly greater weight, while those who had ongoing disease activity tended to lose weight [50•]. In one recent study, it was observed that greater improvements in CRP were associated with greater weight gain, suggesting that successful treatment of the disease may result in reversal of the catabolic pathways [51•]. Previous studies have also shown that initiation of TNF-α inhibitors results in weight gain [52, 53].
Treatments for RA may also have direct effects on weight. Compared to methotrexate, the initiation of prednisone is associated with significantly greater weight gain, while the initiation of leflunomide is associated with greater weight loss. These associations persist even after adjusting for the response to the drug, as measured by the change in CRP [54]. In other words, while treatment of the inflammatory disease appears to be associated with weight gain, certain drugs themselves may also be implicated, independent of their effect on the disease.
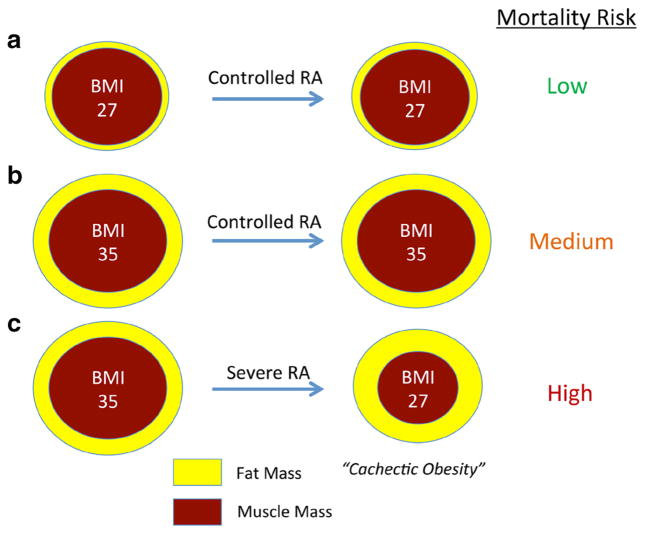
Changes in body composition in active RA make the association between inflammation and BMI even more complicated. Weight is composed of both lean (muscle) and fat mass, each of which is dynamic in chronic inflammatory diseases. Fat mass, or adiposity, likely confers much of the risk of cardiovascular disease and other comorbidities traditionally associated with elevated BMI in the general population. Healthy and intentional weight loss through diet and exercise will reduce fat mass and lower cardiovascular risk. In contrast, weight loss that occurs in the setting of inflammatory diseases such as RA may be primarily observed in the lean compartment [55], resulting in a phenomenon termed “cachectic obesity”—namely low lean (or muscle) mass with high body fat percentage (Fig. 1) [41, 56, 57]. Patients may have an unchanged or reduced BMI but continue to have excess adiposity. Much discussion has ensued about how to best define “obesity” in RA and other inflammatory conditions where BMI may not adequately reflect the extent of adiposity [58]. At the very least, clinicians should be aware that many RA patients with a relatively normal BMI might have substantial adiposity that may still put them at risk for comorbidities traditionally associated with obesity.
Fig. 1.
Hypothetical framework linking weight loss, body composition, and mortality risk in RA. Severe RA leads to a decrease in lean muscle mass more than fat mass, and both rapid weight loss and adiposity are hypothesized to be associated with mortality risk. Shown are a an overweight individual (BMI = 27) with minimal change in weight or body composition; b an obese individual (BMI = 35) with minimal change in weight or body composition; and c an obese individual with severe RA who loses weight with proportionally greater loss in the lean compartment (cachectic obesity)—this latter individual is hypothesized to have the greatest long-term risk of death
Changes in body composition may also include altered fat distribution, including greater accumulation of visceral fat and an increased risk of resulting metabolic syndrome [59–62]. It was also recently observed that RA patients have greater intramuscular fat accumulation [41, 63•]. Intramuscular fat accumulation has been postulated to lead to insulin resistance since skeletal muscle is responsible for handling approximately 40 % of post-prandial glucose uptake. Intramuscular fat has been associated with many long-term outcomes including fracture, cardiovascular disease risk, and death in the general population [64–67]. A normal BMI among RA patients should not preclude measurement of waist circumference, evaluation for insulin resistance, management of blood pressure, and assessment of lipids with the goal of reducing cardiovascular disease risk. While many tools are available to estimate body composition, such as whole-body dual energy absorptiometry (DXA), future research should focus on the most cost-effective and practical ways to evaluate and address body composition alterations in RA.
