Abstract
We have shown in a rodent model of hemorrhagic shock (HS) that fresh frozen plasma (FFP) reduces lung inflammation and injury which are correlated with restitution of syndecan-1. Since the gut is believed to contribute to distant organ injury and inflammation after shock, the current study sought to determine if the protective effects of plasma would extend to the gut and to elucidate the contribution of syndecan-1 to this protective effect. We also examined the potential role of TNFα, and a disintegrin and metalloproteinase (ADAM)-17, both intestinal sheddases of syndecan-1. Wild-type (WT) and syndecan-1−/− (KO) mice were subjected to HS followed by resuscitation with lactated ringers (LR) or FFP and compared to shock alone and shams. Small bowel and blood were obtained after 3 hours for analysis of mucosal injury and inflammation and TNFα and ADAM-17 protein expression and activity. After HS, gut injury and inflammation were significantly increased compared to shams. Resuscitation with LR decreased both injury and inflammation which were further lessened by FFP. KO mice displayed worsened gut injury and inflammation after HS compared to WT mice, and LR and FFP equivalently inhibited injury and inflammation. Both systemic and intestinal TNFα and ADAM-17 followed similar trends, with increases after HS, reduction by LR, and a further decrease by FFP in WT but not KO mice. In conclusion, FFP decreased gut injury and inflammation after hemorrhagic shock, an effect that was abrogated in syndecan-1−/− mice. Plasma also decreased TNFα and ADAM-17, representing a potential mechanistic link to its protection via syndecan-1.
INTRODUCTION
Hemorrhage remains a leading cause of early deaths in severely injured patients. (1) Recent efforts have focused on improved techniques for control of truncal and extremity bleeding while resuscitative strategies are attempting to optimize the timing and ratios of blood component administration.(2) The early and empiric use of fresh frozen plasma (FFP) in hemodynamically unstable patients with bleeding has led to a decrease in early hemorrhagic deaths.(3,4) This decrease in mortality from a plasma-based resuscitation strategy appears to extend beyond its ability to correct trauma-induced coagulopathy and provide hemorrhage control and is hypothesized to involve additional protective effects of plasma on a dysfunctional endothelium that follows trauma and hemorrhage.(5) Endothelial dysfunction leads to not only coagulation abnormalities but also to inflammation and breakdown of organ specific endothelial and epithelial barrier integrity. We have previously shown in a rodent model that hemorrhagic shock results in loss of pulmonary vascular integrity with subsequent hyperpermeability and edema that is mitigated by plasma based resuscitation.(6) Similarly, shock-induced lung inflammation and injury was significantly decreased by plasma. Concurrent with these improvements in lung injury were alterations in pulmonary syndecan-1 and the endothelial glycocalyx.(7)
Syndecans are cell surface or transmembrane co-receptors that modulate binding and signaling of cytokines, chemokines, and adhesion molecules, among other heparin-binding molecules. Syndecan-1 has been a focal area of our investigations into plasma's systemic protective effects on the endothelium. Syndecan-1 is found on both endothelial and epithelial cells although much of the literature refers to shed syndecan-1 as a potential surrogate marker for the endothelial glycocalyx.(8-11) Epithelial cells also possess a glycocalyx structure and pertubations of epithelial syndecan-1 can lead to intestinal inflammation, edema and injury after gut ischemia/reperfusion.(12,13) Therefore, in the current study we investigated the role of intestinal syndecan-1 on gut injury and inflammation after plasma-based resuscitation following trauma and hemorrhage. We hypothesized that plasma would lessen injury to the gut through restoration of syndecan-1 on the intestinal epithelium. We tested this hypothesis using a clinically relevant coagulopathic mouse model of trauma and hemorrhage in WT and Sdc1-KO mice. We also examined the potential role of TNFα and a disintegrin and metalloproteinase (ADAM)-17, as intestinal sheddases of syndecan-1 that may contribute to the loss of syndecan-1 after hemorrhage and restitution by plasma.
