Abstract
Chronic rhinosinusitis (CRS) is a heterogeneous inflammatory condition with a multifactorial basis. Infectious triggers of CRS have been proposed, but demonstration remains elusive. Evolving research suggests that abnormal host mucosal immune responses, rather than specific pathogens themselves, may underlie the chronic inflammatory state. Despite constant contact with airborne particulates and microorganisms, the sinonasal epithelium maintains mucosal homeostasis through innate and adaptive immune mechanisms that eliminate potential threats. Innate immunity encompasses a broad collection of constitutive and inducible processes that can be nonspecific or pathogen directed. Some innate immune pathways are closely intertwined with tissue growth and repair. The persistent inflammation observed in CRS may result from a pathologic imbalance in innate immune interactions between the host and the environment. Impairment of critical innate immune protection renders the sinonasal mucosal surface susceptible to colonization and potential injury, stimulating the prominent adaptive immune response that characterizes CRS.
Introduction
The sinonasal tract is continuously exposed to particulates and potential pathogens that are normally removed by mucociliary clearance without stimulating an inflammatory response. Secreted antimicrobial proteins and peptides in the mucus, as well as immunoglobulins and opsonins assist in the mucosal defense of the airway surface. Sinonasal epithelial cells participate centrally in this process, secreting proteins into the mucus and propelling the mucus blanket out of the nose with coordinated ciliary movement. Much of this activity occurs constitutively, but inductive mechanisms also allow the system to be accelerated or intensified. Epithelial cells may be acted on by endogenous mediators produced by resident and infiltrating cell populations, and by local sensory nerves. Additionally, epithelial cells detect and respond directly to exogenous signals in the airway lumen, often as the first contact between the host and environment. In mucosal homeostasis, local irritants and pathogens are rapidly and efficiently eliminated through innate pathways of mucosal immunity without wider stimulation of the adaptive immune system. At times, these defenses may be overwhelmed, and epithelial cells may recruit a more sustained and directed immune response by interacting with resident immune cells, including dendritic cells, macrophages, and mucosal lymphocytes. The adaptive and innate immune arms work together to control the threat, and homeostasis is ultimately restored.
Chronic rhinosinusitis (CRS) is a disease defined by persistent inflammation of the sinonasal mucosa. Only a small proportion of CRS cases are caused by genetic disorders, such as cystic fibrosis, or identifiable systemic inflammatory disorders, including Wegener’s granulomatosis and sarcoidosis. For clinical research purposes, CRS has been divided into two broad categories based on the presence or absence of nasal polyps. In CRS with nasal polyps (CRSwNP), the inflammation is typically eosinophilic and associated with a T-helper (Th) type 2 cytokine profile that is not prominent in CRS without nasal polyps (CRSsNP). CRSsNP tends to be more Th1 cytokine dominated, although both forms of CRS have a mixed Th1/Th2 profile. The etiology of CRS remains an active subject of debate and speculation. Current evidence suggests that CRS is a heterogeneous condition with a multifactorial basis relating to both the host and the environment. Broadly speaking, CRS is increasingly viewed as a disorder of the sinonasal mucosal immune system. The symptoms and characteristic pathologic features of CRS result largely from sustained inflammation associated with local adaptive immune activation. Specific infectious and noninfectious agents have been implicated as triggers of this inflammation in CRS, but none has proven to act universally. More likely, host predisposition plays the key role, with a variety of external influences having the potential to provoke or modify the disease in a predisposed individual. It is attractive to hypothesize that the underlying causes of CRS are innate immune defects that disrupt normal mucosal homeostasis and permit otherwise innocuous airborne microorganisms and particulates to stimulate an immune response. Further failure of innate immune mechanisms to regulate inflammation and restore homeostasis may result in a chronic inflammatory state, even in the absence of an ongoing trigger.
Innate Immune Molecules of the Sinonasal Tract
The immune system of mammals is divided into innate and adaptive arms that work cooperatively to defend the host against infection. The term innate refers to immune mechanisms that do not require prior exposure to the pathogen. Adaptive immune responses initially take time to develop due to trafficking of immune cells and the expansion of specific lymphocyte populations. Thus, the first lines of immediate defense against airborne microorganisms and particulates are innate in nature. Mucociliary clearance and secretion of endogenous antimicrobials and opsonins comprise the chief innate mechanisms through which microbes are attacked and removed. Compared with the adaptive immune system, genes of the innate immune system are evolutionarily ancient and “hard wired” in the genome. Although this may suggest a lack of refinement and specificity of function, rapidly unfolding research suggests that the innate immune system is remarkably complex. Pattern-recognition receptors (PRRs) have been discovered that permit early detection of individual pathogens or their products, resulting in the prompt induction of defensive responses. Activation of epithelial cell PRRs triggers secretion of antimicrobial peptides and other effectors that are directed against the specific infectious threat. In addition, PRR stimulation induces expression of cytokine mediators that signal to infiltrating leukocytes, dendritic cells, and other resident cell populations. A summary of innate immune molecules derived from the sinonasal tract is shown in Table 1.
Table 1.
