Abstract
Background:
The aetiology of Barrett's oesophagus (BO) and oesophageal cancer is poorly understood. We previously demonstrated that Golgi structure and function is altered in oesophageal cancer cells. A Golgi-associated protein, GOLPH2, was previously established as a tissue biomarker for BO. Cellular functions for GOLPH2 are currently unknown, therefore in this study we sought to investigate functional roles for this Golgi-associated protein in oesophageal disease.
Methods:
Expression, intracellular localisation and secretion of GOLPH2 were identified by immunofluorescence, immunohistochemistry and western blot. GOLPH2 expression constructs and siRNA were used to identify cellular functions for GOLPH2.
Results:
We demonstrate that the structure of the Golgi is fragmented and the intracellular localisation of GOLPH2 is altered in BO and oesophageal adenocarcinoma tissue. GOLPH2 is secreted by oesophageal cancer cells and GOLPH2 expression, cleavage and secretion facilitate cell migration and invasion. Furthermore, exposure of cells to DCA, a bile acid component of gastric refluxate and known tumour promoter for oesophageal cancer, causes disassembly of the Golgi structure into ministacks, resulting in cleavage and secretion of GOLPH2.
Conclusions:
This study demonstrates that GOLPH2 may be a useful tissue biomarker for oesophageal disease. We provide a novel mechanistic insight into the aetiology of oesophageal cancer and reveal novel functions for GOLPH2 in regulating tumour cell migration and invasion, important functions for the metastatic process in oesophageal cancer.
Keywords: bile acids, golgi, Barrett's oesophagus, oesophageal cancer, GOLPH2
Oesophageal adenocarcinoma is a multi-stage progressive disease. In response to chronic exposure of the oesophagus to gastric refluxate, normal squamous epithelial cells undergo metaplasia to a specialised intestinal-like columnar epithelium, a pre-malignant condition called Barrett's oesophagus (BO) (Atherfold and Jankowski, 2006). A number of molecular events are associated with progression from BO metaplasia, low to high grade dysplasia and finally adenocarcinoma, known as the metaplasia–dysplasia–adenocarcinoma sequence (Ransford and Jankowski, 2000). Repeated exposure of the oesophagus to refluxate has been implicated as the main underlying cause of disease progression although the precise aetiology of oesophageal adenocarcinoma is poorly understood (Marshall et al, 1997; Lagergren et al, 1999; Nehra et al, 1999).
Deoxycholic acid (DCA) is a bile acid present in gastric refluxate with known pro-tumorigenic activities, promoting BO and oesophageal adenocarcinoma development in animal models (Chen et al, 2002; Majka et al, 2010) altering p63 and COX2 expression and activating cell signalling pathways associated with carcinogenesis (Shah et al, 2005; Roman et al, 2007; Looby et al, 2009). More recently, we demonstrated that exposure of colonic and oesophageal cells to DCA causes dispersal of cis-Golgi membranes througout the cytoplasm, resulting in impaired protein processing, trafficking and secretion (Byrne et al, 2010, 2012). We identified a mechanism whereby DCA causes over-activation of the membrane fission process at the Golgi via protein kinase Cη/protein kinase D (Byrne et al, 2010).
The Golgi apparatus is responsible for glycolipid biosynthesis and has a critical function in the protein secretory pathway involved in glycosylation, sorting and secretion of newly synthesised proteins received from the endoplasmic reticulum (ER)(Glick and Malhotra, 1998; Glick, 2000; Maccioni et al, 2011). The Golgi comprises a series of cisternae organised in a cis-medial-trans manner. Proteins from the ER are received at the Golgi where they undergo post-translational modifications such as glycosylation and folding. Proteins are sorted at the Trans-Golgi-Network for transport to post-Golgi compartments or the cell surface (Glick, 2000). The structured architecture of the Golgi cisternae is critical for these processes. Abnormal protein glycosylation is a hallmark of cancer although the underlying mechanisms are not fully understood (Zheng et al, 1994; Dennis et al, 1999; Dube and Bertozzi, 2005). Altered processing and expression of complex glycoproteins is observed in patients with reflux disease, BO and oesophageal cancer (Shimamoto et al, 1987; Neumann et al, 2008).
Glycosylation of proteins at the Golgi is crucial for protein trafficking, expression and signalling (Fan et al, 1997; Isaji et al, 2009). DCA impaired glycosylation of E-cadherin and reduced trafficking to the cell-membrane as a consequence of effects on Golgi structure (Byrne et al, 2012).
Since DCA is a tumour promoter for oesophageal adenocarcinoma and impairs protein processing, trafficking and secretion via effects on Golgi structure, we sought to investigate whether Golgi-associated proteins could potentially play a functional role in the pathogenesis of this disease. Using previously published meta-analysis of gene-expression microarray data sets, Golgi-associated proteins altered in BO and oesophageal adenocarcinoma tissue were identified. Increased expression of a Golgi-localised protein, GOLPH2 (GOLM1/GP73), was identified in tissue from patients with BO compared with patients without disease, suggesting this protein may act as a tissue biomarker for BO (Lao-Sirieix et al, 2009; Wang et al, 2009).
GOLPH2 is a 73-kDa type II cis-Golgi-localised protein expressed primarily in cells of epithelial lineage. To date cell-specific functions for GOLPH2 have not been identified; however, mouse models expressing c-terminally truncated GOLPH2 show overall reduced survival and disorganisation of hepatic and renal epithelial cells, demonstrating an essential cellular function (Wright et al, 2008). Although GOLPH2 normally localises to the Golgi, it can cycle to distal compartments such as sorting endosomes and the plasma membrane, but is ultimately retrieved back to the Golgi via a late endosomal-bypass pathway and not secreted from normal cells (Mallet and Maxfield, 1999; Puri et al, 2002). GOLPH2 undergoes cleavage at a Pro-protein convetase (PC) cleavage site for trafficking to distal compartments (Bachert et al, 2007). GOLPH2 expression is highly upregulated in hepatitis viral infection, implicating the potential involvement of GOLPH2 in injury response (Iftikhar et al, 2004). In the diagnosis of hepatocellular carcinoma, GOLPH2 has a comparable accuracy to Alpha-Fetoprotein (Zhou et al, 2012) and is also detectable as a urinary and tissue biomarker for prostate cancer. In hepatocellular cancer, overexpression of GOLPH2 results in saturation of the retrieval system and its secretion from the cell, thereby acting as an effective serum biomarker in this disease (Marrero et al, 2005; Bachert et al, 2007; Ba et al, 2012).
As there are no known functions for GOLPH2, we chose to characterise this protein in terms of its potential role in oesophageal cancer. This study demonstrates that GOLPH2 localises with the Golgi in non-dysplastic tissue but does not localise to the Golgi in intestinal metaplastic or adenocarcinoma patient tissue, where the Golgi structure is fragmented. We sought to investigate the consequences of this altered cytoplasmic localisation, propose a potential mechanism of how this could occur, if GOLPH2 could be used as a potential biomarker for oesophageal disease and identify novel functions for GOLPH2.
Results
Identification of candidate Golgi-associated proteins involved in oesophageal cancer progression
Having previously demonstrated that the structure and function of the Golgi was altered in both colon and oesophageal carcinogenesis (Byrne et al, 2010, 2012), here we sought to investigate whether Golgi-associated proteins may have a role in oesophageal cancer progression. Using an in silico analysis-based approach, we identified two meta-analysis studies which analysed four publicly available microarray data sets of genes differntially expressed in BO and normal oesophagus (Figure 1A). Wang et al (2009) statistically analysed 4 previously published data sets (Van Baal et al, 2005; Hao et al, 2006; Greenawalt et al, 2007; Ostrowski et al, 2007) and identified 68 genes common across the 4 data sets (Wang et al, 2009). Forty of these genes were independently validated by immunohistochemistry (IHC) on normal oesophagus and BO patient tissue (n=6 Normal, n=6 BO) (Wang et al, 2009). One of these candidate genes was a Golgi-associated protein, GOLPH2. Lao-Sirieix et al (2009) also undertook a meta-analysis study of gene-expression microarray data sets, including two of the data sets analysed by Wang et al (2009) (Hao et al, 2006; Greenawalt et al, 2007). In this study, indpendent validation was performed by both RT–PCR and IHC (n=20 Normal and n=20 BO) and 14 genes were verified to be statistically significantly overexpressed in Barrett's tissue compared with normal (Lao-Sirieix et al, 2009). GOLPH2 was identified as one of these candidate genes. The identification of GOLPH2 across multiple gene-expression microarray data sets and independantly verification by two separate research groups suggest it may be a useful biomarker for identifying patients with BO. In this study, we chose to characterise GOLPH2 further, as no funtions for this protein have been identified to date.
