Abstract
Dendritic cells (DCs) are major antigen-presenting cells that can efficiently prime and cross-prime antigen-specific T cells. Delivering antigen to DCs via surface receptors is thus an appealing strategy to evoke cellular immunity. Nonetheless, which DC surface receptor to target to yield the optimal CD8+ and CD4+ T cell responses remains elusive. Herein, we report the superiority of CD40 over 9 different lectins and scavenger receptors at evoking antigen-specific CD8+ T cell responses. However, lectins (e.g., LOX-1 and Dectin-1) were more efficient than CD40 at eliciting CD4+ T cell responses. Common and distinct patterns of subcellular and intracellular localization of receptor-bound αCD40, αLOX-1 and αDectin-1 further support their functional specialization at enhancing antigen presentation to either CD8+ or CD4+ T cells. Lastly, we demonstrate that antigen targeting to CD40 can evoke potent antigen-specific CD8+ T cell responses in human CD40 transgenic mice. This study provides fundamental information for the rational design of vaccines against cancers and viral infections.
Abbreviations: ANOVA, analysis of variance; AP, alkaline phosphatase; APC, antigen-presenting cells; CD, cluster of differentiation; CFSE, carboxyfluorescein succinimidyl ester; Coh, cohesin; CTL, cytotoxic T lymphocyte; DC, dendritic cell; EEA1, early endosome antigen 1; ELISA, enzyme-linked immunosorbent assay; ELISpot, enzyme-linked immunospot; Doc, dockerin; Flu.M1, influenza virus matrix protein 1; GM-CSF, granulocyte-macrophage colony-stimulating factor; HA1, hemagglutinin subunit 1; hCD40Tg, human CD40 transgenic; HLA, human leukocyte antigen; HPV, human papillomavirus; HRP, horseradish peroxidase; IFN, interferon; mAb, monoclonal antibody; IL, interleukin; i.p., intraperitoneal(ly); JaCoP, Just another Colocalization Plugin; LAMP-1, lysosomal-associated membrane protein 1; MART-1, melanoma antigen recognized by T cells 1; mDC, myeloid dendritic cell; MHC, major histocompatibility complex; Mo-DC, monocyte-derived dendritic cell; NHP, non-human primate; NP, nucleoprotein; Poly(I:C), polyinosinic:polycytidylic acid; PBMC, peripheral blood mononuclear cells; PBS, phosphate-buffered saline; pDC, plasmacytoid dendritic cell; PSA, prostate specific antigen; s.c., subcutaneous(ly); TMB, 3,3′,5,5′-tetramethylbenzidine; TLR, toll-like receptor; TNF, tumor necrosis factor
Keywords: Dendritic cell, Cross-presentation, CD40, Lectins, Vaccine
Highlights
-
•
Antigen delivery to DCs via CD40 is more efficient than through nine other receptors at eliciting CD8 T+ cell response.
-
•
Antigen delivery via lectins (e.g., LOX-1 and Dectin-1) is more efficient than CD40 at eliciting CD4+ T cell responses.
The success of an immunotherapeutic vaccine for cancer is largely dependent on its ability to evoke potent cellular immunity. Although targeting antigens to dendritic cells (DCs) has been known to be an efficient strategy to evoke cellular immunity, which targeted receptors yield the optimal cellular immunity remained elusive. We report that targeting CD40, compared to 9 other DC receptors, results in the greatest levels of CD8+ cytotoxic T cell responses, while targeting lectins results in enhanced CD4+ helper T cell responses. The findings of this study will assist us in the rational design of immunotherapeutic vaccines against cancers.
1. Introduction
Dendritic cells (DCs) are professional antigen presenting cells (APCs) that can efficiently prime T cells. Both endogenous and exogenous antigens are efficiently presented by DCs in the context of major histocompatibility complex class I and II (MHC I and II)/peptide complexes. Among various types of APCs, DCs are the most efficient at cross-presenting antigens to T cells (Delamarre and Mellman, 2011, Jung et al., 2002, Segura and Villadangos, 2009), although the types and magnitude of T cell responses largely rely on the functional specialty and plasticity of DC subsets.
T cell-mediated immunity plays crucial roles in therapeutic immunity against cancers and viral infections. The potent ability of DCs to cross-prime CD8+ T cells positions them as novel cellular targets for the rational design of vaccines. In line with this premise, Bonifaz et al., 2002, Bonifaz et al., 2004 demonstrated that the efficiency of antigen cross-presentation by DCs, assessed by measuring the magnitude of antigen-specific CD8+ T cell responses, could be improved over 100-fold by targeting antigens to DEC205 in mice. This seminal observation has led many scientists to further study the biology of DC surface receptors and the use of the “DC-targeting vaccines” against cancers and viral infections.
For more than a decade, researchers have been attempting to optimize DC-targeting vaccines by delivering antigens to different DC surface receptors. These receptors include c-type lectins (e.g., DEC205, DC-SIGN, CD207, LOX-1, DC-ASGPR, Dectin-1, DCIR, DCIR2, CLEC6, CLEC9A, and CLEC12A) (Bonifaz et al., 2004, Caminschi et al., 2008, Carter et al., 2006, Delneste et al., 2002, Dudziak et al., 2007, Duluc et al., 2014, Flacher et al., 2014, Flamar et al., 2013, Idoyaga et al., 2008, Idoyaga et al., 2011, Kastenmuller et al., 2014, Lahoud et al., 2009, Li et al., 2012, Meyer-Wentrup et al., 2008, Ni et al., 2010, Sancho et al., 2008, Tacken et al., 2005, Tacken et al., 2007, Tacken et al., 2011, Weck et al., 2008), as well as non-lectin receptors, including CD40 (Chatterjee et al., 2012, Cohn et al., 2013, Flamar et al., 2013, Rosalia et al., 2015, Williams et al., 2012), mannose receptor (Tsuji et al., 2011), and integrins (Castro et al., 2008). Antigens delivered to DCs via each of these receptors have been reported to elicit certain levels of antigen-specific CD8+ T cell responses in vitro in humans and in vivo in mice or non-human primates (NHPs). However, it still remains unclear which targeted receptors are the most efficient at priming and boosting antigen-specific CD8+ and CD4+ T cell responses. Finding a specific DC surface receptor that permits us to efficiently evoke potent CD8+ and CD4+ T cell responses will be fundamental for the rational design of effective DC-targeting vaccines against cancers and viral infections. Recent preclinical (in NHPs) and clinical data of DEC205-targeting vaccines also suggest that efficient priming and activation of antigen-specific CD8+ cytotoxic T lymphocytes (CTLs) are still major challenges for the success of DC-targeting vaccines for cancer immunotherapy (Kastenmuller et al., 2014). However, it is also important to note that CD4+ T cells are crucial for the longevity of memory CD8+ CTL-mediated immunity (Janssen et al., 2003), which will determine the efficacy of vaccines in many circumstances.
In this study, we first compared nine different human DC surface receptors for their ability to promote antigen cross-presentation to CD8+ T cells. We found that CD40 was the most efficient at priming and boosting antigen-specific CD8+ CTLs that were functional. We then compared CD40 with the two best DC lectins, LOX-1 and Dectin-1, for their ability to present antigens to CD4+ T cells. Interestingly, both LOX-1 and Dectin-1 were superior to CD40 at evoking antigen-specific CD4+ T cell responses. To assess the mechanistic insights of the functional dichotomy of CD40 versus lectins (e.g., LOX-1 and Dectin-1) in antigen presentation to CD8+ and CD4+ T cells, we have examined subcellular and intracellular trafficking of the three different receptor-bound antibodies in DCs. We further investigated the kinetics of antigen cross-presentation by DCs targeted with antigen via different receptors. Lastly, we were able to show that antigen targeting to CD40 results in potent CD8+ T cell responses in vivo using human CD40 transgenic (hCD40Tg) mice. This in vivo model further allowed us to conclude that CD40 is superior to Langerin, another lectin receptor, at evoking antigen-specific CD8+ T cell responses, while targeting antigen to Langerin resulted in greater levels of antigen-specific CD4+ T cell responses than targeting to CD40.
2. Materials and Methods
2.1. Antibodies, Peptides, Tetramers and Other Reagents
Monoclonal antibodies (mAbs) specific to CD4, CD8, CD11c, CD80, CD83, CD86, perforin and interferon (IFN)γ were purchased from BioLegend. mAbs specific to CD3, CD19, CD123, Lin-1, HLA-DR, CD45RA, and CD45RO were purchased from BD Biosciences. mAbs to CD14 and HLA-ABC were purchased from eBioscience. LIVE/DEAD fixable dead cell stain kit and mAbs to granzyme B were from Invitrogen. HLA-A*0201-influenza virus matrix protein 1 (Flu.M1) 58–66, HLA-A*0201-melanoma antigen recognized by T cells 1 (MART-1) 26–35, and H-2Db-human papillomavirus (HPV) 16.E749–57 tetramers were from Beckman Coulter. Flu.M158–66 and MART-126–35 (27L) peptides were synthesized by Bio-Synthesis. Overlapping 15-mer peptides (staggered by 11 amino acids) spanning the entire nucleoprotein (NP) (A/environment/Viet Nam/1203/2004 H5N1) and hemagglutinin subunit 1 (HA1) (A/PR/8/34 H1N1), HPV16.E6 and E7 proteins and human prostate specific antigen (PSA) were purchased from Mimotopes. Carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen) was used for measuring CD8+ T cell proliferation. Human granulocyte-macrophage colony-stimulating factor (GM-CSF) was purchased from the Baylor University Medical Center Investigational Pharmacy. Interleukin (IL)-2, IL-4, IL-7, and IL-15 were purchased from PeproTech.
2.2. DC-targeting mAbs
mAbs specific for the ectodomains of human receptors [αLOX-1 (15C4) (Li et al., 2012), αDC-ASGPR (49C11) (Li et al., 2012), αDCIR (9E8) (Klechevsky et al., 2010), αCD40 (12E12) (Flamar et al., 2013), αDectin-1 (15E2) (Ni et al., 2010), αDEC205 (MG38) (Bonifaz et al., 2002), and αLangerin (4C7)] were used. mAbs specific for the ectodomains of human MARCO (11A8), CLEC6 (9B9), and DC-SIGN/L (16E7) were generated using receptor ectodomain.hIgG (human IgG1 Fc) and human placental alkaline phosphatase (AP), as previously described (Ni et al., 2010). Cloned mAbs were purified by HPLC using MabSelect resin (GE Healthcare). The specificities of mAbs were verified by their specific binding to corresponding receptors expressed on 293F cells transfected with the full-length receptors. The specificities of the mAbs were also confirmed by ELISA by comparing them to the recombinant receptor-Fc and hIgG-Fc fusion proteins (Ni et al., 2010). Chimeric mAbs containing human IgG4 heavy chain with two site mutations (S228P and L235E) (Reddy et al., 2000) were made to further abolish non-specific binding to Fc receptors.