Sinonasal epithelium–derived innate immune molecules
| Secreted antimicrobials |
| Defensins |
| α defensins (HNP1–HNP6) |
| β defensins (HBD1–HBD4) |
| Cathelicidins |
| LL-37 or hCAP |
| Elastase inhibitors |
| SLPI |
| C-type lectins (collectins) |
| Surfactant protein A |
| Surfactant protein B |
| Mannose-binding lectin |
| Lactoferrin |
| Lysozyme |
| Chitinases |
| Acid mammalian chitinase |
| Chitinase-1 |
| Opsonins |
| Complement factors |
| Pentraxins |
| Serum amyloid A |
| Lipocalins |
| Lipocalin-2 (NGAL) |
| PLUNC (?) |
| Cell surface proteins |
| Toll-like receptors |
| S-100 proteins |
| Costimulatory molecules |
| Intracellular proteins |
| NOD receptors |
| RIG-1 |
| MDA-5 |
| Toll-like receptors |
HBD—human β-defensin; hCAP—human cathelicidin antimicrobial peptide; HNP—human neutrophil peptide; MDA-5—melanoma differentiation–associated gene 5; NGAL—neutrophil gelatinase–associated lipocalin; NOD—nucleotide oligomerization domain; PLUNC—palate, lung, and nasal epithelial carcinoma; RIG-1—retinoic acid–induced gene 1; SLPI—secretory leukocyte proteinase inhibitor.
Mucociliary clearance
The continuously flowing mucus blanket is the primary innate immune defense of the sinonasal tract. The nasal cavity filters inspired air, trapping particulates and potential pathogens in the mucus and propelling them harmlessly toward the pharynx. Mucus contains enzymes, immunoglobulins, opsonins, and antimicrobial peptides that limit microbial growth. Proteins in the mucus are derived from plasma transudate, mucus, and serous cells in submucosal glands, goblet cells, Clara cells, epithelial cells, and other cells within the mucosa (plasma cells, mast cells, phagocytes, and fibroblasts). The quantity and viscoelastic properties of the mucus and the ciliary beat frequency determine the effectiveness of mucociliary clearance. Water content, ionic concentration, and pH give the tangled network of mucin molecules their viscoelastic properties. The major components of sinonasal mucus are two distinct mucin genes, MUC5AC and MUC5B, which produce several high molecular weight, heavily glycosylated macromolecules [1].
Pattern-recognition receptors
PRRs are expressed on multiple cell types but play a particularly important defensive role in epithelial cells situated at the interface between the external environment and the mucosal surface of the respiratory, digestive, and urogenital tracts [2]. Activation of PRRs at mucosal surfaces allows defensive immune mechanisms to be initiated rapidly and independent of the adaptive immune system. In the nasal cavity, where microbial interaction is frequent, there is likely to be negative regulation of PRR activation and tolerance of normal upper airway flora. This type of tolerance has not been investigated in the sinonasal tract, but it is known to occur in the intestinal epithelium and is critical to maintaining mucosal homeostasis in the presence of commensal organisms. Sinonasal epithelial cell PRRs play sentinel roles in detecting “danger” signals at the mucosal surface. The innate immune response to a perceived threat is tailored to the specific pattern of PRRs that are activated and may include simultaneous local effector production, inflammatory cytokine signaling, and induction of repair mechanisms for mucosal injury. The balance between normal physiologic PRR signaling and pathologic PRR activation may be an important determinant of the inflammatory state in CRS.
Toll-like receptors (TLRs) are a family of transmembrane PRRs involved in the recognition of conserved motifs associated with pathogens [3]. TLRs were originally identified by their similarity to Drosophila Toll, a protein with a role in embryonic development patterning. There are 11 known TLRs in mammals, all sharing a common structure of an extracellular domain with leucine-rich repeats and an intracellular signaling domain similar to that of the interleukin (IL)-1 receptor family (TIR domain) [4]. Individual TLR proteins recognize distinct pathogen-associated molecular patterns (PAMPs), such as bacterial lipopeptide, endotoxin, flagellin, double-stranded RNA, or bacterial DNA. The signaling pathways of the TLRs differ significantly, but key transduction molecules implicated include MyD88, IL-1 receptor–associated kinase, tumor necrosis factor receptor–associated factor 6, mitogen-activated protein kinases, and nuclear factor-κB (NF-κB).
Nucleotide oligomerization domain–like receptors (NLRs) are a family of 23 proteins in humans that are intracellular, cytoplasmic pathogen sensors. Although they are expressed primarily in immune cells, NLRs are also expressed by epithelial cells. These receptors consist of a central nucleotide oligomerization domain flanked by a C-terminal ligand-binding domain and an N-terminal cell-signaling domain. Nod1 and Nod2 are the best characterized NLRs, which recognize bacterial peptidoglycans. Nod1 is more specific to gram-negative bacteria. Both Nod1 and Nod2 activate NF-κB and mitogen-activated protein kinases, leading to inflammatory gene expression. NLR signaling is synergistic with TLR signaling in this respect, providing a redundant and complementary mechanism for initiating host antimicrobial activity in the face of potential infection.
Retinoic acid–induced gene 1 (RIG-1) and melanoma differentiation–associated gene 5 (MDA-5) are cytosolic proteins that function as RNA helicases while also containing caspase activation and recruitment domains. RIG-1 recognizes noncapped 5′ triphosphated RNAs, and MDA-5 recognizes double-stranded RNA. Like TLRs and NLRs, RIG-1 and MDA-5 activate NF-κB–dependent transcription. The major difference is in the adaptor proteins that initiate the signaling. RIG-1 and MDA-5 use a caspase activation and recruitment domain protein that anchors the protein to the outer mitochondrial membrane and facilitates nuclear translocation of proinflammatory transcription factors.
Innate immune effectors
Airway mucus is a complex mixture of more than 1000 proteins that contribute to host defense of the mucosal surface [5]. In terms of concentration, the predominant antimicrobial proteins are lysozyme, lactoferrin, and secretory leukocyte proteinase inhibitor. In addition to molecules that immobilize or kill microorganisms, epithelial cells of the sinonasal epithelia also secrete cytokine mediators that attract and activate effector cells of the innate and adaptive immune system. Secreted antimicrobials include small cationic peptides such as the β defensins and larger antimicrobial proteins such as lysozyme and lactoferrin [6]. These “endogenous antibiotics” inhibit microbial growth and have direct microbicidal activity, allowing time for the mucociliary apparatus to eliminate the microbial threat or for phagocytic cells to be recruited. Pathologic deficiencies in the antimicrobial properties of airway secretions can lead to colonization by microorganisms and may contribute to adaptive immune activation in CRS.
