Abstract
The NOP receptor (nociceptin/orphanin FQ opioid peptide receptor) is the most recently discovered member of the opioid receptor family and, together with its endogenous ligand, N/OFQ, make up the fourth members of the opioid receptor and opioid peptide family. Because of its more recent discovery, an understanding of the cellular and behavioral actions induced by NOP receptor activation are less well developed than for the other members of the opioid receptor family. All of these factors are important because NOP receptor activation has a clear modulatory role on mu opioid receptor-mediated actions and thereby affects opioid analgesia, tolerance development, and reward. In addition to opioid modulatory actions, NOP receptor activation has important effects on motor function and other physiologic processes. This review discusses how NOP pharmacology intersects, contrasts, and interacts with the mu opioid receptor in terms of tertiary structure and mechanism of receptor activation; location of receptors in the central nervous system; mechanisms of desensitization and downregulation; cellular actions; intracellular signal transduction pathways; and behavioral actions with respect to analgesia, tolerance, dependence, and reward. This is followed by a discussion of the agonists and antagonists that have most contributed to our current knowledge. Because NOP receptors are highly expressed in brain and spinal cord and NOP receptor activation sometimes synergizes with mu receptor-mediated actions and sometimes opposes them, an understanding of NOP receptor pharmacology in the context of these interactions with the opioid receptors will be crucial to the development of novel therapeutics that engage the NOP receptor.
I. Introduction
Shortly after the cloning of the delta, mu, and kappa opioid receptors, a fourth receptor was cloned by homology with the opioid receptors. This fourth receptor, like the opioid receptors, is a seven transmembrane-spanning G protein-coupled receptor (GPCR), which has overall homology with the opioid receptors as high as the three opioid receptors have with each other. Because of this high homology, the cloning was somewhat facile and was accomplished by several laboratories almost simultaneously. The first paper to be published was by Mollereau et al. (1994), and they called this new receptor opioid receptor like receptor 1, ORL1. Other cloning papers followed quickly, and this same receptor was called LC132, XOR1, kappa 3, ROR-C, C3 (Bunzow et al., 1994; Fukuda et al., 1994; Wang et al., 1994; Lachowicz et al., 1995; Pan et al., 1995). Despite the close homology with opioid receptors, this orphan receptor, when transfected into mammalian cells, did not appear to bind or be activated by standard opiate ligands at low concentrations. For lack of a high affinity ligand, there was not an appropriate binding assay to characterize this receptor. Nevertheless, it was activated by high concentrations of the opiate agonist etorphine and inhibited by a high concentration of naloxone (Mollereau et al., 1994). In addition, it was clearly coupled to Gi, like the opioid receptors, because receptor activation still inhibited adenylyl cyclase (Mollereau et al., 1994). Despite the fact that standard opiates did not activate this receptor at low concentrations, this receptor appeared to be in the opioid receptor family.
Approximately 2 years after the discovery of the orphan receptor, at that time generally called ORL1, two groups identified an endogenous neuropeptide that bound with high affinity to ORL1 and activated the receptor, as determined by inhibition of cAMP accumulation in transfected cells (Meunier et al., 1995; Reinscheid et al., 1995). In both cases, the endogenous ligand was discovered by fractionating tissue (in one case rat brain and the other porcine pituitary) based upon ability to inhibit adenylyl cyclase activity in cells transfected with ORL1. These were the first examples of “reverse pharmacology” to identify ligands subsequent to the discovery of the receptor, a process that has been since used many times (Civelli et al., 2013). This 17-amino acid neuropeptide was called nociceptin (for its ability to decrease hot plate latency when administered intracerebroventricularly into mice) (Meunier et al., 1995) and orphanin FQ (Reinscheid et al., 1995) to denote a ligand for an orphan receptor with first and last amino acids Phe and Gln. The heptadecapeptide Phe-Gly-Gly-Phe-Thr-Gly-Ala-Arg-Lys-Ser-Ala-Arg-Lys-Leu-Ala-Asn-Gln is interesting for several reasons. First the Phe-Gly-Gly-Phe amino terminal is obviously reminiscent of the Tyr-Gly-Gly-Phe found in all opioid peptides. Second, this is a highly basic peptide, quite similar to dynorphin in the number of Lys and Arg residues. Third, the gene structure of the prepropeptide is also similar to the opioid peptide genes (Mollereau et al., 1996a; Nothacker et al., 1996). Together these discoveries of ORL1 and nociceptin/orphanin FQ identified the fourth members of the opioid receptor and opioid gene families. IUPHAR nomenclature for this receptor and peptide is now officially NOP (nociceptin opioid peptide) receptor and N/OFQ (Cox et al., 2015). Compounds targeting the NOP receptor were recently advanced to clinical trials, so an understanding of this receptor system has increased clinical relevance. This review will discuss the NOP receptor system and its important modulatory role in several central nervous system (CNS) systems, along with the signaling pathways that mediate its activity and the synthetic compounds that have been instrumental in the identification and validation of many of these activities.
II. Nociceptin Opioid Peptide Receptor
A. Nociceptin Opioid Peptide Receptor Protein
Comparison of the cDNA-derived amino acid sequence of the NOP protein with that of the opioid receptors and other GPCRs shows that it contains several conserved amino acids and motifs, particularly in the transmembrane helices and the intracellular loops, placing the NOP receptor in the GPCR Class A (rhodopsin-like) receptors, like the mu, delta, and kappa opioid receptors. Greater than 70% of the amino acid residues in the second, third, and seventh helices (TM2, TM3, and TM7) are conserved between NOP and the mu, delta, and kappa opioid receptors. However, only 50% of residues are conserved in TM1, TM5, and TM6, whereas in TM4, only 24% of residues are conserved (Meunier et al., 2000). There is high sequence conservation in the intracellular loops (ICL) among the opioid receptor family, particularly in ICL3 (>80%), which connects TM5 and TM6 and is involved in activation and interaction with the G proteins. The extracellular loops (ECL) on the other hand, have very little sequence similarity among the four opioid receptors, NOP being closest to the kappa opioid receptor in containing a significant number of acidic residues in its ECL2. Notably however, the ECL2 in NOP, but not in other opioid receptors, is involved in receptor activation, as discussed below. Nonetheless, the NOP receptor sequence contains all the conserved activation-associated motifs termed "microswitches" found in the TM helices of Class A GPCRs, including the other opioid receptors (Nygaard et al., 2009; Tehan et al., 2014), suggesting that the transmembrane and intracellular amino acid residues involved in conformational changes during receptor activation (microswitches) in NOP are consistent and similar to the other opioid receptors and Class A GPCRs (see section A.2).
1. Nociceptin Opioid Peptide Receptor Tertiary Structure.
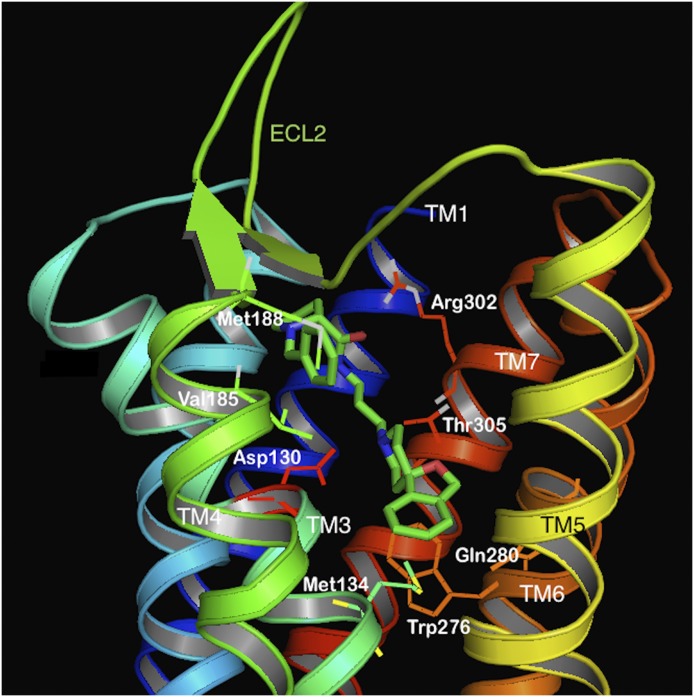
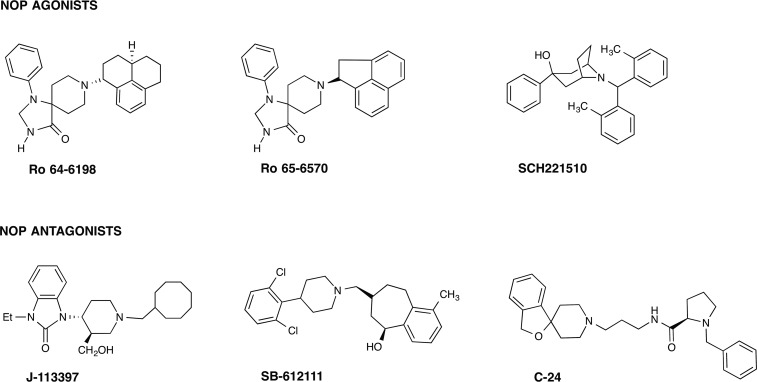
In the rapid explosion of GPCR crystal structure determinations published in the last few years, the structures of all four opioid receptor family members were solved in their inactive, antagonist-bound conformations (Granier et al., 2012; Manglik et al., 2012; Thompson et al., 2012; Wu et al., 2012). These give an atomic-level view into the tertiary structures of the opioid GPCRs and provide confirmation of the several previous homology models of the opioid receptors developed to understand the architecture of these receptors. The NOP receptor was crystallized in its inactive form, bound to the antagonist C-24 (PDB ID: 4EA3, see Fig. 1). As expected, the ligand-binding pocket is contained within the transmembrane helices, with residues from TM3, TM5, TM6, and TM7 interacting with the ligand in the binding pocket. Similarly, molecular modeling of the complex of the peptide agonist N/OFQ with homology models of the NOP receptor (Topham et al., 1998; Akuzawa et al., 2007; Daga and Zaveri, 2012) show that the N-terminal sequence F-G-G-F of N/OFQ binds deep in the transmembrane binding pocket, where the N-terminal amino group of N/OFQ makes an essential anchoring charge interaction with the conserved D1303.32 (superscripts refer to the Ballesteros-Weinstein numbering of the TM helix residue), present in all the opioid receptors as well as in biogenic amine GPCRs (Fig. 2). Although the binding of small-molecule antagonist C-24 in the NOP receptor crystal structure may involve different amino acid residues than those interacting with the peptide agonist N/OFQ in the modeled complex, an extensive array of site-directed mutagenesis studies carried out with NOP show that there are only 4–5 amino acid residues in NOP that afford the exquisite selectivity of N/OFQ for the NOP receptor and precludes binding of small-molecule morphinan opioid ligands. Mutation of the following NOP receptor residues to their corresponding conserved opioid receptor residues (A2165.39 to K, V2796.51–Q2806.52–V2816.53 to I–H–I and T3057.39 to I) confers a functional opioid alkaloid binding site in NOP receptors, which binds opioid antagonists with high affinity, without adversely affecting N/OFQ binding significantly (Meng et al., 1998). This study was consistent with mutagenesis of Q280 in TM6 in NOP to histidine, a TM6 residue conserved in all three opioid receptors, which results in an increase in affinity of opioid agonists lofentanil, etorphine, and dynorphin A and antagonists diprenorphine and nor-BNI, but does not affect N/OFQ binding or potency significantly (Mollereau et al., 1996b). The effect of the Q280H mutation on the binding of small-molecule NOP receptor ligands is not known; however, a Q280A mutation was shown to reduce the potency of receptor activation by N/OFQ and the NOP agonist SCH 221510 by several orders of magnitude (Thompson et al., 2012). Although these five residues (A216, V279, Q280, V281, and T305) serve to preclude binding of opiate ligands to the NOP receptor, no studies have yet explored the reverse question: what residues in the mu, delta, and kappa opioid receptors prevent binding of selective NOP ligands to opioid receptors? Clues for such NOP selectivity-enhancing interactions have come from computer-aided molecular docking studies of the selective NOP agonist Ro 64-6198 into the first active-state NOP receptor homology model, developed by Daga and Zaveri (2012), based on the opsin template (Fig. 3). The amide hydrogen in Ro 64-6198 makes direct hydrogen-bond interaction with T3057.39 (Ile in other opioid receptors) at the extracellular end of the binding pocket, whereas the phenalenyl ring of Ro 64-6198 interacts with the hydrophobic V2796.51 residue inside the binding pocket (Fig. 3) (Daga and Zaveri, 2012). An isoleucine residue, found in the mu, delta, and kappa opioid receptors in this position, would sterically hinder binding of Ro 64-6198 and is possibly responsible for precluding the binding of Ro 64-6198 to these other opioid receptors. The phenalenyl group of Ro 64-6198 is therefore contributing to the excellent selectivity of this ligand for the NOP receptor (Fig. 3) (Daga and Zaveri, 2012).
Fig. 1.
Molecular model of the NOP receptor crystal structure bound to NOP antagonist C-24 (green) (PDB ID: 4EA3). The TM helices are colored in 7 different colors and labeled. The ECL2 loop, between TM4 and TM5 is shown in green. Side chains of amino acids interacting with the antagonist are shown as sticks and labeled.
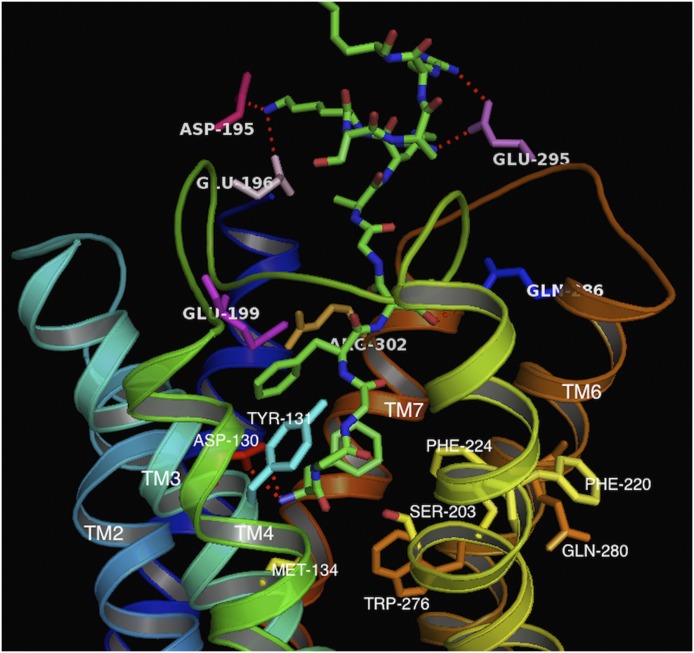
Fig. 2.
N/OFQ (1-13) peptide (green sticks) bound to the active-state homology model of the NOP receptor. The TM helices are in different colors. The side chains of amino acids interacting with the peptide are labeled. Note the acidic residues of the ECL2 loop (D195, E196) interacting with the basic residues (8-13) of N/OFQ.
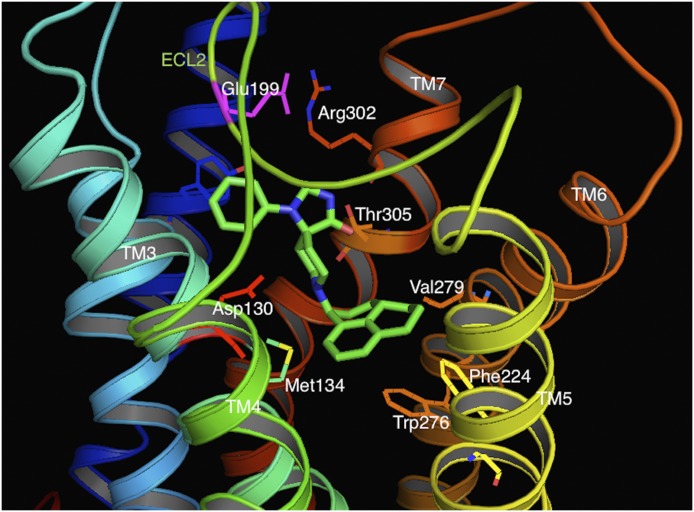
Fig. 3.
NOP agonist Ro 64-6198 (green sticks) bound to the active-state NOP receptor model. The small-molecule NOP agonist interacts with the T305 (orange sticks) and Y309 (blue sticks). The phenalenyl group of the NOP agonist is in close proximity to V279 (orange sticks, labeled) within the transmembrane pocket. This residue is isoleucine in the other opioid receptors, which is likely responsible for the lower affinity of Ro 64-6198 for the other opioid receptors.
Although there is high homology and similarity in functional architecture in the transmembrane and intracellular loops between NOP and other opioid receptors, the ECLs of NOP receptors are distinct in their amino acid sequence, particularly ECL2 that connects the extracellular ends of TM4 and TM5 and ECL3 that connects TM6 and TM7. The ECL2 of NOP has the same residue length as the mu and delta opioid receptors but has almost no sequence similarity. On the other hand, the NOP ECL2 contains several Glu acidic residues, similar to the ECL2 in the kappa receptor, which is three residues longer, and contains mainly Asp residues. Overall, therefore, the NOP ECL2 is unique in its primary structure among the opioid receptors, and its interactions with the amino acids at the extracellular ends of the TM domains (Daga and Zaveri, 2012) play a distinct and critical role in receptor activation, unlike the other three opioid receptors.
Because the recently resolved crystal structure of NOP is bound to an antagonist and is in its inactive form, it does not show molecular interactions of the ECL2 with the bound ligand (Thompson et al., 2012). However, elegant receptor chimera studies, with a NOP–kappa chimera, clearly show that the ECL2 of NOP is an absolute requirement for activation of NOP by N/OFQ, unlike the ECL2 of the kappa receptor, which can be replaced with that of NOP without adversely affecting the activation of the kappa receptor by dynorphin (Mollereau et al., 1999). In fact, replacing the N terminus, ECL1, and ECL2 of the kappa receptor with those of NOP results in a receptor hybrid that has equipotent binding affinity for N/OFQ and dynorphin and, importantly, is activated efficiently by both peptides without a significant loss in potency compared with the native receptors (Mollereau et al., 1999). These studies underscore the importance of the NOP receptor ECL2 for binding and activation of the receptor by NOP agonists.
2. Nociceptin Opioid Peptide Receptor Activation.
The high degree of homology in the TM helices between NOP and other opioid receptors would suggest that the mechanism of receptor activation of the opioid family GPCRs may involve the same residues after ligand binding, resulting in G protein binding and further downstream events. Although the crystal structure of an agonist-bound "active-state" or a constitutively active form of the NOP receptor has not yet been solved, a molecular dynamics simulation of a homology model of the active-state NOP receptor and comparison with the inactive-state receptor suggests that NOP receptor activation is accompanied by movements of the TM helices which are transduced to the intracellular domains, in a manner similar to other Class A GPCRs (Daga and Zaveri, 2012). The intracellular end of TM4 moves toward the helical bundle, whereas that of TM6 moves outward from the helical bundle, creating a binding pocket for the G protein. Activation-associated microswitches (Nygaard et al., 2009) found in all Class A GPCRs are also in their "active" conformations in the active-state NOP homology model. For instance, the conserved "DRY" motif in TM3 (also present in NOP, as Asp1473.49–Arg1483.50–Tyr1493.51) shows no ionic interaction between D147 and R148 in the active-state homology model but has the "ionic lock" between these two residues in the inactive-state conformation (Daga and Zaveri, 2012). In the NOP active-state model, the D1483.50 microswitch shows a H-bonding interaction with Y2355.58, which is an activation-associated interaction involving this conserved DRY motif (Daga and Zaveri, 2012).
The W2766.48 microswitch is part of the CWxP motif in TM6, which undergoes major conformational movement during receptor activation of NOP, as in most other Class A GPCRs. The W276 indole side chain moves from its inactive rotamer conformation to an "active rotamer conformation," in which it interacts with the Phe2245.47 to form an "aromatic lock," an activation-associated conformational movement (Daga and Zaveri, 2012). Another activation-associated microswitch is present in the NPxxY motif in TM7, in which Y7.53 (Y319 in TM7 in NOP) toggles between an inactive rotamer and an active rotamer, which interacts with TM6 residues during activation (Nygaard et al., 2009).
Mutagenesis studies have implicated several residues that are important for the intrinsic efficacy of the endogenous agonist N/OFQ. For instance, mutation of Q2866.58 near the extracellular end of TM6 completely abolishes activation by N/OFQ, without any effect on the binding affinity for the mutated receptor (Mouledous et al., 2000). This suggests a very specific role for this residue during activation after N/OFQ binding, although it does not contribute to binding affinity of N/OFQ. Alanine mutations of W2766.48, the rotamer toggle activation microswitch, and F2245.47 (part of the TM5 "ionic lock" microswitch) have differential effects on activation by structurally different agonists (Mouledous et al., 2000). The W276-A (and F224-A) mutant showed two to fourfold decreased binding by N/OFQ and decreased potency of activation, but no decrease in overall intrinsic efficacy, i.e., the W276-A showed full agonist efficacy, albeit with higher concentrations of N/OFQ. However, with the hexapeptide Ac-RYYKWK-NH2 [a partial agonist at NOP (Dooley et al., 1997)] and lofentanil, the W276-A mutant showed no decrease in binding affinity for these ligands but could not be fully activated by these ligands, producing low efficacy partial agonist activity. It is likely, therefore, that structurally different agonist ligands engage different residues during activation, resulting in multiple "active states," leading to different levels of intrinsic efficacy and possibly functional selectivity (biased signaling) at the intracellular end of the membrane-bound NOP receptor (Wacker et al., 2013; Shukla et al., 2014).
Despite the similarities of NOP receptor activation-associated TM movements to other GPCRs, one feature that sets apart NOP receptor activation from that of the three other opioid receptors and most other GPCRs is the absolute requirement for the ECL2 for activation (Lapalu et al., 1998; Mollereau et al., 1999). Mutation studies of ECL2 residues have not yet been reported, but the NOP ECL2 contains a high number of acidic residues, mainly Glu. Only the kappa receptor ECL2 has similar acidic residues (mainly Asp), but these have not been shown to be critical for receptor activation, as for NOP. Molecular dynamics simulation of the active-state NOP homology model suggests that NOP activation may involve movement of the ECL2 forward toward TM7, where it may participate in interactions with residues at the extracellular end of TM7 and TM3 or even with agonist ligands, resulting in a proposed activation-associated conformational movement of the TM helices (Daga and Zaveri, 2012). Binding of NOP agonist Ro 64-6198 to the active-state NOP model (Fig. 3) shows that TM7 residues such as T3057.39 and Y3097.43 interact with the agonist and with E199ECL2 in an activation-associated network (Daga and Zaveri, 2012). Consistent with its primary structure, the tertiary structure and ligand-induced conformational changes identify the NOP receptor as belonging to the opioid receptor family but nonetheless unique from the other receptors in important ways.
There is a small amount of information pertaining to potential constitutive activity of NOP receptors. Electrophysiological recording of neurons, in which overexpression of the receptor was induced by microinjection of coding cDNA, demonstrated the antagonist C-24 to have inverse agonist activity, indicative of constitutive activation of NOP receptor when overexpressed (Mahmoud et al., 2010). In another study, in which the ability to constitutively activate G-protein-coupled pathways was investigated in a series of NOP receptor point mutations, only the N133W mutant displayed increased ligand-independent signaling (Kam et al., 2002). Interestingly, this mutated residue (N3.35) was recently found to contribute to the network of interactions that establish a sodium binding pocket in the structure of several GPCRs (Katritch et al., 2014), including the delta opioid receptor (Fenalti et al., 2014). The sodium binding pocket collapses upon receptor activation, thus suggesting that presence of sodium may stabilize the receptor in an inactive conformation (Katritch et al., 2014). Very recently using a BRET-based assay to investigate NOP receptor/G-protein interactions, it was demonstrated that GDP was not able to significantly inhibit the baseline BRET ratio (Malfacini et al., 2015). However, in membranes expressing the other opioid receptors, under similar experimental conditions, GDP can suppress the baseline BRET ratio, indicating a reduction in spontaneous receptor/G-protein interactions, with maximal effects 4–5 times greater at delta than mu receptors (Vezzi et al., 2013). Thus these results demonstrate that the propensity to display constitutive activity is much lower for NOP compared with the mu and particularly the delta opioid receptor.
B. Location of Nociceptin Opioid Peptide Receptors
NOP receptors are highly expressed in many brain regions. Although several immunohistochemical studies have been carried out on NOP receptors, the lack of validated antibodies that do not crossreact with brain tissue for NOP receptor knockout [NOP(−/−)] mice has raised considerable concern regarding these results. Nevertheless, in situ hybridization and in vitro autoradiography using [125I]-N/OFQ have provided an adequate representation of NOP receptor localization and in general have been somewhat consistent with the immunohistochemical studies (Neal et al., 1999a; Florin et al., 2000). NOP receptors are expressed in multiple brain regions and are involved in a large number of central processes including pain, learning and memory, emotional states, neuroendocrine control, food intake, and motor control. In many of these neuronal pathways, there is also considerable overlap between the location of the NOP receptor and the peptide N/OFQ, as determined by immunohistochemistry and in situ hybridization (Neal et al., 1999b). Consistent with these findings, intracerebroventricular administration of N/OFQ modulates many of these processes: decreasing spatial learning (Sandin et al., 1997; Sandin et al., 2004), modulating anxiety (Jenck et al., 1997), increasing food intake (Polidori et al., 2000), and although it has no effect on its own, intracerebroventricular N/OFQ modulates opioid reward (Murphy et al., 1999) and nociception (Meunier et al., 1995; Reinscheid et al., 1995). With respect to nociceptive processing, NOP receptors are found in high numbers in pain-related brain regions within both the ascending and descending pain pathways including the periaqueductal gray (PAG), thalamic nuclei, somatosensory cortex, rostral ventral medulla, lateral parabrachial nucleus, spinal cord, and dorsal root ganglia (DRGs) (Neal et al., 1999a; Florin et al., 2000). In each supraspinal location where tested, NOP receptor activation by local injection of N/OFQ appears to block the actions of opiate analgesics (Morgan, 1997; Pan et al., 2000), which explains the anti-opioid effects of N/OFQ when administered intracerebroventricularly. Patch clamp electrophysiological studies have also been used to explain the anti-opioid action of N/OFQ. In the vlPAG, mu receptors can be found on approximately one-third of the neurons, and mu receptor activation blocks the descending pain signal (Vaughan et al., 1997; Connor and Christie, 1998). NOP receptors are found on every cell in the vlPAG and can thereby block the desending analgesic pathway and occlude the actions of mu opiates (Morgan et al., 1997; Connor and Christie, 1998).
NOP receptors are also highly expressed in regions involved in reward and drug abuse. Consistent with brain-mediated anti-opioid effects, NOP agonists attenuate the rewarding effects of opiates and other abused drugs, a topic that is discussed in more detail below. Accordingly, NOP receptors are highly expressed in the mesocorticolimbic drug reward circuitry, including ventral tegmental area (VTA), nucleus accumbens, and prefrontal cortex, as well as the central amygdala, involved in stress and drug relapse, and the medial habenula-interpedunclear nucleus pathway (Neal et al., 1999a), thought to be involved in abuse of nicotine and likely other drugs as well. There are also NOP receptors on hypocretin/orexin-containing cells in the lateral hypothalamus (Xie et al., 2008). In each of these brain regions, NOP receptor activation reduces the release of the neurotransmitters that mediate rewarding effects.
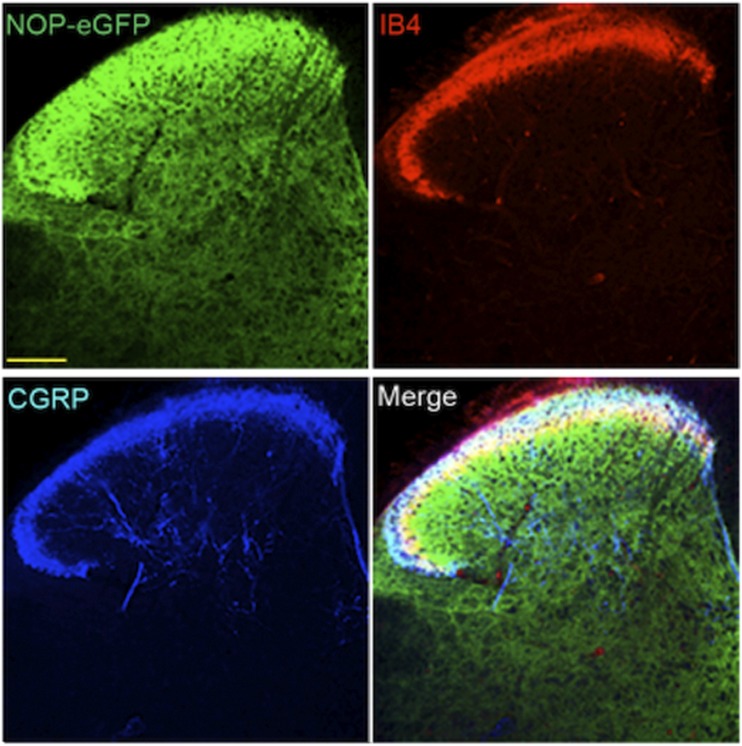
Recently, knock-in mice have been developed with NOP-eGFP receptors in place of the native receptor (Ozawa et al., 2015), similar to knock-in mice containing delta-eGFP and mu-mCherry receptors (Scherrer et al., 2006; Erbs et al., 2015). For each of these mutant mice, the tagged receptor has been valuable in identifying receptor location and trafficking without the need for problematic opioid receptor antibodies, with resolution far superior to in vitro autoradiography. For the NOP receptor, location of the NOP-eGFP receptor in brain is basically similar to what has been described using in vitro autoradiography (Neal et al., 1999a). In addition to the NOP-eGFP expression in the brain, NOP-eGFP receptors can be found in the dorsal horn of the spinal cord and in DRG. To determine the specific lamina location of NOP receptors in the spinal cord, additional immunostaining was performed with lamina markers. NOP-eGFP receptors are present at the most superficial lamina (I and II) and dorsal border of lamina IIinner where calcitonin gene related peptide (CGRP)-positive and IB-4-positive nociceptive primary afferents project (Fig. 4). This intense immunoreactivity also colocalizes with PKCγ positive interneurons in the ventral border of laminae II and III indicating that the NOP receptors might have a regulatory mechanism in the control of chronic mechanical allodynia (Neumann et al., 2008; Basbaum et al., 2009). Therefore, NOP receptors are distributed between laminae I through III in the dorsal horn, regions important for the regulation of pain systems.
Fig. 4.
