Abstract
Angiotensin converting enzyme 2 (ACE2) which breaks down profibrotic peptide angiotensin II to antifibrotic peptide angiotensin-(1–7) is a potential therapeutic target in liver fibrosis. We therefore investigated the long-term therapeutic effect of recombinant ACE2 using a liver-specific adeno-associated viral genome 2 serotype 8 vector (rAAV2/8-ACE2) with a liver-specific promoter in three murine models of chronic liver disease, including carbon tetrachloride-induced toxic injury, bile duct ligation-induced cholestatic injury, and methionine- and choline-deficient diet-induced steatotic injury. A single injection of rAAV2/8-ACE2 was administered after liver disease has established. Hepatic fibrosis, gene and protein expression, and the mechanisms that rAAV2/8-ACE2 therapy associated reduction in liver fibrosis were analyzed. Compared with control group, rAAV2/8-ACE2 therapy produced rapid and sustained upregulation of hepatic ACE2, resulting in a profound reduction in fibrosis and profibrotic markers in all diseased models. These changes were accompanied by reduction in hepatic angiotensin II levels with concomitant increases in hepatic angiotensin-(1–7) levels, resulting in significant reductions of NADPH oxidase assembly, oxidative stress and ERK1/2 and p38 phosphorylation. Moreover, rAAV2/8-ACE2 therapy normalized increased intrahepatic vascular tone in fibrotic livers. We conclude that rAAV2/8-ACE2 is an effective liver-targeted, long-term therapy for liver fibrosis and its complications without producing unwanted systemic effects.
Introduction
Liver cirrhosis and its sequelae of liver failure, portal hypertension, and hepatocellular cancer is now one of the world's leading causes of chronic illness and death.1 As a result, there is a major need to develop antifibrotic therapies which can prevent liver scarring and the development of cirrhosis in patients with chronic liver disease.
There have been many advances in our understanding of the mechanisms involved in liver fibrosis and a number of possible targets for antifibrotic therapy have been identified.2 However, the development of antifibrotic therapies has been hindered by concerns about the lack of liver specificity of treatments which block pathways involved in liver inflammation and fibrosis, and their potential to produce unwanted side effects in other organs.
One possible target for anti-fibrotic therapy is the intrahepatic renin-angiotensin system (RAS). A number of studies suggest that the hepatic RAS plays a key role in liver fibrosis. Angiotensin II (Ang II), a key effector peptide of the classic RAS, drives liver fibrosis via a number of mechanisms including increasing production of reactive oxygen species (ROS) by NADPH.3,4,5 While angiotensin receptor blockers which block the Ang II type 1 receptor, have been shown to ameliorate liver fibrosis in several animal models of liver injury, their effectiveness in man has not been confirmed in randomized studies. Furthermore, there is increasing concern about the safety of angiotensin receptor blockers in patients with established cirrhosis.6,7
Another approach is to target the so-called “alternate arm” of the RAS, which opposes many of the deleterious effects of Ang II of the classic RAS.8 Activity of this arm of the RAS is modulated by angiotensin converting enzyme 2 (ACE2), a homologue of ACE, which breaks down Ang II to angiotensin-(1–7) (Ang-(1–7)), a peptide with anti-fibrotic and vasodilatory activity. Work from our laboratory recently demonstrated that in experimental cholestatic liver disease, Ang-(1–7) infusion inhibits hepatic fibrosis.9 Furthermore, a recent study has shown that liver fibrosis is accelerated in mice lacking ACE2 gene and in the short-term, daily intraperitoneal injections of recombinant human ACE2 protein, commenced at the induction of liver injury, inhibited the initiation of liver fibrosis in mice.10 In cirrhosis, Ang II increases hepatic resistance to portal flow whilst hepatic tone is lowered by Ang-(1–7) which suggests that strategies which increase hepatic ACE2 activity and change the balance of angiotensin peptide levels in the liver, could also be useful in the treatment of portal hypertension.11,12,13 However, given that the study by Osterreicher and colleagues was for only up to 2 weeks and of prophylactic strategy with more invasive in nature, we were interested in a long-term therapeutic and less invasive approach of ACE2 treatment in liver fibrosis.
We therefore wished to determine, in contrast to systemic administration of ACE2 protein,10 whether a gene therapy approach using a liver-trophic recombinant AAV genome 2 serotype 8 vector carrying murine ACE2 (rAAV2/8-ACE2) under the control of a liver-specific human antitrypsin promoter, could be used to produce long-term liver-specific upregulation of the expression of ACE2 in established liver disease. This vector was injected intraperitoneally only once into mice with chronic liver disease induced by three different approaches.
Results
Effects of rAAV2/8-ACE2 therapy on tissue ACE2 mRNA expression and ACE2 activity
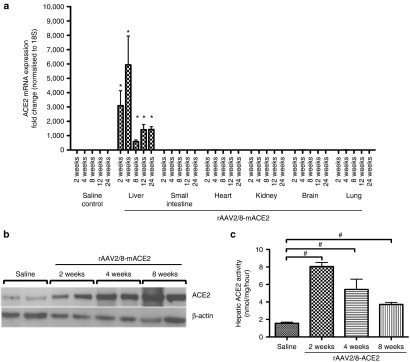
In order to determine the expression levels and organ-specificity / tissue tropism of the viral vector, liver, kidney, lungs, heart, brain, and small intestine were removed at 2, 4, 8, 12, and 24 weeks after a single intraperitoneal injection of rAAV2/8-ACE2 in healthy mice. Quantitative PCR results (Figure 1a) showed that vector-mediated ACE2 expression was liver specific, and significantly increased at all time points in rAAV2/8-ACE2 vector injected mice, and this was accompanied by upregulation of hepatic ACE2 protein (Figure 1b) and activity (Figure 1c) at 2, 4, and 8 weeks.
Figure 1.
ACE2 expression in the liver and other major organs of healthy C57BL/6 mice treated with rAAV2/8-ACE2 gene therapy. ACE2 expression was evaluated by (a) qPCR, (b) western blotting, and (c) activity assay. rAAV2/8-ACE2 significantly increased liver specific ACE2 mRNA expression, ACE2 protein and activity compared to mice treated with saline injection. Each bar represents the mean ± SEM profile from n = 5 mice per treatment group. *P < 0.0001 and #P < 0.01 were calculated by one-way analysis of variance with Tukey comparison test.
