Abstract
During native subsistence hunts from 1987 to 2007, blubber and liver samples from 50 subadult male northern fur seals (Callorhinus ursinus) were collected on St. Paul Island, Alaska. Samples were analyzed for legacy persistent organic pollutants (POPs), recently phased out/current-use POPs, and vitamins. The legacy POPs measured from blubber samples included polychlorinated biphenyl congeners, dichlorodiphenyltrichloroethanes (DDT and metabolites), chlorobenzenes, chlordanes, and mirex. Recently phased-out/current-use POPs included in the blubber analysis were the flame retardants, polybrominated diphenyl ethers, and hexabromocyclododecanes. The chemical surfactants, perfluorinated alkyl acids and vitamins A and E were assessed in the liver samples. Overall, concentrations of legacy POPs are similar to levels seen in seal samples from other areas of the North Pacific Ocean and the Bering Sea. Statistically significant correlations were seen between compounds with similar functions (pesticides, flame retardants, vitamins). With sample collection spanning two decades, the temporal trends in the concentrations of POPs and vitamins were assessed. For these animals, the concentrations of the legacy POPs tend to decrease or stay the same with sampling year; however, the concentrations of the current-use POPs increased with sampling year. Vitamin concentrations tended to stay the same across the sampling years. With the population of northern fur seals from St. Paul Island on the decline, a detailed assessment of exposure to contaminants and the correlations with vitamins fills a critical gap for identifying potential population risk factors that might be associated with health effects.
Introduction
Pinnipeds, fin-footed marine mammals, occupy a high trophic level in the marine environment, making them susceptible to the bioaccumulation of chemical contaminants such as persistent organic pollutants (POPs). Exposure to legacy POPs (including polychlorinated biphenyls (PCBs) and chlorinated pesticides) has been associated with health problems, including immunotoxicity and reproductive impairments in pinnipeds (Beckmen et al. 1999; Hutchinson and Simmonds 1994; Tabuchi et al. 2006). Many of the potential health problems resulting from long-term exposure to legacy POPs are not fully understood in wild populations of pinnipeds (Towell et al. 2006). An assessment of the POPs burden in these animals over the decades may be important to help understand trends in past and current levels of POPs since pinniped populations have recently exhibited a low reproduction rate and POPs concentrations may be associated with this rate (Towell et al. 2006). The Arctic has been shown to be a major sink for a variety of legacy POPs, although the levels of POPs in the Arctic vary depending on the environmental matrix (Muir et al. 1992). While many legacy POPs have been banned from use for decades, in general they have been slow to decline in top predators, especially mammals because of their persistent nature especially in the Arctic (Muir et al. 1992; Riget et al. 2010).
In addition to legacy POPs, pinnipeds are exposed to currently-used (and recently banned) pollutants, including polybrominated diphenyl ethers (PBDEs), hexabromocyclododecanes (HBCDs), and perfluorinated alkyl acids (PFAAs). PBDEs were extensively used as flame retardants in industrial and commercial applications (Darnerud et al. 2001). With the phase out of many PBDE commercial mixtures in Europe and the United States starting in 2006, HBCDs were considered a likely replacement, and they are often used in buildings and upholstery (Covaci et al. 2006). PFAAs were globally used for many applications, mainly as a surface protector for furniture, carpets, paper, and packaging (Prevedouros et al. 2006). PBDEs, HBCDs, and PFAAs are similar to legacy POPs because they are known to be persistent in the environment and have bioaccumulative properties. Many of these current-use POPs have been shown to accumulate in the arctic marine environment (Braune et al. 2005; Butt et al. 2010; Hart et al. 2009; Muir et al. 1992; Smithwick et al. 2006; Tomy et al. 2009; Verreault et al. 2005).
One group of pinnipeds, the northern fur seals (Callorhinus ursinus), are otariid (externally eared) seals that have a wide geographical range throughout the North Pacific Ocean extending from Japan, eastward to Northern California, and northward to the Bering Sea (Gentry 1997). While their habitat is not completely Arctic, in their northern range, northern fur seal habit overlaps with arctic pinnipeds such as the ringed seal (Phoca hispida). The seals are philopatric, having well established breeding colonies at several locations in the Bering Sea including the Pribilof Islands, Alaska. Within the Pribilof Islands, the animals using St. Paul Island have seen a pupping decline of nearly three-fold since the early 1970s (Towell et al. 2006). Hypotheses for the cause of the decline include climate changes, commercial fisheries interactions, and/or predation (Towell et al. 2006). Chemical contamination is another suspected cause for the declining population; however, there have been few studies looking at chemical contaminants in northern fur seals from St. Paul Island (Wang et al. 2010). These studies focus primarily on legacy POPs and have not provided information about current-use POPs in the population.
A potential indicator of population decline could be the reduction or disruption of vitamin homeostasis in northern fur seals. Alterations in vitamin levels are a potential biomarker of exposure to legacy POPs. A possible link between exposure to PCBs and chlorinated pesticides with decrease levels of circulating retinol (vitamin A) and hepatic vitamin E has been observed in beluga whales, ringed seals, grey seals, and harbor seals (Desforges et al. 2013; Kakela et al., 1999; Nyman et al., 2003; Routti et al., 2005; Simms et al. 2000). It has been hypothesized that changes in vitamin A, essential in growth and development, may in turn correlate with population changes (Simms and Ross 2000). Biological factors, including size and reproductive status, may affect vitamin biomarkers (Loseto et al. 2009). However, it is difficult to understand what changes in vitamin levels elicit effects in wild populations because there is a lack of information on the concentrations of vitamins in wildlife, specifically northern fur seals.
The primary aim of this study was to measure concentrations of legacy POPs (PCBs and chlorinated pesticides), recently phased out/current-use POPs (PBDEs, HBCDs, and PFAAs), and vitamins (A and E) in northern fur seal blubber and liver tissue collected from four rookeries on St. Paul Island between 1987 and 2007. In order to assess contaminant-related issues in pinnipeds, an understanding of their exposure to the myriad of POPs and health status markers is critical. Since factors such as age, weight, sex, and body condition, can influence the health and accumulation of POPs, we specifically looked at subadult (2–3 years of age) males for this study. Secondly, since previous studies have hypothesized vitamin levels potentially correlate with POPs concentrations, we examine correlations among different POPs classes and vitamins. Thirdly, the temporal trends of POPs and vitamins were assessed in the samples (from 1987 to 2007), and a comparison among the legacy POPs, recently phased out/current-use POPs, and vitamin concentrations spanning the 20 year time period was performed. With the population of northern fur seals from St. Paul Island on the decline, a detailed assessment of exposure to contaminants, the information for recently phased out/current-use POPs and vitamins fills an important data gap which helps to put in context POPs exposure with other risks affecting this population.