MATERIALS AND METHODS
Mouse model of hemorrhagic shock
All procedures performed were protocols approved by the University of Texas Houston Medical School and the University of Maryland School of Medicine Animal Welfare Committees. The experiments were conducted in compliance with the National Institutes of Health guidelines on the use of laboratory animals. All animals were housed at constant room temperature with a 12:12-h light-dark cycle with access to food and water ad libitum. Male syndecan-1−/− mice (generously provided by Dr. Pyong Park) on the C57BL/6J background and wild type littermates, 8-10 weeks of age and weighing approximately 20 grams, were used for all experiments. An established coagulopathic mouse model of trauma-hemorrhagic shock was utilized.(6) Under isoflurane anesthesia, a midline laparotomy incision was made, the organs inspected, then the incision closed. Bilateral femoral arteries were cannulated for continuous hemodynamic monitoring and blood withdrawal or resuscitation. After 10-minute period of equilibration, mice were bled to a mean arterial pressure (MAP) of 35±5 mmHg and maintained for 90 minutes. Shams underwent anesthesia and placement of catheters but were not subjected to hemorrhagic shock. Mice were resuscitated with either lactated Ringer's (LR) at 3X shed blood volume or fresh frozen plasma at 1× shed blood and compared to animals that underwent shock alone as we have described.(6) Fresh frozen plasma was obtained from Gulf Coast Regional Blood Bank, Houston,TX.
Upon completion of resuscitation, vascular catheters were removed, incisions closed, and the animals were awoken from anesthesia. After three hours, animals were sacrificed by exsanguination under isoflurane anesthesia. Blood was obtained at the time of sacrifice and the distal small intestine was harvested for further analysis. The three hour time point was chosen based on our previous investigation showing partial restoration of the endothelial glycocalyx and pulmonary syndecan-1 by plasma at this time point.(7)
Small intestine mucosal damage
Intestinal tissue was embedded, placed on slides and stained with hematoxylin and eosin. Five random fields with 100-250 villi from each mouse were analyzed in a blinded fashion by means of light microscopy at 100X magnification. Villi were identified as having damage if any one of the following were present: development of a subepithelial space, extension of the subepithelial space with moderate lifting of the epithelial layer from the lamina propria, massive epithelial lifting down the sides of the villi, denuded villi with lamina propria and dilated capillaries and/or digestion and disintegration of the lamina propria with hemorrhage, and ulceration.(14)
Small intestine neutrophil infiltration
Intestinal tissue was placed on slides, and neutrophils were stained using naphthol ASD chloroacetate esterase. Tissue was stained via hematoxylin counterstain and neutrophils counted per area of tissue in a blinded fashion by means of light microscopy at 100X magnification.
Small intestine and plasma TNFα and A disintegrin and metalloproteinase (ADAM)-17
Levels of TNFα and ADAM-17, also known as TNFα converting enzyme (TACE), in plasma and small intestinal tissue were measured by mouse TNFα ELISA kit and mouse ADAM-17 ELISA kit (MyBioSource, San Diego, CA). The activity of ADAM-17 in the intestinal tissue was measured by SensoLyte 520 TACE (α-secretase) activity assay kit (AnaSpec, Fremont, CA ) according to manufacturers’ instructions. Results were normalized to protein concentration, and there were no significant differences between groups (data not shown).
Data Analysis
All data was analyzed by one way analysis of variance (ANOVA) with Bonferroni correction. P values < 0.05 were considered significant. Data are expressed as mean ± SEM, n=8 per group.
RESULTS
Plasma mitigates gut injury and inflammation in wild type but not syndecan-1−/− mice
In wild type mice, gut injury (Figure 1) and inflammation (Figure 2) were significantly increased after hemorrhagic shock (46.3 ±3.7% injury and 62.1± 3.9 inflammation) compared to shams (2.6± 1.2% injury and 5.0± 1.2 and inflammation). Resuscitation with LR decreased both injury (25.6±2.9%) and inflammation (44.3±2.0) and these parameters were further decreased by FFP (15.4±1.8% injury and 29.5±2.5 inflammation). Additionally, KO mice had worsened gut injury (64.4± 3.0%) and inflammation (118± 5.9) after HS compared to WT mice. While LR decreased these parameters in KO mice (45.9±3.0% injury and 88.1±3.2 inflammation), there was no further protection by FFP (40.3 ±3.1% injury and 80.6 ±3.3 inflammation).
Figure 1. Plasma mitigates gut injury in wild type but not syndecan-1 null mice.
