Abstract
Brominated flame retardants (BFRs) are incorporated into various consumer products to prevent flame propagation. These compounds leach into the domestic environment, resulting in chronic exposure and contamination. Pregnancy failure is associated with high levels of BFRs in human follicular fluid, raising serious questions regarding their impact on female reproductive health. The goal of this study is to elucidate the effects of an environmentally relevant BFR mixture on female rat ovarian functions (i.e., folliculogenesis and steroidogenesis). A BFR dietary mixture formulated to mimic the relative BFR congener levels in North American house dust was administered to adult female Sprague-Dawley rats from 2 to 3 wk before mating until Gestational Day 20; these diets were designed to deliver nominal doses of 0, 0.06, 20, or 60 mg/kg/day of the BFR mixture. Exposure to BFRs triggered an approximately 50% increase in the numbers of preantral and antral follicles and an enlargement of the antral follicles in the ovaries of the dams. A significant reduction in the expression of catalase, an antioxidant enzyme, and downregulation of the expression of insulin-like factor 3 (Insl3) and 17alpha-hydroxylase (Cyp17a1) were observed in the ovary. In addition, BFR exposure affected steroidogenesis; we observed a significant decrease in circulating 17-hydroxypregnenolone and an increase in testosterone concentrations in BFR-exposed dams. Thus, BFRs target ovarian function in the rat, adversely affecting both folliculogenesis and steroidogenesis.
Keywords: androgens, brominated flame retardants, catalase, endocrine disrupters, folliculogenesis, HBCDD, insulin-like factor 3, ovary, oxidative stress, PBDE, steroidogenesis, toxicology
INTRODUCTION
Flame retardant chemicals are produced in high volumes, widespread in the environment, and detected in each of us, and they bioaccumulate [1]. These chemicals are incorporated into numerous products that typically are highly flammable, such as plastics, textiles, and foams; they contribute up to 30% of the weight of the components in office and home electronics, synthetic building materials, and furniture or motor vehicles [1]. A major class of flame retardants is represented by brominated flame retardants (BFRs), including polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecane (HBCDD) [1]. With time, these flame retardants leach out into domestic environments, resulting in repetitive exposure through inhalation and ingestion [2, 3]. Because the BFRs are lipophilic and persistent [4], they bioaccumulate in human breast milk, serum, and adipose tissues [5] and biomagnify through food chains [3]. A number of the PBDEs that have been banned in North America for more than 10 years are still detected in human body fluids as well as in domestic dust [6, 7].
Evidence is increasing that the BFRs are endocrine disruptors, affecting androgenic, estrogenic, and thyroid hormone activities [8]. In humans, adverse effects on male reproduction are associated with exposure to BFRs and include changes in the levels of steroid hormones [9–11] and a decrease in sperm count and quality [12–14]. In utero and lactational exposure to PBDEs is associated with an increased incidence of cryptorchidism [15, 16]. In adolescent girls, high serum levels of BFRs are associated with an earlier age of menarche [17–19]. Previous investigations have reported that the detection of high levels of PBDEs in follicular fluid or in serum is associated with a longer time to conceive [20] and with failure of embryo implantation after in vitro fertilization [21].
Most studies on the effects of the BFRs in animal models have focused on the consequences of exposure to individual congeners or commercial mixtures. These studies show that BFRs disrupt the endocrine system [8, 22] and are associated with diabetes, cancer, and neurobehavioral and developmental disorders [23]. The window of exposure is important because perinatal exposure to BFRs results in clear disruptions in the timing of puberty and in gametogenesis in male and female rats [24–27]. One of the difficulties in relating the animal experiments to human health is that human exposure is to the complex mixtures of BFRs found in the environment rather than to specific, individual congeners. We have shown that chronic exposure of adult male rats to an environmentally relevant BFR mixture, mimicking the relative congener levels in house dust, resulted in an enlargement of the liver and kidney and altered thyroid hormone parameters in the absence of effects on the reproductive system [28]. No changes in estrous cyclicity or pregnancy outcomes, such as mating and fecundity indices or numbers of live fetuses, are observed in female rats fed this same BFR mixture before mating and during gestation [29]. However, in vitro studies with porcine antral follicles have shown that steroid hormone production and the expression of steroidogenic enzymes are affected by single PBDE congeners [30], their metabolites [31], or a mixture reflecting the PBDEs detected in human serum [32].
There may be multiple explanations for a divergence in findings in epidemiological studies, in vivo animal experiments, and in vitro studies. One possibility is that species differences exist; however, the BFRs have similar endocrine-disrupting activities in a wide range of species [8]. A second possibility is that the standard testing procedures to assess the effects of such chemicals on female reproduction may not detect important effects on ovarian function. The objective of the present study is to determine whether BFRs target the ovary in the rat. To accomplish this goal, ovarian function was assessed in female Sprague-Dawley rats fed an environmentally relevant BFR mixture before mating and during gestation in which no significant changes in estrous cyclicity, mating, or fecundity were observed [29].
MATERIALS AND METHODS
BFR Mixture Formulation
Formulation of the BFR mixture was described previously [28, 29]. Briefly, three technical PBDE mixtures (DE-71, DE-79, and BDE-209) and one HBCDD mixture were combined to yield a ratio of PBDE congeners and HBCDDs comparable to the median levels observed in Boston household dust [33]. This BFR mixture was incorporated into an isoflavone-free diet (Teklad Global 2019; Harlan Laboratories). Diets were formulated to contain 0, 0.75, 250, or 750 mg of BFR mixture per kilogram. Diet samples from each experimental condition were collected, and BFR content was confirmed by gas chromatography/mass spectrometry as previously described [34]. These BFR-supplemented diets were designed to deliver nominal doses of 0, 0.06, 20, and 60 mg/kg body wt/day, assuming a daily food consumption of 80 g/kg body wt/day. The lowest dose was estimated to be a close approximation of maximum human exposure based on a dust ingestion rate of 100 mg/day in children (16.5 kg body wt) and the scaling of dose from humans to rodents (human to rat body surface area ratio, 1:6.3) [33, 35].
Animals and Treatment
Virgin female Sprague-Dawley rats (wt, 200–250 g) were obtained from Charles River Laboratories. Animals were housed individually at the Animal Resources Centre of McGill University in rooms maintained at 20°C on a 12L:12D photoperiod. Food and water were provided ad libitum. All animal studies were conducted in accordance with the procedures and principles outlined in the Guide to the Care and Use of Experimental Animals prepared by the Canadian Council on Animal Care under protocol 4456. Following 1 wk of acclimatization to the control diet, female rats were randomly assigned to one of four experimental conditions (n = 35–38 per group) and fed a BFR-supplemented diet for 2–4 wk before mating. During this period, estrous cyclicity was evaluated by analyzing vaginal cytology [36]. Females in proestrus were caged with proven breeder male Sprague-Dawley rats (maintained on the control diet) overnight. Gestational Day (GD) 0 was designated as the day a sperm-positive vaginal smear was observed. Following insemination, females continued on their respective diets until euthanasia on GD 20.
Sample Collection
Dams were euthanized by CO2 asphyxiation followed by exsanguination via cardiac puncture on GD 20. Ovaries were removed and weighed. The right ovary was fixed in 4% paraformaldehyde, whereas the left was stored at −80°C for gene expression analysis. Whole blood was transferred to a Vacutainer SST (BD Biosciences), allowed to clot for 30 min at room temperature, then held on ice for no longer than 4 h until centrifugation at 1300 × g for 20 min. Serum was aliquoted and stored at −80°C.
Measurements of BFR Serum Levels
Serum samples (∼1 g) were thawed and weighed into 50-ml glass centrifuge tubes. Each sample was fortified with surrogate standards (twelve 13C12-labeled PBDE congeners [BDE-15, BDE-28, BDE-47, BDE-77, BDE-99, BDE-100, BDE-126, BDE-138, BDE-153, BDE-154, BDE-183, and BDE-209; Cambridge Isotope Laboratories] and 13C12 labeled α-, β-, and γ-HBCDD; Wellington Laboratories). The samples were homogenized, and extracts were cleaned up as previously described [34]. Following the elution of PBDEs from the deactivated Florisil columns (60–100 mesh; Fisher Scientific) with 70 ml of hexane, a second round-bottom flask was placed under the Florisil column, and HBCDD isomers were eluted using 70 ml of dichloromethane:hexane (30:70, v/v). Both fractions were reduced in volume to approximately 1 ml using rotary evaporation. The PBDE fraction was transferred to a v-notch vial, evaporated to dryness using a gentle stream of nitrogen, rediluted in iso-octane, and mixed. The final iso-octane extract was transferred to a chromatographic vial for analysis. The HBCDD fraction was similarly transferred to a v-notch vial but taken only to near dryness (∼50 μl) using a gentle stream of nitrogen before the addition of C122H18Br6 analogs of α-, β-, and γ-HBCDD as performance standards. The samples were then placed in a fume hood, where they remained until dryness was attained. To each dry HBCDD extract, 100 μl of methanol:water (80:20, v/v) were added, and the vial was mixed and transferred to a chromatography vial. Dilution of serum sample extracts was necessary for rats that had been fed high concentrations of PBDEs and HBCDD (e.g., 20 and 60 mg/kg/day) to ensure that accurate concentration measurements could be achieved. Analysis and quality assurance were carried out as previously described [29, 34].