2.3. mAb-Doc, Coh-antigen and Their Conjugates
Recombinant fusion proteins of mAb-dockerin (Doc), cohesin (Coh)-Flu.M158–66, and Coh-MART-126–35 (27L) were previously described (Flamar et al., 2012, Ni et al., 2010). mAb-antigen conjugates were formed by mixing one molar equivalent of mAb-Doc with two molar equivalents of Coh-antigen proteins in 1X PBS with Ca2 + and Mg2 + (Biosources). The Doc and Coh domains self-associate, forming a stable and specific complex.
2.4. Recombinant Fusion Proteins of mAb-Flu.NP, -Flu.HA1 and -HPV16.E6/7
Production of mAb-NP and mAb-HA1 proteins was as previously described (Li et al., 2012, Skinner et al., 2014). Fusion proteins bearing the E6 and E7 proteins of HPV16 were made using the same method.
2.5. Cells
All healthy (cancer-free) blood donors provided a written informed consent prior to inclusion in the study in accordance with the approval by the Institutional Review Boards at Baylor Research Institute. Mo-DCs were prepared by culturing purified blood monocytes from healthy individuals. Briefly, monocytes enriched from fresh peripheral blood mononuclear cells (PBMCs) or frozen elutriated cell fractions were cultured in DC culture medium (CellGenix) in the presence of 100 ng/ml human GM-CSF and 50 ng/ml IL-4 for 6 days. On day 3, culture medium was replaced with fresh medium containing the same concentrations of GM-CSF and IL-4. PBMCs of HLA-A*0201+ healthy donors were fractionated by elutriation. Total CD4+ and CD8+ T cells were enriched using enrichment kits (StemCell Technologies). Naïve CD8+ T cells (CD45RA+ CD45RO−) (purity > 99.2%) were further sorted on a FACSAria II (BD Biosciences). Monocytes and total B cells were purified using enrichment kits (StemCell Technologies). Blood myeloid DCs (mDCs, Lin-1− HLA-DR+ CD11c+ CD123−) and plasmacytoid DCs (pDCs, Lin-1− HLA-DR+ CD11c− CD123+) were pre-enriched using a pan-DC enrichment kit (StemCell Technologies) and then sorted. All flow cytometry data were collected on a FACSCanto II (BD Biosciences) and analyzed with FlowJo v9 (Tree Star).
2.6. T Cell Assays
A total of 5 × 103 monocyte-derived DCs (Mo-DCs) were loaded with the indicated amounts of recombinant proteins or antigens and co-cultured with 2 × 105 purified autologous CFSE-labeled CD8+ T cells for nine days in the presence of 20 units/mL IL-2 and 10 units/mL IL-7. In experiments using PBMCs, 50 units/mL IL-15 was added to the cultures on day 2. RPMI 1640 medium (Gibco), supplemented with 10% heat-inactivated human AB serum (Gemini), 50 unit/mL penicillin, 50 μg/mL streptomycin, 2 mM L-glutamate, non-essential amino acids (Sigma), 25 mM HEPES (Life Technologies), and 1 mM sodium pyruvate (Sigma), was used. CD8+ T cells were then stained with tetramer and αCD8 mAb. In some experiments, CD8+ T cells were stained with tetramer, αGranzyme B and αPerforin mAbs at the same time. To assess intracellular IFNγ expression, T cells were restimulated with the indicated peptides for 6 h in the presence of brefeldin A (BD Biosciences), as per the manufacturer's protocols. To measure cytotoxicity of CD8+ T cells, a 5 h 51Cr-release assay was performed using T2 cells loaded with the indicated peptides. The cytotoxicity of MART-126–35-specific CD8+ T cells was also measured using cell lines (MEL290 and K562) that were grown in complete RPMI 1640 medium containing 10% FCS (Gemini).
2.7. Immunofluorescence
Mo-DCs (2 × 105/well) were plated in 24-well culture plates. αCD40 (12E12), αLOX-1 (15C4), or αDectin-1 (15E2) mAbs conjugated with Alexa Fluor 647 were added at 1 μg/mL followed by a 1-h incubation on ice. For internalization assays, cells were incubated for 1 h in a CO2 incubator at 37 °C. Cells were prefixed with 3% paraformaldehyde (Polysciences) for 30 min on ice and then fixed for 20 min at room temperature. Cells were then stained with Alexa Fluor 488-coupled rabbit anti-human early endosome antigen 1 (EEA1) or anti-human lysosomal-associated membrane protein 1 (LAMP-1) in PBS containing 0.1% saponin. Each optical slice was 0.5 μm thick. Images were acquired on a Leica DMI16000 confocal microscope (Nanterre, France). Image-J software was used to perform image analysis, channel imaging, and surface plotting (3D presentation). For each donor (n = 9) and each labeling antibody (αCD40, αLOX-1, and αDectin-1 mAbs), at least 10 pictures each with more than 10 cells were taken and analyzed. Just another Colocalization Plugin (JaCoP) software was used to calculate Mander's coefficients.
2.8. Animals and Immunization
All mouse experiments were conducted with the approval of the Institutional Animal Care and Use Committee at Baylor Research Institute. Animals were housed in a pathogen-free environment at the animal facility of Baylor Research Institute. All facilities received daily monitoring and care from the animal facility staff under the supervision of a veterinarian. A maximum of 5 mice were housed per cage. hCD40Tg mice (ImmuRx) and wild-type C57BL/6 (Jackson Laboratory) used were 6-to-10-week-old females. Animals were immunized either s.c. or i.p., as indicated, on days 0, 14 and 28, with 100 μL PBS containing 30 μg of either αCD40-HPV16.E6/7 or αLangerin-HPV16.E6/7 and 50 μg polyinosinic:polycytidylic acid [poly(I:C)] (Invivogen). Anesthesia and euthanasia was achieved by cervical dislocation after the mice were made unconscious from exposure to isoflurane. During anesthesia, peripheral blood was collected from the retro-orbital sinus and used for tetramer staining. Spleens were collected after euthanasia and processed into single-cell suspension for ELISpot assays.
2.9. ELISpot Assays
Mouse IFNγ ELISpotPlus pre-coated plates and reagents were obtained from Mabtech. Briefly, purified splenic CD4+ and CD8+ T cells from immunized mice were stimulated with γ-irradiated wild-type splenocytes loaded with the indicated peptide pools (1 μM). After a 40 h incubation, plates were washed and incubated with biotinylated rat anti-mouse IFNγ for 2 h. After washing the plates, streptavidin-horseradish peroxidase (HRP) was added and incubated for 1 h. IFNγ was detected using 3,3′,5,5′-tetramethylbenzidine (TMB). The reaction was terminated once the formation of discrete purple-colored spots was detected. Spots were counted using ELISpot services (Zellnet Consulting).
2.10. Statistics
Statistical significance was determined using the analysis of variance (ANOVA) and Student's t-test with Prism 6 software (GraphPad Software). Significance was set at P < 0.05.
2.11. Accession Codes
GenBank references for mAbs and recombinant proteins are αMARCO (11A8): KP684033 and KP684034; αDC-SIGN (16E7): HQ912690.1 and HQ912691.1; αCLEC6 (9B9): KP684031 and KP684032; αLangerin (4C7): JX002669.
3. Results
3.1. The Superiority of CD40 Over Eight Other Receptors for CD8+ T Cell Cross-priming
Herein, we compared the levels of MART-126–35-specific CD8+ T cell responses primed with Mo-DCs loaded with different mAb-MART-126–35 (27L) conjugates. We used nine mAbs that were specific to different DC surface receptors. All mAbs were engineered as chimeras containing the mouse V-region and human IgG4 Fc with two mutations (S228P and L235E) to further abolish their non-specific binding to Fc receptors (Reddy et al., 2000). mAb-antigen conjugates were made through non-covalent stable interactions between Coh-antigen and mAb-Doc, and they were well suited for targeting antigens to DCs via surface receptors (Flamar et al., 2013, Ni et al., 2010). Mo-DCs generated in serum-free DC culture medium containing GM-CSF and IL-4 expressed CD11c, CD14, costimulatory molecules (CD80, CD83, CD86) and high levels of HLA-ABC and HLA-DR (Supplemental Fig. 1A). However, the expression levels of such surface molecules were variable among Mo-DCs generated with monocytes from different donors (Supplemental Fig. 1B).
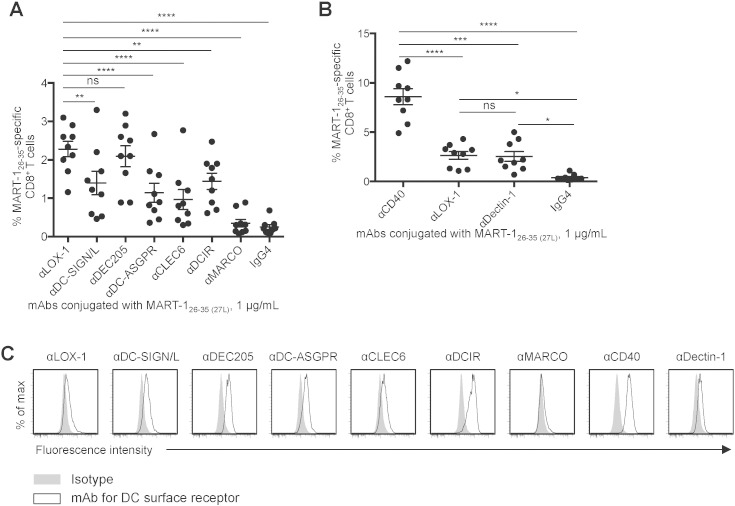
Fig. 1A shows that DCs loaded with any of the eight different mAb-MART-126–35 (27L) conjugates were able to prime various levels of MART-126–35-specific CD8+ T cell responses, as measured by tetramer staining. DCs loaded with conjugates made with αLOX-1 and αDEC205 resulted in similar levels of MART-126–35-specific CD8+ T cell responses, but they were more efficient at priming MART-126–35-specific CD8+ CTLs than conjugates made with other mAbs (αDC-ASGPR, αCLEC6, αMARCO, and control IgG4). Thus, we selected the αLOX-1 conjugate and compared it to αCD40 and αDectin-1 conjugates in the second experiments (Fig. 1B). The αCD40 conjugate was more efficient than the other two at priming MART-126–35-specific naïve CD8+ T cells. Representative tetramer staining data for Fig. 1A and B are presented in Supplemental Fig. 2A andB, respectively.
Fig. 1.
The superiority of CD40 over eight other receptors for CD8+ T cell cross-priming. A and B. Purified naïve CD8+ T cells were co-cultured with Mo-DCs loaded with 1 μg/mL mAb-MART-126–35(27L) for 9 days. CD8+ T cells were then stained with HLA-A*A0201-MART-126–35 tetramer. Dots represent data generated with cells from individual healthy donors (n = 9). Data are presented as mean ± SD, and significance was determined using an ANOVA test. C. Mo-DCs were stained with 1 μg/mL of the indicated fluorescence-labeled mAbs and analyzed by flow cytometry. Representative flow cytometric data out of three experiments are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001; ns, not significant.