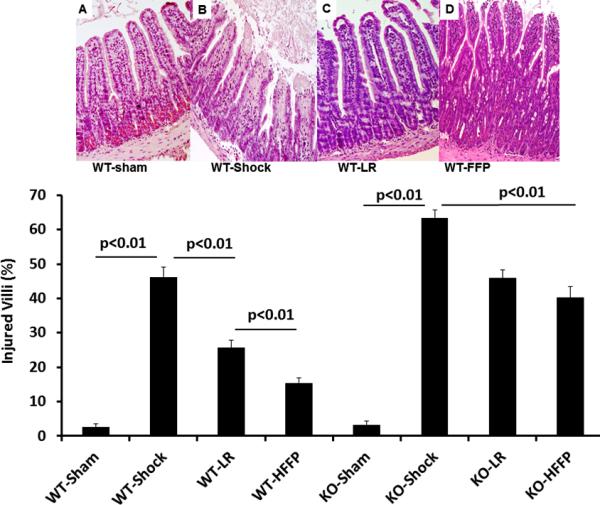
Wild-type and syndecan-1 null mice underwent laparotomy and hemorrhagic shock followed by resuscitation with either lactated Ringers or fresh frozen plasma. After three hours small bowel was harvested and histopathologic injury assessed. Representative images from WT mice are shown for each group: (A) WT shams, (B) WT shock (C) WT LR and (D) WT FFP. The percentage of injured villi are shown in the graph. Data are expressed as mean±SEM, n=8/group with significance indicated by lines over the respective groups. WT= wild type; LR= lactated ringers, FFP= fresh frozen plasma
Figure 2. Plasma mitigates inflammation in wild type but not syndecan-1 null mice.
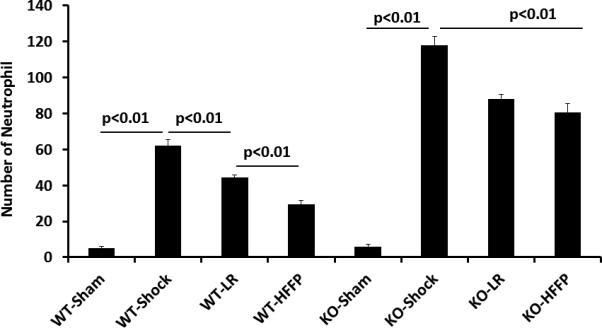
Wild-type and syndecan-1 null mice underwent laparotomy and hemorrhagic shock followed by resuscitation with either lactated Ringers or fresh frozen plasma. After three hours small bowel was harvested for assessment of inflammation by quantifying neutrophilic infiltration. Data are expressed as mean±SEM, n=8/group with significance indicated by lines over the respective groups.
Plasma lessens systemic and gut TNFα and ADAM-17 in wild type but not syndecan-1−/− mice
There was a low but measurable level of systemic TNFα in WT and KO sham animals (WT 20.9±1.9 pg/ml and null 23.9 ±1.5pg/ml) that was significantly increased after hemorrhagic shock in WT (155.8±6.3 pg/ml) mice and further increased in KO mice (207.9±11.9pg/ml) (Figure 3A). TNFα significantly decreased with LR resuscitation in WT (79.7±4.0 pg/ml) and KO (142.8±6.9 pg/ml), though remained significantly higher in KO mice. FFP further decreased TNFα in WT (49.3±1.8 pg/ml) but not KO mice (126.6±7.2 pg/ml FFP).
Figure 3. Plasma lessens systemic TNFα and ADAM-17 protein in wild type but not syndecan-1 KO mice.
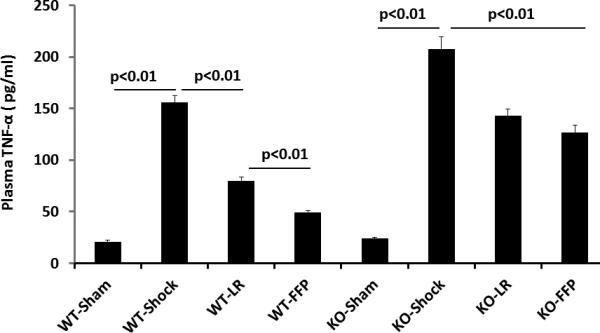
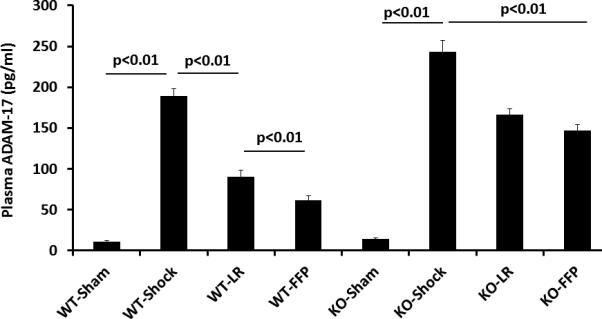
Wild-type and syndecan-1 KO mice underwent laparotomy and hemorrhagic shock followed by resuscitation with either lactated Ringers or fresh frozen plasma. After three hours animals were euthanized and blood obtained for measurement of TNFα and ADAM-17 protein by ELISA. Data are expressed as mean±SEM, n=8/group with significance indicated by lines over the respective groups.
