Abstract
Photodynamic therapy (PDT) was discovered more than 100 years ago, and has since become a well-studied therapy for cancer and various non-malignant diseases including infections. PDT uses photosensitizers (PSs, non-toxic dyes) that are activated by absorption of visible light to initially form the excited singlet state, followed by transition to the long-lived excited triplet state. This triplet state can undergo photochemical reactions in the presence of oxygen to form reactive oxygen species (including singlet oxygen) that can destroy cancer cells, pathogenic microbes and unwanted tissue. The dual-specificity of PDT relies on accumulation of the PS in diseased tissue and also on localized light delivery. Tetrapyrrole structures such as porphyrins, chlorins, bacteriochlorins and phthalocyanines with appropriate functionalization have been widely investigated in PDT, and several compounds have received clinical approval. Other molecular structures including the synthetic dyes classes as phenothiazinium, squaraine and BODIPY (boron-dipyrromethene), transition metal complexes, and natural products such as hypericin, riboflavin and curcumin have been investigated. Targeted PDT uses PSs conjugated to antibodies, peptides, proteins and other ligands with specific cellular receptors. Nanotechnology has made a significant contribution to PDT, giving rise to approaches such as nanoparticle delivery, fullerene-based PSs, titania photocatalysis, and the use of upconverting nanoparticles to increase light penetration into tissue. Future directions include photochemical internalization, genetically encoded protein PSs, theranostics, two-photon absorption PDT, and sonodynamic therapy using ultrasound.
Keywords: naturally occurring photosensitizers, photochemical mechanisms, photodynamic therapy, photosensitizers, synthetic dyes, tetrapyrroles
INTRODUCTION
The concept of photodynamic therapy (PDT) dates back to 1900. A medical student, Oscar Raab, working in Munich, Germany [1], made an accidental discovery that micro-organisms such as paramecia that had been incubated with certain dyes could be killed when exposed to light, but not when they were kept in the dark. When it was subsequently discovered that oxygen in the air was also necessary for this light-mediated killing effect to occur, the term ‘photodynamic action’ was coined. It was not long after these discoveries that the first efforts were made to use this phenomenon as a cancer therapy, by painting dyes on to superficial skin tumours and then exposing them to light. However, the following two world wars and the impressive rise of the pharmaceutical industry in the 1950s and 1960s delayed the further exploration of PDT by over 60 years. The modern era of PDT started in the 1970s in the U.S.A., largely due to the efforts of Dr Thomas Dougherty working at Roswell Park Cancer Institute in Buffalo, NY. The first photosensitizer (PS) that was introduced by Dougherty and co-workers was a water-soluble mixture of porphyrins that was named ‘haematoporphyrin derivative’ (HpD), and a more purified preparation later became known as Photofrin. Although Photofrin is still the most frequently used PS throughout the world today, it has many acknowledged disadvantages including skin photosensitivity that can last for weeks or months and can be highly troubling for patients, and a relatively small absorbance peak at 630 nm making it somewhat inefficient in use, especially for bulky tumours where light penetration is problematic [2]. Since then medicinal chemists have attempted to synthesize and discover molecules that could act as improved PSs, and several hundred compounds have now been proposed as potentially useful to mediate PDT for tackling cancer, infections and many other diseases. In recent years PDT has returned to its earliest roots, and antimicrobial photodynamic inactivation (aPDI) has made something of a comeback. The structures of antimicrobial PSs have some features in common with anti-cancer PSs, but there are also major differences.
Photochemical mechanisms
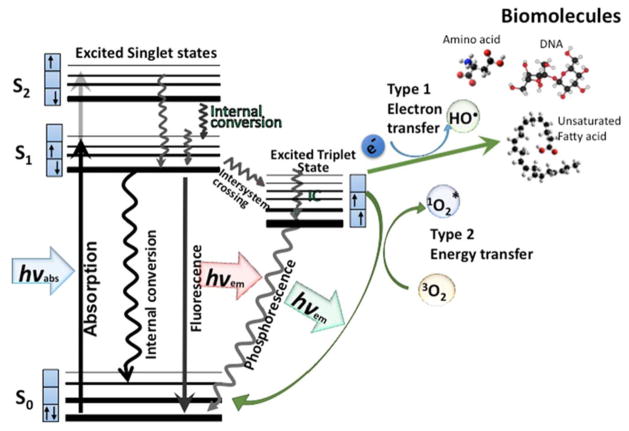
The PS molecule is a singlet in its ground state because it has two electrons with opposite spins. Absorption of a photon of light with the appropriate quantum energy (wavelength) leads to the excitation of one electron into a higher-energy orbital (illustrated by the Jablonski diagram shown in Figure 1). This singlet excited-state PS is very unstable and loses its excess energy either as emission of light (fluorescence) or production heat (internal conversion). However, the excited singlet PS may undergo a process known as ‘intersystem crossing’ to form a more stable excited triplet state with parallel spins. The triplet-state PS molecule can decay back to the ground state (by emitting a phosphorescent photon) but this is a ‘forbidden process’ by the quantum selection rules, so the triplet state is much more stable than the singlet state having a lifetime of microseconds compared with only nanoseconds for the excited singlet. This long lifetime of the triplet state allows it sufficient time to transfer its energy by colliding with molecular oxygen (O2), which is unique in being a molecular triplet in its ground state. This energy-transfer step leads to the formation of singlet oxygen (1O2) (and ground-state PS), and the reaction is referred to as a Type II photochemical process [3]. A Type I photochemical process can also occur whereby the excited-state PS undergoes electron transfer reactions that eventually forms reactive oxygen species (ROS). This mechanism may involve either acquisition or donation of an electron to form the radical cation or radical anion. The radical anion can react with oxygen to produce the superoxide radical anion (O2•−). Dismutation or one-electron reduction of O2•− gives hydrogen peroxide (H2O2), which in turn can undergo another one-electron reduction to form the powerful oxidant hydroxyl radicals (HO•). ROS generation via Type II chemistry is mechanistically much simpler than via Type I, and most PSs used for anti-cancer PDT are believed to operate via the Type II rather than the Type I mechanism.
Figure 1. Jablonski diagram.
When light (hv) is absorbed by the PS, the electron moves from a non-excited low-energy singlet state into a high-energy singlet state. This excited state can lose energy by emitting a photon (fluorescence) or by internal conversion (non-radiative decay). The process known as intersystem crossing involves flipping of the spin of the high-energy electron, leading to a long-lived excited triplet state. In the presence of molecular oxygen, superoxide and hydroxyl radicals are formed in Type I reactions and singlet oxygen in a Type II reaction. These ROS can damage most types of biomolecules (amino acids, lipids, nucleic acids).
Properties of ideal PSs
Most of the PSs used in cancer therapy are based on the tetrapyrrole backbone, a structure similar to that contained in the protoporphyrin prosthetic group contained in haemoglobin. Depending on the precise structure, effective PSs can be synthesized with absorbance bands between 600 and 800 nm. Since the penetration of light into tissue increases with wavelength, agents with strong absorbance in the deep-red spectral region such as chlorins, bacteriochlorins and phthalocyanines tend to make much more efficient PSs although many other factors are also important.
A PS should ideally be a single pure compound to allow manufacturing under good manufacturing practice (GMP) conditions with quality control and low manufacturing costs, and leading to better stability in storage. It should have a strong absorption peak in the red to near-infrared spectral region (between 650 and 800 nm) because absorption of single photons with wavelengths longer than 800 nm does not provide enough energy to excite oxygen to its singlet state. PSs should possess a substantial triplet quantum yield leading to good production of ROS upon irradiation. It should have no dark toxicity and relatively rapid clearance from normal tissues, thereby minimizing the side effects of phototoxicity [4]. Although it used to be considered desirable for the interval between drug administration and irradiation (drug–light interval, DLI) to be as long as possible (up to 4 days), so that the PS was given sufficient time to clear from normal tissues, while remaining concentrated in tumours, many reports now suggest that the tumour response may be substantially better when light is delivered at a much shorter DLI (minutes or hours) when most of the PS is still present in the blood vessels, thus producing marked vascular damage [5]. Some reports have suggested that a pronounced inflammatory response resulting from necrotic cell death after PDT is important because it aids the immune-stimulating function of PDT, whereas other reports have suggested that PDT regimens that produce more apoptosis and less necrosis and inflammation are suitable for applications such as PDT of brain tumours where swelling is undesirable. Previous findings, however, show that certain PDT-induced apoptotic cell death mechanisms are also highly immunogenic and can stimulate antitumour immunity [6]. The light-mediated destruction of the PS (known as photobleaching) was thought to be undesirable, but some reports now suggest that this phenomenon may make light dosimetry during PDT less critical, as over-treatment is avoided when the remaining PS is destroyed during the illumination [7].
The discovery that 5-aminolaevulinic acid (ALA) could function as a biosynthetic precursor of the PS protoporphyrin IX [8] has led to many applications in which ALA or ALA-esters can be topically applied to tissues, or even administered orally. These ALA derivatives are considered to be ‘pro-drugs’, needing to be metabolically converted into protoporphyrin IX in order to become active PSs. Successes in treatment of skin cancer and other dermatologic indications have been reported.
Tumour-targeting in PDT
The most effective PSs tend to be relatively hydrophobic compounds that rapidly diffuse into tumour cells and localize in intracellular membrane structures such as mitochondria and endoplasmic reticulum (ER). More polar compounds tend to be taken up by the active process of adsorptive or fluid phase endocytosis, and this process is slower than passive diffusion, necessitating a longer DLI. Many hypotheses have been proposed to account for the tumour-localizing properties that have long been observed in PDT [9]. These explanations include the occurrence of leaky and tortuous tumour blood vessels that are typical of the neovascularization process occurring in tumour angiogenesis, and together with the absence of lymphatic drainage in tumours are collectively known as the ‘enhanced permeability and retention (EPR) effect’ [10]. Some of the most effective PS compounds have been found preferentially to low-density lipoprotein (LDL) among various serum proteins, and it has been proposed that over-expressed LDL receptors that are sometimes found on tumour cells could be important in tumour localization [11].
Mechanisms of PDT-mediated cytotoxicity
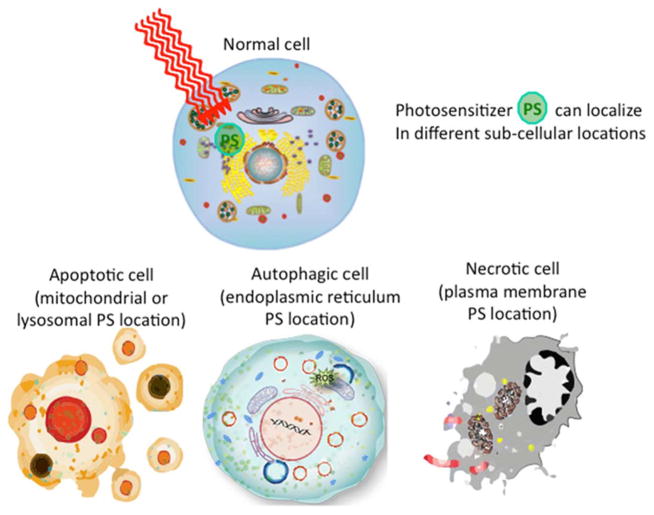
The lifetime of singlet oxygen (1O2) is very short (~10–320 ns), limiting its diffusion to only approximately 10–55 nm in cells [12]. Thus, photodynamic damage is likely to occur very close to the intracellular location of the PS [13]. PDT can kill cells via the three main morphologies of cell death: apoptotic, necrotic and autophagy-associated cell death (Figure 2). It is thought that the subcellular localization of the PS in different organelles (mitochondria, lysosomes, endoplasmic reticulum, plasma membrane, etc.) plays a major role in the type of cell death mechanism that dominates, but other factors such as the overall PDT dose (PS concentration × light fluence) and DLI also play a role. Overall it is accepted that apoptosis is the principal modality of cell death when cells are treated with PDT in vitro.
