Abstract
Objective
The objective of this exploratory study was to evaluate tibiofemoral joint contact point excursions and velocities during downhill gait and assess the relationship between tibiofemoral joint contact mechanics with frontal-plane knee joint motion and lower extremity muscle weakness in patients with knee osteoarthritis (OA).
Methods
Dynamic stereo X-ray was used to quantify tibiofemoral joint contact mechanics and frontal-plane motion during the loading response phase of downhill gait in 11 patients with knee OA and 11 control volunteers. Quantitative testing of the quadriceps and the hip abductor muscles was also performed. Group differences in contact mechanics and frontal-plane motion excursions were compared using analysis of covariance with adjustments for body mass index. Differences in strength were compared using independent sample t-tests. Additionally, linear associations between contact mechanics with frontal-plane knee motion and muscle strength were evaluated using Pearson's correlation coefficients.
Results
Patients with knee OA demonstrated larger medial/lateral joint contact point excursions (p<0.02) and greater heel-strike joint contact point velocities (p<0.05) for the medial and lateral compartments compared to the control group. The peak medial/lateral joint contact point velocity of the medial compartment was also greater for patients with knee OA compared to their control counterparts (p=0.02). Additionally, patients with knee OA demonstrated significantly increased frontal-plane varus motion excursions (p<0.01) and greater quadriceps and hip abductor muscle weakness (p=0.03). In general, increased joint contact point excursions and velocities in patients with knee OA were linearly associated with greater frontal-plane varus motion excursions (p<0.04) but not with quadriceps or hip abductor strength.
Conclusion
Altered contact mechanics in patients with knee OA may be related to compromised frontal-plane joint stability but not with deficits in muscle strength.
Keywords: Kinematics, Contact mechanics, Instability, Quadriceps, Hip Abductors
1. Introduction
An accumulating body of scientific evidence suggests that altered gait mechanics may play a role in onset and progression of knee osteoarthritis (OA).1, 2 To this end, Andriacchi and colleagues1 have previously proposed that the mechanical breakdown of the articular cartilage may be the result of abnormal motions that shift the joint contact point to infrequently loaded areas of the knee joint. Shifts in the areas of load-bearing to regions in the cartilage that have not adapted to the high customary loads of daily activities can cause surface-zone fibrillations and loss of articular cartilage surface lubrication which can lead to increased friction and large tangential shear stresses.3 In response to the elevated shear stress, chondrocytes production of catabolic mediators is upregulated, leading to greater matrix damage and a progressive cascade of cartilage loss.4, 5 Once the OA sequence has begun, the articulating surfaces respond negatively to the cyclical ambulatory compressive loads and shear stresses which lead to further cartilage degradation and disease progression.3 To date, evidence in support of altered knee joint contact patterns during gait in patients with knee OA remains scant due to the technical challenges associated with direct evaluation of in-vivo knee contact mechanics.
Altered patterns of knee joint contact during gait in patients with knee OA could theoretically occur due to age-associated changes in the musculoskeletal system such as deficits in lower extremity muscle strength and/or presence of joint instability. Muscles of the lower extremity have been indicated to play a critical role in the preservation of normal knee joint function by providing dynamic knee joint stability and shock absorption, while maintaining safe transfer of forces across the joint.6, 7 As such, weakness of the quadriceps muscle has long been considered as a strong risk factor for onset8-10 and progression11 of knee OA. More recently, increasing research evidence indicate that the commonly observed impairments of the hip abductor musculature can also contribute to the pathomechanics of knee OA12-15 and a greater likelihood of disease progression.16 However, reports from randomized clinical trials of quadriceps and hip abductor muscle strengthening suggest that despite improvements in pain and function, stronger quadriceps or hip abductor muscles do not reduce the ambulatory compressive loads often associated with the pathomechanics of knee OA.13, 17-20 Therefore, the mechanism(s) by which stronger muscles contribute to the reported clinical improvements in pain and function after muscle strengthening remains unclear.
A potential hypothesis related to the role of stronger muscles in providing clinical benefits for patients with knee OA may be through providing increased dynamic knee joint stability to compensate for the previously reported increases in knee joint laxity in arthritic knees.21, 22 In support of this notion, recent evidence suggests that lower extremity muscle weakness may be associated with self-reports of knee joint instability in patients with knee OA.23, 24 Knee joint instability can be mechanically defined as increased total motion or high velocity displacements and rotations of the tibia with respect to the femur in arthritic compared to healthy knees and has been linked to altered gait mechanics in patients with knee OA.21 Given that healthy knees move through minimal amounts of frontal-plane knee joint motion during weightbearing,25, 26 increased varus/valgus motion of the knee joint during gait has been suggested as a potential sign of compromised knee joint stability.27 Increased varus/valgus motion could contribute to the etiology of knee OA by shifting the location and movements of the tibiofemoral joint contact points, thus altering the patterns of knee joint loading. To date, evidence in support of the associations between increased varus/valgus knee joint motion and altered joint contact patterns in patients with knee OA remains limited.
The primary purpose of this study was to evaluate the differences in tibiofemoral joint contact point excursions and velocities between patients with knee OA compared to a control group of older adults without knee OA during the loading response phase of downhill gait. Downhill gait was selected as a frequently reported problematic task in patients with knee OA that challenges both knee stability and lower extremity muscle strength. Additionally, the secondary aim of this study was to assess the linear association between knee joint contact point excursions and velocities with frontal-plane varus/valgus knee joint motion excursions and quadriceps and hip abductor muscle strength in patients with knee OA. It was hypothesized that compared to the control group, patients with knee OA would demonstrate evidence of greater and more abrupt tibiofemoral joint contact point motion during the loading response phase of downhill gait that are associated with increased varus/valgus motion excursions and quadriceps and hip abductor muscle weakness.
2. Materials and Methods
2.1. Subjects
Eleven patients with symptomatic, medial compartment knee OA participated in this study. All knee OA patients met the American College of Rheumatology classification criteria for knee OA28 and demonstrated primary medial compartment radiographic knee OA of at least grade II or higher according to the Kellgren and Lawrence radiographic severity rating scale.29 A control group of 11 older adults without radiographic evidence of knee OA was recruited to undergo identical testing to the knee OA group. Participants were excluded, regardless of group designation, if they had a past history of traumatic knee injury or knee surgery, lower extremity total joint arthroplasty or if they required use of an assistive device or a rest period to walk a distance of 30.5 meters (100 feet). All participants were informed as to the nature of the study and signed an informed consent form approved by the Institutional Review Board of the University of Pittsburgh.
