Abstract
Background In plants, the products of secretory activity leave the protoplast and cross the plasma membrane by means of transporters, fusion with membranous vesicles or, less commonly, as result of disintegration of the cell. These mechanisms do not address an intriguing question: How do secretory products cross the cell wall? Furthermore, how do these substances reach the external surface of the plant body? Such diverse substances as oils, polysaccharides or nectar are forced to cross the cell wall and, in fact, do so. How are chemical materials that are repelled by the cell wall or that are sufficiently viscous to not cross passively released from plant cells?
Scope and Conclusions I propose a cell-cycle model developed based on observations of different secreting systems, some unpublished results and an extensive literature review, aiming to understand the processes involved in both the secretory process and the release of secretion products. In the absence of facilitated diffusion, a mechanical action of the protoplast is necessary to ensure that some substances can cross the cell wall. The mechanical action of the protoplast, in the form of successive cycles of contraction and expansion, causes the material accumulated in the periplasmic space to cross the cell wall and the cuticle. This action is particularly relevant for the release of lipids, resins and highly viscous hydrophilic secretions. The proposed cell-cycle model and the statements regarding exudate release will also apply to secretory glands not elaborated upon here. Continuous secretion of several days, as observed in extrafloral nectaries, salt glands and some mucilage-producing glands, is only possible because the process is cyclical.
Keywords: cell wall, colleters, mucilage, nectar, plant resins, plant secretions.
INTRODUCTION
The secretory activity of plants is often associated with direct and indirect defence against biotic and abiotic agents. Different types of secretory structures are involved in the synthesis of a highly diverse array of chemical substances, and thus have aroused the interest of many areas of inquiry. Not only are secretory substances important to an individual plant, conferring a greater competitive ability and resistance to environmental adversities, many also possess high economic value. Thus, the secretory processes of plants have been studied by different areas of science; however, despite recent advances, there remains an immeasurable range of questions without adequate answers. One question that has intrigued researchers of plant secretory activity is the way by which secretory products leave the secretory cell and reach the site of accumulation or the external environment.
After synthesis, secretory products leave the protoplast and accumulate in the periplasmic space, from which they then pass through the cell wall and out of the plant cell. This process is common to a wide variety of secretory structures such as nectaries, colleters and resin glands. However, little is known regarding the forces that cause these materials to cross the barrier imposed by the cell wall and cuticle. Some hypotheses, formulated to explain this transit of substances, are not universally accepted and do not apply to all situations or to different natural chemical products, such as lipids and sugars.
What determines that a volume of oil or resin leaves the periplasmic space and crosses the cell wall, hydrophilic in most cases? What forces drive the exudates, causing them to move against a concentration gradient? Answers to questions of this nature are essential for a comprehensive understanding of the process of release of substances produced by secretory cells.
As with animal exocrine glands, plant glands are identified as holocrine or merocrine, depending on how their product is secreted. In holocrine glands, the entire cell disintegrates to release its substances, whereas cells in merocrine glands remain alive and secrete their substances by exocytosis (see Fahn, 1979, and references therein). These terms were first employed at the beginning of the 20th century and seem to be very common for both plant and animal systems. However, in plants these modes of secretion are restricted to the release of substances by the protoplast, and not the entire release processes themselves, which consist of the passage of secretory products across the cell wall. This peculiarity is due to the fact that these terms were first used for animal glandular systems, and thus the cell wall was not considered in these processes. For plant secretory systems, the merocrine process of secretion has two distinct modes, as employed by Fahn (1979): eccrine and granulocrine. In the eccrine mode, substances are transported directly through the plasma membrane, whereas in the granulocrine mode substances are enclosed in specific vesicles for exocytosis.
To say that the mode of secretion is eccrine or granulocrine means little, although for historical reasons most ultrastructural studies do so. Many authors, such as Fahn (2000), resort to these terms to explain the output of materials from the protoplast. However, the modes of eccrine or granulocrine say nothing about how these materials pass through the cell wall and, thereby, leave the secretory cell. Faced with large gaps in understanding the secretory cycles of plants, explaining the way by which the products of secretory cells are released out of a plant or to the lumen of internal secretory structures is highly relevant and can contribute to reducing in the large uncertainty on the subject. Thus, an elaborated model of the cell cycle is proposed based on observations of the different steps of the secretory process in different plant species and in different secretory structures involved in the biosynthesis of oils, resins, polysaccharides and nectar.
MODEL OF THE CELL CYCLE INVOLVED IN SECRETION RELEASE
According to Nepi (2007), nectar secretion refers to the release of nectar from the protoplast. By contrast, nectar release (or exudation) refers to the events that lead to nectar disposal to the nectar consumers, usually out of a nectary (and consequently out of the plant). I believe these concepts of secretion and exudation are useful to other secretory products as well and will thus employ these distinguishing terms throughout this article.
Initial stage of the secretory cycle
Some secretory processes constitute single events concentrated in a short time interval of minutes to a few hours. In many floral nectaries, for example, the secretion of nectar is strongly dependent on starch hydrolysis and the entire process of nectar synthesis occurs in a short period of time; in such cases, the secretory process is not cyclical. However, the secretory process of most secretory structures is cyclical. Colleters, secretory cavities for gums, oils or resins, extrafloral and post-floral nectaries all have secretory processes comprising several cycles with durations that can exceed several months.