NOP-eGFP receptors are highly distributed in laminae I-III and ×. Tissue sections from the spinal cord were incubated with anti-GFP, and –CGRP (laminae I and IIo, panel A). Tissues were also treated with biotinylated IB4 (dorsal border of lamina IIi) and streptavidin. This figure is reprinted with permission from the Journal of Neuroscience.
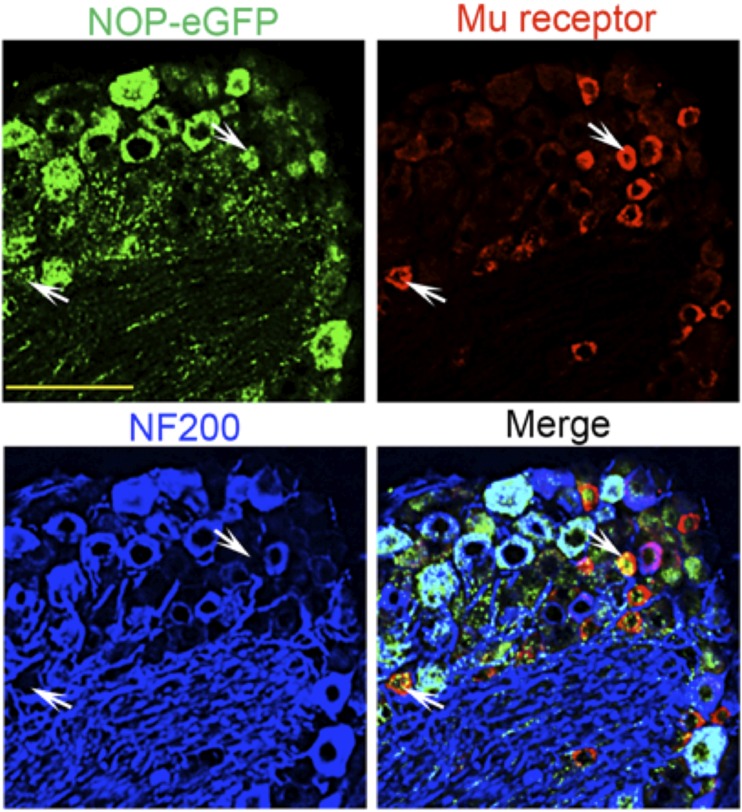
In addition to the spinal cord, NOP receptors are found in a large number of DRG neurons, large and small, myelinated and unmyelinated. Approximately 43% of all DRG neurons express NOP-eGFP, almost evenly split between small and large cell body diameter. The majority of the large diameter neurons are neurofilament 200 (NF200) positive, therefore representing myelinated A-fibers. Approximately one third of the small unmylelinated neurons are also positive for CGRP indicating that NOP-eGFP receptors are present in peptidergic C nociceptors. Peptidergic C-fibers are essential to acute heat pain as well as injury-induced heat hyperalgesia (Cavanaugh et al., 2009) and have been shown to project to laminae I and IIouter of the spinal cord (Basbaum et al., 2009) where robust immunoreactivity of NOP-eGFP is also observed. A smaller proportion of small unmyelinated (NF200-) NOP-eGFP+ DRG neurons bind IB4, indicating that NOP receptors are also present in the non-peptidergic DRG neurons, which are involved in mechanical pain (Basbaum et al., 2009; Cavanaugh et al., 2009; Scherrer et al., 2009; Vrontou et al., 2013; Bardoni et al., 2014). These studies suggest that NOP receptors might regulate the function of two classes of C nociceptors that respond to both heat and mechanical pain. NOP-eGFP receptors also are co-localized with mu opioid receptors in peptidergic C-nociceptors (Fig. 5).(Fig 6). (Fig 7). These results and the similar location of NOP and mu receptors in the spinal cord probably explain the ability of NOP receptor agonists to mediate an antinociceptive response when administered intrathecally (i.t.).
Fig. 5.
Colocalization of NOP-eGFP and mu receptors in DRG neurons. Tissue sections were incubated with anti-GFP (green), anti–mu-receptor (red), and anti-NF200 (blue) antibodies. White arrows show small diameter NOP-eGFP+, Mu+ cells. Scale bars 100 μm. This figure is reprinted with permission from the Journal of Neuroscience (Ozawa et al., 2015).
Fig. 6.
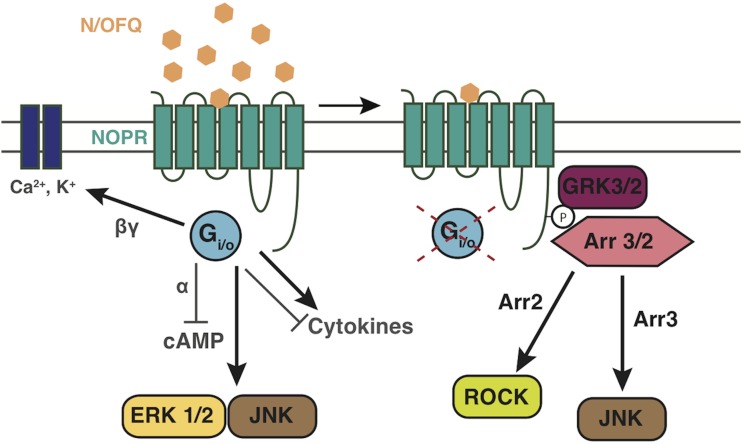
Summary of NOP receptor signaling. Figure cartoons the basic NOP receptor signal transduction and trafficking pathways highlighted in this review, and those that have generally been shown by multiple studies. Figure shows NOP receptor canonical coupling to inhibition of calcium channels, and activation of inward rectifying potassium channels. Figure also highlights recent work showing NOP receptor activation of MAPKs, and desensitization pathways via GRK3 and GRK2, and recent data showing that NOP receptors can both positivity and negatively influence cytokine/inflammatory pathway signaling. Furthermore, the cartoon depicts recent papers showing that NOP receptor activation and arrestin signaling can initiate downstream signaling to JNK and ROCK pathways. Arrows refer to activation steps; T lines refer to blockade or inhibition of function.
Fig. 7.
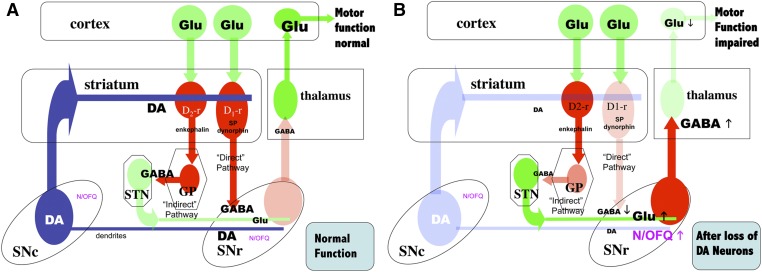
Schematic representation of the organization of basal ganglia regulating motor function and the effects dopamine (DA) depletion on N/OFQ expression and release. DA neurons are represented in blue; glutamate (Glu) neurons in green; GABA neurons in red; color density indicates the relative levels of activity in each system with normal DA neuron function (Panel A, normal function), or after loss of a significant fraction of DA neurons (Panel B). GP, globus pallidus; N/OFQ, nociception/ orphanin FQ; SNc, substantia nigra compacta; SNr, substantia nigra reticulate; STN, subthalamic nucleus, Panel A. With DA neuron function intact, GABA release in the pallido-subthalamic neurons in the “indirect” striato-nigral pathway reduces Glu release from the subthalamic neurons that activate the GABAergic nigrothalamic pathway. With low release of GABA in the thalamus from this pathway, the thalamocortical glutamatergic neurons are active, increasing activity in motor cortex and maintaining normal motor function. N/OFQ levels and release in the SNr are relatively low under these conditions. Panel B. When nigrostriatal DA function is impaired (e.g., after 6-OHDA or MPTP treatment), activity in the subthalamic glutamatergic neurons to the SNr is increased, resulting in activation of the nigrothalamic GABA pathway and inhibition of thalamocortical neurons that facilitate normal motor function. After 6-OHDA or MPTP treatment, ppN/OFQ mRNA and N/OFQ levels and release in SNr are increased (Marti et al., 2005, 2010); N/OFQ release in SNr is also increased by haloperidol treatment (Marti et al., 2010). NOPr antagonists largely reverse the effects of DA depletion by 6-OHDA on GABA release in SNr and thalamus (Marti et al., 2005, 2007, 2010). Treatment with 6-OHDA also reduces NOPr mRNA expression in SNc (Norton et al., 2002; Marti et al., 2005). Arrows indicate the direction of change in N/OFQ, GABA or Glu release after 6-OHDA or MPTP treatment.
C. Regulation of Expression of NOP Receptors
The molecular control of NOP receptor expression is complex and has not been fully elucidated. The human NOP receptor gene is located on chromosome 20. The promotor region of the human NOP receptor gene was analyzed by Palmer and colleagues (Ito et al., 2000; Xie et al., 2000). This region contains a number of predicted regulatory elements, including response elements for the glucocorticoid receptor, metal response elements, and multiple retinoic acid response elements. Retinoic acid, a potent regulator of NOP receptor expression in NT2 cells in culture, also induces differentiation of these cells (Ito et al., 2000). The transcription factor response elements Sp1, AP-2, EGR, Krox-20, ETF, and CP1 or GCF sites are also found in the promotor region of the human NOP gene. No TATA box or CCAAT box was found upstream of the transcription start sites for the NOP receptor protein. The promotor regions of the mu and delta opioid receptor genes also contain response elements for some of these transcription factors (Min et al., 1994; Im et al., 1999).
Xie et al. (Xie et al., 2000) identified two transcription start sites in the human NOP receptor gene with products that differ only in their 5′ upstream non-coding regions. The upstream site leads to the expression of exons 1A, 1B and 2 with an ATG stop codon in exon 2. The second down stream start site leads to the expression on exons 1B and 2, which contain the coding regions for the NOP receptor. In contrast, the mouse NOP receptor gene, located on mouse chromosome 2, contains 5 exons, with the protein-coding region starting in exon 2 and ending in exon 4 (Ito et al., 2000). The expression of NOP receptor splice variants was first reported in rat (Wang et al., 1994) who found at least two variant forms of the receptor mRNA expressed in rat hypothalamus, describing these as long and short (truncated) forms of the receptor. The truncated form of the receptor was missing the fifth, sixth and seventh transmembrane domains and the entire third intracellular loop. Expression studies lead to the conclusion that this truncated form had very weak capacity to bind N/OFQ and an inability to regulate G-protein function. It remains to be determined if they have other functions. In contrast to the rat, in mouse brain, five variant forms of NOP receptor gene transcript have been reported with differential expression of the variant forms across mouse brain regions (Pan et al., 1998). The functional roles of the variant forms remain unclear.
NOP receptor gene transcripts have also been reported in human lymphocytes (Wick et al., 1995) and truncated forms indicative of alternative splicing of the NOP receptor gene product were also identified in human lymphocytes and lymphocyte cell lines (Halford et al., 1995). It is unclear if either the full-length NOP receptor or its truncated forms serve functional roles in lymphocytes, although a possible role for the NOP receptor in mediating the agonist-induced decrease of allergen-induced airway hyper-responsiveness after allergen exposure has been proposed (Sullo et al., 2013).
Interestingly, the gene for human Galpha interacting protein (GAIP, also known as RGS19), a regulator of GPCR signaling (interacting with the Gα subunits of Gi, Go, Gz and Gq), is located upstream of the NOP receptor gene but oriented in the opposite direction and separated by an 83 bp sequence that may function as a bi-directional promotor for both genes (Ito et al., 2000; Xie et al., 2003, 2005). Exon 1A of the human NOP receptor gene appears to function in reverse as a promotor for the GAIP gene. This arrangement suggests that NOP receptor expression may be co-regulated with GAIP and thus serve a modulatory role in GPCR signaling. In some tissues, GAIP and NOP receptor may be co-expressed. However, Ito et al. (Ito et al., 2000) note that NOP receptor and GAIP expression sites do not always co-exist either in tissues or in cell lines, with several identified cell types capable of expressing NOP receptor without GAIP or vice-versa.
Xie et al. identified an alternative transcription site for mouse GAIP, leading to the expression of a truncated GAIP missing an N-terminal domain that is thought to interact with G-proteins (Xie et al., 2003). Co-expression of the full-length mouse GAIP with NOP receptor in COS cells resulted in potentiation of N/OFQ stimulation of GTPase and a reduction of N/OFQ-mediated inhibition of cAMP production (relative to the stimulation when only the NOP receptor gene was expressed) (Xie et al., 2005). When the N-terminally-truncated mouse GAIP transcript was co-expressed with NOP receptors, both the GAIP-induced potentiation of N/OFQ mediated GTPase activity and attenuation of an N/OFQ-mediated reduction in cAMP production were reduced. These results suggest that co-expression of both the full-length GAIP with NOP receptor facilitates receptor regulation of G-protein function. The facilitatory effect of co-expression of full-length GAIP on GTPase activity and inhibition of adenylyl cyclase was relatively selective for NOP receptors; there was less facilitation when full length GAIP was co-expressed with mu, delta or kappa receptors but this selectivity was lost when the truncated GAIP was co-expressed (Xie et al., 2005).
III. Signal Transduction Pathways Activated by NOP Receptor Ligands
A. Classic Gi-Signaling Pathways
For NOP receptors, like all GPCRs, following activation by agonist the Gα and Gβγ subunits dissociate to then act on the various effector pathways (Childers and Snyder, 1978; Childers et al., 1979). Early work in opioid receptor pharmacology demonstrated that guanine nucleotides such as GTP modulate agonist binding to opioid receptors in membrane preparations from brain tissue. It was later determined that GTPase activity is stimulated by opioid agonists (Barchfeld and Medzihradsky, 1984) and NOP receptor activation clearly promotes guanine nucleotide exchange (Sim et al., 1996; Narita et al., 1999). Agonist stimulation of opioid receptors was also shown to inhibit cyclic adenosine monophosphate (cAMP) production in a manner similar to that of other types of GPCR. Several reports have confirmed that NOP receptor activation inhibits adenylyl cyclase activity similarly and it is widely accepted that the NOP receptor couples to pertussis-toxin–sensitive G-proteins, including Gαi, to cause inhibition of cAMP formation (Zhang et al., 2012a). However, it has also been suggested that NOP receptors can promiscuously couple to other G proteins, although this has been less well characterized in physiologically relevant systems, and has only been demonstrated in heterologous expression studies and SH-SY5Y cells (Chan et al., 1998).
Opioid receptors canonically couple to Kir3 and Ca2+ channels via Gβγ pathways. Likewise, NOP receptors also couple to these two channels (Connor et al., 1996b; Connor and Christie, 1998). Channel deactivation for Kir3 interactions happens after GTP to GDP hydrolysis and Gβγ removal from interaction with the channel (Wickman and Clapham, 1995). Opening of Kir channels causes cellular hyperpolarization and inhibits tonic neural activity. When activated, NOP receptors also cause a reduction in Ca2+ currents sensitive to P/Q-type, N-type, and L-type channel blockers (Connor et al., 1996b; Zhang et al., 2012a). NOP receptor inhibition of N-type calcium conductance is likely mediated by binding of the dissociated Gβγ subunit directly to the channel. This binding event is thought to reduce voltage activation of channel pore opening (Zamponi and Snutch, 1998, 2002; Beedle et al., 2004; Yeon et al., 2004; Ruiz-Velasco et al., 2005). Furthermore, it has also been recently reported that NOP receptors use Rho-associated coiled-coil-containing protein kinase (ROCK) and LIM domain kinase (LIMK) in the regulation of voltage-dependent Ca2+ channels (Mittal et al., 2013).
B. NOP Receptors and Kinase Signaling
All known classes of GPCRs couple to various intracellular kinase cascades. In particular, opioid receptors have been demonstrated to couple to protein kinase A and protein kinase C (PKC) pathways, in additional to the more recently appreciated signaling through mitogen-activated protein kinase (MAPK) cassettes. Furthermore, it was discovered in the mid 1990s that the phosphorylated arrestin-bound GPCR complex is not simply inactive but that it recruits alternate signal transduction cascades, including MAPKs (Bruchas and Chavkin, 2010; Whalen et al., 2011; Chang and Bruchas, 2014). Similarly, signaling to MAPK cassettes in opioid receptors and NOP receptors can in part be mediated via this process (Zhang et al., 2012a). NOP receptor activity can induce activation of PKC (Armstead, 2002) as well as activation of phospholipase A2 and C (Fukuda et al., 1998; Yung et al., 1999).
NOP receptor-dependent activation of all three MAPK cassettes has been demonstrated. NOP receptor induced extracellular-signal regulated kinase (ERK) phosphorylation has not been extensively examined; however, two groups have demonstrated that the endogenous agonist N/OFQ will cause NOP receptor-mediated increases in ERK 1/2 phosphorylation levels in heterologous expression systems (COS7, CHO, and HEK293 cells) (Lou et al., 1998; Zhang et al., 2012a). In a recent report, ERK 1/2 signaling via NOP receptors was shown to be independent of receptor phosphorylation and GRK/arrestin signaling (Zhang et al., 2012a). However this requires further examination with other ligands and in alternate model systems.
Opioid receptor activation of p38 MAPK cassettes has gained interest due to the effects of kappa receptor-induced p38 phosphorylation and aversive behaviors (Bruchas and Chavkin, 2010; Bruchas et al., 2011). NOP receptor activation has been linked to phosphorylation of p38 MAPK in vitro. In one report it was demonstrated that NOP receptors activate p38 signaling via protein kinase A and PKC pathways (Zhang et al., 1999). Examination of NOP receptor-mediated p38 signaling in endogenous systems under pathologic conditions, as shown in Armstead (2006), and in various tissues will be important next steps in understanding the coupling of NOP receptors to this MAPK cassette.
Likewise, activation of c-Jun N-terminal kinase (JNK) signaling by opioid receptors has been recently examined for its interesting mu and kappa regulatory properties (Bruchas et al., 2007; Melief et al., 2010; Al-Hasani and Bruchas, 2011). At the NOP receptor, important early studies in NG-108 cells showed that N/OFQ could induce phosphorylation of JNK in a time- and concentration-dependent manner (Chan and Wong, 2000). Furthermore, in this report it was suggested that JNK activation via NOP receptors occurred in both a pertussis toxin (PTX)-sensitive and -insensitive fashion. PTX-insensitive G-proteins, Gz, G12, 14, and 16, were all reported to potentially play a role. Later it was reported that PTX-insensitive NOP-mediated JNK signaling was likely to be mediated through G-protein-coupled receptor kinase 3 (GRK3) and arrestin 3 because of an absence of late phase JNK phosphorylation in cells where GRK and arrestin were selectively knocked down using siRNA approaches (Zhang et al., 2012a). Additional evidence for a GRK/arrestin-mediated effect was provided in cells expressing a C-terminal phosphorylation NOP receptor mutant (S363A). This report also corroborated earlier reports that NOP receptors couple to JNK in a PTX-sensitive fashion during the early phase of activity. NOP receptor signaling is summarized in Figure 6.
C. Nociceptin Opioid Peptide Receptor Desensitization, Downregulation, and Recycling
1. Phosphorylation and Desensitization.
NOP receptors, like the other three opioid receptors, are regulated by homologous desensitization. The receptor is rendered less responsive to repeated or continuous stimulation and exposure to agonist. Receptor desensitization is one of the underlying mechanisms for opioid tolerance, and NOP receptors have been shown to become desensitized after high agonist concentration or repeated sustained exposure to agonists in a number of contexts (Connor et al., 1996a; Mandyam et al., 2000; Thakker and Standifer, 2002a) in both acute and chronic treatment paradigms (for a thorough review on NOP receptor regulation see, Donica et al., 2013). In addition, receptor desensitization to the known NOP receptor downstream signaling cascades including ion channels, kinase signaling, and cAMP have been demonstrated by numerous groups.
The mechanisms of receptor regulation occur in a multistep manner, including phosphorylation, internalization, and downregulation or recycling. NOP receptors are phosphorylated in a similar manner to the three other opioid receptors and GPCRs in general. After the dissociation of the Gα from the Gβγ subunits, Gβγ recruits G-protein receptor kinase (GRK) to the receptor for phosphorylation. The receptor undergoes a shift in conformation, allowing for arrestin docking to the receptor and subsequently the recruitment of the endocytosis machinery. The human, mouse and rat NOP receptors contain multiple serine, tyrosine, and threonine sites within their intracellular loops and C termini that are suitable for GRK or protein kinase A/C phosphorylation (Zhang et al., 2012a; Donica et al., 2013). GRK regulation of NOP receptors has been shown to act at multiple C-terminal sites (Mandyam et al., 2002). GRKs phosphorylate serine residues 334 and 335 on the C-terminal tail of the rat NOP receptor (337 in the human) and mutations of these residues significantly reduce the amount of receptor desensitization (Wang et al., 2006). A recent study showed that mutation of the C-terminal serine 363 to alanine of the human NOP receptor prevented receptor desensitization as measured in coupling to adenylate cyclase inhibition and calcium channel inhibition (Zhang et al., 2012a). It is likely that multiple phosphorylation sites are important for NOP receptor desensitization and that various agonist types may influence the recruitment of one or more GRKs to the receptor, as has been reported recently for mu-opioid receptor regulation REF. In addition, there is some evidence in physiologic studies that opioid receptor localization can dramatically determine its densensitization properties, and thus expression of GRKs locally at pre- or postsynaptic sites might greatly influence NOP receptor regulation in this way (Pennock et al., 2012). In this recent report, it was found that presynaptic NOP and mu receptors in proopiomelanocortin neurons inhibited neurotransmitter release over a sustained period, whereas postsynaptic NOP and mu receptor responses more rapidly desensitized. This is an important consideration that suggests that NOP receptor regulation and desensitization is critically dependent on cellular location, cell type, and agonist type. Future studies using various neuronal types as well as additional tools including receptor mutants, GRK knockdown studies, antibodies, and biased-ligands are required to better understand the differences observed in NOP receptor desensitization.
It is thought that GRK3 and GRK2 play critical roles in the phosphorylation of the NOP receptor. Important work by Thakker and Standifer (2002a) showed that prolonged activation of NOP receptors can ultimately influence the levels of GRK2 and 3 in a PKC-dependent manner. In addition, knockdown of GRK3, but not GRK2 in BE2-C cells, prevented NOP receptor desensitization. This effect was also observed recently in NOP receptor expressing HEK293 cells, whereby GRK3, but not GRK2, was shown to be the critical GRK mediating NOP receptor function (Zhang et al., 2012a). However, it is important to consider the variability and differences in expression systems and receptor species used. It is clear that NOP receptors have putative sites for both GRK2 and GRK3, and in fact both kinases may act to regulate its desensitization. In addition, examining the role of the noncanonical GRKs, 5 and 6, might prove insightful given their recent implications in bias-ligand dependent regulation of other opioid receptors (Glück et al., 2014). It is likely that with the variety of available C terminal and third loop phorphosylation sights on NOP receptors that agonist-dependent and cell type-dependent GRK recruitment occurs, whereby different cellular milleus and agonists can cause engagement of separate GRK mechanisms, thereby effecting desensitization and downstream signaling. In some cell types the expression levels of GRK subtypes will vary, and thus NOP receptor regulation by these kinases might change. Furthermore, a specific bar code for GPCR phosphorylation that is engaged differentially has been suggested for the mu receptor (Williams et al., 2013), but whether similar types of dynamic phosphorytion occur in for the NOP receptor system will need to be tested in a variety endogenously expressing cells and primary neuronal types going forward. Moving our investigations into more physiologically relevant systems that endogenously express NOP receptors will help to resolve these important questions.
2. Nociceptin Opioid Peptide Receptor Internalization, Recycling, and Downregulation.
GPCR internalization is mediated via recruitment of arrestin and typically via either a clathrin-dependent or -independent process. Numerous groups have investigated the many stages of NOP receptor trafficking (for a thorough review, see Donica et al., 2013). Early work in the NOP receptor field had difficulty in finding agonist-induced internalization (Dautzenberg et al., 2001); however, later reports showed that NOP receptors indeed internalized in response to N/OFQ treatment (Spampinato et al., 2001, 2002). Similar to the kappa opioid receptor disparities in internalization conditions (Bruchas and Chavkin, 2010), it is likely that differences reported in the internalization of NOP receptors are due to expression variability and model system used. In most cases, NOP receptors have been shown to start internalizing fairly rapidly, within 5–10 minutes after agonist treatment, with very robust internalization at 1 hour post-treatment in transfected cells (Spampinato et al., 2001; Corbani et al., 2004; Zhang et al., 2012a). As with the mu receptor, the level of internalized receptor depends on the ligand. For NOP receptors, hexapeptide partial agonists did not induce receptor internalization or robust GRK translocation (Spampinato et al., 2001; Corbani et al., 2004). This could be due to the fact that these were partial agonists or potentially due to an intrinsic difference in ligand-stimulated β-arrestin coupling and internalization, as has been demonstrated for mu receptors (Zaki et al., 2000; Bohn et al., 2004). It has been suggested that receptor regulation depends on the agonist examined and that peptide versus small molecule agonists at NOP receptors might influence their regulation via different mechanisms but this hypothesis requires further examination (Donica et al., 2013).
The role of arrestin in NOP receptor internalization and regulation has only been investigated by a few groups. Knockdown of arrestin3 (β-arrestin2), but not arrestin2 (β-arrestin1), resulted in a blockade of NOP receptor internalization after treatment with N/OFQ (Zhang et al., 2012a). Furthermore, mutation of serine 363 prevents arrestin3 recruitment to the cell surface and N/OFQ-induced NOP receptor internalization. Dominant positive arrestin3 R170E, which binds receptors in the absence of phosphorylation at the receptor, was able to rescue a NOP receptor S363A mutant’s internalization (Zhang et al., 2012a). Another recent study demonstrated that NOP receptors can use arrestin2 to regulate downstream signaling (Mittal et al., 2013). How the NOP receptor engages these various arrestins and whether agonists of varying efficacies and potencies can induce differential rates of internalization and divergent arrestin2/3 recruitment remains an active area of study. Recent evidence suggests that compounds acting as partial agonists with respect to NOP/G-protein signaling behave as antagonists with little to no activity in NOP/arrestin coupling (Chang et al., 2015b; Malfacini et al., 2015). In fact, NOP receptors indeed functionally recruit both arrestin2 and arrestin3, yet may recruit arrestin3 in a more efficacious manner (Chang et al., 2015b). NOP ligands also differ in the kinetics of arrestin recruitment as examined using biolumenscence energy transfer (BRET) techniques (Chang et al., 2015b). It is therefore possible that agonist, cell type, and environment will have a large impact on NOP internalization and arrestin recruitment properties. Again, studies in cell lines endogenously expressing the receptor or using mice with tagged NOP receptors will be critical to advancing this area of the field.
NOP receptor recycling has not been extensively examined, although some groups have shown that, in transfected cells, once internalized receptors remain internalized after washout for up to 90 minutes to 2 hours in some reports (Spampinato et al., 2001; Spampinato et al., 2002). Long-term treatment of GPCRs with agonists generally causes them to either become recycled after some critical time window or to become transported to proteasomes and lysosomes. NOP receptors become downregulated to varying levels depending on the agonist used and time period of exposure. Generally, longer exposure times with full agonists such as N/OFQ or Ro 64-6198 result in dramatic reductions in NOP binding sites from 3 to 48 hours (Dautzenberg et al., 2001; McDonald et al., 2003a). The role of receptor density in these regulatory processes has also been extensively examined because of the potential differences in NOP receptor levels from heterologous expression systems to endogenous tissue levels (McDonald et al., 2003a; Barnes et al., 2007). Future work examining receptor recovery using fluorescence recovery after photo-bleaching, or live cell imaging after agonist exposure would facilitate a better understanding of receptor recycling and downregulation (Aguila et al., 2011).
D. Cross Talk with Mu Opioid Receptors
NOP receptors colocalize with mu opioid receptors in many brain regions and share signaling pathways, so perhaps it is not surprising that both cross talk between these receptors with respect to intracellular signaling, and heterodimerization have been investigated both in cell culture and in brain or DRG neurons.
Mu agonists can induce heterologous desensitation of NOP receptors in some cell types that contain both receptors but not in others. A 1 hour treatment of BE(2)-C human neuroblastoma cells with the mu agonist DAMGO reduced N/OFQ-mediated inhibition of cAMP accumulation. Although the same treatment of SH-SY5Y cells was ineffective in reducing N/OFQ signaling (Mandyam et al., 2000, 2003). Likewise, in CHO or HEK 293 overexpressing recombinant NOP and mu receptors, mu receptor activation had no effect on NOP receptor-mediated stimulation of ERK1/2 (Hawes et al., 1998) or inhibition of cAMP (Wang et al., 2005). This is probably due to differences in signal transduction components native to these cell lines. In cells in which the two receptors share specific components of the signaling cascade, such as kinase isoforms, then cross talk, in the form of heterologous desensitization, can result.
Similarly, N/OFQ treatment can affect mu receptor activation in the same cell lines. In BE(2)-C cells, short treatment with N/OFQ induces translocation of PKCα, GRK2, and GRK3 to the plasma membrane. The increase in GRK2 levels at the plasma membrane resulted in enhanced DAMGO-mediated mu receptor phosphorylation and a resultant increased desensitization (Mandyam et al., 2002; Ozsoy et al., 2005). Prolonged N/OFQ treatment reduced the ability of mu agonists to inhibit cAMP accumulation in BE(2)-C and SH-SY5Y cells (Thakker and Standifer, 2002a), although N/OFQ treatment had no effect on the ability of mu agonists to activate ERK1/2 (Thakker and Standifer, 2002b).
Although still a controversial topic, heterodimerization between NOP and mu receptors has also been investigated in cell culture and in DRG neurons. Heterodimerization can potentially play a role in the modulation of NOP or mu receptor activity by altering receptor-ligand interactions, functional activity of the respective receptors, and receptor trafficking. NOP/mu receptor heterodimers have been demonstrated using coimmunoprecipitation (Pan et al., 2002; Wang et al., 2005; Evans et al., 2010) and immunofluorescence microscopy approaches (Evans et al., 2010). Pan et al. (2002) reported a very large (250 fold) increase in the affinity of mu agonists, but not naloxone, for the inhibition of [3H]N/OFQ binding in cells transfected with both receptors. On the other hand, it was also reported that NOP/mu dimers result in a decrease in the potency of DAMGO to inhibit cAMP accumulation or stimulate MAP Kinase (Wang et al., 2005). In both transfected tsA-201 cells and rat dorsal root ganglia, NOP receptors coprecipitated with mu, delta, and kappa opioid receptors, suggesting potential heterodimers with each of the opioid receptors. Consistent with this observation, activation of NOP receptors with N/OFQ or activation of an opioid receptor with its selective ligand induced internalization of both receptors (Evans et al., 2010). These reports suggest that mu and NOP receptors interact in discrete and interesting ways that may alter the pharmacology of these two receptor systems and ultimately their signaling properties. However, it is important to recognize that heterodimerization among Class A GPCRs does still remain controversial, and future studies using total internal reflection fluorescence microscopy in cells expressing the native receptors will shed additional light on these interactions and further explore the effects of mu and NOP coexpression.
