Abstract
Radical prostatectomy (RP) and radiotherapy (RT) are highly effective in improving prostate cancer survival. However, both have a detrimental effect on erectile function (EF). Penile rehabilitation consists of understanding the mechanisms that cause erectile dysfunction (ED) and utilizing pharmacologic agents, devices or interventions to promote male sexual function. For the past decade, many researchers have pursued to define effective treatment modalities to improve ED after prostate cancer treatment. Despite the understanding of the mechanisms and well-established rationale for postprostate treatment penile rehabilitation, there is still no consensus regarding effective rehabilitation programs. This article reviews a contemporary series of trials that assess penile rehabilitation and explore treatment modalities that might play a role in the future. Published data and trials related to penile rehabilitation after RP and RT were reviewed and presented. Although recent trials have shown that most therapies are well-tolerated and aid in some degree on EF recovery, we currently do not have tangible evidence to recommend an irrefutable penile rehabilitation algorithm. However, advancements in research and technology will ultimately create and refine management options for penile rehabilitation.
Keywords: erectile dysfunction, penile rehabilitation, technology
INTRODUCTION
The American Cancer Society estimates that about 233 000 new cases of prostate cancer will be diagnosed in the year 2014.1 Given the improvement in detection and treatment modalities, prostate cancer patients are diagnosed at an earlier stage that can be both cured and controlled. However, despite the improved treatment efficacy, secondary effects such as erectile dysfunction (ED) still remain as a major concern for both physicians and patients. Alemozaffar et al.2 attempted to predict erectile function (EF) after prostate cancer patients undergoing radical prostatectomy (RP), external radiotherapy (RT), and brachytherapy. Pretreatment sexual health-related quality-of-life score, age, serum prostate-specific antigen level, race/ethnicity, body mass index, and intended treatment details were associated with functional erections 2 years after treatment. They found that 48% of patients (n = 1027) with functional erections prior to treatment reported ED 2 years after treatment. In the prostatectomy cohort, 60% of patients with prior functional erections reported ED, along with 42% and 37% of the external RT and brachytherapy cohorts, respectively. The prostate cancer outcomes study revealed 60% of men experienced self-reported ED 18 months after RP, and only 28% of men reported erections firm enough for intercourse at a 5-year follow-up.3 This pernicious effect on sexual function has wider effects on men's quality-of-life and general well-being.4
The etiology of ED after prostate cancer treatment has been found to be multifactorial. There is evidence that changes of neuropraxia, ischemic and hypoxic insults, fibrotic remodeling, and apoptosis contribute to ED even after meticulous dissection in attempt to preserve the neurovascular bundle (NVB) during prostatectomy.5,6 Neuropraxia ensues due to mechanical stretching of cavernous nerves, thermal injury from electrocautery use and inflammation from surgical trauma. The persistent lack of erections after neuropraxia can itself set up a cascade of harmful processes to EF. Wang6 summarized the mechanism of how chronic impotence reduces blood flow to the corporeal bodies, which consequently leads to fibrosis and transformation of the trabecular smooth muscle through collagen, which itself leads to the loss of the veno-occlusive mechanism required to maintain erections. Furthermore, ligation of accessory internal pudendal arteries during prostatectomy decreases arterial inflow which intensifies hypoxia and ultimately leads to apoptosis.6,7
Radiotherapy also causes ED by damaging the NVB, penile vasculature, and cavernosal tissue, although the impact to these components is different. Stember and Mulhall8 reported that there are three mechanisms of injury contributing to the development of ED. The first mechanism is vasculogenic. Radiation precipitates fibrosis and ischemia by damaging endothelial cells in penile arteries and sinusoids of the corpora cavernosum in a dose- and time-dependent manner. Second, neurovascular injury occurs but to a much lesser extent. Zelefsky and Eid9 classified neurogenic injury in 3% of post-RT patients in a penile Duplex Doppler-based study. The third major effect is the dose-dependent ultrastructural changes that generate corporal fibrosis, venous leakage and, therefore, inability to maintain erections. In many occasions, radiation is accompanied by androgen deprivation therapy (ADT) which by itself has been found to decrease EF, ejaculation, and libido.10
Penile rehabilitation consists of understanding these mechanisms and utilizing pharmacologic agents, devices or interventions to promote male sexual function before and after any insult to the penile erectile physiologic axis.11 For the past decade, many researchers have pursued to define effective treatment modalities to improve ED after prostate cancer treatment. Despite the understanding of the mechanisms and well-established rationale for postprostate treatment penile rehabilitation, there is still no consensus regarding effective rehabilitation programs. This article will review a contemporary series of trials and studies pertaining to penile rehabilitation after prostate cancer treatment.
DISCUSSION
Phosphodiesterase 5 inhibitors
Since entering the market in 1998, phosphodiesterase-5 inhibitors (PDE5is) revolutionized the treatment of ED. It is relatively safe profile, and ease of use has made them popular among patients and physicians. PDE5is decrease the breakdown of cyclic guanosine monophosphate (cGMP) that increases the efflux of intracellular calcium ions and result in smooth muscle relaxation and erection. This mechanism is potentiated by nitric oxide production stimulated by cavernous nerves.12,13 Clinical trials using PDEis presented in this review are summarized in Table 1.
Table 1.