ADAM-17, a protein that cleaves TNFα on the cell surface, was found to be present at protein levels that systemically mirrored those of TNFα with an increase after shock (189.1±8.7 pg/ml WT) and a further increase in KO mice (243.4±13.5 pg/ml vs shams 11.0±1.2 pg/ml WT and 14.1±1.5pg/ml null) (Figure 3B). There was a decrease with LR in both WT (90.5±7.4 pg/ml) and KO mice (166.6±7.3 pg/ml) though ADAM-17 remained significantly increased in KO mice compared to WT. Similarly, FFP decreased ADAM-17 in WT mice (61.8±5.2 pg/ml) but not in KO mice (147.0±7.4 pg/ml). Lastly, to ensure that ADAM-17 protein levels accurately reflected ADAM-17 activity, plasma ADAM-17 activity was measured (Figure 4A) and confirmed the protein measurements.
Figure 4. ADAM-17 activity in wild type and syndecan-1 null mice.
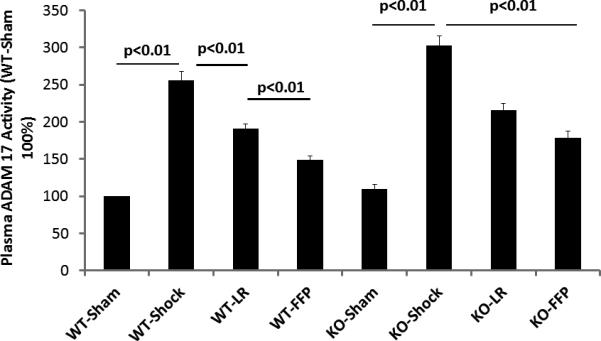
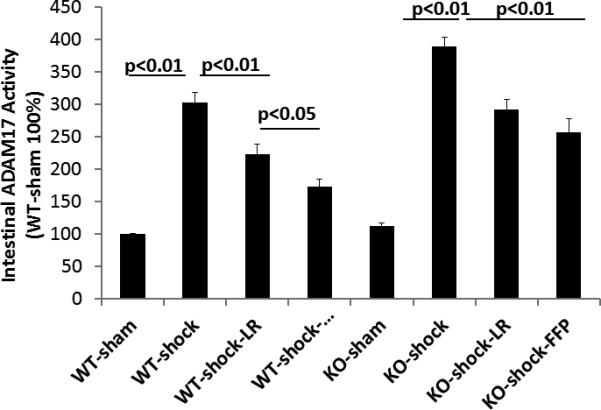
Wild-type and syndecan-1 null mice underwent laparotomy and hemorrhagic shock followed by resuscitation with either lactated Ringers or fresh frozen plasma. After three hours animals were euthanized and small bowel and blood obtained for measurement of ADAM-17 activity. Data are expressed as mean±SEM, n=8/group with significance indicated by lines over the respective groups.
The potential contribution of intestinal TNF α and ADAM-17 to plasma's protection was next assessed. The overall protein levels of both were markedly increased compared to systemic levels but the pattern of change was similar between serum and small intestinal TNFα and ADAM-17 protein (Table 1). ADAM-17 intestinal activity is shown in Figure 4B. In WT mice, ADAM-17 activity was significantly increased after hemorrhagic shock (303±15%), lessened by LR (223±16%), but further decreased by plasma (173±11%). These findings are suggestive of a role of FFP in the regulation of ADAM-17 (or TACE) activity. In KO mice, there was a further increase in ADAM-17 activity after hemorrhagic shock (388±14%) compared to WT, with a reduction by LR (292±15%) but no further decrease by plasma (256±21%).
Table 1.