Materials and Methods
Sample Collection and Preparation
Full-depth blubber (n=50) and liver (n=49) tissue samples from northern fur seals were collected as part of the Alaska Marine Mammal Tissue Archival Project (AMMTAP). The standardized AMMTAP protocols have been described previously (Becker et al. 1993). Briefly, samples were collected during native subsistence hunts from subadult males (2–3 years of age) from three rookeries on St. Paul Island, Alaska, from 1987 to 2007 (Supplemental Information, Table S1). The blubber and liver tissue samples were processed in the field using established protocols designed to minimize sample contamination (Becker and Wise 2006). Then samples were placed in liquid nitrogen vapor-phase shippers and sent to the National Institute of Standards and Technology’s (NIST) Marine Environmental Specimen Bank (Marine ESB) for storage. All samples were stored in liquid nitrogen vapor-phase freezers (−150° C) in a clean room environment prior to further processing and analysis. Once the samples were chosen for analysis, each blubber and liver tissue was cryogenically homogenized using methods described elsewhere in Zeisler, et al. (1983) and Pugh et al. (2007). This process resulted in approximately 20 to 25 aliquots (i.e. sub-samples) of a fresh, frozen powder homogenate, approximately 5g to 6g each.
Analytical Methods
Persistent Organic Pollutants (POPs)
The extraction methods have been previously explained in detail in Bachman et al. (2014). Briefly, blubber samples were mixed with sodium sulfate and transferred to pressurized fluid extraction (PFE) cells for extraction. The internal standard solution was added to the samples, and then samples were extracted with dichloromethane using the PFE. Extracted samples were subsequently cleaned up using size exclusion chromatography, followed by solid phase extraction using acidified silica to fractionate the HBCDs (Bachman et al. 2014). PCBs, pesticides, and PBDEs were quantified by gas chromatography with mass spectrometric detection (GC-MSD) operated in either the electron impact mode or the negative chemical ionization mode with selected ion monitoring. HBCDs were quantified using liquid chromatography tandem mass spectrometry detection (LC-MS/MS) in the multiple reaction monitoring (MRM) mode. Details of the instrumental methods can be found in Kucklick et al. (2013).
Perfluorinated Alkyl Acids (PFAAs)
Extraction and cleanup methods for PFAAs have been previously described in Reiner et al. (2012). Briefly, liver samples were extracted using basic methanol, filtered, and further cleaned using graphitized non-porous carbon solid phase extraction. PFAAs were determined using LC-MS/MS in the MRM mode. Further details of the extraction, cleanup, and instrumental methods can be found in Reiner et al. (2012).
Vitamins
Details of the extraction and cleanup for vitamin analysis can be found in Kucklick et al. (2013). Liver samples were digested using a potassium hydroxide heated (40 °C) extraction method. After extraction, a liquid-liquid extraction was performed three times using a hexane:petroleum ether mixture (1:1 volume fraction). The organic layer was removed, evaporated to dryness, and reconstituted in a 9:1 (volume fraction) ethanol: ethyl acetate mixture. All steps were performed in a room with subdued lighting, and when possible, the samples were fully shielded from the light. Instrumental analysis was performed using LC with ultraviolet/fluorescence detection.
Quality Control
Blanks, calibration solutions, NIST Standard Reference Materials (SRMs), and quality control materials were processed alongside the blubber and liver samples. SRM 1945 Organics in Whale Blubber was used as the control material in blubber sample analyses, and QC97LH2 Beluga Whale Liver Homogenate and SRM 1946 Lake Superior Fish Tissue were used as the control materials for the liver sample analyses. To assess if methods were in control, the POPs measured in SRMs 1945, 1946, and QC97LH2 had to agree with the certified and reference values provided on the Certificates of Analysis. A compound was considered to be significantly above the reporting limit (RL) if the mass of an analyte in the sample was greater than the mean plus three standard deviations of all blanks.
Statistical Methods
Statistical analyses were performed on lipid-normalized data (ng/g lipid mass) for the sums of 64 PCB congeners (8, 18, 28, 31, 44, 49, 52, 56, 66, 74, 79, 87, 92, 95, 99, 101, 105, 110, 112, 114, 118, 119, 121, 127, 128, 132, 137, 138, 146, 149, 151, 153, 156, 158, 157, 159, 163, 167, 170, 172, 174, 175, 176, 177, 178, 180, 183, 185, 187, 191, 193, 194, 195, 196, 197, 199, 200, 201, 202, 205, 206, 207, 208, and 209), six DDT-related compounds (2,4’- and 4,4’-DDE, DDD, and DDT), five chlordanes (oxychlordane, trans-chlordane, cis-chlordane, trans-nonachlor, and cis-nonachlor), three hexachlorocyclohexanes (α-, β-, and γ-HCH), pentachlorobenzene, hexachlorobenzene, mirex, six PBDE congeners (47, 99, 100, 153, 154, and 155), and α-HBCD, on wet mass data (ng/g wet mass) for 15 PFAAs (PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnA, PFDoDA, PFTriA, PFTA, PFBS, PFHxS, PFOS, and PFOSA), and on wet mass data (µg/g wet mass) for vitamin A (retinol) and two vitamin E compounds (α- and γ-tocopherol). Values below the RL were imputed as the limit of quantitation divided by square root of two.
All statistical analyses were performed in Stata/SE 11.2 (more details can be found in the SI). Spearman rank correlation coefficients, or Spearman’s rho (ρ), were run to determine statistical dependence among the analytes. Proportionate percentile parametric quantile regression models (Cox et al. 2007; Pierce et al. 2011; Gribble et al. 2013) were used to summarize the percent change in chemical level per year over 1987–2007 in the seal population of St. Paul Island as a linear function of year. In proportionate percentile models, the percent change ‘trends’ for the median are the same percent change ‘trends’ for all other quantiles of the chemical distribution. Clustering of seals within rookeries was acknowledged by cluster-robust standard errors. Most models assumed lognormal distributions, except for the vitamin E model which was Weibull.
Results and Discussion
Most classes of POPs and vitamins were detected in each of the fur seal samples (Table 1). The exceptions were mirex and α-HBCD, which were detected in 68 % and 96 % of the samples, respectively. Examining the legacy POPs in blubber tissue, the concentrations of Σ64 PCB and Σ6 DDT made up more than 70 % of the total POPs measured in the northern fur seal samples. The Σ64 PCB median concentration was 2130 ng/g lipid mass and the Σ6 DDT median concentration was 1240 ng/g lipid mass. Of the 64 PCBs determined in the northern fur seal samples, concentrations of hexa- and heptachlorobiphenyl, especially PCBs 138, 153, and 180 were found in higher amounts (over 68 % of the total PCB burden) compared to lower chlorinated (tetra- and pentachlorobiphenyl; 27 % of the total PCB burden) PCB homologs (Supplemental Information, Figure S1). The most persistent DDT metabolite, 4,4’-DDE was the dominant DDT compound detected in the northern fur seal blubber samples, making up more than 70 % of the Σ6 DDTs in the samples. Detected in much lower amounts, Σ5 Chlordane median concentration was 652 ng/g lipid mass. Trans-nonachlor, one of the major constituents of the insecticide chlordane, was the highest detected of the Σ5 Chlordanes measured in the northern fur seals. Σ3 HCH median concentration of 331 ng/g lipid mass, pentacholorobenzene median concentration was 3.59 ng/g lipid mass, hexachlorobenzene median concentration was 0.775 ng/g lipid mass, and mirex median concentration was 12.6 ng/g lipid mass.
