Abstract
Here, we describe a fatal serious adverse event observed in a patient infused with autologous T-cell receptor (TCR) transduced T cells. This TCR, originally obtained from a melanoma patient, recognizes the well-described HLA-A*0201 restricted 26–35 epitope of MART-1, and was not affinity enhanced. Patient 1 with metastatic melanoma experienced a cerebral hemorrhage, epileptic seizures, and a witnessed cardiac arrest 6 days after cell infusion. Three days later, the patient died from multiple organ failure and irreversible neurologic damage. After T-cell infusion, levels of IL-6, IFN-γ, C-reactive protein (CRP), and procalcitonin increased to extreme levels, indicative of a cytokine release syndrome or T-cell-mediated inflammatory response. Infused T cells could be recovered from blood, broncho-alveolar lavage, ascites, and after autopsy from tumor sites and heart tissue. High levels of NT-proBNP indicate semi-acute heart failure. No cross reactivity of the modified T cells toward a beating cardiomyocyte culture was observed. Together, these observations suggest that high levels of inflammatory cytokines alone or in combination with semi-acute heart failure and epileptic seizure may have contributed substantially to the occurrence of the acute and lethal event. Protocol modifications to limit the risk of T-cell activation-induced toxicity are discussed.
Introduction
Adoptive cell transfer with tumor infiltrating lymphocytes (TIL) has been shown to induce clinical responses in approximately 50% of melanoma patients in phase 1–2 trials.1 However, the generation of autologous tumor-infiltrating T lymphocytes for adoptive cell therapy has thus far not been feasible for most other human cancers. To address this limitation, infusion of autologous T cells that have been genetically modified with a tumor-reactive TCR—TCR gene therapy—has been developed as an alternative immunotherapeutic strategy. TCR gene therapy has the theoretical advantage that it allows the use of a set of particularly effective TCRs reactive with shared tumor antigens in large patient groups. In addition, as TCR gene therapy entails the genetic modification of naive or memory T cells that are expanded in vitro for only a short period of time, it has the potential to provide patients with T-cell populations with increased capacity for long-term engraftment, as compared to the highly differentiated TIL.
In 2006, the first clinical TCR gene therapy trial was reported, demonstrating that T cells modified with a MART-1-specific T-cell receptor (DMF4) could be detected at low levels in the peripheral blood of melanoma patients for more than 2 months. The clinical response rate in this first trial was low (2/17),2 however, subsequent trials utilizing a MART-1 reactive TCR with a higher affinity (DMF5), or a TCR reactive with the NY-ESO-1 cancer/testis antigen, have shown more encouraging response rates in patients with melanoma (30% for DMF5 and 45% for NY-ESO-1 TCR) and synovial sarcoma (66% for NY-ESO-1 TCR).3,4 Recently, a clinical trial was reported in which MART-1 reactive TCR gene therapy was combined with a peptide pulsed DC vaccine, revealing transient antitumor activity in 9 out of 13 melanoma patients.5
In all four trials, T-cell reinfusion was preceded by nonmyeloablative lymphodepleting conditioning of the patient (cyclophosphamide and fludarabine). Following cell infusion, high-dose bolus IL-2 up to tolerance was given. Infused cell numbers in these trials varied between 1 × 109 and 130 × 109 cells.
Within the NY-ESO-1 and MART-1-DMF4 trials, no substantial T-cell-related toxicity was observed.
