Abstract
Nerve growth factor (NGF) is the firstly discovered and best characterized neurotrophic factor, known to play a critical protective role in the development and survival of sympathetic, sensory and forebrain cholinergic neurons. NGF promotes neuritis outgrowth both in vivo and in vitro and nerve cell recovery after ischemic, surgical or chemical injuries. Recently, the therapeutic property of NGF has been demonstrated on human cutaneous and corneal ulcers, pressure ulcer, glaucoma, maculopathy and retinitis pigmentosa. NGF eye drops administration is well tolerated, with no detectable clinical evidence of systemic or local adverse effects. The aim of this review is to summarize these biological properties and the potential clinical development of NGF.
Keywords: Clinical trials, NGF, NGF-healing action, treatment, NGF receptors, visual system
INTRODUCTION
The Nerve Growth Factor (NGF): A Brief Overview
NGF is a neurotrophic factor discovered in 1950 for its properties of promoting growth and survival of peripheral sensory and sympathetic nerve cells of mammalians, human included [1, 2]. Subsequent studies carried out in laboratory animals have shown that peripherally innervated tissues produce and release NGF which is retrogradelly transported by specific receptors, to finally provide a protective action and a functional neuronal integrity [3]. The biological action of NGF is mediated by two NGF-receptors, the historically recognized high-affinity receptor trkANGFR, having a tyrosine kinase activity, and the low-affinity transmembrane glycoprotein, a non-selective pan-neurotrophin receptor p75NTR that regulates signaling through trkANGFR [4]. Binding of NGF to p75NTR activates additional signaling pathways that, in the absence/reduced expression of co-expressed trkANGFR on NGF-target cell, might trigger apoptosis [4-6]. The effect of NGF on target cells depends on the ratio of these two receptors co-distributed on cell surface [7]. Thus, the reduced transport of NGF, produced by NGF-target/innervated tissues can lead to damage of nervous cells, as observed in peripheral neuropathies [8]. Subsequent studies carried out in animal models showed that exogenous NGF administration can promote peripheral nerve growth and re-establish the functional activity of peripheral nerve fibers and damaged neurons [8-11]. However, the attempts of using exogenous NGF in human neuropathies were not encouraging since intravenously NGF peripheral administration caused unwanted side-effects, in addition to the desired stimulation of nerve fiber outgrowth [12]. The first evidence that NGF might have therapeutic properties, was obtained with the topical administration on human cutaneous, corneal and pressure ulcers [13] while the effects on Glaucoma [14], Maculopathy [15] and Retinitis Pigmentosa represent recent outcomes [16]. The topical application provided successful outcomes and resulted to be well tolerated, since no detectable clinical evidence of systemic or local adverse effects were observed [17, 18]. These results paved the way for the development of clinical trials of NGF in Ophthalmology and cutaneous ulcers.
NGF Contribution in Tissue Healing: A Brief Cellular Overview
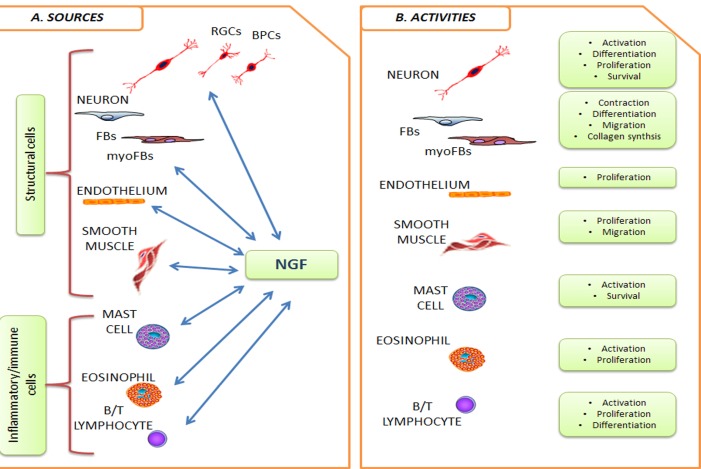
Because of its activities outside the nervous system, NGF is actually recognized as a pleiotropic factor. NGF is produced and utilized by several cell types, including structural (epithelial cells, fibroblasts/myofibroblasts, endothelial cells, smooth muscle cells and hepatocytes), accessory (glial cells, astrocytes and Muller cells) and immune (antigen presenting cells, lymphocytes, granulocytes, mast cells and eosinophils) cells [19, 20]. An overview of the cross-talk between NGF and structural/immune cells is highlighted in Fig. 1. Since the discovery, NGF attracted clinicians for potential application in different fields, firstly in the treatment of neurological disorders and later on as healing-promoting agent in the ulcer management [13]. The reparative properties of NGF have been tested in several conditions of healing. Successful NGF effects were obtained in impaired healing due to experimental injury or autoimmune disorders and during the topical long-term NGF application in experimental as well as human pressure and cutaneous ulcers [21-24]. The profibrogenic activity of NGF was later on confirmed in several in vitro studies, indicating the fibroblast as target cells for the NGF-driven healing [25]. A consistent number of patients suffering from corneal ulcers due to neurotrophic impairments associated with herpetic infections, chemical burns, local surgery, topical anesthetic abuse and diabetes have been treated until now [13, 17]. The topical application of murine NGF promoted a complete healing of the ulcer associated with a recovery of the corneal sensitivity, providing a long term benefit to trigeminal innervations [26]. In a recent in vitro study, our group provided data on NGF as an inducer of myofibroblast apoptosis by shifting the trkANGFR/p75NTR ratio in favor of p75NTR and inducing up-regulation of terminal caspase-3, opening a more fruitful possibility for counteracting myoFBs resistance to apoptosis [27]. This observation is in line with a previous study showing that NGF is expressed during fibrotic liver injury and may regulate the number of activated Hepatic Stellate Cells (myoFBs-like cells) via induction of caspase3-mediated apoptosis [28]. In a pilot study carried out in mice with acute self-limiting pro-fibrotic liver injury, NGF administration allowed recovery from injury by means of myoFB apoptosis and absence of trkANGFR [29]. Due to the lack of effective pharmacological agents for balanced tissue repairs (except for the short-term steroid application), these new findings suggest that NGF might be a suitable therapeutic tool in conditions with impaired tissue healing. These findings were also provided by a previous study on cultured keratocytes, supporting the new therapeutic strategies to overt fibrosis by modulating myoFB survival in fibrotic tissues [30].
Fig. (1).
NGF target cells (NTC). Cell types belonging to nervous and immune system as well as some structural cells, all previously reported to be receptive (NGFR-bearing cells) and producers of NGF, are drawn in panel A. Crosstalk occurs via interplays with the other soluble factors, comprising cytokines, neuropeptides, growth as well as angiogenic/angiostatic factors, and other cell mediators, including soluble receptors. The main recognized functional activities are summarized in B. Abbreviations: RGCs, retinal ganglion cells; BPCs, bipolar cells; FBs/myoFBs, Fibroblast/myofibroblast.
