Abstract
In the United States, >40% of children are either poor or near-poor. As a group, children in poverty are more likely to experience worse health and more developmental delay, lower achievement, and more behavioral and emotional problems than their more advantaged peers; however, there is broad variability in outcomes among children exposed to similar conditions. Building on a robust literature from animal models showing that environmental deprivation or enrichment shapes the brain, there has been increasing interest in understanding how the experience of poverty may shape the brain in humans. In this review, we summarize research on the relationship between socioeconomic status and brain development, focusing on studies published in the last 5 years. Drawing on a conceptual framework informed by animal models, we highlight neural plasticity, epigenetics, material deprivation (eg, cognitive stimulation, nutrient deficiencies), stress (eg, negative parenting behaviors), and environmental toxins as factors that may shape the developing brain. We then summarize the existing evidence for the relationship between child poverty and brain structure and function, focusing on brain areas that support memory, emotion regulation, and higher-order cognitive functioning (ie, hippocampus, amygdala, prefrontal cortex) and regions that support language and literacy (ie, cortical areas of the left hemisphere). We then consider some limitations of the current literature and discuss the implications of neuroscience concepts and methods for interventions in the pediatric medical home.
Approximately 1 in 5 children in the United States lives in poverty, and >40% of children are poor or near-poor.1 In 2013, the American Academy of Pediatrics added child poverty to its Agenda for Children in recognition of poverty’s broad and enduring effects on child health and development.2 As a group, children in poverty are more likely to experience developmental delay, perform worse on cognitive and achievement tests, and experience more behavioral and emotional problems than their more advantaged peers.3–5 In addition, child socioeconomic status (SES) is tied to educational attainment, health, and psychological well-being decades later.6–9 Increasingly, research is focused on understanding the extent to which these long-term outcomes are related to changes in the developing brain.
For 50 years, research in animals has documented that rearing environments affect brain development. “Enriched environments,” including toys, social stimulation, and novelty, induce changes brain structure, function, and gene expression.10 Animals raised in enriched conditions demonstrate better learning and memory and greater capacity for plasticity and behavioral adaptation.10 Although animal models can be difficult to extrapolate to child poverty, these studies provide a basis for the idea that poverty may shape the brain at the molecular, neural, cognitive, and behavioral levels.11
Neuroscience research on poverty and brain development in humans is relatively new. The first studies examined socioeconomic disparities in behavior and cognition using tasks intended to localize to specific brain systems.12–17 Other studies built on this work by directly examining SES differences in brain structure and function18–22 and neural networks and functional connectivity between brain areas.23–25 Despite significant progress, current understandings of how, why, when, and in what individuals poverty shapes the brain remain incomplete.
This review builds on previous reviews11,26–34 to summarize the neuroscience of poverty for pediatric practitioners. We focus on poverty rather than other forms of adversity (eg, abuse/neglect, institutionalization) and on state-of-the-art studies published in the last 5 years. After briefly discussing the measurement of SES, we present an overview of brain development and sensitive periods. We then discuss deprivation and stress as factors hypothesized to shape brain development. Finally, we review what is known about how poverty shapes the brain and consider implications for pediatric practice.
Defining Poverty
Studies of SES and the brain rely on a variety of measures including family income (or income-to-needs ratio), educational attainment, occupational status, neighborhood SES, and perceived social position. (The diversity of these measures is illustrated in Tables 1, 2, 3, and 4, which summarize studies discussed later.) Although SES indicators are intended as proxies for the environments of poverty,35 they provide little insight into how individuals actually experience poverty. In addition, there is no bright line that distinguishes socioeconomic deprivation likely to result in poor outcomes from deprivation less likely to do so. A child living marginally above the federal poverty level is not appreciably better off than one marginally below; indeed, in some cases, families well above this threshold may lack the resources to meet their children’s needs.
TABLE 1.
Studies Included in Left Occipitotemporal and Perisylvian Regions: Language and Reading Section
| Reference | n | Age | Poverty Measure | Method | Main Findings |
|---|---|---|---|---|---|
| D’Angiulli, et al (2012)36 | 28 | 13 y | Adapted Hollingshead index (residential area quality, income, education, occupation); 14 adolescents from low-SES neighborhood, 14 from high-SES neighborhood; compared high- versus low-SES groups | Cross-sectional ERP and EEG study with target detection task; diurnal cortisol collected on day of ERP/EEG | Higher-SES adolescents showed greater ERP/EEG differentiation between attended versus unattended stimuli; no SES-related differences in task performance or accuracy; lower-SES adolescents had slightly higher cortisol levels, but no differences in cortisol reactivity to the ERP/EEG task between the SES groups |
| Jednoróg, et al (2012)18 | 23 | 10 y (8–11) | Weighted average of maternal education and maternal current occupation status | Cross-sectional MRI study | SES positively correlated with literacy, verbal skills, and gray matter in middle temporal gyri, left fusiform gyrus, and right inferior occipitotemporal region; SES positively correlated with gyrification in left hemisphere; SES not related to phonological skills |
| Noble, et al (2012)20 | 60 | 11 y (5–17) | Average years of parental education and family income/needs ratio | Cross-sectional MRI study | Significant parental education × child age interaction for left superior temporal gyrus and left inferior frontal gyrus indicating increasing SES disparities in volume with age (volume decreased with age in lowest SES, was stable in middle SES, and increased in highest SES; n = 44 for this analysis) |
| Noble, et al (2007)16 | 150 | First grade | Composite of parent education, occupation, and income/needs ratio | Cross-sectional study using cognitive tasks of language, visuospatial processing, memory, working memory, cognitive control, and reward processing | SES related to scores on all tasks except reward processing; SES accounted for more variance in language scores than other scores; home/school variables accounted for majority of variance in language scores accounted for by SES |
| Noble, et al (2006)14 | 38 | 8 y (7–9), below–average reading ability | Composite score of parent education, occupation, and income/needs ratio | Cross-sectional fMRI study using pseudoword task and tests of phonological awareness, reading ability, and receptive vocabulary | Significant phonological awareness × SES interactions in left fusiform region (high-SES children with low phonological awareness more likely to increase fusiform activity during phonological task than low-SES children with low phonological awareness); for low-SES children only, strong association between phonological awareness and left fusiform activity; SES not related to reading ability, receptive vocabulary, or accuracy on fMRI task |
| Noble et al (2015)37 | 1099 | 12 y (3–20) | Parent education and family income | Cross-sectional MRI study plus inhibitory control, working memory, picture vocabulary and oral reading recognition tasks | Parent education and family income positively associated with cortical surface area in regions related to language ability (education: left superior, middle, and inferior temporal gyri, inferior frontal gyrus, medial orbito-frontal region, and precuneus; income: bilateral inferior temporal, insula, and inferior frontal gyrus); income associated with surface area in brain regions responsible for language and EF |
| Raizada, et al (2008)24 | 14 | 5 y | Hollingshead index | Cross-sectional fMRI study using a rhyming task and tests of receptive language, phonological ability, language, and IQ | SES positively related to asymmetry in inferior frontal gyrus; relation remained significant after controlling for language scores; rhyming task performance not related to SES or inferior frontal gyrus activity |
| Tomalski, et al (2013)38 | 45 | 7 mo (6–9) | Parental occupation (3 categories) and gross family income | Cross-sectional EEG study | Higher frontal γ-power in infants from higher-income families; significant differences between highest- versus middle and lowest-level maternal job groups; no power differences by paternal occupation |
ERP, event-related potential; fMRI, functional MRI.
TABLE 2.