Lysozyme, an enzyme directed against the peptidoglycan cell wall of bacteria, is highly effective against many common upper-airway, gram-positive species (eg, streptococci). Killing gram-negative bacteria requires cofactors such as lactoferrin, antibody-complement complexes, or ascorbic acid to disrupt the outer membrane so that lysozyme can act on the sensitive peptidoglycan layer [7]. Lactoferrin is an iron-binding protein that inhibits microbial growth by sequestering iron. It is a major component of neutrophil granules but is also stored and released by serous mucosal glands. The N-terminal cationic fragment “lactoferricin” also can be directly microbicidal [8]. Secretory leukocyte proteinase inhibitor is another antibacterial innate immune effector in nasal mucus. It consists of two separate functional domains: the N-terminus, with in vitro activity against both gram-negative and gram-positive bacteria, and the C-terminus, which inhibits neutrophil elastase [9]. Another secreted product identified from methacholine-induced nasal lavage fluid is sPLA2, which has Ca2+-dependent antimicrobial activity against gram-positive and gram-negative bacteria [10].
Cathelicidins and defensins are other large families of antimicrobial peptides produced in the sinonasal tract and the lower airways. Cathelicidins are synergistic with lysozyme and lactoferrin and have broad-spectrum activity against gram-positive and gram-negative bacteria and against fungi and enveloped viruses. The only known human cathelicidin peptide has been named LL-37 [11]. Synthetic LL-37 neutralizes lipopolysaccharide and lipoteichoic acid [12]. Human defensins consist of the α defensins and β defensins 1 to 4, which are peptides with a characteristic six-cysteine/three-disulfide pattern and a three-dimensional fold [13]. The α defensins are contained in granules of neutrophils and Paneth cells of the intestine and in nasal mucosa. The human β defensins 1 to 4 have been demonstrated in the upper and lower airways [14].
Collectins such as surfactant protein (SP)-A and SP-D have roles in innate immune defense beyond surfactant lipid homeostasis. SP-A and SP-D have been demonstrated in the sinonasal mucosa of rabbits and humans [15]. SP-A and SP-D bind and agglutinate nonself structures, including bacteria, fungi, allergens, and environmental inorganic substrates. SP-A and SP-D, as well as the C-type lectin mannose-binding lectin also initiate and enhance immune cell ingestion and killing of targets.
Lipocalins are a diverse family of proteins sharing limited regions of sequence homology and a common tertiary structure architecture. They perform a variety of functions in different tissues, acting generally as transporters of small hydrophobic molecules. One lipocalin, known by the names lipocalin-2, neutrophil gelatinase–associated lipocalin, siderocalin, 24p3, or uterocalin, appears to have a role in innate immunity. Lipocalin-2 production is stimulated by activation of TLRs by bacterial PAMPs and then limits bacterial growth by sequestering a bacterial iron siderophore [12].
Sinonasal epithelial cells also produce acute-phase proteins such as complement components and serum amyloid A [16]. Serum amyloid A is a pentraxin that acts as an important opsonin by binding directly to gram-positive bacteria. RNA for all components of the alternative pathway of complement activation is present in mucosal specimens obtained from the ethmoid sinuses of humans [17]. Cleavage products of complement proteins are potent chemoattractants for granulocytes and act to opsonize particulates for removal by phagocytes.
Sinonasal Epithelial Innate Immune Function
In recent years, investigators have studied in detail the innate immune responses of the sinonasal epithelium. Although the antimicrobial properties of nasal secretions have long been recognized, the specific role of epithelial cells as participants in mucosal immunity is a relatively new concept. In the late 1990s, in vitro studies revealed the capacity of bronchial epithelial cells to produce a wide spectrum of proinflammatory mediators [18]. Several, including IL-6; IL-8; tumor necrosis factor-α; granulocyte-macrophage colony-stimulating factor; regulated on activation, normal T-cell expressed and secreted (RAN-TES); and eotaxin, have been produced by sinonasal epithelial cells as well. Until recently, the sources of innate immune effectors in the nose were believed to be inflammatory cells, serous glands, and plasma exudation [19]. In 2006, Lane et al. [17] showed that messenger RNA for complement components was expressed locally within the sinus mucosa, and also confirmed and extended an earlier finding by Claeys et al. [14] that TLRs were expressed within the epithelium. Since then, other innate immune genes have been identified as being expressed by epithelial cells, including β defensins, the cathelicidin LL-37, and SPs. A highly expressed secreted protein called palate, lung, and nasal epithelial carcinoma associated is also believed to be an innate immune effector derived from epithelial cells of the nose [20].
The expression of immune genes by epithelial cells in vitro can be stimulated by exposure to cytokines or exogenous agents such as pathogen-associated molecules, pollutants, and certain allergens. TLR ligands induce not only antimicrobial peptides but also the expression of cytokines and cell surface costimulatory molecules. Stimulation of TLR3 on nasal epithelial cells with double-stranded RNA in vitro, as well as by rhinovirus in vivo, results in induction of the B7 homologues B7-H1 and B7-DC [21•]. In mice, influenza virus induces expression of β defensin and SP-D by sinonasal epithelial cells [22]. The effect of environmental irritants such as cigarette smoke on innate immunity appears to be mixed. In one study, cigarette smoke extract enhanced TLR3-mediated innate immune responses in CRS patients [23]. However, other published studies indicate that cigarette smoke components decrease epithelial cell expression of innate immune genes [24,25].
