Abstract
Worldwide, HIV care is becoming increasingly decentralized. For patients in care at centralized facilities, this requires down-referral to local clinics for their HIV care. Information on the real-world experience and predictors of retention in care at the time of down-referral is lacking. We sought to evaluate the effect of patient-level factors on retention in care surrounding a period of down-referral to new clinics for patients with and without virologic failure (VF) on their first-line ART. We conducted a secondary analysis of a case-control study of people living with HIV attending the Sinikethemba (SKT) Clinic at McCord Hospital in Durban, South Africa. Cases (VF) and controls (no VF) responded to a questionnaire focused on individual-level factors. Subsequently, participants self-reported either changing service provider (retained in care), were unable to be reached, died or reported not attending a new provider visit (not retained in care). Multivariate logistic regression was conducted with factors associated with not being retained in care in a univariate analysis. In all, 458 patients were enrolled in the parent study (158 cases and 300 controls) with a median age of 38 years old and with 65% women. A total of 436 (95%) participants successfully established care at the down-referral clinic. In the multivariate analysis, not being pleased with the clinic (SKT), lower adherence scores, and shorter duration of ART predicted failure of down-referral. Down-referral was successful even for patients with VF. Individual-level factors could act as predictors for patients at increased risk for poor retention during the down-referral process to a local clinic.
Introduction
Throughout the world increasing numbers of persons living with HIV (PLWH) are eligible for antiretroviral therapy (ART), given the World Health Organization's recommendation for initiating first-line ART in patients with CD4 cell counts of less than 500 cells/μl.1 In accordance with the new guidelines, as many as 28.6 million people may be eligible for ART.2 In 2013, 2 million additional people were initiated on ART (the largest annual increase ever) bringing the global total to 12.9 million, with 11.7 million of those living in low- and middle-income countries.3 An increasing challenge for growing ART programs is to maximize clinic retention prior to and after ART initiation. A Meta-analysis shows median retention rates of only 65% at 36 months in some sub-Saharan Africa settings.4
A variety of demographic and clinical factors are associated with low retention rates across settings.5–9 In Mozambique patients with lower CD4+ T cell lymphocyte counts at presentation were at higher risk of poor retention,5 yet in a rural South African program CD4 counts >200 cells/μl were independently associated with poor retention.9 Among patients in care in Tanzania, Uganda, and Zambia younger age, WHO clinical stage IV disease, weight loss, being bedridden, and/or having a poor functional status were all independent risk factors for poor retention in ART programs.6 A number of studies have elucidated similar findings, but little is known about how individual-level factors (beyond race, gender, age, and basic biomedical data), HIV drug resistance, and virologic failure (VF) influence retention within ART programs. We aim to explore this issue further in this article, with a focus on the risk of poor retention in the midst of down-referral.
The healthcare delivery model for ART is evolving as efforts to maximize the number of patients on ART increase. The unprecedented numbers of patients eligible for ART combined with a stagnant funding stream to support ART programs have shifted increasing responsibility to local governments and the public health sector within countries to provide HIV care. In turn, centralized ART programs rely on task-shifting as well as decentralization of HIV care and treatment outside of program-specific, specialized HIV clinics into local primary healthcare clinic and community health centers, a process known as down-referral.10–12
In health systems undergoing the process of down-referral, retention in care may become even more difficult for patients as the physical location of where they receive their HIV care changes. The challenge as patients change clinics may arise from a disruption in patient–provider continuity as well as the requisite for the patient to become familiar with a new clinic's process of care. However, one distinct advantage is that these down-referrals may actually result in the patient receiving HIV care/ART closer to home. Data are scarce for outcomes of patients having to undergo the process of down-referral, but initial figures suggest that between 70% and 95% of patients who are down-referred do successfully link to their next clinic. Structural-level factors influencing down-referral outcomes have been explored.13 We posit that individual-level factors could assist in predicting who is at greatest risk for poor retention following a down-referral process, in order to provide additional navigational resources for the specific patients at risk.