Fig. 1C shows that DCs expressed higher levels of CD40 and DCIR than other receptors tested, although the αDCIR conjugate was less efficient than the αLOX-1 conjugate at priming MART-126–35-specific naïve CD8+ T cells. DCs also expressed slightly higher levels of DC-SIGN/L, DEC205, and DC-ASGPR than LOX-1, CLEC6, and Dectin-1. These data suggested that the magnitude of antigen-specific CD8+ T cell responses elicited with different mAb-MART-126–35 (27L) conjugates (Fig. 1A and B) does not necessarily correlate with the surface expression levels of the receptors targeted or consequently with antigen loads (Reuter et al., 2015). We thus concluded that the αCD40-MART-126–35 (27L) conjugate was more efficient than eight other mAb conjugates at priming MART-126–35-specific CD8+ T cells.
3.2. CD8+ CTLs Primed with CD40-targeted DCs are Functional
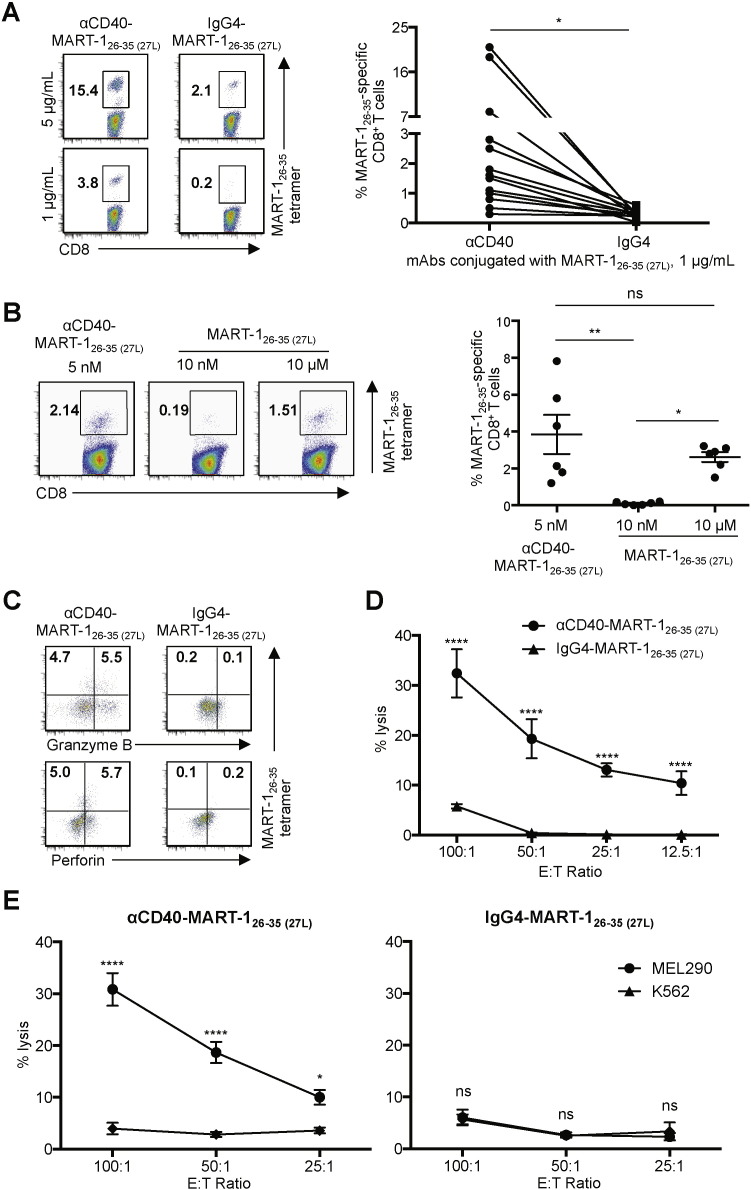
Next, we tested whether αCD40-MART-126–35 (27L) conjugate could target CD40 expressed on DCs. DCs were loaded with two different concentrations of αCD40-MART-126–35 (27L) conjugate and then co-cultured for nine days with autologous naïve CD8+ T cells. As shown in Fig. 2A, DCs loaded with αCD40 conjugate primed MART-126–35-specific CD8+ T cells at both 5 and 1 μg/mL; whereas DCs loaded with 5 μg/mL IgG4 conjugate only resulted in a minimal level of CD8+ T cell priming. Summarized data from 13 independent experiments using cells from different healthy donors are presented (Fig. 2A, right panel). In addition, DCs loaded with 5 nM (1 μg/mL) αCD40-MART-126–35 (27L) conjugate, which contains 10 nM MART-126–35 (27L), were far more efficient than DCs loaded with 10 nM MART-126–35 (27L) peptide (Fig. 2B). Targeting MART-126–35 (27L) to DCs via CD40 was at least 1000 times more efficient at priming MART-126–35-specific CD8+ T cells than the non-targeted loading of MART-126–35 (27L) onto DCs (Fig. 2B). Summarized data generated with cells from six different donors are presented (Fig. 2B, right panel).
Fig. 2.
CD8+ CTLs primed with CD40-targeted DCs are functional. A. Purified naïve CD8 T cells were co-cultured with Mo-DCs loaded with the indicated amounts of αCD40-MART-126–35 (27L) or IgG4-MART-126–35 (27L) conjugates for 9 days. CD8+ T cells were then stained with HLA-A*A0201-MART-126–35 tetramer. Representative flow cytometric data (left) and donor-matched frequencies of MART-126–35-specific CD8+ T cells induced with αCD40-MART-126–35 (27L)- or IgG4-MART-126–35 (27L)-loaded Mo-DCs are shown (right). Dots represent data generated with cells from individual healthy donors (n = 13). Significance was determined using a paired t-test. B. As in A, purified naïve CD8+ T cells were co-cultured with Mo-DCs loaded with the indicated amounts of αCD40-MART-126–35 (27L) conjugate or MART-126–35 (27L) peptide. CD8+ T cells were stained with HLA-A*A0201-MART-126–35 tetramer. Representative flow cytometric data (left) and summarized data (right). Dots represent data generated with cells from individual healthy donors (n = 6). Data are presented as mean ± SD. Significance was determined using an ANOVA test. C. CD8+ T cells in A primed with Mo-DCs loaded with 1 μg/mL mAb-MART-126–35 (27L) were stained for granzyme B and perforin. D. A 5 h 51Cr release assay using T2 cells loaded with 10 μM MART-126–35 peptide were used as target cells. CD8+ T cells primed with Mo-DCs loaded with 1 μg/mL αCD40-MART-126–35 (27L) or IgG4-MART-126–35 (27L) were used as effector cells. E. A 5 h 51Cr release assay using MEL290 and control K562 cell lines as target cells. CD8+ T cells primed with Mo-DCs loaded with 1 μg/mL αCD40-MART-126–35 (27L) (left) or IgG4-MART-126–35 (27L) (right) were used as effector cells. Error bars in D and E indicate SD of triplicate assays. Significance was determined using an ANOVA test. Two independent experiments resulted in similar data. *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001; ns, not significant.
Fractions of MART-126–35-specific CD8+ T cells primed with DCs loaded with αCD40-MART-126–35 (27L) conjugate expressed both granzyme B and perforin (Fig. 2C). They were also able to lyse T2 cells loaded with 10 μM MART-126–35. CD8+ CTLs that were primed with IgG4-MART-126–35 (27L) conjugate-loaded DCs showed minimal killing activity (Fig. 2D). As shown in Fig. 2E, MART-126–35-specific CD8+ CTLs that were primed with αCD40-MART-126–35 (27L) conjugate-loaded DCs could also lyse MEL290 cells (HLA-A*0201+ and MART-1+) but not the control cell line K562 (Fig. 2E, left panel). CD8+ T cells primed with IgG4-MART-126–35 (27L) conjugate-loaded DCs could not specifically lyse MEL290 (Fig. 2E, right panel).
The functional activities of CD8+ CTLs primed with αCD40-MART-126–35 (27L) conjugate-loaded DCs were further compared with those primed with four other mAb-MART-126–35 (27L) conjugates. DCs loaded with αCD40-MART-126–35 (27L) conjugate induced a greater frequency of IFNγ+ and TNFα+ CD8+ T cell responses than the other four (Supplemental Fig. 3A). This was further supported by the data in Supplemental Fig. 3B, showing that CD8+ CTLs primed with αCD40-MART-126–35 (27L) conjugate-loaded DCs were more efficient than those primed with other mAb-MART-126–35 (27L) conjugate-loaded DCs at lysing T2 cells. We therefore concluded that αCD40-MART-126–35 (27L) conjugate targeted CD40 and could thus efficiently prime functional MART-126–35-specific CD8+ CTLs.
3.3. The Superiority of CD40 Over LOX-1 and Dectin-1 for Boosting Memory CD8+ CTLs
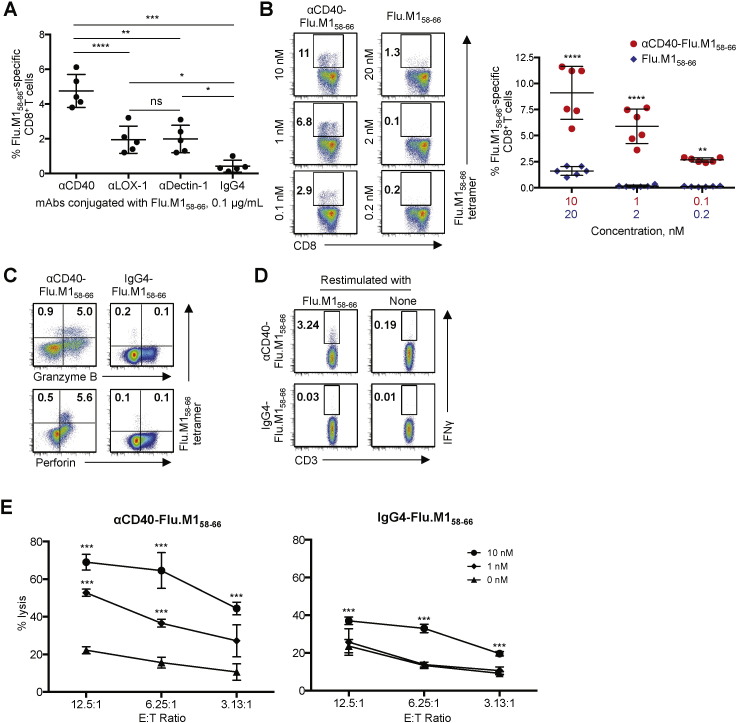
We compared the levels of Flu.M158–66-specific memory CD8+ T cell responses elicited by DCs loaded with αCD40 conjugates with those elicited by αDectin-1 and αLOX-1 conjugates. These mAbs (αDectin-1 and αLOX-1) were selected based on the data in Fig. 1A and B. Fig. 3A shows that DCs loaded with 0.1 μg/mL αCD40-Flu.M158–66 conjugate were more efficient than DCs loaded with the same concentration of αLOX-1- or αDectin-1-Flu.M158–66 conjugate at activating Flu.M158–66-specific CD8+ T cells, as measured by tetramer staining. Similarly, when compared with six other mAb-Flu.M158–66 conjugates, DCs loaded with αCD40-Flu.M158–66 conjugate resulted in the greatest level of Flu.M158–66-specific CD8+ T cell activation (Supplemental Fig. 4A and B). Fig. 3B (left panel) further demonstrates that DCs loaded with αCD40-Flu.M158–66 conjugate are far more efficient than DCs loaded with the equimolar amounts of Flu.M158–66. Data from five independent experiments using cells from different healthy donors (n = 6) are shown in Fig. 3B (right panel).