Table 1.
Concentrations of a) POPs (ng/g lipid mass) in northern fur seal blubber samples and b) PFAAs (ng/g wet mass) and vitamins (µg/g wet mass) in liver samples.
| a. | Polavina blubber (n=23) |
Northeast blubber (n=2) |
Zapadni blubber (n=9) |
Reef blubber (n=16) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Median | n > RL | Range | Median | n > RL | Range | Median | n > RL | Range | Median | n > RL | |
| Σ64PCBs | 923–3650 | 2110 | 23 | 1360–2586 | 1980 | 2 | 1510–2600 | 1850 | 9 | 681–4410 | 2520 | 16 |
| Σ6DDTs | 297–5720 | 1170 | 23 | 453–1100 | 775 | 2 | 1520–5000 | 2980 | 9 | 287–156000 | 867 | 16 |
| Σ5Chlordanes | 305–2720 | 588 | 23 | 577–1050 | 815 | 2 | 649–1760 | 826 | 9 | 221–2080 | 616 | 16 |
| Σ3HCHs | 98.0–725 | 326 | 23 | 420–796 | 608 | 2 | 264–583 | 438 | 9 | 124–736 | 264 | 16 |
| Pentachlorobenzene | 1.87–8.89 | 2.74 | 23 | 3.41–5.86 | 4.63 | 2 | 2.77–6.53 | 4.04 | 9 | 1.81–8.57 | 3.85 | 16 |
| Hexachlorobenzene | <0.135–4.89 | 0.850 | 20 | 1.37–1.59 | 1.48 | 2 | 0.530–3.23 | 0.780 | 9 | <0.135–2.56 | 0.723 | 13 |
| Mirex | <0.0835–107 | 11.8 | 14 | <0.0847–17.3 | 8.67 | 1 | 7.57–23.0 | 12.1 | 9 | <0.0682–27.2 | 18.7 | 9 |
| Σ6PBDEs | 1.46–126 | 35.1 | 23 | 3.33–9.30 | 6.31 | 2 | 11.4–45.1 | 21.6 | 9 | 12.2–132 | 24.5 | 16 |
| α-HBCD | <0.0740–15.1 | 3.02 | 22 | <0.0680–0.513 | 0.0935 | 1 | 0.296–6.99 | 2.90 | 9 | 0.436–30.7 | 1.99 | 16 |
| b. | Polavina liver (n=23) |
Northeast liver (n=1) |
Zapadni liver (n=9) |
Reef liver (n=16) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Median | n > RL | Range | Median | n > RL | Range | Median | n > RL | Range | Median | n > RL | |
| Σ15PFAAs | 6.89–137 | 42.7 | 23 | 27.1 | -- | 1 | 8.59–45.9 | 19.6 | 9 | 4.86–164 | 39.4 | 16 |
| Retinol | 4.80–654 | 134 | 23 | 52.4 | -- | 1 | 11.5–671 | 43.9 | 9 | 11.1–203 | 45.9 | 16 |
| α-Tocopherol | <472–23400 | 11100 | 20 | 4790 | -- | 1 | <472–15300 | 12700 | 8 | <472–16700 | 9820 | 14 |
| γ-Tocopherol | <573–1310 | 738 | 12 | 657 | -- | 1 | <573–1290 | 618 | 3 | <573–2010 | 877 | 12 |
Abbreviations: POPs=persistent organic pollutants, PCBs=polychlorinated biphenyls, DDTs= dichlorodiphenyltrichloroethanes, HCH= hexachlorocyclohexanes, PBDEs=polybrominated diphenyl ethers, α-HBCD=α-hexabromocyclododecanes, PFAAs=perfluorinated alkyl acids
Values shown as “<” a specified number describe the actual reporting limit (RL)
The recently phased out/current-use POPs were detected in concentrations were much lower in concentrations (less than 0.5 % of the total POPs measured) compared to Σ64 PCB and Σ6 DDT concentrations measured in this study. The median concentration in blubber for Σ6 PBDEs in fur seal blubber samples was 26.3 ng/g lipid mass, with a significant contribution of the total coming from BDE 47 (median concentration of BDE 47 16.0 ng/g lipid mass, average percent of total PBDEs 61 %). The only HBCD isomer detected in blubber tissue was α-HBCD with a median concentration of 2.49 ng/g lipid mass. The median hepatic concentration for Σ15 PFAAs was 37.0 ng/g wet mass, with significant contribution to the total coming from six of the longer chain perfluorocarboxcyclic acids (Σ6 PFCAs, median concentration 29.1 ng/g wet mass), especially the odd chain PFCAs perfluorononanic acid (PFNA), perfluoroundecanoic acid (PFUnA), and perfluorotridecanoic acid (PFTriA). One of the most frequently detected PFAA, perfluorooctane sulfonate (PFOS) was found at lower concentrations compared to the long-chain PFCAs (Supplemental Information, Table S2 and Figure S2).
In this study, vitamins A and E were determined in northern fur seal liver samples since in some studies the concentrations measured in blubber, plasma, and liver tissues have been shown to be affected by POP’s exposure in other marine mammal species (Kakela et al. 1999; Nyman et al. 2003; Routti et al. 2005; Desforges et al. 2013). To the authors’ knowledge this is the first time vitamins A and E have been examined in wild northern fur seals. The concentrations of vitamin A (in the form of retinol) ranged from 4.8 µg/g wet mass to 671 µg/g wet mass (median concentration of 59.9 µg/g wet mass). This concentration is within the same range as hepatic concentrations of vitamin A seen in ringed seals, but lower than hepatic concentrations of retinol measured in male juvenile bowhead whales (Kakela et al. 1999; Rosa et al. 2007). The concentration of vitamin E (in the form of α-tocopherol and γ-tocopherol) ranged from <RL to 24100 µg/g wet mass (median concentration of 11300 µg/g wet mass), with the majority of vitamin E quantified being in the form of α-tocopherol. In comparison to vitamin E concentrations measured in ringed seals and bowhead whales, the concentrations measured in this study are higher (Kakela et al. 1999; Rosa et al. 2007).