ACE2 therapy reduces hepatic Ang II and increases Ang-(1–7) levels in the fibrotic liver
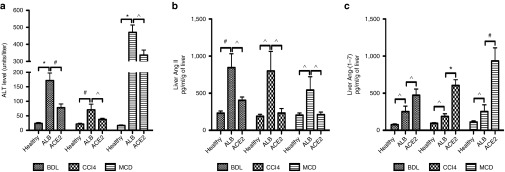
We studied the effects of the therapeutic administration of rAAV2/8-ACE2 in three models of chronic liver injury. At sacrifice, in all three models of liver disease, mice treated with rAAV2/8-ACE2 showed a significant reduction in serum alanine transaminase (ALT) level compared to those that received human serum albumin control vector (rAAV2/8-ALB) (Figure 2a). Moreover, compared with the healthy controls without the vector injection (mean ± SEM: 33 ± 7 U/l), rAAV2/8-ALB vector injected healthy mice after 10 days showed no change in plasma ALT level at different doses (means ± SEM: 26 ± 8, 32 ± 5, 34.5 ± 11.5, and 35 ± 12 U/l in mice injected with 1 × 1010, 5 × 1010, 1 × 1011, and 5 × 1011 gc, respectively), suggesting that the vector itself is unlikely to cause liver injury. In addition, we found no difference in plasma ALT levels in healthy mice injected with rAAV2/8-ACE2 (1 × 1011 gc) for up to 24 weeks (means ± SEM: 29.5 ± 3.7, 28 ± 3.8, 24.2 ± 1.9, 21 ± 2.1, and 26.4 ± 7.8 U/l in mice 2, 4, 8, 12, and 24 weeks after vector injection, respectively) compared with that in healthy mice without rAAV2/8-ACE2 vector (mean ± SEM: 20.2 ± 2.3 U/l). Additionally, we found that the levels of liver Ang-(1–7) peptide in BDL mice without rAAV2/8-ALB vector (mean ± SEM: 365 ± 166 pg/ml/g liver) were not different from those in BDL mice injected with rAAV2/8-ALB vector (mean ± SEM: 248 ± 68 pg/ml/g liver).
Figure 2.
Plasma aminotransferase level (ALT) and hepatic angiotensin peptides levels in the BDL, CCl4, and MCD models with ACE2 gene therapy. rAAV2/8-ACE2 treated animals displayed significant reduction of (a) plasma alanine aminotransferase (ALT). In addition, ACE2 therapy decreased (b) hepatic Ang II concentrations and (c) increased hepatic Ang-(1–7) concentrations. Each bar represents the mean ± SEM profile from n >10 mice per treatment group. *P < 0.0001, #P < 0.01 and ^P < 0.05 and were calculated by one-way analysis of variance with Tukey comparison test.
As expected, hepatic ACE2 mRNA expression was increased up to 2,700-fold in rAAV2/8-ACE2-treated mice with liver disease (BDL: 2,000-, CCl4: 300- and MCD: 2,700-fold increase compared to control vector treated diseased livers). Mechanistically, hepatic ACE2 overexpression would be expected to produce dual benefits; degradation of the potent profibrotic peptide Ang II and generation of the antifibrotic peptide Ang-(1–7).12 This was confirmed by the findings in all three models. Hepatic Ang II peptide concentrations were significantly decreased in animals treated with rAAV2/8-ACE2, compared with those treated with human serum ALB control vector (Figure 2b), and this was associated with markedly increased levels of Ang-(1–7) (Figure 2c).
ACE2 gene therapy decreased experimental liver fibrosis
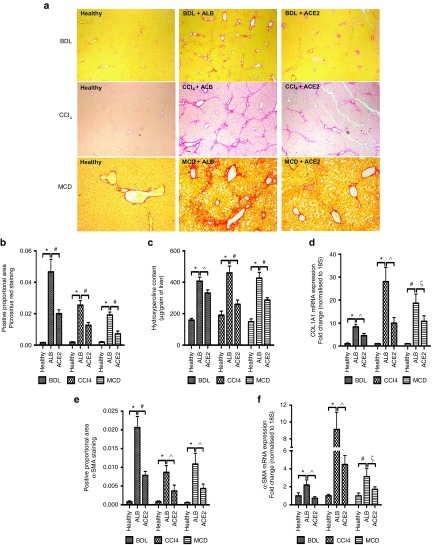
We then assessed the effects of modulation of hepatic angiotensin peptide levels by rAAV2/8-ACE2 treatment on hepatic fibrosis. In comparison with rAAV2/8-ALB-treated mice, hepatic collagen deposition, as assessed by picrosirius red staining (Figure 3a,b), hydroxyproline content (Figure 3c) and collagen type 1a1 (COL1A) mRNA expression (Figure 3d), were significantly reduced by rAAV2/8-ACE2 treatment in all three models. Moreover, as assessed by α-smooth muscle actin (α-SMA) immunohistochemistry (Figure 3e) and qPCR (Figure 3f), hepatic stellate cell (HSC) activation, a major cellular event in the development and progression of liver fibrosis, was significantly reduced in all rAAV2/8-ACE2-treated groups compared with rAAV2/8-ALB-treated controls.
Figure 3.
ACE2 inhibited liver fibrosis and hepatic stellate cell activation. Fibrosis in the BDL, CCl4, and MCD models was evaluated by morphometric analysis of (a and b) liver sections stained with picrosirius red, by (c) liver hydroxyproline content, and by (d) qPCR for COL1A mRNA, showed a significant reduction of hepatic fibrosis by rAAV2/8-ACE2 therapy. ACE2-treated mice also displayed a significantly decreased (e) positive staining of α-SMA, a marker for activated HSCs as well as (f) the mRNA level. Each bar represents the mean ± SEM profile from n >10 mice per treatment group. *P < 0.0001, #P < 0.01, ^P < 0.05, and ξP = 0.06 were calculated by one-way analysis of variance with Tukey comparison test.