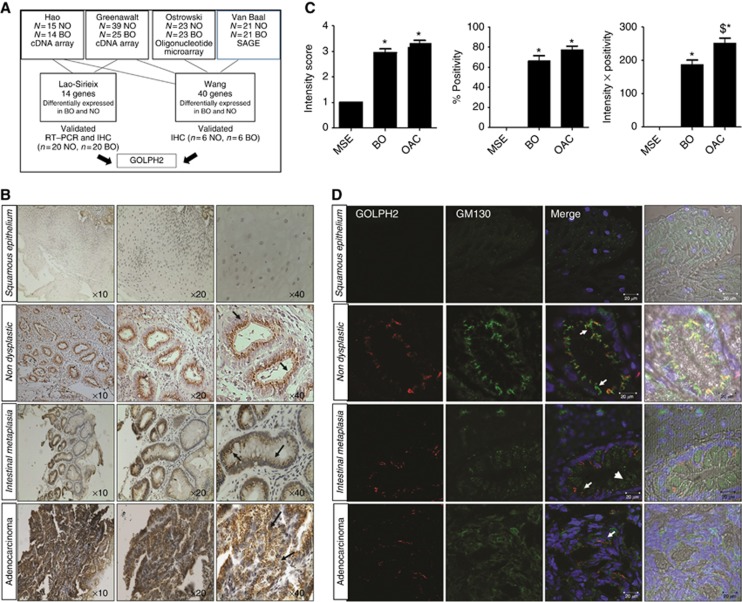
Figure 1.
(A) Identification of candidate Golgi-associated proteins involved in oesophageal cancer progression. An in silico analysis-based approach was used to identify potential candidate Golgi-associated proteins from two meta-analysis studies (Lao-Sirieix et al, 2009, Wang et al, 2009) which analysed four publicly available microarray data sets of genes differentially expressed in Barrett's oesophagus and normal oesophagus. Lao-Sirieix et al (2009) undertook a meta-analysis study of gene-expression microarray data sets, in two data sets ((Hao et al, 2006; Greenawalt et al, 2007). Independent validation was performed by both RT–PCR and IHC (n=20 Normal and n=20 Barrett's oesophagus) and 14 genes were verified to be statistically significantly overexpressed in Barrett's tissue compared with normal tissue (Lao-Sirieix et al, 2009). Wang et al, (2009) statistically analysed 4 data sets (Van Baal et al, 2005; Hao et al, 2006; Greenawalt et al, 2007; Ostrowski et al, 2007) and identified 40 genes common across the 4 data sets, independently validated by IHC on normal oesophagus and BO patient tissue (n=6 Normal, n=6 BO). GOLPH2 was identified as one of these candidate genes common to both of these meta-analyses studies. (B) GOLPH2 localisation in oesophageal tissue. Immunohistochemistry was used to identify GOLPH2 in tissue from patients with adenocarcinoma using an anti-GOLPH2 antibody (arrows). Images represent Squamous epithelium, non-dysplastic glands (from normal oesophagus n=6), Barrett's intestinal metaplasia (n=13) and oesophageal adenocarcinoma (n=15 identified by a consultant histopathologist (SF)). (C) Graphs represent expression intensity levels, % Positivity and Intensity × Positivity (Left to Right, respectively) in Barretts oesophagus (BO), oesophageal adenocarcinoma (OAC) and matched squamous epithelium (MSE) (*P<0.05 vs MSE, $P<0.05 vs BO). (D) Immunofluorescence images representing the typical localisation of GOLPH2 observed in squamous epithelium tissue, non-dysplastic glands, Barrett's intestinal metaplasia and adenocarcinoma. Golgi structure was identified using an antibody to the cis-Golgi-network protein GM130 (green, arrowheads), GOLPH2, using an anti-GOLPH2 antibody (Red, arrows) and nuclei using Hoechst (blue). Images were acquired using a Zeiss LSM510 laser confocal microscope.
Expression and intracellular localisation of the Golgi-associated protein; GOLPH2, in oesophageal tissue
As GOLPH2 was verified to be overexpressed in Barrett's epithlium in the meta-analysis gene-expression studies, we first sought to determine the expression of GOLPH2 in oesophageal tissue compartments. We examined squamous epithelium (SE) and non-dysplastic glands in normal patient tissue (n=6), intestinal metaplasia in BO patient tissue (n=13) and adenocarcinoma in OAC patient tissue (n=15) by IHC. GOLPH2 was not observed in SE (Figure 1B, top row). GOLPH2 was localised in glandular epithelial cells adjacent to the nucleus in non-dysplastic tissue (Figure 1B, second row, arrows). GOLPH2 expression and intracellular localisation was altered in areas of intestinal metaplasia (Figure 1B, third row, arrows) and adenocarcinoma (Figure 1B, bottom row, arrows) with a punctate distribution observed in the cytoplasm. The intensity of GOLPH2 expression and percentage of epithelial cells positive for punctate GOLPH2 structures (% Positivity) were quantified in BO tissue and OAC tissue (n=13 samples BO, n=15 OAC, histology confirmed by consultant histopatholgist SF, and quantified by AMB and SB). Adjacent normal matched SE (MSE) was also assessed. GOLPH2 Intensity and % Positivity were significantly increased in BO tissue and OAC tissue compared with adjacent MSE, but no significant differences were observed in intensity levels or % Positivity between BO and OAC (Figure 1C). Using an Intensity × Percentage positivity score, there was a significant increase observed in OAC compared with BO tissue (Figure 1C). Barrett's oesophageal tissue contains predominantly glandular epithelial cells. Since GOLPH2 was predominantly expressed in glandular epithelial cells, this could provide the reason for increased gene expression observed in the microarray data sets. Although ameanable to use as a biomarker for the presence of BO, this highlights a limitation of using gene-expression microarrays to identify oncoproteins, as biopsies of tissue contain a mixed population of epithelial/stromal tissue. Therefore a more indepth chracterisation of protein expression is critical to identify cell type expression and the function of specific proteins in disease progression.
Since the Golgi structure is fragmented in tissue from patients with colorectal cancer (Byrne et al, 2010), we hypothesised the structure of the Golgi might also be altered in BO and OAC, leading to the observed punctate intracellular localisation of GOLPH2. To look at the co-localisation of GOLPH2 with the Golgi apparatus we used immunofluorescence on MSE, non-dysplastic, intestinal metaplasia and adenocarcinoma tissues. Images are representative of biopsied tissue from normal oesophagus, BO and oesophageal adenocarcinoma patients (n=6 per group).Using cis-Golgi-localised protein GM130 as a Golgi-marker, we saw no staining in SE (Figure 1D, Top row). GOLPH2 was localised at the Golgi next to the nucleus in non-dysplastic tissue (Figure 1D, second row, arrows) but this co-localisation was lost in intestinal metaplasia and adenocarcinoma tissue where the Golgi structure was fragmented (Figure 1D, third and bottom rows, arrows, see also Supplementary Figure 1). This pattern was observed for all six patient biopsy samples in each group. These results indicate that GOLPH2 was localised to an intact Golgi structure in normal and metaplastic tissue, but Golgi localisation of GOLPH2 was lost in intestinal metaplasia and adenocarcinoma tissue where the Golgi membranes were dispersed.
GOLPH2 is secreted by SKGT4 oesophageal adenocarcinoma cells
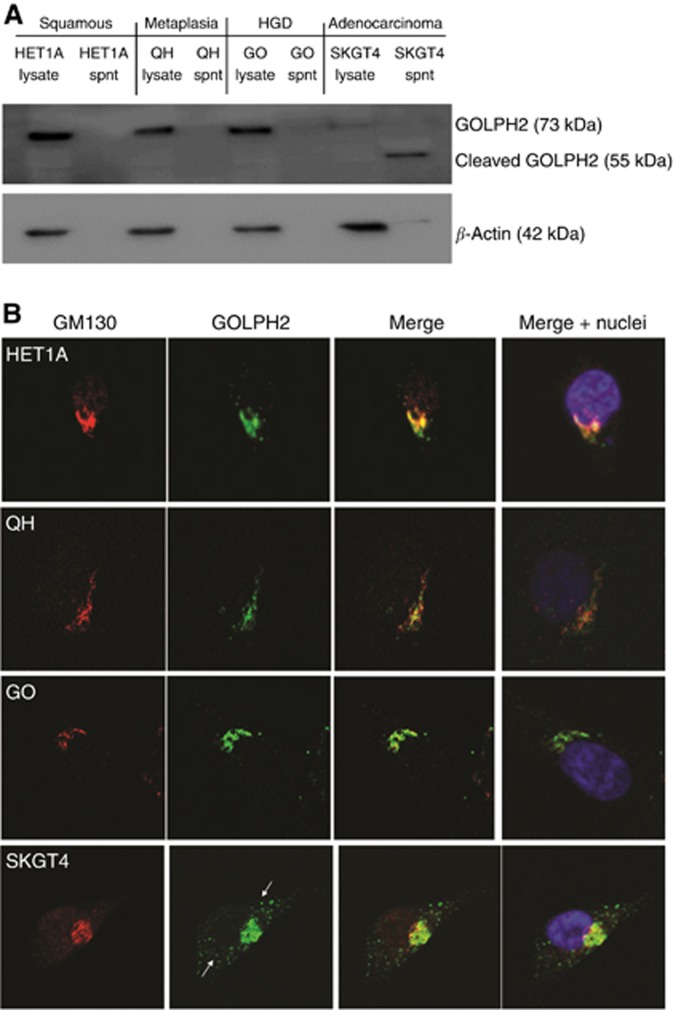
To evaluate the signifcance and potential consequnces of the observed altered intracellular localisation, we used in vitro models of the oesophageal meataplasia dysplasia and adenocarcinoma sequence. Although it is predominantly Golgi-localised, GOLPH2 can cycle distal to the Golgi and has shown to be secreted by hepatocellular cancer cells, therefore we investigated the expression, secretion and intracellular localisation of GOLPH2. All cell lines express GOLPH2, and while it is not secreted from the non neoplastic squamous epithelial HET1A, metaplastic QH or dysplastic GO cells, we demonstrate the cleaved form (55 kDa), previously identified as the secreted form is secreted by the SKGT4 adenocarcinoma cells and detected in the supernatant (Hu et al, 2011) (Figure 2A). β-actin was used as a loading control and to confirm lysis did not occur. To conform the identity of the band as GOLPH2, we silenced GOLPH2 expression using siRNA in SKGT4 cells and show the secretion of GOLPH2 is lost (Supplementary Figure 2). Brefeldin-A, an inhibitor of endocytosis, also inhibited GOLPH2 secretion (Supplementary Figure 2). Treatment of GOLPH2 is localised to the cis-Golgi-network in all cell lines, as indicated by co-localisation with GM130 (Figure 2B). GOLPH2-positive vesicles were observed distal to the Golgi in the cytoplasm of the SKGT4 cells (Figure 2B, bottom panel, arrows).