Thus, weight is not a stable phenomenon over time among individuals with RA and can be influenced by the phenotype of disease, systemic inflammatory burden, response to treatment, direct effects of treatments, as well as diet, physical activity and comorbid conditions. Furthermore, loss of lean body mass in the setting of active disease may result in a lower BMI with continued excessive adiposity.
Obesity and Long-Term Outcomes in RA
Numerous studies have shown that overweight and obese individuals have a lower risk of radiographic progression [42, 44–46]. A recent study demonstrated that an obese BMI was associated with a lower likelihood of radiographic and MRI erosion progression even after adjustment for measures of disease activity and severity, including adjustment for MRI-measured synovitis in the same joints [68]. These results suggest that obesity is associated with less destructive inflammatory disease. In addition, in this study, individuals with lower BMI had greater bone edema on MRI even after adjustment for differences in synovitis, suggesting that these individuals with a low BMI may have a distinct and perhaps more severe phenotype. It may be that this severe phenotype has led to weight loss over time, resulting in a low BMI. Even in the setting of lower radiographic scores, obese subjects are still noted to have greater reported disability, emphasizing the disconnect between increased pain and disability attributable to obesity and the inflammatory disease process [42].
Of great interest is the association between obesity and decreased mortality in patients with RA—the so-called obesity paradox. Despite what is known about the adverse complications of obesity, patients with RA with greater weight consistently appear to have a lower long-term risk of early death [37••, 69, 70]. This observation was recently shown to hold true for all causes of death in RA including cancer, cardiovascular, and lung disease-related deaths [71•].
Again, change in weight may be the crucial factor in explaining the obesity paradox. In a recent study, weight loss, especially rapid weight loss over 1 year, was a strong predictor of death in patients with RA [72•]. The strong association between weight loss and mortality may obscure the association between obesity and mortality in epidemiologic studies. Obesity likely carries the same cardiovascular and overall mortality risk in patients with RA as in the general population. A patient who has lost substantial weight, however, is at very high risk for mortality and therefore will have higher mortality risk than his obese counterpart. In addition, the patient who loses weight is likely to primarily lose lean body mass, resulting in increased mortality risk both from continued adiposity as well as from the underlying process leading to weight loss (Fig. 1).
In fact, the seemingly paradoxical association between obesity and decreased mortality has also been observed in other chronic illnesses and among the elderly [73, 74••, 75, 76]. It has been hypothesized in these conditions that disease-related (or age-related) weight loss may account for this observation as opposed to a biologically beneficial effect of excess weight and adiposity [77].
To the clinician, this phenomenon is important to understand, since a low BMI and especially unintentional weight loss may have prognostic implications and predict the occurrence of many adverse outcomes. The clinician should not interpret these observations to mean that obesity is biologically protective. In fact, quite the opposite is likely true; adiposity likely carries the same risk in RA as in the general population. Weight loss through diet and exercise, reducing fat mass preferentially over lean body mass, may improve cardiovascular outcomes as well as physical function and disability. In contrast, clinicians should think of unintentional weight loss and a low BMI as markers of more inflammatory and destructive disease and a warning of increased mortality risk.
Overall, these observations support greater attention to adiposity and related comorbidities, even among those with normal BMI. For the reasons outlined here, it is likely that previous studies in RA have significantly underestimated the mortality risk that adiposity carries in patients with RA. It is not yet clear how to incorporate new technologies that more accurately estimate adiposity and visceral fat accumulation, but risk stratification tools in RA may be enhanced in the future by understanding the prognostic value of body composition alterations and incorporating this information into new algorithms.
Conclusions
Obesity is a common and critically important factor that impacts the diagnosis, treatment, and assessment of long-term risk among patients with RA. Recent literature supports greater attention to how obesity and alterations in body composition impact the development of the disease, measurement of disease activity, efficacy of treatment, and the risk for long-term adverse outcomes.