Although questions remain pertaining to the involvement of true heterodimerization of NOP receptors, clearly NOP and mu (as well as other opioid receptors) coexist in various brain regions and in individual cells and dimerization or sharing of signal transduction pathways can easily be seen as methods of regulation of both NOP and mu signaling.
IV. Cellular Actions of Nociceptin Opioid Peptide Receptors
A. Electrophysiological Analysis of Nociceptin Opioid Peptide Action in Brain and Spinal Cord
As discussed above, NOP receptors couple to both voltage-dependent calcium channels and inwardly rectifying potassium channels to mediate their inhibitory influence on neuronal function. One of the most extensively examined physiologic systems whereby NOP receptors have been characterized includes their function in sensory neurons. In particular, numerous groups have investigated the role of NOP receptor activity in the DRG, which transmit sensory information from the periphery to the spinal cord. Because mu-opioids act to reduce transmitter release from terminals via suppression of calcium currents presynaptically, the effects of N/OFQ have been investigated in a similar context. N/OFQ-induced suppression of N-type Ca2+ has been observed in DRG neurons (Abdulla and Smith, 1998; Beedle et al., 2004; Murali et al., 2012).
It has also been suggested that NOP receptors can cause internalization of N-type calcium channels to ultimately influence the efficacy of their channel regulatory properties and influence nociceptive behavioral states (Altier et al., 2006). It was suggested that prolonged exposure to N/OFQ (30 minutes) induces internalization of a NOP receptor–N-type calcium channel signaling complex. However, a recent study reported a conflicting finding that in DRG neurons N/OFQ exposure indeed causes a rapid desensitization of the NOP receptor, but that there is no observed functional loss in surface N-type calcium channels (Murali et al., 2012). The reasons for these discrepancies remain unknown, but it is clear that NOP receptors communicate readily with high-voltage activated calcium channel currents within the dorsal root ganglion. Further study is warranted to investigate the additional physiologic effects of NOP receptors in DRG neurons, especially within states of chronic neuropathic pain, where NOP receptors might hold promise for therapeutic benefit.
Because opioid analgesia is at least in part mediated via both presynaptic mechanisms, including reduced transmitter release in the spinal cord, and postsynaptic activation of GIRK channels, it has long been thought that NOP receptors work in a similar manner. Intrathecal injection of N/OFQ into the dorsal horn modulates C-fiber evoked “wind-up” and action potential discharge after repeated stimuli (Stanfa et al., 1996). In addition, N/OFQ suppresses glutamate ventral root potentials in a concentration-dependent manner (Faber et al., 1996), as well as depresses evoked-excitatory postsynaptic potentials (EPSPs) in the substantial gelatinosa neurons of the spinal cord. Studies have demonstrated that the effects of N/OFQ in the spinal cord are almost all presynaptic because of insensitivity of N/OFQ on mini-EPSC amplitude to tetrodotoxin treatment (Liebel et al., 1997). However, other groups have shown that NOP can exert postsynaptic effects within the spinal cord because of its ability to inhibit glutamatergic and kainic-acid evoked currents (Shu et al., 1998). Furthermore, extracellular recordings in the dorsal horn and trigeminal nucleus have demonstrated that N/OFQ inhibits AMPA- and NMDA-mediated responses in a similar manner as the other opioid receptor types (Wang et al., 1996).
NOP receptor-mediated changes in physiologic output within the brain have been extensively examined. The midbrain PAG, rostral ventromedial medulla (RVM), and dorsal raphe nucleus (DRN) have been examined because of the prevailing role of opioids in mediating antinociception through their action in these brain regions (Morgan et al., 2006; Zhao et al., 2007; Land et al., 2009; Connor et al., 2015). N/OFQ has been shown to inhibit IPSCs and EPSCs within the PAG and cause a reduction in the frequency of mIPSCs and mEPSCs, again suggesting a critical role for these receptors at presynaptic sites (Vaughan et al., 1997; Kuo et al., 2008). In the RVM, N/OFQ was shown to inhibit spontaneous neuronal activity, and in the DRN NOP receptors were shown to be coupled to GIRK currents as seen for this receptor in other cell types (Vaughan and Christie, 1996). In the RVM, mu receptors are found on secondary OFF cells, and agonist activation blocks the descending pain signal. Conversely, kappa receptors are found on primary or ON cells. NOP receptors are found on both ON and OFF cells; activation of these receptors blocks mu opiate-mediated antinociceptive activity in naive animals but induces apparent analgesic activity in morphine-tolerant animals (Wang et al., 1996; Pan et al., 2000). These experiments demonstrated how the ultimate result of NOP receptor activation can be state dependent, whereas activation of mu receptors has invariant antinociceptive activity.
NOP receptors have also been shown to inhibit long-term potentiation (LTP) in the hippocampal CA1 region, through depression of field potentials, and reduced spike amplitude. It was also demonstrated that N/OFQ application increased the paired-pulse facilitation (Yoshimura and Jessell, 1989; Yu et al., 1997). Consistent with these findings, NOP(−/−) mice show enhanced LTP in the CA1 region of the hippocampus, suggesting that NOP receptors might influence learning and memory as a result of these physiologic mechanisms (Manabe et al., 1998).
Additional slice electrophysiology studies have reported a diverse array of functional modulation by NOP receptors within the central amygdala, bed nucleus of the stria terminalis, hypothalamus, and limbic structures (Chen et al., 2009; Kallupi et al., 2014). In a few recent reports it was shown that N/OFQ acts to suppress glutamate transmission within the central amygdala and that NOP receptor agonists alter GABAergic transmission. It was very recently proposed that activation of N/OFQ-containing cells and receptors in the central amygdala are important for the mediation of anxiety-like behavior, responses to stress, and drugs of abuse including alcohol (Cruz et al., 2012; Ciccocioppo et al., 2014; Kallupi et al., 2014). Understanding how N/OFQ and NOP receptors influence neuronal activity within these circuits is a critical next step in our uncovering how NOP receptor activation mediates behavioral affective states.
In summary, in almost all neuronal types tested, N/OFQ and its receptor activate inwardly rectifying potassium conductances and inhibit Ca2+ channels. This has been demonstrated in both peripheral and central sites of action at typically presynaptic sites of action. N/OFQ and NOP receptor actions on cellular activity have been studied in numerous brain and spinal sites (see review, Moran et al., 2000, Table 1). In all these reports, they have been shown to elicit varying degrees of effects on EPSC, IPSC, mEPSC, mIPSC amplitude and frequency, in addition to changing LTP and neuronal firing rates. Although these findings are consistent with reports for mu, kappa, and delta opioid receptors, in that activation of NOP receptors results in generalizable neuronal inhibition, there are likely differences in expression, localization, and ultimate circuit output that are uniquely NOP receptor mediated. Future studies examining these differential circuit modulations and in pathologic states are clearly warranted.
TABLE 1.
Effects of N/OFQ, injected into discrete brain regions, or of a NOP-receptor agonist administered systemically, on the function of the dopaminergic nigro-striatal projection in rats
| Treatment | Result |
|---|---|
| N/OFQ, into SNr | 1) reduced firing of SNc DA neurons; effect blocked by UFP-101 or J113397 (Marti et al., 2004) |
| “ | 2) reduced release of DA into microdialysates of dorsal striatum; effect blocked by UFP-101 (Marti et al., 2004) |
| “ | 3) reduced rat motor performance on rotarod; UFP-101 improved performance (Marti et al., 2004) |
| “ | 4) increased release of Glu into microdialysates of SNr; blocked by a NOP receptor antagonist (Marti et al., 2002) |
| N/OFQ, i.c.v. | 1) stimulated locomotor activity at a low doses (10 ng), reduced activity at high dose (10µg) ; stimulatory effect reduced by both D1 and D2 antagonists (Florin et al., 1996) |
| N/OFQ, i.c.v. | 2) dose-dependently reduced L-DOPA-induced dyskinesia in 6-OHDA rats; effect blocked by UFP-101 & J113397 (Marti et al., 2012) |
| N/OFQ, into striatum | reduced L-DOPA-induced abnormal involuntary movements (AIMS) in 6-OHDA rats (Marti et al., 2012) (N/OFQ is less potent in reducing AIMS after administration to SNr). |
| Ro-65-6570 i.p. | dose-dependently reduced L-DOPA-induced dyskinesia in 6-OHDA rats; effect blocked by UFP-101 & J113397 (Marti et al., 2012) |
i.c.v, intracerebroventricular injection; i.p., intraperitoneal administration
B. Effects of Nociceptin Opioid Peptide Receptor Activation On Release of Central Nervous System Neurotransmitters
NOP receptor inhibition of calcium conductance has the immediate effect of reducing and regulating calcium-dependent neurotransmitter release (for an extensive review, see Schlicker and Morari, 2000). As such, NOP receptor regulation of neurotransmitter release has been examined in several contexts including brain slices, synaptosomes, and in vivo using microdialysis. NOP receptor activation results in a general decrease in monoamine release. For example, N/OFQ treatment has been demonstrated to inhibit norepinephrine release in cerebral cortical slices, as well as in cerebellar, hippocampal, and hypothalamic slice preparations (Siniscalchi et al., 1999; Werthwein et al., 1999; Schlicker and Morari, 2000; Lu et al., 2010). Furthermore, NOP receptor activation leads to a decrease in dopamine release in striatal slices, and most recently within the nucleus accumbens and ventral tegemental area in vivo using microdialysis approaches (Murphy et al., 1996; Murphy and Maidment, 1999; Vazquez-DeRose et al., 2013). The regulation of extracellular dopamine by N/OFQ has likely important implications in the NOP receptor regulation of cocaine-induced behaviors including locomotion and reward processing (Murphy and Maidment, 1999; Vazquez-DeRose et al., 2013). Activation of the NOP receptor inhibits tyrosine hydroxylase phosphorylation, dopamine synthesis, and dopamine receptor signaling, suggesting that NOP receptors are poised to tightly regulate dopamine transmission at multiple levels (Olianas et al., 2008). The regulation of dopamine release and neurotransmission by NOP receptors has been suggested to have important implications in Parkinson’s disease, reward, and addiction-related disease states. Finally, it has also been demonstrated in cortical slices and in the DRN that that NOP receptor activation also inhibits serotonin release (Siniscalchi et al., 1999; Fantin et al., 2007; Lu et al., 2010; Nazzaro et al., 2010).
NOP receptors are also poised to regulate glutamate and GABA release, by virtue of their presynaptic localization, and inhibit neuronal firing. Indeed, NOP receptors inhibit glutamate release within the RVM and spinal cord (Lu et al., 2010), as well as decrease glutamate release in rat cortical neurons (Bianchi et al., 2004). Additional studies have reported a role for NOP receptor modulation of glutamate and GABA release within the lateral amygdala and cerebrocortex. N/OFQ and NOP receptors have also been shown to modulate acetylcholine release at cholinergic circuits (Uezu et al., 2005; Hiramatsu et al., 2008) in both pharmacological and genetic knockout studies. It is hypothesized that these effects impact learning and memory-related behaviors via changes in LTP and LTD within the hippocampus.
In general, N/OFQ and NOP receptors act to inhibit the release of monoamine and other neurotransmitters. However, given their widespread expression patterns it is possible that via complex disinhibition and indirect circuit-related effects, activation of the NOP receptor system will result in an increase in transmitter or neuropeptide output. This is possible in the case of opioid disinhibition of GABAergic transmission, as proposed for mu-opioid induced increases in dopaminergic output and release (Johnson and North, 1992). Additional studies to isolate the effect of NOP receptors within discrete cell types and neural circuits are needed along with techniques for measuring transmission with better temporal resolution, such as electrochemical detection methods like fast scan cyclic voltammetry.
C. Nociceptin Opioid Peptide Receptor and Inflammatory Signaling
NOP receptors are widely expressed within the immune system including known expression patterns on lymphocytes, monocytes, B/T cells, and mononuclear cells (Halford et al., 1995; Wick et al., 1995; Peluso et al., 1998; Arjomand et al., 2002). This broad expression pattern of NOP receptors highlights their critical role in modulation of immune function. Several important studies have begun to dissect how NOP receptors regulate function and signaling in these cells and how NOP signal transduction cascades in these cells may overlap with its signaling in other cells and neurons.
Interestingly, NOP receptors appear to bidirectionally regulate cytokine expression and release, again indicating that these receptors are poised to dynamically respond to stimuli in a cell type-dependent and environmentally important context. For example, N/OFQ treatment inhibits the production of proinflammatory cytokines interleukin-6, internleukin-1β, and tumor necrosis factor alpha in a variety of cell types and tissues, including in the spinal cord and astrocytes (Fu et al., 2007; Miller and Fulford, 2007). In contrast, a very recent report found that NOP receptors activate nuclear factorκΒ, providing a possible mechanism for how NOP receptors might engage the immune system (Donica et al., 2011). Furthermore, sustained activation of NOP receptors causes a dramatic upregulation in transcription factor nuclear factor κΒ, activating protein-2, and activating transcription factor-2 (Chan and Wong, 2000). How NOP receptors engage cytokine signaling pathways through G-protein and arrestin signaling pathways is an important future step, although given the properties of NOP MAPK transduction (see section IV) it is likely that there is cross-talk and utilization of these pathways for mobilizing the cytokine cascades. MAPK signaling has a broad array of convergent points with GPCR signaling as do the canonical cytokine pathways (Raman et al., 2007), so it is likely that the NOP-dependent stimulation of JNK and p38 MAPKs converge onto the nuclear factor κB pathways and could in turn elevate cytokine transcription. Future experiments to stimulate NOP receptors in the presence and absence of selective MAPK inhibitors will be key extensions of this work.
The role of NOP receptors in coupling to cytokine pathways remains an important active area of investigation. Because the NOP system is widely implicated in stress-related pathophysiology (Zhang et al., 2012b, 2015) and recent evidence suggests that cykokines can also regulate mood and psychologic responses to stress (Zhu et al., 2010; Moretti et al., 2015), a better understanding how NOP cytokine signaling, extended into nonimmune cell types, will prove critical as we attempt to understand how NOP signaling functions to bidirectionally regulate the stress response.
V. Biologic Actions of Nociceptin Opioid Peptide Receptors
A. Nociceptin Opioid Peptide Receptors and Opiate Activity
1. Analgesia.
The initial studies on the newly discovered peptide N/OFQ found that intracerebroventricular administration in mice led to an unexpected decrease in hot plate and tail flick latencies, indicating that the treated animals had increased sensitivity to heat and the peptide had nociceptive activity rather than the expected antinociceptive activity, as observed for opioid compounds (Meunier et al., 1995; Reinscheid et al., 1995). However, Grandy and colleagues determined that N/OFQ did not actually decrease tail flick latency per se but actually blocked intracerebroventricular injection-induced (stress-induced) analgesia (Mogil et al., 1996a). Further studies in mice indicated that N/OFQ could block the antinociceptive activity of mu, delta, and kappa analgesics, and therefore N/OFQ had antiopiate activity rather than nociceptive activity (Mogil et al., 1996b). This is mediated, at least partly by activation of NOP receptors in the periaqueductal gray (PAG), because direct injection into this brain region can block the antinociceptive actions of either morphine or kainic acid microinjected into the PAG (Morgan et al., 1997). NOP receptor agonists also block stress-induced analgesia. In fact, naloxone only attenuates a portion of stress-induced analgesia, whereas NOP receptor agonists block it completely, indicating that N/OFQ blocks both an endogenous opioid as well as nonopioid components of stress-induced analgesia (Rizzi et al., 2001b). Although the antianalgesic effects of NOP receptor agonists delivered into the brain are very profound, the effect of N/OFQ administration into the spinal cord has the opposite result. Intrathecal administration of N/OFQ produces a direct antinociception and potentiates morphine (Xu et al., 1996; Yamamoto et al., 1997).
An initial hypothesis concerning the action of NOP receptor-active compounds was that if N/OFQ induced pain, antagonists might have antinociceptive activity. The results of such studies are complicated. Although peptide antagonists of NOP receptors have significant antinociceptive activity when administered intracerebroventriuclarly, small molecule antagonists are generally devoid of activity regardless of the route of administration (Di Giannuario et al., 2001; Calo' et al., 2002a; Rizzi et al., 2007a). In fact this observation is not fully consistent in the literature. One NOP receptor antagonist, JTC 801 (N-(4-amino-2-methylquinolin-6-yl)-2-[(4-ethylphenoxy)methyl]benzamide), appears to have naloxone-irreversible antinociceptive activity in both acute and chronic pain models when administered systemically (Yamada et al., 2002; Suyama et al., 2003; Tamai et al., 2005), whereas the majority of selective antagonists do not have any effect on latencies in tail withdrawal assays in naive animals. The actions of individual peptide and small molecule agonists and antagonists will be discussed in detail below.
2. Chronic Pain.
The situation with regard to chronic neuropathic or inflammatory pain appears to be somewhat different. As with acute pain, N/OFQ has antiallodynic and antihyperalgesic activity after intrathecal administration in models of chronic neuropathic and inflammatory pain (Hao et al., 1998; Corradini et al., 2001). However, the levels of NOP receptors and N/OFQ change in chronic or inflammatory pain states, suggesting a sensitization of the NOP system (Andoh et al., 1997; Sun et al., 2001; Briscini et al., 2002; Ma et al., 2005). Furthermore, both preproN/OFQ(−/−) [ppN/OFQ(−/−)] and NOP(−/−)mice display increased inflammatory hyperalgesia in the formalin assay, but not in an acute pain assay (Depner et al., 2003), similar to NOP(−/−) rats (Rizzi et al., 2011). These studies suggest that the NOP system may be recruited differently in different pain modalities. Furthermore the plasticity of the NOP system may mediate some of the sensitivity induced by various chronic pain paradigms. This has been demonstrated using selective synthetic agonists and antagonists, and is discussed in greater detail below in the section on bifunctional NOP/mu compounds.
3. Opioid Tolerance Development.
One of the clinical drawbacks to opiate analgesia is the development of tolerance, leading to escalation of dose and increased risk for overdose. A drug that could be administered with the opiate and prevent tolerance development could potentially reduce the dose of the opiate and provide an improved safety margin. In fact, many drugs have been demonstrated to reduce or reverse morphine tolerance, but none have to date proven useful clinically (Dourish et al., 1988; Trujillo and Akil, 1991; Kolesnikov et al., 1992; Elliott et al., 1994; Davis and Inturrisi, 1999; Lutfy et al., 2001b; Hull et al., 2013). Modulation of NOP receptors can also block the development as well as reverse morphine tolerance in rodents. Although there is some controversy in the literature (Kest et al., 2001; Mamiya et al., 2001), morphine tolerance has been demonstrated to be significantly reduced in mice in which either the NOP receptor or ppN/OFQ has been knocked out (Ueda et al., 1997; Chung et al., 2006). Furthermore, N/OFQ-antibody partially reversed tolerance to chronic morphine (Tian and Han, 2000). These results are consistent with the fact that coadministration of morphine together with the antagonist J-113397 was able to block tolerance development in normal mice (Ueda et al., 1997; Chung et al., 2006). Available evidence suggests that the brain area relevant for the action of endogenous N/OFQ on opioid tolerance could be the ventrolateral periaqueductal gray. In fact, a local injection of J-113397 in this area is able to prevent tolerance to the analgesic action of systemic morphine (Scoto et al., 2010) and of the mu opioid receptor selective agonist DAMGO injected locally (Parenti and Scoto, 2010).
Conversely, intracerebroventricular administration of N/OFQ shortly after a daily systemic administration of morphine also blocked the development of morphine tolerance (Lutfy et al., 2001b). However, after a daily injection of morphine led to the development of tolerance, NOP receptor antagonists, administered just before morphine treatment, increased tail flick latency, indicating that they blocked the expression of tolerance, which will be discussed in greater detail below (Zaratin et al., 2004; Chung et al., 2006). Together these studies suggest that chronic morphine treatment leads to an upregulation of the NOP system in the brain, which attenuates morphine analgesia and in turn can be blocked by treatment with a NOP receptor antagonist. However, the conflicting results with agonists and antagonists clearly indicate that additional studies are required to better understand the involvement of the NOP receptor system in the development and potential attenuation of opioid tolerance.
4. Opioid Addiction Liability and Reward.
Another serious concern relating to the chronic use of opiates is the development of severe physical and psychologic dependence. In animals, abuse liability is measured with several behavioral paradigms including drug self-administration studies, discriminative stimulus experiments, and development of a conditioned place preference (CPP). Initial studies demonstrated that N/OFQ is neither rewarding nor aversive (Devine et al., 1996). In fact, intracerebroventricularly administered N/OFQ can block morphine CPP and CPP induced by cocaine, alcohol, and methamphetamine (Ciccocioppo et al., 2000; Kotlinska et al., 2002, 2003; Zhao et al., 2003; Sakoori and Murphy, 2004). N/OFQ also blocks self-administration of alcohol (Ciccocioppo et al., 2004). These results are consistent with the ability of NOP agonists to reduce extracellular dopamine levels in the nucleus accumbens, as well as their ability to block a drug-induced increase (Murphy et al., 1996; Murphy and Maidment, 1999; Lutfy et al., 2001a). In fact, N/OFQ can block cocaine-induced increase in extracellular dopamine when administered intracerebroventricularly (Lutfy et al., 2001a) or directly into the VTA (Murphy and Maidment, 1999) or when reverse dialized directly into the nucleus accumbens (Vazquez-DeRose et al., 2013). Based upon the ability of N/OFQ to block extracellular dopamine levels and block CPP of so many abused drugs, it is somewhat surprising that N/OFQ was ineffective in attenuating heroin self-administration in rats (Walker et al., 1998).
In fact, the ability of NOP agonists to attenuate self-administration of any abused drug is not clear. N/OFQ and the selective small molecule agonist Ro 64-6198 have both been demonstrated to block ethanol self-administration in rats (Ciccocioppo et al., 1999, 2004; Kuzmin et al., 2007); however, these studies have been generally conducted in alcohol preferring rat strains, some of which, including the Marchigian Sardinian alcohol preferring rat line, showed anomalies in their NOP-N/OFQ system (Economidou et al., 2008). Other studies found NOP agonists to be effective in decreasing alcohol drinking only in rats with a previous history of alcohol dependence but not in unselected or nondependent rat lines (Ciccocioppo et al., 2014; de Guglielmo et al., 2015). A single publication found efficacy in normal Wistar rats (Kuzmin et al., 2007). There are no publications demonstrating that NOP receptor agonists can block self-administration of cocaine or nicotine. Clearly, the effect of NOP receptor activation is different on two standard “drug abuse” paradigms, CPP and self-administration. Why this is the case is unclear. One possibility is that CPP has a very strong learning component, whereas NOP receptor activation is detrimental to spatial learning and decreases long-term potentiation (Sandin et al., 1997; Yu et al., 1997; Manabe et al., 1998). This might explain why NOP receptor agonist Ro 64-6198 blocked the acquisition of CPP but not its expression (Shoblock et al., 2005). In fact, inhibition of self-administration generally means blocking expression of drug taking and perhaps NOP receptor agonists are effective in attenuating acquisition but not expression of drug reward.
B. Nociceptin Opioid Peptide Receptor and Motor Function
Morphine and related drugs are well known to play a modulatory role on motor function, with effects varying markedly across species: facilitation of motor function in horses (Combie et al., 1981; Nugent et al., 1982) and cats (French et al., 1979; Kamata et al., 2012) but inhibition of motor function in dogs (Kamata et al., 2012). Rodents in particular display increased motor function with low doses of opiates as displayed in the “running fit” seen in mice after morphine treatment (Goldstein and Sheehan, 1969), with spasticity and impaired function at higher doses. This response to morphine is under genetic control, with nonresponders to morphine also showing no motor response to amphetamine (Judson and Goldstein, 1978), implicating the nigrostriatal system in these locomotor responses. Mu and delta receptors are expressed in discrete locations within the substantia nigra, the VTA, and both the dorsal and ventral striatum (Mansour et al., 1994). With the discovery of N/OFQ, it was therefore natural that the effects of the peptide and its receptor on motor function would be studied.
Early studies on the properties of N/OFQ noted that administration of the peptide modified motor function in mice and rats. Reinscheid et al. (1995) reported a dose-dependent inhibition of motor function in mice after intracerebroventricular or intrathecal administration of N/OFQ, and this was confirmed in rats by Devine et al. (1996). However, others noted that low doses of the peptide facilitated motor function (Florin et al., 1996; Kuzmin et al., 2004). The location of NOP receptors in relation to the nigrostriatal system and central dopamine pathways was systematically studied by Norton et al. (2002), evaluating the distribution of ppN/OFQ or NOP mRNAs in relation to the mRNA for tyrosine hydroxylase (TH) in the substantia nigra pars compacta (SNc) and VTA of rat brain and changes in their distribution after destruction of the DA neurons by unilateral injection of 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle (MFB). N/OFQ expression was entirely in nondopaminergic neurons. Approximately 50% of the TH-expressing neurons in SNc coexpressed NOP receptor mRNA. However, only about 6–7% of the NOP receptor-positive neurons in SNc also expressed TH; in contrast, 50–60% of the NOP receptor-positive neurons also expressed the mRNA for glutamic acid decarboxylase (GAD65/67), a marker for GABA neurons. (Norton et al., 2002). Injection of 6-OHDA into the MFB resulted in a marked loss of NOP receptor mRNA in SNc and VTA, along with the loss of the TH-positive DA neurons. There was also an apparent compensatory increase in N/OFQ mRNA expression in both the SNc and the VTA. These results indicate that a significant fraction of the SNc DA neurons coexpress NOP receptor and may be subject to regulation by N/OFQ released from non-DA neurons, but a substantial fraction of the NOP receptor expression in SNc and VTA is in non-DA neurons, possibly GABAergic, that may also play a role in modulating the function of the nigrostriatal DA neurons.
Studies with intracerebral injections of N/OFQ revealed complex actions on central dopamine systems regulating motor activity. Low doses of N/OFQ given intracerebroventricularly increased locomotor activity in mice, whereas a high dose reduced locomotor activity. The stimulatory effects of a low dose of N/OFQ was not blocked by naloxone, but was dose dependently inhibited by either the DA D1 receptor antagonist SCH23390 (7-chloro-3-methyl-1-phenyl-1,2,4,5-tetrahydro-3-benzazepin-8-ol) or the D2 receptor antagonist haloperidol, suggesting that the stimulation was mediated by enhanced dopaminergic transmission (Florin et al., 1996). The functional significance of N/OFQ-NOP receptor regulation of nigrostriatal function has been further analyzed by Morari and colleagues at the University of Ferrara in an extensive series of studies. Administration of N/OFQ directly into the substantia nigra pars reticulata (SNr) of rats reduces the firing rate of SNc DA neurons and also the release of DA into microdialysates of the dorsal striatum. These effects were inhibited or blocked dose dependently by coadministration to the SNr of the peptide NOP receptor antagonist UFP-101 (N-(Benzyl)Gly-Gly-Gly-Phe-Thr-Gly-Ala-Arg-Lys-Ser-Ala-Arg-Lys-Arg-Lys-Asn-Gln-NH2) or either intranigral or systemic administration of the small molecule NOP receptor antagonist J-113397 (Marti et al., 2004). Administration of N/OFQ to the SNr impaired the ability of rats to run on a rotarod, whereas administration of UFP-101 by the same route improved motor performance. In the same study, evaluation of the effects of genetic disruption of the NOP receptor gene in mice revealed that NOP(−/−) mice were able to stay on the rotarod for a longer period of time than NOP(+/+) animals, again supporting a role for the N/OFQ-NOP receptor system as a modulator of dopaminergic regulation of motor function. The effects of N/OFQ or a nonpeptide NOP receptor-agonist, Ro 65-6570, on the functions of components of the nigrostriatal dopamine pathway are shown in Table 1.
These results pointed to the possibility that antagonism of NOP receptors might alleviate the symptoms of Parkinsonism. Blockade of NOP receptors with the peptide antagonist UFP-101 or the nonpeptide J-113397 relieved hypokinesia in the 6-hydroxydopamine-treated hemi-parkinsonian rat, and NOP(−/−)- mice were relatively resistant to haloperidol-induced akinesia compared with NOP(+/+) mice (Marti et al., 2005). Unilateral 6-OHDA lesioning of the SNc also caused an increase in N/OFQ mRNA and peptide levels on the side of the lesion. Subsequently, Viaro et al. (2008) reported that J-113397 attenuated the Parkinsonian-like symptoms of MPTP toxicity in macaque monkeys trained to perform a reaching task in which the speed of arm movement was measured without affecting motor function in untreated macaques. However, in both mice and macaques, J-113397 exhibited a bell-shaped dose-response curve with respect to facilitation of motor function, with no effect or possible exacerbation of motor impairment at high doses. Dopamine D2-receptor blockade by haloperiodol in rats offers another experimental model for Parkinson’s disease (PD). Extracellular levels of N/OFQ were elevated in the SNr of haloperidol-treated rats in parallel with the degree of akinesia, and elevated levels of N/OFQ were observed in the cerebral spinal fluid of patients with PD (Marti et al., 2010), emphasizing the connection between elevated N/OFQ levels and impairment of motor function.
Mice with genetic deletion of ppN/OFQ lost fewer SNc DA neurons than wild-type [ppN/OFQ(+/+)] mice after MPTP treatment and retained substantially more TH in the striatal terminals of the DA neurons after an MPTP treatment that reduced the SNc DA cell count by more than 60% in wild-type mice (Marti et al., 2005), suggesting that blockade of NOP receptors exerts a neuroprotective effect against toxic insults to the SNc DA neurons. Deletion of ppN/OFQ did not protect against striatal depletion of DA by another neurotoxin, methamphetamine, which acts primarily on the axonal terminals of the DA neurons, indicating that the protection provided by elimination of the N/OFQ-NOP receptor system was selective for toxicity mediated in the SNr (Brown et al., 2006). MPTP treatment increased the expression of ppN/OFQ mRNA specifically in a subset of TH-negative neurons within the SNr, but did not increase the numbers of neurons expressing ppN/OFQ mRNA in the VTA (Gouty et al., 2010).
