The heart undergoes hypertrophy in response to pressure overload, a response generally considered to be an adaptive mechanism to reduce increased wall stress. When the stress is too great, or other molecular changes are elicited, hypertrophy can decompensate leading to development of heart failure. Macroautophagy (hereafter referred to as autophagy) has been implicated in this process. Autophagy is an intracellular system whereby cytoplasmic components and damaged organelles are sequestered in double-membrane vesicles called autophagosomes and delivered to lysosomes for degradation. Nutrient starvation or cellular stress rapidly induce autophagy, providing amino acids and fatty acids to synthesize proteins and to generate ATP, while also eliminating damaged mitochondria.1-4 Damaged mitochondria are the major source of reactive oxygen species (ROS) contributing to apoptotic and necrotic cell death thus preservation of mitochondrial integrity is critical for cell survival. Mitochondrial quality control can be achieved through autophagy, mitochondria-selective autophagy (mitophagy), fission/fusion and mitochondrial biogenesis, processes that are in many ways interrelated.5 There are, however, contradictory published observations regarding the timing or direction of changes in general autophagy and its functional significance in the cardiac response to pressure overload remains controversial.2-4, 6, 7 Moreover, whether the regulation of mitochondrial quality control by autophagy is beneficial or deleterious in the development of pressure overload-induced hypertrophy or its transition to heart failure has not been extensively studied.
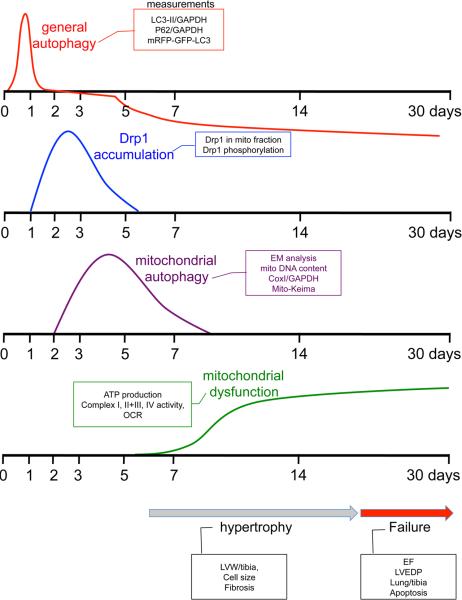
In this issue of Circulation, Shirakabe et al8 provide greater clarity into the role of mitochondrial autophagy in pressure-overload induced cardiac hypertrophy and failure. By analyzing multiple time points from 1 hr to 30 days after transverse aortic constriction (TAC), the authors delineate sequential and ordered changes in autophagy and mitochondrial autophagy that occur at different phases of development of TAC-induced hypertrophy and cardiac dysfunction (Figure 1).8 General autophagy is rapidly increased between 1 and 12 hrs after TAC, normalized at 1 to 3 days, and suppressed to below physiological levels from 5 days to 30 days after TAC. These changes in autophagy are evidenced by increased and decreased autophagic flux assessed by mRFP-GFP-LC3 puncta in the presence or absence of lysosome inhibition in vivo. The observation that autophagy is transiently activated, but normalized prior to and indeed suppressed in concert with the development of hypertrophy, suggests that autophagy is an early adaptive process that either signals, or is not integral to, regulation of hypertrophy and heart failure.
Figure 1.
Time-course of general autophagy, mitochondrial Drp1 accumulation, mitochondrial autophagy and mitochondrial dysfunction following pressure-overload (adapted from Online Figure XB, Shirakabe et al8)
Shirakabe et al8 further analyzed the process they specifically termed mitochondrial autophagy. Their studies included electron microscopic analysis of autophagosomes that contained mitochondria and also changes in mitochondrial DNA and mitochondrial matrix proteins. Interestingly, mitochondrial autophagy was found to be activated at the same time that general autophagy was normalized (it increased at 3 days after TAC) and also to be transient, normalizing at one week after TAC.8 Additional studies used AAV9-mediated co-expression of Mito-Keima and YFP-Lamp1 in the heart to demonstrate increased delivery of mitochondria to the lysosomal compartment at 3-7 days after TAC. The population of foreshortened mitochondria was concomitantly increased. Importantly, the decline of mitochondrial autophagy directly preceded development of mitochondrial dysfunction. Drp1, a mitochondrial fission protein, was genetically deleted in the adult heart by this same laboratory and the cardiac-specific conditional Drp1 knockout was shown to develop ventricular dysfunction and lethality.9 The time course of phosphorylation and mitochondrial translocation of Drp1 examined in the current study was found to peak at 3 days after TAC thus temporally associated with development of mitochondrial autophagy. To directly assess involvement of Drp1 in the sequence of events described above cardiac specific heterozygous Drp1 knockout mice were subject to TAC. Mitochondrial autophagy was suppressed and the increase in the population of the foreshortened mitochondria was largely attenuated. Remarkably, decreasing endogenous Drp1 also lead to a more rapid onset of mitochondrial dysfunction and accelerated TAC induced cardiac hypertrophy and dysfunction. These results strongly suggest that Drp1 regulates the size of mitochondria and mitochondrial autophagy in the heart subjected to TAC and that mitochondrial autophagy serves as a compensatory and adaptive response to maintain mitochondrial quality in the face of pressure-overload.