CRS and Epithelial Innate Immunity
It has become increasingly appreciated that host immune factors likely play a primary role in the pathogenesis of CRS. Accordingly, recent investigations have analyzed innate and adaptive immune profiles in CRSwNP and CRSsNP. CRSwNP is associated with an eosinophilic, Th2-skewed inflammatory process, as opposed to the Th1-dominated milieu of CRSsNP. How the Th1/Th2 balance is established in the sinus mucosa and may be shaped by local innate immune processes is largely unknown. PAMPs are thought to influence the profile of resident lymphocyte populations and perhaps the direction of Th differentiation. In addition to Th1 and Th2, another subclass of Th cells known as Th17 has been identified as being important in mucosal immunity, but its role in CRS pathogenesis is not yet defined. T-regulatory cells (Tregs) are also believed to play a role in downregulating immune responses and mediating tolerance. Van Bruaene et al. [26] recently identified a decrease in the expression of the Treg transcription factor forkhead box P3 in CRSwNP that was accompanied by an increase in the Th1-promoting transcription factor T-bet and Th2-promoting GATA-3. Another study of CRSwNP patients also showed decreased forkhead box P3 and found that the level of its expression was increased with the use of intranasal steroids [27]. The relationship between the innate and adaptive immune processes in CRS pathogenesis is an active area of investigation. Stimulation of epithelial cell PRRs induces expression of cytokines and cell surface proteins that modify the adaptive immune response (Fig. 1). Examples include the B-cell–activating factor, which regulates local immunoglobulin production, and thymic stromal lymphopoietin, which modulates dendritic cell function. Expression of these factors is increased in CRSwNP [28,29].
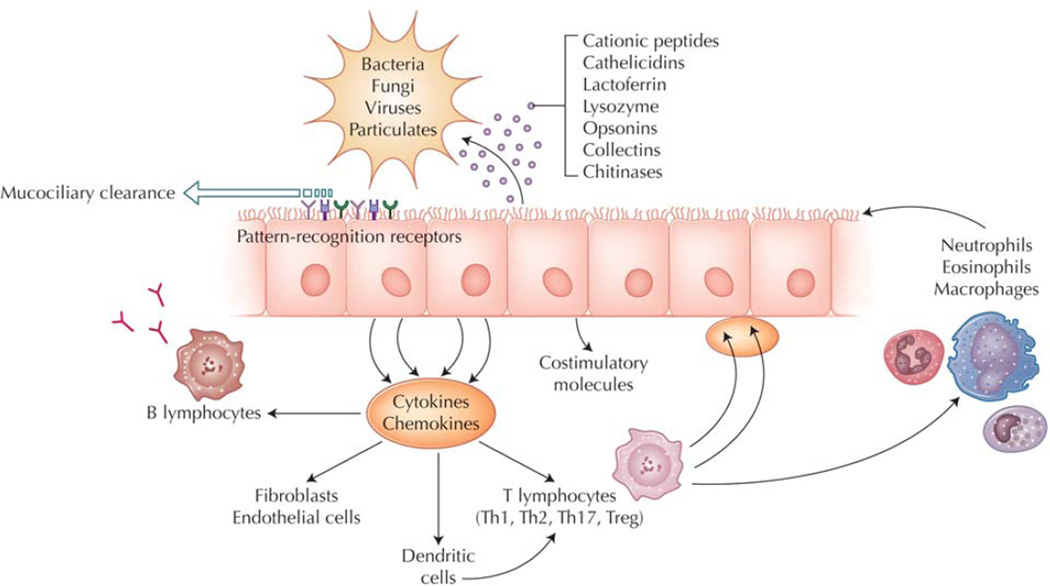
Figure 1.
Innate immunity of the sinonasal tract. The primary mechanism of sinonasal innate immune defense is orderly mucociliary clearance. The mucus blanket, which contains many secreted antimicrobials and opsonins, is continuously propelled to the nasopharynx, providing constitutive, nonspecific protection of the sinonasal mucosal surface. In addition, sinonasal epithelial cells actively participate in innate immunity, using pattern-recognition receptors to detect luminal pathogens and responding directly with selective expression of targeted antimicrobial effectors. At the same time, epithelial cells signal to adaptive immune cells through cytokines and costimulatory molecules to coordinate a vigorous defense of the mucosal surface. Emerging evidence suggests that predominance of certain T-helper (Th) populations (Th1, Th2, and Th17) in the mucosa, as well as the presence of T-regulatory cells (Tregs) may play a role in chronic rhinosinusitis pathogenesis. Epithelial cells guide the recruitment of adaptive immune cells by producing signaling molecules that interact locally with resident dendritic cells and T cells. Cytokines produced by specific T-cell subclasses modulate the innate immune responses of epithelial cells by influencing the pattern of antimicrobial gene expression. Chronic sinonasal inflammatory disease may result from the loss of mucosal homeostasis due to dysregulation of these innate immune pathways.
Several genes associated with innate immunity have been reported to be upregulated in sinus mucosa obtained from patients with CRS. Secretory glycoproteins gp340 and GalNac appear to be elevated and may contribute to the thickened secretions present in the disease [30,31]. Similarly, expression of the mucin genes MUC5A and MUC5B is elevated in CRSwNP patients compared with controls [32,33]. Other recent studies have shown neutrophil gelatinase–associated lipocalin to be upregulated in nasal polyps [34], and the cathelicidin LL-37 to be increased in both eosinophilic CRSwNP and CRSsNP [35,36]. In nasal explants taken from CRS patients, fungal allergens stimulate expression of LL-37 and the collectin SP-D [37,38].
