Abstract
Oil spills represent an overwhelming carbon input to the marine environment that immediately impacts the sea surface ecosystem. Microbial communities degrading the oil fraction that eventually sinks to the seafloor must also deal with hydrostatic pressure, which linearly increases with depth. Piezosensitive hydrocarbonoclastic bacteria are ideal candidates to elucidate impaired pathways following oil spills at low depth. In the present paper, we tested two strains of the ubiquitous Alcanivorax genus, namely A. jadensis KS_339 and A. dieselolei KS_293, which is known to rapidly grow after oil spills. Strains were subjected to atmospheric and mild pressure (0.1, 5 and 10 MPa, corresponding to a depth of 0, 500 and 1000 m, respectively) providing n-dodecane as sole carbon source. Pressures equal to 5 and 10 MPa significantly lowered growth yields of both strains. However, in strain KS_293 grown at 10 MPa CO2 production per cell was not affected, cell integrity was preserved and PO43− uptake increased. Analysis of its transcriptome revealed that 95% of its genes were downregulated. Increased transcription involved protein synthesis, energy generation and respiration pathways. Interplay between these factors may play a key role in shaping the structure of microbial communities developed after oil spills at low depth and limit their bioremediation potential.
Mineralization of hydrocarbons to non-toxic compounds in marine environments is a process known to occur both in natural and anthropogenically-affected ecosystems1. Despite the fact that hydrocarbons are naturally released into the environment through bio- and geological processes2 oil spills have a major environmental impact. Temperature, pH, nutrient and O2 availability are known to affect the structure of seawater microbial communities3 along with the alkane profile of the spilled oil, thereby determining the long-term impact of oil spills. Another critical factor for oil biodegradation at sea is hydrostatic pressure. Pressure increases about 0.1 MPa every ten meters of seawater, meaning that for instance marine life at 1000 m below the water surface experiences a pressure equal to 10 MPa. Oil spills on surface waters are characterized by the release of hydrocarbons in the form of a thin film, mainly constituted by light, water-soluble hydrocarbons4. Eventually, these compounds dissolve in the water column or remain trapped within immiscible oil components, which become emulsified and are dispersed as small droplets5. Oil presence on the seafloor may be due to sinking, which is influenced by natural and anthropogenic factors as association with particulate6 and use of dispersants during oil spill control operation7, or may result from drilling activities, operation or accidents occurring at deep sea. In the recent Deep Water Horizon spill occurred in the Gulf of Mexico the fraction eventually sinking to the seafloor was estimated to be up to 10% of the total spilled oil, covering an area estimated between 3000–8000 km2 8,9.
Information on microbial oil degradation mechanisms under hydrostatic pressures is scarce10,11,12,13 and focuses quite exclusively on high hydrostatic pressure (from 15 to 50 MPa, correspondent to depths >1500 m). Our hypothesis was that a mild increase in pressure (from 0.1 to 10 MPa) might be sufficient to impose significant stress on cells adapted to atmospheric pressure. Pressure increase in a water body follows the linear equation for vertical pressure variation (Equation 1)
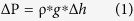 |
where ΔP represents the variation in pressure, ρ is the density of seawater, g is gravity and Δh is the difference in depth. Marine life in the mesopelagic zone (200 to 1000 m) is subjected to mild hydrostatic pressures (2 to 10 MPa). However, the relative pressure increase experienced in this zone is much higher with respect to deeper zones, as pressure doubles 5 times within 800 m. Other critical factors such as pH and salinity are not as remarkably affected by depth as pressure, while temperature varies significantly in this zone although to a lower extent with respect to pressure and depending on latitude and season14.
The study of how microbes residing in the surface of the seawater column respond to mild pressure increases may provide insights on the primary drivers for microbial diversity and their potential function in marine areas under anthropogenically-affected conditions. In the present paper, we focused on the effects exerted by mild pressure increases (0.1, 5 and 10 MPa, corresponding to 0, 500 and 1000 m depth) on two strains belonging to different species of the genus Alcanivorax, namely A. jadensis KS_339 and A. dieselolei KS_293, which were isolated from surface waters of the Mediterranean Sea. As these species are ubiquitous hydrocarbonoclastic marine bacteria detected in a wide range of oil-contaminated environments, from surface to deep sea15, in both seawater and sediments16,17,18, their response to pressure may provide interesting information on actual site environmental constraints following oil spills. Both strains showed a piezosensitive profile, with reduced growth yields under hydrostatic pressures higher than the atmospheric. Cell integrity and activity in A. dieselolei KS_293 was nevertheless preserved despite pressure increases up to 10 MPa and therefore was selected for comparing transcript pathways during growth at 0.1 and 10 MPa.
Results
Growth yields of Alcanivorax species under atmospheric and mild hydrostatic pressure
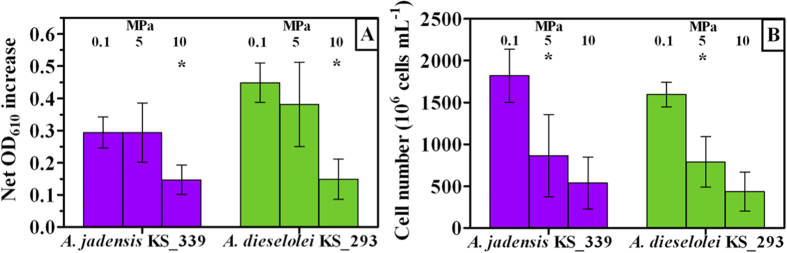
Alcanivorax jadensis KS_339 and A. dieselolei KS_293 were independently incubated at different hydrostatic pressures (0.1, 5 or 10 MPa) providing C12 as sole carbon source. Pressure had a major impact on cell growth, as estimated by optical density (Fig. 1a). At atmospheric pressure (0.1 MPa) A. jadensis had a lower cell density in comparison with A. dieselolei. However, when cells were incubated under the highest pressure (10 MPa) net growth was comparable between the two strains and lower than that observed at 0.1 MPa (P < 0.05) (Fig. 1a). Growth trends estimated by OD were different when cell number was analysed by flow cytometry, as strains were comparable at any given pressure (Fig. 1b). Increases in pressure up to 5 MPa resulted in a significant reduction of cell number (P < 0.05), which was further reduced upon incubation at 10 MPa. Reduction in growth between 0.1 and 10 MPa in both A. jadensis and A. dieselolei was equal to one order of magnitude. Provided that increased hydrostatic pressure would have resulted into a higher O2 solubility, control experiments under ambient pressure were carried out using pure O2 as gas phase (Fig. S2). As no effect on culture growth was detected, the potentially negative impact of increased oxygenation on cell replication was excluded.
Figure 1. Growth of A. jadensis KS_339 (purple) and A. dieselolei KS_293 (green) under atmospheric (0.1 MPa) and mild pressure (5 and 10 MPa).
Bars indicate 95% confidence intervals. A: Net optical density increase; asterisks indicate that values at 10 MPa were significantly lower (P < 0.05) than those observed at 0.1 and 5 MPa. B: Final cell number; asterisks indicate that values at 0.1 MPa were significantly higher (P < 0.05) than those observed at 5 and 10 MPa.
C12 water apparent solubility was determined (Fig. S1) and concentrations changed according to culture conditions and resulting cell yields, being 7.5 g L−1 the amount initially supplied (i.e, 1%, v-v). Detectable C12 in cell suspensions is affected by several phenomena (adsorption to plastic components of the reactor, oil solubility in water, apparent solubilisation by extracellular and transmembrane surfactants, residual C12 concentrations after cell consumption)19. Therefore, analysis of the detectable C12 was only aimed at evaluating its general bioavailability. Lowest C12 concentrations coincided with high cell number (32 and 98 μg L−1 in A. jadensis and A. dieselolei, respectively, at 0.1 MPa, Fig. S1 and Fig. 1b), indicating that access to the carbon source was not limiting. Provided that C12 was the sole carbon source supplied, decreased pH value as a result of CO2 production was considered an indirect measure of C12 degradation. Hence, a final low pH (Fig. 2) was considered as an indication of a high C12 consumption rate. Increased hydrostatic pressure was coincident with a minor change in pH in comparison with control experiments provided with sterile medium (Fig. 2), in agreement with reduced growth rates (Fig. 1b). The reduced amount of CO2 produced per reactor (Fig. S3) is consistent with such a pH increasing trend with pressure (Fig. 2), although it can be assumed that under increased hydrostatic pressure cells actually experienced more acidic pH values than what measured after decompression. In A. jadensis cell activity was increased at 5 MPa but eventually dropped to low values when tested at 10 MPa (CO2 production per cell, Fig. 3a), while A. dieselolei cells showed a constant output irrespective of the pressure applied (Fig. 3a). Thus, in the latter reduced impact on pH variation under pressure was a result of a lower cell number per volume rather than a decrease of the cell respiration rate. Concerning O2 respiration, no significant difference was detected in both strains, although reproducibility was higher when testing A. dieselolei (Fig. 3b). The final amount of O2 transferred to the liquid phase as a result of the higher hydrostatic pressure was also measured (Table S1) and was found to be of the same order of magnitude of cell respiration (Table S2). Strain activity was evaluated by the uptake rate of PO43− (Fig. 3c) whose consumption per cell decreased linearly with pressure in A. jadensis (Fig. 3c), while in A. dieselolei it remained constant (around 25 mg 10−9 cells) and higher than in A. jadensis (P < 0.05). The observed differences between the two strains are in accordance with the intact vs total cell ratio, which was higher for A. dieselolei at any given pressure (P < 0.05, Fig. 4). In A. dieselolei the amount of damaged cells at the highest pressure applied was rather low (about 22% of total cell number, Fig. 4) suggesting that despite the decreased growth rate in this strain some structural or physiological mechanism linked to cell integrity counterbalanced mild hydrostatic pressure.