In order to understand if POPs exposures come from common routes, it is important to examine relationships among the different classes of compounds. There were strong correlations among classes of compounds; many of these pairwise correlations were significant (p<0.05; Table 2). Some of the correlations correspond to similar commercial uses (i.e. pesticides, flame retardants) so the correlations among these chemicals may be partly explained by common routes of application or use. The concentration of Σ64 PCBs were positively correlated with most the concentrations of most legacy POPs, including Σ6 DDTs, Σ5 Chlordanes, Σ3 HCHs, pentachlorobenzene, and mirex (ρPCBs/DDT = 0.3, ρPCBs/chlordanes = 0.7, ρPCBs/HCHs = 0.6, ρPCBs/pentachlorobenzene = 0.4, ρPCBs/mirex = 0.7). Σ64 PCBs was negatively correlated with hexachlorobenzene (ρPCBs/hexachlorobenzene = −0.5), suggesting there are differences in exposure and/or metabolism of these compounds. Similarly, the concentrations of Σ6 DDTs was positively correlated with the concentrations of Σ5 Chlordane (ρDDTs/chlordanes = 0.8), Σ3 HCH (ρDDTs/HCH = 0.5), pentachlorobenzene (ρDDTs/pentachlorobenzene = 0.6), and mirex (ρDDTs/mirex = 0.4), the concentrations of Σ5 Chlordane was positively correlated with the concentrations of Σ3 HCH (ρchlordanes/HCH = 0.8), pentachlorobenzene (ρchlordanes/pentachlorobenzene = 0.6), and mirex (ρchlordanes/mirex = 0.5), and the concentrations of Σ3 HCH was correlated with the concentrations of pentachlorobenzene (ρHCH/pentachlorobenzene = 0.5) and mirex (ρHCH/mirex = 0.5). Since most legacy POPs correlate with each other, one can hypothesize there are common routes of exposure for most legacy POPs.
Table 2.
Spearman correlations of POPs and vitamin concentrations in northern fur seal samples. Asterisks (*) indicate there were significant correlations (p<0.05).
| Σ64PCBs | Σ6DDTs | Σ5Chlordanes | Σ3HCHs | Pentachlorobenzene | Hexachlorobenzene | Mirex | Σ6PBDEs | α-HBCD | Σ15PFAAs | Retinol | α-Tocopherol | γ-Tocopherol | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Σ64PCBs | - | 0.3* | 0.7* | 0.6* | 0.4* | −0.5* | 0.7* | 0.3* | −0.08 | −0.2 | 0.1 | −0.02 | 0.1 |
| Σ6DDTs | - | 0.8* | 0.5* | 0.6* | −0.06 | 0.4* | −0.2 | −0.06 | −0.4* | 0.06 | 0.07 | 0.2 | |
| Σ5Chlordanes | - | 0.8* | 0.6* | −0.1 | 0.5* | −0.03 | 0.06 | −0.4* | 0.05 | 0.02 | 0.2 | ||
| Σ3HCHs | - | 0.5* | −0.09 | 0.5* | −0.2 | −0.2 | −0.5* | 0.2 | 0.002 | 0.04 | |||
| Pentachlorobenzene | - | 0.05 | 0.5* | −0.2 | −0.1 | −0.4 | −0.08 | 0.02 | −0.1 | ||||
| Hexachlorobenzene | - | −0.4* | −0.4* | −0.2 | −0.007 | −0.1 | 0.1 | −0.1 | |||||
| Mirex | - | 0.09 | 0.06 | −0.1 | 0.09 | 0.1 | 0.02 | ||||||
| Σ6PBDEs | - | 0.5* | 0.4* | −0.06 | 0.1 | 0.05 | |||||||
| α-HBCD | - | 0.4* | −0.1 | 0.1 | −0.1 | ||||||||
| Σ15PFAAs | - | −0.06 | −0.04 | −0.1 | |||||||||
| Retinol | - | 0.5 | −0.07 | ||||||||||
| α-Tocopherol | - | 0.01 | |||||||||||
| γ-Tocopherol | - |
The concentrations of the brominated flame retardants (Σ6 PBDEs and α-HBCD) were positively correlated with each other (ρPBDE/α-HBCD = 0.5). Both Σ6 PBDEs and α-HBCD are recent use flame retardants and their correlation with one another suggests similar sources of exposure. Interestingly, Σ6 PBDEs correlated positively with Σ64 PCBs (ρPBDEs/PCBs = 0.3) and negatively with hexachlorobenzene (ρPBDEs/hexachlorobenzene = −0.4). The concentrations of Σ15 PFAAs negatively correlated with concentrations of many legacy POPs, including, Σ6 DDTs, Σ5 Chlordane, Σ3 HCH, and pentachlorobenzene (ρPFAAs/DDT = −0.4, ρPFAAs/chlordanes = −0.4, ρPFAAs/HCHs = −0.5, ρPFAAs/pentachlorobenzene = −0.4), but positively correlated with concentrations of Σ6 PBDEs and α-HBCD (ρPFAAs/PBDEs = 0.4, ρPFAAs/α-HBCD = 0.4). Since Σ15 PFAAs negatively correlate with all legacy POPs, this suggests there are differences in uptake and metabolism. PFAAs are not lipophilic like most legacy POPs therefore it was not expected to see positive correlations among these classes of compounds. With correlations shown among many POPs classes, it is important to consider exposure of multiple POPs groups when assessing animal health and not just one class of compounds.
There have been few studies done examining the influence of POPs on vitamin A and E levels in marine mammals (Kakela et al. 1999; Nyman et al. 2003; Routti et al. 2005; Desforges et al. 2013). Previous studies have shown a decrease in retinol levels in northern elephant seals (Mirounga angustirostris) and harbor seals (Phoca vitulina) associated with PCBs and 4, 4’-DDE (Beckmen et al. 1997; Brouwer et al. 1989; De Swart et al. 1994). It has been suggested by Nyman et al. (2003) that elevated levels of vitamin E could potentially be used as a biomarker of exposure to higher POPs loads. The concentrations of the vitamins retinol and α-tocopherol were positively correlated with one another (ρretinol/α-tocopherol = 0.5); however, vitamins did not correlate with any POPs in this study.
All samples were combined to assess the temporal trends of POPs and vitamins in northern fur seals from St. Paul Island, with the standard errors being corrected for clustering by rookeries (Table 3). The Σ64 PCBs, Σ6 DDTs, and mirex concentrations showed no significant increase or decrease in northern fur seals from 1987 to 2007. When looking at homolog groups for PCBs there is were no significant changes based on homolog group. The concentrations of the other legacy POPs showed a decline in northern fur seals from 1987 to 2007. There was a significant decrease of 5 % per year of Σ5 Chlordanes and hexachlorobenzene concentrations (95 % CI: −7, −1 and −7, −2, respectively), a 4 % decrease annually of Σ3 HCH concentrations (95 % CI: −6, −3), and a 3 % decrease annually of pentachlorobenzene (95 % CI: −4, −2) in the northern fur seal blubber samples (Table 3). The steady state and decrease in legacy POPs, including PCBs and pesticides, has been shown in other marine mammal studies from the Arctic (Hoguet et al. 2013; Lebeuf et al. 2007; Riget et al. 2010).
