Abstract
Although physical activity reduces anxiety in humans, the neural basis for this response is unclear. Rodent models are essential to understand the mechanisms that underlie the benefits of exercise. However, it is controversial whether exercise exerts anxiolytic-like potential in rodents. Evidence is reviewed to evaluate the effects of wheel running, an experimental mode of exercise in rodents, on behavior in tests of anxiety and on norepinephrine and galanin systems in neural circuits that regulate stress. Stress is proposed to account for mixed behavioral findings in this literature. Indeed, running promotes an adaptive response to stress and alters anxiety-like behaviors in a manner dependent on stress. Running amplifies galanin expression in noradrenergic locus coeruleus (LC) and suppresses stress-induced activity of the LC and norepinephrine output in LC-target regions. Thus, enhanced galanin-mediated suppression of brain norepinephrine in runners is supported by current literature as a mechanism that may contribute to the stress-protective effects of exercise. These data support the use of rodents to study the emotional and neurobiological consequences of exercise.
Keywords: Anxiety, Emotion, Enrichment, Exercise, Fear, Galanin, Locus coeruleus, Norepinephrine, Physical activity, Rodent, Stress, Wheel running
1. Anxiety and its treatment
Fear and anxiety-related behavior are adaptive responses that span across the phylum and serve to protect the organism from threat (Belzung and Philippot, 2007). However, mental pathology occurs when these responses are excessive, persistent, and clinically impairing in humans, according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IVR) (American Psychiatric Association, 2000). Anxiety is the most prevalent type of mental disorder in the general population (Kessler et al., 2009). Anxiety defines a class of disorders that contain an assortment of diagnoses (i.e., panic, agoraphobia, phobias, obsessive-compulsive disorders, posttraumatic or acute stress disorder, and generalized anxiety disorder), each of which possess a unique prevalence, pattern of symptoms, course, and treatment (American Psychiatric Association, 2000). Anxiety disorders exact a pervasive toll on the individual and impair numerous aspects of quality-of-life by inducing physical, social, emotional, and occupational dysfunction (Mendlowicz and Stein, 2000). The lifetime prevalence of DSM-IVR anxiety is about 31% and the 1 year prevalence is about 19% in the US alone, according to World Mental Health surveys (Kessler et al., 2009). The annual cost of treating anxiety in the US is $42.3 billion as assessed by the most recent national survey in 1990 (Greenberg et al., 1999; see also Konnopka et al., 2009). Thus, anxiety disorders incur substantial cost to both the individual and society.
Pharmacotherapy is often a first-line of treatment for anxiety (Jameson and Blank, 2010; Weisberg et al., 2007). Yet, current drug therapies for anxiety have many limitations, including the high financial expense, delay in onset, limited efficacy, unwanted side effects, dependence, and stigma associated with consuming and depending on pharmaceuticals (Davidson, 2009; Huffman and Alpert, 2010). A need to develop novel treatments to fulfill these shortcomings exists. Physical inactivity is a risk factor for mental pathology (Abu-Omar et al., 2004; Dunn et al., 2001; Goodwin, 2003) and physical activity improves psychological risk factors (Lavie et al., 2011), which suggests that involvement in physical activity contributes to normal mental health. Exercise may offer additional benefits that leading anxiety therapies cannot (e.g., social acceptance of exercise as a healthy behavior, low financial costs, limited side effects, physical health benefits). Evidence accumulated extensively over the past 30 years suggests that physical activity is a promising candidate for the treatment of anxiety.
Physical activity protects against the onset of anxiety and treats anxiety symptoms in healthy people and medical patients, despite age, sex, or other medical conditions (Herring et al., 2010; U.S. Department of Health and Human Services, 2008). The effectiveness of exercise is comparable to or better than many standard forms of anxiety treatment (Carek et al., 2011; Petruzzello et al., 1991; Wipfli et al., 2008). Quantitative reviews suggest that exercise reduces anxiety with a small-to-moderate magnitude of effectiveness that can be seen after short- and long-term treatment (Conn, 2010; Herring et al., 2010; Long and Van Stavel, 1995; Petruzzello et al., 1991; Wipfli et al., 2008). Population data show that as the weekly frequency of exercise increases the risks for anxiety decreases (Goodwin, 2003). Moderate-to-high intensities of exercise yield larger treatment effects on anxiety than low intensities, as further supported by intervention studies (Conn, 2010). However, a dose-response relation or specific duration or mode of exercise that is especially well suited for treating anxiety is yet to be confirmed using randomized controlled trials, likely because such studies are presently scarce (Dunn et al., 2001; Larun et al., 2006; Wipfli et al., 2008). Nonetheless, there is evidence that acute aerobic exercise and training produces immediate and lasting improvements in anxiety symptoms (Herring et al., 2012; Petruzzello et al., 1991), whereas the beneficial effects of a resistance exercise may depend on characteristics of the exercise regimen such as intensity and duration (Bibeau et al., 2010). Together, these data suggest that physical activity can serve as an alternative or complement to current treatments for anxiety.
The neurobiological mechanisms that support the anxiolytic potential of exercise are unclear. Rodent models of anxiety are essential to permit mechanism-driven investigation into the neural basis of exercise. The contribution of rodent models was key to establish that exercise has neurogenic, neurotrophic, and neuroplastic effects that underlie improvements in learning and memory (for review see van Praag, 2009). However, it is presently controversial whether voluntary exercise reduces anxiety-like behavior in rodents. Evaluation of such evidence is critical at this juncture, as it will help determine whether rodent models should be used to understand the role exercise has on anxiety and its underlying neurobiology. Thus, the primary aim of this review is to evaluate current evidence of voluntary wheel running in behavioral tests of anxiety in rodents. We conclude that rodent models can indeed be used to understand the effects of exercise on anxiety and propose that the behavioral efficacy of exercise depends on stress. We also identify variables that may impact the relationship between voluntary exercise and anxiety-like behavior, while drawing attention to limitations in the literature and recommending research to further understand this relationship. A secondary aim is to examine how wheel running alters neurotransmission involving norepinephrine and galanin in circuits that regulate stress and anxiety. We propose that wheel running promotes an adaptive response to stress via norepinephrine-galanin mediated brain mechanisms.
This review will focus on evidence from studies that used voluntary exercise. Evidence from other experimental paradigms (e.g., treadmill running, swimming) will not be included due to the confounded influence of stress and well-documented difference between free-choice wheel running and forced exercise in motor and affective behavior (Burghardt et al., 2004; Dishman et al., 1996; Forristall et al., 2007; Gorton et al., 2010; Leasure and Jones, 2008; Liu et al., 2009), brain signaling systems (Dishman et al., 1996; Dunn et al., 1996; Leasure and Jones, 2008; Liu et al., 2009; van Praag et al., 1999), and other physiological systems sensitive to stress (Hayes et al., 2008; Moraska et al., 2000). Stress increases wheel running in a manner that is blocked by an anxiolytic drug (Uchiumi et al., 2008), suggesting that voluntary exercise is not itself a stressor. Exercise also protects against stress at the neurobiological, neuroendocrine, and neuroimmune level (for review see Greenwood and Fleshner, 2008, 2011; Sothmann et al., 1996). Although voluntary exercise has qualities of a stressor (e.g., activates sympathetic nervous system, HPA axis, and other stress-responsive brain circuitry), it deserves unique classification and examination from other stressors because it is engaged voluntarily and is neuroprotective, predictable, controllable, and rewarding (Belke and Wagner, 2005; Cotman and Engesser-Cesar, 2002; Greenwood et al., 2011; Stranahan et al., 2008; Werme et al., 2002).
2. Tests and models of anxiety in rodents
The distinction between tests and models of anxiety is an important consideration in this review because their use, alone or in combination, may influence the interpretation of behavioral outcomes produced by voluntary exercise. Tests and models are tools that are user-defined by their application in research and do not possess “hereditary titles” (Kalueff et al., 2007). Tests of anxiety are commonly used once in a study as a bioassay or screen to characterize anxiolytic drugs or to phenotype rodents (e.g., genetic knockouts). Tests of anxiety are optimized under specific environmental parameters, validated mainly by benzodiazepines, and include the Geller and Vogel conflict, defensive burying, elevated plus maze, fear potentiated startle, hole board, open field, social interaction, and ultrasonic vocalization (for review see Lister, 1990; Treit et al., 2010). Although it is relevant to note that many of these tests can become models and induce persistent anxiety-relevant features in studies that measure the lasting consequence of exposure to the test itself, they are more commonly used as a test on one occasion with no further testing. Thus, as generally used in the biomedical literature and in every study examined in the present review, tests of anxiety allow a means to collect dependent variables to characterize behavior (hereafter referred to as baseline responding). Models of anxiety exhibit validity (e.g., construct, etiological, face validity) that tests do not necessarily possess and can produce relatively stable and persistent anxiety-related traits after induction by an experimental manipulation (hereafter referred to as evoked responding). Common stress-evoked models include uncontrollable stress, chronic unpredictable stress, and maternal deprivation. However, tests of anxiety are unfittingly referred to as ‘models’ of psychopathology throughout biomedical when used as a screen (for reviews see Holmes, 2003; van der Staay, 2006). This terminology misuse muddles the theoretical purpose (independent vs. dependent variable) and implicitly assumes that tests possess forms of validity that were not necessarily evoked. Thus, this misnomer may affect how one assigns value to data and places results into logical frameworks.
The bulk of basic research employing voluntary wheel running used tests of anxiety as screens to measure baseline responding (i.e., without the use of a model or assessing evoked responding after exposure to an experimental stressor; Binder et al., 2004; Burghardt et al., 2004; Collins et al., 2009; Dishman et al., 1996; Droste et al., 2007; Dubreucq et al., 2010b; Duman et al., 2008; Falls et al., 2010; Fuss et al., 2010a, b; Garcia-Capdevila et al., 2009; Grace et al., 2009; Hopkins and Bucci, 2010a,b; Leasure and Jones, 2008; Pietropaolo et al., 2006). However, several reports measured the influence of wheel running on evoked responding in tests of anxiety by exposing rodents to a stressor (Dishman et al., 1997; Fox et al., 2008; Greenwood et al., 2005a, 2003a, 2008, 2007; Lancel et al., 2003; Masini et al., 2011; Salam et al., 2009; Sciolino et al., 2012) or stress-based models of anxiety (De Chiara et al., 2010; Maniam and Morris, 2010; Zheng et al., 2006). Based on evidence available to date, we propose that the behavioral efficacy of exercise in tests of anxiety is influenced by stress, including stress-based models of anxiety. The stress response is any event that moves an organism away from homeostasis and can be adaptive when elicited short-term in a threatening environment. However, stress can become maladaptive and contribute to the development/exacerbation of anxiety when excessive, uncontrollable, and persistent (Maier and Watkins, 2005; McEwen et al., 2012; McEwen and Gianaros, 2011). In the present review, we try to avoid making assumptions about emotional states and complex cognitive processes in rats that may not exist and/or cannot be directly measured (Cryan et al., 2005; Holmes, 2003; Steimer, 2011), but focus more on the variables that are manipulated and measured in the experiments. We therefore refer to stress in the context of an independent variable rather than assuming that it has produced anxiety in rodents.
3. Effects of wheel running on anxiety-like behavior and fear learning
3.1. Wheel running offers anxiolytic-like potential in a manner dependent on stress
The effect of exercise is at odds when assessing baseline responding in tests of anxiety (see Table 1). Chronic wheel running produces anxiolytic-like (Binder et al., 2004; Dishman et al., 1996; Dubreucq et al., 2010a; Duman et al., 2008; Falls et al., 2010; Gorton et al., 2010; Hopkins and Bucci, 2010a; Salam et al., 2009), anxiogenic-like (Burghardt et al., 2004; Fuss et al., 2010a, b; Grace et al., 2009), and null effects (Pietropaolo et al., 2006) in rodents. Discrepancies between these reports on exercise and affect can be attributed to differences in experimental parameters, including social rearing conditions (Stranahan et al., 2006; Stranahan et al., 2008), time of day of behavioral testing (Hopkins and Bucci, 2010b), type of sedentary comparison group (no wheel vs. locked wheel) (Dubreucq et al., 2010b), duration or distance of wheel running (Burghardt et al., 2004; Burghardt et al., 2006; Greenwood et al., 2005a, 2007), sex of subjects (Pietropaolo et al., 2008), time of testing relative to the last wheel access (Duman et al., 2008), as well as aversiveness of the testing environment, handling history, and genetic background (Lister, 1990; Takahashi et al., 2008). Although these experimental parameters likely moderate the relationship of wheel running and emotion, no single variable is expected to reliably account for inconsistent effects of exercise across tests of anxiety when stress was not experimentally manipulated. Instead, wheel running produces inconsistent effects on baseline responding in tests of anxiety likely due to a variety of internal and/or external variables that ultimately influence the impact of stressors on the organism.
Table 1.
Summary of the effects of wheel running on baseline responding in tests of anxiety.
| Behavioral test | Exercise is anxiolytic? |
Wheel access (d) |
Distance ran (km/d) |
Sex | Strain/species | Housed | Sed Ctrl | Reference |
|---|---|---|---|---|---|---|---|---|
| Acoustic startle | Y | 14a | 4.5 | M | C57BL/6J | G | L | (Falls et al., 2010) |
| Y | 14 a | 5 | M | C57BL/6N | G | L | (Salam et al., 2009) | |
| – | 28 | 10 | M | C57BL/6NCrl | S | L | (Cacciaglia et al., 2011) | |
| – | 60a | nd | F | C57BL6/J | G | L | (Pietropaolo et al., 2006) | |
| – | 90 | 4 | F, M | WT mice | S | L | (Pietropaolo et al., 2008) | |
| Dark:light box | N | 21 | 8 | M | C57BL/6J | S | L | (Fuss et al., 2010a) |
| Y | 24 | 6–7 | M | C57Bl6/N | S | A/L | (Dubreucq et al., 2010b) | |
| N | 26 | 9–12 | M | C57BL/6J | S | L | (Fuss et al., 2010b) | |
| – | 28a | 3–4 | M,F | 129×C57Bl6 | G | A | (Garcia-Mesa et al., 2011) | |
| Y | 28 | 4 | M | C57BL/6N | S | A | (Binder et al., 2004) | |
| N | 28 | 10 | M | C57BL/6NCrl | S | L | (Cacciaglia et al., 2011) | |
| Defensive withdrawal | – | 21 | 4 | M | Sprague | S | A | (Sciolino et al., 2012) |
| Elevated plus maze | – | 12a | 5 | M,F | Sprague | G | A | (Brocardo et al., 2011) |
| – | 19a | 2 | M | Long Evans | G | A | (Hopkins and Bucci, 2010b) | |
| N | 20 | 1–2 | M | Sprague | S | A | (Grace et al., 2009) | |
| – | 21 | 4 | M | Sprague | S | A | (Sciolino et al., 2012) | |
| Y | 28 | 4 | M | C57BL6/N | S | A | (Binder et al., 2004) | |
| Y | 28b | 5 | M | Sprague | G-S | A | (Hopkins and Bucci, 2010a) | |
| Y | 21–28 | 12–14 | M | C57Bl/6 | S | A | (Duman et al., 2008) | |
| – | 28a,c | <1–5 | M | Wistar | G | A | (Garcia-Capdevila et al., 2009) | |
| Y | 30b | 1.5 | M | C57Bl/6 | G | L | (Gorton et al., 2010) | |
| N | 28 or 56 | 6 | M | Sprague | S | L | (Burghardt et al., 2004) | |
| – | 60a | nd | F | C57BL6/J | G | L | (Pietropaolo et al., 2006) | |
| Y, N | 90 | 4 | F, M | WT mice | S | L | (Pietropaolo et al., 2008) | |
| Elevated zero maze | Nd | 21 | 8 | M | C57BL/6J | S | L | (Fuss et al., 2010a) |
| N | 25 | 9-12 | M | C57BL/6J | S | L | (Fuss et al., 2010b) | |
| N | 28 | 10 | M | C57BL/6NCrl | S | L | (Cacciaglia et al., 2011) | |
| Hole board | – | 28 | 4 | M | C57BL/6N | S | A | (Binder et al., 2004) |
| – | 28a | 3–4 | M,F | 129×C57Bl6 | G | A | (Garcia-Mesa et al., 2011) | |
| Novel cage/container | Y | 28a | 3–4 | M,F | 129×C57Bl6 | G | A | (Garcia-Mesa et al., 2011) |
| Y | 28 | 4-7 | M | Sprague | S | A | (Collins et al., 2009) | |
| Y | 28 | 6 | M | Sprague | S | A | (Droste et al., 2007) | |
| Y | 42 | 2 | M | Sprague | S | A | (Masini et al., 2011) | |
| Novel food | – | 60a | nd | F | C57BL6/J | G | L | (Pietropaolo et al., 2006) |
| Open field | − ↔ | 12a | 5 | M,F | Sprague | G | A | (Brocardo et al., 2011) |
| Y↓ | 14 a | 5 | M | C57BL/6N | G | L | (Salam et al., 2009) | |
| nd↓ | 19 a | 2 | M | Long Evans | G | A | (Hopkins and Bucci, 2010b) | |
| − ↓ | 20 | 1–2 | M | Sprague | S | A | (Grace et al., 2009) | |
| N↓ | 21 | 8 | M | C57BL/6J | S | L | (Fuss et al., 2010a) | |
| N ↓ | 21 | 9–12 | M | C57BL/6J | S | L | (Fuss et al., 2010b) | |
| Ne ↓ e | 21–28 | 12–14 | M | C57BL6/J | S | A | (Duman et al., 2008) | |
| − ↓ | 28a,c | <1–5 | M | Wister | G | A | (Garcia-Capdevila et al., 2009) | |
| nd ↔ | 28a | 3–4 | M,F | 129×C57Bl6 | G | A | (Garcia-Mesa et al., 2011) | |
| −f nd | 28 | 4 | M | C57BL/6 N | S | A | (Binder et al., 2004) | |
| N ↓ | 28 or 56 | 6 | M | Sprague | S | L | (Burghardt et al., 2004) | |
| − ↔ | 37 | 3–5 | M | CB1 KO & WT mice | S | L | (Dubreucq et al., 2010a) | |
| − ↔ | 56b | <1 | F | Long Evans | G | L | (Leasure and Jones, 2008) | |
| Y ↑ | 56 | 3 | M | Sprague | S | A | (Dishman et al., 1996) | |
| − ↔ | 60a | nd | F | C57BL6/J | G | L | (Pietropaolo et al., 2006) | |
| nd ↔ | 90 | 4 | F, M | WT mice | S | L | (Pietropaolo et al., 2008) | |
| Shock probe defensive burying | – | 21 | 4 | M | Sprague | S | A | (Sciolino et al., 2012) |
| Social interaction | Y | 14a | 5 | M | C57BL/6N | G | L | (Salam et al., 2009) |
| – | 56 | 6 | M | Sprague | S | L | (Burghardt et al., 2004) | |
| Stress-induced hyperthermia | Y | 14a | 5 | M | C57BL/6N | G | L | (Salam et al., 2009) |
Abbreviations:–,no effect on anxiety; ↑, increased locomotion; ↓, reduced locomotion; ↔, did not alter locomotion; A, absent wheel controls; CB1, cannabinoid type I receptor; F, female; G, group housing; KO, knockout; L, locked wheel controls; M, male; nd, no data; N, anxiogenic; S, single housing; Sed Ctrl, sedentary controls; WT, wild type; Y, anxiolytic. Footnotes:
wheel was shared;
exercise was restricted;
resistance created in wheel;
effect depends on hippocampal neurogenesis;
effect depends on time of testing relative to last wheel access;
measured object recognition during test. Notes: Open field reported as changes in center time/entries/distance from the walls followed by changes in locomotion. Wheel access is reported at behavioral testing and underlining indicates behaviorally ineffective durations. Mean distance ran is reported around the time of behavioral testing and was divided by the number of subjects per cage when the wheel was shared.