Fig. 3.
The superiority of CD40 over LOX-1 and Dectin-1 for boosting functional memory CD8+ CTLs. A–C. Purified CD8+ T cells were co-cultured with Mo-DCs loaded with the indicated amounts of mAb-Flu.M158–66 conjugates or Flu.M158–66 peptide. CD8+ T cells were then stained with HLA-A*A0201-Flu.M158–66 tetramer. A. Frequencies of Flu.M158–66-specific CD8+ T cells activated by Mo-DCs loaded with 0.1 μg/mL mAb-Flu.M158–66 conjugates. Dots represent data generated with cells from healthy donors (n = 5). B. Frequencies of Flu.M158–66-specific CD8+ T cells elicited by Mo-DCs loaded with αCD40-Flu.M158–66 at 10, 1, 0.1 nM, or with Flu.M158–66 peptide at 20, 2, 0.2 nM. Each Flu.M158–66 conjugate molecule contains two molecules of Flu.M158–66 antigen. Representative flow cytometric data (left) and summarized data (mean ± SD) from five independent experiments (n = 6) are presented. C. CD8+ T cells activated with Mo-DCs loaded with αCD40-Flu.M158–66 or IgG4-Flu.M158–66 in A were further stained for granzyme B and perforin. Three independent experiments showed similar results. Representative flow cytometric data on the frequencies of Flu.M158–66-specific granzyme B+ or perforin+ CD8+ T cells are shown. D. CD8+ T cells activated with Mo-DCs loaded with αCD40-Flu.M158–66 or IgG4-Flu.M158–66 in A were restimulated with 1 μM Flu.M1 peptide, and intracellular IFNγ expression was assessed. Three independent experiments showed similar results. Representative flow cytometric data on the frequencies of Flu.M158–66-specific IFNγ+ CD8+ T cells are shown. E. A 5 h 51Cr release assay using T2 cells loaded with the indicated amounts of Flu.M158–66 peptide. CD8+ T cells activated with Mo-DCs loaded with 0.1 μg/mL αCD40-Flu.M158–66 or IgG4-Flu.M158–66 were used as effector cells. Error bars indicate SD of triplicate assays. Three independent experiments resulted in similar data. Significance in A, B and E was determined using an ANOVA test. *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001; ns, not significant.
Fractions of Flu.M158–66-specific CD8+ CTLs elicited by DCs loaded with αCD40-Flu.M158–66 conjugate expressed granzyme B and perforin (Fig. 3C) as well as IFNγ (Fig. 3D). In line with this, they were also able to lyse T2 cells loaded with Flu.M158–66 peptide at both 10 and 1 nM (Fig. 3E, left panel), while CD8+ CTLs elicited with IgG4-Flu.M158–66 conjugate only lysed target cells loaded with 10 nM Flu.M158–66 peptide (Fig. 3E, right panel). Taken together, we concluded that αCD40-Flu.M158–66 conjugate targeted CD40 and could thus efficiently activate Flu.M158–66-specific memory CD8+ CTLs. In addition, targeting Flu.M158–66 to DCs via CD40 is more efficient at boosting Flu.M158–66-specific CD8+ T cell responses than targeting Flu.M158–66 to other receptors (Supplemental Fig. 4), including LOX-1 or Dectin-1.
3.4. Distinct Functions of CD40 and Lectins (e.g., LOX-1 and Dectin-1) at Eliciting T Cell Responses
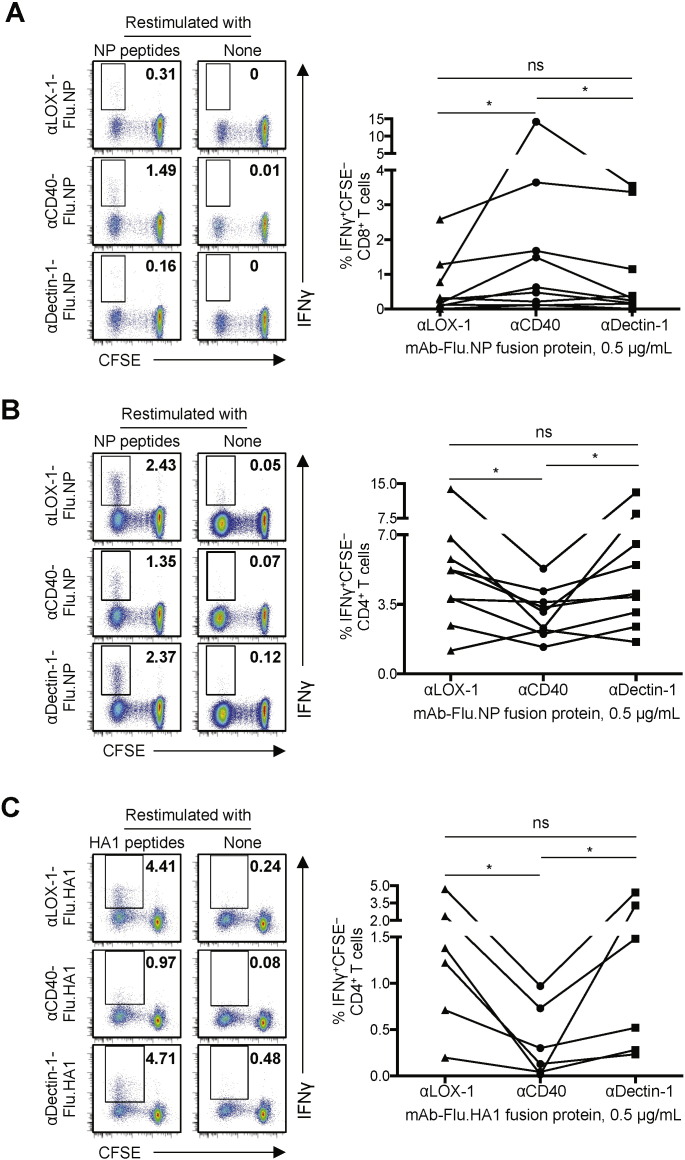
To further confirm the specialized function of CD40 for enhancing antigen cross-presentation to CD8+ T cells, we used recombinant fusion proteins of mAbs and influenza viral nucleoprotein (Flu.NP). Experiments performed with recombinant fusion proteins of mAbs and whole protein antigens (e.g., Flu.NP) are thought to be a more biologically relevant way to assess the ability of DCs to cross-present antigens and subsequently should be utilized for the rational design of vaccines against cancers and microbial infections. It also allows us to assess the multiple repertoires of antigen-specific CD8+ as well as CD4+ T cell responses. CFSE-labeled PBMCs were cultured for eight days with αCD40-Flu.NP, αLOX-1-Flu.NP or αDectin-1-Flu.NP fusion proteins. They were then restimulated with a Flu.NP peptide pool to measure intracellular IFNγ expression. As shown in Fig. 4A (left panel), αCD40-Flu.NP fusion protein was more efficient than αLOX-1-Flu.NP or αDectin-1-Flu.NP fusion protein at activating Flu.NP-specific IFNγ+ CD8+ T cells. Data from nine independent experiments using cells from different healthy donors are summarized in Fig. 4A (right panel). Interestingly, however, αCD40-Flu.NP fusion protein was significantly less efficient than αLOX-1-Flu.NP or αDectin-1-Flu.NP fusion protein at activating Flu.NP-specific IFNγ+ CD4+ T cells (Fig. 4, left panel). Data from nine independent experiments further confirmed this (Fig. 4B, right panel). αLOX-1-Flu.NP and αDectin-1-Flu.NP fusion proteins resulted in similar levels of Flu.NP-specific CD8+ (Fig. 4A) and CD4+ T cell responses (Fig. 4B). The difference between CD40 and the other two receptors at eliciting CD4+ T cell responses was further confirmed by assessing influenza hemagglutinin subunit 1 (Flu.HA1)-specific IFNγ+ CD4+ T cell responses elicited with Flu.HA1 fusion proteins of the three mAbs (Fig. 4C). αCD40-Flu.HA1 fusion protein was less efficient than αLOX-1-Flu.HA1 or αDectin-1-Flu.HA1 fusion protein at eliciting Flu.HA1-specific CD4+ T cell responses. We also measured Flu.HA1-specific CD8+ T cell responses (Supplemental Fig. 5), but there was no significant level of Flu.HA1-specific CD8+ T cell responses to the three mAb-Flu.HA1 fusion proteins. Previous studies (Lee et al., 2008, McMichael et al., 1986, Townsend and Skehel, 1982) have shown that influenza-specific CD8+ memory T cells mostly target internal proteins, including Flu.NP, but not outer membrane proteins, such as Flu.HA1. Supplemental Fig. 6A and B demonstrate that the variability of the magnitude of Flu.NP- and Flu.HA1-specific T cell responses among donors (as observed in Fig. 4) was mainly due to the variability of the frequencies of pre-existing Flu.NP- and Flu.HA1-specific memory T cells of the donors. PBMCs from nine healthy donors were stimulated with Flu.NP or Flu.HA1 peptide pools. The frequencies of Flu.NP- and Flu.HA1-specific CD4+ and CD8+ T cells were measured by intracellular IFNγ staining. Taken together, we concluded that CD40 has a specialized function to promote antigen cross-presentation to CD8+ but not antigen presentation to CD4+ T cells, in contrast to LOX-1 and Dectin-1, which promoted CD4+ but not CD8+ T cells.
Fig. 4.
Functional specialty of CD40 and lectins (e.g., LOX-1 and Dectin-1) in enhancing CD8+ and CD4+ T cell responses, respectively. A–C. CFSE-labeled PBMCs from healthy donors (n ≥ 6) were cultured in the presence of 0.5 μg/mL of the indicated (A and B) mAb-Flu.NP or (C) mAb-Flu.HA1 recombinant fusion proteins for 8 days. Cells were restimulated with NP in A and B or HA1 peptide pool in C at 1 μM (of each peptide), and intracellular IFNγ expression in live (A) CD8+ and (B and C) CD4+ T cells was assessed. Representative flow cytometric data on the frequencies of CFSE− IFNγ+ (A) CD8+ or (B and C) CD4+ T cells (left) and donor-matched frequencies of CFSE− IFNγ+ (A) CD8+ and (B and C) CD4+ T cells (bottom) are shown. Dots represent data generated with cells from individual donors, and significance was determined using a paired t-test. *, P < 0.05; ns, not significant.