Figure 2. Decrease in pH value after incubation of A. jadensis KS_339 (purple) and A. dieselolei KS_293 (green) under atmospheric (0.1 MPa) and mild pressure (5 and 10 MPa).
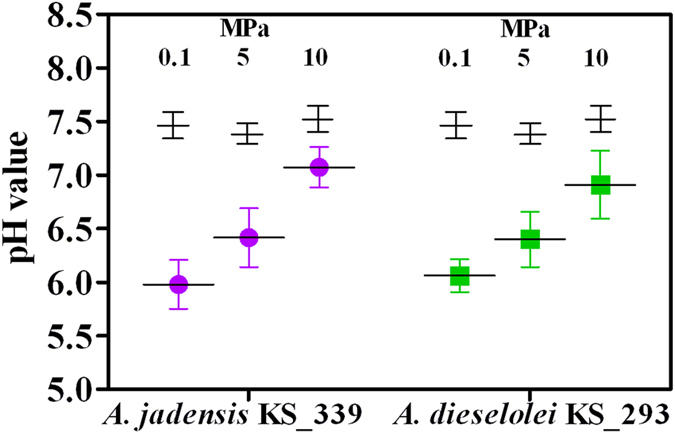
Bars indicate 95% confidence intervals. Black crosses represent sterile controls.
Figure 3. Physiological response in A. jadensis KS_339 (purple) and A. dieselolei KS_293 (green) under atmospheric (0.1 MPa) and mild pressure (5 and 10 MPa).
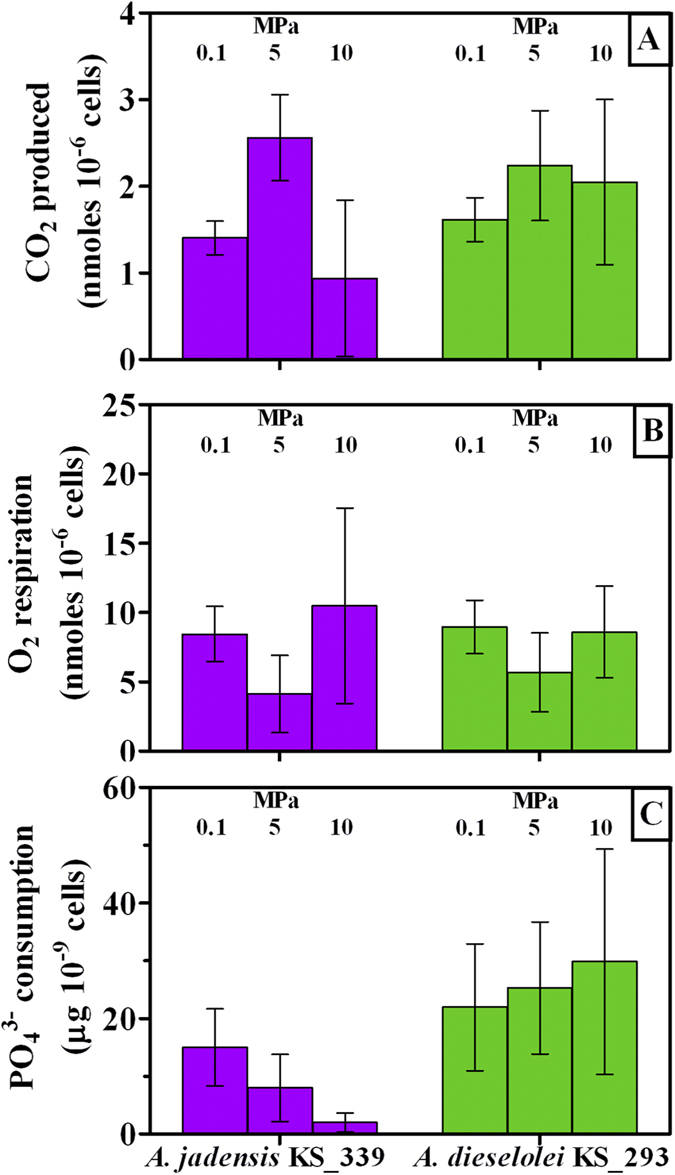
Bars indicate 95% confidence intervals. All values refer to the final cell number and amounts detected at the end of the incubation. (A): CO2 production per cell; (B): O2 respiration per cell; (C): PO43− uptake per cell.
Figure 4. Cell integrity in A. jadensis KS_339 (purple) and A. dieselolei KS_293 (green) under atmospheric (0.1 MPa) and mild pressure (5 and 10 MPa) expressed as percentage of intact cells over total number of cells.
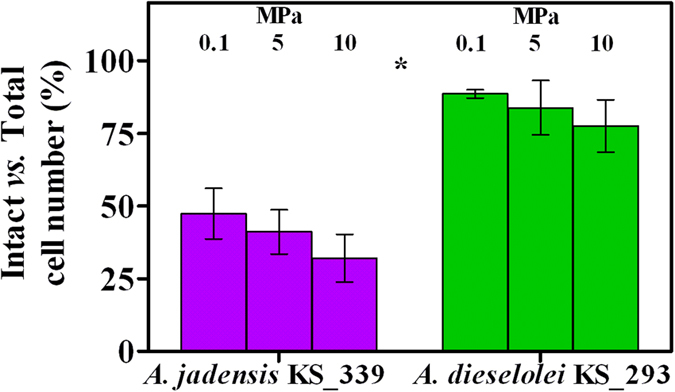
Bars indicate 95% confidence intervals. Asterisk indicates that A. dieselolei KS_293 cells were always significantly higher (P < 0.05) than A. jadensis KS_339 cells at any tested pressure.
Transcriptomic response in A. dieselolei KS_293 at 1 and 10 MPa
To assess the mechanisms determining the observed response to hydrostatic pressure, A. dieselolei KS_293 cells grown under 0.1 and 10 MPa where collected and their transcriptome analyzed. Under 10 MPa the majority of the genes were downregulated as compared to atmospheric pressure (Fig. 5). Gene clusters showing upregulation between 0.1 and 10 MPa (here defined as a log2 fold change higher than 0.50) were only 70 out of 1430 (equal to 4.9% of all SSD gene clusters). This result alone gives a hint of the general impediment imposed over cell metabolism by a pressure of 10 MPa, suggesting that critical metabolic pathways for cell replication and survival were impaired. Clusters of orthologous genes (COG) upregulated under pressure were predominantly associated with translation, ribosomal structure and biogenesis, and energy production and conversion (Fig. 6).
Figure 5. Effect of 4-day incubation at 10 MPa on the gene expression of A. dieselolei KS_293 cells with respect to 0.1 MPa.

Figure 6. Upregulated COG in A. dieselolei KS_293 cells incubated at 10 MPa with respect to expression levels at 0.1 MPa.
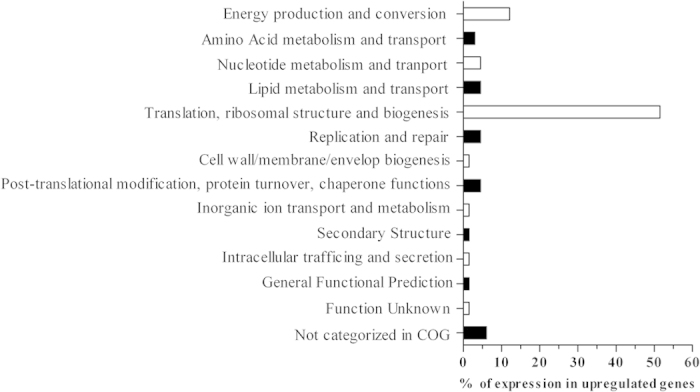
Percentage of expression is normalized on the total number of upregulated gene clusters, as shown in Fig. 5, green bar.
The most represented class of upregulated genes was that associated with ribosomal proteins (28/66, Table 1), in particular genes related with the 50 S subunit (21/28). On the contrary, all RNA ribosomal methyltransferase proteins were downregulated (10/10, Table 1). Genes related with tRNA modification were also upregulated, in particular truB, a tRNA pseudouridine synthase, which was the third most upregulated gene in the whole transcriptome (3.09 log2 FC, Table 2). Translation-related elongation factors were largely upregulated or unaffected (5/6); in particular, one Tu and Ts were upregulated (Table 2). Similarly, post-translational modification proteins such as chaperonins had lower expression (6/8) with the only exception of groEL and groES, which belong to close loci (B5T_03449 and B5T_03450, respectively, Table 2).
