Abstract
Objective:
The purpose of this study was to test an experimental model of the effects of sleep continuity disturbance on sleep architecture and positive mood in order to better understand the mechanisms linking insomnia and depression.
Design:
Participants were randomized to receive 3 consecutive nights of sleep continuity disruption via forced nocturnal awakenings (FA, n = 21), or one of two control conditions: restricted sleep opportunity (RSO, n = 17) or uninterrupted sleep (US, n = 24).
Setting:
The study was set in an inpatient clinical research suite.
Participants:
Healthy, good-sleeping men and women were included.
Measurement and Results:
Polysomnography was used to measure sleep architecture, and mood was assessed via self-report each day. Compared to restricted sleep opportunity controls, forced awakenings subjects had significantly less slow wave sleep (P < 0.05) after the first night of sleep deprivation, and significantly lower positive mood (P < 0.05) after the second night of sleep deprivation. The differential change in slow wave sleep statistically mediated the observed group differences in positive mood (P = 0.002).
Conclusions:
To our knowledge, this is the first human experimental study to demonstrate that, despite comparable reductions in total sleep time, partial sleep loss from sleep continuity disruption is more detrimental to positive mood than partial sleep loss from delaying bedtime, even when controlling for concomitant increases in negative mood. With these findings, we provide temporal evidence in support of a putative biologic mechanism (slow wave sleep deficit) that could help explain the strong comorbidity between insomnia and depression.
Citation:
Finan PH, Quartana PJ, Smith MT. The effects of sleep continuity disruption on positive mood and sleep architecture in healthy adults. SLEEP 2015;38(11):1735–1742.
Keywords: sleep continuity disruption, positive mood, slow wave sleep, insomnia, sleep deprivation
INTRODUCTION
Adequate sleep is a critical component of adaptive mental health functioning. Approximately 10% of the population suffers from insomnia, a chronic sleep disorder with daytime impairments.1 The most commonly reported symptom of insomnia is sleep continuity disruption, whereby inadequate sleep is achieved in fits and starts throughout the night.2 One of the most common daytime symptoms of insomnia is depressed mood.3 However, the mechanisms underlying the interface of insomnia and depression are poorly understood. In the current study, we tested an experimental model of the effects of sleep continuity disturbance on positive and negative mood in order to better understand the mechanisms linking insomnia and depression. We evaluated changes in mood and sleep architecture following a forced awakenings experimental manipulation of sleep continuity (FA) versus a restricted sleep opportunity comparison condition (RSO) that matched the FA condition in overall amount of sleep loss, but permitted well-consolidated sleep.
Several large epidemiological studies have indicated that insomnia increases vulnerability for incident and recurrent depression.4,5 Depression is characterized by poor mood regulation, and patients frequently describe frank deficits in positive mood, a core symptom known as anhedonia. Although the effects of insomnia and poor sleep quality on negative mood have been well characterized,6 emerging literature highlights the possibility that sleep loss and poor sleep quality may be more robustly linked to deficits in positive mood, relative to negative mood.7–10 Mechanisms accounting for the effect of insomnia on positive mood, however, have not been explained.
Evidence suggests that changes in sleep architecture increase risk for depression.11 The sleep architecture changes most commonly associated with depression include decreased latency to rapid eye movement (REM) sleep increased rapid eye movement sleep and increased REM density.12–14 Although a great deal of attention has been placed on REM sleep, reductions in slow wave sleep (SWS) have also been observed in depression.15–17 Despite the extensive literature on sleep architecture and depression, the extent to which changes in sleep architecture are associated with positive relative to negative mood states is not presently known, and it is not clear if one aspect of sleep architecture is more strongly associated with positive mood states than others. One intriguing finding from Walker and Stickgold18,19 revealed that positive emotional memories were more poorly consolidated, whereas negative emotional memories were relatively unchanged following total sleep deprivation. The authors postulated that loss of SWS may have accounted for this effect,18,19 but could not directly test that hypothesis due to their inability to measure SWS in the context of total sleep deprivation. To the extent that sleep continuity disruption impairs the generation of slow waves, it is reasonable to hypothesize that multiple nights of FA may have a greater effect on positive, relative to negative, mood than RSO, in which consolidated sleep is permitted and SWS is expected to be only transiently attenuated.
In the current study, healthy good-sleeping participants were randomized to receive 3 consecutive nights of FA, or one of two control conditions: RSO or uninterrupted sleep (US). We hypothesized that positive mood, but not negative mood, would be more greatly impaired in the FA condition relative to RSO, and that the effect would be statistically mediated by changes in sleep architecture (either REM, REM latency, or SWS).