Acknowledgments
Dr. Baker would like to acknowledge funding through a Veterans Affairs Clinical Science Research & Development Career Development Award (IK2 CX000955). The contents of this work do not represent the views of the Department of the Veterans Affairs or the United States Government.
Funding Dr. Baker is funded by a Veterans Affairs Clinical Science Research & Development Career Development Award (IK2 CX000955). Dr. George is funded by an NIH T32 training grant (5T32AR007442-28).
Footnotes
Conflict of Interest Michael D. George and Joshua F. Baker declare that they have no conflicts of interest.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent All referenced studies performed by the authors were approved by the appropriate institutional review board and were been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010;69(1):70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- 2.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119(6):503, e1–9. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 3.Källberg H, Ding B, Padyukov L, Bengtsson C, Rönnelid J, Klareskog L, et al. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011;70(3):508–11. doi: 10.1136/ard.2009.120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004;50(10):3085–92. doi: 10.1002/art.20553. [DOI] [PubMed] [Google Scholar]
- 5.Klareskog L, Stolt P, Lundberg K, Källberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 6.Lu B, Solomon DH, Costenbader KH, Karlson EW. Alcohol consumption and risk of incident rheumatoid arthritis in women: a prospective study. Arthritis Rheumatol Hoboken NJ. 2014;66(8):1998–2005. doi: 10.1002/art.38634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen M, Jacobsen S, Klarlund M, Frisch M. Socioeconomic status and risk of rheumatoid arthritis: a Danish case-control study. J Rheumatol. 2006;33(6):1069–74. [PubMed] [Google Scholar]
- 8.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 10.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9 · 1 million participants. Lancet Lond Engl. 2011;377(9765):557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–82. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Crowson CS, Matteson EL, Davis JM, Gabriel SE. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Care Res. 2013;65(1):71–7. doi: 10.1002/acr.21660. In this case-control study, a history of obesity was associated with increase risk of incident RA, with a stronger association in patients <60 years old. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voigt LF, Koepsell TD, Nelson JL, Dugowson CE, Daling JR. Smoking, obesity, alcohol consumption, and the risk of rheumatoid arthritis. Epidemiol Camb Mass. 1994;5(5):525–32. [PubMed] [Google Scholar]
- 14.Symmons DP, Bankhead CR, Harrison BJ, Brennan P, Barrett EM, Scott DG, et al. Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis: results from a primary care-based incident case-control study in Norfolk, England. Arthritis Rheum. 1997;40(11):1955–61. doi: 10.1002/art.1780401106. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen M, Jacobsen S, Klarlund M, Pedersen BV, Wiik A, Wohlfahrt J, et al. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res Ther. 2006;8(4):R133. doi: 10.1186/ar2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernández Avila M, Liang MH, Willett WC, Stampfer MJ, Colditz GA, Rosner B, et al. Reproductive factors, smoking, and the risk for rheumatoid arthritis. Epidemiol Camb Mass. 1990;1(4):285–91. doi: 10.1097/00001648-199007000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Cerhan JR, Saag KG, Criswell LA, Merlino LA, Mikuls TR. Blood transfusion, alcohol use, and anthropometric risk factors for rheumatoid arthritis in older women. J Rheumatol. 2002;29(2):246–54. [PubMed] [Google Scholar]
- 18.Rodríguez LAG, Tolosa LB, Ruigómez A, Johansson S, Wallander M-A. Rheumatoid arthritis in UK primary care: incidence and prior morbidity. Scand J Rheumatol. 2009;38(3):173–7. doi: 10.1080/03009740802448825. [DOI] [PubMed] [Google Scholar]