Studies Included in Hippocampus: Learning and Memory Section
| Reference | n | Age, y | Poverty Measure | Method | Main Findings |
|---|---|---|---|---|---|
| Hair, et al (2015)39 | 389 | 12 (4–22) | Family income adjusted for household size using binary and categorical measures | Longitudinal MRI study of normal brain development; scans at 2-y intervals across 3 periods, plus Wechsler Abbreviated Scale of Intelligence and Woodcock-Johnson II Test of Achievement | Low-income children scored lower on tests of cognitive ability and had reductions in gray matter in the frontal and temporal lobes and the hippocampus; differences in gray matter in the hippocampus explained ≤16% of differences in cognitive ability; income effects greatest among the poorest children |
| Hanson, et al (2011)40 | 431 | 11 (SD 4) | Family income, parent (maternal and paternal) education | Cross-sectional MRI study | Positive association between family income and child hippocampal volume, adjusting for parental education; no consistent associations between parent education and hippocampal size, adjusting for family income |
| Hanson, et al (2015)41 | 128 | 12 (9–15) | 4 groups: (1) institutionalized/abandoned children with early neglect (n = 36); (2) low SES (parents unskilled employees with ≤high school education) (n = 20); (3) victims of physical abuse (n = 31); (4) comparison group of middle-SES children (based on Hollingshead 2-factor index) with no maltreatment (n = 41) | Cross-sectional MRI study | Low-SES group had smaller hippocampi than middle-SES group; smaller left hippocampal volume associated with more behavioral problems; cumulative life stress and behavioral problems were inversely associated with hippocampal volume; hippocampal volumes partially mediated relations between early life stress and behavior problems |
| Jednoróg, et al (2012)18 | 23 | 10 (8–11) | Weighted average of maternal education and maternal occupational status | Cross-sectional MRI study | SES positively correlated with hippocampus gray matter volume, but not associated with memory or visuospatial processing. |
| Luby, et al (2013)42 | 145 | 10 (6–12) | Income/needs ratio | Longitudinal study with 3–6 annual assessments of child psychiatric status, stressful life events, caregiver education; assessment of parental support/hostility at age 4–7, child MRI at age 10 | Higher income/needs associated with greater left hippocampal volume, mediated by caregiving support/hostility and life stress |
| Noble, et al (2012)43 | 275 | 40 (17–87) | Years of education | Cross-sectional MRI study | Age-related decreases in hippocampal volume greater for participants with less education (versus those with more education). |
| Noble, et al (2015)37 | 1099 | 12 (3–20) | Parent education, family income | Cross-sectional MRI study; inhibitory control, working memory, picture vocabulary, and oral reading recognition tasks | Parent education positively associated with cortical surface area in regions supporting language, reading, executive function, and spatial skills; income positively associated with performance on cognitive tasks; relation between income and inhibitory control and working memory mediated by cortical surface area; parent education positively associated with left hippocampal volume; relation between hippocampal volume and education was stronger for children with the least educated parents; income not associated with hippocampal volume |
| Noble, et al (2012)20 | 60 | 11 (5–17) | Average years of parental education and family income/needs ratio | Cross-sectional MRI study | SES-related differences in hippocampal volume due to positive relations between hippocampal volume and income/needs (not parental education) |
| Rao, et al (2010)19 | 49 | 14 (13–16) | All participants were African American and exposed to cocaine in utero; did not examine SES; examined effect of parental nurturance and environmental stimulation (HOME scale) | Longitudinal study with assessment of parental nurturance and home environment at 4 and 8 y and MRI at 13–16 y | Parental nurturance at age 4 inversely associated with hippocampal volume at age 13–16; nurturance at age 4 explained 25% of left hippocampal volume; hippocampal volume not related to memory ability; nurturance at age 8 positively associated with memory ability; nurturance at age 8 and environmental stimulation at age 4 and 8 not related to hippocampal volume |
| Sheridan, et al (2013)44 | 33 (19 in fMRI) | 10 (8–12) | Maternal education, family income/needs ratio, and maternal SSS | Cross-sectional fMRI study using Paired Associate Learning task. Social stress task administered outside of scanner, and salivary cortisol assessed | Maternal SSS positively associated with baseline cortisol and hippocampal activation; income/needs not associated with hippocampal activation; SSS, education, and income/needs not associated with child hippocampal volume; no associations between maternal education and income/needs and cortisol; learning task performance not associated with SES measures |
| Staff, et al (2012)45 | 235 | 64 (64–65) | SES at age 11 recalled at age 64 using paternal occupation and home conditions at age 11 | MRI study using mental ability assessments obtained at age 11 and conducted MRI at age 64 | Lower childhood SES associated with less hippocampal volume, adjusting for 11-y-old mental ability, gender, and current occupation and education |
fMRI, functional MRI; HOME, Home Observation for Measurement of the Environment; SSS, subjective social status.
TABLE 3.
Studies Included in Amygdala: Fear and Emotional Processing Section
| Reference | n | Age, y | Poverty Measure | Method | Main Findings |
|---|---|---|---|---|---|
| Gilliam, et al (2015)46 | 165 men | 20 | Did not examine SES effects; participants recruited from urban WIC Nutrition Supplement Centers; sample divided in 3 groups: men with mothers with depression scores that were (1) consistently high; (2) consistently moderate; (3) consistently low; groups did not differ on childhood SES (Hollingshead Index) | Longitudinal study with maternal depression assessed 7 times from when the child was age 1.5 to 10 y; MRI and assessment of child depression, delinquency, and aggression conducted at age 20 | Maternal depression not related to amygdala or hippocampal volume at age 20; men in the moderate depression group had higher amygdala/hippocampal ratio compared with men in the low depression group; amygdala/hippocampal ratio positively associated with aggression (not delinquency or depression) at age 20; maternal depression (low versus moderate) and aggression mediated by amygdala/hippocampal ratio |
| Hanson, et al (2011)40 | 431 | 11 (SD 4) | Family income and parent (maternal and paternal) education level | Cross-sectional MRI study | In models with maternal and paternal education and family income, no significant relations between these SES measures and amygdala volume |
| Hanson, et al (2015)41 | 128 | 12 (9–15) | 4 groups: (1) institutionalized/abandoned children with early neglect (n = 36); (2) low SES (parents unskilled employees with ≤HS education) (n = 20); (3) victims of physical abuse (n = 31); (4) comparison group of middle-SES children (based on Hollingshead 2-factor index) with no maltreatment (n = 41) | Cross-sectional MRI study | Low-SES children and children with history of neglect or abuse had smaller left amygdalae than comparison children; cumulative life stress and behavioral problems inversely associated with left amygdala volume; amygdala volume did not mediate early life stress/behavioral problems relations |
| Kim, et al (2013)47 | 49 | 24 (20–27) | Income/needs ratio | Longitudinal study with SES assessed at age 9, chronic stressors assessed at age 9, 13, and 17; fMRI at age 24 using an emotional regulation task | Low income at age 9 associated with decreased PFC activity and increased amygdala activity; childhood chronic stress mediated the relation between income and PFC activity; at age 9, children from low-income families had positive associations between amygdala and left VLPFC, while children from higher-income families had negative associations between amygdala and left VLPFC during emotional regulation task |
| Luby, et al (2013)42 | 145 | 10 (6–12) | Income/needs ratio | Longitudinal study with 3–6 annual assessments of child psychiatric status, stressful life events, and caregiver education; laboratory task of parental support/hostility at age 4–7; child MRI at age 10 | Higher income/needs associated with greater left amygdala volume; relations between income/needs and amygdala volume not mediated by caregiving behaviors, education, or child life stress |
| Lupien, et al (2011)48 | 38 | 10 | Did not examine SES effects; maternal depression was assessed throughout childhood (17 children with mothers with chronic depression compared with 21 children who were not exposed to depression); groups matched on income | Longitudinal study with maternal depression assessed at 5, 17, 30, 42, 60, 84, 156 mo; MRI at age 10 y; salivary cortisol assessed on arrival at laboratory and before and after MRI | Children with depressed mothers had larger right and left amygdala volumes compared with children with no exposure to depression; positive correlation between mean maternal depressive symptoms and amygdala volume; children with depressed mothers also had greater cortisol output compared with unexposed children |
| Muscatell, et al (2012)49 | 16 | 20 (18–24) | SSS relative to university community | Cross-sectional fMRI study using a social information task | Inverse association between SSS and activity in PFC (DMPFC, MPFC) during social information task |
| 22 | 13 (12–13) | Composite of parental education and family income | Cross-sectional fMRI study using an angry faces processing task | Viewing angry faces associated with increased amygdala activity; inverse relation between SES and activity in DMPFC and left amygdala during processing of angry faces | |
| Moutsiana, et al (2015)50 | 59 | 22 | Did not examine SES; maternal depression and infant attachment assessed | Longitudinal study; infant attachment assessed at 18 mo; depression/anxiety disorders assessed at 8, 13 16, and 22 y. Maternal depression assessed at child ages 18 mo and 5, 8, 16 y; MRI at age 22 | Significant effect of infant attachment on adult amygdala volume; larger amygdalae associated with insecure attachment, controlling for maternal depression |
| Noble, et al (2015)37 | 1099 | 12 (3–20) | Parent education and family income | Cross-sectional MRI study; inhibitory control, working memory, picture vocabulary, and oral reading recognition tasks | Income positively associated with performance on cognitive tasks; education and income not related to amygdala volume |
| Noble, et al (2012)20 | 60 | 11 (5–17) | Average years of parental education and family income/needs ratio | Cross-sectional MRI study | SES-related differences in amygdala volume due to inverse relations between amygdala volume and parent education (not income/needs) |
| Suzuki, et al (2014)51 | 115 | 10 (7–12) | Family income assessed at time of fMRI (age 7–12) | Longitudinal study with depression and stressful/traumatic life events measured annually from ages 3–5 to 7–12 y; fMRI using gender identification task of emotional faces conducted at age 7–12 | Controlling for family income, stressful life events associated with increased activation to fearful faces in the right amygdala; traumatic life events positively associated with left amygdala activity to sad faces |
| Taylor, et al (2006)52 | 30 | (18–36) | Adversity and childhood family environment measured with Risky Families questionnaire | Cross-sectional fMRI study using emotional faces task | Left amygdala activation to negative emotional faces lower in adults from risky families; adults from low-risk families had negative correlation between amygdala and RVLPFC activity, adults from high-risk families had positive correlation between amygdala and RVLPFC activity |
DMPFC, dorsomedial prefrontal cortex; fMRI, functional MRI; MPFC, medial prefrontal cortex; RVLPFC, right ventrolateral prefrontal cortex; SSS, subjective social status.
