Abstract
Blood vessels are exposed to multiple mechanical forces that are exerted on the vessel wall (radial, circumferential and longitudinal forces) or on the endothelial surface (shear stress). The stresses and strains experienced by arteries influence the initiation of atherosclerotic lesions, which develop at regions of arteries that are exposed to complex blood flow. In addition, plaque progression and eventually plaque rupture is influenced by a complex interaction between biological and mechanical factors—mechanical forces regulate the cellular and molecular composition of plaques and, conversely, the composition of plaques determines their ability to withstand mechanical load. A deeper understanding of these interactions is essential for designing new therapeutic strategies to prevent lesion development and promote plaque stabilization. Moreover, integrating clinical imaging techniques with finite element modelling techniques allows for detailed examination of local morphological and biomechanical characteristics of atherosclerotic lesions that may be of help in prediction of future events. In this ESC Position Paper on biomechanical factors in atherosclerosis, we summarize the current ‘state of the art’ on the interface between mechanical forces and atherosclerotic plaque biology and identify potential clinical applications and key questions for future research.
Keywords: Atherosclerosis, Haemodynamics, Blood flow, Mechanotransduction, Endothelial cell, Plaque rupture
Biomechanical forces
This Position Paper is focused on the influence of biomechanical forces on the development, function, and pathophysiology of the vasculature. In each cardiac cycle, blood is transported under pulsatile pressure through the aorta for distribution to the peripheral organs through the branching arterial system. The interactions of pulsatile blood flow with arterial geometries generate complex biomechanical forces on the vessel wall with spatial and temporal variations.
Thus, arteries are exposed to circumferential and longitudinal stresses, i.e. perpendicular and longitudinal forces generated by intraluminal pressure, and axial stress (shear stress), which acts longitudinally on the surface of the arterial wall (Figure 1). Blood vessels alter their morphology and function in response to changes in blood flow that are detected by vascular cells through decentralized mechanotransduction mechanisms.1,2 Endothelial cells (ECs) are exquisitely sensitive to shear stress, the frictional force generated by blood flow. Average wall shear stress in the healthy human aorta varies from 10 to 20 dynes/cm2 and circumferential stress varies from 1 to 2 × 106 dynes/cm2 according to anatomical site. In areas of arterial stenosis (decreased lumen area and thus radius), the same blood volume is pushed through a lower cross-sectional area and thus the blood velocity increases and as a consequence the wall shear stress increases inside the stenotic region. Furthermore, the endothelium downstream the stenosis is exposed to disturbed flow and oscillatory shear stress. Flow simulation studies describe the complex situation near arterial bifurcations and side branches, regions associated with disturbed blood flow showing repetitive phases of flow reversal resulting in steep spatial and temporal gradients in wall shear stress.3
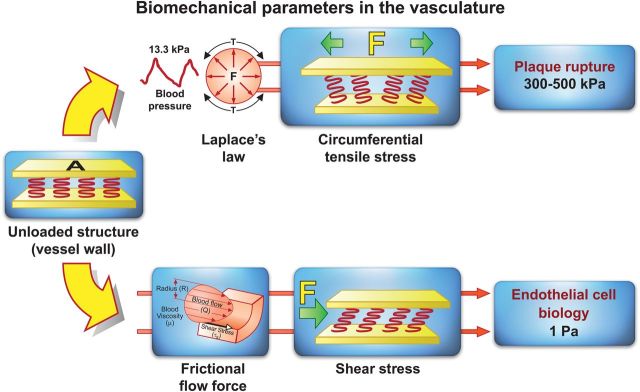
Figure 1.
Biomechanical forces acting on the arterial wall. Blood pressure and blood flow induce forces in the vascular system that deforms the vessel wall. When forces are to be compared, they need to be normalized to area. Force per area is called stress and is expressed in N/m2 or Pascal (Pa). Blood pressure produces a force directed perpendicular to the vessel wall. As a consequence, the cylindrical structure will be stretched circumferentially, resulting in a circumferential stress. Stress in the range of 300–500 kPa is associated with plaque rupture. In contrast, the force induced by a difference in movement of blood and the non-moving vessel wall leads to stress and strain parallel to the surface of endothelial cells. Due to its shearing deformation, this is called a shear stress. This shear stress is of small amplitude (1 Pa) and exerts its main effects through the activation of mechanosensitive receptors and signalling pathways.
Biomechanical regulation of arterial homeostasis
Mechanical forces regulate multiple aspects of vascular physiology and function and play a key role in vascular development and homeostatic mechanisms as well as during arterial disease. In the short term, acute increases in shear stress trigger activation of ECs and the generation of substances such as nitric oxide (NO) and prostacyclin, which promote vasodilation. On the other hand, long-term alterations in flow can lead to structural adjustments to restore vascular and mechanical homeostasis. Arterial remodelling processes including angiogenesis (growth of new blood vessels from pre-existing vessels) and arteriogenesis (collateral artery growth) are highly sensitive to local mechanical conditions.4–6 Raised levels of shear stress represent a major stimulus for exercise-induced angiogenesis, a process that involves NO signalling.7 In addition, increased flow leads to increases in arterial diameter, which promotes tissue perfusion. For example, animal studies revealed that unilateral carotid artery occlusion leads to outward remodelling of the contralateral carotid artery (in response to increased flow) and inward remodelling of the occluded artery (due to reduced flow).8,9 The molecular and cellular mechanisms that accompany arterial remodelling and repair in response to mechanical forces have only been partially defined. Studies of cultured ECs and animals demonstrated that high shear stress activates transcriptional programmes that promote proliferation and matrix remodelling, processes that are intimately involved in structural remodelling of arteries,10,11 as well as survival of ECs by inhibiting the expression of pro-apoptotic factors.12–14 Flow also influences EC migration by regulating actin cytoskeleton remodelling, cell polarity, formation of lamellipodia, and stress fibre contraction; factors that are essential for cell traction.15
While the effects of shear stress on vascular physiology have been studied in detail, the effects of mechanical stretch have received little attention. Thus, although axial and circumferential stretches also play an important role in regulating EC physiology, vascular cell proliferation, and matrix remodelling, the mechanisms involved are not well understood. Mechanical stretch regulates smooth muscle cell (SMC) functions by inducing deformation of the extracellular matrix in which SMCs are embedded, a change that is detected by mechanoreceptors.16 Physiological pulsatile circumferential stress on the arterial wall maintains medial SMCs in their contractile differentiated state.17,18 In contrast, excessive pressure increase due to hypertension or compressive forces produced by balloon angioplasty and/or stent placement stretches the artery and activates SMCs, which subsequently undergo phenotypic adaptation to a dedifferentiated synthetic state.19–21 Thus, mechanical circumferential stress modulates gene expression and SMC functions such as proliferation, survival/apoptosis, migration, and extracellular matrix remodelling through receptor-tyrosine kinases (e.g. platelet-derived growth factor receptor), focal adhesions that link the extracellular matrix and the intracellular cytoskeleton, and ion channels activating complex intracellular signalling pathways including Ras homologue gene family, member A (RhoA)/Rho kinase, mitogen-activated protein kinases (MAPKs), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3 K)/Akt, forkhead transcription factors of the FoxO subfamily, and other signalling pathways.11,19,22–24 Of note, some of these molecular mechanisms have been revealed using in vitro models and now require validation using ex vivo or in vivo systems.25,26
Biomechanical regulation of focal atherosclerosis
Shear stress and plaque initiation