Table 3.
Temporal trend (% change per year) and 95 % confidence intervals of POPs and vitamins in northern fur seals from St. Paul Island from 1987 to 2007.
| Compound | Trend | 95 % CI |
|---|---|---|
| Σ64PCBs | NS | −3, +1 |
| di- and trichlorobiphenyls | NS | −2, +1 |
| tetrachlorobiphenyls | NS | −3, 0 |
| pentachlorobiphenyls | NS | −5, +1 |
| hexachlorobiphenyls | NS | −3, 0 |
| heptachlorobiphenyls | NS | −3, 0 |
| octachlorobiphenyls | NS | −1, +1 |
| Σ6DDTs | NS | −17, +2 |
| 4,4'-DDT | NS | −25, +2 |
| Σ5Chlordanes | 5 % decrease | −7, −1 |
| Σ3HCHs | 4 % decrease | −6, −3 |
| β-HCH | 3 % decrease | −1, −4 |
| Pentachlorobenzene | 3 % decrease | −4, −2 |
| Hexachlorobenzene | 5 % decrease | −7, −2 |
| Mirex | NS | −21, +4 |
| Σ6PBDEs | 9 % increase | +5, +13 |
| BDE 47 | 9 % increase | +5, +13 |
| α-HBCD | 12 % increase | +8, +16 |
| Σ15PFAAs | 9 % increase | +8, +10 |
| PFOS | NS | −2, +16 |
| Retinol | NS | −5, +3 |
| α-Tocopherol | NS | −2, 0 |
| γ-Tocopherol | NS | −2, 0 |
Abbreviations: NS=no significant trend
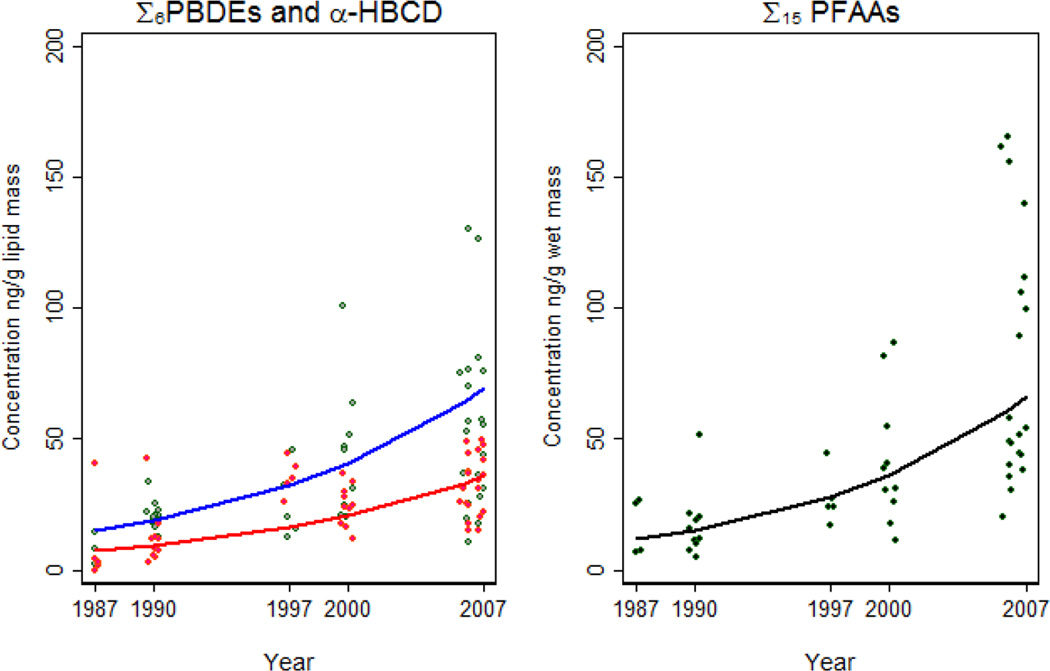
Unlike the legacy POPs, the recently phased out/current-use POPs showed increases over this 20-year study time frame. Σ6 PBDE and α-HBCD concentrations both significantly increased from 1987 to 2007 at an average rate of 9 % (95 % CI: +5, +13) and 12 % (95 % CI: +8, +16) annually, respectively (Table 3; Figure 1). Σ15 PFAA concentrations increased at an average rate of 9 % (95 % CI: +8, +10) annually (Table 3; Figure 1). The increases in Σ6 PBDE concentrations are being driven primarily by the increase in BDE 47. The increase in Σ15 PFAA is being driven by the increases in the PFCAs and there was no significant change in concentrations of PFOS from 1987 to 2007 (Table 3). The increase of the recently phased out/current-use POPs is not unexpected as other studies have shown increases of the concentrations of PBDEs, α-HBCD, and PFAAs in marine mammals from the Alaskan Arctic (Ikonomou et al. 2002; She et al. 2002; Reiner et al. 2011; Hoguet et al. 2013).
Figure 1.
Temporal concentration trends of Σ6PBDEs (blue) and α-HBCD (red) in northern fur seal blubber samples and Σ15PFAAs (black) in northern fur seal liver samples.
This was the first time vitamins have been measured in wild northern fur seals. Temporally, both vitamins A and E showed no significant changes in the northern fur seal samples from 1987 to 2007. Because of the limited knowledge of vitamin concentrations in wild populations, this vitamin information can potentially be used as baseline vitamin A and E measurements for wild northern fur seals. Although no correlations were shown among vitamins and POPs in northern fur seals, knowing typical vitamin A and E levels expected in wildlife can aid future researchers who may want to use these vitamins as potential biomarkers. Additionally, vitamin A and E concentrations in liver tissue are dependent on diet, so these baseline vitamin concentrations may be used to understand potential changes in the northern fur seals feeding habits.