Intestinal TNFα and ADAM-17 protein following hemorrhagic shock and resuscitation.
| TNFα | TNFα | ADAM-17 | ADAM-17 | |
|---|---|---|---|---|
| Group | WT | KO | WT | KO |
| Sham | 20.9 ± 1.0 | 22.0 ± 1.3 | 1.31 ±0.06 | 1.28 ±0.07 |
| HS | 38.8 ± 1.5* | 45.8 ± 0.8* | 2.67 ±0.10* | 3.43 ±0.08* |
| HS-LR | 29.9 ± 0.8*# | 39.3 ± 1.2*# | 1.94 ±0.08*# | 2.37 ±0.10*# |
| HS-FFP | 24.5 ± 1 .0#^ | 36.9 ± 0.8*# | 1.52 ±0.05*#^ | 2.12 ±0.11*# |
WT= wild tye; KO=knock out; HS= hemorrhagic shock; LR=lactated Ringers; FFP=fresh frozen plasma. Results are presented as ng/ml; mean±SEM, n=8/group.
p<0.01 vs sham of respective WT or KO mouse
p<0.01 vs HS of respective WT or KO mouse
p<0.01 vs LR of respective WT or KO mouse
DISCUSSION
The current data demonstrates for the first time that resuscitation with plasma after hemorrhagic shock provides protection to intestinal epithelial cells, an effect that is mediated in part by syndecan-1. Gut injury and inflammation were increased after hemorrhagic shock and lessened by plasma in WT but not syndecan-1 KO mice, suggesting a critical role for syndecan-1 in FFP mediated gut protection. The primary focus of most syndecan-1 studies has been based on its endothelial, rather than epithelial, location.(8-11, 15) However, recent reports have shown that epithelial syndecan-1 is important to intestinal pathologies.(12, 16) We have shown in an in-vitro model of hypoxia/reoxygenation that intestinal epithelial cell syndecan-1 shedding was significantly increased and cell surface syndecan-1 immunostaining reduced.(13) Clinical studies in patients with inflammatory bowel disease have shown that Crohns patients had significantly reduced intestinal syndecan-1 that correlated with disease severity.(17) Yablecovitch et al demonstrated elevated levels of soluble syndecan-1 in serum of patients with both Crohn's and ulcerative colitis.(18) These studies highlight the important and newly appreciated role of syndecan-1 in intestinal pathology. Hemorrhagic shock, though not an intestine specific insult, is clearly a systemic insult that results in vascular and epithelial barrier compromise in multiple organs (19)
In our past work, we have shown in a mouse model of LPS that syndecan-1 shedding protects the host from dysregulated inflammation by facilitating resolution of neutrophilic inflammation.(20) In these studies ablation of syndecan-1 resulted in significantly increased neutrophil accumulation in multiple organs. Syndecan-1 did not affect systemic or local expression of inflammatory factors such as TNFα but specifically facilitated removal of CXC chemokines. This is in distinct contrast to the current findings in a mouse model of hemorrhagic shock where both local (gut) and systemic levels of TNFα were markedly elevated in syndecan-1−/− mice compared to wild type mice. The pro-inflammatory phenotype of null mice after hemorrhagic shock and endotoxic shock suggests that syndecan-1 shedding confers protection, though the precise mechanism remains unclear. We have previously reported in a pilot study of severely injured patients that three pro-inflammatory cytokines with endothelial activity; IFN gamma, fractalkine, and IL-1β, all correlated with systemic levels of shed syndecan-1. (21) We postulated that hemorrhagic shock-induced syndecan-1 shedding which exposes the underlying injured endothelium to these pro-inflammatory cytokines. This in turn can lead to endothelial activation and attraction of pathologic neutrophils to the site of injury. IFN gamma then binds to heparan sulfate moieties on the shed syndecan-1 ectododomain, facilitating resolution of inflammation at the injured endothelium. These data suggest a dual role for syndecan-1 where shedding can be detrimental but also aid in resolution and restoration. Stratt et al recently reported that transfusion of FFP to nonbleeding critically ill patients was associated with a reduction in systemic TNFα and syndecan-1, similar to the results of the current laboratory study. Importantly, FFP did not aggravate the inflammatory response but rather suggested an endothelial stabilizing effect. (22)
Ectodomain shedding of syndecan-1 is regulated by multiple signaling pathways converging on a diverse group of metalloproteinases, referred to as sheddases. The ADAM family comprises a large group of these sheddases.(23) We sought to specifically investigate the role of ADAM17, also known as TNFα converting enzyme (TACE), as a modulator of syndecan-1 shedding. ADAM-17 is co-expressed with syndecan-1 on epithelial cells and has been shown to mediate syndecan-1 shedding in lung epithelial cells.(24, 25) In wild type mice intestinal ADAM 17 was increased after hemorrhagic shock, lessened by resuscitation with LR and further decreased by plasma. Additionally, ADAM-17 protein and activity were higher in syndecan-1 KO mice after shock and resuscitation and not lessened by plasma when compared to LR resuscitation. These data suggest that there may be complex pathways involved that interlink syndecan-1 to ADAM17 expression and activity.