Atherosclerosis is characterized by the accumulation of inflammatory cells, lipids, extracellular matrix, and other materials in the artery wall. Although atherosclerosis is associated with systemic risk factors (e.g. gender, age, and high serum cholesterol), plaques form preferentially at branches and bends in arteries that are exposed to non-uniform, disturbed patterns of blood flow.27 Two mechanisms have been identified, which could explain the link between disturbed blood flow and atherosclerosis development, namely alterations in mass transport and vascular responses to mechanical stimuli.28 The ‘mass transport theory’ states that the transport of certain bioactive substances [e.g. low-density lipoproteins (LDL)] from the circulation to the vessel wall may be promoted at sites of disturbed flow due to prolonged contact between blood and vascular ECs. This differs from the ‘shear stress theory’, which emphasizes the effects of blood flow-induced mechanical forces on vascular physiology. Of note, these theories are not mutually exclusive. Both mass transport and shear stress influence plaque formation, and these factors interact at a functional level, e.g. shear stress alters vessel permeability that, in turn, regulates molecular transport.29 Several lines of evidence suggest that shear stress regulates plaque initiation. First, fluid dynamic studies revealed that the spatial distribution of EC dysfunction, inflammation, and lesion formation in arteries correlates with the magnitude and pattern of shear stress.30–32 For example, regions exposed to low, oscillatory shear in the murine aorta are prone to lesion formation. These sites are also characterized by a highly heterogenous population of ECs that display enhanced expression of inflammatory molecules, higher rates of apoptosis and senescence, and a reduced proliferative reserve, which compromises vascular repair potential.33–41 A second important evidence for the ‘shear stress theory’ was provided in studies demonstrating a causal relationship between shear stress and atherosclerosis by applying a constrictive cuff to generate distinct shear stress environments (low, low/oscillatory, and high shear fields) in carotid arteries in rabbits and mice.42,43 Flow-dependent atherosclerosis in mice has been confirmed with other models inducing disturbed flow by partial ligation or tandem ligations of the carotid artery.44,45 There has been considerable debate over the relative importance of shear stress magnitude, frequency, or direction (e.g. oscillations, tangential shear) in dictating vascular function,46 but it is conceivable that ECs can detect changes in each of these parameters and respond accordingly. This question has been addressed using the shear stress-altering cuff model that demonstrated that low shear and low, oscillatory shear induced different vascular responses.42,43
Mechanoreceptors
Evidence for the ‘shear stress theory’ has also been obtained through the identification and characterization of mechanoreceptors. A large variety of membrane-associated molecules and microdomains have been proposed as potential shear stress sensors including ion channels [e.g. transient receptor potential (TRP) channels and P2X4 receptors], receptor-tyrosine kinases [e.g. vascular endothelial growth factor receptor (VEGFR) and angiopoietin receptor], adhesion molecules (e.g. PECAM-1/VE-cadherin/VEGFR2), the glycocalyx, membrane microdomains (e.g. primary cilia and caveolae), the cytoskeleton, and the lipid bilayer plasma membrane 47–49 (Figure 2). Several mechanoreceptors have pleiotropic functions and, therefore, influence atherosclerosis at multiple levels. For example, bone marrow cell-derived PECAM-1 has been reported to be both pro-atherogenic50 and atheroprotective,51 irrespective of the haemodynamic environment, whereas PECAM-1 in ECs accelerates atherogenesis in low shear environments.50,52 While the exact mechanisms have yet to be elucidated, targeting such receptors therapeutically will require a cell-type and context-specific strategy. Despite these insights, the mechanisms that allow cells to respond specifically to distinct mechanical conditions remain largely unknown. Thus, further studies involving specialized techniques to apply force to specific receptors or discrete regions of the cell (e.g. magnetic tweezers) are required to characterize the mechanisms that regulate the activity and function of mechanoreceptors.53
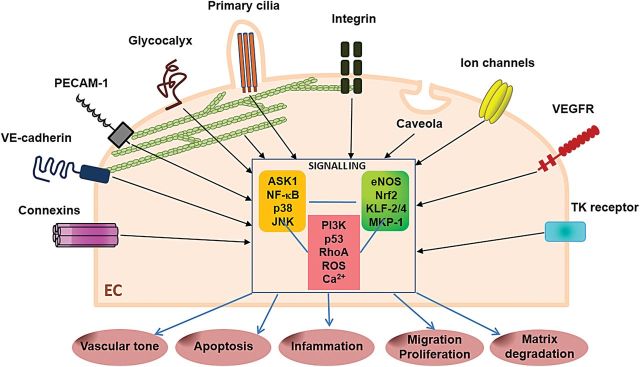
Figure 2.
Mechanoreceptors and intracellular signalling in arterial endothelium. Schematic representation of a large variety of membrane-associated molecules and microdomains that have been proposed as potential shear stress sensors converting a mechanical signal into a chemical response. Shear stress activates receptor-tyrosine kinase, such as the vascular endothelial growth factor receptor and PECAM-1, which regulate leukocyte adhesion and endothelial cell–endothelial cell coupling as well as mechanoresponsiveness. In addition to these mechanoreceptors, shear stress can also activate ion channels, actin filaments, caveolae, the glycocalyx, primary cilia, and adherence or gap junction proteins. Shear stress influences activation of endothelial cells through multiple mechanisms that target the mitogen-activated protein kinases, nuclear factor-kappa-B, and regulators of these pathways including mitogen-activated protein kinase phosphatase-1, Kruppel-like factors-2 and -4, nuclear factor erythroid 2-related factor, and endothelial nitric oxide synthase.
Shear stress and inflammatory signalling
The application of flow to cultured ECs has been used to identify causal relationships between shear stress and EC function and to define the signalling pathways involved. Shear stress influences EC inflammatory responses by modulating the expression of non-coding RNAs as well as mRNAs. Regions with disturbed flow display a focal enrichment and luminal redistribution of endothelial junctional adhesion molecule-A (JAM-A) that promotes mononuclear cell recruitment into the arterial wall. Conversely, atheroprotective laminar flow mediates repression of JAM-A through microRNA (miR)-145.54 These data identify endothelial JAM-A as a crucial effector molecule guiding inflammatory cell entry at predilection sites of atherosclerosis. Low, oscillatory shear stress influences EC expression of adhesion proteins and other inflammatory molecules through multiple mechanisms that target the MAPK pathway and the nuclear factor-kappa-B (NF-κB) pathway.36,55 In contrast, atheroprotective shear stress induces several negative regulators of inflammatory pathways including the transcription factors Kruppel-like family 2 (KLF2) and 4 (KLF4)56–58 and nuclear factor erythroid 2-related factor (Nrf2).59–63 The mechanism for KLF2 activation by shear stress involves ERK5-MEF2 signalling, which activates the KLF2 promoter,58,64–66 and suppression of miR-92a, which is a negative regulator of KLF2 and KLF4 mRNA expressions.67,68 Conversely, miR-92a is expressed by ECs in atheroprone low shear stress regions, increased by hypercholesterolaemia, and in vivo miR-92a blockade by antagomir treatment protects against the development of atherosclerosis.69 High unidirectional shear stress also reduces inflammatory MAP kinases by inhibiting ASK-1 (an inflammatory MAP kinase kinase kinase),70 blocking cleavage of protein kinase C epsilon (PKCζ),71 inducing MAPK phosphatase-1 (MKP-1), a negative regulator of p38 and JNK MAP kinases,37 and via down-regulation of the angiotensin II type 1 receptor.72,73
In contrast, low shear stress enhances NF-κB expression via activation of a JNK1-ATF2 transcriptional programme36 and promotes NF-κB activation via induction of positive regulators [e.g. Toll-like receptors,74 bone morphogenic proteins,75–77 inhibitor of κB kinase 2 (IKK238), and reactive oxygen species55,78,79]. In addition to microRNA control,68 recent epigenetic regulation of pro- and anti-inflammatory gene expression in disturbed flow regions has been demonstrated including altered flow-induced DNA methylation of endothelium mediated by DNA methyltransferases.80–83 Thus, low oscillatory shear stress induces pro-atherogenic epigenetic and transcriptional programmes in EC, whereas high unidirectional shear induces multiple anti-inflammatory processes.
Shear stress and endothelial apoptosis, senescence, and proliferation
Shear stress can also influence EC injury by inducing signalling pathways that regulate apoptosis or senescence (Figure 3). Disturbed flow induces EC apoptosis through multiple mechanisms including activation of PKCζ,40 JNK MAP kinase,84,85 and p53,86 and through up-regulation of an unfolded protein response signalling pathway.39 In contrast, uniform flow suppresses apoptosis via the up-regulation and/or activation of protective signalling pathways involving superoxide dismutase and NO synthase, for example.70,84,87–91
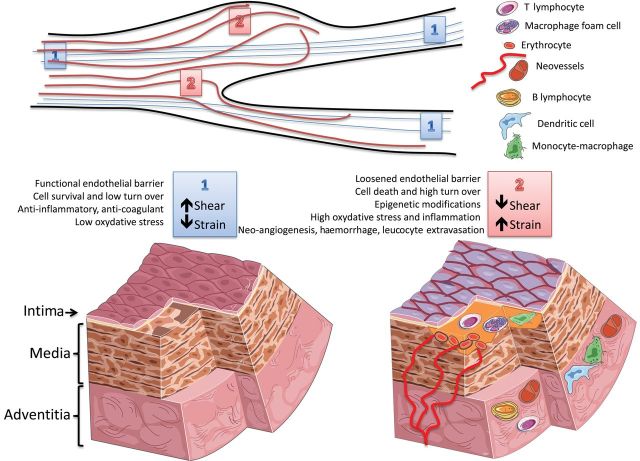
Figure 3.
Effects of shear and strain on the arterial wall. (top) Schematic representation of different biomechanical forces along the arterial tree; 1 = laminar flow (blue lines) imposing a high shear stress parallel to the vascular wall and a low circumferential strain; 2 = arterial regions with a change in the diameter (lack of wall parallelism) and/or proximity to bifurcations (presence of disturbed flow, red line) are subjected to a relatively lower shear stress and higher strain. (bottom left) High shear stress and low strain (‘1’) contribute to maintenance of the physiological properties of the endothelial barrier (anti-coagulant, anti-inflammatory, and anti-oxidant properties) and of the vessel wall (homeostatic cell and matrix turn-over). (bottom right) Low shear and high strain (‘2’) cause endothelial cell death and reduce the physiological endothelial barrier function, thus favouring the formation of atherosclerotic plaques (yellow matter). Plaque progression can also be affected by biomechanical factors inducing an accelerated cell and matrix turn-over, modifications of the vascular stromal cells, inflammation, and intraplaque haemorrhage. This can boost plaque growth and in turn impact on the local flow dynamics, thus generating a vicious circle between biomechanical factors and atherosclerosis.