2.2. Dynamic Stereo X-ray Testing Procedures
Dynamic Stereo X-ray (DSX) methods were used to quantify 3-dimensional (3D) tibiofemoral joint kinematics from biplane radiographic images. The biplane X-ray system contained two X-ray gantries that were configured with their beam paths intersecting at 60° in a plane parallel to the floor. Each gantry contained a 100 kW pulsed X-ray generator (CPX 3100CV; EMD Technologies, Quebec, Canada), a 40 cm image intensifier (Thales, Neuilly-sur-Seine, France), and a high-speed 4 megapixel digital video camera (Phantom v10, Vision Research, Wayne, New Jersey, USA). The X-ray generators were customized to provide short-duration pulses at very high repetition rates. For the current study, radiographs were generated with a 1ms pulse width at 100 Hz, with a maximum radiographic protocol of 90 kVp/200 mA and a 1 second collection time (100 ms total x-ray exposure) per trial.
Participants' knees were imaged during a downhill gait condition (7% grade, 0.75 m/s) on an instrumented treadmill (Bertec Corp., Columbus, OH, USA). The decision to use a downhill gait condition was made based on our previous clinical experience with patients with knee OA who reported frequent difficulty and pain while walking downhill. To this end, downhill walking has been suggested to be more demanding on the knee joint compared to level gait, as it leads to significant increases in knee flexion angle, vertical ground reaction force and knee joint moments.30-33 Given that downhill walking also challenges knee joint stability and lower extremity muscle strength,31, 34 it represents a reasonable model for assessing knee joint biomechanics during high-demanding daily tasks such as going up or down stairs.35 Additionally, a relatively slow gait velocity of 0.75 m/s was chosen for our experimental set up based on the result of our pilot testing demonstrating that most patients with knee OA were unable to walk downhill at higher speeds.
Participants were positioned on a treadmill within the biplane X-ray system so that the knee of interest would remain in the system's 3D imaging volume throughout the loading response phase of gait. Loading response was selected as a critical time period associated with high demands on the knee joint and reports of dynamic alignment change in patients with knee OA.36, 37 For participants with knee OA, the knee in which they reported symptoms or the most painful knee in bilateral cases was designated as the test knee. For control participants, the knee from the dominant lower limb was designated as the test knee. For each subject, data was collected for 3 individual gait trials and averaged for statistical analysis. For each trial, the X-ray system was triggered manually prior to heel contact to record a 200ms time period. The loading response phase was then defined as the first 20% of the stance phase of gait after heel contact, determined from the vertical ground reaction force profile.38
2.3. Quantification of Knee Joint Motion
All participants also underwent computed tomography (CT) imaging of the tibiofemoral joint of interest. The CT field of view was approximately 28 × 28 cm, slice thickness ranged from 0.6 to 1.25 mm, and in-plane resolution was approximately 0.55 mm per pixel. Single slices through the center of the femoral head and tibial plafond were acquired during the same scan to determine the mechanical axis of the lower extremity. The tibia and femur were manually segmented from the CT images and custom software was used to perform feature-based interpolation to create 3D bone models.39 A model-based tracking algorithm was then employed to determine 3D joint motion by matching the radiographic images with projections through the 3D volumetric bone models.40
Local coordinate systems aligned with anatomic axes were established for the tibia and the femur using the patient-specific 3-D bone models generated from CT scans as previously described.39, 41 For the femur, spheres were fitted to the medial and lateral femoral condyles. The medial/lateral femoral axis (y-axis) was defined in the direction of the line connecting the centers of the spheres, and the midpoint of this line was defined as the origin of the femoral coordinate system. The anterior/posterior femoral axis (x-axis) was calculated from the cross-product of the y-axis with a vector from the origin to the center of the femoral head. The proximal/distal axis (z-axis) was determined using the cross-product of the x- and y-axes. The medial/lateral tibial axis (y-axis) was defined along the line connecting the most medial and lateral points of the tibial plateau. The origin of the tibial coordinate system was established at the midpoint of this line. The anterior/posterior axis (x-axis) was calculated from the cross-product between the y-axis and a vector connecting the origin to the center of the tibia plafond. The proximal/distal axis (z-axis) of the tibia was determined from the cross-product of the x- and y-axes.
The body-fixed rotation angles of the tibial anatomical coordinate system relative to the femoral anatomical coordinate system were calculated for each motion frame with neutral rotations (zero values) as the position where the tibia and femoral coordinate systems were aligned. Using this convention, varus/valgus motion occurred about a floating intermediate axis mutually perpendicular to the y-axis defined by the medial and lateral femoral condyles and the z-axis along the anatomically-defined long axis of the tibia.39 The resulting angles corresponded to the rotational components of the joint coordinate system described by Grood and Suntay.42 This experimental approach has shown to have excellent accuracy in terms of measurement bias of 0.11° and measurement precision of 0.31° for varus/valgus rotations.40 Varus/valgus rotation excursions were then computed by subtracting the minimum from the maximum frontal-plane joint angles during the loading response phase of gait. The kinematic data were filtered using a 4th order low-pass butterworth filter at 10 Hz.
The location of the joint contact points were estimated using the distance-weighted centroid of the region of closest proximity between the bony surfaces in both the medial and lateral tibiofemoral joint compartments as previously described.43 Briefly, 3D wireframe meshes were generated from the subchondral bone regions of the tibial and femoral medial and lateral compartment articulating regions, using subject-specific CT scans. The center and area of each triangular mesh element were calculated for each bone surface triangle, and the distance between each surface element and all opposing bone surface elements was then determined for each frame of data. The minimum distances to the opposing bone for each surface element were combined to create overall minimum distance maps for each motion frame. To determine estimated contact points, distance and area-weighted centroids were calculated from the elements making up the closest 200 mm2 area of the medial and lateral tibial surfaces for each motion frame. The resulting joint contact points were expressed in the tibial coordinate system with its origin at the center of the tibial plateau. Anterior/posterior and medial/lateral contact point excursions were computed by subtracting the minimum from the maximum contact point position across all frames (Figure 1). Joint contact point velocities, being the first derivative of the linear joint contact point displacements, were derived from the difference of the contact point position between two consecutive data points divided by the corresponding time interval.
Figure 1.
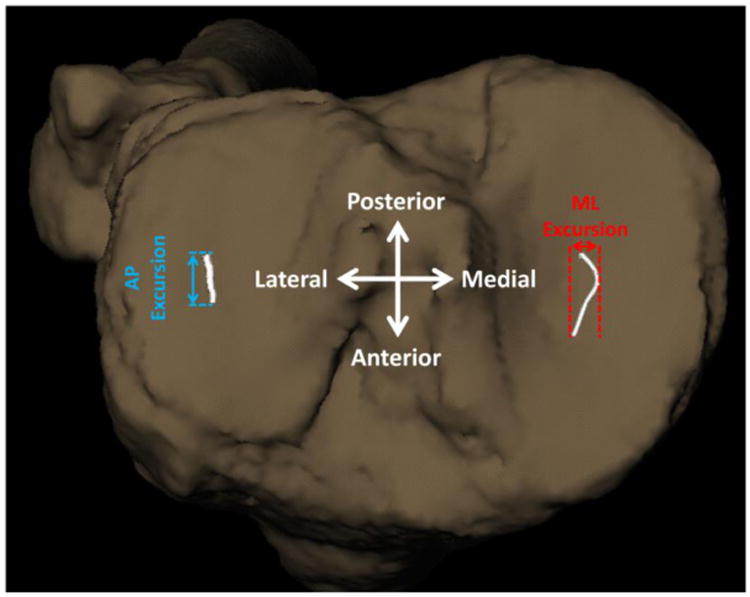
A representative pattern of knee joint contact point excursions. The anteroposterior (AP) and mediolateral (ML) tibiofemoral contact point excursions over the tibial plateau were computed by subtracting the minimum from the maximum contact point positions across all frames during the loading response phase of downhill gait.