ADAM17 has diverse substrates which can include adhesion proteins and receptors, growth factors, and pro-inflammatory cytokines such as TNFα.(26) TNFα is elevated early after hemorrhagic shock in patients and in rodents, as shown in the current study.(27,28) There was a 65 fold increase in systemic TNFα in WT mice after hemorrhagic shock compared to sham mice. Whether the gut merely instigates a systemic response or directly contributes to the systemic response via gut-derived shed TNFα is unknown. There is data in the cancer literature that epithelial tumors shed syndecan systemically, suggesting that in pathologic states shed epithelial ectodomains can enter the vasculature.(29,30) It is therefore conceivable that some portion of cleaved intestinal TNFα contributed to the increase in systemic TNFα. Results of the current study support, but no not prove, syndecan-1 shedding after hemorrhagic shock by TNFα. In addition to functioning as a syndecan-1 sheddase, TNFα can also downregulate syndecan-1 expression. (31,32) Together, these observations suggest that after hemorrhagic shock TNFα induces syndecan-1 shedding in an ADAM17-dependent manner and that FFP inhibits this shedding mechanism.
Wild type mice expressed less gut and systemic TNFα and ADAM 17 than KO mice, consistent with FFP inhibiting sheddase activity or increasing expression of sheddase inhibitors. This does not, however, explain why KO mice expressed more TNFα and ADAM 17 than wild type mice and why plasma did not mitigate expression of TNFα or ADAM 17 in syndecan-1 KO mice. Results at least suggest that plasma is exerting its protection by directly interacting with syndecan-1. It could either restore cell surface syndecan-1 expression by mobilizing preformed syndecan -1 or contain a ligand which binds to syndecan-1 and inhibits subsequent shedding. Plasma contains over 1000 proteins and which, if any, function as effective syndecan-1 ligands has not been investigated. FFP could be exerting its protective effect by inhibiting ADAM-17. It is conceivable that plasma may contain protease inhibitors that could reduce the levels of ADAM-17. We have shown in preliminary studies that a gut protease inhibitor administered after hemorrhagic shock can decrease gut and systemic ADAM-17 (unpublished data).
The current studies represent preliminary studies of the complex interaction of intestinal and systemic syndecan-1, TNFα and ADAM-17, after hemorrhagic shock and their potential role in plasma's protection. Our proposed pathway for hemorrhagic shock-induced syndecan-1 shedding and protection by plasma is shown in Fig. 5. Our results suggest that regulation of syndecan-1 post-shock emerges as a potential therapeutic target for novel drug discovery in the treatment of trauma patients. Further research should focus on the molecular pathways by which FFP elicits its protective effects. Additionally, further biochemical isolation of FFP's constituent proteins and elucidation of their effects upon regulation of syndecan-1 is warranted as is a better understanding of the role of sheddase inhibition by plasma.
Figure 5. Proposed pathway of fresh frozen plasma's protection after hemorrhagic shock.
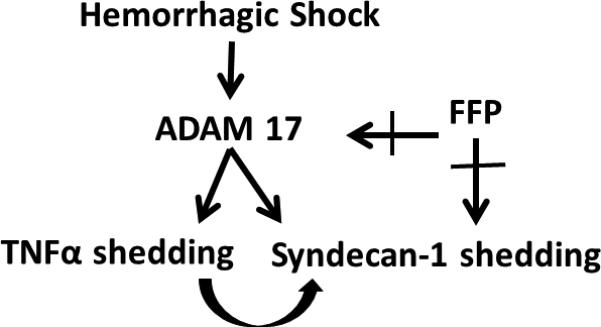
After hemorrhagic shock, ADAM-17 is increased. ADAM-17 is a known sheddase of both TNFα and syndecan-1. Additionally, TNFα is a potent sheddase of syndecan-1. Fresh frozen plasma (FFP) may directly interact with ADAM-17 to reduce its activity and/or interact with syndecan-1 to reconstitute gut epithelial cell syndecan-1 and reduce systemic syndecan-1 shedding.
Acknowledgments
This grant was funded in part by the National Institutes of Health grants RO1GM107482 (RAK) and the Department of Defense W81XWH-11-2-006.
