Abstract
Bone and dentin share similar biochemical compositions and physiological properties. Dentin, a major tooth component, is formed by odontoblasts; in contrast, bone is produced by osteoblasts. Osterix (Osx), a zinc finger-containing transcription factor, has been identified as an essential regulator of osteoblast differentiation and bone formation. However, it has been difficult to establish whether Osx functions in odontoblast differentiation and dentin formation. To understand the role of Osx in dentin formation, we analyzed mice in which Osx was subjected to tissue-specific ablation under the control of either the Col1a1 or the OC promoter. Two independent Osx conditional knockout mice exhibited similar molar abnormalities. Although no phenotype was found in the crowns of these teeth, both mutant lines exhibited short molar roots due to impaired root elongation. Furthermore, the interradicular dentin in these mice showed severe hypoplastic features, which were likely caused by disruptions in odontoblast differentiation and dentin formation. These phenotypes were closely related to the temporospatial expression pattern of Osx during tooth development. These findings indicate that Osx is required for root formation by regulating odontoblast differentiation, maturation, and root elongation. Cumulatively, our data strongly indicate that Osx is a site-specific regulator in tooth root formation.
Keywords: Osx, tooth development, odontoblasts, tooth roots, dentinogenesis, mice
Introduction
Bone and dentin exhibit similar biochemical compositions, although they perform different biological functions (Veis 1993). Although both cell types originate from mesenchymal cells, osteoblasts and odontoblasts display different morphologies and functions. Nevertheless, osteoblasts and odontoblasts are both believed to contribute to skeletal tissue formation through processes such as matrix formation and mineralization.
Runx2 and Osterix (Osx), 2 zinc finger-containing transcription factors, have been identified as master regulators of osteoblast differentiation during bone formation (Komori et al. 1997; Otto et al. 1997; Nakashima et al. 2002). Genetic studies of osteoblast differentiation indicate that Osx acts downstream of Runx2; for example, Runx2 expression is normal in Osx-null mice, while no Osx transcripts are detected in the skeletal elements of Runx2 knockout mice (Nakashima et al. 2002). These findings suggest that Runx2 is required for the differentiation of multipotential mesenchymal progenitor cells into preosteoblasts, whereas Osx is required in a later maturation stage of preosteoblasts into functional osteoblasts (Nakashima and de Crombrugghe 2003).
Tooth formation is regulated by reciprocal interactions between the epithelium and the mesenchyme (Thesleff 2003). Numerous growth factors and transcription factors are known to be involved in the regulation of tooth development (Tummers and Thesleff 2009). During tooth development, Runx2 is expressed in the dental mesenchyme until the late cap stage and is then downregulated in the dental papilla during odontoblast differentiation. In contrast, Osx is not expressed in the dental mesenchyme before odontoblast differentiation; rather, the pattern of Osx expression overlaps with that of Dspp (Chen et al. 2009). Based on the temporospatial expression patterns of Runx2 and Osx, these 2 genes are hypothesized to play roles in tooth morphogenesis and odontoblast differentiation, respectively. Indeed, in Runx2 null mice, tooth germs were arrested at the cap stage; however, no visible tooth abnormalities were observed in the embryos of Osx null mice (D’Souza et al. 1999; Nakashima et al. 2002).
In Osx null mice, mesenchymal cells of skeletal elements are unable to differentiate into osteoblasts, and the bone matrix is not deposited (Nakashima et al. 2002). Since most bone matrix proteins are also found in dentin, Osx has been postulated to play a role in odontoblast differentiation and dentin formation, although this has not been confirmed. Furthermore, the molecular mechanisms underlying odontoblast differentiation and dentin formation are unclear. Here, we investigated the roles of Osx in odontoblast differentiation and dentin formation by analyzing 2 independent mouse lines enabling the odontoblast-specific inactivation of Osx under the control of either the Col1a1 or the OC promoter.
Materials and Methods
Mouse Strains and β-Galactosidase Staining
All experimental procedures were approved by the Animal Welfare Committee of Chonbuk National University. Osx-floxed allele (Osxfl/fl) and Osteocalcin-Cre (OC-Cre) mice have been previously described (Nakashima et al. 2002; Akiyama et al. 2005; Tan et al. 2007). The 3.6-kb Col1a1-Cre (Col1a1-Cre; Liu et al. 2004) and Rosa26 (R26R; Soriano 1999) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). To generate Col1a1-Cre;Osxfl/fl (OsxCol) and OC-Cre;Osxfl/fl (OsxOC) mice, Col1a1-Cre;Osxfl/+ or OC-Cre;Osxfl/+ (control) mice were crossed with Osxfl/fl mice as appropriate. Mouse offspring were genotyped by polymerase chain reaction analysis using previously described primers (Liu et al. 2004; Akiyama et al. 2005; Tan et al. 2007). To analyze the level of Cre activity in Col1a1-Cre and OC-Cre mice, Col1a1-Cre and OC-Cre mice were crossed with R26R mice, and the mandibles of the double transgenic mice were processed for X-gal staining as described previously (Kim et al. 2013). A total of 95 animals were used in this study.