Figure 2.
GOLPH2 expression in oesophageal cell lines. (A) Basal levels of GOLPH2 expression and secretion in whole cell lysates or supernatants were analysed by western blot. (B) Localisation of GOLPH2 was identified using antibodies specific to GOLPH2 (green) and the cis-Golgi-network marker GM130 (Red). Nuclei were identified with Hoechst (Blue). Arrows indicate cytoplasmic vesicles containing GOLPH2 (Green). Images were acquired using a Zeiss LSM510 laser confocal microscope.
Exposure of HET1A cells to DCA causes Golgi structure dissasembly and GOLPH2 secretion
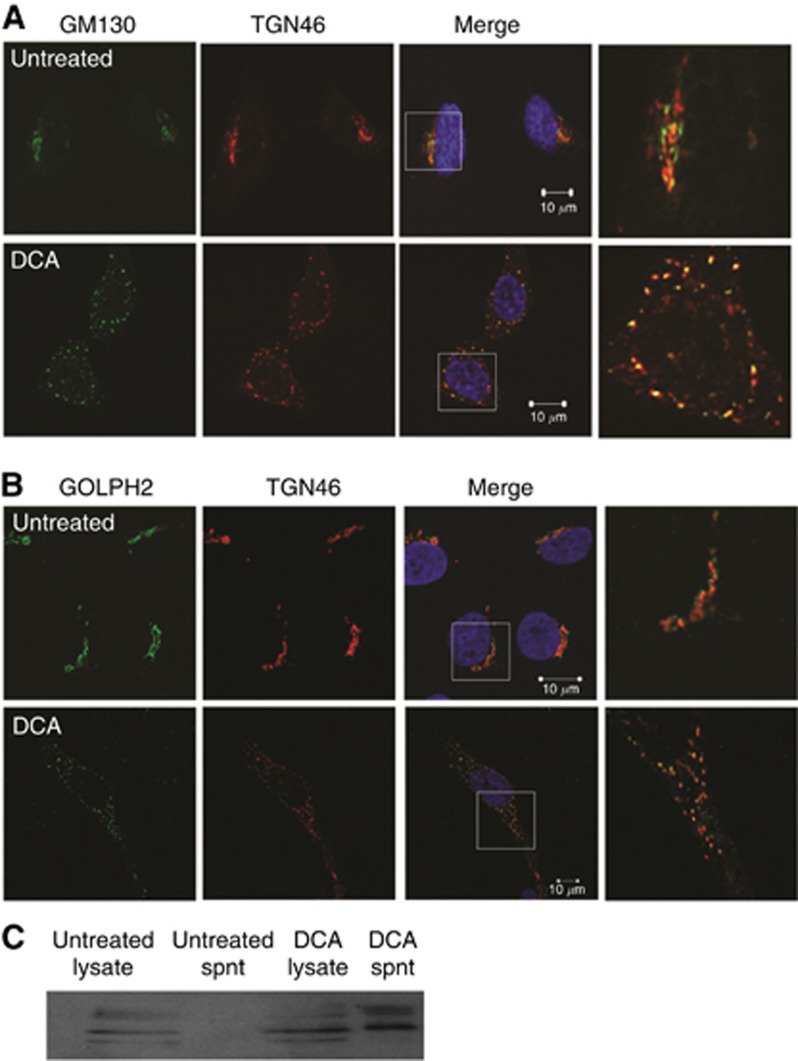
Repeated exposure of the oesophagus to bile acids such as DCA is implicated as the leading cause of oesophageal tumorigenesis. We previously demonstrated that exposure of oesophageal cells to DCA causes dispersal of cis-Golgi membranes throughout the cytoplasm resulting in altered protein trafficking and secretion in a manner independent of apoptosis (Byrne et al, 2012). To determine if our previous observation of dispersal of cis-Golgi membranes was indicitive of complete vesiculation of the Golgi apparatus, or whether Golgi ministacks were formed, we used both the cis-Golgi-network (GM130) and Trans-Golgi-Network (TGN46) markers to visualise Golgi morphology. In untreated cells, GM130 and TGN46 markers co-localised as a single peri-nuclear ribbon structure, indicating a normal intact Golgi structure (Figure 3A top row). Exposure to DCA caused the dissasembly of the Golgi into multiple ministacks where both GM130 and TGN46 remained co-localised (Figure 3A, bottom row). On treatement with DCA, GOLPH2 remained localised with the Golgi ministacks as indicated by co-locolisation with TGN46 (Figure 3B). Having demonstrated that GOLPH2 is constitutively secreted by the adenocarcinoma cell line, we next investigated the effect of DCA on GOLPH2 secretion in the squamous oesophageal epithelial cells (HET1A) and found that in response to DCA GOLPH2 was secreted (Figure 3C). These results indicate that in response to DCA, Golgi ministacks are formed and consequently GOLPH2 was secreted. This secretion is not due to membrane leakage as measured by an LDH assay (data not shown).
Figure 3.
DCA converts the Golgi into ministacks in HET1A cells resulting in GOLPH2 secretion. (A) Cells were treated with 300 μM DCA for 6 h and co-stained for the cis and trans-Golgi markers GM130 (green) and TGN46 (red), respectively, nuclei were identified with Hoechst (Blue). Images were acquired using a Zeiss LSM510 laser confocal microscope. White boxes represent areas that were magnified. (B) Intracellular localisation of GOLPH2 in response to DCA was identified using anti-GOLPH2 and TGN46 antibodies. (C) The effect of DCA on secretion of GOLPH2 from HET1A cells was determined by treating the cells with 300 μM DCA for 6 h and examining the expression of GOLPH2 in lysates and supernatants by western blot.
