Abstract
Aging is characterized by the progressive accumulation of degenerative changes, culminating in impaired function and increased probability of death. It is the major risk factor for many human pathologies – including cancer, type 2 diabetes, and cardiovascular and neurodegenerative diseases – and consequently exerts an enormous social and economic toll. The major goal of aging research is to develop interventions that can delay the onset of multiple age-related diseases and prolong healthy lifespan (healthspan). The observation that enhanced longevity and health can be achieved in model organisms by dietary restriction or simple genetic manipulations has prompted the hunt for chemical compounds that can increase lifespan. Most of the pathways that modulate the rate of aging in mammals have homologs in yeast, flies, and worms, suggesting that initial screening to identify such pharmacological interventions may be possible using invertebrate models. In recent years, several compounds have been identified that can extend lifespan in invertebrates, and even in rodents. Here, we summarize the strategies employed, and the progress made, in identifying compounds capable of extending lifespan in organisms ranging from invertebrates to mice and discuss the formidable challenges in translating this work to human therapies.
Keywords: aging, anti-aging medicine, age-related diseases
Introduction
Aging is characterized by molecular, cellular, and organismal changes that culminate in the inability of an organism to maintain physiological integrity 1. In humans, aging is associated with a greatly increased predisposition to a wide variety of diseases, including cancer, type 2 diabetes (T2D), neurodegeneration, and cardiovascular disease, leading to increased morbidity and mortality 1, 2. The long-term objective of aging research is to develop interventions that can delay the onset of age-associated diseases and promote longevity. With this goal, research in biogerontology is focused on elucidating basic mechanisms of aging. Current evidence suggests that many of these mechanisms are conserved among eukaryotes, from yeast to mammals.
In recent decades, work in diverse organisms has identified cellular signaling pathways that modulate the aging rate 3, 4. Many of these pathways normally function to sense the nutritional status of the organism ( Figure 1) and initiate signaling cascades that modulate specific inter- and intra-cellular pathways and alter target cell physiology accordingly 2. These nutrient-sensing pathways, which include insulin and insulin-like growth factor (IGF) signaling (IIS) 5, target of rapamycin (mTOR) signaling 6, adenosine monophosphate (AMP)-activated protein kinase (AMPK) signaling 7, and sirtuins 8, coordinate cellular growth- and metabolism-related processes and integrate them with levels of nutrients, energy, growth factors, and stress. When nutrient levels and growth cues are reduced, signaling through these pathways is altered. Genetic or, in some cases, pharmacologic manipulation of these pathways can lead to lifespan extension, whereas their age-associated dysregulation may contribute to organismal senescence.
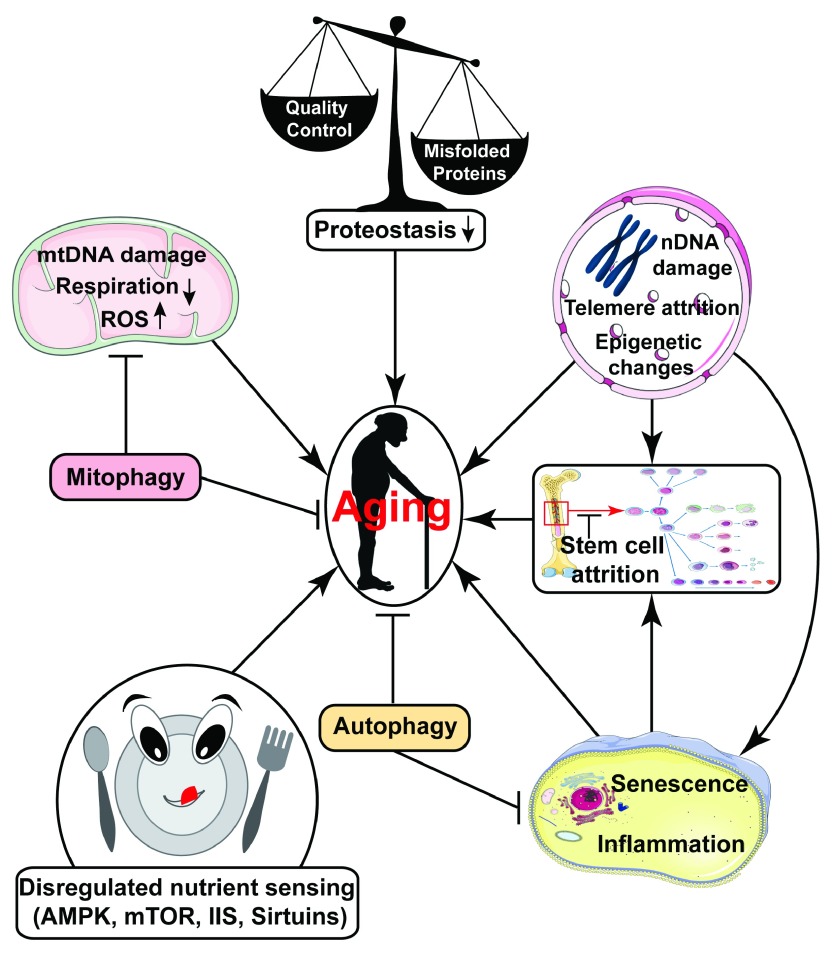
Figure 1. Summary of various factors that may contribute to aging.
Dysregulation of nutrient-sensing pathways, mitochondrial dysfunction, loss of proteostasis, stem cell attrition, accumulated DNA damage, reduced autophagy, accumulation of senescent cells, and increased sterile inflammation are some important pathways thought to drive aging 1.
Dietary restriction (DR), a dietary regimen involving either a reduction in overall calorie ingestion without malnutrition or diminished intake of specific dietary components such as amino acids, is the best-characterized intervention that slows aging and delays disease in a wide range of species 9, 10. Molecular effectors implicated in mediating the remarkable effects of DR include these nutrient-sensing pathways 9. Initial evidence suggests that some of these same pathways may impact aging and disease in humans as well. For example, genetic variants in the FOXO3A gene, encoding a transcription factor downstream of IIS, have been linked to human longevity 11– 16. Individuals with Laron dwarfism have greatly reduced serum IGF1 levels and profound protection from T2D and cancer 17. Pharmacological interventions that partially mimic DR by modulating activities of these nutrient-sensing pathways have the potential to improve healthspan and promote longevity. For example, rapamycin, a specific inhibitor of mTOR, has been proposed to provoke some of the beneficial effects of DR under standard feeding and nutrient conditions 18. Similarly, a handful of other molecules such as metformin and resveratrol have been shown to modulate nutrient signaling and promote healthspan in multiple model organisms and are discussed in detail subsequently.
In addition to dysregulation of nutrient-sensing pathways, other conserved mechanisms implicated in the deleterious manifestations of aging include ( Figure 1) i) mitochondrial dysfunction, leading to impaired respiratory metabolism, increased generation of reactive oxygen species (ROS), as well as potentially other sequelae, ii) increased accumulation of DNA damage, induced by exogenous insults and endogenous hazards including DNA replication errors and ROS, iii) diminished proteostasis associated with increased protein misfolding and aggregation, iv) cellular senescence, contributing to tissue dysfunction, v) increased sterile inflammation, vi) stem cell attrition, and vii) epigenetic alterations 1, 19. For a more complete discussion of conserved aging mechanisms, the reader is referred elsewhere 1. Pharmacological agents targeting some of these changes represent candidate anti-aging drugs. In this review, we will provide an overview of pharmacological interventions with known or potential ability to delay aging and promote late-life health. First, we summarize the major contributions that studies in invertebrate model systems have made towards screening efforts to identify small molecule anti-aging drugs. Then we focus in depth on molecules currently under study for their potential to extend lifespan and delay disease. Finally, challenges in screening for new anti-aging drugs and in translating this work to humans will be discussed.
Invertebrates as model systems to screen pro-longevity small molecules
Due to a variety of factors – notably including ease of genetic manipulation and a physiology similar to that of humans – the mouse has become the pre-eminent mammalian model organism in aging biology 20. However, in light of the high housing costs and relatively long lifespan of mice, large-scale unbiased screening to identify anti-aging medicines is not feasible in this organism. With the realization that many aging-related pathways are evolutionarily conserved, even among widely divergent species, short-lived invertebrate models have instead been employed for such screening. The nematode Caenorhabditis elegans – with its short lifespan of ~3 weeks, ease of culture and genetic manipulation, and well-characterized aging biology – represents a very attractive model system for chemical screening to identify compounds that modulate lifespan and age-related phenotypes. Indeed, several studies have identified a number of candidate anti-aging compounds using C. elegans as a model organism. To date, the most comprehensive small molecule lifespan screen using C. elegans was conducted by Petrascheck et al., who evaluated 88,000 chemicals for their ability to enhance longevity 21. They identified 115 compounds that significantly increased worm lifespan. Interestingly, one of these displayed structural resemblance to human antidepressants that affect signaling by the neurotransmitter serotonin. They subsequently found that mianserin, a serotonin receptor antagonist used as an antidepressant in humans, extends C. elegans lifespan when administered at 50 μM, likely via mechanisms linked to DR 21. In an evaluation of 19 compounds with known effects on human physiology, Evason et al. reported that the anticonvulsants ethosuximide (dosed at 2 and 4 mg/mL), trimethadione (4 mg/mL), and 3,3-diethyl-2-pyrrolidinone (2 mg/mL) delayed age-related changes and increased C. elegans lifespan 22.
Using a bioinformatics approach to identify DR mimetics, Calvert et al. analyzed drugs that induce gene expression changes similar to those associated with DR and identified 11 small molecules with this property 23. Interestingly, among five drugs tested, four – rapamycin (administered at 10 μM), allantoin (250 μM), trichostatin A (100 μM), and LY-294002 (100 μM) – provoked increased lifespan and healthspan in wild-type (WT) C. elegans. Conversely, no longevity effects were observed in the eat-2 mutant background, a genetic DR model, suggesting that the life-extending effects of these drugs may indeed occur via DR-related mechanisms 23.
A study by Alavez et al. reported that amyloid-binding compounds maintain protein homeostasis and extend lifespan in C. elegans 24. Exposure of WT worms to the amyloid-binding dye Thioflavin T (ThT) at either 50 or 100 μM throughout adulthood increased median lifespan by 60% and maximal lifespan by 43–78% 24. ThT treatment reduced Aβ-aggregation and preserved muscle integrity in C. elegans models of Alzheimer’s disease (AD), resulting in a decreased proportion of paralyzed worms. ThT administration also suppressed the toxicity associated with metastable proteins in mutant worms 24. ThT-mediated suppression of protein aggregation and lifespan extension depended upon molecular chaperones, autophagy, proteosomal function, the proteostasis regulator heat shock factor 1 (HSF-1), and the stress resistance and longevity transcription factor SKN-1 24. Compounds with structural similarity to ThT also extended worm lifespan by up to 40%, but at significantly lower concentrations than ThT. Moreover, exposure to other protein-aggregate-binding compounds like curcumin (100 μM) and rifampicin (10–100 μM) extended worm lifespan by up to 45% 24. These results highlight the importance of proteostasis in worm healthspan and lifespan, and provide further impetus for the development of interventions capable of maintaining proteostasis to suppress aging and age-related diseases.
The National Institute of Aging has recently sponsored a pharmacological intervention program using Caenorhabditis as a model system, analogous to similar ongoing efforts in the mouse. The Caenorhabditis Intervention Testing Program (CITP) is a multi-institutional effort aimed at identifying compounds with the ability to extend lifespan and enhance healthspan, using multiple Caenorhabditis species and multiple strains of C. elegans. The identification of compounds that are effective in genetically diverse worm populations may accelerate the discovery of interventions that can extend lifespan/healthspan in other species, potentially including humans.
The fruit fly Drosophila melanogaster represents another model suitable for the screening of anti-aging compounds 25. A wide variety of genetic strains of D. melanogaster are available, with different mean lifespans, useful for validation of compound efficacy across multiple genetic backgrounds. Similar to C. elegans, Drosophila has a short lifespan, and the many genetic tools available in this organism facilitate mechanistic study of lead compounds 25. The first study reporting lifespan extension in Drosophila by administration of a drug was performed by Kang et al., who showed that feeding Drosophila 4-phenylbutyrate at 5–10 mM – a drug with multiple activities, including histone deacetylase inhibition – significantly increased both median and maximum lifespan without negative impacts on locomotion, stress resistance, or reproduction 26. A more recent study described the screening of protein kinase inhibitors for effects on Drosophila lifespan 27. Among the 80 inhibitors tested in this study, 17 significantly increased Drosophila lifespan without affecting food intake or consumption, indicating that the effects of these inhibitors on Drosophila lifespan do not involve DR 27. In this regard, a recent study by Slack et al. reported that attenuation of RAS-Erk-ETS signaling results in reduced IIS and provokes lifespan extension in Drosophila 28. Trametinib (1.56–15.6 μM), a highly specific MEK inhibitor that attenuates signaling downstream of RAS, can prolong median lifespan of female Drosophila by up to 12% (p=1.92 × 10 -10), and at higher doses (156 μM), improves late-life survival 28. Trametinib administration was effective in promoting fly longevity even when administered to middle-aged animals. These and similar findings with other drugs – cf. extension of mouse lifespan by rapamycin treatment initiated in middle age, see below – raise the possibility that anti-aging medicines in humans might be effective even when administered to older individuals, thus avoiding potential developmental side effects of these drugs.
Compounds that modulate aging and age-associated phenotypes in mammals
The mTOR inhibitor rapamycin
mTOR is a conserved serine/threonine kinase that senses and responds to nutrient availability, growth factors, and environmental stress and plays a key role in triggering growth 6, 29. In multicellular eukaryotes, mTOR exists in two distinct multi-protein complexes, mTORC1 and mTORC2, distinguished by their association with regulatory-associated protein of mTOR (RAPTOR) and rapamycin-insensitive companion of mTOR (RICTOR), respectively 30, 31. Rapamycin forms a complex with the FKBP12 protein, which binds to mTORC1 and inhibits its activity 32. Importantly, chronic treatment with rapamycin also inhibits mTORC2 33. mTORC1 activity is regulated by nutrients (glucose and amino acids), cytokines, hormones (insulin or IGF1), energy (ATP levels), and oxidative stress via PI3K, AKT, and AMPK signaling 6. Key downstream mediators of mTORC1 signaling are pathways that control cell growth, proliferation, stress response, and autophagy 29, 34. mTORC1, therefore, critically integrates cellular growth and maintenance with nutrient availability, hormonal cues, and other environmental stimuli.
