Nearly a year ago, UNAIDS launched the ambitious “90–90–90” targets to help end the AIDS epidemic: by 2020, 90% of people living with HIV will be diagnosed, 90% of those diagnosed will be on sustained antiretroviral therapy (ART) with 90% viral suppression in those on ART [1]. A welcome feature of the targets is that they focus not just on expanding access to diagnosis and treatment but also on quality of care in terms of retention and suppression, which are key to optimal HIV outcomes. Perhaps the greatest challenge in achieving these targets will be ensuring that their reach is extended to all populations everywhere. It is therefore encouraging and appropriate that the 90–90–90 targets prioritize equity across populations, with specific focus on their achievement for children and adolescents [1]. The Collaborative Initiative for Pediatric HIV Education and Research has sponsored this supplement of the journal to highlight some of the challenges and ways forward towards attaining 90–90–90 for children and adolescents. Many of these are outlined in the opening paper by Abrams and Strasser [2].
Why are these targets so important for children and adolescents?
Children and adolescents have not gone away
Despite an impressive 40% reduction in mother-to-child HIV transmission (MTCT) in the last five years, there were still an estimated 220,000 new paediatric infections in 2014 [3]. Due to years of failure to prevent MTCT, as well as the success of ART programmes in keeping children alive, we are left with a legacy of an estimated 2.6 million children <15 years living with HIV worldwide currently, nearly 90% of them in sub-Saharan Africa (SSA) [4]. Every day, 410 children die from HIV across the world [3]. By 2020, it is estimated that there will still be well over one million children <15 years old needing ART [5]. However, the burden of paediatric HIV will shift from toddlers and young children not on ART, to older children and adolescents, a growing proportion of whom will have initiated ART [6]. There are currently 2.1 million HIV-infected adolescents aged 10–19 [7] and nearly one-sixth of all new HIV infections are in adolescents aged 15–19 [8]. Until we successfully reduce HIV incidence in adolescents, this population will continue to grow, being a mix of those recently infected together with long-term survivors of perinatal infection.
Children lag in access to diagnosis and treatment
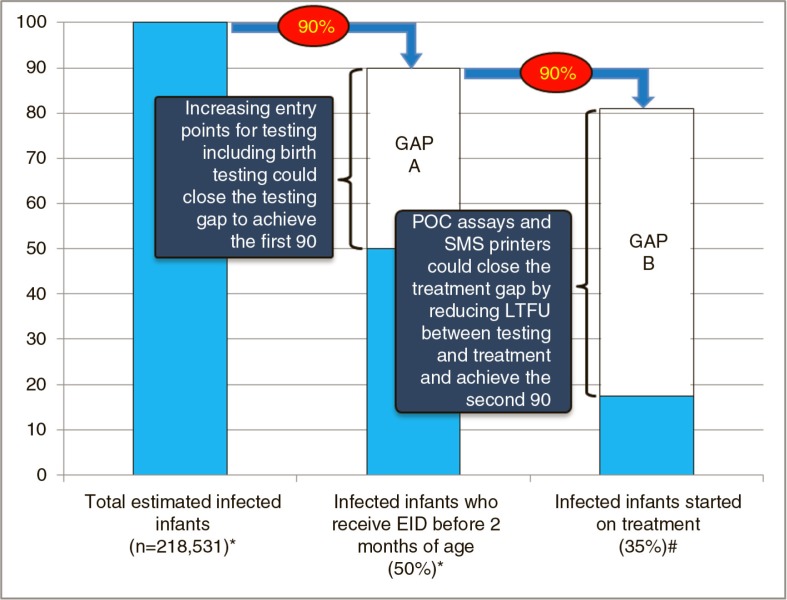
Infants, children and adolescents continue to have the largest gaps in HIV diagnosis and treatment [3,4,8]. Despite encouraging recent scale-up of early infant diagnostic (EID) services, only half of HIV-exposed infants received an EID test before two months of age in 22 Global Plan priority countries during 2014 [9]. In older children, although there is potential for HIV diagnosis within child survival programmes, integration of provider-initiated testing and counselling remains limited [10,11]. A large burden of undiagnosed perinatally acquired HIV-infection in adolescents has been identified in primary care clinics and other services [12–14]. Among older youth aged 15–19 in East and Southern Africa, only one in three girls and one in five boys had ever tested for HIV and received their results [15].
It is well-known that the treatment gap for children remains vast and substantially larger than that of adults, with less than a third of HIV-infected children <15 years receiving ART in 2014 [4]. Given the high pre-ART mortality in infants [16–18], the treatment gap for children would be even larger if the denominator for determining treatment access was all newly infected individuals, rather than just those surviving with HIV [19]. While global data on treatment access for adolescents is lacking, a South African survey suggests that the proportion of HIV-infected adolescents on ART is less than half of that in any other age group [20].
