Abstract
3‐18F‐l‐α‐methyl‐tyrosine ([18F]FAMT), a PET probe for tumor imaging, has advantages of high cancer‐specificity and lower physiologic background. FAMT‐PET has been proved useful in clinical studies for the prediction of prognosis, the assessment of therapy response and the differentiation of malignant tumors from inflammation and benign lesions. The tumor uptake of [18F]FAMT in PET is strongly correlated with the expression of L‐type amino acid transporter 1 (LAT1), an isoform of system L upregulated in cancers. In this study, to assess the transporter‐mediated mechanisms in FAMT uptake by tumors, we examined amino acid transporters for FAMT transport. We synthesized [14C]FAMT and measured its transport by human amino acid transporters expressed in Xenopus oocytes. The transport of FAMT was compared with that of l‐methionine, a well‐studied amino acid PET probe. The significance of LAT1 in FAMT uptake by tumor cells was confirmed by siRNA knockdown. Among amino acid transporters, [14C]FAMT was specifically transported by LAT1, whereas l‐[14C]methionine was taken up by most of the transporters. Km of LAT1‐mediated [14C]FAMT transport was 72.7 μM, similar to that for endogenous substrates. Knockdown of LAT1 resulted in the marked reduction of [14C]FAMT transport in HeLa S3 cells, confirming the contribution of LAT1 in FAMT uptake by tumor cells. FAMT is highly specific to cancer‐type amino acid transporter LAT1, which explains the cancer‐specific accumulation of [18F]FAMT in PET. This, vice versa, further supports the cancer‐specific expression of LAT1. This study has established FAMT as a LAT1‐specific molecular probe to monitor the expression of a potential tumor biomarker LAT1.
Keywords: Amino acid transporter, biomarker, molecular probe, PET, tumor imaging
The most commonly used positron emission tomography (PET) probe for tumor imaging is 2‐18F‐fluoro‐2‐deoxy‐D‐glucose ([18F]FDG), a glucose analog taken up by tumor cells via glucose transporters.1 Although [18F]FDG PET has been successfully used for the diagnosis and staging of malignant tumors, it has been recognized that [18F]FDG is accumulated in inflammatory tissues, benign lesions and some normal tissues such as brain, causing false‐positives and physiologic backgrounds.2 To overcome such disadvantages of [18F]FDG PET, various chemical compounds including amino acid derivatives that generally exhibit more tumor‐selective properties have been developed as candidates for tumor‐specific PET tracers. For example, l‐[11C‐methyl]methionine ([11C]MET) and O‐(2‐[18F]fluoroethyl)‐l‐tyrosine ([18F]FET) (Fig. S1) are, compared with [18F]FDG, less accumulated in brain and inflammatory tissues.3 However, [11C]MET and [18F]FET, as well as most of the other amino acid tracers, still suffer from some false‐positives and physiologic backgrounds in PET.4, 5
An amino acid tracer for single photon emission computed tomography (SPECT), 3‐123I‐l‐α‐methyltyrosine ([123I]IMT) (Fig. S1), is known to accumulate in malignant tumors with less false positives and low brain‐background.3 By substituting 123I of [123I]IMT with 18F, 3‐18F‐l‐α‐methyltyrosine ([18F]FAMT) (Fig. S1) was developed as a PET tracer for tumor imaging.6 In clinical studies, we and others have shown that [18F]FAMT specifically accumulates in tumors and is useful for the prediction of prognosis, the assessment of therapy response and the differentiation of malignant tumors from inflammation and benign lesions.7, 8, 9, 10, 11, 12, 13, 14 It has, therefore, been speculated that [18F]FAMT is taken up by cancer cells via amino acid transporters specifically expressed in cancer cells. The tumor uptake of [18F]FAMT in PET is, in fact, well correlated with the level of expression of L‐type amino acid transporter 1 (LAT1, SLC7A5) in various tumors, including non‐small cell lung cancer, oral squamous cell carcinoma and esophageal cancer.9, 10, 12, 14
LAT1 is one of the isoforms of system L that transports large neutral amino acids in a Na+‐independent manner.15, 16 LAT1 is predominantly expressed in a wide range of tumor cell lines, primary human tumors of various tissue origins, such as brain, lung, pancreas, breast, prostate, oral cavity, esophagus, stomach, liver, biliary tract, ovary, skin and bone, and their metastatic legions, where the expression of LAT1 correlates with tumor cell proliferation, angiogenesis and poor prognosis.12, 14, 15, 17, 19, 20, 21, 22, 23, 24 Because of such clinico‐pathological significance, LAT1 has been regarded as a candidate tumor biomarker.24 Previously, using nonradiolabeled FAMT, we examined the interaction of FAMT with LAT1 in comparison with the other system L transporter LAT2 expressed in normal tissues16 and suggested that FAMT is interacted with LAT1 but less so with LAT2.25 We showed that α‐methyl moiety of FAMT is responsible for its preference for LAT1 to LAT2.25 In the present study, to reveal the transporter‐mediated mechanisms of tumor‐specific uptake of [18F]FAMT and to establish FAMT as a molecular probe to monitor the expression of a potential tumor biomarker LAT1, we have synthesized 14C‐labled FAMT, which is easier to handle in in vitro studies, and used it to obtain direct evidence of FAMT transport by LAT1 and further to examine whether FAMT is transported by the other amino acid transporters.
