Abstract
The cleaning of hard surfaces in hospital rooms is critical for reducing health care–associated infections. This review describes the evidence examining current methods of cleaning, disinfecting, and monitoring cleanliness of patient rooms, as well as contextual factors that may affect implementation and effectiveness. Key informants were interviewed, and a systematic search for publications since 1990 was done with the use of several bibliographic and gray literature resources. Studies examining surface contamination, colonization, or infection with Clostridium difficile, methicillin-resistant Staphylococcus aureus, or vancomycinresistant enterococci were included.
Eighty studies were identified—76 primary studies and 4 systematic reviews. Forty-nine studies examined cleaning methods, 14 evaluated monitoring strategies, and 17 addressed challenges or facilitators to implementation. Only 5 studies were randomized, controlled trials, and surface contamination was the most commonly assessed outcome. Comparative effectiveness studies of disinfecting methods and monitoring strategies were uncommon. Future research should evaluate and compare newly emerging strategies, such as self-disinfecting coatings for disinfecting and adenosine triphosphate and ultraviolet/fluorescent surface markers for monitoring. Studies should also assess patient-centered outcomes, such as infection, when possible. Other challenges include identifying high-touch surfaces that confer the greatest risk for pathogen transmission; developing standard thresholds for defining cleanliness; and using methods to adjust for confounders, such as hand hygiene, when examining the effect of disinfecting methods.
Health care–associated infections (HAIs) are a leading cause of illness and death in the United States and worldwide. In 2011, an estimated 721 800 HAIs occurred in the United States, leading to 75 000 deaths (1). A multifaceted approach to preventing infection is critical to reducing the risk for HAIs, including hand hygiene practices, antimicrobial stewardship, and environmental cleaning and disinfecting.
Several studies demonstrate that health care–associated pathogens frequently contaminate the patient environment, including both porous surfaces (such as curtains) and hard, nonporous surfaces (such as bed rails and medical equipment) (2–4). Contaminated surfaces are a reservoir for transmission of pathogens directly through patient contact with the environment or indirectly through contamination of health care workers' hands and gloves.
Environmental cleaning is important for reducing microbial contamination of surfaces and subsequent risk for HAIs. Environmental cleaning is a complex, multifaceted process and involves the physical action of cleaning surfaces to remove organic and inorganic material, followed by application of a disinfectant, as well as monitoring strategies to ensure the appropriateness of these practices. In addition, contextual factors, such as management tools and organizational structure, and culture can affect the implementation and effectiveness of cleaning, disinfecting, and monitoring strategies. The goal of this review is to provide a systematic overview on environmental cleaning of hospital room surfaces to prevent HAIs. We focus on environmental cleaning of the hard surfaces most frequently touched by patients and health care workers, which are often called high-touch surfaces or objects. We also discuss key health care–associated pathogens for which there is the most evidence for environmental transmission, specifically methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and Clostridium difficile (5–8). Finally, we enumerate the evidence gaps in the literature and propose future research directions.
METHODS
This review is based on a protocol and technical brief produced by the ECRI Institute–Penn Medicine Evidence-based Practice Center for the Agency for Healthcare Research and Quality (AHRQ) (9). The protocol and final report are available at www.effectivehealthcare.ahrq.gov. Twelve key informants with expertise in infectious diseases, infection control, environmental disinfection, hospital epidemiology, microbiology, and management of environmental services staff in health care settings contributed to the protocol and report, including helping to refine the literature search, review limitations in the current evidence, and discuss potential directions for future research.
Data Sources and Search Strategy
We searched several databases and gray literature sources from 1 January 1990 through 4 February 2015. The complete set of databases searched and the search strategy is available in Appendix Tables 1 and 2 (available at www.annals.org).
Study Selection
Titles, abstracts, and full-text articles were screened in duplicate using the database Distiller SR (Evidence Partners). We included studies of any design that addressed our clinical questions; examined any inpatient wards (such as medicine, surgery, and critical care); addressed high-touch surfaces; evaluated environmental contamination, colonization, or infection with C. difficile, MRSA, or VRE or included several unspecified pathogens that were likely to include those infections; and were published in English. Studies were excluded if they took place exclusively in pediatric, ambulatory, operating room, or long-term care settings; addressed only soft, porous surfaces (such as linens or curtains) or transmission routes not inherent to the environmental reservoir (such as caregiver hands, stethoscopes, or invasive medical devices); examined products or processes not available in the United States or not currently being investigated; or were in vitro studies that did not collect samples from actual patient rooms.
Data Extraction and Synthesis
A standardized data extraction form was used by 1 reviewer to collect information on patient populations; pathogens; high-touch surfaces; type of cleaning, disinfecting, monitoring, and implementation strategy; study design; and study outcomes. A random sample of 25% of abstracted data was verified by another reviewer. Descriptions of cleaning/disinfecting and monitoring methods currently used in hospital settings are shown in Appendix Tables 3 and 4 (available at www.annals.org), respectively. We developed an evidence map to synthesize information on the type and depth of research available on cleaning, disinfecting, and monitoring processes. We also highlighted important knowledge gaps in the evidence base.
Role of the Funding Source
This project was funded by AHRQ. A representative from AHRQ served as a contracting officer's technical representative and provided technical assistance and feedback during the conduct of the evidence report. AHRQ did not directly participate in the literature search; determination of study eligibility criteria; data analysis or interpretation; or preparation, review, or approval of the manuscript for publication. This work was also supported in part by the National Institutes of Health, which had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
RESULTS
The literature searches yielded 80 clinical studies for inclusion in the review, 76 of which were primary studies and 4 of which were systematic reviews. The Appendix Figure (available at www.annals.org) shows the study selection process.
Of the 80 clinical studies, 49 (61%) (2 systematic reviews) focused on cleaning or disinfecting, 14 (18%) (2 systematic reviews) focused on monitoring, and 17 (21%) focused on implementation of cleaning or monitoring strategies. No conference abstracts presented within the past 2 years were identified for inclusion. Appendix Tables 5 and 6 (available at www.annals.org) describe identified clinical practice guidelines and clinical trials (ClinicalTrials.gov), respectively.
The primary setting for most studies was the intensive care unit. The most commonly examined hightouch objects included bed rails, call buttons, light switches, side or tray tables, and toilets, but the selection of high-touch objects across studies varied substantially.
Outcomes reported in the 76 primary studies were broadly categorized as surface contamination (such as bacterial burden, number of surfaces cleaned, and positive microbiological cultures), patient colonization (such as new VRE colonization), or infection rate (such as incidence rate expressed per 1000 patient days). Among the primary studies reporting pathogens of interest, the most commonly reported pathogen was C. difficile (n = 40), followed by MRSA (n = 30) and VRE (n = 30). Some studies evaluated several pathogens.
