Abstract
Commercially important Commiphora species are drought-tolerant plants and they are leafless for most of the year. Therefore, it is necessary to develop some molecular marker for the identification. Intended for that, in the present study, species-specific, sequence-characterized amplified regions (SCAR) markers were developed for proficient and precise identification of closely related species Commiphora wightii and C. myrrha, which may ensure the quality, safety, and efficacy of medicines made from these plants through adulterous mixing of these plants. Two species-specific RAPD amplicons were selected, gel-purified, cloned, and sequenced after screening of 20 RAPD primers. The sequence of 979 and 590 nucleotides (Genebank accession numbers K90051 and K90052) was used for development of 4 SCAR markers, namely, Sc1P, Sc1Pm, Sc2P, and Sc2Pm. Out of them, the Sc1Pm was specific for C. wightii, while Sc2P discriminated both the Commiphora species. These markers are first reported and will be useful for rapid identification of closely related Commiphora wightii and C. myrrha species.
1. Introduction
Commiphora spp. of the family Burseraceae is being used as a medicinal plant since ancient times and now rated as an endangered plant species [1]. They are found in the arid to semiarid regions of the world, including the deserts of India, Pakistan, Africa, and Saudi Arabia, while in India, it is found in Rajasthan, Madhya Pradesh, Gujarat, Tamilnadu, Orissa, and Karnataka. About 185 species of Commiphora were found worldwide, out of them C. wightii (synonym C. mukul), C. agallocha, C. stocksiana, C. berryi, and C. myrrha were found in India [2, 3]. In earlier studies about the flora of India, the “Guggul” plant was known as Commiphora mukul (Hook ex Stocks) Engl. or Balsamodendron mukul (Hook ex Stocks). Finally, it was named as C. wightii (Arn.) by Bhandari in 1964.
C. wightii was well-documented medicinal plant since 3000 years ago [4], having exciting biological activities like being anti-inflammatory, antimicrobial, hepatoprotective, muscle relaxing, antiarthritic, hypolipidemic, hypocholesterolemic, antiobesity, antioxidant, antimalarial, antimycobacterial, antischistosomal, larvicidal, and mollucidal [2, 3, 5–19].
C. wightii contains a bitter gum known as Guggul (Myrrh) in stems and leaves. The yellowish gum oozes upon making an incision and solidifies in the hot environment to a hard brownish resin. Guggul is medicinally important and is used in the treatment of hypercholesterolemia and cardiovascular diseases [9, 20]; it is also shown to have anticancerous activity [21]. The extract of gum Guggul, as gugulipid, guggulipid, or guglipid, is reported as a folk remedy in the Unani and Ayurvedic system of medicine. Two trans-isomers of Guggulsterone, namely, Guggulsterones E and Z, were reported in gum Guggul as important active steroid which are used as cholesterol-lowering agents. The pharmacological properties associated with gum Guggul include anti-inflammatory, antibacterial, anticoagulant, antirheumatic, COX inhibitory, and hypolipidemic activities that are mostly due to the presence of these steroids [22, 23]. In 1986, Guggul lipids were granted approval in India for marketing as a lipid-lowering drug [24]. Several products of standardized formulations of C. wightii were already in human use as cholesterol-lowering agents [22, 25].
Commiphora species have been called “taxonomically difficult,” because of being drought-tolerant plants and they are leafless for most of the year [26]. There is resemblance of gum Guggul with gum resin of other species within and outside of the genus, which make high risk of adulteration in commercial samples either deliberately to get more profit or accidentally. Therefore, it is important to validate the C. wightii plants and their gum Guggul in commercial samples due to its various pharmacological significances [27]. Many types of markers, namely, morphological, biochemical, and DNA based molecular markers, are commonly used in the identification of species [28]. Molecular markers were used in the identification of species and individual, their origin, and difference at the molecular level in between them [29]. During the last few decades, the use of molecular markers, revealing polymorphism at the DNA level, has been playing an increasing part in plant biotechnology and their genetic studies. These DNA based markers are differentiated into two types: first is non-PCR based RFLP and second is PCR based markers (RAPD, AFLP, SSR, SNP, etc.) [30]. RAPD is a PCR-based technology, based on enzymatic amplification of target or random DNA segments with arbitrary primers. The main advantage of RAPDs is that they are quick and easy to assay, had no sequence data required for primer construction, randomly distributed throughout the genome, and had a dominant nature [31]. However, RAPD marker is not suitable for the species identification, because of their low reproducibility and dominant nature [32]. A RAPD marker can be converted into a codominant and reproducible marker, that is, Sequence-Characterized Amplified Region (SCAR), which may be applicable for authentication of species.
Looking upon these problems, it is necessary to develop some molecular marker for the identification of C. wightii. In the present study, an attempt has been made for the development of SCAR markers for C. wightii.
2. Materials and Methods
Total 28 accessions of two different species of Commiphora, that is, C. wightii (17) and C. myrrha (11), were collected from Bhopal, Obaidullaganj (Madhya Pradesh), Akola (Maharashtra), Anand (Gujarat), and Jaipur (Rajasthan), and conserved at MPCST Human Herbal Health Care Garden, Bhopal.
2.1. Selection of RAPD Primers and Amplicons
Genomic DNA was isolated from fresh young stem C. wightii and C. myrrha using the method of Sairkar et al. (unpublished). The yield of DNA was measured using a NanoDrop UV-Spectrophotometer (ND-1000). Genomic DNA was amplified by the 20 primers (Table 1). A cocktail of 40 μL reaction volumes was made with 20 μL, 2x red dye PCR mix (Merck), 1 μL primer (10 pM), and 1 μL template DNA (25 ng/μL) and amplification was performed on the gradient automatic thermal cycler (Eppendorf) following Sairkar et al. [33]. The PCR products were separated electrophoretically on 1.5% agarose gel at 5–10 volts/cm of the gel and visualized by ethidium bromide. The specific amplicon, which discriminates between C. wightii and C. myrrha, was selected and processed for the development of SCAR marker.
Table 1.