Histology, Immunohistochemistry, and In Situ Hybridization
For histologic analysis, the mice were sacrificed, and their mandibles were carefully dissected. The dissected tissues were fixed in 4% paraformaldehyde (PFA) and decalcified in 10% EDTA for 2 to 4 wk at 4 °C. The decalcified tissues were dehydrated through a graded ethanol series, embedded in paraffin, and sectioned at 5-µm thickness. Slides were stained with hematoxylin and eosin (H&E) and dichrome staining solution (Sigma-Aldrich, St. Louis, MO, USA).
For immunostaining, sections were treated with 3% hydrogen peroxide and incubated with mouse monoclonal anti–nestin antibody (1:200; Chemicon, Temecula, CA, USA) or rabbit polyclonal antibodies against Osx (1:200; Abcam, Cambridge, MA, USA), Alpl (1:50; Protein Tech, Chicago, IL, USA), Dsp (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), Phex (1:50; Sigma-Aldrich), periostin (1:600; Abcam), or Dmp1 (1:750; Takara Bio, Shiga, Japan). Histostain Plus primary (DAB) kit (Zymed Laboratories, San Francisco, CA, USA) was used according to the manufacturer’s instructions. In situ hybridization of tissue sections was performed as previously described (Kim et al. 2013). Digoxigenin-labeled probes for Dsp, OC, Bsp, and Osx were prepared in house.
Cell Proliferation Assay
To detect the extent of cell proliferation in the developing roots, 5′-bromo-2′deoxyuridine (BrdU) labeling reagent (45 µg/g body weight; Roche, Indianapolis, IN, USA) was injected intraperitoneally into 14-d-old (P14) mice. Two hours after injection, their mandibles were dissected, embedded, and sectioned in the mid-sagittal plane for immunodetection with a BrdU labeling and detection kit (Roche). For statistical analysis, 3 independent littermates were used in each study.
Micro–Computed Tomography
Mandibles were dissected from 4- to 8-wk-old OsxCol, OsxOC, and control mice and fixed in 4% PFA. The jaws were scanned using a desktop scanner (1076 Skyscan Micro-CT; Skyscan, Kontich, Belgium). They were subsequently reconstructed and analyzed with CTscan software (Skyscan).
Teeth Isolation
Mandibular first molars were isolated from the mandibles of 4-wk-old OsxCol, OsxOC, and control mice as previously described (Kim et al. 2013).
Statistical Analysis
All data are presented as means ± SEM. All statistical analyses were done using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). Statistical differences were determined by Student’s t test, and P < 0.05 was considered statistically significant.
Results
Temporospatial Expression of Osx in Odontoblasts during Tooth Development
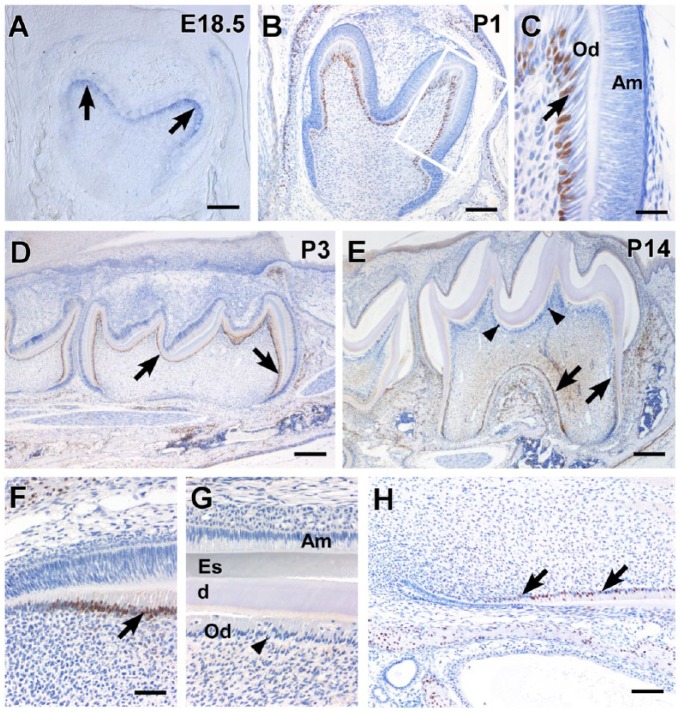
In the developing mandibular molars of E18.5 mouse embryos, Osx messenger RNA (mRNA) was specifically expressed in differentiating odontoblasts below the future cusp area (Fig. 1A). Immunohistochemical staining revealed that Osx was localized in the nuclei of differentiated odontoblasts in the mandibular molar at P1 (Fig. 1B, C). In contrast, Osx was localized in all odontoblasts of the whole crown of the mandibular molar at P3 (Fig. 1D). At P14, Osx was localized in newly differentiated odontoblasts and pulp cells in the root region, including the furcation area. However, Osx was not expressed in the odontoblasts of the crown region (Fig. 1E). The localization of Osx in incisors was similar to that in molars. Before dentin matrix deposition, Osx was expressed in differentiating odontoblasts; in contrast, Osx expression was efficiently downregulated after dentin formation (Fig. 1F–H).