2.4. Muscle Strength Testing Procedures
Muscle force was measured isometrically using a force dynamometer (Nicholas MMT, Lafayette Instruments, Lafayette, USA). A non-elastic adjustable strap was used to firmly hold the dynamometer stationary at a fixed distance for the participant to push against. The quadriceps muscle was tested with the participant in a seated position, with the hip at 90 degrees of flexion and the knee positioned at an angle of 60 degrees of flexion. The dynamometer was positioned distally over the anterior surface of the tibia at a distance of 30 cm from the knee joint line. Hip abductor strength was tested with the participant in sidelying and the hip joint positioned in neutral (i.e. no rotation in any plane) and the dynamometer placed over the lateral aspect of the thigh at a distance of 25 cm distal to the greater trochanter. Each participant performed 3 repetitions of each test with 30 seconds of rest allowed between each muscle contraction. The mean force of the three strength measurements was converted to torque by multiplying the mean force output by the resistance lever arm and then normalized by dividing it by the subject's body mass. A single investigator performed all strength measurements. To determine whether reliable data could be obtained, all strength measurements were repeated on 5 volunteers on 2 separate days at least 7 days apart. This procedure generated an intraclass correlation coefficient (ICC) of 0.96 for strength testing of the quadriceps and 0.95 for the hip abductors.
2.5. Statistical Analysis
Group means and standard deviations for demographics and weight-normalized muscle strength measurements were compared using independent sample t-tests for continuous variables and chi-square test for categorical variables. Analysis of covariance was used to evaluate the differences in joint contact positions/excursions and frontal-plane knee joint angles/motion excursions between groups while adjusting for variations in body mass index (BMI). In addition, the correlations between knee joint contact motion excursions and velocities with frontal-plane knee varus/valgus motion excursion and quadriceps/hip abductor muscle strength within each group were determined using Pearson's correlation coefficients. Given that the current exploratory study was observational and non-confirmatory in nature, corrections for multiple comparisons were not performed. While p-value adjustments for multiple comparisons are often necessary in order to reduce the probability of making type I errors in confirmatory studies when a final conclusion or decision needs to be reached, adjustments for multiple comparisons in observational studies increase the chance of making type II errors that may prevent exploring potentially meaningful leads and may not be appropriate.44, 45 All statistical analyses were performed using a two-tailed significant level of p<0.05 in STATA version 11.2 (StataCorp, College Station, TX).
3. Results
3.1. Demographics
There were no statistically significant differences in terms of age, gender, height, and body weight between groups (Table 1). However, BMI was found to be significantly higher in the knee OA group compared to the control group (mean difference of 5.8 kg/m2; p=0.004).
Table 1.
Subject characteristics.
| Control (n=11) | Knee OA (n=11) | Significance (P-value) | |
|---|---|---|---|
| Age (years) | 67.5±5.0 | 69.6±8.0 | 0.47 |
| Female (%) | 54.5% | 72.7% | 0.38 |
| Height (cm) | 177.2±12.7 | 168.4±8.5 | 0.07 |
| Weight (m) | 77.3±12.1 | 85.8±14.2 | 0.15 |
| Body Mass Index (kg/m2) | 24.6±2.6 | 30.4±5.3 | <0.01 |
All values are expressed as mean ± standard deviations unless otherwise indicated. OA = Osteoarthritis.
3.2. Contact Positions, Excursions, and Velocities
No significant group differences were observed for the AP or ML positions of the joint contact point at the time of heel strike or the peak AP or ML positions during the entire loading response phase of gait for either the medial or lateral compartments (Table 2). Compared to the control group, the knee OA group demonstrated significantly longer medial/lateral joint contact point excursions for both the medial and the lateral compartments (P<0.02; Table 3; Figure 2). The peak medial/lateral contact point velocity was also significantly greater for the medial compartment (P=0.02) in the knee OA group compared to the controls but the group differences for the lateral compartment did not reach statistical significance (P=0.06; Table 3). In addition, the knee OA group demonstrated significant increases in their heel-strike joint contact point velocity for both medial (P=0.04) and lateral (P<0.05) tibiofemoral compartments compared to their control counterparts (Table 3).
Table 2.
Group comparisons of medial and lateral tibiofemoral joint contact point positions during the loading response phase of downhill gait.
| Control (n=11) | Knee OA (n=11) | 95% Confidence Interval for Group Differences | Effect Size (Partial eta2) | Significance (P-value)* | |
|---|---|---|---|---|---|
| Medial Compartment | |||||
| AP Contact Point Position @ Heel-Strike (mm) | -5.7 ± 2.7 | -2.8 ± 5.5 | -7.7 to 2.1 | 0.07 | 0.25 |
| Peak AP Contact Point Position (mm) | -3.6 ± 4.2 | 0.78 ± 5.6 | -10.9 to 0.25 | 0.17 | 0.06 |
| ML Contact Point Position @ Heel-Strike (mm) | 1.2 ± 1.2 | 1.2 ± 3.8 | -2.0 to 4.1 | 0.03 | 0.49 |
| Peak ML Contact Point Position (mm) | 1.3 ± 1.7 | 1.4 ± 3.9 | -3.1 to 3.7 | 0.01 | 0.86 |
| Lateral Compartment | |||||
| AP Contact Point Position @ Heel-Strike (mm) | -2.2 ± 2.9 | 0.88 ± 4.4 | -8.5 to 0.3 | 0.21 | 0.06 |
| Peak AP Contact Point Position (mm) | 2.0 ± 4.2 | 2.9 ± 5.0 | -7.5 to 2.7 | 0.05 | 0.33 |
| ML Contact Point Position @ Heel-Strike (mm) | -3.1 ± 1.2 | -1.3 ± 3.9 | -4.3 to 2.1 | 0.03 | 0.49 |
| Peak ML Contact Point Position (mm) | -2.7 ± 1.2 | -1.0 ± 4.1 | -4.2 to 2.5 | 0.02 | 0.60 |
All values are expressed as mean ± standard deviations with respect to the tibial coordinate system with its origin at the center of the tibial plateau. AP = anterior (+) / posterior (-); ML = medial (+) /lateral (-); OA = Osteoarthritis.