Footnotes
Conflict of Interest: The authors declare no competing conflicts of interest.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the US Department of Defense or the US Government
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Cotton BA, Reddy N, Hatch QM, Lefebrvre E, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Holcomb JB. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254(4):598–605. doi: 10.1097/SLA.0b013e318230089e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, Cotton BA, Matijevic N, Muskat P, Myers JG, Phelan HA, White CE, Zhang J, Rahbar MH, PROMMTT Study Group The prospective, observational, multicenter, massive transfusion study, PROMMTT: comparative effectiveness of a time-varying treatment and competing risks. JAMA Surg. 2013;148(2):127–36. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holcomb JB, Tilley BC, Baraniuk S, Fox EF, Wade CE, Podbielski J, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, Cohen MJ, Cotton BA, Fabian TC, Inaba K, Kerby JD, Muskat P, O'Keeffe T, Rizoli S, Robinson BR, Scalea TM, Schreiber MA, Stein DM, Weinberg JA, Callum JL, Hess JR, Matijevic N, Miller CN, Pittet JF, Hoyt DB, Pearson GD, Leroux B, van Belle G, PROPPR Study Group Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma. The PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkins DH, Rappold JF, Badloe JF, Berséus O, Blackbourne L, Brohi KH, Butler FK, Cap AP, Cohen MJ, Davenport R, DePasquale M, Doughty H, Glassberg E, Hervig T, Hooper TJ, Kozar R, Maegele M, Moore EE, Murdock A, Ness PM, Pati S, Rasmussen T, Sailliol A, Schreiber MA, Sunde GA, van de Watering LM, Ward KR, Weiskopf RB, White NJ, Strandenes G, Spinella PC. THOR position paper on remote damage control resuscitation: definitions, current practice and knowledge gaps. Shock. 2014;41(Suppl 1):3–12. doi: 10.1097/SHK.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng Z, Pati S, Potter D, Brown R, Holcomb JB, Grill R, Wataha K, Park PW, Xue H, Kozar RA. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan-1. Shock. 2013;40(3):195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, Ko TC, Paredes A. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112:1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, Protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254(2):194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 9.Chappell D, Jacob M, Hofmann-Kiefer K, Rehm M, Welsch U, Conzen P, Becker BF. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc Res. 2009;83(2):388–96. doi: 10.1093/cvr/cvp097. [DOI] [PubMed] [Google Scholar]
- 10.Grundmann S, Fink K, Rabadzhieva L, Bourgeois N, Schwab T, Moser M, Bode C, Busch HJ. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation. 2012;83(6):715–20. doi: 10.1016/j.resuscitation.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Bruno R, Peter K, Becker BF. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 12.Bode L, Salvestrini C, Park PW, Li JP, Esko JD, Yamaguchi Y, Murch S, Freeze HH. Heparan sulfate and syndecan-1 are essential in maintaining murine and human intestinal epithelial barrier function. J Clin Invest. 2008;118(1):229–38. doi: 10.1172/JCI32335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng Z, Ban K, Sen A, Grill R, Park PY, Costantini TW, Lin W, Kozar RA. Syndecan-1 plays a novel role in enteral glutamine’s gut protective effects of the post ischemic gut. Shock. 2012;38(1):57–62. doi: 10.1097/SHK.0b013e31825a188a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reino DC, Palange D, Feketeova E, Bonitz RP, Xu da Z, Lu Q, Sheth SU, Peña G, Ulloa L, De Maio A, Feinman R, Deitch EA. Activation of toll-like receptor 4 is necessary for trauma hemorrhagic shock-induced gut injury and polymorphonuclear neutrophil priming. Shock. 2012;38(1):107–14. doi: 10.1097/SHK.0b013e318257123a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung C, Fuernau G, Muench P, Desch S, Eitel I, Schuler G, Adams V, Figulla HR, Thiele H. Impairment of the endothelial glycocalyx in cardiogenic shock and its prognostic relevance. Shock. 2015 Feb 13; doi: 10.1097/SHK.0000000000000329. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Chen Y, Song Y, Zhang S, Xie X, Wang X. Activated syndecan-1 shedding contributes to mice colitis induced by dextran sulfate sodium. Dig Dis Sci. 2011;56:1047–1056. doi: 10.1007/s10620-010-1398-8. [DOI] [PubMed] [Google Scholar]