GOLPH2 is cleaved at the pro-protein convertase (PC) consensus site and secreted in response to DCA
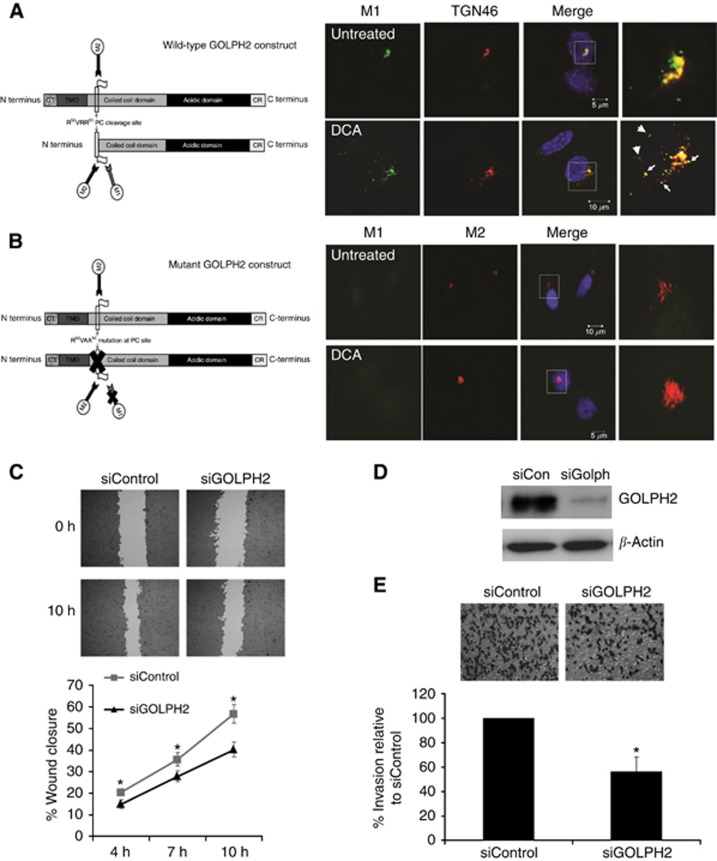
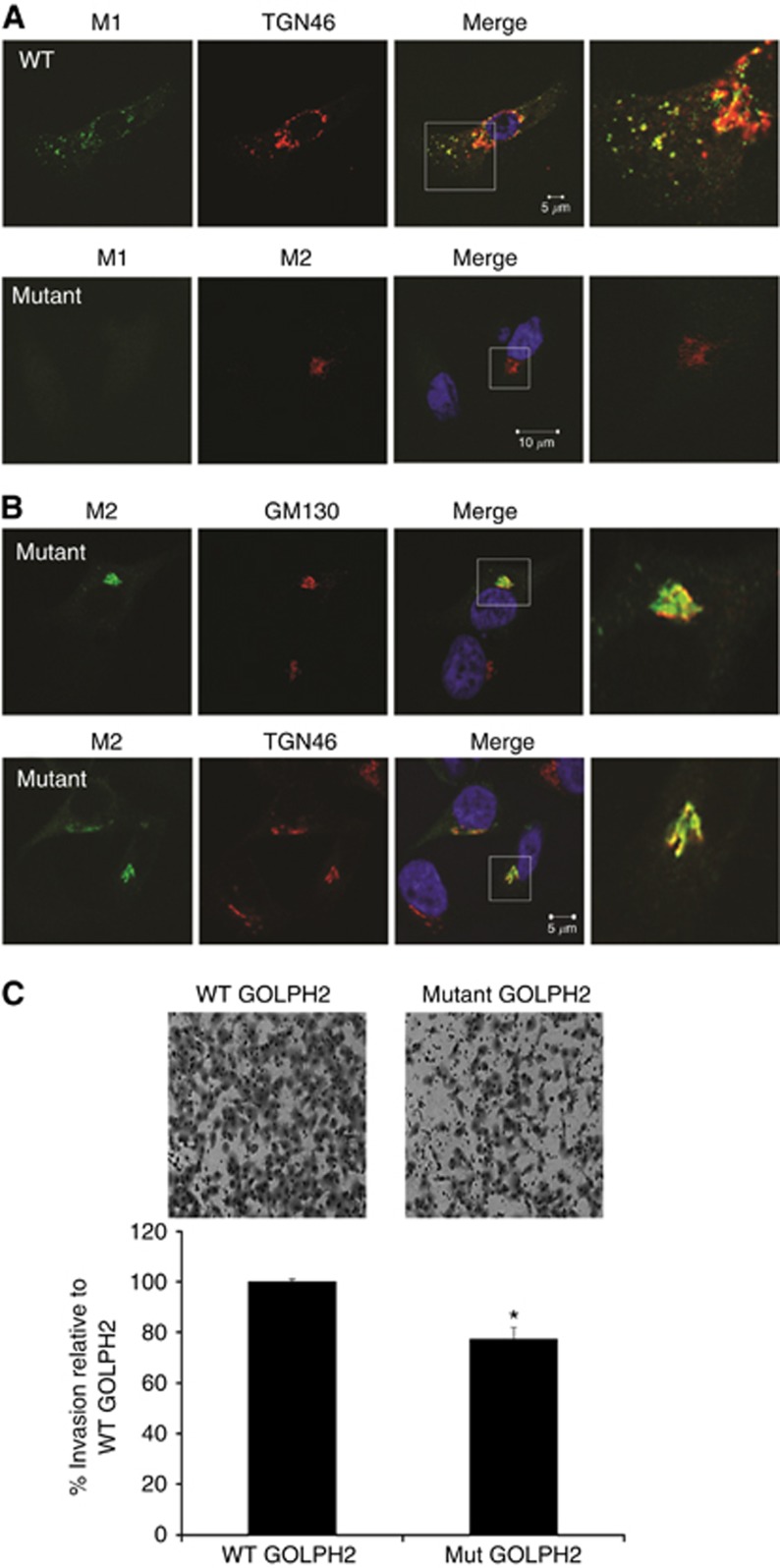
We next proceeded to investigate the mechanism of GOLPH2 secretion in response to DCA. Previous studies demonstrate that GOLPH2 cycles from the cis-Golgi compartment to the TGN and endosomes where it is exposed to proteases such as the PC furin (Bachert et al, 2007). GOLPH2 is cleaved by furin at the R52VRR55 PC consensus site releasing the ectodomain. Cleaved GOLPH2 is generally retreived back to the Golgi, however,when overexpressed as in hepatocellular carcinoma, there is increased localisation of GOLPH2 in TGN vesicles, resulting in exposure to furin and subsequent cleavage. In these circumstances the retrieval system is saturated and GOLPH2 is not recycled back to the early Golgi compartments and is secreted (Bachert et al, 2007). To verify if exposure of oesophageal cells to DCA results in cleavage of GOLPH2 at the PC site, a construct containing a FLAG epitope at the R52VRR55 PC site was transfected into HET1A cells (Figure 4A). Cleavage at this site results in exposure of this epitope, which can be recognised using the M1 anti-FLAG antibody, therefore this antibody recognises GOLPH2 that has been cleaved at the PC site. In untreated cells cleaved GOLPH2 (M1) was observed localised to the TGN next to the nucleus (Figure 4A). Following exposure to DCA, cleaved GOLPH2 (M1) was redistributed throughout the cytoplasm localising with TGN vesicles (Figure 4A arrowhead). To confirm cleavage of GOLPH2 at the PC site, a contruct containing a mutation (R52VAA5) which can not be cleaved was transfected into HET1A cells and then treated with DCA. The M2 anti-FLAG antibody recognises both internal and C/N-terminal FLAG tags therefore M2 can recognise both cleaved and non-cleaved GOLPH2.Cleaved GOLPH2 (M1) was not observed in the cells transfected with mutant GOLPH2 plasmid, demonstrating DCA cleaves GOLPH2 at the R52VRR55 PC site (Figure 4B). To confirm whether it is the cleaved form that is secreted, HET1A cells were transfected with WT and mutant plasmids and probed with an M1 antibody. The cleaved form of GOLPH2 was previously shown to have a molecular weight of 55 kDa (Hu et al, 2011). A band at 110 kDa was observed secreted by the wild type but not in the supernatants from cells transfected with pcDNA or mutant GOLPH2 (Supplementary Figure 3). Both intracellular and secreted GOLPH2 exist as disulfide-bonded dimers and dimerisation is required for secretion (explaining the observed 110-kDa band). These results suggest that in response to DCA, GOLPH2 is cleaved at the PC site, and this cleaved form is secreted. Neither overexpression of WT or mutant GOLPH2 plasmids had an effect on Golgi structure, nor did they prevent DCA-induced Golgi fragmentation (Supplementary Figure 4). Depleting expression of GOLPH2 by siRNA also had no affect on Golgi structure (Supplementary Figure 5). These results rule out a role for GOLPH2 expression/cleavage in Golgi structure maintenance.
Figure 4.
GOLPH2 is cleaved at the PC site and associates with TGN vesicles in response to DCA. (A) A wild-Type GOLPH2 construct with a FLAG tag inserted immediately C-terminal to the RVRR Pro-protein convertase (PC) cleavage site was used to determine whether exposure of HET1A cells to DCA resulted in cleavage of GOLPH2 at the PC site. The M2 antibody detects non-cleaved GOLPH2 as it can recognise internal FLAG tags. The M1 antibody detects cleaved GOLPH2 as it only recognises the FLAG tag when it is free at the N terminus. Once cleavage at the PC site occurs, the internal FLAG tag is exposed at a new N terminus and can be recognised by M1. HET1A cells were transfected with the wild-type GOLPH2 construct and exposed to 300 μM DCA for 6 h. M1 anti-FLAG antibody identified GOLPH2 cleaved at the PC site (Green). Localisation at the TGN was identified using an antibody against TGN46, respectively (red). Arrows indicate co-localisation, arrowheads indicate GOLPH2-containing vesicles. White boxes represent areas that were magnified. (B) A GOLPH2 construct with a mutation in the RVRR PC site was transfected into HET1A cells to confirm cleavage at the PC site. Cleavage of GOLPH was determined using the M1 antibody (green). The M2 antibody (red) was used to identify non-cleaved GOLPH2 (and therefore also identified transfected cells). Nuclei were identified with Hoechst (Blue). Images were acquired using a Zeiss LSM510 laser confocal microscope. GOLPH2 knockdown impairs cell migration and invasion. (D) SKGT4 cells were transfected with GOLPH2 (siGOLPH2) or non-targeting siRNA control (siControl, D) and a scratch wound assay was used to determine the effect on cell migration (C). The wound was imaged at 4, 7 and 10 h using the Cellavista Imaging system. Data represents the mean % Wound closure±s.e.m. for n=4 experiments. The percentage wound closure at the various time points was calculated by expressing the area of the wound at indicated time points as a percentage of the original area of the wound. SKGT4 cells were transfected with GOLPH2 (siGOLPH2) or non-targeting siRNA control (siControl) and a Matrigel boyden chamber invasion assay was used to determine the effect on cell invasion (E). Data represent the mean number of cells invaded±s.e.m. for n=3 experiments (*P<0.05 compared with siControl).
