Abstract
The mechanisms underlying development of ribonucleoprotein (RNP) autoantibodies are unclear. The U1-70K protein is the predominant target of RNP autoantibodies, and the RNA binding domain has been shown to be the immunodominant autoantigenic region of U1-70K, although the specific epitopes are not known. To precisely map U1-70K epitopes, we developed silicon-based peptide microarrays with >5700 features, corresponding to 843 unique peptides derived from the U1-70K protein. The microarrays feature overlapping peptides, with single-amino acid resolution in length and location, spanning amino acids 110–170 within the U1-70K RNA binding domain. We evaluated the serum IgG of a cohort of patients with systemic lupus erythematosus (SLE; n = 26) using the microarrays, and identified multiple reactive epitopes, including peptides 116–121 and 143–148. Indirect peptide ELISA analysis of the sera of patients with SLE (n = 88) revealed that ~14% of patients had serum IgG reactivity to 116–121, while reactivity to 143–148 appeared to be limited to a single patient. SLE patients with serum reactivity to 116–121 had significantly lower SLE Disease Activity Index (SLEDAI) scores at the time of sampling, compared to non-reactive patients. Minimal reactivity to the peptides was observed in the sera of healthy controls (n = 92). Competitive ELISA showed antibodies to 116–121 bind a common epitope in U1-70K (68–72) and the matrix protein M1 of human influenza B viruses. Institutional Review Boards approved this study. Knowledge of the precise epitopes of U1-70K autoantibodies may provide insight into the mechanisms of development of anti-RNP, identify potential clinical biomarkers and inform ongoing clinical trails of peptide-based therapeutics.
Keywords: Silicon-based peptide microarray, systemic lupus erythematosus, SLE, ribonucleoprotein, RNP
Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease that frequently affects multiple tissues, including the joints, skin and kidneys. Multiple serum autoantibodies targeting nuclear antigens, including DNA, Smith, Ro, La, histones and ribonucleoprotein (RNP), are a defining feature of SLE. These autoantibodies can develop years before disease onset, are associated with disease activity, and, in the case of anti-DNA, can initiate early stages of renal disease [1–3]. Development of RNP autoantibodies closely precede diagnosis of SLE, implicating them in the pathogenesis of disease [1].
RNP autoantibodies bind to components of the U1–small nuclear ribonucleoprotein (U1–snRNP) complex, which is one of five snRNPs that make up the spliceosome [4]. Anti-RNPs are known to bind epitopes within three proteins from the U1–snRNP complex, U1-70K, U1-A and U1-C, with U1-70K being the predominant target [5]. Autoantibodies that recognize U1-70K (also called U1-68K, U1-70 and U1-70 kD) are found in the sera of 11–44% of patients with SLE [6–8]. High-titer U1-70K autoantibodies are found in the sera of 95% of patients with mixed connective tissue disease (MCTD), an overlap syndrome sharing clinical features with SLE, systemic sclerosis and polymyositis [5,7,9,10].
Multiple epitopes exist in U1-70K, and the serum reactivity patterns to these epitopes are heterogeneous between patients with U1-70K autoantibodies [11,12]. A number of approaches have been used to map the epitopes of autoantibodies to U1-70K, including analysis of recombinant protein fragments [11–16], overlapping peptides [17–19] and chimeric Drosophila proteins [14,20,21]. Identifying the precise length and location of epitopes with these approaches is a labor-intensive process that often requires multiple iterations of peptide synthesis or protein design and expression. Current approaches also have relatively low resolution, and potentially miss epitopes that rely on specific N- or C-termini. It is important to identify the precise epitopes of U1-70K autoantibodies in order to understand the mechanisms underlying development of anti-RNP. Additionally, identifying epitope reactivity associated with specific clinical manifestations of SLE could enhance clinical autoantibody tests. For example, an apoptosis-induced proteolytic fragment of U1-70K has been associated with skin disease in patients with SLE or MCTD [22].
Recently, our group developed silicon-based peptide microarrays to map SLE patient serum antibody epitopes within the N-terminal tail of histone H2B [23]. We used maskless photolithography to synthesize every possible sub-peptide within the region, with respect to length and location, on the surface of derivatized microprocessor-grade silicon wafers. While traditional overlapping peptide libraries often have an offset, or resolution, of five amino acids, and are constrained to a single-peptide length, our platform enabled single-amino acid resolution in both offset and length. Other advantages of using a silicon substrate include high-feature density, high reproducibility and low-background fluorescence. Using the microarrays, we identified a five amino acid minimum epitope within the tail of H2B that was associated with increased activation of the type I interferon pathway and disease activity in patients with SLE [24].
In the current study, we characterized the epitopes of U1-70K autoantibodies at single-amino acid resolution using silicon-based peptide microarrays with >5700 features, corresponding to 843 unique peptides derived from the U1-70K protein. The features on the microarrays represent overlapping peptides, with single-amino acid resolution in length and location, spanning amino acids 110–170 within the U1-70K RNA binding domain. We focused on the RNA binding domain, as it is the immunodominant autoantigenic region of U1-70K [12], and our lab recently identified a population of autoreactive CD4+ T cells in MRL/lpr mice specific for a peptide (131–150) within the region [25]. Further, a peptide-based therapeutic, rigerimod (IPP-201101, trade name Lupuzor), that features amino acids 131–151 of the U1-70K RNA binding domain is currently starting phase III clinical trials for treatment of SLE [26].
We validated the U1-70K microarrays using two commercial anti-U1-70K antibodies. We then used the microarrays to characterize the epitopes of the serum IgG of patients with SLE. We identified multiple reactive sequences and further investigated the two most reactive epitopes, 116–121 and 143–148 by indirect peptide ELISA. Approximately 14% of patients with SLE had serum reactivity to epitope 116–121, while 143–148 appeared to be a patient-specific epitope. SLE patients with serum reactivity to 116–121 had lower disease activity than non-reactive patients. We showed patient serum antibodies to 116–121 bind a common epitope in U1-70K (68–72) and the matrix protein M1 of human influenza B viruses by competitive ELISA. Identification of epitopes within the U1-70K autoantigen may elucidate how anti-RNP develops, enhance clinical autoantibody tests and inform the development of peptide-based therapeutics.
Methods
Patients
We used serum samples from patients with SLE and normal controls collected as part of the Autoimmune Biomarkers Collaborative Network (ABCoN; Table S1), with approval from the University of Minnesota Institutional Review Board (protocol 0110M09982). All the patients with SLE met American College of Rheumatology revised criteria for classification of SLE [27]. Sera from healthy adult controls (n = 88) were baseline samples from an influenza vaccine study at the Stanford-Lucille Packard Children’s Hospital (LPCH) Vaccine Program in 2008–2009 (Table S2). Inclusion criteria are detailed in Price et al. [28]. The study was approved by the Stanford IRB (protocol 8215). Written consent was obtained from all the individuals referenced in the article.