Figure 2. Cell death mechanisms.
The subcellular localization of the PS in different organelles (mitochondria, lysosomes, endoplasmic reticulum, plasma membrane, etc.) plays a major role in the type of cell death mechanism that dominates, but other factors such as the overall PDT dose (PS concentration × light fluence) and DLI also play a role.
Antimicrobial photoinactivation
It is somewhat ironic that the origins of PDT over 100 years ago were in the killing of micro-organisms, but throughout the period from the 1970s to 2010, cancer was the overwhelming most popular target disease in PDT research. Now with the inexorable rise of multi-drug-resistance among pathogenic microbes, a PDI has made something of a comeback.
The ideal PS structure is very different between anti-cancer drugs and antimicrobial drugs. Anti-cancer PSs tend to be lipophilic with little or no overall charge (either positive or negative). Antimicrobial PSs, on the other hand, should have pronounced cationic charges, and in many cases the more charges the better especially for targeting Gram-negative bacteria. Anti-cancer PSs are usually expected to have long wavelength (farred/near-infrared) absorption bands for good tissue penetration of the exciting light, whereas for antimicrobial PSs, this property is much less important as infections that will be treated by PDT tend to be rather superficial in nature.
TETRAPYRROLE STRUCTURES
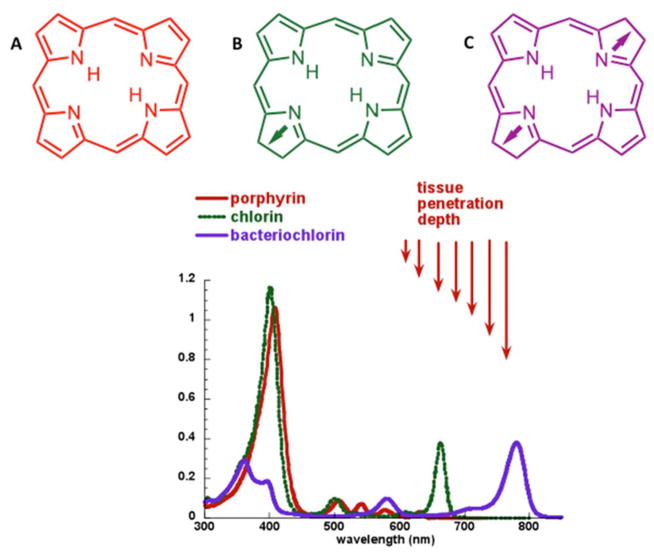
Tetrapyrrole structures make up the largest group of PSs that have been employed for anti-cancer applications. Tetrapyrrole backbones occur naturally in several important biomolecules such as haem, chlorophyll and bacteriochlorophyll. In fact, tetrapyrroles have been termed the ‘pigments of life’ [14]. As the double-bonds are successively reduced when moving from backbones that are porphyrins to chlorins to bacteriochlorins the Q-band is substantially red-shifted and the height of the band also increases (Figure 3). Phthalocyanines also have a large band in the 670 nm region. Broadly speaking, tetrapyrrole PSs (with the exception of bacteriochlorins) tend to produce predominantly Type II singlet oxygen, compared with the Type I ROS (such as hydroxyl radicals) that are often produced by PSs with other structures. The number of tetrapyrrole compounds used as PSs in PDT is too high to even list them all, but in Table 1 we have tried to list the most important compounds and the newest examples.
Figure 3. Structure and absorption spectra of tetrapyrrole photosensitizers.
Tetrapyrrole absorption spectrum showing porphyrins, chlorins and bacteriochlorins.
Table 1.
PSs based on tetrapyrrole backbone
| Class | Name | Structure | λmax | Application | Reference |
|---|---|---|---|---|---|
| Porphyrin | Photofrin (NB actual structure of Photofrin is a complex mixture of ester- and ether-linked dimers and oligomers) |
|
630 nm | Cancer, in vitro, in vivo, clinical | [135] |
| Porphyrin | ALA-induced protoporphyrin IX |
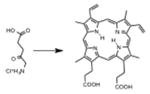
|
635 nm | Cancer, in vitro, in vivo, clinical | [136] |
| Porphyrin | 5,10,15,20-Tetrakis(1-methylpyridinium-4-yl) porphyrin tosylate |
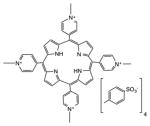
|
White | Cancer, antimicrobial, in vitro, in vivo | [137] |
| Porphyrin | XF70 |
|
White | Antimicrobial, in vitro, in vivo | [138] |
| Chlorin | Foscan, m-tetrahydroxyphenylchlorin |
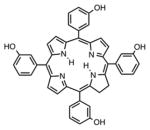
|
652 nm | Cancer, in vitro, in vivo, clinical | [139] |
| Chlorin | Verteporfin, benzoporphyrin derivative mono acid ring A |
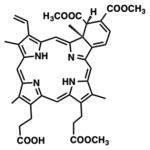
|
690 nm | Cancer, ophthalmology, in vitro, in vivo, clinical | [18] |
| Chlorin | Chlorin(e6) |
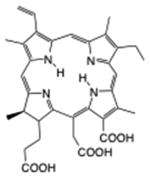
|
660 nm | Cancer, in vitro, in vivo, clinical | [19] |
| Chlorin | Monoaspartyl chlorin(e6), talaporfin sodium |
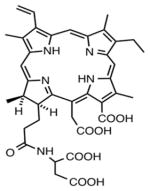
|
660 nm | Cancer, cardiology, in vitro, in vivo, clinical | [140] |
| Chlorin | HPPH |
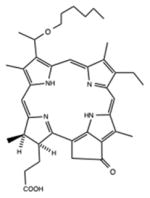
|
660 nm | Cancer, in vitro, in vivo, clinical | [141] |
| Bacteriochlorin | TOOKAD Soluble, WST-11 |
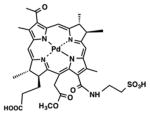
|
753 nm | Cancer, in vitro, in vivo, clinical | [142] |
| Bacteriochlorin | LUZ11 |

|
748 nm | Cancer, in vitro, in vivo, clinical | [27] |
| Bacteriochlorin | BC19 |
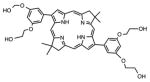
|
732 nm | Cancer, in vitro, in vivo | [28] |
| Bacteriochlorin | BC21 |
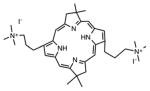
|
732 nm | Antimicrobial, in vitro | [143] |
| Phthalocyanine | Liposomal ZnPC |
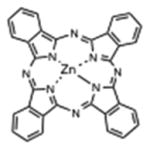
|
670 nm | Cancer, in vitro, in vivo, clinical | [144] |
| Phthalocyanine | Chloroaluminium sulfonated phthalocyanine (CASP) |
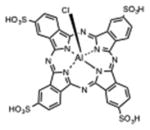
|
670 nm | Cancer, in vitro, in vivo | [145] |
| Phthalocyanine | Silicon phthalocyanine (PC4) |
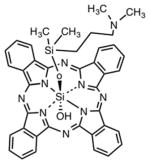
|
675 nm | Cancer, in vitro, in vivo, clinical | [33] |
| Phthalocyanine | RLP068 |
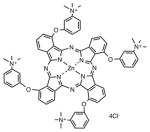
|
690 nm | Antimicrobial, in vitro, in vivo | [146] |
Porphyrins
As mentioned above, HpD and Photofrin were the first PSs to receive regulatory approval, and these and similar preparations are still in widespread use around the world [4]. ALA-induced protoporphyrin IX is, of course, a porphyrin and this methodology is widely used around the world, mainly by dermatologists [15]. There have been some new porphyrin compounds proposed as PSs, but these have been tested mainly in the PDI field [16]. Although porphyrins are most efficiently activated at the Soret band (~400 nm), the presence of several Q-bands extending as far as the 630 nm region means that red light is most often used to activate porphyrins in vivo, although blue, green or white light has been used for more superficial applications.
Chlorins
Chlorins include several of the most important clinical PSs, namely m-tetrahydroxyphenylchlorin (Temoporfin or Foscan) [17], benzoporphyrin derivative (Verteporfin) [18] and Radachlorin (now Bremachlorin) [19]. Chlorin(e6) is derived from naturally occurring chlorophyll and it has been used on its own (formulated either as the trisodium salt known as photodithazine [20] or dissolved in polyvinylpyrrolidone [21]). The monoaspartyl derivative (laserphyrin, taloporphin sodium or LS11) [22] has been used in Japan. Other PSs that have advanced to clinical trials can also be classified as chlorins, including the pyrophaeophorbide derivative HPPH [23] and tin(II) etiopurpurin [24]. Red light between 650 and 700 nm is used to activate chlorins depending on the exact structure.
Bacteriochlorins
The bacteriochlorin group of compounds also contains clinically important PSs. The lead-containing bacteriophaeophorbide derivative known as TOOKAD [25] and its newer water-soluble derivative known as TOOKAD Soluble have been tested in clinical trials for prostate cancer [26]. The new bacteriochlorin derivative known as LUZ11 [27] recently entered clinical trials for head and neck cancer in Portugal (Photodynamic Therapy With LUZ11 in Advanced Head and Neck Cancer, Clinical Trials.Gov NCT02070432). Other bacteriochlorin structures have been tested as PSs for both anti-cancer [28] and antimicrobial applications [29]. Near-infrared light between 700 and 800 nm is used to activate bacteriochlorins and this was shown to be particularly effective against pigmented tumours such as malignant melanoma [30].
Phthalocyanines
Phthalocyanines were some of the earliest compounds studied in the 1980s and 1990s. It should be noted that phthalocyanines are also synthetic dyes and could have also been included in the next section. There used to be a mixture of sulfonated chloroaluminium phthalocyanines known as CASPs that received considerable attention in the PDT field [31]. Unmodified zinc-phthalocyanine was tested in a liposomal formulation in both animal models and in clinical trials [32]. The silicon-substituted phthalocyanine PC4 has also been tested in vivo [33] and in clinical trials [34]. Cationic phthalocyanines such as RLP068 have been studied for antimicrobial applications, both in vivo [35] and in clinical trial for infected diabetic foot ulcers [36]. Phthalocyanines are activated by far-red light in the 670 nm range.
SYNTHETIC DYES
Structures that can be classified as synthetic dyes account for a sizeable fraction of the molecules that are being studied for a PDI and a selection are listed in Table 2.
Table 2.