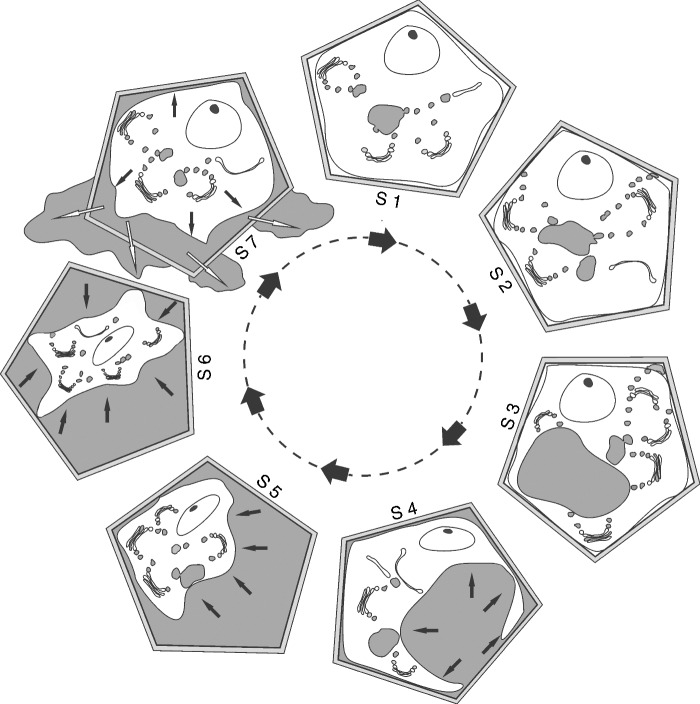
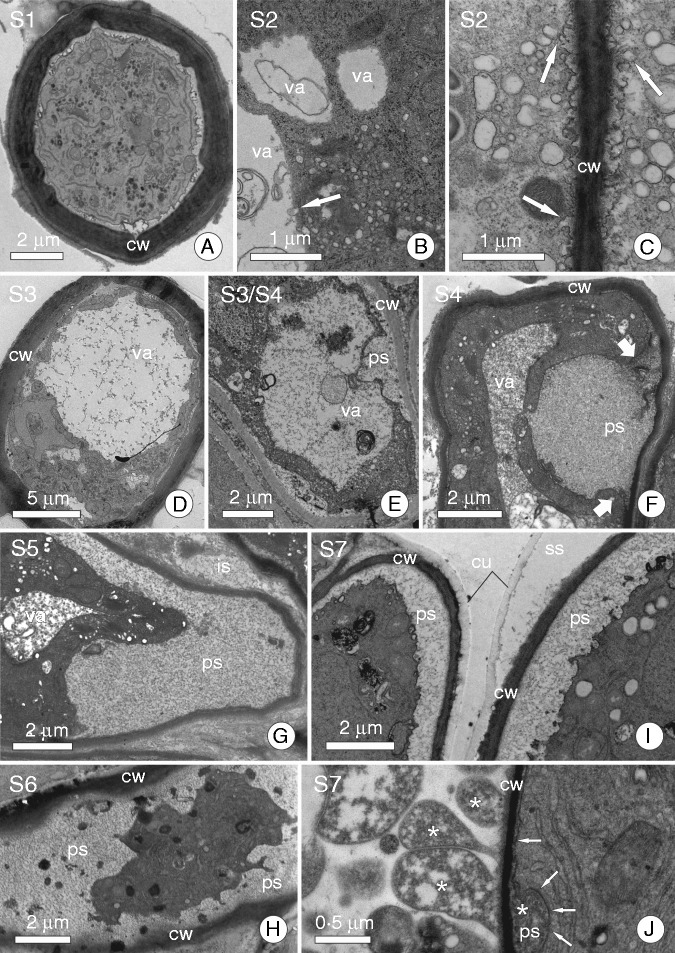
In cyclical secretory processes, a discrete, well-defined secretory cycle can be described. In the initial state of such a secretory cycle (Fig. 1, S1; Fig. 2A), vesicles derived from the Golgi apparatus or the endoplasmic reticulum merge with each other creating microvacuoles or provacuoles (see Marty, 1999; Happel et al., 2004), which then merge with larger vacuoles, within which the secretory product is temporarily accumulated (Fig. 1, S2; Fig. 2B). Similarly, these vesicles can transport secretory products or their precursors to the plasma membrane, fusing with it and releasing the products into the periplasmic space by a granulocrine process (Fig. 1, S2 and S3; Fig. 2C).
Fig. 1.
Model of the secretory cycle in which changes in the volume of the protoplast are responsible for releasing the products of secretory activity initially contained in the periplasmic space. In S1 to S3, note the action of the membranous organelles (Golgi apparatus and endoplasmic reticulum) during the secretory activity, whose products accumulate in small vacuoles or in the central vacuole. In S4, fusion of the vacuolar and plasma membranes releasing the vacuolar contents in the periplasmic space can be seen – this step may be omitted in some secretory systems in which the vesicles derived from the organelle synthesis merge directly with the plasma membrane. In S5 and S6, the substances accumulated in the periplasmic space exert pressure against the protoplast, which becomes dense. In S7, the protoplast increases in volume due to continuation of the synthesis process that produces vesicles and vacuoles, thus generating pressure that compresses the secretory products, forcing them against the cell wall and promoting exudation to the outside of the plant body or into intercellular spaces. Plasmodesmata are present in all stages for almost all secretory cells; even in stage S6 some of these structures remain in order to allow the entrance of new raw materials. Black arrows indicate pressure, white arrows indicate extravasation through the cell wall; S1–S7, successive stages of the secretory process.