Silicon-based peptide microarrays
A detailed description of the fabrication and probing of the silicon-based peptide microarrays is available in a previous manuscript [23]. The sequences of the peptide features present on the microarrays were based on amino acids 110–170 of the human U1–snRNP 70-kDa protein [Genbank:NP_001287998.1, Genbank:NP_003080.2). Maskless photolithography was used to synthesize peptides that were covalently linked to derivatized microprocessor-grade silicon wafers by their C-termini [23]. Briefly, we washed deprotected microarrays in propylene glycol monomethyl ether acetate (PGMEA; Sigma), 2-propanol (Sigma, St. Louis, MO) and then PBS with 0.1% Tween-20 (Sigma; PBST). We then incubated the microarrays with primary antibodies (at the indicated concentrations) or human serum (diluted 1/250) in 3% FCS in PBST. Following incubation with antibodies or serum, we stained the microarrays with Cy3-conjugated goat anti-rabbit IgG (H + L; Invitrogen, Carlsbad, CA) or Cy5-conjugated goat anti-human IgG (Fcy specific; Jackson ImmunoResearch, West Grove, PA) diluted in 20% FCS in PBST. We scanned the microarrays using a GenePix 4000B microarray scanner (Molecular Devices, Sunnyvale, CA). Fluorescent images were gridded using GenePix Pro 6.0 Acquisition and Analysis Microarray Software (Molecular Devices) to determine the median fluorescence intensity (MFI) of each feature.
Indirect peptide ELISA
We used indirect ELISA to measure reactivity of anti-U1-70K polyclonal antibodies, 70R-4091 (Fitzgerald, Acton, MA), to U1-70K peptides 111–124 NYDTTESKLRREFE(K-biotin), 118–124 KLRREFE(K-biotin), 120–124 RREFE(K-biotin), 143–154 PRGYAFIEYEHE(K-biotin). Indirect ELISA was also used to measure human serum IgG binding to U1-70K peptides 116–121 ESKLRR(K-biotin), and 143–148 PRGYAF(K-biotin). Peptides were biotinylated on the C-terminus to match the orientation of peptides on the U1-70K microarrays. All the peptides were synthesized by solid phase synthesis, purified by high-performance liquid chromatography (HPLC; >87%), and verified by mass spectrometry. Streptavidin High-Binding Capacity Coated 96-Well Plates (Pierce, Rockford, IL) were coated with the peptides at 2 μg/ml in PBS. Patient serum was diluted 1/100 (or as otherwise indicated) in PBST with 10 mg/ml BSA. All the measurements were performed on duplicate wells. Dissociation-Enhanced Lanthanide Fluorescent Immunoassay (DELFIA) Europium-labeled goat anti-rabbit IgG or mouse anti-human IgG (Fcγ specific) were used as secondary reagents (PerkinElmer, Waltham, MA). Time-resolved fluorescence was determined using a Wallac Victor model 1420 Multilabel Counter (PerkinElmer). For patient serum ELISAs, the signal of 116–121 was subtracted from that of 143–148, and vice versa, to account for non-specific binding. This could have caused an underestimation of the frequency of epitope-reactive patients, in the case of double-positive patient samples. We did not observe any double-positive patients by microarray, suggesting that the occurrence of double-positive patients is below approximately 0.05%.
Competitive ELISA
Two 116–121 reactive patient serum samples were diluted 1/100 in PBST with 5% FCS, and then incubated with U1-70K peptide 68–72 (ERKRR), a scrambled control peptide (RRERK), or vehicle for 20 min at room temperature (peptide concentration = 16 μg/ml). Both peptides were synthesized by solid phase synthesis, purified by HPLC (490%) and verified by mass spectrometry. Following incubation, duplicate samples were used to probe Streptavidin High-Binding Capacity Coated 96-Well Plates (Pierce) that had been coated with 116–121 ESKLRR(K-biotin) at 2 μg/ml in PBS. DELFIA Europium-labeled mouse anti-human IgG (Fcγ specific) was used as secondary reagent (PerkinElmer), and time-resolved fluorescence was determined using a Wallac Victor model 1420 Multilabel Counter (PerkinElmer).
Statistics
Calculations were performed with R 3.0.2 [29]. Replicate features on the U1-70K microarrays were averaged and median centered prior to analysis. The R package pheatmap was used to create the hierarchically clustered heatmap [30]. Prism 6 software (GraphPad, La Jolla, CA) was used to perform the Mann–Whitney tests.
Results
Layout of U1-70K silicon-based peptide microarrays
To investigate the precise patterns of antibody reactivity to epitopes within the RNA binding domain of U1-70K, we created silicon-based microarrays featuring peptides corresponding to amino acids 110–170 of the human U1-70K protein (Figure 1A). The microarray layout is illustrated in Figure 1(B), and consists of overlapping 3- to 10-mer peptides in triplicate, tiled with a one amino acid offset across the region, and duplicate 21 × 21 feature blocks representing every possible 1- to 21-mer within the sequence ranges. Each 21 × 21 block on the microarrays has a diagonal line of symmetry, highlighted in yellow in Figure 1(C), with duplicate features on each side. Within the lower right half of each block, moving one feature along a row toward the line of symmetry corresponds to the removal of a single amino acid from the C-terminus of the peptide sequence. Similarly, moving one feature up a column corresponds to the removal of a single amino acid from the N-terminus. In all, there are >5700 features, corresponding to 843 unique peptides on each U1-70K microarray.
Figure 1.
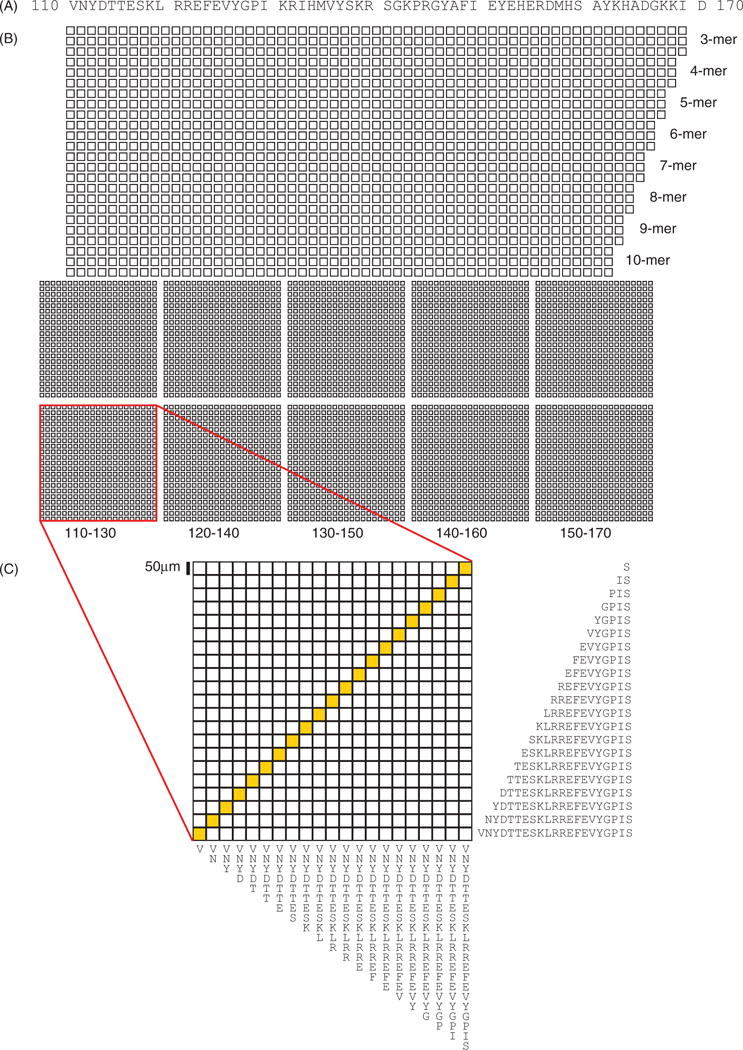
Layout and content of silicon-based U1-70K peptide microarrays. (A) Peptides representing sequences from amino acids 110–170 of the RNA-binding domain of the human U1-70K protein were synthesized using maskless photolithography on the surface of a silicon wafer. (B) The microarray consists of overlapping 3- to 10-mer peptides in triplicate, tiled with a one amino acid offset across the region (upper), and duplicate 21 × 21 feature blocks representing every possible 1- to 21-mer within the sequence ranges shown below (lower). (C) An expanded view of a block featuring peptides derived from amino acid sequence 110–130 is shown. The peptide sequences of features from the bottom row and right-most column are shown for illustration.
