Abstract
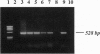
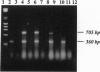
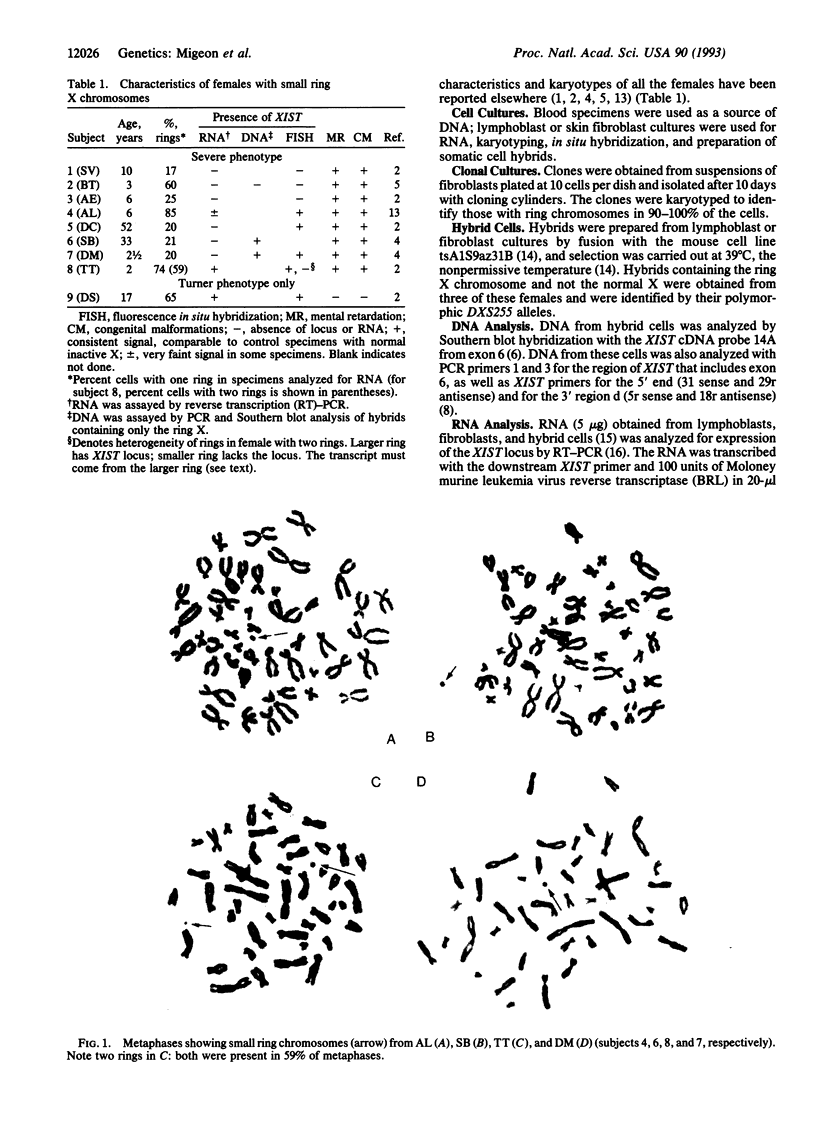
The severe phenotype of human females whose karyotype includes tiny ring X chromosomes has been attributed to the inability of the small ring X chromosome to inactivate. The XIST locus is expressed only from the inactive X chromosome, resides at the putative X inactivation center, and is considered a prime player in the initiation of mammalian X dosage compensation. Using PCR, Southern blot analysis, and in situ hybridization, we have looked for the presence of the XIST locus in tiny ring X chromosomes from eight females who have multiple congenital malformations and severe mental retardation. Our studies reveal heterogeneity within this group; some rings lack the XIST locus, while others have sequences homologous to probes for XIST. However, in the latter, the locus is either not expressed or negligibly expressed, based on reverse transcription-PCR analysis. Therefore, what these tiny ring chromosomes have in common is a level of XIST transcription comparable to an active X. As XIST transcription is an indicator of X chromosome inactivity, the absence of XIST transcription strongly suggests that tiny ring X chromosomes in females with severe phenotypes are mutants in the X chromosome inactivation pathway and that the inability of these rings to inactivate is responsible for the severe phenotypes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballabio A., Willard H. F. Mammalian X-chromosome inactivation and the XIST gene. Curr Opin Genet Dev. 1992 Jun;2(3):439–447. doi: 10.1016/s0959-437x(05)80155-8. [DOI] [PubMed] [Google Scholar]
- Borsani G., Tonlorenzi R., Simmler M. C., Dandolo L., Arnaud D., Capra V., Grompe M., Pizzuti A., Muzny D., Lawrence C. Characterization of a murine gene expressed from the inactive X chromosome. Nature. 1991 May 23;351(6324):325–329. doi: 10.1038/351325a0. [DOI] [PubMed] [Google Scholar]
- Brockdorff N., Ashworth A., Kay G. F., Cooper P., Smith S., McCabe V. M., Norris D. P., Penny G. D., Patel D., Rastan S. Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature. 1991 May 23;351(6324):329–331. doi: 10.1038/351329a0. [DOI] [PubMed] [Google Scholar]
- Brockdorff N., Ashworth A., Kay G. F., McCabe V. M., Norris D. P., Cooper P. J., Swift S., Rastan S. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell. 1992 Oct 30;71(3):515–526. doi: 10.1016/0092-8674(92)90519-i. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Ballabio A., Rupert J. L., Lafreniere R. G., Grompe M., Tonlorenzi R., Willard H. F. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991 Jan 3;349(6304):38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Hendrich B. D., Rupert J. L., Lafrenière R. G., Xing Y., Lawrence J., Willard H. F. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992 Oct 30;71(3):527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Willard H. F. Noninactivation of a selectable human X-linked gene that complements a murine temperature-sensitive cell cycle defect. Am J Hum Genet. 1989 Oct;45(4):592–598. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dennis N. R., Collins A. L., Crolla J. A., Cockwell A. E., Fisher A. M., Jacobs P. A. Three patients with ring (X) chromosomes and a severe phenotype. J Med Genet. 