All analyses were adjusted for group differences in body mass index.
Table 3.
Group comparisons of medial and lateral tibiofemoral joint contact path motion and velocities during the loading response phase of downhill gait.
| Control (n=11) | Knee OA (n=11) | 95% Confidence Interval for Group Differences | Effect Size (Partial eta2) | Significance (P-value)* | |
|---|---|---|---|---|---|
| Medial Compartment | |||||
| Contact Point AP Excursion (mm) | 3.1 ± 1.8 | 4.0 ± 2.7 | -1.6 to 3.6 | 0.03 | 0.43 |
| Contact Point ML Excursion (mm) | 0.5 ± 0.3 | 1.4 ± 1.3 | 0.2 to 2.3 | 0.25 | 0.02 |
| Contact Point Velocity @ Heel-Strike (mm/second) | 31.1 ± 20.0 | 73.6 ± 36.4 | 2.3 to 67.5 | 0.21 | 0.04 |
| Peak AP Contact Point Velocity (mm/second) | 60.9 ± 42.0 | 68.7 ± 67.7 | -56.3 to 71.0 | 0.01 | 0.81 |
| Peak ML Contact Point Velocity (mm/second) | 13.2 ± 6.8 | 30.4 ± 27.2 | 4.1 to 46.9 | 0.25 | 0.02 |
| Lateral Compartment | |||||
| Contact Point AP Excursion (mm) | 3.2 ± 1.9 | 2.8 ± 1.8 | -2.0 to 2.1 | 0.01 | 0.99 |
| Contact Point ML Excursion (mm) | 0.7 ± 0.4 | 1.2 ± 0.8 | 0.30 to 1.6 | 0.33 | <0.01 |
| Contact Point Velocity @ Heel-Strike (mm/second) | 29.1 ± 14.4 | 43.9 ± 23.8 | 0.63 to 43.1 | 0.19 | <0.05 |
| Peak AP Contact Point Velocity (mm/second) | 52.1 ± 32.6 | 45.9 ± 38.3 | -33.0 to 44.0 | 0.01 | 0.75 |
| Peak ML Contact Point Velocity (mm/second) | 17.3 ± 8.6 | 20.9 ± 14.3 | -0.59 to 23.0 | 0.17 | 0.06 |
All values are expressed as mean ± standard deviations. AP = anterior/posterior; ML = medial/lateral; OA = Osteoarthritis.
All analyses were adjusted for group differences in body mass index.
Figure 2.
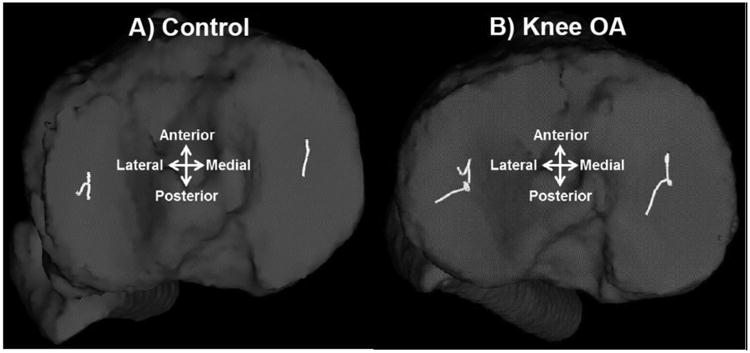
Representative tibiofemoral joint contact profiles of a control knee (A) and a knee with osteoarthritis (B).
3.3. Knee Varus/Valgus Motion Excursion
Patients with knee OA demonstrated greater frontal-plane knee varus motion excursions compared to the control group (P<0.01; Table 4). However, the varus/valgus angles at the time of heel-strike or the peak varus/valgus angles during the entire loading response phase of gait were not different between groups. Correlation analysis revealed non-significant linear associations between frontal-plane varus motion excursion with medial and lateral compartment joint contact point excursions/velocities in the control group (Table 5). On the other hand, a linear positive association was observed between greater frontal-plane knee varus motion excursion with longer medial compartment medial/lateral contact point excursion (r=0.646; P=0.03) and higher medial compartment peak medial/lateral contact point velocity (r=0.628; P=0.04) in the knee OA group. Additionally, greater knee varus motion excursion was also linearly associated with higher lateral compartment contact point velocity at heel-strike (r=0.624; P=0.04) and higher lateral compartment peak medial/lateral contact point velocity (r=0.638; P=0.03) in the knee OA group.
Table 4.
Group comparisons of Varus/Valgus positions, excursions and quadriceps and hip abductor muscle strength between the knee OA and control groups.
| Control (n=11) | Knee OA (n=11) | 95% Confidence interval for Group Difference | Effect Size (Partial eta2) | Significance (P-value) | |
|---|---|---|---|---|---|
| Varus (-)/Valgus (+) angle @ heel contact (°)* | 0.1 ± 3.7 | -0.5 ± 8.3 | -7.7 to 6.8 | 0.01 | 0.90 |
| Peak Varus (-)/Valgus (+) anglef (°)* | -0.8 ± 3.7 | -1.5 ± 9.1 | -8.6 to 7.1 | 0.01 | 0.84 |
| Varus (-)/Valgus (+) Motion Excursion (°)* | -1.0 ±0.3 | -1.8 ± 1.3 | -2.4 to -0.5 | 0.33 | <0.01 |
| Quadriceps Strength (Nm/kg) | 1.0 ± 0.2 | 0.8 ± 0.2 | 0.02 to 0.4 | 0.21 | 0.03 |
| Hip Abductor Strength (Nm/kg) | 0.8 ± 0.2 | 0.6 ± 0.2 | 0.02 to 0.4 | 0.21 | 0.03 |
All values are expressed as mean ± standard deviations. OA = Osteoarthritis
Analysis was adjusted for group differences in body mass index.
Table 5. Pearson correlation coefficients for the associations between knee joint contact mechanics and knee varus/valgus motion excursion, quadriceps muscle strength, and hip.
| Varus/Valgus Excursion | Quadriceps Strength | Hip Abductor Strength | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Control | OA | Control | OA | Control | OA | |
| Medial Compartment | ||||||
| Contact Point AP Excursion | -0.520 | 0.521 | -0.004 | 0.484 | -0.367 | 0.314 |
| Contact Point ML Excursion | -0.321 | 0.646* | -0.302 | 0.054 | -0.453 | -0.299 |
| Contact Point Velocity @ Heel-Strike | -0.373 | 0.343 | -0.162 | 0.170 | -0.391 | 0.270 |
| Peak AP Contact Point Velocity | -0.324 | 0.327 | -0.002 | 0.537 | -0.293 | 0.368 |
| Peak ML Contact Point Velocity | -0.130 | 0.628* | -0.135 | -0.029 | -0.380 | -0.111 |
| Lateral Compartment | ||||||
| Contact Point AP Excursion | -0.022 | 0.548 | -0.386 | 0.786* | -0.363 | 0.244 |
| Contact Point ML Excursion | 0.085 | 0.345 | 0.008 | 0.176 | -0.037 | 0.310 |
| Contact Point Velocity @ Heel-Strike | 0.288 | 0.624* | 0.123 | 0.724* | 0.486 | 0.442 |
| Peak AP Contact Point Velocity | 0.081 | 0.413 | 0.041 | 0.779* | -0.037 | 0.478 |
| Peak ML Contact Point Velocity | -0.022 | 0.638* | -0.123 | 0.227 | -0.202 | -0.011 |
abductor muscle strength.