Atheroprone sites are associated with higher rates of EC proliferation compared with protected regions,33,41,85,92 a feature that may enhance vascular permeability to LDL and other atherogenic molecules. However, a recent study also indicates that low, oscillatory shear stress can induce EC senescence via activation of p53.93 This seemingly paradoxical situation emphasizes the complex heterogenous nature of EC phenotypes at atheroprone sites. The molecular mechanisms linking shear stress with EC mitosis are uncertain, but it has been established that JNK1 positively regulates proliferation at atheroprone sites, whereas the induction of the cyclin-dependent kinase regulator GADD45 promotes quiescence under high shear stress conditions.94 In addition, down-regulation of miR-126-5p by disturbed flow abrogates EC proliferation at atherosusceptible sites by up-regulating the Notch1 inhibitor Dlk1.41 Administration of miR-126-5p rescued EC proliferation at disease-prone sites and limited atherosclerosis, demonstrating the importance of an EC proliferative reserve in the prevention of atherosclerosis and pointing towards a possible therapeutic approach.
Gene discovery platforms for endothelial cell mechanosignalling pathways
The identification of biomarkers for atherosclerosis (e.g. proteins, lipids, or RNA) is an emerging area of research. While classical techniques focus on individual or few factors, the development of high-throughput strategies, i.e. -omics approaches, allows comparisons of protein expression patterns or lipidomic profiles at a broad level in a single experiment.95 Omics studies have been performed to characterize mechanosensitive signalling pathways directly in ECs from arterial sites 33,34 and in many cultured cell experiments.10,96–99 In cultured ECs, 900–1800 genes were regulated by varying levels of fluid flow, whereas studies performed in vivo show a lower number of differentially expressed genes. The gene profiles associated with the differentially regulated genes show high variability depending on experimental conditions, and importantly, the bioinformatics methods used to analyse the data. Despite this high variability, the current data sets can be summarized with the concept of endothelial priming. In this concept, unidirectional high shear stress confers protection by an up-regulation of anti-atherogenic, anti-thrombotic, and anti-inflammatory gene signatures, whereas low oscillatory shear stress induces pro-thrombotic and pro-inflammatory genes.99 Consequently, regions exposed to low shear stress are pre-disposed to atherosclerosis and are more sensitive to high cholesterol levels and inflammatory mediators, whereas regions exposed to high shear stress are protected. Recent studies in intact non-atherosclerotic animals confirmed these in vitro studies and suggest that endothelial priming occurs in vivo, and this might be one of the reasons for the presence of predilection sites.100 New studies focused on obtaining changes in gene networks in ECs during plaque development are under way and will shed new light on how ECs react to a combination of mechanical stimuli and an inflamed sub-endothelium.
Plaque progression and remodelling
Arterial sites with developing atherosclerotic plaques undergo compensatory expansive remodelling to maintain their luminal diameter, a process that presumably normalizes shear stress to a constant level.101,102,103 Although compensatory remodelling is considered as the ‘classical’ remodelling response during plaque growth, constrictive remodelling (defined as shrinkage of the vessel radius)104 and excessive compensatory remodelling105 (defined as over compensation leading to radius increase) are observed in a small percentage of arteries.106 In general, outward remodelling will lead to a persistence of low shear stress, thereby exaggerating lipid uptake and inflammation. Since inflammation has been associated with positive outward remodelling,107 it may contribute to further development of vulnerable plaques. As a result, plaques with a large necrotic core are found at low shear stress locations.105,108–110 Interestingly, regional differences have been observed in vascular remodelling responses, e.g. there is less compensation for plaque growth in arteries in the lower extremities.111
Plaque evolution after remodelling
Upon further progression of plaques, positive remodelling can no longer compensate plaque growth resulting in narrowing of the vessel lumen. In general, lumen narrowing initiates when plaque burden exceeds 40%.101 While the precise mechanism underlying the limitation in outward remodelling is unknown, intraplaque bleeding,112,113 multiple plaque ruptures,114 and a circumferential extension of endothelial dysfunction at the plaque site have been put forward as possible explanations.101 Once atherosclerotic plaques encroach into the lumen, ECs experience a change in local shear stress, i.e. high shear stress at the upstream part and low, oscillatory shear stress at the downstream side of the plaque, where initially low shear stress was present.108 There is a lack of detailed information as to whether ECs covering the advanced atherosclerotic lesion remain responsive to changes in local shear stress. On the one hand, the shear stress-dependent transcription factor KLF2 seems down-regulated,57 cross-talk between ECs via connexins is diminished115 and endothelial nitric oxide synthase expression is decreased at plaques.116 On the other hand, a preferential occurrence of apoptosis of ECs is present in the downstream regions of advanced human carotid lesions.14 In addition, studies of stented arteries suggest that ECs overlaying plaques retain the ability to respond to flow.117–119 In summary, whereas plaque initiation typically occurs in low shear stress regions, plaque progression may be accompanied by (excessive) compensatory remodelling, thereby keeping the lumen open and maintaining the low shear stress exposure to the plaque. Plaques may also encroach into the lumen resulting in exposure to high shear stress for ECs in the upstream region of the plaque.
The influence of biomechanical forces on plaque destabilization and rupture
Pathological studies suggest that a large necrotic core, high macrophage content, reduced collagen levels, and thin fibrous cap are the hallmarks of plaque vulnerability120–123 and thus may be the precursors of plaque rupture. However, a recent study indicates that only 5% of the identified vulnerable plaques (thin-capped fibroatheromas, TCFAs) are associated with plaque rupture and suggests that plaque morphology is not sufficient to predict plaque rupture.124,125 As biomechanical factors are involved in plaque rupture, they might help to identify vulnerable plaques.
The role of wall stress in plaque rupture
A plaque ruptures if the local wall stress (i.e. stress within an atherosclerotic lesion) exceeds the fracture stress (strength) of the fibrous cap. Note that the stress in the wall is caused by a variety of factors, including the blood pressure, local geometry, and local tissue composition and is 1 × 104 to 2 × 106 times higher than the shear stress at the endothelium.126 Moreover, maximal predicted plaque stresses in symptomatic patients are higher than those predicted in asymptomatic patients, suggesting that plaques with higher stresses may be more prone to rupture and thus leading to cardiovascular events.127 Biomechanical stress could therefore potentially act as a useful tool for risk assessment of plaque rupture. However, the threshold value for wall stress to be used for risk prediction is currently under debate.128
Plaque composition influences rupture as it is a key determinant of cap strength. The highest wall stress is typically found at the thinnest areas of the fibrous cap,129,130 a region that co-localizes with increased macrophage density,131 intraplaque haemorrhage,132 and local microcalcifications.133
The role of low and high shear stress in plaque destabilization
The causative role of low shear stress in vulnerable plaque formation was elegantly shown in several animal studies imposing low shear stress in defined arterial regions.42,134 Although these studies clearly demonstrate that low shear stress modulates local inflammation and thereby cap thickness and strength, the majority of such studies have concentrated on relatively few locations in mature arteries and thus may have introduced an underestimation of the variety of mechanical factors involved in disease development as was eloquently pointed out by Peiffer et al.46
The notion that plaque ruptures/ulcerations are most frequently observed at the upstream side of advanced plaques has strengthened the idea that high shear stress may be involved in upstream plaque destabilization.135–137 Moreover, plaque composition at the upstream side of the plaque is markedly different from the downstream side, i.e. enhanced macrophage accumulation and apoptosis, lipid accumulation, intraplaque haemorrhage, and thinner fibrous caps.135,137 As a result, upstream plaque regions that are exposed to high shear stress show an increased strain—a local measure for plaque weakness—implying that those regions are more prone to rupture.138 In vivo studies on the role of shear stress in plaque destabilization confirmed increased vulnerability for the high shear stress plaque regions at 6 months of follow-up.110 High shear stress is known to activate matrix metalloproteinases (MMPs), favouring thinning of the artery wall and eccentric remodelling in an in vivo arteriovenous fistula model.139 If a similar process occurs in the advanced atherosclerotic lesion, this might account for a thin fibrous cap in high shear regions of the stenosis. Clearly, more studies are needed to investigate the potential causative role of high shear stress in plaque destabilization.
Location of plaque rupture
As plaque rupture depends on both the local wall stress and the local strength of the tissue, we would like to propose that co-localization of high wall stress and shear stress-induced plaque weakening will finally lead to plaque rupture. Figure 4 depicts the relationship between shear stress, plaque geometry, the plaque strength—the stress threshold at which a plaque ruptures—and the local wall stress in the process of plaque rupture. The minor co-incidence that wall stress exceeds the local plaque strength and the short time frame may offer an explanation why only 5% of TCFAs rupture. Shear stress is a biomechanical parameter acting for many years on TCFA formation and cap weakening through biological processes. On the other hand, wall stress concentrations are thought to lead to plaque rupture over much shorter time frames. Further experimental and clinical imaging studies are needed to investigate the co-localization of these biomechanical parameters in identifying ‘rupture-prone TCFAs’.