Sequence of SCAR markers designed using 1 kb amplicon.
| Name of SCAR | Name of fragments | Sequence (5′-3′) | Total length | Temp | Size (bp) |
|---|---|---|---|---|---|
| Sc1P | Sc1P (F) | CTGTGAGGCATTTGTATATTTAA | 23 bases | 60°C | 631 |
| Sc1P (R) | CTTGTGGTCTTTCAGTCAATAG | 22 bases | 62°C | ||
|
| |||||
| Sc1Pm | Sc1P (F) | CTGTGAGGCATTTGTATATTTAA | 23 bases | 60°C | 910 |
| Sc1Pm (R) | CTTGAGAACGAAATCTAACAAG | 22 bases | 60°C | ||
2.2. Cloning of Selected RAPD Amplicon
The selected amplicons were eluted using Medox-Easy Spin Column Cleanup Minipreps kit and ligated with the TA cloning vector (pGEM5Z, Promega). The ligated TA vector was transformed into competent cells of E. coli (DH5α), which was prepared using single step ultracompetent cell preparation kit (Medox). The first selection of recombinant clones was based on developed blue and white colonies on LB (Luria Burtani) agar plates containing 0.5 mg/mL ampicillin, 24 μg/mL IPTG, and 30 μg/mL X-gal. The plasmid of white and blue colonies was isolated through Medox-Easy ultrapure spin column plasmid DNA minipreps kit. Three selection steps, that is, clone retardation, restriction digestion, and amplification of plasmid, were adopted to identify positive insert within the plasmid. In retardation step, plasmids were separated electrophoretically to observe the presence of insert within plasmid, while in restriction digestion, plasmids were digested with PvuII enzyme for insert release. In the final step, the plasmids were amplified through the PCR reaction using 50 μL that consist of 25 μL 2x red dye PCR mix (Merck), 1 μL each of forward and reverse M-13 primers (10 pM each), 1 μL of plasmid DNA (25 ng/μL) with a PCR profile of 94°C for 12 minutes, 30 cycles of 30 seconds at 94°C, 30 seconds at 55°C and 45 seconds at 72°C, and final extension on 72°C at 10 minutes using the gradient automatic thermal cycler (Eppendorf).
2.3. Designing and Screening of SCAR Marker
Plasmid having desired amplicon was sequenced by Aristogene Pvt. Ltd., Bangalore, India, using M13 reverse and forward sequencing primers and consensus sequence of amplicons was developed. The homology search of consensus sequences was performed by the NCBI BLAST tool. The primer pairs were designed for these sequences by using PRIMER 3 software [34] and used as a candidate for SCAR primer. Four accessions of each species of C. wightii and C. myrrha were amplified through these primer pairs (synthesized by Aristogene) with a cocktail of 40 μL containing 20 μL of 2x red dye PCR mix (Merck), 1 μL of each of the SCAR primer pair (10 pM each), and 1 μL of template DNA (25 ng/μL). Amplification was performed on the gradient automatic thermal cycler (Eppendorf) with PCR conditions: 94°C for 5 minutes, 30 cycles of 30 seconds at 94°C, 30 seconds at 58°C and 1 minute at 72°C, and final extension on 72°C at 10 minutes. Among the all designed primer pairs, suitable primer pair was selected which discriminate the both species of Commiphora and further screened in all the accessions for validation of SCAR marker.
3. Result
3.1. Identification of RAPD Primer and Amplicon
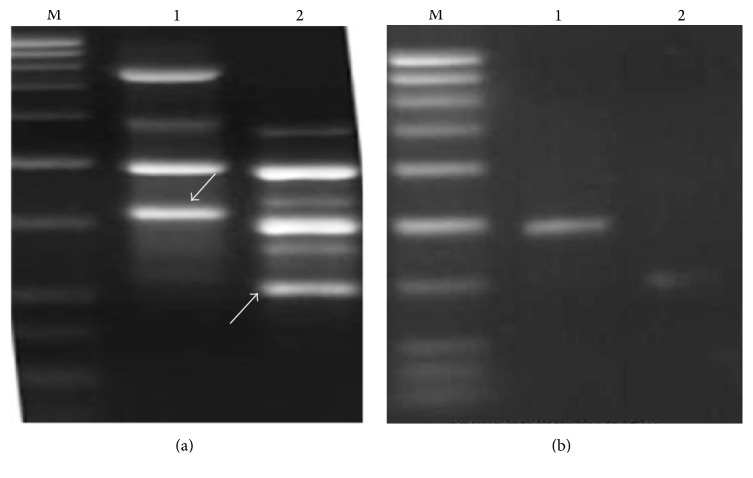
Out of 20 RAPD primers, 1 kb amplicon of OPD-02 and 0.6 kb amplicon of OPD-08 discriminate both Commiphora species as it was present only in C. wightii accessions (Figure 1(a)). Due to specificity of these amplicons, they were cloned, sequenced, and used for SCAR marker development. These bands were elected from agarose gels and gel electrophoresis revealed that they were appropriate for cloning (Figure 1(b)).
Figure 1.
Selection and elution of desired amplicon. (a) PCR product of sample Commiphora wightii on low melting agarose gel. Lanes 1 and 2 amplified by primers OPD-02 and OPD-08. (b) Eluted desired amplicon run on agarose gel. Lanes 1 and 2, fragment sizes 1 kb and 0.6 kb, respectively.
3.2. Cloning and Selection of Positive Clone
White colony of competent cells (E. coli) having T vector with 1 kb and 0.6 kb insert was undertaken for plasmid isolation and three selection criteria were performed for the conformation of positive clone. The screening for retardation checking reveals that 17 positive plasmids for 1 kb insert and 5 positive plasmids for 0.6 kb may have proper insert (Figure 2). These positive plasmids were digested with the restriction endonuclease (PvuII) for insert release. A total of 6 positive plasmids of 1 kb insert and 4 positive plasmids of 0.6 kb insert release their respective insert fragment (Figure 3). In the third stage of selection, 4 positive plasmids for 1 kb insert 3 positive plasmids for 0.6 kb insert were finalized for sequencing after amplify with M-13 primer (Figure 4).
Figure 2.

Retardation checking by plasmid run on agarose gel; Lanes 1 to 21: positive cloned plasmids 1 to 21, B: negative cloned plasmid isolated from blue colony.
Figure 3.
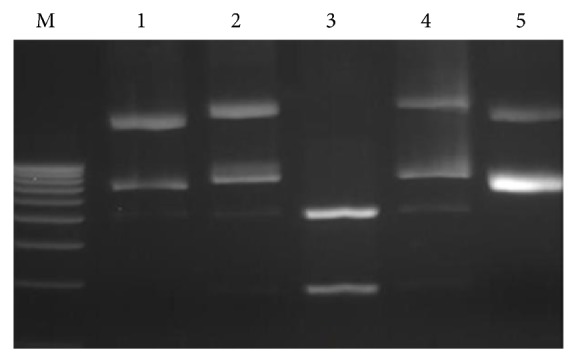
Insert release, digested plasmids by restriction endonuclease PvuII run on agarose gel, plasmid of Lanes 1, 2, 4, and 5 realised 1 kb fragment after digestion.