Follicle Count
Fixed ovaries were paraffin-embedded, serially sectioned (section thickness, 5 μm), and stained with hematoxylin-eosin. Classification of follicle stage was determined based on the morphology and thickness of the granulosa cell layer using previously defined classifications with some modifications [37]. Briefly, primordial follicles were identified as having a single layer of flattened granulosa cells, whereas primary follicles had a single layer comprising at least one rounded granulosa cell. Secondary follicles were identified as follicles with two to four layers of granulosa cells; antral follicles had more than four layers of granulosa cells surrounded by a theca cell layer and an antrum. The presence of pyknotic cells, empty zona pellucida, and/or disorganized granulosa and theca cell layers indicated an atretic follicle. Total follicle numbers per ovary at each stage were estimated as follows: secondary, antral, and atretic follicles with a visible oocyte were counted in every section starting from the first mounted section of the ovary. The total number of growing follicles per ovary was estimated as N = f × NN, where N is the number of secondary, antral, or atretic follicles; f is the fraction of ovarian sections sampled; and NN is the total number of follicles at each stage counted in the sections sampled. Primordial and primary follicles were counted in every fourth section, with N = f × 0.5 × NN, where N is the number of primordial or primary follicles and 0.5 is a correction factor derived from the absolute primordial and primary follicle counts to avoid double counting of the same follicle. Follicles at different stages were enumerated in five ovaries per experimental group and by two independent counters blinded to sample exposure group.
Follicle Morphometric Analyses
The diameters and areas of follicles at all stages, as well as the widths of the granulosa and theca cell layers in antral follicles, were measured in 3–10 follicles per stage per ovary and in five ovaries per experimental group using ImageJ software [38]. Diameters were measured consistently in sections with a visible oocyte and were recorded as the longest distance along a straight line between opposite follicle edges that passes through the oocyte. Area measurements included the theca cell layer. Determinations of the granulosa and theca compartment thicknesses were done following the same criteria as those applied for diameter measurements.
Protein Localization by Immunohistochemistry
Phospho-histone H2A.X (γ-H2AX) and proliferating cell nuclear antigen (PCNA) immunoreactive proteins were localized in paraffin cross-sections of ovarian tissues. The retrieval of antigens was done on deparaffinized and hydrated sections by boiling the slides in 0.01 M citrate buffer (pH 6.0) at 95°C for 10 min. Immunohistochemical reactions were done using VECTASTAIN ABC Systems (Vector Laboratories) following the manufacturer's recommendations. Tissues were incubated overnight at 4°C with 4 μg/ml of a mouse monoclonal PCNA antibody (PC10; ab29; Abcam) or with 2 μg/ml of a rabbit monoclonal γ-H2AX antibody (9718; Cell Signaling). Negative controls were done by replacing the first antibody with mouse immunoglobulin (Ig) G2A (ab91361; Abcam) for the PCNA or with the diluent for γ-H2AX. ImmPACT NovaRED Peroxidase (HRP) Substrate (Vector Laboratories) was used to detect peroxidase activity, and hematoxylin was used to counterstain nuclei. Digital images were obtained at 40× magnification (100-μm scale bar) with a Leica DM LB2 microscope and an Infinity 3 camera (Lumenera Corporation). Quantification of PCNA and γ-H2AX positively stained granulosa cells was done by counting NovaRed positively stained granulosa cells in antral follicles, as previously described [39], using ImageJ software and its plugin for color deconvolution [38]. The number of NovaRed positively stained cells over the area of three fields per follicle was calculated. Three to ten follicles per ovary were analyzed from three females per experimental group.
Quantification of Serum Hormone Levels
Steroid hormones, including pregnenolone, progesterone, 17-OH pregnenolone, 17-OH progesterone, dihydroepiandrosterone, androstenedione, testosterone, and dihydrotestosterone (DHT), were measured in 100-μl serum samples by liquid chromatography with dual mass spectrometry (operated by the company OpAns LLC) using an Agilent 1200 capillary high-performance liquid chromatography system interfaced to an Agilent Triple Quad LC/MS (model 6430). Serum levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were measured on the Luminex 2000IS platform using the Milliplex rat pituitary panel (Millipore) and estradiol by radioimmunoassay (Siemens Medical Solutions Diagnostics) at the Ligand Assay and Analysis Core Laboratory (Center for Research in Reproduction, University of Virginia). Rat anti-Mullerian hormone (AMH) ELISA kits (Cusabio Life Sciences) were used to quantify serum levels of AMH. Quantification of hormone and gonadotropin levels was done in 16–21 serum samples per experimental group.
Protein Quantification by Western Blots
Total protein extracts from whole ovarian tissue samples were obtained by sonication in radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1% NP-40 substitute, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris at pH 7.5). Aliquots of 10 μg of protein extract were separated by electrophoresis in 12% SDS-PAGE and transferred to Amersham Hybond-P hydrophobic polyvinyl difluoride membranes (GE Healthcare) according to the manufacturer's instructions. The presence of proteins on membranes was assessed using Ponceau S staining (Sigma-Aldrich). The blots were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS) single strength for 1 h at room temperature. Blots were probed with 1 μg/ml of a rabbit polyclonal catalase (CAT) antibody (H-300; sc-50508; Santa Cruz Biotechnology), with 5 μg/ml of a rabbit polyclonal superoxide dismutase 1 (SOD1) antibody (Cu/Zn SOD ADI-SOD-100-D; Enzo Lifesciences) for 1 h at room temperature or with 0.6 μg/ml of the mouse monoclonal PCNA antibody overnight at 4°C. After washing in TBS single strength with 0.1% Tween-20, membranes were incubated with ECL anti-rabbit IgG horseradish peroxidase (HRP)-linked donkey whole antibody (CAT and SOD1; GE Healthcare) or with ECL anti-mouse IgG HRP-linked sheep whole antibody (PCNA; GE Healthcare) for 1 h at room temperature. HeLa whole-cell lysate (sc-2200; Santa Cruz Biotechnology) was used as a positive control to validate primary antibody specificity. Immunoreactive bands were detected with Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare) and visualized on Amersham Hyperfilm ECL (GE Healthcare) using an ImageQuant LAS 500 reader (GE Healthcare Life Sciences). The intensities of the bands stained with ECL and Ponceau S were quantified using ImageJ software [38] to estimate the amounts of SOD1, CAT, and PCNA proteins relative to the total protein (Ponceau S) as previously described [40, 41].
Gene Expression Quantification
Total RNA extraction from 30 mg of ovary tissue was done using the RNeasy Plus Mini Kit (Qiagen) following the manufacturer's recommendations. Total RNA quantity and quality were determined using a NanoDrop 2000 (Thermo Scientific). Complementary DNA was synthesized from 1 μg of total RNA using the SuperScript VILO MasterMix (Invitrogen, Life Technologies). Custom TaqMan Array Plates (Life Technologies) were designed to quantify expression of 40 genes specifically selected based on their roles in ovarian functions (Supplemental Table S1; Supplemental Data are available online at www.biolreprod.org). Briefly, 2 μl of cDNA diluted 1:10 (v/v) in RNase-free water, 5 μl of TaqMan Fast Advanced Master Mix (Life Technologies), and 3 μl of RNase-free water were mixed to complete a total reaction volume of 10 μl, which was pipetted into PCR Array plates primed with TaqMan probes designed for rat DNA sequences. Amplifications were done in a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific) with thermal conditions as follow: 50°C for 2 min, 95°C for 20 sec, and 40 cycles of 95°C for 3 sec and 60°C for 30 sec. Quantification of superoxide dismutase 1 (Sod1) and catalase (Cat) mRNAs was done using RT2 qPCR Primer Assays for Rat Sod1 (NM_017050; PPR43506A; Qiagen) and for Cat (NM_012520; PPR42937A; Qiagen), and RT2 SYBRGreen qPCR Mastermix (Qiagen). The total reaction volume of 20 μl was comprised of 2 μl of cDNA diluted 1:10 (v/v), 1 μl of RT2 qPCR Primer, 10 μl of Mastermix, and 7 μl of RNase-free H2O. Thermal conditions of the amplification were 95°C for 10 min and 40 cycles of 95°C for 15 sec and 60°C for 1 min. TaqMan Endogenous Control Assays (Life Technologies) enabled the identification of peptidylprolyl isomerase A (Ppia) as the most stable endogenous gene under our conditions. The expression of each gene was quantified in triplicate in four ovarian samples per experimental group. Results were analyzed using Expression Suite Software (Life Technologies) and the comparative Ct method [42].