PD is accompanied by hyperactivity of the subthalamic nucleus and increased release of glutamate (Glu) in the pathway from the subthalamic nucleus to the SNr (Bergman et al., 1990). In normal freely moving rats, administration of N/OFQ into the SNr resulted in an increased release of Glu into extracellular fluid, as measured by Glu concentrations in microdialysates of the SNr (Marti et al., 2002), suggesting that N/OFQ might activate Glu release in this pathway. The increase in Glu release was reversed by coadministration of an NOP receptor antagonist but not by naloxone, confirming the role of NOP receptors. Increased Glu release was also observed in dialysates of SNr in rats in which SNc neurons had been depleted by 6-OHDA; treatment with the NOP receptor antagonist J-113397 again normalized this release (Marti et al., 2005), confirming a role for NOP receptors activated by the increased levels of endogenous N/OFQ after 6-OHDA treatment. Further studies revealed that J-113397 treatment increased extracellular levels of GABA in the lesioned SNr of 6-OHDA-hemilesioned rats, but did not significantly affect GABA levels on the nonlesioned side (Marti et al., 2007). In contrast, the extracellular levels of GABA in the ventromedial thalamus of 6-OHDA treated rats, a major target of nigrothalamic GABA neurons, were decreased on the lesioned side after J-113397 treatment.
A plausible explanation of the motor facilitation by NOP receptor antagonists in animals in which dopamine function is impaired is that blockade of NOP receptor in SNr enhances GABA release within the SNr with a resulting inhibition of firing in the nigrothalamic GABAergic neurons, and this in turn causes disinhibition in the thalamus. The disinhibited thalamocortical neurons enhance cortical activation and facilitate motor function (Marti et al., 2007). After MPTP treatment, local elevation of N/OFQ levels may inhibit the release of GABA in SNr and elevate local Glu levels to neurotoxic levels in the SNc DA neurons whose dendrites are extensively distributed throughout the SNr. The SNc DA neurons are known to be particularly sensitive to calcium toxicity (Dragicevic et al., 2015). Collectively these results point to a role for endogenous N/OFQ in the SNr, acting through NOP receptors, in dysregulating local control of both Glu and GABA concentrations, particularly when endogenous DA is depleted, with deleterious effects on both the SNc DA neurons and on the nigrothalamic output pathway of the SNr (see Fig. 7).
Both facilitatory and inhibitory motor actions of N/OFQ were abolished in animals in which TH activity was inhibited, indicating that endogenous DA is critical for both actions (Kuzmin et al., 2004). Florin et al. (1996) previously noted that the facilitatory effects of low doses of N/OFQ were abolished by haloperidol treatment, suggesting a role for D2 receptors. More extensive studies by the Morari group (Viaro et al., 2008, 2011) showed that motor facilitation by low doses of N/OFQ or by NOP receptor antagonists was lost in mice with genetic deletion of the D2 receptor (D2−/− mice), or by selective deletion of the long-form of the D2 receptor (D2L−/− mice), indicating the importance of endogenous DA acting on D2 receptors in these actions. Even in the PD animal models where endogenous DA is depleted, it is likely that sufficient DA remains for facilitatory effects on motor function when stimulated by agents enhancing DA release. In contrast to the enhancement of motor function by low doses of NOP receptor antagonist, the inhibitory effects of high concentrations of NOP receptor antagonists were lost in D2−/− mice but not in mice with selective deletion of the long form of the receptor (D2L−/−), indicating that the long form is not required for this action. D2 autoreceptors are thought to be predominantly comprised of the short form of the D2 receptor (Usiello et al., 2000), and the short form is the predominant D2 receptor isoform expressed in SNc DA neurons (Jomphe et al., 2006; Viaro et al., 2013). This suggests the specific involvement of D2 autoreceptors in the inhibitory effects of high doses of NOP receptor antagonists, although again the detailed mechanisms underlying these actions are unclear.
There are also effects of NOP receptor ligands in the striatum. The level of expression of N/OFQ and NOP receptor in striatum is low in rodents (Neal et al., 1999a,b; Florin et al., 2000), but effects of N/OFQ on neurotransmission in striatum, including inhibition of D1 receptor signaling on the GABAergic medium spiny neurons of striatum (Olianas et al., 2008), are reported. These effects were antagonized by a NOP receptor antagonist, confirming a striatal function for NOP receptors in the rat. Higher levels of NOP receptor expression are reported in the primate striatum (Berthele et al., 2003; Bridge et al., 2003). The functional roles of the N/OFQ-NOP receptor system in the striatum are not fully elucidated, but activation of NOP receptor has been shown to reduce the dyskinesias induced by chronic l-DOPA administration in experimental models of PD in both rats and nonhuman primates (Marti et al., 2012).
The mechanisms underlying the induction of l-DOPA-induced dyskinesias (LID) after depletion of striatal dopamine remain controversial. Loss of DA in the nigrostriatal terminals of SNc neurons in PD or after 6-OHDA or MPTP is thought to induce hypersensitivity with adaptive changes in the function of striatal medium spiny neurons (Olianas et al., 2008) and substantial changes in gene expression in striatum (Heiman et al., 2014). There are also marked presynaptic changes, with loss of presynaptic control of DA release after administration on l-DOPA both from the residual DA neurons terminals but also from DA synthesized from l-DOPA in the terminals of serotonergic neurons (Mosharov et al., 2015).
Administration of N/OFQ intracerebroventricularly, or the NOP receptor agonist Ro 65-6570 systemically, to 6-OHDA hemilesioned rats treated with l-DOPA significantly reduced LID incidence and severity (Marti et al., 2012). The reduction of LID was observed without any reduction in basal locomotor activity, probably because the dose of Ro 65-6570 required to reduce LID (0.01 mg/kg, i.p.) is considerably lower than the dose (1 mg/kg, i.p.) required to reduce locomotor activity in rats not pretreated with l-DOPA. Reduction of LID was observed when N/OFQ was administered directly into the dorsolateral striatum and to a lesser extent when administered directly into the SNr. These results suggest that N/OFQ actions in the striatum play an important role in the anti-LID action of NOP receptor agonists. Conversely, NOP receptor antagonists increased the intensity of LID responses in the same experimental paradigms, but this effect required that the antagonist be injected into the SNr; striatal injections of UFP-101 did not alter LID intensity. The beneficial effects of a NOP receptor agonist on LID were also evaluated in MPTP-lesioned macaques monkeys primed with L-DOPA. Ro 65-6570 given intramuscularly attenuated LID after l-DOPA administration without affecting the reduction on parkinsonian symptoms caused by l-DOPA treatment in the monkeys (Marti et al., 2012).
These studies collectively demonstrate that agonists and antagonists at NOP receptors exert significant modulatory effects on the various forms of motor dysfunction associated with PD and with the late-onset side effects of l-DOPA in the treatment of PD. NOP receptor antagonists significantly reduce the impairment of motor performance in experimental models of PD. The magnitude of the improvement in rodents suggests that a similar action in humans with PD would be clinically useful, but this class of drugs has not yet been tested in humans and the extent of any beneficial effect in man is unknown. In contrast, NOP receptor agonists have been shown to alleviate l-DOPA-induced dyskinesia in experimental PD at doses that do not impair motor function in normal rats, suggesting that NOP receptor agonists might alleviate the dyskinesias that torment PD patients in the later stages of their disease. These differential actions of NOP receptor antagonists and agonists may occur at different sites in the DA pathways regulating output from the basal ganglia. The beneficial effects of NOP receptor antagonists on motor function in PD models appears to be primarily mediated by antagonism of endogenous N/OFQ in the substantia nigra reticulata (Marti et al., 2004). In contrast, the alleviation of dyskinesias by NOP receptors agonists after chronic l-DOPA treatment is probably mediated primarily in the striatum (Marti et al., 2012). Thus agonists and antagonists at the same type of receptor may both offer therapeutic benefit in the alleviation of various motor symptoms associated with PD through actions at different sites in the neural pathways regulating motor function. However, despite the fact that much of this evidence has been available for more than 5 years, neither NOP receptor agonists nor NOP receptor antagonists are currently identified in recent reviews of potential new drug therapies for PD (e.g., Hung and Schwarzschild, 2014; Stayte and Vissel, 2014). The reasons for this lack of attention to the potential therapeutic benefits of NOP receptor ligands in the treatment of PD are not entirely clear, but may be related to the biphasic dose-response curves displayed by NOP receptor antagonists in relieving the motor symptoms of PD (Volta et al., 2011) and the potential for NOP receptor antagonists to exacerbate LID in PD patients receiving l-DOPA.
VI. Nociceptin Opioid Peptide Receptor Ligands
As with opioid receptors, a great deal has been learned about the NOP system due to the identification of antagonists (both peptide and small molecules) and of systemically active small-molecule agonists. There are a large number of NOP ligands described in literature. Structure activity relationship (SAR) studies on N/OFQ have generated NOP-selective peptide ligands encompassing full and partial agonist as well as pure antagonist activities. Screening of peptide combinatorial libraries allowed the identification of N/OFQ-unrelated NOP-selective peptide ligands. In the frame of research activities mainly performed in industrial laboratories several different chemical classes of small molecule NOP ligands were discovered including piperidines, spiropiperidines, nortropanes, 4-amino-quinolines, quinazolines, and others. A detailed medicinal chemistry analysis of most if not all the available NOP ligands was recently published (Mustazza and Bastanzio, 2011). This section describes and discusses the pharmacological features of NOP-selective ligands including peptide and nonpeptide compounds that have been most important to the field, particularly with respect to their involvement in pain and drug abuse, thereby leading to a better understanding of the role of the NOP receptor system in these processes. The NOP ligands discussed here have been pharmacologically characterized in detail both in vitro and in vivo, used for investigating the biologic functions under control of the N/OFQ-NOP receptor system, and ultimately have been instrumental for foreseeing the therapeutic potential of innovative drugs interacting with the NOP receptor.
The in vitro pharmacological profile of the NOP receptor agonists and antagonists analyzed in the present section is summarized in Tables 2 and 3, respectively. The ligands were evaluated in membranes of cells expressing the human NOP receptor with classic receptor binding, stimulated [35S]GTPγS binding, and a BRET-based assay measuring NOP/G-protein interaction (Malfacini et al., 2015). These ligands were also tested in calcium mobilization studies performed in whole cells coexpressing the NOP receptor and the chimeric G-protein Gαqi5 (Camarda et al., 2009) and in bioassay experiments performed in N/OFQ-sensitive isolated tissues such as the electrically stimulated mouse (Berzetei-Gurske et al., 1996; Calo' et al., 1996) and rat (Bigoni et al., 1999) vas deferens.
TABLE 2.
In vitro pharmacological profile of NOP selective agonists
| human NOP |
rodent NOP |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| receptor binding |
NOP/G-protein |
[35S]GTPγS |
Ca2+ mobilization |
mVD |
rVD |
|||||||
| pKi | Selectivity | pEC50 | α | pEC50 | α | pEC50 | α | pEC50 | α | pEC50 | α | |
| N/OFQ | 9.91a | >1000 | 8.44b | 1.00 | 8.75a | 1.00 | 9.54c | 1.00 | 7.47d | 1.00 | 6.83e | 1.00 |
| N/OFQ(1-13)-NH2 | 10.24a | 276 | 8.46b | 1.00 | 9.28a | 0.86 | 9.30c | 0.96 | 7.40f | 1.01 | 6.90f | 0.99 |
| UFP-112 | 10.55e | >1000 | 9.35b | 0.98 | 10.55e | 1.03 | 9.05c | 1.04 | 9.24d | 0.97 | 8.34e | 1.14 |
| PWT2-N/OFQ | 10.30g | >1000 | 9.17b | 1.10 | 10.12g | 1.14 | 8.83g | 0.98 | 7.92g | 0.99 | 7.23h | 1.03 |
| [F/G] | 8.00i | 67 | 7.85b | 0.72 | 8.05j | 0.67 | 8.03c | 0.54 | slight transient effect pA2 6.75f | Inactive pA2 6.83f | ||
| UFP-113 | 10.26e | >500 | 9.35b | 0.45 | 9.72e | 0.79 | 7.97c | 0.62 | variable agonist effects pA2 9.10e | variable agonist effects pA2 9.22e | ||
| Ac-RYYRWK-NH2 | 9.01l | >1000 | 8.76h | 0.78 | 8.67l | 0.57 | 8.68c | 0.58 | 8.07m | 0.71 | 7.93h | 0.78 |
| Ro 64-6198 | 9.41n | >100 | 7.76h | 1.01 | 8.09a | 0.89 | 7.98c | 1.07 | 6.84° | 1.05 | 7.24° | 0.95 |
| Ro 65-6570 | 8.25p | 10 | 7.77b | 0.96 | 7.73h | 1.01 | 7.95q | 1.05 | 6.80q | 1.15 | 7.11q | 1.0 |
a (McDonald et al., 2003b), b (Malfacini et al., 2014), c (Camarda et al., 2009), d (Rizzi et al., 2007c), e (Arduin et al., 2007a), f (Bigoni et al., 1999), g (Rizzi et al., 2014), h unpublished results, i(Varani et al., 1999), J (Wright et al., 2003), l (Dooley et al., 1997), m (Rizzi et al., 2002a), n (Jenck et al., 2000), o (Rizzi et al., 2001c), p (Hashiba et al., 2001), q (Molinari et al., 2012), r (Varty et al., 2008). α is the ratio between the Emax of the agonist and the Emax of N/OFQ.
TABLE 3.
In vitro pharmacological profile of NOP selective antagonists
| human NOP |
rodent NOP |
||||||
|---|---|---|---|---|---|---|---|
| receptor binding |
NOP/G-protein |
[35S]GTPγS |
Ca2+ mobilization |
mVD |
rVD |
||
| pKi | selectivity | pKB/pA2 | pKB/pA2 | pKB/pA2 | pKB/pA2 | pKB/pA2 | |
| [Nphe1] | 8.39a | 269 | partial agonistb | 7.33c | 6.29d | 6.04a | 6.16a |
| UFP-101 | 10.24e | > 1000 | 7.66b | 8.85c | 7.66d | 7.29e | 7.30e |
| J-113397 | 9.15f | 147 | 7.95b | 9.08f | 7.32d | 7.81g | 7.77g |
| SB-612111 | 9.18h | > 1000 | 8.96b | 9.70h | 8.16d | 8.50h | 8.20h |
| C-24 | 9.62i | 794 | 9.11b | 9.98i | 8.73i | 8.44i | 8.28i |
a (Calo’ et al., 2000b), b (Malfacini et al., 2015), c (McDonald et al., 2003b), d (Camarda et al., 2009), e (Calo’ et al., 2002b), f (Trapella et al., 2006), g (Bigoni et al., 2000), h (Spagnolo et al., 2007), i (Fischetti et al., 2009b). Underscored data indicate pA2 values obtained from Schild plots.
A. Nociceptin/Orphanin FQ Related Peptides
A large number of SAR studies have been performed on the N/OFQ peptide sequence. These have been analyzed in detail in recent reviews (Mustazza and Bastanzio, 2011; Calo' and Guerrini, 2013). In the following paragraphs we briefly summarize those studies leading to the identification of chemical modifications of the N/OFQ sequence able to increase binding affinity or to modulate agonist efficacy and therefore instrumental in generating useful pharmacological tools.
1. Peptide Full Agonists.
Both alanine scans and peptide truncation studies have been conducted to identify required amino acids and the shortest peptide to maintain activity (Dooley and Houghten, 1996; Reinscheid et al., 1996). N/OFQ(1–13)-NH2 is the shortest truncated version of N/OFQ that maintains maximal affinity for the NOP receptor. N/OFQ(1–13)-NH2 maintains functional activity, both in vitro and in vivo, that is basically indistinguishable from the native peptide (reviewed in Calo' et al., 2000a). Amidation of the C terminal of the endogenous peptide, i.e., N/OFQ-NH2, slightly increased its potency both in vitro and in vivo (reviewed in Calo' et al., 2000c), likely due to lower susceptibility to carboxypeptidases.
Examination of the Phe4 residue (Guerrini et al., 2001) demonstrated that pF introduced into the phenyl ring led to a significant increase in activity (Bigoni et al., 2002; Rizzi et al., 2002b). [(pF)Phe4]N/OFQ(1–13)-NH2 has full agonist activity, being 3- to 10-fold more potent than N/OFQ. This is the case for both in vitro and in vivo assays. In vivo [(pF)Phe4]N/OFQ(1–13)-NH2 was more potent and had longer lasting effects with respect to locomotor activity, pain threshold, and cardiovascular parameters, in mice and food intake in rats.
The two sets of Arg-Lys in N/OFQ are thought to be important because they bind to the acidic residues in ECL2 of the NOP receptor (Topham et al., 1998). Okada et al. (2000) inserted Arg-Lys at different positions throughout the peptide, leading to the identification of [Arg14Lys15]N/OFQ as a highly potent NOP full agonist approximately 10-fold more potent than N/OFQ (Rizzi et al., 2002c). Similar results were obtained in different laboratories in various bioassay and other cellular studies (Rizzi et al., 2002c; Basso et al., 2005; Trombella et al., 2005). Subsequent to intracerebroventricular injection in mice, [Arg14Lys15]N/OFQ acted like N/OFQ, producing pronociceptive effects in the tail-withdrawal assay and inhibiting locomotor activity. Furthermore, [Arg14Lys15]N/OFQ was approximately 30-fold more potent than N/OFQ and produced longer lasting effects (Rizzi et al., 2002c).
NMR investigations (Orsini et al., 2005; Tancredi et al., 2005) and molecular modeling studies (Topham et al., 1998; Thompson et al., 2012) indicated that the C-terminal region of N/OFQ prefers alpha helix conformations. Supporting this proposal, substitution in position 7 and 11 with the alpha helix inducing residue Aib increases N/OFQ potency (Zhang et al., 2002). Therefore, the chemical modifications [(pF)Phe4], [Arg14Lys15], [Aib7], and C terminal amidation were combined in the same molecule generating the peptide [(pF)Phe4Aib7Arg14Lys15]N/OFQ-NH2 (UFP-112) (Rizzi et al., 2007b). UFP-112 is a full agonist at NOP receptors, but is up to 100 fold more potent than N/OFQ in isolated tissues (Table 2), indicating that the combined chemical modifications elicited synergistic rather than additive effects on peptide potency (Calo' et al., 2011). As with N/OFQ, UFP-112 is inactive in tissues isolated from NOP(−/−) mice (D'Agostino et al., 2005; Rizzi et al., 2007b). UFP-112 is also considerably more stable than N/OFQ, exhibiting a plasma t1/2 threefold longer than that of N/OFQ, a difference that was even more pronounced in brain homogenate (Rizzi et al., 2007b).
Although peptides are often considered ineffective as pharmaceuticals, particular for CNS disorders, the very high selectivity of action of peptide NOP agonists makes them valuable research tools. The data produced using peptide agonists has greatly increased our knowledge on the effects of the selective activation of NOP receptors on a large number of peripheral and central systems, including respiratory, gastrointestinal, genitourinary, cardiovascular, and renal systems in the periphery, as well as stress and anxiety, pain, food intake, locomotion, and drug addiction in the CNS, subsequent to intracerebroventricular administration. However, the poor pharmacokinetic properties of peptides, particularly inefficient transport across the blood-brain barrier, limit their usefulness to target CNS disorders. However, peptide NOP agonists may be useful after intrathecal administration, a treatment that is becoming more popular for patients with intractable pain (Kress et al., 2009) Currently, only two drugs are approved for this indication, morphine and the N-type calcium channel blocker, ω-conotoxin analog, ziconotide (Pope and Deer, 2013). However, neither of these is ideal because ziconotide is poorly tolerated, whereas the analgesic effect of morphine displays considerable tolerance liability. The intrathecal administration of N/OFQ or other peptide agonists in rodents produces antinociceptive effects; however, side effects such as hind limbflaccidity have limited the effectiveness of this type of treatment.
Although spinal administration of peptide NOP agonists is problematic in rodents, it might be very different in primates. In rhesus monkeys, spinal N/OFQ produced dose-dependent and behaviorally selective analgesia in the nanomole range (Ko et al., 2006; Ko and Naughton, 2009). Although N/OFQ itself displayed lower potency than morphine, and was relatively short acting, the NOP agonist UFP-112 was more potent than morphine and produced a similar magnitude of analgesia with a similar duration of action. The antinociceptive effects of spinal UFP-112 in monkeys was due to activation of NOP receptors, because it was sensitive to J-113397 but not to naltrexone. Furthermore, subthreshold doses of UFP-112 and morphine, when given in combination intrathecally, produced a robust antinociceptive action. Although tolerance has also been described to the effects of spinal N/OFQ in rats, no cross tolerance with morphine has been observed (Hao et al., 1997; Micheli et al., 2015). Collectively these nonhuman primate studies suggest that peptide NOP receptor agonists have the potential to be developed as innovative spinal analgesics. Because the shift from morphine to a peptide NOP agonist, such as UFP-112, in a patient with a permanent spinal catheter is expected to be a rather simple procedure, it may be possible to alternate the two drugs each time tolerance develops to one of the treatments, possibly resulting in a continuous pain relief, thus making the use of peptide NOP agonists in patients with intractable pain an interesting future possibility.
Recently a novel and facile chemical strategy for the synthesis of tetrabranched peptides named peptide welding technology (PWT; Guerrini et al., 2014) was used to prepare three N/OFQ tetrabranched derivatives containing different cores (PWT1, PWT2, and PWT3). PWT derivatives of N/OFQ behaved as high affinity, potent full agonists with respect to receptor binding, [35S]GTPγS binding, calcium mobilization in cells expressing human NOP receptors, as well as in native animal tissues (electrically stimulated mouse vas deferens bioassay). The in vitro pharmacological profile of PWT2-N/OFQ is summarized in Table 2. In vivo in mice, N/OFQ PWT derivatives mimicked the inhibitory effects exerted by the natural peptide on locomotor activity, showing 40-fold higher potency and much longer lasting action. In fact, although the action of N/OFQ disappears 1 hour after intracerebroventricular injection, that exerted by PWT derivatives lasted up to 24 hours. The inhibitory effects of PWT2-N/OFQ on locomotor activity were no longer present in NOP(−/−) mice (Rizzi et al., 2014). After intrathecal administration in mice, PWT2-N/OFQ produced antinociceptive effects both in nociceptive (tail withdrawal) and neuropathic (chronic constriction injury) pain models, results that were confirmed in nonhuman primates. In fact as with rodent studies, in monkeys PWT2-N/OFQ mimicked the antinociceptive effects of N/OFQ, being approximately 100-fold more potent. In addition, although the effects of 100 nmol N/OFQ lasted for 2.5 hours (Ko et al., 2006), those elicited by 1 nmol PWT2-N/OFQ were still statistically significant 24 hours after the spinal injection (Rizzi et al., 2015). These recent findings demonstrated that that the PWT can be successfully applied to the peptide sequence of N/OFQ to generate tetrabranched derivatives characterized by a pharmacological profile similar to the native peptide and associated with a higher potency and an extraordinary prolongation of in vivo action, suggesting that spinal administration of NOP receptor-active peptides could be of significant clinical value for treatment of chronic pain.
2. Peptide Partial Agonists.
Modifications of the conformational freedom (Guerrini et al., 1998) or of the spatial disposition (Calo' et al., 2000b) of Phe1 relative to Phe4 reduces peptide efficacy. Crystallographic analysis and docking investigations indicate that the Gly2-Gly3 dipeptide acts as a conformation-inducing spacer between the pharmacophores Phe1 and Phe4 and allows the N-terminal nitrogen atom of the peptide to forms an ionic interaction with the Asp130 of the NOP receptor (Daga and Zaveri, 2012; Thompson et al., 2012)
The increase of conformational freedom obtained by reducing the Phe1-Gly2 peptide bond in N/OFQ, i.e., [F/G]N/OFQ(1-13)-NH2, produces a loss of efficacy generating the first N/OFQ-related peptide showing partial agonist efficacy (Calo' et al., 1998; Guerrini et al., 1998). [F/G]N/OFQ(1-13)-NH2 has been extensively evaluated in vitro and in vivo (reviewed in Calo' et al., 2000a). Although initially considered an antagonist, based upon in vitro bioassays (Guerrini et al., 1998), it was later shown both in vitro and in vivo that [F/G]N/OFQ(1-13)-NH2 can behave as a partial or full agonist or even as a pure antagonist, depending on the preparation or the assay. After intracerebroventricular administration in mice, [F/G]N/OFQ(1-13)-NH2 had full agonist activity and thereby mimicked the pronociceptive effect of N/OFQ in the tail withdrawal assay (Calo' et al., 1998). However, it behaved as a partial agonist when measuring locomotor activity (Rizzi et al., 2001a) and a pure antagonist, blocking N/OFQ-induced bradycardia and hypotension (Madeddu et al., 1999). This variable pharmacological activity is most likely due to the low efficacy agonist properties of this ligand whose final effect strongly depends upon the the receptor reserve and the resulting stimulus-response coupling of the preparation/function under study. This interpretation has been confirmed experimentally because the pharmacological activity of [F/G]N/OFQ(1-13)-NH2 has been manipulated to encompass full and partial agonism to pure antagonism, using the same cells by modifying NOP receptor density as the only variable (McDonald et al., 2003a).
The chemical modifications [(pF)Phe4], [Aib7], and [Arg14Lys15] discussed above that increase peptide affinity/potency have also been combined with [F/G] to generate UFP-113 (Arduin et al., 2007), a NOP agonist with 100-fold increase in potency and longer duration of action (Table 2). After intrathecal injection, UFP-113 mimicked N/OFQ action, eliciting dose-dependent (0.001–1 nmol) antinociception in the rat paw pressure test, effects that were no longer evident in NOP(−/−) rats (Micheli et al., 2015).
3. Peptide Antagonists.
[Nphe1]N/OFQ(1–13)-NH2 was the first peptide with consistent antagonist activity reported in literature. [Nphe1]N/OFQ(1–13)-NH2 showed selective binding to recombinant NOP receptors, reversed the inhibitory effects of N/OFQ on cAMP accumulation (Calo' et al., 2000b), and competitively antagonized the contractile effect of N/OFQ but not of endomorphin-1 in the mouse colon (pA2 6.0) (Rizzi et al., 1999). It also antagonized N/OFQ action in electrically stimulated isolated tissues of the mouse, rat, and guinea-pig (pA2 6.0–6.4) (Calo' et al., 2000b). Unlike [F/G]N/OFQ(1–13)-NH2, [Nphe1]N/OFQ(1–13)-NH2 displayed consistent antagonist activity in vivo where it prevented the pronociceptive and antimorphine actions of intracerebroventricular N/OFQ. The antagonist nature of this compound was confirmed in numerous studies performed in different laboratories and was previously reviewed (Calo' et al., 2000a,c).
To increase ligand potency and maintain antagonist activity, the chemical modifications [Nphe1] and [Arg14Lys15] were combined to generate UFP-101 (Calo' et al., 2002b), a pure antagonist with at least 10-fold higher potency than [Nphe1]N/OFQ(1–13)-NH2. UFP-101 has been studied extensively both in vitro and in vivo and has been demonstrated to reverse many of the biologic actions of N/OFQ including locomotor activity, pain transmission, neurochemical actions, food intake, cardiovascular, kidney and gastric functions, memory, drug reward, hypothalamic-pituitary-adrenal axis responses, anxiety, and depression (reviewed in Calo' et al., 2005 and Calo' and Guerrini, 2013). A tritiated version of UFP-101 was found useful for receptor binding studies using recombinant NOP receptors as well as animal tissues (Ibba et al., 2008).
B. Nociceptin/Orphanin FQ Unrelated Peptides
In 1997, Dooley et al. identified, from a large peptide combinatorial library, 15 hexapeptides with high affinity for the NOP receptor, of which 5 were examined for in vitro activity (Dooley et al., 1997). These very basic hexapeptides behaved as potent and selective NOP receptor partial agonists with potency similar to N/OFQ but reduced efficacy in several in vitro assays with α values typically in the range 0.5–0.8 (see Table 1). Ac-RYYRWK-NH2 has been evaluated mainly in vitro (reviewed in Calo' et al., 2000c) where it behaves as full or partial agonist or even as a pure antagonist similar to [F/G]N/OFQ(1–13)-NH2. In fact, the reasons for the differing pharmacological behavior of Ac-RYYRWK-NH2 are similar to those already discussed for [F/G]N/OFQ(1–13)-NH2 and have been proven using a NOP receptor inducible system (McDonald et al., 2003a).
SIP technology was used to generate the NOP ligand ZIP120 (Ac-RYYRWKKKKKKK-NH2) from Ac-RYYRWK-NH2 (Rizzi et al., 2002a). In electrically stimulated mouse and rat vas deferens, ZP120 displayed the same efficacy as Ac-RYYRWK-NH2, but with approximately 10-fold higher potency (Rizzi et al., 2002a; Fischetti et al., 2009a). Interestingly, when measuring calcium mobilization in cells expressing chimeric G-proteins (Camarda et al., 2009), the potency of ZP120 was relatively low, as was the case with several other NOP ligands such as UFP-112, UFP-113, and PWT2-N/OFQ (see Table 2). Each of these compounds is characterized by a slow kinetics of activation of the NOP receptor, as suggested by bioassay experiments in isolated tissues (Rizzi et al., 2002c; Calo' et al., 2011; Guerrini et al., 2014). It is possible that the rapid kinetics that characterize the calcium transient response may be incompatible with the slow kinetics of the ligand receptor interaction (for a detailed discussion of this topic see Camarda et al., 2009; Rizzi et al., 2014). In vivo, however, ZP120 displayed very high potency and long duration of action in locomotor activity and tail withdrawal experiments in mice (Rizzi et al., 2002a), effects that were no longer present in NOP(−/−) mice (Fischetti et al., 2009a). This compound is of particular interest because it was developed specifically to be used in humans by Zealand Pharma (Glostrup, Denmark). ZP120, which has diuretic activity, reached phase II clinical trials for acute decompensated heart failure, but was discontinued due to an unexpected drop in systolic and diastolic blood pressure in patients. However, Serodus Pharmaceuticals (Oslo, Norway) has capitalized on this side effect of ZP120 and is continuing the development of this compound for treatment-resistant systolic hypertension.
NOP-selective peptides were useful in validating the NOP receptor as a possible therapeutic target. Together with results obtained with nonpeptide agonists and antagonists or receptor knockout studies, a large body of evidence has been collected indicating that NOP directed ligands are worthy of development as innovative drugs for the treatment of a potentially large number of syndromes. For such indications the development of orally active, brain-penetrant, nonpeptide molecules is necessary to perform clinical investigations aimed at firmly identifying their effectiveness in patients and eventually their place in therapy. Examples of important non-peptide agonists and antagonists are discussed below.
C. Nonpeptide Nociceptive Opioid Peptide Ligands
1. Nonpeptide Agonists.
Researchers at Hoffman La Roche (Basel, Switzerland) performed a rather large series of SAR studies aimed at the identification of NOP selective agonists (Wichmann et al., 1999). These chemical efforts, nicely reviewed by Shoblock (2007), led to the identification of [(1S,3aS)-8-(2,3,3a,4,5, 6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza- spiro[4.5]decan-4-one] Ro 64-6198 (see chemical structure in Fig. 8) as a highly potent and selective NOP agonist (Wichmann et al., 2000). This compound is the most widely used NOP receptor synthetic agonist and is a very useful tool for NOP receptor target validation studies. In particular, experiments performed with Ro 64-6198 contributed to the identification of anxiety, neuropathic pain, drug abuse, cough, and possibly anorexia as possible therapeutic indications for NOP receptor agonists. This molecule was also extremely useful in the identification of NOP receptor agonist side effects including motor disturbance, impairment of memory, and hypothermia (Shoblock, 2007).