PSs classed as synthetic dyes
| Class | Name | Structure | λmax | Application | Reference |
|---|---|---|---|---|---|
| Phenothiazinium salt | Methylene Blue |
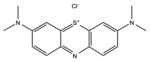
|
660 nm | Cancer, antimicrobial, in vitro, in vivo, clinical | [37] |
| Phenothiazinium salt | Toluidine Blue O |

|
630 nm | Antimicrobial, in vitro, in vivo, clinical | [147] |
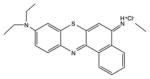
| Phenothiazinium salt | PP904 |
|
630 nm | Antimicrobial, in vitro, in vivo, clinical | [41] |
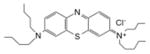
| Benzophenothiazinium salt | EtNBS |
|
670 nm | Cancer, antimicrobial, in vitro, in vivo, clinical | [148] |
| Halogenated xanthene | Rose Bengal |

|
540 nm | Cancer, antimicrobial, tissue bonding, in vitro, in vivo, clinical | [149] |
| Squaraine | ASQI |
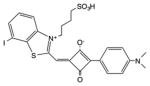
|
610 nm | Antimicrobial, in vitro | [150] |
| BODIPY | Zinc(II)-dipicolylamine di-iodo-BODIPY |
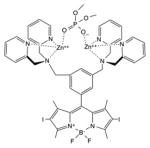
|
540 nm | Antimicrobial, in vitro | [151] |
| BODIPY | DIMPy-BODIPY |
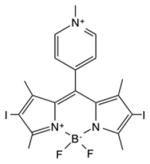
|
530 nm | Antimicrobial, in vitro | [152] |
| Phenalenone | Cationic |
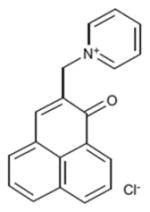
|
380 nm | Antimicrobial, in vitro | [52] |
| Transition metal complex | Ruthenium |
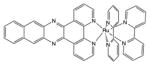
|
450 nm | Antimicrobial, in vitro | [153] |
| Transition metal complex | Rhodium |
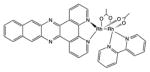
|
450 nm | Cancer, antimicrobial, in vitro | [154] |
| Transition metal complex | Iridium |
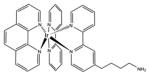
|
600 nm | Cancer, antimicrobial, in vitro | [155] |
Phenothiazinium salts
The two most popular phenothiazinium dyes (Methylene Blue [37] and Toluidine Blue [38]) have been widely studies for both antimicrobial applications [39] and less often for anti-cancer [40]. There are some new phenothiazinium dye structures that have been recently prepared mostly for antimicrobial use. PP904 was clinically tested for infected non-healing leg ulcers [41]. The benzophenothiazinium dye EtNBS has been studied for both antimicrobial [42] and anti-cancer [43] PDT.
Rose Bengal
Rose Bengal is a member of the xanthene class of fluorescent dyes that includes fluorescein (the most popular synthetic fluorophore). The introduction of heavy atoms into the rings, such as the halogen atoms bromine and iodine, increases the triplet yield of the molecule by facilitating intersystem crossing. Rose Bengal has a long history as a photoactive dye and has been explored for antimicrobial applications [44], tissue bonding applications [45] and anti-cancer applications [46].
Squaraines
The squaraine dye structure has a delocalized system of molecular orbitals providing good absorption in the visible range. The four-membered carbon ring is usually stabilized against nucleophilic attack by surrounding it with a rotaxane structure [47,48]. In a similar manner to xanthenes (see above), the introduction of iodine substituents into the ring increases the triplet yield by the heavy atom effect.
BODIPY dyes
The BODIPY (boron-dipyrromethene) dye structure usually contains a BF2 bridging unit, and constitutes a popular class of fluorophores [49]. Addition of heavy halogen atoms in the pyrrole rings increases the triplet yield and allows the molecules to function as PSs [50].
Phenalenones
The parent compound phenalenone was often used as a reference standard for generation of 1O2 [51]. Maisch and co-workers have recently synthesized a quaternized cationic derivative of phenalenone as an antimicrobial PS [52,53].
Transition metal compounds
Transition metal co-ordination compounds are a relatively new class of PS. Ruthenium(II) polypyridyl complexes are perhaps the most studied [54], although other ruthenium ligands [55], rhodium [56] and cyclometalated iridium [57] complexes have also been studied. Rhodium compounds are often in the form of Rh(II)–Rh(II) bridged dimer compounds [58]. There is also evidence that some luminescent platinum(II) and gold(III) compounds can act as PSs [59].
NATURAL PRODUCTS
The idea to use a naturally occurring compound as a PS inherently contains possible contradictions. If plants have evolved over millennia to grow in sunlight, then surely they cannot contain a highly active PS molecule or they would have burned up in the sun? Nevertheless, there are several natural product isolates that have been extensively explored as PSs and some of these are listed in Table 3.
Table 3.
Naturally occurring compounds as PSs
| Class | Name | Structure | λmax | Application | Reference |
|---|---|---|---|---|---|
| Perylenequinone | Hypericin |
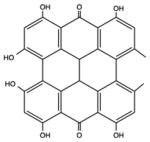
|
570 nm | Cancer, antimicrobial, in vitro, in vivo | [60] |
| Perylenequinone | Hypocrellin |

|
470 nm | Cancer, antimicrobial, in vitro, in vivo, clinical | [63] |
| Flavin | Cationic riboflavin |
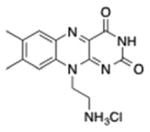
|
UVA/440 nm | Antimicrobial, in vitro | [68] |
| Curcuminoid | Curcumin |
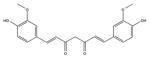
|
420 nm | Antimicrobial, in vitro, in vivo, clinical | [156] |
Hypericin
Hypericin is a perylenequinone isolated from St John’s wort, a long-known medicinal plant [60]. Hypericin is a hydrophobic molecule that requires formulation in a drug-delivery vehicle (liposomes, micelles, nanoparticles) [61]. The peak absorption band of hypericin is 600 nm, so orange light is used. Hypericin has been found to efficiently localize in the endoplasmic reticulum and to cause ER stress after light application, that can produce ‘damage associated molecular patterns’ that efficiently activate the immune system [62].
Hypocrellin
Hypocrellins A and B are another group of perylenequinone pigments which have been isolated from the parasitic fungi Hypocrella bambuase sacc and Shiraia bambusicola P. Heen found in China and other parts of Asia including Sri Lanka [63]. These compounds have been used in PDT and can also benefit from encapsulation in liposomes [64].
Riboflavin
Riboflavin (vitamin B2) has been explored as an antimicrobial PS. It has been tested for antimicrobial [65] and blood product sterilization [66] applications, and also as a photoactivated cross-linker for corneal stiffening [67]. Riboflavin has two peaks in the UVA (360 nm) and blue (440 nm) regions. Maisch et al. [68] have synthesized a cationic version of riboflavin designed as an antimicrobial PS.
Curcumin
Curcumin is a relative newcomer in the PDT field, although it has been known as a spice and medicinal compound for centuries [69]. Again curcumin is a very hydrophobic molecule and requires some kind of formulation vehicle to allow it to be used as a PS [70]. Curcumin is activated by blue light [71]. Curcumin has found most applications as an antimicrobial PS in dentistry to eradicate oral pathogens [72].
TARGETED PDT
There have been a large number of targeting studies in which PSs are covalently attached to various molecules that have some affinity for neoplasia or to receptors expressed on specific tumours [73]. The intention is to rely on the ability of the targeting vehicle to control localization so that the PS can be chosen based on its photochemical properties rather than on its tumour targeting properties, which are often unimpressive. These targeting vehicles include monoclonal antibodies [74], antibody fragments [75], peptides [76], proteins such as transferrin [77], epidermal growth factor [77] and insulin [78], LDL [79], various carbohydrates [80], somatostatin [81], folic acid [82] and many others. Table 4 lists some of these targeting ligand–PS conjugates.
Table 4.
Targeting ligands in PDT
| Class | Ligand | Target | PS | Application | Reference |
|---|---|---|---|---|---|
| Monoclonal antibody | OC125 | Ovarian cancer | Chlorin(e6) | Cancer, in vitro, in vivo | [157] |
| Peptide | Octreotide | Somatostatin receptor | Chlorin(e6) | Leukaemia, in vitro | [81] |
| Peptide | RGD tripeptide | αvβ3 integrin | Phaeophorbide a | Cancer, in vitro | [76] |
| Lipoprotein | Low-density lipoprotein | LDL receptor | Tetra-t-butyl silicon phthalocyanine | Cancer, atherosclerosis, in vitro, in vivo | [158] |
| Serum protein | Transferrin | Transferrin receptor | Hematoporphyrin | Cancer, in vitro | [77] |
| Serum protein | Modified albumin | Scavenger receptor | Chlorin(e6) | Atherosclerosis, in vitro, in vivo | [159] |
| Vitamin | Folic acid | Folate receptor | Phaeophorbide a | Cancer, in vitro | [76] |
| Steroid | Oestradiol | Steroid receptor | Phaeophorbide a | Breast cancer, in vitro | [160] |
| Carbohydrate | Mannose | Mannose receptor | Meso-tetraphenylporphyrin | Cancer, in vitro | [161] |
NANOTECHNOLOGY
The nanotechnology revolution has had a major impact on PDT as it also has had in other areas of biomedicine [83]. The fact that most effective PSs tend to be insoluble, hydrophobic molecules with a high propensity to aggregate means that encapsulation in nano-drug carriers may make a big difference to their performance [84]. Moreover many other nanostructures such as plasmonic gold nanoparticles, mesoporous silica nanoparticles, carbon nanotubes, graphene and upconversion nanoparticles have found uses in PDT [85]. There is another group of nanostructures where the actual nanoparticle itself acts as the PS absorbing light and producing ROS, as in the case of fullerenes [86], titanium dioxide [84] and some types of quantum dots [87].
Nanoparticle PS delivery
There has been an astonishing variety of nanoparticles used to solubilize, encapsulate and deliver PSs to both tumours [88] and microbial cells [89]. As mentioned above, the planar conjugated structures of PSs that are needed to absorb the light means the molecules tend to be hydrophobic and prone to aggregation. Liposomes, micelles, nanoemulsions can all be constructed out of lipids or amphiphilic polymers that self-assemble into delivery vehicles for PSs [90]. These nanovehicles have many advantages, the most important of which are providing a big increase in photochemical efficiency, and the ability to localize in tumours after IV injection due to the EPR effect [91].
Fullerenes
Fullerenes are closed-cage all-carbon nanostructures composed of sp2 hybridized carbon atoms (C60, C70, C84, etc.) and with a large molar absorption coefficient and high triplet yields. In organic solvents or hydrophobic environments, fullerenes are very efficient in producing photoexcited 1O2, whereas in aqueous environments, fullerenes switch the photochemical mechanism to Type 1 producing HO• [92]. Pristine fullerenes are insoluble in water, but the cage can be readily functionalized with polar groups such as carboxylic acids and quaternary amino groups that improve solubility and biological compatibility. Fullerenes have been used to mediate PDT of cancer cells [93] and microbial cells [94] in vitro, to treat tumours [95] and infections [96] in vivo, and there is even one case report of a clinical application.