Materials and Methods
Chemicals
For the synthesis of 3‐fluoro‐l‐α‐methyl[carboxyl‐14C]tyrosine ([14C]FAMT), 3‐fluoro‐4‐methoxyphenylacetone as a starting material was purchased from NARD Institute (Amagasaki, Japan). [14C]FAMT was synthesized by Sekisui Medical (Tokyo, Japan) to obtain high specific radioactivity by Bücherer–Strecker reaction.26 [14C]FAMT was identified by the analysis of 1H‐nuclear magnetic resonance (AV400M; Bruker Biospin, Rheinstetten, Germany), high performance liquid chromatograph (Agilent 1200) and mass spectrum (LTQXL; Thermo Fisher Scientific, Waltham, MA, USA).25 The purity of the [14C]FAMT determined on high‐performance liquid chromatography was 99% and its specific radioactivity was 1.77 GBq/mmol.
l‐[14C]Leucine and l‐[14C]Alanine were purchased from Moravek Biochemicals (Brea, CA, USA). l‐[14C]Methionine and l‐[14C]Cystine were from American Radiolabeled Chemicals (St. Louis, MO, USA). l‐[14C]Glutamine (10.1 GBq/mmol) and l‐[14C]Tyrosine were from PerkinElmer (Boston, MA, USA) and Amersham Biosciences (Buckinghamshire, UK), respectively.
Non‐radiolabeled FAMT was purchased from NARD Institute (Amagasaki, Japan).25 Amino acids and 2‐amino‐2‐norbornanecarboxylic acid (BCH) were from Sigma‐Aldrich (St. Louis, MO, USA). Other general chemicals were from Wako (Osaka, Japan).
Expression in Xenopus laevis oocytes and transport measurements
cDNA for human transporters used in this study are presented in Table S1. cRNA were synthesized in vitro from the linearized plasmids using an mMessage mMachine Kit, polyadenylated with a Poly(A) Tailing Kit and purified with a MEGAclear Kit (Ambion, Austin, TX, USA) according to the manufacturer's protocol.
For Xenopus oocyte expression, defolliculated oocytes were injected with polyadenylated cRNA (25 ng/oocyte).15 For functional expression of LAT1, LAT2, y+LAT1 and y+LAT2, equimolar 4F2hc cRNA was co‐injected.15, 16, 17 For B0AT1, equimolar collectrin cRNA was co‐injected.27
Transport measurements were performed 2–4 days after injection as previously described.15, 17 In brief, the oocytes were incubated at room temperature with 500 μL uptake buffer containing 14C‐labeled compounds. ND96 solution (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, pH 7.5) was used as the uptake buffer for Na+‐dependent transport. For Na+‐free uptake buffer, NaCl was replaced by choline‐Cl. The radioactivity was determined by liquid scintillation counting. Functional expression of each transporter in Xenopus oocytes was confirmed by measuring the transport of its typical substrate as described in the legends to figures. To determine the concentration dependence of transport, the transport rate at each concentration was obtained by subtracting the uptake rate of control oocytes without cRNA injection from that of the oocytes expressing LAT1. The Michaelis constant (K m) and maximal transport rate (V max) were determined by plotting the transport rate against FAMT concentration and fitting to a Michaelis–Menten curve using enzyme kinetics module of Sigma Plot 12.5 (Systat Software, San Jose, CA, USA).
For transport measurements, 5–12 oocytes were used for each measurement. To confirm the reproducibility of the results, three separate experiments using different batches of oocytes were performed. Statistical differences were determined using Student's unpaired t‐test. Differences were considered significant at P < 0.05.
siRNA knockdown of LAT1
Non‐targeting control siRNA#1 (D‐001810‐01‐05) and #2 (D‐001810‐02‐05) were purchased from Thermo Fisher Scientific. LAT1 siRNA#1 (s15653), #2 (s15654) and #3 (s15655) were from Ambion. HeLa S3 cells were seeded in six‐well plates at a density of 2 × 105 cells/well and transfected with 24 nM of siRNA using RNAiMax (Invitrogen, Carlsbad, CA, USA). Two days after transfection, cells were reseeded in a 24‐well plate at 5 × 104 cells/well and in a 6‐cm dish at 4 × 105 cells/dish for [14C]FAMT transport measurement and western blot analysis, respectively.
Transport measurement in HeLa S3 cells
Transport measurement was carried out as described previously,25 2 days after reseeding of HeLa S3 cells treated or not treated with siRNA. Briefly, cells were incubated with 300 μL of Hanks balanced salt solution containing 1 μM [14C]FAMT or l‐[14C]methionine for 1 min at 37°C. Radioactivity was measured by liquid scintillation counting.
Western blot
Two days after reseeding of HeLa S3 cells treated or not treated with siRNA, the cells were lysed in buffer containing 50 mM Tris‐HCl (pH 7.4), 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (pH 8.0), 1 mM phenylmethanesulfonyl fluoride, 1% NP40 and protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany). The western blot was performed as described previously.25 Antihuman LAT1 polyclonal antibody17 and antihuman β‐actin monoclonal antibody (Sigma‐Aldrich, St. Louis, MO, USA) were used as primary antibodies at 1:5000 dilutions.