The antifibrotic effects of ACE2 gene therapy are associated with reduced expression of proinflammatory and profibrotic cytokines
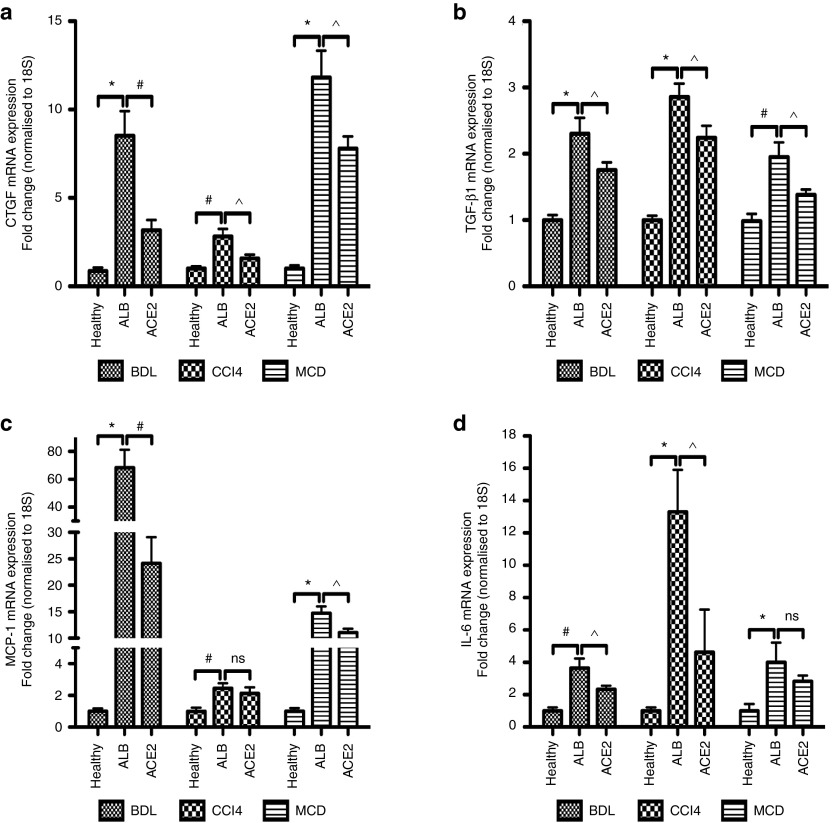
Secretion of extracellular matrix proteins by activated HSCs and myofibroblasts following liver injury is triggered by increased expression of profibrogenic and proinflammatory mediators. We therefore investigated the effects of ACE2 therapy on mRNA expression of connective tissue growth factor (CTGF) (Figure 4a), transforming growth factor-β1 (TGF-β1) (Figure 4b), monocyte chemoattractant protein-1 (MCP-1) (Figure 4c), and interleukin-6 (IL-6) (Figure 4d). These cytokines were significantly downregulated in rAAV2/8-ACE2-treated mice in all three models, when compared to rAAV2/8-ALB-treated mice.
Figure 4.
ACE2 gene therapy reduced profibrotic mediators and proinflammatory cytokines expression. rAAV2/8-ACE2 therapy reduced mRNA levels of profibrotic mediators (a) CTGF, (b) TGF-β1, as well as proinflammatory cytokines (c) MCP-1 and (d) IL-6 in the BDL, CCl4, and MCD models. Each bar represents the mean ± SEM profile from n >10 mice per treatment group. *P < 0.0001, #P < 0.01, ^P < 0.05, and ns = nonsignificant were calculated by one-way analysis of variance with Tukey comparison test.
ACE2 therapy suppresses NADPH oxidase activity, lipid peroxidation, and MAPK signaling
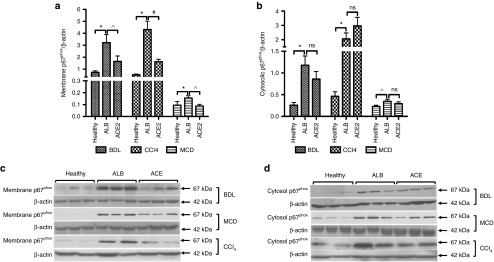
The generation of ROS via activation of NADPH oxidase in HSCs is a key mechanism through which Ang II mediates liver inflammation and fibrosis.5,14,15,16 In order to activate NADPH oxidase, translocation of different NADPH subunits from the cytosol to the membrane is required.14 We therefore measured translocation of p67phox, a rate-limiting subunit of NADPH oxidase activation. As expected, cytosolic and membrane protein levels of p67phox subunit were significantly increased in all animal models. However, in response to ACE2 therapy, p67phox protein level in the membrane was markedly reduced in all three models (Figure 5a,c), whereas ACE2 therapy had no effect on p67phox protein level in the cytosol (Figure 5b,d), suggesting it was membrane translocation of the subunit that was inhibited by ACE2 therapy, but not p67phox subunit protein synthesis. In keeping with these findings, it has been recently shown that the metabolism of Ang II to Ang-(1–7) by ACE2 attenuates Ang II-dependent ROS formation in rats.17
Figure 5.
ACE2 attenuated NADPH oxidase subunit translocation from the membrane to the cytosol. rAAV2/8-ACE2 therapy markedly reduced protein level of NADPH p67phox protein subunit in the membrane (a and c) but had no effect on cytosolic subunit levels (b and d) in the BDL, CCl4, and MCD models, suggesting rAAV2/8-ACE2 inhibited membrane translocation of p67phox subunit. Each bar represents the mean ± SEM profile from n >10 mice per treatment group. *P < 0.0001, #P < 0.01, ^P < 0.05, and ns = nonsignificant were calculated by one-way analysis of variance with Tukey comparison test.
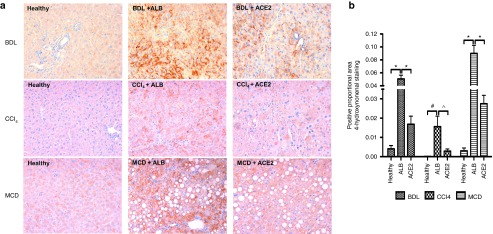
As known, lipid peroxidation induced by generation of ROS contributes to many forms of chronic liver disease. Thus, we assessed hepatic 4-HNE, a reliable biomarker of ROS-initiated lipid peroxidation in liver diseases,18 in all three disease models. We found that 4-HNE adduct formation was markedly reduced in rAAV2/8-ACE2-treated mice compared with rAAV2/8-ALB-treated mice (Figure 6a,b).
Figure 6.
ACE2 inhibited hepatic oxidative stress via reduction of lipid peroxidation. Oxidative stress in the BDL, CCl4, and MCD models was evaluated morphometric analysis of (a and b) liver sections stained with 4-hydroxynonenal, which showed a significant reduction of hepatic oxidative stress by rAAV2/8-ACE2 therapy. Each bar represents the mean ± SEM profile from n > 10 mice per treatment group. *P < 0.0001, #P < 0.01, and ^P < 0.05 were calculated by one-way analysis of variance with Tukey comparison test.