Titanium dioxide
It has long been known that titanium dioxide (TiO2) or titania acts as a large band gap semiconductor. When excited with UVA light, an electron is excited from the valence band into the conductance band leaving behind a positively charged hole. The electron can produce superoxide from oxygen, whereas the hole can produce hydroxyl radicals from water. These ROS have been used in the process of photocatalysis which is used to kill micro-organisms and degrade organic pollutants [97]. In recent times TiO2 nanoparticles have been used as PDT agents often as composites or hybrids [98–100].
Quantum dots
Although many researchers have prepared conjugates between quantum dots and various different PSs to carry out PDT [101–103], a recent study showed that graphene quantum dots could mediate PDT on their own without any added PS [87].
Upconversion nanoparticles
Upconversion is a process in which the sequential absorption of two or more photons leads to the emission of light at shorter wavelength than the excitation wavelength. The mechanism relies on absorption of two consecutive photons, where second near-infrared photon is absorbed by a ‘virtual excited state’ to give the actual excited state that can then emit a much shorter wavelength photon. Lanthanide-doped nanoparticles emerged in the late 1990s and the optical transitions from compounds containing yttrium, ytterbium and erbium were found to be suitable for photon upconversion. In contrast with two-photon absorption (2PA) which needs a very high peak power density provided by femtosecond pulsed lasers, photon upconversion can operate with reasonable efficiency using a CW laser (often at 980 nm) [104]. Near-infrared wavelengths are preferred for their greater tissue penetration, and the shorter wavelengths emitted are able to excite more different PSs, that have been attached to the lanthanide-doped nanoparticles. Recently a NaYbF4:Nd@NaGdF4:Yb/Er@NaGdF4 core–shell–shell nanoparticle loaded with chlorin(e6) and folic acid for targeting was reported that could be excited with 808 nm laser [105].
FUTURE DIRECTIONS
Photochemical internalization (PCI)
The PCI is a new technique in which a highly amphiphilic PS such as aluminium phthalocyanine adjacent disulfonate or Amphinex (meso-tetraphenylchlorin adjacent disulfonate) [106] is administered together with a cytotoxic molecule such as bleomycin [107] or a ribosome-inactivating protein [108]. The cytotoxic molecule is taken up by endocytosis into endosomes which also have the PS in their membranes. When light is delivered, the endosomes are broken open, releasing the cytotoxic molecule into the cytoplasm where its toxicity is much higher. This approach has advanced through in vitro studies [109], to in vivo [110], and into clinical trials for head and neck cancer [111]. PCI can also be used for non-viral delivery of DNA and other nucleic acid-based therapeutics as a form of gene therapy [112].
Genetically encoded proteins
It was previously discovered that some modified fluorescent proteins based on the fundamental chromophore structure present in green fluorescent protein (GFP) can not only emit fluorescence, but also produce ROS upon excitation with light [113]. The most well known of these photodynamically active fluorescent proteins is called KillerRed [114]. The intracellular location of the expressed protein can be controlled by additional genetic elements attached to the DNA sequence for the KillerRed molecule. For instance KillerRed has been targeted to cell membranes [115], to lysosomes [116], to mitochondria [117] and to the nucleus [118]. KillerRed has been used as an optogenetic tool to allow the light-mediated inactivation of specific groups of neurons in Caeorhabditis elegans [119] in transgenic zebrafish [120], in Xenopus laevis embryos [121] and in the mouse retina [122]. Another genetically encoded PS is the flavoprotein ‘miniSOG’ [123].
Theranostics
The term ‘theranostics’ was coined as a combination of diagnostics and therapeutics [124], but should really combine more elements than simply an imaging agent that allows a tumour to be visualized (often by fluorescence), and a therapeutic agent (often photodynamic) that allows a tumour to be destroyed [125]. Theranostic agents could in addition be designed to act as ‘smart drug-delivery vehicles’ that could be activated by a change in the environment of the tumour or the infection such as lower pH [126], redox, enzyme activation [127], elevated temperature or magnetic fields [128]. Furthermore theranostic agents could also be designed to incorporate a method to monitor the effectiveness of treatment, possibly in real-time, and in the days following treatment. Porphysomes (self-assembled nanostructures from lipid-conjugated porphyrins) are an interesting example of a theranostic PDT agent [129].
Two-photon excitation
Another way to use long wavelength near-infrared light that penetrates deeply into tissue to excite PSs with short wavelength absorptions (in addition to upconversion nanoparticles) is 2-PA. Here two separate near-infrared photons have to arrive at the PS molecule virtually simultaneously, and if they are absorbed at the same time they will be equivalent to a single photon of half the wavelength [130]. However, the need for simultaneous absorption of both photons means that an extremely high peak power density (in the order of GW/cm2) is required. Moreover, the efficiency of 2-PA PDT depends strongly on what is known as the 2-PA cross-section of the PS molecule, which is measured in Goeppert-Mayer units (1 GM = 10−50 cm4 ·s·molecule−1·photon−1). Most regular PSs have a 2-PA cross-section between 1 and 100, but specially designed PSs can have values as high as 33 000 [131]. 2-PA PDT has been tested in vivo when the femtosecond laser beam was delivered though the whole body of the mouse to reach the tumour on the other side [132].
Sonodynamic therapy (SDT)
The ability to activate some kinds of PS molecule with ultrasound energy instead of light has been known for some years [133]. The ultrasound employed is usually between 1 and 2 MHz delivered at power densities between 0.5 and 10 W/cm2. In fact, although many sonosensitizers are the same types of molecule as those used as PSs, this is not always the case, and some chemotherapeutic drugs have been shown to be potentiated by ultrasound [134]. The main proposed mechanisms of actions are: (a) generation of flashes of light (sonoluminescence) from collapse of cavitation bubbles that activates the PS to produce singlet oxygen; (b) generation of free radicals which initiate chain peroxidation of membrane lipids via peroxyl and/or alkoxyl radicals; (c) physical destabilization of the cell membrane by the sonosensitizer thereby rendering the cell more susceptible to ultrasound induced shear forces; (d) ultrasound-enhanced drug transport across the cell membrane (sonoporation). The main advantage claimed for SDT is much deeper tissue penetration by ultrasound compared with light. However, it must be mentioned that SDT is still considered controversial due to its use in alternative medicine clinics.
CONCLUSIONS
PDT is a highly multidisciplinary field that involves chemists, physicists, biologists, engineers and physicians. Chemists, of course, are constantly seeking to design, synthesize, purify and characterize new compounds that can be used as PSs. Many significant advances have been made in PS design during the last 20 years, and second-, third- and even fourth-generation PSs have been described. Drug delivery and formulation requirements have increasingly been recognized as being of high importance, as many old-style PSs suffered from sub-optimal formulation. The advent of the nanotechnology revolution has had a big impact on PDT, and is expected to continue to influence the field. New tumour-targeting modalities are being reported frequently, and it is expected that these will be utilized to deliver PSs, as the need for activation by spatially confined light application provides yet another level of selectivity. Many commentators agree that the antimicrobial applications of PDT are now growing faster than the traditional anti-cancer applications. Moreover, it is also possible that other medical applications will emerge, as happened in the past for choroidal neovascularization.
Acknowledgments
We are grateful to Dr Ying-Ying Huang for help with the figures.
FUNDING
This work was supported by the National Institutes of Health [grant number R01AI050875 (to M.R.H.)].
Abbreviations
- ALA
5-aminolaevulinic acid
- aPDI
antimicrobial photodynamic inactivation
- BODIPY
boron-dipyrromethene
- DLI
drug–light interval
- EPR
enhanced permeability and retention
- ER
endoplasmic reticulum
- HpD
haematoporphyrin derivative
- LDL
low-density lipoprotein
- 2PA
two-photon absorption
- PCI
photochemical internalization
- PDT
photodynamic therapy
- PS
photosensitizer
- ROS
reactive oxygen species
- SDT
sonodynamic therapy
References
- 1.Moan J, Peng Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003;23:3591–3600. [PubMed] [Google Scholar]
- 2.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, et al. Photodynamic therapy of cancer: an update. CA: Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foote CS. Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which may be important in biological systems. Science. 1968;162:963–970. doi: 10.1126/science.162.3857.963. [DOI] [PubMed] [Google Scholar]
- 4.Allison RR, Sibata CH. Oncologic photodynamic therapy photosensitizers: a clinical review. Photodiagnosis Photodyn Ther. 2010;7:61–75. doi: 10.1016/j.pdpdt.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Chen B, Roskams T, de Witte PA. Antivascular tumor eradication by hypericin-mediated photodynamic therapy. Photochem Photobiol. 2002;76:509–513. doi: 10.1562/0031-8655(2002)076<0509:atebhm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805:53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Ascencio M, Collinet P, Farine MO, Mordon S. Protoporphyrin IX fluorescence photobleaching is a useful tool to predict the response of rat ovarian cancer following hexaminolevulinate photodynamic therapy. Lasers Surg Med. 2008;40:332–341. doi: 10.1002/lsm.20629. [DOI] [PubMed] [Google Scholar]