AP = anteroposterior; ML = mediolateral; OA = Osteoarthritis.
Indicates significant (p < 0.05) within-group correlation
3.4. Quadriceps and Hip Abductor Strength
Patients with knee OA demonstrated significant quadriceps and hip abductor muscle weakness (P=0.03) compared to the control group (Table 4). Correlation analysis revealed no significant linear associations between medial or lateral compartment contact point excursions or velocities and quadriceps or hip abductor muscle strength in the control group (Table 5). In addition, no significant linear associations were observed between medial compartment contact point excursions or velocities and quadriceps or hip abductor muscle strength in the knee OA group. However, statistically significant linear associations were observed in the knee OA group between greater quadriceps strength and longer lateral compartment anterior/posterior contact point excursion (r=0.786; P<0.01), lateral compartment contact point velocity at heel-strike (r=0.724; P=0.01), and lateral compartment peak anterior/posterior contact point velocity (r=0.779; P<0.01). No significant linear associations were observed between hip abductor muscle strength and lateral compartment contact mechanics in the knee OA group.
4. Discussion
The hypothesis that patients with knee OA demonstrate altered knee joint contact mechanics during downhill gait was supported by the data. Overall, patients with knee OA had significantly longer medial/lateral joint contact point excursions (figure 2) and greater peak medial/lateral joint contact point velocities for both the medial and lateral tibiofemoral compartments compared to their control counterparts. Knee OA patients also demonstrated a significant increase in their medial and lateral compartment joint contact point velocities after heel-strike. The greater and more abrupt movements of the tibiofemoral joint contact point observed in patients with OA compared to the control group is suggestive of dynamic knee joint instability and may represent the inability of the tibiofemoral joint to adequately handle the high demands placed on the knee joint during a task such as downhill walking.
Frank and colleagues46 previously reported an association between increased medial/lateral knee joint translations during the mid-stance phase of gait with cartilage and bone damage in unstable sheep knees 20-weeks after anterior cruciate ligament (ACL)/medial collateral ligament transection. Anderst and colleagues47 also reported that increased velocity of the medial compartment joint contact point in the early stance phase of running is associated with greater medial compartment cartilage damage in unstable canine knees after 2 years. The findings from the above animal studies are relevant to the current study in that similar alterations in knee joint contact point translations and velocities are observed in our cohort of patients with knee OA. Additionally, greater knee joint translations and velocities have also been previously reported for patients with knee OA and self-reported episodic knee joint instability, providing further evidence for presence of mechanical instability at the level of the joint surfaces in patients with knee OA.48 It is plausible that the increased frictional forces49 and elevated contact and shear stresses49, 50 previously associated with greater excursions and higher velocities of sliding joint surfaces could facilitate further cartilage damage in unstable joints of patients with knee OA when they are involved in high demanding functional tasks.
The results of our study also suggest that patients with knee OA move through greater degrees of frontal-plane knee varus motion excursion during the loading response phase of downhill gait compared to their control counterparts. Increased frontal-plane knee joint instability has been previously linked with development and progression of knee OA after meniscectomy and medial collateral ligament tears.51, 52 Previous reports of greater frontal plane knee joint motion due to laxity in the uninvolved knees of patients with OA and as a function of aging further support the concept that greater varus/valgus motion is not merely a consequence of pathologic changes that develop at later stages of disease but rather an important risk factor for disease development and progression.22 It is likely that greater varus excursions in patients with knee OA could lead to more rapid medial/lateral translations of the joint contact points and increase the magnitude of contact forces and shift the load-bearing areas of the joint to cartilage regions that have not adapted to the high customary loads of daily activities to cause greater symptoms and further joint damage. To this end, the use of knee bracing53, 54 and laterally- wedged shoe insoles55, 56 have shown to effectively limit the frontal-plane motion and medial/lateral excursions of the knee joint during weightbearing along with improvements in pain and function. Although it is logical to assume that these treatment options may also be effective in limiting knee joint contact extrusions and velocities in patients with OA, this assertion should be formally investigated in future studies. Additionally, it has been reported that individuals with medial knee OA attempt to stabilize their knees with greater co-contraction of the muscles that cross the knee joint.57-59 However, such strategy is undesirable as it could contribute to higher joint compression and faster progression of the OA disease process.
Knee OA patients in our study also demonstrated significant quadriceps and hip abductor muscle strength deficits compared to their control counterparts. However, our hypothesis that better quadriceps and hip abductor muscles strength will be linearly associated with reduced joint contact point excursions and velocities was not supported by the data. This finding is consistent with previous reports from clinical trials in that greater peak quadriceps or hip abductor muscle strength may not necessarily translate to improvements in the local mechanical environment of the knee joint.13, 17-20 Therefore, strategies geared towards strength maximization of the quadriceps and hip abductor muscles may not provide better dynamic knee joint stability or improved joint contact mechanics in patients with medial compartment disease. Given that the quadriceps and the hip abductor muscles represent the two most commonly targeted muscle groups for rehabilitation of patients with knee OA, our findings have potential clinical implications as conventional exercise regimens recommended for treatment of knee OA are heavily focused on isolated quadriceps and hip abductor muscle strengthening.
An interesting finding of our study was the observed positive linear associations between quadriceps muscle strength and longer lateral compartment anterior/posterior joint contact point excursions and velocities in the OA group (Table 5). This finding may relate to previously reported increases in knee joint laxity in the anterior/posterior direction in OA knees,60 to the point where the anterior pull of a stronger quadriceps contraction at the muscle's tibial insertion could lead to greater and more rapid anterior/posterior joint contact point translations. This observation raises the possibility that the clinical effect of quadriceps strengthening interventions in patients with knee OA may be improved by considering the status of the passive restraint system of the knee joint as previously suggested.61 Alternatively, it could also be argued that knee OA patients with greater quadriceps strength may have moved through greater knee flexion range of motion which could subsequently result in greater anterior/posterior joint contact point excursions. However, a closer inspection of our data revealed that although the control subjects in our study moved through a greater knee flexion excursion during the loading response phase of downhill gait (control = 9.5° ± 4.4° versus knee OA = 6.8° ± 4.1°), their greater quadriceps strength (Table 4) was not associated with increased anterior/posterior contact point excursions (Table 5). It is likely that the adequate joint stability provided by the intact passive restraint system in a healthy knee allows for proper functioning of stronger quadriceps muscles within the knee joint's physiologic range of motion.