Statistical Analyses
Statistical analyses were done using SigmaPlot Software (Systat Software). Data were analyzed by one-way ANOVA followed by post hoc Holm-Sidak test to compare the means of parameters measured in treated versus control animals. When tests for assumptions of homogeneity of variance and normality failed, data were log transformed and retested. When homoscedasticity and normality were still not satisfied after this transformation, data were retested using Kruskal-Wallis ANOVA on ranks. For statistical analysis of follicle counts, primary and secondary follicles were combined and categorized as preantral follicles. Follicle numbers were analyzed using Pearson coefficient. The level of significance was set at P < 0.05.
RESULTS
Serum BFR Levels Were Dose-Dependently Increased in Treated Females
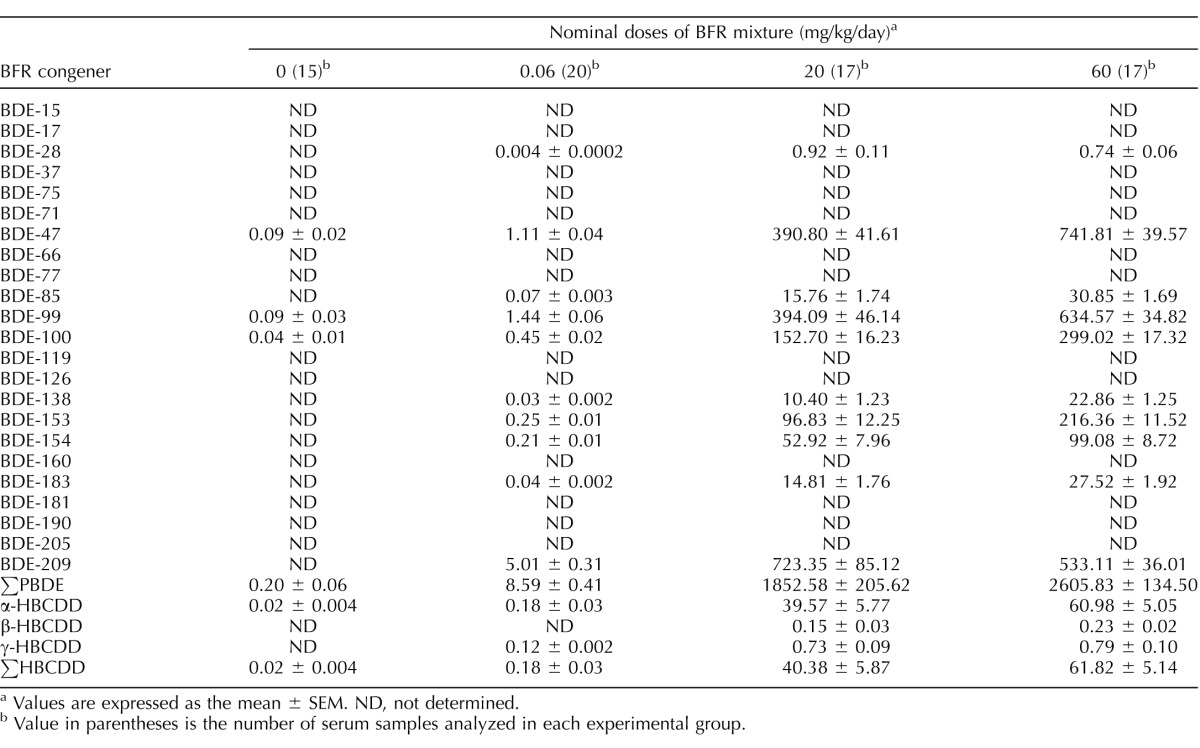
Brominated flame retardants, including PBDE congeners and HBCDD isomers, were detected in serum samples collected on GD 20 from all BFR-exposed females (Table 1). Traces of three PBDE congeners and α-HBCDD were detected in control females. Serum levels of ∑PBDEs and ∑HBCDDs increased in a dose-dependent manner in females exposed to the different dietary mixtures, ranging from 0.2 ± 0.06 to 2605.8 ± 134.5 μg/g lipid and from 0.02 ± 0.004 to 61.8 ± 5.1 μg/g lipid (mean ± SEM), respectively. Concentrations of 13 of the 26 BFR congeners measured were present above the level of detection, with BDE-47, BDE-99, BDE-100, BDE-153, BDE-154, BDE-209, and α-HBCDD being the most abundant in serum collected from females exposed to the BFR mixture. The relative proportion of each congener within each dose group was consistent except for those of BDE-209 and BDE-47, which were dose-dependently decreased and increased, respectively (Supplemental Fig. S1).
TABLE 1.
Serum concentrations of BFR congeners (in μg/g of lipid).
Values are expressed as the mean ± SEM. ND, not determined.
Value in parentheses is the number of serum samples analyzed in each experimental group.
Exposure to an Environmentally Relevant BFR Mixture Increases the Number of Preantral and Antral Follicles in a Dose-Related Manner
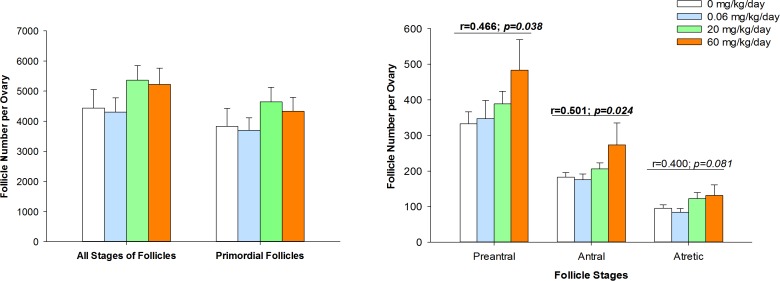
The effects of our BFR mixture on folliculogenesis in females exposed before mating and during gestation were evaluated by estimating the numbers of follicles at different stages of folliculogenesis in the ovaries (Fig. 1). Exposure to the BFR mixture did not significantly affect the total number of follicles per ovary or the number of primordial follicles per ovary (Fig. 1A). In contrast, the numbers of preantral and antral follicles were increased significantly, correlating with increasing doses of the BFR mixture (Fig. 1B). Preantral and antral follicle counts were elevated approximately 1.5-fold in ovaries collected from females exposed to the highest dose of the BFR mixture compared to ovaries from nonexposed females. The number of atretic follicles was not increased significantly by BFR exposure, although there tended to be a positive correlation (Fig. 1B).
FIG. 1.
Increases in follicle number following exposure to an environmentally relevant BFR mixture. The total number of follicles and the number of primordial follicles (A) and of preantral, antral, and atretic follicles (B) were estimated in serial sections obtained from ovaries. Values represent the mean ± SEM (n = 5). r is the Pearson correlation coefficient and p its P-value.