- 8.De Rosa FS, Bentley MV. Photodynamic therapy of skin cancers: sensitizers, clinical studies and future directives. Pharm Res. 2000;17:1447–1455. doi: 10.1023/a:1007612905378. [DOI] [PubMed] [Google Scholar]
- 9.Hamblin MR, Newman EL. On the mechanism of the tumour-localising effect in photodynamic therapy. J Photochem Photobiol B. 1994;23:3–8. doi: 10.1016/s1011-1344(94)80018-9. [DOI] [PubMed] [Google Scholar]
- 10.Iyer AK, Greish K, Seki T, Okazaki S, Fang J, Takeshita K, Maeda H. Polymeric micelles of zinc protoporphyrin for tumor targeted delivery based on EPR effect and singlet oxygen generation. J Drug Target. 2007;15:496–506. doi: 10.1080/10611860701498252. [DOI] [PubMed] [Google Scholar]
- 11.Kessel D. The role of low-density lipoprotein in the biodistribution of photosensitizing agents. J Photochem Photobiol B. 1992;14:261–262. doi: 10.1016/1011-1344(92)85103-2. [DOI] [PubMed] [Google Scholar]
- 12.Dysart JS, Patterson MS. Characterization of Photofrin photobleaching for singlet oxygen dose estimation during photodynamic therapy of MLL cells in vitro. Phys Med Biol. 2005;50:2597–2616. doi: 10.1088/0031-9155/50/11/011. [DOI] [PubMed] [Google Scholar]
- 13.Moan J, Berg K, Kvam E, Western A, Malik Z, Ruck A, Schneckenburger H. Intracellular localization of photosensitizers. Ciba Found Symp. 1989;146:95–107. doi: 10.1002/9780470513842.ch7. [DOI] [PubMed] [Google Scholar]
- 14.Battersby AR. Tetrapyrroles: the pigments of life. Nat Prod Rep. 2000;17:507–526. doi: 10.1039/b002635m. [DOI] [PubMed] [Google Scholar]
- 15.Krammer B, Plaetzer K. ALA and its clinical impact, from bench to bedside. Photochem Photobiol Sci. 2008;7:283–289. doi: 10.1039/b712847a. [DOI] [PubMed] [Google Scholar]
- 16.Alves E, Faustino MA, Neves MG, Cunha A, Tome J, Almeida A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med Chem. 2014;6:141–164. doi: 10.4155/fmc.13.211. [DOI] [PubMed] [Google Scholar]
- 17.Wagner A, Denzer UW, Neureiter D, Kiesslich T, Puespoeck A, Rauws EA, Emmanuel K, Degenhardt N, Frick U, Beuers U, et al. Temoporfin improves efficacy of photodynamic therapy in advanced biliary tract carcinoma: a multicenter prospective phase II study. Hepatology. 2015;62:1456–1465. doi: 10.1002/hep.27905. [DOI] [PubMed] [Google Scholar]
- 18.Chan WM, Lim TH, Pece A, Silva R, Yoshimura N. Verteporfin PDT for non-standard indications – a review of current literature. Graefes Arch Clin Exp Ophthalmol. 2010;248:613–626. doi: 10.1007/s00417-010-1307-z. [DOI] [PubMed] [Google Scholar]
- 19.Biswas R, Moon JH, Ahn JC. Chlorin e6 derivative radachlorin mainly accumulates in mitochondria, lysosome and endoplasmic reticulum and shows high affinity toward tumors in nude mice in photodynamic therapy. Photochem Photobiol. 2014;90:1108–1118. doi: 10.1111/php.12273. [DOI] [PubMed] [Google Scholar]
- 20.Romanko YS, Tsyb AF, Kaplan MA, Popuchiev VV. Effect of photodynamic therapy with photodithazine on morphofunctional parameters of M-1 sarcoma. Bull Exp Biol Med. 2004;138:584–589. doi: 10.1007/s10517-005-0133-5. [DOI] [PubMed] [Google Scholar]
- 21.Chin WW, Heng PW, Bhuvaneswari R, Lau WK, Olivo M. The potential application of chlorin e6-polyvinylpyrrolidone formulation in photodynamic therapy. Photochem Photobiol Sci. 2006;5:1031–1037. doi: 10.1039/b605772a. [DOI] [PubMed] [Google Scholar]
- 22.Nanashima A, Nagayasu T. Current status of photodynamic therapy in digestive tract carcinoma in Japan. Int J Mol Sci. 2015;16:3434–3440. doi: 10.3390/ijms16023434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lobel J, MacDonald IJ, Ciesielski MJ, Barone T, Potter WR, Pollina J, Plunkett RJ, Fenstermaker RA, Dougherty TJ. 2-[1-Hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) in a nude rat glioma model: implications for photodynamic therapy. Lasers Surg Med. 2001;29:397–405. doi: 10.1002/lsm.10001. [DOI] [PubMed] [Google Scholar]
- 24.Rostaporfin: PhotoPoint SnET2, Purlytin, Sn(IV) etiopurpurin, SnET2, tin ethyl etiopurpurin. Drugs R D. 2004;5:58–61. doi: 10.2165/00126839-200405010-00013. authors, No. [DOI] [PubMed] [Google Scholar]
- 25.Gross S, Gilead A, Scherz A, Neeman M, Salomon Y. Monitoring photodynamic therapy of solid tumors online by BOLD-contrast MRI. Nat Med. 2003;9:1327–1331. doi: 10.1038/nm940. [DOI] [PubMed] [Google Scholar]
- 26.Azzouzi AR, Lebdai S, Benzaghou F, Stief C. Vascular-targeted photodynamic therapy with TOOKAD Soluble in localized prostate cancer: standardization of the procedure. World J Urol. 2015;33:937–944. doi: 10.1007/s00345-015-1535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saavedra R, Rocha LB, Dabrowski JM, Arnaut LG. Modulation of biodistribution, pharmacokinetics, and photosensitivity with the delivery vehicle of a bacteriochlorin photosensitizer for photodynamic therapy. ChemMedChem. 2014;9:390–398. doi: 10.1002/cmdc.201300449. [DOI] [PubMed] [Google Scholar]
- 28.Huang YY, Mroz P, Zhiyentayev T, Sharma SK, Balasubramanian T, Ruzie C, Krayer M, Fan D, Borbas KE, Yang E, et al. In Vitro photodynamic therapy and quantitative structure-activity relationship studies with stable synthetic near-infrared-absorbing bacteriochlorin photosensitizers. J Med Chem. 2010;53:4018–4027. doi: 10.1021/jm901908s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang L, Krayer M, Roubil JG, Huang YY, Holten D, Lindsey JS, Hamblin MR. Stable synthetic mono-substituted cationic bacteriochlorins mediate selective broad-spectrum photoinactivation of drug-resistant pathogens at nanomolar concentrations. J Photochem Photobiol B. 2014;141:119–127. doi: 10.1016/j.jphotobiol.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mroz P, Huang YY, Szokalska A, Zhiyentayev T, Janjua S, Nifli AP, Sherwood ME, Ruzie C, Borbas KE, Fan D, et al. Stable synthetic bacteriochlorins overcome the resistance of melanoma to photodynamic therapy. FASEB J. 2010;24:3160–3170. doi: 10.1096/fj.09-152587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts WG, Klein MK, Loomis M, Weldy S, Berns MW. Photodynamic therapy of spontaneous cancers in felines, canines, and snakes with chloro-aluminum sulfonated phthalocyanine. J Natl Cancer Inst. 1991;83:18–23. doi: 10.1093/jnci/83.1.18. [DOI] [PubMed] [Google Scholar]
- 32.Schieweck K, Capraro HG, Isele U, van Hoogevest P, Ochsner M, Maurer T, Batt E. CGP 55847, liposome-delivered zinc(II) phthalocyanine as phototherapeutic agent for tumours. Proc SPIE. 1994;2078:107–118. [Google Scholar]
- 33.Anderson CY, Freye K, Tubesing KA, Li YS, Kenney ME, Mukhtar H, Elmets CA. A comparative analysis of silicon phthalocyanine photosensitizers for in vivo photodynamic therapy of RIF-1 tumors in C3H mice. Photochem Photobiol. 1998;67:332–336. [PubMed] [Google Scholar]
- 34.Kinsella TJ, Baron ED, Colussi VC, Cooper KD, Hoppel CL, Ingalls ST, Kenney ME, Li X, Oleinick NL, Stevens SR, Remick SC. Preliminary clinical and pharmacologic investigation of photodynamic therapy with the silicon phthalocyanine photosensitizer pc 4 for primary or metastatic cutaneous cancers. Front Oncol. 2011;1:14. doi: 10.3389/fonc.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vecchio D, Dai T, Huang L, Fantetti L, Roncucci G, Hamblin MR. Antimicrobial photodynamic therapy with RLP068 kills methicillin-resistant Staphylococcus aureus and improves wound healing in a mouse model of infected skin abrasion PDT with RLP068/Cl in infected mouse skin abrasion. J Biophotonics. 2013;6:733–742. doi: 10.1002/jbio.201200121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mannucci E, Genovese S, Monami M, Navalesi G, Dotta F, Anichini R, Romagnoli F, Gensini G. Photodynamic topical antimicrobial therapy for infected foot ulcers in patients with diabetes: a randomized, double-blind, placebo-controlled study – the D.A.N.T.E (Diabetic ulcer Antimicrobial New Topical treatment Evaluation) study. Acta Diabetol. 2014;51:435–440. doi: 10.1007/s00592-013-0533-3. [DOI] [PubMed] [Google Scholar]
- 37.Wainwright M, Crossley KB. Methylene Blue – a therapeutic dye for all seasons? J Chemother. 2002;14:431–443. doi: 10.1179/joc.2002.14.5.431. [DOI] [PubMed] [Google Scholar]
- 38.Tseng SP, Hung WC, Chen HJ, Lin YT, Jiang HS, Chiu HC, Hsueh PR, Teng LJ, Tsai JC. Effects of toluidine blue O (TBO)-photodynamic inactivation on community-associated methicillin-resistant Staphylococcus aureus isolates. J Microbiol Immunol Infect. 2015 doi: 10.1016/j.jmii.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Tardivo JP, Adami F, Correa JA, Pinhal MA, Baptista MS. A clinical trial testing the efficacy of PDT in preventing amputation in diabetic patients. Photodiagnosis Photodyn Ther. 2014;11:342–350. doi: 10.1016/j.pdpdt.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Graciano TB, Coutinho TS, Cressoni CB, de Freitas CP, Pierre MB, Pereira SA, Shimano MM, Frange RC, Garcia MT. Using chitosan gels as a toluidine blue O delivery system for photodynamic therapy of buccal cancer: in vitro and in vivo studies. Photodiagnosis Photodyn Ther. 2015;12:98–107. doi: 10.1016/j.pdpdt.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Morley S, Griffiths J, Philips G, Moseley H, O’Grady C, Mellish K, Lankester CL, Faris B, Young RJ, Brown SB, Rhodes LE. Phase IIa randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: a new approach to antimicrobial therapy. Br J Dermatol. 2013;168:617–624. doi: 10.1111/bjd.12098. [DOI] [PubMed] [Google Scholar]
- 42.Verma S, Sallum UW, Athar H, Rosenblum L, Foley JW, Hasan T. Antimicrobial photodynamic efficacy of side-chain functionalized benzo[a]phenothiazinium dyes. Photochem Photobiol. 2009;85:111–118. doi: 10.1111/j.1751-1097.2008.00403.x. [DOI] [PubMed] [Google Scholar]
- 43.Frimberger AE, Moore AS, Cincotta L, Cotter SM, Foley JW. Photodynamic therapy of naturally occurring tumors in animals using a novel benzophenothiazine photosensitizer. Clin Cancer Res. 1998;4:2207–2218. [PubMed] [Google Scholar]
- 44.Costa AC, Rasteiro VM, Pereira CA, Rossoni RD, Junqueira JC, Jorge AO. The effects of rose bengal- and erythrosine-mediated photodynamic therapy on Candida albicans. Mycoses. 2012;55:56–63. doi: 10.1111/j.1439-0507.2011.02042.x. [DOI] [PubMed] [Google Scholar]