Validation of microarrays with commercial U1-70K antibodies
We validated the U1-70K microarrays using two commercial polyclonal antibodies raised against regions of U1-70K that are represented on the microarrays (Figure 2A and S1). Antibody 70R-4901 showed high levels of reactivity to peptides corresponding to sequences within its immunogen (91–140), compared to those that were outside of the immunogen (Figure 2A). Two distinct minimal epitopes, 112–115 and 121–124, were identified, and are highlighted in Figure 2A. Antibody GTX101864 also showed reactivity to peptides within its immunogen, with the minimal epitope 147–152 (Figure S1). Both of the respective isotype control antibodies had minimal reactivity. Reactivity of 70R-4901 to the microarrays was highly reproducible across replicates and experimental days (R2>0.98; Figure S2). Further, reactivity of 70R-4901 to peptide 111–130 as well as other closely related peptides on the microarray was substantially reduced by pre-incubating with soluble 111–130, but not 141–160 (Figure S3).
Figure 2.
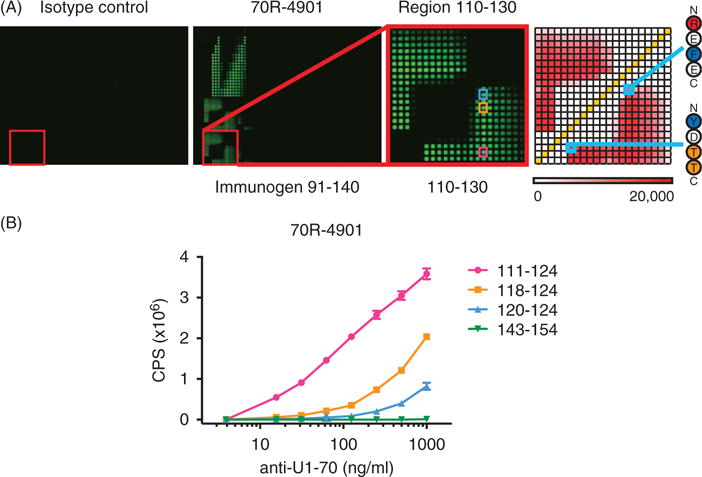
U1-70K peptide microarrays identify the minimal epitopes of an anti-U1-70K polyclonal antibody. (A) An anti-U1-70K polyclonal antibody, 70R-4091, and a nonspecific polyclonal control, were used to probe U1-70K microarrays at 1 μg/ml. Reactivity was detected with a Cy3-conjugated anti-rabbit secondary antibody, and the resulting fluorescent microarray images are shown, including an inset featuring block 110–130 in greater detail. A heatmap (right) shows the Median Fluorescence Intensity (MFI) of each feature in the inset block, and minimal epitopes 112–115 and 121–124 are highlighted. (B) Selected reactive peptides (111–124, 118–124 and 120–124) and a nonreactive control (143–154) were generated as C-terminal lysine-biotinylated peptides using traditional solid-phase synthesis. Indirect peptide ELISA on streptavidin-coated plates was performed using 70R-4091 followed by europium-labeled goat anti-rabbit IgG, and the time-resolved fluorescence counts for each condition are shown (error bars = SD). Reactive peptides used for ELISA are highlighted in the microarray inset in (A).
We confirmed the epitopes of polyclonal 70R-4901 identified using the microarrays by indirect peptide ELISA. We selected a highly reactive sequence spanning both minimal epitopes (111–124), two shorter reactive subsequences (118–124 and 120–124) and a non-reactive sequence (143–154), and generated corresponding biotinylated peptides by traditional solid-phase synthesis. In agreement with the microarrays, we found that peptides 111–124, 118–124 and 120–124 were also reactive by ELISA, while the signal of 143–154 was negligible (Figure 2B). These findings demonstrate that the U1-70K microarray platform is capable of sensitive and specific determination of the minimal epitopes of commercial polyclonal antibodies.
Identification of U1-70K serum autoantibody epitopes at single-amino acid resolution
To identify autoantibody epitopes within the RNA binding domain of U1-70K, we probed U1-70K microarrays with sera from patients with SLE (n = 26) in the Autoimmune Biomarkers Collaborative Network (ABCoN) cohort (Figure 3). We observed diverse binding patterns between SLE patient samples and identified multiple epitopes, including 116–121, 143–148 and 138–142 (Figures 3 and 4).
Figure 3.
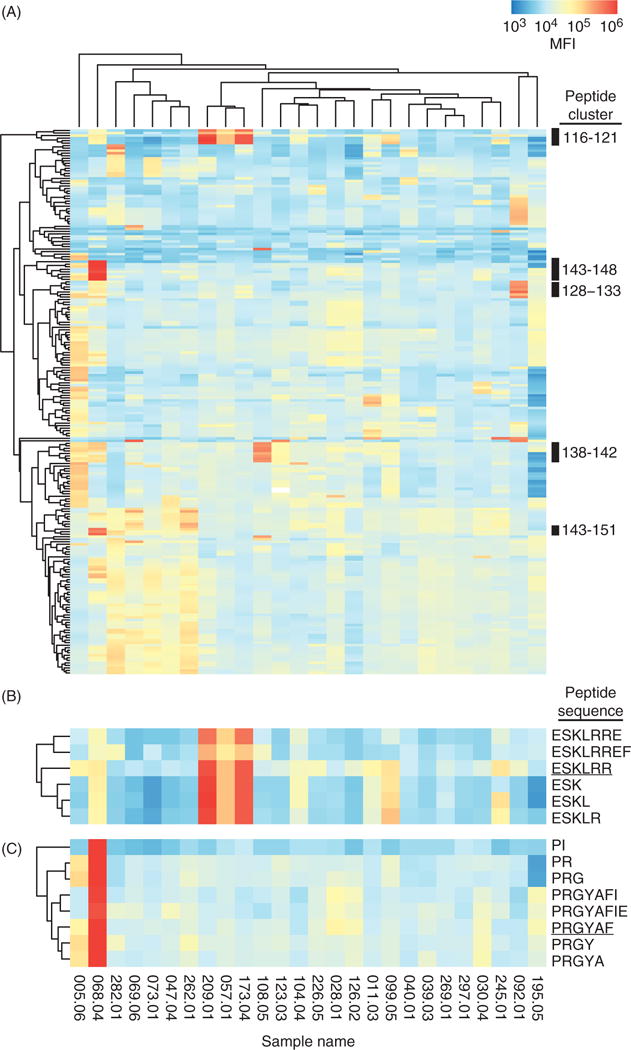
U1-70K peptide microarrays identify the serum antibody epitopes of patients with SLE. (A) Serum samples from patients with SLE (n = 26) were used to probe U1-70K microarrays, and antibody binding was detected using a Cy5-conjugated anti-human IgG (Fcγ specific) secondary antibody. A hierarchically clustered (unsupervised, Euclidean distance) heatmap of 216 peptides, filtered for features with a maximum Z-score <3 is shown. Black bars on the right indicate reactive peptide clusters. Peptide clusters 116–121 and 143–148 are shown in more detail in (B) and (C), respectively. The sequences corresponding to the microarray features are shown on the right, and the peptides used for indirect ELISA are underlined. Sample names are shown at the bottom of each column. The MFIs of each microarray were median centered and log transformed prior to clustering.