NGF AND PERIPHERAL NERVOUS SYSTEM
Studies carried out on laboratory animals and isolated cells have demonstrated the protective action of NGF not only on the survival of degenerating peripheral nerve cells but also on the regulation of neurotransmitters and neuropeptides synthesis of sympathetic and sensory nerve cells [31, 32]. In vivo down-regulation of NGF, through the administration of NGF-antibodies or by peripheral nerve lesion causes a marked decrease in Substance P (SP) and Calcitonin-Gene Related Peptide (CGRP) synthesis, while exogenous NGF administration influences the neuronal plasticity that allows the adult nervous system to modify its structure and functions in response to stimuli [32]. Moreover, it was also demonstrated that the constitutive synthesis of NGF in adult tissues correlates with Peripheral Nervous System (PNS) neurons phenotypic features, such as innervation density, cell body size, axonal terminal sprouting, dendrites arborization, induction and/or inhibition of neuropeptides and neurotransmitters or transmitter-producing enzymes [33]. The therapeutic properties of NGF were firstly explored in patients affected by peripheral neuropathies induced by type I or II Diabetes [34, 35]. The results of these phase I and phase II clinical trials confirmed the protective action of NGF observed in laboratory animals, raising optimism and encouraged to proceed with these tests [33, 36]. However, the phase-III trial did not confirm the results of the previous trials and the clinical studies were thereafter interrupted [12]. No exhaustive explanations were provided for the outcomes of the last clinical trial. One possible hypothesis suggested by the authors comprised the different biological preparations of human recombinant NGF and its biochemical properties, as well as the characteristic of the study (animal and human trials), including age of enrolled patients, onset/severity of neuropathy, clinical history of patients and the placebo group of patients. Indeed, the possibility of a not enough quality NGF protein purification was also suggested as an explanation for the undesired side-effects observed meanwhile the study, such as the peripheral pain [12]. Suggestion for possible future strategies might be the production of highly purified human NGF to avoid undesired NGF effects, different route of administration, utilization of small molecules capable of stimulating endogenous synthesis and release of NGF [12].
NGF AND CENTRAL NERVOUS SYSTEM
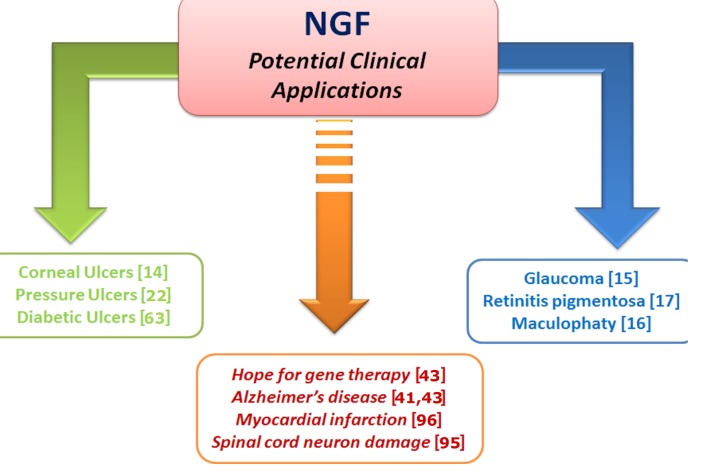
Degenerative disorders of the CNS are characterized by the progressive loss of structure and functions of neurons, most likely the result of not enough synthesis and/or release of neurotrophic factors, including NGF [37]. This hypothesis is supported by different structural and biochemical findings showing that the synthesis/release of NGF as well as brain NGF signaling were markedly affected in brain neuro-degenerative disorders, while exogenous administration of NGF was able to protect degenerating neurons [38]. Moreover, these studies demonstrated that NGF was able to promote survival of Basal Forebrain Cholinergic Neurons (BFCN), known to degenerate in age-related disorders (such as in Alzheimer’s disease (AD)), leading to the hypothesis that intracerebral administration of NGF might reduce or prevent brain neuronal degeneration of these patients. Studies during the early 1980’s showed that NGF intracerebral administration was able to prevent the lesion-induced degeneration of BFCN in experimental rodents and to delay the atrophy in the aged rat brain neurons [39-41]. Few years later, NGF was tested in AD patients, but the results of these studies were associated with systemic pain, weight loss and only mild neurological improvements and the study was stopped [42, 43]. Few years later, NGF was by the intracranial route delivered in a spatial restricted manner, as an ex-vivo gene delivery NGF in human CNS [44]. The phase I clinical trial of NGF gene delivery seems to be a promising experimental approach to assess whether NGF has a potential therapeutic future for AD. However, these observations suggested that different methods of administration and better sources of NGF will be available. In more recent years, other strategies have been identified for the delivery of NGF into the damaged brain neurons and to bypass safely the Brain-Blood-Barrier [45]. It was found that the nose-to-brain route might be a potential useful route for targeting drugs to the brain via the olfactory pathway [46, 47]. Accordingly, it was observed that the nasal NGF administration as well as eye topical one, might be a more safe strategy to deliver NGF into the brain [47]. More recently, the nose-brain and eye-brain pathways have been investigated by different research groups, demonstrating the potential alternative route of delivering NGF into the brain. The first demonstration that NGF could be safely delivered to damaged brain neurons was published by Chen and coworkers, who showed that NGF administered via nasal cavity can protect the damaged BFCN and improves the behavioural performance in AD experimental models [47]. Likewise, we have demonstrated that eye topical NGF administration can reach damaged brain neurons and protect them and improve behaviour performance, as observed on rodents [48]. Thus, the nose-to-brain route for neuroprotective molecule delivery would permit repeated administrations, without producing adverse side-effects and permit a more rapid effect on the brain target [49-51]. The direct delivery of a wide variety of therapeutic agents to the CNS has been demonstrated in mice, rats, primates and humans. Fig. 2 schematises the experimental and clinical evidence of NGF in animal models and humans.
Fig. (2).
NGF and Clinical applications. The scheme summarizes the old (green), recent (blue) and future (brown) NGF applications. Citations are reported in brackets.
NGF AND VISUAL SYSTEM
NGF has been tested for wound healing processes based on its biological activities on both neuronal and non-neuronal cells. Investigation of the potential use of NGF as therapeutic agent has derived impetus from recent clinical research trials for the treatment of human peripheral neuropathies. In line with these observations, it has been hypothesized that NGF might be able to become a pharmacological healing molecule in the visual system. All in vivo and in vitro findings carried out until now are underneath described in details.
