Abstract
Invasive species are one of the greatest threats to biodiversity worldwide, and to successfully manage their introductions is a major challenge for society. Knowledge on the impacts of an invasive species is essential for motivating decision makers and optimally allocating management resources. We use a prominent invasive fish species, the round goby (Neogobius melanostomus) to objectively quantify the state of scientific knowledge on its impacts. Focusing on how native fish species are affected by round goby invasions, we analyzed 113 peer-reviewed papers and found that impacts are highly ecosystem and time scale dependent. We discovered round goby impacts to be profound, but surprisingly complex. Even if identical native species were affected, the impacts remained less comparable across ecosystems than expected. Acknowledging the breadth but also limitations in scientific knowledge on round goby impacts would greatly improve scientists’ ability to conduct further research and inform management measures.
Electronic supplementary material
The online version of this article (doi:10.1007/s13280-015-0718-9) contains supplementary material, which is available to authorized users.
Keywords: Invasive species impact, Management, Prevention, Round goby, Neogobius melanostomus
Introduction
Invasive species pose one of the most serious threats to ecosystems in general and aquatic ecosystems in particular (Strayer 2010). For example, the number of introduced fish species still continues to grow (Blanchett et al. 2009; Ellender and Weyl 2014). Introductions occur either intentionally, e.g., by releasing aquarium fishes or by stocking, or unintentionally, e.g., in ballast water of cargo ships (García-Berthou et al. 2005). Some of these numerous fish introductions are a risk to native ecosystems and, if ecosystem services are compromised, eventually to humans (Gozlan 2008). The increasing number of introductions and the uncertainty about whether introductions will lead to an invasion with ecological impacts poses a challenge to decision makers. Decision makers prioritize management efforts on those species that are expected to have the most adverse impact (Simberloff 2003). Because not all potentially harmful introductions can be simultaneously managed, decision makers have to “maximize the trade-off between accuracy and utility” of a management (Kornis et al. 2013).
The process of a successful management is multidisciplinary and requires at least three main players to efficiently interact on an equal footing: decision makers (in our context especially ecosystem managers), scientists, and the general public (Bayliss et al. 2013; Seidl et al. 2013). The ontology of these interactions has received much scientific attention (Lawrence 2015). In this paper, we focus on the primary contribution of scientists to the process: providing scientific knowledge (Walsh et al. 2015; N’Guyen et al. 2015). One key aspect of scientific knowledge that is relevant to decision makers is the information on how harmful a recently detected non-native species can become. For fish invasions, tools such as the fish invasiveness scoring kit (FISK) have been developed to allow decision makers a risk assessment and to ensure that management actions are commensurate with the level of risk posed by an invader (Copp et al. 2009). FISK assesses the risk of a non-native fish becoming invasive in a certain ecosystem based on 49 questions about the species’ biogeography, invasion history, biology, and ecology.
These tools should help prevent the introduction of potentially harmful species that have had demonstrable ecological impacts elsewhere. Countries such as New Zealand or Australia use risk-assessment tools as basis for the customs authorities to implement import bans of certain species (Keller et al. 2007, 2008; Campbell 2011). These tools, however, are of limited use if a non-native species has already established a localized population and decision makers need to decide whether and how such a potential source population should be managed (Gozlan et al. 2010). For example, in Europe alone, more than one non-native species per year becomes established (EU 2009). Decision makers cannot simultaneously instigate a preventive management against the spread of all non-native species. Rather, they want to know which one will have the most severe impacts, because the most important reason to manage a localized non-native population is to prevent its impacts. The safest way to know whether a non-native species will have impacts in a new ecosystem is knowledge about its impacts in already invaded ecosystems (Daehler and Gordon 1997; Simberloff 2003; Bayliss et al. 2013). Scientists’ primary contribution to a prospective preventive management is knowledge about the impacts a potential invasive species has had elsewhere.
Scientifically, there has long been a call for more structured reviews providing an objective account of invasion processes (Heger et al. 2013). There have been several new approaches put forward that might improve the predictive capabilities of invasion biologists. For example, the analysis and comparison of functional responses of invaders and native species could improve impact assessments because invasive species that are more efficient resource consumers than native species should have more severe impacts (Dick et al. 2014).
Whether any impacts of non-native species become detected is a matter of time. Biological invasions are characterized by time lags: the introduction lags behind vector activity, the population growth lags behind establishment, and so on (see Crooks 2005 for a review on time lags in invasion biology). Eventually, also the impacts of an invasive species lag behind its population increase and its areal distribution. Even the per-capita impact of an invader can change over time. For example, over time, the invader might evolve aggressive behavior or native species might evolve to better cope with the new predator or prey. Thus, an objective analysis of any invaders’ impacts needs to consider temporal aspects of its invasion. Decision makers need to be informed about time lags, too. For example, the decision to spend a lot of resources to contain an invasion in its early stages is informed by the knowledge that population growth lags behind establishment and a population is best managed when it is still in its post-establishment lag phase (Crooks 2005).
Our aim here is to use a topical case study to objectively analyze scientists’ knowledge contribution in the form of peer-reviewed papers to inform a preventive management. Our study species is the round goby (Neogobius melanostomus; Fig. 1). The round goby is a small bottom-living fish native to the Ponto-Caspian region. This species is listed among the 100 worst invasive species in Europe (DAISIE 2015). In 1990, it was found both in the Baltic Sea and in the Laurentian Great Lakes, probably after being introduced by ballast water (Corkum et al. 2004). Since then, it has been spreading rapidly (Kornis et al. 2012). The building of waterways and the increased commercial and recreational shipping across Europe and North America is believed to have accelerated the spread of round goby by providing pathways and vectors for active and passive dispersal (Britton and Gozlan 2013; Roche et al. 2013).
Fig. 1.
a Round goby (Neogobius melanostomus) displaying the characteristic black spot on the first dorsal fin and its fused pelvic fin. b Gobies amassing on an unhooking mat during a recreational fishing event by the Mosel, a river in Germany where round gobies have established and spread. c Study case: the Harbour Kleinhüningen, Switzerland, where round gobies have been first detected in 2012. Photo credits: a Magnus Thorlacius, b Guido Eberhardt, c Philipp E. Hirsch
Round goby was discovered 2012 in the Rhine in Switzerland (Kalchhauser et al. 2013). The Swiss population is currently rather localized to some 15 km of river, but it might spread further into Swiss and German waters such as the River Aare or Lake Constance. This secondary spread concerns scientists and decision makers. It also bothers the general public when, e.g., iconic native fish species are negatively affected by round goby. Therefore, we instigated a transdisciplinary project to prevent the further spread of round goby into Switzerland. A first joint workshop of scientists and decision makers within this project revealed that scientific knowledge on round goby prevention and control would be needed, but is sparse. The vast majority of published knowledge on round goby is about its ecological impacts (N’Guyen et al. 2015). This review aims at objectively quantifying the scientific state of knowledge on round goby impacts. We consider such an objective assessment of scientists’ knowledge contribution as an important basis for a successful management. This successful management includes the prevention of further spread and the control of an established population. To reach any of these goals, a cooperative process bridging disciplines is needed. An objective assessment of each players’ contribution, in our case, scientific knowledge, facilitates such a successful cooperative management across disciplines (Rosendahl et al. 2015).
Given the fact that most scientific papers on round goby are about its impacts on native species, we expected to find clearly demonstrable impacts across invaded ecosystems. We were especially interested whether different studies found similar impacts of round goby on native fish. Therefore, we expect that, ultimately, the knowledge on impacts that round goby had in other ecosystems will improve the chances of a successful preventive management of their secondary spread.