Not only DCs, but also monocytes and B cells express CD40 (Flamar et al., 2013), LOX-1 (Li et al., 2012), and Dectin-1 (Ni et al., 2010). Therefore, both monocytes and B cells targeted with antigens could also contribute to the CD4+ and CD8+ T cell responses observed in Fig. 4. However, we have previously reported that the majority of antigen-specific T cell responses elicited by targeting antigens to CD40 and LOX-1 were due to the roles of DCs (Flamar et al., 2013, Li et al., 2012). Supplemental Fig. 7 further demonstrates that DCs, particularly mDCs, loaded with αDectin-1-Flu.HA1 fusion protein are far more efficient than loaded pDCs, monocytes, or B cells at eliciting Flu.HA1-specific T cell responses.
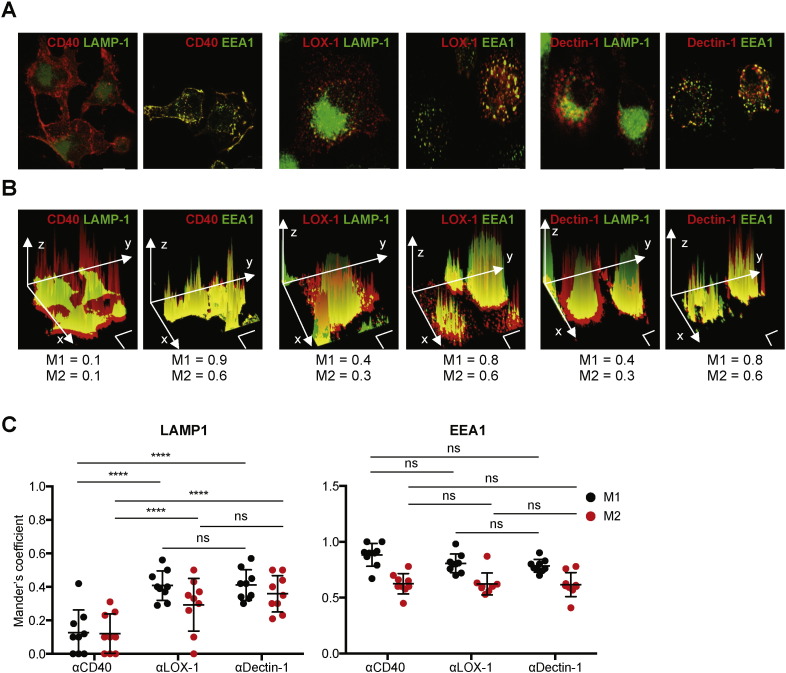
3.5. Distinct Patterns of mAb Localization in Subcellular and Intracellular Compartments
The cellular compartments where antigens are delivered can impact the outcome of antigen cross-presentation by DCs (Belizaire and Unanue, 2009, Burgdorf et al., 2007, Burgdorf et al., 2008, Chatterjee et al., 2012, Cohn et al., 2013, Harding et al., 1991, Zehner et al., 2011). To further understand the functional specialties of CD40, LOX-1, and Dectin-1 that have been observed in this study, we examined subcellular and intracellular localization of receptor-bound αCD40, αLOX-1, and αDectin-1 mAbs in DCs (Fig. 5). We found two major differences between αCD40 and the other two mAbs. First, a large fraction of αCD40 mAb stayed at the plasma membrane of DCs (Fig. 5A, left panels). In contrast, the majority of αLOX-1 and αDectin-1 mAbs were internalized into the cell cytoplasm within 1 h (Fig. 5A, middle and right panels). Second, αCD40 mAb that did internalize into the cytosolic compartment mainly accumulated in the early endosomes, as it co-localized with αEEA1 mAb (Fig. 5A, left panels). In contrast, significant fractions of αLOX-1 (Fig. 5A, middle panels) and αDectin-1 mAbs (Fig. 5A, right panels) localized into both the early and late endosomes, as they co-localized with αEEA1 as well as αLAMP-1 mAb that targets the late endosomes). These observations were further confirmed by the Mander's coefficients acquired by using the Image-J software (Fig. 5B and C). M1 represents the fraction of αEEA1 or αLAMP-1 mAb that overlaps with αCD40, αLOX-1, or αDectin-1 mAb; while M2 represents the fraction of αCD40, αLOX-1, or αDectin-1 that overlaps with αEEA1 or αLAMP-1 mAb. Only ~ 10% of DCs showed co-localization of αCD40 and αLAMP-1 mAbs, while more than 75% of DCs showed co-localization of αCD40 and αEEA-1 mAbs. In contrast to αCD40 mAb, 35–45% of DCs showed co-localization of αLOX-1 and αLAMP-1 mAbs. αDectin-1 mAb showed patterns of subcellular localization that were similar to what were observed with αLOX-1 mAb. Summarized data from nine donors, each with analyses done on at least 100 cells, are shown in Fig. 5C. Taken together, the patterns of subcellular and intracellular localization of CD40-bound αCD40 mAb were distinct from those of αLOX-1 and αDectin-1 mAbs, which showed a high similarity.
Fig. 5.
Distinct patterns of subcellular and intracellular localization of αCD40, αLOX-1 and αDectin-1 mAbs. A–C. Mo-DCs were incubated with fluorescent αCD40, αLOX-1, and αDectin-1 mAbs at 1 μg/mL. DCs were further stained with αLAMP-1 and αEEA1 antibodies. Images were acquired on a Leica DMI16000 confocal microscope (100X). A. Representative merged images of CD40, LOX-1 or Dectin-1 (red) staining and LAMP-1 or EEA-1 (green) staining are shown. Scale bar indicates 10 μm. B. Representative three-dimensional graphs were plotted based on the fluorescence intensity (z-axis) and merged images in A. Scale bars indicate 10 μm on both x-axis and y-axis. Mander's coefficients, M1 and M2, were calculated using the Just Another Colocalization Plugin Software (JaCoP). M1 represents the percentage of αEEA1 or αLAMP-1 mAb that overlaps with αCD40, αLOX-1, or αDectin-1 mAb. M2 represents the percentage of αCD40, αLOX-1, or αDectin-1 that overlaps with αEEA1 or αLAMP-1 mAb. C. Summarized data represent M1 and M2 from 9 donors. For each donor, at least 100 cells from 10 pictures were acquired to calculate the colocalization values. Dots represent individual donors and error bars indicate SD. Significance was determined using an ANOVA test. ****, P < 0.001; ns, not significant.
3.6. CD40 Targeting Leads to Greater and Prolonged Antigen Cross-presentation to CD8+ T Cells
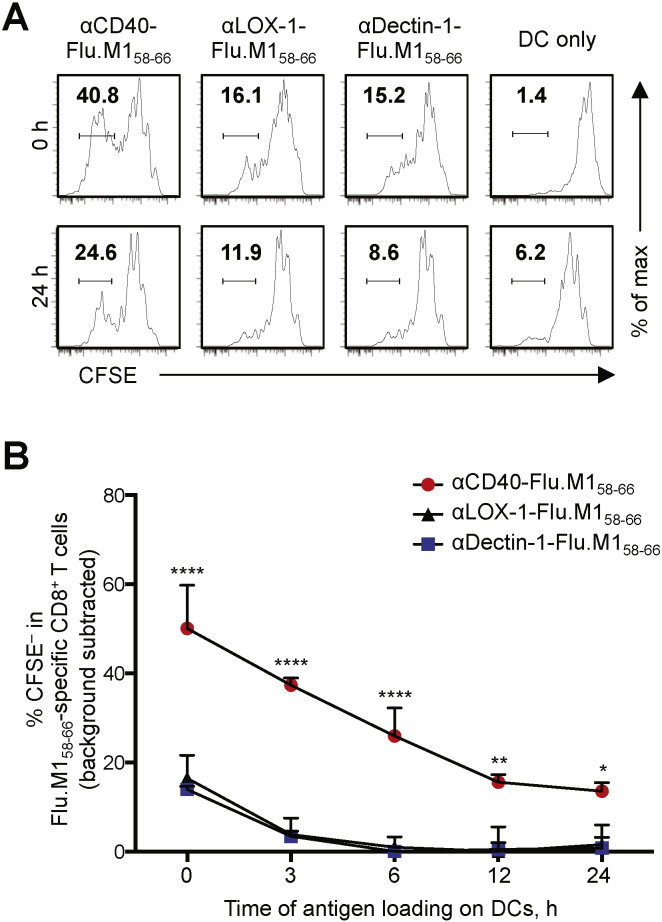
To further understand the mechanistic insights for the superiority of CD40 over other receptors, we investigated the kinetics of antigen presentation by DCs targeted with different mAb-Flu.M158–66 conjugates. CFSE-labeled Flu.M158–66-specific CD8+ T cell lines were co-cultured with DCs incubated for different time periods (0, 3, 6, 12, and 24 h) with the three different mAb-Flu.M158–66 conjugates (Fig. 6). CD8+ T cell proliferation was assessed on day 6 by measuring CFSE dilution. As shown in Fig. 6A and B, DCs loaded with αCD40-Flu.M158–66 conjugate were more efficient than DCs loaded with the other two mAb-Flu.M158–66 conjugates at all time points tested. This indicates that CD40-targeted antigens can be more efficiently cross-presented in the context of MHC class I than LOX-1- or Dectin-1-targeted antigens. It also indicates that DCs targeted with antigens via CD40 are able to present antigens for a longer time period than DCs targeted with antigens via Dectin-1 or LOX-1. This prolonged antigen cross-presentation by CD40-targeted DCs was not due to the activation effects of αCD40 in the fusion protein, as assessed by measuring surface phenotypes of DCs as well as cytokine and chemokine secretion by DCs (data not shown). This was consistent with previously published data (Chatterjee et al., 2012). One clear difference between CD40 and lectins (e.g., LOX-1 and Dectin-1) was in the localization of mAbs (Fig. 5). The majority of αLOX-1 and αDectin-1 was internalized within 1 h, but a large fraction of αCD40 remained at the plasma membrane. Nonetheless, some αCD40 was internalized (mainly to the early endosomes, which is an important path for antigen cross-presentation by DCs to CD8+ T cells) (Burgdorf et al., 2007, Burgdorf et al., 2008, Cohn et al., 2013). In addition, the ability of CD40 to maintain αCD40 on the plasma membrane instead of being degraded after internalization could reflect a continuous release of antigens to early endosomes over an extended period of time for continuous antigen cross-presentation to CD8+ T cells (Fig. 6). Taken together, we concluded that antigen targeting to DCs via CD40 results in greater CD8+ T cell responses than targeting to LOX-1 and Dectin-1. This is due to enhanced antigen cross-presentation by DCs at early time points as well as for an extended time period, as shown in Fig. 6.
Fig. 6.