- 19••.Wesley A, Bengtsson C, Elkan A-C, Klareskog L, Alfredsson L, Wedrén S, et al. Association between body mass index and anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis: results from a population-based case-control study. Arthritis Care Res. 2013;65(1):107–12. doi: 10.1002/acr.21749. This case-control study found a significant association between obesity and incident CCP-negative RA but not CCP-positive RA in women, and an inverse association between obesity and incident CCP-positive RA in men. [DOI] [PubMed] [Google Scholar]
- 20••.Lu B, Hiraki LT, Sparks JA, Malspeis S, Chen C-Y, Awosogba JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Ann Rheum Dis. 2014;73(11):1914–22. doi: 10.1136/annrheumdis-2014-205459. This large cohort study of women in the Nurses Health Study and Nurses Health Study II found an association between obesity and incident RA with a stronger association among women diagnosed with RA at age 55 or younger. BMI measures 4 and 6 years before the diagnosis of RA showed similar associations compared to BMI at the time of diagnosis, and the number of years of obesity was associated with the risk of developing RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harpsøe MC, Basit S, Andersson M, Nielsen NM, Frisch M, Wohlfahrt J, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43(3):843–55. doi: 10.1093/ije/dyu045. [DOI] [PubMed] [Google Scholar]
- 22•.Lahiri M, Luben RN, Morgan C, Bunn DK, Marshall T, Lunt M, et al. Using lifestyle factors to identify individuals at higher risk of inflammatory polyarthritis (results from the European Prospective Investigation of Cancer-Norfolk and the Norfolk Arthritis Register—the EPIC-2-NOAR Study) Ann Rheum Dis. 2014;73(1):219–26. doi: 10.1136/annrheumdis-2012-202481. In this cohort study obesity was non-significantly associated with incident RA overall, but was strongly associated with seronegative polyarthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther. 2015;17(1):86. doi: 10.1186/s13075-015-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turesson C, Bergström U, Pikwer M, Nilsson J-Å, Jacobsson LTH. A high body mass index is associated with reduced risk of rheumatoid arthritis in men, but not in women. Rheumatol Oxf Engl. 2015 Sep 8; doi: 10.1093/rheumatology/kev313. [DOI] [PubMed] [Google Scholar]
- 25.Versini M, Jeandel P-Y, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13(9):981–1000. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Gómez R, Conde J, Scotece M, Gómez-Reino JJ, Lago F, Gualillo O. What’s new in our understanding of the role of adipokines in rheumatic diseases? Nat Rev Rheumatol. 2011;7(9):528–36. doi: 10.1038/nrrheum.2011.107. [DOI] [PubMed] [Google Scholar]
- 27.Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, Avalos I, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008;58(7):2105–12. doi: 10.1002/art.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klaasen R, Wijbrandts CA, Gerlag DM, Tak PP. Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis Rheum. 2011;63(2):359–64. doi: 10.1002/art.30136. [DOI] [PubMed] [Google Scholar]
- 29•.Gremese E, Carletto A, Padovan M, Atzeni F, Raffeiner B, Giardina AR, et al. Obesity and reduction of the response rate to anti-tumor necrosis factor α in rheumatoid arthritis: an approach to a personalized medicine. Arthritis Care Res. 2013;65(1):94–100. doi: 10.1002/acr.21768. In a cohort of 641 patients, obese patients were less likely to have DAS28 remission at 1 year after initiating TNFa antagonists. [DOI] [PubMed] [Google Scholar]
- 30•.Heimans L, van den Broek M, le Cessie S, Siegerink B, Riyazi N, Han KH, et al. Association of high body mass index with decreased treatment response to combination therapy in recent-onset rheumatoid arthritis patients. Arthritis Care Res. 2013;65(8):1235–42. doi: 10.1002/acr.21978. An analysis of the BeST trial found that overweight patients had lower response rates to initial treatment based on change in DAS28 and had higher subjective but not objective disease activity measures. [DOI] [PubMed] [Google Scholar]
- 31••.Sandberg MEC, Bengtsson C, Källberg H, Wesley A, Klareskog L, Alfredsson L, et al. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Ann Rheum Dis. 2014;73(11):2029–33. doi: 10.1136/annrheumdis-2013-205094. Among a cohort with DMARD-naïve RA, patients with obesity were less likely to have low disease activity at 3 and 6 months, driven by differences in subjective components of the DAS28. [DOI] [PubMed] [Google Scholar]