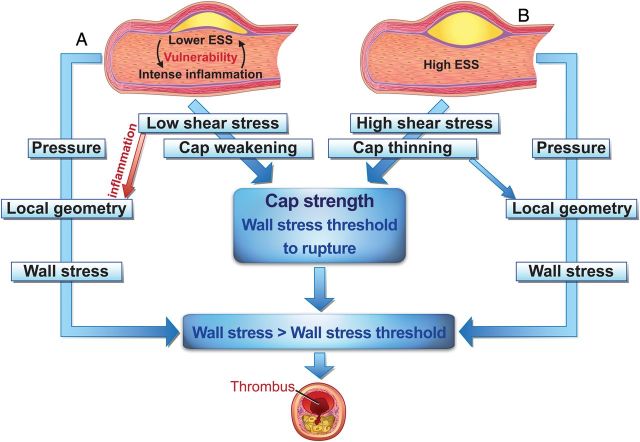
Figure 4.
Concept of the influence of shear stress and wall stress on plaque rupture. Co-localization of peak wall stress and shear stress-induced cap thinning and cap strength will dictate location and timing of plaque rupture. (A) (Excessive) compensatory remodelling induces low shear stress stimulating local inflammation and thereby fibrous cap thinning and plaque weakening, influencing the cap strength, (B) high shear stress induces cap thinning and weakening. Wall stress inside the cap is related to blood pressure and the local cap geometry and thickness. If the local wall stress exceeds the cap strength (the wall stress threshold at which it ruptures), the cap will rupture.
In summary, low shear stress promotes the initiation and progression of atherosclerotic lesions. Non-stenotic vulnerable plaques are typically associated with low shear stress, which can promote inflammation and influence plaque stability. This contrasts with stenotic high-risk plaques that are typically exposed to high shear stress. Evidence is accumulating for a role of high shear stress in plaque destabilization. Finally, co-localization of high wall stress and low plaque strength may be considered as a novel future marker for identification of vulnerable plaques.
Clinical perspectives
Interactions between drugs and mechanoresponses
Biomechanical factors may affect the responsiveness of ECs to pharmacological agents, as demonstrated by the synergy between statins and laminar shear stress in inducing KLF2-dependent atheroprotective signalling in ECs both in vivo and in vitro.140,141 Consequently, the endothelium lining atherosusceptible sites of low shear stress may be less responsive to the pleiotropic effects of statins,140 highlighting the importance of considering biomechanical factors in the development of atheroprotective therapeutics. In addition, changes in biomechanical forces can be used for targeted drug delivery to atherosclerotic lesions.142 Mechanosensitive liposomes can be used to preferentially release preloaded drugs under increased shear stress143 and could thus potentially selectively target the upstream segment of the advanced plaque (before the point of maximal stenosis) that shows an increased incidence of plaque rupture, as discussed above. In silico tests with these 1,3-diaminophospholipid vesicles show promising results,143 but validation in more complex fluids and large animals are mandatory. Another approach includes shear-activated nanotherapeutic aggregates.144 This strategy also uses high shear stress caused by vascular narrowing as a targeting mechanism to deliver drugs to (partially) obstructed blood vessels. Microscale aggregates of nanoparticles coated with tissue plasminogen activator (tPA) break into nanoscale components when exposed to abnormally high fluid shear stress. When administered intravenously in mice, these shear-activated nanotherapeutics induce rapid clot dissolution in a mesenteric injury model.
Diagnostic and prognostic implications of biomechanical factors
The implementation of biomechanical factors in the clinical decision-making for patients with atherosclerosis is today restricted to measurements of flow or pressure alterations. The fractional flow reserve (FFR) of a coronary atherosclerotic lesion can be measured as the pressure fall from the proximal aorta to the coronary segment distal to the lesion, during maximal coronary vasodilatation. According to the latest ESC guidelines, percutaneous coronary intervention (PCI) is indicated if FFR is ≤0.8.145 FFR-guided PCI has been associated with improved clinical outcomes and fewer stents implanted. Likewise, non-invasively measured coronary flow reserve (CFR) by transthoracic Doppler echocardiography of the left anterior descending coronary artery is recommended for patients with suspected coronary microvascular disease.145 However, limiting interventions to only obstructive coronary disease may be insufficient, since plaques not causing haemodynamically significant flow restriction may be prone to rupture. One important clinical application of the above outlined role of biomechanical factors in atherosclerosis could be to identify sites exposed to unfavourable biomechanical forces, associated with high risk of plaque rupture, and to use this information to guide treatment. The development of novel imaging tools has rendered the evaluation of wall shear stress possible in coronary patients, by integrating clinical examination techniques (Doppler ultrasound, CT, IVUS, OCT, MRI, VH) with computational flow dynamics (CFD). Of note, a recent study revealed that rotational coronary angiography and CFD could be used to accurately measure FFR in patients with stable angina, thus informing PCI without the need for invasive catheter-based measurements.146,147 In addition, phase-contrast MRI-based shear stress measurement techniques are currently under development to assess the local wall shear stress distribution in human carotid arteries, likely facilitating in the near future the clinical assessment of local wall shear stress without extensive technical expertise. Finally, intravascular palpography can be used for measures of plaque deformation (strain) during pulsating blood flow.
Flow-mediated dilatation (FMD) of a conduit artery following limb ischaemia is a measure of endothelial function, and an impaired FMD is a sign of endothelial dysfunction in for example diabetes and subclinical atherosclerosis.148 In contrast to the transient increase in shear stress during reactive hyperaemia, exercise-induced elevations of shear stress may be associated with a more sustained FMD increase in the supplying conduit artery.149 In addition to these immediate flow alterations, also long-term effects on FMD have been demonstrated after repetitive exercise, suggesting that exercise-induced changes in shear stress induce beneficial effects in terms of flow-mediated endothelial function and vascular remodelling,150 which may be implicated in the protective value of physical activity in reducing vascular dysfunction and atherosclerosis.
Recently, the PREDICTION study revealed that low shear stress was an independent predictor for luminal obstruction in patients with acute coronary syndrome, but was not associated with a change in plaque area.108 Although clinical events rates were too low to evaluate the effects of shear stress on outcome in terms of acute coronary syndromes,108 this study provides an initial indication that considering biomechanical factors may be clinically relevant for assessing locations with progressive disease.
Knowledge from basic science is increasingly being translated into the clinical setting. A deeper understanding of the effects of mechanical forces on vascular biology will further these developments of novel shear regulated drugs, enhance diagnostic tools and inform clinical decision-making for interventional cardiologists and cardiovascular surgeons. Similarly, innovations in the clinic should feedback to drive new basic science questions in the fields of vascular biology, engineering, and computational modelling.
Funding
This position paper was funded by the European Society of Cardiology.
Conflict of interest: P.F.D. holds the patent 6399311 B2 issued 4 June 2002. R.V.: Abbott Vascular, Biosensors International, Boston Scientific, CeloNova, Cordis J&J, Lutonix, Medtronic, Terumo, Merck Speaker's Bureau, 480 Biomedical, WL Gore, outside the submitted work. C.W. has received grants from BMBF, during the conduct of the study; and grants from DFG and from ERC, outside the submitted work.