Table 1. Ribosomal protein gene expression in A. dieselolei KS_293 cells under 10 and 0.1 MPa.
| Regulation | log2 FC | 10 MPa | 0.1 MPa | Cluster ID | Locus Tag | Description |
|---|---|---|---|---|---|---|
| + | 4.47 | 2536.9 | 114.3 | 1929 | B5T_03774 | 50S ribosomal protein L36 |
| + | 4.12 | 120.0 | 6.9 | 913 | B5T_03782 | 30S ribosomal protein S14 |
| + | 2.52 | 8358.9 | 1461.7 | 334 | B5T_03777 | 50S ribosomal protein L30 |
| + | 2.46 | 2871.8 | 520.6 | 1912 | B5T_03790 | 50S ribosomal protein L22 |
| + | 2.35 | 27.9 | 5.5 | 1800 | B5T_03806 | 50S ribosomal protein L11 |
| + | 2.19 | 809.0 | 177.3 | 1335 | B5T_03781 | 30S ribosomal protein S8 |
| + | 2.17 | 1256.4 | 278.6 | 66 | B5T_03786 | 30S ribosomal protein S17 |
| + | 2.11 | 199.9 | 46.4 | 2176 | B5T_03787 | 50S ribosomal protein L29 |
| + | 1.94 | 651.8 | 169.3 | 808 | B5T_01420 | 50S ribosomal protein L19 |
| + | 1.81 | 117.7 | 33.5 | 2150 | B5T_03778 | 30S ribosomal protein S5 |
| + | 1.58 | 287.3 | 96.2 | 1378 | B5T_03804 | 50S ribosomal protein L10 |
| + | 1.55 | 207.0 | 70.6 | 1281 | B5T_03780 | 50S ribosomal protein L6 |
| + | 1.53 | 201.4 | 69.7 | 180 | B5T_03783 | 50S ribosomal protein L5 |
| + | 1.49 | 42.2 | 15.1 | 2106 | B5T_03779 | 50S ribosomal protein L18 |
| + | 1.42 | 3469.9 | 1300.6 | 1903 | B5T_03803 | 50S ribosomal protein L7/L12 |
| + | 1.19 | 49.2 | 21.6 | 1232 | B5T_02734 | 50S ribosomal protein L32 |
| + | 1.19 | 293.9 | 128.8 | 906 | B5T_03784 | 50S ribosomal protein L24 |
| + | 1.16 | 113.4 | 50.9 | 662 | B5T_03788 | 50S ribosomal protein L16 |
| + | 1.12 | 42.4 | 19.6 | 1059 | B5T_03776 | 50S ribosomal protein L15 |
| + | 1.1 | 302.2 | 140.7 | 1541 | B5T_03789 | 30S ribosomal protein S3 |
| + | 1.03 | 380.9 | 186.7 | 594 | B5T_03987 | 50S ribosomal protein L31 |
| + | 0.96 | 47.0 | 24.2 | 1225 | B5T_03769 | 50S ribosomal protein L17 |
| + | 0.92 | 1029.5 | 543.5 | 2053 | B5T_03202 | 50S ribosomal protein L35 |
| + | 0.88 | 193.8 | 105.1 | 327 | B5T_03728 | 50S ribosomal protein L27 |
| + | 0.75 | 566.0 | 335.5 | 1101 | B5T_00191 | 50S ribosomal protein L28 |
| + | 0.73 | 228.6 | 137.9 | 1759 | B5T_03771 | 30S ribosomal protein S4 |
| + | 0.72 | 734.2 | 446.1 | 848 | B5T_03521 | 50S ribosomal protein L13 |
| + | 0.69 | 190.8 | 118.4 | 1786 | B5T_03855 | 30S ribosomal protein S15 |
| = | 0.42 | 134.5 | 100.4 | 251 | B5T_03794 | 50S ribosomal protein L4 |
| = | 0.35 | 279.4 | 218.7 | 1161 | B5T_03792 | 50S ribosomal protein L2 |
| = | 0.35 | 209.7 | 164.1 | 94 | B5T_03799 | 30S ribosomal protein S7 |
| = | 0.23 | 16.1 | 13.7 | 1356 | B5T_03520 | 30S ribosomal protein S9 |
| = | 0.18 | 764.7 | 672.8 | 258 | B5T_01417 | 30S ribosomal protein S16 |
| = | 0.17 | 107.1 | 94.9 | 1132 | B5T_03725 | 30S ribosomal protein S20 |
| = | 0.1 | 455.0 | 425.9 | 575 | B5T_02974 | 30S ribosomal protein S1 |
| = | 0.1 | 176.3 | 165.0 | 1111 | B5T_03791 | 30S ribosomal protein S19 |
| = | 0.08 | 91.1 | 86.4 | 151 | B5T_03793 | 50S ribosomal protein L23 |
| = | 0.08 | 103.4 | 97.9 | 263 | B5T_01921 | 30S ribosomal protein S2 |
| = | 0.05 | 240.4 | 231.7 | 917 | B5T_00790 | 30S ribosomal protein S6 |
| = | 0.02 | 443.1 | 436.6 | 665 | B5T_00192 | 50S ribosomal protein L33 |
| = | −0.17 | 36.4 | 40.8 | 1365 | B5T_00964 | 30S ribosomal protein S21 |
| = | −0.2 | 255.5 | 293.0 | 1254 | B5T_03772 | 30S ribosomal protein S11 |
| = | −0.22 | 772.1 | 899.1 | 466 | B5T_03796 | 30S ribosomal protein S10 |
| = | −0.31 | 962.3 | 1193.7 | 1397 | B5T_03795 | 50S ribosomal protein L3 |
| = | −0.44 | 11.7 | 15.9 | 610 | B5T_03805 | 50S ribosomal protein L1 |
| = | −0.46 | 4.2 | 5.8 | 1385 | B5T_00793 | 50S ribosomal protein L9 |
| − | −0.53 | 45.1 | 65.1 | 2091 | B5T_03201 | 50S ribosomal protein L20 |
| − | −0.73 | 595.5 | 985.4 | 1647 | B5T_00792 | 30S ribosomal protein S18 |
| − | −0.79 | 82.7 | 143.3 | 1895 | B5T_03773 | 30S ribosomal protein S13 |
| − | −0.8 | 81.8 | 142.0 | 113 | B5T_03866 | Ribosomal RNA large subunit methyltransferase E |
| − | −0.9 | 41.0 | 76.6 | 1244 | B5T_03634 | 50S ribosomal protein L25 |
| − | −0.92 | 7.4 | 14.1 | 489 | B5T_01980 | Ribosomal large subunit pseudouridine synthase E |
| − | −1.12 | 19.0 | 41.3 | 2097 | B5T_03496 | Ribosomal RNA small subunit methyltransferase H |
| − | −1.25 | 9.0 | 21.6 | 1977 | B5T_04427 | Ribosomal RNA small subunit methyltransferase G |
| − | −1.27 | 9.5 | 22.9 | 2158 | B5T_03261 | Ribosomal protein S6 modification protein |
| − | −1.44 | 4.8 | 13.0 | 645 | B5T_00115 | Ribosomal RNA small subunit methyltransferase B |
| − | −1.92 | 20.9 | 79.0 | 518 | B5T_01191 | Ribosomal RNA large subunit methyltransferase N |
| − | −1.94 | 3.1 | 11.8 | 915 | B5T_03262 | Ribosomal protein S6 modification protein |
| − | −2.07 | 39.0 | 163.4 | 2029 | B5T_03800 | 30S ribosomal protein S12 |
| − | −2.12 | 549.9 | 2393.9 | 474 | B5T_03578 | Ribosomal subunit interface protein |
| − | −2.12 | 28.3 | 122.8 | 1883 | B5T_01013 | Ribosomal protein L11 methyltransferase |
| − | −2.6 | 1.4 | 8.5 | 233 | B5T_02829 | Ribosomal RNA large subunit methyltransferase M |
| − | −3.04 | 6.5 | 53.9 | 889 | B5T_00978 | Ribosomal RNA small subunit methyltransferase A |
| − | −3.14 | 1.2 | 10.3 | 895 | B5T_01813 | Ribosomal RNA large subunit methyltransferase L |
| − | −3.31 | 0.3 | 3.2 | 1702 | B5T_03442 | Ribosomal-protein-alanine acetyltransferase |
| − | −4.28 | 2.2 | 41.6 | 2014 | B5T_01081 | Ribosomal RNA large subunit methyltransferase H |
Table 2. Expression of tRNA modification and translation elongation factors, and chaperonin proteins genes in A. dieselolei KS_293 cells under 10 and 0.1 MPa.