Figure 4.

Amplification of plasmid by vector primers (M-13). Lane 1, 2, 3 and 9 produced 1 kb fragment while lanes 4, 5, and 6 produced 0.6 kb fragment.
3.3. Sequencing and In Silico Application
The clones were sequenced and 979 bp and 590 bp consensus sequences were formed for 1 kb insert 0.6 kb insert, respectively (Figures 5 and 6). The BLAST search was performed for the obtained sequences and no significant homologous sequence was found in the NCBI database. This DNA sequences were deposited in the NCBI gene bank database with accession numbers K90051 and K90052. Two candidate SCAR primer pairs for each DNA sequences were designed, that is, primers Sc1P and Sc1Pm from 979 bp sequences and primer Sc2P and Sc1Pm from 590 bp sequences (Tables 1 and 2). These primers were deposited in the NCBI Prob database with accession number Pr031905450 to Pr031905453.
Figure 5.
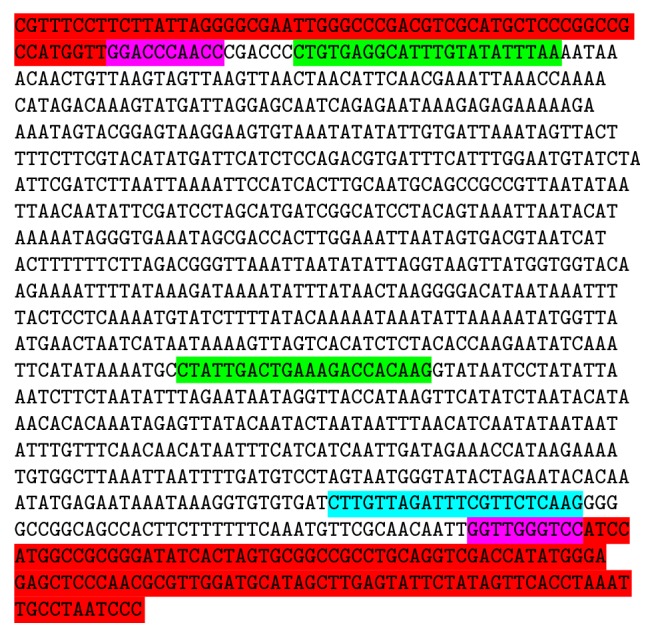
Consensus sequence (979 bp) of 1 kb fragment (Genebank ID K90051). Red highlights: vector sequence. Green highlight: SCAR primer region. Blue highlight: second reverse SCAR primer. Pink highlight: RAPD primer region.
Figure 6.

Consensus sequence (590 bp) of 0.6 kb fragment (Genebank ID K90052). Red highlights: vector sequence. Green highlight: SCAR primer region. Blue highlight: second reverse SCAR primer. Pink highlight: RAPD primer region.
Table 2.
Sequence of SCAR markers designed from 0.6 kb amplicon.
| Name of SCAR | Name of fragments | Sequence (5′-3′) | Total length | Temp. | Size (bp) |
|---|---|---|---|---|---|
| Sc2P | Sc2P (F) | GTACCCAATGTAGTAATATTCC | 22 bases | 60°C | 491 |
| Sc2P (R) | TAGTTAGTTTGATGACCATCACA | 23 bases | 62°C | ||
|
| |||||
| Sc2Pm | Sc2P (F) | GTACCCAATGTAGTAATATTCC | 22 bases | 60°C | 570 |
| Sc2Pm (R) | GTGTGCCCCATTCAACCAAT | 20 bases | 60°C | ||
3.4. Development of SCAR Marker
The candidate SCAR primer pairs were screened with three accessions of each of C. wightii and C. myrrha which revealed that the primer Sc1Pm is highly specific for C. wightii and amplified 910 bp amplicon, while primer Sc1P had a similar banding pattern in all the samples. Primer Sc2P discriminated both the Commiphora species as it gave 491 bp amplicon for C. wightii, and 1200 bp for C. myrrha, while primer Sc2Pm gives 491 and 570 bp amplicon for C. wightii and C. myrrha, respectively (Figure 7).
Figure 7.

Screening of SCAR primers. (a) Lanes 1 to 2: C. myrrha and Lanes 3 to 4: C. wightii amplified by primers Sc1P; Lanes 5 to 6: C. myrrha and C. wightii, amplified by primers Sc2P. (b) Lanes 1 to 2, C. myrrha and Lanes 3 to 4, C. wightii amplified by primers Sc1P; Lanes 5 to 6, C. myrrha and Lanes 7 to 8, C. wightii, amplified by primers Sc2P. (c) Lanes 1 to 4, C. myrrha amplified by primers Sc1P, Sc1Pm, Sc2P, and Sc2Pm; Lanes 5 to 8, C. wightii amplified by primers Sc1P, Sc1Pm, Sc2P, and Sc2Pm.
Based on the above results, primers Sc1Pm and Sc2P were authenticated through amplification of eight accession of each species, that is, C. wightii and C. myrrha (Figure 8). The similar results were observed during this screening as they were discriminated both the species of Commiphora.
Figure 8.
Screening of SCAR primers. (a) Lanes 1 to 8, accessions of C. wightii amplified by primers Sc1Pm. (b) Lanes 1 to 8, accessions of C. myrrha amplified by primers Sc1Pm. (c) Lanes 1 to 8, accessions of C. wightii and Lanes 9 to 16, accessions of C. myrrha amplified by primers Sc2P. M = low range ruler (3000, 2500, 2000, 1500, 1000, 600, 300, 200, and 100 bp). L = 100 bp ladder (1000, 900, 800, 700, 600, 500, 400, 300, 200, and 100 bp).
4. Discussion
Identification of plants at the species level traditionally is a feverish job and needs special care during identification. This work usually needs to be a specially trained expert, after that many human errors were observed. To overcome this problem, in the early 1990s many specific molecular identification technologies were popular which are more reliable [35, 36]. Nowadays molecular taxonomists are engaged in preparation of the nucleotide sequence of a short DNA fragment for all living species on earth, which is called DNA barcodes [37–39].