Innate immune defects in CRSwNP
Although overactivity of sinonasal innate immunity may contribute directly to the chronic inflammatory state through production of proinflammatory mediators, decreased innate immune function may also stimulate inflammation indirectly by failing to inhibit microbial colonization. Mounting evidence indicates that CRSwNP is associated with decreased epithelial cell innate immune function. The level of mRNA expression of multiple TLRs, particularly TLR9, is reduced in recalcitrant cases of CRSwNP [39•,40]. Epithelial cells derived from patients with treatment-unresponsive CRSwNP also display decreased expression of the IL-22 receptor, which may result in a relative defect in epithelial cell antimicrobial responses driven by T cells [41]. In vitro exposure of sinonasal epithelial cells to the Th2 cytokines IL-4 and IL-13 results in decreased expression of TLR9, human β-defensin 2 and SP-A [42]. In aggregate, these findings suggest that innate immune function may be depressed in CRSwNP, or at least blunted by the presence of CRSwNP-associated Th2 cytokines. This may help to explain the observed increase in bacterial and fungal colonization that characterizes eosinophilic CRS [43].
Other epithelial cell–derived immune mediators display increased expression in CRSwNP and may play a direct stimulatory role in disease pathogenesis. Eotaxins 1 to 3 are eosinophil chemoattractants produced by sinonasal epithelial cells that are upregulated in CRSwNP [44]. The expression of eotaxins may be induced in vitro by stimulation with Th2 cytokines [45]. In addition to eotaxins, the chemokine RANTES has been implicated as having increased expression in polyp tissue. Acid mammalian chitinase (AMCase) is a protein produced by airway epithelial cells that has been shown to be associated with Th2 inflammation of the lower airway. Ramanathan et al. [46] demonstrated that AMCase is produced by sinonasal epithelial cells and that its expression is increased in recalcitrant CRSwNP. AMCase is a functional enzyme that breaks down chitin, an abundant biopolymer comprising the major component of the cell walls of fungus, insects, and parasites. In this way, AMCase may be viewed as an “antiparasite” immune effector that is different from other described antimicrobial effectors released upon stimulation of PRRs. In contrast to the downregulation of antimicrobial effectors in CRSwNP, AMCase levels are increased, suggesting activity of an alternative innate immune pathway perhaps directed at parasitic pathogens. It is attractive to postulate that antimicrobial and antiparasite innate immune pathways may be opposing in a fashion analogous to Th1 and Th2 cytokine profiles in adaptive immunity. Many PAMPs are recognized to stimulate antimicrobial effectors and “Th1-like” immunity. Recently, chitin has been shown to elicit expression of AMCase and eotaxin-3 by sinonasal epithelial cells in primary culture [47]. The PRR that recognizes chitin remains unknown, although the mechanism of AMCase induction also appears to involve the epidermal growth factor receptor signal transduction pathway [48].
Innate immunity in the sinonasal tract is closely intertwined with mechanisms of injury and repair. Protease-activated receptors (PARs) in the epithelium respond to environmental proteases present in airborne particulates and microbes entering the nasal cavity and activate overlapping signal transduction pathways also used by PRRs. Sinonasal epithelial cells express PAR2, which is demonstrated to play a role in Staphylococcus-induced expression of chemotactic cytokines IL-8 and growth-regulated oncogene α [49]. Antiproteases are produced at the epithelial surface as a host defense against microbial proteases. In CRSwNP, the antiprotease SPINK5 mRNA and protein have been reported to be reduced, potentially undermining maintenance of the mechanical epithelial barrier. In the same study, S100 genes involved in host defense and epithelial repair also were shown to be decreased in expression in CRS patients compared with controls [50]. Fibroblasts are key players in the epithelial growth and repair process and may serve to promote the inflammatory state in CRS. Fibroblast cultures derived from polyps express the lymphocyte chemokine thymus and activation-regulated chemokine (CCL-17) when stimulated with agonists for TLR2 to TLR5, IL-4, or tumor necrosis factor-α [51•,52,53]. Interestingly, recent evidence suggests that TLRs on epithelial cells may activate signaling cascades using the epidermal growth factor receptor, supporting the tight association between innate immunity and injury repair [54–56].
Relationship between dysregulated innate immunity and chronic sinus infection
The sinonasal epithelium uses increased mucociliary flow as an important nonspecific defense mechanism in response to airborne irritants. Poor mucociliary function promotes chronic stasis of mucus, giving inhaled organisms an opportunity to overcome secreted defenses and grow within the sinonasal cavities, thus yielding infectious inflammation. Patients with inflammatory sinonasal disease have impaired mucociliary clearance as compared with healthy individuals. This is related to the altered viscoelastic properties of the mucus, as well as changes in ciliary dynamics. The ability of sinonasal epithelial cells to adapt their ciliary function to environmental stimuli may be impaired in CRS [57].
Bacteria and other microorganisms are frequently isolated from the sinonasal tract of patients with CRS and thus continue to be implicated in CRS pathogenesis. It is challenging to determine whether the role of such organisms in the disease is one of cause or effect. Certainly, the loss of innate immune homeostasis at the sinonasal mucosal boundary will predispose an individual to microbial colonization. As compared with the lower airway, the sinonasal tract faces a greater burden of airborne particles and microorganisms and is more prone mechanically to chronic mucus stasis. Whereas a cough can propel mucus out of the bronchopulmonary tree, nose blowing does not move air through the sinuses to evacuate them. The sinuses depend entirely on mucociliary clearance; when secretions fail to exit the sinuses, they become vulnerable to microbial colonization. Some bacterial species establish residence in the form of biofilms, which are complex structures providing bacteria with resistance to systemic and topical antibiotics [55,58]. The presence of bacterial biofilms theoretically may provoke a chronic inflammatory reaction. It is intriguing to speculate that bacteria in biofilms produce factors that interfere with host innate immunity to promote their survival. In a recent study, the level of lactoferrin in nasal secretions was observed to be reduced in CRS patients with paranasal sinus biofilms [59]. Future research will be needed to determine whether innate immune deficits can be caused by biofilms, or, conversely, if biofilms occur solely as a consequence of preexisting failed innate immune protection.