Figure 4.
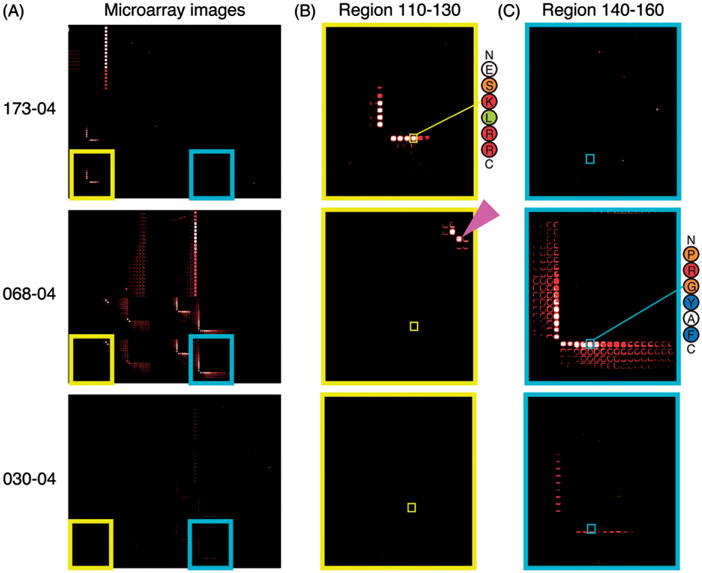
U1-70K microarrays reveal diverse peptide reactivity patterns when probed with SLE patient serum. (A) Fluorescence images from selected microarrays are shown with patient identifiers on the left. Patient samples 173-04 and 068-04 have high reactivity to 116–121 and 143–148, respectively. A less-reactive patient sample, 030-04, is shown for comparison. Insets highlighting reactivity to specific peptide features within the 110–130 (B) and 140–160 (C) blocks are shown. Peptide features corresponding to peptides used for validation by ELISA (116–121 and 143–148) are indicated with smaller squares, and their sequences are shown to the right of the most reactive samples. Peptide 128–129 PI is indicated with a magenta arrow.
Approximately 12% (3/26) of the patients with SLE had serum IgG reactivity to epitope 116–121. Samples 173-04 and 209-01 had high reactivity, while lower levels were observed in 057-01 (Figure 3A). A shorter minimal epitope, 116–118 ESK, was capable of supporting 90% of reactivity to 116–121 (Figure 4B). Reactivity to 116–121 was constrained at the N-terminus to glutamic acid 116, as a gain or loss of a single amino acid at the N-terminus caused a dramatic reduction in reactivity (Figure 4B). Further, a similar peptide on the microarray with a tyrosine in place of the glutamic acid (136–138 YSK) had minimal reactivity, compared to 116–118 ESK. These results show that reactivity to epitope 116–121 is present in the sera of a subset of patients with SLE, is critically dependent on the N-terminal glutamic acid, and can tolerate variation in length at the C-terminus.
Reactivity to epitope 143–148 appeared to be unique to patient sample 068-04 (Figure 3C). Within 143–148, sequences PRGY and PRGYA were the most reactive, while the dipeptide PR was sufficient for 98% of reactivity. Similar to 116–121, a gain or loss of a single amino acid at the N-terminus caused a dramatic reduction in reactivity (Figure 4C). Further, similar dipeptides on the microarray with leucine, glutamic acid, arginine or lysine in place of the N-terminal proline, showed minimal reactivity. A related sequence, 128–129 PI, also showed high levels of reactivity, but reactivity was diminished by the addition of lysine 130 at the C-terminus (Figure 4B, second row). This indicates that the 143–148 epitope is constrained at the N-terminus to proline, allows for some variation at position 2, but shows more sequence specificity if additional amino acids are included at the C-terminus.
Patient sample 108-05 was reactive to region 138–149 (KRSGKPRGYAFI), containing the minimal epitope 138–142 (Figure 3A). Similar to 116–121 and 143–148, binding was constrained at the N-terminus to lysine 138, but allowed for variation in the length of the C-terminus. Peptide 138–149 overlaps with 143–148, but reactivity patterns were distinct between patient samples 108-05 and 068-04. This was likely due to the specificity of the antibodies for their respective epitopes’ unique N-terminal sequences, and emphasizes the advantage of using a platform with single-amino acid resolution.
ELISA confirmation of SLE serum IgG reactivity to U1-70K peptide epitopes
We used indirect peptide ELISA to validate the serum autoantibody epitopes identified with the microarrays. We selected the two most reactive epitopes, 116–121 and 143–148, and synthesized biotinylated peptides corresponding to their sequences by traditional solid-phase synthesis. We assayed patient serum samples 173-04 and 068-04, as they were the most reactive to 116–121 and 143–148 by microarray, respectively. We found there was a high level of agreement with the microarray findings: 173-04 had high levels of IgG reactivity to 116–121, but negligible reactivity to 143–148, while 068-04 showed the opposite pattern (Figure 5A).
Figure 5.