References
- 1.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw A, Xu Q. Biomechanical stress-induced signaling in smooth muscle cells: an update. Curr Vasc Pharmacol. 2003;1:41–58. doi: 10.2174/1570161033386745. [DOI] [PubMed] [Google Scholar]
- 3.Steinman DA. Simulated pathline visualization of computed periodic blood flow patterns. J Biomech. 2000;33:623–628. doi: 10.1016/s0021-9290(99)00205-5. [DOI] [PubMed] [Google Scholar]
- 4.Hoefer IE, den Adel B, Daemen MJ. Biomechanical factors as triggers of vascular growth. Cardiovasc Res. 2013;99:276–283. doi: 10.1093/cvr/cvt089. [DOI] [PubMed] [Google Scholar]
- 5.Chouinard-Pelletier G, Jahnsen ED, Jones EA. Increased shear stress inhibits angiogenesis in veins and not arteries during vascular development. Angiogenesis. 2013;16:71–83. doi: 10.1007/s10456-012-9300-2. [DOI] [PubMed] [Google Scholar]
- 6.Egginton S. In vivo shear stress response. Biochem Soc Trans. 2011;39:1633–1638. doi: 10.1042/BST20110715. [DOI] [PubMed] [Google Scholar]
- 7.Hudlicka O, Brown MD, May S, Zakrzewicz A, Pries AR. Changes in capillary shear stress in skeletal muscles exposed to long-term activity: role of nitric oxide. Microcirculation. 2006;13:249–259. doi: 10.1080/10739680600556951. [DOI] [PubMed] [Google Scholar]
- 8.Masuda H, Zhuang YJ, Singh TM, Kawamura K, Murakami M, Zarins CK, Glagov S. Adaptive remodeling of internal elastic lamina and endothelial lining during flow-induced arterial enlargement. Arterioscler Thromb Vasc Biol. 1999;19:2298–2307. doi: 10.1161/01.atv.19.10.2298. [DOI] [PubMed] [Google Scholar]
- 9.Langille BL, Odonnell F. Reductions in arterial diameter produced by chronic decreases in blood-flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 10.Chen BPC, Li YS, Zhao YH, Chen KD, Li S, Lao JM, Yuan SL, Shyy JYJ, Chien S. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics. 2001;7:55–63. doi: 10.1152/physiolgenomics.2001.7.1.55. [DOI] [PubMed] [Google Scholar]
- 11.Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. P47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res. 2005;97:533–540. doi: 10.1161/01.RES.0000181759.63239.21. [DOI] [PubMed] [Google Scholar]
- 12.Urbich C, Walter DH, Zeiher AM, Dimmeler S. Laminar shear stress upregulates integrin expression: role in endothelial cell adhesion and apoptosis. Circ Res. 2000;87:683–689. doi: 10.1161/01.res.87.8.683. [DOI] [PubMed] [Google Scholar]
- 13.Chen BP, Li YS, Zhao Y, Chen KD, Li S, Lao J, Yuan S, Shyy JY, Chien S. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics. 2001;7:55–63. doi: 10.1152/physiolgenomics.2001.7.1.55. [DOI] [PubMed] [Google Scholar]
- 14.Tricot O, Mallat Z, Heymes C, Belmin J, Lesèche G, Tedgui A. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101:2450–2453. doi: 10.1161/01.cir.101.21.2450. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Butler P, Wang YX, Hu YL, Han DC, Usami S, Guan JL, Chien S. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci USA. 2002;99:3546–3551. doi: 10.1073/pnas.052018099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Int Med. 2006;259:381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 17.Birukov KG, Bardy N, Lehoux S, Merval R, Shirinsky VP, Tedgui A. Intraluminal pressure is essential for the maintenance of smooth muscle caldesmon and filamin content in aortic organ culture. Arterioscler Thromb Vasc Biol. 1998;18:922–927. doi: 10.1161/01.atv.18.6.922. [DOI] [PubMed] [Google Scholar]
- 18.Stegemann JP, Hong H, Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J App Physiol. 2005;98:2321–2327. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- 19.Haga J, Li Y-SJ, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells (vol. 40, p. 947, 2007) J Biomech. 2008;41:2331. doi: 10.1016/j.jbiomech.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Shyu KG. Cellular and molecular effects of mechanical stretch on vascular cells and cardiac myocytes. Clin Sci. 2009;116:377–389. doi: 10.1042/CS20080163. [DOI] [PubMed] [Google Scholar]
- 21.Chaabane C, Otsuka F, Virmani R, Bochaton-Piallat ML. Biological responses in stented arteries. Cardiovasc Res. 2013;99:353–363. doi: 10.1093/cvr/cvt115. [DOI] [PubMed] [Google Scholar]
- 22.Lemarie CA, Tharaux PL, Esposito B, Tedgui A, Lehoux S. Transforming growth factor-alpha mediates nuclear factor kappa B activation in strained arteries. Circ Res. 2006;99:434–441. doi: 10.1161/01.RES.0000237388.89261.47. [DOI] [PubMed] [Google Scholar]
- 23.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abid MR, Yano K, Guo S, Patel VI, Shrikhande G, Spokes KC, Ferran C, Aird WC. Forkhead transcription factors inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia. J Biol Chem. 2005;280:29864–29873. doi: 10.1074/jbc.M502149200. [DOI] [PubMed] [Google Scholar]
- 25.Anwar M, Shalhoub J, Lim C, Gohel M, Davies A. The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. J Vasc Res. 2012;49:463–478. doi: 10.1159/000339151. [DOI] [PubMed] [Google Scholar]
- 26.Qiu J, Zheng Y, Hu J, Liao D, Gregersen H, Deng X, Fan Y, Wang G. Biomechanical regulation of vascular smooth muscle cell functions: from in vitro to in vivo understanding. J R Soc Interface. 2014;11:20130852. doi: 10.1098/rsif.2013.0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 28.Back M, Gasser T, Michel JB, Caligiuri G. Biomechanical factors in the biology of aortic wall and aortic valve diseases. Cardiovasc Res. 2013;99:232–241. doi: 10.1093/cvr/cvt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jo H, Dull RO, Hollis TM, Tarbell JM. Endothelial albumin permeability is shear dependent, time-dependent, and reversible. Am J Physiol. 1991;260:H1992–H1996. doi: 10.1152/ajpheart.1991.260.6.H1992. [DOI] [PubMed] [Google Scholar]
- 30.Davies PF. Endothelial mechanisms of flow-mediated athero-protection and susceptibility. Circ Res. 2007;101:10–12. doi: 10.1161/CIRCRESAHA.107.156539. [DOI] [PubMed] [Google Scholar]
- 31.Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas—implications for atherogenesis. Arterioscler Thromb Vasc Biol. 2007;27:346–351. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]
- 32.Dai GH, Kaazempur-Mofrad MR, Natarajan S, Zhang YZ, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci USA. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foteinos G, Hu YH, Xiao QZ, Metzler B, Xu QB. Rapid endothelial turnover in atherosclerosis-prone areas coincides with stem cell repair in apolipoprotein E-deficient mice. Circulation. 2008;117:1856–1863. doi: 10.1161/CIRCULATIONAHA.107.746008. [DOI] [PubMed] [Google Scholar]
- 34.Zeng LF, Zampetaki A, Margariti A, Pepe AE, Alam S, Martin D, Xiao QZ, Wang W, Jin ZG, Cockerill G, Mori K, Li YSJ, Hu YH, Chien S, Xu QB. Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proc Natl Acad Sci USA. 2009;106:8326–8331. doi: 10.1073/pnas.0903197106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci USA. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuhlmann S, Van der Heiden K, Saliba D, Tremoleda JL, Khalil M, Zakkar M, Chaudhury H, Le AL, Mason JC, Udalova I, Gsell W, Jones H, Haskard DO, Krams R, Evans PC. Disturbed blood flow induces RelA expression via c-Jun N-terminal kinase 1 A novel mode of NF-kappa B regulation that promotes arterial inflammation. Circ Res. 2011;108:950–959. doi: 10.1161/CIRCRESAHA.110.233841. [DOI] [PubMed] [Google Scholar]
- 37.Zakkar M, Chaudhury H, Sandvik G, Enesa K, Luong LA, Cuhlmann S, Mason JC, Krams R, Clark AR, Haskard DO, Evans PC. Increased endothelial mitogen-activated protein kinase phosphatase-1 expression suppresses proinflammatory activation at sites that are resistant to atherosclerosis. Circ Res. 2008;103:726–732. doi: 10.1161/CIRCRESAHA.108.183913. [DOI] [PubMed] [Google Scholar]
- 38.Passerini AG, Polacek DC, Shi CZ, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci USA. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res. 2009;105:453–U127. doi: 10.1161/CIRCRESAHA.109.203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magid R, Davies PF. Endothelial protein kinase C isoform identity and differential activity of PKC xi in an athero-susceptible region of porcine aorta. Circ Res. 2005;97:443–449. doi: 10.1161/01.RES.0000179767.37838.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M, Wang S, Kiessling F, Olson EN, Weber C. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJAP, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 43.Cheng C, van Haperen R, de Waard M, van Damme LCA, Tempel D, Hanemaaijer L, van Cappellen GWA, Bos J, Slager CJ, Duncker DJ, van der Steen AFW, de Crom R, Krams R. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood. 2005;106:3691–3698. doi: 10.1182/blood-2005-06-2326. [DOI] [PubMed] [Google Scholar]