In this analysis we examined the predictive capacity of various individual-level factors on retention, in the context of a down-referral process, for patients with and without VF on first-line ART.
Materials and Methods
Study setting and patient population
This is a secondary analysis of data from the Risk Factor for Virologic Failure (RFVF) study conducted at McCord Hospital (MCH) in Durban, South Africa. RFVF was an unmatched case-control study examining a complex array of factors associated with VF.14 Patients were selected from the Sinikethemba Clinic (SKT) at MCH. This clinic was a semiprivate, government-subsidized clinic with partial President's Emergency Plan for AIDS Relief (PEPFAR) funding since 2004.14,15 Patients paid a monthly fee of about $18 USD, which covered all clinical care and medications.13 At SKT, routine viral load (VL) monitoring was done for all patients 5 months after initiating ART. If the VL was <1, 000 copies/ml (cpm), patients were maintained on their initial regimen and followed with annual VL monitoring. If the VL was >1,000 cpm, a repeat VL was done 1–3 months later. If the VL remained >1,000 cpm, regimen changes were considered based upon the level of adherence. HIV genotypes were available only as part of the RFVF study.
All patients attending the clinic who were ≥18 years of age and on ART for ≥5 months were offered participation in this parent study. Patients were enrolled in the RFVF study from October 2010 to June 2012.
Study design
In the parent study, cases were defined as patients with virologic failure (HIV-1 VL ≥1,000 copies/ml) after being on their first-line ART regimen for ≥5 months. Controls were defined as patients without virologic failure (HIV-1 VL <1,000 copies/ml) after being on first-line ART for ≥5 months. All patients who enrolled in the RFVF parent study completed an extensive questionnaire, administered by trained research staff, which covered demographic, socioeconomic, psychosocial, and biomedical information. SKT closed in 2012 due to expiration of funding, leaving about 5,000 patients to be down-referred to community-based clinics for continued HIV care.
During this emergent down-referral process, each patient enrolled at SKT was contacted to arrange for follow-up HIV care at a new clinic. All patients (n = 458) in the RFVF study were again contacted by study staff and asked a series of questions pertaining to where they were currently receiving HIV care (family members could report for patients who were unavailable or who had died). The patients were contacted over a 3-month period after the last patient was discharged from the RFVF study. Patients were classified as “changed service provider” if they self-reported being actively engaged in care anywhere in the healthcare system (at another MCH clinic or any other clinic). Patients were classified as lost to follow-up (LTFU) if they self-reported being out of care or if they were unable to be reached, and they were classified as dead if a family member reported as such. The primary outcome of interest was whether patients were retained in HIV care following the down-referral process. The primary outcome variable was dichotomized where those patients who changed service provider were considered “retained in care” and those who were LTFU or died were considered “not retained.”
In this secondary analysis, variables from the parent study were evaluated as potential risk factors for not being retained in HIV care after completion of the down-referral process.
Statistical analysis
Statistical tests of independence were utilized to evaluate the association of select demographic, socioeconomic, psychosocial, and biomedical variables with LTFU. Variables in the initial univariate analyses were chosen based on knowledge of predictors from prior linkage and retention in care literature. Additionally, some novel variables, which the investigators hypothesized would be important, were included in the univariate analyses.
Age at enrollment and level of education were analyzed as both continuous and dichotomized variables, while other variables were dichotomized based on distribution of responses. Three additional derived variables from the original study were evaluated in univariate analyses. These variables were as follows: “Access” incorporated the antiretroviral refill dates and quantity dispensed of which the derivation was based on the medication possession ratio (MPR)14,16,17; “Adherence” used a pill count ratio (PCAR). This was calculated using an enrollment pill count and the dispensed pills over the previous 180 days to give a fraction of the pills prescribed that were actually taken14,17,18,19; A “Wealth index” was developed using a principal component analysis on assets and utilities that a patient possessed and was analyzed as a continuous variable.20 “Access” and “Adherence” variables were dichotomized around median scores.