Molecular markers allow the detection of specific DNA sequence differences between tests of individuals of an organism [40]. DNA markers are unlimited in number and are not affected by environmental factors and developmental stages of the plant [41]. The discovery of PCR technology changed the entire molecular biology and a single random oligonucleotide primer (10-bp long) was discovered in 1990 as a universal marker technology called RAPDs [42]. The main advantages of RAPD markers are the following: they are universal and cost-effective and for application of these markers they did not need any genetic information of the target organism and they can map almost completed genomic DNA of the target organism [43]. However, each method analyses different aspects of DNA sequence variation and different regions of the genome. RAPD and AFLP markers appear to frequently target repetitive regions of the genome.
The presence of polysaccharides, polyphenols, and other secondary metabolites in the leaves of Commiphora species creates complications in the DNA process. Haque et al. 2008 [44] and Samantaray et al. [45] used various methods and described the process for DNA isolation. Their isolated DNA showed good PCR amplification; therefore, it can further be used in molecular downstream applications. Molecular variations among accessions collected from different localities of Rajasthan and Gujarat were described by Suthar et al. [46]. Intraspecific variation in Commiphora wightii populations was described by Haque et al. [47] using Internal Transcribed Spacer (ITS1-5.8S-ITS2) Sequences while Harish et al. [48] studied genetic variations on accessions collected from Indian Thar Desert using RAPD and ISSR markers. Molecular variations among different biotypes of Commiphora wightii were done by Vyas and Joshi in 2015 [49] using RAPD markers. Genetic variability among the C. wightii germplasm collected from Rajasthan and Haryana was studied by Kulhari et al. [50]. Samantaray et al. [51] used sixty different random decamer primers and identified three primers which produced specific fragment in the female plant of C. wightii but failed to do so from the male plant DNAs. Their finding was helpful for the breeding practice of C. wightii and our SCAR markers may be useful for identification of C. wightii at species level.
The developed SCAR markers by us were used for identification of C. wightii and discrimination among C. wightii and C. myrrha. SCAR markers maybe are developed using sequence of RAPD fragments which are characterized by many advantages, including their specificity, low cost, ease, fast use, reproducibility, abundance, and being polymorphic in nature targeting specific regions of the genomes [52–54] employed with success in plant and animal species identification [30, 55–58].
In this study, RAPD amplicons were selected for cloning, sequencing, and final development of SCAR markers. Specific characters of RAPD markers entice researchers and usually SCAR markers have been developed from RAPD amplicons [58–61]. Amplicon of other fingerprinting methods like AFLP [62–64] and ISSR (Inter Simple Sequence Repeat) [52] was also used to develop SCAR markers.
Developed Sc1Pm marker in this study produced a 910 bp amplicon with C. wightii, while in other samples no amplification was observed. These results revealed that this SCAR marker might be used in identification and authentication of C. wightii. Many reports are available in which SCAR markers have been used for authentication of medicinal plant species like Panax ginseng [65], bent-grass [66], Bamboo [67], Piper longum [68], Artemisia princeps and A. argyi [69], Phyllanthus emblica [70], strawberry [71] Jatropha curcas [72, 73], Ganoderma lucidum [74], Pueraria tuberosa [75], Dendrobium candidum [76], Sinapis arvensis [77], Cornus officinalis [78], and Scrophularia ningpoensis [79]. Three SCAR markers of Phyllanthus species were developed from three specific RAPD sequences that can identify and differentiate the morphologically similar Phyllanthus species [80].
SCAR markers have been also developed for breeding programs of crops like Rice [81], Citrus tristeza [82], Brassica napus L. [83], Grapevine [84], Wheat [85], Buckwheat [86], Grape [87], Barley [88], Atractylodes japonica and A. macrocephala [89], Diplocarpon rosae [90], Puccinia coronata [91], Puccinia striiformis [92], Thinopyrum elongatum [93], Liriope and Ophiopogon [94], Medicago sativa [95], Triticum turgidum [96], and Miscanthus sacchariflorus [97].
Our marker Sc2P produced a prominent amplicon of 491 bp in the C. wightii, and 1.2 kb in the C. myrrha while other plant samples did not show amplification. The result revealed that this SCAR primer might be used for the discrimination among C. wightii and C. myrrha. Only few reports are present with a single primer discrimination among two closely related species.
Acknowledgments
The authors wish to thank Professor P. K. Verma, Director General, MPCST, and Dr. R. K. Singh, Resource Scientist and Group Head, Advance Research Instrumentation Facility, MPCST, for providing laboratory facilities.
Conflict of Interests
The authors declare that there is no conflict of interests.
Authors' Contribution
Dr. P. K. Sairkar performed wet and dry laboratory work under the supervision of Dr. N. P. Shukla and Professor Anjana Sharma. Professor A. Sharma was cosupervisor of this work and all microbiology related work was performed under her supervision, while Dr. N. P. Shukla was supervisor of this work and probes were developed under his supervision.