Evidence Map
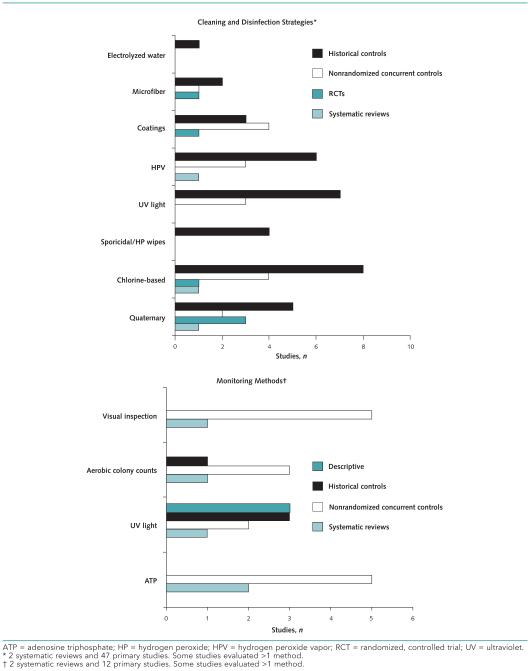
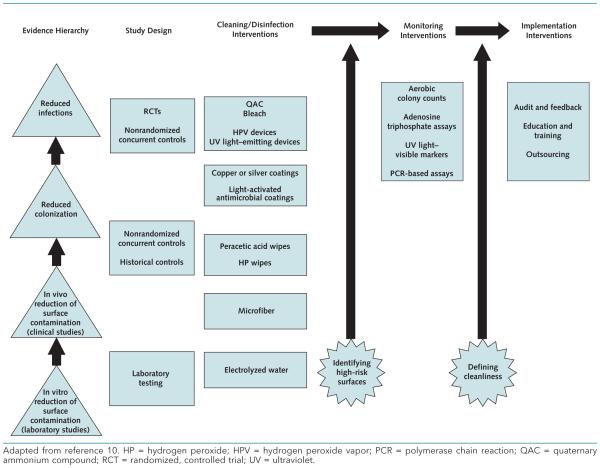
Figure 1 shows the number and research designs of published studies that address major categories of cleaning or disinfection strategies and monitoring methods, respectively. Figure 2 depicts evidence gaps that suggest high-impact areas for future research, as recommended by our key informants or indicated by our analysis of the current evidence base. The interventions are organized in a framework adapted from McDonald and Arduino's recently proposed “evidence hierarchy” for environmental infection control (10). This framework represents the progression of evidence for the effectiveness of environmental interventions, from laboratory studies that measure surface contamination; to clinical studies that assess contamination in realworld settings; to studies that address patient-centered outcomes, such as pathogen colonization and infection.
Figure 1.
Evidence map showing the number and study designs of published studies that address major categories of cleaning and disinfection strategies and monitoring methods.
Figure 2.
Evidence needs for future research in environmental cleaning.
Strategies for Environmental Cleaning
Forty-seven primary studies (11–57) and 2 systematic reviews (58, 59) focusing on cleaning and disinfecting were identified. Of the 47 primary studies, 27 (57%) were done in the United States and the remaining 19 were done in the United Kingdom, Australia, Sweden, Canada, Norway, and Italy. Studies were published between 1998 and September 2014; 28 (60%) were published since 2012, refiecting recently intensified interest in this topic.
Only 5 primary studies (11%) were randomized, controlled trials, and 1 (2%) was a randomized crossover study. Study durations ranged from 4 weeks to 43 months. Most studies (n = 31 [66%]) used a primary outcome of surface contamination. Only 16 studies (34%) reported pathogen colonization or infection rate as a primary outcome, and C. difficile was mostly commonly assessed.
Cleaning and disinfecting methods were generally categorized as surface cleaning or disinfecting, automated processes, or effectiveness of enhanced coatings or surfaces for disinfecting. Studies examining chemical disinfectants reported mixed findings, including reductions in VRE (51) and C. difficile rates (16, 20, 21, 54) with the use of bleach-based disinfectants; decreased C. difficile spore levels with the use of accelerated hydrogen peroxide (48); and ineffectiveness of a chlorine-based product in reducing C. difficile contamination and infection rates (14). Six studies integrating various wipes (such as hydrogen peroxide) into preventive strategies (15, 17, 25–28) reported positive outcomes, including sustained reductions in C. difficile infection rates (15, 27). Seventeen studies implementing no-touch methods (such as ultraviolet [UV] light and hydrogen peroxide vapor) reported positive findings (11, 13, 19, 29 –31, 39, 40, 42, 44 –46, 50, 52, 53, 56), and 3 of these studies specifically found reduced infection rates (29 –31). Seven of 8 studies (88%) evaluating enhanced coatings, such as copper-coated surfaces, reported positive findings (12, 32–37). Appendix Tables 7 and 8 (available at www.annals.org) describe the characteristics of cleaning and disinfecting studies.
Strategies for Monitoring Cleanliness
Two systematic reviews (60, 61) and 12 primary studies (62–73) evaluated strategies for monitoring environmental cleaning and disinfecting. The locations for 11 of the 12 primary studies were reported and included the United States (n = 7 [64%]), United Kingdom (n = 3 [27%]), and Canada (n = 1 [9%]). Studies were published from 2003 to 2013; 3 (25%) were published since 2012.
The most common study design was nonrandomized using concurrent control groups (n = 5 [42%]). Study durations ranged from 4 weeks to 8 months; 4 studies did not report duration. Eight studies (67%) assessed percentage of targets cleaned (62, 65–67) or cleaning rate (63, 64, 68, 69) as the primary outcome. Less commonly reported outcomes included microbial burden counts (71, 73), sensitivity to detect pathogens (70), and number of positive cultures (72). Four studies focused on a single pathogen (63, 66, 68, 72).
Fluorescent/UV surface markers and adenosine triphosphate bioluminescence were the most commonly evaluated monitoring methods. Six of the 8 studies (75%) mainly focusing on fluorescent/UV surface markers (64–69) concluded that these monitoring methods were useful and highly objective and helped achieve substantial improvements in cleaning and disinfecting practices. Visual observation was found to be inferior to various other monitoring methods in 4 of 5 primary studies (80%) (62, 63, 70 –73) and 1 review (100%) (61). Appendix Tables 7 and 9 (available at www.annals.org) describe the characteristics of monitoring studies.
Implementing Cleaning and Monitoring Strategies
Implementation Strategies
Seventeen primary studies focused specifically on implementing infection control interventions and contextual factors (74 –90). These studies were published between 2006 and September 2014; 9 (53%) were published since 2012. Most studies (n = 14 [82%]) were done in the United States, with remaining studies done in Australia and Canada.
Thirteen studies (76%) used historical controls, including before-and-after study designs (n = 9), and interrupted time series (n = 4). Three studies (18%) were nonrandomized using concurrent control groups, and 1 (6%) was an uncontrolled, descriptive study. Study length ranged from 8 weeks to 4 years. Most studies reported a primary outcome of surface contamination. Only 2 studies (12%) reported pathogen acquisition as a primary outcome (83, 90). Clinical infection was reported as a primary and secondary outcome in 3 (80, 83, 90) and 2 (75, 76) studies, respectively. With regard to pathogen type, C. difficile and VRE were the primary focus of 3 (75, 80, 81) and 2 (85, 90) studies, respectively. The remaining studies focused on at least 2 pathogens of interest.