A number of studies have established a link between mTOR signaling pathways and longevity in organisms ranging from yeast to mammals. Inhibition of mTOR signaling by genetic or pharmacologic means extends lifespan in yeast 35– 37, nematodes 38, 39, fruit flies 40, and mice 33, 41– 47. Likewise, genetic deletion in mice of the downstream mTORC1 effector, S6 kinase 1, increases oxidative metabolism, protects against age- and diet-induced obesity, and increases female lifespan 47, 48. Consistently, enhanced activity of the mTORC1 target 4E-BP1 in skeletal muscle results in increased oxidative metabolism and protects mice from diet- and age-induced metabolic dysfunction 49.
In a landmark study, NIA’s Interventions Testing Program (ITP) showed that treatment of a genetically heterogeneous mouse stock with the mTOR inhibitor rapamycin (administered at 14 mg/kg food; 2.24 mg/kg body weight/day) initiated at either 9 months or 20 months of age extended lifespan in both sexes 43, 50. A follow-up study demonstrated that the increase in mouse lifespan induced by rapamycin is dose and sex dependent. At a given chow concentration of rapamycin, female mice showed a greater increase in lifespan than did males, which correlated with higher blood levels of rapamycin achieved in females relative to males 51. Rapamycin treatment induced entirely distinct gene expression changes in males and females, implying the existence of sex-specific responses to mTOR inhibition 51. Furthermore, the expression patterns of xenobiotic-metabolizing enzymes in the livers of rapamycin-treated (14 mg/kg food) mice differed strikingly from those in DR-exposed animals at 12 months of age 51. Indeed, DR is less effective in lifespan extension when initiated later in life 52– 54, while rapamycin treatment extends the lifespan of mice, even when started in middle age 43, 55. Crucially, rapamycin-induced lifespan extension in mice has also been observed in diverse genetic backgrounds 41, 42, 44, 56.
Mechanisms of longevity extension by rapamycin remain a hotly debated topic in aging biology 56, 57. Rapamycin has anti-neoplastic properties 58– 60, and cancer is the major cause of death in most mouse strains that show rapamycin-mediated lifespan extension 43, 61. In this context, one plausible explanation for the extension of mouse lifespan by rapamycin is that this drug suppresses the onset and/or aggressiveness of lethal cancers. However, some investigators have reported that rapamycin also inhibits age-associated phenotypes besides neoplasia 62, 63, strongly suggesting that this drug has broader anti-aging effects. In contrast, a recent exhaustive study by Neff et al. claimed that the effects of rapamycin on aging phenotypes per se were quite limited 56. In this regard, conflicting observations have been made concerning the effects of rapamycin treatment in mouse models of AD 64. Long-term rapamycin treatment led to behavioral improvements in mouse AD models and induced an autophagy-mediated decrease in Aβ and hyperphosphorylated tau levels 65, 66. Conversely, rapamycin has been shown to promote Aβ production 67, 68 and led to an increase in Aβ-induced cell death 69.
Rapamycin has significant side effects – metabolic dysfunction, cataract, and testicular atrophy in particular – that may limit its long-term utility as an anti-aging treatment in humans 70, 71. Most importantly, due to the immunomodulatory effects of mTOR inhibitors, treatment of human patients with the rapamycin-like drug everolimus/RAD001 is associated with a higher incidence of infection in individuals with diseases such as cancer 72, 73 and tuberous sclerosis complex (TSC) 74. Conversely, a recent study showed that short-term administration of everolimus/RAD001 to healthy older individuals enhanced the immunological response to influenza vaccination, with modest side effects 75. Decreased influenza vaccine response is a major clinical challenge in older populations 76. These findings suggest that intermittent or short-term administration of rapamycin or other mTOR inhibitors might suppress certain functionally important effects of aging, such as poor immunization response, while avoiding the negative consequences associated with chronic use of these agents. A recent study in mice is consistent with this view, identifying an intermittent rapamycin administration regimen in mice that minimizes metabolic dysfunction, while maintaining chronic mTORC1 suppression in adipose tissue, though not in other tissues 77. It will be of great interest to evaluate the effects of such intermittent dosing regimens on a wide range of age-associated phenotypes and on lifespan.
Metformin and other biguanides
Metformin, an oral biguanide antiglycemic agent, is the most widely used drug in the treatment of metabolic syndrome and T2D. Metformin’s mechanism of action is not completely understood and is likely to be multi-factorial. It was reported to decrease serum glucose levels by inhibiting respiratory chain Complex I in hepatocytes 78, resulting in reduced ATP production, leading to activation of the LKB1 and AMPK kinases, suppressing hepatic gluconeogenesis 79, 80. Metformin has been reported to activate AMPK in many other tissues, including adipose, skeletal muscle, heart, pancreatic β-cells, and hypothalamus with potential beneficial physiological effects in patients with T2D 81, 82. However, metformin also exerts important effects independent of AMPK and LKB1 83, e.g. by antagonizing the action of glucagon 84. Recently, another AMPK-independent mechanism has been revealed for metformin. A study by Madiraju et al. showed that metformin non-competitively inhibits the redox shuttle enzyme mitochondrial glycerophosphate dehydrogenase, increasing the cytosolic redox state and decreasing the mitochondrial redox state 85. This suppresses hepatic gluconeogenesis by reducing the conversion of lactate and glycerol to glucose 85. Although metformin is currently approved for treatment of T2D, a large literature suggests efficacy of metformin against other conditions, particularly cardiovascular diseases and cancer 78. In this regard, a recent study demonstrated that metformin reduces tumorigenesis by inhibiting mitochondrial Complex I in cancer cells 86.
AMPK activation provokes longevity in flies and worms 87, 88. A number of studies suggest that metformin treatment can recapitulate some effects of DR. In this context, several studies have examined the effects of metformin and other biguanides on lifespan and reported a variety of outcomes. Metformin and other biguanides extend C. elegans lifespan in a dose-dependent manner 89– 91. The increase in C. elegans lifespan by metformin is mediated through inhibition of bacterial folate and methionine metabolism, which in turn alters methionine metabolism in the worm, resulting in reduced S-adenosylmethionine and increased S-adenosylhomocysteine levels 89. However, metformin apparently does not extend longevity in D. melanogaster 92, 93. Indeed, despite robust activation of AMPK, high doses of metformin actually decrease lifespan of both male and female flies 93, perhaps due to disruption of intestinal fluid homeostasis 93. However, metformin treatment suppressed age-related phenotypes in intestinal midgut stem cells 94 and also exerted beneficial effects in a fly obesity model 95. A recent study showed that metformin treatment causes a significant extension in mean and maximal lifespan in both sexes of the cricket Acheta domesticus 96.
Several studies have been performed in rodents to test the effects of metformin and other biguanides on lifespan; the outcomes have varied with genotype, sex, and dose and duration of treatment 97. Chronic treatment with metformin (100 mg/kg in the drinking water) enhanced the mean lifespan of cancer-prone HER-2/neu transgenic, outbred SHR, and inbred 129/Sv female mice by 8% (p<0.05), 37.8% (p<0.01), and 4.4% (p<0.05), respectively 98– 100. Metformin treatment also extended the maximum lifespan of HER-2/neu transgenic and outbred SHR female mice by 9% and 10.3%, respectively, while no effect was observed on maximal lifespan in inbred 129/Sv female mice 98– 100. Conversely, treatment of inbred 129/Sv male mice with a similar dose of metformin actually reduced mean lifespan by 13.4% 100. However, metformin treatment (2 mg/mL in drinking water) in a transgenic mouse model of Huntington disease (HD) prolonged male mean lifespan by 20.1% (p=0.017), but did not affect female survival 101. It has been reported that metformin treatment (100 mg/kg in the drinking water) of female outbred SHR mice initiated at 3 months of age induced a trend towards increased mean lifespan 102. Metformin treatment also postponed the onset of detectable tumors when started at young or middle ages, but not at old age 102. Neonatal metformin treatment of 129/Sv mice (100 mg/kg via subcutaneous injection) led to a 20% (p<0.001) increase in male mean lifespan and also slightly increased maximum lifespan by 3.5% 103. However, in females, the mean and maximum lifespan in metformin-treated groups were decreased by 9.1% and 3.8%, respectively 103. In a recent study by Martin-Montalvo et al., male C57BL/6 mice supplemented with 0.1% metformin in the diet showed a 5.8% increase in mean lifespan (p=0.02, Gehan–Breslow survival test), whereas supplementation with 1% metformin was toxic and reduced mean lifespan by 14.4% 104. However, supplementation of B6C3F1 male mice with 0.1% metformin resulted in extension of mean lifespan only by 4.2% (p=0.064, Gehan–Breslow) 104. Treatment with another biguanide, phenformin (2 mg/mouse in 0.2 mL of drinking water), significantly reduced spontaneous tumor development in female C3H/Sn mice and prolonged mean lifespan by 21% or more (p<0.05) 105, 106 and maximum lifespan by 26% 105. Evaluation of the lifespan effects of metformin in mice by the ITP consortium is ongoing, and the results should be available soon.
In rats, buformin treatment (5 mg/rat in 1 mL of drinking water) led to a non-significant 7.3% increase in mean lifespan of female LIO animals, while phenformin (5 mg/rat in 1 mL of drinking water) had no effect 105. However, administration of both buformin and phenformin increased the maximum lifespan of female LIO rats by 5.5% and 9.8%, respectively 105. Treatment with metformin (300 mg/kg/day) did not increase either mean or maximum lifespan of male F344 rats 107. However, in the same report, a parallel group of male F344 rats exposed to DR also failed to exhibit lifespan extension 107, leaving the metformin results in this study somewhat inconclusive. Mechanistically, treatment with metformin has been proposed to mimic some effects of DR, in particular by increasing AMPK activity and also activating antioxidant responses, leading to a reduction in both oxidative damage accumulation and chronic inflammation 104.
Although no study has formally analyzed the effects of long-term metformin treatment on lifespan in healthy humans, randomized clinical trials of metformin showed beneficial effects on health and survival in overweight/obese patients with T2D, as shown by decreased incidences of cardiovascular disease and cancer and reduced overall mortality 108– 110. However, when combined with sulfonylurea, metformin increased the risk of diabetes-related death and all-cause mortality in a mixed group of non-overweight and overweight/obese individuals with T2D 78, 108. Consistent with these observations, a recent study by Bannister et al. reported that patients with T2D treated with metformin displayed improved survival compared to matched, non-diabetic controls, whereas those treated with sulfonylureas showed reduced survival 111.
Given the relatively promising rodent data, the hints that metformin might suppress cancer and other age-associated conditions in humans, and metformin’s relatively benign safety profile, there is great current interest in formally testing the ability of this drug to delay age-associated disease in humans 112. Indeed, the US Food and Drug Administration (FDA) recently approved a study termed Targeting Aging With Metformin (TAME) for the evaluation of metformin as an anti-aging drug. The TAME project will involve approximately 3000 participants between the ages of 70 years and 80 years who either already have one, two, or all three of the conditions: cancer, heart disease, or cognitive impairment or are at risk of developing them. The trial will take place at roughly 15 centers around the United States over 5–7 years, costing approximately $50 million 113. The goal of the study is to determine whether metformin can prevent the onset of age-associated disease. This landmark trial will represent the first testing of a candidate anti-aging compound in humans.
Resveratrol and other sirtuin-activating compounds
The sirtuins are a family of NAD +-dependent deacetylases/ADP-ribosyltransferases/deacylases implicated in regulating nutrient responses and numerous other aspects of cell biology 8. Overexpression of Sir2, the founding member of the sirtuin family, extends replicative lifespan in the budding yeast Saccharomyces cerevisiae by repressing the accumulation of extrachromosomal rDNA plasmids, promoting segregation of an undamaged proteome to the daughter cell, enforcing subtelomeric silencing, and perhaps other mechanisms 114, 115. Several, though not all, investigators have found that overexpression of sirtuins in worms and flies modestly increases lifespan in these organisms 116– 123. Interestingly, the Sir2 homolog Sir-2.1 can extend C. elegans lifespan in a manner independent of its deacetylase activity 116. Indeed, nicotinamide (NAM), a product of sirtuin activity, and its metabolite, 1-methylnicotinamide (MNA), are capable of extending worm lifespan, potentially by inducing transient ROS signaling 116. In mammals, SIRT1 is the closest Sir2 homolog; overexpression of this protein in the brain (but not the whole organism) extends lifespan 124, probably by enhancing hypothalamic function during aging 125. Global overexpression of another sirtuin, SIRT6, extends mouse lifespan in males specifically, at least in part via suppression of lung cancer, a major cause of death in males of the mouse stock used 126, 127. SIRT2 overexpression stabilizes levels of the mitotic checkpoint protein BubR1 in progeroid BubR1 H/H mice and extends both median and maximum lifespan in male mice of this strain 128. No information is available concerning the potential effects of chronic SIRT2 overexpression in WT animals. Accumulating evidence suggests that NAD + levels may decline during aging, impairing sirtuin activity, and that the ability of sirtuin overexpression to increase lifespan partially counters this effect by maintaining sirtuin function in the face of a diminished NAD + pool in older organisms 129.