References
- 1.IUCN. IUCN Red List of Threatened Species. Version 2012.2, 2012, http://www.iucnredlist.org/
- 2.Kirtikar K. R., Basu B. D. Indian Medicinal Plants. Vol. 1. Dehra Dun, India: Bishen Singh Mahendra Pal Singh; 1935. [Google Scholar]
- 3.Hocking D. Trees for Drylands. New Delhi, India: Oxford and IBH Publishing; 1993. [Google Scholar]
- 4.Joshi A. Ayurvedic Patrikaritaka Has (History of Ayurvedic Publications) Jodhpur, India: Mimeo. Mohan Ayurvedic Pharmacy; 1980. [Google Scholar]
- 5.Chaturvedi G. N., Singh R. H. Experimental studies on anti-arthritic effect of certain indigenous drugs. Indian Journal of Medical Research. 1965;53(1):71–80. [PubMed] [Google Scholar]
- 6.Satyavati G. V., Dwarkanath C., Ttripathi S. N. Experimental studies on the hypocholesterolemic effect of Commiphora mukul Engl. (Guggul) Indian Journal of Medical Research. 1969;57(10):1950–1962. [PubMed] [Google Scholar]
- 7.Kumar S., Shankar V. Medicinal plants of Indian desert: Commiphora wightii (Arnott) Bhand. Journal of Arid Environments. 1982;5:1–11. [Google Scholar]
- 8.Satyavati G. V. Use of plant drugs in Indian traditional systems of medicine and their relevance to primary health care. In: Wagner H., Farnsworth N. R., editors. Economic and Medicinal Plant Research. London, UK: Academic Press; 1990. pp. 39–56. [Google Scholar]
- 9.Singh R. B., Niaz M. A., Ghosh S. Hypolipidemic and antioxidant effects of Commiphora mukul as an adjunct to dietary therapy in patients with hypercholesterolemia. Cardiovascular Drugs and Therapy. 1994;8(4):659–664. doi: 10.1007/bf00877420. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt A. D., Dalal D. G., Shah S. J., et al. Conceptual and methodologic challenges of assessing the short-term efficacy of Guggulu in obesity: data emergent from a naturalistic clinical trial. Journal of Postgraduate Medicine. 1995;41(1):5–7. [PubMed] [Google Scholar]
- 11.Allam A. F., El-Sayed M. H., Kahlil S. S. Laboratory assessment of molluscidal activity of Commiphora molmol (myrrha) on Biomphalaria alexandrina, Bulinus truncates and Lymnea cailliaudi . Journal of the Egyptian Society of Parasitology. 2001;31:683–690. [PubMed] [Google Scholar]
- 12.El Ashry E. S. H., Rashed N., Salama O. M., Saleh A. Components, therapeutic value and uses of myrrh. Pharmazie. 2003;58(3):163–168. [PubMed] [Google Scholar]
- 13.Massoud A. M. A., El Ebiary F. H., Abd-El-Salam N. F. Effect of myrrh extract on the liver of normal and bilharzially infected mice—an ultrastructural study. Journal of the Egyptian Society of Parasitology. 2004;34(1):1–21. [PubMed] [Google Scholar]
- 14.Wang X., Greilberger J., Ledinski G., Kager G., Paigen B., Jürgens G. The hypolipidemic natural product Commiphora mukul and its component guggulsterone inhibit oxidative modification of LDL. Atherosclerosis. 2004;172(2):239–246. doi: 10.1016/j.atherosclerosis.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Abbas F. A., Al-Massarany S. M., Khan S., Al-Howiriny T. A., Mossa J. S., Abourashed E. A. Phytochemical and biological studies on Saudi Commiphora opobalsamum L. Natural Product Research. 2007;21(5):383–391. doi: 10.1080/14786410600942025. [DOI] [PubMed] [Google Scholar]
- 16.Shen T., Wan W., Yuan H., et al. Secondary metabolites from Commiphora opobalsamum and their antiproliferative effect on human prostate cancer cells. Phytochemistry. 2007;68(9):1331–1337. doi: 10.1016/j.phytochem.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Sharma A., Patel V. K., Rawat S., Ramteke P., Verma R. Identification of the antibacterial component of some indian medicinal plants against Klebsiella pneumoniae . International Journal of Pharmacy and Pharmaceutical Sciences. 2010;2(3):123–127. [Google Scholar]
- 18.Goyal C., Ahuja M., Sharma S. K. Preparation and evaluation of anti-inflammatory activity of gugulipid-loaded proniosomal gel. Acta Poloniae Pharmaceutica—Drug Research. 2011;68(1):147–150. [PubMed] [Google Scholar]
- 19.Al-Howiriny T., Al-Sohaibani M., Al-Said M., Al-Yahya M., El-Tahir K., Rafatullah S. Effect of Commiphora opobalsamum (L.) Engl. (Balessan) on experimental gastric ulcers and secretion in rats. Journal of Ethnopharmacology. 2005;98(3):287–294. doi: 10.1016/j.jep.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 20.Deng R., Yang D., Radke A., Yang J., Yan B. The hypolipidemic agent guggulsterone regulates the expression of human bile salt export pump: dominance of transactivation over farsenoid X receptor-mediated antagonism. Journal of Pharmacology and Experimental Therapeutics. 2007;320(3):1153–1162. doi: 10.1124/jpet.106.113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao D., Singh S. V. z-Guggulsterone, a constituent of Ayurvedic medicinal plant Commiphora mukul, inhibits angiogenesis in vitro and in vivo . Molecular Cancer Therapeutics. 2008;7(1):171–180. doi: 10.1158/1535-7163.mct-07-0491. [DOI] [PubMed] [Google Scholar]
- 22.Zhu N., Rafi M. M., DiPaola R. S., et al. Bioactive constituents from gum guggul (Commiphora wightii) Phytochemistry. 2001;56(7):723–727. doi: 10.1016/s0031-9422(00)00485-4. [DOI] [PubMed] [Google Scholar]
- 23.Meselhy M. R. Inhibition of LPS-induced NO production by the oleogum resin of Commiphora wightii and its constituents. Phytochemistry. 2003;62(2):213–218. doi: 10.1016/s0031-9422(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 24.Indian Pharmacopeia 2007. Indian Pharmacopoeia (IP), Government of India, Ministry of Health and Family Welfare; 2007. [Google Scholar]
- 25.Szapary P. O., Wolfe M. L., Bloedon L. T., et al. Guggulipid for the treatment of hypercholesterolemia: a randomized controlled trial. Journal of the American Medical Association. 2003;290(6):765–772. doi: 10.1001/jama.290.6.765. [DOI] [PubMed] [Google Scholar]
- 26.Gachathi F. N. Recent advances on classification and status of the main gum-resin producing species in the family Burseraceae . Proceedings of the Regional Conference for Africa on Conservation, Management, and Utilization of Plant Gums, Resins and Essential Oils; October 1997; Nairobi, Kenya. [Google Scholar]