Three studies (18%) (75, 76, 80) used multicomponent strategies to prevent C. difficile infections and reported positive findings. Five studies (64, 76, 81, 84, 87) reporting on sustainability of preventive strategies described ongoing education, direct feedback, and commitment and flexibility of administrative leaders as key components to successful implementation.
Appendix Table 10 (available at www.annals.org) describes the characteristics of the implementation studies.
Contextual Factors
Contextual factors for implementation strategies examined in the 76 primary studies and identified by key informants included structural organizational characteristics, such as outsourcing of environmental services (80, 91) and organization of environmental services within the administrative hierarchy of a hospital. External factors that affect environmental cleaning efforts included adherence to “evidence-based policies and procedures” from various organizations (such as the Centers for Medicare & Medicaid Services and The Joint Commission). A positive patient safety culture that fosters collaboration and respect among clinical and support services staff, as well as between supervisors and front-line personnel, were examined in 5 studies (77, 80, 84, 87, 92). Implementation and management tools were identified as key contextual factors and include staff education and training, dedicated training time, use of internal audit and feedback, and presence of internal or external persons responsible for implementation. Of the 24 studies (32%) that integrated implementation tools, education was reported as a key component in most (n = 23 [96%]); 5 studies (21%) specifically reported on training staff (13–15, 77, 84) and 5 additional studies (21%), all published since 2012, described use of audits (14, 17, 81, 82, 84).
DISCUSSION
Contamination of high-touch environmental surfaces plays an important role in transmission of pathogens in the acute care hospital setting. Increasing attention has been directed toward the importance of environmental cleaning and disinfecting in the prevention of HAIs. We reviewed 4 systematic reviews and 76 primary studies of environmental cleaning. We found considerable diversity with regard to both study design and cleaning/disinfecting and monitoring methods examined across studies, as well as many limitations in the evidence base. There was a lack of direct, rigorous comparative studies of various methods, with only 5 studies designed as randomized, controlled trials. Our review of the literature also highlighted a limited focus on patient-centered outcomes, such as patient colonization or infection. Instead, surface contamination was the most commonly reported outcome.
The results of these studies, as well as synthesis of key informant input, suggest that evaluating the clinical effectiveness of cleaning and disinfecting methods is challenging. A major limitation is the gap between optimized use of surface cleaning or disinfecting agents in studies and practical implementation in real-world settings (such as appropriate dwell time and type of surface targeted). Manufacturers provide recommendations for proper use of their products, but most studies do not report thoroughness of cleaning or adherence to disinfectant dwell time; this information also remains largely unknown in daily practice. An important related concern is uncertainty by end users about the applicability of some manufacturer recommendations. Guidance that accompanies products may be based on laboratory testing under ideal conditions rather than clinical settings. Recommendations may also be developed based on certain types of pathogens, but users may choose to implement a product or technology for broader effects. Few studies directly compared the effectiveness of different methods; instead, many used before-and-after study designs to assess the effect of a single disinfecting method.
Another challenge to interpreting the results of the current evidence base is determining the specific effect of environmental cleaning and disinfecting interventions in the context of multicomponent infection prevention strategies (93). Infection prevention comprises many critical components in addition to hard surface cleaning, including sterilization of instruments, implementation of appropriate isolation precautions, and proper hand hygiene. These and other elements may sometimes be included as interventions within a larger infection prevention strategy, limiting the ability to discern the specific effect of any single approach. These factors also have the potential to modify the effectiveness of environmental cleaning interventions. Considerable uncertainty also remains about which surfaces, including high-touch objects, should be targeted for cleaning and disinfecting.
Limitations in the evidence base for monitoring methods were also identified, including the lack of direct, rigorous comparative studies of various technologies. Key informants noted that hospitals may be reluctant to adopt such methods as adenosine triphosphate and UV/fluorescent surface markers given the relative absence of data. Another important limitation in the literature is the lack of consensus for thresholds of cleanliness. Specifically, although various cleanliness thresholds with the use of adenosine triphosphate and certain microbiological methods were described across studies, there is no established benchmark for defining a surface as “clean.” The real-world goal of environmental cleaning and disinfecting should be to reduce risk for pathogen transmission rather than establishing a continuously sterile surface. Benchmarks for surface cleanliness that correlate with decreases in pathogen acquisition should therefore be determined. As with studies evaluating cleaning and disinfecting methods, studies on monitoring methods demonstrated considerable variation in high-touch objects selected for evaluation, making it challenging to determine which surfaces are at greatest risk for microbial contamination and pathogen transmission.
Our review has important limitations. First, it provides only an inventory of available evidence and does not appraise the risk of bias of individual studies or provide overall ratings of the strength of evidence for each intervention and outcome examined. Second, the review was restricted to studies of C. difficile, MRSA, and VRE; thus, our findings may not be fully generalizable to interventions aimed at reducing infections due to other organisms (such as gram-negative pathogens). Future research should seek to review the evidence base for other pathogens. Further, many of the studies included in this review were undertaken during outbreaks and may not be representative of the effect of cleaning/disinfecting and monitoring in nonoutbreak settings.
Future research on environmental cleaning and disinfecting to reduce HAIs should address the following key questions: What surfaces, including high-touch objects, should be cleaned and disinfected? How should surfaces be cleaned and disinfected, and what is the comparative effectiveness of different methods? How should cleaning and disinfecting be monitored and measured, and what would be appropriate benchmarks for cleanliness and reduced risk for pathogen transmission? How should interventions be implemented, including in-depth study of facilitators and barriers to real-world implementation?
In summary, our review of the literature indicates an increased interest in environmental cleaning and disinfecting for the prevention of HAIs. However, there are many limitations in the current evidence base. Future research on environmental cleaning that addresses these limitations and evidence gaps will be critical for informing real-world interventions for reducing the risk for HAIs in the hospital setting.
Supplementary Material
Key Summary Points.
Environmental cleaning is an important component of a multifaceted infection control strategy to prevent health care–associated infections.
Emerging technologies have led to increased interest in evaluating environmental cleaning, disinfecting, and monitoring in the acute care hospital setting.
A major limitation of the evidence base is the lack of comparative studies addressing the relative effectiveness of various cleaning, disinfecting, and monitoring strategies.
Few studies assess clinical, patient-centered outcomes, including patient colonization and health care–associated infection rates.
Future studies are needed that directly compare newer disinfecting and monitoring methods, assess the effect of contextual factors on implementation, and evaluate patient-centered outcomes.