| Function | Regulation | log2 FC | 10 MPa | 0.1 MPa | Cluster ID | Locus Tag | Description |
|---|---|---|---|---|---|---|---|
| tRNA modification | |||||||
| + | 3.09 | 231.6 | 27.2 | 789 | B5T_03856 | tRNA pseudouridine synthase B; truB | |
| + | 1.45 | 349.7 | 127.6 | 718 | B5T_01185 | Iron-binding protein IscA; thiolation factor | |
| Translation elongation factors | |||||||
| + | 0.54 | 298.1 | 205.6 | 155 | B5T_03797 | Elongation factor Tu; tuf | |
| + | 0.54 | 74.4 | 51.2 | 930 | B5T_01922 | Elongation factor Ts, tsf | |
| = | 0.43 | 302.0 | 224.3 | 155 | B5T_03809 | Elongation factor Tu; tuf | |
| = | 0.17 | 89.0 | 79.3 | 1100 | B5T_03798 | Elongation factor G, fusA | |
| = | −0.13 | 100.3 | 109.6 | 573 | B5T_00690 | Elongation factor P; efp | |
| − | −2.3 | 15.5 | 76.0 | 453 | B5T_01535 | Elongation factor 4; lepA | |
| Chaperonin proteins | |||||||
| + | 0.62 | 731.7 | 477.7 | 920 | B5T_03449 | 60 kDa chaperonin, groEL | |
| + | 0.59 | 1091.2 | 725.0 | 1897 | B5T_03450 | 10 kDa chaperonin, groES | |
| − | −0.65 | 38.5 | 60.2 | 572 | B5T_02668 | Chaperone protein htpG | |
| − | −1.04 | 39.6 | 81.4 | 342 | B5T_03872 | Chaperone protein dnaJ | |
| − | −1.22 | 506.6 | 1180.5 | 2001 | B5T_03873 | Chaperone protein dnaK | |
| − | −1.59 | 11.1 | 33.3 | 1599 | B5T_03874 | Protein grpE | |
| − | −2.05 | 150.9 | 623.4 | 104 | B5T_03703 | ATP-dependent Clp protease, ATP-binding subunit; ClpB | |
| − | −3.25 | 0.3 | 2.8 | 315 | B5T_00544 | 33 kDa chaperonin, hslO | |
Upregulated COGs related with energy production and conversion were mainly associated with the F-type ATP synthase (Table 3). This enzymatic complex is divided into two major components, a central stalk and a stator, which are constituted by different subunits. All the gene loci related to this enzyme complex are closely located (B5T_04416 to B5T_04423, Table 3) but only 50% of them were upregulated (4/8): in particular genes related with the expression of the stator subunits (3/5) were upregulated or remained unaffected (2/5), indicating that functionality of this component is of primary importance under mild pressure. Despite respiration rates were not affected by increased pressure (Fig. 3b), cytochrome c (16/18) and b (2/2) related genes were downregulated at 10 MPa (Table 3), with the exception of ubiquinol-cytochrome c reductases genes the expression of which was upregulated (2/2, Table 3). Furthermore, we found that a pool of genes encoding for Na+ transporting NADH reductases (from B5T_01840 to B5T_01844) was slightly affected by pressure as transcription of 1/5 was enhanced (0.98, Table 3) and 2/5 remained unaffected, indicating a potential role of this enzyme complex under mild pressure.
Table 3. Expression of some energy production and conversion COG in A. dieselolei KS_293 cells under 10 and 0.1 MPa.
| Function | Regulation | log2 FC | 10 MPa | 0.1 MPa | Cluster ID | Locus Tag | Description |
|---|---|---|---|---|---|---|---|
| ATP synthase | |||||||
| Central stalk | |||||||
| + | 0.64 | 107.8 | 69.3 | 485 | B5T_04418 | ATP synthase gamma chain | |
| − | −1.22 | 31.4 | 73.3 | 1610 | B5T_04422 | ATP synthase subunit c 1 | |
| − | −1.63 | 51.8 | 159.9 | 1581 | B5T_04416 | ATP synthase epsilon chain | |
| Stator | |||||||
| + | 1.05 | 153.4 | 74.3 | 942 | B5T_04420 | ATP synthase subunit delta | |
| + | 1 | 81.6 | 40.7 | 506 | B5T_04421 | ATP synthase subunit b | |
| + | 0.7 | 755.7 | 465.8 | 323 | B5T_04423 | ATP synthase subunit a | |
| = | 0.37 | 41.2 | 31.8 | 163 | B5T_04419 | ATP synthase subunit alpha | |
| = | −0.34 | 101.4 | 128.3 | 1566 | B5T_04417 | ATP synthase F1, beta subunit | |
| Cytochrome c | |||||||
| = | 0.27 | 29.4 | 24.4 | 288 | B5T_04195 | Cytochrome c subfamily, putative | |
| = | −0.21 | 120.1 | 138.7 | 1326 | B5T_04288 | Cytochrome c-type protein | |
| − | −0.91 | 7.8 | 14.7 | 1440 | B5T_04287 | Cytochrome c subfamily, putative | |
| − | −1.02 | 63.0 | 127.8 | 1533 | B5T_01136 | Cytochrome c oxidase assembly protein CtaG/Cox11 | |
| − | −1.24 | 21.7 | 51.2 | 1852 | B5T_01137 | Cytochrome c oxidase, subunit I | |
| − | −2.53 | 13.0 | 75.2 | 386 | B5T_01576 | Cytochrome c-type biogenesis protein CcmE | |
| − | −2.6 | 8.1 | 49.1 | 947 | B5T_01135 | Cytochrome c oxidase, subunit III | |
| − | −2.61 | 15.8 | 96.3 | 1875 | B5T_02284 | Cytochrome c oxidase, cbb3-type, subunit II | |
| − | −2.62 | 8.8 | 54.0 | 110 | B5T_02282 | Cytochrome c oxidase, cbb3-type, subunit III, putative | |
| − | −2.69 | 11.1 | 72.0 | 2031 | B5T_02615 | Cytochrome c subfamily, putative | |
| − | −2.89 | 1.9 | 13.9 | 1575 | B5T_01415 | Cytochrome c assembly protein | |
| − | −3.03 | 2.2 | 18.0 | 1166 | B5T_01572 | Cytochrome c biogenesis ATP-binding export protein CcmA | |
| − | −3.07 | 2.4 | 19.8 | 1142 | B5T_01138 | Cytochrome c oxidase subunit 2 | |
| − | −3.12 | 9.5 | 82.7 | 1284 | B5T_02285 | Cytochrome c oxidase, cbb3-type, subunit I | |
| − | −3.45 | 3.1 | 34.3 | 862 | B5T_02281 | Cytochrome c oxidase accessory protein CcoG | |
| − | −3.87 | 0.4 | 5.5 | 611 | B5T_01554 | Cytochrome c heme lyase subunit CcmL | |
| − | −4.14 | 0.8 | 13.3 | 421 | B5T_01552 | Cytochrome c-type biogenesis protein CcmF | |
| − | −5.07 | 0.4 | 11.7 | 1861 | B5T_01019 | Cytochrome c biogenesis protein transmembrane region | |
| Ubiquinol-Cytochrome c | |||||||
| + | 0.79 | 215.1 | 124.4 | 1648 | B5T_03519 | ubiquinol-cytochrome c reductase iron-sulfur subunit; petA | |
| + | 0.58 | 164.9 | 110.0 | 1172 | B5T_03517 | ubiquinol-cytochrome c reductase cytochrome c1 subunit; petC | |
| − | −2.48 | 18.2 | 101.9 | 1213 | B5T_00990 | ubiquinol oxidase, subunit II, putative | |
| Cytochrome b | |||||||
| − | −0.97 | 25.4 | 49.7 | 285 | B5T_03518 | cytochrome b; petB | |
| − | −0.58 | 102.7 | 153.2 | 1237 | B5T_02687 | succinate dehydrogenase, cytochrome b556 subunit | |
| Na+ translocating NADH reductase | |||||||
| + | 0.98 | 51.8 | 26.3 | 781 | B5T_01844 | Na+-translocating NADH-quinone reductase subunit E | |
| = | −0.32 | 52.3 | 65.5 | 615 | B5T_01843 | Na+-translocating NADH-quinone reductase subunit D | |
| = | −0.33 | 14.8 | 18.6 | 359 | B5T_01842 | Na+-transporting NADH-ubiquinone oxidoreductase subunit C | |
| − | −0.52 | 31.2 | 44.6 | 2010 | B5T_01840 | Na+-translocating NADH-quinone reductase subunit A | |
| − | −0.78 | 24.4 | 42.0 | 1158 | B5T_01841 | Na+-translocating NADH-ubiquinone oxidoreductase subunit B | |
Transcriptional functionality did not appear to be affected by mild pressure as all related genes were either unaffected or downregulated with few exceptions (Table 4). Genes encoding DNA repair proteins such as ruvC, a histone-like bacterial DNA repair and recombination protein and recF were among those with enhanced expression at 10 MPa, although the majority of these genes were generally downregulated (7/11, Table 4). Further, all transcriptional regulators were downregulated (7/9) with the exception of two MarR and LysR family genes (Table 4). Finally, we found that all genes related to the pathway involved in protein export and cell damage were unaffected (2/9) or downregulated (6/9), with the exception of the gene encoding for the YajC subunit (Table 4). Similarly, upregulation of genes related with the osmolite ectoine was not observed (Table 4). This was in agreement with the lack of ectoine detection in cells incubated at both 0.1 and 10 MPa (data not shown), thus confirming our previous observations on the structural resistance by the cells (Fig. 4). Finally, genes related to alkane activation, such as alkane hydroxylase (B5T_00103) or cytochrome P450 (B5T_02075), and fatty acids degradation were all strongly downregulated under mild pressure (Table 5).