Treatment of infants and children is life-saving and prevents later chronic morbidity
In the absence of treatment, perinatally HIV-infected infants experience extraordinarily high mortality, which can be reduced by 75% with immediate ART in children <3 months of age [16–18,21]. This is undoubtedly the strongest evidence for the urgency of paediatric ART and immediate treatment of infants must be a priority. However, the goals of any medical intervention including ART go beyond averting death and severe morbidity, and extend to optimizing wellness. For example, the CHER study demonstrated significantly better neurocognitive outcomes and less comorbidity with immediate compared to deferred ART [22,23]. There is no randomized controlled trial evidence of the benefit of starting ART within the first few weeks of life as addressed by Cotton et al. [24]. However, arguments in favour of diagnosing and treating paediatric HIV soon after birth include the rapid disease progression in early infancy and the potential to lower viral reservoirs with possible later treatment-sparing options [25–27].
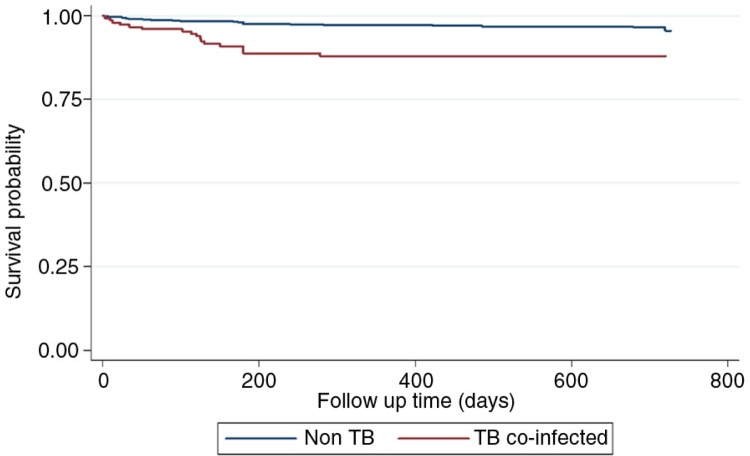
In older children, a causal modelling study showed small but significantly reduced mortality with universal ART in children aged 5–10 years, and studies consistently show better height gain with immediate treatment in children [28–31]. Cohort studies suggest that once stunted, children may not be able to attain normal height after starting ART even if virologically suppressed [32,33]. Furthermore, puberty is delayed with ART initiation at older ages and more severe disease, so deferred ART may result in permanently reduced adult height [34,35]. Immune reconstitution may also be better with earlier ART [36,37]. Similarly, as outlined in the article by Vreeman et al. [38], increased access to ART has been associated with reductions in HIV-associated comorbidities in children, including HIV encephalopathy, HIV-associated nephropathy, anaemia and malignancy. Importantly, manuscripts in this issue by Chamla et al. [39] and Rabie et al. [40] highlight the reduced risk of tuberculosis in children on ART. This is a significant benefit given the exceptionally high risk of infection with both drug-sensitive and -resistant organisms from early infancy onwards in settings where most HIV-infected children live, the complexity of co-treatment especially in young children and the substantial risk of permanent sequelae, especially following tuberculous meningitis [40,41].
Focusing on treatment success in children is critical as they require lifelong treatment
The second and third “90s”, namely retention on ART and achieving viral suppression on first-line therapy, are paramount for children who face lifelong treatment with access to a limited range of alternative drugs. These goals are important to prevent exhausting limited treatment options and to achieve optimal ART outcomes, as well as to prevent transmission of multi-drug resistant viruses when these children grow up with HIV and become sexually active. In addition, sustained virologic suppression, especially from early infancy, is associated with better neurocognitive and growth outcomes as well as reduced viral reservoirs [42–44].
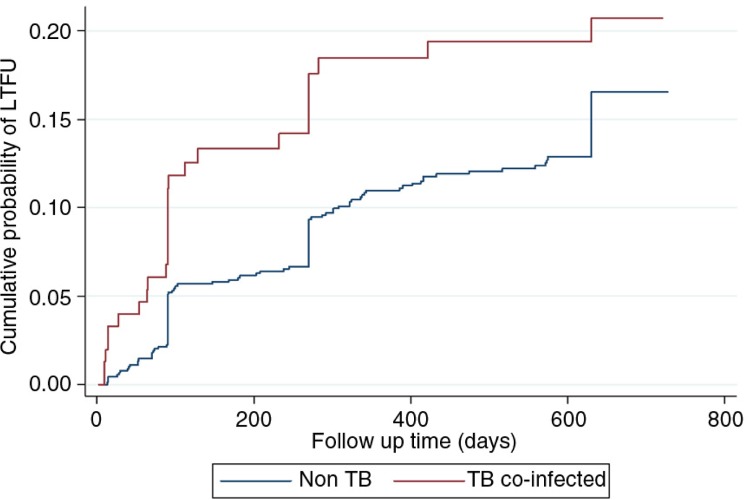
While reports from individual research cohorts suggest that good retention and viral suppression are possible, more routine programmatic data reflects a less optimistic picture [45,46]. In an analysis of routine data of >13,000 children from SSA and Asia, loss to follow-up (LTFU) by 18 months after ART initiation was higher in SSA, ranging from 9.0% in Southern Africa to 21.8% in West Africa [47]. In addition to young age and disease severity, requirement to pay for drugs or services and larger clinic size were associated with higher LTFU [47,48]. Recent systematic reviews of mostly research cohorts suggest that viral failure is higher in children than adults, although comparisons are difficult due to study heterogeneity [49,50]. The same review noted that 90% of children who had failed therapy had at least one resistance mutation, with 76% of children developing resistance within a year of failure. Even in children on lopinavir-based first-line with a high genetic barrier to resistance, 11% had lopinavir mutations [51].