Downregulation of GOLPH2 expression impaired cell migration and invasion
Neither physiological nor pathological roles have been identified for GOLPH2 to date. Using yeast hybridisation, GOLPH2 has recently been found to associate with pre-secretory and secreted forms of clusterin, an apolipoprotein involved in cell migration and invasion of breast and lung cancer (Zhou et al; Cheng et al, 2012; Niu et al, 2012). We therefore investigated a potential role for GOLPH2 in cell migration of the adencoarcinoma cell line SKGT4 using a scratch wound assay (Figure 4C). SKGT4 cells depleted of GOLPH2 expression with siRNA (Figure 4D) migrated at a significantly lower rate compared with cells transfected with control siRNA (Figure 4C), suggesting GOLPH2 plays a role in migration. Using a Matrigel boyden chamber invasion assay, we show that when GOLPH2 expression was depleted with siRNA, the invasive capacity of SKGT4 cells was reduced compared with controls, demonstrating a function for GOLPH2 in tumour cell invasion (Figure 4E). A second siRNA-targeting GOLPH2 (Qiagen, Venlo, Limburg, Netherlands) was used to deplete GOLPH2 expression and also demosntrated a decrease in invasion, indicating the decrease in invasion was not due to non-specific off-target effects (Supplementary Figure 6). The decrease in cell migration and invasion was not due to an effect on cell proliferation, as depletion of GOLPH2 had no effect on cell metabolic activity as measured by an MTT assay (Supplementary Figure 7). Here we show for the first time, functional roles for GOLPH2 in cell migration and invasion.
Expression of a non-cleavable GOLPH2 mutant impairs cell invasion
To determine whether cleavage of GOLPH2 was required for cell invasion, SKGT4 cells were transfected with either wild-type (cleavable) or the mutant (non-cleavable) GOLPH2 plasmids and invasion was measured using the Matrigel boyden chamber invasion assay. Overexpression of the wild-type GOLPH2 leads to cleavage and potentially secretion, whereas cells expressing the mutant non-cleavable GOLPH2 would not get cleaved or secreted. Cleaved GOLPH2 was detected in wild-type cells using the M1 antibody (Figure 5A top panel). In the mutant cells, GOLPH2 was not cleaved as M1 staining was not observed (Figure 5A second panel). Non-cleaved GOLPH2, detected by the M2 antibody, remained localised at the Golgi as visualised by the co-localisation with GM130 and TGN46 markers (Figure 5B). There was a decrease in invasion of the cells expressing the mutant GOLPH2 plasmid compared with the wild-type GOLPH2-expressing cells (Figure 5C). These results indicate that cleavage of GOLPH2 facilitates tumour cell invasion.
Figure 5.
Cleavage of GOLPH2 facilitates cell invasion. (A) SKGT4 cells were transfected with a wild-type (WT) cleavable or mutant non- cleavable GOLPH2 plasmid. Cleaved GOLPH2 was identified using the M1 anti-FLAG antibody (Green) and non-cleaved GOLPH2 was identified using the M2 antibody (red). (B) Localisation of non-cleaved GOLPH2 was identified using M2 (Green), GM130 (Red) and TGN46 (Red) antibodies. Images were acquired using a Zeiss LSM510 laser confocal microscope. White boxes represent areas that were magnified. (C) SKGT4 cells were transfected with WT or mutant plasmids and a Matrigel boyden chamber assay was used to determine the effect on cell invasion. Data represents the mean number of cells invaded±s.e.m. for n=3 experiments (*P<0.05 compared with WT).
Discussion
In this study, we demonstrate that intracellular localisation and secretion of the Golgi-associated protein, GOLPH2, is altered across the oesophageal metaplasia–dysplasia–adenocarcinoma sequence. In oesophageal epithelial cells, the bile acid, DCA, caused disassembly of the Golgi into ministacks, cleavage of GOLPH2 at the PC site and secretion from the cell. Functions for GOLPH2 in regulating migration and invasion suggest a potential role for GOLPH2 in oesophageal cancer progression.
The role of the Golgi in the aetiology of cancer is an emerging field. Here we observed altered Golgi structure at the different stages of oesophageal disease progression. In contrast to non-dysplastic tissue where Golgi structure was intact next to the nucleus, we show the Golgi structure was completely fragmented and dispersed in Barrett's intestinal metaplasia and adenocarcinoma tissue. A study undertaken over two decades ago demonstrated a dilated ER and Golgi at an ultra-structural level in BO tissue compared with normal oesophageal tissue (Levine et al, 1989). These features were attributed to the enhanced secretory functions of glandular epithelial cells. However, since our data demonstrate that DCA perturbs the Golgi structure in vitro, we suggest the observed fragmented Golgi structure in vivo may be due to repeated exposure of the oesophagus to DCA in refluxate. Importantly, perturbations to Golgi structure in response to DCA do not lead to apoptosis in our cell models (Byrne et al, 2012) and the observed fragmented Golgi in adenocarcinoma tissue indicate the Golgi can adapt/retain some functional features necessary for cell survival. Having demonstrated that fragmentation of the Golgi in vitro is associated with secretion of GOLPH2, it is possible to suggest GOLPH2 is secreted from those epithelial cells with inherently fragmented Golgi in vivo. The diffuse cytoplasmic staining observed in the adenocarcinoma tissue (Figure 1B) supports this possibility.
GOLPH2 is a cis-Golgi-localised protein with unknown cell functions. Expression is localised to epithelial cells of intestinal glands, prostatic glands, lung bronchioles and biliary epithelial cells (Kladney et al, 2000). Here we show that GOLPH2 is associated with the Golgi in epithelial cells in vivo in non-dysplastic tissue but this Golgi co-localisation is lost in Intestinal metaplastic and adenocarcinoma tissue where the Golgi structure is fragmented. We show a significant difference in GOLPH2 expression and localisation between intestinal metaplasia and adenocarcinoma tissues (quantified as %Intensity × Positivity), which could act as a useful biomarker to discriminate between these tissue types. GOLPH2 has been shown to be secreted in lung, renal, prostate and hepatocellular carcinoma (Iftikhar et al, 2004; Marrero et al, 2005; Fritzsche et al, 2008; Wei et al, 2008; Zhang et al, 2010). The fact that the adenocarcinoma cells constitutively secrete GOLPH2 in vitro also suggests that the observed dissociation of GOLPH2 from the Golgi in adenocarcinoma tissue could lead to secretion in vivo. Intriguingly, one study demonstrated GOLPH2 localised next to the nucleus in normal and neoplastic prostate glands suggestive of a Golgi localisation. In tissue from prostatic intraepithelial neoplasia (PIN) and invasive carcinoma, GOLPH2 localisation was still observed at the peri-nuclear/Golgi region but additional diffuse cytoplasmic localisation was observed (Kristiansen et al, 2008). Our data suggest that altered (cytoplasmic) GOLPH2 localisation in neoplastic/invasive prostate tissue could be due to either an altered Golgi structure or dissociation from the Golgi. Higher levels of GOLPH2 were detected in urine from patients with PIN (Laxman et al, 2008; Wei et al, 2008), lending further credibility to our hypothesis that the diffuse GOLPH2 staining observed in our oesophageal tissue could be associated with its secretion in vivo, thus suggesting potential use as a serum biomarker for oesophageal disease. The majority of oesophageal cancer patients are asymptomatic and present to the clinic at a very advanced stage with a poor prognosis. A serum biomarker identifying those asymptomatic patients with pre-malignant or early stage malignant disease would allow for early intervention and a better prognosis. On the other hand, patients with a diagnosis of Barrett's are subjected to repeated endoscopy screening, which in the majority of patients is unnecessary, as the risk of patients progressing to develop oesophageal cancer is low and dependant on the associated grade of dysplasia (0.5% for non-dysplastic to 40% with high-grade-dysplastic) (Ong et al, 2010). A prognostic biomarker would be beneficial and studies are currently underway to address whether GOLPH2 could act as a potential prognostic marker to predict progression to cancer.