Table 4. Expression of genes related with DNA processing, protein export and osmolites production in A. dieselolei KS_293 cells under 10 and 0.1 MPa.
| Function | Regulation | log2 FC | 10 MPa | 0.1 MPa | Cluster ID | Locus Tag | Description |
|---|---|---|---|---|---|---|---|
| RNA polymerases | |||||||
| + | 0.54 | 22.8 | 15.7 | 497 | B5T_00166 | DNA-directed RNA polymerase subunit omega | |
| = | 0.35 | 414.0 | 325.0 | 739 | B5T_03770 | DNA-directed RNA polymerase, alpha subunit | |
| = | −0.02 | 80.8 | 81.9 | 1558 | B5T_03801 | DNA-directed RNA polymerase subunit beta' | |
| = | −0.3 | 54.8 | 67.5 | 1782 | B5T_03802 | DNA-directed RNA polymerase subunit beta | |
| DNA repairing system | |||||||
| + | 1.44 | 3.0 | 1.1 | 1577 | B5T_03233 | Crossover junction endodeoxyribonuclease; ruvC | |
| + | 0.78 | 495.3 | 287.9 | 1089 | B5T_02378 | Histone-like bacterial DNA-binding protein | |
| + | 0.76 | 34.2 | 20.3 | 366 | B5T_00003 | DNA replication and repair protein; recF | |
| = | −0.49 | 12.3 | 17.3 | 1956 | B5T_03101 | Recombinase A; recA | |
| − | −2.78 | 3.6 | 24.4 | 324 | B5T_03876 | DNA repair protein; recN | |
| − | −2.82 | 0.6 | 4.0 | 1043 | B5T_03100 | Regulatory protein; recX | |
| − | −2.96 | 2.4 | 18.4 | 1708 | B5T_03007 | Recombination protein; recR | |
| − | −3.36 | 2.2 | 22.1 | 393 | B5T_03250 | ATP-dependent DNA helicase; recQ | |
| − | −3.44 | 0.9 | 10.1 | 478 | B5T_00150 | ATP-dependent DNA helicase; recG | |
| − | −4.34 | 0.9 | 17.7 | 48 | B5T_01518 | Single-stranded-DNA-specific exonuclease; recJ | |
| − | −4.38 | 0.4 | 7.8 | 1179 | B5T_01540 | DNA repair protein; recO | |
| Transcriptional regulators | |||||||
| + | 0.71 | 44.1 | 26.9 | 1768 | B5T_00887 | Transcriptional regulator, MarR family | |
| + | 0.65 | 2.8 | 1.8 | 275 | B5T_02642 | LysR family transcriptional regulator | |
| − | −3.87 | 7.8 | 114.2 | 891 | B5T_02527 | TraR/DksA family transcriptional regulator | |
| − | −4.25 | 1.8 | 33.7 | 200 | B5T_04299 | Transcriptional regulator, AraC family protein | |
| − | −4.34 | 1.5 | 30.8 | 1292 | B5T_01803 | Transcriptional regulator, TetR family | |
| − | −4.45 | 0.2 | 4.2 | 736 | B5T_01483 | Two component transcriptional regulator, winged helix family | |
| − | −4.48 | 0.1 | 3.2 | 1969 | B5T_02708 | putative transcriptional regulator | |
| − | −5.41 | 0.3 | 11.6 | 1735 | B5T_01045 | Two-component transcriptional regulator, LuxR family protein | |
| − | −5.44 | 0.3 | 12.3 | 1975 | B5T_00817 | transcriptional regulator; NrdR | |
| Transcription elongation factors | |||||||
| = | 0.1 | 41.0 | 38.3 | 680 | B5T_03868 | Transcription elongation factor greA 1; greA | |
| − | −0.52 | 114.6 | 164.3 | 2066 | B5T_03859 | Transcription elongation factor; nusA | |
| − | −2.2 | 14.5 | 66.3 | 585 | B5T_04038 | Transcription elongation factor greA 1; greB | |
| Protein export | |||||||
| + | 1.05 | 274.8 | 132.8 | 1399 | B5T_03676 | Preprotein translocase, YajC subunit | |
| = | 0.25 | 296.8 | 250.3 | 869 | B5T_03775 | Preprotein translocase SecY subunit | |
| = | 0.03 | 122.9 | 120.0 | 292 | B5T_00612 | Protein-export protein secB | |
| − | −0.54 | 33.6 | 48.8 | 1233 | B5T_03808 | Preprotein translocase subunit secE | |
| − | −0.65 | 28.5 | 44.6 | 2024 | B5T_03861 | Preprotein translocase SecG subunit, putative | |
| − | −1 | 55.9 | 111.9 | 1490 | B5T_03674 | Preprotein translocase SecF subunit | |
| − | −1.55 | 116.2 | 339.2 | 968 | B5T_03479 | Protein translocase subunit secA | |
| − | −1.97 | 12.1 | 47.5 | 821 | B5T_04447 | Membrane protein oxaA yidC | |
| − | −3.01 | 4.2 | 33.5 | 1027 | B5T_03675 | Preprotein translocase SecD subunit | |
| Ectoine synthesis | |||||||
| = | −0.23 | 115.6 | 135.6 | 471 | B5T_00884 | Ectoine synthase | |
| − | −2.68 | 0.8 | 5.0 | 445 | B5T_04304 | Ectoine hydroxylase | |
Table 5. Expression of genes related with activation of alkanes and fatty acids degradation in A. dieselolei KS_293 cells under 10 and 0.1 MPa.
| Function | Regulation | log2 FC | 10 MPa | 0.1 MPa | Cluster ID | Locus Tag | Description |
|---|---|---|---|---|---|---|---|
| Alkanes activation | |||||||
| − | −1.37 | 1066.6 | 2749.9 | 764 | B5T_02075 | Cytochrome P450 alkane hydroxylase | |
| − | −2.39 | 31.4 | 164.6 | 407 | B5T_00103 | Alkane hydroxylase | |
| Fatty acids degradation | |||||||
| − | −0.62 | 77.7 | 119.0 | 1313 | B5T_01517 | 3-hydroxyacyl-CoA dehydrogenase/short chain enoyl-CoA hydratase | |
| − | −0.88 | 40.8 | 75.1 | 898 | B5T_00925 | Acyl-CoA dehydrogenase | |
| − | −1.04 | 16.6 | 34.1 | 1411 | B5T_00923 | Acyl-CoA dehydrogenase | |
| − | −1.17 | 12.9 | 29.0 | 143 | B5T_01516 | 3-ketoacyl-CoA thiolase | |
| − | −1.2 | 11.6 | 26.8 | 644 | B5T_03556 | Acyl-CoA dehydrogenase | |
| − | −1.34 | 12.6 | 32.0 | 660 | B5T_02905 | 3-hydroxyacyl-CoA dehydrogenase | |
| − | −1.59 | 15.1 | 45.6 | 689 | B5T_01467 | Acyl-CoA dehydrogenase | |
| − | −1.98 | 1.4 | 5.5 | 1976 | B5T_04210 | Putative acetyl-CoA acetyltransferase with thiolase domain | |
| − | −2.09 | 3.3 | 14.2 | 1467 | B5T_01622 | Acyl-CoA dehydrogenase | |
| − | −2.39 | 5.0 | 26.5 | 858 | B5T_01799 | Acyl-CoA dehydrogenase | |
| − | −2.49 | 4.8 | 26.8 | 55 | B5T_03005 | Acyl-CoA dehydrogenase | |
| − | −2.5 | 423.6 | 2388.1 | 914 | B5T_02077 | Pyridine nucleotide-disulfide oxidoreductase domain protein | |
| − | −2.89 | 3.3 | 24.2 | 202 | B5T_02439 | Acyl-CoA dehydrogenase | |
| − | −2.95 | 1.3 | 9.7 | 654 | B5T_01468 | Acyl-CoA dehydrogenase | |
Discussion
Gene expression in high-pressure-adapted microbes from deep-sea has been elucidated over the last decades (reviewed in20). Description of pressure-sensitive pools of genes (the “so-called” stimulons21) and/or individual genes and gene traits revealed that they were regulated in several different microbes. The most notable ones include genes which code for heat or cold shock proteins (Hsp or Csp, respectively), outer membrane proteins (Omp), cell division proteins, ribosomes, elongation factors, cytochromes and DNA repair proteins (sigma factor family)22. Provided that the average depth of the Earth’s oceans is about 3800 m (corresponding to about 38 MPa) a widely shared hypothesis considers that pressure-loving bacteria (piezophiles and hyperpiezophiles) would possibly inhabit niches at this depth and further below23. Hence, the mechanisms involved in high-pressure-resistance such as in vivo protein synthesis have been reviewed for pressure ranges between 55 and 400 MPa24,25,26,27,28,29. On the contrary, little information on the effect of mild hydrostatic pressure on microbial metabolism is available30,31. While the study of piezophiles may reveal novel cellular mechanisms supporting life in the deep biosphere, the study of piezotolerant and piezosensitive bacteria may highlight the most sensitive pathways affected by hydrostatic pressure. Albright and Morita32 found that in the psychrophilic marine bacterium Vibrio marinus the rate of protein and RNA synthesis was affected after being exposed to 20 MPa for 60 min, although 1-atm rates were resumed shortly afterwards. Turley33 observed reduced uptake of 3H-thymidine and 3H-leucine for microbial protein synthesis between 0.1 and 43 MPa, but no effect on cell abundance.
In the present study, the hydrocarbon degraders Alcanivorax dieselolei KS_293 and Alcanivorax jadensis KS_339 showed a piezosensitive profile, with significant growth reduction already at 5 MPa (Fig. 1b), suggesting that their biodegradation potential may decrease with depth. However, under 10 MPa A. dieselolei KS_293 showed unaffected carbon degradation capacity (Fig. 3a), higher PO43− uptake (Fig. 3c) and lower number of damaged cells (Fig. 4) than A. jadensis KS_339, indicating a certain level of resistance to the stressing effects derived from hydrostatic pressure. Analysis of the transcriptome of A. dieselolei KS_293 revealed that expression of the large majority of its genome was downregulated at mild pressure (Fig. 5), while expression of only few pathways preserving critical functions, such as protein synthesis and energy production, was enhanced. Remarkably, most of the known pressure-responsive genes were not upregulated (Table S3) together with those involved in alkanes activation and fatty acids degradation (Table 5). These results suggest that bioremediation capacity of piezosensitive hydrocarbonoclastic bacteria may be already highly impaired at mild pressure, and that the interplay of the upregulated pathways has a role in counteracting mild pressure effects (Fig. 6).