Although muscle strength was not linearly associated with joint contact excursions and velocities in patients with knee OA in our study, the potential influence of lower extremity muscles on knee joint mechanics during gait could not be completely ruled out. For example, promoting more synergistic muscle activity through neuromuscular training aimed at improving timing and coordination of the lower extremity muscle contractions may be beneficial in patients with knee OA for achieving better knee joint stability, decreased muscle co-contraction, and reduced joint shear and compressive forces. To this end, promising results have been reported for the effectiveness of neuromuscular training programs that include balance, perturbation, agility, plyometrics, endurance and functional activity training in providing better joint biomechanics and stability in young athletes at risk for ACL injury.62-65 Gait modification strategies aimed at improving dynamic lower extremity alignment have also shown to effectively reduce the knee adduction moment, which is commonly used as a surrogate measure of medial compartment joint loading, through systematic neuromuscular training with the use of feedback in healthy participants with and without knee varus deformity.66, 67 More recently, several randomized controlled trails have reported on the feasibility of implementing neuromuscular training programs for patients with knee OA,68-70 however whether such programs can improve joint contact mechanics still remains unknown. Also, given that we only considered the possibility of linear associations, non-linear associations between muscle strength and joint contact excursions and velocities may exist that were not explored in the current study. Therefore, future research is warranted to provide additional information regarding the overall role of lower extremity muscles in influencing knee contact mechanics in patients with knee OA.
The results of our study should be considered in light of a number of limitations. First, we acknowledge the potential limitations of the small sample size used in this investigation. The addition of more subjects would have improved the power of the study and may have identified additional differences between groups. Second, the locations of the tibiofemoral joint contact points were determined using the distance-weighted centroid of the region of closest proximity on the subchondral bone surfaces obtained from CT images, which does not take into consideration the irregularities of the articular cartilage thickness or degeneration of the meniscus. However, it was previously demonstrated that the anterior/posterior and medial/lateral excursions of the joint contact points determined using the aforementioned method were not significantly different between participants with distinctly different radiographic knee OA severity which takes into account loss of joint space.48 We also chose to investigate the influence of the two most commonly studied lower extremity muscle groups (i.e. quadriceps and hip abductors) related to the pathomechanics of knee OA. We acknowledge that there are other lower extremity muscle groups that may influence knee joint kinematics and contact mechanics during ambulation which may warrant further investigation in this patient population. Finally, we did not evaluate passive knee joint laxity as a part of our study. Therefore, the influence of passive knee joint laxity on the observed differences in knee joint contact mechanics could not be determined and should be investigated in the future.
5. Conclusions
The findings from this preliminary investigation demonstrate that increased frontal-plane varus knee joint excursion may be related to greater and more abrupt motion of the knee joint articulating surfaces in patients with knee OA. Deficits in quadriceps and hip abductor muscle strength, however, did not seem to influence the contact mechanics of the knee joint in patients with medial compartment knee OA. Verification of these results with larger sample sizes along with longitudinal observations of the impact of altered joint mechanics on knee OA progression should be further considered in future research.
Highlights.
Knee kinematics & muscle strength were examined in patients with osteoarthritis.
Joint contact during downhill gait was assessed using Dynamic stereo x-ray methods.
Patients with osteoarthritis had increased knee contact point excursion & velocity.
Altered knee contact patterns were associated with increased varus knee motion.
However, muscle weakness was not associated with altered knee joint contact.
Acknowledgments
This work was supported by the Pittsburgh Claude D. Pepper Older Americans Independence Center (Grant P30 AG024827) and a National Center Medical Rehabilitation Research, National Institute of Child Health and Human Development / National Institute Neurological Disorders and Stroke, National Institutes of Health career development award (K12 HD055931).
Footnotes
This study was approved by the Health Sciences Institutional Review Board, University of Pittsburgh, Pittsburgh, PA, USA.
Conflict of interest: None of the authors have any financial or personal relationships that would be deemed a conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–57. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage. 2013;21:10–5. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40:215–22. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- 4.Das P, Schurman DJ, Smith RL. Nitric oxide and G proteins mediate the response of bovine articular chondrocytes to fluid-induced shear. J Orthop Res. 1997;15:87–93. doi: 10.1002/jor.1100150113. [DOI] [PubMed] [Google Scholar]
- 5.Lane Smith R, Trindade MC, Ikenoue T, Mohtai M, Das P, Carter DR, et al. Effects of shear stress on articular chondrocyte metabolism. Biorheology. 2000;37:95–107. [PubMed] [Google Scholar]
- 6.Bennell KL, Hunt MA, Wrigley TV, Lim BW, Hinman RS. Role of muscle in the genesis and management of knee osteoarthritis. Rheum Dis Clin North Am. 2008;34:731–54. doi: 10.1016/j.rdc.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Hurley MV. The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum Dis Clin North Am. 1999;25:283–98. doi: 10.1016/s0889-857x(05)70068-5. [DOI] [PubMed] [Google Scholar]
- 8.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 9.Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127:97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Slemenda C, Heilman DK, Brandt KD, Katz BP, Mazzuca SA, Braunstein EM, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41:1951–9. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Segal NA, Torner JC, Felson D, Niu J, Sharma L, Lewis CE, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum. 2009;61:1210–7. doi: 10.1002/art.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alnahdi AH, Zeni JA, Snyder-Mackler L. Muscle impairments in patients with knee osteoarthritis. Sports Health. 2012;4:284–92. doi: 10.1177/1941738112445726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennell KL, Hunt MA, Wrigley TV, Hunter DJ, McManus FJ, Hodges PW, et al. Hip strengthening reduces symptoms but not knee load in people with medial knee osteoarthritis and varus malalignment: a randomised controlled trial. Osteoarthritis Cartilage. 2010;18:621–8. doi: 10.1016/j.joca.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Hinman RS, Hunt MA, Creaby MW, Wrigley TV, McManus FJ, Bennell KL. Hip muscle weakness in individuals with medial knee osteoarthritis. Arthritis Care Res (Hoboken) 2010;62:1190–3. doi: 10.1002/acr.20199. [DOI] [PubMed] [Google Scholar]