Acknowledgment
The authors thank the following persons at the ECRI Institute for their assistance with the preparation of the technical brief: Michele Datko, MS; James Davis, MSN, RN; David Snyder, PhD; Gina Giradi, MS; Luke A. Petosa, MSc; Joann Fontanarosa, PhD; Michael Phillips; Jennifer Dell’Aquila Maslin; Helen Dunn; Lydia Dharia; and Evidence-based Practice Center Director, Karen Schoelles, MD, SM. They also thank the following persons, who served as key informants on the associated technical brief: Michelle Alfa, PhD; Philip Carling, MD; Patti Costello; Mia Gonzales Dean, MBA, MS; Curtis Donskey, MD; Rich Feczko; Elaine Larson, PhD, RN; Luis Ostrosky-Zeichner, MD; William A. Rutala, PhD, MS, MPH; Daniel Schwartz, MD, MBA; and James P. Steinberg, MD. The authors also thank the following persons, who served as peer reviewers on the associated technical brief: Dottie Borton, RN, BSN, CIC; Mary K. Hayden, MD; L. Clifford McDonald, MD; Gina Pugliese, RN, MS; Gary A. Roselle, MD; and Robert A. Weinstein, MD. They also acknowledge Kim Marie Wittenberg, MA, who served as the AHRQ Task Order Officer, and Timothy J. Wilt, MD, MPH, at the Minnesota Evidence-based Practice Center, who served as the Associate Editor for the associated technical brief.
Grant Support: This project was funded under AHRQ (contract HHSA 290-2012-00011-I). This topic was nominated by a member of the 3M Hospital Hygiene Global Advisory Board on behalf of the Board. This work was also supported in part by the National Institutes of Health (K01-AI103028; Dr. Han).
Footnotes
From Perelman School of Medicine, University of Pennsylvania, and Center for Evidence-based Practice, University of Pennsylvania Health System, Philadelphia, and ECRI Institute–Penn Medicine Evidence-based Practice Center, Plymouth Meeting, Pennsylvania.
Disclaimer: This article is based on research conducted by the ECRI Institute–Penn Medicine Evidence-based Practice Center under contract to AHRQ, U.S. Department of Health and Human Services. The findings and conclusions in this document are those of the authors, who are responsible for its contents and should not be construed as endorsement by AHRQ or the U.S. Department of Health and Human Services.
Disclosures: Dr. Han, Ms. Sullivan, Mr. Leas, Dr. Pegues, Ms. Kaczmarek, and Dr. Umscheid report grants from AHRQ during the conduct of the study. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?ms Num=M15-1192.
Current author addresses and author contributions are available at www.annals.org.
Author Contributions: Conception and design: J.H. Han, B.F. Leas, D.A. Pegues, C.A. Umscheid.
Analysis and interpretation of the data: J.H. Han, N. Sullivan, B.F. Leas, D.A. Pegues, C.A. Umscheid.
Drafting of the article: J.H. Han, N. Sullivan, D.A. Pegues.
Critical revision of the article for important intellectual content: J.H. Han, B.F. Leas, D.A. Pegues, C.A. Umscheid.
Final approval of the article: J.H. Han, N. Sullivan, B.F. Leas, D.A. Pegues, J.L. Kaczmarek, C.A. Umscheid.
Provision of study materials or patients: B.F. Leas, C.A. Umscheid.
Obtaining of funding: B.F. Leas, C.A. Umscheid.
Administrative, technical, or logistic support: N. Sullivan, B.F. Leas, J.L. Kaczmarek, C.A. Umscheid.
Collection and assembly of data: J.H. Han, N. Sullivan, B.F. Leas, D.A. Pegues.
Contributor Information
Dr. Jennifer H. Han, Division of Infectious Diseases, Department of Medicine, Hospital of the University of Pennsylvania, 811 Blockley Hall, 423 Guardian Drive, Philadelphia, PA 19104.
Ms. Nancy Sullivan, ECRI Institute Evidence-based Practice Center and Health Technology Assessment Group, ECRI Institute Headquarters, 5200 Butler Pike, Plymouth Meeting, PA 19462-1298.
Mr. Brian F. Leas, Center for Evidence-based Practice, University of Pennsylvania Health System, 3535 Market Street, Suite 50, Philadelphia, PA 19104.
Dr. David A. Pegues, Department of Healthcare Epidemiology, Infection Prevention, and Control, Hospital of the University of Pennsylvania, 3400 Spruce Street, Ground Founders, Philadelphia, PA 19104.
Ms. Janice L. Kaczmarek, ECRI Institute Evidence-based Practice Center and Health Technology Assessment Group, ECRI Institute Headquarters, 5200 Butler Pike, Plymouth Meeting, PA 19462-1298.
Dr. Craig A. Umscheid, Center for Evidence-based Practice, University of Pennsylvania Health System, 3535 Market Street, Suite 50, Philadelphia, PA 19104.
References
- 1.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–208. doi: 10.1056/NEJMoa1306801. [PMID: 24670166] doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagenvoort JH, Sluijsmans W, Penders RJ. Better environmental survival of outbreak vs. sporadic MRSA isolates. J Hosp Infect. 2000;45:231–4. doi: 10.1053/jhin.2000.0757. [PMID: 10896803] [DOI] [PubMed] [Google Scholar]
- 3.Wendt C, Wiesenthal B, Dietz E, Ruüden H. Survival of vancomycin-resistant and vancomycin-susceptible enterococci on dry surfaces. J Clin Microbiol. 1998;36:3734–6. doi: 10.1128/jcm.36.12.3734-3736.1998. [PMID: 9817912] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [PMID: 16914034] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acineto-bacter species. Am J Infect Control. 2010;38:S25–33. doi: 10.1016/j.ajic.2010.04.196. [PMID: 20569853] doi:10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- 6.Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet Infect Dis. 2008;8:101–13. doi: 10.1016/S1473-3099(07)70241-4. [PMID: 17974481] [DOI] [PubMed] [Google Scholar]
- 7.Drees M, Snydman DR, Schmid CH, Barefoot L, Hansjosten K, Vue PM, et al. Prior environmental contamination increases the risk of acquisition of vancomycin-resistant enterococci. Clin Infect Dis. 2008;46:678–85. doi: 10.1086/527394. [PMID: 18230044] doi:10.1086/527394. [DOI] [PubMed] [Google Scholar]
- 8.Weber DJ, Anderson D, Rutala WA. The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis. 2013;26:338–44. doi: 10.1097/QCO.0b013e3283630f04. [PMID: 23743816] doi:10.1097/QCO.0b013 e3283630f04. [DOI] [PubMed] [Google Scholar]
- 9.Leas BF, Sullivan N, Han JH, Pegues DA, Kaczmarek J, Umscheid CA. (Prepared by the ECRI Institute–Penn Medicine Evidence-based Practice Center under contract HHSA290-2012-00011-I) Agency for Healthcare Research and Quality; Rockville, MD: 2015. Environmental Cleaning for the Prevention of Healthcare-Associated Infections (HAI) [PubMed] [Google Scholar]