Exposure to BFRs Affects Antral Follicle Morphology
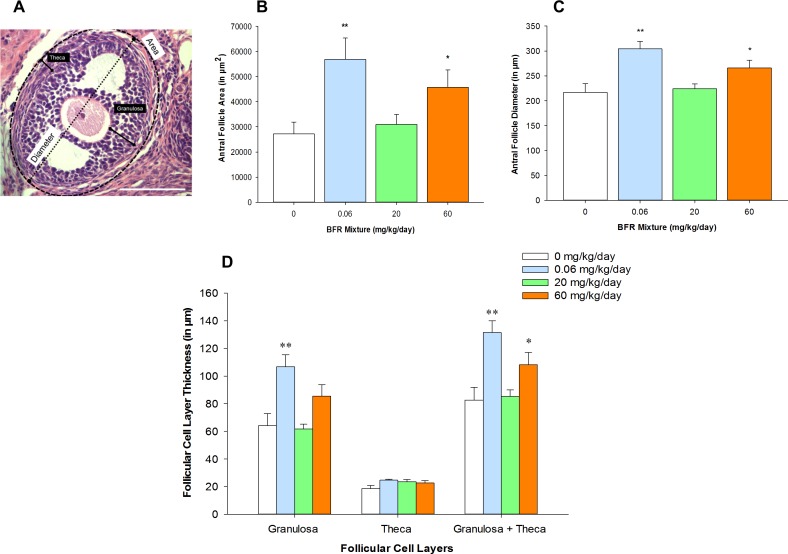
To determine if follicle growth was disrupted by exposure to BFRs, we measured follicular diameters and areas in cross-sections of ovaries (Fig. 2A). The size of primordial and preantral follicles was comparable in ovaries collected from control and BFR-exposed females (Supplemental Fig. S2). However, the diameters and areas of the antral follicles were increased significantly in the treatment groups that received the lowest and the highest doses of the BFR mixture; no effects were observed at the medium dose (Fig. 2, B and C). To elucidate whether this effect on antral follicle growth was a result of the thickening of follicular cell layers, the width of the granulosa and/or theca cell layers was measured. The thickness of this follicular cell layer was increased significantly at the lowest and the highest doses of the BFR mixture (Fig. 2D). Significant effects on the thickness of the granulosa cell layer, but not on the theca cell layer, were also observed in the ovaries collected from females exposed to the lowest dose of the BFR mixture; a tendency toward an increase was found at the highest dose (P = 0.067). Our results reveal that chronic exposure to the BFR mixture increases antral follicle size by acting on the granulosa cell compartment.
FIG. 2.
Increase in antral follicle size associated with exposure to an environmentally relevant BFR mixture. The diameter and area of the granulosa and theca cell compartments in a rat antral follicle are represented (A). Follicle areas (B), diameters (C), and thickness of follicular cell layers (D) were measured in at least three antral follicles per ovary. Values represent the mean ± SEM (n = 5). *P < 0.05, **P < 0.01. Bar = 100 μm.
The expression of a number of genes involved at different stages of folliculogenesis was determined to investigate the mechanism by which exposure to BFRs affects follicle growth (Supplemental Table S2). No significant differences in the expression of a number of the genes that are critical for primordial follicle activation and transition into primary follicles were observed among ovaries collected from control and BFR-exposed females; these genes included Pten, Foxo3a, Sohlh1, Kitlg, Notch2, Lfng, Bax, Bcl2, Ngf, Mis, Ahr, and Arnt [43]. Genes essential for antral follicle growth, including Gdf9, Bmp4, Bmp7, Igf1, Ccnd2 Rxfp2, Ahr, Arnt, Rarres, and Cmlkr1 [44], were also not differentially expressed in ovaries from control compared with BFR-exposed females. In addition, neither the ovarian expression of Mullerian-inhibiting substance (Mis) (Supplemental Table S2) nor the serum levels of the hormone (AMH) (Supplemental Fig. S3), a critical regulator of antral follicle growth [45, 46], were affected by exposure to BFRs.
Exposure to BFRs Influences Expression of Specific Genes in Ovaries
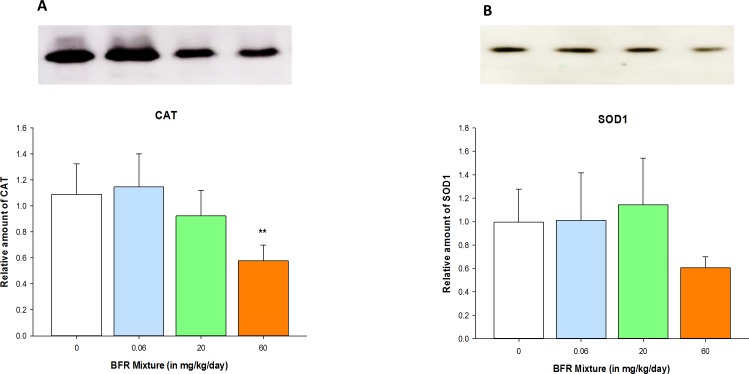
The expression of genes specific to follicular atresia was quantified in ovaries collected from nonexposed and exposed females to assess whether the BFR mixture affected these markers of follicular fate. BFR exposure did not affect the expression of Bax, Bcl2, or Casp2, genes that regulate apoptosis, or of Cat and Sod1, genes that encode for two antioxidant enzymes (Supplemental Table S2). However, CAT protein expression was decreased in the ovaries of females exposed to the highest BFR treatment; there tended to be a decrease in SOD1 protein expression (P = 0.094, ANOVA) (Fig. 3 and Supplemental Fig. S4). These data suggest that the protective system against oxidative stress may be reduced in BFR-exposed ovaries. Because a shift in redox homeostasis may result in an increase in reactive oxygen species (ROS) and, thus, in DNA damage, we performed immunolocalization of γ-H2AX and PCNA, markers of DNA double-strand breaks and DNA repair, respectively, in ovarian sections. Both γ-H2AX and PCNA proteins were localized specifically to follicular cells; however, neither their distribution nor the number of positively stained follicular cells were altered in ovaries from exposed females compared to control females (Supplemental Figs. S5 and S6). The PCNA protein content of ovaries was also not affected by exposure to BFRs (Supplemental Fig. S6, C and D).
FIG. 3.
Reduction of antioxidant enzyme expression in ovaries collected from females exposed to the BFR mixture. The relative amounts of CAT (60 kDa; A) and SOD1 (16 kDa; B) proteins were analyzed in ovarian lysate samples by Western blotting and quantified by densitometry. Values represent the mean ± SEM (n = 4). **P < 0.01.
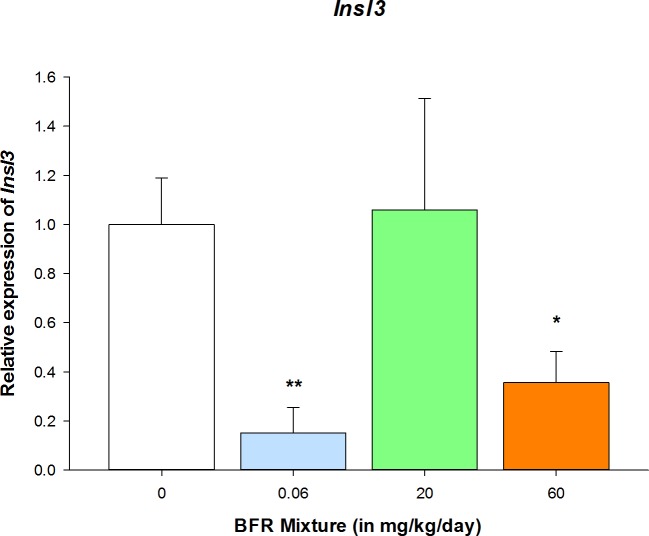
Insulin-like factor 3 (INSL3) plays a major role in intrafollicular signaling between the oocyte and the theca and granulosa cell compartments, leading to regulation of granulosa cell proliferation and growth of antral follicles [47]. Exposure to our BFR mixture significantly reduced Insl3 expression in ovaries collected from females exposed to the lowest and the highest doses in comparison with control group (Fig. 4).
FIG. 4.
Insulin-like factor 3 expression was significantly reduced by BFRs. Expression of the Insl3 gene was quantified in ovarian samples by quantitative PCR, and its expression was shown to be significantly downregulated at the lowest and the highest doses of the BFR mixture. Values represent the mean ± SEM (n = 4). *P < 0.05, **P < 0.01.