Fig. 2.
Ultrastructure of some secretory cells showing different secretory structures in order to demonstrate the stages of the secretory cycle. (A) Aechmea blanchetiana (Bromeliaceae) mucilage-secreting trichome; cell in stage S1. (B) Secretory cell from stem epidermis of Schizolobium parahyba (Fabaceae) showing vesicles from dictyosomes merging with the large vacuole; a characteristic of stage S2. (C) Secretory cell of a floral nectary from Erythrina speciosa (Fabaceae); arrows indicate fusion of small vesicles to the plasma membrane to discharge secretion in stage S2. (D) Aechmea blanchetiana (Bromeliaceae) mucilage-secreting trichome with a large central vacuole full of mucilage (stage S3). (E) Cell from a colleter of Calycophyllum spruceanum (Rubiaceae) in a stage similar to that shown in D (S3), but here we note that the plasma and vacuolar membranes are closer to the periplasmic space. (F) Secretory cell of a colleter from Caryocar brasiliense (Caryocaraceae); arrows indicate edges of a vacuole that has just merged with the plasma membrane and secretion from within the vacuole in the periplasmic space; a characteristic of stage S4. (G, H) Secretory cell of a colleter from C. brasiliense (Caryocaraceae) and Tontelea micrantha (Celastraceae), respectively. Note the secretion is in the periplasmic space and the protoplast has become dark and compressed (stages S5 and S6). (I) Nectar-secreting cell from floral nectary of Luehea grandiflora (Malvaceae) in stage S7; note that the protoplast presses secretion against the cell wall, causing it to cross the cell wall and accumulate in the subcuticular space. (J) Secretory cell of a colleter from Plumeria rubra (Apocynaceae). Arrows indicate the plasma membrane and a rubber-like secretion in the periplasmic space; this secretion is being pressed against the cell wall, causing it to cross into the intercellular space (stage S7). Abbreviations: cw, cell wall; cu, cuticle; ps, periplasmic space; ss, subcuticular space; va, vacuole.
These secretory cycles, however, are not exclusive to the granulocrine mode of secretion, and occur in the same manner when the eccrine mode prevails. When the secretory products are initially accumulated freely in the cytosol, or arising from the plastids, which is common in secretions of hydrophobic nature, the same model applies, but without membrane barriers to limit these substances. This model is easily observed in a variety of cases of the secretion of oils and resins, where the fusion of droplets produces larger droplets until they contact the plasma membrane and extravasation occurs into the periplasmic space (Paiva et al., 2008; Rodrigues and Machado, 2012). In many cases, there is no merging of the droplets and they are directed to the plasma membrane and merge across it. In these cases, the substances are, from the outset, accumulated in the periplasmic space.
With accumulation of substances in the interior of the vacuole, this organelle expands and comes to occupy a large part of the protoplast until the membrane of the vacuole becomes close to the plasma membrane (Fig. 1, S1–S3; Fig. 2D, E). As a result of the large volume of the vacuole, the plasma and vacuolar membranes touch each other and fuse together, promoting exocytosis and the transfer of these products to the periplasmic space (Fig. 1, S4; Fig. 2F). Adopting the definition proposed by Nepi (2007), secretion occurs at this moment.
Overcoming the barrier imposed by the cell wall
For obvious reasons, secretory products previously contained in the protoplast and now accumulated in the periplasmic space continue to occupy space in the lumen of the cell, as they have not yet crossed the barrier imposed by the cell wall. Thus, the protoplast comes to occupy a smaller portion of the lumen due to the accumulation of material in the periplasmic space (Fig. 1, S5 and S6; Fig. 2G, H). This accumulation of substances in the periplasmic space continually increases the pressure on the protoplast so that it becomes compressed and its cytoplasmic matrix becomes denser (Fig. 1, S6; Fig. 2H).
The pressure generated by the substances accumulated in the periplasmic space sometimes causes the rupture of some plasmodesmata, leading to the protoplast moving away from the cell wall, and causing massive expansion of the periplasmic space. Even when the protoplast is compressed by the material accumulated externally, the secretory process is not interrupted and new vesicles carrying substances are released into the cytoplasmic matrix, merging (or not) with new vacuoles and beginning a new cycle of protoplast expansion (Fig. 1, S7; Fig. 2I) or, in cases of secretory products freely accumulated in the cytosol, the accumulation of these substances increases the volume of the protoplast. At this stage, notably, the pressure changes direction because the accumulation of material in the protoplast converts it into a pressure-generating element, which causes the accumulated substances in the periplasmic space to be pressed against the cell wall and forcing them to cross into intercellular spaces (Fig. 2I, J). For secretory epidermis, released substances pass into the subcuticular space or out of the plant body entirely. Thus, the protoplast expands again until it comes close to the cell wall when, in the central vacuole, the accumulation of numerous vesicles or the accumulation of free substances in the cytosol have reached their maximum volume (Fig. 1, S1). At this point, a new sequence of fusions between the compartments that hold the secretory products and the plasma membrane occurs, so that these materials are released into the periplasmic space, thus repeating the entire cycle. This cycle operates independently in each of the secretory cells that comprise a gland and varies in duration among plant species and among stages in the same gland.