Results
[14C]FAMT is transported only by LAT1 among amino acid transporters
To determine the amino acid transporters responsible for FAMT uptake, we examined amino acid transport systems transporting aromatic amino acids such as systems L (LAT1~4), T (TAT1), B0 (B0AT1), B0,+ (ATB0,+), b0,+ and y+L (y+LAT1, y+LAT2) (Table S1).27, 28 In addition, we tested systems ASC (ASCT1, ASCT2) and N/A (SNAT1~5) mainly transporting small neutral amino acids to cover most neutral amino acid transporters (Table S1).27, 28 The transport of [14C]FAMT by each transporter functionally expressed in Xenopus oocytes was measured and compared with that of l‐[14C]methionine.
Among the transporters from system L for large neutral amino acids, LAT1‐expressing oocytes exhibited a high level of [14C]FAMT uptake compared with control oocytes (Fig. 1a). Its transport rate was comparable to that of l‐[14C]leucine, a typical substrate of LAT1. LAT2, LAT3 and LAT4 did not mediate [14C]FAMT transport (Fig. 1b–d). In contrast, l‐[14C]methionine was transported by all the system L transporters (Fig. 1). Other amino acid transporters transporting aromatic amino acids, TAT1, B0AT1, ATB0,+, b0,+, y+LAT1 and y+LAT2, did not transport [14C]FAMT, whereas these transporters except TAT1 transported l‐[14C]methionine (Fig. 2). The transporters for small neutral amino acids, ASCT1, ASCT2, SNAT1, SNAT2, SNAT3, SNAT4 and SNAT5, did not transport [14C]FAMT, whereas l‐[14C]methionine was transported by all except ASCT1 (Fig. S2). The results on the amino acid transporters are summarized in Table 1.
Figure 1.
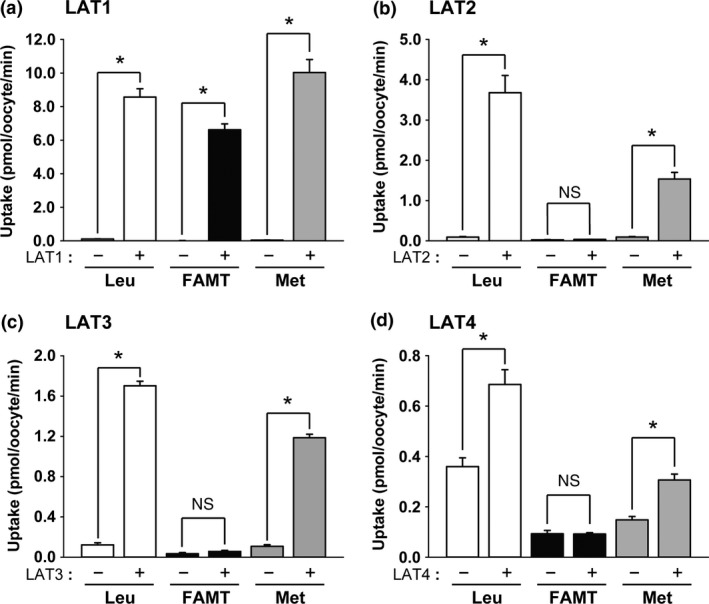
[14C]FAMT transport by system L transporters. The uptakes of 50 μM [14C]FAMT (FAMT) and l‐[14C]methionine (Met) as well as a typical substrate l‐[14C]leucine (Leu) (50 μM) were measured for 15 min for LAT1 (a), LAT2 (b) and LAT3 (c), and for 30 min for LAT4 (d). The uptakes were measured in Na+‐free uptake buffer on the control oocytes (“−”) and the oocytes expressing each transporter (“+”). The uptake rates were expressed as mean ± SEM (n = 8–12). *P < 0.05; n.s., not significant.
Figure 2.