There are few studies looking at legacy POPs in northern fur seals from the Pribilof Islands (Loughlin et al. 2002; Wang et al. 2010). These studies show similar concentrations of PCBs, DDTs, HCHs, and hexachlorobenzene measured in northern fur seal blubber tissue (Table 4). The recently phased out/current-use POPs have not been previously examined in northern fur seals from the Alaskan Arctic; however, PBDEs have been examined in female northern fur seal blubber samples collected from Japan (Kajiwara et al. 2004). The Kajiwara et al. study and ours showed similar PBDEs concentrations (2004). Although α-HBCD has not previously been measured in northern fur seals, α-HBCD has been measured in California sea lions (Zalphophus californianus) blubber samples (Stapleton et al. 2006). The values of α-HBCD reported by Stapleton et al. (2006) tend to be higher in California sea lions. This difference can be explained by proximity of California sea lions to higher ambient sources originating from California watersheds, and subsequently different food web sources. One PFAA, PFOS, has been measured in northern fur seals twice previously (Giesy and Kannan 2001; Kannan et al. 2001). The concentrations of PFOS measured in this study (Supplemental Information, Table S2) are similar to concentrations measured in other northern fur seal liver samples from the Pribilof Islands (Giesy and Kannan 2001; Kannan et al. 2001).
Table 4.
Comparison of mean concentrations of legacy POPs (ng/g lipid mass) in nortern fur seal blubber samples from the Priblof Islands collected as part of this study and published results.
| Pribilof Island Location (Year; sample size) | ΣPCBs | ΣDDTs | ΣChlordanes | ΣHCHs | Pentachlorobenzene | Hexachlorobenzene | Mirex | |
|---|---|---|---|---|---|---|---|---|
| St. Paul Island (1987; n=5) | 2020 | 2110 | 1130 | 526 | 5.22 | 2.35 | 17.2 | This study |
| St. Paul Island (1990; n=10) | 2900 | 32400 | 1370 | 502 | 5.25 | 1.34 | 17 | This study |
| St. George Island (1995–1996; n=10) | 3030 | 3300 | NM | NM | NM | 1.15 | NM | Loughlin et al., 2002 |
| St. Paul Island (1995–1996; n=10) | 2400 | 1990 | NM | NM | NM | 1.36 | NM | Loughlin et al., 2002 |
| St. Paul Island (1997; n=5) | 2000 | 2580 | 950 | 409 | 4.27 | 0.875 | 14.5 | This study |
| St. Paul Island (2000; n=10) | 2350 | 713 | 591 | 384 | 2.47 | 0.788 | 14.1 | This study |
| St. Paul Island (2003–2004; n=10) | 823 | 1090 | NM | 72.1 | NM | 0.42 | NM | Wang et al. 2009 |
| St. Paul Island (2006; n=10) | 2030 | 612 | 468 | 202 | 3.1 | 0.729 | 10.4 | This study |
| St. Paul Island (2007; n=10) | 1900 | 1850 | 721 | 265 | 3.79 | 1.14 | 23.7 | This study |
Abbreviations: NM=not measured
Alaskan natives rely on the annual northern fur seals harvests’ from the Pribilof Islands as part of their subsistence diet. It is important from a human health perspective to consider the meat and blubber from the northern fur seals as a potential route of dietary exposure to legacy and current-use POPs. POPs are primarily sequestered in blubber tissue, so the consumption of marine mammal blubbers has been suggested as a source of POPs to Nunavik Inuits (Dallaire et al. 2009; Weihe et al. 2008). It would be rational to assume Alaskan natives who consume subsistence food, such as northern fur seals, are exposed to many POPs, though the health effects of consuming food with levels reported here is unclear.
Conclusions
This study demonstrates that recently banned and current use POPs are increasing in the arctic region, while the legacy POPs are either declining or remaining constant. Similar trends have been seen in arctic species studied. Monitoring recently banned and current use POPs in future studies will be important to see if these POPs begin to decline in the arctic region. This study also gives baseline information about the concentrations of vitamins A and E in northern fur seals. The identification of POPs in this population of northern fur seals can be used to help understand potential risk factors for this declining population and also fills a critical gap in contaminant information for this sub-arctic species. Although the toxicological implications of the concentrations measured in the blubber and liver of northern fur seals are unknown, their potential impact on both northern fur seal health and human health still exists and should be considered.
Supplementary Material
Acknowledgments
The following are acknowledged for their support of AMMTAP work at St. Paul Island over the years: the Aleut Community of St. Paul Island: Phillip A. Zavadil, Aquilina D. Lestenkof, Pamela Lestenkof, Pat Kozloff, and Darleen Melovidov, and from the NMFS Alaska Regional Office: Mike Williams and Steve Zimmerman. A special acknowledgment goes to Terry Spraker, Colorado State University, who provided special aid at St. Paul Island in obtaining all of the specimens used in this study. The collection and banking of NFS specimens was supported by funding from U.S. Department of Interior’s Minerals Management Service, U.S. Geological Survey Biological Resources Division, and National Marine Fisheries Service Office of Protected Resource’s Marine Mammal Health and Stranding Response Program National Marine Mammal Tissue Bank (Teresa K. Rowles, Program Manager). M. Gribble was supported by a T32 training grant from the National Institute for Environmental Health Sciences (T32ES013678-07).
Footnotes
Disclaimer
Certain commercial equipment, instruments, or materials are identified in this paper to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Bachman MJ, Keller JM, West KL, Jensen BA. Persistent organic pollutant concentrations in blubber of 16 species of cetaceans stranded in the Pacific Islands from 1997 through 2011. Sci Total Environ. 2014;488–489:115–123. doi: 10.1016/j.scitotenv.2014.04.073. [DOI] [PubMed] [Google Scholar]
- Becker PR, Koster BJ, Wise SA, Zeisler R. Biological specimen banking in Arctic research: an Alaska perspective. Sci Total Environ. 1993;139–140:69–95. doi: 10.1016/0048-9697(93)90009-u. [DOI] [PubMed] [Google Scholar]
- Becker PR, Wise SA. The U.S. National biomonitoring specimen bank and the marine environmental specimen bank. J Environ Monit. 2006;8:795–799. doi: 10.1039/b602813f. [DOI] [PubMed] [Google Scholar]