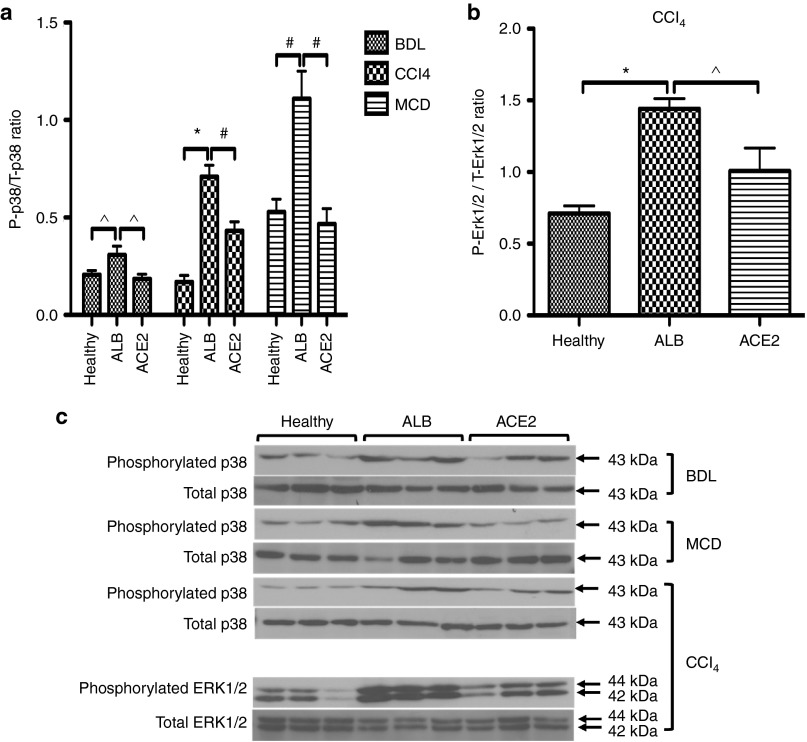
Moreover, there are close links between oxidative stress and activation of mitogen activated protein kinases (MAPKs), including p38 and ERK1/2.19 We hypothesized that sustained elevation of hepatic ACE2 should decrease MAPK phosphorylation and/or increases their dephosphorylation. Western blot analysis showed that in all liver fibrosis models, treatment with rAAV2/8-ACE2 significantly reduced phosphorylated p38 in the liver compared to those in rAAV2/8-ALB-treated mice (Figure 7a,c). Moreover, ACE2 therapy produced a significant reduction in phosphorylated ERK1/2 levels in the CCl4 model compared to those in rAAV2/8-ALB-treated mice (Figure 7b,c).
Figure 7.
ACE2 reduced phosphorylation of MAPKs including (a and c) p38 in the BDL, MCD, and CCl4 models and (b and c) ERK1/2 in the CCl4 model. Phosphorylation of MAPKs was evaluated by western blotting in total liver proteins. Each bar represents the mean ± SEM profile from n > 10 mice per treatment group. *P < 0.0001, #P < 0.01, and ^P < 0.05 were calculated by one-way analysis of variance (ANOVA) with Tukey comparison test.
Effects of ACE2 therapy on methoxamine-mediated hepatic vasoconstriction
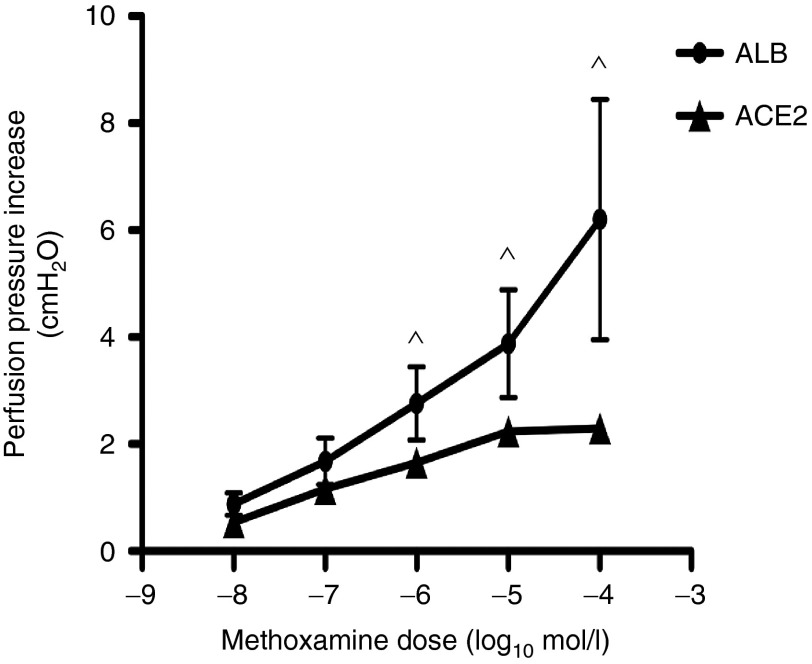
The local production of Ang-(1–7) has been shown to play an important role in the regulation of vascular reactivity to α-adrenergic agonists.20 We therefore wished to generate functional data to support the concept that locally generated Ang-(1–7) could reduce intrahepatic vascular resistance in the fibrotic liver. In Figure 8, we show that the α-adrenergic agonist methoxamine produced a dose-dependent increase in perfusion pressure in in situ perfused livers from rAAV2/8-ALB control vector treated BDL animals. In the livers from rAAV2/8-ACE2-treated BDL mice, there was a marked attenuation of this response to methoxamine, consistent with our previously documented vasodilatory effects of Ang-(1–7) in cirrhotic liver.11,13
Figure 8.
ACE2 significantly reduced hepatic vasoconstriction. Perfusion pressure in the liver was evaluated by in situ perfused mouse liver experiment. rAAV2/8-ACE2 gene therapy reduced methoxamine induced vasoconstriction in the livers of BDL mice, thus improving intrahepatic vascular resistance to portal inflow. Each symbol represents the mean ± SEM profile from n = 4 to 5 per treatment group. ^P < 0.05 was calculated by Student's two-tailed, unpaired t-test.