Figure 1.
Temporospatial expression of Osx in the odontoblasts of developing mouse tooth germs. (A) Specific expression of Osx messenger RNA in differentiating odontoblasts at E18.5. (B–D) Expression of Osx in the nuclei of differentiated odontoblasts in the mouse mandibular molar crowns at P1 and P3. The boxed area shown in (B) is magnified in (C). (E) Downregulation of Osx expression in crown odontoblasts after the initiation of root formation. However, Osx was still expressed in root odontoblasts and pulp cells. (F–H) Expression of Osx in odontoblasts before dentin matrix deposition in the developing maxillary incisors. However, Osx was no longer expressed after dentin formation. The black arrows and black arrowheads indicate Osx expression and the downregulation of Osx expression in odontoblasts, respectively. Od, odontoblasts; Am, ameloblasts; d, dentin; Es, enamel space. Scale bars: 200 µm (A, B, D, E), 100 µm (H), 50 µm (F, G), and 40 µm (C).
Targeted Ablation of Osx in Odontoblasts Leads to Short Molar Roots and Thin Interradicular Dentin
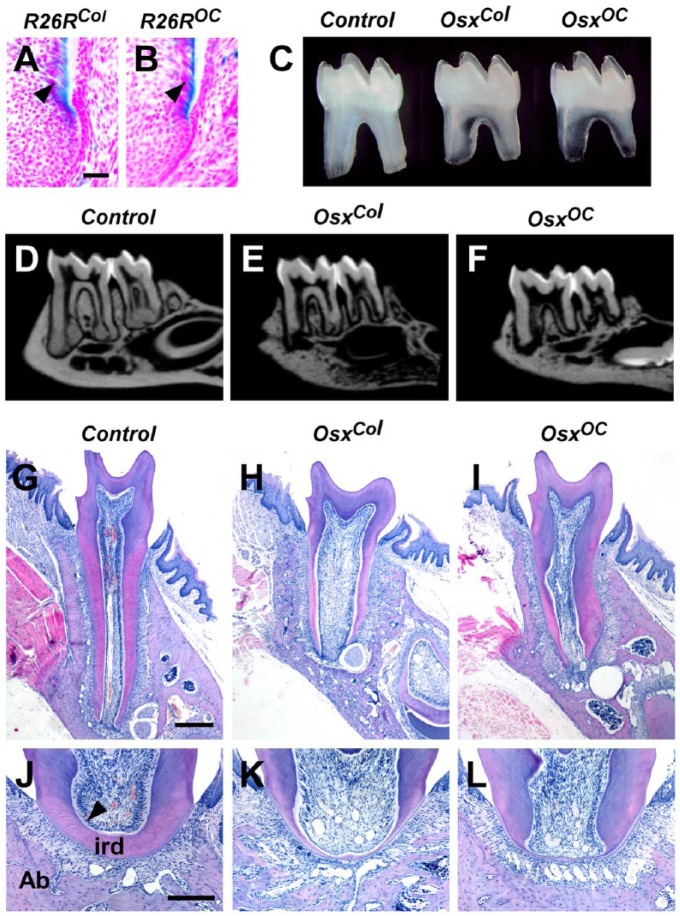
We next confirmed that Cre recombination at the Col1a1-Cre and OC-Cre promoters was active in odontoblasts. In mouse molars at P10, strong β-galactosidase activity was observed in the odontoblasts and differentiating odontoblasts of Col1a1-Cre;R26R and OC-Cre;R26R double transgenic mice (Fig. 2A, B). Stereomicroscopic observation of isolated mandibular first molars revealed short roots (89% penetrance; n = 9 for each genotype), whereas no differences in crown height in either mutant lines were observed at 4 wk (Fig. 2C, Appendix Fig. 1). In addition, translucent regions were found in the furcation areas between the roots in both mutant lines. Consistent with these observations, micro–computed tomography revealed that the mandibular molars of both mutant mice exhibited short roots and hypoplastic interradicular dentin at 8 wk (Fig. 2D–F). These tooth phenotypes were also confirmed in tissue sections of mandibular first molars at 2 and 4 wk (Fig. 2G–L, Appendix Fig. 2). In the sections containing the root elongation area, the root dentin layer was short in the molars obtained from these mutants (Fig. 2G–I). Moreover, the interradicular dentin layer was extremely thin, and odontoblasts were poorly differentiated in the furcation area (Fig. 2J–L).