Exposure to BFRs Disrupts Ovarian Steroidogenesis
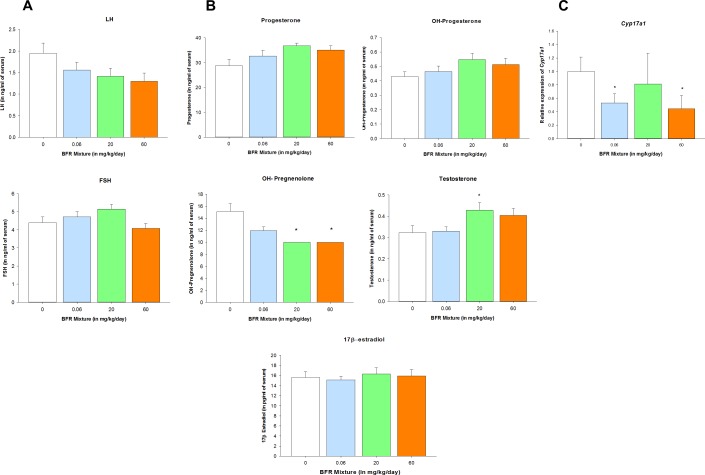
Circulating levels of the gonadotropins FSH and LH were analyzed to determine whether the BFR-induced alterations in folliculogenesis result from perturbations on the hypothalamic-pituitary-ovarian axis (Fig. 5A). No statistically significant differences in the serum concentrations of LH or FSH were observed; however, the concentration of LH tended to decrease, whereas the concentration of FSH tended to increase in serum samples collected from females exposed to BFRs. The ovarian expression of FSH and LH receptors (Fshr and Lhcgr, respectively) was stable among all the experimental groups (Supplemental Table S2).
FIG. 5.
Exposure to BFRs significantly altered ovarian steroidogenesis. Concentrations of FSH and LH (A) and of progesterone, 17-OH-progesterone, 17-OH-pregnenolone, testosterone, and 17β-estradiol in serum (B) were measured in serum samples (n = 14–21). Levels of 17-OH-pregnenolone were below the level of detection in the 20- and 60-mg/kg/day BFR treatment groups. The analysis was done by reporting the value corresponding to the lowest level of detection (10 ng/ml) for 17-OH-pregnenolone as measurements obtained for these two doses. Thus, SEM equals zero, and no error bars for these two sets of samples were represented in the corresponding graph. 17α-Hydroxylase (Cyp17a1) gene expression was quantified by quantitative PCR in ovarian lysate (n = 4) (C). Values represent the mean ± SEM. *P < 0.05.
Ovarian steroidogenesis, the direct upstream regulator of folliculogenesis, was evaluated for potential BFR-induced disruptions (Fig. 5B). A significant decrease in serum concentrations of 17-OH-pregnenolone was observed in females exposed to the 20- and 60-mg/kg/day doses of the BFR mixture. Progesterone concentrations tended to increase in a dose-dependent manner (P = 0.083), and 17-OH-progesterone remained unaffected (P = 0.206) by BFR exposure. No effects on circulating levels of 17β-estradiol were observed. However, androgen synthesis was clearly impacted by BFRs, as revealed by an increase in testosterone at the two higher doses, with statistical significance in the 20-mg/kg/day BFR treatment group. Exposure to BFRs did not modify the expression of steroid hormone receptors (Esr1, Esr2, or Ar) or the expression of steroidogenic enzymes (Star, Tspo, Cyp11a1, Hsd3ß1, Hsd17ß1, Srd5a1, or Cyp19) (Supplemental Table S2). However, the expression of 17α-hydroxylase (Cyp17a1), the rate-limiting enzyme for androgen synthesis, was significantly downregulated in ovaries collected from females exposed to the lowest and the highest doses of the BFR mixture (Fig. 5C).
DISCUSSION
The current study provides clear evidence that steroidogenesis and ovarian folliculogenesis are disrupted in female rats by exposure to an environmentally relevant BFR mixture before mating and during gestation. This mixture reflects the type and the relative proportions of BFRs detected in North American house dust.
Increasing concentrations of PBDE and HBCDD congeners were found in serum samples collected from females exposed to increasing doses of the BFR mixture, confirming exposure. The relative proportion of PBDE congeners detected in serum increased in proportion to the increases in treatment dose for each experimental group with the exception of BDE-209 and BDE-47 [29]. Such discrepancies in the relative distribution of BDE-209 and BDE-47 congeners between the different doses of the same BFR mixture in the diet and in serum samples were described previously in male Sprague-Dawley rats [28]. The effects we observed in our high-dose treatment group (e.g., increased preantral and antral follicles and increased CAT expression) are consistent with a dose–dependent response to an increase in congener concentrations. Other responses, such as the U- or inverted U-shaped responses we observed for follicle area, Insl3 expression, or testosterone concentration, do not correlate with changes in the PBDE or HBCDD congeners.
Significant effects were observed on ovarian morphology and physiology in animals exposed to both the lowest and the highest doses, but not to the middle dose, of our environmentally relevant BFR mixture. This observation is of potential human significance because adverse effects were observed in the environmentally relevant low-dose treatment group that may reflect human exposure. Such a nonmonotonic, U-shaped dose-response pattern has been observed previously for other endocrine-disrupting chemicals [48]. In addition, this same BFR mixture also produced a nonlinear dose response in adult male rats with thyroid gland morphology as the endpoint [28], suggesting that this mixture has complex actions on multiple endocrine-dependent pathways.
Here, we show that the exposure of female rats to a BFR mixture affects folliculogenesis, with significant effects on follicle number and size. These effects on folliculogenesis were not accompanied by changes in estrous cyclicity, fecundity, or litter size [29] (Supplemental Table S3). In rodents, folliculogenesis lasts approximately 60 days [49–51]. The initial recruitment of primordial follicles and the development of primary to preantral follicles takes place during the first 55 days and is gonadotropin-independent [44]. Conversely, the cyclic phase, during which the development of preantral follicles into preovulatory follicles occurs, lasts 2–3 days [49–51]. In our experimental design, BFR exposure started 2–4 wk before mating and continued until GD 20. During the first 2 wk of gestation in rats, gonadotropin levels are low [52, 53] and the cyclic phase of folliculogenesis is silenced, to resume during the last week of gestation concomitant with a rise in FSH in preparation for a postpartum estrus [52–54]. Initial recruitment of primordial follicles is likely to occur throughout gestation as it is gonadotropin-independent. Our data suggest that BFRs target initial follicular recruitment because preantral and antral follicle numbers were increased in a dose-dependent manner.
The significant decrease in expression of the antioxidant enzyme CAT suggests a reduction in the metabolic inactivation of ROS and a decrease in cellular protection against oxidative stress in ovaries collected from females exposed to the highest dose of the BFR mixture. Follicular atresia may be mediated by oxidative stress [55, 56]; CAT has been shown to play an important role in protecting against oxidative stress in rat ovaries [57]. In addition, CAT is a biomarker of age-dependent oxidative stress in human granulosa cells [58]. Evidence indicates that PBDEs, specifically BDE-47 and BDE-99, induce oxidative stress in tissue samples or cells [59–69]. Overproduction of ROS induced by exposure to different BFR congeners was reported in rat liver [63, 64] and in cell culture models, such as human urothelial cells [65] or embryonic kidney cells [66]. Intracellular levels of glutathione were increased in the HTR-8/SVneo human first-trimester extravillous trophoblast cell line following BDE-47 treatment [67], and the oxidized glutathione (GSSG):reduced glutathione (GSH) ratio was significantly increased in rat brain following BDE-99 treatment [68]. The antioxidant enzymes CAT and SOD were downregulated by exposure to BDE-99 in rat brain [68, 69]. To our knowledge, our results are the first to suggest that BFRs induce an oxidative stress response in the ovary. Interestingly, three ovotoxic agents, 4-vinylcyclohexene diepoxide (VCD), methoxychlor (MXC), and 7,12-dimethylbenz[a]anthracene (DMBA), trigger primordial follicle activation in an apparent compensation for the accelerated, toxicant-induced depletion of antral follicles, thus leading to premature ovarian failure [70–72]. Antral follicle atresia induced by MXC results from downregulation of antioxidant enzymes, including SOD1 and CAT, and from an increased level of ROS [73, 74]. This finding is also consistent with a recent model that describes a functional link between the dynamics of primordial follicle depletion and the selection of the antral follicles that develop into ovulatory follicles [75]. Thus, BFR-induced disruption of protective mechanisms against ROS in follicles may lead to increased follicular recruitment as a mechanism of compensation for their adverse effects on antral follicle health.
Our results demonstrate that exposure to an environmentally relevant BFR mixture disrupts androgen synthesis. Interestingly, the increase in serum testosterone levels we observed after BFR exposure was accompanied by the downregulation of ovarian Cyp17a1 gene expression. Thus, we found an inhibition of androgen synthesis in the ovary in concert with an elevation of circulating androgen concentrations. In rats, the placenta, rather than the ovaries or the adrenals, is the major site of androgen synthesis; serum levels of androgen reach a peak at GD 20 and subsequently fall until parturition [76–78]. PBDE congeners were detected previously in rat [79] and human [80, 81] placental samples. In rat placental samples, aromatase activity was inhibited and vasoconstriction was induced by PBDEs [79, 82]. Therefore, the increased serum levels of testosterone observed in the present study may be due to the inhibition of aromatase in the placenta by our BFR mixture.