METHODS
Participants
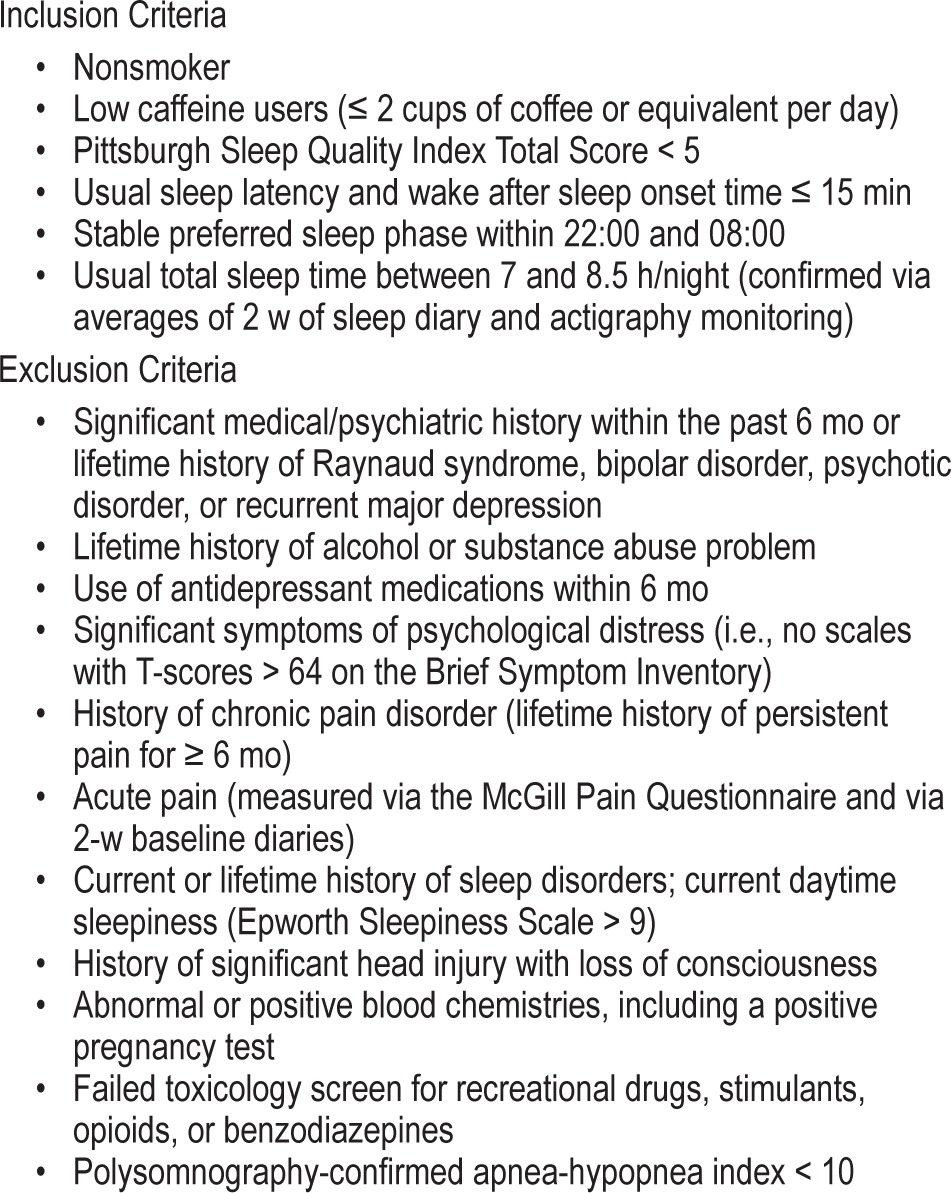
The sample was composed of 62 healthy men and women demonstrating good sleep. The female subjects (n = 39) included in this report participated in a prior study examining the effects of sleep disruption on pain, but did not examine changes in mood.20 Subsequent to that publication we recruited 23 men to participate in the current study on sleep and affect, following identical procedures. Subjects were required to undergo a 2-w washout period of centrally acting agents (including caffeine), prior to starting the study. Eligibility criteria is provided in Table 1. The protocol was approved by the institutional review board and all subjects completed informed consent prior to participation.
Table 1.
General inclusion and exclusion criteria.
Measures
Questionnaires were administered each night prior to the subject's typical bedtime. Exact timing of measurements was not recorded, but technicians were instructed to administer the questionnaires as close as possible to the subject's sleep diary-verified bedtime, which typically occurred between 22:00 and 23:00. The following questionnaires were administered.
Profile of Mood States Bipolar (POMS Bipolar)
The POMS Bipolar is a 72-item, four-point Likert scale self-report measure with 36 positive and 36 negative emotion items. The POMS-Bipolar has good internal consistency and is sensitive to change.21 The measure produces a global positive mood scale and a global negative mood scale, which in our sample correlated at r = −0.45. We used the global indices in primary analyses, and followed up with secondary analyses that investigated group differences in each of the subscales. The Composed/Anxious subscale assesses low activation positive items such as relaxed and serene versus high activation negative items such as anxious and jittery. The Agreeable/ Hostile subscale assesses low activation positive items such as friendly and sympathetic versus high activation negative items, such as annoyed and angry. The Elated/Depressed scale measures high activation positive items such as cheerful and joyful versus low activation negative items such as lonely and dejected. The Confident/Unsure scale measures high activation positive items such as bold and forceful versus low activation negative items such as unsure and timid. The Energetic/Tired scale assesses high activation positive items such as alertness and vigor versus low activation negative items such as sluggishness and exhaustion. Finally, the Clearheaded/Confused subscale assesses high activation positive items such as efficient and attentive versus low activation negative items such as confused and dazed.
Stanford Sleepiness Scale (SSS)
The SSS is a widely used, seven-item, seven-point Likert scale self-report measure, designed to evaluate subjective changes in sleepiness.22 The SSS can be repeatedly administered and is highly sensitive to change.
Nocturnal Polysomnography (PSG)
Participants slept in a private inpatient research room. PSGs were acquired according to standard PSG procedures, using Embla amplifiers (N7000) and Somnologica software, and scored according to established clinical research standards,23 as described in our earlier publication.20
Study Design
Figure 1 depicts the study design, which is adapted from the parent study.20 After completing the 2-w screening period, subjects were admitted to the clinical research unit for a night of polysomnographic screening. Subjects who remained eligible were randomized to one of three conditions: FA, RSO, or US (see endnote A). In all three conditions, subjects were required to remain on the unit throughout the day, and were permitted to move about the unit, interact with other research participants, and spend time in the group recreation room at their leisure. Specific activities were not recorded.
Figure 1.