Materials and methods
We aimed to explore the known impacts of non-native round goby on native species. To this end, we conducted a systematic quantitative literature review. This method allows us to objectively identify overlaps and gaps in current scientific knowledge (Pickering and Byrne 2014). Following the PRISMA statement (Moher et al. 2009), we analyzed the published literature on the ecological impacts of round goby on native species in different ecosystems. We define ecological impact as measurable outcomes of interactions that include any of the following: predation, competition for food or shelter, and availability of a new prey. These interactions must lead to quantitatively measurable changes, but the changes do not have to reach a certain significance level to be considered in our review (Davidson and Hewitt 2014; Ojaveer and Kotta 2015). The literature search was carried out in the web of knowledge database (http://webofknowledge.com) using the search terms ‘round goby,’ ‘Neogobius melanostomus,’ ‘diet,’ ‘predation,’ ‘prey,’ ‘competition,’ separated by Boolean operators ‘AND’ or ‘OR’: (‘round goby’ OR ‘neogobius melanostomus’) AND (‘diet’ OR ‘predation’ OR ‘prey’ OR ‘competition’). The last search was conducted on April 8, 2015.
The resulting list of publications was first screened for duplicates, which were removed. In a second step, papers were screened to identify relevant primary research articles. We included only peer-reviewed studies in English providing a quantitative analysis of round goby interactions with other species based on results from a field study or laboratory experiments, including, e.g., stomach content analysis, stable isotope analysis, or behavioral experiments. All review articles that did not present original research, books, book chapters, and gray literature such as reports were excluded. We acknowledge that these forms of publications might also contain information on round goby impacts. However, our aim was to objectively quantify the scientific knowledge on impacts. Because scientific papers are filed in web of knowledge in a structured and accessible way and because peer review is, despite substantial shortcomings, the highest standard in science, we feel our focus is justified. Reference lists of all papers were screened for additional papers, which entered the same process as the papers found in the web of knowledge.
The information on impacts was extracted from the paper and entered in a personal spreadsheet database (Pickering and Byrne 2014). Studies and species were then structured and grouped with Excel®’s built-in filter function in three categories: impacts on invertebrates, impacts on a specific vertebrate described in one study, and impacts on a specific vertebrate described in more than one study. Here, we focus on round goby impacts on native fish as predator, competitor, or prey. From a management perspective, the impact on native fish is likely to receive the most attention. Fish directly or indirectly provide a variety of important ecosystem services and are of socioeconomic value (Holmlund and Hammer 1999). For example, native brown trout (Salmo trutta) are the most popular game fish in Switzerland, and expensive restoration programs supported by the public have been installed to conserve the native Atlantic salmon (Salmo salar) in the Rhine (Anonymous 1998; Burkhardt-Holm et al. 2002). Because the above-mentioned attractiveness of fish species applies in other countries as well, most of the papers published on round goby impacts in other ecosystems focus on fish. This review is therefore also driven by the concern that iconic freshwater fish species will be affected by round goby invasion and that this effect deserves particular attention when communicating with decision makers and the general public.
Results
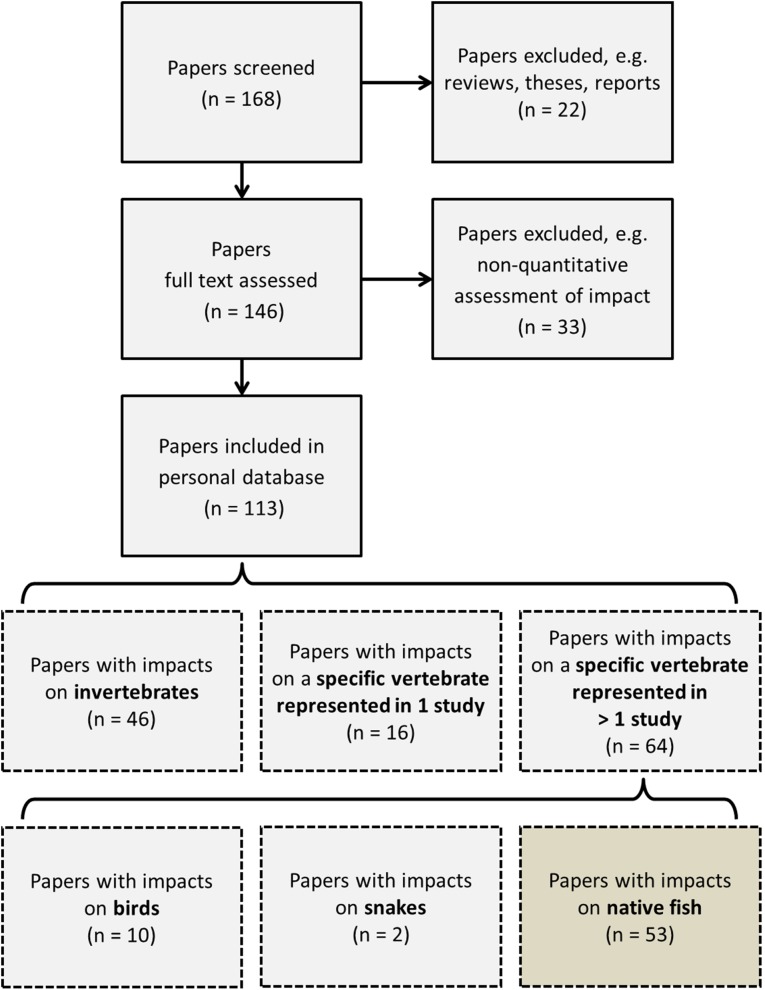
We screened 168 papers according to our criteria to identify relevant primary research articles (Fig. 2). After excluding reviews, theses, reports, and studies not meeting the inclusion criteria (e.g., to provide a quantitative assessment of round goby impact on native species), the results of 113 relevant papers were entered in the personal spreadsheet database. Finally, to analyze whether different studies found the same round goby impacts on the same native species, papers and species were grouped as described above. We show and discuss here only impacts on fish species that are represented in more than one study, to allow a comparison of the impacts between different ecosystems. For full disclosure and to facilitate future data mining, we provide the spreadsheet as electronic supplementary material (Table S1). An exemplary presentation of how this detailed information allows comparing impacts across ecosystems can be found in Table 1, where we present the available information about impacts of round goby on Eurasian perch (Perca fluviatilis) in a structured and comprehensive way. Supplementary Table S2 provides the same information for yellow perch (Perca flavescens).
Fig. 2.
Numbers of screened and included papers for the literature review. Papers can enter several categories in the personal database, e.g., when a paper studied goby diet and goby as prey item, it is included in the category “invertebrates” as well as “vertebrates.”
Table 1.