To further determine whether increasing autophagy could rescue the heart against TAC induced heart failure, the authors used a TAT-Beclin 1 peptide (TB1) which liberates endogenous Beclin 1 from the Golgi apparatus and thereby increases Beclin1 available to regulate of autophagy.10 TB1 was administered on day 7, a time at which autophagy is suppressed and mitochondrial autophagy is declining, and shown to restore these responses at 30 days. Strikingly this also lead to improved mitochondrial function and prevented development of heart failure, as evidenced by decreased myocyte size, inhibition of lung edema, preserved contractile function, and diminished cardiomyocyte cell death. Importantly TB1 could not overcome the deleterious effects of haploinsufficiency of Drp1. Thus Drp1 is required for the protective effect of Beclin 1, supporting the concept that Drp1 plays a crucial role in compensatory autophagic clearance of mitochondria and mitochondrial quality control.
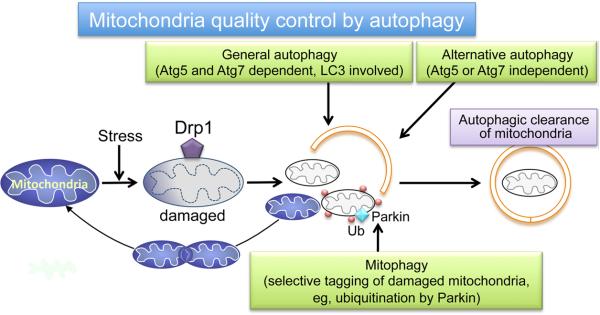
A question that is not conclusively answered in this study is what molecular mechanism is responsible for induction of mitochondrial autophagy by pressure overload. Is this process the same as the previously described mitochondria-selective autophagy (mitophagy), as depicted in Figure 2? Notably two major events in mitophagy, Parkin translocation to mitochondria and ubiquitination of mitochondrial proteins, were examined and found not to occur at the time that mitochondrial autophagy was increased. Accordingly, the established process of Parkin-mediated mitophagy does not appear to be responsible for the mitochondrial autophagy observed following TAC. In addition TAC-induced mitochondrial autophagy does not begin until general autophagy has returned to basal levels, thus general autophagy of mitochondria seems unlikely to assume this function. Shirakabe et al8 speculate that an alternative form of autophagy, which is Atg5- or Atg7-independent and cannot be evaluated by conventional markers such as LC3-II,11 plays a role in mitochondrial autophagy regulated by Drp1 in response to pressure overload. The occurrence of alternative autophagy in the heart has not been well documented thus further study will be required to test this intriguing possibility. More detailed mechanistic information will be needed to dissect and determine the role of general autophagy, alternative autophagy, mitophagy and their contributions to mitochondrial quality control (Figure 2).
Figure 2.
Schematic of potential mechanisms for mitochondrial quality control by autophagy.
Shirakabe et al8 demonstrate at day 2 following TAC that there is increased phosphorylation of Drp1 at Ser616 and concomitantly decreased phosphorylation at Ser637. Published work has identified Cdk1, PKCδ and ERK as kinases that mediate phosphorylation of Drp1 at Ser616 and increase its translocation to mitochondria12, 13 ; PKA, CaMKIα, Pim-1 and ROCK1 have been reported to phosphorylate Drp1 at Ser637, inhibiting Drp1 translocation to mitochondria in some but not all studies.12, 13 Interestingly Ser637 is also a target for calcineurin-dependent dephosphorylation.12 In addition to phosphorylation, Drp1 undergoes multiple post-transcriptional modifications including S-nitrosylation, SUMOylation, O-GlcNAcylation.12 There has been extensive research into the signaling pathways by which pressure overload leads to cardiac hypertrophy and heart failure. Similar delineation of the signals transducing pressure overload to Drp1 mitochondrial translocation may be the next frontier in understanding how mitochondrial quality control is regulated and its cessation contributes to the progression and decompensation of cardiac hypertrophy. Drp1 plays an active role in mitochondrial fission, suggested to be a prerequisite step for mitochondrial autophagy.12, 14 Whether Drp1-dependent fission is mechanistically linked to Drp1-induced mitochondrial autophagy remains to be determined. A dynamic interdependence between fission and mitochondrial autophagy could underlie the disparate time dependent effects of complete Drp1 deletion in the adult heart observed in different laboratories.9, 14-16
Finally, it is of considerable interest that general autophagy is such an early and transient response, and that mitochondrial autophagy is also self-limited, turning off after the first week of pressure overload. In light of the evidence that mitochondrial autophagy seems to prevent development of mitochondrial dysfunction and heart failure, understanding why the process is halted could provide important insights into how to prevent this progression. A decline in mitochondrial quality control is increasingly appreciated as a major factor in development of hypertrophy and heart failure. Accordingly mitochondrial autophagy can be viewed as a means of protecting the heart against the toxic effects of ROS production by dysfunctional mitochondria. 4, 5, 9, 14, 17-20 . The work by Shirakabe et al8 provides new insights into the role of Drp1 mediated mitochondrial autophagy, suggesting that this is part of a compensatory pathway that serves to protect the heart against pressure overload induced maladaptive hypertrophy and limit cardiac decompensation.