Fig. 8.
Chemical structure of non-peptide NOP selective ligands
Ro 64-6198 binds the NOP receptor with subnanomolar affinity, displays high selectivity (>100-fold) over classic opioid receptors, and behaves as a full agonist (Jenck et al., 2000; Wichmann et al., 2000). This basic in vitro pharmacological profile has been confirmed in several studies using different assays (Dautzenberg et al., 2001; Hashiba et al., 2002; McDonald et al., 2003a; McLeod et al., 2004; Camarda et al., 2009) (Table 2). Ro 64-6198 stimulated [35S]GTPγS binding in rat brain sections in a concentration dependent manner with potency close to N/OFQ. In general, the brain distribution of agonist stimulated [35S]GTPγS binding was similar when either Ro 64-6198 or N/OFQ were used (Gehlert et al., 2006). However, other results underlined some differences in Ro 64-6198 versus N/OFQ in vitro actions. Chiou et al. (2004) reported that in rat periaqueductal gray slices, measuring activation of G-protein-coupled inwardly rectifying K+ channels, Ro 64-6198 mimicked N/OFQ effects but with lower maximal effects and, importantly, affecting only a subset of N/OFQ sensitive neurons. Similar results were later reported using a different nonpeptide NOP agonist (Liao et al., 2011). Moreover, in these experiments, Ro 64-6198 displayed a very slow kinetics of action. This slow kinetics of action associated with slowly reversible effects was also reported for Ro 64-6198 in N/OFQ-sensitive electrically stimulated tissues. Antagonist studies demonstrated that in the rat vas deferens, Ro 64-6198 behaved as a NOP selective agonist, in the guinea pig ileum as a NOP/opioid mixed agonist, whereas in the mouse vas deferens, Ro 64-6198 actions could not be fully prevented even using a cocktail of NOP and opioid receptor antagonists, suggesting interaction with an unknown inhibitory site (Rizzi et al., 2001c). Thus Ro 64-6198 selectivity of action seems to be variable depending on species and tissues. This implies that the involvement of the NOP receptor in the vivo actions of Ro 64-6198 should be carefully assessed with receptor antagonists and/or knockout studies.
Ro 64-6198 crucially contributed to our understanding of the anxiolytic-like properties of NOP agonists. Jenck et al. (1997, 2000) demonstrated that Ro 64-6198, given systemically in the 0.3 to 3 mg/kg dose range, mimicked the anxiolytic-like effect of supraspinal N/OFQ in several rat assays, including elevated plus-maze, fear-potentiated startle, and operant conflict. The anxiolytic-like effects of Ro 64-6198 were comparable to those elicited by benzodiazepines. These initial findings were later confirmed and extended in different laboratories using several assays including rat conditioned lick suppression test, isolation-induced vocalizations in rat and guinea pig pups, mouse Geller-Seifter test (Varty et al., 2005), marble burying (Nicolas et al., 2006), rat Vogel conflict punished drinking test, the social approach-avoidance test in Lewis rats, novelty-induced hypophagia and stress-induced hyperthermia in mice (Goeldner et al., 2012). In some of these assays, the action of Ro 64-6198 was demonstrated to be exclusively due to NOP receptor activation using either J-113397 or NOP(−/−) mice. Importantly the anxiolytic-like effects of Ro 64-6198 did not show tolerance liability after 15 days of daily drug exposure (Dautzenberg et al., 2001), These studies also confirmed on-target side effects of Ro 64-6198, particularly inhibition of locomotor activity and sedation. However a therapeutic window is evident between anxiolytic and sedative doses; this is wider in rats than in mice (Higgins et al., 2001; Varty et al., 2005).
As far as drug abuse is concerned, Ro 64-6198 was reported to counteract the rewarding and reinforcing properties of morphine and ethanol. Importantly Ro 64-6198 itself is devoid of rewarding properties as demonstrated by lack of conditioned place preference in rodents (Jenck et al., 2000; Le Pen et al., 2002) and lack of self-administration in monkeys (Ko et al., 2009). In an elegant study of conditioned place preference in mice, Ro 64-6198 (1 mg/kg) inhibited the acute rewarding properties of morphine (Shoblock et al., 2005). Ro 64-6198 was also shown to inhibit the acquisition, expression, and reinstatement of ethanol conditioned place preference (Kuzmin et al., 2003). In a separate study it was demonstrated that Ro 64-6198 is also active in reducing ethanol self-administration and preventing relapse of ethanol drinking (Kuzmin et al., 2007). These results obtained with Ro 64-6198 are in line with a rather large number of findings obtained with N/OFQ, or other NOP ligands, suggesting that NOP agonists are worthy of further exploration as innovative treatments for drug abuse (Zaveri, 2011; Witkin et al., 2014). However, one should keep in mind the observation that NOP receptor agonists appear to be more efficacious for attenuation of CPP than self-administration, as discussed above, once again displaying the complicated nature of the NOP receptor system.
As discussed above, peptide NOP agonists block opioid antinociception when administered intracerebroventricularly but have antinociceptive activity when administered intrathecally. The development of Ro 64-6198 permitted the determination of the result of systemic administration of a NOP agonist on nociception. A number of studies suggest that the systemic injection of Ro 64-6198 does not modify nociceptive pain transmission in rodents, as demonstrated in the tail flick, tail immersion, tactile or cold water stimulation, and foot shock test (Jenck et al., 2000; Obara et al., 2005; Varty et al., 2005; Reiss et al., 2008). However an exception to this rule is the mouse hot plate test where systemic Ro 64-6198 produced modest antinociceptive effects (Reiss et al., 2008; Chang et al., 2015a). These effects were reproduced in NOP(+/+) but not NOP(−/−) mice. In addition, subthreshold doses of Ro 64-6198 and morphine elicited additive antinociceptive effects (Reiss et al., 2008). In contrast to rodent studies, Ro 64-6198 elicits brilliant inhibitory effects on nociceptive pain transmission in nonhuman primates. In fact, Ro 64-6198 (0.001–0.06 mg/kg, s.c.) produced robust antinociception against an acute noxious stimulus (50°C water) and capsaicin-induced allodynia in monkeys. J-113397 (0.01–0.1 mg/kg, s.c.) dose dependently produced rightward shifts of the dose-response curve to Ro 64-6198, whereas naltrexone was inactive. Moreover Ro 64-6198 was devoid of typical opioid side effects such as respiratory depression and itch/scratching responses (Ko et al., 2009). The reasons for this difference in the effects of Ro 64-6198 on nociceptive pain transmission between rodents and monkeys are not known. However, very recent data suggest that, unlike rodents, the supraspinal injection of N/OFQ causes antinociceptive effects in monkeys (Ding et al., 2015). Thus species-specific opposite effects of NOP control on nociceptive pain transmission in the brain, probably due to differences in circuitry, may likely explain the above-mentioned differences of Ro 64-6198 action in rodents and nonhuman primates.
In the rat sciatic nerve injury model, Ro 64-6198 given intrathecally or intraplantarly produced antiallodynic effects that were sensitive to NOP antagonists (Obara et al., 2005). Similar results were obtained in response to systemic injection of Ro 64-6198 in monkeys. In fact, tail injection of carrageenan produced long-lasting thermal hyperalgesia in monkeys. Ro 64-6198 dose dependently attenuated carrageenan-induced thermal hyperalgesia, being much more potent for its antihyperalgesic than antinociceptive effects (Sukhtankar et al., 2014). These findings are in line with considerable literature evidence indicating the NOP agonists elicit more potent and robust antinociceptive effects against neuropathic and inflammatory than nociceptive pain (Schroder et al., 2014).
Another biologic effect of N/OFQ mimicked by the systemic injection of Ro 64-6198 is the inhibition of the cough reflex. In guinea pig studies, aerosolized capsaicin produces a dose-dependent increase in cough number. Ro 64-6198 significantly inhibits capsaicin effects in a dose dependent manner. The antitussive effect of Ro 64-6198 was blocked by J-113397 but not by naltrexone (McLeod et al., 2004). Based upon a large number of studies demonstrating the involvement of the NOP receptor in cough and airway microvasculature, Merck Sharp & Dohme conducted clinical trials on the full NOP receptor agonist SCH 486757. Phase II trials indicated no improvement over the comparator, codeine (McLeod et al., 2011), although the authors made the point that these are difficult clinical trials since the patients often improve spontaneously during the course of the trial.
a. Ro 65-6570.
Among the compounds generated in Roche laboratories and described by Wichmann et al. (1999), the compound 8-(1,2-dihydroacenaphthylen-1-yl)-1-phenyl-1,3,8-triazaspiro[4,5]decan-4-one (Ro 65-6570, see chemical structure in Fig. 8) was identified as the most interesting molecule of the series. Ro 65-6570 bound with subnanomolar affinity to the human NOP receptor, displaying 10-fold selectivity over opioid receptors, and produced a NOP antagonist reversible, concentration-dependent inhibition of cAMP formation with maximal effects similar to N/OFQ and a value of potency 10-fold lower (Hashiba et al., 2001). This compound has similar potency and efficacy as Ro 64-6198 in a variety of in vitro and in vivo paradigms. (Jenck et al., 1997; Byford et al., 2007; Rutten et al., 2010).
2. Nonpeptide Antagonists.
a. J-113397.
1-[(3R,4R)-1-cyclooctylmethyl-3- hydroxymethyl-4-piperidyl]-3-ethyl-1, 3-dihydro-2H-benzimidazol-2-one (J-113397, see chemical structure in Fig. 8) was identified by Banyu researchers at the end of the 1990s the first potent and selective nonpeptide NOP antagonist (Kawamoto et al., 1999). This molecule has been widely used and has contributed to our knowledge of the N/OFQ - NOP receptor system. J-113397 binds with low nanomolar affinity to the NOP receptor and displays high selectivity for NOP over classic opioid receptors, although other laboratories reported significantly less NOP receptor selectivity, particularly with respect to the mu receptor (Zaratin et al., 2004). In [35S]GTPγS binding experiments with the human NOP receptor, J-113397 displayed competitive antagonism with high potency and selectivity for the NOP receptor (Ozaki et al., 2000b). Studies in other laboratories confirmed these initial findings and demonstrated that J-113397 was able to antagonize N/OFQ effects in different preparations including rat brain and spinal cord (Yamada et al., 2003) and smooth muscle preparations (Bigoni et al., 2000).
J-113397 was useful for investigating the neurochemical actions of N/OFQ. In rat and mouse cerebral cortex synaptosomes, N/OFQ inhibited the release of tritiated serotonin and noradrenaline in a J-113397-sensitive manner (Marti et al., 2003; Mela et al., 2004). These inhibitory effects of N/OFQ and antagonist action of J-113397 were confirmed using electrically stimulated human cerebral cortex slices (Rominger et al., 2002; Berger et al., 2006). Interestingly, a microdialysis study demonstrated that N/OFQ inhibited the release of noradrenaline in the basolateral nucleus of the amygdala in awake rats, whereas systemic administration of J-113397 produced opposite effects, thus suggesting that a large part of basal release of noradrenaline in the basolateral nucleus of the amygdala is under tonic inhibitory control by the endogenous N/OFQ-NOP receptor system (Kawahara et al., 2004).
J-113397 was crucial for studying the in vivo biologic functions controlled by the N/OFQ-NOP receptor system and to predict possible therapeutic indications of NOP ligands. As far as pain transmission is concerned, J-113397, administered subcutaneously, dose dependently (3–30 mg/kg) inhibited hyperalgesia elicited by supraspinal administration of N/OFQ in the mouse tail-flick test (Ozaki et al., 2000a). Similar to rodents (Zeilhofer and Calo', 2003), N/OFQ produces inhibitory effects on pain transmission at the peripheral (Ko et al., 2002) and spinal (Ko et al., 2006) level in monkeys; these actions of N/OFQ are fully prevented by the systemic injection of 0.1 mg/kg J-113397.
The ability of NOP agonists to block CPP of abused drugs was discussed above. Perhaps more interesting is the observation that coadministration of J-113397 during conditioning facilitates morphine-induced conditioned place preference. This evidence is consistent with the observation that NOP(−/−) rats are more sensitive to the rewarding effect of morphine than NOP(+/+) animals (Marquez et al., 2008; Rutten et al., 2011). Thus, pharmacological or genetic inactivation of the NOP system rendered rats more susceptible to the rewarding effect of morphine, supporting the hypothesis that the NOP receptor may be a therapeutic target for the treatment of drug abuse and addiction (Zaveri, 2011). J-113397 was also instrumental for studying the relationship between N/OFQ-ergic signaling and cocaine abuse. In fact, N/OFQ injected supraspinally or microinjected into the ventral tegmental area blocked cocaine-induced behavioral sensitization. The effect of the peptide was no longer evident in animals pretreated with J-113397 (Lutfy et al., 2002). Similar to what was found with morphine, NOP(−/−) mice expressed greater conditioned place preference than NOP(+/+) animals. Furthermore, the rewarding action of cocaine was enhanced in wild-type mice treated with 3 mg/kg J-113397. Together, these results strongly suggest that the endogenous N/OFQ-NOP receptor system is involved in the rewarding action of cocaine (Marquez et al., 2008).
In the seminal paper by Redrobe et al. (2002) it was demonstrated that the intracerebroventricular injection of N/OFQ does not modify the behavior of mice in the forced swim test, the standard assay used for screening potential antidepressant drugs. The systemic injection of the universal opioid antagonist naloxone was also inactive. On the contrary, J-113397 given systemically at 20 mg/kg, reduced immobility time, an effect mimicked by the intracerebroventricular injection of [Nphe1]N/OFQ(1–13)-NH2. Importantly open field analysis revealed that treatment with these molecules did not induce significant changes in locomotor activity. Further studies demonstrated that NOP(−/−) mice display an antidepressant phenotype in the mouse forced swim test (Gavioli et al., 2003) and that in these animals the action of J-113397 is no longer evident (Gavioli and Calo', 2006). These initial findings suggesting that NOP selective antagonists are worthy of development as innovative antidepressants have been confirmed with several molecules, various assays, and in different laboratories. The available information in this specific field was recently reviewed (Gavioli and Calo', 2013). Very recently a double-blind, placebo-controlled trial performed with the novel NOP selective antagonist LY2940094 in patients with major depressive disorder provided the first clinical evidence that the blockade of NOP receptor signaling represents a promising strategy for the treatment of depression (Post et al., 2015).
b. SB-612111.
(−)-cis-1-Methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9- tetrahydro-5H-benzocyclohepten-5-ol (SB-612111, see chemical structure in Fig. 8) was reported by Smithkline Beecham (Brentford, UK) researchers as a novel NOP selective antagonist. SB-612111 displayed subnanomolar affinity for the recombinant human NOP and high (>150-fold) selectivity over classic opioid receptors. Compared with J-113397 included in the same set of experiments, SB-612111 showed higher affinity and selectivity. In a whole cell gene reporter assay, SB-612111 antagonized N/OFQ effects showing a competitive mode of interaction. In the same assay performed with cells expressing the mu opioid receptor, SB-612111 was inactive up to micromolar concentrations (Zaratin et al., 2004). SB-612111 competitively antagonized the effects of N/OFQ on [35S]GTPγS binding in CHO-NOP cell membranes and on cAMP accumulation in CHO-NOP cells with high potency, as well as in isolated peripheral tissues of mice, rats, and guinea pigs and in mouse cerebral cortex synaptosomes in which it was found to be 3–10 times more potent than J-113397 (Spagnolo et al., 2007). The in vitro pharmacological actions of SB-612111 were also investigated in electrophysiological studies. For instance, in slices taken from the ventromedial nucleus of the hypothalamus, bath application of N/OFQ stimulated an inwardly rectifying potassium current that was sensitive to G-protein inactivation. Application of SB-612111 blocked this effect of N/OFQ (Chee et al., 2011). Moreover SB-612111 dose dependently antagonized N/OFQ induced G-protein-coupled inwardly rectifying K+ current in periaqueductal gray neurons. SB-612111 has no agonistic activity and does not affect the current stimulated by a selective mu receptor agonist (Liao et al., 2011).
In vivo SB-612111 completely and dose dependently blocked both the pronociceptive and the antimorphine action elicited by intracerebroventricular N/OFQ in the mouse hot-plate test. In line with knockout and J-113397 studies mentioned above, SB-62111 administration can also reverse tolerance to the analgesic effect of morphine (Zaratin et al., 2004). Another study demonstrated that in the mouse tail withdrawal assay, SB-612111 given intraperitoneally up to 3 mg/kg did not modify tail withdrawal latencies per se but was able to prevent the pronociceptive and the antinociceptive action of N/OFQ given intracerebroventricularly and intrathecally, respectively. In food intake studies performed in sated mice, SB-612111 had no effect on food consumption but fully prevented the orexigenic effect of N/OFQ (Rizzi et al., 2007a).
The antiparkinsonian effects of SB-612111 were studied in reserpinized mice and 6-hydroxydopamine hemilesioned rats under both acute and chronic administration protocols. In reserpinized mice SB-612111 provided a dose-dependent antiparkinsonian effect. In 6-hydroxydopamine hemilesioned rats SB-612111 ameliorated motor performance. In addition SB-612111 synergized with levodopa at subthreshold doses. When chronically administrated, SB-612111 maintained its effects over time without modifying baseline activity (Marti et al., 2013).
In line with the results obtained with other NOP selective antagonists and with NOP(−/−) mice and rats (Gavioli and Calo', 2013), in the mouse forced swim and tail suspension tests, SB-612111 (1–10 mg/kg) reduced immobility time. The antidepressant-like effect elicited by SB-612111 in the forced swim test was reversed by the intracerebroventricular injection of N/OFQ and no longer evident in NOP(−/−) mice (Rizzi et al., 2007a).
c. C-24.
In 2006, Banyu (Kitanomaru Square, Japan) researchers described a focused library approach aimed at the identification of novel leads developed as NOP antagonists. Beginning from a compound identified by random screening, a highly focused library was designed based on three-dimensional pharmacophore similarity. A novel D-proline amide class was identified in this library and was found to possess potent NOP antagonistic activity. Among these compounds, 1-benzyl-N-[3-[spiroisobenzofuran-1(3H),4'-piperidin-1-yl]propyl] pyrrolidine-2-carboxamide (C-24, see chemical structure in Fig. 8) demonstrated subnanomolar affinity for the NOP receptor associated with extraordinary selectivity (>9000-fold). In [35S]GTPγS binding studies, C-24 inhibited N/OFQ stimulatory effects with subnanomolar potency (Goto et al., 2006). These initial findings were confirmed and extended in subsequent in vitro studies in transfected cells and smooth muscle preparations (Fischetti et al., 2009a). Moreover in electrophysiological studies, C-24 behaved as a pure antagonist at the native NOP receptors expressed in periaqueductal gray neurons, where it blocked N/OFQ induced G-protein-coupled inwardly rectifying potassium current. However, in this preparation C-24 demonstrated only moderate potency and selectivity (Liao et al., 2009). In native sympathetic neurons, C-24 blocked N/OFQ-mediated Ca2+ current inhibition. Interestingly neurons microinjected with NOP cDNA displayed enhanced tonic inhibition of Ca2+ currents in the absence of agonists that was abolished after pretreatment with pertussis toxin. This strongly suggests constitutively active NOP receptors in transfected neurons. In these neurons C-24 not only antagonized the N/OFQ inhibitory effect but also exerted inverse agonism, as measured by the loss of tonic Ca2+ current inhibition (Mahmoud et al., 2010).
In vivo, C-24 displayed good brain penetration and was able, at 3 mg/kg, to fully prevent the locomotor depressant action of a NOP agonist in mice (Goto et al., 2006). In the mouse tail withdrawal assay, C-24 at 10 mg/kg antagonized the pronociceptive action of N/OFQ given supraspinally. Moreover at the same dose C-24 blocked the antinociceptive effect of spinal N/OFQ while being inactive against the antinociceptive action of endomorphin-1 (Fischetti et al., 2009a). In line with previous antagonist studies (see above), in 6-hydroxydopamine hemilesioned rats, systemically administered C-24 improved motor activity in the 0.1–10 mg/kg dose range (Volta et al., 2011). Importantly, among a large panel of NOP ligands, C-24 imparted the highest thermostability to the NOP receptor. On this basis, C-24 was selected for cocrystallization trials to solve the X-ray structure of the NOP receptor (Thompson et al., 2012).
B. Bifunctional Compounds
N/OFQ administered intracerebroventricularly concurrently with systemic morphine blocks morphine tolerance development (Lutfy et al., 2001b). Furthermore, N/OFQ administered intracerebroventricularly blocks drug-induced increase in extracellular dopamine in the nucleus accumbens and blocks CPP induced by a variety of abused drugs (Murphy et al., 1996; Lutfy et al., 2001a). These results led to the hypothesis that a compound with both mu and NOP agonist activity might retain the mu-mediated analgesia but with reduced tolerance development and reduced reward. Several investigators have identified or synthesized compounds with both mu and NOP receptor agonist activity and examined this hypothesis.
Before the design of novel compounds, one well-known opiate, buprenorphine, was found to activate NOP receptors, which apparently leads to some of the biologic properties of this compound. Although buprenorphine has only moderate affinity for NOP receptors (80–100 nM) some laboratories have demonstrated significant activity in vitro for stimulation of [35S]GTPγS binding, as well as inhibition of adenylyl cyclase using a reporter gene assay, and stimulation of MAP kinase, all in transfected cells (Wnendt et al., 1999; Bloms-Funke et al., 2000; Huang et al., 2001). Other laboratories found significantly less efficacy for buprenorphine in transfected cells and brain membranes (Lester and Traynor, 2006; Khroyan et al., 2009). Nevertheless behavioral results suggest buprenorphine has NOP agonist activity in vivo.
Buprenorphine is a partial agonist at mu opioid receptors and as such has a very shallow dose-response curve for antinociceptive activity in the tail withdrawal assay, and in fact, at appropriate stimulus intensity (for instance warm water temperature) an antinociception dose-response curve results in an inverted U shape, with decreased tail flick latency at higher doses. Lutfy et al. (2003) demonstrated that in NOP(−/−) mice, the efficacy of buprenorphine continued to increase at higher doses. Furthermore, a similar result was found with coadministration of the NOP receptor antagonist J-113397. These results strongly suggest that at higher doses, the NOP agonist activity of buprenorphine can interfere with the analgesic activity of the mu component of buprenorphine.
An analogous experiment was conducted with respect to alcohol consumption. In Marchigian Sardinian alcohol-preferring (msP) rats, buprenorphine also has a biphasic effect on alcohol consumption. In low doses buprenorphine increases alcohol consumption, but at high doses consumption decreases. However, the intracerebroventricular administration of the peptide NOP antagonist UFP-101 reverses the high dose buprenorphine-induced inhibition and results in the continued increase in alcohol consumption (Ciccocioppo et al., 2007). These results are consistent with a mu receptor-mediated increase in alcohol consumption at low buprenorphine doses, which is blocked and reversed by NOP receptor activation at higher doses of buprenorphine. These results suggest that buprenorphine can activate both mu and NOP receptors in situ and that a compound that activates both receptors could maintain analgesic activity with reduced abuse liability.
This was tested more directly with a series of compounds that had various affinities and activities at mu and NOP receptors. Compounds were designed with a NOP scaffold rather than an opioid scaffold and resulted in high affinity at both receptors, unlike buprenorphine, which has significantly higher affinity at the opioid receptors rather than at NOP. The first compound tested was SR16435, which has high affinity and potent partial agonist activity at both mu and NOP receptors (Khroyan et al., 2009). This compound has potent antinociceptive activity in the radian heat tail flick assay. It also has reduced tolerance development compared with morphine when given daily at its antinociceptive EC50 dose. However, this compound induces a CPP equal to that of morphine. To determine whether partial agonist activity at NOP receptors was not sufficient to attenuate the reward induced by the mu component, SR16507 was tested. This compound has equal high affinity at both NOP and mu receptors, but is a full agonist at the NOP receptor and partial agonist at mu. This compound has very potent antinociceptive activity but still induces a modest CPP, although it also attenuated morphine CPP (Toll et al., 2009). SR14150 is a somewhat selective NOP agonist with partial agonist activity at both NOP and mu receptors. This compound, although it is a weak mu agonist, has naloxone reversible antinociceptive activity, but in this case without inducing CPP (Toll et al., 2009). This indicates that a profile can be found with both NOP and mu agonist activity in which antinociceptive activity remains but reward is diminished by the presence of the NOP component. However, in this set of compounds, the presence of NOP agonist activity also attenuates the antinociceptive activity of the mu component, as demonstrated by potentiation of the antinociception by coadministration of the NOP receptor antagonist SB-612111 (Khroyan et al., 2009). SR16835 is also a somewhat selective NOP agonist with weak mu agonist activity but full agonist activity at NOP receptors. This compound does not have acute antinociceptive activity in the tail flick test nor does it induce a CPP. However, the full agonist activity at NOP receptors is sufficient to attenuate morphine CPP (Toll et al., 2009). Taken together, these studies suggest that a NOP/mu profile can be found that produces antinociceptive activity with reduced reward and reduced tolerance development and confirms the observation that a compound with sufficient NOP agonist activity might have potential as a drug abuse medication.
Interestingly, the analgesic properties of these compounds are somewhat different under conditions of chronic pain. In spinal nerve ligated mice, when mechanical allodynia is tested using von Frey filaments, the nonselective mu/NOP partial agonist SR14150 is antiallodynic; however, this activity is blocked by SB-612111, a NOP antagonist, rather than by naloxone. Furthermore, SR16835, which is inactive in blocking tail flick acute pain, was able to attenuate SNL-induced mechanical allodynia, an action also blocked by SB-612111 (Khroyan et al., 2011). These results are consistent with chronic pain-, as well as chronic inflammation-induced changes in the levels of NOP receptor mRNA, N/OFQ peptide levels, and ppN/OFQ mRNA levels in rodents (Andoh et al., 1997; Itoh et al., 2001; Briscini et al., 2002; Witta et al., 2003) and humans (Raffaeli et al., 2006) and once again suggest that NOP agonists might have better success in treatment of chronic or inflammatory rather than nociceptive acute pain.
The results in rodents suggest that mu activity is required for antinociceptive activity after systemic administration, and NOP receptor activation attenuates both analgesia and reward. As discussed above, the results seem to be different in primates. In rhesus monkeys, the antinociceptive activity of buprenorphine appears to be fully reversed by naltrexone, indicating that it is due to mu receptor activation, and this activity is potentiated rather than inhibited by the NOP agonists SCH 221510 and Ro 64-6198. In fact, both compounds acted synergistically with buprenorphine, suggesting that bifunctional NOP/mu compounds may have considerable clinical use (Cremeans et al., 2012). This was further substantiated with the nonselective NOP/mu agonist peptide [Dmt1]N/OFQ(1–13)-NH2, which has very potent antinociceptive activity when administered intrathecally to rhesus monkeys (Molinari et al., 2013). These studies suggest that NOP receptor agonists, or NOP/mu agonists, may be particularly effective in humans for relief of acute, as well as chronic pain. This seems to be borne out because the dual high affinity, high efficacy compound cebranopadol, synthesized by Grunenthal (Aachen, Germany), is now in clinical trials for pain.
Cebranopadol (previously called GRT-6005) is a first in class NOP/mu full agonist that is in Phase II clinical trials for both acute and chronic pain (Schunk et al., 2014; Lambert et al., 2015). This compound has nanomolar affinity at NOP, mu, and kappa receptors, with approximately 20 nM affinity at delta receptors (Linz et al., 2014). In [35S]GTPγS binding experiments it has full efficacy at NOP, mu, and delta receptors, with 67% efficacy at kappa. Cebranopadol has very potent antinociceptive activity in the 5 μgl/kg range when administered intravenously and 25 μgl/kg when administered orally in acute pain models in rats, with similar potency in chronic pain models, in both cases being approximately 1000 times more potent than morphine (Linz et al., 2014). This compound is longer lasting than morphine, with reduced tolerance development in the chronic pain assays. Interestingly there seems to be little effect on either motor coordination or respiration in analgesic doses. The apparent clinical success of cebranopadol, at least to this point, demonstrates the potential clinical usefulness of this particular receptor profile.
VII. Future Directions and New Tools
Based upon the discussion above there are certain important topics that clearly require additional research and new developments.
The dichotomy between analgesic activity in rodents and primates is striking. Selective NOP receptor agonists are poorly analgesic, at best, when administered systemically in rodents exposed to acute pain (Jenck et al., 2000; Obara et al., 2005; Varty et al., 2005; Reiss et al., 2008). Yet in nonhuman primates, several selective NOP receptor agonists have been demonstrated to be equieffective with powerful opiates, but without the well-known opioid induced itching (Ko et al., 2009). This is probably due to NOP receptor circuitry differences in the various species (Ding et al., 2015), and this should be examined carefully because of obvious clinical implications. In this regard, new tools being currently developed will soon be available to study NOP receptor circuitry. NOP-eGFP knock-in mice have already demonstrated utility in identifying NOP receptor-containing cells in the brain, spinal cord, and DRG (Ozawa et al., 2015). Additional genetic models for NOP receptor research are also currently in early stages of research and under development. These include cre-driver mice to target N/OFQ- and NOP receptor-containing neurons to better dissect this systems regulation of endogenous neural circuitry in behavior. Furthermore, conditional knockout lines for both the NOP receptor and ppN/OFQ are in the final stages of development. These new mouse tools will allow for cell-type specific control as has just recently been reported for mu and kappa opioid systems (via optogenetics and chemogenetics) (Al-Hasani et al., 2015; Siuda et al., 2015; Vardy et al., 2015) along with cell type-selective deletion studies to more mechanistically dissect N/OFQ and NOP receptor neural circuits that mediate behavior.
NOP receptor agonists block contextual drug associations (i.e., CPP) of every drug tested, including morphine, cocaine, amphetamine, and alcohol (Ciccocioppo et al., 2000; Kotlinska et al., 2002, 2003; Zhao et al., 2003; Sakoori and Murphy, 2004). This is consistent with a NOP receptor agonist-induced decrease in extracellular dopamine in the NAc (Murphy et al., 1996; Murphy and Maidment, 1999; Vazquez-DeRose et al., 2013). Nevertheless, the ability of NOP receptor agonists to block self-administration of these drugs appears to be far less robust, if effective at all (Walker et al., 1998; Ciccocioppo et al., 2014; de Guglielmo et al., 2015). One difference in the way in which NOP receptor agonists have been tested in these two “drug abuse” paradigms is that NOP receptor agonists block acquisition of CPP but generally have been tested for their ability to block expression of self-administration. Assuming dopamine is a major player in these reward paradigms, this may suggest a fundamental difference in the role of NOP receptors in CPP versus self-administration, or a difference in acquisition versus expression of drug abuse.