Adolescents are an especially vulnerable group
Adolescents experience obstacles to accessing health services on their own, including stigma, lack of youth-friendly services and parental consent policies, making this a key group for targeting 90–90–90 [1,13]. Whether transitioning from paediatric services or initiating HIV care for the first time, adolescents also frequently struggle with the linked domains of adherence, retention, stigma, disclosure and negotiation of sexual relationships [52]. These difficulties are exacerbated in a context of social and structural deprivation described by Cluver et al. [53], and by the complexities of transitioning to adult services as outlined by Lee and Hazra [54]. Adolescents are the only age group in which AIDS-related deaths are increasing [15], with HIV being the leading cause of adolescent deaths in Africa and the second leading cause of death among adolescents globally [55,56]. There is limited experience with transition of adolescents to adult services in resource-limited settings; however, in the UK adolescents experienced a five-fold increased mortality risk after transition to adult health services [57]. Achieving 90–90–90 among adolescents is important not only for their own health but also to prevent transmission. Adolescents have a high lifetime potential of transmitting HIV as HIV risk behaviour tends to be highest at young ages and those adolescents who become horizontally infected earlier generally also engage in sexual and other risk behaviours [58].
A focus on children means focusing on adults too
While the 90–90–90 targets make us think about those already HIV-infected, one of their most important benefits will be in the reduction of new HIV infections [59]. Ending paediatric HIV critically requires improving diagnosis and treatment in adults, both to directly prevent MTCT, but also to prevent incident infections in adults. There is increasing recognition that Option B+ will not achieve virtual elimination of paediatric HIV unless accompanied by reductions in adult HIV incidence, as incident infection in pregnant and breastfeeding women after a first negative antenatal test is one of the key drivers of ongoing mother-to-child transmission [60,61]. Bringing an end to paediatric AIDS therefore means achieving 90–90–90 for children and adults everywhere.
Making the most of the 90–90–90 targets for children and adolescents
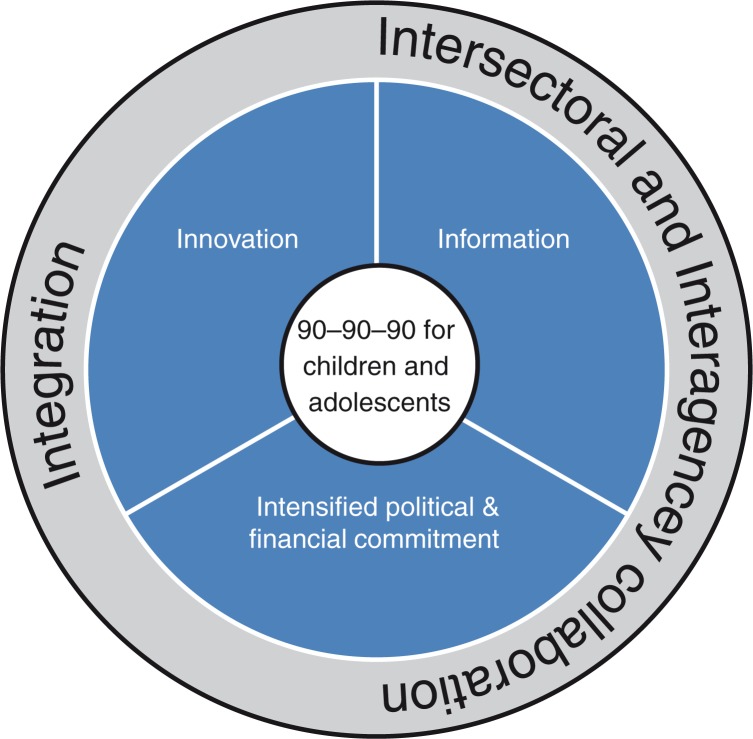
The specific focus on children and adolescents in the UNAIDS 2020 targets, together with alignment of political commitment and financial resources, provides a much-needed opportunity to address previous inequities both in research and service delivery for paediatric HIV. The articles in this issue describe a number of challenges and barriers to achieving the targets, but also important linked strategies for overcoming them, which are represented in the conceptual framework in Figure 1.
Figure 1.
Strategies to achieve 90–90–90 for children and adolescents.
Information
One of the major barriers to improving paediatric HIV care is the paucity of paediatric HIV research. There is frequently little or no high-quality evidence on which to base policies and guidelines. Many paediatric HIV care recommendations in WHO and national guidelines therefore remain conditional, rather than strong, with a risk of less commitment to their implementation [31,62,63]. The effects of limited paediatric research range from the inferior and limited HIV and tuberculosis drugs and formulations available for children in all age groups, highlighted in this supplement by Boerma et al. [64], Cotton et al. [24], Penazzato et al. [65] and Rabie et al. [40], to the lack of evidence-based transition models for adolescents moving to adult HIV care [54]. While paediatric HIV research is not easy for a number of reasons, including the developmental biology of children, decreased number of new paediatric infections, and complex but important regulatory and ethical requirements, it is essential if we are to achieve the 90–90–90 targets. Both clinical and implementation science research is needed to identify more effective and safer ways of treating children, especially newborns [24], adolescents [65] and those failing therapy [64], as well as how best to operationalize and deliver interventions at scale in a range of settings [66].