- 10.McDonald LC, Arduino M. Editorial commentary: climbing the evidentiary hierarchy for environmental infection control [Editorial] Clin Infect Dis. 2013;56:36–9. doi: 10.1093/cid/cis845. [PMID: 23042965] doi:10.1093 /cid/cis845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jinadatha C, Quezada R, Huber TW, Williams JB, Zeber JE, Copeland LA. Evaluation of a pulsed-xenon ultraviolet room disinfection device for impact on contamination levels of methicillin-resistant Staphylococcus aureus. BMC Infect Dis. 2014;14:187. doi: 10.1186/1471-2334-14-187. [PMID: 24708734] doi:10.1186/1471-2334-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt MG, Attaway HH, Sharpe PA, John J, Jr, Sepkowitz KA, Morgan A, et al. Sustained reduction of microbial burden on common hospital surfaces through introduction of copper. J Clin Microbiol. 2012;50:2217–23. doi: 10.1128/JCM.01032-12. [PMID: 22553242] doi:10.1128/JCM.01032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell BG, Digney W, Locket P, Dancer SJ. Controlling methicillin-resistant Staphylococcus aureus (MRSA) in a hospital and the role of hydrogen peroxide decontamination: an interrupted time series analysis. BMJ Open. 2014;4:e004522. doi: 10.1136/bmjopen-2013-004522. [PMID: 24747791] doi: 10.1136/bmjopen-2013-004522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldenberg SD, Patel A, Tucker D, French GL. Lack of enhanced effect of a chlorine dioxide-based cleaning regimen on environmental contamination with Clostridium difficile spores. J Hosp Infect. 2012;82:64–7. doi: 10.1016/j.jhin.2012.06.004. [PMID: 22795136] doi:10.1016/j.jhin.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Carter Y, Barry D. Tackling C. difficile with environmental cleaning. Nurs Times. 2011;107:22–5. [PMID: 21998939] [PubMed] [Google Scholar]
- 16.Whitaker J, Brown BS, Vidal S, Calcaterra M. Designing a protocol that eliminates Clostridium difficile: a collaborative venture. Am J Infect Control. 2007;35:310–4. doi: 10.1016/j.ajic.2006.08.010. [PMID: 17577477] [DOI] [PubMed] [Google Scholar]
- 17.Friedman ND, Walton AL, Boyd S, Tremonti C, Low J, Styles K, et al. The effectiveness of a single-stage versus traditional three-staged protocol of hospital disinfection at eradicating vancomycin-resistant enterococci from frequently touched surfaces. Am J Infect Control. 2013;41:227–31. doi: 10.1016/j.ajic.2012.03.021. [PMID: 22981721] doi:10.1016/j.ajic.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Gillespie E, Wilson J, Lovegrove A, Scott C, Abernethy M, Kot-sanas D, et al. Environment cleaning without chemicals in clinical settings. Am J Infect Control. 2013;41:461–3. doi: 10.1016/j.ajic.2012.07.003. [PMID: 23177456] doi: 10.1016/j.ajic.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Sitzlar B, Deshpande A, Fertelli D, Kundrapu S, Sethi AK, Donskey CJ. An environmental disinfection odyssey: evaluation of sequential interventions to improve disinfection of Clostridium difficile isolation rooms. Infect Control Hosp Epidemiol. 2013;34:459–65. doi: 10.1086/670217. [PMID: 23571361] doi:10.1086/670217. [DOI] [PubMed] [Google Scholar]
- 20.Hacek DM, Ogle AM, Fisher A, Robicsek A, Peterson LR. Significant impact of terminal room cleaning with bleach on reducing nosocomial Clostridium difficile. Am J Infect Control. 2010;38:350–3. doi: 10.1016/j.ajic.2009.11.003. [PMID: 20123150] doi:10.1016/j.ajic.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 21.McMullen KM, Zack J, Coopersmith CM, Kollef M, Dubberke E, Warren DK. Use of hypochlorite solution to decrease rates of Clostridium difficile-associated diarrhea. Infect Control Hosp Epidemiol. 2007;28:205–7. doi: 10.1086/511791. [PMID: 17265404] [DOI] [PubMed] [Google Scholar]
- 22.De Lorenzi S, Finzi G, Parmiggiani R, Cugini P, Cacciari P, Salva-torelli G. Comparison of fioor sanitation methods. J Hosp Infect. 2006;62:346–8. doi: 10.1016/j.jhin.2005.09.021. [PMID: 16376456] [DOI] [PubMed] [Google Scholar]
- 23.Schmidt MG, Anderson T, Attaway HH, 3rd, Fairey S, Kennedy C, Salgado CD. Patient environment microbial burden reduction: a pilot study comparison of 2 terminal cleaning methods. Am J Infect Control. 2012;40:559–61. doi: 10.1016/j.ajic.2011.07.013. [PMID: 21981792] doi:10.1016/j.ajic.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Sjoüberg M, Eriksson M, Andersson J, Noreén T. Transmission of Clostridium difficile spores in isolation room environments and through hospital beds. APMIS. 2014;122:800–3. doi: 10.1111/apm.12218. [PMID: 24475890] doi:10.1111/apm.12218. [DOI] [PubMed] [Google Scholar]
- 25.Hess AS, Shardell M, Johnson JK, Thom KA, Roghmann MC, Netzer G, et al. A randomized controlled trial of enhanced cleaning to reduce contamination of healthcare worker gowns and gloves with multidrug-resistant bacteria. Infect Control Hosp Epidemiol. 2013;34:487–93. doi: 10.1086/670205. [PMID: 23571365] doi:10.1086/670205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyce JM, Havill NL. Evaluation of a new hydrogen peroxide wipe disinfectant. Infect Control Hosp Epidemiol. 2013;34:521–3. doi: 10.1086/670212. [PMID: 23571371] doi:10.1086/670212. [DOI] [PubMed] [Google Scholar]
- 27.Orenstein R, Aronhalt KC, McManus JE, Jr, Fedraw LA. A targeted strategy to wipe out Clostridium difficile. Infect Control Hosp Epidemiol. 2011;32:1137–9. doi: 10.1086/662586. [PMID: 22011546] doi:10.1086/662586. [DOI] [PubMed] [Google Scholar]