Wheel runners are resistant to the toll of stressors or stress-evoked models of anxiety (see Table 2). It is important to note that benefit of exercise on evoked responding in an array of tests of anxiety is mainly due to stress-induced impairment in sedentary, but not exercise rodents (De Chiara et al., 2010; Dishman et al., 1997; Fox et al., 2008; Greenwood et al., 2005a, 2003a, 2008, 2007; Maniam and Morris, 2010; Masini et al., 2011; Sciolino et al., 2012; Zheng et al., 2006). Reliable detection of the beneficial consequences of wheel running may result only when stress is experimentally manipulated because the effects of exercise interact with stress to alter responding in a manner that behavioral screens can detect. Experimental evidence that bolsters this conclusion suggests that differences between exercise and sedentary rats on evoked responding in tests of anxiety emerge after exposure to repeated injection/drug stress, but not in the absence of such stress (Fox et al., 2008; Sciolino et al., 2012). For example, rats that were allowed access to a running wheel for 3 wk exhibited anxiolytic-like behaviors across several tests if the rat had a history of repeated stress, but failed to produce these effects in exercised rats tested under baseline conditions of stress or intense stress evoked by a high dose of an anxiogenic drug (see Fig. 1). The central thesis of this review is that the anxiolytic-like benefit of chronic voluntary exercise emerges after exposure to mild-to-moderate intensity stress, wherein the level of stress an animal experiences is deliberately induced by an experimenter or inherent in the experimental design (e.g., aversiveness of the housing or testing environment, rearing and handling conditions) and/or modified by other factors that influence stressor responsiveness (e.g., genetics, maternal history; see Fig. 2). Although no evidence directly shows that physically active animals are more anxious than sedentary animals following extremely high intensity stress, this is a possibility based on research that shows anxiogenic-like effects of exercise in select populations (e.g., high runners; Fuss et al., 2010a,b). The thesis of this review is further evaluated below by examining the impact of exercise on baseline responding in tests of anxiety, followed by examining the impact of exercise on stress-evoked responding.
Table 2.
Summary of the effects of wheel running on stress-evoked responding in tests of anxiety.
| Behavioral test | Stressor | Stressor regimen | Exercise relieves stressor? |
Wheel access (d) |
Distance ran (km/d) |
Sex | Strain/species | Housing | Sed Ctrl | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Acoustic startle | mCPP (0.1–1 mg/kg) | 1× | Ya | 14b | 4.5 | M | C57BL/6N | G | L | (Fox et al., 2008) |
| Dark:light box | Maternal deprivation | PND 2–14 (180 min/d) | Y | 84b | <1 | F | Sprague | G | nd | (Maniam and Morris, 2010) |
| Defensive withdrawal | FG7142 (30 mg/kg) | 1× | – | 21 | 4 | M | Sprague | S | A | (Sciolino et al., 2012) |
| Saline inject or FG7142 (7.5 mg/kg) | 10 d (1×/d) | Y | 21 | 4 | M | Sprague | S | A | (Sciolino et al., 2012) | |
| Elevated plus maze | FG7142 (30 mg/kg) | 1× | Y | 21 | 4 | M | Sprague | S | A | (Sciolino et al., 2012) |
| Saline inject or FG7142 (7.5 mg/kg) | 10 d (1×/d) | Y | 21 | 4 | M | Sprague | S | A | (Sciolino et al., 2012) | |
| Maternal deprivation | PND 2–14 (180 min/d) | Y | 84b | <1 | F | Sprague | G | nd | (Maniam and Morris, 2010) | |
| Open field | Chronic mild stress | 28 d (1×/d) | −(Y) | 14,28,or 42 | nd | M | Sprague | S | A | (Zheng et al., 2006) |
| Repeated social stress | 3 d (3 hour/d) | Y (Y)d | 15 | nd | M | C57/Bl6 | G | A | (De Chiara et al., 2010) | |
| Uncontrollable tail shocks | 1 session | Y (−) | 42 | 3 | M | Fisher344 | S | nd | (Greenwood and Fleshner, in press) | |
| Shock probe defensive burying |
FG7142 (30 mg/kg) | 1× | – | 21 | 4 | M | Sprague | S | A | (Sciolino et al., 2012) |
| Saline inject or FG7142 (7.5 mg/kg) |
10 d (1×/d) | Y | 21 | 4 | M | Sprague | S | A | (Sciolino et al., 2012) | |
| Shuttle box escape, freezing |
Uncontrollable foot shocks | 1 hour session | Y | 14 or 42 | 2 or 3 | M | Fisher344 | S | A | (Greenwood et al., 2007) |
| Uncontrollable tail shocks | 1 session | Y | 21 or 42 | 2 or 3 | M | Fisher344 | S | L | (Greenwood et al., 2005a) | |
| Uncontrollable tail shocks | 1 session | Y | 42 | 4 | M | Sprague | S | L | (Greenwood et al., 2003a) | |
| Fluoxetine (10 mg/kg) | 1× | Y | 42 | 3 | M | Fisher344 | S | A | (Greenwood et al., 2008) | |
| Uncontrollable foot shocks | 20–45 min session | Y | 63–84e | 1.5 | F | Sprague | S | A | (Dishman et al., 1997) |
Abbreviations:–,did not reduce effects of stressor; A, absent wheel controls; F, female; G, group housing; L, locked wheel controls; M, male; mCPP, metachlorophenylpiperazine; MPTP, 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine; S, single housing; Sed Ctrl, sedentary controls; Y, reduced the toll of stressor. Footnotes:
exercise produces anxiolytic-like response when stressor is not present;
exercise wheel was shared;
cexercise was restricted;
exercise effects were dependent on cannabinoid CB1 receptors;
resistance created in wheel.
Notes: Wheel access is reported at the time of behavioral testing, wherein underlining indicates behaviorally ineffective durations. Mean distance ran is reported at behavioral testing and divided by the number of subjects per cage when the wheel was shared. Open field data are reported first as changes in center time/entries followed by changes in locomotion in parentheses. All drugs listed above were given via an intraperitoneal route.
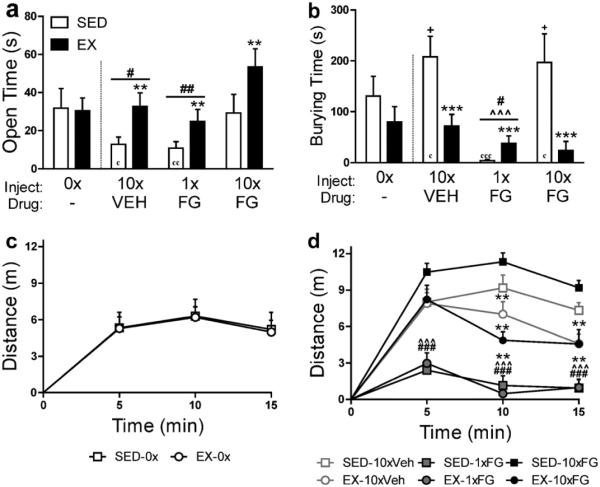
Fig. 1.
Stress alters the benefit of exercise in tests of anxiety. Exercise produces anxiolytic-like behavior in the (a) elevated plus maze and (b) shock probe defensive burying test and facilitates locomotor habituation in the (c–d) open field only after exposure to repeated injection or pharmacological stress using the anxiogenic β-carboline FG7142 (7.5 mg/kg i.p. × 10 days), but not in the absence of these stressors or in the presence of stress induced by a high, acute dose of FG7142 (30 mg/kg i.p. × 1 day). Results obtained from Sciolino et al. (2012). Data are reported as mean ± SEM (n = 8–10). ***p < 0.001, **p < 0.01 vs. sedentary; ###p < 0.001, ##p < 0.01, #p < 0.05 vs. chronic FG7142; +p < 0.05 vs. exercise rats treated with chronic vehicle, both sedentary and exercise rats treated with acute FG7142, and exercise rats treated with chronic FG7142; ˆˆˆp < 0.001 vs. chronic vehicle; cccp < 0.001, ccp < 0.01, cp < 0.05 vs. pooled no inject groups. Abbreviations: EX, exercise; FG, FG7142; SED, sedentary; VEH, vehicle.
Fig. 2.
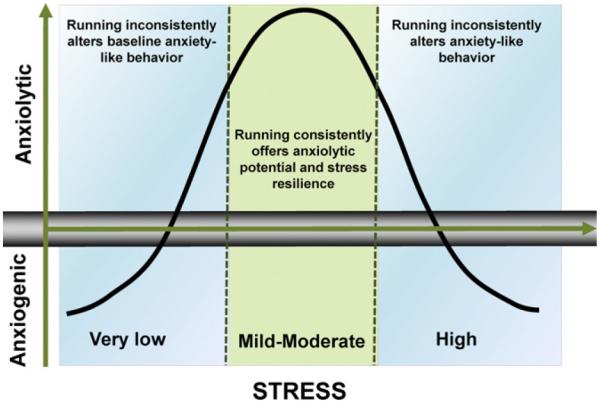
A stress-based model to explain the influence of wheel running in tests of anxiety. The relationship between voluntary wheel running and performance in tests of anxiety is non-monotic and influenced by the level of stress the animal is experiencing. Anxiolytic-like effects of exercise are expected to occur because wheel running interacts with stress to alter behavior. The impact of mild-to-moderate levels of stress reveals anxiolytic-like benefits of exercise, whereas null or anxiogenic findings occur outside of this range (in blue area). The level of stress an animal experiences can be deliberately induced by an experimenter or inherent in the experimental design (e.g., aversiveness of the housing or testing environment, rearing and handling conditions) and/or modified by other factors that influence stressor responsiveness (e.g., genetics, maternal history). This model is adapted from the Yerkes-Dodson law (Broadhurst, 1957; Yerkes and Dodson, 1908).
3.1.1. Effects of wheel running on baseline responding in tests of anxiety
3.1.1.1. Affect-modulated startle
The startle reflex is a muscular contraction to an abrupt stimulus (e.g., tone, light, air puff) that likely serves to avert injury from attack (for review see Koch and Schnitzler, 1997). Select isoforms of anxiety, including posttraumatic stress disorder and obsessive compulsive disorder, are distinguished by exaggerated baseline startle and/or diminished ability to inhibit startle (Braff et al., 2001; Pole, 2007). Emotive-laden stimuli can be used to enhance or diminish startle (Grillon and Baas, 2003; Lang and McTeague, 2009; Risbrough, 2010). Whether acute exercise reduces such measures of startle in humans is presently unclear. An acute exercise session was not sufficient to alter baseline startle or affect-modulated startle in healthy individuals, nor did it alter prepulse inhibition in those with high-trait anxiety (Duley et al., 2007; Smith and O’Connor, 2003; Smith et al., 2002; Tieman et al., 2001). Yet, the impact of a chronic exercise regimen on the startle reflex remains to be elucidated.
While some reports suggest that a history of wheel running reduces baseline startle (Falls et al., 2010; Salam et al., 2009), others show that running does not alter this measure (Cacciaglia et al., 2011; Pietropaolo et al., 2006, 2008). Prepulse inhibition of the acoustic startle response is consistently unaltered by wheel running (Lau et al., 2009; Pietropaolo et al., 2006; Salam et al., 2009). In line with the idea that a history of wheel running produces beneficial effects on startle, mice that ran on a wheel for 2 wks exhibited reduced acoustic startle amplitude relative to sedentary counterparts (Salam et al., 2009). Reductions in baseline startle were not seen after short durations of wheel running (3 days after), but were detected 1 wk after and persisted as long as the mice were allowed to run (up to 12 weeks). Thus, reduced startle after wheel running likely occurs from adaptations that result from repeated running. Further, wheel running and sedentary rodents both exhibit comparable reductions in startle as acoustic stimuli are repeatedly presented, which suggests that wheel running exerts an influence on startle independent of habituation (Pietropaolo et al., 2006; Salam et al., 2009). Reports that show reduced baseline startle after wheel running originate from a laboratory that uniquely tested for startle during the light phase of the light:dark cycle, whereas those showing null effects stem from laboratories that tested during the dark phase; cf. Cacciaglia et al. (2011) and Pietropaolo et al. (2006, 2008). Diurnal variations in startle may explain why these reports are at odds, as startle exhibits circadian rhythmicity (Horlington, 1970; Ison and Foss, 1997; Miller and Gronfier, 2006). Specifically, startle is about half the amplitude in the light versus the dark phase, and the effects of pharmacological agents may be more evident when startle is measured in the light phase (Brick et al., 1984; Chabot and Taylor, 1992a,b; Flood et al., 2007). Thus, testing in the light phase may produce better experimental conditions to reveal treatment-induced reductions in startle by wheel running. However, it is also possible that divergent effects of wheel running are due to inherent or external levels of stress that affect startle (Zhang et al., 2011).
3.1.1.2. Exploration
Most research on exercise characterized anxiety-like behavior using tests that rely heavily on locomotion. Exploration-based tests are time-restricted to elicit a typical response and include examples like the elevated plus and zero mazes, hole board, dark:light box, and open field tests (for review of tests see Lister, 1990; Treit et al., 2010). Responding in exploration-based tests relies on unconditioned, spontaneous behavior in a novel testing apparatus that is designed to elicit approach-avoidance conflict. These tests likely evoke a degree of neophobia, exploration, fear, and motivation, although the weight of each is probably different in each test. Attesting to the differences across tests of exploration, the degree of overlap is suggested to be very low (estimated at approximately <20% overlap; Ramos, 2008). However, an important unifying theme of all exploration-based conflict tests is the reliance on locomotor activity (File, 1985, 2001). File offers particularly useful advice in a review on the use of tests of exploratory behavior to study anxiolytic agents, writing, “…the use of tests of exploratory behavior to screen for new potential “benzodiazepine-like” compounds is somewhat hazardous, unless accompanied by other tests and carefully interpreted” (File, 1985). Applying and extending this advice to understand exercise, we are reminded that such tests are GABA-ergic sensitive (and possibly preferential) and warned against overreliance or oversimplification of anxiety-like behavior from exploration-based tests (as they are often confounded by locomotor activity and result from numerous impinging drives). Nonetheless, exploration-based tests are important to profile anxiolytics, including exercise regimens.
3.1.1.2.1. Dark:light tests
Rodent behavior in the dark:light box is driven by the conflict to avoid brightly illuminated spaces against the need to explore this novel environment (for review see Crawley, 1985; Lister, 1990; Treit et al., 2010). The defensive withdrawal test is a validated variant of the dark:light box, which has a proportionally smaller dark enclosure (Crawley, 1981; Heinrichs et al., 1997; Pare et al., 2001; Pritchard et al., 1991; Roman and Arborelius, 2009; Smagin et al., 1996; Smith et al., 1998; Stone et al., 1995; Takahashi et al., 1989; Yang et al., 1990). Typically, anxiolytic-like responding is defined by decreased latency to enter, increased time spent, and/or increased entries in the lit compartment.
In dark:light tests, wheel running reduces (Binder et al., 2004; Dubreucq et al., 2010b), enhances (Cacciaglia et al., 2011; Fuss et al., 2010a; Fuss et al., 2010b), or does not alter (Garcia-Mesa et al., 2011; Sciolino et al., 2012) baseline anxiety-like behavior. Systematic factors likely account for differing results and may include the control group comparison, amount of wheel running, and level of stress the animal experiences. The sedentary comparison group likely contributes to reliable detection of anxiolytic-like effects of exercise in the dark:light test, as every report demonstrating this effect compared behavior against a sedentary group without a blocked wheel. For example, Chaouloff and colleagues (2010b) showed that the beneficial effects of wheel running in the dark:light box were present only when compared to sedentary controls that did not have a blocked wheel. Comparisons to no-wheel sedentary controls probably maximizes the difference between experimental groups, as a blocked wheel offers some degree of exercise (e.g., hanging, climbing; Koteja et al., 1999) and environmental enrichment (Lehmann and Herkenham, 2011; Schrijver et al., 2002). The amount of wheel running may also contribute to the detection of anxiogenic behavior in the dark:light box, which emerges in mice that ran approximately more 8 km per day (see Table 1). In support of this explanation, qualities of the training regimen (Leasure and Jones, 2008) and the amount of neurogenesis in the hippocampus is a necessary factor that determines the affective consequence of exercise in the dark:light box (Fuss et al., 2010a). Also, strong associations exist between the number of cells exhibiting a marker of hippocampal neurogenesis (DCX) and anxiogenic-like behavior in dark:light test, with an inverse correlation observed between time in the lit side and exits from dark side and positive correlation observed between initial latency to exit and the extent of exploration of the lit side (Fuss et al., 2010b). Together, these data suggest that wheel running has the potential to exert benefits on baseline anxiety-like behavior in the dark:light box, although additional factors likely moderate baseline anxiety (e.g., high amounts of running, sedentary control group, stress).
3.1.1.2.2. Elevated mazes
Rodent behavior in the elevated maze is theorized to be the product of the endogenous drive to avoid unprotected open spaces versus the motivation to explore a novel environment (for review see Dawson and Tricklebank, 1995; Walf and Frye, 2007; Wall and Messier, 2001). The elevated plus and zero maze are comparable in concept, sensitivity to detect anxiolytic/anxiogenic agents, and design, except the elevated zero maze has the O-shape modification that eliminates the potential confound of a central hub (Braun et al., 2011; Kulkarni et al., 2007; Shepherd et al., 1994). Typical anxiolytic-like behavior in the elevated mazes consists of increased open arm time and entries, as well as a concomitant decrease in time spent on the closed arms.