The need for information extends beyond academic research to monitoring and evaluation of routine programmes – we will not know whether we have met the 90–90–90 targets unless we measure them, and we are unlikely to achieve them unless we monitor our progress (or lack thereof) towards them, using the information to improve programmes. In this respect, the lack of access to routine viral load monitoring in many settings is a major obstacle both to achieving 90% suppression and knowing how close or far off we are.
Innovation
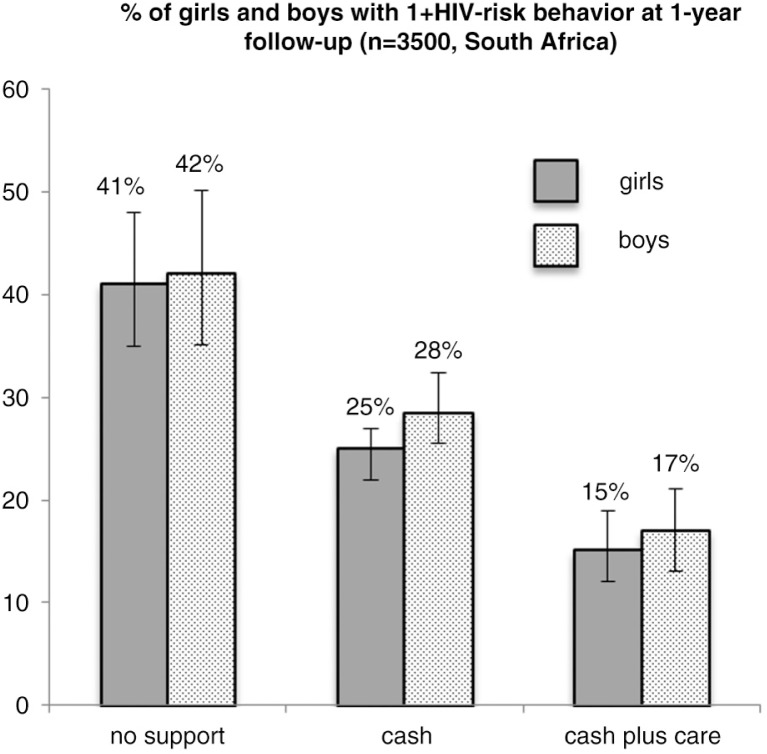
We will not reach 90–90–90 for children with a “business as usual” approach. Many articles in this issue discuss innovations both within and outside the health system that show promise in improving paediatric and adolescent HIV care. For example, Essajee et al. [67] review four innovative approaches to EID, namely point-of-care testing, use of SMS printers to connect laboratories and peripheral facilities, alternative health system entry points for EID and birth testing. Lee and Hazra [54] emphasize the need for innovative transition models that use a public health approach and can be implemented at scale in resource-limited settings, and Abrams and Strasser [2] point out that service delivery innovations such as youth-friendly services and community-, school- and home-based ART are long overdue as we seek to achieve quality ART scale-up for children. Cluver et al. [53] argue that we need to combine biomedical solutions with social protection innovations beyond the health system, including cash transfers, parental and education support (“cash, care, classroom”), to increase uptake of prevention and treatment technologies in adolescents. In addition, Penazzato et al. [65] outline a role for innovative trial design to fast-track comparisons of new drugs in children.
Intensified political and financial commitment
Abrams and Strasser [2] emphasize the need for political commitment and financial resources to chart a steady course to the 90–90–90 targets for children. In securing this commitment, it is helpful that the new Sustainable Development Goals support the UNAIDS targets, including the aim of ending the epidemics of AIDS and tuberculosis by 2030 [68]. UNAIDS has estimated the resource requirements to meet the goal of ending AIDS will increase incrementally, reaching US$18 billion by 2020, with modest declines through to 2030 [1]. While these costs may seem daunting in the context of diminishing global HIV funding, there will likely be severe cost implications for HIV programming beyond 2020 if the necessary investments to accelerate the end of AIDS are not made now [1]. Globally and at country level, we need to continue to scale up advocacy for funding from all sources. At the same time we need to improve and use information about cost-effectiveness and programme efficiency gains, innovative financing mechanisms and broader economic analysis so that finite resources are used in the most efficient way.
Integration
Integration has been a “buzzword” in adult HIV for several years, with emerging promising practices for children and adolescents. The need for integration is highlighted by a number of articles in this supplement. As described by Chamla et al. [66], the rationale for integration includes the conventional goals of improving service delivery, health outcomes and efficiencies as demonstrated by improved outcomes following implementation of the Integrated Management of Childhood Illness (IMCI). Integration can also provide a platform for dissemination of innovations such as point-of-care diagnostics and viral load assays. The double dividend initiative launched in 2013 is one integrating approach intending to catalyse accelerated action towards both ending paediatric HIV and improving child survival [69]. It aims to identify service delivery platforms that provide better care for HIV-affected and - infected children through strategic investments from which all children can benefit. Integration is a promising strategy to address missed opportunities for HIV diagnosis, especially in infants missed or lost from PMTCT programmes, delayed ART initiation and poor retention, treatment of comorbidities and improved adolescent care. Rabie et al. [40] identify components of tuberculosis, HIV, antenatal and IMCI care where linkage and integration would facilitate optimal delivery of tuberculosis preventive and treatment services to HIV-infected children. In a previous CIPHER supplement in this journal, Bekker et al. [58] emphasized the role of integration in adolescent-centred rather than speciality-centred services, with comprehensive peer-guided youth-friendly one-stop shops in a diverse array of community-based settings. Critically, services need to link HIV-testing and diagnosis with prevention and treatment services, address other adolescent health needs, especially sexual and reproductive health, and, as outlined by Lee and Hazra [54], prepare adolescents for transition to adult services.