TABLE 4.
Studies Included in Prefrontal Cortex: Executive Functions Section
| Reference | n | Age | Poverty Measure | Method | Main Findings |
|---|---|---|---|---|---|
| Blair, et al (2011)53 | 1292 | 36 mo | Income/needs ratio; parenting assessed with free play or structured interaction task; household risk assessed (household density, neighborhood sensitively, noise) | Longitudinal study with assessments at age 7, 15, 24, and 36 mo; basal cortisol and parenting assessed at 7, 15, and 24 mo; household risk assessed at 7 and 24 mo; EF assessed at 36 mo | Cortisol inversely related to EF and higher in poor children; parenting related to EF and IQ, household risks inversely related to EF and IQ; maternal education, income/needs not associated with EF or IQ; cortisol inversely related to positive parenting and this relation mediated the effect between positive parenting and EF |
| Blair, et al (2011)54 | 1135 | 48 mo | Income/needs ratio used to create “poor” and “not poor” groups; groups used to create sum score for chronicity of poverty over the assessments; economic need and economic sufficiency assessed with Economic Strain Questionnaire; family stability and housing quality assessed | Longitudinal study with assessments at age 7, 15, 24, 36, and 48 mo; salivary cortisol and parenting assessed at 7, 15, and 24 mo | Duration of life in poverty inversely associated with cortisol; family instability, low economic sufficiency, poor housing quality associated with higher cortisol; positive parenting inversely related to cortisol, but no relation between negative parenting and cortisol |
| Hair, et al (2015)39 | 389 | 12 y (4–22) | Family income adjusted for household size using binary and categorical measures | Longitudinal MRI study of normal brain development; scans at 2-y intervals across 3 periods, plus Wechsler Abbreviated Scale of Intelligence and Woodcock-Johnson II Test of Achievement | Low-income children scored lower on tests of cognitive ability and had reductions in gray matter in frontal and temporal lobes and hippocampus; differences in gray matter in frontal lobe explained ≤16% of differences in cognitive ability; income effects were greatest among the poorest children |
| Hanson, et al (2013)55 | 77 | 0–53 mo | Family income (≤200% FPL vs >200%–400% FPL) | Longitudinal MRI study of normal brain development; average of 3 scans per child ∼6 mo apart | Infants from lower-SES families had reduced frontal-lobe gray matter volume compared with those from higher-SES families; no differences by SES in white matter volume |
| Hanson, et al (2012)56 | 61 | 12 y (SD 2 y) | Maternal education; life stress measured with Youth Life Stress Interview of parents and children | Cross-sectional MRI study with EF battery | Life stress inversely associated with PFC volume in gray matter near the anterior cingulate and frontal poles and in white matter near the forceps minor; life stress also inversely associated with memory; prefrontal volumes mediated relation between life stress and working memory; comparing effect of stressors in past year and cumulative life stressors, cumulative stressors had larger effect on EF |
| Holz, et al (2015)57 | 167 | 25 y | Poverty assessed at age 3 mo using maternal report of income below the poverty level (Germany); dichotomized into exposed (n = 33)/not exposed (n = 134) to early poverty | Longitudinal MRI study; poverty assessed at 3 mo; life stress assessed regulatory from age 3 mo to 25 y; conduct disorder assessed at 8, 11, 15, and 19 y; MRI at 25 y | Adults who experienced early poverty had more conduct disorder symptoms and smaller OFC volumes compared with unexposed adults; relation between poverty and conduct disorder symptoms mediated by OFC volume; life stress and maternal smoking during pregnancy also mediated this relation; OFC volume inversely related to conduct disorder symptoms |
| Lawson, et al (2013)22 | 283 | 11.5 y (SD 4 y) | Family income adjusted for family size and sum of maternal and paternal education | Cross-sectional MRI study | Parental education positively associated with thickness of right anterior cingulate gyrus and left superior frontal gyrus; family income not related to thickness of either area |
| Liberzon, et al (2015)58 | 49 | 23–24 y | Income/needs ratio assessed at age 9; ratio used as continuous variable and dichotomized into low (mean 0.76; n = 23) versus mid-SES (mean 2.7; n = 26) groups | Longitudinal fMRI study using shifted-attention emotion appraisal task; TSST administered before fMRI; cortisol assessed before and after SST; poverty assessed at age 9; fMRI and TSST assessed at 23–24 y | Adults exposed to poverty in middle childhood showed less DLPFC recruitment during emotion regulation task; this pattern mediated the effect of poverty on adult task performance; income/needs positively associated with task accuracy and unrelated to cortisol |
| Lipina, et al (2013)59 | 250 | 5 y (SD 0.5 y) | NES socioeconomic scale: parent education and occupation, dwelling score, overcrowding, health history, preschool attendance, books/reading to children, computer/internet use, effortful control; compared groups with unmet basic needs to those with met basic needs (SES groups) | Cross-sectional study using EF battery | Children with unmet basic needs had lower efficacy and scores on tasks related to prefrontal and executive systems; child literacy activities mediated the relation between SES group and working memory and fluid processing, and computer activities mediated the relation between SES group and fluid processing |
| Noble, et al (2015)37 | 1099 | 12 y (3–20) | Parent education and family income | Cross-sectional MRI study; inhibitory control, working memory, picture vocabulary, and oral reading recognition task | Parent education positively associated with cortical surface area in regions supporting language, reading, executive function, and spatial skills; income positively associated with cortical surface area in regions supporting various language and EF; income positively associated with performance on cognitive tasks; relations between income and inhibitory control and working memory mediated by cortical surface area |
DLPFC= dorsolateral prefrontal cortex; fMRI, functional MRI; OFC, orbitofrontal cortex; RMFG, right middle frontal gyrus; TSST, Trier Social Stress Task.