Round goby interactions with Eurasian perch (Perca fluviatilis) are context dependent (n.a. = not available, TL = total length, SL = standard length)
| Species | Eurasian perch (Perca fluviatilis) | ||
|---|---|---|---|
| Water body | Baltic Sea | Middle Danube | |
| Sampling site | Bay of Gdansk, Poland | Curonian Lagoon, Lithuania | Near Bratislava, Slovakia |
| Abiotic factors | |||
| Salinity | Brackish water | Brackish water | Fresh water |
| Depth (m) | n.a. | n.a. | 0.5–2.5 |
| Min./max./mean temp. (°C) | n.a. | n.a. | n.a. |
| Year(s) of study | 2004 | 2007–2012 | 2004 |
| Season of study | May–August | n.a. | 30 Aug. and 1 Sept. |
| First record round goby | Late 1980s | 2002 | 2003 |
| Age of round goby population | Approx. 15 years | 5–7 years | 1 year |
| Round goby population density | n.a. (“one of the dominant species”) | 1–251 ind./1000 m3 | n.a. |
| Life stage or length of round goby | n.a. | n.a. | 1–4 years |
| Life stage or length of native species | 113–293 mm TL | Small: 8.9 ± 0.3 cm TL Medium: 13.1 ± 1.0 cm TL Large: 27.2 ± 1.4 cm TL |
Age 1: 56.8–63.7 mm SL Age 2: 89.2–109 mm SL Age 3: 78.5–138.3 mm SL Age 4: 104.1–178.1 mm SL |
| Primary data acquisition method | Stomach content and stable isotopes from field samples; comparison between invaded and uninvaded area (around island of Öland) | Stomach content and stable isotopes from field samples | Stomach content and stable isotopes from field samples |
| Sample size of perch | 100 | 9 | 56 |
| Predation on round goby | Competition with round goby | ||
|---|---|---|---|
| Primary interaction | Perch almost exclusively feed on round goby; importance as food organism increases with increasing perch size | Round goby constitutes 17.4 ± 14.3% of large perch diet (estimated from stable isotopes); no isotopic niche overlap with benthivorous perch (small and medium length classes), thus no competition suspected | High diet overlap (except for age 2 or 3 perch [unclear]), due to preference for gammarids |
| Resulting impact on native species | Changes in trophic links; round goby is an energy pathway from mussels to top predators | Change in top predator diets | Potential for competition with small perch |
| Anticipated future effects | Bioaccumulation of toxins via mussels and round goby possible; may contribute to a new link in the energy pathway from bivalves to higher human exploited trophic levels (i.e., fish) | Stabilization of round goby population density due to predation | n.a. |
| Authors | Almqvist et al. (2010) | Rakauskas et al. (2013) | Copp et al. (2008) |
A broad range of methods have been applied in the reviewed papers, including laboratory experiments, manipulative studies under semi-natural conditions, before/after studies in the field, stomach content analysis, and stable isotope analysis. The literature review showed some profound, but ambiguous impacts of round goby on native fish species (Table 2). We summarize and structure these based on a taxonomic grouping of the affected species: native benthic fish, predatory percid fish, predatory gadid fish, and predatory salmonid fish.
Table 2.
Variations of round goby interactions with native species (see text for references). (A) The general type of interactions between round goby and native species varies with interactor life stage. (B) The intensity of the interaction differs between ecosystems and studies. (C) The impact resulting from the interaction differs between ecosystems and studies
| (A) General type of interaction | Studied native species | (B) Differences in interaction intensity with native species | (C) Differences in impacts on native species |
|---|---|---|---|
| Competition | |||
|
Logperch (Percina caprodes) | Non-ambiguous (i.e., competition was found in all studies) | Inter-study differences in round goby impact on logperch abundance |
| Johnny darter (Etheostoma nigrum) and other darter species | Ambiguous: inter-study differences in round goby competition with darter species | Inter-study differences in round goby impact on darter abundance Inter-study differences in round goby impact on darter growth rates |
|
| Competition and predation | |||
|
Mottled sculpin (Cottus bairdii) and other sculpin species | Ambiguous: inter-study differences in round goby competition with sculpin species | Inter-study differences in round goby impact on sculpin abundance |
|
Yellow perch (Perca flavescens) | Ambiguous: inter-study differences in round goby competition with juvenile yellow perch Ambiguous: Inter-study differences in yellow perch predation on round goby |
Inter-study differences in round goby impact on yellow perch body condition |
| Eurasian perch (Perca fluviatilis) | Ambiguous: inter-study differences in round goby competition with juvenile Eurasian perch Ambiguous: Inter-study differences in Eurasian perch predation on round goby |
Not assessed in studies | |
| Predation | |||
|
Burbot (Lota lota) | Ambiguous: Inter-study differences in predation on round goby | Inter-study differences in round goby impact on burbot body condition |
| Lake whitefish (Coregonus clupeaformis) | Ambiguous: Inter-study differences in predation on round goby | Inter-study differences in round goby impact on lake whitefish body condition | |
|
Lake trout (Salvelinus namaycush) | Ambiguous: Inter-study differences in predation on round goby | Inter-study differences in round goby impact on lake trout reproduction |
| Smallmouth bass (Micropterus dolomieu) and other bass species | Ambiguous: Inter-study differences in predation on round goby | Inter-study differences in round goby impact on bass growth and body condition | |
| Walleye (Sander vitreus) | Ambiguous: Inter-study differences in predation on round goby | Inter-study differences in round goby impact on walleye growth and body condition | |
Impacts on native benthic fish
The impacts of round goby on benthic fish have been investigated in 13 of the 53 papers. Logperch (Percina caprodes) and round goby compete for food and shelter under laboratory conditions (Balshine et al. 2005; Bergstrom and Mensinger 2009) and show high diet overlap in the St. Clair River (French and Jude 2001). However, the impact of round goby abundance on logperch abundance in Hamilton Harbour, Lake Ontario remains elusive (Balshine et al. 2005). No impact on logperch abundance has been found in catchments of Lake Michigan (Kornis et al. 2013).
Johnny darter (Etheostoma nigrum) abundance decreased in southern Lake Michigan following round goby invasion. No specific interaction is established as the causal link for the decline (Lauer et al. 2004). In contrast, no change in johnny darter abundance has been found in catchments of Lake Michigan (Kornis et al. 2013). In a tributary river of Lake Michigan, round gobies have invader-density-dependent impacts on growth rates of johnny darter: johnny darter growth rates decreased in an in situ experiment with presence of a few gobies (2.7 individuals m−2), but not with the presence of many gobies (10.7 individuals m−2; Kornis et al. 2014). Other darters such as blackside darter (Percina maculate), fantail darter (Etheostoma flabellare), and rainbow darter (E. caeruleum) are suspected to have diet or habitat overlap with round goby (French and Jude 2001; Poos et al. 2010; Abbett et al. 2013). In tributaries of Lake Erie, no rainbow darters and johnny darters were found in any of the streams containing round goby, whereas they were present in all of the goby-absent streams (Krakowiak and Pennuto 2008).
Mottled sculpins (Cottus bairdii) interact with round gobies in three ways: they compete for food and shelter (Dubs and Corkum 1996), mottled sculpins prey on round goby young-of-the-year (YOY; French and Jude 2001), and round goby prey on mottled sculpin eggs and YOY (French and Jude 2001; Mychek-Londer et al. 2013). These interactions have different impacts on mottled sculpin abundance in different ecosystems. In southern Lake Michigan, mottled sculpin populations were displaced, and their abundance decreased within less than 4 years after the first round goby was caught (4 years: Janssen and Jude 2001; 2–3 years: Lauer et al. 2004). On the other hand, no short-term change or temporal trend in mottled sculpin abundance was observed in Lake Michigan catchments despite increases in round goby abundance (Kornis et al. 2013). Other sculpin species did not show clear-cut responses to round gobies when investigated: round gobies gained more weight during a feeding experiment than slimy sculpins (C. cognatus) or spoonhead sculpins (C. ricei), but the non-native and native species had little physical contact (Bergstrom and Mensinger 2009). In the field, round gobies show no significant diet overlap with deepwater sculpins (Myoxocephalus thompsonii) and slimy sculpins (Mychek-Londer et al. 2013).
Impacts on percid fish
The impact of round goby on native percids have been investigated in 23 out of 53 papers. Round goby impacts on yellow perch (P. flavescens) have been extensively studied in the Great Lakes area (9/53; Table S2). Impacts are life stage dependent and include competition for food in the juvenile stages (Duncan et al. 2011; Crane et al. 2015) or one-sided predation by adult yellow perch on round gobies (Johnson et al. 2005; Lee and Johnson 2005; Truemper and Lauer 2005; Truemper et al. 2006; Campbell et al. 2009; Reyjol et al. 2010; Taraborelli et al. 2010; Crane et al. 2015). The strengths of both interactions depend on the complexity of habitat structure, biotic factors, and round goby density (Reyjol et al. 2010). If predation occurs in the adult stages, round goby as novel food item can be beneficial for yellow perch. Round gobies may provide an energetic advantage over traditional prey: foraging costs should be lower when predators feed on abundant goby prey than on less-abundant and presumably harder-to-catch native prey (Johnson et al. 2005), thus leading to a higher mass-at-length for larger yellow perch (> 27.5 cm total length TL, Crane et al. 2015).