Penile rehabilitation after prostate cancer treatment: summary of clinical trials using oral PDE5i
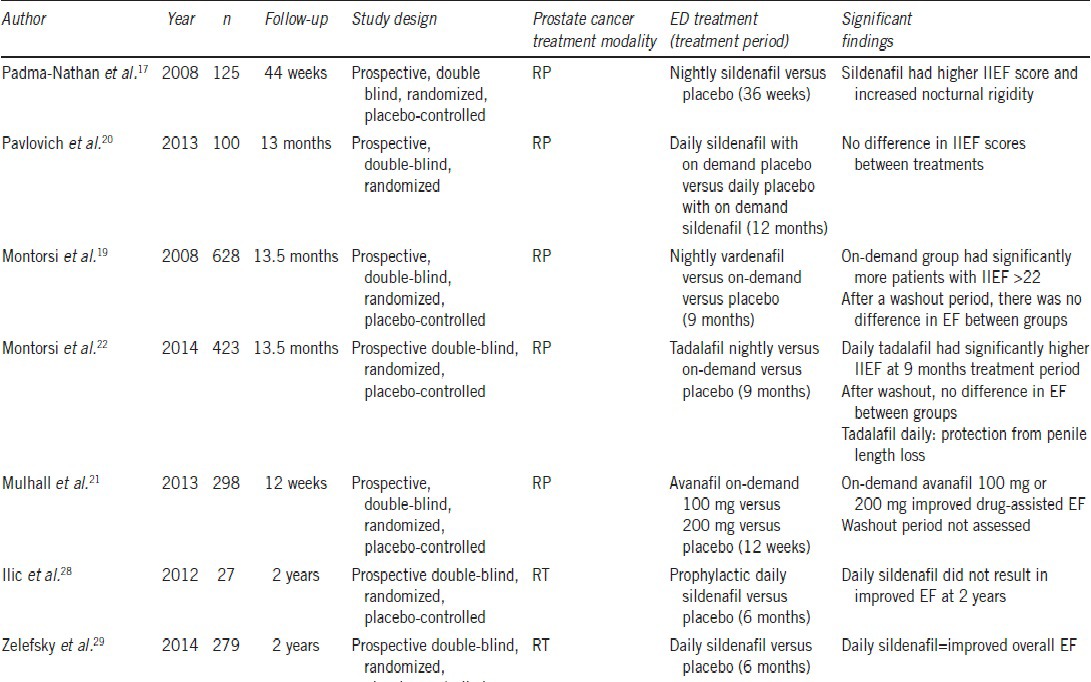
A number of studies have investigated the role of PDE5is in postprostate cancer treatment patients and many of these reported higher International Index of EF (IIEF) scores and spontaneous erection rates.14,15,16,17 Padma-Nathan et al.17 performed the first multicenter, double-blind, randomized, placebo-controlled trial to our knowledge evaluating the effects of nightly sildenafil on EF after bilateral nerve-sparing RP. They randomized 125 patients into three parallel fixed-dose treatment groups: placebo and 50 mg or 100 mg sildenafil. Trial enrollment ended prematurely because of a “lack of treatment effect,” but 82 men completed the trial and 76 completed the postwashout evaluation period. After an 8-week washout period, only one of 25 patients (4%) in the placebo arm was potent, versus 14 of 51 patients (27%) in the sildenafil 50 mg and 100 mg groups combined. The significant difference of P = 0.016 suggested that nightly sildenafil has a benefit for penile rehabilitation after prostatectomy. Critics of this study state that the placebo rate was lower than that specified by the investigators.18 Furthermore, treatment administration began 1 month after surgery, and there was a significant patient dropout rate, which may call into question the statistical power of the study.
Montorsi et al.19 published the REINVENT penile rehabilitation after prostatectomy trial in 2008. REINVENT was a 628- patient multicenter, double-blind placebo-controlled trial in which patients with a baseline IIEF score of >26 were randomized into taking nightly vardenafil, on-demand vardenafil, or placebo for 9 months. Primary outcome was the percentage of patients achieving an EF domain of the IIEF (IIEF-EF) score >21. After the 9-month treatment period, on-demand vardenafil was associated with more patients scoring ≥22 compared to placebo. However, results from this trial did not support nightly vardenafil over on-demand dosing, and as a matter of fact found no improvement in IIEF score after washout for either protocol compared with placebo. Limitations to this study are that dropout rates ranged between 31%–35% in the study arms and there was no defined limit in drug usage in the on-demand arm, therefore creating doubt that the two study arms truly represented different dosage patterns. The authors concluded that their data argue against the use of nightly PDE5i in the treatment of the postprostatectomy ED.
Pavlovich et al.20 found similar results when they randomized 100 preoperatively potent men who had undergone nerve-sparing RP to receive either nightly sildenafil and on-demand placebo (nightly sildenafil group), or on-demand sildenafil and nightly placebo (on-demand sildenafil group; with a maximum on-demand dose of 6 tablets per month) starting the day after surgery for 12 months. All men had previously completed a presurgery IIEF-EF survey and had a score of ≥26 before undergoing nerve-sparing RP. Surgeons prospectively recorded the quality of NVB preservation, and this was quantified using a nerve sparing score (NSS) of one to four, with higher scores representing better preservation. The double-blind study period included quality-of-life assessments at 1, 3, 6, 9, and 12 months after RP, and at 13 months after a washout period. Compliance in returning questionnaires ranged from 60% to 96% per time-point but was balanced between groups. After adjusting for potential confounding factors, no significant differences were found in EF between treatments (nightly vs on-demand sildenafil) at any single time-point after RP. The summary NSS was the only factor that was consistently found to have a significant association with EF outcomes in all longitudinal multivariable models. A 1-unit increase in NSS was associated with an absolute increase in IIEF-EF score of 1.65 (P = 0.005). This study had factors that weakened its findings. First, fearing that patients would not want to be randomized to a placebo-only group, a pure placebo arm was not part of the trial. Ninety percent of subjects were Caucasian, which may not necessarily be generalizable to all populations. Moreover, the study period in the trial was only 13 months, which is short of the 18–24 months duration recommended by some authors.
In a recent study, Mulhall et al.21 found that 3 months of treatment with the newly approved PDE5i avanafil taken on-demand significantly improved drug-assisted EF after prostatectomy. They randomized 298 patients with the post-RP ED of 6 months or more to on-demand 100 mg or 200 mg avanafil or placebo for 12 weeks. At the end of treatment, 100 mg (31%) and 200 mg (41%), avanafil groups responded that the treatment improved their erections when compared to placebo (10.7%). Treatment efficacy was also statistically significant when stratifications by age, ED baseline severity, and type of RP were performed. Dropout rates ranged from 8%–24% between groups, with the largest amount in the placebo group in which 14 of 24 patients discontinued from the study withdrew their consent. This fact raises the possibility that these patients perceived lack of treatment efficacy. Sustained effect on unassisted EF and long-term response to treatment were not assessed in this study.