- Beckmen KB, Lowenstine LJ, Newman J, Hill J, Hanni K, Gerber J. Clinical and pathological characterization of northern elephant seal skin disease. J Wildlife Disease. 1997;33:438–449. doi: 10.7589/0090-3558-33.3.438. [DOI] [PubMed] [Google Scholar]
- Beckmen KB, Ylitalo GM, Towell RG, Krahn MM, O’Hara TM, Blake JE. Factors affecting organochlorine contamnant concnetrations in milk and blood of northern fur seal (Callorhinus ursinus) dams and pups from St. George Island, Alaska. Sci Total Environ. 1999;231:183–200. doi: 10.1016/s0048-9697(99)00094-7. [DOI] [PubMed] [Google Scholar]
- Braune BM, Outridge PM, Fisk AT, Muir DCG, Helm PA, Hobbs K, Hoekstra PF, Kuzyk ZA, Kwan M, Letcher RJ, Lockhart WL, Norstrom RJ, Stern GA, Stirling I. Persistent organic pollutants and mercury in marine biota of the Canadian Arctic: An overview of spatial and temporal trends. Sci Total Environ. 2005;351–352:4–56. doi: 10.1016/j.scitotenv.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Brouwer AK, Reijnders PJH, Koeman JH. Polychlorinated biphenyl (PCB)-contaminanted fish induces vitamin A and thyroid hormone deficiency in the common seal (Phoca vitulina) Aquatic Tox. 1989;15:99–106. [Google Scholar]
- Butt CM, Berger U, Bossi R, Tomy GT. Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci Total Environ. 2010;408:2936–2965. doi: 10.1016/j.scitotenv.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Covaci A, Gerecke AC, Law RJ, Voorspoels S, Kohler M, Heeb NV, Leslie H, Allchin CR, De Boer J. Hexabromocyclododecanes (HBCDs) in the environment and humans: A review. Environ Sci Technol. 2006;40:3679–3688. doi: 10.1021/es0602492. [DOI] [PubMed] [Google Scholar]
- Cox C, Chu H, Schneider MF, Munoz A. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26:4352–4374. doi: 10.1002/sim.2836. [DOI] [PubMed] [Google Scholar]
- Dallaire R, Ayotte P, Pereg D, Dery S, Dumas P, Langlois E, Dewailly E. Determinants of plasma concentrations of perfluorooctanesulfonate and brominated organic compounds in Nunavik Inuit adults (Canada) Environ Sci Technol. 2009;43:5130–5136. doi: 10.1021/es9001604. [DOI] [PubMed] [Google Scholar]
- Darnerun PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109(Suppl 1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Swart RL, Ross PS, Vedder LJ, Timmerman HH, Heisterkamp S, Van Loveren H, Vos JG, Reijnders PJH, Osterhaus ADME. Impairment of immune function in harbor seals (Phoca vitulina) feeding on fish from polluted waters. Ambio. 1994;23:155–159. [Google Scholar]
- Desforges JP, Ross PS, Dangerfield N, Palace VP, Whiticar M, Loseto LL. Vitamin A and E profiles as biomarkers of PCB exposure in beluga whales (Delphinapterus leucas) from the western Canadian Arctic. Aquatic Tox. 2013;142–143:317–328. doi: 10.1016/j.aquatox.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Gentry R. Behavior and Ecology of the Northern Fur Seal. Princeton, New Jersey: Princeton University Press; 1997. [Google Scholar]
- Giesy JP, Kannan K. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ Sci Technol. 2001;35:1339–1342. doi: 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- Gribble MO, Crainiceanu CM, Howard BV, Umans JG, Francesconi KA, Goessler W, Zhang Y, Silbergeld EK, Guallar E, Navas-Acien A. Body composition and arsenic metabolism: a cross-sectional analysis in the Strong Heart Study. Environ Health. 2013;12:107. doi: 10.1186/1476-069X-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart K, Gill VA, Kannan K. Temporal trends (1992–2007) of perfluorinated chemicals in Northern Sea Otters (Enhydra lutris kenyoni) from South-Central Alaska. Arch Environ Contam Toxicol. 2009;56:607–614. doi: 10.1007/s00244-008-9242-2. [DOI] [PubMed] [Google Scholar]
- Hoguet J, Keller JM, Reiner JL, Kucklick JR, Bryan CE, Moors AJ, Pugh RS, Becker PR. Spatial and temporal trends of persistent organic pollutants and mercury in beluga whales (Delphinapterus leucas) from Alaska. Sci Total Environ. 2013;449:285–294. doi: 10.1016/j.scitotenv.2013.01.072. [DOI] [PubMed] [Google Scholar]
- Hutchinson JD, Simmonds MP. Organochlorine Contamination in Pinnipeds. Reviews Environ Contam Tox. 1994;136:123–167. doi: 10.1007/978-1-4612-2656-7_4. [DOI] [PubMed] [Google Scholar]
- Ikonomou MG, Rayne S, Addison RF. Exponential increases of the brominated flame retardants, polybrominated diphenyl ethers, in the Canadian Arctic from 1981 to 2000. Environ Sci Technol. 2002;36:1886–1892. doi: 10.1021/es011401x. [DOI] [PubMed] [Google Scholar]
- Kakela R, Kakela A, Hyvarinen H, Asikainen J, Dahl S. Vitamins A1, A2, and E in minks exposed to polychlorinated biphenyls (Aroclor 1242) and copper, via diet based on freshwater or marine fish. Environ Tox Chem. 1999;18:2595–2599. [Google Scholar]
- Kajiwara N, Ueno D, Takahashi A, Baba N, Tanabe S. Polybrominated diphenyl ethers and organochlorines in archived northern fur seal samples from the Pacific coast of Japan, 1972–1998. Environ Sci Technol. 2004;38:3804–3809. doi: 10.1021/es049540c. [DOI] [PubMed] [Google Scholar]
- Kannan K, Koistinen J, Beckmen K, Evans T, Gorzelany JF, Hansen KJ, Jones PD, Helle E, Nyman M, Giesy JP. Accumulation of perfluorooctane sulfonate in marine mammals. Environ Sci Technol. 2001;35:1593–1598. doi: 10.1021/es001873w. [DOI] [PubMed] [Google Scholar]
- Kucklick J, Reiner JL, Schantz MM, Keller JM, Hoguet J, Rimmer C, Ragland T, Pugh R, Moors A, Rhoderick J, Ness J, Peterson D, Becker P. Persistent Organic Pollutants and Vitamins in Northern Fur Seals (Callorhinus ursinus) Collected from St. Paul Island, Alaska as Part of the Alaska Marine Mammal Tissue Archival Project. NISTIR 7958. 2013 [Google Scholar]
- Lebeuf M, Noel M, Trottier S, Measures L. Temporal trends (1987–2002) of persistent, bioaccumulative and toxic (PBT) chemicals in beluga whales (Delphinapterus leucas) from the St. Lawrence Estuary, Canada. Sci Total Environ. 2007;383:216–231. doi: 10.1016/j.scitotenv.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Loseto LL, Stern F, Connelly T, Prokopowicz A, Lean D, Fortier L, Ferguson S. Summer diet of belug whales inferred by farry acid analysis of the eastern Beaufort Sea food web. J Exper Marine Bio Eco. 2009;374:12–18. [Google Scholar]