- 45.Xu N, Yao M, Farinelli W, Hajjarian Z, Wang Y, Redmond RW, Kochevar IE. Light-activated sealing of skin wounds. Lasers Surg Med. 2015;47:17–29. doi: 10.1002/lsm.22308. [DOI] [PubMed] [Google Scholar]
- 46.Panzarini E, Inguscio V, Fimia GM, Dini L. Rose Bengal acetate photodynamic therapy (RBAc-PDT) induces exposure and release of damage-associated molecular patterns (DAMPs) in human HeLa cells. PLoS One. 2014;9:e105778. doi: 10.1371/journal.pone.0105778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole EL, Arunkumar E, Xiao S, Smith BA, Smith BD. Water-soluble, deep-red fluorescent squaraine rotaxanes. Org Biomol Chem. 2012;10:5769–5773. doi: 10.1039/c2ob06783h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsueh SY, Lai CC, Chiu SH. Squaraine-based [2]rotaxanes that function as visibly active molecular switches. Chemistry. 2010;16:2997–3000. doi: 10.1002/chem.200903304. [DOI] [PubMed] [Google Scholar]
- 49.Boens N, Leen V, Dehaen W. Fluorescent indicators based on BODIPY. Chem Soc Rev. 2012;41:1130–1172. doi: 10.1039/c1cs15132k. [DOI] [PubMed] [Google Scholar]
- 50.Kamkaew A, Lim SH, Lee HB, Kiew LV, Chung LY, Burgess K. BODIPY dyes in photodynamic therapy. Chem Soc Rev. 2013;42:77–88. doi: 10.1039/c2cs35216h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segado M, Reguero M. Mechanism of the photochemical process of singlet oxygen production by phenalenone. Phys Chem Chem Phys. 2011;13:4138–4148. doi: 10.1039/c0cp01827a. [DOI] [PubMed] [Google Scholar]
- 52.Cieplik F, Spath A, Regensburger J, Gollmer A, Tabenski L, Hiller KA, Baumler W, Maisch T, Schmalz G. Photodynamic biofilm inactivation by SAPYR – an exclusive singlet oxygen photosensitizer. Free Radic Biol Med. 2013;65:477–487. doi: 10.1016/j.freeradbiomed.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 53.Spath A, Leibl C, Cieplik F, Lehner K, Regensburger J, Hiller KA, Baumler W, Schmalz G, Maisch T. Improving photodynamic inactivation of bacteria in dentistry: highly effective and fast killing of oral key pathogens with novel tooth-colored type-II photosensitizers. J Med Chem. 2014;57:5157–5168. doi: 10.1021/jm4019492. [DOI] [PubMed] [Google Scholar]
- 54.Gill MR, Thomas JA. Ruthenium(II) polypyridyl complexes and DNA – from structural probes to cellular imaging and therapeutics. Chem Soc Rev. 2012;41:3179–3192. doi: 10.1039/c2cs15299a. [DOI] [PubMed] [Google Scholar]
- 55.Fong J, Kasimova K, Arenas Y, Kaspler P, Lazic S, Mandel A, Lilge L. A novel class of ruthenium-based photosensitizers effectively kills in vitro cancer cells and in vivo tumors. Photochem Photobiol Sci. 2015;14:2014–2023. doi: 10.1039/c4pp00438h. [DOI] [PubMed] [Google Scholar]
- 56.Angeles-Boza AM, Bradley PM, Fu PK, Wicke SE, Bacsa J, Dunbar KR, Turro C. DNA binding and photocleavage in vitro by new dirhodium(II) dppz complexes: correlation to cytotoxicity and photocytotoxicity. Inorg Chem. 2004;43:8510–8519. doi: 10.1021/ic049091h. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Tan CP, Zhang W, He L, Ji LN, Mao ZW. Phosphorescent iridium(III)-bis-N-heterocyclic carbene complexes as mitochondria-targeted theranostic and photodynamic anticancer agents. Biomaterials. 2015;39:95–104. doi: 10.1016/j.biomaterials.2014.10.070. [DOI] [PubMed] [Google Scholar]
- 58.Angeles-Boza AM, Bradley PM, Fu PK, Shatruk M, Hilfiger MG, Dunbar KR, Turro C. Photocytotoxicity of a new Rh2(II,II) complex: increase in cytotoxicity upon irradiation similar to that of PDT agent hematoporphyrin. Inorg Chem. 2005;44:7262–7264. doi: 10.1021/ic0500715. [DOI] [PubMed] [Google Scholar]
- 59.To WP, Zou T, Sun RW, Che CM. Light-induced catalytic and cytotoxic properties of phosphorescent transition metal compounds with a d8 electronic configuration. Philos Trans A Math Phys Eng Sci. 2013;371:20120126. doi: 10.1098/rsta.2012.0126. [DOI] [PubMed] [Google Scholar]
- 60.Theodossiou TA, Hothersall JS, De Witte PA, Pantos A, Agostinis P. The multifaceted photocytotoxic profile of hypericin. Mol Pharm. 2009;6:1775–1789. doi: 10.1021/mp900166q. [DOI] [PubMed] [Google Scholar]
- 61.Liu X, Jiang C, Li Y, Liu W, Yao N, Gao M, Ji Y, Huang D, Yin Z, Sun Z, Ni Y, Zhang J. Evaluation of hypericin: effect of aggregation on targeting biodistribution. J Pharm Sci. 2015;104:215–222. doi: 10.1002/jps.24230. [DOI] [PubMed] [Google Scholar]
- 62.Zheng Y, Yin G, Le V, Zhang A, Lu Y, Yang M, Fei Z, Liu J. Hypericin-based photodynamic therapy induces a tumor-specific immune response and an effective DC-based cancer immunotherapy. Biochem Pharmacol. 2014 doi: 10.1016/j.bcp.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 63.Zhenjun D, Lown JW. Hypocrellins and their use in photosensitization. Photochem Photobiol. 1990;52:609–616. doi: 10.1111/j.1751-1097.1990.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 64.Li T, Hou X, Deng H, Zhao J, Huang N, Zeng J, Chen H, Gu Y. Liposomal hypocrellin B as a potential photosensitizer for age-related macular degeneration: pharmacokinetics, photodynamic efficacy, and skin phototoxicity in vivo. Photochem Photobiol Sci. 2015;14:972–981. doi: 10.1039/c4pp00412d. [DOI] [PubMed] [Google Scholar]
- 65.Makdoumi K, Backman A, Mortensen J, Crafoord S. Evaluation of antibacterial efficacy of photo-activated riboflavin using ultraviolet light (UVA) Graefes Arch Clin Exp Ophthalmol. 2010;248:207–212. doi: 10.1007/s00417-009-1231-2. [DOI] [PubMed] [Google Scholar]
- 66.Ettinger A, Miklauz MM, Bihm DJ, Maldonado-Codina G, Goodrich RP. Preparation of cryoprecipitate from riboflavin and UV light-treated plasma. Transfus Apher Sci. 2012;46:153–158. doi: 10.1016/j.transci.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Chan TC, Lau TW, Lee JW, Wong IY, Jhanji V, Wong RL. Corneal collagen cross-linking for infectious keratitis: an update of clinical studies. Acta Ophthalmol. 2015 doi: 10.1111/aos.12754. [DOI] [PubMed] [Google Scholar]
- 68.Maisch T, Eichner A, Spath A, Gollmer A, Konig B, Regensburger J, Baumler W. Fast and effective photodynamic inactivation of multiresistant bacteria by cationic riboflavin derivatives. PLoS One. 2014;9:e111792. doi: 10.1371/journal.pone.0111792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghosh S, Banerjee S, Sil PC. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: a recent update. Food Chem Toxicol. 2015;83:111–124. doi: 10.1016/j.fct.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 70.Wikene KO, Hegge AB, Bruzell E, Tonnesen HH. Formulation and characterization of lyophilized curcumin solid dispersions for antimicrobial photodynamic therapy (aPDT): studies on curcumin and curcuminoids LII. Drug Dev Ind Pharm. 2015;41:969–977. doi: 10.3109/03639045.2014.919315. [DOI] [PubMed] [Google Scholar]
- 71.Bernd A. Visible light and/or UVA offer a strong amplification of the anti-tumor effect of curcumin. Phytochem Rev. 2014;13:183–189. doi: 10.1007/s11101-013-9296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leite DP, Paolillo FR, Parmesano TN, Fontana CR, Bagnato VS. Effects of photodynamic therapy with blue light and curcumin as mouth rinse for oral disinfection: a randomized controlled trial. Photomed Laser Surg. 2014;32:627–632. doi: 10.1089/pho.2014.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sibani SA, McCarron PA, Woolfson AD, Donnelly RF. Photosensitiser delivery for photodynamic therapy. Part 2: systemic carrier platforms. Expert Opin Drug Deliv. 2008;5:1241–1254. doi: 10.1517/17425240802444673. [DOI] [PubMed] [Google Scholar]
- 74.Sato K, Nagaya T, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy in the treatment of pleural disseminated NSCLC: preclinical experience. Theranostics. 2015;5:698–709. doi: 10.7150/thno.11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamblin MR, Miller JL, Hasan T. Effect of charge on the interaction of site-specific photoimmunoconjugates with human ovarian cancer cells. Cancer Res. 1996;56:5205–5210. [PubMed] [Google Scholar]
- 76.You H, Yoon HE, Jeong PH, Ko H, Yoon JH, Kim YC. Pheophorbide – a conjugates with cancer-targeting moieties for targeted photodynamic cancer therapy. Bioorg Med Chem. 2015;23:1453–1462. doi: 10.1016/j.bmc.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 77.Hamblin MR, Newman EL. Photosensitizer targeting in photodynamic therapy. I. Conjugates of haematoporphyrin with albumin and transferrin. J Photochem Photobiol B. 1994;26:45–56. doi: 10.1016/1011-1344(94)85035-6. [DOI] [PubMed] [Google Scholar]
- 78.Sobolev AS, Akhlynina TV, Yachmenev SV, Rosenkranz AA, Severin ES. Internalizable insulin-BSA-chlorin E6 conjugate is a more effective photosensitizer than chlorin E6 alone. Biochem Int. 1992;26:445–450. [PubMed] [Google Scholar]
- 79.Hamblin MR, Newman EL. Photosensitizer targeting in photodynamic therapy. II. Conjugates of haematoporphyrin with serum lipoproteins. J Photochem Photobiol B. 1994;26:147–157. doi: 10.1016/1011-1344(94)07036-9. [DOI] [PubMed] [Google Scholar]
- 80.Park SY, Baik HJ, Oh YT, Oh KT, Youn YS, Lee ES. A smart polysaccharide/drug conjugate for photodynamic therapy. Angew Chem Int Ed Engl. 2011;50:1644–1647. doi: 10.1002/anie.201006038. [DOI] [PubMed] [Google Scholar]
- 81.Kascakova S, Hofland LJ, De Bruijn HS, Ye Y, Achilefu S, van der Wansem K, van der Ploeg-van den Heuvel A, van Koetsveld PM, Brugts MP, van der Lelij AJ, et al. Somatostatin analogues for receptor targeted photodynamic therapy. PLoS One. 2014;9:e104448. doi: 10.1371/journal.pone.0104448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li D, Li P, Lin H, Jiang Z, Guo L, Li B. A novel chlorin-PEG-folate conjugate with higher water solubility, lower cytotoxicity, better tumor targeting and photodynamic activity. J Photochem Photobiol B. 2013;127:28–37. doi: 10.1016/j.jphotobiol.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 83.Staggers N, McCasky T, Brazelton N, Kennedy R. Nanotechnology: the coming revolution and its implications for consumers, clinicians, and informatics. Nurs Outlook. 2008;56:268–274. doi: 10.1016/j.outlook.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 84.Brown EM, Allen LL, Pyles H, Solis J, Wileman TA, Willadsen GB. Advancements in using TiO2 bionanoconjugates for precision degradation of intracellular biological structures. J Biomed Nanotechnol. 2013;9:539–550. doi: 10.1166/jbn.2013.1564. [DOI] [PubMed] [Google Scholar]