The REACTT conducted by Montorsi et al.22 trial attempted to compare the efficacy of tadalafil daily and on demand versus placebo in improving unassisted EF and reducing loss of penile length following nerve-sparing RP. Four hundred twenty-three patients were randomized into 9 months of treatment with tadalafil 5 mg once daily, tadalafil 20 mg on demand, or placebo followed by a 6-week washout period and 3 months open-label tadalafil once daily (all patients). Dropout rates were 14%–18% between groups. They found that after 9 months of treatment, there was a significant difference in reaching the target IIEF-EF ≥22 in the tadalafil once daily group compared to placebo. Nonetheless, after the drug free washout period, there was no significant difference in EF between groups with 20.9%, 16.9%, and 19.1% of patients reaching target IIEF-EF in the tadalafil once daily, on demand and placebo groups, respectively. After the open-label tadalafil once daily period IIEF-EF scores increased in all treatment groups. Regarding penile length, there was significantly less shrinkage of penile length observed in the daily tadalafil group (2.2 mm) compared to other groups (7.9 mm on demand, 6.3 mm placebo) at 9 months of treatment. These data suggest that PDE5is may play a role in the preservation of cavernosal integrity by protecting against structural changes after postprostatectomy neuropraxia.22,23,25
As stated earlier, the etiology of post-RT ED appears to be more related to vascular (endothelial) dysfunction rather than neural injury. There are studies in which patients treated with sildenafil or tadalafil had improved flow-mediated dilation, and some authors suggest these medications have a protective effect in the vascular endothelium.26,27 Ilic et al.28 examined whether early prophylactic sildenafil is effective in reducing long-term ED in prostate cancer patients treated with radiation. A total of 27 men were randomized at a 1:1 ratio to receive either sildenafil citrate 50 mg or placebo every night starting 1 month after completion of RT for a total of 6 months. The primary outcome of this trial was EF measured at 2 years posttreatment using IIEF-5 score. The trial was closed after 32 months due to a poor accrual of patients. There was no significant difference in treatment compliance between groups with 95.1% and 96% compliance of men in sildenafil and placebo groups, respectively. They found a significant difference in IIEF-5 scores by week 4 of the study and at 6 months (P = 0.02 and P = 0.02, respectively). However, there was no difference in EF between groups at 2 years after treatment (P = 0.48), therefore suggesting that regular use of sildenafil after RT for prostate cancer does not improve the long-term EF. This study was grossly underpowered by its small study cohort size and early study termination.
A larger study conducted by Zelefsky et al.29 investigated if adjuvant daily sildenafil preserved EF during and after RT. They randomized 279 patients who were to undergo radiation therapy with or without neo-adjuvant ADT into taking prophylactic daily sildenafil 50 mg versus placebo. Treatment was initiated 3 days pretreatment and continued daily for 6 months, and outcomes were analyzed for 24 months after RT. As expected, ADT patients tended to experience worse EF outcomes than non-ADT patients. Among patients not receiving ADT (90%) there was a significant difference in EF and IIEF scores during RT and 24 months between groups. At 24 months, 81.6% of the sildenafil group and 56% of placebo patients reported functional erections. There were no differences in EF outcomes among external beam radiation therapy compared with brachytherapy or a combined-modality treatment. The greatest improvement in IIEF scores and overall EF was at 6 and 12 months after treatment, suggesting that a longer course of PDE5i may be required to provide even better functional outcomes.
More recently, Pisansky et al.30 conducted the first multicenter, stratified, placebo-controlled, double-blinded, parallel-group study to our knowledge to evaluate the tadalafil for ED prevention in men treated with RT for prostate cancer. Two hundred forty-two men with localized prostate adenocarcinoma and intact EF scheduled to receive RT were randomized 1:1 into receiving 5 mg of tadalafil daily versus placebo. Baseline as well as RT characteristics were balanced well, with no significant differences between groups. Treatment was started within 7 days after initiation of external RT or the date of brachytherapy and was to continue for 24 consecutive weeks. Participants reported IIEF scores before RT and up to 1 year after treatment. Intensity modulation was used in 98% of patients treated with external RT with a median dose of 78.0 Gy. Eighty five percent of patients undergoing brachytherapy received iodine 125, and palladium 103 was used for the remainder. At weeks 28 and 30, they found no statistically significant difference between patients receiving tadalafil (79%) and those receiving placebo (74%). Moreover, tadalafil did not result in a statistically significant difference in improved sexual function at 1 year when compared to placebo. They concluded that daily use of tadalafil did not result in improved EF when compared to placebo. However, testing for other tadalafil dosing schedules, larger study cohorts, and longer follow-ups could yield different results.
The question still remains on whether the use of PDE5is as a penile rehabilitation regimen would improve spontaneous EF in patients after prostate cancer treatment. We found only one meta-analysis and systematic review on the use of oral PDE5is for treating ED after nerve-sparing RP. Wang et al.31 screened 77 studies of which eight randomized controlled trials met inclusion criteria for their review. Some of these studies were analyzed in our review.17,21,22 The meta-analysis showed that PDE5is were effective for treating nerve-sparing RP ED compared to placebo. They also found a trend that responsiveness to PDE5is was associated with longer treatment duration, higher dosage, on-demand dosing, sildenafil, and preoperative mild ED. Although the data provide compelling evidence for the use of PDE5is as a primary treatment for post-RP ED, there remains an opportunity for the development of appropriately designed RTCs with sufficiently long-term follow-up to address PDE5i use in penile rehabilitation.