Figure 2.
Tooth abnormalities in OsxCol and OsxOC mice. (A, B) β-Galactosidase activity in the odontoblasts of Col1a1-Cre;R26R and OC-Cre;R26R mice at P10. (C) Mandibular molars, isolated from both mutants at 4 wk, showing short roots with translucent furcation areas. (D–F) Micro–computed tomography scans of mandibles obtained from mutant mice at 8 wk showing short molar roots and thin interradicular dentin. (G–I) Frontal sections of mesial roots obtained from OsxCol and OsxOC mice at 4 wk, revealing short roots and thin root dentin. (J–L) Images of the furcation regions of molars obtained from OsxCol and OsxOC mice, revealing extremely thin interradicular dentin and severely impaired odontoblast differentiation. The black arrowheads indicate odontoblasts. Ab, alveolar bone; ird, interradicular dentin. Scale bars: 20 µm (A–B), 300 µm (G–I), and 200 µm (J–L).
Impaired Odontoblast Maturation Disturbs Root Elongation
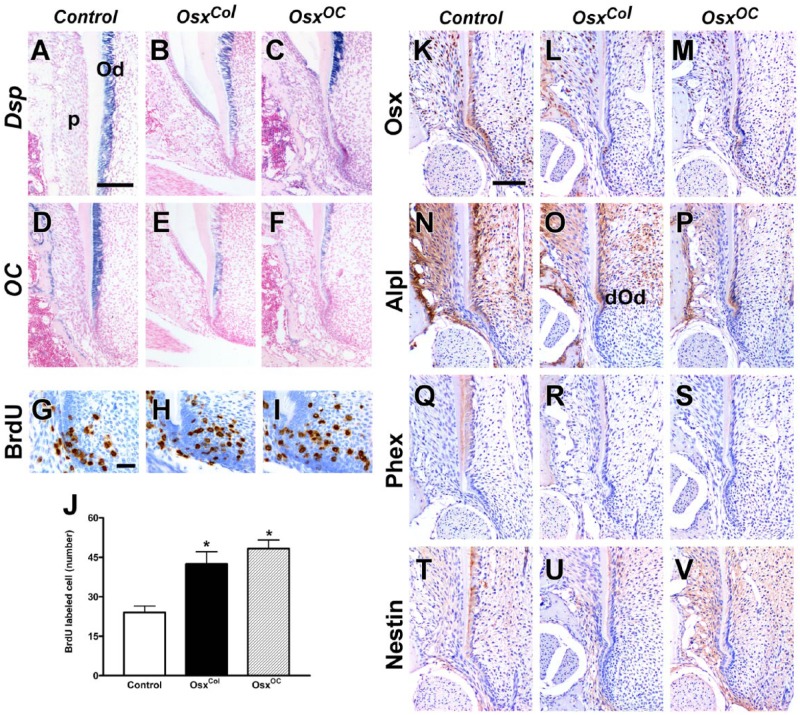
To assess the gene expression changes following the ablation of Osx in odontoblasts during root formation, in situ hybridization was performed. In molars obtained from control mice at P14, Dsp was expressed at high levels in the mature odontoblasts of developing roots; however, the root odontoblasts from OsxCol and OsxOC mice exhibited lower levels of Dsp expression (Fig. 3A–C). In contrast to Dsp, OC was expressed in both mature odontoblasts and differentiating odontoblasts in molars from control mice. Furthermore, both differentiating and mature odontoblasts from OsxCol and OsxOC mice exhibited decreased expression of OC compared with control odontoblasts (Fig. 3D–F). Cell proliferating assays using BrdU-labeling revealed that proliferating cells were mainly located in the dental mesenchyme of the root apex in control mice. BrdU-labeled cells were increased in molars from OsxCol and OsxOC mice (Fig. 3G–J). Immunohistochemistry revealed that Osx was expressed in the root odontoblasts and cells of periodontium in control. However, Osx expression was abolished in the root odontoblasts except some preodontoblasts close to HERS (Fig. 3K–M). Alpl was highly expressed in the control odontoblasts. In contrast, expression of Alpl was downregulated in mature odontoblasts and was not observed in differentiating odontoblasts obtained from OsxCol and OsxOC mice (Fig. 3N–P). Moreover, although Phex and nestin, 2 markers of mature odontoblasts, were expressed in mature control odontoblasts, these markers were almost completely absent from the root odontoblasts obtained from OsxCol and OsxOC mice (Fig. 3Q–V).
Figure 3.