Round goby impacts on Eurasian perch (P. fluviatilis) are known from several sites in Europe and are life stage dependent (3/53; Table 1). Round goby compete with juvenile benthivorous perch not only for food (Copp et al. 2008), but also serve as a prey for larger piscivorous perch, albeit with varying importance (Almqvist et al. 2010; Rakauskas et al. 2013).
Adult smallmouth bass (Micropterus dolomieu), largemouth bass (M. salmoides), rock bass (Ambloplites rupestris), and white bass (Morone chrysops) prey on round goby (Johnson et al. 2005; Dietrich et al. 2006; Hogan et al. 2007; Campbell et al. 2009; Taraborelli et al. 2010; Brownscombe and Fox 2013; Crane et al. 2015). Smallmouth bass predation on round gobies is higher in areas with earlier goby invasion, which can be explained by predator-learning ability (Brownscombe and Fox 2013). In Lake Erie and Lake Ontario, increases in smallmouth bass growth and condition following round goby invasion have been found (Steinhart et al. 2004b; Reyjol et al. 2010; Crane et al. 2015). For white bass, there has been no consistent trend in increased growth after round goby invasion (Johnson et al. 2005). However, round goby impacts on bass are life stage specific. Round gobies have been described as egg predators of smallmouth bass in Lake Erie, where they ate the complete offspring of an unguarded smallmouth bass nest within 15 min in an experiment in the field, in which nest-guarding bass were caught from the nest and later released again (Steinhart et al. 2004a).
Round goby impacts on walleye (Sander vitreus) are predominantly manifested in round goby becoming a prey, but impacts are partly life stage dependent, and inconsistent impacts on predator growth and condition are found. In Lake Ontario, the largest walleye length class benefitted from improved condition, but not the smaller-length classes (pre-invasion period 1993–2004 compared with post-invasion period 2005–2012, Crane et al. 2015). In Lake Erie, walleye condition did not change after round goby invasion (pre-invasion period 1993–1998 compared with post-invasion period 1999–2012, Crane et al. 2015). Some walleye eggs were found in Lake Erie round goby stomachs, but the authors suggest that these eggs were ingested by accident by round gobies foraging on dreissenids (Roseman et al. 2006). The contribution of round goby to walleye diet ranges from around 10% of diet in Lake Erie (Johnson et al. 2005), 30% frequency of occurrence in Lake Ontario (Taraborelli et al. 2010) and Lake Huron (Roseman et al. 2014), to around 50% frequency of occurrence in Lake St. Pierre in the St. Lawrence River (Reyjol et al. 2010).
Impacts on gadid fish
Round goby is an important diet item for burbot (Lota lota) in the Great Lakes area (Johnson et al. 2005; Stapanian et al. 2007; Hensler et al. 2008; Jacobs et al. 2010; Madenjian et al. 2011; Stapanian et al. 2011; Crane et al. 2015). However, the impact of round goby on burbot is life stage and ecosystem dependent. Round goby contribution to burbot diet varies across different ecosystems, and not all burbot size classes benefit from this novel prey. In Lake Erie, round goby is the most important food organism for particularly older burbot by wet weight (Madenjian et al. 2011), and by dry mass (Johnson et al. 2005). A significant improvement in condition of burbot feeding on round goby has recently been detected only for individuals of the smallest length class (375 mm TL), which were in poor-to-median condition prior to round goby invasion. For individuals in the greatest length class (743 mm TL), a significant decrease in condition has been found (pre-invasion period 1993–1998 compared with post-invasion period 1999–2012, Crane et al. 2015). In Lakes Michigan and Huron, burbot with a high amount of round gobies in their diets showed lower growth than those with a lower amount of round goby. The authors suggest that “burbot have not eaten round gobies long enough to affect increases in growth” without further specifying the underlying mechanisms (Hensler et al. 2008).
Impacts on salmonid fish
Round goby impacts on lake trout (Salvelinus namaycush) are life stage and ecosystem dependent: round goby prey on lake trout eggs and fry, thus negatively affecting lake trout reproduction (Chotkowski and Marsden 1999). Adult lake trout prey on round gobies, but their importance as food item varies across ecosystems. Round goby is not an important food item for lake trout in Lake Michigan, although consumed in small numbers (Jacobs et al. 2010). In contrast, round goby is the most important lake trout food organism in Lake Huron (Roseman et al. 2014), and the second most important food item for large lake trout in Lake Ontario in 2004 (Dietrich et al. 2006). Another study in Lake Ontario, conducted four years later, found that round goby contributed substantially to the diet of all length classes of adult lake trout (Rush et al. 2012). Predation on round goby has potentially positive impacts on lake trout reproduction, because round gobies contain relatively high concentrations of thiamine (vitamin B1). High consumption rates of round goby by lake trout could mitigate the thiamine deficiency that might otherwise impair reproduction in trout (Fitzsimons et al. 2009). However, negative impacts of round goby predation on lake trout eggs in the Great Lakes are speculated to overweigh these positive impacts: when round goby overwinter on spawning reefs or forage along river banks, they are believed to decrease recruitment by interstitial predation on lake trout eggs (Chotkowski and Marsden 1999; Fitzsimons et al. 2006, 2009).
Lake whitefish (Coregonus clupeaformis) have been found to use round goby as a new prey item. In Lake Michigan, round gobies are the most important food organisms for lake whitefish in winter (Lehrer-Brey and Kornis 2014). In Lake Huron, their importance during the whole year ranges from low to high depending on the region of the lake (Pothoven and Madenjian 2013). However, despite increased piscivory, the condition of whitefish foraging on round goby did not clearly improve (Pothoven and Madenjian 2013). Our literature review did not find any articles investigating the effects of round goby on the European trout (Salmo trutta) or whitefish (Coregonus lavaretus) species flock.
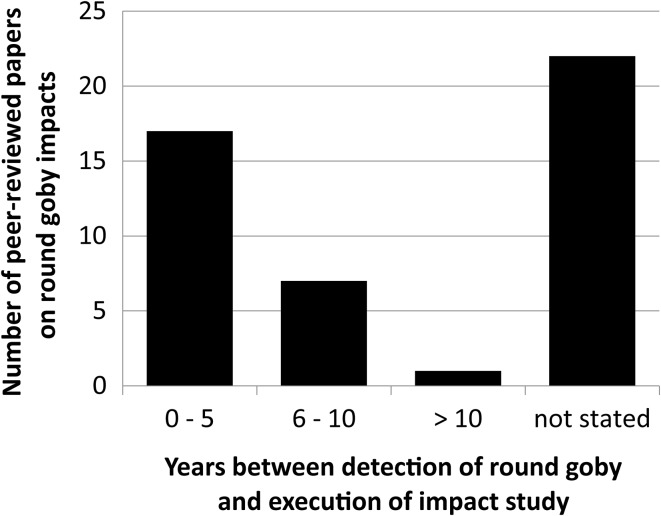
Temporal aspects are usually not addressed
When assessing the time since first detection across studies analyzing the impacts of round goby on native fish species, we found that many studies give no information at all (Fig. 3). The majority of studies are undertaken within 5 years after detection of round goby as an invasive species, and only one study assessed the long-term impacts (more than 10 years).
Fig. 3.
Most peer-reviewed papers about round goby impacts on native fish species do not state the years between detection of round goby and execution of the impact study
Discussion
Round goby impacts are profound, but variable across ecosystems, life stages, and time scales
In our literature review, we found 53 papers demonstrating that round gobies interact with native fish species (Fig. 2). Affected species respond in a variety of ways to this new predator, competitor, or prey. The directions, i.e., whether native species individuals or populations showed positive or negative responses, frequently differed across studies (Table 2). We did not find that round goby had the same clearly demonstrable, comparable impacts on a specific native species across all studies.