- 17.Yablecovitch D, Stein A, Shabat-Simon M, Naftali T, Gabay G, Laish I, Oren A, Konikoff FM. Soluble syndecan-1 levels are elevated in patients with inflammatory bowel disease. Dig Dis Sci. 2015 Feb 22; doi: 10.1007/s10620-015-3589-9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Qing Q, Wang Q, Xu J, Zhi F, Park PW, Zhang Y, Chen Y. Syndecan-1 and heparanase: potential markers for activity evaluation and differential diagnosis of Crohn's disease. Inflamm Bowel Dis. 2013;19(5):1025–33. doi: 10.1097/MIB.0b013e318280298f. [DOI] [PubMed] [Google Scholar]
- 19.Fishman JE, Sheth SU, Levy G, Alli V, Lu Q, Xu D, Qin Y, Qin X, Deitch EA. Intraluminal nonbacterial intestinal components control gut and lung injury after trauma hemorrhagic shock. Ann Surg. 2014;260(6):1112–20. doi: 10.1097/SLA.0000000000000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114(14):3033–43. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, Wang WW, Zaske AM, Menge T, Kozar RA. Modulation of Syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS ONE. 2011;6(8):e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Straat M, Müller MC, Meijers JC, Arbous MS, Spoelstra-de Man AM, Beurskens CJ, Vroom MB, Juffermans NP. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: a prospective substudy of a randomized trial. Crit Care. 2015;19:163. doi: 10.1186/s13054-015-0828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gooz M. ADAM-17: The enzyme that does it all. Crit Rev Biochem Mol Biol. 2010;45(2):146–169. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruessmeyer J, Martin C, Hess FM, Schwartz N, Schmidt S, Kogel T, Hoettecke N, Schmidt B, Sechi A, Uhlig S, Ludwig A. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J Biol Chem. 2010;285(1):555–64. doi: 10.1074/jbc.M109.059394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cesaro A, Abakar-Mohamat A, Brest P, Lassalle S, Selva E, Filippi J, Hebuterne X, Hugot JP, Doglio A, Galland F, et al. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009;296(6):G1332–43. doi: 10.1152/ajpgi.90641.2008. [DOI] [PubMed] [Google Scholar]
- 26.Dusterhoft S, Hobel K, Oldefest M, Lokau J, Waetzig GH, Chalaris A, Garbers C, Scheller J, Rose-John S, Lorenzen I, Grotzinger J. A disintegrin and metalloprotease 17 dynamic interaction sequence, the sweet tooth for the human interleukin 6 receptor. J Biol Chem. 2014;289(23):16336–48. doi: 10.1074/jbc.M114.557322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jastrow K, Gonzalez EA, McGuire MF, Suliburk JW, Kozar RA, Iyengar S, Motschall DA, McKinley BA, Moore FA, Mercer DW. Early cytokine production risk stratifies trauma patients for multiple organ failure. J Am Coll Surg. 2009;209:320–331. doi: 10.1016/j.jamcollsurg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Namas R, Ghuma A, Torres A, Polanco P, Gomez H, Barclay D, Gordon L, Zenker S, Kim HK, Hermus L, et al. An adequately robust early TNF-alpha response is a hallmark of survival following trauma/hemorrhage. PLoS One. 2009;4(12):e8406. doi: 10.1371/journal.pone.0008406. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, MacLeod V, Miao HQ, Theus A, Zhan F, Shaughnessy JD, Jr, Sawyer J, Li JP, Zcharia E, Vlodavsky I, Sanderson RD. Heparanase enhances sydnecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007;282:13326–13333. doi: 10.1074/jbc.M611259200. [DOI] [PubMed] [Google Scholar]
- 30.Purushothaman A, Chen L, Yang Y, Sanderson RD. Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. J Biol Chem. 2008:32628–32636. doi: 10.1074/jbc.M806266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry-Stanley MJ, Zhang B, Erlandsen SL, Wells CL. Synergistic effect of tumor necrosis factor-alpha and interferon-gamma on enterocyte shedding of syndecan-1 and associated decreases in internalization of Listeria monocytogenes and Staphylococcus aureus. Cytokine. 2006;34(5-6):252–9. doi: 10.1016/j.cyto.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Kainulainen V, Nelimarkka L, Jarvelainen H, Laato M, Jalkanen M, Elenius K. Suppression of syndecan-1 expression in endothelial cells by tumor necrosis factor-alpha. J Biol Chem. 1996;271(31):18759–66. doi: 10.1074/jbc.271.31.18759. 2. [DOI] [PubMed] [Google Scholar]