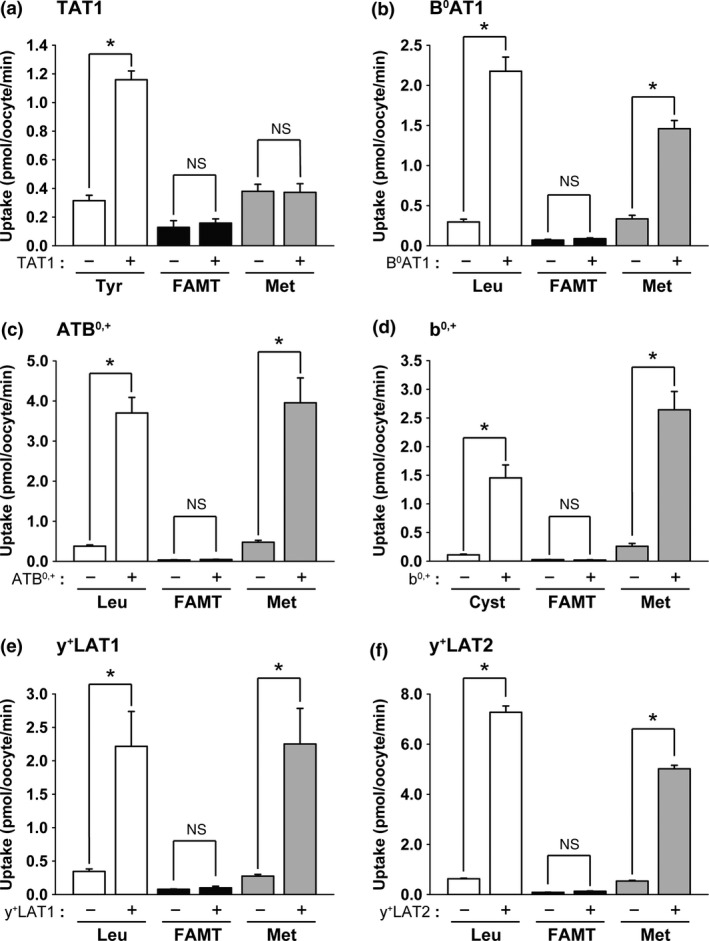
[14C]FAMT transport by neutral amino acid transporters other than system L that transport aromatic amino acids. The uptakes of 50 μM l‐[14C]tyrosine (Tyr), [14C]FAMT (FAMT) and l‐[14C]methionine (Met) by TAT1 (a), the uptakes of 50 μM l‐[14C]leucine (Leu), [14C]FAMT (FAMT) and l‐[14C]methionine (Met) by B0 AT1 (b), ATB 0,+ (c), y+ LAT1 (e) and y+ LAT2 (f), and the uptakes of 50 μM l‐[14C]cystine (Cyst), [14C]FAMT (FAMT) and l‐[14C]methionine (Met) by b0,+ (d) were measured on the control oocytes (“−”) and the oocytes expressing each transporter (“+”). For the functional expression of b0,+ in (d), rBAT, an auxiliary subunit of system b0,+ transporter strongly inducing system b0,+ activity in Xenopus oocytes, was expressed in Xenopus oocytes, therefore, the induced system b0,+ activity was not that of human but of Xenopus although human rBAT was expressed.16 l‐[14C]Tyrosine and l‐[14C]cystine were used as typical substrates of TAT1 and b0,+, respectively. l‐[14C]Leucine was used as a typical substrate of B0 AT1, ATB 0,+, y+ LAT1 and y+ LAT2. Na+‐free uptake buffer was used for TAT1 and b0,+, whereas ND96 solution was used for the others. Uptakes were measured for 30 min for B0 AT1 and for 15 min for the others. Uptake rates were expressed as mean ± SEM (n = 5–10). *P < 0.05; n.s., not significant.
Table 1.
Transport of [14C]FAMT and [14C]Met by amino acid transporters
| Amino acid transport system | Transporter | Gene | [14C]FAMT | [14C]Met |
|---|---|---|---|---|
| L | LAT1 | SLC7A5 | +† | +‡ |
| L | LAT2 | SLC7A8 | − | + |
| L | LAT3 | SLC43A1 | − | + |
| L | LAT4 | SLC43A2 | − | + |
| T | TAT1 | SLC16A10 | − | − |
| B0 | B0AT1 | SLC6A19 | − | + |
| B0,+ | ATB0,+ | SLC6A14 | − | + |
| b0,+† | − | + | ||
| y+L | y+LAT1 | SLC7A7 | − | + |
| y+L | y+LAT2 | SLC7A6 | − | + |
| ASC | ASCT1 | SLC1A4 | − | − |
| ASC | ASCT2 | SLC1A5 | − | + |
| N/A | SNAT1 | SLC38A1 | − | + |
| N/A | SNAT2 | SLC38A2 | − | + |
| N/A | SNAT3 | SLC38A3 | − | + |
| N/A | SNAT4 | SLC38A4 | − | + |
| N/A | SNAT5 | SLC38A5 | − | + |
†“+” indicates that [14C]FAMT or [14C]Met is transported by the designated transporter, whereas “−” indicates that [14C]FAMT or [14C]Met is not transported. ‡b0,+ activity was induced by expressing rBAT (SLC3A1), an auxiliary subunit of system b0,+ transporter, in Xenopus oocytes (see the legend to Fig. 2).
Concentration‐dependent [14C]FAMT transport by LAT1
Kinetic properties of [14C]FAMT transport by LAT1 were determined. As shown in Figure 3, LAT1‐mediated [14C]FAMT transport was saturable and followed Michaelis–Menten kinetics. Its Km and Vmax values were 72.7 ± 11.0 μM and 12.1 ± 0.5 pmol/oocyte/min, respectively.
Figure 3.