Interagency and intersectoral collaboration
There is a huge diversity of role players and stakeholders in paediatric and adolescent HIV, both within and outside the health service. Stakeholders include funding agencies, policy makers, researchers, implementing partners, ministries of health, industry (pharmaceutical and diagnostic), health care workers, non-profit and community-based organizations, as well as, importantly, children, adolescents and caregivers themselves. Like previous targets, the 90–90–90 agenda provides an opportunity for these groups to work towards a common goal, facilitating collaboration. For example, Chamla et al. [66] highlight that integration has tended to focus at the level of service delivery, but needs to step up to full integration across numerous health system domains including governance, human resources, information and financing. The Pediatric HIV Treatment Initiative (PHTI) (discussed by Penazzato et al. [65]) is an important multi-stakeholder activity that aims to accelerate development of and access to WHO-recommended paediatric antiretroviral formulations by co-ordinating drug development and engaging industry to ensure sharing of intellectual property rights to facilitate formulation development. The Interagency Task Team on prevention and treatment of HIV infection in pregnant women mothers and children (IATT) is a collaboration that provides formulary guidance on optimal paediatric antiretrovirals [65]. Demand for different drugs is consolidated through endorsement of this formulary by major implementers and purchasers. Another example of intersectoral collaboration in drug development has been the recognition in 2010 of paediatric HIV as a “neglected disease” by the Drugs for Neglected Diseases initiative (DNDi) [70]. In consultation with experts from countries where HIV is endemic, major research institutions, and international and non-governmental organizations, DNDi has developed “ideal” and “acceptable” specifications for desired formulations/combinations of paediatric antiretrovirals and identified priorities for acceleration of clinical studies.
Intersectoral collaboration needs to extend beyond the health system and its traditional partners. Cluver et al. [53] point out that key HIV risk behaviours as well as treatment adherence or non-adherence do not happen in the clinic, but in social and family spaces where children and adolescents live. While we clearly need health system and clinical innovations to achieve 90–90–90, treatment and prevention interventions will be far more effective if they take into account the social and structural context that drives the decisions and behaviours of children, adolescents and their caregivers.
Final comments
There can be no keener revelation of a society's soul than the way it treats its children.
– Nelson Mandela
There are many challenges to reaching the 90–90–90 targets for children and adolescents. They require a range of linked activities by multiple players working together with concerted effort at many levels within and beyond the health system. While targets can be criticized, they drive progress and help to consolidate and renew financial and political commitment to HIV prevention and treatment. These targets therefore offer the global community an opportunity to focus on children, and the very real and remarkable possibility of ending paediatric HIV.
References
- 1.UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS; 2014. JC2684. [Google Scholar]
- 2.Abrams E, Strasser S. 90-90-90 – Charting a steady course to end the pediatric HIV epidemic. J Int AIDS Soc. 2015;18(Suppl 6):20296. doi: 10.7448/IAS.18.7.20296. doi: http://dx.doi.org/10.7448/IAS.18.7.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. How AIDS changed everything. 2015. [cited 2015 Sep 6]. Available from: http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf.
- 4.UNAIDS. HIV Factsheet 2015. 2015. [cited 2015 Sep 6]. Available from: http://www.unaids.org/sites/default/files/media_asset/20150714_FS_MDG6_Report_en.pdf.
- 5.Penazzato M, Bendaud V, Nelson L, Stover J, Mahy M. Estimating future trends in paediatric HIV. AIDS. 2014;28(Suppl 4):S445–51. doi: 10.1097/QAD.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson L, Davies M, Moultrie H, Sherman G, Bland R, Rehle T, et al. The effect of early initiation of antiretroviral treatment in infants on pediatric AIDS mortality in South Africa – a model-based analysis. Pediatr Infec Dis J. 2012;31:474–80. doi: 10.1097/INF.0b013e3182456ba2. [DOI] [PubMed] [Google Scholar]
- 7.UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2013. 2013. [cited 2015 Jun 15]. Available from: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Global_Report_2013_en_1.pdf.
- 8.United Nations Children's Fund. Towards and AIDS-free Generation: Sixth Stocktaking Report. 2013. [cited 2015 Sep 2]. Available from: http://www.childrenandaids.org/files/str6_full_report_29-11-2013.pdf.
- 9.World Health Organization. Global update on the health sector response to HIV, 2014. 2014. [cited 2014 Nov 4]. Available from: www.who.int.