Kinetics of antigen cross-presentation of DCs targeted via CD40, LOX-1, or Dectin-1. A and B. CFSE-labeled Flu.M158–66-specific CD8+ T cell lines were co-cultured with Mo-DCs pre-incubated for the indicated time periods with 1 nM (0.1 μg/ml) mAb-Flu.M158–66 fusion proteins. On day 6, CD8+ T cell proliferation was assessed by flow cytometry. A. Representative flow cytometric data from 0 and 24 h. B. Summarized data are presented as mean ± SD of triplicate assays. Significance was determined using an ANOVA test. Two independent experiments resulted in similar data. *, P < 0.05; ***, P < 0.005; ****, P < 0.001.
3.7. CD40 Targeting Evokes Potent CD8+ T Cell Responses in hCD40Tg Mice
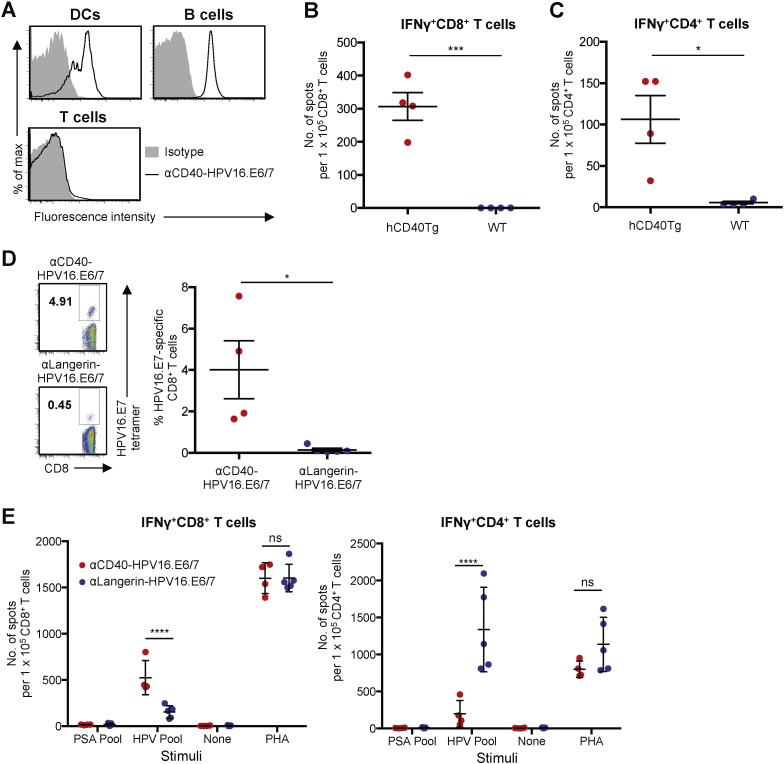
Recombinant fusion proteins of αCD40 mAb (clone 12E12) and HPV16.E6/7 proteins (αCD40-HPV16.E6/7) were generated as described before (Flamar et al., 2013, Joo et al., 2014, Li et al., 2012). As shown in Fig. 7A, αCD40-HPV16.E6/7 bound to splenic CD11c+ DCs and B220+ B cells but not to CD3+ T cells from the hCD40Tg animals. αCD40-HPV16.E6/7 did not bind to any of the cell types from wild-type C57BL/6 mice (data not shown). Consistently, only spleens of hCD40Tg animals showed HPV16.E6/7-specific IFNγ+ CD8+ (Fig. 7B) and CD4+ T cell responses (Fig. 7C), as assessed by IFNγ ELISpot assay, after receiving three doses of αCD40-HPV16.E6/7 (30 μg/dose) plus poly(I:C) (50 μg/dose) (Bonifaz et al., 2002, Gurer et al., 2008). Without poly(I:C), αCD40-HPV16.E6/7 could not elicit E6/7-specific T cell responses (data not shown).
Fig. 7.
Antigen targeting to CD40 can efficiently elicit antigen-specific CD8+ T cell responses in hCD40Tg mice. A. Binding of αCD40-HPV16.E6/7 (1 μg/mL) to splenic CD11c+ DCs, B220+ B cells, and CD3+ T cells of hCD40Tg mouse. B and C. hCD40Tg or WT animals (n = 4 per group) were immunized s.c. with a combination of αCD40-HPV16.E6/7 (30 μg/dose) and poly(I:C) (50 μg/dose) in 100 μL PBS. Animals were boosted twice with the same vaccine at two-week intervals and were sacrificed 7 days after the second boost. IFNγ ELISpot assays were performed on (B) CD8+ and (C) CD4+ T cells purified from splenocytes with HPV16.E6/7 peptide pool at 1 μM as stimulus. D and E. hCD40Tg animals were immunized i.p. with a combination of poly(I:C) (50 μg/dose) and αCD40-HPV16.E6/7 (30 μg/dose) or αLangerin-HPV16.E6/7 (30 μg/dose) in 100 μL PBS (n = 4 per group). Animals were boosted twice with the same vaccine at two-week intervals and were sacrificed 7 days after the second boost. D. CD8+ T cells in peripheral blood were stained with H-2Db-HPV16.E7RAHYNIVTF tetramer. Left, representative flow cytometry data. Right, summarized data. E. IFNγ ELISpot assays were performed on CD8+ (left) and CD4+ (right) T cells purified from splenocytes. Dots represent data generated with individual animals. All data are presented as mean ± SD. Significance was determined using a t-test in (B–D) or ANOVA test in (E). *, P < 0.05; ****, P < 0.001; ns, not significant.
Using the hCD40Tg animals, we were also able to compare CD40 and Langerin, another lectin receptor, for their ability to evoke antigen-specific CD8+ and CD4+ T cell responses in vivo. αLangerin mAb (clone 4C7) binds to both human and murine Langerin (Igyarto et al., 2011). In addition, αLangerin mAb injected intraperitoneally (i.p.) effectively targeted Langerin+ cells in mice (Igyarto et al., 2011). We thus immunized animals with combinations of poly(I:C) (50 μg) plus either 30 μg αCD40-HPV16.E6/7 or αLangerin-HPV16.E6/7 by i.p. three times at two-week intervals. Seven days after the second boosting, blood E7-specific CD8+ T cells were assessed by H-2Db-RAHYNIVTF tetramer staining (Fig. 7D). Compared to animals immunized with αLangerin-HPV16.E6/7, those immunized with αCD40-HPV16.E6/7 had a higher percentage of tetramer+ CD8+ T cells. IFNγ ELISpot assays using CD8+ and CD4+ T cells purified from splenocytes also showed that animals immunized with αCD40-HPV16.E6/7 had more IFNγ+ CD8+ T cells than those immunized with αLangerin-HPV16.E6/7 (Fig. 7E, left panel). However, αLangerin-HPV16.E6/7 was significantly more efficient than αCD40-HPV16.E6/7 at eliciting IFNγ+ CD4+ T cell responses (Fig. 7E, right panel). It is also of note that animals immunized with αCD40-HPV16.E6/7 had more (> 2-fold on average) CD8+ than CD4+ T cells that are specific for HPV16.E6/7, while those immunized with αLangerin-HPV16.E6/7 had more (> 5-fold on average) CD4+ than CD8+ T cells. We also assessed HPV16.E6/7-specific T cell responses elicited after immunizing animals s.c. with the two recombinant fusion proteins. Supplemental Fig. 8A shows that αCD40-HPV16.E6/7 was significantly more effective than αLangerin-HPV16.E6/7 at eliciting HPV16.E7-specific CD8+ T cell responses, as measured by staining CD8+ T cells in the blood with tetramer. ELISpot data generated with purified CD8+ and CD4+ T cells from splenocytes also showed that αCD40-HPV16.E6/7 was more efficient than αLangerin-HPV16.E6/7 at eliciting E6/7-specific IFNγ+ CD8+ T cell responses (Supplemental Fig. 8B, left panel), while αLangerin-HPV16.E6/7 was more efficient than αCD40-HPV16.E6/7 at eliciting IFNγ+ CD4+ T cell responses (Supplemental Fig. 8B, right panel). A previous study (Idoyaga et al., 2011) demonstrated that antigen targeting to Langerin or DEC205 resulted in comparable levels of antigen-specific IFNγ+ CD8+ T cell responses in mice. Our human in vitro data (Fig. 1, Fig. 2) demonstrate that CD40 targeting is significantly more efficient than LOX-1, Dectin-1 or DEC205 targeting that showed similar levels of CD8+ T cell responses. Taking all of these findings together, we concluded that targeting antigen to CD40 is an efficient strategy to evoke antigen-specific CD8+ T cell responses.
4. Discussion
Understanding the biology of human DC surface receptors and the functional consequences of the actions of individual receptors is fundamental for the rational design of medicines for cancers, inflammatory diseases (including autoimmune diseases) and microbial infections. Of the many different receptors expressed on the surface of DCs, lectin-like receptors are considered to be one of the major pattern-recognition receptor families. Some of these receptors, Dectin-1 (Duluc et al., 2014, Joo et al., 2015, LeibundGut-Landmann et al., 2007), DCIR (Fujikado et al., 2008), DC-SIGN (Geijtenbeek and Gringhuis, 2009), LOX-1 (Joo et al., 2014), and DC-ASGPR (Li et al., 2012), are known to play important roles in shaping the quality and quantity of host immune responses. However, the ability of these receptors to capture antigens and deliver them to intracellular compartments makes them novel targets for DC antigen delivery to enhance antigen cross-presentation to T cells. Nonetheless, one major question still remains: “Which targeted receptor results in optimal antigen cross-presentation to T cells?” This study has demonstrated that CD40 is superior to nine other lectins and scavenger receptors at cross-presenting antigen to CD8+ T cells. This was confirmed with both a tumor-associated self antigen and different forms of viral antigens. Interestingly, however, DC lectins (e.g., LOX-1 Dectin-1 and Langerin) were superior to CD40 at presenting antigens to CD4+ T cells.
To further understand such functional specialization of CD40, we examined the subcellular and intracellular localization of receptor-bound mAbs in DCs. Previous studies (Burgdorf et al., 2007, Burgdorf et al., 2008) showed that early endosomes are essential for the cross-presentation of antigens. Recently, Cohn (Cohn et al., 2013) and Chatterjee (Chatterjee et al., 2012) also showed that antigen delivery to early endosomes could result in enhanced antigen cross-presentation to CD8+ T cells, although antigens in late endosomes and lysosomes can also be cross-presented. However, these late compartments are far less efficient for cross-presentation of some antigens. This was due to a higher concentration of lysosomal enzymes, which degrade antigens before they can be released into the cytosol. In line with this, inhibiting proteolysis enhances the ability of the late compartments to cross-present accumulated antigens (Chatterjee et al., 2012). In this study, we found that significant fractions of receptor-bound αLOX-1 and αDectin-1 mAbs also localized to the early endosomes, although targeting CD40 was far more efficient at eliciting CD8+ T cell responses than targeting LOX-1 or Dectin-1. Quantitative analysis of the intracellular compartments across nine different donors further revealed that αCD40 mAb localized mainly to the early endosomes, but αLOX-1 and αDectin-1 localized to both the early and late endosomes. This suggested that there could be other critical factors in addition to the roles of early endosomes that can further influence the efficiency of antigen cross-presentation by DCs via MHC class I molecules. Accordingly, we showed that a large fraction of αCD40 mAb remained at the plasma membrane even after a 1-h incubation at 37 °C, whereas the majority of both αLOX-1 and αDectin-1 mAbs were internalized into endosomal vesicles. Slow internalization to early endosomes or rapid antigen recycling, as speculated previously (Chatterjee et al., 2012, Cohn et al., 2013), could result in increased antigen stability, followed by prolonged antigen presentation and enhanced CD8+ T cell responses, as we have demonstrated in Fig. 6.