- 27.Ahmed H. A., MacLeod E. T., Hide G., Welburn S. C., Picozzi K. The best practice for preparation of samples from FTA®cards for diagnosis of blood borne infections using African trypanosomes as a model system. Parasites and Vectors. 2011;4, article 68 doi: 10.1186/1756-3305-4-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tautz D., Arctander P., Minelli A., Thomas R. H., Vogler A. P. A plea for DNA taxonomy. Trends in Ecology and Evolution. 2003;18(2):70–74. doi: 10.1016/S0169-5347(02)00041-1. [DOI] [Google Scholar]
- 29.Techen N., Crockett S. L., Khan I. A., Sheffler B. E. Authentication of medicinal plants using molecular biology techniques to compliment conventional methods. Current Medicinal Chemistry. 2004;11(11):1391–1401. doi: 10.2174/0929867043365206. [DOI] [PubMed] [Google Scholar]
- 30.Kumar P., Gupta V. K., Misra A. K., Modi D. R., Pandey B. K. Potential of molecular markers in plant biotechnology. Plant Omics Journal. 2009;2(4):141–162. [Google Scholar]
- 31.Williams J. G. K., Hanafey M. K., Rafalski J. A., Tingey S. V. Methods in Enzymology. Vol. 218. New York, NY, USA: Academic Press; 1993. Genetic analysis using random amplified polymorphic DNA markers; pp. 704–740. [DOI] [PubMed] [Google Scholar]
- 32.Ganiea S. H., Upadhyaya P., Dasa S., Sharmab M. P. Authentication of medicinal plants by DNA markers. Plant Gene. 2015;4:83–99. doi: 10.1016/j.plgene.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sairkar P., Vijay N., Silawat N., et al. Inter-species association of Ocimum genus as revealed through random amplified polymorphic DNA fingerprinting. Science Secure Journal of Biotechnology. 2012;1(1):1–8. [Google Scholar]
- 34.Rozen S., Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods in Molecular Biology. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 35.Cheung K.-S., Kwan H.-S., But P. P.-H., Shaw P.-C. Pharmacognostical identification of American and Oriental ginseng roots by genomic fingerprinting using Arbitrarily Primed Polymerase Chain Reaction (AP-PCR) Journal of Ethnopharmacology. 1994;42(1):67–69. doi: 10.1016/0378-8741(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 36.Mizukami H., Ohbayashi K., Kitamutra Y., Ikenaga T. Restriction fragment length polymorphisms (RFLPs) of medicinal plants and crude drugs. I. RFLP probes allow clear identification of Duboisia interspecific hybrid genotypes in both fresh and dried tissues. Biological and Pharmaceutical Bulletin. 1993;16(4):388–390. doi: 10.1248/bpb.16.388. [DOI] [PubMed] [Google Scholar]
- 37.Hebert P. D. N., Cywinska A., Ball S. L., deWaard J. R. Biological identification through DNA barcodes. Proceedings of the Royal Society of London. Series B, Biological Sciences. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savolainen V., Cowan R. S., Vogler A. P., Roderick G. K., Lane R. Towards writing the encyclopaedia of life: an introduction to DNA barcoding. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360(1462):1805–1811. doi: 10.1098/rstb.2005.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ratnasingham S., Hebert P. D. N. BOLD: the barcode of life data system (http://www.barcodinglife.org) Molecular Ecology Notes. 2007;7(3):355–364. doi: 10.1111/j.1471-8286.2007.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langridge P., Chalmers K. The principle: identification and application of molecular markers. In: Lorz H., Wenzel G., editors. Molecular Marker Systems in Plant Breeding and Crop Improvement. Vol. 49. Berlin, Germany: Springer; 2004. pp. 129–149. (Biotechnology in Agriculture and Forestry). [Google Scholar]
- 41.Winter P., Kahl G. Molecular marker technologies for plant improvement. World Journal of Microbiology & Biotechnology. 1995;11(4):438–448. doi: 10.1007/BF00364619. [DOI] [PubMed] [Google Scholar]
- 42.Williams J. G. K., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Research. 1990;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tingey S. V., del Tufo J. P. Genetic analysis with random amplified polymorphic DNA markers. Plant Physiology. 1993;101(2):349–352. doi: 10.1104/pp.101.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haque I., Bandopadhyay R., Mukhopadhyay K. An optimised protocol for fast genomic DNA isolation from high secondary metabolites and gum containing plants. Asian Journal of Plant Sciences. 2008;7(3):304–308. doi: 10.3923/ajps.2008.304.308. [DOI] [Google Scholar]
- 45.Samantaray S., Hidayath K. P., Maiti S. An isolation protocol of genomic DNA from Commiphora wightii (Arnott.) Bhandari: an endangered medicinal plant. International Journal of Integrative Biology. 2009;6(3):127–131. [Google Scholar]
- 46.Suthar S., Thul S., Kukreja A. K., Ramawat K. G. RAPD markers reveal polymorphism in Commiphora wightii, an endangered medicinal tree. Journal of Cell and Tissue Research. 2008;8(2):1477–1480. [Google Scholar]
- 47.Haque I., Bandopadhyay R., Mukhopadhyay K. Intraspecific variation in Commiphora wightii populations based on internal transcribed spacer (ITS1-5.8S-ITS2) sequences of rDNA. Diversity. 2009;1(2):89–101. doi: 10.3390/d1020089. [DOI] [Google Scholar]
- 48.Harish, Gupta A. K., Phulwaria M., Rai M. K., Shekhawat N. S. Conservation genetics of endangered medicinal plant Commiphora wightii in Indian Thar Desert. Gene. 2014;535(2):266–272. doi: 10.1016/j.gene.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 49.Vyas P., Joshi R. Assessment of molecular variations among different biotypes of Commiphora wightii (Arnott.) Bhandari, using RAPD markers. International Journal of Innovative Science, Engineering & Technology. 2015;2(6):328–338. [Google Scholar]
- 50.Kulhari A., Singh R., Chaudhury A., Dhawan A. K., Kalia R. K. Assessment of genetic variability through ISSR and RAPD markers in Commiphora wightii (Arn.) Bhandari. Acta Physiologiae Plantarum. 2015;37, article 113 doi: 10.1007/s11738-015-1859-y. [DOI] [Google Scholar]