We identified three main explanations for why the literature did not reveal a more straightforward picture: First, round goby interactions with the same native species vary with the life stage of the interactor (Table 2A). For example, round gobies act as predators of eggs, compete with juveniles, or act as novel prey for adults of the same species (e.g., mottled sculpin or smallmouth bass). Second, the intensity of the interactions (e.g., intensity of competition or predation) differs across ecosystems (Table 2B). In some ecosystems, the interaction is very strong; in other ecosystems, the interaction between round goby and the same native species is not observed at all. For example, the intensity of competition, e.g., measured as diet overlap between native species and round gobies, varies in different ecosystems (e.g., logperch and Eurasian perch). Similarly, round goby contribution to predator diet is different for the same predatory species in different ecosystems (e.g., burbot). Third, not only the intensity of the interaction, but also how round goby impacts are reflected in native species’ growth rate and abundance differ across studies (Table 2C). For example, although competition with round goby can lead to a decreased abundance of the native species in some ecosystems, no change in the abundance of the same native species has been observed in other ecosystems (e.g., johnny darter). Similarly, predation on round gobies can lead to better condition factor or growth rate in predators in one ecosystem, whereas in another ecosystem no change in predator condition or growth can be observed (e.g., yellow perch).
Reasons for impact variations across ecosystems, life stages, and time scales
Species invasions are natural processes. The impacts of an invasive species can therefore be as complex as the impacts of any other species in the ecosystem (Crooks 2005). Against this background, it is not surprising that round goby impacts vary across ecosystems.
To further complicate things, finding impacts of invasive species depends on the temporal scale that is applied in searching for them (Strayer et al. 2006). Investigating impacts of a recently established population can reveal entirely different results from those obtained when investigating impacts of a longer established population. Unfortunately, despite our efforts to explore the time dependency of impacts, we could not investigate this question; too few studies did even state the age of the round goby population investigated (Fig. 3). The remaining studies did not allow for a quantification of impacts across population age. Scaling impacts from severe to weak or positive to negative alone would be a daunting task, so that a relationship between invasion time and impact scale would be rather arbitrary. We can say, however, that scientists should be better aware of the time dependency in biological invasions. If scientists appreciate frequently occurring lag phases in invasion research, then we can eventually arrive at a more thorough understanding of the relationship between time and impact.
This is all the more important as evolutionary processes can influence biological invasions on timescales that were previously not appreciated—the so-called contemporary time scales (Stockwell et al. 2003). Some traits which cause a non-native species to become invasive have evolved in a new system on timescales less than ten years (Whitney and Gabler 2008). This also holds good for the native species responding to invasive species. If, for example, native predators adapt to invasive species as a new prey, then native predator populations can increase over time, whereas invasive species populations decrease (Sheehy and Lawton 2014). In the case of round goby, Brownscombe and Fox (2013) tested for how readily native predators forage upon this newly available prey species: predation rates on round gobies were lower in the recently invaded systems compared to systems in which predators had time to learn to capture and consume this novel prey species.
Eventually, biological invasions can even result in entirely new species (Lee 2002; Lee et al. 2007). Processes such as hybridization with native or other invasive species can tremendously alter the ecological interactions and congruent impacts in any invaded ecosystem. In lower stretches of the River Rhine, for example, the round goby has been found to hybridize with monkey goby (Neogobius fluviatilis), a confamilial invasive goby species (Lindner et al. 2013). Which impacts are in store when invasive goby species hybridize will be even harder to predict than when clearly defined species boundaries exist.
The state of the scientific knowledge needs to be communicated to decision makers
It becomes clear that, if we want to inform a preventive management, we cannot wait until conclusive evidence for comparable impacts of round goby is available. We propose to communicate the knowledge on impacts scientists already have accrued, despite our inabilities to predict and generalize. On the onset of our project, we expected that impacts on specific native species, which re-occur across different studies, could help decision makers to prioritize if and how to instigate management measures against round goby. To this end, our focus was to scrutinize the broadest body of knowledge that scientists possess concerning round goby: knowledge on impacts. We believe the available scientific information on round goby impacts, albeit ecosystem dependent, can still be relevant to inform decision makers about potential threats. Paradoxically, the chances for successful management of a non-native species are best when we know least about its impacts: at the time when it has just established (Kriticos et al. 2003). We argue that the lack of comparable impacts of round goby on native species is no reason to conclude that there will be no impacts of round goby in a newly invaded ecosystem.
Decision makers want timely and relevant information if and how a potentially invasive non-native species should be managed (Walsh et al. 2015). Therefore, in a management context, it is more important to rapidly disseminate the current knowledge than to improve our epistemic knowledge and ability to predict round goby impacts in a particular system. In the context of preventing an approaching invader, a central task for scientists is to communicate that incomplete knowledge on negative impacts is no reason to neglect possible future impacts, i.e., the absence of evidence for negative impacts is not an evidence for the absence of negative impacts (Ojaveer and Kotta 2015). Importantly, the appreciation of time lags will improve the decision making at different stages of the invasion to more effectively make the right management choices. Along these lines, it also needs to be appreciated that an approaching invader can cross the country-borders in the course of its spread. In the case of round goby, this means that if the High Rhine and adjoining Lake Constance are invaded, three or more Central European countries will be affected. Managing such an invasion requires cooperation across borders. Institutions such as the International Commission for the Protection of the Rhine or the International Commission for the Protection of Lake Constance (ICPR 2015; IGKB 2015) provide an existing framework for this kind of cooperation. This literature review advances our ability to objectively assess what we as scientists can contribute to this cooperation and how to more effectively instigate an effective management of one of the 100 worst invaders in Europe.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Catherine Cornaz for her help with the literature review and two anonymous reviewers for helpful comments on an earlier version of this manuscript. This project was funded by a special grant from the Federal Office for the Environment, Switzerland, the Research Centre for Sustainable Energy and Water Supply (FoNEW), the canton BS, plus cantonal lottery funds of AG, BL, SO.
Biographies
Philipp E. Hirsch
is a Postdoctoral Fellow at the Department of Environmental Sciences, and associated to the Research Centre for Sustainable Energy and Water Supply, University of Basel, Switzerland. His research interests include ecological and evolutionary processes in biological invasions and how society can find ways to sustainably manage biological invasion.
Anouk N’Guyen
is a PhD Student at the Department of Environmental Sciences, University of Basel, Switzerland. Her research interests include inter- and transdisciplinary processes to solve complex human–environment problems such as managing invasive species.
Irene Adrian-Kalchhauser
is a Postdoctoral Fellow at the Department of Environmental Sciences, University of Basel, Switzerland. Her research interests include invasion genetics, the epigenetics of adaptation, and the applications of molecular methods in invasive species management.
Patricia Burkhardt-Holm
is a Professor of Ecology at the Department of Environmental Sciences, University of Basel, Switzerland. Her research focuses on aquatic ecosystems, particularly on fish and the impact of natural and anthropogenic factors.
Footnotes
Philipp E. Hirsch and Anouk N’Guyen shared first authorship.
Contributor Information
Philipp E. Hirsch, Phone: 0041 61 26704 07, Email: philipp.hirsch@unibas.ch
Anouk N’Guyen, Phone: 0041 61 267 04 11, Email: anouk.nguyen@unibas.ch.
Irene Adrian-Kalchhauser, Email: irene.adrian-kalchhauser@unibas.ch.
Patricia Burkhardt-Holm, Email: patricia.holm@unibas.ch.
References
- Abbett R, Waldt EM, Johnson JH, McKenna JE, Jr, Dittman DE. Interactions between invasive round gobies (Neogobius melanostomus) and fantail darters (Etheostoma flabellare) in a tributary of the St. Lawrence River, New York, USA. Journal of Freshwater Ecology. 2013;28:529–537. doi: 10.1080/02705060.2013.794165. [DOI] [Google Scholar]
- Almqvist G, Strandmark AK, Appelberg M. Has the invasive round goby caused new links in Baltic food webs? Environmental Biology of Fishes. 2010;89:79–93. doi: 10.1007/s10641-010-9692-z. [DOI] [Google Scholar]
- Anonymous. 1998. Exhibition of July: Salmon 2000: Preliminary success of the renaturalization programme. Natur und Museum (Frankfurt am Main) 128: 220–223.