- 85.Huang YY, Sharma SK, Dai T, Chung H, Yaroslavsky A, Garcia-Diaz M, Chang J, Chiang LY, Hamblin MR. Can nanotechnology potentiate photodynamic therapy? Nanotechnol Rev. 2012;1:111–146. doi: 10.1515/ntrev-2011-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mroz P, Tegos GP, Gali H, Wharton T, Sarna T, Hamblin MR. Photodynamic therapy with fullerenes. Photochem Photobiol Sci. 2007;6:1139–1149. doi: 10.1039/b711141j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ge J, Lan M, Zhou B, Liu W, Guo L, Wang H, Jia Q, Niu G, Huang X, Zhou H, et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat Commun. 2014;5:4596. doi: 10.1038/ncomms5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Master A, Livingston M, Sen Gupta A. Photodynamic nanomedicine in the treatment of solid tumors: perspectives and challenges. J Control Release. 2013;168:88–102. doi: 10.1016/j.jconrel.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perni S, Prokopovich P, Pratten J, Parkin IP, Wilson M. Nanoparticles: their potential use in antibacterial photodynamic therapy. Photochem Photobiol Sci. 2011;10:712–720. doi: 10.1039/c0pp00360c. [DOI] [PubMed] [Google Scholar]
- 90.Sadasivam M, Avci P, Gupta GK, Lakshmanan S, Chandran R, Huang YY, Kumar R, Hamblin MR. Self-assembled liposomal nanoparticles in photodynamic therapy. Eur J Nanomed. 2013;5:115–129. doi: 10.1515/ejnm-2013-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Avci P, Erdem SS, Hamblin MR. Photodynamic therapy: one step ahead with self-assembled nanoparticles. J Biomed Nanotechnol. 2014;10:1937–1952. doi: 10.1166/jbn.2014.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamakoshi Y, Umezawa N, Ryu A, Arakane K, Miyata N, Goda Y, Masumizu T, Nagano T. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2-* versus 1O2. J Am Chem Soc. 2003;125:12803–12809. doi: 10.1021/ja0355574. [DOI] [PubMed] [Google Scholar]
- 93.Mroz P, Pawlak A, Satti M, Lee H, Wharton T, Gali H, Sarna T, Hamblin MR. Functionalized fullerenes mediate photodynamic killing of cancer cells: Type I versus Type II photochemical mechanism. Free Radic Biol Med. 2007;43:711–719. doi: 10.1016/j.freeradbiomed.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, Hamblin MR. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol. 2005;12:1127–1135. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mroz P, Xia Y, Asanuma D, Konopko A, Zhiyentayev T, Huang YY, Sharma SK, Dai T, Khan UJ, Wharton T, Hamblin MR. Intraperitoneal photodynamic therapy mediated by a fullerene in a mouse model of abdominal dissemination of colon adenocarcinoma. Nanomedicine. 2011;7:965–974. doi: 10.1016/j.nano.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu Z, Dai T, Huang L, Kurup DB, Tegos GP, Jahnke A, Wharton T, Hamblin MR. Photodynamic therapy with a cationic functionalized fullerene rescues mice from fatal wound infections. Nanomedicine (Lond) 2010;5:1525–1533. doi: 10.2217/nnm.10.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Foster HA, Ditta IB, Varghese S, Steele A. Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Appl Microbiol Biotechnol. 2011;90:1847–1868. doi: 10.1007/s00253-011-3213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sette A, Spadavecchia J, Landoulsi J, Casale S, Haye B, Crociani O, Arcangeli A. Development of novel anti-Kv 11.1 antibody-conjugated PEG-TiO nanoparticles for targeting pancreatic ductal adenocarcinoma cells. J Nanopart Res. 2013;15:2111. doi: 10.1007/s11051-013-2111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng X, Zhang S, Lou X. Controlling silica coating thickness on TiO2 nanoparticles for effective photodynamic therapy. Colloids Surf B Biointerfaces. 2013;107:220–226. doi: 10.1016/j.colsurfb.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 100.Kotagiri N, Sudlow GP, Akers WJ, Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat Nanotechnol. 2015;10:370–379. doi: 10.1038/nnano.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maksimov EG, Gvozdev DA, Strakhovskaya MG, Paschenko VZ. Hybrid structures of polycationic aluminum phthalocyanines and quantum dots. Biochemistry (Mosc) 2015;80:323–331. doi: 10.1134/S0006297915030074. [DOI] [PubMed] [Google Scholar]
- 102.Shao L, Gao Y, Yan F. Semiconductor quantum dots for biomedicial applications. Sensors (Basel) 2011;11:11736–11751. doi: 10.3390/s111211736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou L, Zhou L, Ge X, Zhou J, Wei S, Shen J. Multicolor imaging and the anticancer effect of a bifunctional silica nanosystem based on the complex of graphene quantum dots and hypocrellin A. Chem Commun (Camb) 2015;51:421–424. doi: 10.1039/c4cc06968d. [DOI] [PubMed] [Google Scholar]
- 104.Dou QQ, Rengaramchandran A, Selvan ST, Paulmurugan R, Zhang Y. Core-shell upconversion nanoparticle – semiconductor heterostructures for photodynamic therapy. Sci Rep. 2015;5:8252. doi: 10.1038/srep08252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ai F, Ju Q, Zhang X, Chen X, Wang F, Zhu G. A core-shell-shell nanoplatform upconverting near-infrared light at 808 nm for luminescence imaging and photodynamic therapy of cancer. Sci Rep. 2015;5:10785. doi: 10.1038/srep10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berg K, Nordstrand S, Selbo PK, Tran DT, Angell-Petersen E, Hogset A. Disulfonated tetraphenyl chlorin (TPCS2a), a novel photosensitizer developed for clinical utilization of photochemical internalization. Photochem Photobiol Sci. 2011;10:1637–1651. doi: 10.1039/c1pp05128h. [DOI] [PubMed] [Google Scholar]
- 107.Selbo PK, Weyergang A, Hogset A, Norum OJ, Berstad MB, Vikdal M, Berg K. Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules. J Control Release. 2010;148:2–12. doi: 10.1016/j.jconrel.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 108.Weyergang A, Selbo PK, Berstad ME, Bostad M, Berg K. Photochemical internalization of tumor-targeted protein toxins. Lasers Surg Med. 2011;43:721–733. doi: 10.1002/lsm.21084. [DOI] [PubMed] [Google Scholar]
- 109.Dietze A, Bonsted A, Hogset A, Berg K. Photochemical internalization enhances the cytotoxic effect of the protein toxin gelonin and transgene expression in sarcoma cells. Photochem Photobiol. 2003;78:283–289. doi: 10.1562/0031-8655(2003)078<0283:pietce>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 110.Norum OJ, Gaustad JV, Angell-Petersen E, Rofstad EK, Peng Q, Giercksky KE, Berg K. Photochemical internalization of bleomycin is superior to photodynamic therapy due to the therapeutic effect in the tumor periphery. Photochem Photobiol. 2009;85:740–749. doi: 10.1111/j.1751-1097.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- 111.Jerjes W, Upile T, Radhi H, Hopper C. Photodynamic therapy vs. photochemical internalization: the surgical margin. Head Neck Oncol. 2011;3:53. doi: 10.1186/1758-3284-3-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boe SL, Hovig E. Enhancing nucleic acid delivery by photochemical internalization. Ther Deliv. 2013;4:1125–1140. doi: 10.4155/tde.13.78. [DOI] [PubMed] [Google Scholar]
- 113.Mueller G, Waldeck W, Braun K. From green to red–to more dead? Autofluorescent proteins as photosensitizers. J Photochem Photobiol B. 2010;98:95–98. doi: 10.1016/j.jphotobiol.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 114.Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. A genetically encoded photosensitizer. Nat Biotechnol. 2006;24:95–99. doi: 10.1038/nbt1175. [DOI] [PubMed] [Google Scholar]
- 115.Liao ZX, Li YC, Lu HM, Sung HW. A genetically-encoded KillerRed protein as an intrinsically generated photosensitizer for photodynamic therapy. Biomaterials. 2014;35:500–508. doi: 10.1016/j.biomaterials.2013.09.075. [DOI] [PubMed] [Google Scholar]
- 116.Serebrovskaya EO, Ryumina AP, Boulina ME, Shirmanova MV, Zagaynova EV, Bogdanova EA, Lukyanov SA, Lukyanov KA. Phototoxic effects of lysosome-associated genetically encoded photosensitizer KillerRed. J Biomed Opt. 2014;19:071403. doi: 10.1117/1.JBO.19.7.071403. [DOI] [PubMed] [Google Scholar]
- 117.Williams DC, Bejjani RE, Ramirez PM, Coakley S, Kim SA, Lee H, Wen Q, Samuel A, Lu H, Hilliard MA, Hammarlund M. Rapid and permanent neuronal inactivation in vivo via subcellular generation of reactive oxygen with the use of KillerRed. Cell Rep. 2013;5:553–563. doi: 10.1016/j.celrep.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Serebrovskaya EO, Gorodnicheva TV, Ermakova GV, Solovieva EA, Sharonov GV, Zagaynova EV, Chudakov DM, Lukyanov S, Zaraisky AG, Lukyanov KA. Light-induced blockage of cell division with a chromatin-targeted phototoxic fluorescent protein. Biochem J. 2011;435:65–71. doi: 10.1042/BJ20101217. [DOI] [PubMed] [Google Scholar]
- 119.Lee H, Kim SA, Coakley S, Mugno P, Hammarlund M, Hilliard MA, Lu H. A multi-channel device for high-density target-selective stimulation and long-term monitoring of cells and subcellular features in C. elegans. Lab Chip. 2014;14:4513–4522. doi: 10.1039/c4lc00789a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Teh C, Chudakov DM, Poon KL, Mamedov IZ, Sek JY, Shidlovsky K, Lukyanov S, Korzh V. Optogenetic in vivo cell manipulation in KillerRed-expressing zebrafish transgenics. BMC Dev Biol. 2010;10:110. doi: 10.1186/1471-213X-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jewhurst K, Levin M, McLaughlin KA. Optogenetic control of apoptosis in targeted tissues of Xenopus laevis embryos. J Cell Death. 2014;7:25–31. doi: 10.4137/JCD.S18368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Byrne LC, Khalid F, Lee T, Zin EA, Greenberg KP, Visel M, Schaffer DV, Flannery JG. AAV-mediated, optogenetic ablation of Muller Glia leads to structural and functional changes in the mouse retina. PLoS One. 2013;8:e76075. doi: 10.1371/journal.pone.0076075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ryumina AP, Serebrovskaya EO, Shirmanova MV, Snopova LB, Kuznetsova MM, Turchin IV, Ignatova NI, Klementieva NV, Fradkov AF, Shakhov BE, et al. Flavoprotein miniSOG as a genetically encoded photosensitizer for cancer cells. Biochim Biophys Acta. 2013;1830:5059–5067. doi: 10.1016/j.bbagen.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 124.Vatansever F, Chandran R, Sadasivam M, Chiang LY, Hamblin MR. Multi-functionality in theranostic nanoparticles: is more always better? J Nanomed Nanotechnol. 2012;3:e120. doi: 10.4172/2157-7439.1000e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rai P, Mallidi S, Zheng X, Rahmanzadeh R, Mir Y, Elrington S, Khurshid A, Hasan T. Development and applications of photo-triggered theranostic agents. Adv Drug Deliv Rev. 2010;62:1094–1124. doi: 10.1016/j.addr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang S, Zhang L, Dong C, Su L, Wang H, Chang J. Smart pH-responsive upconversion nanoparticles for enhanced tumor cellular internalization and near-infrared light-triggered photodynamic therapy. Chem Commun (Camb) 2015;51:406–408. doi: 10.1039/c4cc08178a. [DOI] [PubMed] [Google Scholar]