Exposure to a mixture of BFRs induced significant antral follicle growth, specifically increasing the thickness of the granulosa cell layer. Androgen synthesis and signaling are critical to ovarian development and function [83]. Testosterone and DHT stimulate primordial follicle activation in the mouse [84]; mouse preantral follicle development is enhanced by androgen treatment, with rapid granulosa cell proliferation and amplified responsiveness to FSH [85]. Increased numbers and the enlargement of follicles are also observed in rat polycystic ovary syndrome models that are generated by creating a condition of hyperandrogenism induced by the administration of DHT or letrozole, an aromatase inhibitor [86]. An increase in follicular wall thickness resulting from the formation of a vascularized and luteinized granulosa layer was observed in letrozole-treated females [86]. Enhanced vascularization of the granulosa cell layer might explain our observation that exposure to BFRs induces an increase in granulosa cell thickness independently of granulosa cell proliferation. Concomitant with this effect on antral follicle growth, exposure to BFRs downregulated ovarian Insl3 gene expression. INSL3 was initially reported to be a critical factor in androgen-regulated testicular descent in men [87]; endocrine disrupters have been reported to target INSL3, leading to an increase in abnormal testis development such as cryptorchidism [88, 89]. In females, INSL3 is important for preantral to antral follicle growth, granulosa cell proliferation, and corpus luteum maintenance [47, 90, 91]. INSL3 is expressed in the theca cell layer; it is regulated by and regulates androgen synthesis in a positive autoregulatory loop [47, 90, 92]. Downregulation of Insl3 gene expression is consistent with downregulation of Cyp17a1 expression in the ovary. This may indicate that exposure to BFRs causes an increase in the production of androgens, specifically of testosterone, in a site other than the ovary (e.g., the placenta) and that these androgens induce an increase in granulosa cell thickness and follicle enlargement.
Although previous studies have evaluated the effects of exposure to individual BFR congeners or commercial mixtures on the ovarian functions of female offspring [25, 27], the present study is the first, to our knowledge, to investigate the impact of exposing adult female rats before mating and during gestation to a BFR mixture. Estrous cyclicity was not affected by exposure to this BFR mixture over the premating period [29] (Supplemental Table S3). Litter sizes were normal among the different experimental groups [29] (Supplemental Table S3), suggesting that the formation of preovulatory follicles and ovulation were not disrupted by BFRs during the 2–4 wk of exposure. The fact that significant effects on folliculogenesis and steroidogenesis were observed in the dam at the end of gestation suggests that a longer window of exposure to BFRs may be required for these adverse effects to be manifested. We do not rule out the possibility that the observed effects are dependent on the pregnant status of the dam; further experiments involving nonpregnant rats could clarify this point. However, our results show clearly that exposure to BFRs before the mating period and during gestation affects the formation of the antral follicles that will generate mature oocytes for ovulation during the postpartum estrus and impacts maternal steroidogenesis. Effects on maternal steroidogenesis may disrupt fetal ovarian development—namely, germ cell nest formation and primordial follicle assembly. These two processes are sensitive to steroid hormone levels and are critical to the establishment of the ovarian reserve [93]. Such conditions may have detrimental consequences on ovarian function during adulthood [94].
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge Mr. Donald Demers, Animal Resources Division Health Canada, for food pelleting and Dr. Chunwei Huang, Lydia Goff, and Marie-Eve Ruest, McGill University, for their assistance.
Footnotes
Supported by grant RHF100625 from the Canadian Institutes of Health Research (CIHR) Institute for Human Development, Child, and Youth Health. R.G.B. is the recipient of an NSERC Create award from the Réseau Québécois en Reproduction and PLCL of a Fonds de recherche du Québec–Santé fellowship. B.R. and B.F.H. are James McGill Professors. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) grant U54-HD28934.
REFERENCES
- Segev O, Kushmaro A, Brenner A. Environmental impact of flame retardants (persistence and biodegradability) Int J Environ Res Public Health. 2009;6:478–491. doi: 10.3390/ijerph6020478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besis A, Samara C. Polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments—a review on occurrence and human exposure. Environ Pollut. 2012;169:217–229. doi: 10.1016/j.envpol.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Johnson-Restrepo B, Kannan K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere. 2009;76:542–548. doi: 10.1016/j.chemosphere.2009.02.068. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Sjödin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ Health Perspect. 2012;120:1049–1054. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XM, Bennett DH, Moran RE, Sjodin A, Jones RS, Tancredi DJ, Tulve NS, Clifton MS, Colon M, Weathers W, Hertz-Picciotto I. Polybrominated diphenyl ether serum concentrations in a Californian population of children, their parents, and older adults: an exposure assessment study. Environ Health. 2015;14:23. doi: 10.1186/s12940-015-0002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnerud PO. Brominated flame retardants as possible endocrine disrupters. Int J Androl. 2008;31:152–160. doi: 10.1111/j.1365-2605.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Stapleton HM, Mukherjee B, Hauser R, Meeker JD. Associations between brominated flame retardants in house dust and hormone levels in men. Sci Total Environ. 2013;445–446:177–184. doi: 10.1016/j.scitotenv.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk ME, Persky VW, Imm P, Knobeloch L, Chatterton R, Anderson HA. Hormone disruption by PBDEs in adult male sport fish consumers. Environ Health Perspect. 2008;116:1635–1641. doi: 10.1289/ehp.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Johnson PI, Camann D, Hauser R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci Total Environ. 2009;407:3425–3429. doi: 10.1016/j.scitotenv.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Stapleton HM. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ Health Perspect. 2010;118:318–323. doi: 10.1289/ehp.0901332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelouahab N, Ainmelk Y, Takser L. Polybrominated diphenyl ethers and sperm quality. Reprod Toxicol. 2011;31:546–550. doi: 10.1016/j.reprotox.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Akutsu K, Takatori S, Nozawa S, Yoshiike M, Nakazawa H, Hayakawa K, Makino T, Iwamoto T. Polybrominated diphenyl ethers in human serum and sperm quality. Bull Environ Contam Toxicol. 2008;80:345–350. doi: 10.1007/s00128-008-9370-4. [DOI] [PubMed] [Google Scholar]
- Small CM, DeCaro JJ, Terrell ML, Dominguez C, Cameron LL, Wirth J, Marcus M. Maternal exposure to a brominated flame retardant and genitourinary conditions in male offspring. Environ Health Perspect. 2009;117:1175–1179. doi: 10.1289/ehp.0800058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main KM, Kiviranta H, Virtanen HE, Sundqvist E, Tuomisto JT, Tuomisto J, Vartiainen T, Skakkebaek NE, Toppari J. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspect. 2007;115:1519–1526. doi: 10.1289/ehp.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanck HM, Marcus M, Tolbert PE, Rubin C, Henderson AK, Hertzberg VS, Zhang RH, Cameron L. Age at menarche and Tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology. 2000;11:641–647. doi: 10.1097/00001648-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Chen A, Chung E, DeFranco EA, Pinney SM, Dietrich KN. Serum PBDEs and age at menarche in adolescent girls: analysis of the National Health and Nutrition Examination Survey 2003–2004. Environ Res. 2011;111:831–837. doi: 10.1016/j.envres.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassinari R, Mancini FR, Mantovani A, Busani L, Maranghi F. Pilot study on the dietary habits and lifestyles of girls with idiopathic precocious puberty from the city of Rome: potential impact of exposure to flame retardant polybrominated diphenyl ethers. J Pediatr Endocrinol Metab. 2015;28:1369–1372. doi: 10.1515/jpem-2015-0116. [DOI] [PubMed] [Google Scholar]