- 28.Wiemken TL, Curran DR, Pacholski EB, Kelley RR, Abdelfattah RR, Carrico RM, et al. The value of ready-to-use disinfectant wipes: compliance, employee time, and costs. Am J Infect Control. 2014;42:329–30. doi: 10.1016/j.ajic.2013.09.031. [PMID: 24581022] doi:10.1016/j.ajic.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 29.Levin J, Riley LS, Parrish C, English D, Ahn S. The effect of portable pulsed xenon ultraviolet light after terminal cleaning on hospital-associated Clostridium difficile infection in a community hospital. Am J Infect Control. 2013;41:746–8. doi: 10.1016/j.ajic.2013.02.010. [PMID: 23685092] doi:10.1016/j.ajic.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Haas JP, Menz J, Dusza S, Montecalvo MA. Implementation and impact of ultraviolet environmental disinfection in an acute care setting. Am J Infect Control. 2014;42:586–90. doi: 10.1016/j.ajic.2013.12.013. [PMID: 24837107] doi:10.1016/j.ajic.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Manian FA, Griesnauer S, Bryant A. Implementation of hospital-wide enhanced terminal cleaning of targeted patient rooms and its impact on endemic Clostridium difficile infection rates. Am J Infect Control. 2013;41:537–41. doi: 10.1016/j.ajic.2012.06.014. [PMID: 23219675] doi:10.1016/j.ajic.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Salgado CD, Sepkowitz KA, John JF, Cantey JR, Attaway HH, Freeman KD, et al. Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect Control Hosp Epidemiol. 2013;34:479–86. doi: 10.1086/670207. [PMID: 23571364] doi:10.1086/670207. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt MG, Attaway HH, Fairey SE, Steed LL, Michels HT, Salgado CD. Copper continuously limits the concentration of bacteria resident on bed rails within the intensive care unit. Infect Control Hosp Epidemiol. 2013;34:530–3. doi: 10.1086/670224. [PMID: 23571374] doi:10.1086/670224. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton D, Foster A, Ballantyne L, Kingsmore P, Bedwell D, Hall TJ, et al. Performance of ultramicrofibre cleaning technology with or without addition of a novel copper-based biocide. J Hosp Infect. 2010;74:62–71. doi: 10.1016/j.jhin.2009.08.006. [PMID: 19819583] doi:10.1016/j.jhin.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Casey AL, Adams D, Karpanen TJ, Lambert PA, Cookson BD, Nightingale P, et al. Role of copper in reducing hospital environment contamination. J Hosp Infect. 2010;74:72–7. doi: 10.1016/j.jhin.2009.08.018. [PMID: 19931938] doi: 10.1016/j.jhin.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Hedin G, Rynbaück J, Loreé B. Reduction of bacterial surface contamination in the hospital environment by application of a new product with persistent effect. J Hosp Infect. 2010;75:112–5. doi: 10.1016/j.jhin.2010.02.007. [PMID: 20381907] doi:10.1016/j.jhin.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Karpanen TJ, Casey AL, Lambert PA, Cookson BD, Nightingale P, Miruszenko L, et al. The antimicrobial efficacy of copper alloy furnishing in the clinical environment: a crossover study. Infect Control Hosp Epidemiol. 2012;33:3–9. doi: 10.1086/663644. [PMID: 22173515] doi:10.1086 /663644. [DOI] [PubMed] [Google Scholar]
- 38.Byers KE, Durbin LJ, Simonton BM, Anglim AM, Adal KA, Farr BM. Disinfection of hospital rooms contaminated with vancomycin- resistant Enterococcus faecium. Infect Control Hosp Epidemiol. 1998;19:261–4. doi: 10.1086/647806. [PMID: 9605276] [DOI] [PubMed] [Google Scholar]
- 39.Best EL, Parnell P, Thirkell G, Verity P, Copland M, Else P, et al. Effectiveness of deep cleaning followed by hydrogen peroxide decontamination during high Clostridium difficile infection incidence. J Hosp Infect. 2014;87:25–33. doi: 10.1016/j.jhin.2014.02.005. [PMID: 24746230] doi:10.1016/j.jhin.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Anderson DJ, Gergen MF, Smathers E, Sexton DJ, Chen LF, Weber DJ, et al. Decontamination of targeted pathogens from patient rooms using an automated ultraviolet-C-emitting device. Infect Control Hosp Epidemiol. 2013;34:466–71. doi: 10.1086/670215. [PMID: 23571362] doi:10.1086/670215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sigler V, Hensley S. Persistence of mixed staphylococci assemblages following disinfection of hospital room surfaces. J Hosp Infect. 2013;83:253–6. doi: 10.1016/j.jhin.2012.12.009. [PMID: 23374288] doi:10.1016/j.jhin.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Passaretti CL, Otter JA, Reich NG, Myers J, Shepard J, Ross T, et al. An evaluation of environmental decontamination with hydrogen peroxide vapor for reducing the risk of patient acquisition of multidrug-resistant organisms. Clin Infect Dis. 2013;56:27–35. doi: 10.1093/cid/cis839. [PMID: 23042972] doi:10.1093/cid/cis839. [DOI] [PubMed] [Google Scholar]
- 43.Kundrapu S, Sunkesula V, Jury LA, Sitzlar BM, Donskey CJ. Daily disinfection of high-touch surfaces in isolation rooms to reduce contamination of healthcare workers' hands. Infect Control Hosp Epidemiol. 2012;33:1039–42. doi: 10.1086/667730. [PMID: 22961024] doi:10.1086/667730. [DOI] [PubMed] [Google Scholar]
- 44.Sexton JD, Tanner BD, Maxwell SL, Gerba CP. Reduction in the microbial load on high-touch surfaces in hospital rooms by treatment with a portable saturated steam vapor disinfection system. Am J Infect Control. 2011;39:655–62. doi: 10.1016/j.ajic.2010.11.009. [PMID: 21641089] doi:10.1016/j.ajic.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Chan HT, White P, Sheorey H, Cocks J, Waters MJ. Evaluation of the biological efficacy of hydrogen peroxide vapour decontamination in wards of an Australian hospital. J Hosp Infect. 2011;79:125–8. doi: 10.1016/j.jhin.2011.06.009. [PMID: 21824681] doi:10.1016/j.jhin.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Boyce JM, Havill NL, Moore BA. Terminal decontamination of patient rooms using an automated mobile UV light unit. Infect Control Hosp Epidemiol. 2011;32:737–42. doi: 10.1086/661222. [PMID: 21768755] doi:10.1086/661222. [DOI] [PubMed] [Google Scholar]
- 47.Wilson AP, Smyth D, Moore G, Singleton J, Jackson R, Gant V, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med. 2011;39:651–8. doi: 10.1097/CCM.0b013e318206bc66. [PMID: 21242793] doi:10.1097/CCM.0b013e318206bc66. [DOI] [PubMed] [Google Scholar]
- 48.Alfa MJ, Lo E, Wald A, Dueck C, DeGagne P, Harding GK. Improved eradication of Clostridium difficile spores from toilets of hospitalized patients using an accelerated hydrogen peroxide as the cleaning agent. BMC Infect Dis. 2010;10:268. doi: 10.1186/1471-2334-10-268. [PMID: 20843348] doi: 10.1186/1471-2334-10-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersen BM, Rasch M, Kvist J, Tollefsen T, Lukkassen R, Sandvik L, et al. Floor cleaning: effect on bacteria and organic materials in hospital rooms. J Hosp Infect. 2009;71:57–65. doi: 10.1016/j.jhin.2008.09.014. [PMID: 19013671] doi: 10.1016/j.jhin.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 50.Mahida N, Vaughan N, Boswell T. First UK evaluation of an automated ultraviolet-C room decontamination device (Tru-D™) J Hosp Infect. 2013;84:332–5. doi: 10.1016/j.jhin.2013.05.005. [PMID: 23846236] doi:10.1016/j.jhin.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Grabsch EA, Mahony AA, Cameron DR, Martin RD, Heland M, Davey P, et al. Significant reduction in vancomycin-resistant entero-coccus colonization and bacteraemia after introduction of a bleach-based cleaning-disinfection programme. J Hosp Infect. 2012;82:234–42. doi: 10.1016/j.jhin.2012.08.010. [PMID: 23103245] doi:10.1016/j.jhin.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Havill NL, Moore BA, Boyce JM. Comparison of the microbiological efficacy of hydrogen peroxide vapor and ultraviolet light processes for room decontamination. Infect Control Hosp Epidemiol. 2012;33:507–12. doi: 10.1086/665326. [PMID: 22476278] doi:10.1086/665326. [DOI] [PubMed] [Google Scholar]