1993 Jun;30(6):482–486. doi: 10.1136/jmg.30.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumbach M. M., Morishima A., Taylor J. H. HUMAN SEX CHROMOSOME ABNORMALITIES IN RELATION TO DNA REPLICATION AND HETEROCHROMATINIZATION. Proc Natl Acad Sci U S A. 1963 May;49(5):581–589. doi: 10.1073/pnas.49.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P., Turner B. M. The inactive X chromosome in female mammals is distinguished by a lack of histone H4 acetylation, a cytogenetic marker for gene expression. Cell. 1993 Jul 30;74(2):281–289. doi: 10.1016/0092-8674(93)90419-q. [DOI] [PubMed] [Google Scholar]
- Kushnick T., Irons T. G., Wiley J. E., Gettig E. A., Rao K. W., Bowyer S. 45X/46X,r(X) with syndactyly and severe mental retardation. Am J Med Genet. 1987 Nov;28(3):567–574. doi: 10.1002/ajmg.1320280304. [DOI] [PubMed] [Google Scholar]
- LYON M. F. Sex chromatin and gene action in the mammalian X-chromosome. Am J Hum Genet. 1962 Jun;14:135–148. [PMC free article] [PubMed] [Google Scholar]
- Lafrenière R. G., Brown C. J., Rider S., Chelly J., Taillon-Miller P., Chinault A. C., Monaco A. P., Willard H. F. 2.6 Mb YAC contig of the human X inactivation center region in Xq13: physical linkage of the RPS4X, PHKA1, XIST and DXS128E genes. Hum Mol Genet. 1993 Aug;2(8):1105–1115. doi: 10.1093/hmg/2.8.1105. [DOI] [PubMed] [Google Scholar]
- Lengauer C., Green E. D., Cremer T. Fluorescence in situ hybridization of YAC clones after Alu-PCR amplification. Genomics. 1992 Jul;13(3):826–828. doi: 10.1016/0888-7543(92)90160-t. [DOI] [PubMed] [Google Scholar]
- Lindgren V., Chen C. P., Bryke C. R., Lichter P., Page D. C., Yang-Feng T. L. Cytogenetic and molecular characterization of marker chromosomes in patients with mosaic 45,X karyotypes. Hum Genet. 1992 Feb;88(4):393–398. doi: 10.1007/BF00215672. [DOI] [PubMed] [Google Scholar]
- Richler C., Soreq H., Wahrman J. X inactivation in mammalian testis is correlated with inactive X-specific transcription. Nat Genet. 1992 Nov;2(3):192–195. doi: 10.1038/ng1192-192. [DOI] [PubMed] [Google Scholar]
- Salido E. C., Yen P. H., Mohandas T. K., Shapiro L. J. Expression of the X-inactivation-associated gene XIST during spermatogenesis. Nat Genet. 1992 Nov;2(3):196–199. doi: 10.1038/ng1192-196. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Migeon B. R. Asynchronous replication of homologous loci on human active and inactive X chromosomes. Proc Natl Acad Sci U S A. 1990 May;87(10):3685–3689. doi: 10.1073/pnas.87.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke D. L., Wiktor A., Palmer C. G., Miller D. A., Witt M., Babu V. R., Worsham M. J., Roberson J. R., Weiss L. Ullrich-Turner syndrome with a small ring X chromosome and presence of mental retardation. Am J Med Genet. 1992 Aug 1;43(6):996–1005. doi: 10.1002/ajmg.1320430617. [DOI] [PubMed] [Google Scholar]
- Van Dyke D. L., Wiktor A., Roberson J. R., Weiss L. Mental retardation in Turner syndrome. J Pediatr. 1991 Mar;118(3):415–417. doi: 10.1016/s0022-3476(05)82159-6. [DOI] [PubMed] [Google Scholar]
- Walker C. L., Cargile C. B., Floy K. M., Delannoy M., Migeon B. R. The Barr body is a looped X chromosome formed by telomere association. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6191–6195. doi: 10.1073/pnas.88.14.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]