Conclusions
Situated at the interface between the host and the environment, the sinonasal tract plays a critical role in the immune defense against airborne pathogens. The recent literature has begun to characterize the physiology of sinonasal mucosal innate immunity and how it may contribute to CRS pathogenesis. Both overactivity and underactivity of normal innate immune processes can lead to a loss of mucosal homeostasis. Although the trigger of chronic inflammation in CRS remains unknown, it is likely that any imbalance in the dynamic interaction between the host and environment will stimulate a local inflammatory response. Certain forms of eosinophilic polypoid CRS appear to be associated with diminished antimicrobial innate immunity and a predisposition to colonization. In other cases, abnormal patterns of mucosal innate immune gene expression may persistently stimulate the adaptive immune system and interfere with healthy resolution and repair. Further research is necessary to understand how the sinonasal mucosal innate immune system maintains homeostasis in the face of continuous microbial exposure and how dysregulation of this essential function may relate to chronic inflammatory sinus disease. Despite the heterogeneity in CRS phenotypes, identification of common inflammatory pathways involving the innate immune system may point the way toward novel treatments.
Footnotes
Disclosure
No potential conflict of interest relevant to this article was reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol. 2004;286:L887–L892. doi: 10.1152/ajplung.00323.2003. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 4.Janssens S, Beyaert R. Role of Toll-like receptors in pathogen recognition. Clin Microbiol Rev. 2003;16:637–646. doi: 10.1128/CMR.16.4.637-646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindahl M, Stahlbom B, Tagesson C. Newly identified proteins in human nasal and bronchoalveolar lavage fluids: potential biomedical and clinical applications. Electrophoresis. 1999;20:3670–3676. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3670::AID-ELPS3670>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 6.Lee SH, Kim JE, Lim HH, et al. Antimicrobial defensin peptides of the human nasal mucosa. Ann Otol Rhinol Laryngol. 2002;111:135–141. doi: 10.1177/000348940211100205. [DOI] [PubMed] [Google Scholar]
- 7.Ellison RT, 3rd, Giehl TJ. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991;88:1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuwata H, Yip TT, Yip CL, et al. Bactericidal domain of lactoferrin: detection, quantitation, and characterization of lactoferricin in serum by SELDI affinity mass spectrometry. Biochem Biophys Res Commun. 1998;245:764–773. doi: 10.1006/bbrc.1998.8466. [DOI] [PubMed] [Google Scholar]
- 9.Zhu J, Nathan C, Ding A. Suppression of macrophage responses to bacterial lipopolysaccharide by a non-secretory form of secretory leukocyte protease inhibitor. Biochim Biophys Acta. 1999;1451:219–223. doi: 10.1016/s0167-4889(99)00111-1. [DOI] [PubMed] [Google Scholar]
- 10.Aho HJ, Grenman R, Sipila J, et al. Group II phospholipase A2 in nasal fluid, mucosa and paranasal sinuses. Acta Otolaryngol. 1997;117:860–863. doi: 10.3109/00016489709114215. [DOI] [PubMed] [Google Scholar]
- 11.Sorensen OE, Follin P, Johnsen AH, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 12.Vonk MJ, Hiemstra PS, Grote JJ. An antimicrobial peptide modulates epithelial responses to bacterial products. Laryngoscope. 2008;118:816–820. doi: 10.1097/MLG.0b013e31816422d7. [DOI] [PubMed] [Google Scholar]
- 13.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 14.Claeys S, de Belder T, Holtappels G, et al. Human beta-defensins and Toll-like receptors in the upper airway. Allergy. 2003;58:748–753. doi: 10.1034/j.1398-9995.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 15.Woodworth BA, Lathers D, Neal JG, et al. Immunolocalization of surfactant protein A and D in sinonasal mucosa. Am J Rhinol. 2006;20:461–465. doi: 10.2500/ajr.2006.20.2892. [DOI] [PubMed] [Google Scholar]
- 16.Sha Q, Truong-Tran AQ, Plitt JR, et al. Activation of airway epithelial cells by Toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 17.Lane AP, Truong-Tran QA, Myers A, et al. Serum amyloid A, properdin, complement 3, and Toll-like receptors are expressed locally in human sinonasal tissue. Am J Rhinol. 2006;20:117–123. [PMC free article] [PubMed] [Google Scholar]
- 18.Polito AJ, Proud D. Epithelia cells as regulators of airway inflammation. J Allergy Clin Immunol. 1998;102:714–718. doi: 10.1016/s0091-6749(98)70008-9. [DOI] [PubMed] [Google Scholar]
- 19.Kaliner MA. Human nasal host defense and sinusitis. J Allergy Clin Immunol. 1992;90:424–430. doi: 10.1016/0091-6749(92)90162-u. [DOI] [PubMed] [Google Scholar]
- 20.Kim CH, Kim K, Jik Kim H, et al. Expression and regulation of PLUNC in human nasal epithelium. Acta Otolaryngol. 2006;126:1073–1078. doi: 10.1080/00016480600606749. [DOI] [PubMed] [Google Scholar]
- 21. Heinecke L, Proud D, Sanders S, et al. Induction of B7-H1 and B7-DC expression on airway epithelial cells by the Toll-like receptor 3 agonist double-stranded RNA and human rhinovirus infection: in vivo and in vitro studies. J Allergy Clin Immunol. 2008;121:1155–1160. doi: 10.1016/j.jaci.2008.02.009. This study found that nasal epithelial cell expression of costimulatory molecules is increased in response to a TLR3 agonist in vitro.