Disrupted odontoblast maturation and root elongation during root formation in OsxCol and OsxOC mice at P14. Tissue-specific ablation of Osx interferes with odontoblast maturation in root formation but not with odontoblast differentiation in the root apex. (A–F) Downregulation of Dsp and OC expression in root odontoblasts of OsxCol and OsxOC mice at P14. (G–J) Increased cell proliferation in the dental mesenchyme near the molar root apex in OsxCol and OsxOC mice. (K–M) Lack of Osx expression in the root odontoblasts of OsxCol and OsxOC mice at P14. (N–P) Decreased Alp1 expression in odontoblasts, except for differentiating odontoblasts, in the developing molar roots of OsxCol and OsxOC mice at P14. (Q–V) Downregulation of Phex and nestin, 2 markers of mature odontoblasts, in root odontoblasts. Od, odontoblasts; dOd, differentiating odontoblasts; p, periodontium. *P < 0.05. Scale bars: 200 µm (A–F), 40 µm (G–I), and 100 µm (K–V).
Extremely Thin Interradicular Dentin Is Caused by Impaired Odontoblast Differentiation
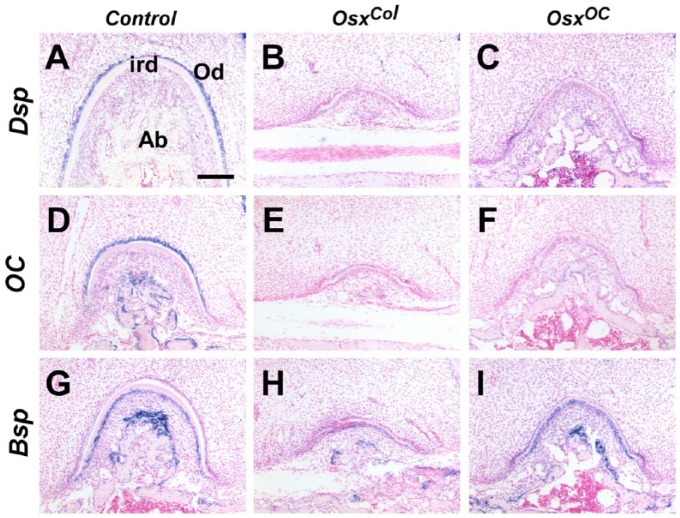
Since the interradicular dentin was severely hypoplastic in the molars of OsxCol and OsxOC mice, we compared the gene expression changes in the furcation areas of the developing mandibular first molars at P14. In control molars, both Dsp and OC were specifically expressed in odontoblasts inside the interradicular dentin, whereas Bsp was expressed in cementoblasts outside the interradicular dentin (Fig. 4A, D, G). In contrast, Dsp and OC expression was not detected in the odontoblasts inside the interradicular dentin of OsxCol and OsxOC mice (Fig. 4B, C, E, F). Although no changes in Bsp expression were observed in OsxOC mice, a low level of Bsp expression was observed in the cementoblasts of OsxCol mice (Fig. 4H, I).
Figure 4.
Gene expression changes in odontoblasts of the furcation areas of OsxCol and OsxOC mice. (A–F) Specific expression of Dsp and OC in odontoblasts of the furcation areas of control mice. In contrast, Dsp and OC were downregulated in the odontoblasts of the furcation areas of OsxCol and OsxOC mice. (G–I) Expression of Bsp in the cementoblasts aligned outside the interradicular dentin and osteoblasts in control and OsxOC mice. In contrast, OsxCol mice exhibited decreased expression of Bsp. Ab, alveolar bone; ird, interradicular dentin; Od, odontoblasts. Scale bar: 150 µm (A–I).
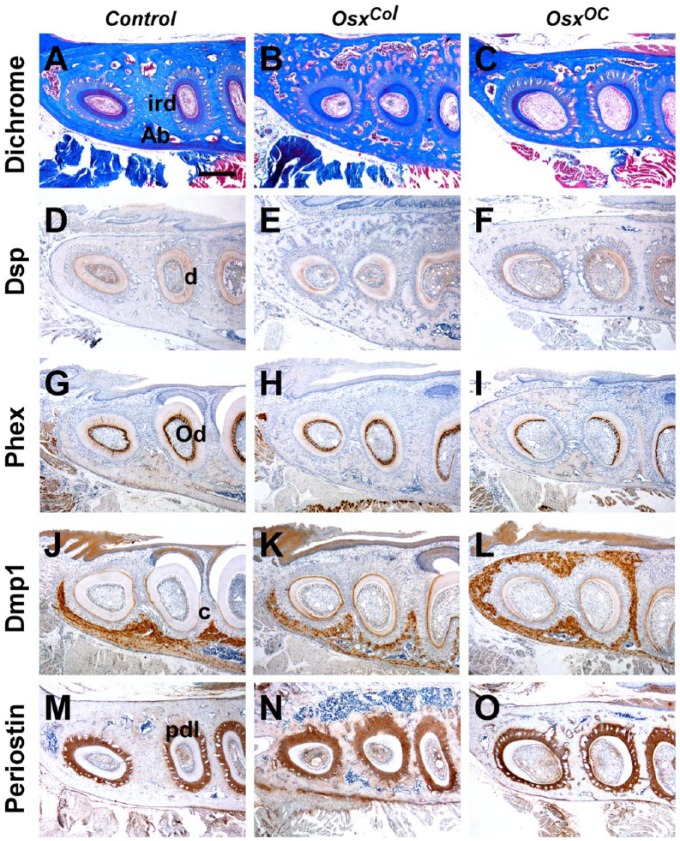
Root abnormalities were clearly observed in the horizontal sections crossing the middle third of the molar root. Dichrome staining revealed that the mandibular first molars of OsxCol and OsxOC mice exhibited remarkably thinner interradicular dentin. In contrast, the distance between the mesial and distal root was increased, and this area was filled with alveolar bone (Fig. 5A–C). Moreover, Dsp expression was slightly decreased in the root dentin of OsxCol and OsxOC mice (Fig. 5D–F), and Phex expression was downregulated in odontoblasts inside the interradicular dentin in OsxCol and OsxOC mice (Fig. 5G–I). Dmp1 was localized in the cementum and alveolar bone of OsxCol and OsxOC mice, similar to its localization in molars from control mice (Fig. 5J–L). Periostin was specifically localized in the periodontium, and periodontal spaces were slightly increased in the molar roots of OsxCol and OsxOC mice (Fig. 5M–O).