- 32.Ellerby N, Mattey DL, Packham J, Dawes P, Hider SL. Obesity and comorbidity are independently associated with a failure to achieve remission in patients with established rheumatoid arthritis. Ann Rheum Dis. 2014;73(11):e74. doi: 10.1136/annrheumdis-2014-206254. [DOI] [PubMed] [Google Scholar]
- 33.Ottaviani S, Gardette A, Tubach F, Roy C, Palazzo E, Gill G, et al. Body mass index and response to infliximab in rheumatoid arthritis. Clin Exp Rheumatol. 2015;33(4):478–83. [PubMed] [Google Scholar]
- 34.Iannone F, Fanizzi R, Notarnicola A, Scioscia C, Anelli MG, Lapadula G. Obesity reduces the drug survival of second line biological drugs following a first TNF-α inhibitor in rheumatoid arthritis patients. Jt Bone Spine Rev Rhum. 2015;82(3):187–91. doi: 10.1016/j.jbspin.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Ottaviani S, Gardette A, Roy C, Tubach F, Gill G, Palazzo E, et al. Body mass index and response to rituximab in rheumatoid arthritis. Jt Bone Spine Rev Rhum. 2015 Jul 13; doi: 10.1016/j.jbspin.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Pers Y-M, Godfrin-Valnet M, Lambert J, Fortunet C, Constant E, Mura T, et al. Response to tocilizumab in rheumatoid arthritis is not influenced by the body mass index of the patient. J Rheumatol. 2015;42(4):580–4. doi: 10.3899/jrheum.140673. [DOI] [PubMed] [Google Scholar]
- 37••.Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res. 2012;64(10):1471–9. doi: 10.1002/acr.21627. High and low BMI were associated with higher HAQ, patient global, fatigue, quality of life, and other measures in a large longitudinal cohort with RA. All cause mortality was increased in patients with BMI <18.5 kg/m2. [DOI] [PubMed] [Google Scholar]
- 38•.Ajeganova S, Andersson ML, Hafström I BARFOT Study Group. Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: a long-term follow-up from disease onset. Arthritis Care Res. 2013;65(1):78–87. doi: 10.1002/acr.21710. Patients with obesity had higher HAQ, pain, and global health scores with no differences in inflammatory markers. [DOI] [PubMed] [Google Scholar]
- 39.Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbidity of obesity and pain in a general population: results from the Southern Pain Prevalence Study. J Pain Off J Am Pain Soc. 2007;8(5):430–6. doi: 10.1016/j.jpain.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Peltonen M, Lindroos AK, Torgerson JS. Musculoskeletal pain in the obese: a comparison with a general population and long-term changes after conventional and surgical obesity treatment. Pain. 2003;104(3):549–57. doi: 10.1016/S0304-3959(03)00091-5. [DOI] [PubMed] [Google Scholar]
- 41.Baker JF, Von Feldt J, Mostoufi-Moab S, Noaiseh G, Taratuta E, Kim W, et al. Deficits in muscle mass, muscle density, and modified associations with fat in rheumatoid arthritis. Arthritis Care Res. 2014;66(11):1612–8. doi: 10.1002/acr.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker JF, George M, Baker DG, Toedter G, Von Feldt JM, Leonard MB. Associations between body mass, radiographic joint damage, adipokines and risk factors for bone loss in rheumatoid arthritis. Rheumatol Oxf Engl. 2011;50(11):2100–7. doi: 10.1093/rheumatology/ker294. [DOI] [PubMed] [Google Scholar]
- 43.Katz PP, Yazdany J, Trupin L, Schmajuk G, Margaretten M, Barton J, et al. Sex differences in assessment of obesity in rheumatoid arthritis. Arthritis Care Res. 2013;65(1):62–70. doi: 10.1002/acr.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westhoff G, Rau R, Zink A. Radiographic joint damage in early rheumatoid arthritis is highly dependent on body mass index. Arthritis Rheum. 2007;56(11):3575–82. doi: 10.1002/art.23033. [DOI] [PubMed] [Google Scholar]
- 45.van der Helm-van Mil AHM, van der Kooij SM, Allaart CF, Toes REM, Huizinga TWJ. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis. 2008;67(6):769–74. doi: 10.1136/ard.2007.078832. [DOI] [PubMed] [Google Scholar]
- 46.Kaufmann J, Kielstein V, Kilian S, Stein G, Hein G. Relation between body mass index and radiological progression in patients with rheumatoid arthritis. J Rheumatol. 2003;30(11):2350–5. [PubMed] [Google Scholar]