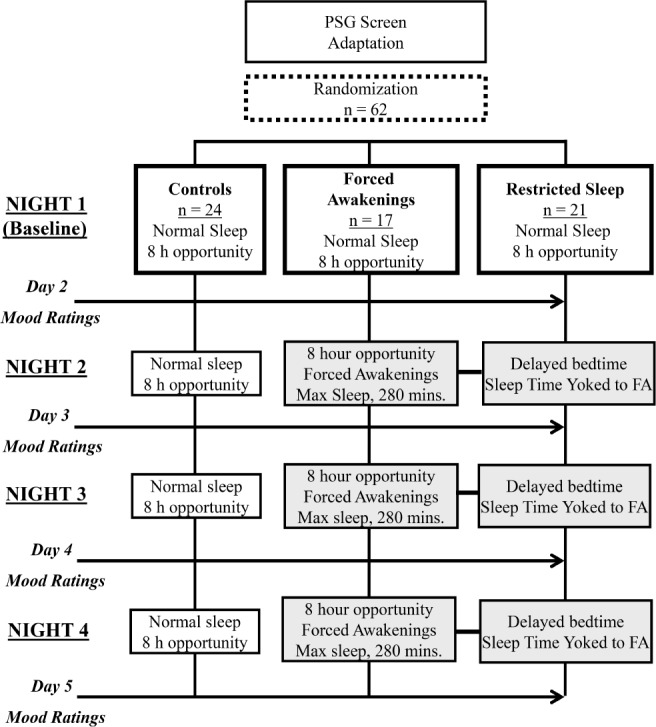
Study design.
US
Subjects continued to sleep undisturbed with an 8-h sleep opportunity for the remaining 5 nights.
FA
Subjects underwent 3 consecutive nights (Nights 2–4) of partial sleep deprivation via a forced awakening protocol. The night was divided into eight 1-h intervals. One of the hour-long intervals was randomly determined as a 60-min forced awakening, during which no sleep was permitted. The remaining seven, 60-min intervals were subdivided into thirds (20-min intervals), and one 20-min interval was randomly selected as a forced awakening period. During assigned forced awakening periods, nursing staff awakened subjects and keep them awake for the entire interval. Subjects were asked to sit up in bed with the lights on to reduce the chance of microsleep. The maximum total sleep time possible was 280 min. Polysomnographic monitoring was maintained for the entire sleep opportunity period. Subjects were not permitted to the leave the inpatient unit during sleep deprivation periods and were under continuous nursing supervision/monitoring (day and night) to prevent naps and ensure safety.
RSO
During Nights 2–4 of RSO, subjects' total sleep opportunity period was restricted and yoked to the amount of total sleep time achieved by a matched subject in the FA group. This was accomplished by delaying the RSO subject's bedtime and keeping a fixed wake time. For example, if an FA subject achieved 210 min of total sleep time on Night 2, the yoked RSO subject would be provided a 210-min opportunity for undisturbed sleep (bedtime 03:30, wake time 07:00) on Night 2. Subjects in the RSO condition were monitored polysomnographically for an entire 8-h period.
Data Analytic Plan
Prior to conducting primary analyses, FA and RSO groups were compared to US as a manipulation check on total sleep time and sleepiness. Primary analyses were then limited to FA and RSO groups. The primary hypotheses were that positive mood, but not negative mood, would be significantly lower over time in the FA condition relative to RSO, and that the effect would be statistically mediated by changes in REM, REM latency, or SWS. To test these hypotheses, we first calculated residualized change scores for SWS, REM, and REM latency (referred to as ΔSWS, ΔREM, and ΔREM latency, respectively). Next, separate multilevel growth models were fit to include Group (FA versus RSO), Time (Day 2 through Day 5), the residualized change in sleep architecture (ΔSWS, ΔREM, and ΔREM latency, respectively) (see endnote B), and all two- and three-way interactions therein. Multilevel models were tested according to specification from Singer and Willett.24 Negative mood was covaried in all models to partial outshared variance between affective valences. Sex was covaried due to the imbalance between males and females, and prior evidence for sex differences in sleep architecture.15 Sleepiness was covaried to account for shared variance with positive mood. The same models were then repeated, with negative mood as the outcome and positive mood as the covariate to determine if effects were differential by valence.
Several methods for testing statistical mediation have been proposed,25 and study design is a key factor in choosing which method to employ. Due to the temporal sequencing of the target variable, the mediator, and the outcome, we elected to employ the MacArthur approach26 to test mediation. This approach specifies that two criteria be met to infer mediation: (1) the target variable must temporally precede the mediator, and (2) the target variable and mediator must be associated. In our model, the outcome was change in positive mood, the target variable was the Group × Time interaction because we were examining group differences in the change in positive mood over time, and the mediators were ΔSWS, ΔREM, and ΔREM latency, respectively. Because the sleep manipulation preceded any change in sleep architecture, mediation was inferred from either a statistically significant (P < 0.05) interaction of Group × Time × sleep architecture (ΔSWS, ΔREM, or ΔREM), or a main effect of one of the sleep architecture terms.26
RESULTS
Table 2 provides demographic information on all participants by Group. The sample was comprised of a majority of females due to an initial recruitment strategy that targeted only females, and subsequently was expanded to include both sexes for the current ancillary project investigating mood. All other demographic indicators were evenly distributed between groups.
Table 2.
Sample demographics.
Manipulation Check
Polysomnographic measures of sleep continuity [total sleep time (TST) and sleep efficiency (SE)] and a subjective measure of daytime sleepiness were used to verify the integrity of the partial sleep deprivation conditions relative to US. Descriptive statistics for these measures are available in Table 3.
Table 3.
Manipulation check on sleep continuity and sleepiness.