Figure 5.
Molecular changes in the middle third of the root and in the periodontium in OsxCol and OsxOC mice. (A–C) Dichrome staining images revealing a remarkable decrease in dentin thickness in the interradicular areas of OsxCol and OsxOC mice. Moreover, the distance between the 2 roots was increased, and this space was often filled with alveolar bone. (D–F) Expression of Dsp in control root dentin. In contrast, Dsp expression was slightly decreased in root dentin of OsxCol and OsxOC mice. (G–I) Specific expression of Phex in control odontoblasts. In contrast, Phex expression was somewhat downregulated in odontoblasts inside the interradicular dentin of OsxCol and OsxOC mice. (J–L) Localization of Dmp1 to the cementum and alveolar bone of OsxCol mice, OsxOC mice, and control mice. (M–O) Localization of periostin to the periodontium in control mice. The periodontal spaces were slightly enlarged in roots of OsxCol and OsxOC mice. Ab, alveolar bone; ird, interradicular dentin; d, dentin; Od, odontoblasts; c, cementum; pdl, periodontal ligament. Scale bar: 400 µm (A–O).
Discussion
In this study, we investigated the functional significance of Osx in odontoblasts during dentin formation. Two independent Osx conditional knockout mouse lines, OsxCol and OsxOC, exhibited similar tooth phenotypes characterized by short molar roots, extremely thin interradicular dentin, and poorly differentiated odontoblasts. These abnormalities were closely associated with the temporospatial expression of Osx and impairment of odontoblast differentiation.
It is generally accepted that tooth formation is regulated by numerous signaling molecules and transcription factors that mediate epithelial-mesenchymal interactions (Tummers and Thesleff 2009). Although it is well established that tooth shape and size are controlled by reciprocal interactions between the dental epithelium and mesenchyme, the molecular mechanisms underlying cellular differentiation and mineralized tissue formation in tooth development are largely uncharacterized. Signaling molecules originating from the dental epithelium, such as Shh, Tgf-β, and Fgf, have been postulated to induce differentiation of mesenchymal cells into odontoblasts in the dental papilla (Lesot et al. 2001; Huang et al. 2009; Li et al. 2014). In addition, several transcription factors, such as Runx2, C/ebpβ, Nrf1, and NF-Y, have been reported to regulate odontoblast differentiation in some in vitro studies (Narayanan et al. 2004; Chen et al. 2005, 2008). However, neither the molecular regulatory mechanisms nor the critical regulatory factors in odontoblast differentiation have been previously identified.
Although bone and dentin exhibit different structural and functional features, these 2 tissues share many similarities with skeletal tissue. In addition, the temporospatial expression patterns of Runx2 and Osx indicate that these genes may play a role in tooth formation (Chen et al. 2009). We found that Osx was specifically expressed in differentiating crown odontoblasts at early postnatal periods; however, Osx expression was efficiently downregulated in crown odontoblasts upon root formation. In contrast, Osx was expressed in root odontoblasts and the furcation region. These results suggest that Osx may play a role only in early odontoblast differentiation during crown formation, whereas Osx is involved in both root odontoblast differentiation and root formation. Consistent with the expression pattern of Osx, 2 independent mutant mouse lines, each with conditional ablation of Osx, exhibited molar root abnormalities, including short roots and severe hypoplastic interradicular dentin. However, no significant differences were observed between mutant and control crown odontoblasts or dentin in a recent report (Zhang et al. 2014). These findings indicate that Osx may not be required for the differentiation of crown odontoblasts but may play an essential role in root odontoblast differentiation, particularly in the interradicular area. Therefore, our results suggest that Osx might regulate odontoblast differentiation in a site-specific manner.