- Loughlin TR, Castellini MA, Ylitalo G. Spatial aspects of organochlorine contamination in northern fur seal tissues. Mar Pollut Bull. 2002;44:1024–1034. doi: 10.1016/s0025-326x(02)00149-2. [DOI] [PubMed] [Google Scholar]
- Muir DCG, Wagemann R, Hargrave BT, Thomas DJ, Peakall DB, Norstrom RJ. Arctic Marine Ecosystem Contamination. Sci Total Environ. 1992;122:75–134. doi: 10.1016/0048-9697(92)90246-o. [DOI] [PubMed] [Google Scholar]
- Nyman M, Bergknut M, Fant ML, Raunio H, Jestoi M, Bengs C, Murk A, Koistinen J, Bäckman C, Pelkonen O, Tysklind M, Hirvi T, Helle E. Contaminant exposure and effects in Baltic ringed and grey seals as assessed by biomarkers. Marine Environ Research. 2003;55:73–99. doi: 10.1016/s0141-1136(02)00218-0. [DOI] [PubMed] [Google Scholar]
- Pierce CB, Cox C, Saland JM, Furth SL, Munoz A. Methods for characterizing differences in longitudinal glomerular filtration rate changes between children with glomerular chronic kidney disease and those with nonglomerular chronic kidney disease. Am J Epidemiol. 2011;174:604–612. doi: 10.1093/aje/kwr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, Fate and Transport of Perfluorocarboxylates. Environ Sci Technol. 2006;40:32–44. doi: 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- Pugh RS, Ellisor MB, Christopher SJ, Moors AJ, Porter BJ, Becker PR. Marine Environmental Specimen Bank: Clean Room and Specimen Bank Protocols. NISTIR 7389. 2007 [Google Scholar]
- Reiner JL, O’Connell S, Butt C, Mabury S, Small J, Silva A, Muir D, Delinsky A, Strynar M, Lindstrom A, Reagen W, Malinsky M, Schafer S, Kwadijk CAF, Schantz M, Keller J. Determination of perfluorinated alkyl acid concentrations in biological standard reference materials. Anal Bioanaly Chem. 2012;404:2683–2692. doi: 10.1007/s00216-012-5943-5. [DOI] [PubMed] [Google Scholar]
- Reiner JL, O’Connell SG, Moors AJ, Kucklick JR, Becker PR, Keller JM. Spatial and temporal trends of perfluorinated compounds in Beluga Whales (Delphinapterus leucas) from Alaska. Environ Sci Technol. 2011;45:8129–8136. doi: 10.1021/es103560q. [DOI] [PubMed] [Google Scholar]
- Riget F, Bignert A, Braune B, Stow J, Wilson S. Temporal trends of legacy POPs in Arctic biota, an update. Sci Total Environ. 2010;408:2874–2884. doi: 10.1016/j.scitotenv.2009.07.036. [DOI] [PubMed] [Google Scholar]
- Rosa C, Blake JE, Mazzaro L, Hoekstra P, Ylitalo GM, O’Hara TM. Vitamin A and E tissue distribution with comparisons to organochlorine concentrations in the serum, blubber and liver of the bowhead whale (Balaena mysticetus) Compar Biochem Physio Part B. 2007;148:454–462. doi: 10.1016/j.cbpb.2007.07.087. [DOI] [PubMed] [Google Scholar]
- Routti H, Nyman M, Bäckman C, Koistinen J, Helle E. Accumulation of dietary organochlorines and vitamins in Baltic seals. Marine Environ Research. 2005;60:267–287. doi: 10.1016/j.marenvres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- She J, Petreas M, Winkler J, Visita P, Mckinney M, Kopec D. PBDEs in the San Francisco Bay Area: measurements in harbor seal blubber and human breast adipose tissue. Chemosphere. 2002;46:697–707. doi: 10.1016/s0045-6535(01)00234-x. [DOI] [PubMed] [Google Scholar]
- Simms W, Jeffries SJ, Ikonomou MG, Ross PS. Contaminant related disruption of vitamin A dynamics in free-ranging harbor seal (Phoca vitulina) pups from British Columbia, Canada and Wahsington State, USA. Environ Tox Chem. 2000;19:2844–2849. [Google Scholar]
- Simms W, Ross PS. Vitamin A as a biomarker of contaminant-related effects. Tox Industrial Health. 2000;16:291–302. doi: 10.1177/074823370001600706. [DOI] [PubMed] [Google Scholar]
- Smithwick M, Norstrom RJ, Mabury SA, Solomon K, Evans TJ, Stirling I, Taylor MK, Muir DC. Temporal trends of perfluoroalkyl contaminants in polar bears (Ursus maritimus) from two locations in the North American Arctic, 1972–2002. Environ Sci Technol. 2006;40:1139–1143. doi: 10.1021/es051750h. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Dodder NG, Kucklick JR, Reddy CM, Schantz MM, Becker PR, Gulland F, Porter BJ, Wise SA. Determination of HBCD, PBDEs and MeO-BDEs in California sea lions (Zalophus californianus) stranded between 1993 and 2003. Marine Poll Bull. 2006;52:522–531. doi: 10.1016/j.marpolbul.2005.09.045. [DOI] [PubMed] [Google Scholar]
- Tabuchi M, Veldhoen A, Dangerfiel N, Jeffries S, Helbing CC, Ross PS. PCB-related alteration of thyroid hormones and thyroid hormone receptor gene expression in free-ranging Harbor seals (Phoca vitulina) Environ Health Perspectives. 2006;114:1024–1031. doi: 10.1289/ehp.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomy GT, Pleskach K, Ferguson SH, Hare J, Stern G, Macinnis G, Marvin CH, Loseto L. Trophodynamics of Some PFCs and BFRs in a Western Canadian Arctic Marine Food Web. Environ Sci Technol. 2009;43:4076–4081. doi: 10.1021/es900162n. [DOI] [PubMed] [Google Scholar]
- Towell RG, Ream RR, York AE. Decline in northern fur seal (Callorhinus ursinus) pup production on the Pribilof Islands. Marine Mammal Science. 2006;22:486–491. [Google Scholar]
- Verreault J, Houde M, Gabrielsen GW, Berger U, Haukas M, Letcher RJ, Muir DCG. Perfluorinated Alkyl Substances in Plasma, Liver, Brain, and Eggs of Glaucous Gulls (Larus hyperboreus) from the Norwegian Arctic. Environ Sci Technol. 2005;39:7439–7445. doi: 10.1021/es051097y. [DOI] [PubMed] [Google Scholar]
- Wang D, Shelver WL, Atkinson S, Mellish JA, Li QX. Tissue distribution of polychlorinated biphenyls and organochlorine pesticides and potential toxicity to Alaskan northern fur seals assessed using PCBs congener specific mode of action schemes. Arch Environ Contam Toxicol. 2010;58:478–488. doi: 10.1007/s00244-009-9396-6. [DOI] [PubMed] [Google Scholar]
- Weihe P, Kato K, Calafat AM, Nielsen F, Wanigatunga AA, Needham LL, Grandjean P. Serum concentrations of polyfluoroalkyl compounds in Faroese whale meat consumers. Environ Sci Technol. 2008;42:6291–6295. doi: 10.1021/es800695m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisler R, Langland JK, Harrison SH. Cryogenic homogenization of biological tissues. Anal Chem. 1983;14:2431–2434. doi: 10.1021/ac00264a055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.