- 47.Lemmey AB, Jones J, Maddison PJ. Rheumatoid cachexia: what is it and why is it important? J Rheumatol. 2011;38(9):2074. doi: 10.3899/jrheum.110308. author reply 2075. [DOI] [PubMed] [Google Scholar]
- 48.Roubenoff R, Roubenoff RA, Ward LM, Holland SM, Hellmann DB. Rheumatoid cachexia: depletion of lean body mass in rheumatoid arthritis. Possible association with tumor necrosis factor. J Rheumatol. 1992;19(10):1505–10. [PubMed] [Google Scholar]
- 49.Roubenoff R. Rheumatoid cachexia: a complication of rheumatoid arthritis moves into the 21st century. Arthritis Res Ther. 2009;11(2):108. doi: 10.1186/ar2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Jurgens MS, Jacobs JWG, Geenen R, Bossema ER, Bakker MF, Bijlsma JWJ, et al. Increase of body mass index in a tight controlled methotrexate-based strategy with prednisone in early rheumatoid arthritis: side effect of the prednisone or better control of disease activity? Arthritis Care Res. 2013;65(1):88–93. doi: 10.1002/acr.21797. In this study, weight change was shown to be dependent on the changes seen in disease activity. In other words, the greater improvement in disease control, the greater the weight gain. [DOI] [PubMed] [Google Scholar]
- 51•.Baker JF, Cannon GW, Ibrahim S, Haroldsen C, Caplan L, Mikuls TR. Predictors of longterm changes in body mass index in rheumatoid arthritis. J Rheumatol. 2015;42(6):920–7. doi: 10.3899/jrheum.141363. In this study, predictors of weight loss included greater age, greater CRP, less improvement in CRP, and active smoking. Thus, those with greater disease activity and lesser response to treatment were observed to have greater odds of weight loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engvall I-L, Tengstrand B, Brismar K, Hafström I. Infliximab therapy increases body fat mass in early rheumatoid arthritis independently of changes in disease activity and levels of leptin and adiponectin: a randomised study over 21 months. Arthritis Res Ther. 2010;12(5):R197. doi: 10.1186/ar3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan E, Baker C, Foley P. Weight gain and tumour necrosis factor-alpha inhibitors in patients with psoriasis. Australas J Dermatol. 2013;54(4):259–63. doi: 10.1111/ajd.12044. [DOI] [PubMed] [Google Scholar]
- 54.Baker Joshua F, Sauer Brian C, Cannon Grant T, Teng Chia-Chen, Michaud Kaleb, Ibrahim Said, et al. Changes in body mass related to the initiation of disease modifying therapies in rheumatoid arthritis. American College of Rheumatology. 2015 Nov; doi: 10.1002/art.39647. Accepted Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giles JT, Ling SM, Ferrucci L, Bartlett SJ, Andersen RE, Towns M, et al. Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum. 2008;59(6):807–15. doi: 10.1002/art.23719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Summers GD, Deighton CM, Rennie MJ, Booth AH. Rheumatoid cachexia: a clinical perspective. Rheumatol Oxf Engl. 2008;47(8):1124–31. doi: 10.1093/rheumatology/ken146. [DOI] [PubMed] [Google Scholar]
- 57.Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol. 2002;85(1):89–99. doi: 10.1016/s0167-5273(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 58.Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Nevill AM, Douglas KM, Jamurtas A, et al. Redefining overweight and obesity in rheumatoid arthritis patients. Ann Rheum Dis. 2007;66(10):1316–21. doi: 10.1136/ard.2006.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305(24):2525–31. doi: 10.1001/jama.2011.878. [DOI] [PubMed] [Google Scholar]
- 60.Solomon DH, Garg R, Lu B, Todd DJ, Mercer E, Norton T, et al. Effect of hydroxychloroquine on insulin sensitivity and lipid parameters in rheumatoid arthritis patients without diabetes mellitus: a randomized, blinded crossover trial. Arthritis Care Res. 2014;66(8):1246–51. doi: 10.1002/acr.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solomon DH, Love TJ, Canning C, Schneeweiss S. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69(12):2114–7. doi: 10.1136/ard.2009.125476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gremese E, Ferraccioli G. The metabolic syndrome: the crossroads between rheumatoid arthritis and cardiovascular risk. Autoimmun Rev. 2011;10(10):582–9. doi: 10.1016/j.autrev.