- 10.Weigel R, Kamthunzi P, Mwansambo C, Phiri S, Kazembe PN. Effect of provider-initiated testing and counselling and integration of ART services on access to HIV diagnosis and treatment for children in Lilongwe, Malawi: a pre- post comparison. BMC Pediatr. 2009;9:80. doi: 10.1186/1471-2431-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fergusson P, Tomkins A. HIV prevalence and mortality among children undergoing treatment for severe acute malnutrition in sub-Saharan Africa: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2009;103:541–8. doi: 10.1016/j.trstmh.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Ferrand RA, Munaiwa L, Matsekete J, Bandason T, Nathoo K, Ndhlovu CE, et al. Undiagnosed HIV infection among adolescents seeking primary health care in Zimbabwe. Clin Infect Dis. 2010;51:844–51. doi: 10.1086/656361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kranzer K, Meghji J, Bandason T, Dauya E, Mungofa S, Busza J, et al. Barriers to provider-initiated testing and counselling for children in a high HIV prevalence setting: a mixed methods study. PLoS Med. 2014;11:e1001649. doi: 10.1371/journal.pmed.1001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrand RA, Bandason T, Musvaire P, Larke N, Nathoo K, Mujuru H, et al. Causes of acute hospitalization in adolescence: burden and spectrum of HIV-related morbidity in a country with an early-onset and severe HIV epidemic: a prospective survey. PLoS Med. 2010;7:e1000178. doi: 10.1371/journal.pmed.1000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Idele P, Gillespie A, Porth T, Suzuki C, Mahy M, Kasedde S, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66(Suppl 2):S144–53. doi: 10.1097/QAI.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 16.Little K, Thorne C, Luo C, Bunders M, Ngongo N, McDermott P, et al. Disease progression in children with vertically-acquired HIV infection in sub-Saharan Africa: reviewing the need for HIV treatment. Curr HIV Res. 2007;5:139–53. doi: 10.2174/157016207780077002. [DOI] [PubMed] [Google Scholar]
- 17.Marston M, Becquet R, Zaba B, Moulton LH, Gray G, Coovadia H, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011;40:385–96. doi: 10.1093/ije/dyq255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becquet R, Marston M, Dabis F, Moulton LH, Gray G, Coovadia HM, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One. 2012;7:e28510. doi: 10.1371/journal.pone.0028510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson LF, Boulle A. How should access to antiretroviral treatment be measured? Bull World Health Organ. 2011;89:157–60. doi: 10.2471/BLT.10.080911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shisana O, Rehle T, Simbayi L, Zuma K, Jooste S, Zungu N, et al. South African national HIV prevalence incidence and behaviour survey 2012. 2014. [cited 2015 Sep 1]. Available from: http://www.hsrc.ac.za/en/research-data/view/6871. [DOI] [PubMed]
- 21.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laughton B, Cornell M, Grove D, Kidd M, Springer PE, Dobbels E, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS. 2012;26:1685–90. doi: 10.1097/QAD.0b013e328355d0ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabie H, Violari A, Duong T, Madhi SA, Josipovic D, Innes S, et al. Early antiretroviral treatment reduces risk of bacille Calmette-Guerin immune reconstitution adenitis. Int J Tuberc Lung Dis. 2011;15:1194–200, i. doi: 10.5588/ijtld.10.0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotton M, Holgate SL, Nelson A, Rabie H, Wedderburn C, Mirochnik M. The last and first frontier – emerging challenges for HIV treatment and prevention in the first week of life with emphasis on premature and low birth weight infants. J Int AIDS Soc. 2015;18(Suppl 6):20271. doi: 10.7448/IAS.18.7.20271. doi: http://dx.doi.org/10.7448/IAS.18.7.20271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourne DE, Thompson M, Brody LL, Cotton M, Draper B, Laubscher R, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS. 2009;23:101–6. doi: 10.1097/qad.0b013e32831c54bd. [DOI] [PubMed] [Google Scholar]
- 26.Persaud D, Palumbo PE, Ziemniak C, Hughes MD, Alvero CG, Luzuriaga K, et al. Dynamics of the resting CD4(+) T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. AIDS. 2012;26:1483–90. doi: 10.1097/QAD.0b013e3283553638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M, Jr, Chun TW, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369:1828–35. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puthanakit T, Saphonn V, Ananworanich J, Kosalaraksa P, Hansudewechakul R, Vibol U, et al. Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre, randomised, open-label trial. Lancet Infect Dis. 2012;12:933–41. doi: 10.1016/S1473-3099(12)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schomaker M, Egger M, Ndirangu J, Phiri S, Moultrie H, Technau K, et al. When to start antiretroviral therapy in children aged 2–5 years: a collaborative causal modelling analysis of cohort studies from southern Africa. PLoS Med. 2013;10:e1001555. doi: 10.1371/journal.pmed.1001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schomaker M, Davies M, Malateste K, Renner L, Sawry S, N'Gbeche S, et al. Growth and mortality outcomes for different antiretroviral therapy initiation criteria in children aged 1–5 years: a causal modelling analysis from West and Southern Africa. Epidemiology. 2015 doi: 10.1097/EDE.0000000000000412. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015. [cited 2015 Sep 30]. Available from: http://who.int/hiv/en/ [PubMed]
- 32.Gsponer T, Weigel R, Davies MA, Bolton C, Moultrie H, Vaz P, et al. Variability of growth in children starting antiretroviral treatment in southern Africa. Pediatrics. 2012;130:e966–77. doi: 10.1542/peds.2011-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabue MM, Kekitiinwa A, Maganda A, Risser JM, Chan W, Kline MW. Growth in HIV-infected children receiving antiretroviral therapy at a pediatric infectious diseases clinic in Uganda. AIDS Patient Care STDS. 2008;22:245–51. doi: 10.1089/apc.2007.0049. [DOI] [PubMed] [Google Scholar]