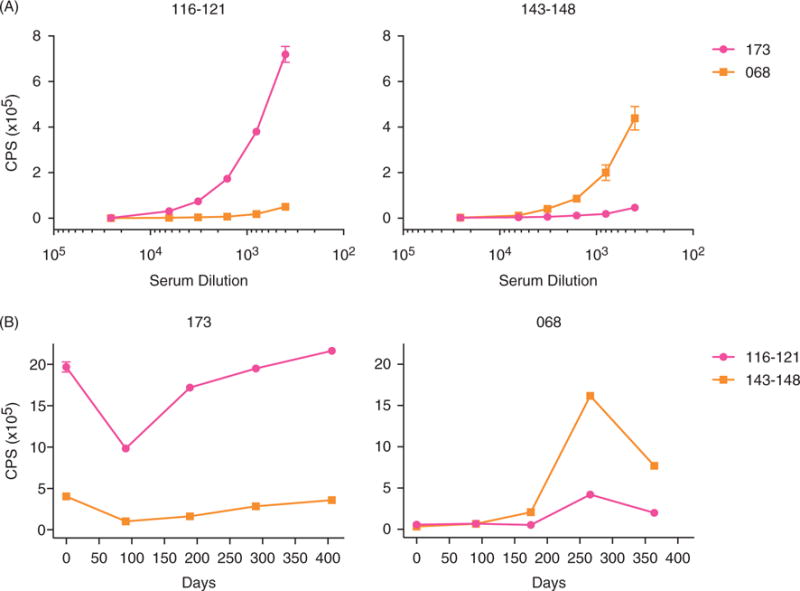
ELISA analysis confirms serum reactivity to U1-70K peptide epitopes, and reveals distinct longitudinal patterns of reactivity between patients. (A) Selected reactive peptide sequences (116–121 and 143–148) were generated as C-terminal lysine-biotinylated peptides using traditional solid-phase synthesis, and were used to perform indirect peptide ELISA. The peptides were bound to streptavidin-coated plates, and the plates were then probed with a dilution series of serum samples from known reactive patients (173 and 068). Binding of the europium-labeled mouse anti-human IgG (Fcγ specific) secondary was quantified by time-resolved fluorescence. (B) Longitudinal serum samples, collected approximately every 3 months and corresponding to 1 year of clinic visits, were assayed by ELISA (error bars = SD).
Longitudinal analysis of reactivity to U1-70K peptide epitopes
To investigate IgG reactivity to peptide epitopes 116–121 and 143–148 over time, we performed peptide ELISA on sera obtained at 5 patient visits each from patients 173 and 068 (Figure 5B). There was an average of 96 days between visits, and the time course had an interval of up to 406 days. We found that patient 173 had relatively stable, high levels of reactivity to 116–121. This corresponded with stable SLE Disease Activity Index (SLEDAI) scores <3 across the visits. Patient 068 had minimal reactivity to 143–148 in the first two visits, followed by a peak at day 266. The increase in reactivity was coincident with an arthritic and hematologic flare at day 266 (SLEDAI of 0 for all the visits except day 266, when it was 7), suggesting that reactivity to 143–148 might be associated with increased disease activity. No other notable clinical features appeared to be associated with reactivity changes to the peptides.
U1-70K epitope reactivity is specific to SLE, and patients with less-active disease
To determine whether reactivity to the peptide epitopes 116–121 and 143–148 was specific to patients with SLE compared to healthy controls, we performed indirect peptide ELISA (Figure 6A). We used sera from patients with SLE (n = 88) and healthy adult controls (n = 92) collected as part of the ABCoN and Stanford-LPCH Vaccine Program cohorts. We found that ~14% (13/88) of patients with SLE had reactivity to 116–121 (using the maximum healthy control signal as a cutoff). Only patient sample 068-04 was positive for 143–148. We compared the SLEDAI scores at the time of sample collection between SLE patients with and without serum reactivity to epitope 116–121, and found that reactive patients had significantly lower scores than non-reactive patients (p = 0.048). While a larger study would be necessary to confirm this finding, it suggests that reactivity to 116–121 may be associated with reduced disease activity (Figure 6B).
Figure 6.
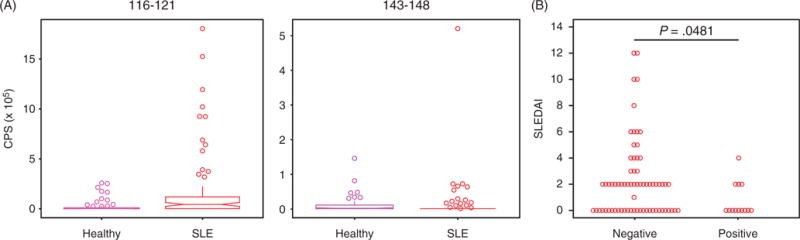
Peptide ELISA reveals a subset of SLE patients with serum IgG reactivity to U1-70K epitope 116–121, and reactivity to 116–121 is associated with decreased disease activity. (A) Biotinylated peptides 116–121 and 143–148 were used to coat 96-well streptavidin coated ELISA plates. Serum samples from patients with SLE (n = 88) and healthy volunteers (n = 92) were used to probe the plates in duplicate, and Europium-labeled anti-human IgG (Fcγ specific) was used as a secondary reagent. Boxplots of the time-resolved fluorescent counts are shown above (vertical bars show 75th percentile + 1.58 × IQR × n−1/2). (B) Patients with SLE were defined as positive or negative if their reactivity to peptide 116–121 was greater or less than the maximum reactivity of the healthy controls, respectively. A Mann–Whitney test was used to compare SLEDAI scores at the time of serum collection between groups.
To investigate reactivity to epitope 116–121 further, we probed a U1-70K microarray with patient serum sample 216-03 (Figure 7). Patient 216 was selected because this subject’s serum samples were consistently reactive to 116–121 across multiple patient visits, as measured by ELISA (coincident with SLEDAI scores of 0 across all the visits, data not shown). In agreement with ELISA, microarray analysis showed that 216-03 was reactive to 116–121. Interestingly, 216-03 had a slightly longer minimum epitope (ESKLR) and would be predicted to have higher specificity to U1-70K compared to the SLE patients who were previously analyzed (ESK). Sample 216-03 also had reactivity to peptide 129–137 (IKRIHMVYS), with minimal epitope 131–136. Unlike the other epitopes we identified, reactivity appeared to allow for variation in length at the N-terminus, but was constrained at the C-terminus to tyrosine or serine at positions 136 or 137, respectively.
Figure 7.
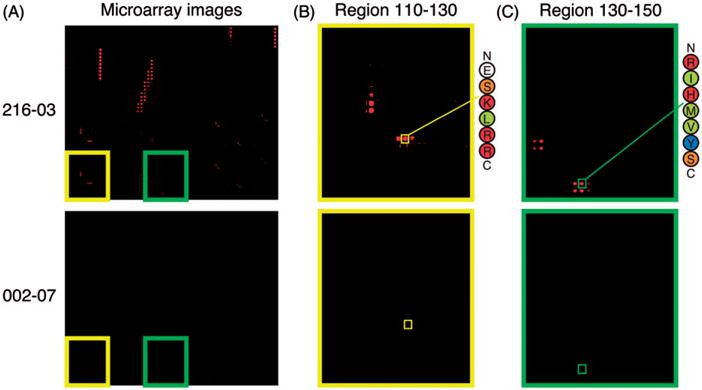
U1-70K microarray analysis of a 116–121 reactive SLE patient serum identified by ELISA. (A) Selected fluorescence images from microarrays are shown with patient identifiers on the left. Patient sample 216-03 was predicted to have high levels of reactivity to 116–121 by peptide ELISA. Patient 002-07 was predicted to be negative to both epitopes by peptides ELISA, and was used as a negative control. Insets highlighting reactivity to specific peptide features with the 110–130 (B) and 130–150 (C) blocks are shown. Sequences of peptides 116–121 and 131–137 are indicated with smaller yellow and green squares, respectively.