Resveratrol and certain other polyphenols were originally identified as Sir2/SIRT1 activators that extended the average and maximal lifespan of yeast 130. It is important to note that resveratrol is a highly promiscuous drug and exerts functionally important effects on many cellular targets 131. Treatment of worms and flies with resveratrol (dosed at 100 μM in worms and 10–100 μM in flies) has also been reported to extend lifespan, dependent on the presence of functional Sir-2.1 and dSir2, respectively 132. However, a study by Bass et al. claimed that resveratrol treatment (1–1000 μM) had no significant effects on Drosophila lifespan 133. The same study also reported that resveratrol treatment at 100 μM induced only a slight and sporadic increase in C. elegans lifespan in both WT and sir-2.1 mutant animals, suggesting that these small increases in C. elegans lifespan induced by resveratrol may be Sir-2.1 independent 133. Resveratrol protects worms from oxidative stress, radiation-induced damage, and amyloid toxicity 134– 136 and also induces radioprotection in flies 137. Resveratrol treatment increases mean and maximum lifespan in the honeybee 138 and the short-lived fishes Nothobranchius furzeri and Nothobranchius guentheri 139– 141.
It was reported that resveratrol and other sirtuin-activating compounds (STACs) activate Sir2/SIRT1 allosterically 130. However, other groups have found that these compounds were unable to enhance SIRT1 activity towards native peptides in vitro 142, 143. In this context, it has been suggested that increased SIRT1 activity induced by resveratrol depends on the presence of a non-native fluorophore conjugated to the peptide sequence originally used in screening for SIRT1 activators 142, 143. Recent reports, however, have shown that resveratrol and other STACs directly bind to SIRT1 and allosterically enhance its deacetylase activity towards non-tagged peptide substrates 144, 145. Resveratrol has also been reported to inhibit the catalytic activity of human tyrosyl transfer-RNA (tRNA) synthetase (TyrRS), resulting in its nuclear translocation and stimulation of NAD +-dependent activation of poly (ADP-ribose) polymerase 1 (PARP1) 146. PARP1 plays important roles in both DNA repair and transcription 147.
In mice, resveratrol is protective against some damaging effects of high-fat/high-calorie diets 148– 151, substantially reduces the growth and development of multiple types of cancers 152– 154, and delays or prevents the onset of AD 155, 156. Moreover, in rodents and humans, resveratrol is protective against both type 1 diabetes and T2D 157, 158 and cardiovascular disease 159 and possesses anti-inflammatory 160 and anti-viral activities 161. Resveratrol supplementation (either at 0.016–0.1% of diet or 25 mg/kg/day) has been reported to increase lifespan in mouse models of obesity 148, AD 162, HD 163, and amyotrophic lateral sclerosis 164, 165. Resveratrol treatment (2–8 mg/kg/day) increases the lifespan of LPS-treated mice 166 and attenuates catecholamine-induced mortality in obese rats (20 mg/kg/day) 167. Furthermore, resveratrol (10 mg/mL, intraperitoneal injection) prolongs survival in a mouse model of sepsis-induced acute kidney injury and restores renal microcirculation 168. Resveratrol administration (18 mg/kg/day in the diet) also improves survival in a rat hypertension model 169. Importantly, however, resveratrol treatment (100–1200 mg/kg food) does not increase lifespan in normal chow-fed mice 50, 170, 171. Resveratrol supplementation induces gene expression changes in several tissues that resemble those associated with calorie restriction in mice 171, 172.
In humans, 30-day resveratrol supplementation (150 mg/day) in obese men induced metabolic changes, including reductions in sleeping and resting metabolic rate, intrahepatic lipid content, circulating glucose levels, inflammatory markers, and systolic blood pressure 173. Skeletal muscle from resveratrol-treated objects displayed increased AMPK activity, increased SIRT1 and PGC-1α protein levels, and improved mitochondrial respiration of fatty acids 173. In contrast, 12 weeks’ supplementation with resveratrol (75 mg/day) in non-obese, postmenopausal women with normal glucose tolerance induced no apparent change in body composition, insulin sensitivity, resting metabolic rate, plasma lipids, or inflammatory markers 174. Moreover, resveratrol supplementation had no effect on its putative molecular targets, including AMPK, SIRT1, NAMPT, and PPARGC1A, in either skeletal muscle or adipose tissue 174.
An important recent study by Cai et al. demonstrated a non-linear dose response for the protective effects of resveratrol in humans and mice 175. When co-administered with high-fat diet (HFD), low-dose resveratrol (~0.07 mg/kg/day) appeared to be more efficacious than high-dose (14 mg/kg/day) in reducing adenoma number and decreasing overall tumor burden in Apc min mice, a model of intestinal carcinogenesis. Interestingly, female mice on the lower dose of resveratrol exhibited significantly higher expression and activation of AMPK in intestinal mucosa than those in the high-dose group 175. Consistently, human colorectal tissues exposed to low dietary concentrations (0.01 to 0.1 μM) of resveratrol ex vivo displayed rapid AMPK activation and increased autophagy at low concentrations and a less pronounced or even no effect at higher doses (1 to 10 μM) 175. This unusual effect may help rationalize the conflicting reports of resveratrol’s efficacies in humans, and future human studies using resveratrol must be designed with careful attention paid to dosage and serum levels and to a thorough assessment of effects on resveratrol’s putative molecular targets.
Other STACs have been synthesized and are reported to enhance healthspan and extend lifespan in mice. The STAC SRT1720 (100 mg/kg/day) has been reported to extend mean lifespan of adult male C57BL/6J mice fed a standard diet by 8.8% (p=0.096), and up to 21.7% (p=0.0193) on a HFD, without increasing maximal lifespan in either context 176, 177. SRT1720 treatment improved physiological parameters in HFD-fed animals, reducing liver steatosis, increasing insulin sensitivity, enhancing locomotor activity, and also inducing a gene expression profile similar to that associated with a standard diet 176. SRT1720 supplementation inhibited pro-inflammatory gene expression in liver and muscle of mice fed a standard chow diet and delayed the onset of age-related metabolic disease 177. Similarly, dietary supplementation (100 mg/kg) with SRT2104, another synthetic STAC, increased both mean and maximal lifespan of male C57BL/6J mice fed a chow diet by 9.7% (p<0.05) and 4.9% (p<0.001), respectively, and increased insulin sensitivity and motor coordination while reducing inflammation 178. Short-term treatment with SRT2104 preserves bone and muscle mass in an experimental atrophy model 178. These findings indicate that resveratrol and other STACs can exert beneficial effects on health, particularly in the context of HFD, and that some STACs can modestly extend lifespan under normal feeding conditions; however, additional studies are warranted to better evaluate their effects on longevity in females and other strains of mice. In this regard, there is great current interest in evaluating the effects of NAD + precursors as therapies for metabolic disease and candidate anti-aging drugs 129.
Other potential candidate anti-aging drugs
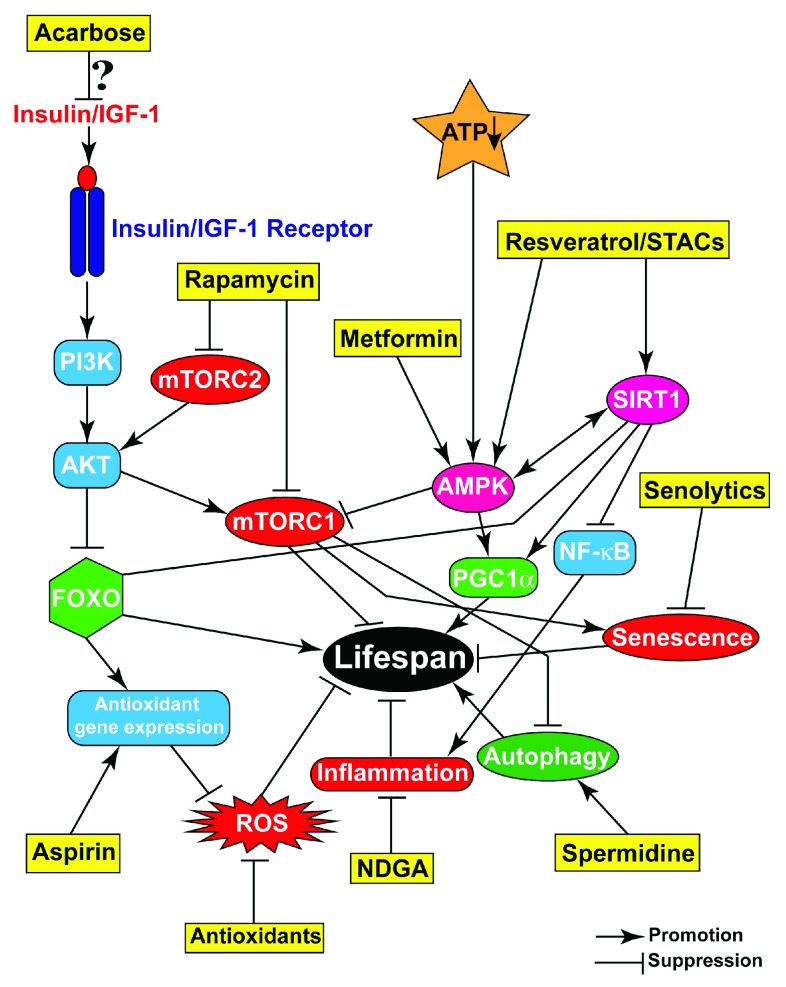
In recent decades, numerous compounds with pro-healthspan and -longevity effects have been identified. Due to space limitations, we restrict our discussion to a few key small molecules that have shown beneficial effects, from invertebrate models to mice ( Figure 2).
Figure 2. Pharmacological interventions targeting aging-related pathways and processes.
Representative compounds (yellow boxes) target various processes or pathways that contribute to aging and either promote or suppress their activities/progression, resulting in improved health and enhanced lifespan.
Spermidine is a member of the polyamine family, involved in numerous critical cellular processes including DNA stability, transcription, translation, apoptosis, cell proliferation, and cell growth 179. In multiple organs, levels of polyamines have been reported to decline with age 180, 181. Indeed, a study by Pucciarelli et al. suggested that maintaining high levels of spermidine during aging might promote longevity 182. Administration of exogenous spermidine extended the lifespan of yeast, flies, worms, and cultured human peripheral blood mononuclear cells 183. Spermidine also reduces the age-related decline of locomotor performance in flies 184. Furthermore, it has been reported that a polyamine-rich diet reduced age-related pathology and increased lifespan in Jcl:ICR male mice 185. Conversely, depletion of endogenous spermidine by genetic manipulation of the polyamine pathway shortens lifespan in yeast 183 and mice 186. Spermidine supplementation reduces levels of age-related oxidative damage in mice 183 and also increases stress resistance in yeast 183 and flies 187. The beneficial effects of spermidine are mediated mainly via induction of autophagy 183, 187, allowing the regulated degradation and recycling of dysfunctional cellular components 188. Defective autophagy prevented the onset of spermidine supplementation-associated benefits 183, 187.
Aspirin, a derivative of salicylic acid, is the prototypical cyclooxygenase inhibitor and non-steroidal anti-inflammatory agent 189. Aspirin is a versatile drug, with antithrombotic and antioxidant properties 190, 191. Indeed, chronic aspirin use in humans reduces the risk of mortality from a variety of age-associated diseases, including atherosclerosis, diabetes, and a variety of cancers 192– 196. Aspirin use has been reported to be associated with increased survival in extreme old age in humans 197. In a recent study by Ayyadevara et al., aspirin was shown to upregulate the expression of antioxidant genes (superoxide dismutase, catalases, and glutathione-S-transferases), resulting in attenuation of endogenous ROS levels and extension of C. elegans lifespan 198. Another study showed that aspirin treatment leads to lifespan extension in the cricket A. domesticus 96. In studies by the ITP, aspirin treatment (21 mg/kg diet) led to an increase in the mean lifespan of male mice, but there was no effect in females 199.
Nordihydroguaiaretic acid (NDGA), also known as masoprocol, is a naturally occurring dicatechol, with antioxidant, antiviral, antineoplastic, and anti-inflammatory activities 200. It has been reported to be a potent antagonist of the inflammatory cytokine TNFα. Dietary administration with NDGA delayed motor deterioration in a mouse model of amyotrophic lateral sclerosis and significantly extended lifespan 201. Consistently, the ITP reported that NDGA (2500 mg/kg diet) increased the lifespan of UM-HET3 male mice 199, 202. Lifespan extension by NDGA was not observed in female mice, even at a dose that produced blood levels equivalent to those in males 202. One possible explanation for this sex discrepancy could be that male controls in this study showed a somewhat short lifespan at two of the three ITP testing sites 202. Additional studies will be required to fully address this issue.
Acarbose is an inhibitor of α-glucosidases, intestinal enzymes that convert complex carbohydrates into simple sugars to facilitate their absorption 203. Acarbose treatment thus impairs carbohydrate digestion and inhibits the normal postprandial glucose rise 203. The ITP found that acarbose administration (1000 mg/kg diet) induced a significant increase in median and maximal lifespan in both sexes, although the impact was much more pronounced in males 202. Acarbose treatment increased male median lifespan by 22% (p<0.0001), but female median lifespan by only 5% (p=0.01). Similarly, maximum lifespan extension in males and females was 11% (p<0.001) and 9% (p=0.001), respectively 202. Acarbose-treated mice had a significant increase in levels of serum fibroblast growth factor 21 (FGF21) and also a mild reduction in IGF1 levels 202. FGF21 plays important roles in the regulation of glucose, lipid, and energy homeostasis 204. Transgenic mice with constitutive FGF21 secretion displayed an increase in both mean and maximal lifespan, probably occurring via reduced IIS 205, 206.
17-α-estradiol is a non-feminizing estrogen, with reduced binding affinity for estrogen receptors 202. It inhibits the activity of the enzyme 5α-reductase, responsible for the reduction of testosterone to the more potent androgen dihydrotestosterone 207, which has higher affinity for the androgen receptor than does testosterone 208. 17-α-estradiol has been reported to be neuroprotective against cerebral ischemia, Parkinson’s disease, and cerebrovascular disease 209– 211. Recently, it has been shown to diminish metabolic and inflammatory impairment in old male mice by reducing calorie intake and altering nutrient sensing and inflammatory pathways in visceral white adipose tissues, without inducing feminization 212. In ITP studies, administration of 17-α-estradiol (4.8 mg/kg diet) from 10 months of age increased male median lifespan by 12%, without significant effect on maximum lifespan or effects on female lifespan 202. Similar to NDGA, the relatively short lifespan of male controls might contribute to this apparent sex discrepancy 202 and further longevity studies are warranted using this drug.