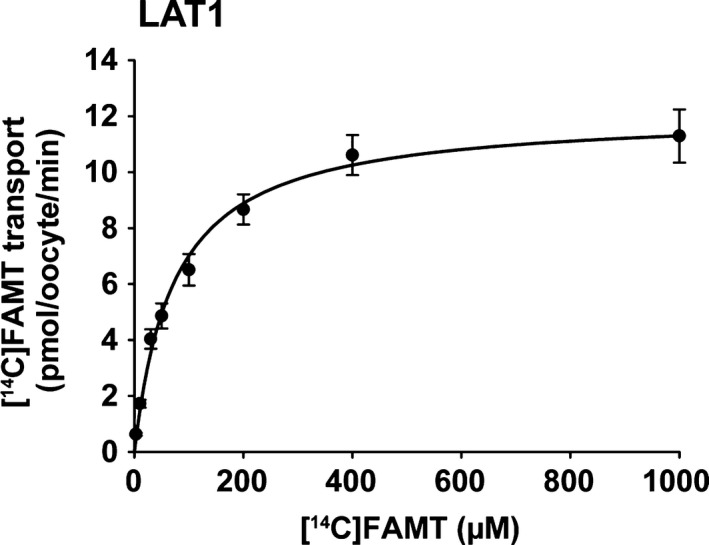
Concentration dependence of [14C]FAMT transport. Concentration dependence of [14C]FAMT transport mediated by LAT1 was determined. LAT1‐mediated transport of [14C]FAMT at each concentration was measured for 15 min in Na+‐free uptake buffer. Transport rates were expressed as means ± SEM (n = 7–9) and fit to Michaelis–Menten curve.
LAT1 knockdown confirms the contribution of LAT1 in [14C]FAMT uptake
To confirm the role of LAT1 in FAMT uptake in cancer cells, we examined the effects of LAT1 knockdown with siRNA on the [14C]FAMT uptake in HeLa S3 cells. As shown in Figure 4a, LAT1 protein expression was highly reduced by the treatment with LAT1 siRNA in HeLa S3 cells, whereas control siRNA had no effect on LAT1 protein level. Accordingly, significant reduction in [14C]FAMT uptake was obtained by LAT1 knockdown (Fig. 4b). The uptake of l‐[14C]methionine was also reduced, although the reduction of [14C]methoinine uptake by LAT1 knockdown was less than that of [14C]FAMT uptake (Fig. 4c).
Figure 4.
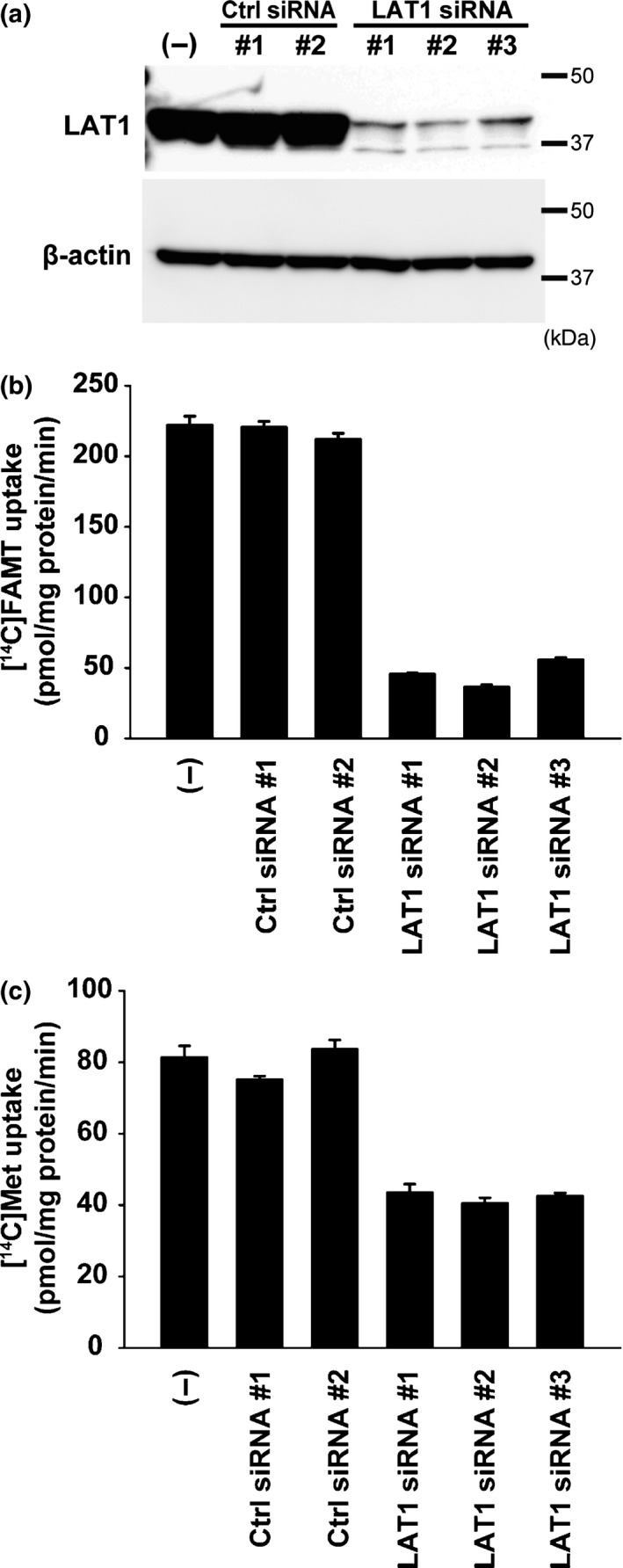
Effect of LAT1 knockdown on the uptake of [14C]FAMT and l‐[14C]methionine in HeLa S3 cells. HeLa S3 cells were transfected with LAT1 siRNA (LAT1 siRNA#1, #2, #3) or non‐targeting control siRNA (Ctrl siRNA#1, #2) and compared with non‐treated control (“(−)”). Knockdown of LAT1 with siRNA highly reduced LAT1 protein level in HeLa S3 cells in western blot (a). Accordingly, [14C]FAMT uptake was decreased by the treatment with LAT1 siRNA (b). The uptake of l‐[14C]methionine (Met) was also reduced, although the reduction of [14C]methoinine uptake by LAT1 knockdown was less than that of [14C]FAMT uptake (c). Control siRNA did not affect the levels of LAT1 protein and the uptake of [14C]FAMT and l‐[14C]methionine (a–c).