Brain Development and Sensitive Periods
Brain development is complex and ongoing throughout childhood and adolescence, with a time course that varies depending on the outcome considered. Parts of the neural tube are developed just 5 weeks after conception, and development of the cortex is evident by midgestation.60 From late gestation to age ∼2 years, there is substantial brain growth, followed by a more gradual increase in the number of neurons.60 The number of synapses in the cerebral cortex peaks within the first few years of life and then plateaus and declines in later childhood and adolescence. Throughout childhood and adolescence, myelination gradually occurs, insulating axons and increasing the speed and synchronization of neural processing.61 In addition, these general processes occur at different rates across the brain. For example, the prefrontal cortex (PFC), which supports cognitive self-regulation and executive functions, develops rapidly in the first 2 years of life, at 7 to 9 years of age, and again in the midteens, with continued myelination into the third decade.60,62 Subcortical structures such the amygdala, which supports emotion processing, and the hippocampus, which supports memory and helps coordinate the stress response, increase in volume until age ∼30 years, at which point they plateau and then gradually decline.60
In general, sensitivity to environmental stimuli, positive or negative, is heightened during periods of rapid brain development. Changes in the brain induced by environmental stimuli are broadly termed “plasticity.” Sensitive periods are those during which plasticity is greatest. Different neural systems have different sensitive periods,61 and animal studies suggest that when a sensitive period closes depends on a variety of factors such as the function and complexity of the circuits involved and the experiences of the individual, rather than age alone.63,64
Brain development is driven by both genetic and environmental influences, as well as the interaction between the two.65 Importantly, the extent to which cognitive and brain development depend on genetic and environmental input may vary by SES. Studies have found that genes explain more of the variance in cognition and brain structure in high-SES individuals than in low-SES individuals.66,67 In addition, behavioral genetics research suggests that genetic variation confers vulnerability or resilience to specific environments and helps explain individual differences in the impact of poverty on brain and cognitive development.68–71 A number of studies have found support for the differential susceptibility hypothesis, which posits that some genetic variants (or “plasticity alleles”) confer greater vulnerability to environmental stimuli, regardless of whether those stimuli are positive or negative.68,72–74 In this way, outcomes among children who share a particular genetic variant may vary substantially based on the nature of environments in which they are raised.”
Epigenetic research demonstrates that environments play an important role in how the genetic code itself is expressed. Although epigenetic influences are increasingly considered central to the relationship between early adversity and later outcomes, they are only just beginning to be understood.26,75,76 One example of research in this area is evidence that maternal care regulates gene expression in the brain.18 Rat pups exposed to high levels of maternal care, regardless of whether they are biologically related to the dam, demonstrate more glucocorticoid receptor expression in the hippocampus and more efficient regulation of negative feedback on the hypothalamic–pituitary–adrenal (HPA) axis. This enables a more modest, well-regulated stress response and better cognitive performance.18,75,76 In addition, epigenetic modifications in response to variations in maternal care can be transmitted across generations.75 Although still limited and confined to individuals exposed to abuse, some evidence is emerging to support a similar role of caregiving in regulating gene expression in the human brain.59,77
Environmental Mediators: Material Deprivation and Stress
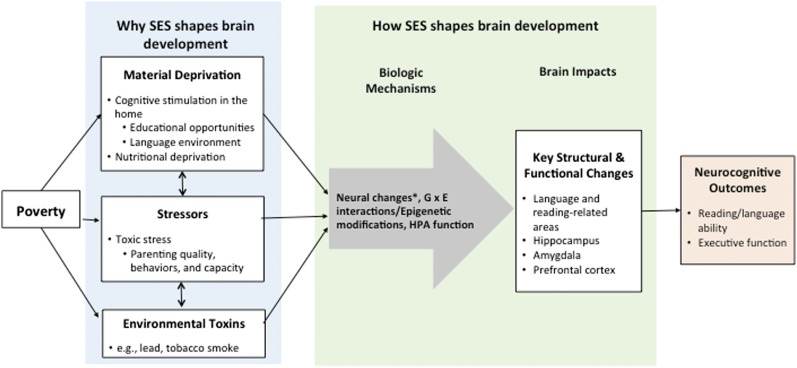
Material deprivation and stress are factors related to SES that may function as environmental mediators59 of the SES–brain development relationship. Figure 1 draws on a framework based on animal neuroscience research advanced by Sheridan and McLaughlin, which posits that the environments of poverty shape neurodevelopment by depriving the brain of key stimuli and increasing its exposure to negative input.77 Children from advantaged backgrounds may also lack cognitive stimulation and experience high levels of stress; however, poor children typically experience more adversities and may have fewer buffering resources.78
FIGURE 1.
A framework based on animal neuroscience research. G × E, gene–environment interaction. *Neural changes = changes in neural plasticity, pruning, synaptic connections, dendritic branching, myelination.
Material Deprivation
Cognitive Stimulation in the Home
For children growing up in poverty, constrained resources may limit parents’ access to the tools needed to provide cognitive stimulation in the home, including toys, books, and educational opportunities.59,79,80 SES may also shape patterns of communication and language.80–82 Research suggests that, relative to their higher-SES peers, children from low-SES families are often exposed to fewer words and conversations and less complex and more directive speech.80–82
Nutritional Deprivation
Micronutrients are critical for healthy brain development, particularly during late gestation and early infancy.60 Because of factors such as food insecurity, low-income infants and children are more likely to experience nutrient deficiencies.83,84 Micronutrients such as vitamin B12, folate, retinoic acid, omega-3 fatty acids, zinc, and iron play a role in regulating gene expression that guides brain development and in modulating neuroplasticity, dendritic arborization, synaptogenesis, and myelination.85 The impact of these deficiencies on brain development and behavior varies based on the neural processes developing at the time and the severity of the deficiency.86 For example, early childhood iron deficiency is associated with poor academic performance; cognitive, emotional, and attention problems; and less educational attainment in adulthood.87,88
Many deficiencies may be prevented or treated with supplementation.60,89,90 The effectiveness of supplementation varies by nutrient, level of deficiency, and age of the child at the time of deficiency and supplementation.60 For example, a meta-analysis concluded that the cognitive effects of iron deficiency in infants and very young children may not be amenable to short-term supplementation, whereas supplementation in school-aged children and adolescents with anemia may yield substantial improvements in cognition.60
Stress
Children growing up in low-SES families are more likely to experience stressors including family conflict, separation, household crowding, and neighborhood disorder.91,92 The term “toxic stress” was coined to highlight similarities between chronic stress and exposure to other toxins for children’s health.65 The stress response system, particularly the HPA axis, has been a focus of research of the health and developmental effects of early adversity.27,93 Evidence from animals and humans suggests that prenatal stress can “program” the HPA, leading to excessive glucocorticoid secretion.93 In humans, postnatal chronic stress can lead to both hyper- and hypoactivity in the HPA, depending on the nature, timing, duration, and severity of the stressor, individuals’ previous experiences, and genetic variation.93,94
Both animals and humans show stress-related changes in brain areas associated with the HPA stress response, including PFC, amygdala, and hippocampus.93 Excessive glucocorticoid exposure can affect neuroplasticity, thereby affecting subsequent stress response and behavioral and emotional regulation.95 In animals, chronic HPA activation reduces synaptic plasticity and neurogenesis in the hippocampus, which, in turn, affects memory and the ability to cope with future stressors.95,96 Taken together, the evidence shows that excessive stress hormones can affect the brain in ways that undermine cognition and mental health if they occur under the right conditions; however, relatively little is known about the specific neural mediators that link poverty to these outcomes.93,95
Disruptions to the parent-child relationship (eg, maternal depression or anxiety, extended separation) are potent sources of chronic stress for children, regardless of SES. Stress may impact parents’ emotional, behavioral, and relational functioning, including their parenting behaviors.79,97 Children raised in poverty are more likely to experience inconsistent and harsh discipline and less nurturing and responsiveness.79,97 Most research in this area has focused on extreme conditions (eg, institutionalization, maltreatment). These studies have linked negative parenting experiences with smaller gray- and white-matter volume in childhood and smaller hippocampal volume in adulthood.98 Importantly, however, individuals vary in their susceptibility to parenting; this susceptibility may be a function of factors such as temperament, physiologic reactivity, and genetics.99,100
Seminal studies in rodents show that maternal caregiving can regulate gene expression in the brain, including genes that govern glucocorticoid receptor expression in the hippocampus, transcription of neural growth factor, and sensitivity to stress hormones.101 Rat pups exposed to high levels of maternal care demonstrate more glucocorticoid receptor expression in the hippocampus and more efficient regulation of negative feedback on the HPA axis.34,102,103 Preliminary studies suggest that humans exposed to abuse and maltreatment show reductions in glucocorticoid receptor expression in the brain, but more evidence is needed to better understand how animal research can be extrapolated to human parenting.104,105
Environmental Toxins
Poor children are more likely live in neighborhoods in which they are exposed to environmental toxins.106,107 In addition, environmental factors associated with poverty may amplify the effect of some toxins.108 For example, children from low-SES families are at higher risk of iron deficiencies, and low iron levels increase the body’s absorption of one of the most well-documented neurotoxins, lead.89 Lead alters the transmission of glutamate and dopamine, resulting in changes in neuronal plasticity and synaptic communication, with particular effects on PFC, hippocampus, and cerebellum.109 Even low levels of lead are related to worse performance on cognitive tasks and reduced auditory recognition ability.110,111 Similarly, environmental tobacco smoke has greater effects on children’s cognitive outcomes among children from lower SES backgrounds relative to their higher SES peers.112
How SES Shapes Brain Development: Evidence for Brain Impacts
Brain Structure and Function
Material deprivation, stress, and environmental toxins are environmental mediators that may link SES with brain development through a set of biologic mechanisms. Brain regions that process and respond to threat, regulate the stress response, and support language, literacy, and executive functions may be particularly vulnerable to these SES-related factors.29,93,113 The protracted development of brain areas supporting these cognitive processes (eg, temporal lobe language regions, amygdala, hippocampus, PFC) makes these areas particularly vulnerable to environmental input.114–116 Here we briefly summarize key findings about the association between SES and the structure and function of these brain areas. Acknowledging that multiple brain areas and networks support higher-level processes, and noting that differences in structure do not necessarily correlate with differences in cognitive ability, we group brain areas and processes together in our discussion to provide a richer understanding of the relations between poverty and physical and cognitive development. Tables 1, 2, 3, and 4 summarize the design, sample, SES measures, and findings for the studies referenced in this section.