- Balshine S, Verma A, Chant V, Theysmeyer T. Competitive Interactions between Round Gobies and Logperch. Journal of Great Lakes Research. 2005;31:68–77. doi: 10.1016/S0380-1330(05)70238-0. [DOI] [Google Scholar]
- Bayliss HR, Stewart GB, Wilcox A, Randall NP. A perceived gap between invasive species research and stakeholder priorities. NeoBiota. 2013;19:67–82. doi: 10.3897/neobiota.19.4897. [DOI] [Google Scholar]
- Bergstrom MA, Mensinger AF. Interspecific resource competition between the invasive round goby and three native species: Logperch, slimy sculpin, and spoonhead sculpin. Transactions of the American Fisheries Society. 2009;138:1009–1017. doi: 10.1577/T08-095.1. [DOI] [Google Scholar]
- Blanchett S, Leprieur F, Beauchard O, Staes J, Oberdorff T, Brosse S. Broad-scale determinants of non-native fish species richness are context-dependent. Proceedings of the Royal Society B-Biological Sciences. 2009;276:2385–2394. doi: 10.1098/rspb.2009.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JR, Gozlan RE. Geo-politics and freshwater fish introductions: How the Cold War shaped Europe’s fish allodiversity. Global Environmental Change-Human and Policy Dimensions. 2013;23:1566–1574. doi: 10.1016/j.gloenvcha.2013.09.017. [DOI] [Google Scholar]
- Brownscombe JW, Fox MG. Living at the edge of the front; reduced predation risk to invasive round goby in a Great Lakes tributary. Hydrobiologia. 2013;707:199–208. doi: 10.1007/s10750-012-1427-z. [DOI] [Google Scholar]
- Burkhardt-Holm P, Peter A, Segner H. Decline of fish catch in Switzerland—Project Fishnet: A balance between analysis and synthesis. Aquatic Sciences. 2002;64:36–54. doi: 10.1007/s00027-002-8053-1. [DOI] [Google Scholar]
- Campbell LM, Thacker R, Barton D, Muir DCG, Greenwood D, Hecky RE. Re-engineering the eastern Lake Erie littoral food web: The trophic function of non-indigenous Ponto-Caspian species. Journal of Great Lakes Research. 2009;35:224–231. doi: 10.1016/j.jglr.2009.02.002. [DOI] [Google Scholar]
- Campbell ML. Assessing biosecurity risk associated with the importation of non-indigenous microalgae. Environmental Research. 2011;111:989–998. doi: 10.1016/j.envres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Chotkowski MA, Marsden JE. Round goby and mottled sculpin predation on lake trout eggs and fry: Field predictions from laboratory experiments. Journal of Great Lakes Research. 1999;25:26–35. doi: 10.1016/S0380-1330(99)70714-8. [DOI] [Google Scholar]
- Copp GH, Kováč V, Zweimüller I, Dias A, Nascimento M, Balážová M. Preliminary study of dietary interactions between invading Ponto-Caspian gobies and some native fish species in the River Danube near Bratislava (Slovakia) Aquatic Invasions. 2008;3:193–200. doi: 10.3391/ai.2008.3.2.10. [DOI] [Google Scholar]
- Copp GH, Vilizzi L, Mumford J, Fenwick GV, Godard MJ, Gozlan RE. Calibration of FISK, an invasiveness screening tool for nonnative freshwater fishes. Risk Analysis. 2009;29:457–467. doi: 10.1111/j.1539-6924.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- Corkum LD, Sapota MR, Skora KE. The round goby, Neogobius melanostomus, a fish invader on both sides of the Atlantic Ocean. Biological Invasions. 2004;6:173–181. doi: 10.1023/B:BINV.0000022136.43502.db. [DOI] [Google Scholar]
- Crane DP, Farrell JM, Einhouse DW, Lantry JR, Markham JL. Trends in body condition of native piscivores following invasion of Lakes Erie and Ontario by the round goby. Freshwater Biology. 2015;60:111–124. doi: 10.1111/fwb.12473. [DOI] [Google Scholar]
- Crooks JA. Lag times and exotic species: The ecology and management of biological invasions in slow-motion. Ecoscience. 2005;12:316–329. doi: 10.2980/i1195-6860-12-3-316.1. [DOI] [Google Scholar]
- Daehler C, Gordon DR. To introduce or not to introduce trade-offs of non-indigenous organisms. Trends in Ecology & Evolution. 1997;12:424–425. doi: 10.1016/S0169-5347(97)01206-8. [DOI] [PubMed] [Google Scholar]
- DAISIE. 2015. European Invasive Alien Species Gateway: 100 of the worst. Retrieved 24 June, 2015, from http://www.europe-aliens.org/speciesTheWorst.do.
- Davidson AD, Hewitt CL. How often are invasion-induced ecological impacts missed? Biological Invasions. 2014;16:1165–1173. doi: 10.1007/s10530-013-0570-4. [DOI] [Google Scholar]
- Dick JTA, Alexander ME, Jeschke JM, Ricciardi A, MacIsaac HJ, Robinson TB, Kumschick S, Weyl OLF, et al. Advancing impact prediction and hypothesis testing in invasion ecology using a comparative functional response approach. Biological Invasions. 2014;16:735–753. doi: 10.1007/s10530-013-0550-8. [DOI] [Google Scholar]
- Dietrich JP, Morrison BJ, Hoyle JA. Alternative ecological pathways in the Eastern Lake Ontario food web—round goby in the diet of lake trout. Journal of Great Lakes Research. 2006;32:395–400. doi: 10.3394/0380-1330(2006)32[395:AEPITE]2.0.CO;2. [DOI] [Google Scholar]
- Dubs DOL, Corkum LD. Behavioral interactions between round gobies (Neogobius melanostomus) and mottled sculpins (Cottus bairdi) Journal of Great Lakes Research. 1996;22:838–844. doi: 10.1016/S0380-1330(96)71005-5. [DOI] [Google Scholar]
- Duncan JM, Marschner CA, González MJ. Diet partitioning, habitat preferences and behavioral interactions between juvenile yellow perch and round goby in nearshore areas of Lake Erie. Journal of Great Lakes Research. 2011;37:101–110. doi: 10.1016/j.jglr.2010.11.015. [DOI] [Google Scholar]
- Ellender BR, Weyl OLF. A review of current knowledge, risk and ecological impacts associated with non-native freshwater fish introductions in South Africa. Aquatic Invasions. 2014;9:117–132. doi: 10.3391/ai.2014.9.2.01. [DOI] [Google Scholar]
- EU. 2009. Invasive alien species: Nature and biodiversity. Retrieved June 24, 2015, from http://ec.europa.eu/environment/pubs/pdf/factsheets/Invasive%20Alien%20Species/Invasive_Alien_EN.pdf.