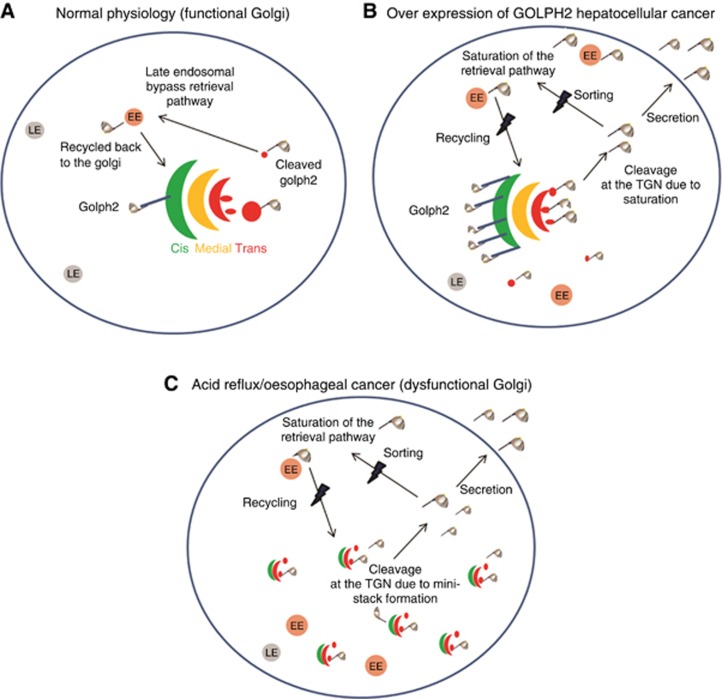
GOLPH2 is a cycling protein and in normal circumstances, it is efficiently retrieved back to the cis-Golgi (Figure 6A). In hepatocellular cancer, the mechanism of secretion is attributed to overexpression of GOLPH2, leading to increased localisation at the TGN, TGN-associated vesicles and sorting endosomes (Figure 6B) (Bachert et al, 2007). These vesicles contain furin, which cleaves GOLPH2 at its PC cleavage site. Saturation of the retrieval system occurs where GOLPH2 can no longer be retrieved back to the Golgi, resulting in secretion from the cell (Bachert et al, 2007). In our cell model, the endogenous secretion of GOLPH2 from the SKGT4 cells is likely to be mediated by trafficking to the cell surface in TGN-associated vesicles, as cleaved GOLPH2 is observed predominantly in these compartments (Figure 4A top row). It is unclear why more GOLPH2 vesicles are observed in the SKGT4 cells compared with HET1A, (Figure 2) but increased protein trafficking and secretion are being recognised as new hallmarks of cancer (Dejeans et al, 2014). The identity of the cleaved GOLPH2-positive vesicles was investigated previously by Bachert et al, who were unable to detect co-localisation of GOLPH2 vesicles with sorting (transferrin) or recycling (RME1) endosomes, but did find some co-localisation with late endosomes (FITC-dextran) (Bachert et al, 2007). We found that GOLPH2 partially co-localised with RAB11, previously shown to be involved in TGN–plasma membrane transport (Bhuin and Roy, 2014), but saw no co-localisation with lysosomes (degradation) (Supplementary Figure 8). We concur with the model proposed by Bachert et al (2007) that close proximity and localisation at the TGN likely lead to increased cleavage and secretion from the SKGT4 cells; however, in our model this is not due to overexpression of GOLPH2 in the adenocarcinoma cells, in fact there is less intracellular GOLPH2 observed in the SKGT4 by western blot (Figure 2A). It is possible that levels of furin are higher in SKGT4 cells, leading to enhanced basal levels of GOLPH2 cleavage. DCA exposure leads to formation of Golgi ministacks in the HET1A cells, causing a redistribution of GOLPH2 into predominantly TGN-associated vesicles and secretion from the cell. GOLPH2 is cleaved at the PC site following exposure of HET1A cells to DCA, as demonstrated by our GOLPH2 wild-type and mutant plasmids. The formation of Golgi ministacks in response to DCA brings the cis-localised GOLPH2 into closer proximity to the TGN and TGN-localised furin potentially resulting in cleavage, redistribution to vesicles and secretion. Alterations to the Golgi structure in response to DCA may also impair the retrieval of GOLPH2 to the early cis-Golgi compartment leading to secretion. Due to effects on Golgi structure, DCA may affect GOLPH2 sorting leading to increased cycling and secretion. There are three main sorting steps in the cell at which DCA may be affecting GOLPH2 cycling, (i) sorting of GOLPH2 into retrieval carriers at the Golgi, (ii) sorting of escaped GOLPH2 at the cell membrane perhaps by affecting clatherin-mediated endocytosis or (iii) sorting into vesicles that move back to the TGN/Golgi. Proteins that are trafficked back to the TGN generally undergo a COP1/Rab6-mediated retrieval mechanism to restore their localisation at the cis-Golgi-network (Puri et al, 2002). Further studies are required to fully elucidate the step(s) at which DCA is affecting GOLPH2 trafficking.
Figure 6.
Proposed mechanism of action. (A) GOLPH2 is normally resident at the cis-Golgi-network and can traffic to distal parts of the cell including sorting endosomes (SE) and the Trans-Golgi-Network (TGN). GOLPH2 is efficiently retrieved from distal compartments back to the Golgi via an early endosome (EE)-dependant, late endosome (LE)-independent pathway. (B) Overexpression of GOLPH2 in Hepatocellular carcinoma saturates the retrieval system resulting in secretion. (C) Golgi structure is altered in cancer and in response to acid reflux (containing DCA) resulting in GOLPH2 localisation at the TGN and cleavage at the PC site resulting in secretion.
As GOLPH2 was found to be overexpressed in BO by two independent meta-analysis studies, we sought to determine its expression in oesophageal adenocarcinoma tissue, and identify physiological and potential oncogenic functions for this protein. Since many cancer types secrete GOLPH2, we investigated whether GOLPH2 was secreted by oesophageal cells using an in vitro model of the metaplasia–dysplasia–adenocarcinoma sequence. GOLPH2 was found to associate with pre-secretory and secreted forms of clusterin, an apolipoprotein involved in cell migration and invasion of breast and lung cancer (Zhou et al; Cheng et al, 2012; Niu et al, 2012), and in this study we demonstrate that GOLPH2 facilitates cell migration. To put these results into a physiological context, wound healing is an important response to the effects of chronic acid reflux, where stratified SE lining the oesophagus is sloughed away revealing the basement membrane epithelial cells. These epithelial cells need to migrate to facilitate wound closure. We demonstrate a reduced rate of cell migration when GOLPH2 expression was depleted, suggesting expression/secretion of GOLPH2 is involved in facilitating wound healing. Localisation at the Golgi would suggest GOLPH2 plays a role in regulation of post-translational protein modification or protein sorting (potentially of pro-migratory factors). As the oesophageal tumour develops, it invades the underlying submucosa. Here we show that depletion of GOLPH2 expression impairs the invasive capacity of SKGT4 cancer cells into an extracellular matrix. Expression of a non-cleavable GOLPH2 reduces the invasive capacity of SKGT4 cells. One possible reason is due to its interaction with clusterin (Zhou et al, 2011). Cleaved GOLPH2 interacts with both intracellular and extracellular secretory clusterin, suggesting GOLPH2 could potentially mediate cell migration and invasion intracellularly or act on factors in the extracellular microenvironment (Zhou et al, 2011). Cells expressing mutant GOLPH2 would not interact with extracellular secretory clusterin, providing a possible explanation for the less invasive phenotype observed in the cells expressing the mutant non-cleavable GOLPH2. The observation of predominantly punctate cytoplasmic vesicles containing GOLPH2 in the high-grade-dysplastic and adenocarcinoma tissue could be associated with an invasive phenotype. Therefore, GOLPH2 has two potential roles in oesophageal cells: (i) participating in wound healing in response to reflux (2D migration) and (ii) invasion of cells into the submucosa and subsequent metastases as the tumour develops (3D migration).
In summary, we chose to investigate the cellular functions for GOLPH2, a Golgi-associated protein previously identified and verified as a tissue-based biomarker for BO. We show fragmented Golgi structure and altered intracellular localisation of GOLPH2 in oesophageal disease, which could potentially be used as a tissue biomarker. This altered intracellular localisation is associated with secretion of GOLPH2 from oesophageal adenocarcinoma cells. Having demonstrated that the tumour promoter present in acid reflux, DCA, perturbs Golgi structure and leads to cleavage and secretion of GOLPH2, we provide a plausible explanation for how this could occur. Finally, novel functions for GOLPH2 in cell migration and invasion demonstrate the importance of this Golgi-associated protein in these processes. Given that Golgi structure and Golgi-associated proteins are altered in oesophageal disease and that the Golgi apparatus is the fundamental core regulator of protein processing and secretion, Golgi-associated proteins represent novel candidates for identification of biomarkers, therapeutic targets and mechanisms underlying the pathogenesis of this disease.
Materials and Methods
Cell culture and reagents
HET1A squamous oesophageal epithelial cells (ATCC, Rockville, MD, USA), QH Barrett's metaplastic cells and GO Barrett's dysplastic cells (also designated CP-A and CP-C, respectively, kindly provided by Professor Rabinovich, the University of Washington) were cultured in bronchial epithelial cell basal medium with supplements (Lonza, Basel, Switzerland). For the QH and GO cell lines, medium was further supplemented with 5%(v/v) foetal calf serum (Gibco-BRL, Grand Island, NY, USA). SKGT4 oesophageal adenocarcinoma cells (ATCC) were cultured in RPMI with 10% (v/v) foetal calf serum. GM130, β-actin, M1, M2 antibodies and all other chemicals were obtained from Sigma-Aldrich Chemical Company (St Louis, MO, USA). TGN46 antibody was obtained from AbD Serotec (Oxford, UK). GOLPH2 antibody was obtained from Abnova (Abnova Corp,Taipei, Taiwan). Horseradish peroxidase-conjugated secondary antibodies, AlexaFluor-conjugated secondary antibodies and Hoechst were obtained from Invitrogen (Carlsbad, CA, USA).