We have to a lesser extent discussed the role of endogenous N/OFQ in the regulation of the NOP system. Like other neuropeptides, how, when, and where N/OFQ is released in response to stress, fear, pain, etc. is poorly understood. Circulating N/OFQ is increased in patients undergoing a migraine as well as in chronic pain patients. Probably N/OFQ release or overexpression has a role in the development or maintanence of chronic pain, as well as other affective disorders and additional investigations into endogenous ligand regulation are greatly needed.
The investigation into NOP receptor biased signaling is in its infacy. Initial studies have demonstrated that partial agonists for G-protein coupling are very poor in β-arrestin-mediated signal transduction (Chang et al., 2015b; Malfacini et al., 2015). New techniques in resonance energy transfer and genetic tools (i.e., conditional arrestin knockout mice) will help characterize new ligands to better understand how NOP receptor biased signaling translates to their in vivo actions.
Medicinal chemistry efforts should be directed to identify novel NOP ligands displaying large bias toward G-protein and arrestin. This will be facilitated by the rapid growth of structural biology techniques and therefore a structure-based approach to NOP drug design. We anticipate that in the next few years NOP biased agonists will be designed based on the structure of the active NOP receptor in complex with G-proteins and arrestins. These compounds together with the knowledge regarding the relative involvement of G-protein and arrestin signaling in the befeficial as well as unwanted actions of NOP ligands will allow the selection of the best molecules for individual indications, thus optimizing innovative drugs acting at the NOP receptor.
VIII. Concludng Remarks
The NOP receptor was first cloned 20 years ago and quickly determined to be a member of the opioid receptor family. Although pharmacologically distinct from the opioid receptors with respect to the affinity of the endogenous peptides, the binding pocket is similar enough so that compounds with affinity at NOP and the opioid receptors can be readily identified. In addition, many of the signal transduction pathways and physiologic actions are common to all of the receptors in this family. Although many behavioral processes, such as pain, reward, anxiety, etc., are common to NOP and the other opioid receptors, the actions of NOP receptors are still less well characterized in various species and under various pathologic conditions. In addition, many new biologic tools have been available to better dissect the role of the NOP receptor and N/OFQ system in central and peripheral circuits. Advances in optogenetics, new mouse lines, viral approaches, chemistry, and biosensors will allow the next decade to be a fruitful effort in uncovering the key sites of action of the N/OFQ-NOP system. So far, a few NOP receptor-targeted compounds have advanced to clinical trials, and this receptor system maintains great promise as a novel target for several clinical indications.
Disclosures
G.C. is one of the inventors of the patent application (WO2006087340) that includes UFP-112 and of the patent application (EP13162532.9) focused on PWT peptides and founder of the University of Ferrara spin off company UFPeptides s.r.l., the assignee of such patents. B.M.C. is required to state that the opinions or assertions expressed in this review are those of the authors; they should not be construed as official or reflecting the views of the Uniformed Services University, the Department of Defense, or the Government of the United States.
ABBREVIATIONS
- 6-OHDA
6-hydroxydopamine
- AP2
activating protein-2
- ATF2
activating transcription factor-2
- BNST
bed nucleus of the stria terminalis
- CGRP
calcitonin gene related peptide
- CPP
conditioned place preference
- DRG
dorsal root ganglia
- DRN
dorsal raphe nucleus
- ECL
extracellular loop
- eGFP
enhanced green fluorescent protein
- EPSC
excitatory post-synaptic current
- EPSP
excitatory post-synaptic potentials
- ERK
extracellular signal-regulated kinase
- FRAP
fluorescence recovery after photo-bleaching
- GAIP
Galpha interacting protein
- GIRK
G-protein inwardly rectifying potassium channel
- GP
globus pallidus
- GPCR
G Protein Coupled Receptor
- GRE
glucocorticoid receptor response element
- GRK
G-protein receptor kinase
- ICL
intracellular loop
- IPSC
inhibitory post-synaptic current
- JNK
c-jun N-terminal kinase
- LID
L-DOPA-induced dyskinesias
- LTP
long-term potentiation
- MAPK
mitogen-activated protein kinase
- MFB
medial forebrain bundle
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MRE
metal response element
- NF200
neurofilament 200
- NFκΒ
nuclear factorκΒ N/OFQ, nociceptin/orphanin FQ
- NOP receptor
Nociceptin/Orphanin FQ Opioid Peptide receptor
- PD
Parkinson's disease
- ppN/OFQ
preproN/OFQ
- PWT
peptide welding technology
- RARE
retinoic acid response element
- ROCK
Rho-associated coiled-coil-containing protein kinase
- RVM
rostral ventromedial medulla
- SAR
structure activity relationship
- SNc
substantia nigra pars compacta
- SNr
substantia nigra pars reticulate
- SP
substance P
- STN
subthalamic nucleus
- TH
tyrosine hydroxylase
- TM
transmembrane
- TNFα
tumor necrosis factor alpha
- vlPAG
ventral lateral periaqueductal gray
- VTA
ventral tegmental area
Authorship Contributions.
Wrote or contributed to the writing of the manuscript: Toll, Bruchas, Calo', Cox, Zaveri.
Footnotes
This work was supported by National Institutes of Health National Institute of Drug Abuse [Grant R01DA023281] (L.T.), Grant R21DA034929 (M.R.B.), Grants R01DA014026 and R01DA027811 (N.T.Z.)] and by the FAR grant of the University of Ferrara (G.C.).
References
- Abdulla FA, Smith PA. (1998) Axotomy reduces the effect of analgesic opioids yet increases the effect of nociceptin on dorsal root ganglion neurons. J Neurosci 18:9685–9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguila B, Simaan M, Laporte SA. (2011) Study of G protein-coupled receptor/β-arrestin interactions within endosomes using FRAP. Methods Mol Biol 756:371–380. [DOI] [PubMed] [Google Scholar]
- Akuzawa N, Takeda S, Ishiguro M. (2007) Structural modelling and mutation analysis of a nociceptin receptor and its ligand complexes. J Biochem 141:907–916. [DOI] [PubMed] [Google Scholar]
- Al-Hasani R, Bruchas MR. (2011) Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115:1363–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park SI, Marcinkiewcz CM, Crowley NA, et al. (2015) Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron 87:1063–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, Chen L, Beedle AM, Ferguson SS, Mezghrani A, et al. (2006) ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci 9:31–40. [DOI] [PubMed] [Google Scholar]
- Andoh T, Itoh M, Kuraishi Y. (1997) Nociceptin gene expression in rat dorsal root ganglia induced by peripheral inflammation. Neuroreport 8:2793–2796. [DOI] [PubMed] [Google Scholar]
- Arduin M, Spagnolo B, Calò G, Guerrini R, Carrà G, Fischetti C, Trapella C, Marzola E, McDonald J, Lambert DG, et al. (2007) Synthesis and biological activity of nociceptin/orphanin FQ analogues substituted in position 7 or 11 with Calpha,alpha-dialkylated amino acids. Bioorg Med Chem 15:4434–4443. [DOI] [PubMed] [Google Scholar]
- Arjomand J, Cole S, Evans CJ. (2002) Novel orphanin FQ/nociceptin transcripts are expressed in human immune cells. J Neuroimmunol 130:100–108. [DOI] [PubMed] [Google Scholar]
- Armstead W. (2002) NOC/oFQ activates PKC and generates superoxide to impair hypotensive cerebrovasodilation after hypoxia/ischemia. Med Sci Monit 8:BR8–BR14. [PubMed] [Google Scholar]
- Armstead WM. (2006) Differential activation of ERK, p38, and JNK MAPK by nociceptin/orphanin FQ in the potentiation of prostaglandin cerebrovasoconstriction after brain injury. Eur J Pharmacol 529:129–135. [DOI] [PubMed] [Google Scholar]
- Barchfeld CC, Medzihradsky F. (1984) Receptor-mediated stimulation of brain GTPase by opiates in normal and dependent rats. Biochem Biophys Res Commun 121:641–648. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Tawfik VL, Wang D, François A, Solorzano C, Shuster SA, Choudhury P, Betelli C, Cassidy C, Smith K, et al. (2014) Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron 81:1312–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes TA, McDonald J, Rowbotham DJ, Duarte TL, Lambert DG. (2007) Effects of receptor density on Nociceptin/OrphaninFQ peptide receptor desensitisation: studies using the ecdysone inducible expression system. Naunyn Schmiedebergs Arch Pharmacol 376:217–225. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso M, Risse PA, Naline E, Calo G, Guerrini R, Regoli D, Advenier C. (2005) Nociceptin/orphanin FQ inhibits electrically induced contractions of the human bronchus via NOP receptor activation. Peptides 26:1492–1496. [DOI] [PubMed] [Google Scholar]
- Beedle AM, McRory JE, Poirot O, Doering CJ, Altier C, Barrere C, Hamid J, Nargeot J, Bourinet E, Zamponi GW. (2004) Agonist-independent modulation of N-type calcium channels by ORL1 receptors. Nat Neurosci 7:118–125. [DOI] [PubMed] [Google Scholar]
- Berger B, Rothmaier AK, Wedekind F, Zentner J, Feuerstein TJ, Jackisch R. (2006) Presynaptic opioid receptors on noradrenergic and serotonergic neurons in the human as compared to the rat neocortex. Br J Pharmacol 148:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. (1990) Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249:1436–1438. [DOI] [PubMed] [Google Scholar]
- Berthele A, Platzer S, Dworzak D, Schadrack J, Mahal B, Büttner A, Assmus HP, Wurster K, Zieglgänsberger W, Conrad B, et al. (2003) [3H]-nociceptin ligand-binding and nociceptin opioid receptor mrna expression in the human brain. Neuroscience 121:629–640. [DOI] [PubMed] [Google Scholar]
- Berzetei-Gurske IP, Schwartz RW, Toll L. (1996) Determination of activity for nociceptin in the mouse vas deferens. Eur J Pharmacol 302:R1–R2. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Marani L, Barbieri M, Marino S, Beani L, Siniscalchi A. (2004) Effects of nociceptin/orphanin FQ and endomorphin-1 on glutamate and GABA release, intracellular [Ca2+] and cell excitability in primary cultures of rat cortical neurons. Neuropharmacology 47:873–883. [DOI] [PubMed] [Google Scholar]
- Bigoni R, Calo' G, Rizzi A, Guerrini R, De Risi C, Hashimoto Y, Hashiba E, Lambert DG, Regoli D. (2000) In vitro characterization of J-113397, a non-peptide nociceptin/orphanin FQ receptor antagonist. Naunyn Schmiedebergs Arch Pharmacol 361:565–568. [DOI] [PubMed] [Google Scholar]
- Bigoni R, Giuliani S, Calo' G, Rizzi A, Guerrini R, Salvadori S, Regoli D, Maggi CA. (1999) Characterization of nociceptin receptors in the periphery: in vitro and in vivo studies. Naunyn Schmiedebergs Arch Pharmacol 359:160–167. [DOI] [PubMed] [Google Scholar]
- Bigoni R, Rizzi D, Rizzi A, Camarda V, Guerrini R, Lambert DG, Hashiba E, Berger H, Salvadori S, Regoli D, et al. (2002) Pharmacological characterisation of [(pX)Phe4]nociceptin(1-13)amide analogues. 1. In vitro studies. Naunyn Schmiedebergs Arch Pharmacol 365:442–449. [DOI] [PubMed] [Google Scholar]
- Bloms-Funke P, Gillen C, Schuettler AJ, Wnendt S. (2000) Agonistic effects of the opioid buprenorphine on the nociceptin/OFQ receptor. Peptides 21:1141–1146. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. (2004) Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol 66:106–112. [DOI] [PubMed] [Google Scholar]
- Bridge KE, Wainwright A, Reilly K, Oliver KR. (2003) Autoradiographic localization of (125)i[Tyr(14)] nociceptin/orphanin FQ binding sites in macaque primate CNS. Neuroscience 118:513–523. [DOI] [PubMed] [Google Scholar]
- Briscini L, Corradini L, Ongini E, Bertorelli R. (2002) Up-regulation of ORL-1 receptors in spinal tissue of allodynic rats after sciatic nerve injury. Eur J Pharmacol 447:59–65. [DOI] [PubMed] [Google Scholar]
- Brown JM, Gouty S, Iyer V, Rosenberger J, Cox BM. (2006) Differential protection against MPTP or methamphetamine toxicity in dopamine neurons by deletion of ppN/OFQ expression. J Neurochem 98:495–505. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C. (2010) Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 210:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. (2007) Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci 27:11614–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, et al. (2011) Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron 71:498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. (1994) Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett 347:284–288. [DOI] [PubMed] [Google Scholar]
- Byford AJ, Anderson A, Jones PS, Palin R, Houghton AK. (2007) The hypnotic, electroencephalographic, and antinociceptive properties of nonpeptide ORL1 receptor agonists after intravenous injection in rodents. Anesth Analg 104:174–179. [DOI] [PubMed] [Google Scholar]
- Calò G, Bigoni R, Rizzi A, Guerrini R, Salvadori S, Regoli D. (2000a) Nociceptin/orphanin FQ receptor ligands. Peptides 21:935–947. [DOI] [PubMed] [Google Scholar]
- Calò G, Guerrini R. (2013) Medicinal chemistry, pharmacology, and biological actions of peptide ligands selective for teh nociceptin/orphanin FQ receptor, in Research and Development of Opioid-Related Ligands (Ko MC, Husbands SM, eds) pp 275–325, Oxford University Press, Washington, DC. [Google Scholar]
- Calò G, Guerrini R, Bigoni R, Rizzi A, Bianchi C, Regoli D, Salvadori S. (1998) Structure-activity study of the nociceptin(1-13)-NH2 N-terminal tetrapeptide and discovery of a nociceptin receptor antagonist. J Med Chem 41:3360–3366. [DOI] [PubMed] [Google Scholar]
- Calò G, Guerrini R, Bigoni R, Rizzi A, Marzola G, Okawa H, Bianchi C, Lambert DG, Salvadori S, Regoli D. (2000b) Characterization of [Nphe(1)]nociceptin(1-13)NH(2), a new selective nociceptin receptor antagonist. Br J Pharmacol 129:1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calò G, Guerrini R, Rizzi A, Salvadori S, Burmeister M, Kapusta DR, Lambert DG, Regoli D. (2005) UFP-101, a peptide antagonist selective for the nociceptin/orphanin FQ receptor. CNS Drug Rev 11:97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calò G, Guerrini R, Rizzi A, Salvadori S, Regoli D. (2000c) Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br J Pharmacol 129:1261–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calò G, Rizzi A, Bigoni R, Guerrini R, Salvadori S, Regoli D. (2002a) Pharmacological profile of nociceptin/orphanin FQ receptors. Clin Exp Pharmacol Physiol 29:223–228. [DOI] [PubMed] [Google Scholar]
- Calò G, Rizzi A, Bogoni G, Neugebauer V, Salvadori S, Guerrini R, Bianchi C, Regoli D. (1996) The mouse vas deferens: a pharmacological preparation sensitive to nociceptin. Eur J Pharmacol 311:R3–R5. [DOI] [PubMed] [Google Scholar]
- Calò G, Rizzi A, Cifani C, Micioni Di Bonaventura MV, Regoli D, Massi M, Salvadori S, Lambert DG, Guerrini R. (2011) UFP-112 a potent and long-lasting agonist selective for the Nociceptin/Orphanin FQ receptor. CNS Neurosci Ther 17:178–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calò G, Rizzi A, Rizzi D, Bigoni R, Guerrini R, Marzola G, Marti M, McDonald J, Morari M, Lambert DG, et al. (2002b) [Nphe1,Arg14,Lys15]nociceptin-NH2, a novel potent and selective antagonist of the nociceptin/orphanin FQ receptor. Br J Pharmacol 136:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarda V, Fischetti C, Anzellotti N, Molinari P, Ambrosio C, Kostenis E, Regoli D, Trapella C, Guerrini R, Severo S, et al. (2009) Pharmacological profile of NOP receptors coupled with calcium signaling via the chimeric protein G alpha qi5. Naunyn Schmiedebergs Arch Pharmacol 379:599–607. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. (2009) Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci USA 106:9075–9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AS, Wong YH. (2000) Regulation of c-Jun N-terminal kinase by the ORL(1) receptor through multiple G proteins. J Pharmacol Exp Ther 295:1094–1100. [PubMed] [Google Scholar]
- Chan JS, Yung LY, Lee JW, Wu YL, Pei G, Wong YH. (1998) Pertussis toxin-insensitive signaling of the ORL1 receptor: coupling to Gz and G16 proteins. J Neurochem 71:2203–2210. [DOI] [PubMed] [Google Scholar]
- Chang SD, Brieaddy LE, Harvey JD, Lewin AH, Mascarella SW, Seltzman HH, Reddy PA, Decker AM, McElhinny CJ, Zhong D, et al. (2015a) Novel synthesis and Pharmacological characterization of NOP receptor agonist 8-(1S,3alphaS)-(2,3,3a,4,5,6-hexahydro-1H-phenalin-1-yl)-1-phenyl-1,3,8-triazaspi ro[4,5]decan-4-one (Ro 64-6198). ACS Chem Neurosci 2015. 6:1956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SD, Bruchas MR. (2014) Functional selectivity at GPCRs: new opportunities in psychiatric drug discovery. Neuropsychopharmacology 39:248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SD, Mascarella SW, Spangler SM, Gurevich VV, Navarro HA, Carroll FI, Bruchas MR. (2015b) Quantitative signaling and structure-activity analyses demonstrate functional selectivity at the nociceptin/orphanin FQ opioid receptor. Mol Pharmacol 88:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MJ, Price CJ, Statnick MA, Colmers WF. (2011) Nociceptin/orphanin FQ suppresses the excitability of neurons in the ventromedial nucleus of the hypothalamus. J Physiol 589:3103–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-L, Li AH, Yeh T-H, Chou A-H, Wang H-L. (2009) Nocistatin and nociceptin exert opposite effects on the excitability of central amygdala nucleus-periaqueductal gray projection neurons. Mol Cell Neurosci 40:76–88. [DOI] [PubMed] [Google Scholar]
- Childers SR, Creese I, Snowman AM, Synder SH. (1979) Opiate receptor binding affected differentially by opiates and opioid peptides. Eur J Pharmacol 55:11–18. [DOI] [PubMed] [Google Scholar]
- Childers SR, Snyder SH. (1978) Guanine nucleotides differentiate agonist and antagonist interactions with opiate receptors. Life Sci 23:759–761. [DOI] [PubMed] [Google Scholar]
- Chiou LC, Chuang KC, Wichmann J, Adam G. (2004) Ro 64-6198 [(1S,3aS)-8-(2,3,3a,4,5,6-Hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one] acts differently from nociceptin/orphanin FQ in rat periaqueductal gray slices. J Pharmacol Exp Ther 311:645–651. [DOI] [PubMed] [Google Scholar]
- Chung S, Pohl S, Zeng J, Civelli O, Reinscheid RK. (2006) Endogenous orphanin FQ/nociceptin is involved in the development of morphine tolerance. J Pharmacol Exp Ther 318:262–267. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. (2000) Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol 404:153–159. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. (2004) Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 172:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Rimondini R, Sommer W, Massi M, Heilig M. (2007) Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol Psychiatry 61:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. (1999) Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 141:220–224. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Stopponi S, Economidou D, Kuriyama M, Kinoshita H, Heilig M, Roberto M, Weiss F, Teshima K. (2014) Chronic treatment with novel brain-penetrating selective NOP receptor agonist MT-7716 reduces alcohol drinking and seeking in the rat. Neuropsychopharmacology 39:2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O, Reinscheid RK, Zhang Y, Wang Z, Fredriksson R, Schiöth HB. (2013) G protein-coupled receptor deorphanizations. Annu Rev Pharmacol Toxicol 53:127–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combie J, Shults T, Nugent EC, Dougherty J, Tobin T. (1981) Pharmacology of narcotic analgesics in the horse: selective blockade of narcotic-induced locomotor activity. Am J Vet Res 42:716–721. [PubMed] [Google Scholar]
- Connor M, Bagley EE, Chieng BC, Christie MJ. (2015) β-Arrestin-2 knockout prevents development of cellular μ-opioid receptor tolerance but does not affect opioid-withdrawal-related adaptations in single PAG neurons. Br J Pharmacol 172:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Christie MJ. (1998) Modulation of Ca2+ channel currents of acutely dissociated rat periaqueductal grey neurons. J Physiol 509:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Vaughan CW, Chieng B, Christie MJ. (1996a) Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurones in vitro. Br J Pharmacol 119:1614–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Yeo A, Henderson G. (1996b) The effect of nociceptin on Ca2+ channel current and intracellular Ca2+ in the SH-SY5Y human neuroblastoma cell line. Br J Pharmacol 118:205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbani M, Gonindard C, Meunier JC. (2004) Ligand-regulated internalization of the opioid receptor-like 1: a confocal study. Endocrinology 145:2876–2885. [DOI] [PubMed] [Google Scholar]
- Corradini L, Briscini L, Ongini E, Bertorelli R. (2001) The putative OP(4) antagonist, [Nphe(1)]nociceptin(1-13)NH(2), prevents the effects of nociceptin in neuropathic rats. Brain Res 905:127–133. [DOI] [PubMed] [Google Scholar]
- Cox BM, Christie MJ, Devi L, Toll L, Traynor JR. (2015) Challenges for opioid receptor nomenclature: IUPHAR Review 9. Br J Pharmacol 172: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremeans CM, Gruley E, Kyle DJ, Ko MC. (2012) Roles of μ-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J Pharmacol Exp Ther 343:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, Roberto M. (2012) Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biol Psychiatry 71:666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino B, Marrocco G, De Nardo M, Calò G, Guerrini R, Gallelli L, Advenier C, Rossi F. (2005) Activation of the nociceptin/orphanin FQ receptor reduces bronchoconstriction and microvascular leakage in a rabbit model of gastroesophageal reflux. Br J Pharmacol 144:813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daga PR, Zaveri NT. (2012) Homology modeling and molecular dynamics simulations of the active state of the nociceptin receptor reveal new insights into agonist binding and activation. Proteins 80:1948–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautzenberg FM, Wichmann J, Higelin J, Py-Lang G, Kratzeisen C, Malherbe P, Kilpatrick GJ, Jenck F. (2001) Pharmacological characterization of the novel nonpeptide orphanin FQ/nociceptin receptor agonist Ro 64-6198: rapid and reversible desensitization of the ORL1 receptor in vitro and lack of tolerance in vivo. J Pharmacol Exp Ther 298:812–819. [PubMed] [Google Scholar]
- Davis AM, Inturrisi CE. (1999) d-Methadone blocks morphine tolerance and N-methyl-D-aspartate-induced hyperalgesia. J Pharmacol Exp Ther 289:1048–1053. [PubMed] [Google Scholar]
- de Guglielmo G, Martin-Fardon R, Teshima K, Ciccocioppo R, Weiss F. (2015) MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addict Biol 20:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depner UB, Reinscheid RK, Takeshima H, Brune K, Zeilhofer HU. (2003) Normal sensitivity to acute pain, but increased inflammatory hyperalgesia in mice lacking the nociceptin precursor polypeptide or the nociceptin receptor. Eur J Neurosci 17:2381–2387. [DOI] [PubMed] [Google Scholar]
- Devine DP, Reinscheid RK, Monsma FJ, Jr, Civelli O, Akil H. (1996) The novel neuropeptide orphanin FQ fails to produce conditioned place preference or aversion. Brain Res 727:225–229. [DOI] [PubMed] [Google Scholar]
- Di Giannuario A, Rizzi A, Pieretti S, Guerrini R, Bertorelli R, Salvadori S, Regoli D, Calo G. (2001) Studies on the antinociceptive effect of [Nphe1]nociceptin(1-13)NH2 in mice. Neurosci Lett 316:25–28. [DOI] [PubMed] [Google Scholar]
- Ding H, Hayashida K, Suto T, Sukhtankar DD, Kimura M, Mendenhall V, Ko MC. (2015) Supraspinal actions of nociceptin/orphanin FQ, morphine and substance P in regulating pain and itch in non-human primates. Br J Pharmacol 172:3302–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donica CL, Awwad HO, Thakker DR, Standifer KM. (2013) Cellular mechanisms of nociceptin/orphanin FQ (N/OFQ) peptide (NOP) receptor regulation and heterologous regulation by N/OFQ. Mol Pharmacol 83:907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donica CL, Ramirez VI, Awwad HO, Zaveri NT, Toll L, Standifer KM. (2011) Orphanin FQ/nociceptin activates nuclear factor kappa B. J Neuroimmune Pharmacol 6:617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley CT, Houghten RA. (1996) Orphanin FQ: Receptor binding and analog structure activity relationships in rat brain. Life Sci 59:PL 23–29. [DOI] [PubMed] [Google Scholar]
- Dooley CT, Spaeth CG, Berzetei-Gurske IP, Craymer K, Adapa ID, Brandt SR, Houghten RA, Toll L. (1997) Binding and in vitro activities of peptides with high affinity for the nociceptin/orphanin FQ receptor, ORL1. J Pharmacol Exp Ther 283:735–741. [PubMed] [Google Scholar]
- Dourish CT, Hawley D, Iversen SD. (1988) Enhancement of morphine analgesia and prevention of morphine tolerance in the rat by the cholecystokinin antagonist L-364,718. Eur J Pharmacol 147:469–472. [DOI] [PubMed] [Google Scholar]
- Dragicevic E, Schiemann J, Liss B. (2015) Dopamine midbrain neurons in health and Parkinson’s disease: emerging roles of voltage-gated calcium channels and ATP-sensitive potassium channels. Neuroscience 284:798–814. [DOI] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, et al. (2008) Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry 64:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K, Hynansky A, Inturrisi CE. (1994) Dextromethorphan attenuates and reverses analgesic tolerance to morphine. Pain 59:361–368. [DOI] [PubMed] [Google Scholar]
- Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, Koch M, Kessler P, Hentsch D, Birling MC, et al. (2015) A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct 220:677–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, You H, Hameed S, Altier C, Mezghrani A, Bourinet E, Zamponi GW. (2010) Heterodimerization of ORL1 and opioid receptors and its consequences for N-type calcium channel regulation. J Biol Chem 285:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Chambers JP, Evans RH, Henderson G. (1996) Depression of glutamatergic transmission by nociceptin in the neonatal rat hemisected spinal cord preparation in vitro. Br J Pharmacol 119:189–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin M, Fischetti C, Trapella C, Morari M. (2007) Nocistatin inhibits 5-hydroxytryptamine release in the mouse neocortex via presynaptic Gi/o protein linked pathways. Br J Pharmacol 152:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenalti G, Giguere PM, Katritch V, Huang XP, Thompson AA, Cherezov V, Roth BL, Stevens RC. (2014) Molecular control of δ-opioid receptor signalling. Nature 506:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti C, Camarda V, Rizzi A, Pelà M, Trapella C, Guerrini R, McDonald J, Lambert DG, Salvadori S, Regoli D, et al. (2009) Pharmacological characterization of the nociceptin/orphanin FQ receptor non peptide antagonist Compound 24. Eur J Pharmacol 614:50–57. [DOI] [PubMed] [Google Scholar]