Acknowledgments
Funding Sources: This work was supported by National Institutes of Health Grants HL028143, HL080101 and HL085577 to Joan Heller Brown and R56 HL097037 and American heart Association 15GRNT 2297009 to Shigeki Miyamoto.
Footnotes
Disclosures: None.
References
- 1.Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. J Clin Invest. 2015;125:55–64. doi: 10.1172/JCI73943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maejima Y, Chen Y, Isobe M, Gustafsson AB, Kitsis RN, Sadoshima J. Recent progress in research on molecular mechanisms of autophagy in the heart. Am J Physiol Heart Circ Physiol. 2015;308:H259–268. doi: 10.1152/ajpheart.00711.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishida K, Otsu K. Autophagy during cardiac remodeling. J Mol Cell Cardiol. 2015 Dec 8;:S0022–2828(15)30142-5. doi: 10.1016/j.yjmcc.2015.12.003. doi: 10.1016/j.yjmcc.2015.12.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Dorn GW, 2nd, Kitsis RN. The Mitochondrial Dynamism-Mitophagy-Cell Death Interactome: Multiple Roles Performed by Members of a Mitochondrial Molecular Ensemble. Circ Res. 2015;116:167–182. doi: 10.1161/CIRCRESAHA.116.303554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirakabe A, Zhai P, Ikeda Y, Saito T, Maejima Y, Hsu CP, Nomura M, Egashira K, Levine B, Sadoshima J. Drp1-dependent mitochondrial autophagy plays a protective role against pressure-overloadinduced mitochondrial dysfunction and heart failure. Circulation. 2016;133:XX–XXX. doi: 10.1161/CIRCULATIONAHA.115.020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res. 2015;116:264–278. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 10.Shoji-Kawata S, Sumpter R, Leveno M, Campbell GR, Zou Z, Kinch L, Wilkins AD, Sun Q, Pallauf K, MacDuff D, Huerta C, Virgin HW, Helms JB, Eerland R, Tooze SA, Xavier R, Lenschow DJ, Yamamoto A, King D, Lichtarge O, Grishin NV, Spector SA, Kaloyanova DV, Levine B. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013;494:201–206. doi: 10.1038/nature11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 12.Hall AR, Burke N, Dongworth RK, Hausenloy DJ. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol. 2014;171:1890–1906. doi: 10.1111/bph.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Din S, Mason M, Volkers M, Johnson B, Cottage CT, Wang Z, Joyo AY, Quijada P, Erhardt P, Magnuson NS, Konstandin MH, Sussman MA. Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation. Proc Natl Acad Sci U S A. 2013;110:5969–5974. doi: 10.1073/pnas.1213294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW., 2nd. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21:273–285. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorn GW., 2nd. Gone fission...:diverse consequences of cardiac Drp1 deficiency. Circ Res. 2015;116:225–228. doi: 10.1161/CIRCRESAHA.114.305672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Hoke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014;33:2798–2813. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westenbrink BD, Ling H, Divakaruni AS, Gray CB, Zambon AC, Dalton ND, Peterson KL, Gu Y, Matkovich SJ, Murphy AN, Miyamoto S, Dorn GW, 2nd, Heller Brown J. Mitochondrial reprogramming induced by CaMKIIdelta mediates hypertrophy decompensation. Circ Res. 2015;116:e28–39. doi: 10.1161/CIRCRESAHA.116.304682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornfeld OS, Hwang S, Disatnik MH, Chen CH, Qvit N, Mochly-Rosen D. Mitochondrial reactive oxygen species at the heart of the matter: new therapeutic approaches for cardiovascular diseases. Circ Res. 2015;116:1783–1799. doi: 10.1161/CIRCRESAHA.116.305432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, Jimenez R, Petrosyan S, Murphy AN, Gustafsson AB. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem. 2013;288:915–926. doi: 10.1074/jbc.M112.411363. [DOI] [PMC free article] [PubMed] [Google Scholar]