The effects of wheel running in the elevated maze are mixed when baseline responding is measured in tests of anxiety. For instance, baseline anxiety-like behavior in the elevated mazes was reduced (Binder et al., 2004; Duman et al., 2008; Gorton et al., 2010; Hopkins and Bucci, 2010a; Pietropaolo et al., 2008), increased (Burghardt et al., 2004; Cacciaglia et al., 2011; Grace et al., 2009; Pietropaolo et al., 2008), or not changed (Brocardo et al., 2011; Garcia-Capdevila et al., 2009; Hopkins and Bucci, 2010b; Pietropaolo et al., 2006; Sciolino et al., 2012) in runners that were not exposed to an experimental stressor. It is possible that inconsistent evidence in the elevated maze results from effects of exercise on locomotion or differences across studies in running distance. A subset of studies showed that wheel running reduces locomotor activity in the elevated maze (e.g., distance traveled, number of total, closed, or full arm entries; Binder et al., 2004; Cacciaglia et al., 2011; Duman et al., 2008; Fuss et al., 2010b; Gorton et al., 2010), and traditionally doses of drugs that impair locomotion confound interpretation of anxiety-like properties (Rodgers et al., 1997). However, the effect of exercise on baseline anxiety-like behavior in the elevated plus maze is still mixed even after excluding studies with locomotor confounds. High amounts of running likely contribute to detection of anxiogenic behavior in the elevated maze (see also section 4.1.1.1 Cacciaglia et al., 2011; Fuss et al., 2010a,b), although a minority of reports also show anxiolytic-like effects after high amounts of running (Duman et al., 2008). Collectively, we conclude that wheel running exerts anxiolytic potential in the elevated mazes, but the effect of exercise is likely influenced by additional factors (e.g., distance of wheel running, stress).
Comparing the effects of wheel running across studies using the elevated maze is difficult because the behaviors measured are diverse, such that reports that conclude the same effect of wheel running produce different alterations in dependent variables. Therefore, it is recommended that future studies demonstrate alterations in complementary behaviors in the elevated mazes (e.g., increased open arm time corresponds with decrease closed arm time), which will add confidence in conclusions about exercise that are based on data generated from these tests. Future studies interested in teasing apart the affective consequences of exercise in the elevated maze should establish a dose-response relationship by testing log-base distance and durations of wheel running. Such research would add valuable insight to evaluate whether there is a threshold or an optimal level of characteristics (e.g., distance, duration, frequency) that define exercise that are needed to acquire beneficial emotional consequences. Indeed, a minimal duration of wheel running is necessary to see changes in the elevated maze (Burghardt et al., 2004) and restricted wheel access is also effective in reducing inherent levels of anxiety-like behavior in the elevated plus maze (Gorton et al., 2010; Hopkins and Bucci, 2010a). The efficiency the elevated maze offers allows the “dose-response” question of exercise to be tested with relative ease, which is of high translational relevance in recommending exercise regimens.
3.1.1.2.3. Hole board
The hole board test permits quantification of both directed (towards holes in floor board of arena) and general (in entire arena) exploratory activity in a novel testing arena (Casarrubea et al., 2011; Crawley, 1985; File and Wardill, 1975; Kliethermes and Crabbe, 2006; Lister, 1990; Ohl et al., 2001). An anxiolytic-like response in this test is generally defined by an enhancement of hole-directed behavior. To date, only a couple of reports tested the effects of wheel running in the hole board test, both of which reported no effect of 4 weeks of wheel running in this assay. Wheel runners and sedentary controls were not different in the expression of head dipping in the Bossier’s four hole board test (Garcia-Mesa et al., 2011). In the modified hole board test that contains 23 centrally-located holes, exercised mice did not reliably differ on anxiety-related measures relative to sedentary mice as measured by the time spent or entries on the hole board (Binder et al., 2004). Exercised mice also exhibited reduced line crosses in the hole board test, which suggests that locomotor effects can be dissociated from head-dipping exploration (Binder et al., 2004). Not enough data are available from the hole board test to credibly interpret the effects of wheel running. It remains to be determined whether behavior in this test is systematically altered by stress and/or characteristics of running (e.g., duration, frequency).
3.1.1.2.4. Open field
The open field is a spacious arena used to characterize spontaneous locomotor activity and exploratory behaviors relevant to the study of anxiety (for review see Calabrese, 2008; Crawley, 1985; Lister, 1990). Similar to other approach-avoidance tests, rodent behavior in the open field is speculated to result from the need to avoid the center, unprotected portion of arena versus the impetus to explore a new environment. Most investigations show that rodents given access to a running wheel later exhibit reduced locomotor activity in the novel open field (Burghardt et al., 2004; Duman et al., 2008; Fuss et al., 2010a,b; Garcia-Capdevila et al., 2009; Grace et al., 2009; Hopkins and Bucci, 2010b; Salam et al., 2009), although some reports show no effect on locomotion upon initial exposure to the open field (Brocardo et al., 2011; Dubreucq et al., 2010a; Garcia-Mesa et al., 2011; Leasure and Jones, 2008; Pietropaolo et al., 2006; Pietropaolo et al., 2008). Comparing across studies, running-induced decreases in locomotion in the open field persist across characteristics of the subject (species, strain, sex, housing), exercise regimen (duration, distance ran, restricted, shared, resistance), and experimental test (duration, lighting, measure of locomotion). Evidence does not suggest that the open field is more aversive to wheel runners (De Chiara et al., 2010; Salam et al., 2009; Zheng et al., 2006). Among the reports that show wheel running does not alter locomotion, wheel running failed to alter anxiety-relevant behaviors like center time or entries in the open field relative to sedentary controls (Dubreucq et al., 2010a; Leasure and Jones, 2008; Pietropaolo et al., 2006). Reduced locomotion in the open field is not likely due to running-induced fatigue because runners resume exercise after behavioral testing (unpublished observation), exhibit enhanced performance in the rotorod test (Salam et al., 2009), and are no different from sedentary controls on locomotion in an activity or home cage (Dubreucq et al., 2010a; Fuss et al., 2010a). Also, a strong positive correlation between open field locomotor activity and running distance exists during the active portion of the day, such that increases in locomotion are associated with increases in running distance (Pietropaolo et al., 2008). The fact that wheel running decreases locomotor activity limits meaningful interpretation of the effects of exercise on emotion-relevant behavior in the open field. Indeed, it is well accepted that inferring emotion from exploratory behavior is inaccurate when confounds in locomotion exist. Thus, we suggest that the open field is not well suited to infer the emotional consequences of wheel running, but is appropriate to observe alterations in locomotion or demonstrate locomotor confounds that could influence other tests of emotion.
3.1.1.3. Novelty
Though all of the paradigms reviewed above typically involve an element of novelty as an aversive stimulus, some paradigms place particular emphasis on novelty as the independent variable, and therefore fit appropriately into a separate category of tests. Novelty is speculated to provoke fear in rodents as measured by reduced exploration and enhanced avoidance in tests that evoke an approach-avoidance conflict (Blanchard et al., 1974; Montgomery, 1955; Montgomery and Monkman, 1955). Several reports suggest that wheel running minimizes the effects of novelty on spontaneous behavior (Collins et al., 2009; Droste et al., 2007; Garcia-Mesa et al., 2011; Masini et al., 2011). During exposure to a small novel cage or container, exercised rats exhibit more resting (i.e., more lying and/or stationary behaviors) and less non-resting behaviors (i.e., rearing, walking, grooming), all of which are displayed in an undisturbed rodent during the daytime (Collins et al., 2009; Droste et al., 2007; Garcia-Mesa et al., 2011; Masini et al., 2011). Exercise also reduced the effects of novelty on HPA and autonomic functioning, as rats allowed to run exhibited reduced plasma ACTH and corticosterone, heart rate, and body temperature after exposure to a novel cage/container compared to sedentary rats (Droste et al., 2007, 2003; Masini et al., 2011). However, runners and sedentary controls did not statistically differ in the latency to consume novel chocolate pellets (Pietropaolo et al., 2006). Interpretation of this result is limited because the stress of novelty per se was not induced (i.e., similar mean latency to consume standard chow and novel chocolate in exercise and sedentary conditions). Further, the mean latency to consume the novel chocolate tended to be lower in the exercise condition relative to a sedentary control, which may be meaningful because the research was based on a small sample (n = 5–6) and statistical analyses that included other groups. More research is necessary to determine whether wheel running reduces food neophobia, preferably as assessed by measures with less nutritional/energetic confounds (e.g., latency to approach the novel food). Together these data suggest that wheel running promotes adaptive coping to the stress of a novel environment.
3.1.1.4. Social interaction
Under normal conditions, rodents spontaneously engage in social interaction, whereas isolation produces an array of behavioral and neurochemical abnormalities (for review see Fone and Porkess, 2008; Hall, 1998; Olsson and Westlund, 2007). In the social interaction test, a pair of rodents is allowed to interact in an arena and the time spent engaged in active social behaviors with an unfamiliar mate is measured (File and Hyde, 1978; Lapiz-Bluhm et al., 2008). Anxiogenic drugs reduce social interaction, whereas this behavior is increased by anxiolytics (File, 1980; File and Baldwin, 1987). Wheel running also has the potential to increase social interaction. For example, Salam et al. (2009) showed that exercised mice that were group housed exhibited increased time/frequency sniffing, following, grooming, and climbing a novel conspecific, relative to non-exercising mice. However, Burghardt et al. (2004) showed that exercise and sedentary rats kept singly housed were no different in the time spent in contact or active pursuit of a novel conspecific. Differences in the social history of the subject (single vs. group housed) or social mate (potentially non-matched vs. matched for social history) may account for differences in social interaction after wheel running. Indeed, isolation in the juvenile period or adulthood increases aggression and alters social interaction and exploratory behavior in rodents (Arakawa, 2005; Douglas et al., 2004; Fone and Porkess, 2008; Van Den Berg et al., 1999). Adolescent rodents that were socially reared prefer a compartment previously paired with similarly housed partners, whereas isolates do not exhibit this preference (Douglas et al., 2004). In any case, although data on wheel running and social interaction is limited, they are consistent with the conclusion that exercise offers anxiolytic potential.
3.1.1.5. Structured threat
Threat initiates defensive behaviors that are analogous across human and non-human animals (Blanchard et al., 2001a,b; Shuhama et al., 2007). Defensive behaviors are speculated to be perturbed in those with anxiety disorder, and accordingly are modified by anxiolytics (Archer, 1979; Griebel et al., 1995a,b; Marks, 1977; Treit et al., 1986; Treit et al., 1981). Structured tests of threat like the shock probe test and anxiety/defense battery initiate an array of defensive behaviors that are not necessarily measured in standard tests of anxiety (Blanchard and Blanchard, 2003; De Boer and Koolhaas, 2003; Treit et al., 1986). Consistent with the idea that the effects of exercise and stress interact, evidence shows that exercise failed to reliably alter measured behavior in the shock probe defensive burying test in rats that were exposed to no experimental stressor (Sciolino et al., 2012). More research is needed to comprehensively understand the conditions under which exercise alters defensive behavior and structured tests of threat should prove useful.
3.1.2. Effects of wheel running on stress-evoked responding in tests of anxiety
3.1.2.1. Affect-modulated startle
Wheel running consistently produces a stress-protective effect in the acoustic startle test of anxiety. For example, wheel running mitigated light-induced and mCPP-induced potentiation of acoustic startle in mice (Fox et al., 2008; Salam et al., 2009). Startle data are consistent with the hypothesis that the anxiolytic-like benefit of wheel running emerges after exposure to mild-to-moderate intensity stress. For example, exercise-induced reductions in startle were dependent on the dose of the anxiogenic agent mCPP, such that only the highest 1 mg/kg i.p. dose increased startle in exercised mice relative to vehicle (Fox et al., 2008). Since wheel running alters factors that modulate startle, such as arousal (Edgar et al., 1991; Hanagasioglu and Borbely, 1982; Welsh et al., 1988), attention (Hopkins et al., 2009; Robinson et al., 2011), and motivation (Eisenstein and Holmes, 2007; Greenwood et al., 2011; Lett et al., 2001; Rozeske et al., 2011; Werme et al., 2002), it is particularly relevant to determine whether these factors influence the effects of wheel running on startle. Collectively, these data suggest that chronic wheel running has the ability to modulate startle in a manner that is beneficial and potentially stress-dependent.
3.1.2.2. Exploration
The ability of wheel running to ameliorate the effects of stress in exploration-based tests of anxiety is clear. In the dark:light box, wheel running prevented the effects of maternal deprivation on anxiety-like behavior as measured by the time spent and entries in the lit area (Maniam and Morris, 2010). Wheel running also facilitated locomotor habituation in the defensive withdrawal test in rats exposed to repeated injection stress or pharmacological stress using the anxiogenic β-carboline FG7142 (7.5 mg/kg × 10 days) (Sciolino et al., 2012). However, a high dose of FG7142 (30 mg/kg i.p. × 1 day) dramatically suppressed locomotor activity and produced intense immobility and avoidance in this test regardless of whether rats ran on a wheel, which could imply that the beneficial effects of exercise are not sufficient to overcome intense stressors. In the elevated plus maze, sedentary rats exhibited anxiety-like behavior after exposure to either repeated injection stress or maternal deprivation as measured by reduced open arm time, open arm entries, and head dips, whereas exercise rats were resilient to this effect of stress (Maniam and Morris, 2010; Sciolino et al., 2012). In the open field, wheel running mitigated the deficits in locomotion induced by chronic mild stress or social stress (De Chiara et al., 2010; Zheng et al., 2006). Uncontrollable tailshock stress increased the time spent in the center of the open field in wheel runners (relative to no-stress), whereas stress produced an opposite or anxiety-like effect in sedentary rats (Greenwood and Fleshner, in press). These data clearly demonstrate that wheel running offers stress resilience in an array of exploration-based tests of anxiety.
3.1.2.3. Shuttle box escape and freezing after shock-elicited fear
Uncontrollable or inescapable stress is a model of anxiety that evokes deficits of shuttle box escape and exaggerated freezing in tests conducted 24–72 hour later, whereas controllable or escapable stress does not (for reviews see Maier and Watkins, 1998; Maier and Watkins, 2005). Uncontrollable stress induced by shock (e.g., 100 shocks/session) produces behavioral sequelae that generalize to environments separate from the fear context and sensitize neural systems that mediate fear. Wheel running does not alter shock-elicited fear per se, but blocks the behavioral impairment later displayed after uncontrollable stress (for review see Greenwood and Fleshner, 2011). Wheel running is repeatedly shown to ameliorate the effects of uncontrollable stress induced by shock on shuttle box escape and freezing (Dishman et al., 1997; Greenwood and Fleshner, 2008; Greenwood et al., 2005a, 2003a, 2008). Of note, the benefit of exercise after uncontrollable stress is displayed after 6 weeks of wheel running, but not before then (Greenwood et al., 2005a, 2003a, 2008, 2007), which suggests that some benefits of exercise become apparent after long durations. Wheel running also protected against the shuttle box escape deficit and exaggerated freezing produced by an acute dose of the selective serotonin reuptake inhibitor fluoxetine (Greenwood et al., 2008). The effects of wheel running after shock-elicited fear are robust and suggest that exercise offers stress resilience.
3.1.2.4. Structured threat
Burying in the shock probe test is an active defensive behavior that is increased by stress or anxiogenic manipulations (Lapiz-Bluhm et al., 2008). The advantage of this measure, in contrast with the majority of those discussed above, is that it assesses active as well as passive behavioral responses to aversive stimuli. We observed that wheel runners do not exhibit the increase in burying that sedentary rats display after repeated injection stress or pharmacological stress using the anxiogenic β-carboline FG7142 (Sciolino et al., 2012). Furthermore, the effect of exercise in this paradigm may depend on the level of evoked stress. A high dose of FG7142 (30 mg/kg i.p. × 1 day) produced intense immobility and hindered other defensive behaviors in the shock probe test regardless of whether rats ran on a wheel (see Fig. 1). These findings once again support the model proposed herein that the anxiolytic potential of exercise depends on stress.
3.2. Wheel running improves fear learning
Exercise improves learning and memory and prevents cognitive decline in humans and non-human animals (for review see Dishman et al., 2006; Lista and Sorrentino, 2010). Fear conditioning is associative learning that permits an organism to use relevant cues in the environment to predict threat (for reviews see Ehrlich et al., 2009; Fanselow and Poulos, 2005; LeDoux, 2003; McNally and Westbrook, 2006). In the context of this review, it is relevant to evaluate whether the effects of exercise on fear drive or produce the enhancement of aversively-motivated learning. Collectively, the effects of exercise on aversively-motivated learning are separable from fear/anxiety-relevant behaviors. Therefore, we conclude that wheel running enhances fear conditioning across paradigms through learning and memory (see Table 3), and not fear processes per se.
Table 3.
Summary of the effects of wheel running on fear learning.
| Behavioral test | Exercise improves fear conditioning? |
Wheel access (d) |
Distance ran (km/d) |
Sex | Strain/species | Housed | Sed Ctrl | Reference |
|---|---|---|---|---|---|---|---|---|
| Fear potentiated startle | Y | 14a | 4.5 | M | C57BL/6J | G | L | (Falls et al., 2010) |
| Passive avoidance | Y | 28a | 1b | M | C57BL/6J | G | L | (Samorajski et al., 1985) |
| – | 28a | 2–3c | M | BALB/c | G | L | (Liu et al., 2009) | |
| Fear conditioned freezing | Yd,f and –e | 19a | 2 | M | Long Evans | G | A | (Hopkins and Bucci, 2010b) |
| Yd,g | 21 | 7 | M | Long Evans | S | A | (Van Hoomissen et al., 2004) | |
| Yd,h | 22 | 7 | M | Long Evans | S | A | (Van Hoomissen et al., 2011) | |
| Yd,i | 26 | 6–7 | M | C57Bl6/N | S | A/L | (Dubreucq et al., 2010b) | |
| – d | 28 | 10 | M | C57BL/6NCrl | S | L | (Cacciaglia et al., 2011) | |
| Yd and –e | 30 | 2–5 | M | Long Evans | S | A | (Baruch et al., 2004) | |
| Yd | 42 | 4 | M | Fisher 344 | S | A | (Greenwood et al., 2009) | |
| – e,j | 38–41 | 5 | M | CB1 WT mice | S | L | (Dubreucq et al., 2010a) | |
| – d,e,k | 46 | 6–7 | M | Long Evans | S | nd | (Wojtowicz et al., 2008) | |
| Yd,l | 54 | 6 | M, F | C57BL6/J | S | A | (Clark et al., 2008) | |
| – d | 56 | 6 | M | Sprague | S | L | (Burghardt et al., 2004) | |
| Yd,m | 56 | 8 | M | Sprague | S | L | (Burghardt et al., 2006) | |
| – d,e | 60a | Nd | F | C57BL6/J | G | L | (Pietropaolo et al., 2006) |
Abbreviations:–, did not alter fear conditioning; A, absent wheel controls; CB1, cannabinoid type I receptor; F, female; G, group housing; L, locked wheel controls; M, male; N, impaired fear conditioning; nd, no data; S, single housing; Sed Ctrl, sedentary controls; WT, wild type; Y, improved fear conditioning. Footnotes:
wheel was shared;
km/hour;
km/12 hour;
conditioning to context;
conditioning to cue;
effect reversed when testing occurred in PM or end of light portion of the light:dark cycle;
effect abolished by non-selective β-adrenergic receptor blocker propranolol;
effect was reversed by olfactory bulbectomy;
effect present only when compared to no wheel controls, not locked wheel controls;
improved deficits of CB1 receptor knockout mice;
trended towards improving fear conditioning, wherein time spent freezing was positively correlated with the number of cells expressing the young neuron marker PSA-NCAM in the dentate gyrus;
effect was not dependent on hippocampal irradiation;
effect present only after high, but not low running. Notes: Wheel access is reported at behavioral testing. Distance ran is reported as the mean at behavioral testing and was divided by the number of subjects per cage when the wheel was shared.