- 34.Williams PL, Abzug MJ, Jacobson DL, Wang J, Van Dyke RB, Hazra R, et al. Pubertal onset in children with perinatal HIV infection in the era of combination antiretroviral treatment. AIDS. 2013;27:1959–70. doi: 10.1097/QAD.0b013e328361195b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szubert AJ, Musiime V, Bwakura-Dangarembizi M, Nahirya-Ntege P, Kekitiinwa A, Gibb DM, et al. Pubertal development in HIV-infected African children on first-line antiretroviral therapy. AIDS. 2015;29:609–18. doi: 10.1097/QAD.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krogstad P, Patel K, Karalius B. Incomplete immune reconstitution despite virologic suppression in HIV-1 infected children and adolescents. AIDS. 2015;29:683–93. doi: 10.1097/QAD.0000000000000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel K, Hernan MA, Williams PL, Seeger JD, McIntosh K, Dyke RB, et al. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin Infect Dis. 2008;46:1751–60. doi: 10.1086/587900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vreeman R, Scanlon M, McHenry M, Nyandiko W. The physical and psychological effects of HIV infection on perinatally HIV-infected children in the HAART era. J Int AIDS Soc. 2015;18(Suppl 6):20258. doi: 10.7448/IAS.18.7.20258. doi: http://dx.doi.org/10.7448/IAS.18.7.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chamla D, Asadu C, Davies A, De Wagt A, Ilesanmi O, Adeyinka D, et al. Patching the Gaps towards the 90-90-90 targets: outcomes of children receiving antiretroviral treatment co-infected with Tuberculosis in Nigeria. J Int AIDS Soc. 2015;18(Suppl 6):20251. doi: 10.7448/IAS.18.7.20251. doi: http://dx.doi.org/10.7448/IAS.18.7.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabie H, Frigati LJ, Hesseling AC, Garcia-Prats A. Tuberculosis: opportunities and challenges for the 90-90-90 targets in HIV-infected children. J Int AIDS Soc. 2015;18(Suppl 6):20236. doi: 10.7448/IAS.18.7.20236. doi: http://dx.doi.org/10.7448/IAS.18.7.20236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Well GT, Paes BF, Terwee CB, Springer P, Roord JJ, Donald PR, et al. Twenty years of pediatric tuberculous meningitis: a retrospective cohort study in the western cape of South Africa. Pediatrics. 2009;123:e1–8. doi: 10.1542/peds.2008-1353. [DOI] [PubMed] [Google Scholar]
- 42.Shiau S, Arpadi S, Strehlau R, Martens L, Patel F, Coovadia A, et al. Initiation of antiretroviral therapy before 6 months of age is associated with faster growth recovery in South African children perinatally infected with human immunodeficiency virus. J Pediatr. 2013;162:1138–45. 1145.e1131–2. doi: 10.1016/j.jpeds.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luzuriaga K, Tabak B, Garber M, Chen YH, Ziemniak C, McManus MM, et al. HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis. 2014;210:1529–38. doi: 10.1093/infdis/jiu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crowell C, Huo Y, Tassiopoulos K, Malee K, Yogev R, Hazra R, et al. Early viral suppression improves neurocognitive outcomes in HIV-infected children. Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2014. Abstract 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peacock-Villada E, Richardson BA, John-Stewart GC. Post-HAART outcomes in pediatric populations: comparison of resource-limited and developed countries. Pediatrics. 2011;127:e423–41. doi: 10.1542/peds.2009-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–89. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 47.Leroy V, Malateste K, Rabie H, Lumbiganon P, Ayaya S, Dicko F, et al. Outcomes of antiretroviral therapy in children in Asia and Africa: a comparative analysis of the IeDEA pediatric multiregional collaboration. J Acquir Immune Defic Syndr. 2013;62:208–19. doi: 10.1097/QAI.0b013e31827b70bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNairy ML, Lamb MR, Carter RJ, Fayorsey R, Tene G, Mutabazi V, et al. Retention of HIV-infected children on antiretroviral treatment in HIV care and treatment programs in Kenya, Mozambique, Rwanda, and Tanzania. J Acquir Immune Defic Syndr. 2013;62:e70–81. doi: 10.1097/QAI.0b013e318278bcb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sigaloff KC, Calis JC, Geelen SP, van Vugt M, de Wit TF. HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: a systematic review. Lancet Infect Dis. 2011;11:769–79. doi: 10.1016/S1473-3099(11)70141-4. [DOI] [PubMed] [Google Scholar]
- 50.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010;10:155–66. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 51.Meyers T, Sawry S, Wong JY, Moultrie H, Pinillos F, Fairlie L, et al. Virologic failure among children taking lopinavir/ritonavir-containing first-line antiretroviral therapy in South Africa. Pediatr Infect Dis J. 2015;34:175–9. doi: 10.1097/INF.0000000000000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51:65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cluver L, Hodes R, Sherr L, Orkin MF, Meinck F, Lim ah Ken P, et al. Social protection: potential for improving HIV outcomes among adolescents. J Int AIDS Soc. 2015;18(Suppl 6):20299. doi: 10.7448/IAS.18.7.20260. doi: http://dx.doi.org/10.7448/IAS.18.7.20260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S, Hazra R. Achieving 90-90-90 in pediatric HIV: adolescence as the touchstone for transition success. J Int AIDS Soc. 2015;18(Suppl 6):20257. doi: 10.7448/IAS.18.7.20257. doi: http://dx.doi.org/10.7448/IAS.18.7.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.World Health Organization. Fact sheet on adolescent health. 2014. [cited 2015 Sep 4]. Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/factsheet/2012/20120417_FS_adolescentsyoungpeoplehiv_en.pdf.