- 44.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–H1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen YC, Bui AV, Diesch J, Manasseh R, Hausding C, Rivera J, Haviv I, Agrotis A, Htun NM, Jowett J, Hagemeyer CE, Hannan RD, Bobik A, Peter K. A novel mouse model of atherosclerotic plaque instability for drug testing and mechanistic/therapeutic discoveries using gene and microRNA expression profiling. Circ Res. 2013;113:252–265. doi: 10.1161/CIRCRESAHA.113.301562. [DOI] [PubMed] [Google Scholar]
- 46.Peiffer V, Sherwin SJ, Weinberg PD. Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? A systematic review. Cardiovasc Res. 2013;99:242–250. doi: 10.1093/cvr/cvt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ando J, Yamamoto K. Flow detection and calcium signalling in vascular endothelial cells. Cardiovasc Res. 2013;99:260–268. doi: 10.1093/cvr/cvt084. [DOI] [PubMed] [Google Scholar]
- 48.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao GY, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 49.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell-surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 50.Harrison M, Smith E, Ross E, Krams R, Segers D, Buckley CD, Nash GB, Rainger G. The role of platelet-endothelial cell adhesion molecule-1 in atheroma formation varies depending on the site-specific hemodynamic environment. Arterioscler Thromb Vasc Biol. 2013;33:694–701. doi: 10.1161/ATVBAHA.112.300379. [DOI] [PubMed] [Google Scholar]
- 51.Goel R, Schrank BR, Arora S, Boylan B, Fleming B, Miura H, Newman PJ, Molthen RC, Newman DK. Site-specific effects of PECAM-1 on atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1996–2002. doi: 10.1161/ATVBAHA.108.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harry BL, Sanders JM, Feaver RE, Lansey M, Deem TL, Zarbock A, Bruce AC, Pryor AW, Gelfand BD, Blackman BR, Schwartz MA, Ley K. Endothelial cell PECAM-1 promotes atherosclerotic lesions in areas of disturbed flow in ApoE-deficient mice. Arterioscler Thromb Vasc Biology. 2008;28:2003–2008. doi: 10.1161/ATVBAHA.108.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collins C, Guilluy C, Welch C, O'Brien ET, Hahn K, Superfine R, Burridge K, Tzima E. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr Biol. 2012;22:2087–2094. doi: 10.1016/j.cub.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitt MMN, Megens RTA, Zernecke A, Bidzhekov K, van den Akker NM, Rademakers T, van Zandvoort MA, Hackeng TM, Koenen RR, Weber C. Endothelial junctional adhesion molecule-A guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation. 2014;129:66–76. doi: 10.1161/CIRCULATIONAHA.113.004149. [DOI] [PubMed] [Google Scholar]
- 55.Xiao H, Lu M, Lin TY, Chen Z, Chen G, Wang WC, Marin T, Shentu Tp, Wen L, Gongol B, Sun W, Liang X, Chen J, Huang HD, Pedra JH, Johnson DA, Shyy JYJ. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128:632–642. doi: 10.1161/CIRCULATIONAHA.113.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJG. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 57.Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJ. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol. 2005;167:609–618. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parmar KM, Larman HB, Dai GH, Zhang YH, Wang ET, Moorthy SN, Kratz JR, Lin ZY, Jain MK, Gimbrone MA, Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fledderus JO, Boon RA, Volger OL, Hurttila H, Yla-Herttuala S, Pannekoek H, Levonen AL, Horrevoets AJ. KLF2 Primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1339–1346. doi: 10.1161/ATVBAHA.108.165811. [DOI] [PubMed] [Google Scholar]
- 60.Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA. Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/akt-dependent activation of Nrf2. Circ Res. 2007;101:723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 61.Hosoya T, Maruyama A, Kang MI, Kawatani Y, Shibata T, Uchida K, Itoh K, Yamamoto M. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem. 2005;280:27244–27250. doi: 10.1074/jbc.M502551200. [DOI] [PubMed] [Google Scholar]
- 62.Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells—a novel anti-inflammatory mechanism. J Biol Chem. 2003;278:703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 63.Zakkar M, Van der Heiden K, Luong LA, Chaudhury H, Cuhlmann S, Hamdulay SS, Krams R, Edirisinghe I, Rahman I, Carlsen H, Haskard DO, Mason JC, Evans PC. Activation of nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol. 2009;29:1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- 64.Yan C, Takahashi M, Okuda M, Lee JD, Berk BC. Fluid shear stress stimulates big mitogen-activated protein kinase 1 (BMK1) activity in endothelial cells—dependence on tyrosine kinases and intracellular calcium. J Biol Chem. 1999;274:143–150. doi: 10.1074/jbc.274.1.143. [DOI] [PubMed] [Google Scholar]
- 65.Yan C, Luo HL, Lee JD, Abe JI, Berk BC. Molecular cloning of mouse ERK5/BMK1 splice variants and characterization of ERK5 functional domains. J Biol Chem. 2001;276:10870–10878. doi: 10.1074/jbc.M009286200. [DOI] [PubMed] [Google Scholar]
- 66.Young A, Wu W, Sun W, Larman HB, Wang N, Li YS, Shyy JY, Chien S, Garcia-Cardena G. Flow activation of AMP-activated protein kinase in vascular endothelium leads to Kruppel-like factor 2 expression. Arterioscler Thromb Vasc Biol. 2009;29:1902–1908. doi: 10.1161/ATVBAHA.109.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu W, Xiao H, Laguna-Fernandez A, Villarreal G, Jr, Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J, Li YS, Chien S, Garcia-Cardena G, Shyy JYJ. Flow-dependent regulation of Kruppel-like factor 2 is mediated by microRNA-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol. 2012;32:979–987. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loyer X, Potteaux S, Vion AC, Guérin CL, Boulkroun S, Rautou PE, Ramkhelawon B, Esposito B, Dalloz M, Paul JL, Julia P, Maccario J, Boulanger CM, Mallat Z, Tedgui A. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res. 2014;114:434–443. doi: 10.1161/CIRCRESAHA.114.302213. [DOI] [PubMed] [Google Scholar]
- 70.Liu YM, Yin GY, Surapisitchat J, Berk BC, Min W. Laminar flow inhibits TNF-induced ASK1 activation by preventing dissociation of ASK1 from its inhibitor 14-3-3. J Clin Invest. 2001;107:917–923. doi: 10.1172/JCI11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Traub O, Monia BP, Dean NM, Berk BC. PKC-epsilon is required for mechano-sensitive activation of ERK1/2 in endothelial cells. J Biol Chem. 1997;272:31251–31257. doi: 10.1074/jbc.272.50.31251. [DOI] [PubMed] [Google Scholar]
- 72.Ramkhelawon B, Vilar J, Rivas D, Mees B, de Crom R, Tedgui A, Lehoux S. Shear stress regulates angiotensin type 1 receptor expression in endothelial cells. Circ Res. 2009;105:869–875. doi: 10.1161/CIRCRESAHA.109.204040. [DOI] [PubMed] [Google Scholar]
- 73.Ramkhelawon B, Rivas D, Lehoux S. Shear stress activates extracellular signal-regulated kinase 1/2 via the angiotensin II type 1 receptor. FASEB J. 2013;27:3008–3016. doi: 10.1096/fj.12-222299. [DOI] [PubMed] [Google Scholar]
- 74.Mullick AE, Soldau K, Kiosses WB, Bell TA, Tobias PS, Curtiss LK. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem. 2003;278:31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 76.Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries—role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation. 2007;116:1258–1266. doi: 10.1161/CIRCULATIONAHA.106.683227. [DOI] [PubMed] [Google Scholar]
- 77.Ni CW, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, Visvader JE, Jo H. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116:E66–E73. doi: 10.1182/blood-2010-04-278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mohan S, Koyoma K, Thangasamy A, Nakano H, Glickman RD, Mohan N. Low shear stress preferentially enhances IKK activity through selective sources of ROS for persistent activation of NF-kappa B in endothelial cells. Am J Physiol Cell Physiol. 2007;292:C362–C371. doi: 10.1152/ajpcell.00535.2005. [DOI] [PubMed] [Google Scholar]