Converging evidence suggest that rodents with a history of wheel running exhibit improved aversively-motivated learning in a contextual fear conditioning paradigm, as assessed by increased freezing to a context that was previously paired with shock (Baruch et al., 2004; Burghardt et al., 2006; Clark et al., 2008; Dubreucq et al., 2010a; Dubreucq et al., 2010b; Greenwood et al., 2009; Hopkins and Bucci, 2010b; Van Hoomissen et al., 2011, 2004). Enhanced contextual fear conditioning occurs across wheel running durations that range from ~2 to 8 weeks, which suggest that the learning effects of exercise are long-lasting. However, exercise-induced adaptations may need to be established prior to contextual fear conditioning, as wheel running (1, 4, or 6 weeks) did not alter freezing to the shock-paired context if it occurred after fear-conditioning (Cacciaglia et al., 2011; Greenwood et al., 2009). Further, wheel running (1 or 6 weeks) did not alter extinction of fear-conditioned freezing, regardless of whether running was pre- or post-fear conditioning (Greenwood et al., 2009). Van Hoomissen and colleagues proposed that wheel running alters the speed of memory retrieval comparable to exercise-training in humans (Smith et al., 2010), as running selectively increased freezing to context in the beginning of the fear conditioning test (Van Hoomissen et al., 2011, 2004).
A minority of reports did not generate an enhancement of contextual fear conditioning after running (Burghardt et al., 2004; Cacciaglia et al., 2011; Pietropaolo et al., 2006; Wojtowicz et al., 2008). Of these Wojtowicz et al. (2008) trended towards demonstrating exercise-induced facilitation of fear conditioning. However, the lack of an effect observed in the other two reports are likely explained by factors previously shown to alter fear conditioning and wheel running, such as the time of testing (Hopkins and Bucci, 2010b) or distance ran (high vs. low running; (Burghardt et al., 2006). Higher amounts of freezing are selectively exhibited in sedentary controls when tests of contextual fear conditioning occur at the beginning relative to the end of the light cycle (Hopkins and Bucci, 2010b). As such, increased freezing in sedentary mice in Pietropaolo et al. (2006) may not be specific to learning because testing was uniquely conducted in the dark of the light:dark cycle, which could preclude detection of enhanced freezing in wheel runners that is indicative of learned fear. The null finding in Burghardt et al. (2004) may be attributed to large individual variation in running, which is supported by subsequent data from the authors showing variation in running concealed gains in contextual fear learning (Burghardt et al., 2006).
Several lines of evidence support the conclusion that wheel running increases fear-conditioned freezing due to associative learning of the shock-context pair. First, wheel running reduced or did not alter freezing to a novel context never paired with shock, but selectively increased freezing to the context paired with shock (Greenwood et al., 2009; Van Hoomissen et al., 2011; Zheng et al., 2006). Second, enhanced contextual freezing after exercise cannot be attributed to confounds in freezing or nociceptive detection/sensitivity because wheel running and sedentary animals exhibit similar pre-conditioning freezing, shock reactivity, and activity burst durations (Baruch et al., 2004; Burghardt et al., 2006; Cacciaglia et al., 2011; Falls et al., 2010; Greenwood et al., 2009; Van Hoomissen et al., 2004). Third, wheel running facilitated learning under non-optimal conditions (e.g., minimal duration of context pre-exposure), and in a manner independent of freezing to a context not paired with an aversive stimulus (Greenwood et al., 2009). Although intrinsic differences in fear may influence fear learning in exercised rodents (Burghardt et al., 2006), the reviewed evidence suggests that it is unlikely that differences in fear per se produce the enhancement of fear learning after exercise. These data suggest that wheel running enhances contextual fear conditioning via learning and memory processes.
Wheel running also enhances fear learning in tests of passive avoidance (see also Liu et al., 2009; Samorajski et al., 1985) and fear-potentiated startle (Falls et al., 2010). Mice given access to a running wheel exhibit enhanced startle amplitude to a tone previously paired with shock relative to sedentary mice (Falls et al., 2010). Wheel running may particularly influence learning and consolidation, as wheel runners exhibit improved fear-potentiated startle when running is restricted to periods most likely to affect learning (2 week before conditioning) or consolidation (2 week after conditioning), but not retrieval or performance (2 week before testing) compared to sedentary counterparts (Falls et al., 2010). However, wheel running does not alter freezing to a tone previously paired with shock (Baruch et al., 2004; Dubreucq et al., 2010a; Hopkins and Bucci, 2010b; Pietropaolo et al., 2006; Wojtowicz et al., 2008). Differences between cued conditioned freezing and other forms of fear conditioning may result from several factors, including the behavioral measure of fear learning (freezing vs. startle), strength of conditioning, or strength of input from different neural regions mediating these responses (e.g., hippocampus, regions of the amygdala, locus coeruleus, dorsal raphe), as previously hypothesized (Burghardt et al., 2006; Falls et al., 2010; Greenwood et al., 2009; Van Hoomissen et al., 2004). Because anxiety is characterized by an inability to inhibit fear responding and a bias to attend to threat-related cues (Garakani et al., 2006; Luyten et al., 2011; for review see Rothbaum and Davis, 2003), it is important for future research to focus on whether wheel running assists in extinguishing learned fear and distinguishing safety signals from threat.
3.3. Conclusions and future directions
Rodents are sensitive to the benefits of voluntary wheel running across tests of anxiety, which supports the utility of rodent models to investigate the mechanisms underlying the benefits of exercise on emotion. The evidence reviewed herein shows a clear benefit of exercise on evoked responding (i.e., after exposure to a stressor or stress-based model of anxiety) and mixed effects for the benefit of exercise on baseline responding in tests of anxiety. Although it remains possible that conflicting evidence of exercise on baseline responding results from variation in behavior across tests of anxiety or laboratories, we identify specific variables that could contribute inconsistent effects in the literature. Further evaluation of the experimental variables (e.g., manipulated stressors, non-manipulated variables that act as stressors) that influence the effects of exercise will be warranted using meta-analysis as research accumulates. Further, wheel running improves fear conditioning through learning and memory processes, which minimizes the possibility that exercise-induced alterations in fear per se drives such learning. In sum, evidence to date suggests that wheel running exerts anxiolytic potential in a manner that depends on stress (Fig. 2).
The important influence of stress in the reviewed data is in line with previous evidence showing that stress is a risk factor for anxiety and comorbid disorders (Cerda et al., 2010; Nugent et al., 2011). The influence of specific types of stress (physical vs. psychological) or intensities of stress (no, mild, moderate, and severe) on exercise outcomes remains to be validated by meta-analytic techniques. Induction of persistent anxiety is an essential design element that is needed to further characterize the ability of exercise to buffer the toll of stressful life events. Thus far, only a few reports investigated the effects of wheel running in an established stress-evoked of anxiety (i.e., chronic mild stress, repeated social stress, maternal deprivation, uncontrollable stress; De Chiara et al., 2010; Maniam and Morris, 2010; Zheng et al., 2006). An extensive review of the advantages and disadvantages of preclinical models of anxiety can be found elsewhere (van der Staay, 2006). Models that are genetically (e.g., High Anxiety Behavior strain, Syracuse strain, serotonin transporter knockouts) and pharmacologically based are particularly well-suited to offer mechanistic insight into the protective effects offered by exercise (Brush, 2003; Jaggi et al., 2011; Kalueff et al., 2010; Neumann et al., 2010; Pego et al., 2010; Wigger et al., 2001). For translational purposes, it will be relevant to explore the biological underpinning of short- and long-access running, as they may have distinct affective consequences (Belke and Garland, 2007).
The absence of a clear dose-response of exercise (intensity, duration, frequency) on anxiety deserves consideration. In humans, a dose-response relationship between exercise and anxiety has yet to be established (Dunn et al., 2001). Similarly, as assessed by correlation in rodents, no reliable association exists between running distance and responding in tests of anxiety, including the elevated plus maze (Burghardt et al., 2004; Pietropaolo et al., 2008), open field (Burghardt et al., 2004; see also Pietropaolo et al., 2008), prepulse inhibition of acoustic startle (Pietropaolo et al., 2008), or shuttle box escape and freezing after uncontrollable stress (Greenwood et al., 2003a). This does not preclude the idea that a dose-response exists for wheel running and anxiety, but forces one to examine whether this response is linear and/or affected by other factors such as stress, reward, attention, or learning.
The reviewed evidence supports the use of wheel running as a tool in the study of exercise and anxiety. Understanding the specific neurobiological mechanisms for exercise-mediated improvements in anxiety should focus on specific behaviors that are well defined operationally. The evidence reviewed above indicates that measures of acoustic startle in fear-potentiated paradigms, defensive burying in the shock probe test, and freezing and shuttle box escape in uncontrollable stress paradigms show particular promise. A symptom-driven approach will show clear links between the neural alterations of wheel running and specific anxiety-relevant behavior. Recognizing that rodent models are limited in their ability to reproduce the collection of symptoms seen in humans with anxiety will minimize anthropomorphic leaps and encourage a coherent understanding of the functional neurobiology underlying exercise.
4. Effects of wheel running on neurotransmission in regions controlling stress and anxiety
Several plausible neural mechanisms have been proposed to mediate the affective consequence of wheel running, including alterations in monoamine (Dishman, 1997; Dunn et al., 1996; Gorton et al., 2010; Greenwood et al., 2003a,b, 2005a,b; Maniam and Morris, 2010; Soares et al., 1999), endocannabinoid (De Chiara et al., 2010), glutamate (Dietrich et al., 2005; Makatsori et al., 2003), GABA (Dishman et al., 1996; Hill et al., 2010), and galanin (Soares et al., 1999; Van Hoomissen et al., 2004) systems. A summary of alterations induced by wheel running in neural circuitry controlling stress and anxiety is provided (see Table 4). Neuroanatomical structures that transmit the stress-protective benefit of wheel running may be elucidated by measures of immediate early gene expression (see Table 5). In particular, wheel running attenuates stress-induced elevations of cFos in stress-responsive circuitry, including the prelimbic and infralimbic cortex, lateral septum, subiculum, bed nucleus of the stria terminalis, periventricular nucleus, preoptic area, dorsal medial hypothalamus, dorsal raphe, cuneiform nucleus, and locus coeruleus (Campeau et al., 2010; Greenwood et al., 2003a,b, 2005a).
Table 4.
Summary of the effects of wheel running on neurotransmission in regions controlling stress and anxiety.
| Cannabinoid (CB) Potentiated reductions in striatal sIPSC, but not sEPSC, frequency that were induced by a cannabinoid agonist (HU210), through presynaptic action and in a manner and dependent on exercise duration (De Chiara et al., 2010); these effects were slowly reversible after discontinuation of running (De Chiara et al., 2010). Potentiated reductions in striatal sIPSC frequency that were induced by the group I metabotropic glutamate receptor agonist S-DHPG in a manner dependent on the CB1 cannabinoid receptors (De Chiara et al., 2010). |
| Dopamine (DA) Dopamine levels were reduced in the Arc, but unchanged in the LC, DR, CeA, BLA, CA1, PVN, PAG, NAc, CPu, and PFC (Dishman et al., 1997; Gorton et al., 2010). DOPAC levels or the ratio of DOPAC to DA levels were not different in the LC, DR, CeA, CA1, Arc, PAG, or PFC whereas only DOPAC/DA levels were reduced in the PVN (Dishman et al., 1997; Soares et al., 1999). |
| Galanin (Gal) Prepro-Gal mRNA was increased in LC (Holmes et al., 2006; Van Hoomissen et al., 2004) and hippocampus (Tong et al., 2001). Prepro-galanin mRNA expression in LC was altered after footshock or chronic pharmacological stress (Sciolino et al., 2012; Soares et al., 1999). |
| Gamma-aminobutyric acid (GABA) GAD67 levels were regionally increased (CA1-3, DG, BNST, motor cortex, NAc core) or decreased (Pir), but unaltered in the PL, IL, sensory cortex, NAc shell, CPu, LS, and amygdala (Hill et al., 2010). GABA levels were unaltered in striatum (Dishman et al., 1996). GABAA receptor density was reduced in striatum (Dishman et al., 1996). Downregulated gene expression of GABAA and glutamate decarboxylase GAD65 in the hippocampus (Molteni et al., 2002). mRNA for GABAA receptor subunits were increased in hippocampal CA1 (α5, β1), CA2 (α5, β1, δ), CA3 (α5), and DG (α5, β1) (Hill et al., 2010). mRNA for select GABAA receptor subunits were reduced in PL (β3), Pir(β3, γ2), IL(α2), NAc core and shell (α.2), CPu (α2), LS (α.2), BNST (γ2), PVN (α.2), and CA3 (α2) (Hill et al., 2010). mRNA for select GABAA receptor subunits were not different in the BLA and CeA (α2, β3, γ2) or sensory cortex (α2, β3) (Hill et al., 2010). |
| Glutamate (Glu) AMPA GluR1 mRNA was decreased and increased in VTA after 1 and 23 d of exercise, respectively (Makatsori et al., 2003). AMPA receptor (GluR1, GluR2/3) and Glu receptor anchoring protein (SAP-97, GRIP-1, PSD-95) immunocontent was increased in cortical postsynaptic density, whereas immunocontent for kainite (GluR6/7) and NMDA receptors (Dietrich et al., 2005) was not altered. NMDA receptor subunit NR1 was unaltered in VTA (Makatsori et al., 2003). Phosphorlyated NMDA subunits (phosphor-NMDAR1, NMDAR2B) and binding of the NMDA receptor antagonist MK801 were increased in cortical postsynaptic densities (Dietrich et al., 2005). Unregulated gene expression of NMDAR2A and NMDAR2B in the hippocampus (Molteni et al., 2002). |
| Norepinephrine (NE) Reduced the number of cFos immunoreactive cells after uncontrollable stress that were colocalized with tyrosine hydroxylase in the LC, A5 cell group, and rostral ventrolateral medulla (Greenwood et al., 2003b). TH mRNA in LC was unaltered (Soares et al., 1999). NE levels were increased in spinal cord and pons medulla (Dunn et al., 1996) and LC and DR (Dishman et al., 1997), which was correlated with increased freezing in contextual fear conditioning. NE levels were no different in the CeA, hippocampus, Arc, PVN, and PAG after footshock (Dishman et al., 1997). Reduced footshock-induced increases in NE levels in the PFC (Soares et al., 1999). MHPG and DHPG levels were unaltered in spinal cord, pons medulla, hippocampus, & frontal cortex (Dunn et al., 1996). α1B mRNA was increased in DRN regions, not in the MR, depending on exercise length; this effect was not correlated with distance ran (Greenwood et al., 2005b). α2 receptor mRNA was unaltered in the locus coeruleus (Greenwood and Fleshner, 2008). β adrenergic BMAX (lower receptor number) and Kd (enhanced affinity/sensitivity) were decreased in the frontal cortex at baseline, but these effects were reversed after footshock (Yoo et al., 1999). |
| Serotonin (5-HT) 5-HT levels were reduced in the CeA, but unchanged in the LC, DR, CA1, Arc, PVN, PAG, NAc, CPu, PFC, and BLA (Dishman et al., 1997; Gorton et al., 2010). Attenuated tail shock-induced activity of 5-HT neurons in the rostral-mid DRN in a manner dependent on the duration of exercise (Greenwood et al., 2003a, 2005a). 5-HIAA levels were reduced in the CeA, but not different in the LC, DR, Arc, PVN, and PAG after both uncontrollable and controllable stress (Dishman et al., 1997). 5-HIAA levels were reduced in CA1 only after uncontrollable stress (Dishman et al., 1997). Ratio of 5-HIAA to 5-HT levels were reduced in PVN, but not different in the LC, DR, CeA, CA1, Arc, and PAG (Dishman et al., 1997). 5-HT transporter mRNA in MR and DRN subregions was reduced; this effect was not correlated with distance ran (Greenwood et al., 2005b). 5-HT1A receptor mRNA was increased in the MR and in subregions of the dorsal and lateral DRN in a manner that was dependent on exercise length, whereas 5-HT1A receptor mRNA was not altered in ventral DRN subregions; these effects were not correlated with distance ran (Greenwood et al., 2003a, 2005b). Reversed maternal deprivation induced reductions in 5-HT1A mRNA in hippocampus (Maniam and Morris, 2010). 5-HT1B receptor mRNA was reduced in select ventral DRN subregions in a manner dependent on exercise length, whereas 5-HT1B receptor mRNA was not changed in the MR or dorsal/lateral DRN; this effect was not correlated with distance ran (Greenwood et al., 2005b). |
Abbreviations: 5-HIAA, 5-hydroxyindoleacetic acid; AMPA, alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate; Arc, arcuate; BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CeA, central amygdala; CPu, caudate putamen; DG, dentate gyrus; DHPG, 3, 4-dihydroxyphenylglycol; S-DHPG,S-3,5-dihydroxyphenylglycine; DOPAC, 3,4-dihydroxyphenylacetic acid; DRN, dorsal raphe nucleus; GAD67, glutamic acid decarboxylase; IL, infralimbic cortex; LC, locus coeruleus; LS, lateral septum; MHPG, 3-methoxy-4-hydroxyphenylglycol; MR, median raphe; NAc, nucleus accumbens; NMDA, N-methyl-D-aspartate; PAG, periaqueductal gray; PFC, prefrontal cortex; Pir, piriform cortex; PL, prelimbic cortex; PVN, paraventricular nucleus; sEPSC, spontaneous excitatory postsynaptic current; sIPSC, spontaneous inhibitory post synaptic current; SubC, subcoeruleus; TH, tyrosine hydroxylase; VLM, ventral lateral medulla; VTA, ventral tegmental area.