Patient tissue and histology
Full ethical approval was granted by the AMNCH/St James's Hospital ethical committee to use patient samples for this study. Informed consent was obtained from each subject and anonymity protected. Tissue status was confirmed by a consultant pathologist (SF). Following endoscopy or surgery, tissue specimens were fixed in 1% formalin and embedded in paraffin. GOLPH2 expression was identified by IHC in tissue from individuals without oesophageal disease (n=6), BO with intestinal metaplasia (n=13) and oesophageal adenocarcinoma (n=15). Adjacent MSE was also used for quantification. Intensity levels were quantified as follows: No staining (1), Weak (2), Moderate (3) and Strong (4). The % Positivity for punctate structures was scored as 0, 25, 50, 75 and 100%. Samples were blinded to tissue status and scored by two independent researchers (AMB and SB). Data were analysed by calculating the average Intensity, average % Positivity and also using an Intensity × Positivity score. Co-localisation of GOLPH2 with the Golgi was investigated by immunofluorescence using GM130 and GOLPH2 antibodies in tissues from normal individuals without oesophageal disease (n=6), BO with intestinal metaplasia (n=6) and oesophageal adenocarcinoma (n=6). Normal individuals are defined as patients undergoing investigative endoscopy having no history of oesophageal disease and whose biopsy samples showed no evidence of disease.
GOLPH2 plasmid transfection
GOLPH2 expression plasmids were a kind gift from Prof A Lindstedt (the University of Pittsburg, Pittsburg, PA, USA). Transient transfections were carried out using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's protocols. A concentration of 50 ng DNA and a 1 : 3 ratio of DNA: Lipofectamine transfection reagent was used for transfection. Cells were treated with DCA 48 h post transfection.
siRNA transfection
Two different smartpools of GOLPH2 siRNA were used in this study: ON-TARGETplus GOLPH2 siRNA or ON-TARGETplus non-targeting control siRNA was obtained from Dharmacon (Lafayette, CO, USA), Flexitube GeneSolution GOLPH2 siRNA and AllStars negative control siRNA from Qiagen SKGT4 cells were reverse transfected at a concentration of 30 nM siRNA using Lipofectamine RNAiMAX transfection reagent for 72 h.
Immunofluorescence staining
Cell lines were treated with DCA in serum and supplement-free medium at time points and concentrations were indicated and fixed with 4% paraformaldehyde. Cells were probed with GM130, TGN46, GOLPH2, M1 or M2 antibodies overnight followed by AlexaFluor-488-conjugated secondary antibodies. Nuclei were stained using Hoechst 3328. Cells were imaged using a Nikon T800 confocal fluorescent microscope (Carl Zeiss, Thornwood, NY, USA).
Protein precipitation from cell culture supernatants
Protein precipitation was carried out using the carrier-assisted trichloroacetic acid method (TCA) (Chevallet et al, 2007). A total volume of 3 ml of serum-free medium was collected following a 16-h incubation. Medium was cooled in ice and sodium lauroyl sarcosinate was added to a concentration of 0.05%. After mixing, TCA was added to a final concentration of 7.5% and precipitated on ice for 2 h. The precipitate was concentrated by centrifugation at 20 000 g for 15 min at 4 °C. The supernatant was removed and the pellet was resuspended in 150 μl of pre-cooled Tetrahydrofuran and centrifuged as above. Following a second tetrahydrofuran wash, 20 μl of Laemmli sample loading buffer was added. If the sample loading buffer appeared yellow, this indicated an acidic pH, which was adjusted by adding 1 μl Tris-HCL pH (8.8). Samples were analysed by western blot.
Western blotting
Cells were treated with 300 μM DCA for 6 h, a dose and time point previously demonstrated to cause Golgi fragmentation, in serum-free medium then lysed using RIPA buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA 1 mM EGTA, 1% NP-40 and 1% sodium deoxycholate) containing protease inhibitor cocktail (Sigma). Total cell protein was quantified by BCA Protein Assay (Thermo Fischer Scientific, Waltham, MA, USA). To assess the secretion of GOLPH2, protein was precipitated from tissue culture supernatant as above. Equal concentrations of protein were separated by SDS–PAGE gel electrophoresis, transferred to PVDF and probed with GOLPH2 and β-actin as loading control.
Wound healing assay
Confluent monolayers of cells transfected with scrambled siRNA or GOLPH2 siRNA were scratched using a 200-μl pipette tip. Whole-well images were taken at 4, 7, 10 and 24 h using a Cellavista cell imaging system (Roche, Basel, Switzerland). Wound areas were calculated at the different time points using the Cellavista imaging software. The percentage wound closure at the various time points was calculated by expressing the area of the wound at indicated time points as a percentage of the original area of the wound.
Transwell invasion assay
The transwell matrigel invasion assay kit (BD Biosciences, Franklin Lakes, NJ, USA) was used to determine effects on cell invasion as previously described. SKGT4 cells were seeded into transwells 72 h post siRNA transfection or 48 h post plasmid transfection, using 10% FCS as a chemoattractant and incubated for 24 h. Membranes were removed, stained with heamotoxylin and eosin, and images were taken with a × 4 objective using the EVOS microscope system (Electron Microscopy Sciences, Hatfield, PA, USA). Cells were counted using the ImageJ cell counting plugin (National Institutes of Health, Bethesda, MD, USA). Data represent the average total number of invaded cells expressed as a percentage of controls, in duplicate wells for n=3 experiments.
Statistical analysis
Statistical comparison between groups was carried out using paired t-test or One-way analysis of variance with Tukey honestly significant difference post hoc correction to examine differences between groups. Data are graphically represented as the mean±s.e.m. All P-values are two-sided and P-values of <0.05 were considered statistically significant in all analyses. Data were analysed using the SPSS statistical software package SPSS Statistics for Windows, Version 17.0 (SPSS Inc., Chicago, IL, USA).
Acknowledgments
This work was funded by the Irish Cancer Society Research fellowship grant number CRF12BYR. We would also like to acknowledge Dr J Conroy and Dr A Davies for technical assistance, Dr M Freeley for critical review of the manuscript and Prof V Malhotra for useful discussions.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Atherfold PA, Jankowski JA (2006) Molecular biology of Barrett's cancer. Best Pract Res Clin Gastroenterol 20: 813–827. [DOI] [PubMed] [Google Scholar]
- Ba M, Long H, Tang Y, Cui S (2012) GP73 expression and its significance in the diagnosis of hepatocellular carcinoma: a review. Int J Clin Exp Pathol 5: 874–881. [PMC free article] [PubMed] [Google Scholar]
- Bachert C, Fimmel C, Linstedt AD (2007) Endosomal trafficking and proprotein convertase cleavage of cis Golgi protein GP73 produces marker for hepatocellular carcinoma. Traffic 8: 1415–1423. [DOI] [PubMed] [Google Scholar]
- Bhuin T, Roy JK (2014) Rab proteins: the key regulators of intracellular vesicle transport. Exp Cell Res 328: 1–19. [DOI] [PubMed] [Google Scholar]
- Byrne A-M, Foran E, Sharma R, Davies A, Mahon C, O'Sullivan J, O'Donoghue D, Kelleher D, Long A (2010) Bile acids modulate the Golgi membrane fission process via a protein kinase Ceta and protein kinase D-dependent pathway in colonic epithelial cells. Carcinogenesis 31: 737–744. [DOI] [PubMed] [Google Scholar]
- Byrne A-M, Sharma R, Duggan G, Kelleher D, Long A (2012) Deoxycholic acid impairs glycosylation and fucosylation processes in esophageal epithelial cells. Glycobiology 22: 638–648. [DOI] [PubMed] [Google Scholar]
- Chen X, Ding Y, Liu CG, Mikhail S, Yang CS (2002) Overexpression of glucose-regulated protein 94 (Grp94) in esophageal adenocarcinomas of a rat surgical model and humans. Carcinogenesis 23: 123–130. [DOI] [PubMed] [Google Scholar]
- Cheng C-Y, Cherng S-H, Wu W-J, Yang T-Y, Huang X-Y, Liao F-T, Wu M-F, Sheu G-T (2012) Regulation of chemosensitivity and migration by clusterin in non-small cell lung cancer cells. Cancer Chemother Pharmacol 69: 145–154. [DOI] [PubMed] [Google Scholar]
- Chevallet M, Diemer H, Van Dorssealer A, Villiers C, Rabilloud T (2007) Toward a better analysis of secreted proteins: the example of the myeloid cells secretome. Proteomics 7: 1757–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejeans N, Manié S, Hetz C, Bard F, Hupp T, Agostinis P, Samali A, Chevet E (2014) Addicted to secrete - novel concepts and targets in cancer therapy. Trends Mol Med 20: 242–250. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Granovsky M, Warren CE (1999) Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta 1473: 21–34. [DOI] [PubMed] [Google Scholar]
- Dube DH, Bertozzi CR (2005) Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nat Rev Drug Discov 4: 477–488. [DOI] [PubMed] [Google Scholar]