- Harley KG, Marks AR, Chevrier J, Bradman A, Sjodin A, Eskenazi B. PBDE concentrations in women's serum and fecundability. Environ Health Perspect. 2010;118:699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Altshul L, Cramer DW, Missmer SA, Hauser R, Meeker JD. Serum and follicular fluid concentrations of polybrominated diphenyl ethers and in-vitro fertilization outcome. Environ Int. 2012;45:9–14. doi: 10.1016/j.envint.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legler J. New insights into the endocrine disrupting effects of brominated flame retardants. Chemosphere. 2008;73:216–222. doi: 10.1016/j.chemosphere.2008.04.081. [DOI] [PubMed] [Google Scholar]
- Kim YR, Harden FA, Toms LM, Norman RE. Health consequences of exposure to brominated flame retardants: a systematic review. Chemosphere. 2014;106:1–19. doi: 10.1016/j.chemosphere.2013.12.064. [DOI] [PubMed] [Google Scholar]
- van der Ven LT, van de Kuil T, Leonards PE, Slob W, Lilienthal H, Litens S, Herlin M, Hakansson H, Canton RF, van den Berg M, Visser TJ, van Loveren H, et al. Endocrine effects of hexabromocyclododecane (HBCD) in a one-generation reproduction study in Wistar rats. Toxicol Lett. 2009;185:51–62. doi: 10.1016/j.toxlet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Ema M, Fujii S, Hirata-Koizumi M, Matsumoto M. Two-generation reproductive toxicity study of the flame retardant hexabromocyclododecane in rats. Reprod Toxicol. 2008;25:335–351. doi: 10.1016/j.reprotox.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Talsness CE, Shakibaei M, Kuriyama SN, Grande SW, Sterner-Kock A, Schnitker P, de Souza C, Grote K, Chahoud I. Ultrastructural changes observed in rat ovaries following in utero and lactational exposure to low doses of a polybrominated flame retardant. Toxicol Lett. 2005;157:189–202. doi: 10.1016/j.toxlet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Lilienthal H, Hack A, Roth-Härer A, Grande SW, Talsness CE. Effects of developmental exposure to 2,2′,4,4′,5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ Health Perspect. 2006;114:194–201. doi: 10.1289/ehp.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernest SR, Wade MG, Lalancette C, Ma YQ, Berger RG, Robaire B, Hales BF. Effects of chronic exposure to an environmentally relevant mixture of brominated flame retardants on the reproductive and thyroid system in adult male rats. Toxicol Sci. 2012;127:496–507. doi: 10.1093/toxsci/kfs098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger RG, Lefevre PL, Ernest SR, Wade MG, Ma YQ, Rawn DF, Gaertner DW, Robaire B, Hales BF. Exposure to an environmentally relevant mixture of brominated flame retardants affects fetal development in Sprague-Dawley rats. Toxicology. 2014;320:56–66. doi: 10.1016/j.tox.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Karpeta A, Rak-Mardyla A, Jerzak J, Gregoraszczuk EL. Congener-specific action of PBDEs on steroid secretion, CYP17, 17β-HSD and CYP19 activity and protein expression in porcine ovarian follicles. Toxicol Lett. 2011;206:258–263. doi: 10.1016/j.toxlet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Karpeta A, Barc J, Ptak A, Gregoraszczuk EL. The 2,2′,4,4′-tetrabromodiphenyl ether hydroxylated metabolites 5-OH-BDE-47 and 6-OH-BDE-47 stimulate estradiol secretion in the ovary by activating aromatase expression. Toxicology. 2013;305:65–70. doi: 10.1016/j.tox.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Karpeta A, Gregoraszczuk E. Mixture of dominant PBDE congeners (BDE-47, -99, -100 and -209) at levels noted in human blood dramatically enhances progesterone secretion by ovarian follicles. Endocr Regul. 2010;44:49–55. doi: 10.4149/endo_2010_02_49. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol. 2008;42:6910–6916. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- Poon S, Wade MG, Aleksa K, Rawn DF, Carnevale A, Gaertner DW, Sadler A, Breton F, Koren G, Ernest SR, Lalancette C, Robaire B, et al. Hair as a biomarker of systemic exposure to polybrominated diphenyl ethers. Environ Sci Technol. 2014;48:14650–14658. doi: 10.1021/es502789h. [DOI] [PubMed] [Google Scholar]
- Allen JG, McClean MD, Stapleton HM, Webster TF. Critical factors in assessing exposure to PBDEs via house dust. Environ Int. 2008;34:1085–1091. doi: 10.1016/j.envint.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN, Midgley AR., Jr Morphometric analysis of follicular development in the rat. Biol Reprod. 1978;19:597–605. doi: 10.1095/biolreprod19.3.597. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH. Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sysel AM, Valli VE, Nagle RB, Bauer JA. Immunohistochemical quantification of the vitamin B12 transport protein (TCII), cell surface receptor (TCII-R) and Ki-67 in human tumor xenografts. Anticancer Res. 2013;33:4203–4212. [PMC free article] [PubMed] [Google Scholar]
- Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Martinez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010;401:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Gilda JE, Gomes AV. Stain-Free total protein staining is a superior loading control to β-actin for Western blots. Anal Biochem. 2013;440:186–188. doi: 10.1016/j.ab.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438–464. doi: 10.1210/er.2008-0048. [DOI] [PubMed] [Google Scholar]
- Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- Sahambi SK, Visser JA, Themmen AP, Mayer LP, Devine PJ. Correlation of serum anti-Mullerian hormone with accelerated follicle loss following 4-vinylcyclohexene diepoxide-induced follicle loss in mice. Reprod Toxicol. 2008;26:116–122. doi: 10.1016/j.reprotox.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Xue K, Kim JY, Liu JY, Tsang BK. Insulin-like 3-induced rat preantral follicular growth is mediated by growth differentiation factor 9. Endocrinology. 2014;155:156–167. doi: 10.1210/en.2013-1491. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- Fortune JE. Ovarian follicular growth and development in mammals. Biol Reprod. 1994;50:225–232. doi: 10.1095/biolreprod50.2.225. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Greenwald GS. Ovarian follicular development and pituitary FSH and LH content in the pregnant rat. Endocrinology. 1966;79:572–578. doi: 10.1210/endo-79-3-572. [DOI] [PubMed] [Google Scholar]
- Nagai S, Kunii H. Gonadotrophic fluctuation in the rat during pregnancy, labor and early stage of lactation. Tohoku J Exp Med. 1972;108:199–206. doi: 10.1620/tjem.108.199. [DOI] [PubMed] [Google Scholar]
- Osman P. Morphometric analysis of follicular dynamics in pregnant and pseudopregnant rats. J Reprod Fertil. 1986;76:11–22. doi: 10.1530/jrf.0.0760011. [DOI] [PubMed] [Google Scholar]
- Luderer U. Ovarian toxicity from reactive oxygen species. Vitam Horm. 2014;94:99–127. doi: 10.1016/B978-0-12-800095-3.00004-3. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012;86:27. doi: 10.1095/biolreprod.111.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parshad RK, Guraya SS. Changes in catalase activity during follicular growth, atresia and luteinization in rat ovary. Indian J Exp Biol. 1993;31:109–111. [PubMed] [Google Scholar]
- Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, Marci R, Pandolfi A, Ragnelli AM, Amicarelli F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol Hum Reprod. 2006;12:655–660. doi: 10.1093/molehr/gal080. [DOI] [PubMed] [Google Scholar]
- Costa LG, Pellacani C, Dao K, Kavanagh TJ, Roque PJ. The brominated flame retardant BDE-47 causes oxidative stress and apoptotic cell death in vitro and in vivo in mice. Neurotoxicology. 2015;48:68–76. doi: 10.1016/j.neuro.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferri S, Caglieri A, Goldoni M, Pinelli S, Alinovi R, Poli D, Pellacani C, Giordano G, Mutti A, Costa LG. Low concentrations of the brominated flame retardants BDE-47 and BDE-99 induce synergistic oxidative stress-mediated neurotoxicity in human neuroblastoma cells. Toxicol In Vitro. 2010;24:116–122. doi: 10.1016/j.tiv.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Blanco J, Mulero M, Domingo JL, Sánchez DJ. Gestational exposure to BDE-99 produces toxicity through upregulation of CYP isoforms and ROS production in the fetal rat liver. Toxicol Sci. 2012;127:296–302. doi: 10.1093/toxsci/kfs082. [DOI] [PubMed] [Google Scholar]