Table 5.
Summary of the effects of wheel running on immediate early gene expression in brain regions controlling stress and anxiety.
| Altered Fos immunoreactivity in a region dependent manner by increasing Fos in the CeA and DG and decreasing Fos in the BLA, without changing this measure in CA1 or CA3; these effects were not colocalized with enkephalin or parvalbumin (Burghardt et al., 2006). |
| Did not alter the amount of cFos mRNA in LC (Soares et al., 1999), the number of cFos immunoreactive neurons in the NAc or PVN (Collins et al., 2009), nor the amount of cFos immunoreactivity in the A7 region, subcoeruleus, CeA, BLA, or lateral habenula (Greenwood et al., 2003b, 2005a). |
| Increased cFos immunoreactivity in the DG, but not CA1 or CA3 (Fuss et al., 2010a). |
| Reduced cFos mRNA in PVN after a saline injection (Campeau et al., 2010). |
| Regionally attenuated footshock stress induced increases in cFos expressing cells/immunoreactivity in DRN, PVN, Bar, LC, A5, ventral medial and lateral medulla, and caudal raphe nucleus (Greenwood et al., 2003a,b, 2005a). Regionally influenced the expression of cFos immunoreactivity in the BNST in a manner that was dependent on the duration of wheel running (Greenwood et al., 2005a). |
| Attenuated auditory stress induced increases in cFos mRNA in PVN, rostral and ventral LS, antereoventral area of anterior BNST, medial POA, DM, Me, ventral subiculum, DR, CnF, PL, and IL (Campeau et al., 2010). |
| Augmented both novelty and forced swim induced increases in the number of cFos immunoreactive neurons in the DG (Collins et al., 2009). |
Abbreviations: Bar, Barrington’s nucleus; BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CeA, central amygdala; CnF, cuneiform nucleus; DG, dentate gyrus; DM, dorsal medial hypothalamic nucleus; DRN, dorsal raphe nucleus; IL, infralimbic cortex; LC, locus coeruleus; LS, lateral septal nucleus; Me, medial amygdaloid nucleus; mRNA, messenger ribonucleic acid; NAc, nucleus accumbens; PL, prelimbic cortex; POA, preoptic area; PVN, paraventricular nucleus; SubC, subcoeruleus. Note that most effects from Campeau et al. (2010) were seen after intermittent and continuous exercise.
Although many mechanisms have been proposed, only a single report to our knowledge provides causal evidence for the effects of exercise on anxiety. For example, exercise-induced alterations in anxiety-like behavior in the open field, dark:light test, and elevated O-maze were reversed by blockade of hippocampal neurogenesis (Fuss et al., 2010a). In contrast, irradiation of the hippocampus did not block the enhancement of contextual fear conditioning that was exhibited in runners, which suggests that intact hippocampal neurogenesis is not required to mediate fear learning (Clark et al., 2008). The enhancement of contextual fear conditioning after wheel running was prevented by either chronic administration of the non-selective β-adrenergic receptor blocker propranolol (Van Hoomissen et al., 2004) or removal of cortical afferents to basal limbic structures (Van Hoomissen et al., 2011). These studies bolster the idea that enhanced fear learning after exercise is likely attributed to improved learning capacity and not fear per se. Although beyond the scope of the present review, it is important to point out that voluntary exercise also exerts stress resilience through neuroendocrine (Stranahan et al., 2008), neuroimmune (Fleshner, 2005), and neuroplastic and neuroprotective (Cotman and Engesser-Cesar, 2002; Stranahan et al., 2009) mechanisms, as reviewed in detail elsewhere. For a comprehensive discussion, the remainder of this review will focus on evidence showing that wheel running alters norepinephrine and galanin systems in circuitry controlling stress and anxiety, as other well-supported mechanisms of exercise are reviewed elsewhere (i.e., serotonergic; Greenwood and Fleshner, 2008, 2011). We propose that a noradrenergic-galaninergic mechanism plays an important role in conferring the stress buffering capacity of exercise. To support this hypothesis, it is important to briefly review how alterations in norepinephrine and galanin systems contribute to anxiety in a manner dependent on stress.
4.1. Norepinephrine and galanin systems control anxiety-like responding in a manner dependent on stress
Norepinephrine is implicated in the pathogenesis of anxiety, and similarly drugs that target the norepinephrine system (i.e., norepinephrine serotonin reuptake inhibitors) are currently a popular first-line of therapy for anxiety (Hoffman and Mathew, 2008; Kalk et al., 2011). Norepinephrine putatively influences anxiety in a manner that depends on conditions of stress (Goddard et al., 2010). Norepinephrine can induce both anxiogenic and anxiolytic effects, and therefore a balanced noradrenergic tone produces appropriate vigilance and stressor responsiveness. Norepinephrine is a neuromodulatory transmitter that likely influences anxiety by optimizing excitatory and inhibitory tone in the brain via action at adrenergic α1, α2, and β receptor subtypes (Goddard et al., 2010; Morilak et al., 2005). The ascending noradrenergic system contains cell bodies in the medulla and pons that project throughout the brain (Moore and Card, 1984). The locus coeruleus is an important noradrenergic nucleus that projects to numerous other regions that regulate stress and anxiety, including the frontal and cingulate cortices, amygdala, olfactory bulb, septum, hippocampus, hypothalamus, periaquiductal gray, and raphe nuclei (Aston-Jones, 2004; Dahlstroem and Fuxe, 1964; Itoi, 2008).
The majority of norepinephrine neurons in the locus coeruleus also contain the peptide/trophic factor galanin (Holets et al., 1988; Holmes and Crawley, 1995; Melander et al., 1986; Skofitsch and Jacobowitz, 1985). Under conditions of high activation, amperometric experiments show that the soma of locus coeruleus neurons release norepinephrine from large dense core vesicles (Huang et al., 2007), which presumably co-contain the peptide galanin. In situ hybridization evaluated at the light and electron microscope level coupled with tannic-acid experiments show that galanin is synthesized and released in large dense-core vesicles from dendrites in the locus coeruleus (Vila-Porcile et al., 2009). Galanin acts on at least three G-protein coupled galanin receptors in the brain noted as GalR1-3, all of which are present in the locus coeruleus, and are differentially expressed in midbrain and limbic structures that respond to stress (Burazin et al., 2000; Hawes and Picciotto, 2004; Hohmann et al., 2003; Kolakowski et al., 1998; Mennicken et al., 2002; O’Donnell et al., 1999; Pang et al., 1998; Wang et al., 1997; Waters and Krause, 2000). Activation of galanin receptors is predominantly inhibitory and induces hyperpolarization/outward current by increasing K+ or decreasing Ca++ conductance (for review see Xu et al., 2005). Electrophysiological data show that galanin inhibits activity of the locus coeruleus in brainstem slice preparations of the rat (Pieribone et al., 1995; Seutin et al., 1989; Sevcik et al., 1993; Xu et al., 2001). Collectively, these data support an auto-inhibitory role of galanin on locus coeruleus neurons.
Stress and rodent models of pathology alter prepro-galanin expression and galanin receptor density in the locus coeruleus as well as the amygdala and hypothalamus (Holmes et al., 1995; Holmes and Crawley, 1996; Makino et al., 1999; O’Neal et al., 2001; Sweerts et al., 1999, 2000). Additionally, polymorphisms in the prepro-galanin promoter are associated with the severity of symptoms in women with anxiety disorders (Unschuld et al., 2008; Unschuld et al., 2010). Both galanin and the non-selective galanin receptor agonist galnon dose-dependently reduce anxiety-like behavior in several rodent assays when administered systemically or intracerebroventricularly (Bing et al., 1993; Rajarao et al., 2007). Whereas a non-specific galanin receptor antagonist like M35 or M40 blocks these effects or produces the opposite effect by increasing anxiety-like behavior (Lyudyno et al., 2008; Rajarao et al., 2007). Interestingly, systemic administration of the selective GalR3 receptor antagonist SNAP37889 or, SNAP398299 produced anxiolytic-like behavior in several rodent assays (Swanson et al., 2005), which suggests that galanin receptor subtypes each uniquely influence anxiety. Although galanin modulates anxiety, the effects of galanin may depend on the brain region of interest, test used to measure anxiety, and the degree of stress the animal experiences (for review see Barrera et al., 2005; Holmes and Picciotto, 2006; Rotzinger et al., 2010).
Accumulated evidence suggests that the anxiety-related effects of galanin are specifically evoked under conditions of high stress or norepinephrine activity. For example, galanin administered intracerebroventricularly failed to alter anxiety-like behavior under non-stressed conditions in C57BL/6J mice in tests of exploration (Karlsson et al., 2005). Administration of the galanin receptor antagonist M40 in the central nucleus of the amygdala was unable to alter behavior in rodents tested for baseline responding in tests of anxiety or evoked responding after experimental exposure to acute restraint stress or the α2 adrenergic receptor antagonist yohimbine (Khoshbouei et al., 2002a). However, restraint stress combined with yohimbine was necessary to increase galanin levels in the central nucleus of the amygdala, and only under these conditions did M40 block the effect of stress on anxiety-like behavior (Khoshbouei et al., 2002a). M40 injected into the lateral septum or bed nucleus of the stria terminalis blocked anxiety-like behaviors in the shock probe defensive burying, elevated plus maze, and social interaction tests in rats that were exposed to an electrified shock prod or restraint stress (Echevarria et al., 2005; Khoshbouei et al., 2002b). Further, transgenic mice that overexpress galanin in norepinephrine and epinephrine-synthesizing neurons exhibit normal behaviors in an array of tests of anxiety under baseline conditions, but after exposure to yohimbine stress exhibit an anxiolytic-like behavior in the dark:light task compared to wild type controls (Holmes et al., 2002). These data support the conclusion that enhanced galanin drive in noradrenergic circuitry is induced by stress to dampen enhanced noradrenergic drive. It is notable that the anxiolytic potential of galanin and wheel running bear a striking similarity: both manipulations do not reliably alter baseline levels of anxiety, yet consistently reduce stress-evoked responding in tests of anxiety-like behavior. This parallels data presented in the first part of the review and highlights the interesting possibility that wheel running exerts anxiolytic-like potential via neural mechanisms that involve interaction between the norepinephrine and galanin systems.
4.2. A proposed noradrenergic-galaninergic brain mechanism underlying the stress protective effects of wheel running
Research from our laboratory and a few others show that wheel running regulates norepinephrine activity. Suggesting that chronic exercise inhibits noradrenergic locus coeruleus neurons, rats allowed regular access to a running wheel exhibit reduced expression of cFos in locus coeruleus neurons that express tyrosine hydroxylase immunoreactivity after uncontrollable stress (Greenwood et al., 2003b). In vivo microdialysis data show that wheel running dampens the enhanced norepinephrine release after footshock stress in the frontal cortex (Soares et al., 1999). As the cortex receives norepinephrine terminals exclusively from the locus coeruleus (Jones and Moore, 1977; Mason and Fibiger, 1979; Ungerstedt, 1971), it is reasonable to assume that such changes after exercise result from alterations in locus coeruleus projection terminals. However, runners were not different from sedentary counterparts in the expression of the rate-limiting norepinephrine synthetic enzyme tyrosine hydroxylase in the locus coeruleus or other regions like the subcoeruleus, A5, or ventral lateral medulla (Greenwood et al., 2003b; Murray et al., 2010; O’Neal et al., 2001; Soares et al., 1999), which supports an important role of galanin in mediating the effects of exercise on the locus coeruleus. Wheel running also failed to alter mRNA expression for the α2 adrenergic autoreceptor in the locus coeruleus after six weeks of running (Greenwood and Fleshner, 2008). The lack of such an effect does not preclude the possibility that wheel running constrains locus coeruleus noradrenergic activity via this autoreceptor, as alterations in locus coeruleus adrenergic α2 receptors may occur after transcription (e.g., increased α2 adrenergic receptor affinity and/or expression of this protein after wheel running). However, it does suggest that exercise-induced inhibition of locus coeruleus activity may involve other, non-noradrenergic mechanisms. Further, wheel running increased β adrenergic receptor binding in the frontal cortex after footshock stress, yet the opposite effect was seen in the absence of an experimental stressor (Yoo et al., 1999). These data show that wheel running alters noradrenergic signaling in neural circuits that are sensitive to stress and support the possibility that wheel running exerts anxiolytic-like potential through use of the neuromodulator galanin in these circuits.
We have repeatedly observed that galanin expression is augmented in the locus coeruleus by wheel running. Rats that ran on a wheel for 3–4 weeks exhibited increased density of prepro-galanin mRNA in the locus coeruleus relative to sedentary rats (Holmes et al., 2006; Murray et al., 2010; Reiss et al., 2009; Sciolino et al., 2012; Van Hoomissen et al., 2004). It is interesting to note that both voluntary and forced exercise increase prepro-galanin mRNA in the locus coeruleus (O’Neal et al., 2001). Supporting the functional relevance of these data, plasma galanin secretion is also increased after an acute bout of exercise in humans (Legakis et al., 2000). Increases in galanin expression in the locus coeruleus is correlated with increases in the distance ran on a wheel, suggesting a dose-dependent effect of exercise on brain galanin (Eisenstein and Holmes, 2007; Holmes et al., 2006; Sciolino et al., 2012). Galanin expression in the locus coeruleus is also increased by acute and chronic stress (Holmes et al., 1995; Kuteeva et al., 2008), an animal model of depression (Holmes and Crawley, 1996), psychotherapeutic treatment (Austin et al., 1990; Holmes et al., 2006; Kadowaki and Emson, 1992), and opiate administration and withdrawal (Holmes et al., 2011), which suggests that galanin is recruited as a counter-regulatory mechanism to dampen noradrenergic tone. Elevated galanin levels after exercise may remain elevated after stressor exposure, as exercised rats that were exposed to shock exhibited increased prepro-galanin mRNA in the locus coeruleus relative to sedentary rats (Soares et al., 1999). These data collectively show that wheel running increases galanin in noradrenergic brain circuits that are sensitive to stress.
We propose that enhanced galanin resulting from wheel running constrains activation of locus coeruleus neurons during stress to beneficially alter anxiety-like behavior (see Fig. 3). Specifically, we suspect that enhanced galanin signal after exercise acts at somatic or somatodendritic galanin receptors in the locus coeruleus to dampen norepinephrine release to target areas that influence anxiety, including the frontal cortex and amygdala. Indeed, in vitro data shows that galanin inhibits the firing rate of locus coeruleus mainly through somatodendritic GalR1 and GalR3 receptors (Ma et al., 2001; Pieribone et al., 1995), and to a lesser extent GalR2 (Hawes and Picciotto, 2004; Lu et al., 2005; Ma et al., 2001; O’Donnell et al., 1999). Wheel running may also exert anxiolytic potential via galanin-mediated inhibition of norepinephrine neurons in regions downstream to the locus coeruleus. The fact that wheel running induced galanin gene expression in the hippocampus of rats supports the idea that multiple levels of the neural axis mediate the anxiolytic-like potential of wheel running (Tong et al., 2001). Indeed, presynaptic GalR1 and GalR2 receptors are expected to modulate release of norepinephrine in forebrain regions, including the hippocampus and cortex (Ma et al., 2001; Xu et al., 1998; Yoshitake et al., 2004). Wheel running alters norepinephrine levels and increases mRNA expression for the α1b adrenergic receptor in the dorsal raphe nucleus in a time-dependent manner compared to sedentary conditions (Dishman et al., 1997; Greenwood et al., 2005b), which lends further evidence to conclude that wheel running-induced adaptations are mediated via noradrenergic signaling in target regions of the locus coeruleus. These results demonstrate that wheel running alters norepinephrine and galanin systems in neural circuits that are sensitive to stress, but the functional significance is yet to be elucidated.
Fig. 3.
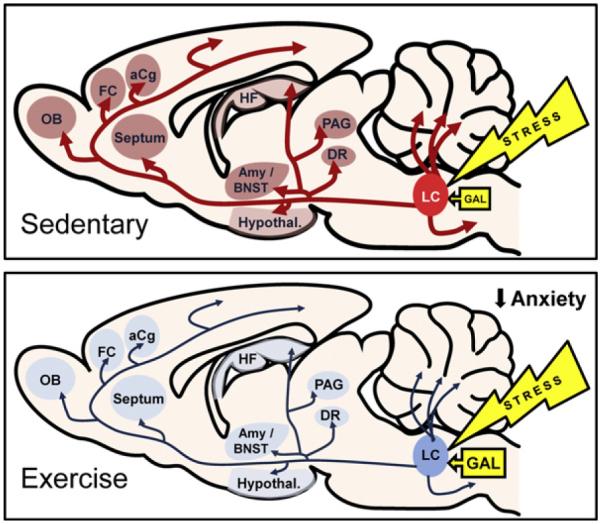
A stress-based neural model of functional noradrenergic circuitry that is implicated in mediating the anxiolytic-like potential of wheel running. Stress increases norepineprhine output from the locus coeruleus to brain circuitry controlling anxiety-like behavior in sedentary rodents. However, rodents given repeated access to a running wheel exhibit increased expression of galanin, a peptide colocalized with norepineprhine in the locus coeruleus relative to sedentary controls. Therefore, we propose that enhanced galanin-mediated suppression of noradrenergic output from the locus coeruleus in wheel runners is a mechanism that can account for the attenuation of anxiety-like behavior after stressor exposure. Abbreviations: aCg, anterior cingulate; Amy, amygdala; BNST, bed nucleus of the stria terminalis; DR, dorsal raphe; FC, frontal cortex; GAL, galanin; HF, hippocampal formation; Hypothal., hypothalamus; LC, locus coeruleus; OB, olfactory bulb; PAG, periaquiductal gray area.
It is also possible that the anxiolytic potential of wheel running is influenced by galanin-mediated alterations in 5-HT. The dorsal raphe may be an especially important site for serotonergic-galaninergic interactions after wheel running, because galanin is synthesized in these neurons (Melander et al., 1986). The functional consequences of exercise on the novel receptor combinations of GalR1 receptor homodimers (Wirz et al., 2005) and heterodimers with monoamine receptors (Borroto-Escuela et al., 2010; Diaz-Cabiale et al., 2010) in regions controlling stress remains to be explored. Future studies should establish whether the anxiolytic potential of wheel running can be attenuated in a manner that is necessary and/or sufficient for brain galanin and whether such effects are time-course specific. Experiments elucidating the anxiolytic potential of wheel running could utilize selective galanin receptor ligands, galanin receptor knockout mice, and galanin overexpressing mice (e.g., driven by a dopamine β-hydroxylase promoter).