NGF and Corneal Ulcer
In recent years, numerous studies have provided evidence that NGF exerts effects on various non-neural cells. NGF plays crucial roles in various biological activities by modulating cell proliferation, migration, differentiation, maintenance/survival, function and plasticity of cells from the anterior to the posterior eye segment [52]. NGF is produced and released by the corneal cells of rodents and humans, while exogenous NGF administration has been shown to play a critical role in migration and proliferation of these cells [53, 54]. Recently, NGF has gained attention for the treatment of human patients with chronic epithelial defects, as topical eye NGF administration restores corneal integrity in human patients with immune or neurotrophic corneal ulcers [55, 56]. Corneal epithelial cells are among the most densely innervated cells of the mammalian organism and alteration of their innervation can cause corneal damages, including deficit of the ocular surface, visual impairments and systemic diseases [57, 58]. However, the first clear evidence that NGF exerts such a protective role was published by Lambiase and co-workers who demonstrated that topic eye NGF administration in humans affected by corneal ulcer stimulate corneal healing [13]. The studies demonstrated that the healing action of NGF was not impaired by the severity of corneal ulcer, the depth of stromal lesion or by the clinical history of the patients [59, 60]. The healing action was equally effective and the eye topical NGF administration induced a complete healing after three-to-six weeks of treatment. Moreover, the NGF eye topical administration enhanced both corneal transparency and tear film production, improving the visual function. Taken together, these results indicate that eye topical NGF treatment acts as a pleiotropic factor for damaged corneal surface through different mechanisms, such as stimulation of corneal innervation and healing, modulation of corneal stem cells, refining of both stroma and endothelial cells [59, 60]. Moreover, eye NGF topical administration enhanced tear release in humans and bulldogs suffering of dry eye, a chronic ocular surface disease characterized by the impairment of tear film quantity and/or quality [61]. The healing action of NGF appears not limited to corneal ulcer, since corroborating studies have shown that topical skin NGF application accelerated the rate of healing of human cutaneous ulcers induced by Diabetes [62], Rheumatoid Arthritis [63], pressure ulcers in humans [64] as well as cutaneous ulcers in domestic (goat) animals [65].
NGF and Glaucoma
One of the first evidences that NGF might be involved in the protective action of retinal cells was published in 1979 by Turner and co-workers, who reported that NGF was able to stimulate the regeneration of axotomized optic nerve in gold fish [66]. Subsequent studies in cats and rodents confirmed and extended this observation by demonstrating that NGF can modulate the neuroplasticity of neurons in the visual cortex and geniculate nucleus [67], promotes functional recovery of Retinal Ganglion Cells (RGCs) after ischemia [68], survival of rat RGCs following optic nerve section [69] prevents changes in ocular dominance distribution induced by monocular deprivation and promotes stabilization of geniculate-cortical synapsis during postnatal development [70], while intracerebral anti-NGF administration in rodents caused a delayed consolidation of the visual cortex synapses [71]. Moreover, other studies have shown that NGF administration stimulated recovery of axotomized optic nerve, ocular ischemia and ocular hypertension [72], while the NGF content in the optic nerve of post-mortem samples of animalsand human subjects died from Multiple Sclerosis is significantly reduced [73]. Together these observations lead to the hypothesis as whether ocular NGF administration would protect damaged retinal cells in glaucomatous patients. Glaucoma is a group of ocular disorders, frequently associated with elevated intraocular pressure (IOP) and characterized by the loss of RGCs and optic nerve damage [74, 75]. Using a rodent model of Glaucoma, induced through injection of hypertonic saline into the episcleral vein [76], we observed that the levels of NGF and NGF-receptors in the retina were significantly down regulated while eye NGF administration reduced this loss and promoted recovery of damaged RGCs [77, 78]. These experimental results obtained in laboratory rodents suggested testing the effects of NGF eye-drop administration in three patients suffering of Glaucoma with progressive visual field defects [14]. Before treatment, the patients underwent baseline psychofunctional and electro functional tests and then followed weekly during the three months of NGF treatment. After 7 weeks of elevated IOP, RGCs of untreated patients were reduced by nearly 40%, whereas a significantly less RGC reduction was observed in NGF treated patients, associated with the inhibition of cell death by apoptosis. These results indicate that patients undergoing NGF treatment resulted in long-lasting improvements in visual field, optic nerve function, contrast sensitivity and visual acuity [14]. Concomitant investigations using the same NGF doses and batch in glaucomatous rabbits (experimental ocular hypertension) indicated that the NGF treatment exerted neuroprotective effects by reducing RGC death, as observed through a reduced apoptotic mechanism, in line with the improvement of all visual function parameters monitored in advanced glaucoma patients [72].
NGF and Retinitis Pigmentosa
In 1990, Faktorovich and coworkers reported for the first time that neurotrophic factors, including NGF, might be involved in the protection action of retinal cell degeneration in rodents with RP, a genetic disorder characterized by the progressive photoreceptor degeneration leading to the loss of vision [79, 80]. A resolute therapy to prevent or arrest the degenerative course of RP has not yet been identified, though several clinical approaches to treat different forms of RP have been suggested and few clinical trials have been taken into consideration [81]. One of the first indications of NGF ability to protect photoreceptors was published in 1996 using the mouse strain C3H with RP characterized by the progressive photoreceptor degeneration during early postnatal life, which demonstrated that intravitreal NGF administration was able to delay photoreceptor degeneration [82]. These observations and the NGF action on retinal cells were confirmed and extended in the Royal College of Surgeon (RCS) rat model, a strain characterized by autosomal recessive mutation of the retinal dystrophy gene [83]. Studies carried out in mice and rats affected by RP showed that the administration of topical NGF administration is able to delay photoreceptor degeneration [16, 83]. The protective action of NGF on RCS photoreceptors was also indicated by findings showing that eye NGF administration enhances both gene and receptor expression in visual cortex and geniculate nucleus [84].
NGF and Macular Degeneration
The Maculopathy is a human ocular pathology characterized by an oval-shaped highly pigmented yellow spot in the macula (the center of retina) [85, 86]. Macular degeneration is usually age-related and progressive with age [86]. Since retinal cells are known to express NGF-receptors and retinal cells are receptive to the action of NGF [14], the effect of NGF topical application was tested in a 94 years old female patient with Maculopathy by several years and unable to respond to all current available therapies and close to complete vision lost [15]. The visual acuity of this patient was progressively worsening in spite of previous surgical and medical treatments and the patient asked to be treated with NGF eye drops. After signing the informed consent, the patient was treated with eye drops for 6 consecutive months. Daily comments from the patient and weekly clinical examination from the ophthalmologist revealed the absence of NGF related undesired effects. Moreover, a clear improvement of visual acuity and of electro functional parameters was clearly monitored 3 months after beginning of the treatment [15]. These observations were consistent with previous findings regarding the neuroprotective action of NGF on retinal cells of glaucomatous patients and the absence of side effects following NGF administration, even after years of follow-up. Cumulating, topical eye NGF treatment seems to be a potential effective therapy for human Maculopathy.