Toxicity in the MART-1-DMF5 trial was however more prominent, consisting of erythematous skin rash (14/20 patients), anterior uveitis (11/20), and hearing loss (10/20). The nature of these toxicities is consistent with on-target recognition of the MART-I antigen that is expressed at these sites, and these toxicities could effectively be treated by topical use of corticosteroids. Severe on-target toxicity was also observed in a trial utilizing T cells transduced with a high avidity murine carcinoembryonic antigen (CEA) reactive TCR. In all three treated patients, a severe but transient inflammatory colitis was induced within a week after cell infusion,6 probably due to lymphocyte recognition of physiological levels of CEA expression within colonic mucosa. More recently, severe neurological toxicity was witnessed in a trial using anti-MAGE-A3 TCR-engineered T cells. The affinity enhanced TCR used in this trial was known to recognize multiple related epitopes within the MAGE-A family (including MAGE-A3/A9/A12) and the observed toxicity was explained by low-level expression of MAGE-A12 within the brain.7
Evidence for the potential occurrence of off-target recognition upon administration of TCR-modified T cells has also been obtained in preclinical and clinical studies. Specifically, we have previously shown the occurrence of lethal autoimmune pathology in mouse models of TCR gene therapy that is driven by mispairing of the introduced and endogenous TCR chains (thereby generating so called “mixed dimers”). In this animal model, marked cachexia developed around 14 days after T-cell infusion. This mixed dimer-induced toxicity can largely be prevented by adjustments in the design of TCR vectors (including those present within the vector used in this study, as described below).8 To our knowledge, mixed dimer-mediated toxicity has not been observed in clinical trials so far. However, a second type of off-target recognition has recently been observed in a trial using an affinity enhanced MAGE-A3 TCR. Adoptive cell transfer of T cells engineered to express this TCR resulted in severe cardiac toxicity, which was mapped to cross-reactivity with an epitope within titin, a protein present in cardiac myocytes.9,10 Finally, within the MART-1/ DC vaccination trial, 2 out of 13 patients experienced serious adverse events (SAE) within 1 week of cell infusion that consisted of acute respiratory distress requiring intubation. This toxicity was associated with patchy pulmonary infiltrates and was accompanied with elevated levels of cytokines in peripheral blood (such as IFN-γ, IL-10, and especially IL-6).5 Also, in this study, patients recovered upon steroid administration.
In parallel to trials that involve administration of TCR-modified T cells, a number of clinical trials have been carried out with T cells transduced with chimeric antigen receptors (CARs). In most of these trials, the B-cell lineage antigen CD19 that is also expressed on B-cell lymphomas, has been targeted. Profound clinical responses using anti-CD19 CAR-modified T cells have been observed in progressive B-cell malignancies, but sometimes at the cost of concomitant toxicity, including tumor lysis syndrome,11 fever, fatigue, rigors, dyspnea, hypotension, renal failure, and reduction in alertness.12,13 In at least some patients, this toxicity was shown to coincide with increased serum levels of inflammatory cytokines such as IFN-γ, TNF-α and IL-6.13,14,15 Although direct evidence is lacking, it is generally assumed that the toxicities observed in the anti-CD19 CAR trials is the result of massive activation of infused CAR-transduced T cells by (on-target) recognition of the CD19 antigen on B lineage cells, including the malignant cells.
In summary, evidence for on-target and off-target toxicity has been observed in TCR-modified T-cell trials. In addition, cytokine release–related toxicity has been described within TCR- and CAR-based trials.
This report describes a SAE observed during the treatment of the first patient in a two-center study aiming to assess the feasibility, safety, and objective response rate of the adoptive transfer of autologous T cells modified with a nonaffinity enhanced MART-1-specific TCR in advanced stage melanoma patients.
Clinical trial design
The TCR used in this study, 1D3HMCys, recognizes the 26–35 epitope of MART-1 presented by HLA-A*0201. MART-1 is a melanosomal protein, expressed in melanocytes, and the vast majority (80%) of melanomas. The 1D3 TCR was originally derived from a T-cell clone of a melanoma patient vaccinated with the MART-126–35 (EAAGIGILTV) peptide,16 and was modified in several ways in order to increase expression and to enhance preferential pairing between its α- and β-chain.17,18 Specifically, the constant domains in the α- and β-chains were replaced by murine constant domains, in order to improve cell surface expression and reduce formation of mixed TCR dimers.19 In addition, the genes encoding the 1D3 α- and β-chain were codon optimized and an additional cysteine residue was introduced in both chains to allow formation of a non-native interchain disulfide bridge.20,21 Finally, a 2A peptide cleavage site encoding sequence was used to link the TCR α- and β-chain genes, in order to obtain equimolar expression of the introduced TCR α- and β-chain from a single translation product.22 None of the above modifications involved alterations within the peptide-MHC exposed area of the TCR α- and β-chains. The optimized 1D3 TCR (1D3HMCys) was introduced into the retroviral MP71 vector for T-cell transductions.
Strategies for retroviral modification of human T cells rely on their in vitro activation and subsequent proliferation. To date, most TCR gene therapy trials have utilized anti-CD3 monoclonal antibody (OKT-3) and IL-2 for these purposes. However, work by Bonini and colleagues in preclinical models has demonstrated that the engraftment potential and function of T cells that are activated by anti-CD3/CD28 beads in the presence of IL-7/IL-15 is significantly stronger, and based on these data, we set up a Good Manufacturing Practice T-cell production process, in which autologous T cells are activated under these conditions.23,24,25 In a direct comparison between expansion with CD3/CD28 beads and IL-7/IL-15 or OKT-3 with IL-2, no major differences in cytokine production upon in vitro stimulation with tumor cells was observed (in three independent donors, Supplementary Figure S1). Bead-activated cells tend to produce less IFN-γ, in accordance with a “less-differentiated” phenotype.