- Fan G, Goldsmith PK, Collins R, Dunn CK, Krapcho KJ, Rogers KV, Spiegel AM (1997) N-linked glycosylation of the human Ca2+ receptor is essential for its expression at the cell surface. Endocrinology 138: 1916–1922. [DOI] [PubMed] [Google Scholar]
- Fritzsche FR, Riener M-O, Dietel M, Moch H, Jung K, Kristiansen G (2008) GOLPH2 expression in renal cell cancer. BMC Urol 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS (2000) Organization of the Golgi apparatus. Curr Opin Cell Biol 12: 450–456. [DOI] [PubMed] [Google Scholar]
- Glick BS, Malhotra V (1998) The curious status of the Golgi apparatus. Cell 95: 883–889. [DOI] [PubMed] [Google Scholar]
- Greenawalt DM, Duong C, Smyth GK, Ciavarella ML, Thompson NJ, Tiang T, Murray WK, Thomas RJS, Phillips WA (2007) Gene expression profiling of esophageal cancer: comparative analysis of Barrett's esophagus, adenocarcinoma, and squamous cell carcinoma. Int J Cancer 120: 1914–1921. [DOI] [PubMed] [Google Scholar]
- Hao Y, Triadafilopoulos G, Sahbaie P, Young HS, Omary MB, Lowe AW (2006) Gene expression profiling reveals stromal genes expressed in common between Barrett's esophagus and adenocarcinoma. Gastroenterology 131: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Li L, Xie H, Gu Y, Peng T (2011) The Golgi localization of GOLPH2 (GP73/GOLM1) is determined by the transmembrane and cytoplamic sequences. PLoS One 6: e28207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftikhar R, Kladney RD, Havlioglu N, Schmitt-Graff A, Gusmirovic I, Solomon H, Luxon BA, Bacon BR, Fimmel CJ (2004) Disease- and cell-specific expression of GP73 in human liver disease. Am J Gastroenterol 99: 1087–1095. [DOI] [PubMed] [Google Scholar]
- Isaji T, Sato Y, Fukuda T, Gu J (2009) N-glycosylation of the I-like domain of β1 integrin is essential for β1 integrin expression and biological function. J Biol Chem 284: 12207–12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kladney RD, Bulla GA, Guo L, Mason AL, Tollefson AE, Simon DJ, Koutoubi Z, Fimmel CJ (2000) GP73, a novel Golgi-localized protein upregulated by viral infection. Gene 249: 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen G, Fritzsche FR, Wassermann K, Jaeger C, Toelle A, Lein M, Stephan C, Jung K, Pilarsky C, Dietel M, Moch H (2008) GOLPH2 protein expression as a novel tissue biomarker for prostate cancer: implications for tissue-based diagnostics. Br J Cancer 99: 939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagergren J, Bergstrom R, Lindgren A, Nyren O (1999) Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 340: 825–831. [DOI] [PubMed] [Google Scholar]
- Lao-Sirieix P, Boussioutas A, Kadri SR, O'Donovan M, Debiram I, Das M, Harihar L, Fitzgerald RC (2009) Non-endoscopic screening biomarkers for Barrett's oesophagus: from microarray analysis to the clinic. Gut 58: 1451–1459. [DOI] [PubMed] [Google Scholar]
- Laxman B, Morris DS, Yu J, Siddiqui J, Cao J, Mehra R, Lonigro RJ, Tsodikov A, Wei JT, Tomlins SA, Chinnaiyan AM (2008) A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res 68: 645–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DS, Reid BJ, Haggitt RC, Rubin CE, Rabinovitch PS (1989) Correlation of ultrastructural aberrations with dysplasia and flow cytometric abnormalities in Barrett's epithelium. Gastroenterology 96: 355–367. [DOI] [PubMed] [Google Scholar]
- Looby E, Abdel-Latif MMM, Athié-Morales V, Duggan S, Long A, Kelleher D (2009) Deoxycholate induces COX-2 expression via Erk1/2-, p38-MAPK and AP-1-dependent mechanisms in esophageal cancer cells. BMC Cancer 9: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni HJF, Quiroga R, Spessott W (2011) Organization of the synthesis of glycolipid oligosaccharides in the Golgi complex. FEBS Lett 585: 1691–1698. [DOI] [PubMed] [Google Scholar]
- Majka J, Rembiasz K, Migaczewski M, Budzynski A, Ptak-Belowska A, Pabianczyk R, Urbanczyk K, Zub-Pokrowiecka A, Matlok M, Brzozowski T (2010) Cyclooxygenase-2 (COX-2) is the key event in pathophysiology of Barrett's esophagus. Lesson from experimental animal model and human subjects. J Physiol Pharmacol 61: 409–418. [PubMed] [Google Scholar]
- Mallet WG, Maxfield FR (1999) Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J Cell Biol 146: 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, Comunale MA, D'Amelio A, Lok AS, Block TM (2005) GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol 43: 1007–1012. [DOI] [PubMed] [Google Scholar]
- Marshall RE, Anggiansah A, Owen WA, Owen WJ (1997) The relationship between acid and bile reflux and symptoms in gastro-oesophageal reflux disease. Gut 40: 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehra D, Howell P, Williams CP, Pye JK, Beynon J (1999) Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut 44: 598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Wex T, Monkemuller K, Vieth M, Fry LC, Malfertheiner P (2008) Lectin UEA-I-binding proteins are specifically increased in the squamous epithelium of patients with Barrett's esophagus. Digestion 78: 201–207. [DOI] [PubMed] [Google Scholar]
- Niu Z, Li X, Hu B, Li R, Wang L, Wu L, Wang X (2012) Small interfering RNA targeted to secretory clusterin blocks tumor growth, motility, and invasion in breast cancer. Acta Biochim Biophys Sin 44: 991–998. [DOI] [PubMed] [Google Scholar]
- Ong CAJ, Lao-Sirieix P, Fitzgerald RC (2010) Biomarkers in Barrett's esophagus and esophageal adenocarcinoma: Predictors of progression and prognosis. World J Gastroenterol 16: 5669–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski J, Mikula M, Karczmarski J, Rubel T, Wyrwicz LS, Bragoszewski P, Gaj P, Dadlez M, Butruk E, Regula J (2007) Molecular defense mechanisms of Barrett's metaplasia estimated by an integrative genomics. J Mol Med 85: 733–743. [DOI] [PubMed] [Google Scholar]
- Puri S, Bachert C, Fimmel CJ, Linstedt AD (2002) Cycling of early Golgi proteins via the cell surface and endosomes upon lumenal pH disruption. Traffic 3: 641–653. [DOI] [PubMed] [Google Scholar]
- Ransford RA, Jankowski JA (2000) Genetic versus environmental interactions in the oesophagitis-metaplasia–dysplasia–adenocarcinoma sequence (MCS) of Barrett's oesophagus. Acta Gastroenterol Belg 63: 18–21. [PubMed] [Google Scholar]
- Roman S, Petre A, Thepot A, Hautefeuille A, Scoazec JY, Mion F, Hainaut P (2007) Downregulation of p63 upon exposure to bile salts and acid in normal and cancer esophageal cells in culture. Am J Physiol Gastrointest Liver Physiol 293: G45–G53. [DOI] [PubMed] [Google Scholar]
- Shah SA, Looby E, Volkov Y, Long A, Kelleher D (2005) Ursodeoxycholic acid inhibits translocation of protein kinase C in human colonic cancer cell lines. Eur J Cancer 41: 2160–2169. [DOI] [PubMed] [Google Scholar]
- Shimamoto C, Weinstein WM, Boland CR (1987) Glycoconjugate expression in normal, metaplastic, and neoplastic human upper gastrointestinal mucosa. J Clin Invest 80: 1670–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baal JWPM, Milano F, Rygiel AM, Bergman JJGHM, Rosmolen WD, Van Deventer SJH, Wang KK, Peppelenbosch MP, Krishnadath KK (2005) A comparative analysis by SAGE of gene expression profiles of Barrett's esophagus, normal squamous esophagus, and gastric cardia. Gastroenterology 129: 1274–1281. [DOI] [PubMed] [Google Scholar]
- Wang J, Qin R, Ma Y, Wu H, Peters H, Tyska M, Shaheen NJ, Chen X (2009) Differential gene expression in normal esophagus and Barrett's esophagus. J Gastroenterol 44: 897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Dunn TA, Isaacs WB, De Marzo AM, Luo J (2008) GOLPH2 and MYO6: putative prostate cancer markers localized to the Golgi apparatus. Prostate 68: 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright LM, Yong S, Picken MM, Rockey D, Fimmel CJ (2008) Decreased survival and hepato-renal pathology in mice with C-terminally truncated GP73 (GOLPH2). Int J Clin Exp Pathol 2: 34–47. [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gu Y, Li X, Wang W, He J, Peng T (2010) Upregulated Golgi phosphoprotein 2 (GOLPH2) expression in lung adenocarcinoma tissue. Clin Biochem 43: 983–991. [DOI] [PubMed] [Google Scholar]
- Zheng M, Fang H, Hakomori S (1994) Functional role of N-glycosylation in alpha 5 beta 1 integrin receptor. De-N-glycosylation induces dissociation or altered association of alpha 5 and beta 1 subunits and concomitant loss of fibronectin binding activity. J Biol Chem 269: 12325–12331. [PubMed] [Google Scholar]
- Zhou Y, Li L, Hu L, Peng T. Golgi phosphoprotein 2 (GOLPH2/GP73/GOLM1) interacts with secretory clusterin. Mol Biol Rep 38: 1457–1462. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Li L, Hu L, Peng T (2011) Golgi phosphoprotein 2 (GOLPH2/GP73/GOLM1) interacts with secretory clusterin. Mol Biol Rep 38: 1457–1462. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ying J, Yin X, Zhang B (2012) Golgi protein 73 versus alpha-fetoprotein as a biomarker for hepatocellular carcinoma: a diagnostic meta-analysis. BMC Cancer 12: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.