- 15.Costa RA, Oliveira LM, Watanabe SH, Jones A, Natour J. Isokinetic assessment of the hip muscles in patients with osteoarthritis of the knee. Clinics (Sao Paulo) 2010;65:1253–9. doi: 10.1590/S1807-59322010001200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang A, Hayes K, Dunlop D, Song J, Hurwitz D, Cahue S, et al. Hip abduction moment and protection against medial tibiofemoral osteoarthritis progression. Arthritis Rheum. 2005;52:3515–9. doi: 10.1002/art.21406. [DOI] [PubMed] [Google Scholar]
- 17.McQuade KJ, de Oliveira AS. Effects of progressive resistance strength training on knee biomechanics during single leg step-up in persons with mild knee osteoarthritis. Clin Biomech (Bristol, Avon) 2011;26:741–8. doi: 10.1016/j.clinbiomech.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim BW, Hinman RS, Wrigley TV, Sharma L, Bennell KL. Does knee malalignment mediate the effects of quadriceps strengthening on knee adduction moment, pain, and function in medial knee osteoarthritis? A randomized controlled trial. Arthritis Rheum. 2008;59:943–51. doi: 10.1002/art.23823. [DOI] [PubMed] [Google Scholar]
- 19.Foroughi N, Smith RM, Lange AK, Baker MK, Fiatarone Singh MA, Vanwanseele B. Lower limb muscle strengthening does not change frontal plane moments in women with knee osteoarthritis: A randomized controlled trial. Clin Biomech (Bristol, Avon) 2011;26:167–74. doi: 10.1016/j.clinbiomech.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Sled EA, Khoja L, Deluzio KJ, Olney SJ, Culham EG. Effect of a home program of hip abductor exercises on knee joint loading, strength, function, and pain in people with knee osteoarthritis: a clinical trial. Phys Ther. 2010;90:895–904. doi: 10.2522/ptj.20090294. [DOI] [PubMed] [Google Scholar]
- 21.Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage. 2004;12:745–51. doi: 10.1016/j.joca.2004.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma L, Lou C, Felson DT, Dunlop DD, Kirwan-Mellis G, Hayes KW, et al. Laxity in healthy and osteoarthritic knees. Arthritis Rheum. 1999;42:861–70. doi: 10.1002/1529-0131(199905)42:5<861::AID-ANR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Felson DT, Niu J, McClennan C, Sack B, Aliabadi P, Hunter DJ, et al. Knee buckling: prevalence, risk factors, and associated limitations in function. Ann Intern Med. 2007;147:534–40. doi: 10.7326/0003-4819-147-8-200710160-00005. [DOI] [PubMed] [Google Scholar]
- 24.Knoop J, van der Leeden M, van der Esch M, Thorstensson CA, Gerritsen M, Voorneman RE, et al. Association of lower muscle strength with self-reported knee instability in osteoarthritis of the knee: results from the Amsterdam Osteoarthritis cohort. Arthritis Care Res (Hoboken) 2012;64:38–45. doi: 10.1002/acr.20597. [DOI] [PubMed] [Google Scholar]
- 25.Perry J. Gait analysis : normal and pathological function. Thorofare, N.J.: SLACK inc.; 1992. [Google Scholar]
- 26.Farrokhi S, Tashman S, Gil AB, Klatt BA, Fitzgerald GK. Are the kinematics of the knee joint altered during the loading response phase of gait in individuals with concurrent knee osteoarthritis and complaints of joint instability? A dynamic stereo X-ray study. Clinical biomechanics. 2012;27:384–9. doi: 10.1016/j.clinbiomech.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Esch M, Steultjens M, Harlaar J, Wolterbeek N, Knol DL, Dekker J. Knee varus-valgus motion during gait--a measure of joint stability in patients with osteoarthritis? Osteoarthritis Cartilage. 2008;16:522–5. doi: 10.1016/j.joca.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 29.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Annals of the rheumatic diseases. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redfern MS. Biomechanics of descending ramps. Gait Posture. 1997;6:119–25. [Google Scholar]
- 31.Kuster M, Sakurai S, Wood GA. Kinematic and kinetic comparison of downhill and level walking. Clin Biomech (Bristol, Avon) 1995;10:79–84. doi: 10.1016/0268-0033(95)92043-l. [DOI] [PubMed] [Google Scholar]
- 32.Lay AN, Hass CJ, Gregor RJ. The effects of sloped surfaces on locomotion: a kinematic and kinetic analysis. Journal of biomechanics. 2006;39:1621–8. doi: 10.1016/j.jbiomech.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 33.McIntosh AS, Beatty KT, Dwan LN, Vickers DR. Gait dynamics on an inclined walkway. Journal of biomechanics. 2006;39:2491–502. doi: 10.1016/j.jbiomech.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 34.Kuster M, Wood GA, Sakurai S, Blatter G. 1994 Nicola Cerulli Young Researchers Award. Downhill walking: a stressful task for the anterior cruciate ligament? A biomechanical study with clinical implications. Knee Surg Sports Traumatol Arthrosc. 1994;2:2–7. doi: 10.1007/BF01552646. [DOI] [PubMed] [Google Scholar]
- 35.Liikavainio T, Isolehto J, Helminen HJ, Perttunen J, Lepola V, Kiviranta I, et al. Loading and gait symmetry during level and stair walking in asymptomatic subjects with knee osteoarthritis: importance of quadriceps femoris in reducing impact force during heel strike? Knee. 2007;14:231–8. doi: 10.1016/j.knee.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Astephen JL, Deluzio KJ. Changes in frontal plane dynamics and the loading response phase of the gait cycle are characteristic of severe knee osteoarthritis application of a multidimensional analysis technique. Clin Biomech (Bristol, Avon) 2005;20:209–17. doi: 10.1016/j.clinbiomech.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–9. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 38.Perry J, Burnfield JM. Gait analysis : normal and pathological function. Thorofare, NJ: SLACK; 2010. [Google Scholar]
- 39.Tashman S, Anderst W. In-vivo measurement of dynamic joint motion using high speed biplane radiography and CT: application to canine ACL deficiency. Journal of biomechanical engineering. 2003;125:238–45. doi: 10.1115/1.1559896. [DOI] [PubMed] [Google Scholar]
- 40.Anderst W, Zauel R, Bishop J, Demps E, Tashman S. Validation of three-dimensional model-based tibio-femoral tracking during running. Medical engineering & physics. 2009;31:10–6. doi: 10.1016/j.medengphy.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goyal K, Tashman S, Wang JH, Li K, Zhang X, Harner C. In vivo analysis of the isolated posterior cruciate ligament-deficient knee during functional activities. The American journal of sports medicine. 2012;40:777–85. doi: 10.1177/0363546511435783. [DOI] [PubMed] [Google Scholar]