Serum antibodies to 116–121 bind a common epitope in U1-70K and influenza B virus
Guldner et al. [31] reported serum antibodies of MCTD and SLE patients bound an epitope (ERKRR) that is common to amino acids 68–72 of U1-70K and the matrix protein M1 of human influenza B viruses. To investigate whether serum antibodies to 116–121 (ESKLRR) also bound to ERKRR, we used competitive ELISA. We selected serum samples from two patients (173 and 216) based on their high reactivity to 116–121. After incubating the serum samples with peptide ERKRR, a scrambled control peptide or vehicle, we measured the remaining IgG reactivity to 116–121 by indirect ELISA. We found that incubation with ERKRR caused substantial reduction in reactivity to 116–121, compared to scrambled peptide and vehicle controls, suggesting antibodies to 116–121 also bind the common epitope found in U1-70K and M1 protein of influenza B (Figure 8).
Figure 8.
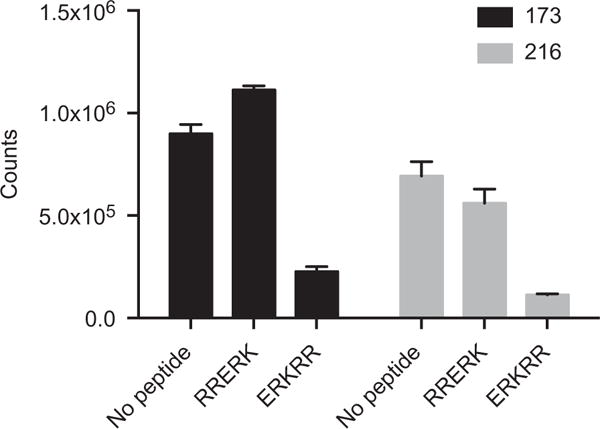
Antibodies to 116–121 bind a common epitope in U1-70K and the matrix protein M1 of human influenza B viruses. Serum samples from two patients, selected based on high reactivity to 116–121, were analyzed using competitive ELISA. The samples were incubated with U1-70K peptide 68–72 (ERKRR), a scrambled control peptide (RRERK) or vehicle. Biotinylated peptide 116–121 was used to coat a 96-well streptavidin coated ELISA plate. Following incubation, the samples were used to probe the plate in duplicate, and Europium-labeled anti-human IgG (Fcγ specific) was used as a secondary reagent. A bar graph of the time-resolved fluorescent counts is shown. Error bars represent SD of duplicate wells.
Discussion and conclusions
In the current study, we characterized the epitopes of serum autoantibodies to U1-70K at single-amino acid resolution using silicon-based microarrays featuring sequences from the RNA binding domain of U1-70K. We validated the microarrays using two commercial U1-70K antibodies, and found that the microarrays were capable of mapping two distinct minimal B cell epitopes within a 13-amino acid long region (Figure 2A). Characterization of antibodies at this level of detail increases their utility as research reagents, and could be used to screen clones for those with desired reactivity profiles.
Microarray analysis of U1-70K autoantibodies in the sera of patients with SLE revealed multiple epitopes, including 116–121 and 143–148, and a high level of epitope diversity between patients. Reactivity to 116–121 was observed in ~14% of SLE patients’ sera, and associated with lower disease activity. Other reports have mapped U1-70K autoantibody reactivity to overlapping regions with 116–121, including 99–128 [20] and 99–120 [11,12]. Our analysis identified a considerably more precise epitope within this region. Netter et al. [12] observed that deletion of residues 116–119 from U1-70K fragment 116–194 resulted in a 50% reduction in the number of reactive patient samples. It was concluded that the region likely contained a discontinuous epitope [12]. Our results show that the fragment contained multiple epitopes, and suggest that the reduction may have resulted from deletion of critical residues within epitope 116–121.
The minimal epitope within 116–121 differed between patients, with reactivity observed to either ESK or ESKLR. Similar inter-patient variability in the length and location of the minimal sequence required for U1-70K autoantibody reactivity, referred to as “microheterogeneity”, has been observed previously [12]. Further, we found reactivity to 116–121 was constrained to glutamic acid 116 at the N-terminus. Traditional epitope mapping techniques would likely miss such subtle examples of microheterogeneity, or epitopes with specific N- or C-termini. Antibodies to the shorter minimal epitope are likely cross-reactive with other proteins or fragments containing ESK at the N-terminus. Future investigation of whether the minimal epitopes of U1-70K autoantibodies change over time, or during an SLE flare, could give additional insight into the unpredictable course of SLE [32].
The mechanisms of development of anti-RNP remain unclear. Retroviruses and influenza viruses have been implicated in triggering autoimmunity to U1-70K through molecular mimicry. Query et al. [33] showed that antibodies to U1-70K were cross-reactive with p30gag proteins of mammalian type C retroviruses, and that anti-RNP was produced by immunization with p30gag. Guldner et al. [31] identified a five amino acid long epitope 68–72 (ERKRR) in U1-70K that was cross-reactive with M1 matrix protein of influenza B viruses. The sequence ERKRR shares similarities with 116–121 (ESKLRR), including an N-terminal glutamic acid as well as multiple basic amino acids, which were identified as a common feature of reactive peptides in RNP+ serum [17]. We found serum antibodies to 116–121 also bound to the M1 matrix protein epitope, further implicating molecular mimicry in the development of anti-RNP.
Evidence suggests that epitope spreading, or the amplification and diversification of immune response through development of reactivity to additional epitopes or molecules, functions in the development of anti-RNP [34]. Greidinger et al. [22] demonstrated that antibodies to components of the U1–snRNP complex emerged in an orderly pattern, often starting with U1-70K and followed by U1-A and U1-C. We showed that antibodies to 116–121 bound epitope 68–72 in U1-70K, indicative of intramolecular epitope spreading. Further, James et al. [17,35] observed SLE serum IgG antibodies to an epitope in the RNA binding domain of U1-A were cross-reactive with ERKRR. Our results indicate intermolecular spreading may also occur between 116–121 in U1-70K and U1-A.
Post-translational modification, including proteolytic cleavage, has also been proposed as a mechanism of breakdown of tolerance to self-proteins [36,37]. We observed that reactivity to 116–121 was critically dependent on its N-terminus, suggesting that it may be a neoepitope created by proteolytic cleavage. Indeed, U1-70K is cleaved in a caspase-3 dependent manner in cells undergoing apoptosis, creating a 40-kDa fragment with an autoantigenic epitope that is not exposed in the full-length protein [38,39].
Previous reports have shown that serum levels of anti-U1-70K increased with disease flare [5], and were greatly reduced in periods of disease remission in patients with MCTD [40]. However, another report found no association between anti-U1-70K and disease activity [41], and RNP is typically not used to monitor disease activity clinically. We observed that reactivity to 116–121 was associated with low disease activity in longitudinal analysis of two patients with consistently high levels of serum IgG reactivity. Further, in a cross-sectional analysis, we found that SLE patients with high levels of serum reactivity to 116–121 had significantly lower disease activity than patients with low levels of serum reactivity. While serum reactivity to 143–148 was limited to a single patient (068), our analysis suggested that it might be associated with increased disease activity. These differences in clinical associations between epitopes highlight the importance of identifying autoantibody epitopes, and the potential to improve clinical autoantibody tests.