β-adrenergic receptor (β-AR) antagonists bind to β-ARs (β1, 2, and 3-AR) and block the action of the endogenous catecholamines epinephrine and norepinephrine. Increased activity of β-ARs may hasten the development of age-related pathologies and increase mortality in genetically modified mice 213– 218. Consistently, chronic administration of β-AR agonists leads to increased mortality and morbidity 219. In humans, increased production of β2-AR due to specific genetic variants is associated with reduced lifespan 220. Conversely, dietary administration of β-AR blockers metoprolol (1.1 g/kg in the diet) and nebivolol (0.27 g/kg in the diet) increased the median lifespan of C3B6F1 male mice by 10% (p=0.016) and 6.4% (p=0.023), respectively, without affecting food intake or utilization 221. However, no effect was observed on maximal lifespan. Consistently, treatment with metoprolol (5 mg/mL diet) and nebivolol (100 μg/mL diet) extended the median lifespan of Drosophila by 23% (p≤0.0001) and 15% (p≤0.001), respectively, without impact on food intake or locomotion 221. Similar to β-AR blockers, an α1-AR antagonist, doxazosin mesylate, which inhibits the binding of norepinephrine to α1-AR on the membrane of vascular smooth muscle cells, extends C. elegans lifespan by 15% 222. Given that some of these agents are routinely administered clinically as antihypertensives and their safety profiles are well characterized, they may warrant further evaluation in humans specifically for their potential anti-aging effects.
Antioxidants, compounds conferring resistance to oxidative stress, have in some cases also proven successful in increasing lifespan, particularly in lower organisms. Dietary supplementation with the glutathione precursor N-acetylcysteine (NAC) increased resistance to oxidative stress, heat stress, and UV irradiation and significantly extended both the mean and the maximum lifespan of C. elegans 223 and D. melanogaster 224. Furthermore, treatment with EUK-134 and EUK-8, small molecule synthetic catalytic mimetics of superoxide dismutase (SOD) and catalase, was reported to extend C. elegans lifespan 225; however, as discussed by Gems and Doonan, other groups have not observed this effect 226. Treatment of a mixed group of male and female C57BL/6 mice with another SOD mimetic, carboxyfullerene (C3, at 10 mg/kg/day), reduced age-associated oxidative stress and mitochondrial superoxide production and modestly extended mean lifespan 227. Consistently, oral administration of carboxyfullerene (C60; 4 mg/kg/day) dissolved in olive oil to male Wistar rats leads to a 90% increase in median lifespan as compared to water-treated controls 228. Similarly, some other studies have shown an ability of antioxidants to extend lifespan in multiple organisms 229, 230.
Conversely, there are many reports that do not support the idea that dietary supplementation with antioxidants can increase the lifespan of healthy animals or humans as a general rule. Dietary supplementation with either vitamin E (α-tocopherol) or vitamin C (ascorbic acid) significantly shortened the lifespan of short-tailed field voles 231. Similarly, treatment of male mice with a nutraceutical mixture enriched in antioxidants was ineffective in extending lifespan 232. Moreover, as described in a recent review by Bjelakovic et al., systematic review and meta-analyses of a large number of randomized clinical trials evaluating the effects of dietary supplementation with various anti-oxidants (β-carotene, vitamin A, vitamin C, vitamin E, and selenium) in humans did not reveal any overall benefit; indeed, in some cases, there was evidence for increased mortality occurring in response to these agents 233. Deleterious effects of antioxidant supplementation may result from inappropriate suppression of the normal signaling functions ROS play in cells, including in crucial cell populations such as stem cells 234.
Selective deletion of senescent cells by senolytic drugs
Cellular senescence refers to permanent cellular growth arrest, which can be induced by multiple stressors, including serial passage, telomere attrition, inappropriate mitotic stimuli, and genotoxic insult 235. Senescence is thought to play an important role in tumor suppression in mammals 236, 237. However, senescent cells develop an altered secretory phenotype (termed the SASP) characterized by the release of factors such as proteases, growth factors, interleukins, chemokines, and extracellular remodeling proteins 238. With advancing age, senescent cells accumulate in various tissues 239– 241 and potentially contribute to pathological states, as factors they secrete induce chronic inflammation, loss of function in progenitor cells, and extracellular matrix dysfunction 236, 242. The functional impact of senescent cells in vivo has been a hotly debated topic in aging biology for many years. Recently, genetic approaches to delete senescent cells in mice have been described, via activation of a drug-inducible “suicide gene” 243. Depleting senescent cells in a progeroid mouse model substantially delayed the onset of multiple age-related phenotypes, including lordokyphosis (a measure of sarcopenia in this model), cataract, loss of adipose tissue, and impaired muscle function 243. However, the overall survival of these mice was not extended substantially by deletion of senescent cells, perhaps because the suicide gene was not expressed in the heart or aorta; cardiac failure is thought to represent a major cause of mortality in this strain 243. A recent landmark study by Baker et al. showed that clearance of naturally occurring senescence cells in non-progeroid mice maintained the functionality of several organs with age, delayed lethal tumorigenesis, and extended median lifespan in mixed and pure C57BL/6 genetic backgrounds by 27% (p<0.001) and 24% (p<0.001), respectively 244. This study provides very strong evidence that age-associated accumulation of senescent cells contributes to age-associated pathologies and shortens lifespan in WT animals.
Pharmacologic, as opposed to genetic, approaches to deplete senescent cells have posed a major technical and conceptual challenge. A recent study showed that senescent cells display increased expression of pro-survival factors, responsible for their well-known resistance to apoptosis 245. Interestingly, small interfering RNA (siRNA)-mediated silencing of many of these factors (ephrins, PI3Kδ, p21, BCL-xL, and others) selectively killed senescent cells but left dividing and quiescent cells unaffected. These siRNAs were termed “senolytic” siRNAs 245. Small molecules (senolytic drugs) targeting the same factors also selectively killed senescent cells. Out of 46 agents tested, dasatinib and quercetin were particularly effective in eliminating senescent cells. Dasatinib, used in cancer treatment, is an inhibitor of multiple tyrosine kinases 246. Quercetin is a natural flavonol that inhibits PI3K, other kinases, and serpins 247, 248. Dasatinib preferentially eliminated senescent human preadipocytes, while quercetin was more effective against senescent human endothelial cells and senescent bone marrow-derived murine mesenchymal stem cells (BM-MSCs). The combination of dasatinib and quercetin was effective in selective killing of senescent BM-MSCs, human preadipocytes, and endothelial cells 245. The combination was more effective in killing senescent mouse embryonic fibroblasts compared to either drug alone. Treatment of chronologically aged WT mice, radiation-exposed WT mice, and progeroid Ercc1 hypomorphic mice with the combination of dasatinib and quercetin reduced the burden of senescent cells. Following drug treatment, old WT mice showed improved cardiac function and carotid vascular reactivity, irradiated mice displayed improved exercise capacity, and progeroid Ercc1 -/Δ mutants demonstrated delay of age-related symptoms and pathologies 245. Similarly, a recent study by Chang et al. identified ABT263 (Navitoclax, a specific inhibitor of the anti-apoptotic proteins BCL-2 and BCL-xL) as another potent senolytic agent 249. ABT263, which is used for the treatment of multiple cancers 250– 252, induced apoptosis and selectively killed senescent cells in a manner independent of cell type or species 249. In culture, senescent human lung fibroblasts (IMR90), human renal epithelial cells, and mouse embryo fibroblasts (MEFs) were more sensitive to ABT263 treatment than their non-senescent counterparts 249. In contrast, another study found that ABT263 is not a broad-spectrum senolytic; instead it acts in a cell type-specific manner 253. In this study, ABT263 was found to be senolytic in human umbilical vein cells (HUVECs), IMR90 cells, and MEFs, but not in human primary preadipocytes 253.
Treatment of either irradiated or naturally aged mice with ABT263 not only reduced the burden of senescent cells, including those among bone marrow hematopoietic stem cell (HSC) and muscle stem cell (MuSC) populations, but also suppressed the expression of several SASP factors and rejuvenated the function of aged HSCs and MuSCs 249. These results, together with the impressive results obtained in genetic models described previously, indicate that senolytic drugs may have a role in improving tissue function during aging. However, some senolytic drugs are associated with toxic side effects, like thrombocytopenia and neutropenia in the case of ABT263, which are major potential hurdles in their use as anti-aging therapies. These toxicities may be mitigated somewhat if these drugs can be administered intermittently, rather than chronically, to achieve their senolytic effects.
Major results concerning the small molecules discussed in this review are summarized in Figure 2.
From model organisms to humans: the challenges of screening for anti-aging drugs
Several drugs have demonstrated great promise in the laboratory setting in enhancing the healthspan and lifespan of multiple species, including mice, raising the possibility that efficacious pharmacologic anti-aging therapy in people may be possible. However, screening for novel small molecules with anti-aging effects in mammals in an unbiased fashion represents an enormous, potentially insurmountable challenge. Alternatively, since it is clear that several cellular pathways affect longevity in an evolutionarily conserved manner, invertebrate models may be quite useful for such screening endeavors. However, some known molecular factors with major effects on mammalian lifespan ( e.g. GH) are not well conserved between invertebrates and mammals. Consequently, small molecule screening efforts relying exclusively on the use of invertebrates will likely miss drugs with potent effects on mammalian aging. Moreover, many of the key physiologic features of humans and other mammals are not well modeled in invertebrates, as the latter lack specific tissues like heart and kidney and complex endocrine, nervous, and circulatory systems that are crucial targets of mammalian aging and age-related pathologies. Most invertebrate aging models possess limited regenerative capabilities and incompletely recapitulate processes such as stem cell renewal, which are required for tissue repair mechanisms that maintain tissue homeostasis in mammals, in order to sustain organ function over years and decades.
The development of new, shorter-lived vertebrate aging systems could be tremendously beneficial in screening for drugs with anti-aging activities. In this context, several features of the naturally short-lived vertebrate African turquoise killfish ( N. furzeri) make this organism an attractive model system to study various aspects of vertebrate aging and potentially as a drug-screening system 254– 258. Recently, using a de novo-assembled genome and CRISPR/Cas9 technology, Harel et al. described a genotype-to-phenotype platform in N. furzeri, opening up the possibility of screening for gene mutations and drugs that increase lifespan in this organism in an integrative fashion 259. One current major limitation of N. furzeri is the need for individual housing in aging studies, greatly increasing husbandry costs. Moreover, it is possible that some of the factors modulating aging in fish and other cold-blooded vertebrates may be dissimilar to those in mammals.
Although mice faithfully recapitulate many aspects of human aging and age-associated diseases, their use in primary screening/testing of a large number of potential anti-aging compounds is not feasible because of the high associated costs. The use of progeroid models, such as Ercc1 hypomorphs or Lmna mutants, with accelerated pathology and short lifespan, might allow the evaluation of many more compounds than could be reasonably tested in WT mice 260, 261; however, whether or not such animals suffer from aging per se is a hotly debated topic 262, 263. Likewise, it is possible that rigorous delineation of appropriate surrogate markers of aging – e.g. increased p16 expression 264 or altered DNA methylation (DNAm) 265 – may allow initial evaluation of a large number of compounds in mice for potential anti-aging effects, without the need to perform costly and lengthy lifespan studies on many different cohorts, each treated with different candidate anti-aging compounds. In this regard, the Horvath group has developed an approach that allows estimation of the age of most tissues and cell types based on age-associated alterations in DNAm levels at 353 CpG sites 266. To the author’s knowledge, longevity screens using surrogate markers such as DNAm have not been attempted in mice.
To date, the discovery of anti-aging compounds has so far been carried out via two basic approaches. One of these is phenotypic, defined as the screening of compounds in cellular or animal models to identify drugs conferring desired biological effects, i.e. lifespan extension 267, 268. Although this approach has proven enormously valuable in many areas of biochemical research, identifying drugs that can modulate lifespan is more time consuming, complex, and expensive than for many other phenotypes 267, 268. Moreover, elucidating the mechanism of action of agents identified in such phenotypic, “black box” screens represents a formidable challenge, though the powerful genetic tools available in invertebrate models can facilitate such efforts. One currently underutilized system with respect to small molecule-based longevity screens is the budding yeast, S. cerevisiae. Two distinct forms of aging have been characterized in this organism, replicative and chronological (population based) 269. In principle, either might serve as the basis for screens for anti-aging compounds, though chronological aging is far more amenable to high-throughput analysis. A complementary approach involves target-based screening for modulators of pathways known or strongly suspected to modulate the aging rate 267. However, by definition, such efforts are unlikely to identify novel cellular factors and pathways involved in longevity.