VACUUM ERECTION DEVICES
The vacuum erection device (VED) has gained popularity among patients and physicians due to its low complication rates, few side-effects, and cost-effectiveness when compared to other penile rehabilitation modalities. VED causes erection by creating negative pressure around the penis and drawing both venous and arterial blood into the corpus cavernosum. Recent animal model studies demonstrated that VED therapy preserves EF by alleviating tissue hypoxia. This helps inhibit apoptosis and prevent cavernous tissue fibrosis.32 Welliver et al.33 confirmed these findings by showing that the use of VED significantly increased both glanular and corporal oximetry, hence improving penile overall oxygen saturation. The VED device contains a constriction ring used at the base of the penis that aids in maintaining erections for intercourse. However, it also decreases oxygen saturation after 30 min of use. Therefore, the use of the constriction band is not recommended in penile rehabilitation.34,35
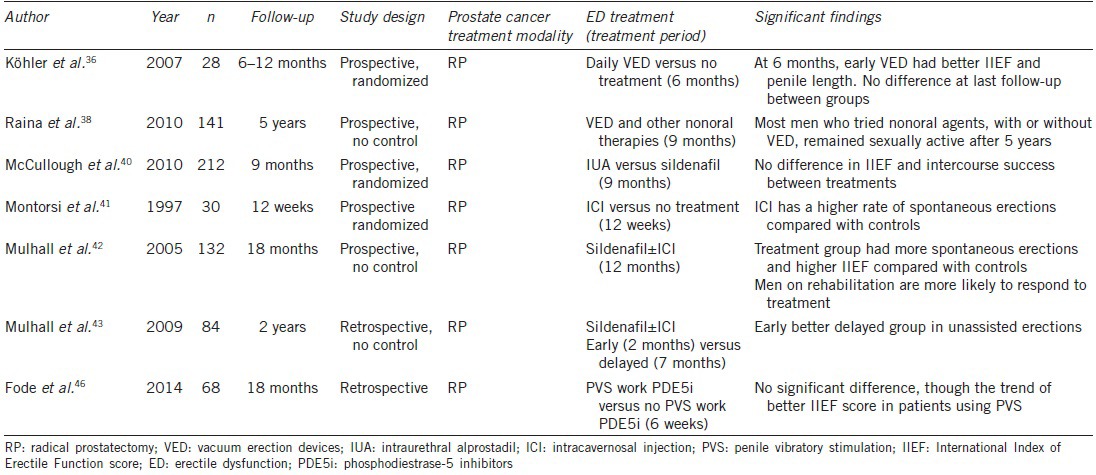
Few clinical trials were found to evaluate the effect of VED after prostate cancer treatment. Köhler et al.36 randomized 28 men with baseline IIEF scores of >11, to start daily VED use 1 month after nerve-sparing RP or start VED on-demand prior to intercourse 6 months after surgery. They found that men who had completed early VED use had significantly greater IIEF scores and stretched penile length (2 cm) compared to the on-demand group. However, at last follow-up (mean 9.5 months, 6–12 months) there was no significant difference in outcomes, and none of the patients reported unassisted EF sufficient for intercourse. This in turn suggests the need for longer rehabilitation periods and the importance of neural pathway regeneration for successful penile rehabilitation.
A prospective clinical trial by Raina et al.37 of 109 patients randomized into using daily VED versus no treatment found that at the end of 9 months, 80% of those using VED had erections sufficient for intercourse and were less likely to report penile shrinkage (85% vs 23%, respectively). Another prospective study by Raina et al. evaluated the long-term effects in EF after RP with the early use of VED and other nonoral ED treatments. One hundred forty-one patients who had undergone nerve-sparing RP were motivated to participate in early penile rehabilitation. At 1- and 5-year follow-up, 80% and 62% of men, respectively, were sexually active. After 5 years, 71% of sexually active men had natural erections sufficient for penetration without assistance. Most patients used either VED alone or in combination with another ED treatment modality. Unfortunately, this study has major limitations, as there was no control group, and protocol details or the nerve-sparing status were not revealed. However, it does recognize VED as a valuable and effective treatment in penile rehabilitation.38
A retrospective study by Basal et al.39 reviewed 203 patients who underwent bilateral nerve-sparing RP and utilized PDE5is, VED, the combination of PDE5i and VED or no treatment for penile rehabilitation. They attempted to study the EF recovery period (EFRP) in patients with mild, moderate or no preoperative ED. Patients with mild ED yielded the shortest EFRP with a mean recovery period of 8.73 ± 5.67 months after surgery. Only PDE5is and combination PDE5is and VED groups had a beneficial effect on EFRP.39 We believe VED has an important role in penile rehabilitation. Unlike other therapies, VED can ensure multiple erections on a daily basis early in the penile rehabilitation period and thus prevent early penile hypoxia which may lead to fibrosis and consequently a decrease in stretch penile length and long-term ED. It's mechanism causing erections works independently of the neural pathway and thus overcomes problems generated by neuropraxia. Most importantly, VED can be used safely with other treatment modalities to achieve better EF results. A summary of clinical trials that assess VED and other nonoral modalities is presented in Table 2.
Table 2.
Penile rehabilitation after prostate cancer treatment: Summary of clinical trials using nonoral modalities
INTRAURETHRAL THERAPY
Intraurethral alprostadil (IUA) is a urethral suppository that delivers prostaglandin E1 (PGE1). PGE1 acts locally by increasing levels of cyclic adenosine 3’,5’-cyclinc monophosphate (cAMP) within the erectile tissue. IUA acts indirectly in the erectile tissue through the urethra.13,40 This urethral suppository bypasses the neural pathway in the corpora cavernosum and generally does not cause systemic side-effects. The most common side-effect is urethral burning and penile pain.
McCullough et al.40 reported the first randomized, prospective trial to study the effect of nightly Medicated Urethral System for Erection (MUSE, Vivus Inc., Mountain View, CA, USA). A total of 212 men were randomized into taking IUA or sildenafil nightly. IUA was titrated from 125 to 250 μg after 1 month of treatment for better toleration. Dropout rates were 19% for the sildenafil group and 30% for IUA group. Most dropouts occurred with the increase in IUA dosage secondary to pain. Compliance rates were 98% and 79% for sildenafil and IUA groups, respectively. IIEF scores increased in the IUA and SC groups from a mean of 9.9 and 10.4 at 1 month to 15.28 and 17.65 at study end, respectively. At 9 months, there were no statistically significant differences in the IIEF-EF score and successful intercourse rates. The only statistically significant difference between groups in erections, assessed by the global assessment question, occurred at 6 months in favor of IUA (76% vs 60%). It is possible this benefit occurred in the period of neuropraxia when PDE5i are not expected to be effective.