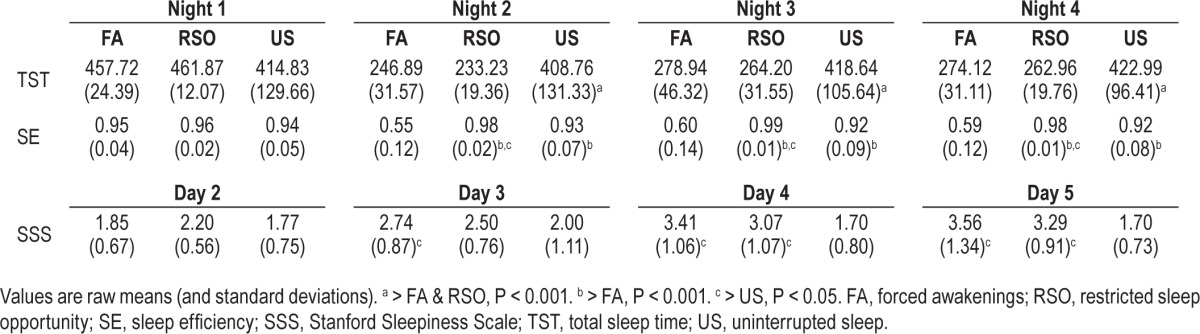
Together, these findings confirmed that participants in US did not experience disruptions of sleep continuity or sleepiness relative to FA or RSO. In addition, as expected, US subjects did not significantly change in positive mood (P = 0.15) or negative mood (P = 0.43) across days. Given these data, US was not included in the primary analyses, and all Group main and interaction effects below refer to the contrast between FA and RSO.
Group Differences in Positive and Negative Mood
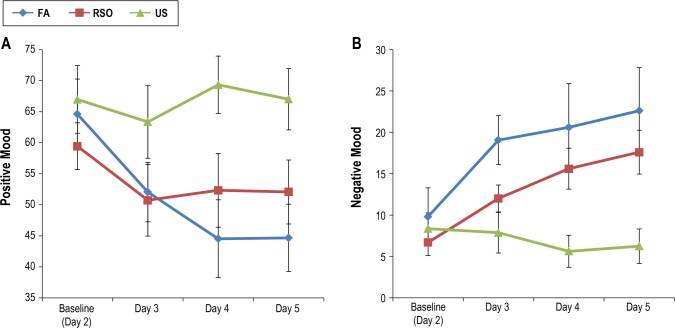
Table 4 shows means and standard deviations for positive and negative mood across days. Groups (FA versus RSO) were not significantly different in positive or negative mood on any day. Figure 2A demonstrates that positive mood decreased for all subjects randomized to RSO or FA. However, a mixed-effects model revealed a significant Group × Time interaction (t = −2.23, df = 38, P = 0.03), indicating that FA subjects evidenced a significantly greater reduction in positive mood from baseline through Day 5 than RSO subjects. This model was significant (P < 0.05) with and without negative mood and sleepiness entered as covariates, suggesting the group effect on positive mood is above and beyond the shared variance between mood valences and sleepiness.
Table 4.
Descriptive statistics for sleep architecture variables and mood.
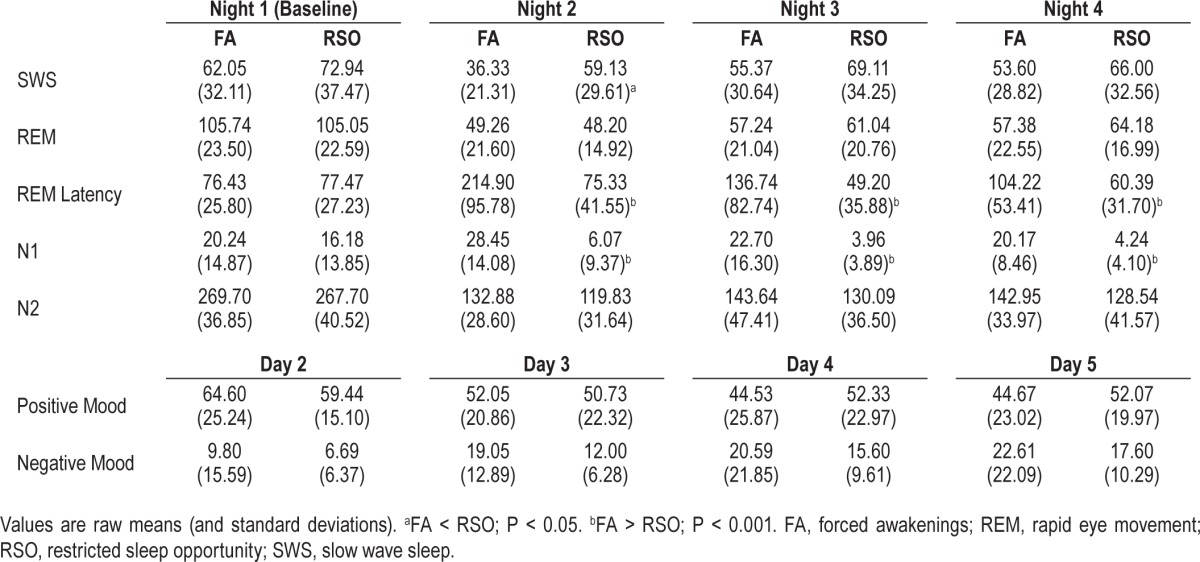
Figure 2.
Means and standard errors of positive mood (A) and negative mood (B) by group over time. FA, forced awakenings; RSO, restricted sleep opportunity; US, uninterrupted sleep.