In addition to such distinct properties of antibody-bound CD40 versus the lectins (LOX-1 and Dectin-1) described above, one may also consider the possible contribution of αCD40-mediated activation signals in the enhanced antigen cross-presentation after targeting CD40. Previous studies in mice (Bennett et al., 1998, Ridge et al., 1998, Schoenberger et al., 1998) showed that interactions between APCs (including DCs) and helper T cells via CD40–CD40L has been suggested to activate APCs to become fully competent for CD8+ T cell priming. Recent studies have also shown that other DC activators, including toll-like receptor (TLR) ligands and type 1 IFN, can also promote antigen cross-presentation (Datta et al., 2003, Maurer et al., 2002, Schulz et al., 2005, Watts et al., 2007, Wei et al., 2010). However, recombinant fusion proteins of αCD40 and antigens used in this study were not able to induce DCs to secrete cytokines or chemokines or induce surface phenotype maturation. This was in line with the previous observation (Chatterjee et al., 2012) that the enhanced antigen cross-presentation by CD40-targeted human DCs was not due to the CD40-mediated activation signals. Nonetheless, questions regarding the possible contribution of CD40 signaling in enhanced antigen cross-presentation may need to be more carefully studied in the future. Apart from the question on mechanistic insights, we may also need to consider the possible effects of αCD40 bound to CD40 on CD40–CD40L interaction in vivo, although this may not be a critical issue if a proper DC activator is included as an adjuvant in the CD40 targeting vaccines, as most likely it would be (Fig. 7).
In vivo data generated using hCD40Tg animals further demonstrate that antigen targeting to CD40 is an efficient way to evoke antigen-specific CD8+ T cell responses in vivo. Although we could not compare CD40 with LOX-1 or Dectin-1 in this animal model due to the limited specificities of mAbs (αLOX-1 and αDectin-1) to human receptors, we were able to verify that CD40 targeting was significantly more efficient than Langerin (another lectin receptor) targeting for the elicitation of antigen-specific CD8+ T cell responses in vivo. In addition, our in vivo data further demonstrate that targeting CD40 results in greater CD8+ than CD4+ T cell responses, while Langerin targeting results in greater CD4+ than CD8+ T cell responses. A previous study (Chatterjee et al., 2012) has already demonstrated that antigen targeting to three different lectin receptors, Langerin, DEC205, and Clec9A, resulted in comparable levels of antigen-specific IFNγ+ CD8+ T cell responses in mice. Data (Fig. 1, Fig. 2) from this study illustrated that targeting LOX-1, Dectin-1 or DEC205 resulted in comparable levels of antigen-specific CD8+ T cell responses, but they were less efficient than targeting CD40. Taking all of these data together, CD40 targeting is more efficient than targeting the lectin receptors tested in this study. Consistent with both LOX-1 and Dectin-1, antigen targeting to Langerin, a c-type lectin receptor expressed on Langerhans cells as well as fractions of dermal DCs (Bonifaz et al., 2004, Delamarre et al., 2003) and CD8α+ DCs (Delneste, 2004) in mice, resulted in greater levels of antigen-specific CD4+ T cell responses.
In summary, this study reports specialized functions of CD40 versus lectins (e.g., Dectin-1, LOX-1 and Langerin) expressed on the surface of DCs. Data from this study also provide fundamental information for the rational design of vaccines against cancers and viral infections. In spite of recent success with the inhibitors of immune checkpoints (e.g., αCTLA4, αPD-1, and αPD-L1 antibodies), particularly in cancer immunotherapy, there is still a need for boosting tumor-specific immunity for better treatment outcomes. CD40 targeting vaccines could thus be combined with such checkpoint inhibitors to provide cancer patients with better clinical benefit that need to be tested in the near future.
Author Contributions
W.Y., L.G., D.L., D.D., H.J., K.U., C.G., R.O., J.R.K, L.N., Y.X., Z.W., S.Z., and J.-P.G. performed experiments. W.Y., L.G., D.L., L.N., D.D., H.J., J.-P.G., G.Z., and S.O. designed the experiments and analyzed the data. W.Y. and S.O. wrote the manuscript. S.O. supervised the study.
Conflict of Interest
The authors have no conflicting financial interests, except that D.L., G.Z., S.Z., and S.O. are named inventors of patents relating to DC targeting that are held by Baylor Research Institute.
Acknowledgments
We thank Carson Harrod (BIIR) for reading this manuscript, Xiao-Hua Li for characterizing mAbs, and Clay Beauregard and LuAnn Snipe for helping in the animal study. This study was funded by the American Cancer Society (RSG-12-075-01-LIB), the National Institutes of Health (U19 AI057234), and a collaborative research grant from Roche.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.01.029.
Appendix A. Supplementary data
Supplementary figures.
References
- Belizaire R., Unanue E.R. Targeting proteins to distinct subcellular compartments reveals unique requirements for MHC class I and II presentation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17463–17468. doi: 10.1073/pnas.0908583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett S.R., Carbone F.R., Karamalis F., Flavell R.A., Miller J.F., Heath W.R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- Bonifaz L., Bonnyay D., Mahnke K., Rivera M., Nussenzweig M.C., Steinman R.M. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifaz L.C., Bonnyay D.P., Charalambous A., Darguste D.I., Fujii S., Soares H., Brimnes M.K., Moltedo B., Moran T.M., Steinman R.M. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf S., Kautz A., Bohnert V., Knolle P.A., Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- Burgdorf S., Scholz C., Kautz A., Tampe R., Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat. Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- Caminschi I., Proietto A.I., Ahmet F., Kitsoulis S., Shin Teh J., Lo J.C., Rizzitelli A., Wu L., Vremec D., van Dommelen S.L., Campbell I.K., Maraskovsky E., Braley H., Davey G.M., Mottram P., van de Velde N., Jensen K., Lew A.M., Wright M.D., Heath W.R., Shortman K., Lahoud M.H. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112:3264–3273. doi: 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R.W., Thompson C., Reid D.M., Wong S.Y., Tough D.F. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J. Immunol. 2006;177:2276–2284. doi: 10.4049/jimmunol.177.4.2276. [DOI] [PubMed] [Google Scholar]
- Castro F.V., Tutt A.L., White A.L., Teeling J.L., James S., French R.R., Glennie M.J. CD11c provides an effective immunotarget for the generation of both CD4 and CD8 T cell responses. Eur. J. Immunol. 2008;38:2263–2273. doi: 10.1002/eji.200838302. [DOI] [PubMed] [Google Scholar]
- Chatterjee B., Smed-Sorensen A., Cohn L., Chalouni C., Vandlen R., Lee B.C., Widger J., Keler T., Delamarre L., Mellman I. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood. 2012;120:2011–2020. doi: 10.1182/blood-2012-01-402370. [DOI] [PubMed] [Google Scholar]
- Cohn L., Chatterjee B., Esselborn F., Smed-Sorensen A., Nakamura N., Chalouni C., Lee B.C., Vandlen R., Keler T., Lauer P., Brockstedt D., Mellman I., Delamarre L. Antigen delivery to early endosomes eliminates the superiority of human blood BDCA3+ dendritic cells at cross presentation. J. Exp. Med. 2013;210:1049–1063. doi: 10.1084/jem.20121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S.K., Redecke V., Prilliman K.R., Takabayashi K., Corr M., Tallant T., DiDonato J., Dziarski R., Akira S., Schoenberger S.P., Raz E. A subset of toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J. Immunol. 2003;170:4102–4110. doi: 10.4049/jimmunol.170.8.4102. [DOI] [PubMed] [Google Scholar]
- Delamarre L., Mellman I. Harnessing dendritic cells for immunotherapy. Semin. Immunol. 2011;23:2–11. doi: 10.1016/j.smim.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Delamarre L., Holcombe H., Mellman I. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation. J. Exp. Med. 2003;198:111–122. doi: 10.1084/jem.20021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delneste Y. Scavenger receptors and heat-shock protein-mediated antigen cross-presentation. Biochem. Soc. Trans. 2004;32:633–635. doi: 10.1042/BST0320633. [DOI] [PubMed] [Google Scholar]
- Delneste Y., Magistrelli G., Gauchat J., Haeuw J., Aubry J., Nakamura K., Kawakami-Honda N., Goetsch L., Sawamura T., Bonnefoy J., Jeannin P. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- Dudziak D., Kamphorst A.O., Heidkamp G.F., Buchholz V.R., Trumpfheller C., Yamazaki S., Cheong C., Liu K., Lee H.W., Park C.G., Steinman R.M., Nussenzweig M.C. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Duluc D., Joo H., Ni L., Yin W., Upchurch K., Li D., Xue Y., Klucar P., Zurawski S., Zurawski G., Oh S. Induction and activation of human Th17 by targeting antigens to dendritic cells via dectin-1. J. Immunol. 2014;192:5776–5788. doi: 10.4049/jimmunol.1301661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flacher V., Tripp C.H., Mairhofer D.G., Steinman R.M., Stoitzner P., Idoyaga J., Romani N. Murine langerin + dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross-tolerance. EMBO Mol. Med. 2014;6:1638. doi: 10.15252/emmm.201303283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamar A.L., Xue Y., Zurawski S.M., Montes M., King B., Sloan L., Oh S., Banchereau J., Levy Y., Zurawski G. Targeting concatenated HIV antigens to human CD40 expands a broad repertoire of multifunctional CD4+ and CD8+ T cells. AIDS. 2013;27:2041–2051. doi: 10.1097/QAD.0b013e3283624305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamar A.L., Zurawski S., Scholz F., Gayet I., Ni L., Li X.H., Klechevsky E., Quinn J., Oh S., Kaplan D.H., Banchereau J., Zurawski G. Noncovalent assembly of anti-dendritic cell antibodies and antigens for evoking immune responses in vitro and in vivo. J. Immunol. 2012;189:2645–2655. doi: 10.4049/jimmunol.1102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikado N., Saijo S., Yonezawa T., Shimamori K., Ishii A., Sugai S., Kotaki H., Sudo K., Nose M., Iwakura Y. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat. Med. 2008;14:176–180. doi: 10.1038/nm1697. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Gringhuis S.I. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurer C., Strowig T., Brilot F., Pack M., Trumpfheller C., Arrey F., Park C.G., Steinman R.M., Munz C. Targeting the nuclear antigen 1 of Epstein–Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses. Blood. 2008;112:1231–1239. doi: 10.1182/blood-2008-03-148072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C.V., Collins D.S., Slot J.W., Geuze H.J., Unanue E.R. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 1991;64:393–401. doi: 10.1016/0092-8674(91)90647-h. [DOI] [PubMed] [Google Scholar]