A final variable, HIV drug resistance (HIVDR), was derived using the World Health Organization's classification of HIV drug resistance.21 HIVDR was categorized into “HIVDR Prevention,” “HIVDR Possible,” and “HIVDR.” “HIVDR Prevention” included those who had no evidence of any virologic failure. “HIVDR Possible” included those who had virologic failure, but at the time of screening for resistance mutations either insufficient virus was present for amplification or no mutations were detected. Finally, “HIVDR” were those who had genetic mutations present that conferred resistance to any of the nucleos(t)ide reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, or protease inhibitors.
Variables that achieved statistical significance in univariate analyses were included in a multivariate (MV) model. Additionally, variables with epidemiological or contextual significance were forced into the model. Due to the interest in the case-control variable and HIVDR variable, three multivariate analyses were performed and reported here. One model contained the case-control variable as a covariate and excluded HIVDR (MV1). A second model contained the HIVDR variable categorized into three outcomes (HIVDR, possible HIVDR, and prevention) and excluded the case-control variable (MV2). The third model contained the HIVDR variable as a binary variable (HIVDR versus possible drug resistance + prevention) and excluded the case-control variable (MV3). All statistical analyses were performed with SAS (SAS Institute, Version 9.3, Cary, NC). The RFVF study was approved by the ethics committee at McCord Hospital and the institutional review board at Emory University in Atlanta, Georgia.
Results
The study cohort consisted of 458 patients, 158 with VF prior to RFVF enrollment (one patient had missing follow-up data and was excluded from this analysis) and 300 with no evidence of VF prior to RFVF enrollment. The median age was 38.4 years old and 65% were women. Eighty-four percent had received at least an eighth-grade education, 79% had an income, 50% owned or rented a home, and 35% were either married or living with their partner. Fourteen percent used their own car or a friend's car to travel to the clinic and 98% of patients relied on themselves or family to pay for antiretroviral (ARV) medications. At the time of the study enrollment, the mean (SD) duration of ART use was 30.2 months (24.3) and the median (IQR) CD4 count was 300.5 cells/μl (183.5–448.0). Tuberculosis was the most frequent opportunistic infection (55%). The median distance from the patient's home to SKT (prior to down-referral) was 11.37 km (IQR 8.16–17.54) while after down-referral the median distance to their clinic was 5.56 km (IQR 2.54–10.46) (for those who successfully down-referred).
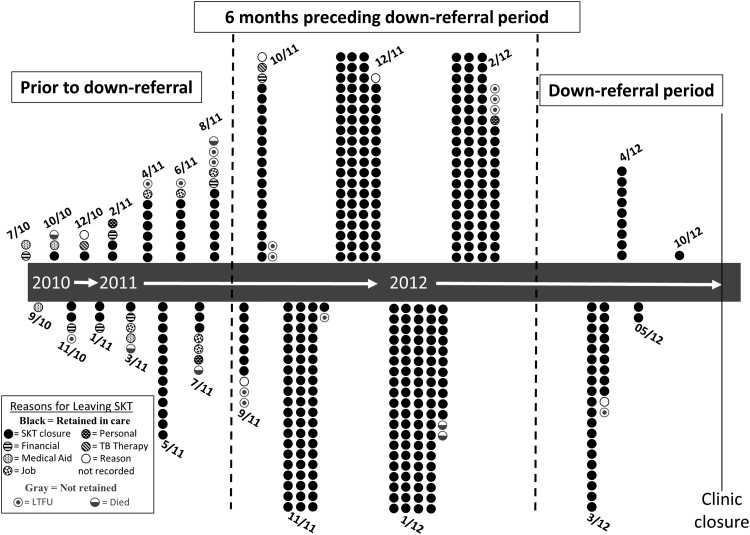
Following down-referral, 436 (95%) patients were retained in care and 21 (5%) were not retained in care (17 were alive and LTFU and four had died). Figure 1 illustrates when each patient attended his or her last clinic visit at SKT in relation to when the actual down-referral period happened. Additionally, Fig. 1 depicts the reasons that patients left SKT. The majority left due to closure of the clinic, but numerous other reasons were provided, particularly for those patients whose last clinic visit at SKT predated the actual down-referral period by more than 6 months. We highlight this 6-month period because patients were routinely scheduled for follow-up clinic visits at least every 6 months. Therefore all patients with routine follow-up should have had a final visit to the clinic during this time frame.