Negative mood significantly increased throughout the 3-day partial sleep deprivation period for all subjects (irrespective of FA or RSO), controlling for positive mood (t = 3.56, df = 103, P = 0.001). FA and RSO groups did not differ in the rate of change of negative mood (P = 0.36). Figure 2B displays means and standard errors.
Together, these findings indicate that (1) FA had a more robust effect on positive mood compared to RSO; 2) the effect is observed on the day after the second night of sleep deprivation and maintained through the day after a subsequent night of sleep manipulation; and (3) the RSO and FA conditions did not differentially influence negative mood.
Secondary analyses on POMS-BI subscales were conducted to increase the granularity of the findings. Significant Group × Time interactions were observed for the Agreeable/Hostile (P = 0.02) and Clearheaded/Confused (P = 0.002) subscales, whereas groups did not differ over time on other subscales (Ps > 0.17). These data suggest that there was not a clear pattern of effects favoring low versus high activation positive mood items, and support the decision to use the global positive mood index as the primary dependent measure. Most importantly, these secondary analyses suggest that the primary group differences on positive mood were not driven solely by a sense of fatigue, but rather represented multiple dimensions of positive mood.
Group Differences in SWS, REM, and REM Latency
Means for each sleep stage (in minutes) by group (FA versus RSO) can be found in Table 4. FA and RSO did not differ in the duration of stage N2 on any night. FA participants had more stage N1 sleep on Night 2 (F = 28.36, df = 34, P < 0.001), Night 3 (F = 17.61, df = 32, P < 0.001), and Night 4 (F = 41.85, df = 31, P < 0.001). Notably, in addition to the increase in stage N1 on Night 2, FA subjects had significantly lower SWS (F = 7.03, df = 34, P = 0.01) and significantly higher REM latency (F = 27.76, df = 34, P < 0.001) observed on that night. Because increased stage N1 might be considered an inherent artifact of the FA condition (i.e., subjects typically reinitiate sleep via N1), we entered stage N1 as a covariate in subsequent models.
SWS
Across subjects, minutes of SWS significantly decreased (t = −2.87, df = 99, P = 0.005; see Figure 3). Groups did not significantly differ in the rates of change over all nights (P = 0.17). However, a post-hoc analysis indicated that the rate of change in SWS from Night 1 to Night 2 was significantly greater for the FA group compared to the RSO group (F = 4.74, df = 33, P = 0.04).
Figure 3.
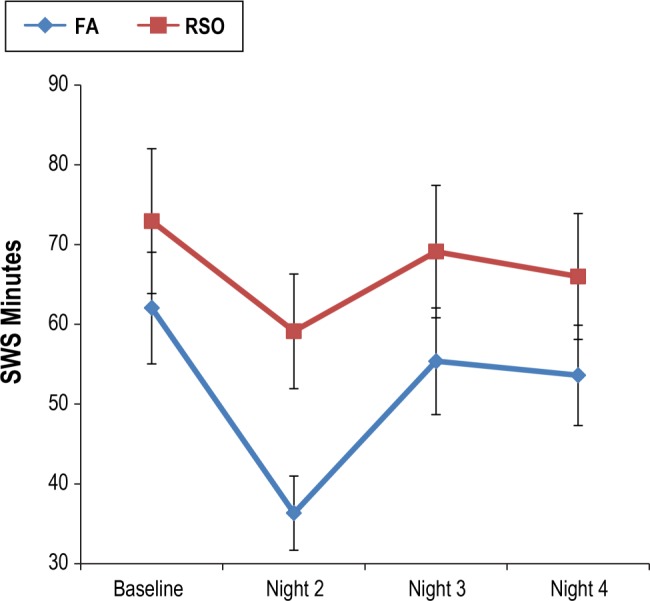
Means and standard errors in slow wave sleep by group over time. FA, forced awakenings; RSO, restricted sleep opportunity; SWS, slow wave sleep.
These findings indicate that FA has a stronger immediate effect on SWS compared to RSO. Although not statistically significant, a trend in the means (see: Table 2) indicates that FA subjects did not fully recover this relative SWS deficit even as sleep pressure increased on the second night of FA.
REM
Groups did not differ in REM on any experimental night. Across subjects, minutes of REM significantly decreased (t = −10.49, df = 101, P < 0.001), and the groups did not significantly differ in the rate of change (P = 0.76).
REM Latency
FA subjects had significantly greater REM latency on each experimental night, and a greater change over time from baseline, relative to RSO (t = 4.85, df = 97, P < 0.001). A post-hoc comparison indicated that the rate of change in REM latency from baseline to Night 2 was significantly greater for the FA group compared to the RSO group (F = 27.34, df = 33, P < 0.001).
Do Changes in SWS or REM Latency Mediate the Effect of Forced Awakenings on Positive Mood?
Based on the aforementioned findings, we created residualized change scores for SWS and REM Latency from baseline to Night 2, and included them as mediators in separate mediation models. Because there were no group differences in REM change over time, it was not eligible to test as a mediator.