NGF and Diabetic Retinopathy
As estimated, DR is one of the leading causes of adult blindness and the most common complication of diabetes [87]. It affects more than 90% of people, leading to retinal edema, neovascularization and vision lost in a number of patients [87]. Since Diabetes alters retinal vascular distribution, inflammatory response and Vascular Endothelial Growth Factor (VEGF) levels, we studied the effect of eye NGF administration in an experimental-induced diabetes in adult rat. It was found that the eye topical administration of NGF in diabetic rats protects from retinal and vascular endothelial cells degeneration [88, 89], while the intraocular injection of anti-NGF antibody exacerbates the retinal degenerative events [89]. Our working hypothesis, currently under investigation, is that the early NGF increase/release represents an endogenous response for protecting RGCs, unable to maintain the degenerative signals induced by diabetes continues. As VEGF is considered as a pro-inflammtory signal for the diabetic retina, the role of NGF is less clear.
Several data indicate that NGF, a well-known product of inflammation, exerts an anti-inflammatory action, as observed in a number of inflammatory disorders and in eye NGF administration, as eye drops promote corneal healing exerting both anti-inflammatory and immuno-modulatory action on corneal endothelial cells [53].
It is therefore possible that NGF might play a pivotal protective role by modulating the deleterious effects of the inflammatory signals induced by VEGF. In line, VEGF has been shown to exert both inflammatory and neurotrophic activities, suggesting synergistic actions on DR [90, 91]. It might also be hypothesized that NGF might exert both neuroprotective and anti-inflammatory action on DR, down regulating the pro-inflammatory action of VEGF [92].
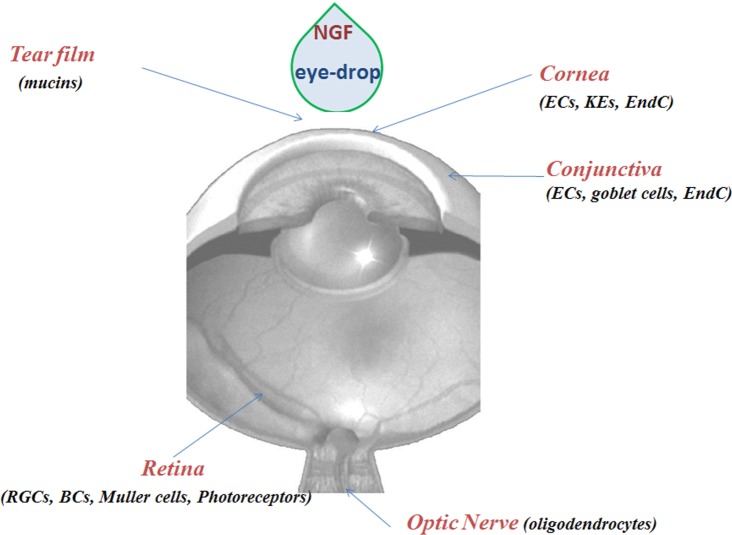
The available evidence of the healing action of NGF on visual tissue targets is indicated in Fig. 3.
Fig. (3).
NGF and visual system. NGF eye-drop application results in different effects from the anterior to the posterior segment. The scheme summarizes all the cells responsive to NGF eye-drop instillation. Abbreviations: ECs, epithelial cells; EndC, endothelial cell; RGCs, retinal ganglion cells; BCs, bipolar cells; KEs, keratocytes.
CONCLUSION
Since NGF discovery, numerous studies performed in laboratory animals and in primary cultures of nerve cells have documented the neuroprotective role of NGF on damaged cells of the peripheral and central nervous system. During the last two decades, a number of evidences have been accumulated supporting the hypothesis that NGF possesses potential therapeutic properties on cells of the visual system, cutaneous cells and most likely on certain disorders of the central nervous system. Other studies are currently under investigations to test the hypothesis whether the human recombinant NGF administered in a safe manner and in absence of side effects, may serve to reduce/protect/prevent human disorders, such peripheral neuropathies, AD, cardiomyopathy and myocardial ischemia, as reported in Table 1 AB.
Table 1.
NGF tissue protection in animal models and humans.
A. NGF Therapeutic Potential Based on Animal Studies
| Type of Disorder | Species | Treatment | Outcome | Refs. |
|---|---|---|---|---|
| Postischemic Coronary Innervation | Dogs | 10ng | Protection | [93] |
| Wound healing | Mice | 1ug | Healing | [21] |
| Hypoxic-Ischemic | Young rats | 15ug | Protection | [94] |
| Encephalomyelitis | Marmosets | 100ug | Protection | [95] |
| Heart Failure | Rats | 9.15nmole | Protection | [96] |
| Nasal delivery | Rats | 10ng | Protection | [48] |
| Glaucoma | Rats | 10ug | Protection | [15] |
B. NGF Therapeutic Potential Based on Human Diseases
| Disease | Dose | Treatment | Side Effects | Outcome | Refs. |
|---|---|---|---|---|---|
| Neurotrophic keratitis | 10μg* | 4 weeks | None | Healed | [54] |
| Immune cornea ulcer | 10μg* | 6-8 weeks | None | Healed | [14] |
| Glaucoma | 10μg* | 12-15 weeks | None | Protective | [15] |
| Maculopathy | 10μg* | 15-20 weeks | None | Protective | [16] |
| Crush syndrome | 10μg** | 1 week | None | Protective | [97] |
| Vasculitic ulcer | 20μg** | 20 weeks | None | Healed | [63] |
| Pressure ulcer | 20μg** | 10 weeks | None | Healed | [22] |
| Other | |||||
| Diabetic neuropathy | 0.3μg*** | 3 weeks | Local pain | Protection? | [9] |
ACKNOWLEDGEMENTS
The Authors’ work summarized in this review was supported in part by the National Italian Research Council (CNR) and Association NGF-ONLUS to Luigi Aloe. Bijorn Omar Balzamino and Alessandra Micera thank the Ministry of Health and Fondazione Roma for financial support.