One of the most affected functions in cells exposed to pressure is translation. In vitro, ribosomal proteins associated with mRNA and tRNA show improved stability and can resist up to 100 MPa, while uncharged ribosomes dissociated at 60 MPa34. Correlation between loss of cell viability and ribosome integrity at high pressure has been postulated23. In the present study, enhanced transcription of 30S and 50S ribosomal proteins was observed in A. dieselolei KS_293 when pressure was increased from 0.1 to 10 MPa (Table 1). Despite pressure reduced growth yields (Fig. 1b) cell viability was not affected (Fig. 4), indicating that cells division occurred at a slower pace. Limited growth has been proposed to be linked with impaired protein synthesis in microbes adapted to atmospheric pressure35,36.
One of the most pressure-sensitive steps affecting translation capacity is binding of aminoacyl-tRNA to ribosomes26 as it determines a conformational change leading to an increase in volume, the latter being an unfavored process under pressure37. In A. dieselolei KS_293, the majority of the translational elongation factors were either unaffected or upregulated (Table 2), in particular Tu and Ts, which have the role of binding the aminoacyl-tRNA to the A-site of the ribosome (Table 2). This further supports the hypothesis that translation capacity was affected, as also observed at much higher pressure (45 MPa) in short-term (30 min) experiments with Lactobacillus sanfranciscensis38.
However, expression of genes related to tRNA modifying proteins was among the most enhanced ones in the whole transcriptome (Table 2), as for truB. The latter expresses a pseudouridine synthase known for producing a pseudouridine in tRNAs, carrying RNA chaperone activity and assisting in the correct folding of tRNA, a process giving selective advantage (though not essential) for cell growth39. Translation accuracy was also potentially affected by pressure in A. dieselolei KS_293, as suggested by the upregulation of chaperonins such as GroEL and GroES (Table 2)40. The reason why only this type of chaperonins/small Hsp (Table 2 and S3) were upregulated under pressure remains unclear.
Adenosine triphosphate (ATP) is synthesized by the F-type ATP synthase (or F1F0 ATP synthase). This transmembrane, proton-force driven complex is known to be the smallest motor enzyme in nature, and uses a transmembrane electrochemical gradient of protons (or Na+ ions) to manufacture ATP from adenosine diphosphate (ADP) and inorganic PO43− 41. It is constituted by a central stalk (made of gamma and epsilon subunits in the F1 and c subunit ring in the F0 part), which rotates relative to a stator (composed of alpha, beta, delta, a and b subunits42) during ATP hydrolysis and synthesis. Genes related with the stator were either unaffected or upregulated in A. dieselolei KS_293 (Table 3) suggesting that functionality of this transmembrane unit was affected at mild pressure. Upregulation of these genes may have been linked to enhanced ATP generation necessary for coping with sustained cell maintenance when pressure increased. Previous reports revealed that unbalanced ATP generation and demand in Streptococcus faecalis cells exposed to 408 atm (equivalent to 40.8 MPa) actually reduced growth rates, but that pressure-volume work, turnover of proteins, peptidoglycan, or stable RNA were not related with the use of ATP43. However, we did not observe higher PO43− consumption per cell (Fig. 3c), cell integrity was negligibly affected (Fig. 4), production of osmolites was not activated (Table 4) and genes related with protein export and cell maintenance were only marginally affected by pressure variation (Table 4). While ATP generation was potentially impacted, upregulation of genes related only to the stator could be attributed to structural problems that this transmembrane component faced under 10 MPa.
Microbes dealing with temperature variations optimize ion permeability, such as proton translocation and ATP synthesis, for bio-energetic purposes44. We found that the pool of genes expressing the Na+ -translocating NADH reductase complex (RNF-NQR) was slightly affected under mild pressure (Table 3). NADH can be oxidized by the respiratory chain of bacteria via NADH:quinone oxidoreductases that belong to 3 distinct enzyme families: NDH-1, NDH-2, and NQR45. The NQR-type enzymes are Na+ -motive NADH-quinone oxidoreductases consisting of 6 subunits and several cofactors. The NADH-quinone oxidoreductase activity of these enzymes is stimulated by Na+ ions and is coupled with pumping of Na+ rather than H+. As explained by van de Vossenberg et al.44, the proton motive force in bacteria dealing with temperature changes is sustained by increasing the rate of proton pumping, by altering the membrane composition to reduce permeability to ions, or by coupling proton-driven processes to less permeable ions such as Na+. A similar process has been proposed as alternative respiration pathway in Shewanella benthica grown under 60 MPa46,47. In particular, a NADH-oxidoreductase would reduce a quinone and this will reduce a quinone oxidase, which in turn would use O2 as the final electron acceptor. This pathway would not need cytochromes c and b as observed in A. dieselolei KS_293, where these genes were downregulated, and would rather use quinone-oxidoreductases, which are mostly upregulated under mild pressure (Table 3). By preferring the quinol-oxidoreductase pathway bacteria are known to maintain efficient respiration, which indeed in the present study was not affected at higher pressure than atmospheric (Fig. 3b).
Surface waters and deep sea are two distinguished niches divided by a large area, the mesopelagic zone, where hydrostatic pressure critically increases within short depths. The metabolic functions of piezosensitive bacteria may result influenced as an attempt to survive in this zone. Response to mild pressure increase differentially impacts hydrocarbonoclastic isolates belonging to the same genus. In the structurally resistant A. dieselolei KS_293 growth rates appear to be lowered by the interplay between impaired protein synthesis and energy production. Furthermore, normal respiration pathways at atmospheric pressure may be affected already under 10 MPa. The response to these critical steps potentially shapes the structure of microbial communities developed after oil spills at sea, therefore playing a role in successful bioremediation strategies.
Materials and Methods
Strains, culture media and growth conditions
Alcanivorax jadensis KS_339 and Alcanivorax dieselolei KS_293 were isolated from surface waters collected in the Gibraltar Strait and the Levantine basin (35° 54′, −7° 00′ and 34° 04′, 34° 00′, respectively) performing enrichment cultures on ONR7a medium48 supplemented with 1% (v:v) diesel oil as sole carbon source.
After isolation, strains were axenically cultivated in static glass bottles of 250 mL (operating volume 100 mL), using ONR7a medium, at initial pH 7.6, for 4 to 7 days at room temperature, providing n-dodecane (Sigma Aldrich, Belgium) 1% (v:v) as sole carbon source.
Mild hydrostatic pressure experiments
Growing cultures were collected, centrifuged at 4000 rpm for 10 min at 4 °C (Sorval RC5c PLUS, Beckman, Suarlée, Belgium) and resuspended in fresh ONR7a medium at initial optical density (OD610) of 0.100. Then, 3.5 mL of liquid culture suspension was transferred into sterile 10 mL syringes. Gas phase (equal to 6.5 mL) was constituted of air, which provided O2 to the cells during the subsequent incubation. n-dodecane (C12) 1% (v:v) was supplied as the sole carbon source. Syringes were closed using a sterile three-way valve, and placed in a 1 L T316 stainless steel high-pressure reactor (HPR) (Parr, USA). Reactor was filled with deionized water and hydrostatic pressure was increased up to 5 or 10 MPa by pumping water with a high-pressure pump (HPLC pump series III, SSI, USA). Pressure was transmitted to the cultures through the piston of the syringe. Experiments at atmospheric pressure (0.1 MPa) were run adjacent to the HPR. Control experiments were constituted by sterile syringes supplied only with sterile medium. Reactors were incubated in a temperature-controlled room at 20 °C for 4 days. At the end of the experiments, pressure was gently released up to 0.1 MPa and syringes were set aside for 30–60 min before running analyses.
Control experiments were conducted under ambient pressure using the same set up described above, with the only exception that air was substituted with pure, sterile O2 (Linde, Schiedam, The Netherlands). Both strains A. jadensis KS_339 and A. dieselolei KS_293 were tested in 3 independent replicates).
Microbial analyses
Optical density was measured at 610 nm with a spectrophotometer (Isis 9000, Dr Lange, Germany). Cell count and intact/damaged cell count was performed by flow cytometry. SYBR® Green I and Propidium Iodide (PI) were used in combination to discriminate cells with intact and damaged cytoplasmic membranes49,50. Staining solution was prepared as follows: PI (20 mM in dimethyl sulfoxide [DMSO], LIVE/DEAD BacLight Kit, Invitrogen, Belgium) was diluted 50 times and SYBR® Green I (10,000 times concentrate in DMSO, Invitrogen) was diluted 100 times in 0.22 μm-filtered-DMSO. Water samples were stained with 10 μL/mL staining solution and 10 μL/mL EDTA (pH 8, 500 mM) for outer membrane permeabilization. Before staining, samples of 1 mL were maintained at room temperature for 30 min to minimize staining temperature effect. Prior to flow cytometric analysis, stained samples were incubated for 13 min in the dark at 37 °C.