Left Occipitotemporal and Perisylvian Regions: Language and Reading
The left occipitotemporal and left perisylvian regions support language and reading.14 Language ability is among the most strongly associated with childhood SES.16 Early work found that higher-SES children tend to display greater neural specialization in reading-related brain areas, and, when reading ability is compromised, higher-SES children may recruit compensatory brain areas for reading.14,24 More recently, socioeconomic factors have been linked to the volume18 and surface area37 of language-related brain areas. Consistent with these findings, as early as infancy, children from lower-SES homes show differences in the electrophysiological signature of language development.36,38 It is possible that differences in the cumulative quality and quantity of language exposure, beginning very early in childhood, may result in differences in the development and specialization of the neural network for language and reading.
Hippocampus: Learning and Memory
The hippocampus supports learning and memory. It is dense with glucocorticoid receptors, making it particularly vulnerable to the effects of stress.96 In animals, excessive glucocorticoid exposure impedes hippocampal development and maturation.96 Neuroimaging studies of family SES and child/adolescent hippocampal size have evaluated these changes at the structural level. Studies of the relationship between family SES (ie, parent occupation/education, income/income-to-needs) and child hippocampal size generally find that higher-SES children have larger hippocampi.18,20,37,40–42 The relationship between childhood poverty and hippocampal volume appears persistent; low childhood SES is associated with smaller hippocampi measured 5 decades later, even when adjusting for adult socioeconomic circumstances.45
Accumulating evidence from studies using longitudinal designs suggests that parenting and chronic stress are environmental mediators of the relationship between family SES and child hippocampal structure. Less supportive and more hostile parenting in preschool may mediate the relationship between lower family income-to-needs ratio and smaller child hippocampal volume 3 to 6 years later.42 Building on this work, recent evidence from a longitudinal study of children and adolescents followed for 6 years suggests that the relationship between family income and neurocognitive performance is mediated by hippocampal volume differences.39
Different timing of assessments may yield different insights into the relationship between SES and hippocampal size. For example, in a longitudinal study of low-income children whose mothers had a history of substance use during pregnancy, 4-year-old children who experienced more parental nurturance had, on average, smaller hippocampal volumes in adolescence.19 Because adolescence marks the beginning of a wave of hippocampal pruning, this suggests that children deprived of parental nurturance in early life may experience delayed hippocampal maturation.19 Some evidence suggest that education itself is related to age-related hippocampal volume decreases across the lifespan; specifically, volume decreases appear more marked among individuals with less education compared with those with more education.43 Together, these studies point to sensitive periods during which both material resources and parental nurturance may have a formative impact on the development of the hippocampus.
Amygdala: Fear and Emotional Processing
The amygdala is involved in emotional learning, motivation, and emotion and threat processing.61 In contrast to the hippocampus, studies of amygdala structure and childhood poverty are more equivocal.20,37,40–42 Functional studies are most consistent; lower childhood SES and risky family environments are associated with greater or less-regulated amygdala activation during emotion processing tasks.47,49,51,52 Chronic stress appears to be a factor in the relationship between childhood poverty and amygdala activity,47 and studies have highlighted the role of parent functioning. For example, threats to the parent–child bond, including maternal depression and insecure infant attachment, have been associated with larger amygdalae in childhood and young adulthood,48,50 as well as higher amygdala-hippocampal volume ratios, a risk factor for emotional dysregulation.46 Together, these findings illustrate the importance of early-life caregiving experiences in shaping the structure and function of the amygdala, the neural foundation of emotion regulation.
Prefrontal Cortex: Executive Functions
The PFC supports cognitive processes including higher-order planning, reasoning, and decision-making. Material deprivation, and specifically lack of cognitive stimulation, may contribute to alterations in PFC function and deficits in neurocognitive functions subserved by the PFC. Less family language complexity is a potential mediator in the relationship between SES and PFC function.17 Similarly, variation in home literacy activities and access to computers has been shown to mediate the relationship between lower SES and poorer child executive functioning.59
In addition to material deprivation, stress and negative parenting behaviors are associated with reductions in PFC volume and surface area.22,37,55,56 Evidence is accumulating that these structural changes help explain the relationship between poverty, chronic stress, and cognitive and behavioral outcomes.39,43,46–52,55–58 For example, younger adolescents exposed to high cumulative life stress during childhood have been shown to demonstrate poorer executive functioning related to smaller PFC volumes.56 Similarly, 1 longitudinal study found that the relationship between early-life poverty and conduct disorder symptoms later in life was mediated by volume reductions in the orbitofrontal cortex.57
Consistent with the hypothesis that excessive glucocorticoids link poverty-related negative input and PFC volume, there is some evidence that children from low-SES families are more likely to exhibit altered cortisol production and related deficits in cognitive functioning. In prospective studies with low-income rural children, material deprivation and stress (including poor housing quality, low economic sufficiency, and family instability) have been related to higher child basal cortisol, whereas positive parenting has been associated with lower cortisol.54 Lower cortisol levels have been shown to mediate the relationship between positive parenting and better executive function (EF), as well as the relationship between higher SES and better child EF.53 These findings thus suggest that, above and beyond material deprivation, exposure to family stress, and resultant effects on the HPA axis, could contribute to alterations in PFC development.
Limitations of Current Literature
Although there is increasing interest in how poverty affects the brain, there are several shortcoming of the current literature. First, little is known about the role of timing and chronicity of poverty on brain structure.31 In fact, there is relatively sparse evidence to illuminate the impact of poverty on the development of the brain per se, because few studies evaluate the brain at >1 point in time. Those that do typically evaluate outcomes over short periods of time.39,59 The paucity of longitudinal studies is related to several methodological challenges, which include rapid changes in brain-imaging technologies across time and a lack of measures and tasks that are equivalent across populations and development.29 Nonetheless, such studies are critical to advancing the field. Longitudinal designs can shed light on sensitive periods in neural processes, which can guide interventions and help refute concerns about irreversibility that could stigmatize children in poverty.26
To inform intervention programs, it is important to differentiate the effects of different SES indicators (eg, income, education, subjective social status). In addition, socioeconomic deprivation rarely occurs in isolation. It is estimated that low-income children experience 5 times more psychosocial risks than higher-income children.117 Consequently, the effects ascribed to low SES likely reflect the impact of a variety of highly correlated factors (eg, nutrition, community violence, parenting quality) that change over time. To illuminate the relationship between poverty and brain development, longitudinal studies with comprehensive measurement of many potential environmental mediators are needed. Perhaps most urgently, experimental studies that assess the impact of changing SES on brain development are needed to determine causal links.