Normal therapeutic doses of IUA range between 500 and 1000 μg. Nonetheless, we found no clinical trials that assess these doses in patients undergoing prostate cancer treatment. Although IUA can improve erections in patients with ED, its use after prostate cancer treatment is limited in the medical community. This is most likely secondary to cost, and the lack of quality randomized controlled trials to prove its overall effectiveness in patients undergoing prostate cancer treatment.
INTRACAVERNOSAL INJECTION
Intracavernosal injection (ICI) consists of PGE1 alprostadil alone or in combination with papaverine and phentolamine. Phentolamine is an α-blocker that causes smooth muscle relaxation, and papaverine is a nonspecific phosphodiesterase inhibitor that increases both cAMP and cGMP in the cavernous tissue. These agents in combination act as vasoactive agents that aid in increasing blood flow to the corpus cavernosum, hence, causing erections and penile engorgement.
The first treatment studied as a penile rehabilitation strategy was ICI by Montorsi et al.41 in 1997. Thirty patients who underwent bilateral nerve-sparing RP were randomized to either receive alprostadil injections 3 times per week for 12 weeks versus no treatment. After 6 months, 67% of men in the treatment group versus 20% in the control group achieved spontaneous erections sufficient for penetration. The researchers concluded that the injections of alprostadil decreased the hypoxia-induced tissue damage. Reported complications were minor, and the therapy proved to be well-tolerated.
Mulhall et al.42 published the only long-term follow-up prospective study that assessed ICI in penile rehabilitation. Men with preoperative erections who underwent RP were treated with early sildenafil and those who did not respond were switched to ICI 3 times per week. A total of 58 patients received penile rehabilitation treatment versus 74 who were allowed to have treatment on-demand off-protocol. Patients who were not compliant with therapy for at least 12 months were excluded from the study group. IIEF scores were assessed for 18 months. At 18 months after prostatectomy, 52% of the rehabilitation group versus 19% of the control group reported unassisted spontaneous erections. Ninety-five percent of patients responded to ICI and 64% to sildenafil in the study group, versus 76% and 24% in the control group, respectively. Limitations of this study include lack of randomization and intention-to-treat and selection bias.
In a similar study in 2009, Mulhall et al.43 attempted to define if EF outcomes were better with early institution of penile rehabilitation. Forty-eight patients in the early group and 36 patients in the delayed group were instructed to obtain three erections per week using sildenafil initially, and if unsuccessful, then intracavernous injections. Penile rehabilitation started at a mean time of 2 months and 7 months after RP in the early and delayed groups, respectively. At 2 years after surgery, 48% of the early group and 30% of the delayed group had unassisted erections hard enough for penetration. There was also a statistical significant difference in those achieving an IIEF-EF score >25 between groups. Even though this is a retrospective study and selection bias could have altered results, this study unveils evidence that timing of penile rehabilitation is of paramount importance.
Intracavernosal injection has been proven effective for the treatment of ED after prostate cancer treatment. However, we are still in need of clinical trials with long-term follow-up that assess its role in penile rehabilitation.
VIBRATORY STIMULATION
The use of penile vibratory stimulation (PVS) was first described by Sobrero et al. in 1965.44 Advancements in technology and technique led to the development of devices that stimulate penile erection in men with ED and ejaculation in men with spinal cord injury. PVS works through the stimulation of branches of the pudendal nerves along the penile shaft. The stimulation causes a reflex parasympathetic erection through the activation of nerve terminal endings that release nitric oxide and inhibit sympathetic fibers. The resultant effect is the liberation of cGMP and cAMP. Both of these cause cavernosal smooth muscle dilation and penile engorgement.45
Fode et al. presented the first prospective randomized study aimed to examine the effect of PVS on penile rehabilitation in patients undergoing nerve-sparing RP. Sixty-eight patients were randomized into using PVS with oral PDE5is versus oral PDE5is without the use of PVS. Patients in the study group were instructed in stimulating the frenulum once daily for at least 1-week before surgery and after catheter removal for a period of 6 weeks. IIEF scores were evaluated at 3, 6, and 12 months after surgery. Results showed that IIEF scores were higher in the PVS group at all times though no difference reached significance. At 12 months after surgery, 53% had reached an IIEF score of at least 18, compared to 32% of patients in the control group (P = 0.07).46 Patient compliance and PDE5i type, frequency, or dosages were not presented in this study. Furthermore, stopping PVS use after 6 weeks raises the question if a longer treatment period would have yielded different outcomes. Although this study had significant limitations, it showed that PVS is both acceptable and tolerable for patients. Most importantly, it also pioneers the use of PVS as an agent in ED after nerve-sparing RP.
LOW-INTENSITY EXTRACORPOREAL SHOCKWAVE THERAPY
The use of low-intensity extracorporeal shockwave (LI-ESW) attempts to alter the underlying pathophysiology of the erectile mechanism. Shockwaves (SWs) applied to the targeted tissue cause mechanical stress and micro-trauma that catalyze a set of biological reactions that result in neovascularization of the tissue.47 Even though this mechanism is not completely understood, recent animal studies revealed that corpora harvested from rats treated with LI-ESWT result in increased smooth muscle and endothelial content, along with upregulation of vascular endothelial growth factor, neuronal NO synthase and von Willebrand factor.48
Human clinical studies have seen a high tolerability and an increase of IIEF-EF scores in patients and high with mild and severe ED treated with this noninvasive modality.49,50 These led Vardi et al. to develop the first randomized, double-blind, sham controlled clinical trial to evaluate the use of LI-ESWT in ED.51 The sham treatment in this study consisted of an identical probe that looked, sounded and felt the same but did not produce any SWs to the targeted tissue. IIEF-EF scores and penile hemodynamics were assessed at 3 and 6 months in 67 randomized participants with ED, who could previously achieve erections with PDE5is. A 4-week PDE5i washout period was performed prior to the initiation of a 9-week treatment period, consisting 2 sessions per week for 3 weeks that were repeated after a 3-week no-treatment interval. Results showed that the overall satisfaction, ability to penetrate, and mean IIEF-EF scores of patients in the treatment group were significantly higher than those in the control group. Penile hemodynamics also revealed a significantly improved resting and maximal postischemic penile blood flow in LI-ESWT participants. Although the study cohort was relatively small, and prostate cancer treatment patients were excluded, this study demonstrated that this modality may serve as an adjunct to penile rehabilitation in the near future.