- 51.Samantaray S., Geetha K. A., Hidayath K. P., Maiti S. Identification of RAPD markers linked to sex determination in guggal [Commiphora wightii (Arnott.)] Bhandari. Plant Biotechnology Reports. 2010;4(1):95–99. doi: 10.1007/s11816-009-0113-8. [DOI] [Google Scholar]
- 52.Zietkiewicz E., Rafalski A., Labuda D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics. 1994;20(2):176–183. doi: 10.1006/geno.1994.1151. [DOI] [PubMed] [Google Scholar]
- 53.Bornet B., Branchard M. Nonanchored Inter Simple Sequence Repeat (ISSR) markers: reproducible and specific tools for genome fingerprinting. Plant Molecular Biology Reporter. 2001;19(3):209–215. doi: 10.1007/bf02772892. [DOI] [Google Scholar]
- 54.Bornet B., Muller C., Paulus F., Branchard M. Highly informative nature of Inter Simple Sequence Repeat (ISSR) sequences amplified using tri- and tetra-nucleotide primers from DNA of cauliflower (Brassica oleracea var. botrytis L.) Genome. 2002;45(5):890–896. doi: 10.1139/g02-061. [DOI] [PubMed] [Google Scholar]
- 55.Parent J.-G., Pagé D. Identification of raspberry cultivars by sequence characterized amplified region DNA analysis. HortScience. 1998;33(1):140–142. [Google Scholar]
- 56.Mariniello L., Sommella M. G., Sorrentino A., Forlani M., Porta R. Identification of Prunus armeniaca cultivars by RAPD and SCAR markers. Biotechnology Letters. 2002;24(10):749–755. doi: 10.1023/a:1015516712754. [DOI] [Google Scholar]
- 57.Yau F. C. F., Wong K. L., Shaw P. C., But P. P. H., Wang J. Authentication of snakes used in Chinese medicine by sequence characterized amplified region (SCAR) Biodiversity and Conservation. 2002;11(9):1653–1662. doi: 10.1023/a:1016836017903. [DOI] [Google Scholar]
- 58.Bautista R., Crespillo R., Cánovas F. M., Claros M. G. Identification of olive-tree cultivars with SCAR markers. Euphytica. 2003;129(1):33–41. doi: 10.1023/a:1021528122049. [DOI] [Google Scholar]
- 59.Parasnis A. S., Gupta V. S., Tamhankar S. A., Ranjekar P. K. A highly reliable sex diagnostic PCR assay for mass screening of papaya seedlings. Molecular Breeding. 2000;6(3):337–344. doi: 10.1023/A:1009678807507. [DOI] [Google Scholar]
- 60.Koveza O. V., Kokaeva Z. G., Gostimsky S. A., Petrova T. V., Osipova E. S. Creation of a SCAR marker in pea Pisum sativum L. using RAPD analysis. Russian Journal of Genetics. 2001;37(4):464–466. doi: 10.1023/a:1016627131572. [DOI] [PubMed] [Google Scholar]
- 61.Arnedo-Andrés M. S., Gil-Ortega R., Luis-Arteaga M., Hormaza J. I. Development of RAPD and SCAR markers linked to the Pvr4 locus for resistance to PVY in pepper (Capsicum annuum L.) Theoretical and Applied Genetics. 2002;105(6-7):1067–1074. doi: 10.1007/s00122-002-1058-2. [DOI] [PubMed] [Google Scholar]
- 62.Negi M. S., Devic M., Delseny M., Lakshmikumaran M. Identification of AFLP fragments linked to seed coat colour in Brassica juncea and conversion to a SCAR marker for rapid selection. Theoretical and Applied Genetics. 2000;101(1-2):146–152. doi: 10.1007/s001220051463. [DOI] [Google Scholar]
- 63.Xu M., Korban S. S. AFLP-derived SCARs facilitate construction of a 1.1 Mb sequence-ready map of a region that spans the Vf locus in the apple genome. Plant Molecular Biology. 2002;50(4-5):803–818. doi: 10.1023/A:1019912419709. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt H., Ehrmann M., Vogel R. F., Taniwaki M. H., Niessen L. Molecular typing of Aspergillus ochraceus and construction of species specific SCAR-primers based on AFLP. Systematic and Applied Microbiology. 2003;26(1):138–146. doi: 10.1078/072320203322337434. [DOI] [PubMed] [Google Scholar]
- 65.Wang J., Ha W.-Y., Ngan F.-N., But P. P.-H., Shaw P.-C. Application of sequence characterized amplified region (SCAR) analysis to authenticate Panax species and their adulterants. Planta Medica. 2001;67(8):781–783. doi: 10.1055/s-2001-18340. [DOI] [PubMed] [Google Scholar]
- 66.Scheef E. A., Casler M. D., Jung G. Development of species-specific SCAR markers in bentgrass. Crop Science. 2003;43(1):345–349. doi: 10.2135/cropsci2003.3450. [DOI] [Google Scholar]
- 67.Das M., Bhattacharya S., Pal A. Generation and characterization of SCARs by cloning and sequencing of RAPD products: a strategy for species-specific marker development in bamboo. Annals of Botany. 2005;95(5):835–841. doi: 10.1093/aob/mci088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manoj P., Banerjee N. S., Ravichandran P. Development of sex-associated SCAR markers in Piper longum L. Plant Genetic Resources Newsletter. 2005;141:44–50. [Google Scholar]
- 69.Lee M. Y., Doh E. J., Park C. H., et al. Development of SCAR marker for discrimination of Artemisia princeps and A. argyi from other Artemisia herbs. Biological and Pharmaceutical Bulletin. 2006;29(4):629–633. doi: 10.1248/bpb.29.629. [DOI] [PubMed] [Google Scholar]
- 70.Dnyaneshwar W., Preeti C., Kalpana J., Bhushan P. Development and application of RAPD-SCAR marker for identification of Phyllanthus emblica Linn. Biological and Pharmaceutical Bulletin. 2006;29(11):2313–2316. doi: 10.1248/bpb.29.2313. [DOI] [PubMed] [Google Scholar]
- 71.Rugienius R., Siksnianas T., Stanys V., Gelvonauskiene D., Bendokas V. Use of RAPD and SCAR markers for identification of strawberry genotypes carrying red stele (Phytophtora fragariae) resistance gene Rpf1 . Agronomy Research. 2006;4:335–339. [Google Scholar]
- 72.Basha S. D., Sujatha M. Inter and intra-population variability of Jatropha curcas (L.) characterized by RAPD and ISSR markers and development of population-specific SCAR markers. Euphytica. 2007;156(3):375–386. doi: 10.1007/s10681-007-9387-5. [DOI] [Google Scholar]
- 73.Basha S. D., Francis G., Makkar H. P. S., Becker K., Sujatha M. A comparative study of biochemical traits and molecular markers for assessment of genetic relationships between Jatropha curcas L. germplasm from different countries. Plant Science. 2009;176(6):812–823. doi: 10.1016/j.plantsci.2009.03.008. [DOI] [Google Scholar]