This trial involves the first administration of T cells modified with the 1D3HMCys TCR and to our knowledge also the first administration of TCR-modified T cells activated in the presence of the homeostatic cytokines IL-7 and IL-15.25
Results
In vitro characteristics of TCR-transduced cell product of patient 1
Autologous apheresis product from patient 1 was used as a T-cell source. After selection and activation with anti-CD3/CD28 beads in medium containing IL-7 and IL-15, cells were retrovirally transduced with the 1D3HMCys TCR, as described in the Materials and Methods section.
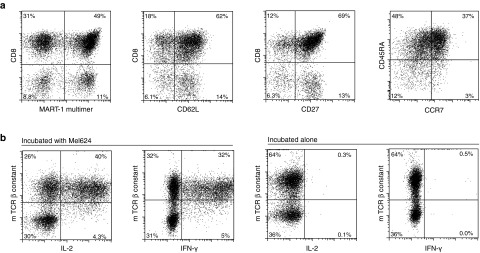
The resulting cells were analyzed for expression of the introduced TCR by MHC multimer staining, and for the expression of CD3, CD4, CD8, CD27, CD62L, CD45RA, and CCR7 at the day of infusion (Figure 1). These analyses revealed a transduction efficiency of 61%, with a similar percentage MHC multimer-positive cells within the CD8+ and CD4+ subsets. The infusion product was 100% CD3 positive. The majority of the transduced cells coexpress CD62L and CD45RA, and express CD27 and CCR7, indicating a central memory or memory stem-like phenotype. The infused cell product for this patient contained 7.52 × 109 cells, of which 4.56 × 109 expressed the 1D3HMCys TCR. Cell viability was 94.8% and the infusion product was sterile.
Figure 1.
Characteristics of the final infusion product. (a) Phenotypic analysis of infusion product, gated on propidium iodide negative lymphocytes. (b) Functional analysis of T-cell receptor (TCR)-transduced cells. TCR-transduced cells were cultured alone or incubated with HLA-A2+ MART-1+ Mel624 cells. Intracellular cytokine production was determined after 15 hours of incubation.
Samples of the final TCR transduced T-cell product of this patient were also used to test whether the cells were capable of recognizing an HLA-A2+, MART-1+ melanoma cell line (mel624), by analysis of IFN-γ and IL-2 production. As shown in Figure 1b, TCR-transduced T cells produced effector cytokines when incubated with mel624 but did not show reactivity when incubated in the absence of target cells. These data are in line with prior validation runs, showing that T-cell activation is only observed upon incubation with HLA-A2+ MART-1+ melanoma cell line and not upon incubation with melanoma lines that lack HLA-A2 (data not shown), demonstrating that the T-cell recognition is restricted to the relevant MHC I-peptide complex.
To test the polyclonality of the final T-cell product, cells were stained for the TCR Vβ repertoire of human T lymphocytes by flow cytometry. As can be appreciated from Supplementary Figure S2, both the starting cells and infusion product contained a similar and broad TCR repertoire, indicating a polyclonal infusion product. No differences were observed between transduced and untransduced cells.
Clinical course
Patient 1 was a 43-year-old woman with cutaneous metastastic melanoma. After progression upon treatment with ipilimumab and an experimental MEK inhibitor, she was included in the 1D3HMCys trial. Immunohistochemistry of a tumor biopsy confirmed MART-1 expression by the tumor cells and the patient was HLA-A*0201 positive. The patient had bulky disease with large bilateral retroperitoneal metastases with a diameter of 18 cm, and pelvic metastases of which the largest had a diameter of 16 cm. In addition, she had multiple pulmonary, subcutaneous, and lymph node metastases of 1–3 cm in diameter and one small asymptomatic brain lesion of 8 mm. At start of treatment, a large volume of ascites was present (10L), most likely due to peritoneal metastases, which was removed via paracentesis during chemotherapy.