Currently, there are no clinical trials that assess LI-ESWT in patients undergoing prostate cancer treatment. Although there are more questions than answers regarding the mechanism and therapeutic use of LI-ESWT for improvement of EF, this modality could one day take part in penile rehabilitation programs.
CONCLUSION
The majority of studies available assess the use of PDE5i in penile rehabilitation, most likely because they are well-known and widely available. PDE5s are well-tolerated and have been proven to improve early assisted sexual function when compared to placebo. Nonetheless, the use of PDE5is has not been proven to significantly improve in unassisted erections in the long-term when compared to placebo. Many clinical trials studying other treatment modalities presented in this review lacked placebo control. However, due to the nature of these modalities such as PVS, ICI, and VEDs, it is difficult to create a believable hoax modality to eliminate the placebo effect.
Currently, there is no standard treatment algorithm or clinical guideline for EF recovery after prostate cancer treatment. Limited robust studies exist for post-RP patients and even less for post-RT. A survey demonstrated that these factors have not hindered American Urological Association urologists from including penile rehabilitation programs in their practices.52 Although today's treatment options are limited, advancements in research and technology will ultimately create and refine management options for penile rehabilitation. Recent therapeutic advances such as PVS, LI-ESWT, impulse magnetic field therapy, nanotechnology, and endovascular treatment may open new ways that can revolutionize treatment of ED.45 Penile rehabilitation pioneers and researchers all over the world may gather this information to launch clinical trials that one day will delineate an algorithm for ED after prostate cancer treatment.
EDITORIAL COMMENT—(BY DR JOHN W DAVIS, DEPARTMENT OF UROLOGY, THE UNIVERSITY OF TEXAS, MD ANDERSON CANCER CENTER, HOUSTON, TEXAS, USA)
Radical prostatectomy surgeons, regardless of technique, have long identified thermal and traction injuries as contributors to post-operative erectile dysfunction, and devised many strategies to reduce them. Given that optimal techniques that limit such injuries can give widely different success rates, especially varying by patient age and pre-operative function, then the focus could shift to peri-operative management to achieve the next level of success. As a surgeon, I certainly wish the data on penile rehabilitation were more solid and transformed into a uniform pathway. In fact, the lack of uniform pathways generates significant variance in participation by third party payors in the U.S. circumstance, and my clinical team spends considerable efforts appealing coverage for these therapies on behalf of our patients. Nevertheless, Drs. Clavell-Hernandez and Wang provide a state-of-the-art summary of the existing literature, with several key take home messages in Tables 1 and 2. My personal practice pattern is to refer radical prostatectomy patients to an ED specialist such as Dr. Wang at the 2-3 week post-operative interval and to encourage use of as many of these methods as they will accept. Patient compliance with initial visits is very high, but will remain a challenge to translate into a study.
AUTHOR CONTRIBUTIONS
JCH and RW contributed to the concept and design of the study and drafted, revised, and approved the manuscript. JCH contributed through the acquisition and analysis of data.
COMPETING INTERESTS
None.
REFERENCES
- 1.What are the Key Statistics about Prostate Cancer? American Cancer Society. [Last accessed on 2014 Apr 08]. Available from: http://www.cancer.org/cancer/prostatecancer/detailedguide/prostatecancer-key-statistics .
- 2.Alemozaffar M, Regan MM, Cooperberg MR, Wei JT, Michalski JM, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011;306:1205–14. doi: 10.1001/jama.2011.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penson DF, McLerran D, Feng Z, Li L, Albertsen PC, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the Prostate Cancer Outcomes Study. J Urol. 2008;179:S40–4. doi: 10.1016/j.juro.2008.03.136. [DOI] [PubMed] [Google Scholar]
- 4.Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 5.McCullough AR. Rehabilitation of erectile function following radical prostatectomy. Asian J Androl. 2008;10:61–74. doi: 10.1111/j.1745-7262.2008.00366.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang R. Penile rehabilitation after radical prostatectomy: where do we stand and where are we going? J Sex Med. 2007;4(4 Pt 2):1085–97. doi: 10.1111/j.1743-6109.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 7.Mulhall JP, Slovick R, Hotaling J, Aviv N, Valenzuela R, et al. Erectile dysfunction after radical prostatectomy: hemodynamic profiles and their correlation with the recovery of erectile function. J Urol. 2002;167:1371–5. doi: 10.1016/s0022-5347(05)65303-7. [DOI] [PubMed] [Google Scholar]
- 8.Stember DS, Mulhall JP. The concept of erectile function preservation (penile rehabilitation) in the patient after brachytherapy for prostate cancer. Brachytherapy. 2012;11:87–96. doi: 10.1016/j.brachy.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Zelefsky MJ, Eid JF. Elucidating the etiology of erectile dysfunction after definitive therapy for prostatic cancer. Int J Radiat Oncol Biol Phys. 1998;40:129–33. doi: 10.1016/s0360-3016(97)00554-3. [DOI] [PubMed] [Google Scholar]