The fact that vacuoles, in the proposed model (see Fig. 1, S1–S4; Fig. 2D–F), serve as transient sites for the accumulation of substances is compatible with the functions of these organelles and is supported by data in the literature. Vacuoles are known to be dynamic, multifunctional organelles; they act in maintaining cellular turgescence and osmotic control, as well as serve as sites for the accumulation of reserve compounds, among other functions. Marty (1999) defined vacuoles ‘as the intracellular compartments that arise as a terminal product of the secretory pathway in plant cells’. In colleters of Psychotria kirkii, Miller et al. (1983) defined vacuoles containing products of secretory activity as storage vacuoles, since they are fused to the membrane and release products into the periplasmic space.
There are several reports of the fusion between vesicles or vacuoles and the plasma membrane to release substances stored in the vacuole or vesicles, which supports the hypothesis presented here (Gedalovich and Kuijt, 1987; Echeverría, 2000; Paiva and Martins, 2011; Mercadante-Simões and Paiva, 2013). According to Echeverría (2000), there is a ‘vesicle-mediated system for metabolite transport from the vacuole to the cell membrane’, thus providing additional evidence that the cyclical model presented here occurs widely.
APPLICATION OF THE PROPOSED MODEL TO SOME SECRETORY SYSTEMS
Secretion of polysaccharides
Many ultrastructural investigations have concluded that the Golgi apparatus is involved in the production of polysaccharides, which is corroborated by cytological, cytochemical and physiological evidence as pointed by Lüttge and Schnepf (1976). In fact, in plant structures involved in polysaccharide secretion, such as colleters, the presence of a massive Golgi apparatus is commonly reported (Meyberg, 1988; Paiva, 2009a; Mercadante-Simões and Paiva, 2013), including in bryophytes (Ligrone, 1986). Immunogold labelling demonstrates that Golgi stacks are correlated with polysaccharide production (Young et al., 2008). Dictyosome-derived vesicles, filled with polysaccharides, move towards the cell periphery and fuse with the plasma membrane, releasing the mucilage into the periplasmic space, as described by Fahn (1988). The pressure exerted by the expanding protoplast, in cycles as proposed in Fig. 1, provides the driving force for the material accumulated in the periplasmic space to cross the cell wall. Crossing the cell wall does not occur passively with substances of high molecular weight, of high viscosity or of hydrophobic character, for example, such as is the case for polysaccharides or mixed secretions produced by colleters.
In Ipomoea cairica (Convolvulaceae), Paiva and Martins (2011) described trichomes involved in the secretion of acid polysaccharides, which act for long periods and extend from ovaries in flower buds until the beginning of fruit maturation, thus allowing the action of successive cycles of release of the substance from the protoplast. Retraction of the protoplast, promoted by the accumulation of materials in the periplasmic space, was observed by Trachtenberg and Fahn (1981) in mucilage-producing cells of Opuntia ficus-indica (Cactaceae) and by Ligrone (1986) in the mucilage hairs of the gametophytes of Timmiella barbuloides (bryophyte).
Note that the secretory cycle proposed herein is grounded on the observations of different secretory structures in different species, although it is most easily observed in the secretion of polysaccharides produced by the action of the Golgi apparatus, as reported by Paiva and Martins (2011) and by Mercadante-Simões and Paiva (2013). A similar explanation for the release of polysaccharides was presented by Lüttge and Schnepf (1976), who stated ‘that extruded polysaccharide is transported passively through the cell wall to the exterior, moved only by the turgor pressure of the cell’. But how can one explain the release of secretions over several days by a cyclical process? In order for the turgor pressure exerted by the protoplast to eliminate discharged substances in the periplasmic space, and thus causing them to pass through the cell wall, it is essential that the contraction and expansion cycles of the protoplast occur repeatedly.
In most mucilage idioblasts studied, the substances produced are accumulated in the periplasmic space, which is expanded so that at the end of the secretory process the protoplast is reduced to a small, central, collapsed mass (Mauseth, 1980; Bakker and Gerritsen, 1992). In these cases, it is likely that there is no counter pressure from the protoplast to expel substances from the periplasmic space toward the outside of the cell, and so they are contained in the lumen where they remain until after cell death. An important consideration is that even if the protoplast exerts pressure against the substances accumulated in the periplasmic space, they do not traverse the cell wall due to the physical and chemical characteristics of both the cell wall and the secreted substances.
The degree of hydration of the polysaccharides comprising the secretion and, of course, the viscosity and fluidity thereof, appears to be, next to its chemical nature, an important factor allowing or preventing passage through the cell wall. In Araucaria angustifolia (Araucariaceae) Mastroberti and Mariath (2008) described a distinct pattern of accumulation of material in mucilage idioblasts, in which the secretion products are accumulated in the protoplast wherein, at the end of the process, cell death occurs and this material remains in the lumen – in this case there is no moment during the ontogenetic process when there is sufficient pressure for these materials to break the barrier imposed by the cell wall.