To date, several signaling molecules have been reported to be associated with site-specific regulation of odontoblast differentiation. Nfic, a DNA-binding transcription factor, has been shown to regulate odontoblast differentiation during root formation; consistent with this finding, Nfic-knockout mice exhibit short molar roots (Steele-Perkins et al. 2003). In addition, Bmp2, Tgfbr2, Smad4, and Ptc1 have been reported to be associated with root elongation (Nakatomi et al. 2006; Gao et al. 2009; Rakian et al. 2013; Wang et al. 2013). Ablation of these genes in the dental mesenchyme also results in short molar roots. Furthermore, we previously observed that ablation of β-catenin in the dental mesenchyme leads to complete absence of roots (Kim et al. 2013). In these genetic mouse models, tooth abnormalities were prominent in the root and were accompanied by impaired bone formation. Previous reports have suggested that crown and root odontoblast differentiation may be controlled by different molecular mechanisms; furthermore, the molecular mechanisms of root dentin and bone formation may share some similarities.
Since most gene-targeted mice showed root abnormality accompanied by HERS defects, interactions between the dental mesenchyme and HERS may play important roles in the regulation of root development (Huang and Chai 2012). In the developing molar roots of OsxCol and OsxOC mice, elongation disturbance was relatively mild and HERS was intact. These results suggest that Osx may not participate directly in the epithelial-mesenchymal interactions during root formation. In addition, proliferating cells were increased in the apical mesenchyme of OsxCol and OsxOC mouse molars. It seems to result from differentiation failure due to disruption of Osx in the differentiating odontoblasts, located just above the proliferating cells in root apex.
Numerous studies previously reported the fundamental differences in crown and root dentin (Beertsen et al. 1985; Takagi et al. 1998). In addition, we recently found the cellular heterogeneity in odontoblasts of crown and root (Bae et al. 2013). The cellular heterogeneity and differences in dentin between crown and root may be associated with site-specific regulation of odontoblast differentiation. These observations suggest that there are differences in odontoblasts and their molecular regulation between crown and roots. In this context, it is interesting to note that dysplastic dentin is usually found in the roots of human patients (MacDougall et al. 2006). This root-specific prevalence of dysplastic dentin may be closely related to the site-specific regulation of odontoblast differentiation.
Taken together, our results demonstrate that targeted ablation of Osx in odontoblasts leads to short molar roots and extremely thin interradicular dentin; moreover, this phenotype is strongly associated with the temporospatial expression of Osx in odontoblasts during tooth formation. Thus, our results strongly suggest that Osx may play as a site-specific regulator of odontoblast differentiation and maturation during tooth root formation. These findings may contribute to further understanding the molecular mechanisms underlying tooth root formation and regeneration.
Author Contributions
T.H. Kim, C.H. Bae, E.S. Cho, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; J.C. Lee, contributed to conception, data acquisition, and analysis, drafted and critically revised the manuscript; J.E. Kim, X. Yang, B. de Crombrugghe, contributed to design and data interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (No. 2013R1A2A1A01007642).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, Zhang Z, Martin JF, Behringer RR, Nakamura T, et al. 2005. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA. 102(41):14665–14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae CH, Kim TH, Chu JY, Cho ES. 2013. New population of odontoblasts responsible for tooth root formation. Gene Expr Patterns. 13(5-6):197–202. [DOI] [PubMed] [Google Scholar]
- Beertsen W, Niehof A, Everts V. 1985. Effects of 1-hydroxyethylidene-1, 1-bisphosphonate (HEBP) on the formation of dentin and the periodontal attachment apparatus in the mouse. Am J Anat. 174(1):83–103. [DOI] [PubMed] [Google Scholar]
- Chen S, Gluhak-Heinrich J, Martinez M, Li T, Wu Y, Chuang HH, Chen L, Dong J, Gay I, MacDougall M. 2008. Bone morphogenetic protein 2 mediates dentin sialophosphoprotein expression and odontoblast differentiation via NF-Y signaling. J Biol Chem. 283(28):19359–19370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Gluhak-Heinrich J, Wang YH, Wu YM, Chuang HH, Chen L, Yuan GH, Dong J, Gay I, MacDougall M. 2009. Runx2, Osx, and Dspp in tooth development. J Dent Res. 88(10):904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Rani S, Wu Y, Unterbrink A, Gu TT, Gluhak-Heinrich J, Chuang HH, MacDougall M. 2005. Differential regulation of dentin sialophosphoprotein expression by Runx2 during odontoblast cytodifferentiation. J Biol Chem. 280(33):29717–29727. [DOI] [PubMed] [Google Scholar]