- Fitzsimons J, Williston B, Williston G, Bravener G, Jonas JL, Claramunt RM, Marsden JE, Ellrott BJ. Laboratory estimates of salmonine egg predation by round gobies (Neogobius melanostomus), sculpins (Cottus cognatus and C. bairdi), and crayfish (Orconectes propinquus) Journal of Great Lakes Research. 2006;32:227–241. doi: 10.3394/0380-1330(2006)32[227:LEOSEP]2.0.CO;2. [DOI] [Google Scholar]
- Fitzsimons JD, Clark M, Keir M. Addition of round gobies to the prey community of Lake Ontario and potential implications to thiamine status and reproductive success of lake trout. Aquatic Ecosystem Health & Management. 2009;12:296–312. doi: 10.1080/14634980903136453. [DOI] [Google Scholar]
- French JRP, Jude DJ. Diets and diet overlap of nonindigenous gobies and small benthic native fishes co-inhabiting the St. Clair River. Michigan. Journal of Great Lakes Research. 2001;27:300–311. doi: 10.1016/S0380-1330(01)70645-4. [DOI] [Google Scholar]
- García-Berthou E, Alcaraz C, Pou-Rovira Q, Zamora L, Coenders G, Feo C. Introduction pathways and establishment rates of invasive aquatic species in Europe. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:453–463. doi: 10.1139/f05-017. [DOI] [Google Scholar]
- Gozlan RE. Introduction of non-native freshwater fish: Is it all bad? Fish and Fisheries. 2008;9:106–115. doi: 10.1111/j.1467-2979.2007.00267.x. [DOI] [Google Scholar]
- Gozlan RE, Britton JR, Cowx I, Copp GH. Current knowledge on non-native freshwater fish introductions. Journal of Fish Biology. 2010;76:751–786. doi: 10.1111/j.1095-8649.2010.02566.x. [DOI] [Google Scholar]
- Heger T, Pahl AT, Botta-Dukát Z, Gherardi F, Hoppe C, Hoste I, Jax K, Lindström L, et al. Conceptual frameworks and methods for advancing invasion ecology. Ambio. 2013;42:527–540. doi: 10.1007/s13280-012-0379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler SR, Jude DJ, He JiX. Burbot growth and diets in lakes Michigan and Huron: An ongoing shift from native species to round gobies. In: Paragamian VL, Bennett DH, editors. Burbot: Ecology, management, and culture. Bethesda: American Fisheries Society; 2008. pp. 91–107. [Google Scholar]
- Hogan LS, Marschall E, Folt C, Stein RA. How non-native species in Lake Erie influence trophic transfer of mercury and lead to top predators. Journal of Great Lakes Research. 2007;33:46–61. doi: 10.3394/0380-1330(2007)33[46:HNSILE]2.0.CO;2. [DOI] [Google Scholar]
- Holmlund CM, Hammer M. Ecosystem services generated by fish populations. Ecological Economics. 1999;29:253–268. doi: 10.1016/S0921-8009(99)00015-4. [DOI] [Google Scholar]
- ICPR. 2015. The Rhine. Retrieved September 7, 2015, from http://www.iksr.org/en/rhine/index.html.
- IGKB. 2015. The Organisation. Retrieved September 7, 2015, from http://www.igkb.org/die-igkb/die-organisation/ (in German).
- Jacobs GR, Madenjian CP, Bunnell DB, Holuszko JD. Diet of lake trout and burbot in northern Lake Michigan during spring: Evidence of ecological interaction. Journal of Great Lakes Research. 2010;36:312–317. doi: 10.1016/j.jglr.2010.02.007. [DOI] [Google Scholar]
- Janssen J, Jude DJ. Recruitment failure of mottled sculpin Cottus bairdi in Calumet Harbor, Southern Lake Michigan, induced by the newly introduced round goby Neogobius melanostomus. Journal of Great Lakes Research. 2001;27:319–328. doi: 10.1016/S0380-1330(01)70647-8. [DOI] [Google Scholar]
- Johnson TB, Bunnell DB, Knight CT. A potential new energy pathway in central Lake Erie: The round goby connection. Journal of Great Lakes Research. 2005;31:238–251. doi: 10.1016/S0380-1330(05)70317-8. [DOI] [Google Scholar]
- Kalchhauser I, Mutzner P, Hirsch PE, Burkhardt-Holm P. Arrival of round goby Neogobius melanostomus (Pallas, 1814) and bighead goby Ponticola kessleri (Günther, 1861) in the High Rhine (Switzerland) BioInvasions Records. 2013;2:79–83. doi: 10.3391/bir.2013.2.1.14. [DOI] [Google Scholar]
- Keller RP, Frang K, Lodge DM. Preventing the spread of invasive species: Economic benefits of intervention guided by ecological predictions. Conservation Biology. 2008;22:80–88. doi: 10.1111/j.1523-1739.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- Keller RP, Lodge DM, Finnoff DC. Risk assessment for invasive species produces net bioeconomic benefits. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:203–207. doi: 10.1073/pnas.0605787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornis MS, Carlson J, Lehrer-Brey G, Vander Zanden JM. Experimental evidence that ecological effects of an invasive fish are reduced at high densities. Oecologia. 2014;175:325–334. doi: 10.1007/s00442-014-2899-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornis MS, Mercado-Silva N, Vander Zanden JM. Twenty years of invasion: A review of round goby Neogobius melanostomus biology, spread and ecological implications. Journal of Fish Biology. 2012;80:235–285. doi: 10.1111/j.1095-8649.2011.03157.x. [DOI] [PubMed] [Google Scholar]
- Kornis MS, Sharma S, Vander Zanden JM, Ricciardi A. Invasion success and impact of an invasive fish, round goby, in Great Lakes tributaries. Diversity and Distributions. 2013;19:184–198. doi: 10.1111/ddi.12001. [DOI] [Google Scholar]
- Krakowiak PJ, Pennuto CM. Fish and macroinvertebrate communities in tributary streams of eastern Lake Erie with and without round gobies (Neogobius melanostomus, Pallas 1814) Journal of Great Lakes Research. 2008;34:675–689. doi: 10.1016/S0380-1330(08)71610-1. [DOI] [Google Scholar]
- Kriticos DJ, Sutherst RW, Brown JR, Adkins SW, Maywald GF. Climate change and biotic invasions: A case history of a tropical woody vine. Biological Invasions. 2003;5:147–165. doi: 10.1023/A:1026193424587. [DOI] [Google Scholar]
- Lauer TE, Allen PJ, McComish TS. Changes in mottled sculpin and johnny darter trawl catches after the appearance of round gobies in the Indiana waters of Lake Michigan. Transactions of the American Fisheries Society. 2004;133:185–189. doi: 10.1577/T02-123. [DOI] [Google Scholar]
- Lawrence RJ. Advances in transdisciplinarity: Epistemologies, methodologies and processes. Futures. 2015;65:1–9. doi: 10.1016/j.futures.2014.11.007. [DOI] [Google Scholar]
- Lee CE. Evolutionary genetics of invasive species. Trends in Ecology & Evolution. 2002;17:386–391. doi: 10.1016/S0169-5347(02)02554-5. [DOI] [Google Scholar]
- Lee CE, Remfert J, Chang Y-M. Response to selection and evolvability of invasive populations. Genetica. 2007;129:179–192. doi: 10.1007/s10709-006-9013-9. [DOI] [PubMed] [Google Scholar]
- Lee VA, Johnson TB. Development of a bioenergetics model for the round goby (Neogobius melanostomus) Journal of Great Lakes Research. 2005;31:125–134. doi: 10.1016/S0380-1330(05)70244-6. [DOI] [Google Scholar]
- Lehrer-Brey G, Kornis MS. Winter distributional overlap facilitates Lake Whitefish (Coregonus clupeaformis) piscivory on invasive round gobies (Neogobius melanostomus) in Green Bay, Lake Michigan. Journal of Freshwater Ecology. 2014;29:153–156. doi: 10.1080/02705060.2013.815663. [DOI] [Google Scholar]
- Lindner K, Cerwenka AF, Brandner J, Gertzen S, Borcherding J, Geist J, Schliewen UK. First evidence for interspecific hybridization between invasive goby species Neogobius fluviatilis and Neogobius melanostomus (Teleostei: Gobiidae: Benthophilinae) Journal of Fish Biology. 2013;82:2128–2134. doi: 10.1111/jfb.12127. [DOI] [PubMed] [Google Scholar]