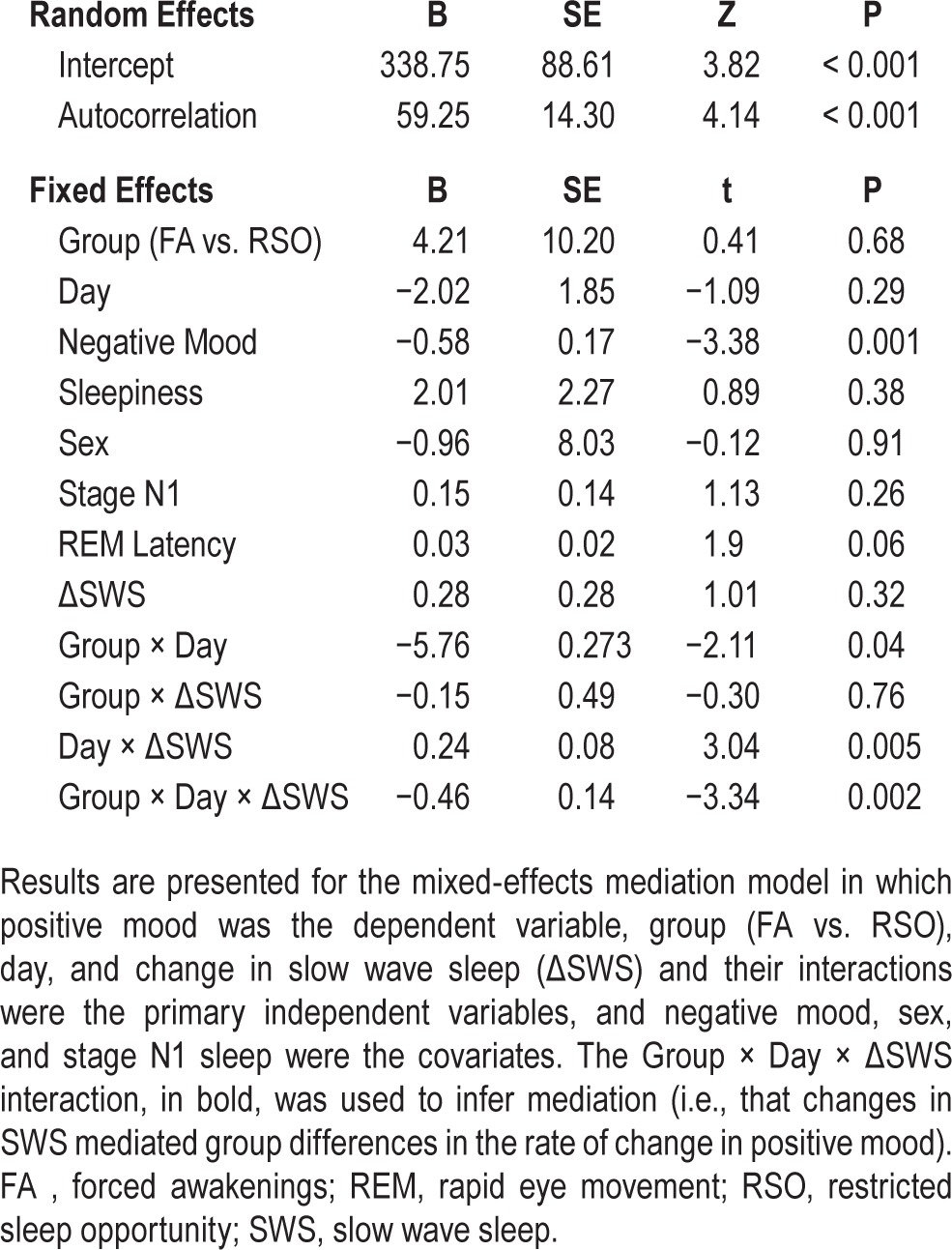
SWS Mediation Model
The SWS mediation model tested whether change in SWS from baseline to Night 2 mediated the linear Group × Time effect on positive mood. The results for this model are included in Table 5. Notably, the significant interaction of Group × Time × ΔSWS (t = −3.34, df = 34, P = 0.002) provides evidence that the change in SWS from baseline to Night 2 mediated the group differences (FA > RSO) in the rate of change in positive mood across nights of partial sleep deprivation. To determine how much variation in positive mood was explained by the mediation effect, a pseudo R2 was calculated from the residual variance components from successive models with and without the Group × Time × ΔSWS interaction term. The pseudo R2 for this effect was 0.14, indicating that the Group × Time × ΔSWS interaction accounted for 14% of the explainable variance in positive mood.
Table 5.
Primary mixed-effects mediation model.
REM Latency Mediation Model
Results from REM latency mediation model indicated that neither the main effect of change in REM latency nor the interaction of Group × Time × ΔREM latency was statistically significant (Ps > 0.95). Thus, the evidence indicated that the change in REM latency did not mediate the group differences in the rate of change of positive mood.
DISCUSSION
This study revealed the following key findings: (1) 3 nights of forced nocturnal awakenings (FA) reduced positive mood to a significantly greater extent than 3 nights of restricted sleep (RSO) in a sleep duration-yoked experimental design; (2) FA produced a significantly larger reduction in SWS and increase in REM latency than the RSO condition; and (3) the change in SWS, but not REM latency, significantly mediated the effect of FA on positive mood.
The current study introduces several novel findings. To our knowledge, this is the first human experimental study to demonstrate that, despite comparable reductions in TST, partial sleep loss from sleep continuity disruption (i.e., FA) is more detrimental to positive mood than partial sleep loss from delaying bedtime (i.e., RSO), even when controlling for concomitant increases in negative mood. Notably, the group difference emerged after the second night of sleep manipulation and was maintained through the next day, indicating that the effect of sleep continuity disruption on positive mood may accumulate over time. The FA paradigm was initially developed as an experimental model for sleep maintenance problems, which are characterized by repeated nocturnal awakenings resulting in elevated levels of wake after sleep onset. Sleep maintenance difficulty is the most commonly reported symptom of insomnia,1,2 and is a common feature of sleep in a variety of other contexts, such as early parenthood, combat, and on-call health care work. As such, the ecological validity of the FA paradigm enhances the generalizability of the present findings. Future studies will be needed to determine the extent to which the acute effects of experimental sleep continuity disruption correlate with the daily effects of sleep maintenance insomnia, which may be less severe than those experienced through our manipulation.