Flow cytometry was performed using a CyAn™ ADP LX flow cytometer (Dakocytomation, Heverlee, Belgium) equipped with a 50-mW Sapphire solid-state diode laser (488 nm). Stability and performance was performed using the Cyto-Cal Alignment Beads and Cyto-Cal multifluor Fluorescent Intensity Calibrator (Distrilab, Leusden, The Netherlands). Green and red fluorescence were collected with photomultiplier tubes using 530/40 and 613/20 bandpass filters respectively. Forward (FS) and side light scatter (SS) were collected with a 488/10 bandpass filter. Milli-Q water was used as the sheath fluid. All samples were collected as logarithmic signals triggered on the green fluorescence channel. Data for 20,000 events for each sample run was collected.
Chemical analyses
O2 respiration and CO2 production were assessed by comparing the head space biogas composition of syringes inoculated with Alcanivorax species and sterile controls at the end of the incubation. Gas phase was analyzed with a Compact GC (Global Analyser Solutions, Breda, The Netherlands), equipped with a Molsieve 5 A pre-column and two channels. In channel 1, a Porabond column detected CH4, O2, H2 and N2. In channel 2, a Rt-Q-bond pre-column and column detected CO2, N2O and H2S. Concentrations of gases were determined with a thermal conductivity detector. pH was measured using a pH meter (Herisau, Metrohm, Switzerland). Sulphate and phosphate were quantified with a Compact Ion Chromatograph (Herisau, Metrohm, Switzerland) equipped with a conductivity detector. Dodecane concentration was evaluated using a GC equipped with a flame ionized detector (FID) (Agilent Technologies, Santa Clara, USA), equipped with a HP-5 capillary column (30 m; 0.25 mm), following these conditions: initial temperature, 60 °C; isothermal for 1 min; temperature rate, 10 °C min−1; final temperature, 320 °C; isothermal for 5 min. The injector (splitless mode) was at 270 °C, FID at 320 °C; carrier gas (N2) flow rate was 60 mL min−1 and injected sample volume was 1 μL. First, 0.7 mL of culture were removed from syringes and extracted from the water-phase using 1:1 n-hexane. Samples were vigorously shaken for 1 min and set aside for 15 min. The upper layer of hexane and extracted dodecane was collected and injected into the GC-FID.
Transcriptomic analysis in A. dieselolei KS_293
Ten independent cultures of A. dieselolei KS_293 were grown at both 0.1 and 10 MPa as described above. At the end of the experiments, pressure was gently released and cultures pooled together in order to have enough cell material and to average the response to pressure in independently grown cultures. Cells were recovered by centrifugation at 4 °C for 5 min at 13000 rpm (Sorval RC5c PLUS, Beckman, Suarlée, Belgium) and pellets stored at −80 °C for further RNA extraction.
RNA extraction and QC
RNA was isolated from pelleted cells using the Rneasy Mini Kit (Qiagen, Antwerp, Belgium) following manufacturer’s instructions. On-column DNase digestion was performed during RNA extraction. RNA concentration was determined using the NanoDrop 2000 UV-Vis spectrophotometer (Thermo Scientific, Waltham, MA, USA). Pellets originated from at 0.1 and 10 MPa had 615.2 and 311.5 ng/μL, respectively. RNA quality control was performed using the 2100 Bioanalyzer microfluidic gel electrophoresis system (Agilent Technologies, Santa Clara, USA).
RNA library prep and sequencing
Libraries for RNA-sequencing were prepared using the ScriptSeq Complete (Bacteria) sample prep kit (Epicentre – Illumina, San Diego, CA, USA). Starting material (1000 ng) of total RNA was depleted of rRNAs using Ribo-Zero magnetic bead based capture-probe system (Illumina, Hayward, USA). Remaining RNA (including mRNAs, lin-cRNAs and other RNA species) was subsequently purified (Agencourt RNA- Clean XP, Beckman Coulter, Brea, CA, USA) and fragmented using enzymatic fragmentation. First strand synthesis and second strand synthesis were performed and double stranded cDNA was purified (AgencourtAMPure XP, Beckman Coulter). RNA stranded libraries were pre-amplified and purified (AgencourtAMPure XP, Beckman Coulter). Library size distribution was validated and quality inspected using the 2100 Bioanalyzer (high sensitivity DNA chip, Agilent Technologies). High quality libraries were quantified using the Qubit Fluorometer (Life Technologies, Carlsbad, CA, USA), concentration normalized and samples pooled according to number of reads. Sequencing was performed on a NextSeq500 instrument using Mid Output sequencing kit (150 cycles) according to manufacturer’s instructions (Illumina, Hayward, USA).
Data processing workflow
Data analysis pipeline was based on the Tuxedo software package (Oracle, Redwood Shores, CA, USA). Components of the RNA-seq analysis pipeline included Bowtie2 (v. 2.2.2), TopHat (v2.0.11) and Cufflinks (v2.2.1) and are described in detail below. TopHat is a fast splice junction mapper for RNA-Seq reads, which aligns sequencing reads to the reference genome using the sequence aligner Bowtie2. TopHat uses sequence alignments to identify splice junctions for both known and novel transcripts. Cufflinks takes the alignment results from TopHat to assemble the aligned sequences into transcripts, constructing a map of the transcriptome. A previously reported transcript annotation was used to guide the assembly process51.
Data analysis
Genes were grouped according to orthologous clusters using the database provided by Ortholuge DB52. Only clusters classified as supporting-species-divergence (SSD) were considered and the rest were discarded (Borderline-SSD, Divergent-SSD, Similar Non-SSD and unevaluated orthologs [RBB]). Up and downregulation analysis was expressed on a log2 basis, indicating fold changes in fragments per kilobase of transcript per million mapped reads (FPKM) between samples at 0.1 and 10 MPa. Gene clusters were arbitrarily considered up-regulated at mild pressure when their log2 fold change was higher than 0.5 between 0.1 and 10 MPa. On the contrary, it was considered that downregulated genes had a −0.50 fold change between the same range of pressures. All the gene clusters that were expressed between −0.5 and 0.5 were considered to be unaffected by the increase in pressure. Hence, the ±0.5 log2 fold change was established in order to have a reasonable compromise in the definition of both upregulated and unaffected genes, provided that a higher threshold may be more appropriate to assess upregulation but would result into an over estimation of unaffected genes. Final analysis of up and down-regulated genes, and clusters of orthologous gene (COG) category was done using the database provided by KEGG (www.genome.jp/kegg).
Statistical analysis
Results were expressed as mean values of experiments made in 4 to 20 independent replicates. Bars in the graphs indicate a 95% confidence interval (95% CI) calculated using a Student t-test with a two-sided distribution. Statistical significance was assessed using a nonparametric test (Mann–Whitney test) which considered a two-sided distribution with 95% CI.
Additional Information
How to cite this article: Scoma, A. et al. Microbial oil-degradation under mild hydrostatic pressure (10MPa): which pathways are impacted in piezosensitive hydrocarbonoclastic bacteria? Sci. Rep. 6, 23526; doi: 10.1038/srep23526 (2016).
Supplementary Material
Acknowledgments
This work was funded by FP-7 project Kill Spill (No. 312139, “Integrated Biotechnological Solutions for Combating Marine Oil Spills”). The authors thank the support of King Abdullah University of Science and Technology (baseline research funds to D.D.). F.M. was supported by Università degli Studi di Milano, DeFENS, European Social Found (FSE) and Regione Lombardia (contract “Dote Ricerca”). Mr. Benjamin Buysschaert and Ms. Nicole Hahn are kindly acknowledged for their help with flow cytometry analyses.
Footnotes
Author Contributions A.S. and M.B. designed and performed the experiments, analysed the data and wrote the paper. E.H.S. analysed the data and co-wrote the paper. F.M. co-wrote the paper. S.B. and D.D. conceived the project and co-wrote the paper. N.B. conceived the project, designed the experiments and co-wrote the paper.