- Shao J, White CC, Dabrowski MJ, Kavanagh TJ, Eckert ML, Gallagher EP. The role of mitochondrial and oxidative injury in BDE 47 toxicity to human fetal liver hematopoietic stem cells. Toxicol Sci. 2008;101:81–90. doi: 10.1093/toxsci/kfm256. [DOI] [PubMed] [Google Scholar]
- Blanco J, Mulero M, Domingo JL, Sanchez DJ. Perinatal exposure to BDE-99 causes decreased protein levels of cyclin D1 via GSK3β activation and increased ROS production in rat pup livers. Toxicol Sci. 2014;137:491–498. doi: 10.1093/toxsci/kft257. [DOI] [PubMed] [Google Scholar]
- Choi JS, Lee YJ, Kim TH, Lim HJ, Ahn MY, Kwack SJ, Kang TS, Park KL, Lee J, Kim ND, Jeong TC, Kim SG, et al. Molecular mechanism of tetrabromobisphenol A (TBBPA)-induced target organ toxicity in Sprague-Dawley male rats. Toxicol Res. 2011;27:61–70. doi: 10.5487/TR.2011.27.2.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Kuester RK, Gallegos A, Sipes IG. Induction of DNA damage in human urothelial cells by the brominated flame retardant 2,2–bis(bromomethyl)-1,3-propanediol: role of oxidative stress. Toxicology. 2011;290:271–277. doi: 10.1016/j.tox.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Cao L, Li F, Lian P, Zhao J. Multiple biomarkers of the cytotoxicity induced by BDE-47 in human embryonic kidney cells. Chemosphere. 2015;126:32–39. doi: 10.1016/j.chemosphere.2015.01.055. [DOI] [PubMed] [Google Scholar]
- Park HR, Loch-Caruso R. Protective effect of nuclear factor E2-related factor 2 on inflammatory cytokine response to brominated diphenyl ether-47 in the HTR-8/SVneo human first trimester extravillous trophoblast cell line. Toxicol Appl Pharmacol. 2014;281:67–77. doi: 10.1016/j.taap.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albina ML, Alonso V, Linares V, Belles M, Sirvent JJ, Domingo JL, Sanchez DJ. Effects of exposure to BDE-99 on oxidative status of liver and kidney in adult rats. Toxicology. 2010;271:51–56. doi: 10.1016/j.tox.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Belles M, Alonso V, Linares V, Albina ML, Sirvent JJ, Domingo JL, Sanchez DJ. Behavioral effects and oxidative status in brain regions of adult rats exposed to BDE-99. Toxicol Lett. 2010;194:1–7. doi: 10.1016/j.toxlet.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Sobinoff AP, Mahony M, Nixon B, Roman SD, McLaughlin EA. Understanding the villain: DMBA-induced preantral ovotoxicity involves selective follicular destruction and primordial follicle activation through PI3K/Akt and mTOR signaling. Toxicol Sci. 2011;123:563–575. doi: 10.1093/toxsci/kfr195. [DOI] [PubMed] [Google Scholar]
- Sobinoff AP, Pye V, Nixon B, Roman SD, McLaughlin EA. Adding insult to injury: effects of xenobiotic-induced preantral ovotoxicity on ovarian development and oocyte fusibility. Toxicol Sci. 2010;118:653–666. doi: 10.1093/toxsci/kfq272. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Sipes IG, Hoyer PB. Initiation of delayed ovotoxicity by in vitro and in vivo exposures of rat ovaries to 4-vinylcyclohexene diepoxide. Reprod Toxicol. 2004;19:71–77. doi: 10.1016/j.reprotox.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci. 2006;93:382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Schuh RA, Fiskum G, Flaws JA. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol Appl Pharmacol. 2006;216:436–445. doi: 10.1016/j.taap.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Monniaux D, Clement F, Dalbies-Tran R, Estienne A, Fabre S, Mansanet C, Monget P. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: what is the link? Biol Reprod. 2014;90:85. doi: 10.1095/biolreprod.113.117077. [DOI] [PubMed] [Google Scholar]
- Gibori G, Chatterton RT, Jr, Chien JL. Ovarian and serum concentrations of androgen throughout pregnancy in the rat. Biol Reprod. 1979;21:53–56. doi: 10.1095/biolreprod21.1.53. [DOI] [PubMed] [Google Scholar]
- Macdonald GJ, Matt DW. Adrenal and placental steroid secretion during pregnancy in the rat. Endocrinology. 1984;114:2068–2073. doi: 10.1210/endo-114-6-2068. [DOI] [PubMed] [Google Scholar]
- Gibori G, Sridaran R. Sites of androgen and estradiol production in the second half of pregnancy in the rat. Biol Reprod. 1981;24:249–256. doi: 10.1095/biolreprod24.2.249. [DOI] [PubMed] [Google Scholar]
- Du P, Li Z, Du L, Zhang H, Zhou Y, Sun W, Xiao X, He Y, Sun B, Yu Y, Chen D. The effects of PBDE-209 exposure during pregnancy on placental ET-1 and eNOS expression and the birth weight of offspring. Int J Dev Neurosci. 2015;43:86–91. doi: 10.1016/j.ijdevneu.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Doucet J, Tague B, Arnold DL, Cooke GM, Hayward S, Goodyer CG. Persistent organic pollutant residues in human fetal liver and placenta from Greater Montreal, Quebec: a longitudinal study from 1998 through 2006. Environ Health Perspect. 2009;117:605–610. doi: 10.1289/ehp.0800205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ruan X, Li Y, Yan M, Qin Z. Polybrominated diphenyl ethers (PBDEs) in aborted human fetuses and placental transfer during the first trimester of pregnancy. Environ Sci Technol. 2013;47:5939–5946. doi: 10.1021/es305349x. [DOI] [PubMed] [Google Scholar]
- Canton RF, Scholten DE, Marsh G, de Jong PC, van den Berg M. Inhibition of human placental aromatase activity by hydroxylated polybrominated diphenyl ethers (OH-PBDEs) Toxicol Appl Pharmacol. 2008;227:68–75. doi: 10.1016/j.taap.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Walters KA. Role of androgens in normal and pathological ovarian function. Reproduction. 2015;149:R193–R218. doi: 10.1530/REP-14-0517. [DOI] [PubMed] [Google Scholar]
- Yang JL, Zhang CP, Li L, Huang L, Ji SY, Lu CL, Fan CH, Cai H, Ren Y, Hu ZY, Gao F, Liu YX. Testosterone induces redistribution of forkhead box-3a and down-regulation of growth and differentiation factor 9 messenger ribonucleic acid expression at early stage of mouse folliculogenesis. Endocrinology. 2010;151:774–782. doi: 10.1210/en.2009-0751. [DOI] [PubMed] [Google Scholar]
- Wang H, Andoh K, Hagiwara H, Xiaowei L, Kikuchi N, Abe Y, Yamada K, Fatima R, Mizunuma H. Effect of adrenal and ovarian androgens on type 4 follicles unresponsive to FSH in immature mice. Endocrinology. 2001;142:4930–4936. doi: 10.1210/endo.142.11.8482. [DOI] [PubMed] [Google Scholar]
- Manneras L, Cajander S, Holmang A, Seleskovic Z, Lystig T, Lonn M, Stener-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–3791. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- Bay K, Andersson AM. Human testicular insulin-like factor 3: in relation to development, reproductive hormones and andrological disorders. Int J Androl. 2011;34:97–109. doi: 10.1111/j.1365-2605.2010.01074.x. [DOI] [PubMed] [Google Scholar]
- Anand-Ivell R, Ivell R. INSL3 as a monitor of endocrine disruption. Reproduction 2014; 147:R87–R95. doi: 10.1530/REP-13-0486. [DOI] [PubMed] [Google Scholar]
- Bay K, Anand-Ivell R. Human testicular insulin-like factor 3 and endocrine disrupters. Vitam Horm. 2014;94:327–348. doi: 10.1016/B978-0-12-800095-3.00012-2. [DOI] [PubMed] [Google Scholar]
- Xue K, Liu JY, Murphy BD, Tsang BK. Orphan nuclear receptor NR4A1 is a negative regulator of DHT-induced rat preantral follicular growth. Mol Endocrinol. 2012;26:2004–2015. doi: 10.1210/me.2012-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanel-Borowski K, Schafer I, Zimmermann S, Engel W, Adham IM. Increase in final stages of follicular atresia and premature decay of corpora lutea in Insl3-deficient mice. Mol Reprod Dev. 2001;58:281–286. doi: 10.1002/1098-2795(200103)58:3<281::AID-MRD6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Glister C, Satchell L, Bathgate RA, Wade JD, Dai Y, Ivell R, Anand-Ivell R, Rodgers RJ, Knight PG. Functional link between bone morphogenetic proteins and insulin-like peptide 3 signaling in modulating ovarian androgen production. Proc Natl Acad Sci U S A. 2013;110:E1426–E1435. doi: 10.1073/pnas.1222216110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Padmanabhan V, Dumesic DA. Contributions of androgen and estrogen to fetal programming of ovarian dysfunction. Reprod Biol Endocrinol. 2006;4:17. doi: 10.1186/1477-7827-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.