FIG. 1.
Depiction of when patients attended their last clinic visit at Sinikethemba (SKT), the reason why they left, and whether they were retained in care or not. Most patients left due to SKT closure and were seen in the 6 months preceding the clinic closure.
Univariate analyses presented in Table 1 show variables achieving statistical significance and variables the investigators posited could be potentially important predictors of being out of care. Variables that met significance were as follows: patient sentiment toward attending SKT,a case-control status, HIVDR status, duration of ART, and Access (MPR) and Adherence scores (PCAR). Of the 300 patients who had no prior virologic failure (HIVDR Prevention) only 2.3% were not retained, whereas 7.8% of the 51 patients with HIVDR Possible were not retained and 9.4% of the 106 patients with HIVDR were not retained (p = 0.0045).
Table 1.
Characteristics of Patients Who Were Retained Versus Not Retained After Down-Referral
| Domain/variable | Retained in care | Not retained | p-value |
|---|---|---|---|
| Total (N) | 436 | 21 | |
| Demographics | |||
| Age at enrollment | |||
| Median [IQR] | 38.5 [33.2–45.4] | 35.6 [32.4–44.4] | 0.23 |
| <35 years old (% yes) | 28.9% | 38.1% | 0.46 |
| Gender (% female) | 64.7% | 61.9% | 0.82 |
| Race (% black) | 99.1% | 95.2% | 0.21 |
| Socioeconomic | |||
| Education | |||
| ≥9 years (% yes) | 83.3% | 95.2% | 0.22 |
| Income (% yes) | 78.9% | 71.4% | 0.42 |
| Own or rent home (% yes) | 50.5% | 42.9% | 0.51 |
| Transport to clinic | |||
| Own car or walk (% yes) | 15.4% | 4.8% | 0.34 |
| Own car or use friend's car (% yes) | 13.8% | 4.8% | 0.34 |
| Payer for ARVs | |||
| Patient alone (% yes) | 78.7% | 76.2% | 0.79 |
| Patient or family (% yes) | 97.5% | 100.0% | 1.00 |
| Wealth index 1 (mean ± SD) | 0 ± 1.9 | 0 ± 1.4 | 0.80 |
| Wealth index 2 (mean ± SD) | 0 ± 1.4 | 0.3 ± 1.3 | 0.54 |
| Psychosocial | |||
| Pleased with clinic (% yes) | 81.7% | 52.4% | 0.0029 |
| Active in faith (% yes) | 59.2% | 47.1% | 0.33 |
| Ever used traditional meds (% yes) | 58.3% | 47.6% | 0.37 |
| Marital status | |||
| Married (% yes) | 20.2% | 19.0% | 1.00 |
| Married or lives with partner (% yes) | 34.2% | 42.9% | 0.48 |
| Alcohol use (% social usea) | 4.1% | 9.5% | 0.23 |
| HIV education (% with much or some) | 98.6% | 100% | 0.49 |
| Depression (% with any level depression) | 38.3% | 47.6% | 0.49 |
| Biomedical | |||
| Tuberculosis (% yes) | 45.0% | 57.1% | 0.37 |
| Any AIDS condition (% yes) | 65.1% | 81.0% | 0.16 |
| CD4 at enrollment (% <200 cell/μl) | 28.0% | 38.1% | 0.33 |
| Prior virologic failure (% yes) | 67.2% | 33.3% | 0.0035 |
| HIV drug resistance (HIVDR) | 0.0045 | ||
| HIVDR | 22.0% | 47.6% | |
| HIVDR Possible | 10.8% | 19.0% | |
| HIVDR Prevention | 67.2% | 33.3% | |
| Medications | |||
| Access scoreb (% below the median) | 52% | 76% | 0.04 |
| Adherence scorec [median (IQR)] | 1.1 [1.1–1.2] | 1.0 [1.0–1.1] | 0.0053 |
| Adherence score (% below the median) | 50% | 81% | 0.0067 |
| ART duration (mean ± SD) | 30.7 ± 24.5 | 17.5 ± 15.5 | 0.0041 |
Social alcohol use was considered a frequency of one use per month up to using each weekend.