- Idoyaga J., Cheong C., Suda K., Suda N., Kim J.Y., Lee H., Park C.G., Steinman R.M. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J. Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- Idoyaga J., Lubkin A., Fiorese C., Lahoud M.H., Caminschi I., Huang Y., Rodriguez A., Clausen B.E., Park C.G., Trumpfheller C., Steinman R.M. Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc. Natl. Acad. Sci. U. S. A. 2011;108:2384–2389. doi: 10.1073/pnas.1019547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igyarto B.Z., Haley K., Ortner D., Bobr A., Gerami-Nejad M., Edelson B.T., Zurawski S.M., Malissen B., Zurawski G., Berman J., Kaplan D.H. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen E.M., Lemmens E.E., Wolfe T., Christen U., von Herrath M.G., Schoenberger S.P. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- Joo H., Li D., Dullaers M., Kim T.W., Duluc D., Upchurch K., Xue Y., Zurawski S., Le Grand R., Liu Y.J., Kuroda M., Zurawski G., Oh S. C-type lectin-like receptor LOX-1 promotes dendritic cell-mediated class-switched B Cell responses. Immunity. 2014;41:592–604. doi: 10.1016/j.immuni.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo H., Upchurch K., Zhang W., Ni L., Li D., Xue Y., Li X.H., Hori T., Zurawski S., Liu Y.J., Zurawski G., Oh S. Opposing roles of dectin-1 expressed on human plasmacytoid dendritic cells and myeloid dendritic cells in Th2 polarization. J. Immunol. 2015;195:1723–1731. doi: 10.4049/jimmunol.1402276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S., Unutmaz D., Wong P., Sano G., De los Santos K., Sparwasser T., Wu S., Vuthoori S., Ko K., Zavala F., Pamer E.G., Littman D.R., Lang R.A. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmuller W., Kastenmuller K., Kurts C., Seder R.A. Dendritic cell-targeted vaccines—hope or hype? Nat. Rev. Immunol. 2014;14:705–711. doi: 10.1038/nri3727. [DOI] [PubMed] [Google Scholar]
- Klechevsky E., Flamar A.L., Cao Y., Blanck J.P., Liu M., O'Bar A., Agouna-Deciat O., Klucar P., Thompson-Snipes L., Zurawski S., Reiter Y., Palucka A.K., Zurawski G., Banchereau J. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoud M.H., Proietto A.I., Ahmet F., Kitsoulis S., Eidsmo L., Wu L., Sathe P., Pietersz S., Chang H.W., Walker I.D., Maraskovsky E., Braley H., Lew A.M., Wright M.D., Heath W.R., Shortman K., Caminschi I. The C-type lectin Clec12A present on mouse and human dendritic cells can serve as a target for antigen delivery and enhancement of antibody responses. J. Immunol. 2009;182:7587–7594. doi: 10.4049/jimmunol.0900464. [DOI] [PubMed] [Google Scholar]
- Lee L.Y., Ha do L.A., Simmons C., de Jong M.D., Chau N.V., Schumacher R., Peng Y.C., McMichael A.J., Farrar J.J., Smith G.L., Townsend A.R., Askonas B.A., Rowland-Jones S., Dong T. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J. Clin. Invest. 2008;118:3478–3490. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeibundGut-Landmann S., Gross O., Robinson M.J., Osorio F., Slack E.C., Tsoni S.V., Schweighoffer E., Tybulewicz V., Brown G.D., Ruland J., Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- Li D., Romain G., Flamar A.L., Duluc D., Dullaers M., Li X.H., Zurawski S., Bosquet N., Palucka A.K., Le Grand R., O'Garra A., Zurawski G., Banchereau J., Oh S. Targeting self- and foreign-antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J. Exp. Med. 2012;209:109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer T., Heit A., Hochrein H., Ampenberger F., O'Keeffe M., Bauer S., Lipford G.B., Vabulas R.M., Wagner H. CpG-DNA aided cross-presentation of soluble antigens by dendritic cells. Eur. J. Immunol. 2002;32:2356–2364. doi: 10.1002/1521-4141(200208)32:8<2356::AID-IMMU2356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- McMichael A.J., Michie C.A., Gotch F.M., Smith G.L., Moss B. Recognition of influenza A virus nucleoprotein by human cytotoxic T lymphocytes. J. Gen. Virol. 1986;67(Pt 4):719–726. doi: 10.1099/0022-1317-67-4-719. [DOI] [PubMed] [Google Scholar]
- Meyer-Wentrup F., Benitez-Ribas D., Tacken P.J., Punt C.J., Figdor C.G., de Vries I.J., Adema G.J. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–4253. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- Ni L., Gayet I., Zurawski S., Duluc D., Flamar A.L., Li X.H., O'Bar A., Clayton S., Palucka A.K., Zurawski G., Banchereau J., Oh S. Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses. J. Immunol. 2010;185:3504–3513. doi: 10.4049/jimmunol.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M.P., Kinney C.A., Chaikin M.A., Payne A., Fishman-Lobell J., Tsui P., Dal Monte P.R., Doyle M.L., Brigham-Burke M.R., Anderson D., Reff M., Newman R., Hanna N., Sweet R.W., Truneh A. Elimination of Fc receptor-dependent effector functions of a modified IgG4 monoclonal antibody to human CD4. J. Immunol. 2000;164:1925–1933. doi: 10.4049/jimmunol.164.4.1925. [DOI] [PubMed] [Google Scholar]
- Reuter A., Panozza S.E., Macri C., Dumont C., Li J., Liu H., Segura E., Vega-Ramos J., Gupta N., Caminschi I., Villadangos J.A., Johnston A.P., Mintern J.D. Criteria for dendritic cell receptor selection for efficient antibody-targeted vaccination. J. Immunol. 2015;194:2696–2705. doi: 10.4049/jimmunol.1402535. [DOI] [PubMed] [Google Scholar]
- Ridge J.P., Di Rosa F., Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Rosalia R.A., Cruz L.J., van Duikeren S., Tromp A.T., Silva A.L., Jiskoot W., de Gruijl T., Lowik C., Oostendorp J., van der Burg S.H., Ossendorp F. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials. 2015;40:88–97. doi: 10.1016/j.biomaterials.2014.10.053. [DOI] [PubMed] [Google Scholar]
- Sancho D., Mourao-Sa D., Joffre O.P., Schulz O., Rogers N.C., Pennington D.J., Carlyle J.R., Reis e Sousa C. Tumor therapy in mice via antigen targeting to a novel, DC-Restricted C-type lectin. J. Clin. Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberger S.P., Toes R.E., van der Voort E.I., Offringa R., Melief C.J. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Schulz O., Diebold S.S., Chen M., Naslund T.I., Nolte M.A., Alexopoulou L., Azuma Y.-T., Flavell R.A., Liljestrom P., Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- Segura E., Villadangos J.A. Antigen presentation by dendritic cells in vivo. Curr. Opin. Immunol. 2009;21:105–110. doi: 10.1016/j.coi.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Skinner J.A., Zurawski S.M., Sugimoto C., Vinet-Oliphant H., Vinod P., Xue Y., Russel-Lodrigue K., Albrecht R.A., Garcia-Sastre A., Salazar A.M., Roy C.J., Kuroda M.J., Oh S., Zurawski G. Immunologic characterization of a rhesus macaque H1N1 challenge model for candidate influenza vaccine assessment. Clin. Vaccine Immunol. 2014 doi: 10.1128/CVI.00547-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacken P.J., de Vries I.J., Gijzen K., Joosten B., Wu D., Rother R.P., Faas S.J., Punt C.J., Torensma R., Adema G.J., Figdor C.G. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood. 2005;106:1278–1285. doi: 10.1182/blood-2005-01-0318. [DOI] [PubMed] [Google Scholar]
- Tacken P.J., de Vries I.J., Torensma R., Figdor C.G. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- Tacken P.J., Ginter W., Berod L., Cruz L.J., Joosten B., Sparwasser T., Figdor C.G., Cambi A. Targeting DC-SIGN via its neck region leads to prolonged antigen residence in early endosomes, delayed lysosomal degradation, and cross-presentation. Blood. 2011;118:4111–4119. doi: 10.1182/blood-2011-04-346957. [DOI] [PubMed] [Google Scholar]
- Townsend A.R., Skehel J.J. Influenza A specific cytotoxic T-cell clones that do not recognize viral glycoproteins. Nature. 1982;300:655–657. doi: 10.1038/300655a0. [DOI] [PubMed] [Google Scholar]
- Tsuji T., Matsuzaki J., Kelly M.P., Ramakrishna V., Vitale L., He L.Z., Keler T., Odunsi K., Old L.J., Ritter G., Gnjatic S. Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+ and CD4+ T cell responses with broad antigen specificity. J. Immunol. 2011;186:1218–1227. doi: 10.4049/jimmunol.1000808. [DOI] [PubMed] [Google Scholar]
- Watts C., Zaru R., Prescott A.R., Wallin R.P., West M.A. Proximal effects of toll-like receptor activation in dendritic cells. Curr. Opin. Immunol. 2007;19:73–78. doi: 10.1016/j.coi.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Weck M.M., Appel S., Werth D., Sinzger C., Bringmann A., Grunebach F., Brossart P. hDectin-1 is involved in uptake and cross-presentation of cellular antigens. Blood. 2008;111:4264–4272. doi: 10.1182/blood-2006-10-051375. [DOI] [PubMed] [Google Scholar]
- Wei J., Waithman J., Lata R., Mifsud N.A., Cebon J., Kay T., Smyth M.J., Sadler A.J., Chen W. Influenza A infection enhances cross-priming of CD8+ T Cells to cell-associated antigens in a TLR7- and type I IFN-dependent fashion. J. Immunol. 2010;185:6013–6022. doi: 10.4049/jimmunol.1002129. [DOI] [PubMed] [Google Scholar]
- Williams B.J., Bhatia S., Adams L.K., Boling S., Carroll J.L., Li X.L., Rogers D.L., Korokhov N., Kovesdi I., Pereboev A.V., Curiel D.T., Mathis J.M. Dendritic cell based PSMA immunotherapy for prostate cancer using a CD40-targeted adenovirus vector. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehner M., Chasan A.I., Schuette V., Embgenbroich M., Quast T., Kolanus W., Burgdorf S. Mannose receptor polyubiquitination regulates endosomal recruitment of p97 and cytosolic antigen translocation for cross-presentation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9933–9938. doi: 10.1073/pnas.1102397108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.