Discussion
Glucose analogues and amino acid derivatives have been used as PET tracers for tumor imaging on the basis that tumor cells take up more nutrients to compensate for increased cellular metabolism.1, 2, 3 The upregulation of transporters responsible for the uptake of glucose and amino acids in tumor cells ensures the usefulness of such PET tracers in the diagnosis of malignant tumors.1, 2, 3 Therefore, the assessment of the interaction of PET tracers with transporters could be beneficial to understand the mechanisms of their uptake in tumors and non‐tumor tissues in PET. In this regard, many studies have been conducted on tumor cell lines to reveal amino acid transporters responsible for the uptake of amino acid tracers. Amino acid transport machineries on the plasma membrane were once distinguished and defined as amino acid transport systems such as L, A, B0,+ and so on by means of inhibitors and based on Na+‐dependence.28 Now it has been revealed that each transport system consists of more than one isoform, which cannot, in general, be distinguished by the classic maneuvers due to the limited specificity of inhibitors.27 Therefore, in the previous study, to examine the transporter responsible for FAMT uptake, we used the culture cells stably expressing LAT1 or LAT2 and looked at the effects of cold FAMT on the uptake and efflux of a substrate of system L, l‐[14C]leucine.25 However, except LAT1 and LAT2, culture cells are not appropriate for the exogenous expression of amino acid transporters due to high‐background amino acid uptake. In the present study, to examine whether FAMT is transported by each amino acid transporter, we have expressed it individually into Xenopus oocytes, which have an advantage of low‐background amino acid uptake.
Previously, based on the observations that non‐labeled FAMT competitively inhibited LAT1‐medicted l‐[14C]leucine uptake and that FAMT induced the efflux of l‐[14C]leucine mediated by LAT1 via an exchange mechanism, we suggested that FAMT is transported by LAT1.25 In the present study, using radiolabeled FAMT, we directly showed that FAMT is transported by LAT1 (Fig. 1) and confirmed the significant contribution of LAT1 in FAMT uptake in tumor cells by means of knockdown of LAT1 (Fig. 4). The Km of FAMT transport determined by direct measurement of [14C]FAMT uptake was 72.7 μM, which is a little higher than 27.5 μM obtained by measuring FAMT‐induced 14C‐leucine efflux in the previous study.25 This is probably because of the underestimation of the transport based on the efflux measurement due to its limited linearity. The Km of LAT1‐mediated [14C]FAMT transport is similar to that of known LAT1 substrates,17 which supports that FAMT is transported by LAT1 efficiently in vivo. Besides LAT1, FAMT was not transported by the other system L isoforms LAT2, LAT3 and LAT4 (Fig. 1). In the previous study, cold FAMT (40 μM) induced a small but significant amount of l‐[14C]leucine efflux in LAT2‐expressing culture cells, which suggested that FAMT is transported by LAT2 at a low level,25 whereas [14C]FAMT (50 μM) was not transported by LAT2 expressed in Xenopus oocytes in the present study (Fig. 1). This discrepancy is due to the residual endogenous LAT1 remaining in LAT2‐expressing culture cells, which we confirmed using a LAT1‐specific inhibitor (data not shown).
In the present study, we furthermore examined transporters for aromatic amino acids and small neutral amino acids and provided direct evidence that FAMT is transported only by LAT1 among amino acid transporters (Table 1). Among them, it is intriguing that B0AT1 and ATB0,+, the broad scope transporters for system B0 and system B0,+, respectively, did not transport FAMT (Fig. 2). By using inhibitors and based on Na+‐dependence, systems B0 and B0,+ were suggested to transport a SPECT tracer [123I]IMT structurally identical to FAMT except for the substitution in position 3 of an aromatic ring (Fig. S1).29, 30 At the moment, it is not clear whether this is because of the inaccuracy in assigning transport systems using less specific inhibitors in culture cells or the fundamental difference between IMT and FAMT in the interaction with B0AT1 and ATB0,+. Interestingly, it has recently been reported that a therapeutic drug for Parkinson's disease l‐DOPA, in which a hydroxyl group is added to position 3 of the aromatic ring of l‐tyrosine, is not transported by B0AT1, although l‐tyrosine is well transported.31 Thus, it may be possible that the presence of fluorine or iodine in position 3 affects the interaction, particularly with B0AT1.