A limitation of our approach is that a portion of anti-U1-70K likely was not detected by our microarrays. While the RNA binding domain is the immunodominant autoantigenic region of U1-70K, previously reported U1-70K epitopes outside of this region would not be detected by our microarrays. Evidence suggests that a fraction of U1-70K antibodies bind to conformational epitopes, including a subset of MCTD patients’ antibodies that specifically bind U1-70K in complex with U1-RNA [12,13,42]. Peptide microarrays are not well suited to the identification of conformational or discontinuous epitopes. In instances where it is critical to survey epitopes across a larger region, a potential solution could be to create multiple arrays, corresponding to contiguous subregions. In future generations of the microarrays, some conformational epitopes could be created through the use of “stapled” or cyclic peptides, which can stabilize α-helix or β-turn conformations, respectively [43,44].
Mapping epitopes is often a labor-intensive process, requires multiple iterations of peptide synthesis or protein design and expression, and has relatively low resolution. Silicon-based peptide microarrays have the following advantages: single-amino acid resolution, high-feature content, high reproducibility and low-background fluorescence. The U1-70K microarrays feature a region of U1-70K that is nearly triple the length (61 amino acids) of our previous H2B silicon-based microarrays (21 amino acids). The ability to synthesize regions of this length expands the range of proteins amenable to this technology, and will streamline the precise mapping of antibody epitopes in immune responses. Further, incorporating an underlying integrated circuit into the microprocessor-grade silicon wafer of the microarrays, combined with use of magnetically labeled secondary antibodies, could allow for real-time measurement of antibody binding [45].
In conclusion, we characterized the epitopes of serum autoantibodies to U1-70K at single-amino acid resolution using silicon-based microarrays featuring sequences from the RNA binding domain of U1-70K. The analysis revealed multiple epitopes, including 116–121 and 143–148, with greater precision than previous approaches. Reactivity to 116–121 was observed in ~14% of SLE patients’ sera, and associated with decreased disease activity. Antibodies to 116–121 bound an epitope common to U1-70K (68–72), U1-A and M1 matrix protein of influenza B, implicating molecular mimicry and epitope spreading in the development of anti-RNP. High-resolution mapping of U1-70K epitopes will be critical to understanding the pathogenesis of SLE, enhancing clinical autoantibody tests and designing new peptide therapeutics.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the patients and volunteers who participated in this study. Alex Kuo for critical discussions relating to the article. Rohit Gupta, Robin Castel Navarro and the members of the Utz lab for support.
M.V. and G.C. are employees of Intel Inc. P.J.U. is the recipient of a Donald E. and Delia B. Baxter Foundation Career Development Award and is supported by National Heart, Lung, and Blood Institute (NHLBI) Proteomics contract HHSN288201000034C, Proteomics of Inflammatory Immunity and Pulmonary Arterial Hypertension; grants from the NIH (5 U19-AI082719, 5 U19-AI050864, 5 U19-AI056363, 1 U19 AI090019 and 4 U19 AI090019); Canadian Institutes of Health Research (2 OR-92141); Alliance for Lupus Research (grant number 21858); a gift from the Ben May Trust and a gift from the Floren Family Trust. The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement number 261382. C.L.L. is a recipient of an NIH National Research Service Award Fellowship (5 F32 AI-080086-02). D.J.H. was supported by the Canadian Institutes of Health Research (CIHR). The trial in healthy volunteers enrolled by the Stanford-LPCH Vaccine Program was supported by NIH/NIAID U19AI090019 (M. Davis, P.I.) and an NIH/NCRR CTSA award number UL1 RR025744.
Footnotes
Declaration of interest
All the other authors declare that they have no competing interests.
References
- 1.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 2.Ehrenstein MR, Katz DR, Griffiths MH, et al. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 1995;48:705–711. doi: 10.1038/ki.1995.341. [DOI] [PubMed] [Google Scholar]
- 3.ter Borg EJ, Horst G, Hummel EJ, et al. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study Arthritis Rheum. 1990;33:634–643. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]
- 4.Kattah NH, Kattah MG, Utz PJ. The U1-snRNP complex: structural properties relating to autoimmune pathogenesis in rheumatic diseases. Immunol Rev. 2010;233:126–145. doi: 10.1111/j.0105-2896.2009.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habets WJ, de Rooij DJ, Hoet MH, et al. Quantitation of anti-RNP and anti-Sm antibodies in MCTD and SLE patients by immunoblotting. Clin Exp Immunol. 1985;59:457–466. [PMC free article] [PubMed] [Google Scholar]
- 6.Reichlin M, Van Venrooij WJ. Autoantibodies to the URNP particles: relationship to clinical diagnosis and nephritis. Clin Exp Immunol. 1991;83:286–290. doi: 10.1111/j.1365-2249.1991.tb05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habets WJ, de Rooij DJ, Salden MH, et al. Antibodies against distinct nuclear matrix proteins are characteristic for mixed connective tissue disease. Clin Exp Immunol. 1983;54:265–276. [PMC free article] [PubMed] [Google Scholar]
- 8.St Clair EW, Query CC, Bentley R, et al. Expression of autoantibodies to recombinant (U1) RNP-associated 70K antigen in systemic lupus erythematosus. Clin Immunol Immunopathol. 1990;54:266–280. doi: 10.1016/0090-1229(90)90088-8. [DOI] [PubMed] [Google Scholar]
- 9.Sharp GC, Irvin WS, Tan EM, et al. Mixed connective tissue disease – an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA) Am J Med. 1972;52:148–159. doi: 10.1016/0002-9343(72)90064-2. [DOI] [PubMed] [Google Scholar]
- 10.Sharp GC, Irvin WS, May CM, et al. Association of antibodies to ribonucleoprotein and Sm antigens with mixed connective-tissue disease, systematic lupus erythematosus and other rheumatic diseases. N Engl J Med. 1976;295:1149–1154. doi: 10.1056/NEJM197611182952101. [DOI] [PubMed] [Google Scholar]
- 11.Guldner HH, Netter HJ, Szostecki C, et al. Epitope mapping with a recombinant human 68-kDa (U1) ribonucleoprotein antigen reveals heterogeneous autoantibody profiles in human autoimmune sera. J Immunol. 1988;141:469–475. [PubMed] [Google Scholar]
- 12.Netter HJ, Guldner HH, Szostecki C, Will H. Major autoantigenic sites of the (U1) small nuclear ribonucleoprotein-specific 68-kDa protein. Scand J Immunol. 1990;32:163–176. doi: 10.1111/j.1365-3083.1990.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 13.Cram DS, Fisicaro N, Coppel RL, et al. Mapping of multiple B cell epitopes on the 70-kilodalton autoantigen of the U1 ribonucleoprotein complex. J Immunol. 1990;145:630–635. [PubMed] [Google Scholar]