- 56.World Health Organization. Health for the world's adolescents: a second chance in the second decade. 2014. [cited 2015 Sep 30]. Available from: http://www.who.int/maternal_child_adolescent/topics/adolescence/second-decade/en/
- 57.Fish R, Judd A, Jungmann E, O'Leary C, Foster C. Mortality in perinatally HIV-infected young people in England following transition to adult care: an HIV Young Persons Network (HYPNet) audit. HIV Med. 2014;15:239–44. doi: 10.1111/hiv.12091. [DOI] [PubMed] [Google Scholar]
- 58.Bekker LG, Johnson L, Wallace M, Hosek S. Building our youth for the future. J Int AIDS Soc. 2015;18:20027. doi: 10.7448/IAS.18.2.20027. doi: http://dx.doi.org/10.7448/IAS.18.2.20027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen MS, Smith MK, Muessig KE, Hallett TB, Powers KA, Kashuba AD. Antiretroviral treatment of HIV-1 prevents transmission of HIV-1: where do we go from here? Lancet. 2013;382:1515–24. doi: 10.1016/S0140-6736(13)61998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson L, Stinson K, Newell ML, Bland R, Moultrie H, Davies M, et al. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2012;59:417–25. doi: 10.1097/QAI.0b013e3182432f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson L, Chiu C, Bekker LG, Myer L, Davies M, Dorrington RE, et al. What will it take to achieve virtual elimination of HIV transmission in South Africa?. 8th International AIDS Soicety Conference on HIV Pathogenesis, Treatment and Prevention; Vancouver, Canada. 2015. Abstract MOPEC442. [Google Scholar]
- 62.WHO. Antiretroviral therapy for HIV infection in infants and children: towards universal access. Recommendations for a public health approach: 2010 revision. 2010. [cited 2010 Oct 19]. Available from: http://www.who.int/hiv/pub/paediatric/infants2010/en/index.html. [PubMed]
- 63.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Summary of key features and recommendations. 2013. [cited 2013 Nov 10]. Available from: http://www.who.int/hiv/pub/guidelines/arv2013/short_summary/en/index.html. [PubMed]
- 64.Boerma RS, Boender TS, Boele van Hensbroek M, Rinke de Wit T, Sigaloff KC. Sequencing pediatric antiretroviral therapy in the context of a public health approach. J Int AIDS Soc. 2015;18(Suppl 6):20265. doi: 10.7448/IAS.18.7.20265. doi: http://dx.doi.org/10.7448/IAS.18.7.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Penazzato M, Lee J, Capparelli E, Essajee S, Ford N, Ojoo A, et al. Optimizing drugs to reach treatment targets for children and adolescents living with HIV. J Int AIDS Soc. 2015;18(Suppl 6):20270. doi: 10.7448/IAS.18.7.20270. doi: http://dx.doi.org/10.7448/IAS.18.7.20270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chamla D, Essajee S, Young M, Kellerman S, Lovich R, Sugandhi N, et al. Integration of HIV in child survival platforms: a novel programmatic pathway towards the 90-90-90 targets. J Int AIDS Soc. 2015;18(Suppl 6):20250. doi: 10.7448/IAS.18.7.20250. doi: http://dx.doi.org/10.7448/IAS.18.7.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Essajee S, Vojnov L, Penazzato M, Jani I, Siberry G, Fiscus S, et al. Reducing mortality in HIV-infected infants and achieving the 90–90–90 target through innovative diagnosis approaches. J Int AIDS Soc. 2015;18(Suppl 6):20299. doi: 10.7448/IAS.18.7.20299. doi: http://dx.doi.org/10.7448/IAS.18.7.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.United Nations. Sustainable development goals. 2015. [cited 2015 Sep 25]. Available from: http://www.un.org/sustainabledevelopment/sustainable-development-goals/
- 69.UNICEF, WHO, Foundation EGPA. The double dividend. 2013. [cited 2015 Sep 5]. Available from: http://www.unicef.org/aids/files/Action_Framework_Final.pdf.
- 70.Lallemant M, Chang S, Cohen R, Pecoul B. Pediatric HIV–a neglected disease? N Engl J Med. 2011;365:581–3. doi: 10.1056/NEJMp1107275. [DOI] [PubMed] [Google Scholar]