Implications for Pediatric Practice
Although young people are particularly vulnerable to the negative effects of poverty, their systems are also likely more malleable in response to intervention. The success of interventions such as the Perry Preschool Program demonstrate that the impact of poverty may be preventable or reversible at cognitive and behavioral levels.118 The Perry Preschool Program, which randomized low-income 3- and 4-year-olds to a high-quality preschool program or a comparison group that received no preschool, demonstrated positive and sustained impacts on achievement test scores, educational attainment, and social skills (but not IQ) among children in the experimental group.118 In addition, preliminary evidence, such as a recent randomized trial of a family-based intervention delivered in Head Start preschools, suggests that improvements at the neural level (eg electrophysiological measures of brain functions that support selective attention) in response to intervention are also possible.119 Although research on reversibility is in its infancy, carefully tailored neuroscience-informed interventions might ultimately enhance practice-based approaches to reduce SES disparities in health and achievement.
The American Academy of Pediatrics has highlighted the need to build pediatricians’ capacity to address poverty in their practices.2 Bright Futures guidelines suggest that primary care providers evaluate and address social needs such as housing, employment, education, and food.120,121 Barriers remain to screening and referral, including time and financial pressures and inadequate capacity and quality of community-based resources.65 Screening for psychosocial needs has been shown to increase utilization of community resources.120 To date, however, the impact of primary care screening and referral on child cognitive, behavioral, or neural development has not been evaluated. It is conceivable that extending screening programs to include environmental mediators of neurodevelopment described above (eg, parenting stress, cognitive stimulation) could promote child neurodevelopment across the socioeconomic spectrum.122
Primary care provides a population-based setting for interventions to mitigate the impact of poverty early in life, as evidenced by programs like Reach Out and Read, which promotes early literacy.65,123 For example, in the Video Interaction Project, delivered alongside well child care, child development specialists provide parent-child interaction coaching and support play and shared reading. In randomized trials, the Video Interaction Project is associated with improvements in parenting quality and parent–child interaction, better cognition, and more shared reading.123–125 Partnerships between clinicians and neuroscientists offer the opportunity to evaluate whether effective programs are also associated with changes at the neural level.
Conclusions and Future Directions
To meaningfully improve child health at the population level, child health professionals must invest in efforts to reduce socioeconomic disparities in health and achievement.65 Pediatricians’ support and advocacy is a critical to expanding high-quality community resources for families, as well as coordinated systems to implement them.65
Children raised in poverty vary substantially with respect to adverse environments and their susceptibility to these environments. Attributing risk based on socioeconomic resources alone may unnecessarily stigmatize families and communities whose children are thriving despite constrained resources. On the other hand, pediatricians may serve as ideal advocates for programs and supports that provide financial benefits to poor families and have been associated with remarkable differences in long-term cognitive and health outcomes.126
In summary, although significant gaps remain, evidence from neuroscience is converging with evidence from epidemiology, developmental psychology, and genetics to underscore the role that social systems play in shaping developing biological systems. Partnering with neuroscientists to incorporate conceptual frameworks and methods into pediatric research could help explicate the neural mechanisms by which adversity affects children’s life chances and target and evaluate programs to ameliorate these effects.
Glossary
- EF
executive function
- HPA
hypothalamic–pituitary–adrenal
- PFC
prefrontal cortex
- SES
socioeconomic status
Footnotes
Drs Johnson and Riis conceptualized the study; and all authors wrote the manuscript and approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose. Funded by the National Institutes of Health (NIH).
FUNDING: This research was supported by KO1DA027229 from NIH/National Institute on Drug Abuse to Dr Johnson.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.DeNavas-Walt C, Proctor BD, US Census Bureau . Income and Poverty in the United States: 2013, Current Population Reports. Washington, D.C.: US Government Printing Office; 2014:60–249 [Google Scholar]
- 2.American Academy of Pediatrics Agenda for Children—Strategic Plan 2014. Available at: www.aap.org/en-us/about-the-aap/aap-facts/AAP-Agenda-for-Children-Strategic-Plan/pages/AAP-Agenda-for-Children-Strategic-Plan.aspx. Accessed January 20, 2014
- 3.Duncan GJ, Brooks-Gunn J, Klebanov PK Economic deprivation and early childhood development. Child Dev 1994;65(2 Spec No):296–318 [PubMed]
- 4.Ackerman BP, Brown ED, Izard CE. The relations between persistent poverty and contextual risk and children’s behavior in elementary school. Dev Psychol. 2004;40(3):367–377 [DOI] [PubMed] [Google Scholar]
- 5.Brooks-Gunn J, Duncan GJ. The effects of poverty on children. Future Child. 1997;7(2):55–71 [PubMed] [Google Scholar]
- 6.Al Hazzouri AZ, Haan MN, Galea S, Aiello AE. Life-course exposure to early socioeconomic environment, education in relation to late-life cognitive function among older Mexicans and Mexican Americans. J Aging Health. 2011;23(7):1027–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guralnik JM, Butterworth S, Wadsworth MEJ, Kuh D. Childhood socioeconomic status predicts physical functioning a half century later. J Gerontol A Biol Sci Med Sci. 2006;61(7):694–701 [DOI] [PubMed] [Google Scholar]
- 8.Minkler M, Fuller-Thomson E, Guralnik JM. Gradient of disability across the socioeconomic spectrum in the United States. N Engl J Med. 2006;355(7):695–703 [DOI] [PubMed] [Google Scholar]
- 9.Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proc Natl Acad Sci USA. 2009;106(16):6545–6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirase H, Shinohara Y. Transformation of cortical and hippocampal neural circuit by environmental enrichment. Neuroscience. 2014;280:282–298 [DOI] [PubMed] [Google Scholar]
- 11.Lipina SJ, Simonds J, Segretin MS. Recognizing the child in child poverty. Vulnerable Child Youth Stud. 2011;6(1):8–17 [Google Scholar]
- 12.Farah MJ, Shera DM, Savage JH, et al. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110(1):166–174 [DOI] [PubMed] [Google Scholar]
- 13.Noble KG, Farah MJ, McCandliss BD. Socioeconomic background modulates cognition-achievement relationships in reading. Cogn Dev. 2006;21(3):349–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noble KG, Wolmetz ME, Ochs LG, Farah MJ, McCandliss BD. Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev Sci. 2006;9(6):642–654 [DOI] [PubMed] [Google Scholar]
- 15.Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev Sci. 2005;8(1):74–87 [DOI] [PubMed] [Google Scholar]
- 16.Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci. 2007;10(4):464–480 [DOI] [PubMed] [Google Scholar]
- 17.Sheridan MA, Sarsour K, Jutte D, D’Esposito M, Boyce WT. The impact of social disparity on prefrontal function in childhood. PLoS One. 2012;7(4):e35744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jednoróg K, Altarelli I, Monzalvo K, et al. The influence of socioeconomic status on children’s brain structure. PLoS One. 2012;7(8):e42486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao H, Betancourt L, Giannetta JM, et al. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. Neuroimage. 2010;49(1):1144–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 2012;15(4):516–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavanagh J, Krishnadas R, Batty GD, et al. Socioeconomic status and the cerebellar grey matter volume. Data from a well-characterised population sample. Cerebellum. 2013;12(6):882–891 [DOI] [PubMed] [Google Scholar]
- 22.Lawson GM, Duda JT, Avants BB, Wu J, Farah MJ. Associations between children’s socioeconomic status and prefrontal cortical thickness. Dev Sci. 2013;16(5):641–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Angiulli A, Herdman A, Stapells D, Hertzman C. Children’s event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology. 2008;22(3):293–300 [DOI] [PubMed] [Google Scholar]