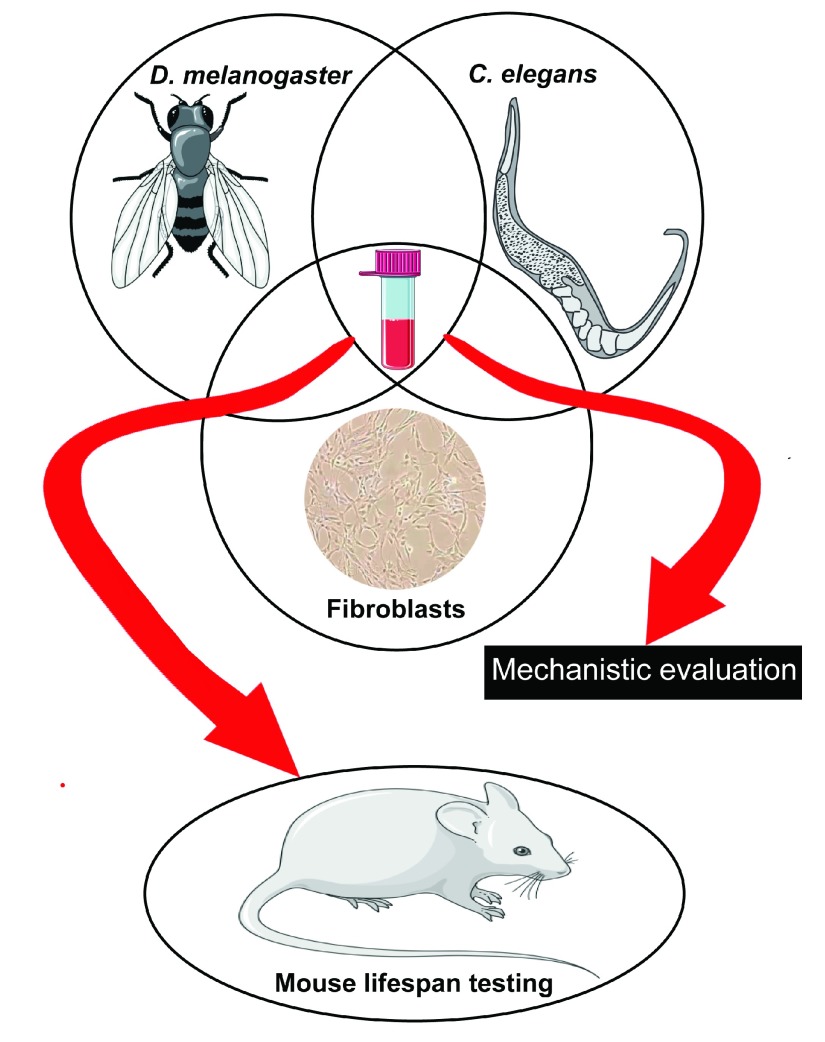
To address these complications, a holistic approach, involving complementary efforts in invertebrates, mammalian cells, and mice, might represent a powerful combination in the quest for anti-aging compounds. With the important caveats noted above, invertebrates can be efficiently used for primary screening of thousands of compounds to identify a few selected candidates with potential anti-aging effects for further testing in mice. In this context, in our Center ( http://www.med.umich.edu/geriatrics/research/glenn/), supported by the Glenn Foundation for Medical Research, compounds are screened for their ability to increase healthspan and lifespan in Drosophila and C. elegans and for enhancement of stress resistance in mammalian fibroblasts, a correlate of longevity in mammals 270. Compounds that are efficacious in all of these assays are candidates for more in-depth mechanistic evaluation and for further testing in mice ( Figure 3).
Figure 3. Approach being followed at University of Michigan for identification of compounds with potential anti-aging effects.
Drugs identified for their ability to increase healthspan and lifespan in Drosophila and Caenorhabditis elegans and to enhance stress resistance in mammalian fibroblasts are potential candidates for further in-depth mechanistic evaluation and testing in mice.
A related challenge in aging research at present is the lack of primate model systems with reasonably short lifespan for preclinical testing of candidate anti-aging drugs. The most commonly used model, the rhesus monkey, lives for three to four decades 20. Another primate, the common marmoset, has several advantages over rhesus monkeys in terms of size, availability, and other biological characteristics 271. Because of their small size, marmosets generally cost less to feed and house in comparison with the rhesus monkey. Furthermore, the marmoset has a gestation period of ~147 days and usually gives birth to 2–3 offspring per delivery. Some marmoset traits more closely resemble those of humans than do those of rhesus, including their disease susceptibility profile. In Europe, the marmoset is used as a non-rodent species for drug safety assessment and toxicology 271. In this regard, in a recent report, Tardif et al. described the dosing procedure, pharmacokinetics, and downstream signaling changes for rapamycin administration to marmosets 272. However, their maximal lifespan is ~17 years – shorter than the rhesus monkey, but still highly impractical for testing pharmacological interventions aimed at extending longevity. The development of new mammalian aging models besides the mouse would be extremely helpful to better elucidate the biological processes underlying mammalian aging and to expedite the translation of pharmacological interventions from the laboratory to actual clinical use in humans.
One model to consider in this regard is dogs, which share their social environment with humans 273. Furthermore, dogs are relatively well understood with regard to aging and disease, exhibit great heterogeneity in body size and lifespan, and provide a large pool of genetic diversity. Dogs might represent a relatively inexpensive model system, particularly if some dog owners were willing to test candidate lifespan-extending drugs that had previously been validated in invertebrate and rodent models. Indeed, identifying interventions that can promote healthspan and lifespan in dogs may represent an excellent entrée to achieving the same goals in humans. In this context, Matthew Kaeberlein and Daniel Promislow at the University of Washington in Seattle have launched a pilot trial involving 30 dogs aimed at testing the efficacy of rapamycin in improving overall health and extending lifespan in large dogs that usually survive for 8 to 10 years 274.
Testing candidate anti-aging compounds in humans represents an enormous challenge 112. It is highly unlikely that pharmaceutical companies can be persuaded to engage in decades-long clinical trials of candidate anti-aging medicines with lifespan as an endpoint. The evaluation of shorter-term surrogate phenotypes, such as molecular markers or age-associated defects such as impaired responses to vaccination 75, may permit initial clinical evaluation of candidate anti-aging compounds in a more reasonable timeframe.
Conclusion
Since ancient times, humanity has dreamed of interventions to slow the aging process and prolong lifespan. However, only in the modern era has biological aging research progressed to the point where interventions that delay human aging may eventually represent a real possibility. Accumulating work in invertebrate models and rodents has identified an ever-growing list of molecules with the ability to extend lifespan and promote late-life health in mammals. Given the intimate link between aging and disease, such drugs may dramatically improve human health if the major challenges in their testing and deployment can be overcome.
Acknowledgements
We thank Dr Richard A. Miller and the peer reviewers for critical comments on this manuscript and apologize to investigators whose work was not cited due to space limitations.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Dudley Lamming, Department of Medicine, University of Wisconsin-Madison, Madison, WI, USA
Joseph Baur, University of Pennsylvania, Philadelphia, PA, USA
Michael Petrascheck, The Scripps Research Institute, La Jolla, CA, USA
Funding Statement
Work in our laboratory is supported by the Glenn Foundation for Medical Research, National Institutes of Health grant R01GM101171 (DL), Department of Defense grant OC140123 (DL), the National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1TR000433, and the John S. and Suzanne C. Munn Cancer Fund of the University of Michigan Comprehensive Cancer Center. Some graphics in the figures were obtained and modified from Servier Medical Art from Servier ( http://www.servier.com/Powerpoint-image-bank).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. López-Otín C, Blasco MA, Partridge L, et al. : The hallmarks of aging. Cell. 2013;153(6):1194–217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Niccoli T, Partridge L: Ageing as a risk factor for disease. Curr Biol. 2012;22(17):R741–52. 10.1016/j.cub.2012.07.024 [DOI] [PubMed] [Google Scholar]
- 3. Fontana L, Partridge L, Longo VD: Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321–6. 10.1126/science.1172539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kenyon CJ: The genetics of ageing. Nature. 2010;464(7288):504–12. 10.1038/nature08980 [DOI] [PubMed] [Google Scholar]
- 5. Rincon M, Muzumdar R, Atzmon G, et al. : The paradox of the insulin/IGF-1 signaling pathway in longevity. Mech Ageing Dev. 2004;125(6):397–403. 10.1016/j.mad.2004.03.006 [DOI] [PubMed] [Google Scholar]
- 6. Caron A, Richard D, Laplante M: The Roles of mTOR Complexes in Lipid Metabolism. Annu Rev Nutr. 2015;35:321–48. 10.1146/annurev-nutr-071714-034355 [DOI] [PubMed] [Google Scholar]
- 7. Hardie DG, Ross FA, Hawley SA: AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–62. 10.1038/nrm3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giblin W, Lombard DB: Sirtuins, healthspan, and longevity in mammals. London: Elsevier;2015. [Google Scholar]
- 9. Speakman JR, Mitchell SE: Caloric restriction. Mol Aspects Med. 2011;32(3):159–221. 10.1016/j.mam.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 10. Fontana L, Partridge L: Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161(1):106–18. 10.1016/j.cell.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anselmi CV, Malovini A, Roncarati R, et al. : Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 2009;12(2):95–104. 10.1089/rej.2008.0827 [DOI] [PubMed] [Google Scholar]
- 12. Flachsbart F, Caliebe A, Kleindorp R, et al. : Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009;106(8):2700–5. 10.1073/pnas.0809594106 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Li Y, Wang WJ, Cao H, et al. : Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum Mol Genet. 2009;18(24):4897–904. 10.1093/hmg/ddp459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pawlikowska L, Hu D, Huntsman S, et al. : Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8(4):460–72. 10.1111/j.1474-9726.2009.00493.x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Soerensen M, Dato S, Christensen K, et al. : Replication of an association of variation in the FOXO3A gene with human longevity using both case-control and longitudinal data. Aging Cell. 2010;9(6):1010–7. 10.1111/j.1474-9726.2010.00627.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Willcox BJ, Donlon TA, He Q, et al. : FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105(37):13987–92. 10.1073/pnas.0801030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. : Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3(70):70ra13. 10.1126/scitranslmed.3001845 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Ingram DK, Roth GS: Calorie restriction mimetics: can you have your cake and eat it, too? Ageing Res Rev. 2015;20:46–62. 10.1016/j.arr.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 19. Haigis MC, Yankner BA: The aging stress response. Mol Cell. 2010;40(2):333–44. 10.1016/j.molcel.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kennedy BK, Berger SL, Brunet A, et al. : Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–13. 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Petrascheck M, Ye X, Buck LB: An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature. 2007;450(7169):553–6. 10.1038/nature05991 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Evason K, Huang C, Yamben I, et al. : Anticonvulsant medications extend worm life-span. Science. 2005;307(5707):258–62. 10.1126/science.1105299 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Calvert S, Tacutu R, Sharifi S, et al. : A network pharmacology approach reveals new candidate caloric restriction mimetics in C. elegans. Aging Cell. 2016;15(2):256–66. 10.1111/acel.12432 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Alavez S, Vantipalli MC, Zucker DJ, et al. : Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature. 2011;472(7342):226–9. 10.1038/nature09873 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Jafari M: Drosophila melanogaster as a model system for the evaluation of anti-aging compounds. Fly (Austin). 2010;4(3):253–7. 10.4161/fly.4.3.11997 [DOI] [PubMed] [Google Scholar]
- 26. Kang HL, Benzer S, Min KT: Life extension in Drosophila by feeding a drug. Proc Natl Acad Sci U S A. 2002;99(2):838–43. 10.1073/pnas.022631999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spindler SR, Li R, Dhahbi JM, et al. : Novel protein kinase signaling systems regulating lifespan identified by small molecule library screening using Drosophila. PLoS One. 2012;7(2):e29782. 10.1371/journal.pone.0029782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slack C, Alic N, Foley A, et al. : The Ras-Erk-ETS-Signaling Pathway Is a Drug Target for Longevity. Cell. 2015;162(1):72–83. 10.1016/j.cell.2015.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Johnson SC, Sangesland M, Kaeberlein M, et al. : Modulating mTOR in aging and health. Interdiscip Top Gerontol. 2015;40:107–27. 10.1159/000364974 [DOI] [PubMed] [Google Scholar]
- 30. Hara K, Maruki Y, Long X, et al. : Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110(2):177–89. 10.1016/S0092-8674(02)00833-4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Sarbassov DD, Ali SM, Kim DH, et al. : Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–302. 10.1016/j.cub.2004.06.054 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Yip CK, Murata K, Walz T, et al. : Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol Cell. 2010;38(5):768–74. 10.1016/j.molcel.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Lamming DW, Ye L, Katajisto P, et al. : Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–43. 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Pan Y, Nishida Y, Wang M, et al. : Metabolic regulation, mitochondria and the life-prolonging effect of rapamycin: a mini-review. Gerontology. 2012;58(6):524–30. 10.1159/000342204 [DOI] [PubMed] [Google Scholar]
- 35. Fabrizio P, Pozza F, Pletcher SD, et al. : Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292(5515):288–90. 10.1126/science.1059497 [DOI] [PubMed] [Google Scholar]
- 36. Powers RW, 3rd, Kaeberlein M, Caldwell SD, et al. : Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20(2):174–84. 10.1101/gad.1381406 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Kaeberlein M, Powers RW, 3rd, Steffen KK, et al. : Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310(5751):1193–6. 10.1126/science.1115535 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Vellai T, Takacs-Vellai K, Zhang Y, et al. : Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426(6967):620. 10.1038/426620a [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Jia K, Chen D, Riddle DL: The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131(16):3897–906. 10.1242/dev.01255 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Kapahi P, Zid BM, Harper T, et al. : Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14(10):885–90. 10.1016/j.cub.2004.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Bokov A, Gelfond J, et al. : Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014;69(2):119–30. 10.1093/gerona/glt056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fok WC, Chen Y, Bokov A, et al. : Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS One. 2014;9(1):e83988. 10.1371/journal.pone.0083988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harrison DE, Strong R, Sharp ZD, et al. : Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–5. 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Anisimov VN, Zabezhinski MA, Popovich IG, et al. : Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10(24):4230–6. 10.4161/cc.10.24.18486 [DOI] [PubMed] [Google Scholar]
- 45. Anisimov VN, Zabezhinski MA, Popovich IG, et al. : Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176(5):2092–7. 10.2353/ajpath.2010.091050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu JJ, Liu J, Chen EB, et al. : Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013;4(5):913–20. 10.1016/j.celrep.2013.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Selman C, Tullet JM, Wieser D, et al. : Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326(5949):140–4. 10.1126/science.1177221 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Um SH, Frigerio F, Watanabe M, et al. : Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431(7005):200–5. 10.1038/nature02866 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Tsai S, Sitzmann JM, Dastidar SG, et al. : Muscle-specific 4E-BP1 signaling activation improves metabolic parameters during aging and obesity. J Clin Invest. 2015;125(8):2952–64. 10.1172/JCI77361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miller RA, Harrison DE, Astle CM, et al. : Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66(2):191–201. 10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Miller RA, Harrison DE, Astle CM, et al. : Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13(3):468–77. 10.1111/acel.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Weindruch R, Walford RL: Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215(4538):1415–8. 10.1126/science.7063854 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Speakman JR, Hambly C: Starving for life: what animal studies can and cannot tell us about the use of caloric restriction to prolong human lifespan. J Nutr. 2007;137(4):1078–86. [DOI] [PubMed] [Google Scholar]