Access is a derived variable from medication possession ratio, represented by the ratio of total days supply of medication over the study interval.
Adherence is a derived variable representing the fraction of prescribed pills that were actually taken; the variable was dichotomized around the median for analysis.
SD, standard deviation; ARV, antiretroviral.
In the multivariate analyses, not feeling pleased about coming to the clinic, duration of ART, and Adherence score remained significant in their association with poor retention in care in each model.b Patients who were “not pleased with clinic” had an odds ratio (OR) of 3.07 (95% CI; 1.13–8.38) of not being retained. Poor adherence remained significant as a predictor of poor retention, with an odds ratio of 3.89 (95% CI; 1.21–12.48). A longer duration of ART was inversely associated with poor retention with an OR of 0.96 (95% CI; 0.9–0.99). The exact ORs varied slightly based on whether the model contained the covariate case-control, HIVDR (binary), or HIVDR (three categories), but the overall trends in statistics and the ultimate message remained consistent across the models. The three final multivariable models are shown in Table 2.
Table 2.
Multivariable Logistic Regression for Predictors of Being Out of Care
| MV1 | MV2 | MV3 | |
|---|---|---|---|
| Domain/variable | Odds ratio (95% confidence interval) | ||
| Demographics | |||
| Age at enrollment (5 year increments) | 1.01 (0.95, 1.08) | 1.01 (0.95, 1.08) | 1.01 (0.95, 1.07) |
| Female gender | 0.72 (0.27, 1.92) | 0.71 (0.27, 1.92) | 0.75 (0.28, 1.99) |
| Socioeconomic | |||
| <9 years of education | 0.26 (0.03, 2.45) | 0.27 (0.03, 2.51) | 0.27 (0.03, 2.50) |
| Psychosocial | |||
| Not pleased with clinic | 3.07a (1.13, 8.34) | 3.04 (1.12, 8.31) | 3.36 (1.25, 9.05) |
| Biomedical | |||
| Absence of any AIDS condition | 0.34 (0.10, 1.10) | 0.34 (0.10, 1.11) | 0.34 (0.10, 1.11) |
| CD4 < 200 cells/μl at enrollment | 0.59 (0.21, 1.71) | 0.55 (0.17, 1.76) | 0.55 (0.17, 1.75) |
| Previous virologic failureb | 0.45 (0.15, 1.40) | — | — |
| HIVDR Possible (reference HIVDR) | — | 0.80 (0.20, 3.25) | — |
| HIVDR Prevention (reference HIVDR) | — | 0.41 (.11, 1.49) | — |
| HIVDR Possible or Prevention (reference HIVDR) | — | — | 0.53 (0.16, 1.69) |
| Medications | |||
| Poor adherenceb (below the median) | 3.89c (1.21, 12.48) | 3.93 (1.23, 12.58) | 4.29 (1.36, 13.51) |
| ART duration (1 month increments) | 0.96c(0.93, 0.99) | 0.96 (0.93, 0.99) | 0.96 (0.93, 0.99) |
These were cases in the original RFVF study, defined as patients with VL ≥1,000 copies/ml after being on first line ART for ≥5 months.