- 14.Degen WG, Pieffers M, Welin-Henriksson E, et al. Characterization of recombinant human autoantibody fragments directed toward the autoantigenic U1-70K protein. Eur J Immunol. 2000;30:3029–3038. doi: 10.1002/1521-4141(200010)30:10<3029::AID-IMMU3029>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Nyman U, Lundberg I, Hedfors E, Pettersson I. Recombinant 70-kD protein used for determination of autoantigenic epitopes recognized by anti-RNP sera. Clin Exp Immunol. 1990;81:52–58. doi: 10.1111/j.1365-2249.1990.tb05290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda Y, Nyman U, Winkler A, et al. Antigenic domains on the U1 small nuclear ribonucleoprotein-associated 70K polypeptide: a comparison of regions selectively recognized by human and mouse autoantibodies and by monoclonal antibodies. Clin Immunol Immunopathol. 1991;61:55–68. doi: 10.1016/s0090-1229(06)80007-3. [DOI] [PubMed] [Google Scholar]
- 17.James JA, Scofield RH, Harley JB. Basic amino acids predominate in the sequential autoantigenic determinants of the small nuclear 70K ribonucleoprotein. Scand J Immunol. 1994;39:557–566. doi: 10.1111/j.1365-3083.1994.tb03413.x. [DOI] [PubMed] [Google Scholar]
- 18.Monneaux F, Briand JP, Muller S. B and T cell immune response to small nuclear ribonucleoprotein particles in lupus mice: autoreactive CD4(+) T cells recognize a T cell epitope located within the RNP80 motif of the 70K protein. Eur J Immunol. 2000;30:2191–2200. doi: 10.1002/1521-4141(2000)30:8<2191::AID-IMMU2191>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 19.Poole BD, Schneider RI, Guthridge JM, et al. Early targets of nuclear RNP humoral autoimmunity in human systemic lupus erythematosus. Arthritis Rheum. 2009;60:848–859. doi: 10.1002/art.24306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriksson EW, Pettersson I. Autoepitope-mapping of the U1-70K protein with human-Drosophila chimeric proteins. J Autoimmun. 1997;10:559–568. doi: 10.1006/jaut.1997.0163. [DOI] [PubMed] [Google Scholar]
- 21.Welin Henriksson E, Wahren-Herlenius M, Lundberg I, et al. Key residues revealed in a major conformational epitope of the U1-70K protein. Proc Natl Acad Sci USA. 1999;96:14487–14492. doi: 10.1073/pnas.96.25.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greidinger EL, Hoffman RW. The appearance of U1 RNP antibody specificities in sequential autoimmune human antisera follows a characteristic order that implicates the U1-70 kd and B′/B proteins as predominant U1 RNP immunogens. Arthritis Rheum. 2001;44:368–375. doi: 10.1002/1529-0131(200102)44:2<368::AID-ANR55>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Price JV, Tangsombatvisit S, Xu G, et al. On silico peptide microarrays for high-resolution mapping of antibody epitopes and diverse protein–protein interactions. Nat Med. 2012;18:1434–1440. doi: 10.1038/nm.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kattah NH, Newell EW, Jarrell JA, et al. Tetramers reveal IL-17-secreting CD4+ T cells that are specific for U1-70 in lupus and mixed connective tissue disease. Proc Natl Acad Sci USA. 2015;112:3044–3049. doi: 10.1073/pnas.1424796112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmer R, Scherbarth HR, Rillo OL, et al. Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled phase IIb clinical trial. Ann Rheum Dis. 2013;72:1830–1835. doi: 10.1136/annrheumdis-2012-202460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 28.Price JV, Jarrell JA, Furman D, et al. Characterization of influenza vaccine immunogenicity using influenza antigen microarrays. PLoS One. 2013;8:e64555. doi: 10.1371/journal.pone.0064555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 30.Kolde R. Pretty Heatmaps. 0.7.7. R package; 2013. [Google Scholar]
- 31.Guldner HH, Netter HJ, Szostecki C, et al. Human anti-p68 autoantibodies recognize a common epitope of U1 RNA containing small nuclear ribonucleoprotein and influenza B virus. J Exp Med. 1990;171:819–829. doi: 10.1084/jem.171.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham KL, Robinson WH, Steinman L, Utz PJ. High-throughput methods for measuring autoantibodies in systemic lupus erythematosus and other autoimmune diseases. Autoimmunity. 2004;37:269–272. doi: 10.1080/08916930410001710686. [DOI] [PubMed] [Google Scholar]
- 33.Query CC, Keene JD. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell. 1987;51:211–220. doi: 10.1016/0092-8674(87)90148-6. [DOI] [PubMed] [Google Scholar]
- 34.Monneaux F, Muller S. Key sequences involved in the spreading of the systemic autoimmune response to spliceosomal proteins. Scand J Immunol. 2001;54:45–54. doi: 10.1046/j.1365-3083.2001.00942.x. [DOI] [PubMed] [Google Scholar]
- 35.James JA, Harley JB. Human lupus anti-spliceosome A protein autoantibodies bind contiguous surface structures and segregate into two sequential epitope binding patterns. J Immunol. 1996;156:4018–4026. [PubMed] [Google Scholar]
- 36.Utz PJ, Anderson P. Posttranslational protein modifications, apoptosis, and the bypass of tolerance to autoantigens. Arthritis Rheum. 1998;41:1152–1160. doi: 10.1002/1529-0131(199807)41:7<1152::AID-ART3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 37.Doyle HA, Yang ML, Raycroft MT, et al. Autoantigens: novel forms and presentation to the immune system. Autoimmunity. 2014;47:220–233. doi: 10.3109/08916934.2013.850495. [DOI] [PubMed] [Google Scholar]
- 38.Casciola-Rosen LA, Miller DK, Anhalt GJ, Rosen A. Specific cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell death. J Biol Chem. 1994;269:30757–30760. [PubMed] [Google Scholar]
- 39.Degen WG, Aarssen Y, Pruijn GJ, et al. The fate of U1 snRNP during anti-Fas induced apoptosis: specific cleavage of the U1 snRNA molecule. Cell Death Differ. 2000;7:70–79. doi: 10.1038/sj.cdd.4400617. [DOI] [PubMed] [Google Scholar]
- 40.Burdt MA, Hoffman RW, Deutscher SL, et al. Long-term outcome in mixed connective tissue disease: longitudinal clinical and serologic findings. Arthritis Rheum. 1999;42:899–909. doi: 10.1002/1529-0131(199905)42:5<899::AID-ANR8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 41.Margaux J, Hayem G, Palazzo E, et al. Clinical usefulness of antibodies to U1snRNP proteins in mixed connective tissue disease and systemic lupus erythematosus. Rev Rhum Engl Ed. 1998;65:378–386. [PubMed] [Google Scholar]
- 42.Murakami A, Kojima K, Ohya K, et al. A new conformational epitope generated by the binding of recombinant 70-kd protein and U1 RNA to anti-U1 RNP autoantibodies in sera from patients with mixed connective tissue disease. Arthritis Rheum. 2002;46:3273–3282. doi: 10.1002/art.10677. [DOI] [PubMed] [Google Scholar]
- 43.Stewart ML, Fire E, Keating AE, Walensky LD. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat Chem Biol. 2010;6:595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorow DS, Shi PT, Carbone FR, et al. Two large immunogenic and antigenic myoglobin peptides and the effects of cyclisation. Mol Immunol. 1985;22:1255–1264. doi: 10.1016/0161-5890(85)90044-6. [DOI] [PubMed] [Google Scholar]
- 45.Osterfeld SJ, Yu H, Gaster RS, et al. Multiplex protein assays based on real-time magnetic nanotag sensing. Proc Natl Acad Sci USA. 2008;105:20637–20640. doi: 10.1073/pnas.0810822105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