The action of the protoplast in the release of materials deposited external to the plasma membrane has been proposed by Morré et al. (1967) for mucilage-producing cells in roots of Zea mays (Poaceae). According to these authors, the extrusion of polysaccharide through the cell wall appears to be a passive process influenced by the degree of hydration of the polysaccharide and by cell turgor. I use these same statements here, but disagree regarding an essential point: this process is not passive because the pressure due to this turgor characterizes an energy-based process of the cell.
Secretion of nectar and other hydrophilic substances
In the case of nectar secretion, where the eccrine mode of secretion seems to prevail (see Lüttge and Schnepf, 1976; Vassilyev, 2010), the release of sugars to the periplasmic space occurs predominantly by membrane transporters against a concentration gradient. In this case, what forces the sugars to pass through the cell wall?
Independent of the mode by which nectar leaves the protoplast, by means of vacuoles or vesicles with a plasma membrane or by means of transporters or channels present in the membrane, the pressure exerted by the protoplast appears not to be important for nectar to cross the barrier imposed by the cell wall. Nectar, or some of the substances that compose it, has facilitated diffusion, which is achieved by hydrophilic substances that, in addition to having low viscosity, provide great affinity for cell-wall components, thus facilitating transport across it.
Regarding secretion of carbohydrates of low molecular weight and that are highly soluble in water, such as nectar components, there is a reduction in ‘water potential” in the region of the periplasmic space as a result of the accumulation of these substances. According to Ren et al. (2007) and Nepi et al. (2011), the hydrolysis of starch in the nectary results in a dramatic decline in water potential, thus triggering an influx of water into the tissue of the nectary and consequently an increase in hydrostatic pressure. The concentration of sugars in a given location, such as the periplasmic space, establishes, according to Lüttge and Schnepf (1976), the water potential gradient required for secretion and the fluid is eliminated by pressure. Note that in this case, pressure is not necessarily generated by the mechanical action of the protoplast, but instead by an osmotic process, as pointed by Nepi et al. (2011). A similar explanation for the release of nectar was formulated by Findlay and Mercer (1971) for nectariferous trichomes of Abutilon, in which the hydrostatic pressure not only explains the release of nectar from secretory cells, but also explains its passage through the cuticular barrier.
In salt-secreting glands, the saline solution can be primarily stored in small vacuoles and vesicles which appear to discharge their contents towards the periplasmic space (Somaru et al., 2002) or they can be secreted by an eccrine process (see Fahn, 2000). According to Zouhaier et al. (2015) the fusion of vacuoles with the plasma membrane ensures the salt excretion process in Limoniastrum guyonianum (Poaceae). Regardless of the processes involved in crossing the plasma membrane, salt accumulation outside the protoplast causes an increase of hydrostatic pressure (see Kobayashi, 2008) once water moves into this region to achieve osmotic equilibrium. Similar to the pressure exerted by the protoplast, as I have explained for other substances, the hydrostatic pressure due to salt accumulation is important in permitting the saline solution to cross the cell wall. In fact, in salt glands of several Poaceae, this pressure creates a subcuticular chamber in which saline solution accumulates (Naidoo and Naidoo, 1998; Somaru et al., 2002; Oi et al., 2012; Céccoli et al., 2015). It is important to consider that the saline solutions, as with nectar, have facilitated diffusion and low viscosity, thus producing a great affinity for cell-wall components. Therefore, some hydrostatic pressure or protoplast action is needed simply to facilitate and direct flow across the cell wall and towards the plant surface.
Secretion of hydrophobic substances – oils and resin
Although this model emphasizes secretion products whose intracellular transport is mediated by vesicles and vacuoles, it can also be applied to substances whose intracellular transport is achieved without the participation of membranous envelopes, as with many resinous or lipidic secretions. In this case, these substances, which are initially free in the cytosol, undergo the process of exocytosis and are released into the periplasmic space where their accumulation generates pressure against the protoplast, which consequently reacts as suggested in other cases (see Fig. 1).
In structures that secrete terpenic resins, the model of secretion proposed herein explains, in a manner similar to that observed for the secretion of polysaccharides, the mode of release of the secretory products. The main difference results from the fact that resin components can leave the protoplast also by an eccrine process (Paiva et al., 2008), by a granulocrine process (Batt, 1987; Milani et al., 2012) or by both (Rodrigues and Machado, 2012; Sá-Haiad et al., 2015). In the resiniferous trichomes of Betula pendula (Betulaceae), Raatikainen et al. (1992) observed that the synthesized products initially accumulate in vesicles that fuse to vacuoles, and these are released into the periplasmic space, from where they diffuse through the cell wall and into the subcuticular space. Although these authors do not describe the action of the protoplast in relation to the movement of resin through the cell wall, such a process is implied.
Is there any other way to explain the fact that viscous and hydrophobic substances, such as oils and resins, that leave the protoplast and pass through the cell wall are recognized as hydrophilic in most cases? As there is no possibility of diluting these accumulated materials in the periplasmic space, nor is there the possibility of facilitated diffusion by the cell wall, we are left accepting the most likely hypothesis of mechanical action of the protoplast. By expanding, the protoplast presses the hydrophobic material accumulated in the periplasmic space and forces it to cross the cell wall, in a manner similar to that shown in Fig. 1. Although not describing the manner in which the resin passes through the cell wall, Sá-Haiad et al. (2015) suggest that pressure is the driving force for the release of resin in two species of Clusia (Clusiaceae), when they state: ‘rupture points viewed on the cuticle surface are a result of pressure exerted by the accumulated secretions in the intercellular spaces’.