Adherence is a derived variable representing the fraction of prescribed pills that were actually taken; the variable was dichotomized around the median for analysis.
p < 0.05.
ART, antiretroviral therapy; MV1, multivariable analysis #1; MV2, multivariable analysis #2; MV3, multivariable analysis #3.
Discussion
Despite inherent limitations in any secondary data analysis, the strength of our current study is that all data were collected prospectively and the data collection occurred prior to patients being out of care. Therefore a true temporal relationship exists for predictors of poor retention following down-referral. The overall rate of not being retained in care in this cohort of patients after down-referral was lower (5%) than reports from other ART programs across sub-Sahara Africa.4,5,8,9,22,23 The high rate of retention is likely multifactorial. We believe this stems partly from an initial selection bias toward patients who tend to remain in care, as participants in the parent case-control study had a median duration of ART of just over 2½ years. Additionally, the clinic itself was relatively well resourced as a hospital-based clinic and the patients could afford to pay a monthly fee, which may distinguish this program and these patients from the public sector. Clinic level factors that may have contributed to patients remaining in care included having a team of service providers dedicated to continuity of care and improving patient–provider relationships as well as patients receiving ongoing education and communication about keeping future appointments. Additionally, all RFVF study participants engaged in an hour-long, detailed psychosocial questionnaire, which may have aided them in processing challenges to care for themselves.
Despite these aspects in favor of patients remaining in care, one-third of the patients did have previous virologic failure with poor adherence during this period.17 Even with high rates of retention we were still able to garner a few important lessons about risk factors for poor retention in care following a down-referral process. We believe that these factors would be magnified only in usual populations initiating ART in the public sector.
It is tempting to claim “victory” with such a low attrition rate, but we urge caution in not dismissing the 5% who failed to remain in care (either LTFU or died). In a country with over 6.3 million infections and over 2 million on ART even a small percentage experiencing treatment interruptions may have significant individual-level and public health implications.
Though a number of variables in the univariate analyses appeared to be predictors of poor retention, only patient sentiment toward visiting the SKT clinic, Adherence scores, and duration of ART proved significant in the multivariable analysis. Patient sentiments toward the clinic and Adherence score played the largest role in predicting who was not retained upon down-referral. Patients who did not feel pleased about visiting the clinic had greater odds of being out of care post down-referral. Finally, lower Adherence scores predicted being out of care emphasizing that “adherence to clinic” is closely associated with adherence to medications. Factors that affect adherence and the ability to remain in care are likely highly correlated.24,25 In fact, among the same study population patient report of anything other than being “pleased” with clinic was associated with poor adherence by one measure.17
The first step of adherence is traveling to the clinic for medication pick-up (usually monthly); thereby patient-level or structural factors that affect transportation may also influence retention. The median distance from patient home to clinic was cut by about 50% post down-referral but we were unable to assess distance to clinic as a predictor for poor retention as patients not retained did not have a clinic location. Further study should be undertaken to better understand which predictors are unique to adherence versus retention and which are truly correlated with both factors.
Perhaps most interesting is that neither prior virologic failure (cases) nor the spectrum of HIVDR was predictive of poor retention after adjusting for other significant variables. It would be convenient if HIV drug resistance early warning indicators could double as the same predictors for those patients who are at highest risk of being lost to follow-up. Yet from this analysis it appears that the processes resulting in poor retention are somewhat distinct from those processes that result in virologic failure and ultimately HIVDR. In this analysis those who had virologic failure without resistance (HIVDR Possible) or no history of virologic failure (HIVDR Prevention) were less likely than those with HIVDR to be out of care (not statistically significant). This was unexpected because patients with HIVDR Possible are presumably less adherent than the HIVDR population. Based on that presumption we would have expected patients with HIVDR to be more likely to be retained in care. We believed this because a prior analysis from the same clinic population found a higher rate of mortality and loss to follow-up (unpublished) in HIVDR possible than in HIVDR.25 Moving forward it will be extremely important to differentiate the predisposing factors for developing drug resistance and for falling out of care.