FA and RSO were both associated with decreases in positive mood and increases in negative mood. However, whereas FA more strongly affected positive mood, the groups were equivalent in their effect on negative mood. This suggests that sleep continuity disruption is especially harmful for positive mood, compared to restricted sleep loss. Additionally, the effect of FA on positive mood was present even when negative mood was covaried, suggesting that FA has unique effects on positive relative to negative mood. These findings support the results of several recent studies that have revealed: (1) a cross-sectional association between self-reported sleep quality and lower positive mood, independent of negative mood, in depressed patients7; (2) a stronger lagged daily association between self-reported sleep quality and lower positive, relative to negative, mood9; (3) a larger change in positive, relative to negative, mood following total sleep deprivation10; and (4) a failure to consolidate positive, but not negative, emotional memories following total sleep deprivation.18 Notably, the current results are in contrast to those demonstrating transient bursts in positive mood following therapeutic total sleep deprivation in depressed patients.27 These conflicting findings are likely due to differences in sleep manipulation (e.g., FA versus total sleep deprivation), and sample constituency (e.g., healthy subjects versus depressed patients), and should be resolved in future investigations with clinical subjects.
The current findings fit the arc of a narrative describing a putative pathway from insomnia to depression in individuals without preexisting mood disturbance. Notably, secondary analyses on mood subscales indicated that the effects of FA on positive mood were not limited to aspects of energy and vigilance, but rather extended to low activation positive emotions such as agreeableness. This supports our findings' relevance for depression; indeed, a deficit in the ability to experience positive emotions is the critical element of anhedonia, one of the core symptoms of depression. Poor positive emotion regulation is associated with cardiovascular disease,28 chronic pain,29 and inflammation,30–32 all of which are comorbid with depression. Furthermore, positive mood promotes resilience to day-to-day spikes in physical and social stressors,33–36 which commonly precipitate depressive episodes.37 Thus, our findings may help future research to clarify sleep related mechanisms by which individuals become vulnerable versus resilient to developing depression and other comorbid chronic illnesses. Additionally, our findings may help explain the high rate of depression remission observed in Manber et al.'s38 clinical trial of cognitive-behavioral therapy for insomnia in patients with comorbid depression and insomnia. Understanding valence-specific effects of sleep continuity disruption on mood, and their corresponding mechanisms, could lead to an improvement in the specificity of treatments available to patients with insomnia.
With these findings, we provide temporal evidence in support of a putative biologic mechanism (SWS deficit) that could explain the strong comorbidity between insomnia and depression. Both groups demonstrated similar levels of partial SWS loss (compared to baseline) for the second and third nights of sleep deprivation. However, because SWS is under robust homeostatic control, the FA group carried forward a larger relative deficit in SWS compared to RSO, which in turn mediated the differential deficit in positive mood. In terms of effect size, the initial change in SWS accounted for 14% of the variance in the divergent linear slopes of positive mood, indicating that the mediation effect was of moderate to large size. Interestingly, the group divergence in positive mood was lagged by 1 day from the group divergence in SWS. These data may indicate that the effects of SWS loss take time to unfold, and may be particularly harmful in the context of chronic sleep continuity disruption.
It remains to be determined whether the relationship between SWS and positive mood changes over the course of daily life with chronic sleep maintenance insomnia and comorbid depression. With the advent of emerging ambulatory sleep electroencephalography technology, it will be imperative to gather longitudinal measurements of sleep architecture, along with sleep-maintenance insomnia symptoms and positive mood, to determine if the present experimental findings can be replicated in a naturalistic setting.
This study was limited in a few respects. First, because the parent study introduced total sleep deprivation before participants engaged in recovery sleep, we were unable to study recovery sleep associated changes in sleep architecture and mood without confound. Future investigations should seek to replicate our findings and include recovery sleep immediately following FA and RSO. Second, the current findings are limited to self-reported mood, and therefore may be influenced by reporting biases. Future studies should include psycho-physiological and/or neurobiological measures of emotion to support and extend the present results. Third, mood was only sampled once per day, which prevents our ability to examine the dynamic changes in mood that may be expected to occur throughout the day as a result of circadian variation.39 Finally, although our findings provide temporal evidence that SWS mediates the effect of sleep continuity disruption on positive mood, we cannot conclude that the effect is causal because the FA condition was an indirect manipulation of SWS. Future studies that selectively disrupt SWS versus REM would extend the current results.
In summary, the current study introduces the novel finding that sleep continuity disruption reduces positive mood via disruption of SWS, and adds texture to an emerging body of literature that highlights the ramifications of insomnia on the regulation of positive emotions. Together, these emergent data provide a compelling rationale for a putative pathway linking insomnia and depression.
ENDNOTES
A: The parent study protocol 20 also included a night of total sleep deprivation and a night of recovery sleep, both of which followed the partial sleep deprivation period. Data associated with those 2 nights were not included in the current analyses for two reasons. First, our hypotheses were specific to the effects of FA on sleep architecture. Second, the night of total sleep deprivation confounded our ability to examine the effects of recovery sleep on sleep architecture and mood.
B: The original manuscript reported a significant difference between FA and RSO on SWS. However, since 21 additional male subjects were recruited for the present analyses, we left open the hypothesis that groups would be different on that parameter, and elected to test all three sleep architecture variables as mediators in the event of significant group differences.
DISCLOSURE STATEMENT
This was not an industry supported study. Funding sources: NIH K23 DA035915 and NIH P30 NR014131 to Dr. Finan; R21 NS051771, and K23 NS47168 both to Dr. Smith. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.National Sleep Foundation. Sleep in America poll 2002. http://sleepfoundation.org/sleep-polls-data/sleep-in-america-poll/2002-adult-sleep-habits.