LIST OF ABBREVIATIONS
- NGF
Nerve Growth Factor
- NTC
NGF Target Cells
- PNS
Peripheral Nervous System
- CNS
Central Nervous System
- VS
Visual System
- AD
Alzheimer disease
- RP
Retinitis Pigmentosa
- DR
Diabetic Retinopathy
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 2.Aloe L., Calzà L. NGF and Related Molecules in Health and Disease. Prog. Brain Res. 2004:146. [Elsevier Editor.]. [Google Scholar]
- 3.Reichardt L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang E.J., Reichardt L.F. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 5.Sofroniew M.V., Howe C.L., Mobley W.C. Nerve growth factor signaling neuroprotection, and neural repair. Ann. Rev. Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 6.Ebendal T. Function and evolution in the NGF family and its receptors. J. Neurosci. Res. 1992;32(4):461–470. doi: 10.1002/jnr.490320402. [DOI] [PubMed] [Google Scholar]
- 7.Micera A., Lambiase A., Stampachiacchiere B., Bonini S., Bonini S., Levi-Schaffer F. Nerve growth factor and tissue repair remodeling: trkA(NGFR) and p75(NTR), two receptors one fate. Cytokine Growth Factor Rev. 2007;18(3-4):245–256. doi: 10.1016/j.cytogfr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Hellweg R., Raivich G., Hartung H.D., Hock C., Kreutzberg G.W. Axonal transport of endogenous nerve growth factor (NGF) and NGF receptor in experimental diabetic neuropathy. Exp. Neurol. 1994;130:24–30. doi: 10.1006/exnr.1994.1181. [DOI] [PubMed] [Google Scholar]
- 9.Apfel S.C., Kessler J.A. Neurotrophic factors in the therapy of peripheral neuropathy. Baillieres Clin. Neurol. 1995;4(3):593–606. [PubMed] [Google Scholar]
- 10.Riaz S.S., Tomlinson D.R. Neurotrophic factors in peripheral neuropathies: pharmacological strategies. Prog. Neurobiol. 1996;49(2):125–143. doi: 10.1016/0301-0082(96)00010-X. [DOI] [PubMed] [Google Scholar]
- 11.McMahon S.B., Priestley J.V. Peripheral neuropathies and neurotrophic factors: animal models and clinical perspectives. Curr. Opin. Neurobiol. 1995;5(5):616–624. doi: 10.1016/0959-4388(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 12.Apfel S.C. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int. Rev. Neurobiol. 2002;50:393–413. doi: 10.1016/S0074-7742(02)50083-0. [DOI] [PubMed] [Google Scholar]
- 13.Lambiase A., Rama P., Bonini S., Caprioglio G., Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N. Engl. J. Med. 1998;338(17):1174–1180. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- 14.Lambiase A., Aloe L., Centofanti M., Parisi V., Báo S.N., Mantelli F., Colafrancesco V., Manni G.L., Bucci M.G., Bonini S., Levi-Montalcini R. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc. Natl. Acad. Sci. USA. 2009;106(32):13469–13474. doi: 10.1073/pnas.0906678106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambiase A., Coassin M., Tirassa P., Mantelli F., Aloe L. Nerve growth factor eye drops improve visual acuity and electrofunctional activity in age-related macular degeneration: a case report. Ann. Ist. Super. Sanita. 2009;45(4):439–442. doi: 10.1590/S0021-25712009000400014. [DOI] [PubMed] [Google Scholar]
- 16.Lenzi L., Coassin M., Lambiase A., Bonini S., Amendola T., Aloe L. Effect of exogenous administration of nerve growth factor in the retina of rats with inherited retinitis pigmentosa. Vision Res. 2005;45:1491–1500. doi: 10.1016/j.visres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Lambiase A., Coassin M., Sposato V., Micera A., Sacchetti M., Bonini S., Aloe L. NGF topical application in patients with corneal ulcer does not generate circulating NGF antibodies. Pharmacol. Res. 2007;56(1):65–69. doi: 10.1016/j.phrs.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari M.P., Mantelli F., Sacchetti M., Antonangeli M.I., Cattani F., D’Anniballe G., Sinigaglia F., Ruffini P.A., Lambiase A. Safety and pharmacokinetics of escalating doses of human recombinant nerve growth factor eye drops in a double-masked, randomized clinical trial. BioDrugs. 2014;28(3):275–283. doi: 10.1007/s40259-013-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Micera A., Puxeddu I., Aloe L., Levi-Schaffer F. New insights on the involvement of Nerve Growth Factor in allergic inflammation and fibrosis. Cytokine Growth Factor Rev. 2003;14(5):369–374. doi: 10.1016/S1359-6101(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 20.Lambiase A., Micera A., Sgrulletta R., Bonini S., Bonini S. Nerve growth factor and the immune system: old and new concepts in the cross-talk between immune and resident cells during pathophysiological conditions. Curr. Opin. Allergy Clin. Immunol. 2004;4(5):425–430. doi: 10.1097/00130832-200410000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda H., Koyama H., Sato H., Sawada J., Itakura A., Tanaka A., Matsumoto M., Konno K., Ushio H., Matsuda K. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J. Exp. Med. 1998;187(3):297–306. doi: 10.1084/jem.187.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernabei R., Landi F., Bonini S., Onder G., Lambiase A., Pola R., Aloe L. Effect of topical application of nerve-growth factor on pressure ulcers. Lancet. 1999;354(9175):307. doi: 10.1016/S0140-6736(99)02784-1. [DOI] [PubMed] [Google Scholar]
- 23.Landi F., Aloe L., Russo A., Cesari M., Onder G., Bonini S., Carbonin P.U., Bernabei R. Topical treatment of pressure ulcers with nerve growth factor: a randomized clinical trial. Ann. Intern. Med. 2003;139(8):635–641. doi: 10.7326/0003-4819-139-8-200310210-00006. [DOI] [PubMed] [Google Scholar]
- 24.Aloe L., Tirassa P., Lambiase A. The topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcers. Pharmacol. Res. 2008;57(4):253–258. doi: 10.1016/j.phrs.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Micera A., Vigneti E., Pickholtz D., Reich R., Pappo O., Bonini S., Maquart F.X., Aloe L., Levi-Schaffer F. Nerve growth factor displays stimulatory effects on human skin and lung fibroblasts, demonstrating a direct role for this factor in tissue repair. Proc. Natl. Acad. Sci. USA. 2001;98(11):6162–6167. doi: 10.1073/pnas.101130898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambiase A., Aloe L., Mantelli F., Sacchetti M., Perrella E., Bianchi P., Rocco M.L., Bonini S. Capsaicin-induced corneal sensory denervation and healing impairment are reversed by NGF treatment. Invest. Ophthalmol. Vis. Sci. 2012;53(13):8280–8287. doi: 10.1167/iovs.12-10593. [DOI] [PubMed] [Google Scholar]