- Florin S, Meunier J, Costentin J. (2000) Autoradiographic localization of [3H]nociceptin binding sites in the rat brain. Brain Res 880:11–16. [DOI] [PubMed] [Google Scholar]
- Florin S, Suaudeau C, Meunier JC, Costentin J. (1996) Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur J Pharmacol 317:9–13. [DOI] [PubMed] [Google Scholar]
- French ED, Vasquez SA, George R. (1979) Behavioral changes produced in the cat by acute and chronic morphine injection and naloxone precipitated withdrawal. Eur J Pharmacol 57:387–397. [DOI] [PubMed] [Google Scholar]
- Fu X, Zhu ZH, Wang YQ, Wu GC. (2007) Regulation of proinflammatory cytokines gene expression by nociceptin/orphanin FQ in the spinal cord and the cultured astrocytes. Neuroscience 144:275–285. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, Miyata T, Houtani T, Sugimoto T. (1994) cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett 343:42–46. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Shoda T, Morikawa H, Kato S, Mima H, Mori K. (1998) Activation of phospholipase A2 by the nociceptin receptor expressed in Chinese hamster ovary cells. J Neurochem 71:2186–2192. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Calo’ G. (2006) Antidepressant- and anxiolytic-like effects of nociceptin/orphanin FQ receptor ligands. Naunyn Schmiedebergs Arch Pharmacol 372:319–330. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Calo’ G. (2013) Nociceptin/orphanin FQ receptor antagonists as innovative antidepressant drugs. Pharmacol Ther 140:10–25. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Marzola G, Guerrini R, Bertorelli R, Zucchini S, De Lima TC, Rae GA, Salvadori S, Regoli D, Calo G. (2003) Blockade of nociceptin/orphanin FQ-NOP receptor signalling produces antidepressant-like effects: pharmacological and genetic evidences from the mouse forced swimming test. Eur J Neurosci 17:1987–1990. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Gackenheimer SL, Shaw JL. (2006) Distribution of nociceptin and Ro64-6198 activated [35S]-GTPgammaS binding in the rat brain. Neuropeptides 40:95–105. [DOI] [PubMed] [Google Scholar]
- Glück L, Loktev A, Moulédous L, Mollereau C, Law P-Y, Schulz S. (2014) Loss of morphine reward and dependence in mice lacking G protein-coupled receptor kinase 5. Biol Psych 76: 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeldner C, Spooren W, Wichmann J, Prinssen EP. (2012) Further characterization of the prototypical nociceptin/orphanin FQ peptide receptor agonist Ro 64-6198 in rodent models of conflict anxiety and despair. Psychopharmacology (Berl) 222:203–214. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Sheehan P. (1969) Tolerance to opioid narcotics. I. Tolerance to the “running fit” caused by levorphanol in the mouse. J Pharmacol Exp Ther 169:175–184. [PubMed] [Google Scholar]
- Goto Y, Arai-Otsuki S, Tachibana Y, Ichikawa D, Ozaki S, Takahashi H, Iwasawa Y, Okamoto O, Okuda S, Ohta H, et al. (2006) Identification of a novel spiropiperidine opioid receptor-like 1 antagonist class by a focused library approach featuring 3D-pharmacophore similarity. J Med Chem 49:847–849. [DOI] [PubMed] [Google Scholar]
- Gouty S, Brown JM, Rosenberger J, Cox BM. (2010) MPTP treatment increases expression of pre-pro-nociceptin/orphanin FQ mRNA in a subset of substantia nigra reticulata neurons. Neuroscience 169:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. (2012) Structure of the δ-opioid receptor bound to naltrindole. Nature 485:400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini R, Caló G, Bigoni R, Rizzi D, Rizzi A, Zucchini M, Varani K, Hashiba E, Lambert DG, Toth G, et al. (2001) Structure-activity studies of the Phe(4) residue of nociceptin(1-13)-NH(2): identification of highly potent agonists of the nociceptin/orphanin FQ receptor. J Med Chem 44:3956–3964. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Calo G, Rizzi A, Bigoni R, Bianchi C, Salvadori S, Regoli D. (1998) A new selective antagonist of the nociceptin receptor. Br J Pharmacol 123:163–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini R, Marzola E, Trapella C, Pela’ M, Molinari S, Cerlesi MC, Malfacini D, Rizzi A, Salvadori S, Calo’ G. (2014) A novel and facile synthesis of tetra branched derivatives of nociceptin/orphanin FQ. Bioorg Med Chem 22:3703–3712. [DOI] [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJJ. (1995) Functional role and sequence analysis of a lymphocyte orphan opioid receptor. J Neuroimmunol 59:91–101. [DOI] [PubMed] [Google Scholar]
- Hao JX, Wiesenfeld-Hallin Z, Xu XJ. (1997) Lack of cross-tolerance between the antinociceptive effect of intrathecal orphanin FQ and morphine in the rat. Neurosci Lett 223:49–52. [DOI] [PubMed] [Google Scholar]
- Hao JX, Xu IS, Wiesenfeld-Hallin Z, Xu XJ. (1998) Anti-hyperalgesic and anti-allodynic effects of intrathecal nociceptin/orphanin FQ in rats after spinal cord injury, peripheral nerve injury and inflammation. Pain 76:385–393. [DOI] [PubMed] [Google Scholar]
- Hashiba E, Harrison C, Galo’ G, Guerrini R, Rowbotham DJ, Smith G, Lambert DG. (2001) Characterisation and comparison of novel ligands for the nociceptin/orphanin FQ receptor. Naunyn Schmiedebergs Arch Pharmacol 363:28–33.11191833 [Google Scholar]
- Hashiba E, Lambert DG, Jenck F, Wichmann J, Smith G. (2002) Characterisation of the non-peptide nociceptin receptor agonist, Ro64-6198 in Chinese hamster ovary cells expressing recombinant human nociceptin receptors. Life Sci 70:1719–1725. [DOI] [PubMed] [Google Scholar]
- Hawes BE, Fried S, Yao X, Weig B, Graziano MP. (1998) Nociceptin (ORL-1) and mu-opioid receptors mediate mitogen-activated protein kinase activation in CHO cells through a Gi-coupled signaling pathway: evidence for distinct mechanisms of agonist-mediated desensitization. J Neurochem 71:1024–1033. [DOI] [PubMed] [Google Scholar]
- Heiman M, Heilbut A, Francardo V, Kulicke R, Fenster RJ, Kolaczyk ED, Mesirov JP, Surmeier DJ, Cenci MA, Greengard P. (2014) Molecular adaptations of striatal spiny projection neurons during levodopa-induced dyskinesia. Proc Natl Acad Sci USA 111:4578–4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Grottick AJ, Ballard TM, Richards JG, Messer J, Takeshima H, Pauly-Evers M, Jenck F, Adam G, Wichmann J. (2001) Influence of the selective ORL1 receptor agonist, Ro64-6198, on rodent neurological function. Neuropharmacology 41:97–107. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Miwa M, Hashimoto K, Kawai S, Nomura N. (2008) Nociceptin/orphanin FQ reverses mecamylamine-induced learning and memory impairment as well as decrease in hippocampal acetylcholine release in the rat. Brain Res 1195:96–103. [DOI] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY. (2001) Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther 297:688–695. [PubMed] [Google Scholar]
- Hull LC, Gabra BH, Bailey CP, Henderson G, Dewey WL. (2013) Reversal of morphine analgesic tolerance by ethanol in the mouse. J Pharmacol Exp Ther 345:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AY, Schwarzschild MA. (2014) Treatment of Parkinson’s disease: what’s in the non-dopaminergic pipeline? Neurotherapeutics 11:34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibba M, Kitayama M, McDonald J, Calo G, Guerrini R, Farkas J, Toth G, Lambert DG. (2008) Binding of the novel radioligand [(3)H]UFP-101 to recombinant human and native rat nociceptin/orphanin FQ receptors. Naunyn Schmiedebergs Arch Pharmacol 378:553–561. [DOI] [PubMed] [Google Scholar]
- Im HJ, Kang SW, Loh HH. (1999) Opioid receptor gene: cytokine response element and the effect of cytokines. Brain Res 829:174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito E, Xie G, Maruyama K, Palmer PP. (2000) A core-promoter region functions bi-directionally for human opioid-receptor-like gene ORL1 and its 5′-adjacent gene GAIP. J Mol Biol 304:259–270. [DOI] [PubMed] [Google Scholar]
- Itoh M, Takasaki I, Andoh T, Nojima H, Tominaga M, Kuraishi Y. (2001) Induction by carrageenan inflammation of prepronociceptin mRNA in VR1-immunoreactive neurons in rat dorsal root ganglia. Neurosci Res 40:227–233. [DOI] [PubMed] [Google Scholar]
- Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Jr, Nothacker HP, Civelli O. (1997) Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci USA 94:14854–14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, Lundstrom K, Cesura AM, Poli SM, Roever S, et al. (2000) A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci USA 97:4938–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomphe C, Tiberi M, Trudeau LE. (2006) Expression of D2 receptor isoforms in cultured neurons reveals equipotent autoreceptor function. Neuropharmacology 50:595–605. [DOI] [PubMed] [Google Scholar]
- Judson BA, Goldstein A. (1978) Genetic control of opiate-induced locomotor activity in mice. J Pharmacol Exp Ther 206:56–60. [PubMed] [Google Scholar]
- Kallupi M, Oleata CS, Luu G, Teshima K, Ciccocioppo R, Roberto M. (2014) MT-7716, a novel selective nonpeptidergic NOP receptor agonist, effectively blocks ethanol-induced increase in GABAergic transmission in the rat central amygdala. Front Integr Neurosci 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam KW, New DC, Wong YH. (2002) Constitutive activation of the opioid receptor-like (ORL1) receptor by mutation of Asn133 to tryptophan in the third transmembrane region. J Neurochem 83:1461–1470. [DOI] [PubMed] [Google Scholar]
- Kamata M, Nagahama S, Kakishima K, Sasaki N, Nishimura R. (2012) Comparison of behavioral effects of morphine and fentanyl in dogs and cats. J Vet Med Sci 74:231–234. [DOI] [PubMed] [Google Scholar]
- Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. (2014) Allosteric sodium in class A GPCR signaling. Trends Biochem Sci 39:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Hesselink MB, van Scharrenburg G, Westerink BH. (2004) Tonic inhibition by orphanin FQ/nociceptin of noradrenaline neurotransmission in the amygdala. Eur J Pharmacol 485:197–200. [DOI] [PubMed] [Google Scholar]
- Kawamoto H, Ozaki S, Itoh Y, Miyaji M, Arai S, Nakashima H, Kato T, Ohta H, Iwasawa Y. (1999) Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[(3R,4R)-1-cyclooctylmethyl-3- hydroxymethyl-4-piperidyl]-3-ethyl-1, 3-dihydro-2H-benzimidazol-2-one (J-113397). J Med Chem 42:5061–5063. [DOI] [PubMed] [Google Scholar]
- Kest B, Hopkins E, Palmese CA, Chen ZP, Mogil JS, Pintar JE. (2001) Morphine tolerance and dependence in nociceptin/orphanin FQ transgenic knock-out mice. Neuroscience 104:217–222. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Jiang F, Zaveri NT, Toll L. (2009) Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonists. J Pharmacol Exp Ther 331:946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Orduna J, Montenegro J, Jiang F, Zaveri NT, Toll L. (2011) Differential effects of nociceptin/orphanin FQ (NOP) receptor agonists in acute versus chronic pain: studies with bifunctional NOP/μ receptor agonists in the sciatic nerve ligation chronic pain model in mice. J Pharmacol Exp Ther 339:687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN. (2009) Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys. J Pain 10:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Terner J, Hursh S, Woods JH, Winger G. (2002) Relative reinforcing effects of three opioids with different durations of action. J Pharmacol Exp Ther 301:698–704. [DOI] [PubMed] [Google Scholar]
- Ko MC, Wei H, Woods JH, Kennedy RT. (2006) Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectrometric studies. J Pharmacol Exp Ther 318:1257–1264. [DOI] [PubMed] [Google Scholar]
- Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP. (2009) Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology 34:2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov YA, Pick CG, Pasternak GW. (1992) NG-nitro-L-arginine prevents morphine tolerance. Eur J Pharmacol 221:399–400. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Rafalski P, Biala G, Dylag T, Rolka K, Silberring J. (2003) Nociceptin inhibits acquisition of amphetamine-induced place preference and sensitization to stereotypy in rats. Eur J Pharmacol 474:233–239. [DOI] [PubMed] [Google Scholar]
- Kotlińska J, Wichmann J, Legowska A, Rolka K, Silberring J. (2002) Orphanin FQ/nociceptin but not Ro 65-6570 inhibits the expression of cocaine-induced conditioned place preference. Behav Pharmacol 13:229–235. [DOI] [PubMed] [Google Scholar]
- Kress HG, Simpson KH, Marchettini P, Ver Donck A, Varrassi G. (2009) Intrathecal therapy: what has changed with the introduction of ziconotide. Pain Pract 9:338–347. [DOI] [PubMed] [Google Scholar]
- Kuo C-J, Liao Y-Y, Guerrini R, Calo’ G, Chiou L-C. (2008) Quantitative study of [(pF)Phe4,Arg14,Lys15]nociceptin/orphanin FQ-NH2 (UFP-102) at NOP receptors in rat periaqueductal gray slices. Eur J Pharmacol 579:110–115. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Kreek MJ, Bakalkin G, Liljequist S. (2007) The nociceptin/orphanin FQ receptor agonist Ro 64-6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology 32:902–910. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. (2003) Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther 304:310–318. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. (2004) Evidence in locomotion test for the functional heterogeneity of ORL-1 receptors. Br J Pharmacol 141:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowicz JE, Shen Y, Monsma FJ, Jr, Sibley DR. (1995) Molecular cloning of a novel G protein-coupled receptor related to the opiate receptor family. J Neurochem 64:34–40. [DOI] [PubMed] [Google Scholar]
- Lambert DG, Bird MF, Rowbotham DJ. (2015) Cebranopadol: a first in-class example of a nociceptin/orphanin FQ receptor and opioid receptor agonist. Br J Anaesth 114:364–366. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. (2009) Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci USA 106:19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapalu S, Moisand C, Butour JL, Mollereau C, Meunier JC. (1998) Different domains of the ORL1 and kappa-opioid receptors are involved in recognition of nociceptin and dynorphin A. FEBS Lett 427:296–300. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Wichmann J, Moreau JL, Jenck F. (2002) The orphanin receptor agonist RO 64-6198 does not induce place conditioning in rats. Neuroreport 13:451–454. [DOI] [PubMed] [Google Scholar]
- Lester PA, Traynor JR. (2006) Comparison of the in vitro efficacy of mu, delta, kappa and ORL1 receptor agonists and non-selective opioid agonists in dog brain membranes. Brain Res 1073-1074:290–296. [DOI] [PubMed] [Google Scholar]
- Liao YY, Jiang F, Chiou LC. (2011) Quantitative study of the antagonistic effect of (-)-cis-1-Methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB-612111) on nociceptin/orphanin FQ-mediated potassium channel activation in rat periaqueductal gray slices. Eur J Pharmacol 657:84–88. [DOI] [PubMed] [Google Scholar]
- Liao YY, Trapella C, Chiou LC. (2009) 1-Benzyl-N-[3-[spiroisobenzofuran-1(3H),4′-piperidin-1-yl]propyl]pyrrolidine-2-carboxamide (Compound 24) antagonizes NOP receptor-mediated potassium channel activation in rat periaqueductal gray slices. Eur J Pharmacol 606:84–89. [DOI] [PubMed] [Google Scholar]
- Liebel JT, Swandulla D, Zeilhofer HU. (1997) Modulation of excitatory synaptic transmission by nociceptin in superficial dorsal horn neurones of the neonatal rat spinal cord. Br J Pharmacol 121:425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz K, Christoph T, Tzschentke TM, Koch T, Schiene K, Gautrois M, Schröder W, Kögel BY, Beier H, Englberger W, et al. (2014) Cebranopadol: a novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J Pharmacol Exp Ther 349:535–548. [DOI] [PubMed] [Google Scholar]
- Lou LG, Zhang Z, Ma L, Pei G. (1998) Nociceptin/orphanin FQ activates mitogen-activated protein kinase in Chinese hamster ovary cells expressing opioid receptor-like receptor. J Neurochem 70:1316–1322. [DOI] [PubMed] [Google Scholar]
- Lü N, Han M, Yang ZL, Wang YQ, Wu GC, Zhang YQ. (2010) Nociceptin/Orphanin FQ in PAG modulates the release of amino acids, serotonin and norepinephrine in the rostral ventromedial medulla and spinal cord in rats. Pain 148:414–425. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Do T, Maidment NT. (2001a) Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl) 154:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, et al. (2003) Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci 23:10331–10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Hossain SM, Khaliq I, Maidment NT. (2001b) Orphanin FQ/nociceptin attenuates the development of morphine tolerance in rats. Br J Pharmacol 134:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Khaliq I, Carroll FI, Maidment NT. (2002) Orphanin FQ/nociceptin blocks cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 164:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Xie H, Dong ZQ, Wang YQ, Wu GC. (2005) Expression of ORL1 mRNA in some brain nuclei in neuropathic pain rats. Brain Res 1043:214–217. [DOI] [PubMed] [Google Scholar]
- Madeddu P, Salis MB, Milia AF, Emanueli C, Guerrini R, Regoli D, Calò G. (1999) Cardiovascular effects of nociceptin in unanesthetized mice. Hypertension 33:914–919. [DOI] [PubMed] [Google Scholar]
- Mahmoud S, Margas W, Trapella C, Caló G, Ruiz-Velasco V. (2010) Modulation of silent and constitutively active nociceptin/orphanin FQ receptors by potent receptor antagonists and Na+ ions in rat sympathetic neurons. Mol Pharmacol 77:804–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfacini D, Ambrosio C, Gro’ MC, Sbraccia M, Trapella C, Guerrini R, Bonora M, Pinton P, Costa T, Calo’ G. (2015) Pharmacological Profile of Nociceptin/Orphanin FQ Receptors Interacting with G-Proteins and β-Arrestins 2. PLoS One 10:e0132865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya T, Noda Y, Ren X, Nagai T, Takeshima H, Ukai M, Nabeshima T. (2001) Morphine tolerance and dependence in the nociceptin receptor knockout mice. J Neural Transm (Vienna) 108:1349–1361. [DOI] [PubMed] [Google Scholar]
- Manabe T, Noda Y, Mamiya T, Katagiri H, Houtani T, Nishi M, Noda T, Takahashi T, Sugimoto T, Nabeshima T, et al. (1998) Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature 394:577–581. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Altememi GF, Standifer KM. (2000) beta-Funaltrexamine inactivates ORL1 receptors in BE(2)-C human neuroblastoma cells. Eur J Pharmacol 402:R1–R37. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Thakker DR, Christensen JL, Standifer KM. (2002) Orphanin FQ/nociceptin-mediated desensitization of opioid receptor-like 1 receptor and mu opioid receptors involves protein kinase C: a molecular mechanism for heterologous cross-talk. J Pharmacol Exp Ther 302:502–509. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Thakker DR, Standifer KM. (2003) Mu-opioid-induced desensitization of opioid receptor-like 1 and mu-opioid receptors: differential intracellular signaling determines receptor sensitivity. J Pharmacol Exp Ther 306:965–972. [DOI] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. (2012) Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature 485:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. (1994) Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol 350:412–438. [DOI] [PubMed] [Google Scholar]
- Marquez P, Nguyen AT, Hamid A, Lutfy K. (2008) The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine. Neuropharmacology 54:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Guerrini R, Beani L, Bianchi C, Morari M. (2002) Nociceptin/orphanin FQ receptors modulate glutamate extracellular levels in the substantia nigra pars reticulata. A microdialysis study in the awake freely moving rat. Neuroscience 112:153–160. [DOI] [PubMed] [Google Scholar]
- Marti M, Mela F, Budri M, Volta M, Malfacini D, Molinari S, Zaveri NT, Ronzoni S, Petrillo P, Calò G, et al. (2013) Acute and chronic antiparkinsonian effects of the novel nociceptin/orphanin FQ receptor antagonist NiK-21273 in comparison with SB-612111. Br J Pharmacol 168:863–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Mela F, Fantin M, Zucchini S, Brown JM, Witta J, Di Benedetto M, Buzas B, Reinscheid RK, Salvadori S, et al. (2005) Blockade of nociceptin/orphanin FQ transmission attenuates symptoms and neurodegeneration associated with Parkinson’s disease. J Neurosci 25:9591–9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Mela F, Guerrini R, Calò G, Bianchi C, Morari M. (2004) Blockade of nociceptin/orphanin FQ transmission in rat substantia nigra reverses haloperidol-induced akinesia and normalizes nigral glutamate release. J Neurochem 91:1501–1504. [DOI] [PubMed] [Google Scholar]
- Marti M, Rodi D, Li Q, Guerrini R, Fasano S, Morella I, Tozzi A, Brambilla R, Calabresi P, Simonato M, et al. (2012) Nociceptin/orphanin FQ receptor agonists attenuate L-DOPA-induced dyskinesias. J Neurosci 32:16106–16119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Sarubbo S, Latini F, Cavallo M, Eleopra R, Biguzzi S, Lettieri C, Conti C, Simonato M, Zucchini S, et al. (2010) Brain interstitial nociceptin/orphanin FQ levels are elevated in Parkinson’s disease. Mov Disord 25:1723–1732. [DOI] [PubMed] [Google Scholar]
- Marti M, Stocchi S, Paganini F, Mela F, De Risi C, Calo’ G, Guerrini R, Barnes TA, Lambert DG, Beani L, et al. (2003) Pharmacological profiles of presynaptic nociceptin/orphanin FQ receptors modulating 5-hydroxytryptamine and noradrenaline release in the rat neocortex. Br J Pharmacol 138:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Trapella C, Viaro R, Morari M. (2007) The nociceptin/orphanin FQ receptor antagonist J-113397 and L-DOPA additively attenuate experimental parkinsonism through overinhibition of the nigrothalamic pathway. J Neurosci 27:1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J, Barnes TA, Okawa H, Williams J, Calo’ G, Rowbotham DJ, Lambert DG. (2003a) Partial agonist behaviour depends upon the level of nociceptin/orphanin FQ receptor expression: studies using the ecdysone-inducible mammalian expression system. Br J Pharmacol 140:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J, Calo G, Guerrini R, Lambert DG. (2003b) UFP-101, a high affinity antagonist for the nociceptin/orphanin FQ receptor: radioligand and GTPgamma(35)S binding studies. Naunyn Schmiedebergs Arch Pharmacol 367:183–187. [DOI] [PubMed] [Google Scholar]
- McLeod RL, Jia Y, Fernandez X, Parra LE, Wang X, Tulshian DB, Kiselgof EJ, Tan Z, Fawzi AB, Smith-Torhan A, et al. (2004) Antitussive profile of the NOP agonist Ro-64-6198 in the guinea pig. Pharmacology 71:143–149. [DOI] [PubMed] [Google Scholar]
- McLeod RL, Tulshian DB, Sadeh J. (2011) Where are the new cough treatments: a debriefing of recent clinical proof-of-concept trials with the NOP agonist SCH 486757. Pharmacology 88:50–54. [DOI] [PubMed] [Google Scholar]
- Mela F, Marti M, Ulazzi L, Vaccari E, Zucchini S, Trapella C, Salvadori S, Beani L, Bianchi C, Morari M. (2004) Pharmacological profile of nociceptin/orphanin FQ receptors regulating 5-hydroxytryptamine release in the mouse neocortex. Eur J Neurosci 19:1317–1324. [DOI] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C. (2010) Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci USA 107:11608–11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Ueda Y, Hoversten MT, Taylor LP, Reinscheid RK, Monsma FJ, Watson SJ, Civelli O, Akil H. (1998) Creating a functional opioid alkaloid binding site in the orphanin FQ receptor through site-directed mutagenesis. Mol Pharmacol 53:772–777. [DOI] [PubMed] [Google Scholar]
- Meunier J, Mouledous L, Topham CM. (2000) The nociceptin (ORL1) receptor: molecular cloning and functional architecture. Peptides 21:893–900. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377:532–535. [DOI] [PubMed] [Google Scholar]
- Micheli L, Di Cesare Mannelli L, Guerrini R, Trapella C, Zanardelli M, Ciccocioppo R, Rizzi A, Ghelardini C, Calò G. (2015) Acute and subchronic antinociceptive effects of nociceptin/orphanin FQ receptor agonists infused by intrathecal route in rats. Eur J Pharmacol 754:73–81. [DOI] [PubMed] [Google Scholar]
- Miller TR, Fulford AJ. (2007) Regulation of nociceptin/orphaninFQ secretion by immune cells and functional modulation of interleukin-2. Peptides 28:2243–2252. [DOI] [PubMed] [Google Scholar]
- Min BH, Augustin LB, Felsheim RF, Fuchs JA, Loh HH. (1994) Genomic structure analysis of promoter sequence of a mouse mu opioid receptor gene. Proc Natl Acad Sci USA 91:9081–9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal N, Roberts K, Pal K, Bentolila LA, Fultz E, Minasyan A, Cahill C, Pradhan A, Conner D, DeFea K, et al. (2013) Select G-protein-coupled receptors modulate agonist-induced signaling via a ROCK, LIMK, and β-arrestin 1 pathway. Cell Reports 5:1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK. (1996a) Orphanin FQ is a functional anti-opioid peptide. Neuroscience 75:333–337.8930999 [Google Scholar]
- Mogil JS, Grisel JE, Zhangs G, Belknap JK, Grandy DK. (1996b) Functional antagonism of mu-, delta- and kappa-opioid antinociception by orphanin FQ. Neurosci Lett 214:131–134. [DOI] [PubMed] [Google Scholar]
- Molinari S, Camarda V, Rizzi A, Marzola G, Salvadori S, Marzola E, Molinari P, McDonald J, Ko MC, Lambert DG, et al. (2013) [Dmt1]N/OFQ(1-13)-NH2: a potent nociceptin/orphanin FQ and opioid receptor universal agonist. Br J Pharmacol 168:151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari S, Malfacini D, Camarda V, Trapella C, Guerrini R, Mustazza C, and Calo G (2012) In vitro pharmacological characterization of the non peptide NOP receptor agonists Ro 65-6570, SCH-221510 and Compound 6d, in BPS Focused Meeting on Neuropeptides, http://www.pa2online.org, King's College London, UK.