Following completion of lymphodepleting chemotherapy (cyclophosphamide day -7 and -6 and fludarabine day -5 to -1, see Materials and Methods), patient 1 received the T-cell product at the end of day 0. Starting at the day of T-cell infusion, the patient received daily subcutaneous injections of low-dose IL-2 (2 × 106 IU). Protocolled cultures of a feces specimen from that day (taken before cell infusion) revealed Extended Spectrum βlactamase (ESBL) Escherichia coli. At day 1 and 2 after T-cell infusion, she developed high fever (> 40 °C) and chills, but no other signs of sepsis. Blood cultures taken from day 1 revealed the same ESBL E. coli. Broad-spectrum antibiotics were initiated (imipenem and vancomycin). Because colonization of the central venous catheter (CVC) by E. coli could possibly be a continuous source of bacteremia, it was removed at the end of day 2 (culture of the catheter tip remained however sterile). The next day (day 3), the patient improved clinically, but kept developing fever (between 38–40 °C) following every IL-2 injection. From day 3 to 5, the patient had normal vital signs and did not show any signs of (severe) sepsis. All blood culture taken from day 2 and onward remained sterile. Because the ESBL E. coli was sensitive to imipenem, but not to vancomycin, the latter was discontinued on day 4, since the CVC was already removed. Following T-cell infusion and IL-2 injections, the patient displayed a substantial weight gain (10 kg relative to the day of T-cell infusion) due to severe fluid retention, including ascites, which was attributed to the IL-2 treatment and the malignant peritonitis. The patient's heart rate was increased the days following T-cell infusion (>100 bpm, starting directly after infusion).
On the morning of day 6, the patient was found sitting in bed, not responsive to verbal stimuli. Her vital signs were normal at that time, although she had a fever of 39.5 °C. Twenty minutes later, she was found cyanotic, but still responsive to stimuli, with a Glasgow Coma Scale of 14. Her blood pressure was normal, but pulse remained high (110/minute). Since neck stiffness, altered mental status and fever were present, infectious meningitis was suspected. Oxygen therapy was initiated and she was admitted to the intensive care unit (ICU). During transportation, she had a generalized tonic-clonic seizure and a witnessed cardiac arrest. (pulseless electrical activity) with no palpable radial pulse. At this point, the patient was intubated and resuscitated for about 10 minutes. The antibiotic regimen was changed to meropenem, vancomycin, and amoxicillin. In the next hours, she stabilized at the ICU, requiring mechanical ventilation with 100% O2 and norepinephrine support. Therapeutic hypothermia (32–34 °C) was initiated postresuscitation in order to minimize brain damage. Prior to the lumbar puncture, a brain and thorax CT was performed. The brain CT showed no signs of intracerebral pressure, but did show an increase in size of the solitary and hemorrhagic brain metastasis with perifocal edema, and general brain edema reminiscent of cerebral damage due to the cardiac arrest. A pulmonary CT-scan showed an enlarged heart (heart-to-lung ratio: 3/5, which was normal before treatment) and bilateral atelectasis of the basal lung areas. The pulmonary metastases were unchanged. A lumbar puncture was performed, revealing a high cerebral spinal fluid pressure (43 cm H2O), but no other abnormalities (normal glucose and protein, no cells or bacteria), thereby ruling out infectious meningitis. Severe sepsis in an immunocompromised patient remained the primary diagnosis and voriconazol was added to the antibiotic regimen. However, all cultures (sputum, blood cultures, broncho-alveolar lavage (BAL), ascites, viral DNA in liquor) remained sterile. The ECG did not show signs of myocardial ischemia or infarction. After cessation of therapeutic hypothermia, sedation was stopped but the patient remained comatose (EMV 3). Subsequently, she developed acute anuric renal insufficiency. She died on day 9 following T-cell infusion due to multiple organ failure.
Pathology and immunohistochemistry
Postmortem examination showed signs of multiple organ failure. Brain autopsy revealed a 2 cm hemorrhage in a metastasis in her right temporal lobe and two additional small metastases. There were no signs of meningitis.