5. Final remarks
The evidence reveals that voluntary wheel running offers anxiolytic potential and stress resiliency. Specific neural mechanisms that underlie such benefits are offered. Wheel running increases galanin in norepinephrine systems that are sensitive to stress. Precisely how wheel running affects interactions between the brain galanin and norepinephrine systems to alter anxiety is under speculation, but alterations likely occur at multiple levels of stress responsive circuitry. We hypothesize that the impact of exercise on galanin in the locus coeruleus is particularly relevant for noradrenergic output to stress responsive targets and subsequent anxiety-like behavior. Candidate norepinephrine and galanin receptor subtypes that transmit the beneficial effect of wheel running are elucidated based on functional neuroanatomical evidence and descriptive reports of wheel running on norepinephrine and galanin neurotransmitter systems. Evidence to date support the use of rodent models to investigate these and other neural mechanisms underlying the emotional consequences of exercise.
Acknowledgements
This research was supported by National Institute of Drug Abuse (NIDA) grant DA027535A (to Philip V. Holmes) and NIDA Diversity Supplement (to DA027535AS1 for Natale R. Sciolino).
References
- Abu-Omar K, Rutten A, Lehtinen V. Mental health and physical activity in the European Union. Soz Praventivmed. 2004;49:301–309. doi: 10.1007/s00038-004-3109-8. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. IVR Washington, D.C.: 2000. [Google Scholar]
- Arakawa H. Interaction between isolation rearing and social development on exploratory behavior in male rats. Behavioural Processes. 2005;70:223–234. doi: 10.1016/j.beproc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Archer J. Behavioural aspects of fear. In: Stuckin W, editor. Fear in Animals and Man. Van Nostrand Reinhold; New York: 1979. pp. 56–85. [Google Scholar]
- Aston-Jones G. Locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The Rat Nervous System. third Elsevier Academic Press; Amsterdam, Boston: 2004. pp. 259–294. [Google Scholar]
- Austin MC, Cottingham SL, Paul SM, Crawley JN. Tyrosine hydroxylase and galanin mRNA levels in locus coeruleus neurons are increased following reserpine administration. Synapse. 1990;6:351–357. doi: 10.1002/syn.890060407. [DOI] [PubMed] [Google Scholar]
- Barrera G, Echevarria DJ, Poulin J-F, Laforest S, Drolet G, Morilak DA. One for all or one for one: does co-transmission unify the concept of a brain galanin system or clarify any consistent role in anxiety? Neuropeptides. 2005;39:289–292. doi: 10.1016/j.npep.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behavioral Neuroscience. 2004;118:1123–1127. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- Belke TW, Garland T., Jr. A brief opportunity to run does not function as a reinforcer for mice selected for high daily wheel-running rates. Journal of the Experimental Analysis of Behaviour. 2007;88:199–213. doi: 10.1901/jeab.2007.62-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW, Wagner JP. The reinforcing property and the rewarding after-effect of wheel running in rats: a combination of two paradigms. Behavioural Processes. 2005;68:165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Belzung C, Philippot P. Anxiety from a phylogenetic perspective: is there a qualitative difference between human and animal anxiety? Neural Plasticity. 2007:59676. doi: 10.1155/2007/59676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibeau WS, Moore JB, Mitchell NG, Vargas-Tonsing T, Bartholomew JB. Effects of acute resistance training of different intensities and rest periods on anxiety and affect. Journal of Strength & Conditioning Research. 2010;24:2184–2191. doi: 10.1519/JSC.0b013e3181ae794b. [DOI] [PubMed] [Google Scholar]
- Binder E, Droste SK, Ohl F, Reul JM. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behavioral Brain Research. 2004;155:197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Bing O, Moller C, Engel JA, Soderpalm B, Heilig M. Anxiolytic-like action of centrally administered galanin. Neuroscience Letters. 1993;164:17–20. doi: 10.1016/0304-3940(93)90846-d. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neuroscience and Biobehavioral Reviews. 2001a;25:205–218. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Hynd AL, Minke KA, Minemoto T, Blanchard RJ. Human defensive behaviors to threat scenarios show parallels to fear- and anxiety-related defense patterns of non-human mammals. Neuroscience and Biobehavioral Reviews. 2001b;25:761–770. doi: 10.1016/s0149-7634(01)00056-2. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Bringing natural behaviors into the laboratory: a tribute to Paul MacLean. Physiology and Behavior. 2003;79:515–524. doi: 10.1016/s0031-9384(03)00157-4. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Kelley MJ, Blanchard DC. Defensive reactions and exploratory behavior in rats. Journal of Comparative and Physiological Psychology. 1974;87:1129–1133. [Google Scholar]
- Borroto-Escuela DO, Narvaez M, Marcellino D, Parrado C, Narvaez JA, Tarakanov AO, Agnati LF, Diaz-Cabiale Z, Fuxe K. Galanin receptor-1 modulates 5-hydroxtryptamine-1A signaling via heterodimerization. Biochemical and Biophysical Research Communications. 2010;393:767–772. doi: 10.1016/j.bbrc.2010.02.078. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berlin) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braun AA, Skelton MR, Vorhees CV, Williams MT. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague-Dawley rats: effects of anxiolytic and anxiogenic agents. Pharmacology Biochemistry and Behavior. 2011;97:406–415. doi: 10.1016/j.pbb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brick J, Pohorecky LA, Faulkner W, Adams MN. Circadian variations in behavioral and biological sensitivity to ethanol. Alcoholism: Clinical & Experimental Research. 1984;8:204–211. doi: 10.1111/j.1530-0277.1984.tb05840.x. [DOI] [PubMed] [Google Scholar]
- Broadhurst PL. Emotionality and the Yerkes-Dodson Law. Journal of Experimental Psychology. 1957;54:345–352. doi: 10.1037/h0049114. [DOI] [PubMed] [Google Scholar]
- Brocardo PS, Boehme F, Patten A, Cox A, Gil-Mohapel J, Christie BR. Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exercise. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Brush FR. Selection for differences in avoidance learning: the Syracuse strains differ in anxiety, not learning ability. Behavior Genetics. 2003;33:677–696. doi: 10.1023/a:1026135231594. [DOI] [PubMed] [Google Scholar]
- Burazin TC, Larm JA, Ryan MC, Gundlach AL. Galanin-R1 and -R2 receptor mRNA expression during the development of rat brain suggests differential subtype involvement in synaptic transmission and plasticity. European Journal of Neuroscience. 2000;12:2901–2917. doi: 10.1046/j.1460-9568.2000.00184.x. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Research. 2004;1019:84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Pasumarthi RK, Wilson MA, Fadel J. Alterations in fear conditioning and amygdalar activation following chronic wheel running in rats. Pharmacology Biochemistry and Behavior. 2006;84:306–312. doi: 10.1016/j.pbb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Cacciaglia R, Krause-Utz A, Vogt MA, Schmahl C, Flor H, Gass P. Voluntary exercise does not ameliorate context memory and hyperarousal in a mouse model for post-traumatic stress disorder (PTSD) World Journal of Biological Psychiatry. 2011 doi: 10.3109/15622975.2011.583270. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. An assessment of anxiolytic drug screening tests: hormetic dose responses predominate. Critical Reviews in Toxicology. 2008;38:489–542. doi: 10.1080/10408440802014238. [DOI] [PubMed] [Google Scholar]
- Campeau S, Nyhuis TJ, Sasse SK, Kryskow EM, Herlihy L, Masini CV, Babb JA, Greenwood BN, Fleshner M, Day HE. Hypothalamic pituitary adrenal axis responses to low-intensity stressors are reduced after voluntary wheel running in rats. Journal of Neuroendocrinology. 2010;22:872–888. doi: 10.1111/j.1365-2826.2010.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. International Journal of Psychiatry in Medicine. 2011;41:15–28. doi: 10.2190/PM.41.1.c. [DOI] [PubMed] [Google Scholar]
- Casarrubea M, Sorbera F, Magnusson MS, Crescimanno G. T-pattern analysis of diazepam-induced modifications on the temporal organization of rat behavioral response to anxiety in hole board. Psychopharmacology (Berlin) 2011;215:177–189. doi: 10.1007/s00213-010-2123-1. [DOI] [PubMed] [Google Scholar]
- Cerda M, Sagdeo A, Johnson J, Galea S. Genetic and environmental influences on psychiatric comorbidity: a systematic review. Journal of Affective Disorders. 2010;126:14–38. doi: 10.1016/j.jad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot CC, Taylor DH. Circadian modulation of the rat acoustic startle response. Behavioral Neuroscience. 1992a;106:846–852. doi: 10.1037//0735-7044.106.5.846. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Taylor DH. Daily rhythmicity of the rat acoustic startle response. Physiology & Behavior. 1992b;51:885–889. doi: 10.1016/0031-9384(92)90131-k. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6 J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Collins A, Hill LE, Chandramohan Y, Whitcomb D, Droste SK, Reul JM. Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLoS One. 2009;4:e4330. doi: 10.1371/journal.pone.0004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS. Anxiety outcomes after physical activity interventions: meta-analysis findings. Nursing Research. 2010;59:224–231. doi: 10.1097/NNR.0b013e3181dbb2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exercise and Sport Sciences Reviews. 2002;30:75–79. doi: 10.1097/00003677-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacology Biochemistry and Behavior. 1981;15:695–699. doi: 10.1016/0091-3057(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Exploratory behavior models of anxiety in mice. Neuroscience & Biobehavioral Reviews. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience & Biobehavioral Reviews. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Dahlstroem A, Fuxe K. Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. ACTA Physiologica Scandinavica Supplement. 1964;(SUPPL 232):231–255. [PubMed] [Google Scholar]
- Davidson JR. First-line pharmacotherapy approaches for generalized anxiety disorder. Journal of Clinical Psychiatry. 2009;70(Suppl. 2):25–31. doi: 10.4088/jcp.s.7002.05. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Tricklebank MD. Use of the elevated plus maze in the search for novel anxiolytic agents. Trends in Pharmacological Science. 1995;16:33–36. doi: 10.1016/s0165-6147(00)88973-7. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. European Journal of Pharmacology. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- De Chiara V, Errico F, Musella A, Rossi S, Mataluni G, Sacchetti L, Siracusano A, Castelli M, Cavasinni F, Bernardi G, Usiello A, Centonze D. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology. 2010;35:374–387. doi: 10.1038/npp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Cabiale Z, Parrado C, Narvaez M, Millon C, Puigcerver A, Fuxe K, Narvaez JA. Neurochemical modulation of central cardiovascular control: the integrative role of galanin. EXS. 2010;102:113–131. doi: 10.1007/978-3-0346-0228-0_9. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Mantese CE, Porciuncula LO, Ghisleni G, Vinade L, Souza DO, Portela LV. Exercise affects glutamate receptors in postsynaptic densities from cortical mice brain. Brain Research. 2005;1065:20–25. doi: 10.1016/j.brainres.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Medical Science and Sports Exercise. 1997;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of Exercise. Obesity (Silver Spring) 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Dunn AL, Youngstedt SD, Davis JM, Burgess ML, Wilson SP, Wilson MA. Increased open field locomotion and decreased striatal GABAA binding after activity wheel running. Physiology and Behavior. 1996;60:699–705. doi: 10.1016/0031-9384(96)00102-3. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Renner KJ, Youngstedt SD, Reigle TG, Bunnell BN, Burke KA, Yoo HS, Mougey EH, Meyerhoff JL. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Research Bulletin. 1997;42:399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Developmental Psychobiology. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Droste SK, Chandramohan Y, Hill LE, Linthorst AC, Reul JM. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 2007;86:26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- Droste SK, Gesing A, Ulbricht S, Muller MB, Linthorst AC, Reul JM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144:3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. CB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviours and hippocampal neurogenesis. Experimental Neurology. 2010a;224:106–113. doi: 10.1016/j.expneurol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Marsicano G, Chaouloff F. Emotional consequences of wheel running in mice: Which is the appropriate control? Hippocampus. 2010 doi: 10.1002/hipo.20778. [DOI] [PubMed] [Google Scholar]
- Duley AR, Hillman CH, Coombes S, Janelle CM. Sensorimotor gating and anxiety: prepulse inhibition following acute exercise. International Journal of Psychophysiology. 2007;64:157–164. doi: 10.1016/j.ijpsycho.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Research. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AL, Reigle TG, Youngstedt SD, Armstrong RB, Dishman RK. Brain norepinephrine and metabolites after treadmill training and wheel running in rats. Medical Science and Sports Exercise. 1996;28:204–209. doi: 10.1097/00005768-199602000-00008. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Medical Science and Sports Exercise. 2001;33:S587–S597. doi: 10.1097/00005768-200106001-00027. discussion 609–510. [DOI] [PubMed] [Google Scholar]
- Echevarria DJ, Hernandez A, Diogenes A, Morilak DA. Administration of the galanin antagonist M40 into lateral septum attenuates shock probe defensive burying behavior in rats. Neuropeptides. 2005;39:445–451. doi: 10.1016/j.npep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Kilduff TS, Martin CE, Dement WC. Influence of running wheel activity on free-running sleep/wake and drinking circadian rhythms in mice. Physiology & Behavior. 1991;50:373–378. doi: 10.1016/0031-9384(91)90080-8. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Eisenstein SA, Holmes PV. Chronic and voluntary exercise enhances learning of conditioned place preference to morphine in rats. Pharmacology Biochemistry and Behavior. 2007;86:607–615. doi: 10.1016/j.pbb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Falls WA, Fox JH, MacAulay CM. Voluntary exercise improves both learning and consolidation of cued conditioned fear in C57 mice. Behavioral Brain Research. 2010;207:321–331. doi: 10.1016/j.bbr.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual Review of Psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. Journal of Neuroscience Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- File SE. What can be learned from the effects of benzodiazepines on exploratory behavior? Neuroscience & Biobehavioral Reviews. 1985;9:45–54. doi: 10.1016/0149-7634(85)90031-4. [DOI] [PubMed] [Google Scholar]
- File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behavioral Brain Research. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- File SE, Baldwin HA. Effects of beta-carbolines in animal models of anxiety. Brain Research Bulletin. 1987;19:293–299. doi: 10.1016/0361-9230(87)90097-9. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? British Journal of Pharmacology. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Wardill AG. The reliability of the hole-board apparatus. Psychopharmacologia. 1975;44:47–51. doi: 10.1007/BF00421183. [DOI] [PubMed] [Google Scholar]
- Fleshner M. Physical activity and stress resistance: sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exercise and Sport Sciences Reviews. 2005;33:120–126. doi: 10.1097/00003677-200507000-00004. [DOI] [PubMed] [Google Scholar]
- Flood DG, Gasior M, Marino MJ. Variables affecting prepulse inhibition of the startle reflex and the response to antipsychotics in DBA/2NCrl mice. Psychopharmacology (Berlin) 2007;195:203–211. doi: 10.1007/s00213-007-0894-9. [DOI] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neuroscience & Biobehavioral Reviews. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Forristall JR, Hookey BL, Grant VL. Conditioned taste avoidance induced by forced and voluntary wheel running in rats. Behavioral Processes. 2007;74:326–333. doi: 10.1016/j.beproc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Fox JH, Hammack SE, Falls WA. Exercise is associated with reduction in the anxiogenic effect of mCPP on acoustic startle. Behavioral Neuroscience. 2008;122:943–948. doi: 10.1037/0735-7044.122.4.943. [DOI] [PubMed] [Google Scholar]
- Fuss J, Ben Abdallah NM, Hensley FW, Weber KJ, Hellweg R, Gass P. Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss J, Ben Abdallah NM, Vogt MA, Touma C, Pacifici PG, Palme R, Witzemann V, Hellweg R, Gass P. Voluntary exercise induces anxiety-like behavior in adult C57BL/6 J mice correlating with hippocampal neurogenesis. Hippocampus. 2010b;20:364–376. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. Mount Sinai Journal of Medicine. 2006;73:941–949. [PubMed] [Google Scholar]
- Garcia-Capdevila S, Portell-Cortes I, Torras-Garcia M, Coll-Andreu M, Costa-Miserachs D. Effects of long-term voluntary exercise on learning and memory processes: dependency of the task and level of exercise. Behavioral Brain Research. 2009;202:162–170. doi: 10.1016/j.bbr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Garcia-Mesa Y, Lopez-Ramos JC, Gimenez-Llort L, Revilla S, Guerra R, Gruart A, Laferla FM, Cristofol R, Delgado-Garcia JM, Sanfeliu C. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. Journal of Alzheimers Disease. 2011;24:421–454. doi: 10.3233/JAD-2011-101635. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, Shekhar A. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety. 2010;27:339–350. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Preventive Medicine. 2003;36:698–703. doi: 10.1016/s0091-7435(03)00042-2. [DOI] [PubMed] [Google Scholar]
- Gorton LM, Vuckovic MG, Vertelkina N, Petzinger GM, Jakowec MW, Wood RI. Exercise effects on motor and affective behavior and catecholamine neurochemistry in the MPTP-lesioned mouse. Behavioral Brain Research. 2010;213:253–262. doi: 10.1016/j.bbr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace L, Hescham S, Kellaway LA, Bugarith K, Russell VA. Effect of exercise on learning and memory in a rat model of developmental stress. Metabolic Brain Disease. 2009;24:643–657. doi: 10.1007/s11011-009-9162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, Ballenger JC, Fyer AJ. The economic burden of anxiety disorders in the 1990. Journal of Clinical Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Medicine. 2008;10:81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exercise and Sport Sciences Reviews. 2011;39:140–149. doi: 10.1097/JES.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Mechanisms underlying the relationship between physical activity and anxiety: Animal data. In: Panteleimon E, editor. Routledge Handbook of Physical Activity and Mental Health. New York: 2013. in press. ISBN: 978-0-415-78299-9. [Google Scholar]
- Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Research. 2005a;1033:164–178. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, Fleshner M. Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha 1b-adrenergic receptor mRNA in the rat raphe nuclei. Biological Psychiatry. 2005b;57:559–568. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. Journal of Neuroscience. 2003a;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behavioral Brain Research. 2011;217:354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HE, Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience. 2003b;120:269–281. doi: 10.1016/s0306-4522(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Brooks L, Fleshner M. Anxiety-like behaviors produced by acute fluoxetine administration in male Fischer 344 rats are prevented by prior exercise. Psychopharmacology (Berlin) 2008;199:209–222. doi: 10.1007/s00213-008-1167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Dorey AA, Fleshner M. Therapeutic effects of exercise: wheel running reverses stress-induced interference with shuttle box escape. Behavioral Neuroscience. 2007;121:992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2009;19:988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Blanchard DC, Agnes RS, Blanchard RJ. Differential modulation of antipredator defensive behavior in Swiss-Webster mice following acute or chronic administration of imipramine and fluoxetine. Psychopharmacology (Berlin) 1995;120:57–66. doi: 10.1007/BF02246145. [DOI] [PubMed] [Google Scholar]
- Griebel G, Blanchard DC, Jung A, Lee JC, Masuda CK, Blanchard RJ. Further evidence that the mouse defense test battery is useful for screening anxiolytic and panicolytic drugs: effects of acute and chronic treatment with alprazolam. Neuropharmacology. 1995b;34:1625–1633. doi: 10.1016/0028-3908(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Critical Reviews in Neurobiology. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Hanagasioglu M, Borbely AA. Effect of voluntary locomotor activity on sleep in the rat. Behavioral Brain Research. 1982;4:359–368. doi: 10.1016/0166-4328(82)90060-2. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Picciotto MR. Characterization of GalR1, GalR2, and GalR3 immunoreactivity in catecholaminergic nuclei of the mouse brain. Journal of Comparative Neurology. 2004;479:410–423. doi: 10.1002/cne.20329. [DOI] [PubMed] [Google Scholar]
- Hayes K, Sprague S, Guo M, Davis W, Friedman A, Kumar A, Jimenez DF, Ding Y. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathology. 2008;115:289–296. doi: 10.1007/s00401-008-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Lapsansky J, Lovenberg TW, De Souza EB, Chalmers DT. Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regulatory Peptides. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- Herring MP, Jacob ML, Suveg C, Dishman RK, O’Connor PJ. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychotherapy and Psychosomatics. 2012;81:21–28. doi: 10.1159/000327898. [DOI] [PubMed] [Google Scholar]
- Herring MP, O’Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Archives of Internal Medicine. 2010;170:321–331. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- Hill LE, Droste SK, Nutt DJ, Linthorst AC, Reul JM. Voluntary exercise alters GABA(A) receptor subunit and glutamic acid decarboxylase-67 gene expression in the rat forebrain. Journal of Psychopharmacology. 2010;24:745–756. doi: 10.1177/0269881108096983. [DOI] [PubMed] [Google Scholar]
- Hoffman EJ, Mathew SJ. Anxiety disorders: a comprehensive review of pharmacotherapies. Mount Sinai Journal of Medicine. 2008;75:248–262. doi: 10.1002/msj.20041. [DOI] [PubMed] [Google Scholar]
- Hohmann JG, Jureus A, Teklemichael DN, Matsumoto AM, Clifton DK, Steiner RA. Distribution and regulation of galanin receptor 1 messenger RNA in the forebrain of wild type and galanin-transgenic mice. Neuroscience. 2003;117:105–117. doi: 10.1016/s0306-4522(02)00798-4. [DOI] [PubMed] [Google Scholar]
- Holets VR, Hokfelt T, Rokaeus A, Terenius L, Goldstein M. Locus coeruleus neurons in the rat containing neuropeptide Y, tyrosine hydroxylase or galanin and their efferent projections to the spinal cord, cerebral cortex and hypothalamus. Neuroscience. 1988;24:893–906. doi: 10.1016/0306-4522(88)90076-0. [DOI] [PubMed] [Google Scholar]
- Holmes A, Picciotto MR. Galanin: a novel therapeutic target for depression, anxiety disorders and drug addiction? CNS & Neurological Disorders Drug Targets. 2006;5:225–232. doi: 10.2174/187152706776359600. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Crawley JN. Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. Journal of Molecular Neuroscience. 2002;18:151–165. doi: 10.1385/JMN:18:1-2:151. [DOI] [PubMed] [Google Scholar]
- Holmes FE, Armenaki A, Iismaa TP, Einstein EB, Shine J, Picciotto MR, Wynick D, Zachariou V. Galanin negatively modulates opiate withdrawal via galanin receptor 1. Psychopharmacology (Berlin) 2011 doi: 10.1007/s00213-011-2515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes PV. Rodent models of depression: reexamining validity without anthropomorphic inference. Critical Reviews in Neurobiology. 2003;15:143–174. doi: 10.1615/critrevneurobiol.v15.i2.30. [DOI] [PubMed] [Google Scholar]
- Holmes PV, Blanchard DC, Blanchard RJ, Brady LS, Crawley JN. Chronic social stress increases levels of preprogalanin mRNA in the rat locus coeruleus. Pharmacology Biochemistry and Behavior. 1995;50:655–660. doi: 10.1016/0091-3057(94)00334-3. [DOI] [PubMed] [Google Scholar]
- Holmes PV, Crawley JN. Coexisting neurotransmitters in central noradrenergic neurons. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 347–353. [Google Scholar]
- Holmes PV, Crawley JN. Olfactory bulbectomy increases prepro-galanin mRNA levels in the rat locus coeruleus. Molecular Brain Research. 1996;36:184–188. doi: 10.1016/0169-328x(95)00295-4. [DOI] [PubMed] [Google Scholar]
- Holmes PV, Yoo HS, Dishman RK. Voluntary exercise and clomipramine treatment elevate prepro-galanin mRNA levels in the locus coeruleus in rats. Neuroscience Letters. 2006;408:1–4. doi: 10.1016/j.neulet.2006.04.057. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, Bucci DJ. BDNF expression in perirhinal cortex is associated with exercise-induced improvement in object recognition memory. Neurobiology of Learning and Memory. 2010a;94:278–284. doi: 10.1016/j.nlm.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins ME, Bucci DJ. Interpreting the effects of exercise on fear conditioning: the influence of time of day. Behavioral Neuroscience. 2010b;124:868–872. doi: 10.1037/a0021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins ME, Sharma M, Evans GC, Bucci DJ. Voluntary physical exercise alters attentional orienting and social behavior in a rat model of attention-deficit/hyperactivity disorder. Behavioral Neuroscience. 2009;123:599–606. doi: 10.1037/a0015632. [DOI] [PubMed] [Google Scholar]
- Horlington M. Startle response circadian rhythm in rats: lack of correlation with motor activity. Physiology & Behavior. 1970;5:49–53. doi: 10.1016/0031-9384(70)90012-0. [DOI] [PubMed] [Google Scholar]
- Huang HP, Wang SR, Yao W, Zhang C, Zhou Y, Chen XW, Zhang B, Xiong W, Wang LY, Zheng LH, Landry M, Hokfelt T, Xu ZQ, Zhou Z. Long latency of evoked quantal transmitter release from somata of locus coeruleus neurons in rat pontine slices. Proceedings of National Academy of Science of the United States of America. 2007;104:1401–1406. doi: 10.1073/pnas.0608897104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JC, Alpert JE. An approach to the psychopharmacologic care of patients: antidepressants, antipsychotics, anxiolytics, mood stabilizers, and natural remedies. Medical Clinics of North America. 2010;94:1141–1160. doi: 10.1016/j.mcna.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Ison JR, Foss JA. Coordinate diurnal variation in the strength of startle elicitation and of startle modification in the rat. Psychobiology. 1997;25:158–162. [Google Scholar]
- Itoi K. Ablation of the central noradrenergic neurons for unraveling their roles in stress and anxiety. Annals of New York Academy of Science. 2008;1129:47–54. doi: 10.1196/annals.1417.012. [DOI] [PubMed] [Google Scholar]
- Jaggi AS, Bhatia N, Kumar N, Singh N, Anand P, Dhawan R. A review on animal models for screening potential anti-stress agents. Neurological Sciences. 2011;32:993–1005. doi: 10.1007/s10072-011-0770-6. [DOI] [PubMed] [Google Scholar]
- Jameson JP, Blank MB. Diagnosis and treatment of depression and anxiety in rural and nonrural primary care: national survey results. Psychiatric Service. 2010;61:624–627. doi: 10.1176/ps.2010.61.6.624. [DOI] [PubMed] [Google Scholar]
- Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Research. 1977;127:25–53. [PubMed] [Google Scholar]
- Kadowaki K, Emson PC. Increase in galanin gene expression in locus coeruleus neurones of the rat following reserpine treatment. Molecular Brain Research. 1992;15:156–160. doi: 10.1016/0169-328x(92)90164-7. [DOI] [PubMed] [Google Scholar]
- Kalk NJ, Nutt DJ, Lingford-Hughes AR. The role of central noradrenergic dysregulation in anxiety disorders: evidence from clinical studies. Journal of Psychopharmacology. 2011;25:3–16. doi: 10.1177/0269881110367448. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Olivier JD, Nonkes LJ, Homberg JR. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neuroscience & Biobehavioral Reviews. 2010;34:373–386. doi: 10.1016/j.neubiorev.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Wheaton M, Murphy DL. What’s wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behavioral Brain Research. 2007;179:1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Holmes A, Heilig M, Crawley JN. Anxiolytic-like actions of centrally-administered neuropeptide Y, but not galanin, in C57BL/6 J mice. Pharmacology Biochemistry and Behavior. 2005;80:427–436. doi: 10.1016/j.pbb.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, Ustun TB, Wang PS. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiologia e Psichiatria Sociale. 2009;18:23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Dove S, Javors M, Morilak DA. Behavioral reactivity to stress: amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacology Biochemistry and Behavior. 2002a;71:407–417. doi: 10.1016/s0091-3057(01)00683-9. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Morilak DA. Modulatory effects of galanin in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuropsychopharmacology. 2002b;27:25–34. doi: 10.1016/S0893-133X(01)00424-9. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Crabbe JC. Pharmacological and genetic influences on hole-board behaviors in mice. Pharmacology Biochemistry and Behavior. 2006;85:57–65. doi: 10.1016/j.pbb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats–circuits mediating evocation, inhibition and potentiation. Behavioral Brain Research. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Kolakowski LF, Jr., O’Neill GP, Howard AD, Broussard SR, Sullivan KA, Feighner SD, Sawzdargo M, Nguyen T, Kargman S, Shiao LL, Hreniuk DL, Tan CP, Evans J, Abramovitz M, Chateauneuf A, Coulombe N, Ng G, Johnson MP, Tharian A, Khoshbouei H, George SR, Smith RG, O’Dowd BF. Molecular characterization and expression of cloned human galanin receptors GALR2 and GALR3. Journal of Neurochemistry. 1998;71:2239–2251. doi: 10.1046/j.1471-4159.1998.71062239.x. [DOI] [PubMed] [Google Scholar]
- Konnopka A, Leichsenring F, Leibing E, Konig HH. Cost-of-illness studies and cost-effectiveness analyses in anxiety disorders: a systematic review. Journal of Affective Disorders. 2009;114:14–31. doi: 10.1016/j.jad.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Koteja P, Garland T, Jr., Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Animal Behavior. 1999;58:1307–1318. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Singh K, Bishnoi M. Elevated zero maze: a paradigm to evaluate antianxiety effects of drugs. Methods & Findings in Experimental & Clinical Pharmacology. 2007;29:343–348. doi: 10.1358/mf.2007.29.5.1117557. [DOI] [PubMed] [Google Scholar]
- Kuteeva E, Wardi T, Lundstrom L, Sollenberg U, Langel U, Hokfelt T, Ogren SO. Differential role of galanin receptors in the regulation of depression-like behavior and monoamine/stress-related genes at the cell body level. Neuropsychopharmacology. 2008;33:2573–2585. doi: 10.1038/sj.npp.1301660. [DOI] [PubMed] [Google Scholar]
- Lancel M, Droste SK, Sommer S, Reul JM. Influence of regular voluntary exercise on spontaneous and social stress-affected sleep in mice. European Journal of Neuroscience. 2003;17:2171–2179. doi: 10.1046/j.1460-9568.2003.02658.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, McTeague LM. The anxiety disorder spectrum: fear imagery, physiological reactivity, and differential diagnosis. Anxiety Stress Coping. 2009;22:5–25. doi: 10.1080/10615800802478247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Bondi CO, Doyen J, Rodriguez GA, Bedard-Arana T, Morilak DA. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. Journal of Neuroendocrinology. 2008;20:1115–1137. doi: 10.1111/j.1365-2826.2008.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larun L, Nordheim LV, Ekeland E, Hagen KB, Heian F. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database of Systematic Reviews. 2006;3:CD004691. doi: 10.1002/14651858.CD004691.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BW, Yau SY, Lee TM, Ching YP, Tang SW, So KF. Intracerebroventricular infusion of cytosine-arabinoside causes prepulse inhibition disruption. Neuroreport. 2009;20:371–377. doi: 10.1097/WNR.0b013e328324edcd. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Milani RV, O’Keefe JH, Lavie TJ. Impact of exercise training on psychological risk factors. Progress in Cardiovascular Diseases. 2011;53:464–470. doi: 10.1016/j.pcad.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156:456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis IN, Mantzouridis T, Saramantis A, Phenekos C, Tzioras C, Mountokalakis T. Human galanin secretion is increased upon normal exercise test in middle-age individuals. Endocrine Research. 2000;26:357–364. doi: 10.3109/07435800009066173. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Herkenham M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. Journal of Neuroscience. 2011;31:6159–6173. doi: 10.1523/JNEUROSCI.0577-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Koh MT. Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiology & Behavior. 2001;72:355–358. doi: 10.1016/s0031-9384(00)00427-3. [DOI] [PubMed] [Google Scholar]
- Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cellular and Molecular Neurobiology. 2010;30:493–503. doi: 10.1007/s10571-009-9488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacology & Therapeutics. 1990;46:321–340. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- Liu YF, Chen HI, Wu CL, Kuo YM, Yu L, Huang AM, Wu FS, Chuang JI, Jen CJ. Differential effects of treadmill running and wheel running on spatial or aversive learning and memory: roles of amygdalar brain-derived neurotrophic factor and synaptotagmin I. Journal of Physiology. 2009;587:3221–3231. doi: 10.1113/jphysiol.2009.173088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long BC, Van Stavel R. Effects of exercise training on anxiety: A meta-analysis. Journal of Applied Sport Psychology. 1995;7:167–189. [Google Scholar]
- Lu X, Mazarati A, Sanna P, Shinmei S, Bartfai T. Distribution and differential regulation of galanin receptor subtypes in rat brain: effects of seizure activity. Neuropeptides. 2005;39:147–152. doi: 10.1016/j.npep.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Luyten L, Vansteenwegen D, van Kuyck K, Gabriels L, Nuttin B. Contextual conditioning in rats as an animal model for generalized anxiety disorder. Cognitive, Affective & Behavioral Neuroscience. 2011;11:228–244. doi: 10.3758/s13415-011-0021-6. [DOI] [PubMed] [Google Scholar]
- Lyudyno VI, Abdurasulova IN, Klimenko VM. The role of the neuropeptide galanin in forming type-specific behavioral characteristics. Neuroscience & Behavioral Physiology. 2008;38:93–98. doi: 10.1007/s11055-008-0013-3. [DOI] [PubMed] [Google Scholar]
- Ma X, Tong YG, Schmidt R, Brown W, Payza K, Hodzic L, Pou C, Godbout C, Hokfelt T, Xu ZQ. Effects of galanin receptor agonists on locus coeruleus neurons. Brain Research. 2001;919:169–174. doi: 10.1016/s0006-8993(01)03033-5. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor Controllability, Anxiety, and Serotonin. Cognitive Therapy and Research. 1998;22:595–613. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience & Biobehavioral Reviews. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Makatsori A, Duncko R, Schwendt M, Moncek F, Johansson BB, Jezova D. Voluntary wheel running modulates glutamate receptor subunit gene expression and stress hormone release in Lewis rats. Psychoneuroendocrinology. 2003;28:702–714. doi: 10.1016/s0306-4530(02)00062-8. [DOI] [PubMed] [Google Scholar]
- Makino S, Asaba K, Nishiyama M, Hashimoto K. Decreased type 2 corticotropin-releasing hormone receptor mRNA expression in the ventrome-dial hypothalamus during repeated immobilization stress. Neuroendocrinology. 1999;70:160–167. doi: 10.1159/000054472. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: Role of hippocampus. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Marks IM. Phobias and obsessions: Clinical phenomena in search of a laboratory model. In: Seligman M, Maser D, editors. Psychopathology: Experimental Models. Freeman; San Francisco: 1977. pp. 174–213. [Google Scholar]
- Masini CV, Nyhuis TJ, Sasse SK, Day HE, Campeau S. Effects of voluntary wheel running on heart rate, body temperature, and locomotor activity in response to acute and repeated stressor exposures in rats. Stress. 2011;14:324–334. doi: 10.3109/10253890.2010.548013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason ST, Fibiger HC. Regional topography within noradrenergic locus coeruleus as revealed by retrograde transport of horseradish peroxidase. Journal of Comparative Neurology. 1979;187:703–724. doi: 10.1002/cne.901870405. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annual Reviews of Medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Predicting danger: the nature, consequences, and neural mechanisms of predictive fear learning. Learning Memory. 2006;13:245–253. doi: 10.1101/lm.196606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander T, Hokfelt T, Rokaeus A, Cuello AC, Oertel WH, Verhofstad A, Goldstein M. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. Journal of Neuroscience. 1986;6:3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendlowicz MV, Stein MB. Quality of life in individuals with anxiety disorders. American Journal of Psychiatry. 2000;157:669–682. doi: 10.1176/appi.ajp.157.5.669. [DOI] [PubMed] [Google Scholar]
- Mennicken F, Hoffert C, Pelletier M, Ahmad S, O’Donnell D. Restricted distribution of galanin receptor 3 (GalR3) mRNA in the adult rat central nervous system. Journal of Chemical Neuroanatomy. 2002;24:257–268. doi: 10.1016/s0891-0618(02)00068-6. [DOI] [PubMed] [Google Scholar]
- Miller MW, Gronfier C. Diurnal variation of the startle reflex in relation to HPA-axis activity in humans. Psychophysiology. 2006;43:297–301. doi: 10.1111/j.1469-8986.2006.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. European Journal of Neuroscience. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. Journal of Comparative & Physiological Psychology. 1955;48:254–260. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- Montgomery KC, Monkman JA. The relation between fear and exploratory behavior. Journal of Comparative & Physiological Psychology. 1955;48:132–136. doi: 10.1037/h0048596. [DOI] [PubMed] [Google Scholar]
- Moore RY, Card JP. Noradrenaline-containing neuron systems, Classical Transmitters in the CNS. In: Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy. third Amsterdam; Elsevier: 1984. pp. 123–156. [Google Scholar]
- Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2000;279:R1321–R1329. doi: 10.1152/ajpregu.2000.279.4.R1321. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Progress in Neuropsychopharmacology and Biological Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Murray PS, Groves JL, Pettett BJ, Britton SL, Koch LG, Dishman RK, Holmes PV. Locus coeruleus galanin expression is enhanced after exercise in rats selectively bred for high capacity for aerobic activity. Peptides. 2010;31:2264–2268. doi: 10.1016/j.peptides.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Wegener G, Homberg JR, Cohen H, Slattery DA, Zohar J, Olivier JD, Mathe AA. Animal models of depression and anxiety: What do they tell us about human condition? Progress in Neuropsychopharmacology & Biological Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.11.028. [DOI] [PubMed] [Google Scholar]
- Nugent NR, Tyrka AR, Carpenter LL, Price LH. Gene-environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacology (Berlin) 2011;214:175–196. doi: 10.1007/s00213-010-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell D, Ahmad S, Wahlestedt C, Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. Journal of Comparative Neurology. 1999;409:469–481. [PubMed] [Google Scholar]
- O’Neal HA, Van Hoomissen JD, Holmes PV, Dishman RK. Prepro-galanin messenger RNA levels are increased in rat locus coeruleus after treadmill exercise training. Neuroscience Letters. 2001;299:69–72. doi: 10.1016/s0304-3940(00)01780-8. [DOI] [PubMed] [Google Scholar]
- Ohl F, Holsboer F, Landgraf R. The modified hole board as a differential screen for behavior in rodents. Behavior Research Methods, Instruments, & Computers. 2001;33:392–397. doi: 10.3758/bf03195393. [DOI] [PubMed] [Google Scholar]
- Olsson IAS, Westlund K. More than numbers matter: The effect of social factors on behaviour and welfare of laboratory rodents and non-human primates. Applied Animal Behaviour Science. 2007;103:229–254. [Google Scholar]
- Pang L, Hashemi T, Lee HJ, Maguire M, Graziano MP, Bayne M, Hawes B, Wong G, Wang S. The mouse GalR2 galanin receptor: genomic organization, cDNA cloning, and functional characterization. Journal of Neurochemistry. 1998;71:2252–2259. doi: 10.1046/j.1471-4159.1998.71062252.x. [DOI] [PubMed] [Google Scholar]
- Pare WP, Tejani-Butt S, Kluczynski J. The emergence test: effects of psychotropic drugs on neophobic disposition in Wistar Kyoto (WKY) and Sprague Dawley rats. Progress in Neuropsychopharmacology & Biological Psychiatry. 2001;25:1615–1628. doi: 10.1016/s0278-5846(01)00204-4. [DOI] [PubMed] [Google Scholar]
- Pego JM, Sousa JC, Almeida OF, Sousa N. Stress and the neuroendocrinology of anxiety disorders. Current Topics in Behavioral Neurosciences. 2010;2:97–117. doi: 10.1007/7854_2009_13. [DOI] [PubMed] [Google Scholar]
- Petruzzello SJ, Landers DM, Hatfield BD, Kubitz KA, Salazar W. A meta-analysis on the anxiety-reducing effects of acute and chronic exercise. Outcomes and mechanisms. Sports Medicine. 1991;11:143–182. doi: 10.2165/00007256-199111030-00002. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Xu ZQ, Zhang X, Grillner S, Bartfai T, Hokfelt T. Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience. 1995;64:861–874. doi: 10.1016/0306-4522(94)00450-j. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Feldon J, Alleva E, Cirulli F, Yee BK. The role of voluntary exercise in enriched rearing: a behavioral analysis. Behavioral Neuroscience. 2006;120:787–803. doi: 10.1037/0735-7044.120.4.787. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Sun Y, Li R, Brana C, Feldon J, Yee BK. The impact of voluntary exercise on mental health in rodents: a neuroplasticity perspective. Behavioral Brain Research. 2008;192:42–60. doi: 10.1016/j.bbr.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychological Bulletin. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Pritchard GA, Galpern WR, Lumpkin M, Miller LG. Chronic benzo-diazepine administration. VIII. Receptor upregulation produced by chronic exposure to the inverse agonist FG-7142. Journal of Pharmacology and Experimental Therapeutics. 1991;258:280–285. [PubMed] [Google Scholar]
- Rajarao SJ, Platt B, Sukoff SJ, Lin Q, Bender CN, Nieuwenhuijsen BW, Ring RH, Schechter LE, Rosenzweig-Lipson S, Beyer CE. Anxiolytic-like activity of the non-selective galanin receptor agonist, galnon. Neuropeptides. 2007;41:307–320. doi: 10.1016/j.npep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Ramos A. Animal models of anxiety: do I need multiple tests? Trends in Pharmacological Science. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Reiss JI, Dishman RK, Boyd HE, Robinson JK, Holmes PV. Chronic activity wheel running reduces the severity of kainic acid-induced seizures in the rat: possible role of galanin. Brain Research. 2009;1266:54–63. doi: 10.1016/j.brainres.2009.02.030. [DOI] [PubMed] [Google Scholar]
- Risbrough V. Behavioral correlates of anxiety. In: Stein MB, Steckler T, editors. Behavioral Neurobiology of Anxiety and Its Treatment. Springer; Berlin Heidelberg: 2010. pp. 205–228. [Google Scholar]
- Robinson AM, Hopkins ME, Bucci DJ. Effects of physical exercise on ADHD-like behavior in male and female adolescent spontaneously hypertensive rats. Developmental Psychobiology. 2011;53:383–390. doi: 10.1002/dev.20530. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cao BJ, Dalvi A, Holmes A. Animal models of anxiety: an ethological perspective. Brazilian Journal of Medical and Biological Research. 1997;30:289–304. doi: 10.1590/s0100-879x1997000300002. [DOI] [PubMed] [Google Scholar]
- Roman E, Arborelius L. Male but not female Wistar rats show increased anxiety-like behaviour in response to bright light in the defensive withdrawal test. Behavioral Brain Research. 2009;202:303–307. doi: 10.1016/j.bbr.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Annals of the New York Academy of Sciences. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Rotzinger S, Lovejoy DA, Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides. 2010;31:736–756. doi: 10.1016/j.peptides.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Greenwood BN, Fleshner M, Watkins LR, Maier SF. Voluntary wheel running produces resistance to inescapable stress-induced potentiation of morphine conditioned place preference. Behavioral Brain Research. 2011;219:378–381. doi: 10.1016/j.bbr.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam JN, Fox JH, Detroy EM, Guignon MH, Wohl DF, Falls WA. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behavioral Brain Research. 2009;197:31–40. doi: 10.1016/j.bbr.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Samorajski T, Delaney C, Durham L, Ordy JM, Johnson JA, Dunlap WP. Effect of exercise on longevity, body weight, locomotor performance, and passive-avoidance memory of C57BL/6 J mice. Neurobiology of Aging. 1985;6:17–24. doi: 10.1016/0197-4580(85)90066-1. [DOI] [PubMed] [Google Scholar]
- Schrijver NC, Bahr NI, Weiss IC, Wurbel H. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacology Biochemistry and Behavior. 2002;73:209–224. doi: 10.1016/s0091-3057(02)00790-6. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Dishman RK, Holmes PV. Voluntary exercise offers anxiolytic potential and amplifies galanin gene expression in the locus coeruleus of the rat. Behavioural Brain Research. 2012;233:191–200. doi: 10.1016/j.bbr.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seutin V, Verbanck P, Massotte L, Dresse A. Galanin decreases the activity of locus coeruleus neurons in vitro. European Journal of Pharmacology. 1989;164:373–376. doi: 10.1016/0014-2999(89)90481-0. [DOI] [PubMed] [Google Scholar]
- Sevcik J, Finta EP, Illes P. Galanin receptors inhibit the spontaneous firing of locus coeruleus neurones and interact with mu-opioid receptors. European Journal of Pharmacology. 1993;230:223–230. doi: 10.1016/0014-2999(93)90806-s. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated zero-maze as an animal model of anxiety. Psychopharmacology (Berlin) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Shuhama R, Del-Ben CM, Loureiro SR, Graeff FG. Animal defense strategies and anxiety disorders. The Anais da Academia Brasileira de Ciências. 2007;79:97–109. doi: 10.1590/s0001-37652007000100012. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6:509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Harris RB, Ryan DH. Corticotropin-releasing factor receptor antagonist infused into the locus coeruleus attenuates immobilization stress-induced defensive withdrawal in rats. Neuroscience Letters. 1996;220:167–170. doi: 10.1016/s0304-3940(96)13254-7. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Smith JC, O’Connor PJ. Physical activity does not disturb the measurement of startle and corrugator responses during affective picture viewing. Biological Psychology. 2003;63:293–310. doi: 10.1016/s0301-0511(03)00072-3. [DOI] [PubMed] [Google Scholar]
- Smith JC, O’Connor PJ, Crabbe JB, Dishman RK. Emotional responsiveness after low- and moderate-intensity exercise and seated rest. Medical Science and Sports Exercise. 2002;34:1158–1167. doi: 10.1097/00005768-200207000-00017. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosomatic Medicine. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J, Holmes PV, Renner KJ, Edwards GL, Bunnell BN, Dishman RK. Brain noradrenergic responses to footshock after chronic activity-wheel running. Behavioral Neuroscience. 1999;113:558–566. doi: 10.1037//0735-7044.113.3.558. [DOI] [PubMed] [Google Scholar]
- Sothmann MS, Buckworth J, Claytor RP, Cox RH, White-Welkley JE, Dishman RK. Exercise training and the cross-stressor adaptation hypothesis. Exercise and Sport Sciences Reviews. 1996;24:267–287. [PubMed] [Google Scholar]
- Steimer T. Animal models of anxiety disorders in rats and mice: some conceptual issues. Dialogues in Clinical Neuroscience. 2011;13:495–506. doi: 10.31887/DCNS.2011.13.4/tsteimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Najimi M, Quartermain D. Potentiation by propranolol of stress-induced changes in passive avoidance and open-field emergence tests in mice. Pharmacology Biochemistry and Behavior. 1995;51:297–300. doi: 10.1016/0091-3057(94)00381-r. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature Neuroscience. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Mattson MP. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromolecular Medicine. 2008;10:118–127. doi: 10.1007/s12017-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Zhou Y, Martin B, Maudsley S. Pharmacomimetics of exercise: novel approaches for hippocampally-targeted neuroprotective agents. Current Medicinal Chemistry. 2009;16:4668–4678. doi: 10.2174/092986709789878292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Blackburn TP, Zhang X, Zheng K, Xu ZQ, Hokfelt T, Wolinsky TD, Konkel MJ, Chen H, Zhong H, Walker MW, Craig DA, Gerald CP, Branchek TA. Anxiolytic- and antidepressant-like profiles of the galanin-3 receptor (Gal3) antagonists SNAP 37889 and SNAP 398299. Proceedings of National Academy of Science of the United States of America. 2005;102:17489–17494. doi: 10.1073/pnas.0508970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweerts BW, Jarrott B, Lawrence AJ. Expression of preprogalanin mRNA following acute and chronic restraint stress in brains of normotensive and hypertensive rats. Brain Research Molecular Brain Research. 1999;69:113–123. doi: 10.1016/s0169-328x(99)00095-9. [DOI] [PubMed] [Google Scholar]
- Sweerts BW, Jarrott B, Lawrence AJ. Acute and chronic restraint stress: effects on [125I]-galanin binding in normotensive and hypertensive rat brain. Brain Research. 2000;873:318–329. doi: 10.1016/s0006-8993(00)02558-0. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Nishi A, Ishii A, Shiroishi T, Koide T. Systematic analysis of emotionality in consomic mouse strains established from C57BL/6 J and wild-derived MSM/Ms. Genes Brain and Behavior. 2008;7:849–858. doi: 10.1111/j.1601-183X.2008.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK, Kalin NH, Vanden Burgt JA, Sherman JE. Corticotropin-releasing factor modulates defensive-withdrawal and exploratory behavior in rats. Behavioral Neuroscience. 1989;103:648–654. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- Tieman JG, Peacock LJ, Cureton KJ, Dishman RK. Acoustic startle eyeblink response after acute exercise. International Journal of Neuroscience. 2001;106:21–33. doi: 10.3109/00207450109149735. [DOI] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiology of Disease. 2001;8:1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- Treit D, Engin E, McEown K. Animal models of anxiety and anxiolytic drug action. Current Topics in Behavioral Neurosciences. 2010;2:121–160. doi: 10.1007/7854_2009_17. [DOI] [PubMed] [Google Scholar]
- Treit D, Lolordo VM, Armstrong DE. The effects of diazepam on fear reactions in rats are modulated by environmental constraints on the rat’s defensive repertoire. Pharmacology Biochemistry and Behavior. 1986;25:561–565. doi: 10.1016/0091-3057(86)90141-3. [DOI] [PubMed] [Google Scholar]
- Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacology Biochemistry and Behavior. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services Physical activity guidelines advisory committee report. 2008. [DOI] [PubMed]
- Uchiumi K, Aoki M, Kikusui T, Takeuchi Y, Mori Y. Wheel-running activity increases with social stress in male DBA mice. Physiology & Behavior. 2008;93:1–7. doi: 10.1016/j.physbeh.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiologica Scandinavica Supplementum. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Unschuld PG, Ising M, Erhardt A, Lucae S, Kohli M, Kloiber S, Salyakina D, Thoeringer CK, Kern N, Lieb R, Uhr M, Binder EB, Muller-Myhsok B, Holsboer F, Keck ME. Polymorphisms in the galanin gene are associated with symptom-severity in female patients suffering from panic disorder. Journal of Affective Disorders. 2008;105:177–184. doi: 10.1016/j.jad.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Unschuld PG, Ising M, Roeske D, Erhardt A, Specht M, Kloiber S, Uhr M, Muller-Myhsok B, Holsboer F, Binder EB. Gender-specific association of galanin polymorphisms with HPA-axis dysregulation, symptom severity, and antidepressant treatment response. Neuropsychopharmacology. 2010;35:1583–1592. doi: 10.1038/npp.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berg CL, Van Ree JM, Spruijt BM. Sequential analysis of juvenile isolation-induced decreased social behavior in the adult rat. Physiology & Behavior. 1999;67:483–488. doi: 10.1016/s0031-9384(99)00062-1. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ. Animal models of behavioral dysfunctions: basic concepts and classifications, and an evaluation strategy. Brain Research Reviews. 2006;52:131–159. doi: 10.1016/j.brainresrev.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen J, Kunrath J, Dentlinger R, Lafrenz A, Krause M, Azar A. Cognitive and locomotor/exploratory behavior after chronic exercise in the olfactory bulbectomy animal model of depression. Behavioral Brain Research. 2011;222:106–116. doi: 10.1016/j.bbr.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen JD, Holmes PV, Zellner AS, Poudevigne A, Dishman RK. Effects of beta-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophic factor. Behavioral Neuroscience. 2004;118:1378–1390. doi: 10.1037/0735-7044.118.6.1378. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends in Neuroscience. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vila-Porcile E, Xu ZQ, Mailly P, Nagy F, Calas A, Hokfelt T, Landry M. Dendritic synthesis and release of the neuropeptide galanin: morphological evidence from studies on rat locus coeruleus neurons. Journal of Comparative Neurology. 2009;516:199–212. doi: 10.1002/cne.22105. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature Protocols. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PM, Messier C. Methodological and conceptual issues in the use of the elevated plus-maze as a psychological measurement instrument of animal anxiety-like behavior. Neuroscience & Biobehavioral Reviews. 2001;25:275–286. doi: 10.1016/s0149-7634(01)00013-6. [DOI] [PubMed] [Google Scholar]
- Wang S, He C, Maguire MT, Clemmons AL, Burrier RE, Guzzi MF, Strader CD, Parker EM, Bayne ML. Genomic organization and functional characterization of the mouse GalR1 galanin receptor. FEBS Letters. 1997;411:225–230. doi: 10.1016/s0014-5793(97)00695-9. [DOI] [PubMed] [Google Scholar]
- Waters SM, Krause JE. Distribution of galanin-1, -2 and -3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience. 2000;95:265–271. doi: 10.1016/s0306-4522(99)00407-8. [DOI] [PubMed] [Google Scholar]
- Weisberg RB, Dyck I, Culpepper L, Keller MB. Psychiatric treatment in primary care patients with anxiety disorders: a comparison of care received from primary care providers and psychiatrists. American Journal of Psychiatry. 2007;164:276–282. doi: 10.1176/appi.ajp.164.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh D, Richardson GS, Dement WC. Effect of running wheel availability on circadian patterns of sleep and wakefulness in mice. Physiology & Behavior. 1988;43:771–777. doi: 10.1016/0031-9384(88)90375-7. [DOI] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, Brene S. Delta FosB regulates wheel running. Journal of Neuroscience. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigger A, Loerscher P, Weissenbacher P, Holsboer F, Landgraf R. Cross-fostering and cross-breeding of HAB and LAB rats: a genetic rat model of anxiety. Behavior Genetics. 2001;31:371–382. doi: 10.1023/a:1012222402346. [DOI] [PubMed] [Google Scholar]
- Wipfli BM, Rethorst CD, Landers DM. The anxiolytic effects of exercise: a meta-analysis of randomized trials and dose-response analysis. Journal of Sport & Exercise Psychology. 2008;30:392–410. doi: 10.1123/jsep.30.4.392. [DOI] [PubMed] [Google Scholar]
- Wirz SA, Davis CN, Lu X, Zal T, Bartfai T. Homodimerization and internalization of galanin type 1 receptor in living CHO cells. Neuropeptides. 2005;39:535–546. doi: 10.1016/j.npep.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. European Journal of Neuroscience. 2008;27:1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Shi TJ, Hokfelt T. Galanin/GMAP- and NPY-like immunoreactivities in locus coeruleus and noradrenergic nerve terminals in the hippocampal formation and cortex with notes on the galanin-R1 and -R2 receptors. Journal of Comparative Neurology. 1998;392:227–251. doi: 10.1002/(sici)1096-9861(19980309)392:2<227::aid-cne6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Tong YG, Hokfelt T. Galanin enhances noradrenaline-induced outward current on locus coeruleus noradrenergic neurons. Neuroreport. 2001;12:1779–1782. doi: 10.1097/00001756-200106130-00052. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Zheng K, Hokfelt T. Electrophysiological studies on galanin effects in brain–progress during the last six years. Neuropeptides. 2005;39:269–275. doi: 10.1016/j.npep.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ. The involvement of central noradrenergic systems and corticotropin-releasing factor in defensive-withdrawal behavior in rats. Journal of Pharmacology and Experimental Therapeutics. 1990;255:1064–1070. [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology. 1908;18:459–482. [Google Scholar]
- Yoo H, O’Neal HA, Hong S, Tackett RL, Dishman RK. Brain β-adrenergic responses to footshock after wheel running. Medicine and Science in Sports and Exercise. 1999;31:1433. [Google Scholar]
- Yoshitake T, Wang FH, Kuteeva E, Holmberg K, Yamaguchi M, Crawley JN, Steiner R, Bartfai T, Ogren SO, Hokfelt T, Kehr J. Enhanced hippocampal noradrenaline and serotonin release in galanin-overexpressing mice after repeated forced swimming test. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:354–359. doi: 10.1073/pnas.0307042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hu XZ, Li H, Li X, Smerin S, Benedek DM, Ursano R. Startle response related genes. Medical Hypotheses. 2011;77:685–691. doi: 10.1016/j.mehy.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G, Halberg F. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behavioral Brain Research. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]