- 74.Su H., Wang L., Ge Y., Feng E., Sun J., Liu L. Development of strain-specific SCAR markers for authentication of Ganoderma lucidum . World Journal of Microbiology and Biotechnology. 2008;24(7):1223–1226. doi: 10.1007/s11274-007-9579-0. [DOI] [Google Scholar]
- 75.Devaiah K. M., Venkatasubramanian P. Development of SCAR marker for authentication of Pueraria tuberosa (Roxb. ex. Willd.) DC. Current Science. 2008;94(10):1306–1309. [Google Scholar]
- 76.Jin B., Jiang F.-S., Yu J., Ding Z.-S., Chen S.-H., Lv G.-Y. Study on sequence characterized amplified region (SCAR) markers in Dendrobium candidum . Zhong Yao Cai. 2010;33(3):343–346. [PubMed] [Google Scholar]
- 77.Pankin A. A., Khavkin E. E. Genome-specific scar markers help solve taxonomy issues: a case study with Sinapis arvensis (Brassiceae, Brassicaceae) American Journal of Botany. 2011;98(3):e54–e57. doi: 10.3732/ajb.1000422. [DOI] [PubMed] [Google Scholar]
- 78.Chen S., Lu X., Wang L. Study on sequence characterized amplified region (SCAR) markers of Cornus officinalis . Zhongguo Zhong Yao Za Zhi. 2011;36(9):1145–1149. doi: 10.4268/cjcmm20110908. [DOI] [PubMed] [Google Scholar]
- 79.Chen C., Duan L.-N., Zhou X.-L., Chen B.-L., Fu C.-X. Molecular authentication of geo-authentic Scrophularia ningpoensis . Journal of Zhejiang University: Science B. 2011;12(5):393–398. doi: 10.1631/jzus.b1000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Theerakulpisut P., Kanawapee N., Maensiri D., Bunnag S., Chantaranothai P. Development of species-specific SCAR markers for identification of three medicinal species of Phyllanthus . Journal of Systematics and Evolution. 2008;46(4):614–621. doi: 10.3724/sp.j.1002.2008.07123. [DOI] [Google Scholar]
- 81.Naqvi N. I., Chattoo B. B. Development of a sequence characterized amplified region (SCAR) based indirect selection method for a dominant blast-resistance gene in rice. Genome. 1996;39(1):26–30. doi: 10.1139/g96-004. [DOI] [PubMed] [Google Scholar]
- 82.Deng Z., Huang S., Xiao S., Gmitter F. G., Jr. Development and characterization of SCAR markers linked to the Citrus tristeza virus resistance gene from Poncirus trifoliata . Genome. 1997;40(5):697–704. doi: 10.1139/g97-792. [DOI] [PubMed] [Google Scholar]
- 83.Barret P., Delourme R., Foisset N., Renard M. Development of a SCAR (sequence characterised amplified region) marker for molecular tagging of the dwarf BREIZH (Bzh) gene in Brassica napus L. Theoretical and Applied Genetics. 1998;97(5):828–833. doi: 10.1007/s001220050962. [DOI] [PubMed] [Google Scholar]
- 84.Lahogue F., This P., Bouquet A. Identification of a codominant SCAR marker linked to the seedlessness character in grapevine. Theoretical and Applied Genetics. 1998;97(5-6):950–959. doi: 10.1007/s001220050976. [DOI] [Google Scholar]
- 85.Hernández P., Martín A., Dorado G. Development of SCARs by direct sequencing of RAPD products: a practical tool for the introgression and marker-assisted selection of wheat. Molecular Breeding. 1999;5(3):245–253. doi: 10.1023/a:1009637928471. [DOI] [Google Scholar]
- 86.Aii J., Nagano M., Woo S. H., Campbell C., Adachi T. Development of the SCAR markers linked to the Sh gene in buckwheat. Fagopyrum. 1999;16:19–22. [Google Scholar]
- 87.Adam-Blondon A.-F., Lahogue-Esnault F., Bouquet A., Boursiquot J. M., This P. Usefulness of two SCAR markers for marker-assisted selection of seedless grapevine cultivars. Vitis. 2001;40(3):147–155. [Google Scholar]
- 88.Ardiel G. S., Grewal T. S., Deberdt P., Rossnagel B. G., Scoles G. J. Inheritance of resistance to covered smut in barley and development of a tightly linked SCAR marker. Theoretical and Applied Genetics. 2002;104(2):457–464. doi: 10.1007/s001220100696. [DOI] [PubMed] [Google Scholar]
- 89.Huh M. K., Bang K. H. Identification of Atractylodes japonica and A. macrocephala by RAPD analysis and SCAR markers. Silvae Genetica. 2006;55(3):101–105. [Google Scholar]
- 90.Whitaker V. M., Bradeen J. M., Debener T., Biber A., Hokanson S. C. Rdr3, a novel locus conferring black spot disease resistance in tetraploid rose: genetic analysis, LRR profiling, and SCAR marker development. Theoretical and Applied Genetics. 2010;120(3):573–585. doi: 10.1007/s00122-009-1177-0. [DOI] [PubMed] [Google Scholar]
- 91.McCartney C. A., Stonehouse R. G., Rossnagel B. G., et al. Mapping of the oat crown rust resistance gene Pc91 . Theoretical and Applied Genetics. 2011;122(2):317–325. doi: 10.1007/s00122-010-1448-9. [DOI] [PubMed] [Google Scholar]
- 92.Hu L., Li G., Zhan H., Liu C., Yang Z. New St-chromosome-specific molecular markers for identifying wheat—Thinopyrum intermedium derivative lines. Journal of Genetics. 2011;91(2):e69–e74. [PubMed] [Google Scholar]
- 93.Xu G. H., Su W. Y., Shu Y. J., Cong W. W., Wu L., Guo C. H. RAPD and ISSR-assisted identification and development of three new SCAR markers specific for the Thinopyrum elongatum E (Poaceae) genome. Genetics and Molecular Research. 2012;11(2):1741–1751. doi: 10.4238/2012.june.29.7. [DOI] [PubMed] [Google Scholar]
- 94.Li G., Park Y.-J. SCAR markers for discriminating species of two genera of medicinal plants, Liriope and Ophiopogon . Genetics and Molecular Research. 2012;11(3):2987–2996. doi: 10.4238/2012.may.18.14. [DOI] [PubMed] [Google Scholar]
- 95.Wang Y., Bi B., Yuan Q. H., Li X. L., Gao J. M. Association of AFLP and SCAR markers with common leafspot resistance in autotetraploid alfalfa (Medicago sativa) Genetics and Molecular Research. 2012;11(1):606–616. doi: 10.4238/2012.march.14.4. [DOI] [PubMed] [Google Scholar]
- 96.Liu Z., Zhu J., Cui Y., et al. Identification and comparative mapping of a powdery mildew resistance gene derived from wild emmer (Triticum turgidum var. dicoccoides) on chromosome 2BS. Theoretical and Applied Genetics. 2012;124(6):1041–1049. doi: 10.1007/s00122-011-1767-5. [DOI] [PubMed] [Google Scholar]
- 97.Kim J. K., An G. H., Ahn S.-H., et al. Development of SCAR marker for simultaneous identification of Miscanthus sacchariflorus, M. sinensis and M. x giganteus . Bioprocess and Biosystems Engineering. 2012;35(1-2):55–59. doi: 10.1007/s00449-011-0592-1. [DOI] [PubMed] [Google Scholar]