- 27.Micera A., Puxeddu I., Balzamino B.O., Bonini S., Levi-Schaffer F. Chronic nerve growth factor exposure increases apoptosis in a model of in vitro induced conjunctival myofibroblasts. PLoS One. 2012;7:e47316. doi: 10.1371/journal.pone.0047316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oakley F., Trim N., Constandinou C.M., Ye W., Gray A.M., Frantz G., Hillan K., Kendall T., Benyon R.C., Mann D.A., Iredale J.P. Hepatocytes express nerve growth factor during liver injury: evidence for paracrine regulation of hepatic stellate cell apoptosis. Am. J. Pathol. 2003;163(5):1849–1858. doi: 10.1016/S0002-9440(10)63544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trim N., Morgan S., Evans M., Issa R., Fine D., Afford S., Wilkins B., Iredale J. Hepatic stellate cells express the low affinity nerve growth factor receptor p75 and undergo apoptosis in response to nerve growth factor stimulation. Am. J. Pathol. 2000;156(4):1235–1243. doi: 10.1016/S0002-9440(10)64994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur H., Chaurasia S.S., Agrawal V., Suto C., Wilson S.E. Corneal myofibroblast viability: opposing effects of IL-1 and TGF beta1. Exp. Eye Res. 2009;89(2):152–158. doi: 10.1016/j.exer.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindsay R.M., Harmar A.J. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337(6205):362–364. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- 32.Verge V.M., Richardson P.M., Wiesenfeld-Hallin Z., Hökfelt T. Differential influence of nerve growth factor on neuropeptide expression in vivo: a novel role in peptide suppression in adult sensory neurons. J. Neurosci. 1995;15(3 Pt 1):2081–2096. doi: 10.1523/JNEUROSCI.15-03-02081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otten U., Schwab M., Gagnon C., Thoenen H. Selective induction of tyrosine hydroxylase and dopamine beta-hydroxylase by nerve growth factor: comparison between adrenal medulla and sympathetic ganglia of adult and newborn rats. Brain Res. 1977;133(2):291–303. doi: 10.1016/0006-8993(77)90765-X. [DOI] [PubMed] [Google Scholar]
- 34.Apfel S.C., Kessler J.A., Adornato B.T., Litchy W.J., Sanders C., Rask C.A., NGF Study Group Recombinant human nerve growth factor in the treatment of diabetic polyneuropathy. Neurology. 1998;51(3):695–702. doi: 10.1212/WNL.51.3.695. [DOI] [PubMed] [Google Scholar]
- 35.Apfel S.C. Neurotrophic factors in the therapy of diabetic neuropathy. Am. J. Med. 1999;107(2B):34S–42S. doi: 10.1016/S0002-9343(99)00011-X. [DOI] [PubMed] [Google Scholar]
- 36.McArthur J.C., Yiannoutsos C., Simpson D.M., Adornato B.T., Singer E.J., Hollander H., Marra C., Rubin M., Cohen B.A., Tucker T., Navia B.A., Schifitto G., Katzenstein D., Rask C., Zaborski L., Smith M.E., Shriver S., Millar L., Clifford D.B., Karalnik I.J. A phase II trial of nerve growth factor for sensory neuropathy associated with HIV infection. AIDS Clinical Trials Group Team 291. Neurology. 2000;54(5):1080–1088. doi: 10.1212/WNL.54.5.1080. [DOI] [PubMed] [Google Scholar]
- 37.Connor B., Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res. Brain Res. Rev. 1998;27(1):1–39. doi: 10.1016/S0165-0173(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 38.McAllister A.K. Neurotrophins and neuronal differentiation in the central nervous system. Cell. Mol. Life Sci. 2001;58(8):1054–1060. doi: 10.1007/PL00000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dreyfus C.F. Effects of nerve growth factor on cholinergic brain neurons. Trends Pharmacol. Sci. 1989;10(4):145–149. doi: 10.1016/0165-6147(89)90166-1. [DOI] [PubMed] [Google Scholar]
- 40.Bäckman C., Rose G.M., Hoffer B.J., Henry M.A., Bartus R.T., Friden P., Granholm A.C. Systemic administration of a nerve growth factor conjugate reverses age-related cognitive dysfunction and prevents cholinergic neuron atrophy. J. Neurosci. 1996;16(17):5437–5442. doi: 10.1523/JNEUROSCI.16-17-05437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer W., Wictorin K., Björklund A., Williams L.R., Varon S., Gage F.H. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature. 1987;329(6134):65–68. doi: 10.1038/329065a0. [DOI] [PubMed] [Google Scholar]
- 42.Eriksdotter Jönhagen M., Nordberg A., Amberla K., Bäckman L., Ebendal T., Meyerson B., Olson L., Seiger, Shigeta M., Theodorsson E., Viitanen M., Winblad B., Wahlund L.O. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 1998;9(5):246–257. doi: 10.1159/000017069. [DOI] [PubMed] [Google Scholar]
- 43.Tuszynski M.H., Thal L., Pay M., Salmon D.P., UH S., Bakay R., Patel P., Blesch A., Vahlsing H.L., Ho G., Tong G., Potkin S.G., Fallon J., Hansen L., Mufson E.J., Kordower J.H., Gall C., Conner J. A phase 1 clinical trial of nerve growth factor Alzheimer’s disease for Alzheimer disease. Nat. Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 44.Granholm A.C., Bäckman C., Bloom F., Ebendal T., Gerhardt G.A., Hoffer B., Mackerlova L., Olson L., Söderström S., Walus L.R., et al. NGF and anti-transferrin receptor antibody conjugate: short and long-term effects on survival of cholinergic neurons in intraocular septal transplants. J. Pharmacol. Exp. Ther. 1994;268(1):448–459. doi: 10.1515/REVNEURO.1998.9.1.31. [DOI] [PubMed] [Google Scholar]
- 45.Granholm A.C., Albeck D., Bäckman C., Curtis M., Ebendal T., Friden P., Henry M., Hoffer B., Kordower J., Rose G.M., Söderström S., Bartus R.T. A non-invasive system for delivering neural growth factors across the blood brain barries: a review. Rev. Neurosci. 1998. [DOI] [PubMed]
- 46.Thorne R.G., Emory C.R., Ala T.A., Frey W.H., II Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995;692(1-2):278–282. doi: 10.1016/0006-8993(95)00637-6. [DOI] [PubMed] [Google Scholar]