- 22.Chong KT, Thangavel RR, Tang X. Enhanced expression of murine beta-defensins (MBD-1, -2,- 3, and -4) in upper and lower airway mucosa of influenza virus infected mice. Virology. 2008;380:136–143. doi: 10.1016/j.virol.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Yamin M, Holbrook EH, Gray ST, et al. Cigarette smoke combined with Toll-like receptor 3 signaling triggers exaggerated epithelial regulated upon activation, normal T-cell expressed and secreted/CCL5 expression in chronic rhinosinusitis. J Allergy Clin Immunol. 2008;122:1145–1153. doi: 10.1016/j.jaci.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 24.MacRedmond RE, Greene CM, Dorscheid DR, et al. Epithelial expression of TLR4 is modulated in COPD and by steroids, salmeterol and cigarette smoke. Respir Res. 2007;8:84. doi: 10.1186/1465-9921-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee WK, Ramanathan M, Jr, Spannhake EW, Lane AP. The cigarette smoke component acrolein inhibits expression of the innate immune components IL-8 and human beta-defensin 2 by sinonasal epithelial cells. Am J Rhinol. 2007;21:658–663. doi: 10.2500/ajr.2007.21.3094. [DOI] [PubMed] [Google Scholar]
- 26.Van Bruaene N, Perez-Novo CA, Basinski TM, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–1441. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 27.Li HB, Cai KM, Liu Z, et al. Foxp3+ T regulatory cells (Tregs) are increased in nasal polyps (NP) after treatment with intranasal steroid. Clin Immunol. 2008;129:394–400. doi: 10.1016/j.clim.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 28.Kato A, Peters A, Suh L, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–1392. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauer J, Damm M, Rosenbohm J, Jurk T. Gene expression of thymic stromal lymphopoietin in nasal mucosa and nasal polyps. Otolaryngol Head Neck Surg. 2004;131:190. [Google Scholar]
- 30.Kim TH, Lee SH, Lee HM, et al. Increased expression of glycoprotein 340 in the ethmoid sinus mucosa of patients with chronic sinusitis. Arch Otolaryngol Head Neck Surg. 2007;133:1111–1114. doi: 10.1001/archotol.133.11.1111. [DOI] [PubMed] [Google Scholar]
- 31.Berger G, Kogan T, Ophir D, et al. Glycoconjugate expression of sinus mucosa in chronic rhinosinusitis: a lectin histochemical study. Am J Rhinol. 2008;22:349–355. doi: 10.2500/ajr.2008.22.3185. [DOI] [PubMed] [Google Scholar]
- 32.Ding GQ, Zheng CQ. The expression of MUC5AC and MUC5B mucin genes in the mucosa of chronic rhinosinusitis and nasal polyposis. Am J Rhinol. 2007;21:359–366. doi: 10.2500/ajr.2007.21.3037. [DOI] [PubMed] [Google Scholar]
- 33.Viswanathan H, Brownlee IA, Pearson JP, Carrie S. MUC5B secretion is up-regulated in sinusitis compared with controls. Am J Rhinol. 2006;20:554–557. doi: 10.2500/ajr.2006.20.2935. [DOI] [PubMed] [Google Scholar]
- 34.Woo HJ, Min JK, Bai CH, et al. Expression of neutrophil gelatinase-associated lipocalin in nasal polyps. Arch Otolaryngol Head Neck Surg. 2008;134:1182–1186. doi: 10.1001/archotol.134.11.1182. [DOI] [PubMed] [Google Scholar]
- 35.Ooi EH, Wormald PJ, Carney AS, et al. Human cathelicidin antimicrobial peptide is up-regulated in the eosinophilic mucus subgroup of chronic rhinosinusitis patients. Am J Rhinol. 2007;21:395–401. doi: 10.2500/ajr.2007.21.3048. [DOI] [PubMed] [Google Scholar]
- 36.Kim ST, Cha HE, Kim DY, et al. Antimicrobial peptide LL-37 is upregulated in chronic nasal inflammatory disease. Acta Otolaryngol. 2003;123:81–85. doi: 10.1080/0036554021000028089. [DOI] [PubMed] [Google Scholar]
- 37.Ooi EH, Wormald PJ, Carney AS, et al. Surfactant protein D expression in chronic rhinosinusitis patients and immune responses in vitro to Aspergillus and alternaria in a nasal explant model. Laryngoscope. 2007;117:51–57. doi: 10.1097/01.mlg.0000243196.75418.6f. [DOI] [PubMed] [Google Scholar]
- 38.Ooi EH, Wormald PJ, Carney AS, et al. Fungal allergens induce cathelicidin LL-37 expression in chronic rhinosinusitis patients in a nasal explant model. Am J Rhinol. 2007;21:367–372. doi: 10.2500/ajr.2007.21.3025. [DOI] [PubMed] [Google Scholar]
- 39. Lane AP, Truong-Tran QA, Schleimer RP. Altered expression of genes associated with innate immunity and inflammation in recalcitrant rhinosinusitis with polyps. Am J Rhinol. 2006;20:138–144. This article examines expression of TLR and innate immune effectors in treatment-unresponsive CRSwNP.