Secretion of oils, resin and other hydrophobic substances is commonly verified in elaiophores and in a wide variety of resin-secreting glands, but similar substances can also be released from osmophores and glands that produce a mixed secretion. Therefore, a discussion of the ways that hydrophobic substances cross the cell wall can be useful for understanding the release of exudates from a wide variety of glands.
Endogenous and intracellular secretion
Regarding secretion by structures whose secretory product is endogenous, intracellular or remains within the cell, as with many latex and mucilage cells, there are two possible modes of excretion: they are either accumulated in the periplasmic space or they are accumulated in the protoplast (the latter, predominantly in latex cells). In these cases, cell death occurs at the end of the process, as is common in mucilage cells, or the secretory product is contained in the protoplast itself, such as in latex cells. Note that in many mucilage cells, the accumulation of materials occurs in the periplasmic space and there is no reversal of the process of protoplast compression, the protoplast dying after the secretory phase as observed in Opuntia polyacantha (Cactaceae), in which the secretory material remains in the cell lumen (see Mauseth, 1980). As there was expansion of the protoplast, as described in stage 7 (Fig. 1, S7), the secretory products do not remain within the cell for a very simple reason: two bodies cannot occupy the same space!
HOW TO CROSS THE CUTICULAR BARRIER OF SOME SECRETING CELLS?
The plant cuticle, by its lipid nature, presents, in some species, separate diffusion paths for lipophilic non-electrolytes and hydrated ionic compounds (Schönherr, 2006). These ionic pathways are pores ranging from 0.45 to 1.18 nm in size and constitute aqueous pathways across cuticles (Schönherr, 2006). While these hydrophilic pathways occur universally, and are present in the cuticle of some secretory structures, their dimensions do not permit the rate of transport commonly observed (e.g. in nectaries, in which droplets of nectar are released in a fraction of a second).
The accumulation of secretion products inside subcuticular space can promote a pressure that permits secretion flux to cross a cuticular barrier, in most cases by cuticle rupture. Thus, the secreted substances can be released, without a requirement of energy, even against a concentration gradient, as pointed-out by Lüttge and Schnepf (1976) for nectar, by Paiva (2009a) for a polysaccharide secretion and by Possobom et al. (2015) for oil secretion. According to Paiva (2009b), in nectar-secreting trichomes of Erythrina speciosa (Fabaceae), the pressure caused by the accumulation of nectar in the trichome secretory head, as a consequence of the Casparian strip that prevents the apoplastic flux, can explain the transport across the cuticle. The action of the endodermal barrier directing the flux of substances is well documented in trichomes (Fahn, 1979).
However, there are cells that do not form a subcuticular space. In these cases, the secretion must cross the cuticle by the way of pores or hydrophilic canals. The presence of cuticular canals was described by Miller (1985) in leaves of many species, although in most glands these structures seem to be quite rare. In some nectaries, these canals are fibrillar outgrowths of the outer cell wall that cross the cuticle, as described by Stpiczynska (2003) for Platanthera chlorantha (Orchidaceae). Cuticular pores were described for osmophores (Pridgeon and Stern, 1983; Stpiczynska, 2001), protein- and mucilage-secreting cells (Davies and Stpiczynska, 2014), and nectaries (Vassilyev and Koteyeva, 2005) although they are unusual in the last named.
For viscous, hydrophilic secretions, such as pectins and gums, as in colleters, there is no barrier to apoplastic transport; in this case one must consider that the secretory product is viscous, which seems to hinder reflux of these secretions toward internal tissue. Furthermore, most secretory cells are in contact with, or close to, the external environment so that the path to the release of exudates is shorter. There are many reports of the accumulation of substances in the subcuticular space of colleters (see Mercadante-Simões and Paiva, 2013), where they are released by cuticular rupture in a manner similar to that discussed above.
In some species and secretory systems, the accumulated lipid secretion in the subcuticular space is not sufficient to break the cuticle and the process of secretion is interrupted. In this manner, the secretion remains protected by the cuticle and volatile components are not lost. Rupture and release of secretion occurs as a result of the action of herbivores, whose mechanical action breaks the cuticle, thus releasing the secretion as a deterrent. This is also the case with glandular trichomes and other surface glands (see Pichersky and Gershenzon, 2002).
Stomata are another common passage for exudate release, but the model presented here is focused on individual or groups of cells from which exudates are released.
FINAL REMARKS
In crossing the plasma membrane, many secretion products reach only part of the way along the path leading them to their final destination. The mechanisms by which these substances cross the plasma membrane to be deposited in the periplasmic space are relatively well known. However, the mechanical actions of the protoplast, performed through successive cycles of contraction and expansion, are fundamental to the transport of some materials accumulated in the periplasmic space across the cell wall and the cuticle. The mechanical action of the protoplast is especially relevant for the release of lipidic, resinous and highly viscous hydrophilic secretions. However, the participation of the protoplast as a mechanical agent is not the case for very fluid hydrophilic substances, for which facilitated diffusion is the process by which they cross the cell wall; yet, the pressure generated by the protoplast on the substances contained in periplasmic and intercellular spaces can be important in the release of these substances.