- Madenjian CP, Stapanian MA, Witzel LD, Einhouse DW, Pothoven SA, Whitford HL. Evidence for predatory control of the invasive round goby. Biological Invasions. 2011;13:987–1002. doi: 10.1007/s10530-010-9884-7. [DOI] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Mychek-Londer JG, Bunnell DB, Stott W, Diana JS, French JRP, III, Chriscinske MA. Using diets to reveal overlap and egg predation among benthivorous fishes in Lake Michigan. Transactions of the American Fisheries Society. 2013;142:492–504. doi: 10.1080/00028487.2012.756431. [DOI] [Google Scholar]
- N’Guyen, A., P.E. Hirsch, I. Adrian-Kalchhauser, and P. Burkhardt-Holm. 2015. Improving invasive species management by integrating priorities and contributions of scientists and decision makers. Ambio. doi:10.1007/s13280-015-0723-z [DOI] [PMC free article] [PubMed]
- Ojaveer H, Kotta J. Ecosystem impacts of the widespread non-indigenous species in the Baltic Sea: Literature survey evidences major limitations in knowledge. Hydrobiologia. 2015;750:171–185. doi: 10.1007/s10750-014-2080-5. [DOI] [Google Scholar]
- Pickering C, Byrne J. The benefits of publishing systematic quantitative literature reviews for PhD candidates and other early-career researchers. Higher Education Research & Development. 2014;33:534–548. doi: 10.1080/07294360.2013.841651. [DOI] [Google Scholar]
- Poos M, Dextrase AJ, Schwalb AN, Ackerman JD. Secondary invasion of the round goby into high diversity Great Lakes tributaries and species at risk hotspots: Potential new concerns for endangered freshwater species. Biological Invasions. 2010;12:1269–1284. doi: 10.1007/s10530-009-9545-x. [DOI] [Google Scholar]
- Pothoven SA, Madenjian CP. Increased piscivory by Lake Whitefish in Lake Huron. North American Journal of Fisheries Management. 2013;33:1194–1202. doi: 10.1080/02755947.2013.839973. [DOI] [Google Scholar]
- Rakauskas V, Pūtys Ž, Dainys J, Lesutienė J, Ložpys L, Arbačiauskas K. Increasing population of the invader round goby, Neogobius melanostomus (Actinopterygii: Perciformes: Gobiidae), and its trophic role in the Curonian Lagoon, SE Baltic Sea. Acta Ichthyologica et Piscatoria. 2013;43:95–108. doi: 10.3750/AIP2013.43.2.02. [DOI] [Google Scholar]
- Reyjol Y, Brodeur P, Mailhot Y, Mingelbier M, Dumont P. Do native predators feed on non-native prey? The case of round goby in a fluvial piscivorous fish assemblage. Journal of Great Lakes Research. 2010;36:618–624. doi: 10.1016/j.jglr.2010.09.006. [DOI] [Google Scholar]
- Roche KF, Janač M, Jurajda P. A review of Gobiid expansion along the Danube-Rhine corridor—geopolitical change as a driver for invasion. Knowledge and Management of Aquatic Ecosystems. 2013;411:1. doi: 10.1051/kmae/2013066. [DOI] [Google Scholar]
- Roseman EF, Schaeffer JS, Bright E, Fielder DG. Angler-caught piscivore diets reflect fish community changes in Lake Huron. Transactions of the American Fisheries Society. 2014;143:1419–1433. doi: 10.1080/00028487.2014.945659. [DOI] [Google Scholar]
- Roseman EF, Taylor WW, Hayes DB, Jones AL, Francis JT. Predation on walleye eggs by fish on reefs in western Lake Erie. Journal of Great Lakes Research. 2006;32:415–423. doi: 10.3394/0380-1330(2006)32[415:POWEBF]2.0.CO;2. [DOI] [Google Scholar]
- Rosendahl J, Zanella MA, Rist S, Weigelt J. Scientists’ situated knowledge: Strong objectivity in transdisciplinarity. Futures. 2015;65:17–27. doi: 10.1016/j.futures.2014.10.011. [DOI] [Google Scholar]
- Rush SA, Paterson G, Johnson TB, Drouillard KG, Haffner GD, Hebert CE, Arts MT, McGoldrick DJ, et al. Long-term impacts of invasive species on a native top predator in a large lake system. Freshwater Biology. 2012;57:2342–2355. doi: 10.1111/fwb.12014. [DOI] [Google Scholar]
- Seidl R, Brand FS, Stauffacher M, Krütli P, Le QB, Spörri A, Meylan G, Moser C, et al. Science with society in the anthropocene. Ambio. 2013;42:5–12. doi: 10.1007/s13280-012-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy E, Lawton C. Population crash in an invasive species following the recovery of a native predator: The case of the American grey squirrel and the European pine marten in Ireland. Biodiversity and Conservation. 2014;23:753–774. doi: 10.1007/s10531-014-0632-7. [DOI] [Google Scholar]
- Simberloff D. How much information on population biology is needed to manage introduced species? Conservation Biology. 2003;17:83–92. doi: 10.1046/j.1523-1739.2003.02028.x. [DOI] [Google Scholar]
- Stapanian MA, Edwards WH, Witzel LD. Recent changes in burbot growth in Lake Erie. Journal of Applied Ichthyology. 2011;27:57–64. doi: 10.1111/j.1439-0426.2011.01845.x. [DOI] [Google Scholar]
- Stapanian MA, Madenjian CP, Tost J. Regional differences in size-at-age of the recovering burbot (Lota lota) population in Lake Erie. Journal of Great Lakes Research. 2007;33:91–102. doi: 10.3394/0380-1330(2007)33[91:RDISOT]2.0.CO;2. [DOI] [Google Scholar]
- Steinhart GB, Marschall EA, Stein RA. Round goby predation on smallmouth bass offspring in nests during simulated catch-and-release angling. Transactions of the American Fisheries Society. 2004;133:121–131. doi: 10.1577/T03-020. [DOI] [Google Scholar]
- Steinhart GB, Stein RA, Marschall EA. High growth rate of young-of-the-year smallmouth bass in Lake Erie: A result of the round goby invasion? Journal of Great Lakes Research. 2004;30:381–389. doi: 10.1016/S0380-1330(04)70355-X. [DOI] [Google Scholar]
- Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends in Ecology & Evolution. 2003;18:94–101. doi: 10.1016/S0169-5347(02)00044-7. [DOI] [Google Scholar]
- Strayer DL. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshwater Biology. 2010;55:152–174. doi: 10.1111/j.1365-2427.2009.02380.x. [DOI] [Google Scholar]
- Strayer DL, Eviner VT, Jeschke JM, Pace ML. Understanding the long-term effects of species invasions. Trends in Ecology & Evolution. 2006;21:645–651. doi: 10.1016/j.tree.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Taraborelli AC, Fox MG, Johnson TB, Schaner T. Round goby (Neogobius melanostomus) population structure, biomass, prey consumption and mortality from predation in the Bay of Quinte, Lake Ontario. Journal of Great Lakes Research. 2010;36:625–632. doi: 10.1016/j.jglr.2010.07.011. [DOI] [Google Scholar]
- Truemper HA, Lauer TE. Gape limitation and piscine prey size-selection by yellow perch in the extreme southern area of Lake Michigan, with emphasis on two exotic prey items. Journal of Fish Biology. 2005;66:135–149. doi: 10.1111/j.0022-1112.2005.00588.x. [DOI] [Google Scholar]
- Truemper HA, Lauer TE, McComish TS, Edgell RA. Response of yellow perch diet to a changing forage base in southern Lake Michigan, 1984–2002. Journal of Great Lakes Research. 2006;32:806–816. doi: 10.3394/0380-1330(2006)32[806:ROYPDT]2.0.CO;2. [DOI] [Google Scholar]
- Walsh JC, Dicks LV, Sutherland WJ. The effect of scientific evidence on conservation practitioners’ management decisions. Conservation Biology. 2015;29:88–98. doi: 10.1111/cobi.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney KD, Gabler CA. Rapid evolution in introduced species, ‘invasive traits’ and recipient communities: Challenges for predicting invasive potential. Diversity and Distributions. 2008;14:569–580. doi: 10.1111/j.1472-4642.2008.00473.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.