- 79.Wang C, Baker BM, Chen CS, Schwartz MA. Endothelial cell sensing of flow direction. Arterioscler Thromb Vasc Biol. 2013;33:2130–2136. doi: 10.1161/ATVBAHA.113.301826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang Y-Z, Jiménez JM, Ou K, McCormick ME, Davies PF. Differential DNA methylation of endothelial Kruppel-like factor 4 (KLF4) promoter in response to hemodynamic disturbed flow in vitro and in vivo. Circ Res. 2014;115:32–43. doi: 10.1161/CIRCRESAHA.115.303883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dunn J, Qiu H, Kim S, Jjingo D, Hoffman R, Kim CW, Jang I, Son DJ, Kim D, Pan C, Fan Y, Jordan IK, Jo H. Flow alters genome-wide methylation, regulating gene expression and atherosclerosis. J Clin Invest. 2014;124:3187–3199. doi: 10.1172/JCI74792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou J, Li Y-S, Wang K-C, Chien S. Epigenetic mechanism in regulation of endothelial function by disturbed flow: induction of DNA hypermethylation by DNMT1. Cell Mol Bioeng. 2014;7:218–224. doi: 10.1007/s12195-014-0325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davies PF, Manduchi E, Stoeckert CJ, Jiménez JM, Jiang Y-Z. Emerging topic: flow-related epigenetic regulation of endothelial phenotype through DNA methylation. Vascul Pharmacol. 2014;62:88–93. doi: 10.1016/j.vph.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garin G, Abe JI, Mohan A, Lu W, Yan C, Newby AC, Rhaman A, Berk BC. Flow antagonizes TNF-alpha signaling in endothelial cells by inhibiting caspase-dependent PKC zeta processing. Circ Res. 2007;101:97–105. doi: 10.1161/CIRCRESAHA.107.148270. [DOI] [PubMed] [Google Scholar]
- 85.Chaudhury H, Zakkar M, Boyle J, Cuhlmann S, van der Heiden K, Luong LA, Davis J, Platt A, Mason JC, Krams R, Haskard DO, Clark AR, Evans PC. c-Jun N-terminal kinase primes endothelial cells at atheroprone sites for apoptosis. Arterioscler Thromb Vasc Biol. 2010;30:546–553. doi: 10.1161/ATVBAHA.109.201368. [DOI] [PubMed] [Google Scholar]
- 86.Heo KS, Lee H, Nigro P, Thomas T, Le NT, Chang E, McClain C, Reinhart-King CA, King MR, Berk BC, Fujiwara K, Woo CH, Abe JI. PKC zeta mediates disturbed flow-induced endothelial apoptosis via p53 SUMOylation. J Cell Biol. 2011;193:867–884. doi: 10.1083/jcb.201010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.dela Paz NG, Walshe TE, Leach LL, Saint-Geniez M, D'Amore PA. Role of shear-stress-induced VEGF expression in endothelial cell survival. J Cell Sci. 2012;125:831–843. doi: 10.1242/jcs.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 89.Dimmeler S, Hermann C, Galle J, Zeiher AM. Upregulation of superoxide dismutase and nitric oxide synthase mediates the apoptosis-suppressive effects of shear stress on endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:656–664. doi: 10.1161/01.atv.19.3.656. [DOI] [PubMed] [Google Scholar]
- 90.Jin X, Mitsumata M, Yamane T, Yoshida Y. Induction of human inhibitor of apoptosis protein-2 by shear stress in endothelial cells. FEBS Lett. 2002;529:286–292. doi: 10.1016/s0014-5793(02)03361-6. [DOI] [PubMed] [Google Scholar]
- 91.Taba Y, Miyagi M, Miwa Y, Inoue H, Takahashi-Yanaga F, Morimoto S, Sasaguri T. 15-Deoxy-Delta(12,14)-prostaglandin J(2) and laminar fluid shear stress stabilize c-IAP1 in vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2003;285:H38–H46. doi: 10.1152/ajpheart.01037.2002. [DOI] [PubMed] [Google Scholar]
- 92.Hansson GK, Chao S, Schwartz SM, Reidy MA. Aortic endothelial-cell death and replication in normal and lipopolysaccharide-treated rats. Am J Path. 1985;121:123–127. [PMC free article] [PubMed] [Google Scholar]
- 93.Warboys CM, de Luca A, Amini N, Luong L, Duckles H, Hsiao S, White A, Biswas S, Khamis R, Chong CK, Cheung WM, Sherwin SJ, Bennett MR, Gil J, Mason JC, Haskard DO, Evans PC. Disturbed flow promotes endothelial senescence via a p53-dependent Pathway. Arterioscler Thromb Vasc Biol. 2014;34:985–995. doi: 10.1161/ATVBAHA.114.303415. [DOI] [PubMed] [Google Scholar]
- 94.Lin K, Hsu PP, Chen BP, Yuan S, Usami S, Shyy JYJ, Li YS, Chien S. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc Natl Acad Sci USA. 2000;97:9385–9389. doi: 10.1073/pnas.170282597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Didangelos A, Stegemann C, Mayr M. The -omics era: proteomics and lipidomics in vascular research. Atherosclerosis. 2012;221:12–17. doi: 10.1016/j.atherosclerosis.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 96.White SJ, Hayes EM, Lehoux S, Jeremy JY, Horrovoets AJ, Newby AC. Characterization of the differential response of endothelial cells exposed to normal and elevated laminar shear stress. J Cell Physiol. 2011;226:2841–2848. doi: 10.1002/jcp.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Butcher JT, Tressel S, Johnson T, Turner D, Sorescu G, Jo H, Nerem RM. Transcriptional profiles of valvular and vascular endothelial cells reveal phenotypic differences—influence of shear stress. Arterioscler Thromb Vasc Biol. 2006;26:69–77. doi: 10.1161/01.ATV.0000196624.70507.0d. [DOI] [PubMed] [Google Scholar]
- 98.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. 2010;298:H367–H374. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frueh J, Maimari N, Homma T, Bovens SM, Pedrigi RM, Towhidi L, Krams R. Systems biology of the functional and dysfunctional endothelium. Cardiovasc Res. 2013;99:334–341. doi: 10.1093/cvr/cvt108. [DOI] [PubMed] [Google Scholar]
- 100.Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res. 2013;99:315–327. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary-arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 102.Zarins CK, Zatina MA, Giddens DP, Ku DN, Glagov S. Shear-stress regulation of artery lumen diameter in experimental atherogenesis. J Vasc Surg. 1987;5:413–420. [PubMed] [Google Scholar]
- 103.Inaba S, Mintz GS, Shimizu T, Weisz G, Mehran R, Marso SP, Xu K, de Bruyne B, Serruys PW, Stone GW, Maehara A. Compensatory enlargement of the left main coronary artery: insights from the PROSPECT study. Coronary Artery Dis. 2014;25:98–103. doi: 10.1097/MCA.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 104.Pasterkamp G, Wensing PJW, Post MJ, Hillen B, Mali WPTM, Borst C. Paradoxical arterial-wall shrinkage may contribute to luminal narrowing of human atherosclerotic femoral arteries. Circulation. 1995;91:1444–1449. doi: 10.1161/01.cir.91.5.1444. [DOI] [PubMed] [Google Scholar]
- 105.Chatzizisis YS, Jonas M, Coskun AU, Beigel R, Stone BV, Maynard C, Gerrity RG, Daley W, Rogers C, Edelman ER, Feldman CL, Stone PH. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress—an intravascular ultrasound and histopathology natural history study. Circulation. 2008;117:993–1002. doi: 10.1161/CIRCULATIONAHA.107.695254. [DOI] [PubMed] [Google Scholar]
- 106.Koskinas KC, Feldman CL, Chatzizisis YS, Coskun AU, Jonas M, Maynard C, Baker AB, Papafaklis MI, Edelman ER, Stone PH. Natural history of experimental coronary atherosclerosis and vascular remodeling in relation to endothelial shear stress. Circulation. 2010;121:2092–2101. doi: 10.1161/CIRCULATIONAHA.109.901678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation. 2002;105:939–943. doi: 10.1161/hc0802.104327. [DOI] [PubMed] [Google Scholar]
- 108.Stone PH, Saito S, Takahashi S, Makita Y, Nakamura S, Kawasaki T, Takahashi A, Katsuki T, Nakamura S, Namiki A, Hirohata A, Matsumura T, Yamazaki S, Yokoi H, Tanaka S, Otsuji S, Yoshimachi F, Honye J, Harwood D, Reitman M, Coskun AU, Papafaklis MI, Feldman CL. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION study. Circulation. 2012;126:172–181. doi: 10.1161/CIRCULATIONAHA.112.096438. [DOI] [PubMed] [Google Scholar]
- 109.Wentzel JJ, Schuurbiers JC, Lopez NG, Gijsen FJ, van der Giessen G, Groen HC, Dijkstra J, Garcia-Garcia HM, Serruys PW. In vivo assessment of the relationship between shear stress and necrotic core in early and advanced coronary artery disease. Eurointervention. 2013;9:989–995. doi: 10.4244/EIJV9I8A165. [DOI] [PubMed] [Google Scholar]
- 110.Samady H, Eshtehardi P, McDaniel MC, Suo J, Dhawan SS, Maynard C, Timmins LH, Quyyumi AA, Giddens DP. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124:779–788. doi: 10.1161/CIRCULATIONAHA.111.021824. [DOI] [PubMed] [Google Scholar]
- 111.Pasterkamp G, Schoneveld AH, vanWolferen W, Hillen B, Clarijs RJG, Haudenschild CC, Borst C. The impact of atherosclerotic arterial remodeling on percentage of luminal stenosis varies widely within the arterial system—a postmortem study. Arterioscler Thromb Vasc Biol. 1997;17:3057–3063. doi: 10.1161/01.atv.17.11.3057. [DOI] [PubMed] [Google Scholar]
- 112.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 113.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 114.Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R. Healed plaque ruptures and sudden coronary death—evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–940. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 115.Pfenniger A, Wong C, Sutter E, Cuhlmann S, Dunoyer-Geindre S, Mach F, Horrevoets AJ, Evans PC, Krams R, Kwak BR. Shear stress modulates the expression of the atheroprotective protein Cx37 in endothelial cells. J Mol Cell Cardiol. 2012;53:299–309. doi: 10.1016/j.yjmcc.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 116.Gambillara V, Chambaz C, Montorzi G, Roy S, Stergiopulos N, Silacci P. Plaque-prone hemodynamics impair endothelial function in pig carotid arteries. Am J Physiol Heart Circ Physiol. 2006;290:H2320–H2328. doi: 10.1152/ajpheart.00486.2005. [DOI] [PubMed] [Google Scholar]