The model presented here can describe the different ways that secretory products are able to cross the cell wall and be released into intercellular spaces or, in some cases, to the outside of the plant. Even for secretory glands not elaborated upon here, this model can be applied when the exudates, after crossing the plasma membrane, have to cross the cell wall, sometimes with a cuticle, in order to exert their function.
ACKNOWLEDGEMENTS
I would like to acknowledge the Center of Microscopy at the Universidade Federal de Minas Gerais for providing the equipment and technical support for experiments involving electron microscopy. Special thanks go to Dr D. M. T. Oliveira for her helpful comments. E. Paiva receives a research grant from CNPq (308589/2011-4).
LITERATURE CITED
- Bakker ME, Gerritsen AF. 1992. The development of mucilage cells in Hibiscus schizopetalus. Acta Botanica Neerlandica 41: 31–42. [Google Scholar]
- Batt JR. 1987. Development and structure of primary secretory ducts in the stem of Commiphora wightii (Burseraceae). Annals of Botany 60: 405–416. [Google Scholar]
- Céccoli G, Ramos J, Pilatti V, et al. 2015. Salt glands in the Poaceae family and their relationship to salinity tolerance. Botanical Review 81: 162–178. [Google Scholar]
- Davies KL, Stpiczynska M. 2014. Labellar anatomy and secretion in Bulbophyllum Thouars (Orchidaceae: Bulbophyllinae) sect. Racemosae Benth. & Hook. f. Annals of Botany 114: 889–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverría E. 2000. Vesicle-mediated solute transport between the vacuole and the plasma membrane. Plant Physiology 123: 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn A. 1979. Secretory tissues in plants. New York: Academic Press. [Google Scholar]
- Fahn A. 1988. Secretory tissues in vascular plants. New Phytologist 108: 229–257. [DOI] [PubMed] [Google Scholar]
- Fahn A. 2000. Structure and function of secretory cells. Advances in Botanical Research 31: 37–75. [Google Scholar]
- Findlay N, Mercer FV. 1971. Nectar production in Abutilon – I. Movement of nectar through the cuticle. Australian Journal of Biological Sciences 24: 647–656. [Google Scholar]
- Gedalovich E, Kuijt J. 1987. An ultrastructural study of the viscin tissue of Phthirusa pyrifolia (H.B.K.) Eichler (Loranthaceae). Protoplasma 137: 145–155. [Google Scholar]
- Happel N, Höning S, Neuhaus JM, Paris N, Robinson DG, Holstein SHE. 2004. Arabdopsis µA-adaptin interacts with the tyrosine motif of the vacuolar sorting receptor VSR-PS1. The Plant Journal 37: 678–693. [DOI] [PubMed] [Google Scholar]
- Kobayashi H. 2008. Ion secretion via salt glands in Poaceae. Japanese Journal of Plant Science 2: 1–8. [Google Scholar]
- Ligrone R. 1986. Structure, development and cytochemistry of mucilage-secreting hairs in the moss Timmiella barbuloides (Brid.) Moenk. Annals of Botany 58: 859–868. [Google Scholar]
- Lüttge U, Schnepf E. 1976. Elimination processes by glands. Organic substances In: Lüttge U, Pitman MG. eds. Transport in plants II, Encyclopedia of Plant Physiology, New Series, Vol. 2B. New York: Springer, 244–277. [Google Scholar]
- Marty F. 1999. Plant vacuoles. Plant Cell 11: 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroberti AA, Mariath JEA. 2008. Development of mucilage cells of Araucaria angustifolia (Araucariceae). Protoplasma 232: 233–245. [DOI] [PubMed] [Google Scholar]
- Mauseth JD. 1980. A stereological morphometric study of the ultrastructure of mucilage cells in Opuntia polyacantha (Cactaceae). Botanical Gazette 141: 374–378. [Google Scholar]
- Mercadante-Simões MO, Paiva EAS. 2013. Leaf colleters in Tontelea micrantha (Celastraceae, Salacioideae): ecological, morphological and structural aspects. Comptes Rendus Biologies 336: 400–406. [DOI] [PubMed] [Google Scholar]
- Meyberg M. 1988. Cytochemistry and ultrastructure of the mucilage secreting trichomes of Nymphoides peltata (Menyanthaceae). Annals of Botany 62: 537–547. [Google Scholar]
- Milani JF, Rocha JF, Teixeira SP. 2012. Oleoresin glands in copaíba (Copaifera trapezifolia Hayne: Leguminosae), a Brazilian rainforest tree. Trees Structure and Function 26: 769–775. [Google Scholar]
- Miller IM, Scott A, Gardner IC. 1983. The development, structure and function of dendroid colleters in Psychotria kirkii Hiern Rubiaceae. Annals of Botany 51: 621–630. [Google Scholar]
- Miller RH. 1985. The prevalence of pores and canals in leaf cuticular membranes. Annals of Botany 55: 459–471. [Google Scholar]
- Morré DJ, Jones DD, Mollenhauer HH. 1967. Golgi apparatus mediated polysaccharide secretion by outer root cap cells of Zea mays. I. Kinetics and secretory pathway. Planta 74: 286–301. [DOI] [PubMed] [Google Scholar]