- 40.Ramanathan M, Jr, Lee WK, Dubin MG, et al. Sinonasal epithelial cell expression of Toll-like receptor 9 is decreased in chronic rhinosinusitis with polyps. Am J Rhinol. 2007;21:110–116. doi: 10.2500/ajr.2007.21.2997. [DOI] [PubMed] [Google Scholar]
- 41.Ramanathan M, Jr, Spannhake EW, Lane AP. Chronic rhinosinusitis with nasal polyps is associated with decreased expression of mucosal interleukin 22 receptor. Laryngoscope. 2007;117:1839–1843. doi: 10.1097/MLG.0b013e31811edd4f. [DOI] [PubMed] [Google Scholar]
- 42.Ramanathan M, Jr, Lee WK, Spannhake EW, Lane AP. Th2 cytokines associated with chronic rhinosinusitis with polyps down-regulate the antimicrobial immune function of human sinonasal epithelial cells. Am J Rhinol. 2008;22:115–121. doi: 10.2500/ajr.2008.22.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferguson BJ, Seethala R, Wood WA. Eosinophilic bacterial chronic rhinosinusitis. Laryngoscope. 2007;117:2036–2040. doi: 10.1097/MLG.0b013e318123f2d7. [DOI] [PubMed] [Google Scholar]
- 44.Olze H, Forster U, Zuberbier T, et al. Eosinophilic nasal polyps are a rich source of eotaxin, eotaxin-2 and eotaxin-3. Rhinology. 2006;44:145–150. [PubMed] [Google Scholar]
- 45.Lezcano-Meza D, Davila-Davila B, Vega-Miranda A, et al. Interleukin (IL)-4 and to a lesser extent either IL-13 or interferon-gamma regulate the production of eotaxin-2/CCL24 in nasal polyps. Allergy. 2003;58:1011–1017. doi: 10.1034/j.1398-9995.2003.00174.x. [DOI] [PubMed] [Google Scholar]
- 46.Ramanathan M, Jr, Lee WK, Lane AP. Increased expression of acidic mammalian chitinase in chronic rhinosinusitis with nasal polyps. Am J Rhinol. 2006;20:330–335. doi: 10.2500/ajr.2006.20.2869. [DOI] [PubMed] [Google Scholar]
- 47.Laleker A, Nkrumah L, Lee WK, et al. Chitin stimulates expression of acidic mammalian chitinase and eotaxin 3 by human sinonasal epithelial cells in vitro. Am J Rhinol. 2009 doi: 10.2500/ajra.2009.23.3256. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartl D, He CH, Koller B, et al. Acidic mammalian chitinase is secreted via an ADAM17/EGFR-dependent pathway and stimulates chemokine production by pulmonary epithelial cells. J Biol Chem. 2008;283:33472–33482. doi: 10.1074/jbc.M805574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudack C, Steinhoff M, Mooren F, et al. PAR-2 activation regulates IL-8 and GRO-alpha synthesis by NF-kappaB, but not RANTES, IL-6, eotaxin or TARC expression in nasal epithelium. Clin Exp Allergy. 2007;37:1009–1022. doi: 10.1111/j.1365-2222.2007.02686.x. [DOI] [PubMed] [Google Scholar]
- 50.Richer SL, Truong-Tran AQ, Conley DB, et al. Epithelial genes in chronic rhinosinusitis with and without nasal polyps. Am J Rhinol. 2008;22:228–234. doi: 10.2500/ajr.2008.22.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nonaka M, Ogihara N, Fukumoto A, et al. Toll-like receptor 2, 3, 4, 5 ligands and interleukin-4 synergistically induce TARC production in nasal polyp fibroblasts. Auris Nasus Larynx. 2008;35:515–520. doi: 10.1016/j.anl.2008.02.001. This study identifies polyp fibroblasts as innate immune participants contributing to Th2 inflammation by producing thymus and activation-regulated chemokine when exposed in combination to IL-4 and TLR agonists.
- 52.Fukumoto A, Nonaka M, Ogihara N, Pawankar R. Induction of TARC production by lipopolysaccharide and interleukin-4 in nasal fibroblasts. Int Arch Allergy Immunol. 2008;145:291–297. doi: 10.1159/000110085. [DOI] [PubMed] [Google Scholar]
- 53.Nonaka M, Ogihara N, Fukumoto A, et al. Combined stimulation of nasal polyp fibroblasts with poly IC, interleukin 4, and tumor necrosis factor alpha potently induces production of thymus- and activation-regulated chemokine. Arch Otolaryngol Head Neck Surg. 2008;134:630–635. doi: 10.1001/archotol.134.6.630. [DOI] [PubMed] [Google Scholar]
- 54.Koff JL, Shao MX, Ueki IF, Nadel JA. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1068–L1075. doi: 10.1152/ajplung.00025.2008. [DOI] [PubMed] [Google Scholar]
- 55.Burgel PR, Nadel JA. Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. Eur Respir J. 2008;32:1068–1081. doi: 10.1183/09031936.00172007. [DOI] [PubMed] [Google Scholar]
- 56.Ding GQ, Zheng CQ, Bagga SS. Up-regulation of the mucosal epidermal growth factor receptor gene in chronic rhinosinusitis and nasal polyposis. Arch Otolaryngol Head Neck Surg. 2007;133:1097–1103. doi: 10.1001/archotol.133.11.1097. [DOI] [PubMed] [Google Scholar]
- 57.Chen B, Shaari J, Claire SE, et al. Altered sinonasal ciliary dynamics in chronic rhinosinusitis. Am J Rhinol. 2006;20:325–329. doi: 10.2500/ajr.2006.20.2870. [DOI] [PubMed] [Google Scholar]
- 58.Hunsaker DH, Leid JG. The relationship of biofilms to chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2008;16:237–241. doi: 10.1097/MOO.0b013e3282fdc6d5. [DOI] [PubMed] [Google Scholar]
- 59.Psaltis AJ, Wormald PJ, Ha KR, Tan LW. Reduced levels of lactoferrin in biofilm-associated chronic rhinosinusitis. Laryngoscope. 2008;118:895–901. doi: 10.1097/MLG.0b013e31816381d4. [DOI] [PubMed] [Google Scholar]