- 24.Raizada RDS, Richards TL, Meltzoff A, Kuhl PK. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. Neuroimage. 2008;40(3):1392–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens C, Fanning J, Coch D, Sanders L, Neville H. Neural mechanisms of selective auditory attention are enhanced by computerized training: electrophysiological evidence from language-impaired and typically developing children. Brain Res. 2008;1205:55–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipina SJ, Segretin MS. Strengths and weakness of neuroscientific investigations of childhood poverty: future directions. Front Hum Neurosci. 2015;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipina SJSJ, Posner MI. The impact of poverty on the development of brain networks. Front Hum Neurosci. 2012;6:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13(2):65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gianaros PJ, Hackman DA. Contributions of neuroscience to the study of socioeconomic health disparities. Psychosom Med. 2013;75(7):610–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raizada RDS, Kishiyama MM. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Front Hum Neurosci. 2010;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brito NH, Noble KG. Socioeconomic status and structural brain development. Front Neurosci 2014;4(8):276 [DOI] [PMC free article] [PubMed]
- 32.Tomalski P, Johnson MH. The effects of early adversity on the adult and developing brain. Curr Opin Psychiatry. 2010;23(3):233–238 [DOI] [PubMed] [Google Scholar]
- 33.Lipina SJ, Colombo JA. Poverty and Brain Development During Childhood: An Approach From Cognitive Psychology and Neuroscience. Washington, D.C.: American Psychological Association; 2009 [Google Scholar]
- 34.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11(9):651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan GJ, Magnuson K. Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdiscip Rev Cogn Sci. 2012;3(3):377–386 [DOI] [PubMed] [Google Scholar]
- 36.D’Angiulli A, Van Roon PM, Weinberg J, et al. Frontal EEG/ERP correlates of attentional processes, cortisol and motivational states in adolescents from lower and higher socioeconomic status. Front Hum Neurosci. 2012;6:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noble KG, Houston SM, Brito NH, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomalski P, Moore DG, Ribeiro H, et al. Socioeconomic status and functional brain development—associations in early infancy. Dev Sci. 2013;16(5):676–687 [DOI] [PubMed] [Google Scholar]
- 39.Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 2015;169(9):822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PLoS One. 2011;6(5):e18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanson JL, Nacewicz BM, Sutterer MJ, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry. 2015;77(4):314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luby J, Belden A, Botteron K, et al. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167(12):1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noble KG, Grieve SM, Korgaonkar MS, et al. Hippocampal volume varies with educational attainment across the life-span. Front Hum Neurosci. 2012;6:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheridan MA, How J, Araujo M, Schamberg MA, Nelson CA. What are the links between maternal social status, hippocampal function and HPA axis function in children?. Dev Sci. 2013;16(5):1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: childhood socioeconomic status influences adult hippocampal size. Ann Neurol. 2012;71(5):653–660 [DOI] [PubMed] [Google Scholar]
- 46.Gilliam M, Forbes EE, Gianaros PJ, Erickson KI, Brennan LM, Shaw DS. Maternal depression in childhood and aggression in young adulthood: evidence for mediation by offspring amygdala—hippocampal volume ratio. J Child Psychol Psychiatry. 2015;56(10):1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim P, Evans GW, Angstadt M, et al. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci USA. 2013;110(46):18442–18447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lupien SJ, Parent S, Evans AC, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA. 2011;108(34):14324–14329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muscatell KA, Morelli SA, Falk EB, et al. Social status modulates neural activity in the mentalizing network. Neuroimage. 2012;60(3):1771–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moutsiana C, Johnstone T, Murray L, et al. Insecure attachment during infancy predicts greater amygdala volumes in early adulthood. J Child Psychol Psychiatry. 2015;56(5):540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki H, Luby JL, Botteron KN, Dietrich R, McAvoy MP, Barch DM. Early life stress and trauma and enhanced limbic activation to emotionally valenced faces in depressed and healthy children. J Am Acad Child Adolesc Psychiatry. 2014;53(7):800–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry. 2006;60(3):296–301 [DOI] [PubMed] [Google Scholar]
- 53.Blair C, Granger DA, Willoughby M, et al. ; FLP Investigators . Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Dev. 2011;82(6):1970–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blair C, Raver CC, Granger D, Mills-Koonce R, Hibel L; Family Life Project Key Investigators . Allostasis and allostatic load in the context of poverty in early childhood. Dev Psychopathol. 2011;23(3):845–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanson JL, Hair N, Shen DG, et al. Family poverty affects the rate of human infant brain growth. PLoS One. 2013;8(12):e80954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanson JL, Chung MK, Avants BB, et al. Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. J Neurosci. 2012;32(23):7917–7925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holz NE, Boecker R, Hohm E, et al. The long-term impact of early life poverty on orbitofrontal cortex volume in adulthood: results from a prospective study over 25 years. Neuropsychopharmacology. 2015;40(4):996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liberzon I, Okada G, Ho SS, Swain JE, Gary W, Function AB. Childhood poverty and recruitment of adult emotion regulatory neurocircuitry. Soc Cogn Affect Neurosci. 2015;10(11):1596–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lipina S, Segretin S, Hermida J, et al. Linking childhood poverty and cognition: environmental mediators of non-verbal executive control in an Argentine sample. Dev Sci. 2013;16(5):697–707 [DOI] [PubMed] [Google Scholar]
- 60.Nyaradi A, Li J, Hickling S, Foster J, Oddy WH. The role of nutrition in children’s neurocognitive development, from pregnancy through childhood. Front Hum Neurosci. 2013;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bangalore L. In: Chudler EH, ed. Brain Development, vol. 1 New York, NY: Chelsea House; 2007 [Google Scholar]
- 62.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718–729 [DOI] [PubMed] [Google Scholar]
- 63.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10(9):647–658 [DOI] [PubMed] [Google Scholar]
- 64.Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30(45):14964–14971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shonkoff JP, Garner AS; Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics . The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1). Available at: www.pediatrics.org/cgi/content/full/129/1/e232 [DOI] [PubMed] [Google Scholar]
- 66.Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychol Sci. 2003;14(6):623–628 [DOI] [PubMed] [Google Scholar]
- 67.Chiang M-C, McMahon KL, de Zubicaray GI, et al. Genetics of white matter development: a DTI study of 705 twins and their siblings aged 12 to 29. Neuroimage. 2011;54(3):2308–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simons RL, Lei MK, Beach SRH, Brody GH, Philibert RA, Gibbons FX. Social environmental variation, plasticity genes, and aggression: evidence for the differential susceptibility hypothesis. Am Sociol Rev. 2011;76(6):833–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary—neurodevelopmental theory. Dev Psychopathol. 2011;23(1):7–28 [DOI] [PubMed] [Google Scholar]
- 70.Brody GH, Yu T, Chen YF, et al. Supportive family environments, genes that confer sensitivity, and allostatic load among rural African American emerging adults: a prospective analysis. J Fam Psychol. 2013;27(1):22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holz N, Boecker R, Buchmann AF, et al. Evidence for a sex-dependent MAOA x childhood stress interaction in the neural circuitry of aggression. Cereb Cortex. 2014;pii:bhu249 [DOI] [PubMed] [Google Scholar]
- 72.Simons RL, Lei MK, Stewart EA, et al. Social adversity, genetic variation, street code, and aggression: A geneticlly informed model of violent behavior. Youth Violence Juv Justice. 2012;10(1):3–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Belsky J, Beaver KM. Cumulative-genetic plasticity, parenting and adolescent self-regulation. J Child Psychol Psychiatry. 2011;52(5):619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Ijzendoorn MH, Belsky J, Bakermans-Kranenburg MJ. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Transl Psychiatry. 2012;2(8):e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Essex MJ, Boyce WT, Hertzman C, et al. Epigenetic vestiges of early developmental adversity: childhood stress exposure and DNA methylation in adolescence. Child Dev. 2013;84(1):58–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roth TL, Sweatt JD. Annual Research Review: Epigenetic mechanisms and environmental shaping of the brain during sensitive periods of development. J Child Psychol Psychiatry. 2011;52(4):398–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheridan MA, McLaughlin KA. Dimensions of early experience and neural development: deprivation and threat. Trends Cogn Sci. 2014;18(11):580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Evans GW, Li D, Sepanski Whipple S. Cumulative risk and child development. Psychol Bull 2013.139(6):1342–1396 [DOI] [PubMed]