- 47.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000;11(1):1–18. doi: 10.1016/S0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 48.Chen X.Q., Fawcett J.R., Rahman Y.E., Ala T.A., Frey W.H., II Delivery of Nerve Growth Factor to the Brain via the Olfactory Pathway. J. Alzheimers Dis. 1998;1(1):35–44. doi: 10.3233/jad-1998-1102. [DOI] [PubMed] [Google Scholar]
- 49.Di Fausto V., Fiore M., Tirassa P., Lambiase A., Aloe L. Eye drop NGF administration promotes the recovery of chemically injured cholinergic neurons of adult mouse forebrain. Eur. J. Neurosci. 2007;26(9):2473–2480. doi: 10.1111/j.1460-9568.2007.05883.x. [DOI] [PubMed] [Google Scholar]
- 50.Miwa T., Moriizumi T., Horikawa I., Uramoto N., Ishimaru T., Nishimura T., Furukawa M. Role of nerve growth factor in the olfactory system. Microsc. Res. Tech. 2002;58(3):197–203. doi: 10.1002/jemt.10149. [DOI] [PubMed] [Google Scholar]
- 51.Vaka S.R., Murthy S.N., Balaji A., Repka M.A. Delivery of brain-derived neurotrophic factor via nose-to-brain pathway. Pharm. Res. 2012;29(2):441–447. doi: 10.1007/s11095-011-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Micera A., Lambiase A., Aloe L., Bonini S., Levi-Schaffer F., Bonini S. Nerve growth factor involvement in the visual system: implications in allergic and neurodegenerative diseases. Cytokine Growth Factor Rev. 2004;15(6):411–417. doi: 10.1016/j.cytogfr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 53.You L., Kruse F.E., Völcker H.E. Neurotrophic factors in the human cornea. Invest. Ophthalmol. Vis. Sci. 2000;41(3):692–702. [PubMed] [Google Scholar]
- 54.Lambiase A., Manni L., Bonini S., Rama P., Micera A., Aloe L. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest. Ophthalmol. Vis. Sci. 2000;41(5):1063–1069. [PubMed] [Google Scholar]
- 55.Lambiase A., Tirassa P., Micera A., Aloe L., Bonini S. Pharmacokinetics of conjunctivally applied nerve growth factor in the retina and optic nerve of adult rats. Invest. Ophthalmol. Vis. Sci. 2005;46(10):3800–3806. doi: 10.1167/iovs.05-0301. [DOI] [PubMed] [Google Scholar]
- 56.Müller L.J., Marfurt C.F., Kruse F., Tervo T.M. Corneal nerves: structure, contents and function. Exp. Eye Res. 2003;76(5):521–542. doi: 10.1016/S0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 57.Lambiase A., Manni L., Bonini S., Rama P., Micera A., Aloe L. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest. Ophthalmol. Vis. Sci. 2000;41(5):1063–1069. [PubMed] [Google Scholar]
- 58.Lambiase A., Micera A., Pellegrini G., Merlo D., Rama P., De Luca M., Bonini S., Bonini S. In vitro evidence of nerve growth factor effects on human conjunctival epithelial cell differentiation and mucin gene expression. Invest. Ophthalmol. Vis. Sci. 2009;50(10):4622–4630. doi: 10.1167/iovs.08-2716. [DOI] [PubMed] [Google Scholar]
- 59.Micera A., Lambiase A., Puxeddu I., Aloe L., Stampachiacchiere B., Levi-Schaffer F., Bonini S., Bonini S. Nerve growth factor effect on human primary fibroblastic-keratocytes: possible mechanism during corneal healing. Exp. Eye. Res., . 2006;83:747–757. doi: 10.1016/j.exer.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 60.Micera A., Puxeddu I., Lambiase A., Antonelli A., Bonini S., Bonini S., Aloe L., Pe'er J., Levi-Schaffer F. The pro-fibrogenic effect of nerve growth factor on conjunctival fibroblasts is mediated by transforming growth factor-beta. Clin. Exp. Allergy. 2005;35:650–656. doi: 10.1111/j.1365-2222.2005.02241.x. [DOI] [PubMed] [Google Scholar]
- 61.Sornelli F., Lambiase A., Mantelli F., Aloe L. NGF and NGF-receptor expression of cultured immortalized human corneal endothelial cells. Mol. Vis. 2010;16:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- 62.Coassin M., Lambiase A., Costa N., De Gregorio A., Sgrulletta R., Sacchetti M., Aloe L., Bonini S. Efficacy of topical nerve growth factor treatment in dogs affected by dry eye. Graefes Arch. Clin. Exp. Ophthalmol. 2005;243(2):151–155. doi: 10.1007/s00417-004-0955-2. [DOI] [PubMed] [Google Scholar]
- 63.Generini S., Tuveri M.A., Matucci Cerinic M., Mastinu F., Manni L., Aloe L. Topical application of nerve growth factor in human diabetic foot ulcers. A study of three cases. Exp. Clin. Endocrinol. Diabetes. 2004;112(9):542–544. doi: 10.1055/s-2004-821313. [DOI] [PubMed] [Google Scholar]
- 64.Tuveri M., Generini S., Matucci-Cerinic M., Aloe L. NGF, a useful tool in the treatment of chronic vasculitic ulcers in rheumatoid arthritis. Lancet. 2000;356(9243):1739–1740. doi: 10.1016/S0140-6736(00)03212-8. [DOI] [PubMed] [Google Scholar]
- 65.Costa N., Fiore M., Aloe L. Healing action of nerve growth factor on lameness in adult goats. Ann. Ist. Super. Sanita. 2002;38(2):187–194. [PubMed] [Google Scholar]
- 66.Turner J.E., Delaney R.K. Retinal ganglion cell response to axotomy and nerve growth factor in the regenerating visual system of the newt (Notophthalmus viridescens): an ultrastructural morphometric analysis. Brain Res. 1979;171(2):197–212. doi: 10.1016/0006-8993(79)90327-5. [DOI] [PubMed] [Google Scholar]
- 67.Maffei L., Berardi N., Domenici L., Parisi V., Pizzorusso T. Nerve growth factor (NGF) prevents the shift in ocular dominance distribution of visual cortical neurons in monocularly deprived rats. J. Neurosci. 1992;12(12):4651–4662. doi: 10.1523/JNEUROSCI.12-12-04651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siliprandi R., Canella R., Carmignoto G. Nerve growth factor promotes functional recovery of retinal ganglion cells after ischemia. Invest. Ophthalmol. Vis. Sci. 1993;34(12):3232–3245. [PubMed] [Google Scholar]
- 69.Carmignoto G., Maffei L., Candeo P., Canella R., Comelli C. Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J. Neurosci. 1989;9(4):1263–1272. doi: 10.1523/JNEUROSCI.09-04-01263.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Domenici L., Berardi N., Carmignoto G., Vantini G., Maffei L. Nerve growth factor prevents the amblyopic effects of monocular deprivation. Proc. Natl. Acad. Sci. USA. 1991;88(19):8811–8815. doi: 10.1073/pnas.88.19.8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Domenici L., Cellerino A., Berardi N., Cattaneo A., Maffei L. Antibodies to nerve growth factor (NGF) prolong the sensitive period for monocular deprivation in the rat. Neuroreport. 1994;5(16):2041–2044. doi: 10.1097/00001756-199410270-00013. [DOI] [PubMed] [Google Scholar]