The consequences of each of these events are different for the individual patient and the public health of the community; subsequently the interventions and types of support necessary to prevent those two distinct endpoints will likely differ. To effectively discern the difference between risk factors for poor retention and for the HIVDR spectrum, a cohort of ART-naive patients initiating ART would need to be followed longitudinally for these outcomes. This would avoid the selection bias that our population contains, already having been on therapy for at least 5 months.
In conclusion, in the context of down-referral, patient sentiments toward attending the clinic and poor adherence may predict who is most likely to fall out of care. If these results are validated in larger studies, we could foresee simple screening questions assessing sentiments or comfort level toward attending clinic visits being combined with a pill count ratio calculation to identify the patients in the clinic most in need of greater support services to increase the likelihood of remaining in care. These types of tools will become even more essential as millions more are initiated on ART within systems in which resources (both human and financial) are already scarce. The ability to a priori predict those most likely to fall out of care in order to prioritize resource allocation toward improving their retention could serve to benefit the individual patient, the ART program, and the community as a whole.
Acknowledgments
We would like to extend our gratitude to the patients of the Sinikithemba Clinic at McCord Hospital in Durban, South Africa for their commitment to improve patient care through supporting research. The study would not have been possible without the efforts of the counselors, medical records staff, nurses, and medical officers at the clinic. We acknowledge the significant contributions of Sifiso Shange, Melisha Pertab, and Dr. Jane Hampton.
Support was provided by Emory University CFAR (P30AI050409) and NIH (R01 AI098558-01A1), Elizabeth Glaser Pediatric AIDS Foundation as part of Project HEART, the Gilead Foundation, Research and Health Sciences IT Division grant support (UL1RR025008).
Data were presented at the Conference on Retroviruses and Opportunistic Infections; Seattle, Washington, February 23–26, 2015.
Author Disclosure Statement
No competing financial interests exist.
Patient sentiment toward visiting the clinic was assessed by asking “how do you feel about coming to clinic” with possible responses as pleased, worried, ashamed, neutral, or other. For analysis the variable was dichotomized into “pleased” and “not pleased.” This question was asked in the context of stigma-related questions.
Each model was also run with Access in place of Adherence (data not shown), which yielded a trend in results similar to what is presented here with Adherence. We present the final models with Adherence rather than Access because they are highly correlated and in prior analyses using the same data set Adherence has been a more stable/reliable predictor than Access.14,17
References
- 1.WHO: Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva, 2013 [PubMed] [Google Scholar]
- 2.Stover J, Gopalappa C, Mahy M, et al. : The impact and cost of the 2013 WHO recommendations on eligibility for antiretroviral therapy. AIDS 2014;28(Suppl 2):S225–230 [DOI] [PubMed] [Google Scholar]
- 3.WHO: Global update on health sector response to HIV, 2014. Executive Summary. July 2014. Accessed on September2, 2014
- 4.Fox MP. and Rosen S: Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health 2010;15(Suppl 1):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palladino C, Briz V, Bellon JM, et al. : Predictors of attrition and immunological failure in HIV-1 patients on highly active antiretroviral therapy from different healthcare settings in Mozambique. PLoS One 2013;8(12):e82718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koole O, Tsui S, Wabwire-Mangen F, et al. : Retention and risk factors for attrition among adults in antiretroviral treatment programmes in Tanzania, Uganda and Zambia. Trop Med Int Health 2014;19:1397–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thida A, Tun ST, Zaw SK, et al. : Retention and risk factors for attrition in a large public health