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 63•.Kramer HR, Fontaine KR, Bathon JM, Giles JT. Muscle density in rheumatoid arthritis: associations with disease features and functional outcomes. Arthritis Rheum. 2012;64(8):2438–50. doi: 10.1002/art.34464. Low muscle density (or greater intramuscular fat accumulation) was largely observed among patients with RA who had greater IL-6 levels, greater HAQ, used prednisone, and did not use hydroxychloroquine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miljkovic I, Kuipers AL, Cauley JA, Prasad T, Lee CG, Ensrud KE, et al. Greater skeletal muscle fat infiltration is associated with higher all-cause and cardiovascular mortality in older men. J Gerontol A Biol Sci Med Sci. 2015;70(9):1133–40. doi: 10.1093/gerona/glv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB, et al. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res Off J Am Soc Bone Miner Res. 2010;25(3):513–9. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Therkelsen KE, Pedley A, Speliotes EK, Massaro JM, Murabito J, Hoffmann U, et al. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2013;33(4):863–70. doi: 10.1161/ATVBAHA.112.301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brumbaugh DE, Crume TL, Nadeau K, Scherzinger A, Dabelea D. Intramyocellular lipid is associated with visceral adiposity, markers of insulin resistance, and cardiovascular risk in prepubertal children: the EPOCH study. J Clin Endocrinol Metab. 2012;97(7):E1099–105. doi: 10.1210/jc.2011-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baker JF, Ostergaard M, George M, Shults J, Emery P, Baker DG, et al. Greater body mass independently predicts less radiographic progression on X-ray and MRI over 1–2 years. Ann Rheum Dis. 2014;73(11):1923–8. doi: 10.1136/annrheumdis-2014-205544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Escalante A, Haas RW, del Rincón I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165(14):1624–9. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 70.Mikuls TR, Fay BT, Michaud K, Sayles H, Thiele GM, Caplan L, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatol Oxf Engl. 2011;50(1):101–9. doi: 10.1093/rheumatology/keq232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.England BR, Sayles H, Michaud K, Caplan L, Davis LA, Cannon GW, et al. Cause-specific mortality in US veteran men with rheumatoid arthritis. Arthritis Care Res. 2015 Jun 19; This study recently demonstrated that obesity was associated with a lower risk of several causes of death including cardiovascular, cancer-related, and lung disease-related deaths. [Google Scholar]
- 72•.Baker JF, Billig E, Michaud K, Ibrahim S, Caplan L, Cannon GW, et al. Weight loss, the obesity paradox, and the risk of death in rheumatoid arthritis. Arthritis Rheumatol Hoboken NJ. 2015;67(7):1711–7. doi: 10.1002/art.39136. In this study, the apparently lower odds of death among obese subjects appeared to be largely explained by an increased risk of death among those individuals who lost weight. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doehner W, Erdmann E, Cairns R, Clark AL, Dormandy JA, Ferrannini E, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol. 2012;162(1):20–6. doi: 10.1016/j.ijcard.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 74••.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. This large study observed paradoxically lower risks of death among individuals in the overweight category, leading to controversy about whether this observation was related to a causally protective role of overweight versus an epidemiologic artifact of illness-related weight loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clark AL, Fonarow GC, Horwich TB. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2014;56(4):409–14. doi: 10.1016/j.pcad.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 76.Tobias DK, Pan A, Jackson CL, O’Reilly EJ, Ding EL, Willett WC, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370(3):233–44. doi: 10.1056/NEJMoa1304501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Preston SH, Stokes A. Obesity paradox: conditioning on disease enhances biases in estimating the mortality risks of obesity. Epidemiol Camb Mass. 2014;25(3):454–61. doi: 10.1097/EDE.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]