- D’Souza RN, Aberg T, Gaikwad J, Cavender A, Owen M, Karsenty G, Thesleff I. 1999. Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth development in mice. Development. 126(13):2911–2920. [DOI] [PubMed] [Google Scholar]
- Gao Y, Yang G, Weng T, Du J, Wang X, Zhou J, Wang S, Yang X. 2009. Disruption of Smad4 in odontoblasts causes multiple keratinocystic odontogenic tumors and tooth malformation in mice. Mol Cell Biol. 29(21):5941–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Xu X, Bringas P, Jr, Hung YP, Chai Y. 2009. Smad4-Shh-Nfic signaling cascade-mediated epithelial-mesenchymal interaction is crucial in regulating tooth root development. J Bone Miner Res. 25(5):1167–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XF, Chai Y. 2012. Molecular regulatory mechanism of tooth root development. Int J Oral Sci. 4(4):177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Bae CH, Lee JC, Ko SO, Yang X, Jiang R, Cho ES. 2013. β-Catenin is required in odontoblasts for tooth root formation. J Dent Res. 92(3):215–221. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al. 1997. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 89(5):755–764. [DOI] [PubMed] [Google Scholar]
- Lesot H, Lisi S, Peterkova R, Peterka M, Mitolo V, Ruch JV. 2001. Epigenetic signals during odontoblast differentiation. Adv Dent Res. 15:8–13. [DOI] [PubMed] [Google Scholar]
- Li CY, Prochazka J, Goodwin AF, Klein OD. 2014. Fibroblast growth factor signaling in mammalian tooth development. Odontology. 102(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Woitge HW, Braut A, Kronenberg MS, Lichtler AC, Mina M, Kream BE. 2004. Expression and activity of osteoblast-targeted Cre recombinase transgenes in murine skeletal tissues. Int J Dev Biol. 48(7):645–653. [DOI] [PubMed] [Google Scholar]
- MacDougall M, Dong J, Acevedo AC. 2006. Molecular basis of human dentin diseases. Am J Med Genet A. 140(23):2536–2546. [DOI] [PubMed] [Google Scholar]
- Nakashima K, de Crombrugghe B. 2003. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 19(8):458–466. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. 2002. The novel zinc finger–containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 108(1):17–29. [DOI] [PubMed] [Google Scholar]
- Nakatomi M, Morita I, Eto K, Ota MS. 2006. Sonic hedgehog signaling is important in tooth root development. J Dent Res. 85(5):427–431. [DOI] [PubMed] [Google Scholar]
- Narayanan K, Ramachandran A, Peterson MC, Hao J, Kolstø AB, Friedman AD, George A. 2004. The CCAAT enhancer-binding protein (C/EBP) beta and Nrf1 interact to regulate dentin sialophosphoprotein (DSPP) gene expression during odontoblast differentiation. J Biol Chem. 279(44):45423–45432. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, et al. 1997. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 89(5):765–771. [DOI] [PubMed] [Google Scholar]
- Rakian A, Yang WC, Gluhak-Heinrich J, Cui Y, Harris MA, Villarreal D, Feng JQ, MacDougall M, Harris SE. 2013. Bone morphogenetic protein-2 gene controls tooth root development in coordination with formation of the periodontium. Int J Oral Sci. 5(2):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 21(1):70–71. [DOI] [PubMed] [Google Scholar]
- Steele-Perkins G, Butz KG, Lyons GE, Zeichner-David M, Kim HJ, Cho MI, Gronostajski RM. 2003. Essential role for NFI-C/CTF transcription-replication factor in tooth root development. Mol Cell Biol. 23(3):1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Nagai H, Sasaki S. 1998. Difference in noncollagenous matrix composition between crown and root dentin of bovine incisor. Calcif Tissue Int. 42(2):97–103. [DOI] [PubMed] [Google Scholar]
- Tan X, Weng T, Zhang J, Wang J, Li W, Wan H, Lan Y, Cheng X, Hou N, Liu H, et al. 2007. Smad4 is required for maintaining normal murine postnatal bone homeostasis. J Cell Sci. 120(Pt 13):2162–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff I. 2003. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 116(Pt 9):1647–1648. [DOI] [PubMed] [Google Scholar]
- Tummers M, Thesleff I. 2009. The importance of signal pathway modulation in all aspects of tooth development. J Exp Zool B Mol Dev Evol. 312B(4):309–319. [DOI] [PubMed] [Google Scholar]
- Veis A. 1993. Mineral-matrix interactions in bone and dentin. J Bone Miner Res. 8(Suppl 2):S493–S497. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cox MK, Coricor G, MacDougall M, Serra R. 2013. Inactivation of Tgfbr2 in Osterix-Cre expressing dental mesenchyme disrupts molar root formation. Dev Biol. 382(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Jiang Y, Qin C, Liu Y, Ho SP, Feng JQ. 2014. Essential role of Osterix for tooth root but not crown dentin formation. J Bone Miner Res [Epub ahead of print Oct 27 2014] in press. doi:10.1002/jbmr.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.