- 2.Rosenberg RP. Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies. Ann Clin Psychiatry. 2006;18:49–56. doi: 10.1080/10401230500464711. [DOI] [PubMed] [Google Scholar]
- 3.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31:473–80. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76:255–9. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 5.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010;14:227–38. doi: 10.1016/j.smrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Bower B, Bylsma LM, Morris BH, Rottenberg J. Poor reported sleep quality predicts low positive affect in daily life among healthy and mood-disordered persons. J Sleep Res. 2010;19:323–32. doi: 10.1111/j.1365-2869.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- 8.Dagys N, McGlinchey EL, Talbot LS, Kaplan KA, Dahl RE, Harvey AG. Double trouble? The effects of sleep deprivation and chronotype on adolescent affect. J Child Psychol Psychiatry. 2012;53:660–7. doi: 10.1111/j.1469-7610.2011.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Wild-Hartmann JA, Wichers M, van Bemmel AL, et al. Day-to-day associations between subjective sleep and affect in regard to future depression in a female population-based sample. Br J Psychiatry. 2013;202:407–12. doi: 10.1192/bjp.bp.112.123794. [DOI] [PubMed] [Google Scholar]
- 10.Talbot LS, McGlinchey EL, Kaplan KA, Dahl RE, Harvey AG. Sleep deprivation in adolescents and adults: changes in affect. Emotion. 2010;10:831. doi: 10.1037/a0020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 12.Kupfer DJ. REM latency: a psychobiologic marker for primary depressive disease. Biol Psychiatry. 1976;11:159–74. [PubMed] [Google Scholar]
- 13.Giles DE, Jarrett RB, Roffwarg HP, Rush AJ. Reduced rapid eye movement latency: a predictor of recurrence in depression. Neuropsychopharmacology. 1987;1:33–9. doi: 10.1016/0893-133x(87)90007-8. [DOI] [PubMed] [Google Scholar]
- 14.Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D. REM sleep dysregulation in depression: state of the art. Sleep Med Rev. 2013;17:377–90. doi: 10.1016/j.smrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Armitage R, Hoffmann R, Trivedi M, Rush AJ. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 2000;95:201–13. doi: 10.1016/s0165-1781(00)00178-5. [DOI] [PubMed] [Google Scholar]
- 16.Plante DT, Landsness EC, Peterson MJ, et al. Sex-related differences in sleep slow wave activity in major depressive disorder: a high-density EEG investigation. BMC Psychiatry. 2012;12:146. doi: 10.1186/1471-244X-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann R, Hendrickse W, Rush AJ, Armitage R. Slow-wave activity during non-REM sleep in men with schizophrenia and major depressive disorders. Psychiatry Res. 2000;95:215–25. doi: 10.1016/s0165-1781(00)00181-5. [DOI] [PubMed] [Google Scholar]
- 18.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–66. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 19.Walker MP. The role of slow wave sleep in memory processing. J Clin Sleep Med. 2009;5(2 Suppl):S20–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 21.Lorr M, McNair DM. Profile of mood states: bipolar form. San Diego, CA: Educational and Industrial Testing Service; 1988. [Google Scholar]
- 22.Hoddes E, Dement W, Zarcone V. The development and use of the Stanford sleepiness scale (SSS) Psychophysiology. 1972;9:150. [Google Scholar]
- 23.Rechtschaffen A, Kales A. Washington, DC: US Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 24.Singer JD, Willet JB. London, UK: Oxford University Press; 2003. Applied longitudinal data analysis: modeling change and event occurrence. [Google Scholar]
- 25.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychol. 2008;27:S101–8. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry. 1999;46:445–53. doi: 10.1016/s0006-3223(99)00125-0. [DOI] [PubMed] [Google Scholar]
- 28.Fredrickson L, Levenson RW. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cogn Emotion. 1998;12:191–220. doi: 10.1080/026999398379718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finan PH, Garland EL. The role of positive affect in pain and its treatment. Clin J Pain. 2015;31:177–87. doi: 10.1097/AJP.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steptoe A, O'Donnell K, Marmot M, Wardle J. Positive affect, psychological well-being, and good sleep. J Psychosom Res. 2008;64:409–15. doi: 10.1016/j.jpsychores.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Steptoe A, Dockray S, Wardle J. Positive affect and psychobiological processes relevant to health. J Pers. 2009;77:1747–76. doi: 10.1111/j.1467-6494.2009.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis MC, Zautra AJ, Reich JW. Vulnerability to stress among women in chronic pain from fibromyalgia and osteoarthritis. Ann Behav Med. 2001;23:215–26. doi: 10.1207/S15324796ABM2303_9. [DOI] [PubMed] [Google Scholar]
- 34.Ong AD, Zautra AJ, Reid MC. Psychological resilience predicts decreases in pain catastrophizing through positive emotions. Psychol Aging. 2010;25:516–23. doi: 10.1037/a0019384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong AD, Bergeman CS, Bisconti TL, Wallace KA. Psychological resilience, positive emotions, and successful adaptation to stress in later life. J Pers Soc Psychol. 2006;91:730. doi: 10.1037/0022-3514.91.4.730. [DOI] [PubMed] [Google Scholar]
- 36.Zautra AJ, Affleck GG, Tennen H, Reich JW, Davis MC. Dynamic approaches to emotions and stress in everyday life: Bolger and Zuckerman reloaded with positive as well as negative affects. J Pers. 2005;73:1511–38. doi: 10.1111/j.0022-3506.2005.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 38.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasler BP, Germain A, Nofzinger EA, et al. Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. J Sleep Res. 2012;21:515–26. doi: 10.1111/j.1365-2869.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]