ART program in Myanmar: A retrospective cohort analysis. PLoS One 2014;9(9):e108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clouse K, Pettifor AE, Maskew M, et al. : Patient retention from HIV diagnosis through one year on antiretroviral therapy at a primary health care clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr 2013;62(2):e39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyles TH, Wilkinson LS, Leisegang R, and Maartens G: Factors influencing retention in care after starting antiretroviral therapy in a rural South African programme. PLoS One 2011;6(5):e19201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goosby E, Von Zinkernagel D, Holmes C, et al. : Raising the bar: PEPFAR and new paradigms for global health. J Acquir Immune Defic Syndr 2012;60(Suppl 3):S158–162 [DOI] [PubMed] [Google Scholar]
- 11.Katz IT, Bassett IV, and Wright AA: PEPFAR in transition—implications for HIV care in South Africa. N Engl J Med 2013;369(15):1385–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kavanagh MM: The politics and epidemiology of transition: PEPFAR and AIDS in South Africa. J Acquir Immune Defic Syndr 2014;65(3):247–250 [DOI] [PubMed] [Google Scholar]
- 13.Cloete C, Regan S, Giddy J, et al. : The linkage outcomes of a large-scale, rapid transfer of HIV-infected patients from hospital-based to community-based clinics in South Africa. Open Forum Infect Dis 2014;1(2):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marconi VC, Wu B, Hampton J, et al. : Early warning indicators for first-line virologic failure independent of adherence measures in a South African urban clinic. AIDS Patient Care STDS 2013;27(12):657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassett IV: Large scale, rapid transfer of HIV-infected patients from hospital-based to primary health clinics in South Africa: An assessment of self-reported linkage to care. Paper presented at the 8th International Conference on HIV Treatment and Prevention Adherence, June2–4, 2013, Miami [Google Scholar]
- 16.Leslie R: Calculating medication compliance, adherence, and persistence in administrative pharmacy claims databases. Pharmaceut Program 2008;1:13–19 [Google Scholar]
- 17.Wu P, Johnson BA, Nachega J, et al. : The combination of pill count and self-reported adherence is a strong predictor of first-line ART failure for adults in South Africa. Curr HIV Res 2014;12(5):366–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ndubuka NO. and Ehlers VJ: Adult patients' adherence to anti-retroviral treatment: A survey correlating pharmacy refill records and pill counts with immunological and virological indices. Int J Nurs Stud 2011;48(11):1323–1329 [DOI] [PubMed] [Google Scholar]
- 19.Lee JK, Grace KA, Foster TG, et al. : How should we measure medication adherence in clinical trials and practice? Ther Clin Risk Manag 2007;3(4):685–690 [PMC free article] [PubMed] [Google Scholar]
- 20.Hare AQ, Ordonez CE, Johnson BA, et al. : Gender-specific risk factors for virologic failure in KwaZulu-Natal: Automobile ownership and financial insecurity. AIDS Behav 2014;18(11):2219–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan MR, Bennett DE, Bertagnolio S, et al. : World Health Organization surveys to monitor HIV drug resistance prevention and associated factors in sentinel antiretroviral treatment sites. Antivir Ther 2008;13(Suppl 2):15–23 [PubMed] [Google Scholar]
- 22.Sanne IM, Westreich D, Macphail AP, et al. : Long term outcomes of antiretroviral therapy in a large HIV/AIDS care clinic in urban South Africa: A prospective cohort study. J Int AIDS Soc 2009;12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltzer K, Ramlagan S, Khan MS, and Gaede B: The social and clinical characteristics of patients on antiretroviral therapy who are 'lost to follow-up' in KwaZulu-Natal, South Africa: A prospective study. SAHARA J 2011;8(4):179–186 [DOI] [PubMed] [Google Scholar]
- 24.Murphy RA, Sunpath H, Castilla C, et al. : Second-line antiretroviral therapy: Long-term outcomes in South Africa. J Acquir Immune Defic Syndr 2012;61(2):158–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy RA, Sunpath H, Lu Z, et al. : Outcomes after virologic failure of first-line ART in South Africa. AIDS 2010;24(7):1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]