- 54. Leontieva OV, Paszkiewicz GM, Blagosklonny MV: Fasting levels of hepatic p-S6 are increased in old mice. Cell Cycle. 2014;13(7):2656–9. 10.4161/15384101.2014.949150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen C, Liu Y, Liu Y, et al. : mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2(98):ra75. 10.1126/scisignal.2000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Neff F, Flores-Dominguez D, Ryan DP, et al. : Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123(8):3272–91. 10.1172/JCI67674 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Richardson A: Rapamycin, anti-aging, and avoiding the fate of Tithonus. J Clin Invest. 2013;123(8):3204–6. 10.1172/JCI70800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garber K: Rapamycin's resurrection: a new way to target the cancer cell cycle. J Natl Cancer Inst. 2001;93(20):1517–9. 10.1093/jnci/93.20.1517 [DOI] [PubMed] [Google Scholar]
- 59. Hidalgo M, Rowinsky EK: The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene. 2000;19(56):6680–6. 10.1038/sj.onc.1204091 [DOI] [PubMed] [Google Scholar]
- 60. Kopelovich L, Fay JR, Sigman CC, et al. : The mammalian target of rapamycin pathway as a potential target for cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1330–40. 10.1158/1055-9965.EPI-07-0045 [DOI] [PubMed] [Google Scholar]
- 61. Blackwell BN, Bucci TJ, Hart RW, et al. : Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol Pathol. 1995;23(5):570–82. 10.1177/019262339502300503 [DOI] [PubMed] [Google Scholar]
- 62. Majumder S, Caccamo A, Medina DX, et al. : Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and enhancing NMDA signaling. Aging Cell. 2012;11(2):326–35. 10.1111/j.1474-9726.2011.00791.x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Wilkinson JE, Burmeister L, Brooks SV, et al. : Rapamycin slows aging in mice. Aging Cell. 2012;11(4):675–82. 10.1111/j.1474-9726.2012.00832.x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Santos RX, Correia SC, Cardoso S, et al. : Effects of rapamycin and TOR on aging and memory: implications for Alzheimer's disease. J Neurochem. 2011;117(6):927–36. 10.1111/j.1471-4159.2011.07262.x [DOI] [PubMed] [Google Scholar]
- 65. Caccamo A, Majumder S, Richardson A, et al. : Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285(17):13107–20. 10.1074/jbc.M110.100420 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Spilman P, Podlutskaya N, Hart MJ, et al. : Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5(4):e9979. 10.1371/journal.pone.0009979 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Zhang S, Salemi J, Hou H, et al. : Rapamycin promotes beta-amyloid production via ADAM-10 inhibition. Biochem Biophys Res Commun. 2010;398(3):337–41. 10.1016/j.bbrc.2010.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yu WH, Cuervo AM, Kumar A, et al. : Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol. 2005;171(1):87–98. 10.1083/jcb.200505082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lafay-Chebassier C, Pérault-Pochat MC, Page G, et al. : The immunosuppressant rapamycin exacerbates neurotoxicity of Abeta peptide. J Neurosci Res. 2006;84(6):1323–34. 10.1002/jnr.21039 [DOI] [PubMed] [Google Scholar]
- 70. Lamming DW: Inhibition of the Mechanistic Target of Rapamycin (mTOR) - Rapamycin and Beyond. Olshansky SJ, Martin GM, Kirkland JL, editors. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press;2016. Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Powell JD, Pollizzi KN, Heikamp EB, et al. : Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. 10.1146/annurev-immunol-020711-075024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Motzer RJ, Escudier B, Oudard S, et al. : Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–65. 10.1002/cncr.25219 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Beaver JA, Park BH: The BOLERO-2 trial: the addition of everolimus to exemestane in the treatment of postmenopausal hormone receptor-positive advanced breast cancer. Future Oncol. 2012;8(6):651–7. 10.2217/fon.12.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Trelinska J, Dachowska I, Kotulska K, et al. : Complications of mammalian target of rapamycin inhibitor anticancer treatment among patients with tuberous sclerosis complex are common and occasionally life-threatening. Anticancer Drugs. 2015;26(4):437–42. 10.1097/CAD.0000000000000207 [DOI] [PubMed] [Google Scholar]
- 75. Mannick JB, Del Giudice G, Lattanzi M, et al. : mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6(268):268ra179. 10.1126/scitranslmed.3009892 [DOI] [PubMed] [Google Scholar]
- 76. Lambert ND, Ovsyannikova IG, Pankratz VS, et al. : Understanding the immune response to seasonal influenza vaccination in older adults: a systems biology approach. Expert Rev Vaccines. 2012;11(8):985–94. 10.1586/erv.12.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Arriola Apelo SI, Neuman JC, Baar EL, et al. : Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell. 2016;15(1):28–38. 10.1111/acel.12405 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Pryor R, Cabreiro F: Repurposing metformin: an old drug with new tricks in its binding pockets. Biochem J. 2015;471(3):307–22. 10.1042/BJ20150497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhou G, Myers R, Li Y, et al. : Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74. 10.1172/JCI13505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shaw RJ, Lamia KA, Vasquez D, et al. : The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–6. 10.1126/science.1120781 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Lim CT, Kola B, Korbonits M: AMPK as a mediator of hormonal signalling. J Mol Endocrinol. 2010;44(2):87–97. 10.1677/JME-09-0063 [DOI] [PubMed] [Google Scholar]
- 82. Coughlan KA, Valentine RJ, Ruderman NB, et al. : AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. 2014;7:241–53. 10.2147/DMSO.S43731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Foretz M, Hébrard S, Leclerc J, et al. : Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120(7):2355–69. 10.1172/JCI40671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Miller RA, Chu Q, Xie J, et al. : Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494(7436):256–60. 10.1038/nature11808 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Madiraju AK, Erion DM, Rahimi Y, et al. : Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–6. 10.1038/nature13270 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Wheaton WW, Weinberg SE, Hamanaka RB, et al. : Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife. 2014;3:e02242. 10.7554/eLife.02242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mair W, Morantte I, Rodrigues AP, et al. : Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470(7334):404–8. 10.1038/nature09706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ulgherait M, Rana A, Rera M, et al. : AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014;8(6):1767–80. 10.1016/j.celrep.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cabreiro F, Au C, Leung K, et al. : Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153(1):228–39. 10.1016/j.cell.2013.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 90. Bakaev V: Effect of 1-butylbiguanide hydrochloride on the longevity in the nematoda Caenorhabditis elegans. Biogerontology. 2002;3:23–4. [Google Scholar]
- 91. Onken B, Driscoll M: Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5(1):e8758. 10.1371/journal.pone.0008758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jafari M, Khodayari B, Felgner J, et al. : Pioglitazone: an anti-diabetic compound with anti-aging properties. Biogerontology. 2007;8(6):639–51. 10.1007/s10522-007-9105-7 [DOI] [PubMed] [Google Scholar]
- 93. Slack C, Foley A, Partridge L: Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS One. 2012;7(10):e47699. 10.1371/journal.pone.0047699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Na HJ, Park JS, Pyo JH, et al. : Mechanism of metformin: inhibition of DNA damage and proliferative activity in Drosophila midgut stem cell. Mech Ageing Dev. 2013;134(9):381–90. 10.1016/j.mad.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 95. Shirazi F, Farmakiotis D, Yan Y, et al. : Diet modification and metformin have a beneficial effect in a fly model of obesity and mucormycosis. PLoS One. 2014;9(9):e108635. 10.1371/journal.pone.0108635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hans H, Lone A, Aksenov V, et al. : Impacts of metformin and aspirin on life history features and longevity of crickets: trade-offs versus cost-free life extension? Age (Dordr). 2015;37(2):31. 10.1007/s11357-015-9769-x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Anisimov VN: Metformin: do we finally have an anti-aging drug? Cell Cycle. 2013;12(22):3483–9. 10.4161/cc.26928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Anisimov VN, Berstein LM, Egormin PA, et al. : Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40(8–9):685–93. 10.1016/j.exger.2005.07.007 [DOI] [PubMed] [Google Scholar]
- 99. Anisimov VN, Berstein LM, Egormin PA, et al. : Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7(17):2769–73. 10.4161/cc.7.17.6625 [DOI] [PubMed] [Google Scholar]
- 100. Anisimov VN, Piskunova TS, Popovich IG, et al. : Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging (Albany NY). 2010;2(12):945–58. 10.18632/aging.100245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ma TC, Buescher JL, Oatis B, et al. : Metformin therapy in a transgenic mouse model of Huntington's disease. Neurosci Lett. 2007;411(2):98–103. 10.1016/j.neulet.2006.10.039 [DOI] [PubMed] [Google Scholar]
- 102. Anisimov VN, Berstein LM, Popovich IG, et al. : If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY). 2011;3(2):148–57. 10.18632/aging.100273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Anisimov VN, Popovich IG, Zabezhinski MA, et al. : Sex differences in aging, life span and spontaneous tumorigenesis in 129/Sv mice neonatally exposed to metformin. Cell Cycle. 2015;14(1):46–55. 10.4161/15384101.2014.973308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. : Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4: 2192. 10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. Anisimov VN, Semenchenko AV, Yashin AI: Insulin and longevity: antidiabetic biguanides as geroprotectors. Biogerontology. 2003;4(5):297–307. 10.1023/A:1026299318315 [DOI] [PubMed] [Google Scholar]
- 106. Dilman VM, Anisimov VN: Effect of treatment with phenformin, diphenylhydantoin or L-dopa on life span and tumour incidence in C3H/Sn mice. Gerontology. 1980;26(5):241–6. 10.1159/000212423 [DOI] [PubMed] [Google Scholar]
- 107. Smith DL, Jr, Elam CF, Jr, Mattison JA, et al. : Metformin supplementation and life span in Fischer-344 rats. J Gerontol A Biol Sci Med Sci. 2010;65(5):468–74. 10.1093/gerona/glq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. UKPDS: Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–65. 10.1016/S0140-6736(98)07037-8 [DOI] [PubMed] [Google Scholar]
- 109. Wu JW, Boudreau DM, Park Y, et al. : Commonly used diabetes and cardiovascular medications and cancer recurrence and cancer-specific mortality: a review of the literature. Expert Opin Drug Saf. 2014;13(8):1071–99. 10.1517/14740338.2014.926887 [DOI] [PubMed] [Google Scholar]
- 110. Holman RR, Paul SK, Bethel MA, et al. : 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 111. Bannister CA, Holden SE, Jenkins-Jones S, et al. : Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab. 2014;16(11):1165–73. 10.1111/dom.12354 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 112. Longo VD, Antebi A, Bartke A, et al. : Interventions to Slow Aging in Humans: Are We Ready? Aging Cell. 2015;14(4):497–510. 10.1111/acel.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Check Hayden E: Anti-ageing pill pushed as bona fide drug. Nature. 2015;522(7556):265–6. 10.1038/522265a [DOI] [PubMed] [Google Scholar]
- 114. Longo VD, Kennedy BK: Sirtuins in aging and age-related disease. Cell. 2006;126(2):257–68. 10.1016/j.cell.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 115. Kaeberlein M, McVey M, Guarente L: The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13(19):2570–80. 10.1101/gad.13.19.2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Schmeisser K, Mansfeld J, Kuhlow D, et al. : Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat Chem Biol. 2013;9(11):693–700. 10.1038/nchembio.1352 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 117. Rizki G, Iwata TN, Li J, et al. : The evolutionarily conserved longevity determinants HCF-1 and SIR-2.1/SIRT1 collaborate to regulate DAF-16/FOXO. PLoS Genet. 2011;7(9):e1002235. 10.1371/journal.pgen.1002235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Viswanathan M, Guarente L: Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature. 2011;477(7365):E1–2. 10.1038/nature10440 [DOI] [PubMed] [Google Scholar]
- 119. Mouchiroud L, Houtkooper RH, Moullan N, et al. : The NAD +/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154(2):430–41. 10.1016/j.cell.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 120. Tissenbaum HA, Guarente L: Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410(6825):227–30. 10.1038/35065638 [DOI] [PubMed] [Google Scholar]
- 121. Burnett C, Valentini S, Cabreiro F, et al. : Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477(7365):482–5. 10.1038/nature10296 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 122. Rogina B, Helfand SL: Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101(45):15998–6003. 10.1073/pnas.0404184101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rogina B, Helfand SL, Frankel S: Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science. 2002;298(5599):1745. 10.1126/science.1078986 [DOI] [PubMed] [Google Scholar]
- 124. Herranz D, Muñoz-Martin M, Cañamero M, et al. : Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1: 3. 10.1038/ncomms1001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 125. Satoh A, Brace CS, Rensing N, et al. : Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18(3):416–30. 10.1016/j.cmet.2013.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lombard DB, Miller RA: Ageing: Sorting out the sirtuins. Nature. 2012;483(7388):166–7. 10.1038/nature10950 [DOI] [PubMed] [Google Scholar]