- 79.Conger RD, Conger KJ, Martin MJ. Socioeconomic status, family processes, and individual development. J Marriage Fam. 2010;72(3):685–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hart B, Risley TR. Meaningful Differences in the Everyday Experience of Young American Children. Baltimore, MD: Paul H. Brookes; 1995 [Google Scholar]
- 81.Weisleder A, Fernald A. Talking to children matters: early language experience strengthens processing and builds vocabulary. Psychol Sci. 2013;24(11):2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuhl PK. Brain mechanisms in early language acquisition. Neuron. 2010;67(5):713–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Magge H, Sprinz P, Adams WG, Drainoni ML, Meyers A. Zinc protoporphyrin and iron deficiency screening: trends and therapeutic response in an urban pediatric center. JAMA Pediatr. 2013;167(4):361–367 [DOI] [PubMed] [Google Scholar]
- 84.Kant AK, Graubard BI. Race-ethnic, family income, and education differentials in nutritional and lipid biomarkers in US children and adolescents: NHANES 2003-2006. Am J Clin Nutr. 2012;96(3):601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Black MM. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull. 2008;29(2 Suppl):S126–S131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosales FJ, Reznick JS, Zeisel SH. Understanding the role of nutrition in the brain and behavioral development of toddlers and preschool children: identifying and addressing methodological barriers. Nutr Neurosci. 2009;12(5):190–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105(4). Available at: www.pediatrics.org/cgi/content/full/105/4/E51 [DOI] [PubMed] [Google Scholar]
- 88.Lozoff B, Smith JB, Kaciroti N, Clark KM, Guevara S, Jimenez E. Functional significance of early-life iron deficiency: outcomes at 25 years. J Pediatr. 2013;163(5):1260–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baker RD, Greer FR; Committee on Nutrition American Academy of Pediatrics . Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics. 2010;126(5):1040–1050 [DOI] [PubMed] [Google Scholar]
- 90.Rogan WJ, Paulson JA, Baum C, et al. ; Council on Environmental Health . Iodine deficiency, pollutant chemicals, and the thyroid: new information on an old problem. Pediatrics. 2014;133(6):1163–1166 [DOI] [PubMed] [Google Scholar]
- 91.Evans GW, Brooks-Gunn J, Klebanov PK. Stressing out the poor: chronic physiological stress and the income-achievement gap. Community Investments. 2011;23(2):22–27 [Google Scholar]
- 92.Evans GW, English K. The environment of poverty: multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Dev. 2002;73(4):1238–1248 [DOI] [PubMed] [Google Scholar]
- 93.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445 [DOI] [PubMed] [Google Scholar]
- 94.Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychol Rev. 2010;117(1):134–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186(1):190–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frodl T, O’Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis. 2013;52:24–37 [DOI] [PubMed] [Google Scholar]
- 97.Conger RD, Conger KJ. Resilience in midwestern families: selected findings from the first decade of a prospective, longitudinal study. J Marriage Fam. 2002;64:361–373 [Google Scholar]
- 98.Belsky J, de Haan M. Annual research review: parenting and children’s brain development: the end of the beginning. J Child Psychol Psychiatry. 2011;52(4):409–428 [DOI] [PubMed] [Google Scholar]
- 99.Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135(6):885–908 [DOI] [PubMed] [Google Scholar]
- 100.Obradović J, Boyce WT. Individual differences in behavioral, physiological, and genetic sensitivities to contexts: implications for development and adaptation. Dev Neurosci. 2009;31(4):300–308 [DOI] [PubMed] [Google Scholar]
- 101.Zhang T-Y, Meaney MJ. Epigenetics and the environmental regulation of the genome and its function. Annu Rev Psychol 2010;61:439–466, C1–C3 [DOI] [PubMed]
- 102.Francis D, Diorio J, Liu D, Meaney M Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 1999;286(5442):1155–1158 [DOI] [PubMed]
- 103.Zhang TY, Labonté B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology. 2013;38(1):111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McGowan PO, Sasaki A, D’Alessio AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suderman M, McGowan PO, Sasaki A, et al. Conserved epigenetic sensitivity to early life experience in the rat and human hippocampus. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17266–17272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu J, Lewis G. Environmental toxicity and poor cognitive outcomes in children and adults. J Environ Health. 2014;76(6):130–138 [PMC free article] [PubMed] [Google Scholar]
- 107.Gray SC, Edwards SE, Miranda ML. Race, socioeconomic status, and air pollution exposure in North Carolina. Environ Res. 2013;126:152–158 [DOI] [PubMed] [Google Scholar]
- 108.McEwen BS, Tucker P. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. Am J Public Health. 2011;101(Suppl 1):S131–S139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sanders T, Liu Y, Buchner V, Tchounwou PB. Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health. 2009;24(1):15–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Geng F, Mai X, Zhan J, et al. Low-level prenatal lead exposure alters auditory recognition memory in 2-month-old infants: an event-related potentials (ERPs) study. Dev Neuropsychol. 2014;39(7):516–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jedrychowski W, Perera FP, Jankowski J, et al. Very low prenatal exposure to lead and mental development of children in infancy and early childhood: Krakow prospective cohort study. Neuroepidemiology. 2009;32(4):270–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol. 2004;26(3):373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69–73 [DOI] [PubMed] [Google Scholar]
- 114.Uematsu A, Matsui M, Tanaka C, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One. 2012;7(10):e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wierenga L, Langen M, Ambrosino S, van Dijk S, Oranje B, Durston S. Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. Neuroimage. 2014;96:67–72 [DOI] [PubMed] [Google Scholar]
- 116.Giedd JN, Blumenthal J, Jeffries NO, et al. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(4):571–588 [DOI] [PubMed] [Google Scholar]
- 117.Evans GW, Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Ann N Y Acad Sci. 2010;1186:174–189 [DOI] [PubMed] [Google Scholar]
- 118.Heckman J, Pinto R, Savelyev P. Understanding the mechanisms through which an influential early childhood program boosted adult outcomes. Am Econ Rev. 2013;103(6):2052–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neville H, Stevens C, Pakulak E, Bell TA. Commentary: Neurocognitive consequences of socioeconomic disparities. Dev Sci. 2013;16(5):708–712 [DOI] [PubMed] [Google Scholar]
- 120.Garg A, Jack B, Zuckerman B. Addressing the social determinants of health within the patient-centered medical home: lessons from pediatrics. JAMA. 2013;309(19):2001–2002 [DOI] [PubMed] [Google Scholar]
- 121.Garg A, Marino M, Vikani AR, Solomon BS. Addressing families’ unmet social needs within pediatric primary care: the health leads model. Clin Pediatr (Phila). 2012;51(12):1191–1193 [DOI] [PubMed] [Google Scholar]
- 122.Milgrom J, Newnham C, Anderson PJ, et al. Early sensitivity training for parents of preterm infants: impact on the developing brain. Pediatr Res. 2010;67(3):330–335 [DOI] [PubMed] [Google Scholar]
- 123.Mendelsohn AL, Huberman HS, Berkule SB, Brockmeyer CA, Morrow LM, Dreyer BP. Primary care strategies for promoting parent-child interactions and school readiness in at-risk families: the Bellevue Project for Early Language, Literacy, and Education Success. Arch Pediatr Adolesc Med. 2011;165(1):33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mendelsohn AL, Valdez PT, Flynn V, et al. Use of videotaped interactions during pediatric well-child care: impact at 33 months on parenting and on child development. J Dev Behav Pediatr. 2007;28(3):206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mendelsohn AL, Dreyer BP, Flynn V, et al. Use of videotaped interactions during pediatric well-child care to promote child development: a randomized, controlled trial. J Dev Behav Pediatr. 2005;26(1):34–41 [PMC free article] [PubMed] [Google Scholar]
- 126.Dahl GB, Lochner L. The impact of family income on child achievement: evidence from the earned income tax credit. Am Econ Rev. 2012;102(5):1927–1956 [Google Scholar]