- 127.Fu XJ, Zhu YQ, Peng YB, Chen YS, Hu YP, Lu HX, Yu WR, Fang Y, Du JZ, Yao M. Enzyme activated photodynamic therapy for methicillin-resistant Staphylococcus aureus infection both inv itro and in vivo. J. Photochem. Photobiol. B. 2014;136:72–80. doi: 10.1016/j.jphotobiol.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 128.Silva AK, Kolosnjaj-Tabi J, Bonneau S, Marangon I, Boggetto N, Aubertin K, Clement O, Bureau MF, Luciani N, Gazeau F, Wilhelm C. Magnetic and photoresponsive theranosomes: translating cell-released vesicles into smart nanovectors for cancer therapy. ACS Nano. 2013;7:4954–4966. doi: 10.1021/nn400269x. [DOI] [PubMed] [Google Scholar]
- 129.Charron DM, Chen J, Zheng G. Theranostic lipid nanoparticles for cancer medicine. Cancer Treat Res. 2015;166:103–127. doi: 10.1007/978-3-319-16555-4_5. [DOI] [PubMed] [Google Scholar]
- 130.Aggarwal A, Thompson S, Singh S, Newton B, Moore A, Gao R, Gu X, Mukherjee S, Drain CM. Photophysics of glycosylated derivatives of a chlorin, isobacteriochlorin and bacteriochlorin for photodynamic theragnostics: discovery of a two-photon-absorbing photosensitizer. Photochem Photobiol. 2014;90:419–430. doi: 10.1111/php.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ogawa K, Kobuke Y. Two-photon photodynamic therapy by water-soluble self-assembled conjugated porphyrins. Biomed Res Int. 2013;2013:125658. doi: 10.1155/2013/125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Starkey JR, Rebane AK, Drobizhev MA, Meng F, Gong A, Elliott A, McInnerney K, Spangler CW. New two-photon activated photodynamic therapy sensitizers induce xenograft tumor regressions after near-IR laser treatment through the body of the host mouse. Clin Cancer Res. 2008;14:6564–6573. doi: 10.1158/1078-0432.CCR-07-4162. [DOI] [PubMed] [Google Scholar]
- 133.Rosenthal I, Sostaric JZ, Riesz P. Sonodynamic therapy – a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;11:349–363. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 134.Sadanala KC, Chaturvedi PK, Seo YM, Kim JM, Jo YS, Lee YK, Ahn WS. Sono-photodynamic combination therapy: a review on sensitizers. Anticancer Res. 2014;34:4657–4664. [PubMed] [Google Scholar]
- 135.Kessel D. Photosensitization with derivatives of haematoporphyrin. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;49:901–907. doi: 10.1080/09553008514553131. [DOI] [PubMed] [Google Scholar]
- 136.Lee PK, Kloser A. Current methods for photodynamic therapy in the US: comparison of MAL/PDT and ALA/PDT. J Drugs Dermatol. 2013;12:925–930. [PubMed] [Google Scholar]
- 137.Liu AH, Sun X, Wei XQ, Zhang YZ. Efficacy of multiple low-dose photodynamic TMPYP4 therapy on cervical cancer tumour growth in nude mice. Asian Pac J Cancer Prev. 2013;14:5371–5374. doi: 10.7314/apjcp.2013.14.9.5371. [DOI] [PubMed] [Google Scholar]
- 138.Maisch T, Bosl C, Szeimies RM, Lehn N, Abels C. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrob Agents Chemother. 2005;49:1542–1552. doi: 10.1128/AAC.49.4.1542-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Senge MO, Brandt JC. Temoporfin (Foscan(R), 5,10,15,20-tetra(m-hydroxyphenyl)chlorin) – a second-generation photosensitizer. Photochem Photobiol. 2011;87:1240–1296. doi: 10.1111/j.1751-1097.2011.00986.x. [DOI] [PubMed] [Google Scholar]
- 140.Muragaki Y, Akimoto J, Maruyama T, Iseki H, Ikuta S, Nitta M, Maebayashi K, Saito T, Okada Y, Kaneko S, et al. Phase II clinical study on intraoperative photodynamic therapy with talaporfin sodium and semiconductor laser in patients with malignant brain tumors. J Neurosurg. 2013;119:845–852. doi: 10.3171/2013.7.JNS13415. [DOI] [PubMed] [Google Scholar]
- 141.Shafirstein G, Rigual NR, Arshad H, Cooper MT, Bellnier DA, Wilding G, Tan W, Merzianu M, Henderson BW. Photodynamic therapy with 3-(1′-hexyloxyethyl) pyropheophorbide-a for early-stage cancer of the larynx: phase Ib study. Head Neck. 2015 doi: 10.1002/hed.24003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moore CM, Azzouzi AR, Barret E, Villers A, Muir GH, Barber NJ, Bott S, Trachtenberg J, Arumainayagam N, Gaillac B, et al. Determination of optimal drug dose and light dose index to achieve minimally invasive focal ablation of localised prostate cancer using WST11-vascular-targeted photodynamic (VTP) therapy. BJU Int. 2015;116:888–896. doi: 10.1111/bju.12816. [DOI] [PubMed] [Google Scholar]
- 143.Huang L, Huang YY, Mroz P, Tegos GP, Zhiyentayev T, Sharma SK, Lu Z, Balasubramanian T, Krayer M, Ruzie C, et al. Stable synthetic cationic bacteriochlorins as selective antimicrobial photosensitizers. Antimicrob Agents Chemother. 2010;54:3834–3841. doi: 10.1128/AAC.00125-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Soriano J, Villanueva A, Stockert JC, Canete M. Vehiculization determines the endocytic internalization mechanism of Zn(II)-phthalocyanine. Histochem Cell Biol. 2013;139:149–160. doi: 10.1007/s00418-012-1012-6. [DOI] [PubMed] [Google Scholar]
- 145.Stern SJ, Flock ST, Small S, Thomsen S, Jacques S. Photodynamic therapy with chloroaluminum sulfonated phthalocyanine in the rat window chamber. Am J Surg. 1990;160:360–364. doi: 10.1016/s0002-9610(05)80543-4. [DOI] [PubMed] [Google Scholar]
- 146.Simonetti O, Cirioni O, Orlando F, Alongi C, Lucarini G, Silvestri C, Zizzi A, Fantetti L, Roncucci G, Giacometti A, et al. Effectiveness of antimicrobial photodynamic therapy with a single treatment of RLP068/Cl in an experimental model of Staphylococcus aureus wound infection. Br J Dermatol. 2011;164:987–995. doi: 10.1111/j.1365-2133.2011.10232.x. [DOI] [PubMed] [Google Scholar]
- 147.Jajarm HH, Falaki F, Sanatkhani M, Ahmadzadeh M, Ahrari F, Shafaee H. A comparative study of toluidine blue-mediated photodynamic therapy versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus: a randomized clinical controlled trial. Lasers Med Sci. 2015;30:1475–1480. doi: 10.1007/s10103-014-1694-1. [DOI] [PubMed] [Google Scholar]
- 148.Cincotta L, Foley JW, MacEachern T, Lampros E, Cincotta AH. Novel photodynamic effects of a benzophenothiazine on two different murine sarcomas. Cancer Res. 1994;54:1249–1258. [PubMed] [Google Scholar]
- 149.Ali MF. Topical delivery and photodynamic evaluation of a multivesicular liposomal Rose Bengal. Lasers Med Sci. 2011;26:267–275. doi: 10.1007/s10103-010-0859-9. [DOI] [PubMed] [Google Scholar]
- 150.Shafeekh KM, Soumya MS, Rahim MA, Abraham A, Das S. Synthesis and characterization of near-infrared absorbing water soluble squaraines and study of their photodynamic effects in DLA live cells. Photochem Photobiol. 2014;90:585–595. doi: 10.1111/php.12236. [DOI] [PubMed] [Google Scholar]
- 151.Rice DR, Gan H, Smith BD. Bacterial imaging and photodynamic inactivation using zinc(ii)-dipicolylamine BODIPY conjugates. Photochem Photobiol Sci. 2015;14:1271–1281. doi: 10.1039/c5pp00100e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carpenter BL, Situ X, Scholle F, Bartelmess J, Weare WW, Ghiladi RA. Antiviral, antifungal and antibacterial activities of a BODIPY-based photosensitizer. Molecules. 2015;20:10604–10621. doi: 10.3390/molecules200610604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lei W, Zhou Q, Jiang G, Zhang B, Wang X. Photodynamic inactivation of Escherichia coli by Ru(II) complexes. Photochem Photobiol Sci. 2011;10:887–890. doi: 10.1039/c0pp00275e. [DOI] [PubMed] [Google Scholar]
- 154.Knoll JD, Turro C. Control and utilization of ruthenium and rhodium metal complex excited states for photoactivated cancer therapy. Coord Chem Rev. 2015;282–283:110–126. doi: 10.1016/j.ccr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maggioni D, Galli M, D’Alfonso L, Inverso D, Dozzi MV, Sironi L, Iannacone M, Collini M, Ferruti P, Ranucci E, D’Alfonso G. A luminescent poly(amidoamine)-iridium complex as a new singlet-oxygen sensitizer for photodynamic therapy. Inorg Chem. 2015;54:544–553. doi: 10.1021/ic502378z. [DOI] [PubMed] [Google Scholar]
- 156.Tonon CC, Paschoal MA, Correia M, Spolidorio DM, Bagnato VS, Giusti JS, Santos-Pinto L. Comparative effects of photodynamic therapy mediated by curcumin on standard and clinical isolate of Streptococcus mutans. J Contemp Dent Pract. 2015;16:1–6. doi: 10.5005/jp-journals-10024-1626. [DOI] [PubMed] [Google Scholar]
- 157.Goff BA, Hermanto U, Rumbaugh J, Blake J, Bamberg M, Hasan T. Photoimmunotherapy and biodistribution with an OC125-chlorin immunoconjugate in an in vivo murine ovarian cancer model. Br J Cancer. 1994;70:474–480. doi: 10.1038/bjc.1994.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Demidova TN, Hamblin MR. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob Agents Chemother. 2005;49:2329–2335. doi: 10.1128/AAC.49.6.2329-2335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Demidova TN, Hamblin MR. Macrophage-targeted photodynamic therapy. Int J Immunopathol Pharmacol. 2004;17:117–126. doi: 10.1177/039463200401700203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.El-Akra N, Noirot A, Faye JC, Souchard JP. Synthesis of estradiol-pheophorbide a conjugates: evidence of nuclear targeting, DNA damage and improved photodynamic activity in human breast cancer and vascular endothelial cells. Photochem Photobiol Sci. 2006;5:996–999. doi: 10.1039/b606117f. [DOI] [PubMed] [Google Scholar]
- 161.Ballut S, Makky A, Chauvin B, Michel JP, Kasselouri A, Maillard P, Rosilio V. Tumor targeting in photodynamic therapy. From glycoconjugated photosensitizers to glycodendrimeric one. Concept, design and properties. Org Biomol Chem. 2012;10:4485–4495. doi: 10.1039/c2ob25181g. [DOI] [PubMed] [Google Scholar]