- 127. Kanfi Y, Naiman S, Amir G, et al. : The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483(7388):218–21. 10.1038/nature10815 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 128. North BJ, Rosenberg MA, Jeganathan KB, et al. : SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. EMBO J. 2014;33(13):1438–53. 10.15252/embj.201386907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Imai S, Guarente L: NAD + and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464–71. 10.1016/j.tcb.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Howitz KT, Bitterman KJ, Cohen HY, et al. : Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–6. 10.1038/nature01960 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 131. Bhullar KS, Hubbard BP: Lifespan and healthspan extension by resveratrol. Biochim Biophys Acta. 2015;1852(6):1209–18. 10.1016/j.bbadis.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 132. Wood JG, Rogina B, Lavu S, et al. : Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–9. 10.1038/nature02789 [DOI] [PubMed] [Google Scholar]
- 133. Bass TM, Weinkove D, Houthoofd K, et al. : Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128(10):546–52. 10.1016/j.mad.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 134. Yin H, Si J, Xu H, et al. : Resveratrol-loaded nanoparticles reduce oxidative stress induced by radiation or amyloid-beta in transgenic Caenorhabditis elegans. J Biomed Nanotechnol. 2014;10(8):1536–44. 10.1166/jbn.2014.1897 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 135. Ye K, Ji CB, Lu XW, et al. : Resveratrol attenuates radiation damage in Caenorhabditis elegans by preventing oxidative stress. J Radiat Res. 2010;51(4):473–9. 10.1269/jrr.10009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 136. Chen W, Rezaizadehnajafi L, Wink M: Influence of resveratrol on oxidative stress resistance and life span in Caenorhabditis elegans. J Pharm Pharmacol. 2013;65(5):682–8. 10.1111/jphp.12023 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 137. Chang CL, Follett P: Resveratrol modifies tephritid fruit fly response to radiation but not nutritional stress. Int J Radiat Biol. 2012;88(4):320–6. 10.3109/09553002.2012.647234 [DOI] [PubMed] [Google Scholar]
- 138. Rascón B, Hubbard BP, Sinclair DA, et al. : The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging (Albany NY). 2012;4(7):499–508. 10.18632/aging.100474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yu X, Li G: Effects of resveratrol on longevity, cognitive ability and aging-related histological markers in the annual fish Nothobranchius guentheri. Exp Gerontol. 2012;47(12):940–9. 10.1016/j.exger.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 140. Valenzano DR, Terzibasi E, Genade T, et al. : Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16(3):296–300. 10.1016/j.cub.2005.12.038 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 141. Genade T, Lang DM: Resveratrol extends lifespan and preserves glia but not neurons of the Nothobranchius guentheri optic tectum. Exp Gerontol. 2013;48(2):202–12. 10.1016/j.exger.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 142. Kaeberlein M, McDonagh T, Heltweg B, et al. : Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280(17):17038–45. 10.1074/jbc.M500655200 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 143. Borra MT, Smith BC, Denu JM: Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280(17):17187–95. 10.1074/jbc.M501250200 [DOI] [PubMed] [Google Scholar]
- 144. Hubbard BP, Gomes AP, Dai H, et al. : Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339(6124):1216–9. 10.1126/science.1231097 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 145. Lakshminarasimhan M, Rauh D, Schutkowski M, et al. : Sirt1 activation by resveratrol is substrate sequence-selective. Aging (Albany NY). 2013;5(3):151–4. 10.18632/aging.100542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sajish M, Schimmel P: A human tRNA synthetase is a potent PARP1-activating effector target for resveratrol. Nature. 2015;519(7543):370–3. 10.1038/nature14028 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 147. Ko HL, Ren EC: Functional Aspects of PARP1 in DNA Repair and Transcription. Biomolecules. 2012;2(4):524–48. 10.3390/biom2040524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Baur JA, Pearson KJ, Price NL, et al. : Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–42. 10.1038/nature05354 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 149. Jimenez-Gomez Y, Mattison JA, Pearson KJ, et al. : Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013;18(4):533–45. 10.1016/j.cmet.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Mattison JA, Wang M, Bernier M, et al. : Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20(1):183–90. 10.1016/j.cmet.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Andrade JM, Paraíso AF, de Oliveira MV, et al. : Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30(7–8):915–9. 10.1016/j.nut.2013.11.016 [DOI] [PubMed] [Google Scholar]
- 152. Liu T, Liu PY, Marshall GM: The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res. 2009;69(5):1702–5. 10.1158/0008-5472.CAN-08-3365 [DOI] [PubMed] [Google Scholar]
- 153. Oberdoerffer P, Michan S, McVay M, et al. : SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135(5):907–18. 10.1016/j.cell.2008.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Carter LG, D'Orazio JA, Pearson KJ: Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer. 2014;21(3):R209–25. 10.1530/ERC-13-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Marambaud P, Zhao H, Davies P: Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem. 2005;280(45):37377–82. 10.1074/jbc.M508246200 [DOI] [PubMed] [Google Scholar]
- 156. Karuppagounder SS, Pinto JT, Xu H, et al. : Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem Int. 2009;54(2):111–8. 10.1016/j.neuint.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Baur JA, Sinclair DA: Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. 10.1038/nrd2060 [DOI] [PubMed] [Google Scholar]
- 158. Ku CR, Lee HJ, Kim SK, et al. : Resveratrol prevents streptozotocin-induced diabetes by inhibiting the apoptosis of pancreatic β-cell and the cleavage of poly (ADP-ribose) polymerase. Endocr J. 2012;59(2):103–9. 10.1507/endocrj.EJ11-0194 [DOI] [PubMed] [Google Scholar]
- 159. Wu JM, Hsieh TC: Resveratrol: a cardioprotective substance. Ann N Y Acad Sci. 2011;1215:16–21. 10.1111/j.1749-6632.2010.05854.x [DOI] [PubMed] [Google Scholar]
- 160. Park DW, Kim JS, Chin BR, et al. : Resveratrol inhibits inflammation induced by heat-killed Listeria monocytogenes. J Med Food. 2012;15(9):788–94. 10.1089/jmf.2012.2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Campagna M, Rivas C: Antiviral activity of resveratrol. Biochem Soc Trans. 2010;38(Pt 1):50–3. 10.1042/BST0380050 [DOI] [PubMed] [Google Scholar]
- 162. Porquet D, Casadesús G, Bayod S, et al. : Dietary resveratrol prevents Alzheimer's markers and increases life span in SAMP8. Age (Dordr). 2013;35(5):1851–65. 10.1007/s11357-012-9489-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Gerhardt E, Gräber S, Szego EM, et al. : Idebenone and resveratrol extend lifespan and improve motor function of HtrA2 knockout mice. PLoS One. 2011;6(12):e28855. 10.1371/journal.pone.0028855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Mancuso R, del Valle J, Modol L, et al. : Resveratrol improves motoneuron function and extends survival in SOD1 G93A ALS mice. Neurotherapeutics. 2014;11(2):419–32. 10.1007/s13311-013-0253-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Song L, Chen L, Zhang X, et al. : Resveratrol ameliorates motor neuron degeneration and improves survival in SOD1 G93A mouse model of amyotrophic lateral sclerosis. Biomed Res Int. 2014;2014: 483501. 10.1155/2014/483501 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 166. Sebai H, Sani M, Ghanem-Boughanmi N, et al. : Prevention of lipopolysaccharide-induced mouse lethality by resveratrol. Food Chem Toxicol. 2010;48(6):1543–9. 10.1016/j.fct.2010.03.022 [DOI] [PubMed] [Google Scholar]
- 167. Avila PR, Marques SO, Luciano TF, et al. : Resveratrol and fish oil reduce catecholamine-induced mortality in obese rats: role of oxidative stress in the myocardium and aorta. Br J Nutr. 2013;110(9):1580–90. 10.1017/S0007114513000925 [DOI] [PubMed] [Google Scholar]
- 168. Holthoff JH, Wang Z, Seely KA, et al. : Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int. 2012;81(4):370–8. 10.1038/ki.2011.347 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 169. Rimbaud S, Ruiz M, Piquereau J, et al. : Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS One. 2011;6(10):e26391. 10.1371/journal.pone.0026391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Strong R, Miller RA, Astle CM, et al. : Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2013;68(1):6–16. 10.1093/gerona/gls070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Pearson KJ, Baur JA, Lewis KN, et al. : Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8(2):157–68. 10.1016/j.cmet.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Barger JL, Kayo T, Vann JM, et al. : A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3(6):e2264. 10.1371/journal.pone.0002264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Timmers S, Konings E, Bilet L, et al. : Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612–22. 10.1016/j.cmet.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Yoshino J, Conte C, Fontana L, et al. : Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16(5):658–64. 10.1016/j.cmet.2012.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 175. Cai H, Scott E, Kholghi A, et al. : Cancer chemoprevention: Evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci Transl Med. 2015;7(298):298ra117. 10.1126/scitranslmed.aaa7619 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 176. Minor RK, Baur JA, Gomes AP, et al. : SRT1720 improves survival and healthspan of obese mice. Sci Rep. 2011;1: 70. 10.1038/srep00070 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 177. Mitchell SJ, Martin-Montalvo A, Mercken EM, et al. : The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 2014;6(5):836–43. 10.1016/j.celrep.2014.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 178. Mercken EM, Mitchell SJ, Martin-Montalvo A, et al. : SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell. 2014;13(5):787–96. 10.1111/acel.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Minois N, Carmona-Gutierrez D, Madeo F: Polyamines in aging and disease. Aging (Albany NY). 2011;3(8):716–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Nishimura K, Shiina R, Kashiwagi K, et al. : Decrease in polyamines with aging and their ingestion from food and drink. J Biochem. 2006;139(1):81–90. 10.1093/jb/mvj003 [DOI] [PubMed] [Google Scholar]
- 181. Scalabrino G, Ferioli ME: Polyamines in mammalian ageing: an oncological problem, too? A review. Mech Ageing Dev. 1984;26(2–3):149–64. 10.1016/0047-6374(84)90090-3 [DOI] [PubMed] [Google Scholar]
- 182. Pucciarelli S, Moreschini B, Micozzi D, et al. : Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res. 2012;15(6):590–5. 10.1089/rej.2012.1349 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 183. Eisenberg T, Knauer H, Schauer A, et al. : Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11(11):1305–14. 10.1038/ncb1975 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 184. Minois N, Rockenfeller P, Smith TK, et al. : Spermidine feeding decreases age-related locomotor activity loss and induces changes in lipid composition. PLoS One. 2014;9(7):e102435. 10.1371/journal.pone.0102435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Soda K, Dobashi Y, Kano Y, et al. : Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp Gerontol. 2009;44(11):727–32. 10.1016/j.exger.2009.08.013 [DOI] [PubMed] [Google Scholar]
- 186. Suppola S, Heikkinen S, Parkkinen JJ, et al. : Concurrent overexpression of ornithine decarboxylase and spermidine/spermine N(1)-acetyltransferase further accelerates the catabolism of hepatic polyamines in transgenic mice. Biochem J. 2001;358(Pt 2):343–8. 10.1042/bj3580343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Minois N, Carmona-Gutierrez D, Bauer MA, et al. : Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and -independent pathways. Cell Death Dis. 2012;3:e401. 10.1038/cddis.2012.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Kobayashi S: Choose Delicately and Reuse Adequately: The Newly Revealed Process of Autophagy. Biol Pharm Bull. 2015;38(8):1098–103. 10.1248/bpb.b15-00096 [DOI] [PubMed] [Google Scholar]
- 189. Vane SJ: Aspirin and other anti-inflammatory drugs. Thorax. 2000;55(Suppl 2):S3–9. 10.1136/thorax.55.suppl_2.S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Shi X, Ding M, Dong Z, et al. : Antioxidant properties of aspirin: characterization of the ability of aspirin to inhibit silica-induced lipid peroxidation, DNA damage, NF-kappaB activation, and TNF-alpha production. Mol Cell Biochem. 1999;199(1–2):93–102. 10.1023/A:1006934612368 [DOI] [PubMed] [Google Scholar]
- 191. Weissmann G: Aspirin. Sci Am. 1991;264(1):84–90. [DOI] [PubMed] [Google Scholar]
- 192. Chan AT, Giovannucci EL, Meyerhardt JA, et al. : Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology. 2008;134(1):21–8. 10.1053/j.gastro.2007.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Jacobs EJ, Rodriguez C, Mondul AM, et al. : A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst. 2005;97(13):975–80. 10.1093/jnci/dji173 [DOI] [PubMed] [Google Scholar]
- 194. Johnson TW, Anderson KE, Lazovich D, et al. : Association of aspirin and nonsteroidal anti-inflammatory drug use with breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1586–91. [PubMed] [Google Scholar]
- 195. Lim SS, Gaziano TA, Gakidou E, et al. : Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries: health effects and costs. Lancet. 2007;370(9604):2054–62. 10.1016/S0140-6736(07)61699-7 [DOI] [PubMed] [Google Scholar]
- 196. Ong G, Davis TM, Davis WA: Aspirin is associated with reduced cardiovascular and all-cause mortality in type 2 diabetes in a primary prevention setting: the Fremantle Diabetes study. Diabetes Care. 2010;33(2):317–21. 10.2337/dc09-1701 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 197. Agüero-Torres H, Viitanen M, Fratiglioni L, et al. : The effect of low-dose daily aspirin intake on survival in the Finnish centenarians cohort. J Am Geriatr Soc. 2001;49(11):1578–80. 10.1046/j.1532-5415.2001.4911264.x [DOI] [PubMed] [Google Scholar]
- 198. Ayyadevara S, Bharill P, Dandapat A, et al. : Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxid Redox Signal. 2013;18(5):481–90. 10.1089/ars.2011.4151 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 199. Strong R, Miller RA, Astle CM, et al. : Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7(5):641–50. 10.1111/j.1474-9726.2008.00414.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Lü JM, Nurko J, Weakley SM, et al. : Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives: an update. Med Sci Monit. 2010;16(5):RA93–100. [PMC free article] [PubMed] [Google Scholar]
- 201. West M, Mhatre M, Ceballos A, et al. : The arachidonic acid 5-lipoxygenase inhibitor nordihydroguaiaretic acid inhibits tumor necrosis factor alpha activation of microglia and extends survival of G93A-SOD1 transgenic mice. J Neurochem. 2004;91(1):133–43. 10.1111/j.1471-4159.2004.02700.x [DOI] [PubMed] [Google Scholar]