- 42.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. Journal of biomechanical engineering. 1983;105:136–44. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 43.Anderst WJ, Les C, Tashman S. In vivo serial joint space measurements during dynamic loading in a canine model of osteoarthritis. Osteoarthritis Cartilage. 2005;13:808–16. doi: 10.1016/j.joca.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 44.Bender R, Lange S. Adjusting for multiple testing--when and how? Journal of clinical epidemiology. 2001;54:343–9. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 45.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. [PubMed] [Google Scholar]
- 46.Frank CB, Beveridge JE, Huebner KD, Heard BJ, Tapper JE, O'Brien EJ, et al. Complete ACL/MCL deficiency induces variable degrees of instability in sheep with specific kinematic abnormalities correlating with degrees of early osteoarthritis. J Orthop Res. 2012;30:384–92. doi: 10.1002/jor.21549. [DOI] [PubMed] [Google Scholar]
- 47.Anderst WJ, Tashman S. The association between velocity of the center of closest proximity on subchondral bones and osteoarthritis progression. J Orthop Res. 2009;27:71–7. doi: 10.1002/jor.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farrokhi S, Voycheck CA, Klatt BA, Gustafson JA, Tashman S, Fitzgerald GK. Altered tibiofemoral joint contact mechanics and kinematics in patients with knee osteoarthritis and episodic complaints of joint instability. Clin Biomech (Bristol, Avon) 2014;29:629–35. doi: 10.1016/j.clinbiomech.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drummond C, Israelachvili J, Richetti P. Friction between two weakly adhering boundary lubricated surfaces in water. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;67:066110. doi: 10.1103/PhysRevE.67.066110. [DOI] [PubMed] [Google Scholar]
- 50.Waldman SD, Bryant JT. Dynamic contact stress and rolling resistance model for total knee arthroplasties. Journal of biomechanical engineering. 1997;119:254–60. doi: 10.1115/1.2796089. [DOI] [PubMed] [Google Scholar]
- 51.Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee--the contributions of the supporting structures. A quantitative in vitro study. J Bone Joint Surg Am. 1976;58:583–94. [PubMed] [Google Scholar]
- 52.Kannus P, Jarvinen M. Osteoarthrosis in a knee joint due to chronic posttraumatic insufficiency of the medial collateral ligament. Nine-year follow-up. Clin Rheumatol. 1988;7:200–7. doi: 10.1007/BF02204455. [DOI] [PubMed] [Google Scholar]
- 53.Ramsey DK, Briem K, Axe MJ, Snyder-Mackler L. A mechanical theory for the effectiveness of bracing for medial compartment osteoarthritis of the knee. The Journal of bone and joint surgery American volume. 2007;89:2398–407. doi: 10.2106/JBJS.F.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Draganich L, Reider B, Rimington T, Piotrowski G, Mallik K, Nasson S. The effectiveness of self-adjustable custom and off-the-shelf bracing in the treatment of varus gonarthrosis. J Bone Joint Surg Am. 2006;88:2645–52. doi: 10.2106/JBJS.D.02787. [DOI] [PubMed] [Google Scholar]
- 55.Ogata K, Yasunaga M, Nomiyama H. The effect of wedged insoles on the thrust of osteoarthritic knees. Int Orthop. 1997;21:308–12. doi: 10.1007/s002640050174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hinman RS, Bowles KA, Metcalf BB, Wrigley TV, Bennell KL. Lateral wedge insoles for medial knee osteoarthritis: effects on lower limb frontal plane biomechanics. Clin Biomech (Bristol, Avon) 2012;27:27–33. doi: 10.1016/j.clinbiomech.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Childs JD, Sparto PJ, Fitzgerald GK, Bizzini M, Irrgang JJ. Alterations in lower extremity movement and muscle activation patterns in individuals with knee osteoarthritis. Clin Biomech (Bristol, Avon) 2004;19:44–9. doi: 10.1016/j.clinbiomech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Schmitt LC, Rudolph KS. Muscle stabilization strategies in people with medial knee osteoarthritis: the effect of instability. J Orthop Res. 2008;26:1180–5. doi: 10.1002/jor.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewek MD, Ramsey DK, Snyder-Mackler L, Rudolph KS. Knee stabilization in patients with medial compartment knee osteoarthritis. Arthritis Rheum. 2005;52:2845–53. doi: 10.1002/art.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wada M, Imura S, Baba H, Shimada S. Knee laxity in patients with osteoarthritis and rheumatoid arthritis. Br J Rheumatol. 1996;35:560–3. doi: 10.1093/rheumatology/35.6.560. [DOI] [PubMed] [Google Scholar]
- 61.Sharma L, Hayes KW, Felson DT, Buchanan TS, Kirwan-Mellis G, Lou C, et al. Does laxity alter the relationship between strength and physical function in knee osteoarthritis? Arthritis Rheum. 1999;42:25–32. doi: 10.1002/1529-0131(199901)42:1<25::AID-ANR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 62.Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24:765–73. doi: 10.1177/036354659602400611. [DOI] [PubMed] [Google Scholar]
- 63.Hurd WJ, Chmielewski TL, Snyder-Mackler L. Perturbation-enhanced neuromuscular training alters muscle activity in female athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14:60–9. doi: 10.1007/s00167-005-0624-y. [DOI] [PubMed] [Google Scholar]
- 64.Myer GD, Ford KR, Palumbo JP, Hewett TE. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. J Strength Cond Res. 2005;19:51–60. doi: 10.1519/13643.1. [DOI] [PubMed] [Google Scholar]
- 65.Chappell JD, Limpisvasti O. Effect of a neuromuscular training program on the kinetics and kinematics of jumping tasks. Am J Sports Med. 2008;36:1081–6. doi: 10.1177/0363546508314425. [DOI] [PubMed] [Google Scholar]
- 66.Barrios JA, Crossley KM, Davis IS. Gait retraining to reduce the knee adduction moment through real-time visual feedback of dynamic knee alignment. J Biomech. 2010;43:2208–13. doi: 10.1016/j.jbiomech.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wheeler JW, Shull PB, Besier TF. Real-time knee adduction moment feedback for gait retraining through visual and tactile displays. Journal of biomechanical engineering. 2011;133:041007. doi: 10.1115/1.4003621. [DOI] [PubMed] [Google Scholar]
- 68.Ageberg E, Link A, Roos EM. Feasibility of neuromuscular training in patients with severe hip or knee OA: the individualized goal-based NEMEX-TJR training program. BMC Musculoskelet Disord. 2010;11:126. doi: 10.1186/1471-2474-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fitzgerald GK, Piva SR, Gil AB, Wisniewski SR, Oddis CV, Irrgang JJ. Agility and perturbation training techniques in exercise therapy for reducing pain and improving function in people with knee osteoarthritis: a randomized clinical trial. Phys Ther. 2011;91:452–69. doi: 10.2522/ptj.20100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knoop J, Dekker J, van der Leeden M, van der Esch M, Thorstensson CA, Gerritsen M, et al. Knee joint stabilization therapy in patients with osteoarthritis of the knee: a randomized, controlled trial. Osteoarthritis Cartilage. 2013;21:1025–34. doi: 10.1016/j.joca.2013.05.012. [DOI] [PubMed] [Google Scholar]