- 10.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 11.Hakky TS, Baumgarten AS, Parker J, Zheng Y, Kongnyuy M, et al. Penile rehabilitation: the evolutionary concept in the management of erectile dysfunction. Curr Urol Rep. 2014;15:393. doi: 10.1007/s11934-014-0393-6. [DOI] [PubMed] [Google Scholar]
- 12.Gontero P, Kirby R. Proerectile pharmacological prophylaxis following nerve-sparing radical prostatectomy (NSRP) Prostate Cancer Prostatic Dis. 2004;7:223–6. doi: 10.1038/sj.pcan.4500737. [DOI] [PubMed] [Google Scholar]
- 13.Alba F, Wang R. Current status of penile rehabilitation after radical prostatectomy. CML Urol. 2010;16:93–101. [Google Scholar]
- 14.McCullough AR, Levine LA, Padma-Nathan H. Return of nocturnal erections and erectile function after bilateral nerve-sparing radical prostatectomy in men treated nightly with sildenafil citrate: subanalysis of a longitudinal randomized double-blind placebo-controlled trial. J Sex Med. 2008;5:476–84. doi: 10.1111/j.1743-6109.2007.00700.x. [DOI] [PubMed] [Google Scholar]
- 15.Bannowsky A, Schulze H, van der Horst C, Hautmann S, Jünemann KP. Recovery of erectile function after nerve-sparing radical prostatectomy: improvement with nightly low-dose sildenafil. BJU Int. 2008;101:1279–83. doi: 10.1111/j.1464-410X.2008.07515.x. [DOI] [PubMed] [Google Scholar]
- 16.Pace G, Del Rosso A, Vicentini C. Penile rehabilitation therapy following radical prostatectomy. Disabil Rehabil. 2010;32:1204–8. doi: 10.3109/09638280903511594. [DOI] [PubMed] [Google Scholar]
- 17.Padma-Nathan H, McCullough AR, Levine LA, Lipshultz LI, Siegel R, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008;20:479–86. doi: 10.1038/ijir.2008.33. [DOI] [PubMed] [Google Scholar]
- 18.Lim KB, Deyoung L, Brock G. Chronic PDE5 inhibitor altered cavernosal protein expression-use in identifying protein biomarker for erectile recovery post trauma. J Sex Med. 2006;3(Suppl 5):383. [Google Scholar]
- 19.Montorsi F, Brock G, Lee J, Shapiro J, Van Poppel H, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;54:924–31. doi: 10.1016/j.eururo.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 20.Pavlovich CP, Levinson AW, Su LM, Mettee LZ, Feng Z, et al. Nightly vs on-demand sildenafil for penile rehabilitation after minimally invasive nerve-sparing radical prostatectomy: results of a randomized double-blind trial with placebo. BJU Int. 2013;112:844–51. doi: 10.1111/bju.12253. [DOI] [PubMed] [Google Scholar]
- 21.Mulhall JP, Burnett AL, Wang R, McVary KT, Moul JW, et al. A phase 3, placebo controlled study of the safety and efficacy of avanafil for the treatment of erectile dysfunction after nerve sparing radical prostatectomy. J Urol. 2013;189:2229–36. doi: 10.1016/j.juro.2012.11.177. [DOI] [PubMed] [Google Scholar]
- 22.Montorsi F, Brock G, Stolzenburg JU, Mulhall J, Moncada I, et al. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: a randomised placebo-controlled study (REACTT) Eur Urol. 2014;65:587–96. doi: 10.1016/j.eururo.2013.09.051. [DOI] [PubMed] [Google Scholar]
- 23.Berookhim BM, Nelson CJ, Kunzel B, Mulhall JP, Narus JB. Prospective analysis of penile length changes after radical prostatectomy. BJU Int. 2014;113:E131–6. doi: 10.1111/bju.12443. [DOI] [PubMed] [Google Scholar]
- 24.Nelson CJ, Scardino PT, Eastham JA, Mulhall JP. Back to baseline: erectile function recovery after radical prostatectomy from the patients’ perspective. J Sex Med. 2013;10:1636–43. doi: 10.1111/jsm.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostafa ME, Senbel AM, Mostafa T. Effect of chronic low-dose tadalafil on penile cavernous tissues in diabetic rats. Urology. 2013;81:1253–9. doi: 10.1016/j.urology.2012.12.068. [DOI] [PubMed] [Google Scholar]
- 26.Desouza C, Parulkar A, Lumpkin D, Akers D, Fonseca VA. Acute and prolonged effects of sildenafil on brachial artery flow-mediated dilatation in type 2 diabetes. Diabetes Care. 2002;25:1336–9. doi: 10.2337/diacare.25.8.1336. [DOI] [PubMed] [Google Scholar]
- 27.Rosano GM, Aversa A, Vitale C, Fabbri A, Fini M, et al. Chronic treatment with tadalafil improves endothelial function in men with increased cardiovascular risk. Eur Urol. 2005;47:214–20. doi: 10.1016/j.eururo.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Ilic D, Hindson B, Duchesne G, Millar JL. A randomised, double-blind, placebo-controlled trial of nightly sildenafil citrate to preserve erectile function after radiation treatment for prostate cancer. J Med Imaging Radiat Oncol. 2013;57:81–8. doi: 10.1111/j.1754-9485.2012.02461.x. [DOI] [PubMed] [Google Scholar]
- 29.Zelefsky MJ, Shasha D, Branco RD, Kollmeier M, Baser RE, et al. Prophylactic sildenafil citrate improves select aspects of sexual function in men treated with radiotherapy for prostate cancer. J Urol. 2014;192:868–74. doi: 10.1016/j.juro.2014.02.097. [DOI] [PubMed] [Google Scholar]