- 202. Harrison DE, Strong R, Allison DB, et al. : Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–82. 10.1111/acel.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. DiNicolantonio JJ, Bhutani J, O'Keefe JH: Acarbose: safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart. 2015;2(1):e000327. 10.1136/openhrt-2015-000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Zhang J, Li Y: Fibroblast Growth Factor 21 Analogs for Treating Metabolic Disorders. Front Endocrinol (Lausanne). 2015;6:168. 10.3389/fendo.2015.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Mendelsohn AR, Larrick JW: Fibroblast growth factor-21 is a promising dietary restriction mimetic. Rejuvenation Res. 2012;15(6):624–8. 10.1089/rej.2012.1392 [DOI] [PubMed] [Google Scholar]
- 206. Zhang Y, Xie Y, Berglund ED, et al. : The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife. 2012;1:e00065. 10.7554/eLife.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 207. Schriefers H, Wright MC, Rozman T, et al. : [Inhibition of testosterone metabolism by 17-alpha-estradiol in rat liver slices]. Arzneimittelforschung. 1991;41(11):1186–9. [PubMed] [Google Scholar]
- 208. Rehman Y, Rosenberg JE: Abiraterone acetate: oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer. Drug Des Devel Ther. 2012;6:13–8. 10.2147/DDDT.S15850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Dykens JA, Moos WH, Howell N: Development of 17alpha-estradiol as a neuroprotective therapeutic agent: rationale and results from a phase I clinical study. Ann N Y Acad Sci. 2005;1052:116–35. 10.1196/annals.1347.008 [DOI] [PubMed] [Google Scholar]
- 210. Liu R, Wen Y, Perez E, et al. : 17beta-Estradiol attenuates blood-brain barrier disruption induced by cerebral ischemia-reperfusion injury in female rats. Brain Res. 2005;1060(1–2):55–61. 10.1016/j.brainres.2005.08.048 [DOI] [PubMed] [Google Scholar]
- 211. Perez E, Liu R, Yang SH, et al. : Neuroprotective effects of an estratriene analog are estrogen receptor independent in vitro and in vivo. Brain Res. 2005;1038(2):216–22. 10.1016/j.brainres.2005.01.026 [DOI] [PubMed] [Google Scholar]
- 212. Stout MB, Steyn FJ, Jurczak MJ, et al. : 17α-Estradiol Alleviates Age-related Metabolic and Inflammatory Dysfunction in Male Mice Without Inducing Feminization. J Gerontol A Biol Sci Med Sci. 2016; pii: glv309. 10.1093/gerona/glv309 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 213. Antos CL, Frey N, Marx SO, et al. : Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase a. Circ Res. 2001;89(11):997–1004. 10.1161/hh2301.100003 [DOI] [PubMed] [Google Scholar]
- 214. Du XJ, Gao XM, Wang B, et al. : Age-dependent cardiomyopathy and heart failure phenotype in mice overexpressing beta(2)-adrenergic receptors in the heart. Cardiovasc Res. 2000;48(3):448–54. 10.1016/S0008-6363(00)00187-5 [DOI] [PubMed] [Google Scholar]
- 215. Engelhardt S, Hein L, Wiesmann F, et al. : Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A. 1999;96(12):7059–64. 10.1073/pnas.96.12.7059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Iwase M, Bishop SP, Uechi M, et al. : Adverse effects of chronic endogenous sympathetic drive induced by cardiac GS alpha overexpression. Circ Res. 1996;78(4):517–24. 10.1161/01.RES.78.4.517 [DOI] [PubMed] [Google Scholar]
- 217. Liggett SB, Tepe NM, Lorenz JN, et al. : Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101(14):1707–14. 10.1161/01.CIR.101.14.1707 [DOI] [PubMed] [Google Scholar]
- 218. Yan L, Vatner DE, O'Connor JP, et al. : Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130(2):247–58. 10.1016/j.cell.2007.05.038 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 219. Ho D, Yan L, Iwatsubo K, et al. : Modulation of beta-adrenergic receptor signaling in heart failure and longevity: targeting adenylyl cyclase type 5. Heart Fail Rev. 2010;15(5):495–512. 10.1007/s10741-010-9183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Zhao L, Yang F, Xu K, et al. : Common genetic variants of the β2-adrenergic receptor affect its translational efficiency and are associated with human longevity. Aging Cell. 2012;11(6):1094–101. 10.1111/acel.12011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Spindler SR, Mote PL, Li R, et al. : β1-Adrenergic receptor blockade extends the life span of Drosophila and long-lived mice. Age (Dordr). 2013;35(6):2099–109. 10.1007/s11357-012-9498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 222. Ye X, Linton JM, Schork NJ, et al. : A pharmacological network for lifespan extension in Caenorhabditis elegans. Aging Cell. 2014;13(2):206–15. 10.1111/acel.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Aydin MH, Sibai AM: Life stressors and psychological well-being. Does Access to Health Care Help the Older Lebanese? J Med Liban. 2015;63(1):34–41. 10.12816/0009917 [DOI] [PubMed] [Google Scholar]
- 224. Brack C, Bechter-Thüring E, Labuhn M: N-acetylcysteine slows down ageing and increases the life span of Drosophila melanogaster. Cell Mol Life Sci. 1997;53(11–12):960–6. 10.1007/PL00013199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Melov S, Ravenscroft J, Malik S, et al. : Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289(5484):1567–9. 10.1126/science.289.5484.1567 [DOI] [PubMed] [Google Scholar]
- 226. Gems D, Doonan R: Antioxidant defense and aging in C. elegans: is the oxidative damage theory of aging wrong? Cell Cycle. 2009;8(11):1681–7. 10.4161/cc.8.11.8595 [DOI] [PubMed] [Google Scholar]
- 227. Quick KL, Ali SS, Arch R, et al. : A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol Aging. 2008;29(1):117–28. 10.1016/j.neurobiolaging.2006.09.014 [DOI] [PubMed] [Google Scholar]
- 228. Baati T, Bourasset F, Gharbi N, et al. : The prolongation of the lifespan of rats by repeated oral administration of [60]fullerene. Biomaterials. 2012;33(19):4936–46. 10.1016/j.biomaterials.2012.03.036 [DOI] [PubMed] [Google Scholar]
- 229. Peng C, Wang X, Chen J, et al. : Biology of ageing and role of dietary antioxidants. Biomed Res Int. 2014;2014: 831841. 10.1155/2014/831841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Sadowska-Bartosz I, Bartosz G: Effect of antioxidants supplementation on aging and longevity. Biomed Res Int. 2014;2014: 404680. 10.1155/2014/404680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Selman C, McLaren JS, Collins AR, et al. : Deleterious consequences of antioxidant supplementation on lifespan in a wild-derived mammal. Biol Lett. 2013;9(4): 20130432. 10.1098/rsbl.2013.0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Spindler SR, Mote PL, Flegal JM: Lifespan effects of simple and complex nutraceutical combinations fed isocalorically to mice. Age (Dordr). 2014;36(2):705–18. 10.1007/s11357-013-9609-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Bjelakovic G, Nikolova D, Gluud C: Antioxidant supplements and mortality. Curr Opin Clin Nutr Metab Care. 2014;17(1):40–4. [DOI] [PubMed] [Google Scholar]
- 234. Holmström KM, Finkel T: Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15(6):411–21. 10.1038/nrm3801 [DOI] [PubMed] [Google Scholar]
- 235. Childs BG, Durik M, Baker DJ, et al. : Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21(12):1424–35. 10.1038/nm.4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Rodier F, Campisi J: Four faces of cellular senescence. J Cell Biol. 2011;192(4):547–56. 10.1083/jcb.201009094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Campisi J, d'Adda di Fagagna F: Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–40. 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- 238. Kozlowski M, Ladurner AG: ATM, MacroH2A.1, and SASP: The Checks and Balances of Cellular Senescence. Mol Cell. 2015;59(5):713–5. 10.1016/j.molcel.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 239. Waaijer ME, Parish WE, Strongitharm BH, et al. : The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell. 2012;11(4):722–5. 10.1111/j.1474-9726.2012.00837.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Tchkonia T, Zhu Y, van Deursen J, et al. : Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123(3):966–72. 10.1172/JCI64098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Campisi J: Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–22. 10.1016/j.cell.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 242. Kirkland JL, Tchkonia T: Clinical strategies and animal models for developing senolytic agents. Exp Gerontol. 2015;68:19–25. 10.1016/j.exger.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Baker DJ, Wijshake T, Tchkonia T, et al. : Clearance of p16 Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–6. 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 244. Baker DJ, Childs BG, Durik M, et al. : Naturally occurring p16 Ink4a-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–9. 10.1038/nature16932 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 245. Zhu Y, Tchkonia T, Pirtskhalava T, et al. : The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58. 10.1111/acel.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 246. Montero JC, Seoane S, Ocaña A, et al. : Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res. 2011;17(17):5546–52. 10.1158/1078-0432.CCR-10-2616 [DOI] [PubMed] [Google Scholar]
- 247. Olave NC, Grenett MH, Cadeiras M, et al. : Upstream stimulatory factor-2 mediates quercetin-induced suppression of PAI-1 gene expression in human endothelial cells. J Cell Biochem. 2010;111(3):720–6. 10.1002/jcb.22760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Bruning A: Inhibition of mTOR signaling by quercetin in cancer treatment and prevention. Anticancer Agents Med Chem. 2013;13(7):1025–31. 10.2174/18715206113139990114 [DOI] [PubMed] [Google Scholar]
- 249. Chang J, Wang Y, Shao L, et al. : Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22(1):78–83. 10.1038/nm.4010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 250. Wendt MD: Discovery of ABT-263, a Bcl-family protein inhibitor: observations on targeting a large protein-protein interaction. Expert Opin Drug Discov. 2008;3(9):1123–43. 10.1517/17460441.3.9.1123 [DOI] [PubMed] [Google Scholar]
- 251. Vogler M, Dinsdale D, Dyer MJ, et al. : Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2009;16(3):360–7. 10.1038/cdd.2008.137 [DOI] [PubMed] [Google Scholar]
- 252. Billard C: BH3 mimetics: status of the field and new developments. Mol Cancer Ther. 2013;12(9):1691–700. 10.1158/1535-7163.MCT-13-0058 [DOI] [PubMed] [Google Scholar]
- 253. Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, et al. : Identification of a Novel Senolytic Agent, Navitoclax, Targeting the Bcl-2 Family of Anti-Apoptotic Factors. Aging Cell. 2015. 10.1111/acel.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 254. Di Cicco E, Tozzini ET, Rossi G, et al. : The short-lived annual fish Nothobranchius furzeri shows a typical teleost aging process reinforced by high incidence of age-dependent neoplasias. Exp Gerontol. 2011;46(4):249–56. 10.1016/j.exger.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 255. Genade T, Benedetti M, Terzibasi E, et al. : Annual fishes of the genus Nothobranchius as a model system for aging research. Aging Cell. 2005;4(5):223–33. 10.1111/j.1474-9726.2005.00165.x [DOI] [PubMed] [Google Scholar]
- 256. Terzibasi E, Valenzano DR, Benedetti M, et al. : Large differences in aging phenotype between strains of the short-lived annual fish Nothobranchius furzeri. PLoS One. 2008;3(12):e3866. 10.1371/journal.pone.0003866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Terzibasi E, Valenzano DR, Cellerino A: The short-lived fish Nothobranchius furzeri as a new model system for aging studies. Exp Gerontol. 2007;42(1–2):81–9. 10.1016/j.exger.2006.06.039 [DOI] [PubMed] [Google Scholar]
- 258. Valenzano DR, Terzibasi E, Cattaneo A, et al. : Temperature affects longevity and age-related locomotor and cognitive decay in the short-lived fish Nothobranchius furzeri. Aging Cell. 2006;5(3):275–8. 10.1111/j.1474-9726.2006.00212.x [DOI] [PubMed] [Google Scholar]
- 259. Harel I, Benayoun BA, Machado B, et al. : A platform for rapid exploration of aging and diseases in a naturally short-lived vertebrate. Cell. 2015;160(5):1013–26. 10.1016/j.cell.2015.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 260. Ramos FJ, Chen SC, Garelick MG, et al. : Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103. 10.1126/scitranslmed.3003802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Zhao J, Li X, McGowan S, et al. : NF-κB activation with aging: characterization and therapeutic inhibition. Methods Mol Biol. 2015;1280:543–57. 10.1007/978-1-4939-2422-6_32 [DOI] [PubMed] [Google Scholar]
- 262. Hasty P, Vijg J: Rebuttal to Miller: 'Accelerated aging': a primrose path to insight?' Aging Cell. 2004;3(2):67–9. 10.1111/j.1474-9728.2004.00087.x [DOI] [PubMed] [Google Scholar]
- 263. Miller RA: 'Accelerated aging': a primrose path to insight? Aging Cell. 2004;3(2):47–51. 10.1111/j.1474-9728.2004.00081.x [DOI] [PubMed] [Google Scholar]
- 264. Sharpless NE, Sherr CJ: Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15(7):397–408. 10.1038/nrc3960 [DOI] [PubMed] [Google Scholar]
- 265. Jones MJ, Goodman SJ, Kobor MS: DNA methylation and healthy human aging. Aging Cell. 2015;14(6):924–32. 10.1111/acel.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Horvath S: DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 267. Carretero M, Gomez-Amaro RL, Petrascheck M: Pharmacological classes that extend lifespan of Caenorhabditis elegans. Front Genet. 2015;6:77. 10.3389/fgene.2015.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Prior M, Chiruta C, Currais A, et al. : Back to the future with phenotypic screening. ACS Chem Neurosci. 2014;5(7):503–13. 10.1021/cn500051h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Longo VD, Shadel GS, Kaeberlein M, et al. : Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab. 2012;16(1):18–31. 10.1016/j.cmet.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Miller RA: Cell stress and aging: new emphasis on multiplex resistance mechanisms. J Gerontol A Biol Sci Med Sci. 2009;64(2):179–82. 10.1093/gerona/gln072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Kishi N, Sato K, Sasaki E, et al. : Common marmoset as a new model animal for neuroscience research and genome editing technology. Dev Growth Differ. 2014;56(1):53–62. 10.1111/dgd.12109 [DOI] [PubMed] [Google Scholar]
- 272. Tardif S, Ross C, Bergman P, et al. : Testing efficacy of administration of the antiaging drug rapamycin in a nonhuman primate, the common marmoset. J Gerontol A Biol Sci Med Sci. 2015;70(5):577–87. 10.1093/gerona/glu101 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 273. Kaeberlein M: The Biology of Aging: Citizen Scientists and Their Pets as a Bridge Between Research on Model Organisms and Human Subjects. Vet Pathol. 2016;53(2):291–8. 10.1177/0300985815591082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Check Hayden E: Pet dogs set to test anti-ageing drug. Nature. 2014;514(7524):546. 10.1038/514546a [DOI] [PubMed] [Google Scholar]