The high specificity of FAMT to LAT1 as described above allows FAMT to image LAT1. LAT1 is predominantly expressed in malignant tumors and metastases but not in benign lesions,23, 24 so that [18F]FAMT is beneficial for imaging malignant tumors. In contrast, in the present study, we showed that l‐[14C]methionine is transported by most amino acid transporters widely expressed in the body (Table 1), which could result in the uptake by non‐tumor tissues such as liver and inflammatory lesions in [11C]MET PET.4 In the LAT1‐knockdown experiments, the reduction of [14C]methoinine uptake was less than that of [14C]FAMT uptake (Fig. 4), which is consistent with the results summarized in Table 1 showing that [14C]methionine is transported by multiple transporters, whereas [14C]FAMT is transported by only LAT1. The high specificity of FAMT to LAT1 could explain the cancer‐specific accumulation and low physiologic background in [18F]FAMT PET. This, vice versa, further supports the cancer‐specificity in the expression of LAT1. Because LAT1 is a potential therapeutic target as well as a diagnosis biomarker of cancer, the inhibitors of LAT1 have been developed to suppress tumor growth.32 LAT1 can also be used for tumor‐targeted delivery of anti‐tumor agents such as melphalan17, 33 and para‐boronophenylalanine in boron neutron capture therapy.34 Therefore, the LAT1‐specific probe established in the present study could also be useful for the selection of subjects and the monitoring of therapeutic effects in the LAT1‐targeting therapies.
Disclosure Statement
The authors declare no conflict of interest.
Supporting information
Fig. S1 Chemical structures of FAMT, IMT, FET and Met.
Fig. S2 [14C]FAMT transport by amino acid transporters for small neutral amino acids.
Table S1 Human cDNA and their constructs for Xenopus oocytes expression.
Acknowledgments
This work was supported by a grant from the Japan Society for the Promotion of Science, the Advanced Research for Medical Products Mining Program of the National Institute of Biomedical Innovation and Japan Regional Innovation Strategy Support Program of the Ministry of Education, Culture, Sports, Science and Technology.
Cancer Sci 107 (2016) 347–352
Funding Information
Grants‐in‐Aid for Scientific Research from the Japan Society for the Promotion of Science, the Advanced Research for Medical Products Mining Program of the National Institute of Biomedical Innovation and Japan Regional Innovation Strategy Support Program of the Ministry of Education, Culture, Sports, Science and Technology.
References
- 1. Plathow C, Weber WA. Tumor cell metabolism imaging. J Nucl Med 2008; 49(Suppl 2): 43S–63S. [DOI] [PubMed] [Google Scholar]
- 2. Cook GJ, Maisey MN, Fogelman I. Normal variants, artefacts and interpretative pitfalls in PET imaging with 18‐fluoro‐2‐deoxyglucose and carbon‐11 methionine. Eur J Nucl Med 1999; 26: 1363–78. [DOI] [PubMed] [Google Scholar]
- 3. Jager PL, Vaalburg W, Pruim J, de Vries EG, Langen KJ, Piers DA. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med 2001; 42: 432–45. [PubMed] [Google Scholar]
- 4. Deloar HM, Fujiwara T, Nakamura T et al Estimation of internal absorbed dose of l‐[methyl‐11C]methionine using whole‐body positron emission tomography. Eur J Nucl Med 1998; 25: 629–33. [DOI] [PubMed] [Google Scholar]
- 5. Pauleit D, Floeth F, Herzog H et al Whole‐body distribution and dosimetry of O‐(2‐18F‐fluoroethyl)‐l‐tyrosine. Eur J Nucl Med Mol Imaging 2003; 30: 519–24. [DOI] [PubMed] [Google Scholar]
- 6. Tomiyoshi K, Amed K, Muhammad S et al Synthesis of isomers of 18F‐labelled amino acid radiopharmaceutical: position 2‐ and 3‐l‐18F‐α‐methyltyrosine using a separation and purification system. Nucl Med Commun 1997; 18: 169–75. [DOI] [PubMed] [Google Scholar]
- 7. Kaira K, Oriuchi N, Otani Y et al Diagnostic usefulness of fluorine‐18‐α‐methyltyrosine positron emission tomography in combination with 18F‐fluorodeoxyglucose in sarcoidosis patients. Chest 2007; 131: 1019–27. [DOI] [PubMed] [Google Scholar]
- 8. Inoue T, Koyama K, Oriuchi N et al Detection of malignant tumors: whole‐body PET with fluorine 18 α‐methyl tyrosine versus FDG—Preliminary study. Radiology 2001; 220: 54–62. [DOI] [PubMed] [Google Scholar]
- 9. Kaira K, Oriuchi N, Otani Y et al Fluorine‐18‐α‐methyltyrosine positron emission tomography for diagnosis and staging of lung cancer: a clinicopathologic study. Clin Cancer Res 2007; 13: 6369–78. [DOI] [PubMed] [Google Scholar]
- 10. Kaira K, Oriuchi N, Shimizu K et al Evaluation of thoracic tumors with 18F‐FMT and 18F‐FDG PET‐CT: a clinicopathological study. Int J Cancer 2009; 124: 1152–60. [DOI] [PubMed] [Google Scholar]
- 11. Kim M, Achmad A, Higuchi T et al Effects of intratumoral inflammatory process on 18F‐FDG uptake: pathologic and comparative study with 18F‐fluoro‐α‐methyltyrosine PET/CT in oral squamous cell carcinoma. J Nucl Med 2015; 56: 16–21. [DOI] [PubMed] [Google Scholar]