- 30.Pisansky TM, Pugh SL, Greenberg RE, Pervez N, Reed DR, et al. Tadalafil for prevention of erectile dysfunction after radiotherapy for prostate cancer: the Radiation Therapy Oncology Group [0831] randomized clinical trial. JAMA. 2014;311:1300–7. doi: 10.1001/jama.2014.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Wang X, Liu T, He Q, Wang Y, et al. Systematic review and meta-analysis of the use of phosphodiesterase type 5 inhibitors for treatment of erectile dysfunction following bilateral nerve-sparing radical prostatectomy. PLoS One. 2014;9:e91327. doi: 10.1371/journal.pone.0091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin HC, Yang WL, Zhang JL, Dai YT, Wang R. Penile rehabilitation with a vacuum erectile device in an animal model is related to an antihypoxic mechanism: blood gas evidence. Asian J Androl. 2013;15:387–90. doi: 10.1038/aja.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welliver RC, Jr, Mechlin C, Goodwin B, Alukal JP, McCullough AR. A pilot study to determine penile oxygen saturation before and after vacuum therapy in patients with erectile dysfunction after radical prostatectomy. J Sex Med. 2014;11:1071–7. doi: 10.1111/jsm.12445. [DOI] [PubMed] [Google Scholar]
- 34.Monga M, Utz W, Reddy P, Kohler T, Hendlin K, et al. Early Use of the Vacuum Constriction Device Following Radical Retropubic Prostatectomy: a Randomized Clinical Trial. South Central Section of the AUA 85 th Annual Meeting. 2006:98. Abstract 50, abstract book. [Google Scholar]
- 35.Bosshardt RJ, Farwerk R, Sikora R, Sohn M, Jakse G. Objective measurement of the effectiveness, therapeutic success and dynamic mechanisms of the vacuum device. Br J Urol. 1995;75:786–91. doi: 10.1111/j.1464-410x.1995.tb07392.x. [DOI] [PubMed] [Google Scholar]
- 36.Köhler TS, Pedro R, Hendlin K, Utz W, Ugarte R, et al. A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU Int. 2007;100:858–62. doi: 10.1111/j.1464-410X.2007.07161.x. [DOI] [PubMed] [Google Scholar]
- 37.Raina R, Agarwal A, Ausmundson S, Lakin M, Nandipati KC, et al. Early use of vacuum constriction device following radical prostatectomy facilitates early sexual activity and potentially earlier return of erectile function. Int J Impot Res. 2006;18:77–81. doi: 10.1038/sj.ijir.3901380. [DOI] [PubMed] [Google Scholar]
- 38.Raina R, Pahlajani G, Agarwal A, Jones S, Zippe C. Long-term potency after early use of a vacuum erection device following radical prostatectomy. BJU Int. 2010;106:1719–22. doi: 10.1111/j.1464-410X.2010.09360.x. [DOI] [PubMed] [Google Scholar]
- 39.Basal S, Wambi C, Acikel C, Gupta M, Badani K. Optimal strategy for penile rehabilitation after robot-assisted radical prostatectomy based on preoperative erectile function. BJU Int. 2013;111:658–65. doi: 10.1111/j.1464-410X.2012.11487.x. [DOI] [PubMed] [Google Scholar]
- 40.McCullough AR, Hellstrom WG, Wang R, Lepor H, Wagner KR, et al. Recovery of erectile function after nerve sparing radical prostatectomy and penile rehabilitation with nightly intraurethral alprostadil versus sildenafil citrate. J Urol. 2010;183:2451–6. doi: 10.1016/j.juro.2010.01.062. [DOI] [PubMed] [Google Scholar]
- 41.Montorsi F, Guazzoni G, Strambi LF, Da Pozzo LF, Nava L, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J Urol. 1997;158:1408–10. [PubMed] [Google Scholar]
- 42.Mulhall J, Land S, Parker M, Waters WB, Flanigan RC. The use of an erectogenic pharmacotherapy regimen following radical prostatectomy improves recovery of spontaneous erectile function. J Sex Med. 2005;2:532–40. doi: 10.1111/j.1743-6109.2005.00081_1.x. [DOI] [PubMed] [Google Scholar]
- 43.Mulhall JP, Parker M, Waters BW, Flanigan R. The timing of penile rehabilitation after bilateral nerve-sparing radical prostatectomy affects the recovery of erectile function. BJU Int. 2010;105:37–41. doi: 10.1111/j.1464-410X.2009.08775.x. [DOI] [PubMed] [Google Scholar]
- 44.Sobrero AJ, Stearns HE, Blair JH. Technic for the induction of ejaculation in humans. Fertil Steril. 1965;16:765–7. doi: 10.1016/s0015-0282(16)35767-3. [DOI] [PubMed] [Google Scholar]
- 45.Stein MJ, Lin H, Wang R. New advances in erectile technology. Ther Adv Urol. 2014;6:15–24. doi: 10.1177/1756287213505670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fode M, Borre M, Ohl DA, Lichtbach J, Sønksen J. Penile vibratory stimulation in the recovery of urinary continence and erectile function after nerve-sparing radical prostatectomy: a randomized, controlled trial. BJU Int. 2014;114:111–7. doi: 10.1111/bju.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gruenwald I, Appel B, Kitrey ND, Vardi Y. Shockwave treatment of erectile dysfunction. Ther Adv Urol. 2013;5:95–9. doi: 10.1177/1756287212470696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J, Zhou F, Li GY, Wang L, Li HX, et al. Evaluation of the effect of different doses of low energy shock wave therapy on the erectile function of streptozotocin (STZ)-Induced Diabetic Rats. Int J Mol Sci. 2013;14:10661–73. doi: 10.3390/ijms140510661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vardi Y, Appel B, Jacob G, Massarwi O, Gruenwald I. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol. 2010;58:243–8. doi: 10.1016/j.eururo.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Gruenwald I, Appel B, Vardi Y. Low-intensity extracorporeal shock wave therapy – A novel effective treatment for erectile dysfunction in severe ED patients who respond poorly to PDE5 inhibitor therapy. J Sex Med. 2012;9:259–64. doi: 10.1111/j.1743-6109.2011.02498.x. [DOI] [PubMed] [Google Scholar]
- 51.Vardi Y, Appel B, Kilchevsky A, Gruenwald I. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol. 2012;187:1769–75. doi: 10.1016/j.juro.2011.12.117. [DOI] [PubMed] [Google Scholar]
- 52.Tal R, Teloken P, Mulhall JP. Erectile function rehabilitation after radical prostatectomy: practice patterns among AUA members. J Sex Med. 2011;8:2370–6. doi: 10.1111/j.1743-6109.2011.02355.x. [DOI] [PubMed] [Google Scholar]