- 72.Lambiase A., Centofanti M., Micera A., Manni G.L., Mattei E., De Gregorio A., de Feo G., Bucci M.G., Aloe L. Nerve growth factor (NGF) reduces and NGF antibody exacerbates retinal damage induced in rabbit by experimental ocular hypertension. Graefes Arch. Clin. Exp. Ophthalmol. 1997;235(12):780–785. doi: 10.1007/BF02332863. [DOI] [PubMed] [Google Scholar]
- 73.Micera A., Lambiase A., Rama P., Aloe L. Altered nerve growth factor level in the optic nerve of patients affected by multiple sclerosis. Mult. Scler. 1999;5(6):389–394. doi: 10.1177/135245859900500i604. [DOI] [PubMed] [Google Scholar]
- 74.Quigley H.A. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kingman S. Glaucoma is second leading cause of blindness globally. Bull. World Health Organ. 2004;82(11):887–888. [PMC free article] [PubMed] [Google Scholar]
- 76.Morrison J.C., Moore C.G., Deppmeier L.M., Gold B.G., Meshul C.K., Johnson E.C. A rat model of chronic pressure-induced optic nerve damage. Exp. Eye Res. 1997;64(1):85–96. doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- 77.Sposato V., Bucci M.G., Coassin M., Russo M.A., Lambiase A., Aloe L. Reduced NGF level and TrkA protein and TrkA gene expression in the optic nerve of rats with experimentally induced glaucoma. Neurosci. Lett. 2008;446(1):20–24. doi: 10.1016/j.neulet.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 78.Sposato V., Parisi V., Manni L., Antonucci M.T., Di Fausto V., Sornelli F., Aloe L. Glaucoma alters the expression of NGF and NGF receptors in visual cortex and geniculate nucleus of rats: effect of eye NGF application. Vision Res. 2009;49(1):54–63. doi: 10.1016/j.visres.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 79.Faktorovich E.G., Steinberg R.H., Yasumura D., Matthes M.T., LaVail M.M. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990;347(6288):83–86. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- 80.Busskamp V., Picaud S., Sahel J.A., Roska B. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 2012;19(2):169–175. doi: 10.1038/gt.2011.155. [DOI] [PubMed] [Google Scholar]
- 81.Jacobson S.G., Cideciyan A.V. Treatment possibilities for retinitis pigmentosa. N. Engl. J. Med. 2010;363(17):1669–1671. doi: 10.1056/NEJMcibr1007685. [DOI] [PubMed] [Google Scholar]
- 82.Lambiase A., Aloe L. Nerve growth factor delays retinal degeneration in C3H mice. Graefes Arch. Clin. Exp. Ophthalmol. 1996;234(Suppl. 1):S96–S100. doi: 10.1007/BF02343055. [DOI] [PubMed] [Google Scholar]
- 83.Amendola T., Aloe L. Developmental expression of nerve growth factor in the eye of rats affected by inherited retinopathy: correlative aspects with retinal structural degeneration. Arch. Ital. Biol. 2002;140(2):81–90. [PubMed] [Google Scholar]
- 84.Amendola T., Fiore M., Aloe L. Postnatal changes in nerve growth factor and brain derived neurotrophic factor levels in the retina, visual cortex, and geniculate nucleus in rats with retinitis pigmentosa. Neurosci. Lett. 2003;345(1):37–40. doi: 10.1016/S0304-3940(03)00491-9. [DOI] [PubMed] [Google Scholar]
- 85.Yuan D., Yuan D., Yuan S., Liu Q. The age-related maculopathy susceptibility 2 polymorphism and polypoidal choroidal vasculopathy in Asian populations: a meta-analysis. Ophthalmology. 2013;120(10):2051–2057. doi: 10.1016/j.ophtha.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 86.Jager R.D., Mieler W.F., Miller J.W. Age-related macular degeneration. N. Engl. J. Med. 2008;358(24):2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 87.Antonetti D.A., Klein R., Gardner T.W. Diabetic retinopathy. N. Engl. J. Med. 2012;366(13):1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 88.Hammes H.P., Federoff H.J., Brownlee M. Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol. Med. 1995;1(5):527–534. [PMC free article] [PubMed] [Google Scholar]
- 89.Mantelli F., Lambiase A., Colafrancesco V., Rocco M.L., Macchi I., Aloe L. NGF and VEGF effects on retinal ganglion cell fate: new evidence from an animal model of diabetes. Eur. J. Ophthalmol. 2014;24:247–253. doi: 10.5301/ejo.5000359. [DOI] [PubMed] [Google Scholar]
- 90.Sondell M., Sundler F., Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur. J. Neurosci. 2000;12(12):4243–4254. doi: 10.1046/j.0953-816X.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 91.Nishijima K., Ng Y.S., Zhong L., Bradley J., Schubert W., Jo N., Akita J., Samuelsson S.J., Robinson G.S., Adamis A.P., Shima D.T. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol. 2007;171(1):53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whitmire W., Al-Gayyar M.M., Abdelsaid M., Yousufzai B.K., El-Remessy A.B. Alteration of growth factors and neuronal death in diabetic retinopathy: what we have learned so far. Mol. Vis. 2011;17:300–308. [PMC free article] [PubMed] [Google Scholar]
- 93.Abe T., Morgan D.A., Gutterman D.D. Protective role of nerve growth factor against postischemic dysfunction of sympathetic coronary innervation. Circulation. 1997;95(1):213–220. doi: 10.1161/01.CIR.95.1.213. [DOI] [PubMed] [Google Scholar]
- 94.Holtzman D.M., Sheldon R.A., Jaffe W., Cheng Y., Ferriero D.M. Nerve growth factor protects the neonatal brain against hypoxic-ischemic injury. Ann. Neurol. 1996;39(1):114–122. doi: 10.1002/ana.410390117. [DOI] [PubMed] [Google Scholar]
- 95.Villoslada P., Genain C.P. Role of nerve growth factor and other trophic factors in brain inflammation. Prog. Brain Res. 2004;146:403–414. doi: 10.1016/S0079-6123(03)46025-1. [DOI] [PubMed] [Google Scholar]
- 96.Kaye D.M., Vaddadi G., Gruskin S.L., Du X.J., Esler M.D. Reduced myocardial nerve growth factor expression in human and experimental heart failure. Circ. Res. 2000;86(7):E80–E84. doi: 10.1161/01.RES.86.7.e80. [DOI] [PubMed] [Google Scholar]
- 97.Chiaretti A., Falsini B., Aloe L., Pierri F., Fantacci C., Riccardi R. Neuroprotective role of nerve growth factor in hypoxicischemic injury. From brain to skin. Arch. Ital. Biol. 2011;149(2):275–282. doi: 10.4449/aib.v149i2.1364. [DOI] [PubMed] [Google Scholar]