- 12. Nobusawa A, Kim M, Kaira K et al Diagnostic usefulness of 18F‐FAMT PET and L‐type amino acid transporter 1 (LAT1) expression in oral squamous cell carcinoma. Eur J Nucl Med Mol Imaging 2013; 40: 1692–700. [DOI] [PubMed] [Google Scholar]
- 13. Kaira K, Oriuchi N, Yanagitani N et al Assessment of therapy response in lung cancer with 18F‐α‐methyl tyrosine PET. Am J Roentgenol 2010; 195: 1204–11. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki S, Kaira K, Ohshima Y et al Biological significance of fluorine‐18‐α‐methyltyrosine (FAMT) uptake on PET in patients with oesophageal cancer. Br J Cancer 2014; 110: 1985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanai Y, Segawa H, Miyamoto KI, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem 1998; 273: 23629–32. [DOI] [PubMed] [Google Scholar]
- 16. Fotiadis D, Kanai Y, Palacín M. The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med 2013; 34: 139–58. [DOI] [PubMed] [Google Scholar]
- 17. Yanagida O, Kanai Y, Chairoungdua A et al Human L‐type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta 2001; 1514: 291–302. [DOI] [PubMed] [Google Scholar]
- 18. Nawashiro H, Otani N, Shinomiya N et al L‐type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int J Cancer 2006; 119: 484–92. [DOI] [PubMed] [Google Scholar]
- 19. Kaira K, Oriuchi N, Imai H et al Prognostic significance of L‐type amino acid transporter 1 expression in resectable stage I‐III nonsmall cell lung cancer. Br J Cancer 2008; 98: 742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaira K, Sunose Y, Arakawa K et al Prognostic significance of L‐type amino‐acid transporter 1 expression in surgically resected pancreatic cancer. Br J Cancer 2012; 107: 632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toyoda M, Kaira K, Ohshima Y et al Prognostic significance of amino‐acid transporter expression (LAT1, ASCT2 and xCT) in surgically resected tongue cancer. Br J Cancer 2014; 110: 2506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaira K, Sunose Y, Ohshima Y et al Clinical significance of L‐type amino acid transporter 1 expression as a prognostic marker and potential of new targeting therapy in biliary tract cancer. BMC Cancer 2013; 13: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaira K, Oriuchi N, Imai H et al L‐type amino acid transporter 1 and CD98 expression in primary and metastatic sites of human neoplasms. Cancer Sci 2008; 99: 2380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Q, Holst J. L‐type amino acid transport and cancer: targeting the mTORC1 pathway to inhibit neoplasia. Am J Cancer Res 2015; 5: 1281–94. [PMC free article] [PubMed] [Google Scholar]
- 25. Wiriyasermkul P, Nagamori S, Tominaga H et al Transport of 3‐fluoro‐l‐α‐methyl‐tyrosine by tumor‐upregulated L‐type amino acid transporter 1: a cause of the tumor uptake in PET. J Nucl Med 2012; 53: 1253–61. [DOI] [PubMed] [Google Scholar]
- 26. Halldin C, Schoeps KO, Stone‐Elander S, Wiesel FA. The Bücherer‐Strecker synthesis of d‐ and l‐(1‐11C)tyrosine and the in vivo study of l‐(1‐11C)tyrosine in human brain using positron emission tomography. Eur J Nucl Med 1987; 13: 288–91. [DOI] [PubMed] [Google Scholar]
- 27. Bröer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 2008; 88: 249–86. [DOI] [PubMed] [Google Scholar]
- 28. Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev 1990; 70: 43–77. [DOI] [PubMed] [Google Scholar]
- 29. Shikano N, Kawai K, Nakajima S et al Renal accumulation and excretion of radioiodinated 3‐iodo‐α‐methyl‐l‐tyrosine. Ann Nucl Med 2004; 18: 263–70. [DOI] [PubMed] [Google Scholar]
- 30. Riemann B, Kopka K, Stogbauer F et al Kinetic parameters of 3‐[123I]iodo‐L‐α‐methyl tyrosine ([123I]IMT) transport in human GOS3 glioma cells. Nucl Med Biol 2001; 28: 293–7. [DOI] [PubMed] [Google Scholar]
- 31. Camargo SM, Vuille‐dit‐Bille RN, Mariotta L et al The molecular mechanism of intestinal levodopa absorption and its possible implications for the treatment of Parkinson's disease. J Pharmacol Exp Ther 2014; 351: 114–23. [DOI] [PubMed] [Google Scholar]
- 32. Oda K, Hosoda N, Endo H et al L‐type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci 2010; 101: 173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim DK, Kanai Y, Choi HW et al Characterization of the system L amino acid transporter in T24 human bladder carcinoma cells. Biochim Biophys Acta 2002; 1565: 112–21. [DOI] [PubMed] [Google Scholar]
- 34. Wongthai P, Hagiwara K, Miyoshi Y et al Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2. Cancer Sci 2015; 106: 279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Chemical structures of FAMT, IMT, FET and Met.
Fig. S2 [14C]FAMT transport by amino acid transporters for small neutral amino acids.
Table S1 Human cDNA and their constructs for Xenopus oocytes expression.