Discussion
In this study, we demonstrate for the first time that a single dose of rAAV2/8-ACE2 vector produces sustained overexpression of ACE2 in the liver. This increase in hepatic ACE2 activity which produced a marked reduction in the ratio of Ang II to Ang-(1–7) in the liver, resulting in reductions of HSC activation, oxidative stress, release of proinflammatory and profibrotic mediators, and the development of liver fibrosis in three different models of liver disease. Importantly, in two of the models (CCl4 and MCD), these changes were sustained for up to 8 weeks after disease initiation. Given the sustained gene expression of ACE2 for up to 6 months achieved in healthy mice (Figure 1a), it is likely that a single dose of this ACE2 viral vector will provide much longer-term inhibition of experimental liver injury and fibrosis.
The Ang II-dependent arm of the RAS is upregulated in chronic liver disease and is thought to be a primary pathway responsible for the pathogenesis of liver fibrosis.3,21 Recently, we showed that the alternate ACE2-dependent arm is also upregulated and postulated that this represents a counter-regulatory tissue protective response, in which ACE2 not only breaks down the profibrotic vasoconstrictor Ang II but also generates the antifibrotic vasodilatory peptide Ang-(1–7).12,22 Indeed, several studies have shown that Ang II infusion exacerbates,3 while Ang-(1–7) infusion ameliorates liver fibrosis.9 Other studies have shown that Ang-(1–7) treatment improves cardiac fibrosis and function caused by Ang II infusion.23,24 Moreover, a number of studies have shown that short-term treatment with recombinant ACE2 protein ameliorates experimental tissue injury in different organs including the liver,10 but none of them have evaluated its effects on local peptide levels, a mechanistically important aspect of ACE2 therapy. The major reduction in hepatic Ang II levels and simultaneous increase in hepatic Ang-(1–7) levels observed in the current study provides a likely mechanism for the beneficial effects of ACE2 therapy. Although our therapy was liver-specific, it should be noted that we did not measure circulating angiotensin peptide levels due to unavailability of sufficient plasma volumes from mice for the assays.
Previous studies in isolated HSCs have shown that Ang II activates the enzyme NADPH oxidase, leading to ROS generation and oxidative stress.5 In these cells, Ang II increases the phosphorylation of p47phox, a rate limiting protein subunit of NADPH oxidase, which upon phosphorylation, is translocated into the membrane for NADPH enzyme assembly. In addition, mice lacking the p47phox protein subunit display a blunted response to Ang II and are protected from liver fibrosis induced by bile duct ligation.5 In the present study, we found that ACE2 therapy significantly reduced protein levels of another rate limiting NADPH oxidase protein subunit, p67phox, in the cell membrane fraction (Figure 5a,c). However, there was no difference in p67phox subunit protein levels in the cytosol (Figure 5b,d), suggesting that it is not subunit protein turnover that is affected by ACE2, but rather its translocation from the cytosol to the membrane. This, in turn, would be expected to reduce ROS generation.
In all three liver disease models used in the current study, 4-HNE adduct formation, a marker of oxidative stress, was markedly reduced by rAAV2/8-ACE2 treatment (Figure 6a,b). These findings are consistent with other studies which showed that ACE2-deficient mice have elevated NADPH oxidase activity in the kidney,25 and that Ang-(1–7) infusion suppresses ROS formation via attenuation of NADPH activity in mice with diabetic nephropathy.26 Collectively, our data suggest that the breakdown of Ang II to Ang-(1–7), catalyzed by ACE2, leads to inhibition of p67phox translocation from the cytosol to the cell membrane and subsequently reduces NADPH oxidase activity and oxidative stress in diseased livers.
One important downstream consequence of ROS generation is the phosphorylation of MAPKs, including p38 and ERK1/2.19 Ang II treatment has been shown to increase MAPK phosphorylation by increasing NADPH oxidase activity,5 whereas Ang-(1–7) decreased MAPK phosphorylation in a fatty liver disease model,27 and inhibited Ang II-induced phosphorylation of MAPK in proximal tubular cells.28 Ang-(1–7) also increases dephosphorylation of MAPKs via its positive effect on MAPK phosphatase activity.29 The present study showed that ACE2 therapy reduced ERK1/2 phosphorylation in CCl4-induced liver fibrosis compared with ALB treated CCl4 livers. However, this effect was not observed in BDL and MCD diet induced liver fibrosis, suggesting ERK1/2 may be differentially regulated, depending on the fibrogenic stimulus. In addition, ACE2 therapy greatly reduced p38 phosphorylation in all three models of liver fibrosis. In contrast, in mouse HSCs Ang-(1–7) blocked Ang II-induced phosphorylation of ERK1/2 without affecting p38 phosphorylation.10 It should be noted, however, that we determined MAPK phosphorylation in whole livers from rAAV2/8-ACE2-treated mice which might better reflect the regulation of hepatic MAPK pathways by ACE2 therapy, as compared to studies in isolated HSCs exposed to Ang II and Ang-(1–7).10
In cirrhosis, intrahepatic vascular tone and portal hypertension are exacerbated by potent vasoconstrictors such as Ang II, α-adrenergic agonists of the sympathetic nervous system and endothelin-1 from cirrhotic animals and cirrhotic patients.30,31,32,33 Because Ang-(1–7) has been shown to regulate responsiveness to these agonists,20 we wished to generate functional data to support the concept that ACE2 therapy could reduce intrahepatic vascular resistance in the fibrotic liver. The α-adrenergic agonist methoxamine which produced a dose-dependent increase in perfusion pressure in perfused control livers, was markedly attenuated in ACE2-treated livers, consistent with our previously documented vasodilatory effects of Ang-(1–7) in cirrhosis.11,13 This suggests that ACE2 therapy could also potentially be used to reduce intrahepatic vascular tone and thus lower portal pressure in cirrhosis.
In this study, we used a liver-specific AAV2 genome pseudotyped with liver-specific serotype 8 (AAV2/8) carrying ACE2 gene under the transcriptional control of a strong liver-specific promoter, apoliprotein E/human α1-antitrypsin, as previously described by us.34 AAV serotype 8 (AAV8), a member of the AAV family isolated from non-human primates, has been an attractive candidate for hepatic gene transfer application because it only elicits minimal immune response35 and it has high transduction efficiency in the liver,36 as shown in the current study, with persistent ACE2 gene expression for at least 6 months. Moreover, recent human clinical trial using a recombinant AAV2/8 vector to increase hepatic factor IX production in hemophilia B found that gene transfer with AAV was safe and well tolerated and partially corrected coagulopathy.37
In conclusion, our results show for the first time that AAV2/8 vector technology can be used therapeutically in diseased livers to produce long-term liver-specific overexpression of ACE2, an antifibrotic mediator. Hepatic overexpression of ACE2 produced by a single intraperitoneal injection of rAAV2/8-ACE2 changed the intrahepatic balance of angiotensin peptides, thus reducing liver injury, oxidative stress, inflammation, fibrosis, and vascular reactivity in chronic liver disease models. Therefore, the use of AAV2/8 to deliver ACE2 therapy to the liver may offer a novel long-term therapeutic approach for the treatment of hepatic fibrosis and its complications.