- 53.Rutala WA, Gergen MF, Weber DJ. Room decontamination with UV radiation. Infect Control Hosp Epidemiol. 2010;31:1025–9. doi: 10.1086/656244. [PMID: 20804377] doi:10.1086/656244. [DOI] [PubMed] [Google Scholar]
- 54.Wilcox MH, Fawley WN, Wigglesworth N, Parnell P, Verity P, Freeman J. Comparison of the effect of detergent versus hypochlo-rite cleaning on environmental contamination and incidence of Clostridium difficile infection. J Hosp Infect. 2003;54:109–14. doi: 10.1016/s0195-6701(02)00400-0. [PMID: 12818583] [DOI] [PubMed] [Google Scholar]
- 55.Boyce JM, Havill NL, Guercia KA, Schweon SJ, Moore BA. Evaluation of two organosilane products for sustained antimicrobial activity on high-touch surfaces in patient rooms. Am J Infect Control. 2014;42:326–8. doi: 10.1016/j.ajic.2013.09.009. [PMID: 24406256] doi:10.1016/j.ajic.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Nerandzic MM, Cadnum JL, Eckart KE, Donskey CJ. Evaluation of a hand-held farultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens. BMC Infect Dis. 2012;12:120. doi: 10.1186/1471-2334-12-120. [PMID: 22591268] doi:10.1186/1471-2334-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart M, Bogusz A, Hunter J, Devanny I, Yip B, Reid D, et al. Evaluating use of neutral electrolyzed water for cleaning near-patient surfaces. Infect Control Hosp Epidemiol. 2014;35:1505–10. doi: 10.1086/678595. [PMID: 25419773] doi:10.1086/678595. [DOI] [PubMed] [Google Scholar]
- 58.Falagas ME, Thomaidis PC, Kotsantis IK, Sgouros K, Samonis G, Karageorgopoulos DE. Airborne hydrogen peroxide for disinfection of the hospital environment and infection control: a systematic review. J Hosp Infect. 2011;78:171–7. doi: 10.1016/j.jhin.2010.12.006. [PMID: 21392848] doi:10.1016 /j.jhin.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Dettenkofer M, Wenzler S, Amthor S, Antes G, Motschall E, Daschner FD. Does disinfection of environmental surfaces infiuence nosocomial infection ratesfi A systematic review. Am J Infect Control. 2004;32:84–9. doi: 10.1016/j.ajic.2003.07.006. [PMID: 15057199] [DOI] [PubMed] [Google Scholar]
- 60.Amodio E, Dino C. Use of ATP bioluminescence for assessing the cleanliness of hospital surfaces: a review of the published literature (1990 –2012) J Infect Public Health. 2014;7:92–8. doi: 10.1016/j.jiph.2013.09.005. [PMID: 24231159] doi:10.1016/j.jiph.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Mitchell BG, Wilson F, Dancer SJ, McGregor A. Methods to evaluate environmental cleanliness in healthcare facilities. Healthc Infect. 2013;18:23–30. [Google Scholar]
- 62.Snyder GM, Holyoak AD, Leary KE, Sullivan BF, Davis RB, Wright SB. Effectiveness of visual inspection compared with non-microbiologic methods to determine the thoroughness of post-discharge cleaning. Antimicrob Resist Infect Control. 2013;2:26. doi: 10.1186/2047-2994-2-26. [PMID: 24088298] doi:10.1186/2047-2994-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mulvey D, Redding P, Robertson C, Woodall C, Kingsmore P, Bedwell D, et al. Finding a benchmark for monitoring hospital clean-liness. J Hosp Infect. 2011;77:25–30. doi: 10.1016/j.jhin.2010.08.006. [PMID: 21129820] doi:10.1016/j.jhin.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Munoz-Price LS, Ariza-Heredia E, Adams S, Olivier M, Francois L, Socarras M, et al. Use of UV powder for surveillance to improve environmental cleaning. Infect Control Hosp Epidemiol. 2011;32:283–5. doi: 10.1086/658666. [PMID: 21460514] doi:10.1086/658666. [DOI] [PubMed] [Google Scholar]
- 65.Carling PC, Parry MF, Bruno-Murtha LA, Dick B. Improving environmental hygiene in 27 intensive care units to decrease multidrug-resistant bacterial transmission. Crit Care Med. 2010;38:1054–9. doi: 10.1097/CCM.0b013e3181cdf705. [PMID: 20081531] doi:10.1097/CCM.0b013e3181cdf705. [DOI] [PubMed] [Google Scholar]
- 66.Blue J, O’Neill C, Speziale P, Revill J, Ramage L, Ballantyne L. Use of a fiuorescent chemical as a quality indicator for a hospital cleaning program. Can J Infect Control. 2008;23:216–9. [PMID: 19350998] [PubMed] [Google Scholar]
- 67.Carling PC, Briggs J, Hylander D, Perkins J. An evaluation of patient area cleaning in 3 hospitals using a novel targeting methodology. Am J Infect Control. 2006;34:513–9. doi: 10.1016/j.ajic.2005.09.001. [PMID: 17015157] [DOI] [PubMed] [Google Scholar]
- 68.Alfa MJ, Dueck C, Olson N, Degagne P, Papetti S, Wald A, et al. UV-visible marker confirms that environmental persistence of Clostridium difficile spores in toilets of patients with C. difficile-associated diarrhea is associated with lack of compliance with cleaning protocol.e. BMC Infect Dis. 2008;8:64. doi: 10.1186/1471-2334-8-64. [PMID: 18474086] doi:10.1186 /1471-2334-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carling PC, Parry MF, Von Beheren SM. Healthcare Environmental Hygiene Study Group. Identifying opportunities to enhance environmental cleaning in 23 acute care hospitals. Infect Control Hosp Epidemiol. 2008;29:1–7. doi: 10.1086/524329. [PMID: 18171180] doi:10.1086/524329. [DOI] [PubMed] [Google Scholar]
- 70.Luick L, Thompson PA, Loock MH, Vetter SL, Cook J, Guerrero DM. Diagnostic assessment of different environmental cleaning monitoring methods. Am J Infect Control. 2013;41:751–2. doi: 10.1016/j.ajic.2012.09.019. [PMID: 23380380] doi:10.1016/j.ajic.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 71.Smith PW, Gibbs S, Sayles H, Hewlett A, Rupp ME, Iwen PC. Observations on hospital room contamination testing. Healthc Infect. 2013;18:10–3. [Google Scholar]