References
- Hawley E. R. et al. Metagenomic analysis of microbial consortium from natural crude oil that seeps into the marine ecosystem offshore Southern California. Stand Genom Sci 9, 1259 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAS in Oil in the sea III: inputs, fates, and effects (National Academy Press, 2003). [PubMed]
- Bargiela R. et al. Bacterial population and biodegradation potential in chronically crude oil-contaminated marine sediments are strongly linked to temperature. Sci Rep 5, doi: 10.1038/srep11651 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines R. B., Frysinger G. S., Hendrick-Smith M. S. & Stuart J. D. Oil spill source identification by comprehensive two-dimensional gas chromatography. Env Sci Technol 33, 2106–2112 (1999). [Google Scholar]
- Tkalich P., Huda K. & Hoong Gin K. Y. A multiphase oil spill model. J Hyd Res 41, 115–125 (2003). [Google Scholar]
- Parinos C. et al. Occurrence, sources and transport pathways of natural and anthropogenic hydrocarbons in deep-sea sediments of the Eastern Mediterranean Sea. Biogeosci 10, 6069–6089 (2013). [Google Scholar]
- Fu J., Gong Y., Zhao X., O’Reilly S. E. & Zhao D. Effects of oil and dispersant on formation of marine oil snow and transport of oil hydrocarbons. Env Sci Technol 48, 14392–14399 (2014). [DOI] [PubMed] [Google Scholar]
- Valentine D. L. et al. Fallout plume of submerged oil from Deepwater Horizon. PNAS 111, 15906–15911 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanton J. et al. Using natural abundance radiocarbon to trace the flux of petrocarbon to the seafloor following the Deepwater Horizon oil spill. Env Sci Technol 49, 847–854 (2014). [DOI] [PubMed] [Google Scholar]
- Schwarz J. R., Walker J. D. & Colwell R. R. Deep-sea bacteria: growth and utilization of hydrocarbons at ambient and in situ pressure. Appl Microbiol 28, 982–986 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Walker J. D. & Colwell R. R. Deep-sea bacteria: growth and utilization of n-hexadecane at in situ temperature and pressure. Canad J Microbiol 21, 682–687 (1975). [DOI] [PubMed] [Google Scholar]
- Grossi V. et al. Hydrostatic pressure affects membrane and storage lipid compositions of the piezotolerant hydrocarbon‐degrading Marinobacter hydrocarbonoclasticus strain# 5. Env microbiol 12, 2020–2033 (2010). [DOI] [PubMed] [Google Scholar]
- Schedler M., Hiessl R., Juárez A. G. V., Gust G. & Müller R. Effect of high pressure on hydrocarbon-degrading bacteria. AMB express 4, 77 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison T. In Oceanography: an invitation to marine science (Cengage Learning, 2015). [Google Scholar]
- Lai Q., Li W. & Shao Z. Complete genome sequence of Alcanivorax dieselolei type strain B5. J Bacteriol 194, 6674–6674 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostka J. E. et al. Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the Deepwater Horizon oil spill. App Env Microbiol 77, 7962–7974 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang W., Lai Q. & Shao Z. Gene diversity of CYP153A and AlkB alkane hydroxylases in oil degrading bacteria isolated from the Atlantic Ocean. Env Microbiol 12, 1230–1242 (2010). [DOI] [PubMed] [Google Scholar]
- Bruns A. & Berthe-Corti L. Fundibacter jadensis gen. nov., sp. nov., a new slightly halophilic bacterium, isolated from intertidal sediment. Int J Syst Bacteriol 49, 441–448 (1999). [DOI] [PubMed] [Google Scholar]
- Ron E. Z. & Rosenberg E. Biosurfactants and oil bioremediation. Curr Opin Biotechnol 13, 249–252 (2002). [DOI] [PubMed] [Google Scholar]
- Bartlett D. H., Kato C. & Horikoshi K. High pressure influences on gene and protein expression. Res Microbiol 146, 697–706 (1995). [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Ingraham J. L. & Schaechter M. Physiology of the bacterial cell: a molecular approach. Cell 7, 341–342 (1990). [Google Scholar]
- Bartlett D. H. Pressure effects on in vivo microbial processes. Biochim Biophys Acta (BBA)-Prot Struct Mol Enzymol 1595, 367–381 (2002). [DOI] [PubMed] [Google Scholar]
- Abe F. Exploration of the effects of high hydrostatic pressure on microbial growth, physiology and survival: perspectives from piezophysiology. Biosci Biotechnol Biochem, 71, 2347–2357 (2007). [DOI] [PubMed] [Google Scholar]
- Pollard E. C. & Weller P. K. The effect of hydrostatic pressure on the synthetic processes in bacteria. Biochim Biophys Acta (BBA)-Biophys Photosynt 112, 573–580 (1966). [DOI] [PubMed] [Google Scholar]
- Yayanos A. A. & Pollard E. C. A study of the effects of hydrostatic pressure on macromolecular synthesis in Escherichia coli. Biophys J 9, 1464 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R. & Landau J. V. Inhibition of cell-free protein synthesis by hydrostatic pressure. J Bacteriol 112, 1222–1227 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch T. J., Farewell A., Neidhardt F. C. & Bartlett D. H. Stress response of Escherichia coli to elevated hydrostatic pressure. J Bacteriol 175, 7170–7177 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammi M. J. et al. Hydrostatic pressure-induced changes in cellular protein synthesis. Biorheol 41, 309–314 (2004). [PubMed] [Google Scholar]
- Jofré A. et al. Protein synthesis in lactic acid and pathogenic bacteria during recovery from a high pressure treatment. Res Microbiol 158, 512–520 (2007). [DOI] [PubMed] [Google Scholar]
- Nogi Y., Masui N. & Kato C. Photobacterium profundum sp. nov., a new, moderately barophilic bacterial species isolated from a deep-sea sediment. Extrem 2, 1–8 (1998). [DOI] [PubMed] [Google Scholar]
- Pradel N. et al. The first genomic and proteomic characterization of a deepsea sulfate reducer: insights into the piezophilic lifestyle of Desulfovibrio piezophilus. PloS one 8 ,doi: 10.1371/journal.pone.0055130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright L. J. & Morita R. Y. Effect of hydrostatic pressure on synthesis of protein, ribonucleic acid, and deoxyribonucleic acid by the psychrophilic marine bacterium, Vibrio marinus. Limnol Oceanograph 13, 637–643 (1968). [DOI] [PubMed] [Google Scholar]
- Turley C. M. The effect of pressure on leucine and thymidine incorporation by free-living bacteria and by bacteria attached to sinking oceanic particles. Deep Sea Res Part I: Ocean Res Papers 40, 2193–2206 (1993). [Google Scholar]
- Gross M., Lehle K., Jaenicke R. & Nierhaus K. H. Pressure‐induced dissociation of ribosomes and elongation cycle intermediates. Eur J Biochem 218, 463–468 (1993). [DOI] [PubMed] [Google Scholar]
- Pope D. H. & Berger L. R. Inhibition of metabolism by hydrostatic pressure: what limits microbial growth? Archiv für Mikrobiologie 93, 367–370 (1973). [DOI] [PubMed] [Google Scholar]
- Landau J. V. & Pope D. H. Recent advances in the area of barotolerant protein synthesis in bacteria and implications concerning barotolerant and barophilic growth. Adv Aquat Microbiol 2, 49–76 (1980). [Google Scholar]
- Somero G. N. Adaptations to high hydrostatic pressure. Ann Rev Physiol 54, 557–577 (1992) [DOI] [PubMed] [Google Scholar]
- Pavlovic M., Hörmann S., Vogel R. F. & Ehrmann M. A. Transcriptional response reveals translation machinery as target for high pressure in Lactobacillus sanfranciscensis. Arc Microbiol 184, 11–17 (2005). [DOI] [PubMed] [Google Scholar]
- Gutgsell N. et al. Deletion of the Escherichia coli pseudouridine synthase gene truB blocks formation of pseudouridine 55 in tRNA in vivo, does not affect exponential growth, but confers a strong selective disadvantage in competition with wild-type cells. Rna 6, 1870–1881 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew M. et al. Protein folding in the central cavity of the GroEL–GroES chaperonin complex. Nature 379, 420–426 (1996). [DOI] [PubMed] [Google Scholar]
- Deckers-Hebestreit G. & Altendorf K. The F0F1-type ATP synthases of bacteria: structure and function of the F0 complex. Ann Rev Microbiol 50, 791–824 (1996). [DOI] [PubMed] [Google Scholar]
- Sambongi Y. et al. Mechanical rotation of the c subunit oligomer in ATP synthase (F0F1): direct observation. Science 286, 1722–1724 (1999). [DOI] [PubMed] [Google Scholar]
- Matsumura P. & Marquis R. E. Energetics of streptococcal growth inhibition by hydrostatic pressure. Appl Env Microbiol 33, 885–892 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Vossenberg J. L. C. M., Ubbink-Kok T., Elferink M. G., Driessen A. J. & Konings W. N. Ion permeability of the cytoplasmic membrane limits the maximum growth temperature of bacteria and archaea. Mol Microbiol 18, 925–932 (1995). [DOI] [PubMed] [Google Scholar]
- Bertsova Y. V. & Bogachev A. V. The origin of the sodium-dependent NADH oxidation by the respiratory chain of Klebsiella pneumoniae. FEBS Lett 563, 207–212 (2004). [DOI] [PubMed] [Google Scholar]
- Kato C. & Qureshi M. H. Pressure response in deep-sea piezophilic bacteria. J Mol Microbiol Biotechnol 1, 87–92 (1999). [PubMed] [Google Scholar]
- Abe F., Kato C. & Horikoshi K. Pressure-regulated metabolism in microorganisms. Trends Microbiol 7, 447–453 (1999). [DOI] [PubMed] [Google Scholar]
- Dyksterhouse S. E., Gray J. P., Herwig R. P., Lara J. C. & Staley J. T. Cycloclasticus pugetii gen. nov., sp. nov., an aromatic hydrocarbon-degrading bacterium from marine sediments. Int J Sys Bacteriol 45, 116–123 (1995). [DOI] [PubMed] [Google Scholar]
- De Roy K., Clement L., Thas O., Wang Y. & Boon N. Flow cytometry for fast microbial community fingerprinting. Water Res 46, 907–919 (2012). [DOI] [PubMed] [Google Scholar]
- Van Nevel S., Koetzsch S., Weilenmann H. U., Boon N. & Hammes F. Routine bacterial analysis with automated flow cytometry. J Microbiol Meth 94, 73–76 (2013). [DOI] [PubMed] [Google Scholar]
- Liu C. & Shao Z. Alcanivorax dieselolei sp. nov., a novel alkane-degrading bacterium isolated from sea water and deep-sea sediment. Int J Sys Evol Microbiol 55, 1181–1186 (2005). [DOI] [PubMed] [Google Scholar]
- Whiteside M. D., Winsor G. L., Laird M. R. & Brinkman F. S. OrtholugeDB: a bacterial and archaeal orthology resource for improved comparative genomic analysis. Nuc Acid Res 41 (D1), D366–D376 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.