Materials and Methods
Animal models of liver injury. Liver fibrosis in male C57BL/6 mice (8 to 10 weeks old) was induced by bile duct ligation (BDL), carbon tetrachloride (CCl4) treatment, or methionine and choline deficient (MCD) diet. BDL was performed as previously described.13 For the CCl4 treatment model, animals were given an i.p. injection of 1:1 mixture of CCl4 and olive oil twice weekly at a dose of (0.5 ml/kg of body weight) for a duration of 8 weeks. For MCD dietary model of NASH, animals were fed the MCD diet for duration of 8 weeks. ACE2 therapy was administered to animals at following time points: (i) at 1 week of BDL, (ii) at 2 weeks of CCl4 injection, (iii) at 2 weeks of MCD diet feeding, based on a time course study which showed active fibrosis at these time points. Experimental procedures were approved by the Animal Welfare and Ethics Committee of Austin Health and performed according to the NHMRC of Australia Guidelines for animal experimentation.
Vector construction. A rAAV2-LSP1 vector,34 constructed using the pAM backbone, was used for producing rAAV2-LSP1-ACE2. The sequence of mouse ACE2, with optimized Kozak sequence, was inserted as an EcoRI/EcoRV fragment under the transcriptional control of the human antitrypsin promoter downstream of hepatic control region of the human ApoE. Control vector (rAAV-LSP1-ALB) was constructed containing human serum albumin (ALB) gene in place of the mouse ACE2 gene. Both vectors were pseudo-serotyped with the liver-specific AAV8 capsid using p5E18-VD2/8 plasmid.
AAV production, purification, titration, and administration. Vectors were packaged by triple plasmid transfection of HEK293T cells with either rAAV2/8-LSP1-ACE2 (ACE2 treatment) or rAAV2/8-LSP1-ALB (control treatment), p5E18-VD2/8, and the adenoviral helper plasmid pXX6 by calcium phosphate/DNA coprecipitation, and the viral particles were purified from cell lysate using standard cesium chloride gradient centrifugation. Vector genomes were titered using real-time qPCR as described previously.34 One week after BDL and 2 weeks after commencing CCl4 treatment or the MCD diet, mice were given a single i.p. injection of rAAV2/8-ACE2 (1011 genome copies) or rAAV2/8-ALB (1011 genome copies) viral particles. One week (BDL group) or 6 weeks (CCl4 and MCD groups) after respective viral vector injections, mice were sacrificed to harvest blood and liver tissue for analysis.
Liver biochemistry. Plasma ALT was measured by an auto-analyzer (Beckman Coulter, Brea, CA), as described previously.9
Quantification of liver fibrosis. Hepatic collagen content was determined colorimetrically by measurement of hydroxyproline content in hydrolyzed liver samples, as described previously.9 In addition, 4-µm thick paraffin sections of liver mounted on silane-coated glass slides were stained with picrosirius red (BioScientific, Sydney, Australia), as described previously.9 Picrosirius red staining was quantified (10 fields/liver section) at ×40 magnification for BDL, CCl4 and at ×200 magnification for MCD using computerized image capture (MCID, Imaging Research, Ontario, Canada), as described previously.9
Immunohistochemistry. Immunohistochemistry was performed on 4 µm sections of paraffin-embedded liver tissue mounted on silane-coated glass slides. Staining for α-smooth muscle actin (α-SMA) and 4-hydroxynonenal (4-HNE) was performed as described previously.38 The positive staining in each section (10 fields/liver section) was determined at ×200 magnification using MCID.
Quantitative polymerase chain reaction analysis. Total RNA was extracted from all livers using TRI reagent (Life Technologies, Carlsbad, CA). QPCR was performed using multiplexing, as described previously.9
Western blotting. Preparation of membrane and cytosolic fractions: Liver tissue samples were homogenized in approximately 0.5 ml of ice-cold lysis buffer and 1× PhosSTOP Phosphatase Inhibitor Cocktail Tablets solution (Hoffmann-La Roche, Basel, Switzerland) and then centrifuged at 45,000 rpm using Optima TLX ultracentrifuge (Beckman Coulter) for 1 hour at 4 °C. After centrifugation, the resultant supernatant was kept as cytosolic p67phox subunit samples, whereas the pellet was resuspended and sonicated in 0.5ml ice-cold lysis buffer and kept as membrane p67phox subunit samples. Membrane specific polyclonal rabbit Na/K-ATPase antibody and cytosolic specific polyclonal rabbit HSP90 antibody (Cell Signaling, Boston, MA) were used to confirm enrichment of the membrane and cytosolic protein fractions.
Liver tissues (~200 μg) from all mice were subjected to western blotting. Polyclonal rabbit ACE2 antibody (Aviva System Biology, San Diego, CA), polyclonal rabbit β-actin antibody (Abcam, Cambridge, UK), polyclonal rabbit p67phox antibody (Sapphire Bioscience, Australia), monoclonal rabbit phosphorylated/total p38 antibody (Cell Signaling, Boston, MA) and polyclonal rabbit phosphorylated/total extracellular signal-regulated kinase (ERK1/2) antibody (Cell Signaling, Boston, MA) were used in liver samples. Thereafter, polyvinylidene fluoride membranes were incubated with polyclonal goat anti-rabbit HRP secondary antibody (Agilent Technologies, Santa Clara, CA). Total p38, total ERK1/2 and β-actin antibodies were used as loading controls. Blots were developed in enhanced chemiluminescence reagent (Thermo Fisher Scientific, Waltham, MA). Intensities of the digitally detected bands were evaluated densitometrically using Gel Doc XR System (BioRad, Hercules, CA).