- 72.Al-Hamad A, Maxwell S. How clean is clean? Proposed methods for hospital cleaning assessment. J Hosp Infect. 2008;70:328–34. doi: 10.1016/j.jhin.2008.08.006. [PMID: 18848370] doi:10.1016/j.jhin.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 73.Malik RE, Cooper RA, Griffith CJ. Use of audit tools to evaluate the efficacy of cleaning systems in hospitals. Am J Infect Control. 2003;31:181–7. doi: 10.1067/mic.2003.34. [PMID: 12734526] [DOI] [PubMed] [Google Scholar]
- 74.Branch-Elliman W, Robillard E, McCarthy G, Jr, Gupta K. Direct feedback with the ATP luminometer as a process improvement tool for terminal cleaning of patient rooms. Am J Infect Control. 2014;42:195–7. doi: 10.1016/j.ajic.2013.08.012. [PMID: 24485376] doi:10.1016/j.ajic.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 75.Koll BS, Ruiz RE, Calfee DP, Jalon HS, Stricof RL, Adams A, et al. Prevention of hospital-onset Clostridium difficile infection in the New York metropolitan region using a collaborative intervention model. J Healthc Qual. 2014;36:35–45. doi: 10.1111/jhq.12002. [PMID: 23294050] doi:10.1111/jhq.12002. [DOI] [PubMed] [Google Scholar]
- 76.Ramphal L, Suzuki S, McCracken IM, Addai A. Improving hospital staff compliance with environmental cleaning behavior. Proc (Bayl Univ Med Cent) 2014;27:88–91. doi: 10.1080/08998280.2014.11929065. [PMID: 24688183] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rupp ME, Fitzgerald T, Sholtz L, Lyden E, Carling P. Maintain the gain: program to sustain performance improvement in environmental cleaning. Infect Control Hosp Epidemiol. 2014;35:866–8. doi: 10.1086/676873. [PMID: 24915215] doi:10.1086/676873. [DOI] [PubMed] [Google Scholar]
- 78.Rupp ME, Huerta T, Cavalieri RJ, Lyden E, Van Schooneveld T, Carling P, et al. Optimum outlier model for potential improvement of environmental cleaning and disinfection. Infect Control Hosp Epidemiol. 2014;35:721–3. doi: 10.1086/676431. [PMID: 24799650] doi:10.1086/676431. [DOI] [PubMed] [Google Scholar]
- 79.Smith PW, Beam E, Sayles H, Rupp ME, Cavalieri RJ, Gibbs S, et al. Impact of adenosine triphosphate detection and feedback on hospital room cleaning. Infect Control Hosp Epidemiol. 2014;35:564–9. doi: 10.1086/675839. [PMID: 24709726] doi:10.1086/675839. [DOI] [PubMed] [Google Scholar]
- 80.Brakovich B, Bonham E, VanBrackle L. War on the spore: Clostridium difficile disease among patients in a long-term acute care hospital. J Healthc Qual. 2013;35:15–21. doi: 10.1111/j.1945-1474.2011.00182.x. [PMID: 22304334] doi:10.1111/j.1945-1474.2011.00182.x. [DOI] [PubMed] [Google Scholar]
- 81.Trajtman AN, Manickam K, Macrae M, Bruning NS, Alfa MJ. Continuing performance feedback and use of the ultraviolet visible marker to assess cleaning compliance in the healthcare environment. J Hosp Infect. 2013;84:166–72. doi: 10.1016/j.jhin.2013.03.004. [PMID: 23631799] doi:10.1016/j.jhin.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 82.Ragan K, Khan A, Zeynalova N, McKernan P, Baser K, Muller MP. Use of audit and feedback with fiuorescent targeting to achieve rapid improvements in room cleaning in the intensive care unit and ward settings. Am J Infect Control. 2012;40:284–6. doi: 10.1016/j.ajic.2011.04.003. [PMID: 21820762] doi:10.1016/j.ajic.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Datta R, Platt R, Yokoe DS, Huang SS. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch Intern Med. 2011;171:491–4. doi: 10.1001/archinternmed.2011.64. [PMID: 21444840] doi:10.1001/archinternmed.2011.64. [DOI] [PubMed] [Google Scholar]
- 84.Murphy CL, Macbeth DA, Derrington P, Gerrard J, Faloon J, Kenway K, et al. An assessment of high touch object cleaning thoroughness using a fiuorescent marker in two Australian hospitals. Healthc Infect. 2011;16:156–63. [Google Scholar]
- 85.Hota B, Blom DW, Lyle EA, Weinstein RA, Hayden MK. Interventional evaluation of environmental contamination by vancomycin-resistant enterococci: failure of personnel, product, or procedurefi. J Hosp Infect. 2009;71:123–31. doi: 10.1016/j.jhin.2008.10.030. [PMID: 19108932] doi:10.1016/j.jhin.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 86.Po JL, Burke R, Sulis C, Carling PC. Dangerous cows: an analysis of disinfection cleaning of computer keyboards on wheels. Am J Infect Control. 2009;37:778–80. doi: 10.1016/j.ajic.2009.02.005. [PMID: 19457585] doi:10.1016/j.ajic.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 87.Carling PC, Parry MM, Rupp ME, Po JL, Dick B, Von Beheren S. Healthcare Environmental Hygiene Study Group. Improving cleaning of the environment surrounding patients in 36 acute care hospitals. Infect Control Hosp Epidemiol. 2008;29:1035–41. doi: 10.1086/591940. [PMID: 18851687] doi:10.1086/591940. [DOI] [PubMed] [Google Scholar]
- 88.Goodman ER, Platt R, Bass R, Onderdonk AB, Yokoe DS, Huang SS. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol. 2008;29:593–9. doi: 10.1086/588566. [PMID: 18624666] doi:10.1086/588566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eckstein BC, Adams DA, Eckstein EC, Rao A, Sethi AK, Yadavalli GK, et al. Reduction of Clostridium Difficile and vancomycin-resistant enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect Dis. 2007;7:61. doi: 10.1186/1471-2334-7-61. [PMID: 17584935] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hayden MK, Bonten MJ, Blom DW, Lyle EA, van de Vijver DA, Weinstein RA. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis. 2006;42:1552–60. doi: 10.1086/503845. [PMID: 16652312] [DOI] [PubMed] [Google Scholar]
- 91.Feczko R, Polizzi T, Schweon SJ, Shamash M, Alameda T. Crothall Healthcare's Strategic Initiatives for Reducing Healthcare-Associated Infections. 2012 Accessed at http://media.crothall.com/global/Crothall%20IP%20White%20Paper%20-%20May%202012%20(FINAL).pdf on 27 July 2015. [Google Scholar]
- 92.Sodexo Quality of Life Services . Health Care. Sodexo; Gaithersburg, MD: Accessed at www.sodexo.com/en/services/on-site/healthcare/offer.aspx on 9 October 2014. [Google Scholar]
- 93.Guise JM, Chang C, Viswanathan M, Glick S, Treadwell J, Umscheid CA, et al. Agency for Healthcare Research and Quality Evidence-based Practice Center methods for systematically reviewing complex multicomponent health care interventions. J Clin Epide-miol. 2014;67:1181–91. doi: 10.1016/j.jclinepi.2014.06.010. [PMID: 25438663] doi:10.1016/j.jclinepi.2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.