- 117.Van der Heiden K, Gijsen FJ, Narracott A, Hsiao S, Halliday I, Gunn J, Wentzel JJ, Evans PC. The effects of stenting on shear stress: relevance to endothelial injury and repair. Cardiovasc Res. 2013;99:269–275. doi: 10.1093/cvr/cvt090. [DOI] [PubMed] [Google Scholar]
- 118.Nakazawa G, Yazdani SK, Finn AV, Vorpahl M, Kolodgie FD, Virmani R. Pathological findings at bifurcation lesions the impact of flow distribution on atherosclerosis and arterial healing after stent implantation. J Am Coll Cardiol. 2010;55:1679–1687. doi: 10.1016/j.jacc.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 119.Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol. 2012;9:439–453. doi: 10.1038/nrcardio.2012.64. [DOI] [PubMed] [Google Scholar]
- 120.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee On Vascular Lesions of the Council On Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 121.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists view. Eur Heart J. 2013;34:719–728. doi: 10.1093/eurheartj/ehs411. [DOI] [PubMed] [Google Scholar]
- 122.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 123.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 124.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 125.Tanaka A, Imanishi T, Kitabata H, Kubo T, Takarada S, Tanimoto T, Kuroi A, Tsujioka H, Ikejima H, Ueno S, Kataiwa H, Okouchi K, Kashiwaghi M, Matsumoto H, Takemoto K, Nakamura N, Hirata K, Mizukoshi M, Akasaka T. Morphology of exertion-triggered plaque rupture in patients with acute coronary syndrome: an optical coherence tomography study. Circulation. 2008;118:2368–2373. doi: 10.1161/CIRCULATIONAHA.108.782540. [DOI] [PubMed] [Google Scholar]
- 126.Humphrey JD. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. 2002. p. i-757. Springer Science & Business Media. [Google Scholar]
- 127.Li ZY, Howarth SP, Tang T, Graves MJ, King-Im J, Trivedi RA, Kirkpatrick PJ, Gillard JH. Structural analysis and magnetic resonance imaging predict plaque vulnerability: a study comparing symptomatic and asymptomatic individuals. J Vasc Surg. 2007;45:768–775. doi: 10.1016/j.jvs.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 128.Holzapfel GA, Mulvihill JJ, Cunnane EM, Walsh MT. Computational approaches for analyzing the mechanics of atherosclerotic plaques: a review. J Biomech. 2014;47:859–869. doi: 10.1016/j.jbiomech.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 129.Ohayon J, Dubreuil O, Tracqui P, Le Floc'h S, Rioufol G, Chalabreysse L, Thivolet F, Pettigrew RI, Finet G. Influence of residual stress/strain on the biomechanical stability of vulnerable coronary plaques: potential impact for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol. 2007;293:H1987–H1996. doi: 10.1152/ajpheart.00018.2007. [DOI] [PubMed] [Google Scholar]
- 130.Akyildiz AC, Speelman L, van Brummelen H, Gutierrez MA, Virmani R, van der Lugt A, van der Steen AF, Wentzel JJ, Gijsen FJ. Effects of intima stiffness and plaque morphology on peak cap stress. Biomed Eng Online. 2011;10:25. doi: 10.1186/1475-925X-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lendon CL, Davies MJ, Born GVR, Richardson PD. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis. 1991;87:87–90. doi: 10.1016/0021-9150(91)90235-u. [DOI] [PubMed] [Google Scholar]
- 132.Sadat U, Teng Z, Young VE, Zhu C, Tang TY, Graves MJ, Gillard JH. Impact of plaque haemorrhage and its age on structural stresses in atherosclerotic plaques of patients with carotid artery disease: an MR imaging-based finite element simulation study. Int J Cardiovasc Imag. 2011;27:397–402. doi: 10.1007/s10554-010-9679-z. [DOI] [PubMed] [Google Scholar]
- 133.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci USA. 2006;103:14678–14683. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koskinas KC, Sukhova GK, Baker AB, Papafaklis MI, Chatzizisis YS, Coskun AU, Quillard T, Jonas M, Maynard C, Antoniadis AP, Shi GP, Libby P, Edelman ER, Feldman CL, Stone PH. Thin-capped atheromata with reduced collagen content in pigs develop in coronary arterial regions exposed to persistently low endothelial shear stress. Arterioscler Thromb Vasc Biol. 2013;33:1494–1504. doi: 10.1161/ATVBAHA.112.300827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cicha I, Woerner A, Urschel K, Beronov K, Goppelt-Struebe M, Verhoeven E, Daniel WG, Garlichs CD. Carotid plaque vulnerability: a positive feedback between hemodynamic and biochemical mechanisms. Stroke. 2011;42:3502–3510. doi: 10.1161/STROKEAHA.111.627265. [DOI] [PubMed] [Google Scholar]
- 136.de Weer TT, Cretier S, Groen HC, Homburg P, Cakir H, Wentzel JJ, Dippel DW, van der Lugt A. Atherosclerotic plaque surface morphology in the carotid bifurcation assessed with multidetector computed tomography angiography. Stroke. 2009;40:1334–1340. doi: 10.1161/STROKEAHA.108.538439. [DOI] [PubMed] [Google Scholar]
- 137.Dirksen MT, van der Wal AC, van den Berg FM, van der Loos CM, Becker AE. Distribution of inflammatory cells in atherosclerotic plaques relates to the direction of flow. Circulation. 1998;98:2000–2003. doi: 10.1161/01.cir.98.19.2000. [DOI] [PubMed] [Google Scholar]
- 138.Gijsen FJ, Wentzel JJ, Thury A, Mastik F, Schaar JA, Schuurbiers JC, Slager CJ, van der Giessen WJ, de Feyter PJ, van der Steen AF, Serruys PW. Strain distribution over plaques in human coronary arteries relates to shear stress. Am J Physiol Heart Circ Physiol. 2008;295:H1608–H1614. doi: 10.1152/ajpheart.01081.2007. [DOI] [PubMed] [Google Scholar]
- 139.Tronc F, Mallat Z, Lehoux S, Wassef M, Esposito B, Tedgui A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler Thromb Vasc Biol. 2000;20:120–126. doi: 10.1161/01.atv.20.12.e120. [DOI] [PubMed] [Google Scholar]
- 140.Ali F, Zakkar M, Karu K, Lidington EA, Hamdulay SS, Boyle JJ, Zloh M, Bauer A, Haskard DO, Evans PC, Mason JC. Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow. J Biol Chem. 2009;284:18882–18892. doi: 10.1074/jbc.M109.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van Thienen JV, Fledderus JO, Dekker RJ, Rohlena J, van IJzendoorn GA, Kootstra NA, Pannekoek H, Horrevoets AJG. Shear stress sustains atheroprotective endothelial KLF2 expression more potently than statins through mRNA stabilization. Cardiovasc Res. 2006;72:231–240. doi: 10.1016/j.cardiores.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 142.Saxer T, Zumbuehl A, Mueller B. The use of shear stress for targeted drug delivery. Cardiovasc Res. 2013;99:328–333. doi: 10.1093/cvr/cvt102. [DOI] [PubMed] [Google Scholar]
- 143.Holme MN, Fedotenko IA, Abegg D, Althaus J, Babel L, Favarger F, Reiter R, Tanasescu R, Zaffalon PL, Ziegler A, Mueller B, Saxer T, Zumbuehl A. Shear-stress sensitive lenticular vesicles for targeted drug delivery. Nat Nanotech. 2012;7:536–543. doi: 10.1038/nnano.2012.84. [DOI] [PubMed] [Google Scholar]
- 144.Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, Ghosh K, Jurek S, Bencherif SA, Bhatta D, Coskun AU, Feldman CL, Wagner DD, Ingber DE. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science. 2012;337:738–742. doi: 10.1126/science.1217815. [DOI] [PubMed] [Google Scholar]
- 145.Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJM, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 146.Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve scientific basis. J Am Coll Cardiol. 2013;61:2233–2241. doi: 10.1016/j.jacc.2012.11.083. [DOI] [PubMed] [Google Scholar]
- 147.Morris PD, Ryan D, Morton AC, Lycett R, Lawford PV, Hose D, Gunn JP. Virtual fractional flow reserve from coronary angiography: modeling the significance of coronary lesions results from the VIRTU-1 (VIRTUal Fractional Flow Reserve from Coronary Angiography) study. JACC Cardiovasc Interv. 2013;6:149–157. doi: 10.1016/j.jcin.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 148.Rafnsson A, Back M. Urinary leukotriene E-4 is associated with renal function but not with endothelial function in type 2 diabetes. Dis Markers. 2013;35:475–480. doi: 10.1155/2013/370461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pyke K, Poitras V, Tschakovsky M. Brachial artery flow-mediated dilation during handgrip exercise: evidence for endothelial transduction of the mean shear stimulus. Am J Physiol Heart Circ Physiol. 2008;294:H2669–H2679. doi: 10.1152/ajpheart.01372.2007. [DOI] [PubMed] [Google Scholar]
- 150.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable N, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55:312–318. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]