Tissue ACE2 activity assay. Enzyme activity of ACE2 in homogenized liver samples was measured as described previously.12 In brief, by using specific quenched fluorogenic substrates (Auspep, Melbourne, Australia), which comprised of a fluorophore, 7-methoxycoumarin-4-acetyl, and a quencher, N-2,4-dinitrophenyl separated by a short peptide chain (alanine, proline and lysine), this proline-lysine bond was cleaved by ACE2, allowing the quencher to separate from the fluorophore, resulting in increased fluorescence emission of 7-methoxycoumarin-4-acetyl. The fluorescence was then measured by using a FLUOstar Optima plate reader (BMG LABTECH GmBH, Ortenberg, Germany). The specific activity of ACE2 was determined using a specific ACE2 inhibitor - MLN4760 (Millennium Pharmaceutical, Cambridge, MA).
Radioimmunoassay to determine hepatic angiotensin peptide levels. Approximately 0.01–0.02 g of fresh liver tissue was chopped into small pieces in a solution of 5 ml of 4M guanidine thiocyanate with 1% trifluroacetic acid (Sigma Aldrich, St Louis, MO) and homogenized. The tissue homogenates were then centrifuged at 50,000 × g for 20 minutes at 4 °C and supernatants were harvested and the peptides were extracted using polymeric reversed phase strata cartridges (Phenomenex, Torrance, CA). Eluted angiotensin peptides were dried using speed vacuum centrifugation followed by resuspension in assay buffer. Hepatic concentrations of Ang II and Ang-(1–7) were measured by radioimmunoassay (ProSearch International, Melbourne, Australia), as described previously.12
In situ perfused mouse liver preparation. BDL mice treated with rAAV2/8-ACE2 or rAAV2/8-ALB were anesthetized and in situ perfused, as described previously.13 Briefly, the portal vein and subdiaphragmatic inferior vena cava were cannulated using 22-guage cannula and the liver was flushed with heparinized (400 IU) saline. Livers were then perfused through the portal vein with carbogenated Krebs-Henseleit solution with 1% bovine serum albumin and 0.1% dextrose in a nonrecirculating system. Portal flow was kept constant at 3 ml per minutes. Once pressure had stabilized after 15 minutes different concentrations (10−8M to 10−4M) of methoxamine (Sigma Aldrich) was infused into the liver by adding methoxamine directly to the reservoir.
Statistical analysis. Means between groups were compared using either a two-tailed, unpaired student's t-test or one-way analysis of variance with Tukey post-hoc test. All data are expressed as mean ± standard error of mean (SEM). All statistical analyses were carried out using the computer package PRISM (GraphPad Prism 4.0). P < 0.05 was considered statistically significant.
Acknowledgments
We gratefully acknowledge Mohan Raizada (University of Florida, USA) for providing mouse ACE2 gene sequence, James Wilson (University of Pennsylvania, USA) for providing AAV serotype 8 vector (p5E18-VD2/8), and Zhiyuan Jia for skilled technical assistance. This work has been done in Heidelberg, Victoria, Australia. This work was supported by Australian National Health and Medical Research Council (NHMRC) project grant (APP1008252). The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript. K.M., P.A., and C.H. assisted with concept and design of the experiments. K.M. performed, acquired, analyzed, and interpreted the data statistically from those experiments. K.M., R.C., S.C., M.H., J.T., A.S., I.A., P.W., and C.H. provided technical and material support for the experiments. K.M. drafted the manuscript and K.M., R.C., S.C., J.T., A.S., I.A., P.W., and C.H. revised and approved the manuscript.
References
- Bosetti, C, Levi, F, Lucchini, F, Zatonski, WA, Negri, E and La Vecchia, C (2007). Worldwide mortality from cirrhosis: an update to 2002. J Hepatol 46: 827–839. [DOI] [PubMed] [Google Scholar]
- Schuppan, D and Kim, YO (2013). Evolving therapies for liver fibrosis. J Clin Invest 123: 1887–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller, R, Gäbele, E, Parsons, CJ, Morris, T, Yang, L, Schoonhoven, R et al. (2005). Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology 41: 1046–1055. [DOI] [PubMed] [Google Scholar]
- Bataller, R, Ginès, P, Nicolás, JM, Görbig, MN, Garcia-Ramallo, E, Gasull, X et al. (2000). Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology 118: 1149–1156. [DOI] [PubMed] [Google Scholar]
- Bataller, R, Schwabe, RF, Choi, YH, Yang, L, Paik, YH, Lindquist, J et al. (2003). NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest 112: 1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenero, J, Bataller, R, Sancho-Bru, P, Domínguez, M, Moreno, M, Forns, X et al. (2009). Effects of losartan on hepatic expression of nonphagocytic NADPH oxidase and fibrogenic genes in patients with chronic hepatitis C. Am J Physiol Gastrointest Liver Physiol 297: G726–G734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terui, Y, Saito, T, Watanabe, H, Togashi, H, Kawata, S, Kamada, Y et al. (2002). Effect of angiotensin receptor antagonist on liver fibrosis in early stages of chronic hepatitis C. Hepatology 36(4 Pt 1): 1022. [DOI] [PubMed] [Google Scholar]
- Santos, RA and Ferreira, AJ (2007). Angiotensin-(1-7) and the renin-angiotensin system. Curr Opin Nephrol Hypertens 16: 122–128. [DOI] [PubMed] [Google Scholar]
- Lubel, JS, Herath, CB, Tchongue, J, Grace, J, Jia, Z, Spencer, K et al. (2009). Angiotensin-(1-7), an alternative metabolite of the renin-angiotensin system, is up-regulated in human liver disease and has antifibrotic activity in the bile-duct-ligated rat. Clin Sci (Lond) 117: 375–386. [DOI] [PubMed] [Google Scholar]
- Osterreicher, CH, Taura, K, De Minicis, S, Seki, E, Penz-Osterreicher, M, Kodama, Y et al. (2009). Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology 50: 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace, JA, Klein, S, Herath, CB, Granzow, M, Schierwagen, R, Masing, N et al. (2013). Activation of the MAS receptor by angiotensin-(1-7) in the renin-angiotensin system mediates mesenteric vasodilatation in cirrhosis. Gastroenterology 145: 874–884.e5. [DOI] [PubMed] [Google Scholar]