- Mollereau C, Simons M-J, Soularue P, Liners F, Vassart G, Meunier J-C, Parmentier M. (1996a) Structure, tissue distribution, and chromosomal localization of the prepronociceptin gene. Proc Natl Acad Sci USA 93:8666–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollereau C, Moisand C, Butour JL, Parmentier M, Meunier JC. (1996b) Replacement of Gln280 by His in TM6 of the human ORL1 receptor increases affinity but reduces intrinsic activity of opioids. FEBS Lett 395:17–21. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Mouledous L, Lapalu S, Cambois G, Moisand C, Butour JL, Meunier JC. (1999) Distinct mechanisms for activation of the opioid receptor-like 1 and kappa-opioid receptors by nociceptin and dynorphin A. Mol Pharmacol 55:324–331. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. (1994) ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett 341:33–38. [DOI] [PubMed] [Google Scholar]
- Moran TD, Abdulla FA, Smith PA. (2000) Cellular neurophysiological actions of nociceptin/orphanin FQ. Peptides 21:969–976. [DOI] [PubMed] [Google Scholar]
- Moretti M, Budni J, Freitas AE, Neis VB, Ribeiro CM, de Oliveira Balen G, Rieger DK, Leal RB, Rodrigues AL. (2015) TNF-α-induced depressive-like phenotype and p38(MAPK) activation are abolished by ascorbic acid treatment. Eur Neuropsychopharmacol 25:902–912. [DOI] [PubMed] [Google Scholar]
- Morgan DO. (1997) Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 13:261–291. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Fossum EN, Levine CS, Ingram SL. (2006) Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol Biochem Behav 85:214–219. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Grisel JE, Robbins CS, Grandy DK. (1997) Antinociception mediated by the periaqueductal gray is attenuated by orphanin FQ. Neuroreport 8:3431–3434. [DOI] [PubMed] [Google Scholar]
- Mosharov EV, Borgkvist A, Sulzer D. (2015) Presynaptic effects of levodopa and their possible role in dyskinesia. Mov Disord 30:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouledous L, Topham CM, Moisand C, Mollereau C, Meunier JC. (2000) Functional inactivation of the nociceptin receptor by alanine substitution of glutamine 286 at the C terminus of transmembrane segment VI: evidence from a site-directed mutagenesis study of the ORL1 receptor transmembrane-binding domain. Mol Pharmacol 57:495–502. [DOI] [PubMed] [Google Scholar]
- Murali SS, Napier IA, Rycroft BK, Christie MJ. (2012) Opioid-related (ORL1) receptors are enriched in a subpopulation of sensory neurons and prolonged activation produces no functional loss of surface N-type calcium channels. J Physiol 590:1655–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NP, Lee Y, Maidment NT. (1999) Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res 832:168–170. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Ly HT, Maidment NT. (1996) Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience 75:1–4. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Maidment NT. (1999) Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem 73:179–186. [DOI] [PubMed] [Google Scholar]
- Mustazza C, Bastanzio G. (2011) Development of nociceptin receptor (NOP) agonists and antagonists. Med Res Rev 31:605–648. [DOI] [PubMed] [Google Scholar]
- Narita M, Mizoguchi H, Oji DE, Dun NJ, Hwang BH, Nagase H, Tseng LF. (1999) Identification of the G-protein-coupled ORL1 receptor in the mouse spinal cord by [35S]-GTPgammaS binding and immunohistochemistry. Br J Pharmacol 128:1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzaro C, Barbieri M, Varani K, Beani L, Valentino RJ, Siniscalchi A. (2010) Swim stress enhances nociceptin/orphanin FQ-induced inhibition of rat dorsal raphe nucleus activity in vivo and in vitro: role of corticotropin releasing factor. Neuropharmacology 58:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ., Jr (1999a) Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol 412:563–605. [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr (1999b) Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol 406:503–547. [PubMed] [Google Scholar]
- Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. (2008) Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci 28:7936–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas LB, Kolb Y, Prinssen EP. (2006) A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur J Pharmacol 547:106–115. [DOI] [PubMed] [Google Scholar]
- Norton CS, Neal CR, Kumar S, Akil H, Watson SJ. (2002) Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J Comp Neurol 444:358–368. [DOI] [PubMed] [Google Scholar]
- Nothacker HP, Reinscheid RK, Mansour A, Henningsen RA, Ardati A, Monsma FJ, Jr, Watson SJ, Civelli O. (1996) Primary structure and tissue distribution of the orphanin FQ precursor. Proc Natl Acad Sci USA 93:8677–8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent TE, Combie JD, Weld JM, Burns P, Tobin T. (1982) Effects of enkephalins versus opiates on locomotor activity of the horse. Res Commun Chem Pathol Pharmacol 35:405–419. [PubMed] [Google Scholar]
- Nygaard R, Frimurer TM, Holst B, Rosenkilde MM, Schwartz TW. (2009) Ligand binding and micro-switches in 7TM receptor structures. Trends Pharmacol Sci 30:249–259. [DOI] [PubMed] [Google Scholar]
- Obara I, Przewlocki R, Przewlocka B. (2005) Spinal and local peripheral antiallodynic activity of Ro64-6198 in neuropathic pain in the rat. Pain 116:17–25. [DOI] [PubMed] [Google Scholar]
- Okada K, Sujaku T, Chuman Y, Nakashima R, Nose T, Costa T, Yamada Y, Yokoyama M, Nagahisa A, Shimohigashi Y. (2000) Highly potent nociceptin analog containing the Arg-Lys triple repeat. Biochem Biophys Res Commun 278:493–498. [DOI] [PubMed] [Google Scholar]
- Olianas MC, Dedoni S, Boi M, Onali P. (2008) Activation of nociceptin/orphanin FQ-NOP receptor system inhibits tyrosine hydroxylase phosphorylation, dopamine synthesis, and dopamine D(1) receptor signaling in rat nucleus accumbens and dorsal striatum. J Neurochem 107:544–556. [DOI] [PubMed] [Google Scholar]
- Orsini MJ, Nesmelova I, Young HC, Hargittai B, Beavers MP, Liu J, Connolly PJ, Middleton SA, Mayo KH. (2005) The nociceptin pharmacophore site for opioid receptor binding derived from the NMR structure and bioactivity relationships. J Biol Chem 280:8134–8142. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Kawamoto H, Itoh Y, Miyaji M, Azuma T, Ichikawa D, Nambu H, Iguchi T, Iwasawa Y, Ohta H. (2000a) In vitro and in vivo pharmacological characterization of J-113397, a potent and selective non-peptidyl ORL1 receptor antagonist. Eur J Pharmacol 402:45–53. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Kawamoto H, Itoh Y, Miyaji M, Iwasawa Y, Ohta H. (2000b) A potent and highly selective nonpeptidyl nociceptin/orphanin FQ receptor (ORL1) antagonist: J-113397. Eur J Pharmacol 387:R17–R18. [DOI] [PubMed] [Google Scholar]
- Ozawa A, Brunori G, Mercatelli D, Wu J, Cippitelli A, Zou B, Xie XS, Williams M, Zaveri NT, Low S, et al. (2015) Knock-In Mice with NOP-eGFP Receptors Identify Receptor Cellular and Regional Localization. J Neurosci 35:11682–11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsoy HZ, Thakker DR, Standifer KM. (2005) Orphanin FQ/nociceptin potentiates [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin-Induced mu-opioid receptor phosphorylation. Mol Pharmacol 68:447–456. [DOI] [PubMed] [Google Scholar]
- Pan YX, Bolan E, Pasternak GW. (2002) Dimerization of morphine and orphanin FQ/nociceptin receptors: generation of a novel opioid receptor subtype. Biochem Biophys Res Commun 297:659–663. [DOI] [PubMed] [Google Scholar]
- Pan YX, Cheng J, Xu J, Rossi G, Jacobson E, Ryan-Moro J, Brooks AI, Dean GE, Standifer KM, Pasternak GW. (1995) Cloning and functional characterization through antisense mapping of a κ 3-related opioid receptor. Mol Pharmacol 47:1180–1188. [PubMed] [Google Scholar]
- Pan YX, Xu J, Wan BL, Zuckerman A, Pasternak GW. (1998) Identification and differential regional expression of KOR-3/ORL-1 gene splice variants in mouse brain. FEBS Lett 435:65–68. [DOI] [PubMed] [Google Scholar]
- Pan Z, Hirakawa N, Fields HL. (2000) A cellular mechanism for the bidirectional pain-modulating actions of orphanin FQ/nociceptin. Neuron 26:515–522. [DOI] [PubMed] [Google Scholar]
- Parenti C, Scoto GM. (2010) The functional antiopioid action of the ventrolateral periaqueductal gray nociceptin/orphanin FQ and nociceptin receptor system underlies DAMGO analgesic tolerance. Pharmacology 86:138–144. [DOI] [PubMed] [Google Scholar]
- Peluso J, LaForge KS, Matthes HW, Kreek MJ, Kieffer BL, Gavériaux-Ruff C. (1998) Distribution of nociceptin/orphanin FQ receptor transcript in human central nervous system and immune cells. J Neuroimmunol 81:184–192. [DOI] [PubMed] [Google Scholar]
- Pennock RL, Dicken MS, Hentges ST. (2012) Multiple inhibitory G-protein-coupled receptors resist acute desensitization in the presynaptic but not postsynaptic compartments of neurons. J Neurosci 32:10192–10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polidori C, de Caro G, Massi M. (2000) The hyperphagic effect of nociceptin/orphanin FQ in rats. Peptides 21:1051–1062. [DOI] [PubMed] [Google Scholar]
- Pope JE, Deer TR. (2013) Ziconotide: a clinical update and pharmacologic review. Expert Opin Pharmacother 14:957–966. [DOI] [PubMed] [Google Scholar]
- Post A, Smart TS, Krikke-Workel J, Dawson GR, Harmer CJ, Browning M, Jackson K, Kakar R, Mohs R, Statnick M, et al. (2015) A Selective Nociceptin Receptor Antagonist to Treat Depression: Evidence from Preclinical and Clinical Studies. Neuropsychopharmacology, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaeli W, Samolsky Dekel BG, Landuzzi D, Caminiti A, Righetti D, Balestri M, Montanari F, Romualdi P, Candeletti S. (2006) Nociceptin levels in the cerebrospinal fluid of chronic pain patients with or without intrathecal administration of morphine. J Pain Symptom Manage 32:372–377. [DOI] [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH. (2007) Differential regulation and properties of MAPKs. Oncogene 26:3100–3112. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Calo’ G, Regoli D, Quirion R. (2002) Nociceptin receptor antagonists display antidepressant-like properties in the mouse forced swimming test. Naunyn Schmiedebergs Arch Pharmacol 365:164–167. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Ardati A, Monsma FJ, Jr, Civelli O. (1996) Structure-activity relationship studies on the novel neuropeptide orphanin FQ. J Biol Chem 271:14163–14168. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270:792–794. [DOI] [PubMed] [Google Scholar]
- Reiss D, Wichmann J, Tekeshima H, Kieffer BL, Ouagazzal AM. (2008) Effects of nociceptin/orphanin FQ receptor (NOP) agonist, Ro64-6198, on reactivity to acute pain in mice: comparison to morphine. Eur J Pharmacol 579:141–148. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Bigoni R, Caló G, Guerrini R, Salvadori S, Regoli D. (1999) [Nphe(1)]nociceptin-(1-13)-NH(2) antagonizes nociceptin effects in the mouse colon. Eur J Pharmacol 385:R3–R5. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Bigoni R, Marzola G, Guerrini R, Salvadori S, Regoli D, Calo’ G. (2001a) Characterization of the locomotor activity-inhibiting effect of nociceptin/orphanin FQ in mice. Naunyn Schmiedebergs Arch Pharmacol 363:161–165. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Gavioli EC, Marzola G, Spagnolo B, Zucchini S, Ciccocioppo R, Trapella C, Regoli D, Calò G. (2007a) Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol]: in vivo studies. J Pharmacol Exp Ther 321:968–974. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Malfacini D, Cerlesi MC, Ruzza C, Marzola E, Bird MF, Rowbotham DJ, Salvadori S, Guerrini R, Lambert DG, et al. (2014) In vitro and in vivo pharmacological characterization of nociceptin/orphanin FQ tetrabranched derivatives. Br J Pharmacol 171:4138–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi A, Marzola G, Bigoni R, Guerrini R, Salvadori S, Mogil JS, Regoli D, Calò G. (2001b) Endogenous nociceptin signaling and stress-induced analgesia. Neuroreport 12:3009–3013. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Molinari S, Marti M, Marzola G, Calo’ G. (2011) Nociceptin/orphanin FQ receptor knockout rats: in vitro and in vivo studies. Neuropharmacology 60:572–579. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Rizzi D, Marzola G, Regoli D, Larsen BD, Petersen JS, Calo’ G. (2002a) Pharmacological characterization of the novel nociceptin/orphanin FQ receptor ligand, ZP120: in vitro and in vivo studies in mice. Br J Pharmacol 137:369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi A, Salis MB, Ciccocioppo R, Marzola G, Bigoni R, Guerrini R, Massi M, Madeddu P, Salvadori S, Regoli D, et al. (2002b) Pharmacological characterisation of [(pX)Phe4]nociceptin(1-13)NH2 analogues. 2. In vivo studies. Naunyn Schmiedebergs Arch Pharmacol 365:450–456. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Spagnolo B, Wainford RD, Fischetti C, Guerrini R, Marzola G, Baldisserotto A, Salvadori S, Regoli D, Kapusta DR, et al. (2007b) In vitro and in vivo studies on UFP-112, a novel potent and long lasting agonist selective for the nociceptin/orphanin FQ receptor. Peptides 28:1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi A, Sukhtankar DD, Ding H, Hayashida K, Ruzza C, Guerrini R, Calo G, Ko MC. (2015) Spinal antinociceptive effects of the novel NOP receptor agonist PWT2-nociceptin/orphanin FQ in mice and monkeys. Br J Pharmacol 172:3661–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi D, Bigoni R, Rizzi A, Jenck F, Wichmann J, Guerrini R, Regoli D, Calo G. (2001c) Effects of Ro 64-6198 in nociceptin/orphanin FQ-sensitive isolated tissues. Naunyn Schmiedebergs Arch Pharmacol 363:551–555. [DOI] [PubMed] [Google Scholar]
- Rizzi D, Rizzi A, Bigoni R, Camarda V, Marzola G, Guerrini R, De Risi C, Regoli D, Calo’ G. (2002c) [Arg(14),Lys(15)]nociceptin, a highly potent agonist of the nociceptin/orphanin FQ receptor: in vitro and in vivo studies. J Pharmacol Exp Ther 300:57–63. [DOI] [PubMed] [Google Scholar]
- Rominger A, Förster S, Zentner J, Dooley DJ, McKnight AT, Feuerstein TJ, Jackisch R, Vlaskovska M. (2002) Comparison of the ORL1 receptor-mediated inhibition of noradrenaline release in human and rat neocortical slices. Br J Pharmacol 135:800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Velasco V, Puhl HL, Fuller BC, Sumner AD. (2005) Modulation of Ca2+ channels by opioid receptor-like 1 receptors natively expressed in rat stellate ganglion neurons innervating cardiac muscle. J Pharmacol Exp Ther 314:987–994. [DOI] [PubMed] [Google Scholar]
- Rutten K, De Vry J, Bruckmann W, Tzschentke TM. (2010) Effects of the NOP receptor agonist Ro65-6570 on the acquisition of opiate- and psychostimulant-induced conditioned place preference in rats. Eur J Pharmacol 645:119–126. [DOI] [PubMed] [Google Scholar]
- Rutten K, De Vry J, Bruckmann W, Tzschentke TM. (2011) Pharmacological blockade or genetic knockout of the NOP receptor potentiates the rewarding effect of morphine in rats. Drug Alcohol Depend 114:253–256. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. (2004) Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology (Berl) 172:129–136. [DOI] [PubMed] [Google Scholar]
- Sandin J, Georgieva J, Schött PA, Ogren SO, Terenius L. (1997) Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur J Neurosci 9:194–197. [DOI] [PubMed] [Google Scholar]
- Sandin J, Ogren SO, Terenius L. (2004) Nociceptin/orphanin FQ modulates spatial learning via ORL-1 receptors in the dorsal hippocampus of the rat. Brain Res 997:222–233. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao Y-Q, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. (2009) Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137:1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Tóth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gavériaux-Ruff C, et al. (2006) Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci USA 103:9691–9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E, Morari M. (2000) Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides 21:1023–1029. [DOI] [PubMed] [Google Scholar]
- Schröder W, Lambert DG, Ko MC, Koch T. (2014) Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists. Br J Pharmacol 171:3777–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunk S, Linz K, Hinze C, Frormann S, Oberbörsch S, Sundermann B, Zemolka S, Englberger W, Germann T, Christoph T, et al. (2014) Discovery of a Potent Analgesic NOP and Opioid Receptor Agonist: Cebranopadol. ACS Med Chem Lett 5:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoto GM, Aricò G, Iemolo A, Ronsisvalle G, Parenti C. (2010) Selective inhibition of the NOP receptor in the ventrolateral periaqueductal gray attenuates the development and the expression of tolerance to morphine-induced antinociception in rats. Peptides 31:696–700. [DOI] [PubMed] [Google Scholar]
- Shoblock JR. (2007) The pharmacology of Ro 64-6198, a systemically active, nonpeptide NOP receptor (opiate receptor-like 1, ORL-1) agonist with diverse preclinical therapeutic activity. CNS Drug Rev 13:107–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Wichmann J, Maidment NT. (2005) The effect of a systemically active ORL-1 agonist, Ro 64-6198, on the acquisition, expression, extinction, and reinstatement of morphine conditioned place preference. Neuropharmacology 49:439–446. [DOI] [PubMed] [Google Scholar]
- Shu YS, Zhao ZQ, Li MY, Zhou GM. (1998) Orphanin FQ/nociceptin modulates glutamate- and kainic acid-induced currents in acutely isolated rat spinal dorsal horn neurons. Neuropeptides 32:567–571. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Westfield GH, Xiao K, Reis RI, Huang LY, Tripathi-Shukla P, Qian J, Li S, Blanc A, Oleskie AN, et al. (2014) Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature 512:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Xiao R, Childers SR. (1996) Identification of opioid receptor-like (ORL1) peptide-stimulated [35S]GTP gamma S binding in rat brain. Neuroreport 7:729–733. [DOI] [PubMed] [Google Scholar]
- Siniscalchi A, Rodi D, Beani L, Bianchi C. (1999) Inhibitory effect of nociceptin on [3H]-5-HT release from rat cerebral cortex slices. Br J Pharmacol 128:119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuda ER, Copits BA, Schmidt MJ, Baird MA, Al-Hasani R, Planer WJ, Funderburk SC, McCall JG, Gereau RW, 4th, Bruchas MR. (2015) Spatiotemporal control of opioid signaling and behavior. Neuron 86:923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo B, Carrà G, Fantin M, Fischetti C, Hebbes C, McDonald J, Barnes TA, Rizzi A, Trapella C, Fanton G, et al. (2007) Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol]: in vitro studies. J Pharmacol Exp Ther 321:961–967. [DOI] [PubMed] [Google Scholar]
- Spampinato S, Di Toro R, Alessandri M, Murari G. (2002) Agonist-induced internalization and desensitization of the human nociceptin receptor expressed in CHO cells. Cell Mol Life Sci 59:2172–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato S, Di Toro R, Qasem AR. (2001) Nociceptin-induced internalization of the ORL1 receptor in human neuroblastoma cells. Neuroreport 12:3159–3163. [DOI] [PubMed] [Google Scholar]
- Stanfa LC, Chapman V, Kerr N, Dickenson AH. (1996) Inhibitory action of nociceptin on spinal dorsal horn neurones of the rat, in vivo. Br J Pharmacol 118:1875–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stayte S, Vissel B. (2014) Advances in non-dopaminergic treatments for Parkinson’s disease. Front Neurosci 8:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Lee H, Rice KC, Ko MC. (2014) Differential effects of opioid-related ligands and NSAIDs in nonhuman primate models of acute and inflammatory pain. Psychopharmacology (Berl) 231:1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullo N, Roviezzo F, Matteis M, Ianaro A, Calò G, Guerrini R, De Gruttola L, Spaziano G, Cirino G, Rossi F, et al. (2013) Nociceptin/orphanin FQ receptor activation decreases the airway hyperresponsiveness induced by allergen in sensitized mice. Am J Physiol Lung Cell Mol Physiol 304:L657–L664. [DOI] [PubMed] [Google Scholar]
- Sun RQ, Wang Y, Zhao CS, Chang JK, Han JS. (2001) Changes in brain content of nociceptin/orphanin FQ and endomorphin 2 in a rat model of neuropathic pain. Neurosci Lett 311:13–16. [DOI] [PubMed] [Google Scholar]
- Suyama H, Kawamoto M, Gaus S, Yuge O. (2003) Effect of JTC-801 (nociceptin antagonist) on neuropathic pain in a rat model. Neurosci Lett 351:133–136. [DOI] [PubMed] [Google Scholar]
- Tamai H, Sawamura S, Takeda K, Orii R, Hanaoka K. (2005) Anti-allodynic and anti-hyperalgesic effects of nociceptin receptor antagonist, JTC-801, in rats after spinal nerve injury and inflammation. Eur J Pharmacol 510:223–228. [DOI] [PubMed] [Google Scholar]
- Tancredi T, Carrà G, Guerrini R, Arduin M, Calò G, Regoli D, Salvadori S, Temussi PA. (2005) The interaction of highly helical structural mutants with the NOP receptor discloses the role of the address domain of nociceptin/orphanin FQ. Chemistry 11:2061–2070. [DOI] [PubMed] [Google Scholar]
- Tehan BG, Bortolato A, Blaney FE, Weir MP, Mason JS. (2014) Unifying family A GPCR theories of activation. Pharmacol Ther 143:51–60. [DOI] [PubMed] [Google Scholar]
- Thakker DR, Standifer KM. (2002a) Induction of G protein-coupled receptor kinases 2 and 3 contributes to the cross-talk between mu and ORL1 receptors following prolonged agonist exposure. Neuropharmacology 43:979–990. [DOI] [PubMed] [Google Scholar]
- Thakker DR, Standifer KM. (2002b) Orphanin FQ/nociceptin blocks chronic morphine-induced tyrosine hydroxylase upregulation. Brain Res Mol Brain Res 105:38–46. [DOI] [PubMed] [Google Scholar]
- Thompson AA, Liu W, Chun E, Katritch V, Wu H, Vardy E, Huang XP, Trapella C, Guerrini R, Calo G, et al. (2012) Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature 485:395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JH, Han JS. (2000) Functional studies using antibodies against orphanin FQ/nociceptin. Peptides 21:1047–1050. [DOI] [PubMed] [Google Scholar]
- Toll L, Khroyan TV, Polgar W, Jiang F, Olsen C, Zaveri NT. (2009) Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/mu-opioid receptor ligands: implications for therapeutic applications. J Pharmacol Exp Ther 331:954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham CM, Moulédous L, Poda G, Maigret B, Meunier JC. (1998) Molecular modelling of the ORL1 receptor and its complex with nociceptin. Protein Eng 11:1163–1179. [DOI] [PubMed] [Google Scholar]
- Trapella C, Guerrini R, Piccagli L, Calo’ G, Carra’ G, Spagnolo B, Rubini S, Fanton G, Hebbes C, McDonald J, et al. (2006) Identification of an achiral analogue of J-113397 as potent nociceptin/orphanin FQ receptor antagonist. Bioorg Med Chem 14:692–704. [DOI] [PubMed] [Google Scholar]
- Trombella S, Vergura R, Falzarano S, Guerrini R, Calo G, Spisani S. (2005) Nociceptin/orphanin FQ stimulates human monocyte chemotaxis via NOP receptor activation. Peptides 26:1497–1502. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. (1991) Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 251:85–87. [DOI] [PubMed] [Google Scholar]
- Ueda H, Yamaguchi T, Tokuyama S, Inoue M, Nishi M, Takeshima H. (1997) Partial loss of tolerance liability to morphine analgesia in mice lacking the nociceptin receptor gene. Neurosci Lett 237:136–138. [DOI] [PubMed] [Google Scholar]
- Uezu K, Sano A, Sei H, Toida K, Houtani T, Sugimoto T, Suzuki-Yamamoto T, Takeshima H, Ishimura K, Morita Y. (2005) Enhanced hippocampal acetylcholine release in nociceptin-receptor knockout mice. Brain Res 1050:118–123. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. (2000) Distinct functions of the two isoforms of dopamine D2 receptors. Nature 408:199–203. [DOI] [PubMed] [Google Scholar]
- Varani K, Rizzi A, Calo G, Bigoni R, Toth G, Guerrini R, Gessi S, Salvadori S, Borea PA, Regoli D. (1999) Pharmacology of [Tyr1]nociceptin analogs: receptor binding and bioassay studies. Naunyn Schmiedebergs Arch Pharmacol 360:270–277. [DOI] [PubMed] [Google Scholar]
- Vardy E, Robinson JE, Li C, Olsen RH, DiBerto JF, Giguere PM, Sassano FM, Huang XP, Zhu H, Urban DJ, et al. (2015) A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron 86:936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varty GB, Hyde LA, Hodgson RA, Lu SX, McCool MF, Kazdoba TM, Del Vecchio RA, Guthrie DH, Pond AJ, Grzelak ME, et al. (2005) Characterization of the nociceptin receptor (ORL-1) agonist, Ro64-6198, in tests of anxiety across multiple species. Psychopharmacology (Berl) 182:132–143. [DOI] [PubMed] [Google Scholar]
- Varty GB, Lu SX, Morgan CA, Cohen-Williams ME, Hodgson RA, Smith-Torhan A, Zhang H, Fawzi AB, Graziano MP, Ho GD, et al. (2008) The anxiolytic-like effects of the novel, orally active nociceptin opioid receptor agonist 8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol (SCH 221510). J Pharmacol Exp Ther 326:672–682. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. (1996) Increase by the ORL1 receptor (opioid receptor-like1) ligand, nociceptin, of inwardly rectifying K conductance in dorsal raphe nucleus neurones. Br J Pharmacol 117:1609–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Christie MJ. (1997) Actions of the ORL1 receptor ligand nociceptin on membrane properties of rat periaqueductal gray neurons in vitro. J Neurosci 17:996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-DeRose J, Stauber G, Khroyan TV, Xie XS, Zaveri NT, Toll L. (2013) Retrodialysis of N/OFQ into the nucleus accumbens shell blocks cocaine-induced increases in extracellular dopamine and locomotor activity. Eur J Pharmacol 699:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzi V, Onaran HO, Molinari P, Guerrini R, Balboni G, Calò G, Costa T. (2013) Ligands raise the constraint that limits constitutive activation in G protein-coupled opioid receptors. J Biol Chem 288:23964–23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaro R, Calcagno M, Marti M, Borrelli E, Morari M. (2013) Pharmacological and genetic evidence for pre- and postsynaptic D2 receptor involvement in motor responses to nociceptin/orphanin FQ receptor ligands. Neuropharmacology 72:126–138. [DOI] [PubMed] [Google Scholar]
- Viaro R, Sanchez-Pernaute R, Marti M, Trapella C, Isacson O, Morari M. (2008) Nociceptin/orphanin FQ receptor blockade attenuates MPTP-induced parkinsonism. Neurobiol Dis 30:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volta M, Viaro R, Trapella C, Marti M, Morari M. (2011) Dopamine-nociceptin/orphanin FQ interactions in the substantia nigra reticulata of hemiparkinsonian rats: involvement of D2/D3 receptors and impact on nigro-thalamic neurons and motor activity. Exp Neurol 228:126–137. [DOI] [PubMed] [Google Scholar]
- Vrontou S, Wong AM, Rau KK, Koerber HR, Anderson DJ. (2013) Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature 493:669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker D, Wang C, Katritch V, Han GW, Huang XP, Vardy E, McCorvy JD, Jiang Y, Chu M, Siu FY, et al. (2013) Structural features for functional selectivity at serotonin receptors. Science 340:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JR, Spina M, Terenius L, Koob GF. (1998) Nociceptin fails to affect heroin self-administration in the rat. Neuroreport 9:2243–2247. [DOI] [PubMed] [Google Scholar]
- Wang H-L, Kuo Y-L, Hsu C-Y, Huang P-C, Li AH, Chou A-H, Yeh T-H, Chen Y-L. (2006) Two C-terminal amino acids, Ser(334) and Ser(335), are required for homologous desensitization and agonist-induced phosphorylation of opioid receptor-like 1 receptors. Cell Signal 18:670–678. [DOI] [PubMed] [Google Scholar]
- Wang HL, Hsu CY, Huang PC, Kuo YL, Li AH, Yeh TH, Tso AS, Chen YL. (2005) Heterodimerization of opioid receptor-like 1 and mu-opioid receptors impairs the potency of micro receptor agonist. J Neurochem 92:1285–1294. [DOI] [PubMed] [Google Scholar]
- Wang JB, Johnson PS, Imai Y, Persico AM, Ozenberger BA, Eppler CM, Uhl GR. (1994) cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett 348:75–79. [DOI] [PubMed] [Google Scholar]
- Wang XM, Zhang KM, Mokha SS. (1996) Nociceptin (orphanin FQ), an endogenous ligand for the QRL1 (opioid-receptor-like1) receptor; modulates responses of trigeminal neurons evoked by excitatory amino acids and somatosensory stimuli. J Neurophysiol 76:3568–3572. [DOI] [PubMed] [Google Scholar]
- Werthwein S, Bauer U, Nakazi M, Kathmann M, Schlicker E. (1999) Further characterization of the ORL1 receptor-mediated inhibition of noradrenaline release in the mouse brain in vitro. Br J Pharmacol 127:300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen EJ, Rajagopal S, Lefkowitz RJ. (2011) Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med 17:126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann J, Adam G, Röver S, Cesura AM, Dautzenberg FM, Jenck F. (1999) 8-acenaphthen-1-yl-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one derivatives as orphanin FQ receptor agonists. Bioorg Med Chem Lett 9:2343–2348. [DOI] [PubMed] [Google Scholar]
- Wichmann J, Adam G, Röver S, Hennig M, Scalone M, Cesura AM, Dautzenberg FM, Jenck F. (2000) Synthesis of (1S,3aS)-8-(2,3,3a,4,5, 6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4. 5]decan-4-one, a potent and selective orphanin FQ (OFQ) receptor agonist with anxiolytic-like properties. Eur J Med Chem 35:839–851. [DOI] [PubMed] [Google Scholar]
- Wick MJ, Minnerath SR, Roy S, Ramakrishnan S, Loh HH. (1995) Expression of alternate forms of brain opioid ‘orphan’ receptor mRNA in activated human peripheral blood lymphocytes and lymphocytic cell lines. Brain Res Mol Brain Res 32:342–347. [DOI] [PubMed] [Google Scholar]
- Wickman K, Clapham DE. (1995) Ion channel regulation by G proteins. Physiol Rev 75:865–885. [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ. (2013) Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65:223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, Tucker RC, Ciccocioppo R. (2014) The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol Ther 141:283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witta J, Buzas B, Cox BM. (2003) Traumatic brain injury induces nociceptin/orphanin FQ expression in neurons of the rat cerebral cortex. J Neurotrauma 20:523–532. [DOI] [PubMed] [Google Scholar]
- Wnendt S, Krüger T, Janocha E, Hildebrandt D, Englberger W. (1999) Agonistic effect of buprenorphine in a nociceptin/OFQ receptor-triggered reporter gene assay. Mol Pharmacol 56:334–338. [DOI] [PubMed] [Google Scholar]
- Wright KE, McDonald J, Barnes TA, Rowbotham DJ, Guerrini R, Calo' G, Lambert DG. (2003) Assessment of the activity of a novel nociceptin/orphanin FQ analogue at recombinant human nociceptin/orphanin FQ receptors expressed in Chinese hamster ovary cells. Neurosci Lett 346:145–148. [DOI] [PubMed] [Google Scholar]
- Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, et al. (2012) Structure of the human κ-opioid receptor in complex with JDTic. Nature 485:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Ito E, Maruyama K, Pietruck C, Sharma M, Yu L, Pierce Palmer P. (2000) An alternatively spliced transcript of the rat nociceptin receptor ORL1 gene encodes a truncated receptor. Brain Res Mol Brain Res 77:1–9. [DOI] [PubMed] [Google Scholar]
- Xie GX, Han X, Ito E, Yanagisawa Y, Maruyama K, Sugano S, Suzuki Y, Wang Y, Gabriel A, Stevens SK, et al. (2003) Gene structure, dual-promoters and mRNA alternative splicing of the human and mouse regulator of G protein signaling GAIP/RGS19. J Mol Biol 325:721–732. [DOI] [PubMed] [Google Scholar]
- Xie GX, Yanagisawa Y, Ito E, Maruyama K, Han X, Kim KJ, Han KR, Moriyama K, Palmer PP. (2005) N-terminally truncated variant of the mouse GAIP/RGS19 lacks selectivity of full-length GAIP/RGS19 protein in regulating ORL1 receptor signaling. J Mol Biol 353:1081–1092. [DOI] [PubMed] [Google Scholar]
- Xie X, Wisor JP, Hara J, Crowder TL, LeWinter R, Khroyan TV, Yamanaka A, Diano S, Horvath TL, Sakurai T, et al. (2008) Hypocretin/orexin and nociceptin/orphanin FQ coordinately regulate analgesia in a mouse model of stress-induced analgesia. J Clin Invest 118:2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XJ, Hao JX, Wiesenfeld-Hallin Z. (1996) Nociceptin or antinociceptin: potent spinal antinociceptive effect of orphanin FQ/nociceptin in the rat. Neuroreport 7:2092–2094.8930965 [Google Scholar]
- Yamada H, Nakamoto H, Suzuki Y, Ito T, Aisaka K. (2002) Pharmacological profiles of a novel opioid receptor-like1 (ORL(1)) receptor antagonist, JTC-801. Br J Pharmacol 135:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Kusaka T, Urayama A, Kimura R, Watanabe Y. (2003) In vitro and ex vivo effects of a selective nociceptin/orphanin FQ (N/OFQ) peptide receptor antagonist, CompB, on specific binding of [3H]N/OFQ and [35S]GTPgammaS in rat brain and spinal cord. Br J Pharmacol 139:1462–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Kimura S. (1997) Analgesic effect of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, in the rat formalin test. Neuroscience 81:249–254. [DOI] [PubMed] [Google Scholar]
- Yeon K-Y, Sim M-Y, Choi S-Y, Lee SJ, Park K, Kim JS, Lee J-H, Lee K-M, Oh SB. (2004) Molecular mechanisms underlying calcium current modulation by nociceptin. Neuroreport 15:2205–2209. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. (1989) Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol 62:96–108. [DOI] [PubMed] [Google Scholar]
- Yu TP, Fein J, Phan T, Evans CJ, Xie CW. (1997) Orphanin FQ inhibits synaptic transmission and long-term potentiation in rat hippocampus. Hippocampus 7:88–94. [DOI] [PubMed] [Google Scholar]
- Yung LY, Joshi SA, Chan RY, Chan JS, Pei G, Wong YH. (1999) GalphaL1 (Galpha14) couples the opioid receptor-like1 receptor to stimulation of phospholipase C. J Pharmacol Exp Ther 288:232–238. [PubMed] [Google Scholar]
- Zaki PA, Keith DE, Jr, Brine GA, Carroll FI, Evans CJ. (2000) Ligand-induced changes in surface mu-opioid receptor number: relationship to G protein activation? J Pharmacol Exp Ther 292:1127–1134. [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. (1998) Modulation of voltage-dependent calcium channels by G proteins. Curr Opin Neurobiol 8:351–356. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. (2002) Modulating modulation: crosstalk between regulatory pathways of presynaptic calcium channels. Mol Interv 2:476–478. [DOI] [PubMed] [Google Scholar]
- Zaratin PF, Petrone G, Sbacchi M, Garnier M, Fossati C, Petrillo P, Ronzoni S, Giardina GA, Scheideler MA. (2004) Modification of nociception and morphine tolerance by the selective opiate receptor-like orphan receptor antagonist (-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB-612111). J Pharmacol Exp Ther 308:454–461. [DOI] [PubMed] [Google Scholar]
- Zaveri NT. (2011) The nociceptin/orphanin FQ receptor (NOP) as a target for drug abuse medications. Curr Top Med Chem 11:1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Calò G. (2003) Nociceptin/orphanin FQ and its receptor--potential targets for pain therapy? J Pharmacol Exp Ther 306:423–429. [DOI] [PubMed] [Google Scholar]
- Zhang C, Miller W, Valenzano KJ, Kyle DJ. (2002) Novel, potent ORL-1 receptor agonist peptides containing alpha-Helix-promoting conformational constraints. J Med Chem 45:5280–5286. [DOI] [PubMed] [Google Scholar]
- Zhang NR, Planer W, Siuda ER, Zhao H-C, Stickler L, Chang SD, Baird MA, Cao Y-Q, Bruchas MR. (2012a) Serine 363 is required for nociceptin/orphanin FQ opioid receptor (NOPR) desensitization, internalization, and arrestin signaling. J Biol Chem 287:42019–42030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gandhi PR, Standifer KM. (2012b) Increased nociceptive sensitivity and nociceptin/orphanin FQ levels in a rat model of PTSD. Mol Pain 8:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Simpson-Durand CD, Standifer KM. (2015) Nociceptin/orphanin FQ peptide receptor antagonist JTC-801 reverses pain and anxiety symptoms in a rat model of post-traumatic stress disorder. Br J Pharmacol 172:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xin SM, Wu GX, Zhang WB, Ma L, Pei G. (1999) Endogenous delta-opioid and ORL1 receptors couple to phosphorylation and activation of p38 MAPK in NG108-15 cells and this is regulated by protein kinase A and protein kinase C. J Neurochem 73:1502–1509. [DOI] [PubMed] [Google Scholar]
- Zhao RJ, Woo RS, Jeong MS, Shin BS, Kim DG, Kim KW. (2003) Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport 14:2383–2385. [DOI] [PubMed] [Google Scholar]
- Zhao Z-Q, Gao Y-J, Sun Y-G, Zhao C-S, Gereau RW, 4th, Chen Z-F. (2007) Central serotonergic neurons are differentially required for opioid analgesia but not for morphine tolerance or morphine reward. Proc Natl Acad Sci USA 104:14519–14524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C-B, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. (2010) Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 35:2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]