- Naidoo Y, Naidoo G, 1998. Sporobolus virginicus leaf salt glands: morphology and ultrastructure. South African Journal of Botany 64: 198–204. [Google Scholar]
- Nepi M. 2007. Nectary structure and ultrastructure In: Nicolson SW, Nepi M, Pacini E, eds. Nectaries and nectar. Dordrecht: Springer, 129–167. [Google Scholar]
- Nepi M, Cresti L, Guarnieri M, Pacini E. 2011. Dynamics of nectar production and nectar homeostasis in male flowers of Cucurbita pepo L. International Journal of Plant Sciences 172: 183–190. [Google Scholar]
- Oi T, Taniguchi M, Miyake H. 2012. Morphology and ultrastructure of the salt glands on the leaf surface of Rhodes grass (Chloris gayana Kunth). International Journal of Plant Sciences 173: 454–463. [Google Scholar]
- Paiva EAS. 2009a. Occurrence, structure and functional aspects of the colleters of Copaifera langsdorffii Desf. (Fabaceae, Caesalpinioideae). Comptes Rendus Biologies 332: 1078–1084. [DOI] [PubMed] [Google Scholar]
- Paiva EAS. 2009b. Ultrastructure and post-floral secretion of the pericarpial nectaries of Erythrina speciosa (Fabaceae). Annals of Botany 104: 937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva EAS, Martins LC. 2011. Calycinal trichomes in Ipomoea cairica (Convolvulaceae): ontogenesis, structure and functional aspects. Australian Journal of Botany 59: 91–98. [Google Scholar]
- Paiva EAS, Oliveira DMT, Machado SR. 2008. Anatomy and ontogeny of the pericarp of Pterodon emarginatus Vogel (Fabaceae, Faboideae), with emphasis on secretory ducts. Anais da Academia Brasileira de Ciências 80: 455–465. [DOI] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J. 2002. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Current Opinion in Plant Biology 5: 237–243. [DOI] [PubMed] [Google Scholar]
- Pridgeon AM, Stern WL. 1983. Ultrastructure of osmophores in Restrepia (Orchidaceae). American Journal of Botany 70: 1233–1243. [Google Scholar]
- Possobom CCF, Guimarães E, Machado SR. 2015. Structure and secretion mechanisms of floral glands in Diplopterys pubipetala (Malpighiaceae), a neotropical species. Flora 211: 26–39. [Google Scholar]
- Raatikainen OJ, Taipale HT, Pelttari A, Lapinjoki SP. 1992. An electron microscope study of resin secretion and production by the glands of seedlings of Betula pendula. New Phytologist 122: 537–543. [DOI] [PubMed] [Google Scholar]
- Ren G, Healy RA, Klyne AM, Horner HT, James MG, Thornburg RW. 2007. Transient starch metabolism in ornamental tobacco floral nectaries regulates nectar composition and release. Plant Science 173: 277–290. [Google Scholar]
- Rodrigues TM, Machado SR. 2012. Oil glands in Pterodon pubescens Benth. (Leguminosae-Papilionoideae): distribution, structure, and secretion mechanisms. International Journal of Plant Sciences 173: 984–992. [Google Scholar]
- Sá-Haiad B, Silva CP, Paula RCV, Rocha JF, Machado SR. 2015. Androecia in two Clusia species: development, structure and resin secretion. Plant Biology 17: 816–824. [DOI] [PubMed] [Google Scholar]
- Schönherr J. 2006. Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. Journal of Experimental Botany 57: 2471–2491. [DOI] [PubMed] [Google Scholar]
- Somaru R, Naidoo Y, Naidoo G. 2002. Morphology and ultrastructure of the leaf salt glands of Odyssea paucinervis (Stapf) (Poaceae). Flora 197: 67–75. [Google Scholar]
- Stpiczynska M. 2001. Osmophores of the fragrant orchid Gymnadenia conopsea L. (Orchidaceae). Acta Societatis Botanicorum Poloniae 70: 91–96. [Google Scholar]
- Stpiczynska M. 2003. Nectar resorption in the spur of Platanthera chlorantha Custer (Rchb.) Orchidaceae – structural and microautoradiographic study. Plant Systematics and Evolution 238: 119–126. [Google Scholar]
- Trachtenberg S, Fahn A. 1981. The mucilage cells of Opuntia ficus-indica (L.) Mill.: development, ultrastructure, and mucilage secretion. Botanical Gazette 142: 206–213. [Google Scholar]
- Vassilyev AE. 2010. On the mechanisms of nectar secretion: revisited. Annals of Botany 105: 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilyev AE, Koteyeva NK. 2005. The characterization of nectar pathways from the apoplasm to the nectary surface. Botanicheskii Zhurnal 90: 854–864. [Google Scholar]
- Young RE, McFarlane HE, Hahn MG, Western TL, Haughn GW, Samuels AL. 2008. Analysis of the Golgi apparatus in Arabidopsis seed coat cells during polarized secretion of pectin-rich mucilage. The Plant Cell 20: 1623–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouhaier B, Abdallah A, Najla T, et al. 2015. Scanning and transmission electron microscopy and X-ray analysis of leaf salt glands of Limoniastrum guyonianum Boiss. under NaCl salinity. Micron 78: 1–9. [DOI] [PubMed] [Google Scholar]