- Herath, CB, Lubel, JS, Jia, Z, Velkoska, E, Casley, D, Brown, L et al. (2009). Portal pressure responses and angiotensin peptide production in rat liver are determined by relative activity of ACE and ACE2. Am J Physiol Gastrointest Liver Physiol 297: G98–G106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath, CB, Mak, K, Burrell, LM and Angus, PW (2013). Angiotensin-(1-7) reduces the perfusion pressure response to angiotensin II and methoxamine via an endothelial nitric oxide-mediated pathway in cirrhotic rat liver. Am J Physiol Gastrointest Liver Physiol 304: G99–108. [DOI] [PubMed] [Google Scholar]
- De Minicis, S and Brenner, DA (2008). Oxidative stress in alcoholic liver disease: role of NADPH oxidase complex. J Gastroenterol Hepatol 23 Suppl 1: S98–103. [DOI] [PubMed] [Google Scholar]
- Paik, YH, Iwaisako, K, Seki, E, Inokuchi, S, Schnabl, B, Osterreicher, CH et al. (2011). The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology 53: 1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes, RP and Kreuzer, J (2005). Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res 65: 16–27. [DOI] [PubMed] [Google Scholar]
- Lo, J, Patel, VB, Wang, Z, Levasseur, J, Kaufman, S, Penninger, JM et al. (2013). Angiotensin-converting enzyme 2 antagonizes angiotensin II-induced pressor response and NADPH oxidase activation in Wistar-Kyoto rats and spontaneously hypertensive rats. Exp Physiol 98: 109–122. [DOI] [PubMed] [Google Scholar]
- Poli, G, Biasi, F and Leonarduzzi, G (2008). 4-Hydroxynonenal-protein adducts: A reliable biomarker of lipid oxidation in liver diseases. Mol Aspects Med 29: 67–71. [DOI] [PubMed] [Google Scholar]
- Kyriakis, JM and Avruch, J (1996). Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays 18: 567–577. [DOI] [PubMed] [Google Scholar]
- Lemos, VS, Côrtes, SF, Silva, DM, Campagnole-Santos, MJ and Santos, RA (2002). Angiotensin-(1-7) is involved in the endothelium-dependent modulation of phenylephrine-induced contraction in the aorta of mRen-2 transgenic rats. Br J Pharmacol 135: 1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paizis, G, Cooper, ME, Schembri, JM, Tikellis, C, Burrell, LM and Angus, PW (2002). Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology 123: 1667–1676. [DOI] [PubMed] [Google Scholar]
- Herath, CB, Warner, FJ, Lubel, JS, Dean, RG, Jia, Z, Lew, RA et al. (2007). Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1-7) levels in experimental biliary fibrosis. J Hepatol 47: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobe, JL, Mecca, AP, Lingis, M, Shenoy, V, Bolton, TA, Machado, JM et al. (2007). Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7). Am J Physiol Heart Circ Physiol 292: H736–H742. [DOI] [PubMed] [Google Scholar]
- McCollum, LT, Gallagher, PE and Ann Tallant, E (2012). Angiotensin-(1-7) attenuates angiotensin II-induced cardiac remodeling associated with upregulation of dual-specificity phosphatase 1. Am J Physiol Heart Circ Physiol 302: H801–H810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki, J, Ortiz-Melo, DI, Mattocks, NK, Xu, K, Prescott, J, Evora, K et al. (2014). ACE2 deficiency increases NADPH-mediated oxidative stress in the kidney. Physiol Rep 2: e00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, J, Patel, VB, Ramprasath, T, Alrob, OA, DesAulniers, J, Scholey, JW et al. (2014). Angiotensin 1-7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicity. Am J Physiol Renal Physiol 306: F812–F821. [DOI] [PubMed] [Google Scholar]
- Santos, SH, Andrade, JM, Fernandes, LR, Sinisterra, RD, Sousa, FB, Feltenberger, JD et al. (2013). Oral Angiotensin-(1-7) prevented obesity and hepatic inflammation by inhibition of resistin/TLR4/MAPK/NF-κB in rats fed with high-fat diet. Peptides 46: 47–52. [DOI] [PubMed] [Google Scholar]
- Su, Z, Zimpelmann, J and Burns, KD (2006). Angiotensin-(1-7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int 69: 2212–2218. [DOI] [PubMed] [Google Scholar]
- Gallagher, PE, Ferrario, CM and Tallant, EA (2008). MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am J Physiol Cell Physiol 295: C1169–C1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartleb, M, Moreau, R, Cailmail, S, Gaudin, C and Lebrec, D (1994). Vascular hyporesponsiveness to endothelin 1 in rats with cirrhosis. Gastroenterology 107: 1085–1093. [DOI] [PubMed] [Google Scholar]
- Heller, J, Schepke, M, Gehnen, N, Molderings, GJ, Müller, A, Erhard, J et al. (1999). Altered adrenergic responsiveness of endothelium-denuded hepatic arteries and portal veins in patients with cirrhosis. Gastroenterology 116: 387–393. [DOI] [PubMed] [Google Scholar]
- Hennenberg, M, Biecker, E, Trebicka, J, Jochem, K, Zhou, Q, Schmidt, M et al. (2006). Defective RhoA/Rho-kinase signaling contributes to vascular hypocontractility and vasodilation in cirrhotic rats. Gastroenterology 130: 838–854. [DOI] [PubMed] [Google Scholar]
- Hennenberg, M, Trebicka, J, Kohistani, AZ, Heller, J and Sauerbruch, T (2009). Vascular hyporesponsiveness to angiotensin II in rats with CCl(4)-induced liver cirrhosis. Eur J Clin Invest 39: 906–913. [DOI] [PubMed] [Google Scholar]
- Cunningham, SC, Dane, AP, Spinoulas, A, Logan, GJ and Alexander, IE (2008). Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol Ther 16: 1081–1088. [DOI] [PubMed] [Google Scholar]
- Lu, Y and Song, S (2009). Distinct immune responses to transgene products from rAAV1 and rAAV8 vectors. Proc Natl Acad Sci U S A 106: 17158–17162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dane, AP, Wowro, SJ, Cunningham, SC and Alexander, IE (2013). Comparison of gene transfer to the murine liver following intraperitoneal and intraportal delivery of hepatotropic AAV pseudo-serotypes. Gene Ther 20: 460–464. [DOI] [PubMed] [Google Scholar]
- Nathwani, AC, Tuddenham, EG, Rangarajan, S, Rosales, C, McIntosh, J, Linch, DC et al. (2011). Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 365: 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin, M, Herath, C, Jia, Z, Leung, C, Coughlan, MT, Forbes, J et al. (2013). Advanced glycation end products augment experimental hepatic fibrosis. J Gastroenterol Hepatol 28: 369–376. [DOI] [PubMed] [Google Scholar]