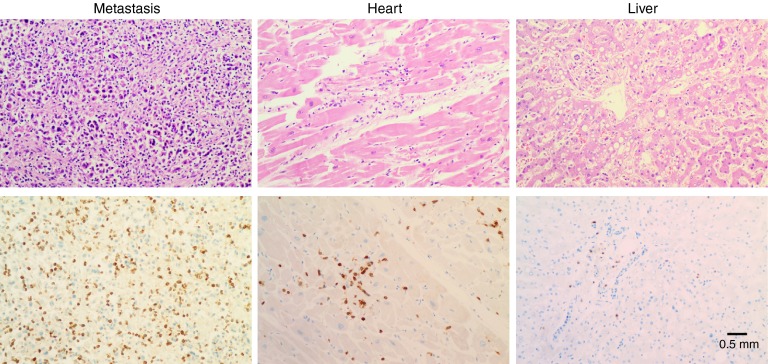
A lymphocytic myocarditis was observed, with influx of CD3/CD8/GranzymeB+ cells that were partly PD-1+ (see Figure 2 for CD3 stain). Parvovirus B19 but not HSV type 1 and 2, CMV, HHV6, and HHV8, was detected by PCR. The presence of Parvovirus can be associated with a viral myocarditis but is not conclusive as viral DNA can also be detected in heart tissue without a lymphocytic myocarditis. There was no evidence of myocardial infarction. IHC showed no expression of MART-1 on the myocard. The multiple (retro)peritoneal metastases showed extensive infiltration of CD3/CD8/granzyme B+ cells (also partly PD-1+) and were partly necrotic (see Figure 2 for CD3 stain). Lymphocytes within the heart and metastases stained partly positive with anti-Vβ14 antibody that recognizes the Vβ segment used by the introduced 1D3HMCys T-cell receptor. Minor infiltration of T cells in other organs (liver, lung, adrenal gland) was also observed. There was no evidence for T-cell influx in the brain.
Figure 2.
Analysis of T-cell infiltration in metastasis, heart, and liver. Upper panel shows H&E staining, lower panel shows CD3 staining.
The expression patterns of granzyme B and PD-1 within CD3-positive T cells in the metastases and heart was the same as expression on cytospin material of either the infusion product, or infusion product activated by coincubation with mel624 cell line (MART-1 and HLA-A*0201 positive). This indicates that granzyme B and PD-1 expression, as measured by immunohistochemistry, are not useful as markers for recent lymphocyte activation under these conditions in this specific patient.
Microbiological analysis and clinical chemistry
Blood, ascites, BAL, and liquor all taken at day 6 were cultured and remained sterile. S100B levels showed a significant increase (from 4 to 39 µg/l, reference value <0.1 μg/l) upon T-cell infusion, and procalcitonin levels were highly elevated (up to 330 µg/l, reference value < 0.05 µg/l) at the time of ICU admission and remained high afterwards. Ferritin levels also increased steadily upon T-cell infusion, from 1,900 µg/l 1 day after infusion up to >37,324 µg/l during ICU admission (reference <160 µg/l for women).
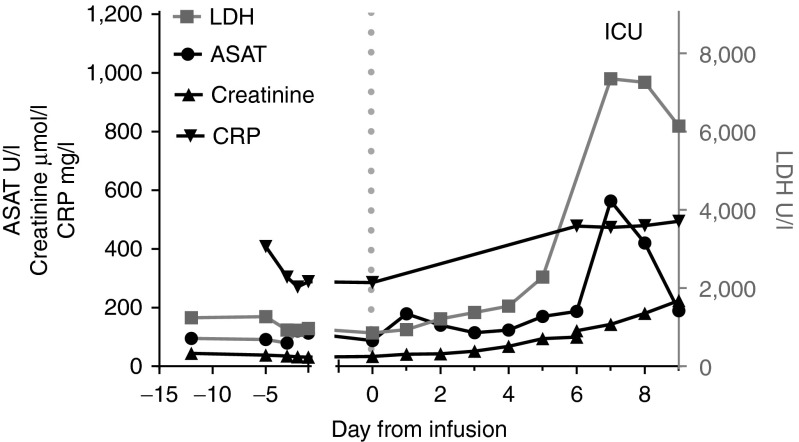
CRP levels were already elevated before T-cell infusion (Figure 3). Furthermore, levels of lactate dehydrogenase (LDH) and aspartate aminotransferase (ASAT) increased directly after deterioration, at day 6 (Figure 3). Creatinine levels rose slowly after T-cell infusion (Figure 3).
Figure 3.
Blood values during treatment. Dotted line indicates the day of T-cell infusion. Normal reference values are <247 U/l LDH, <31 U/l ASAT, 40–95 µmol/l creatinine, and <8 mg/l for C-reactive protein. ASAH, aspartate aminotransferase; LDH, lactate dehydrogenase.
The serum level of NT-proBNP, a marker for heart failure, was slightly elevated just before T-cell infusion (55 pmol/l, the reference value for women is below 18 pmol/l). At day 3, the levels of NT-proBNP reached 2037 pmol/l and during ICU admission levels were above 7,000 pmol/L. Also, the level of Troponine T was elevated to 155 ng/l (reference below 50 ng/l) at the time of ICU admission, both most likely a result of the cardiac resuscitation. The patient accumulated fluids, resulting in edema of legs and sacral areas, which may have been caused by heart failure instead of the assumed IL-2 toxicity. CK-MB levels remained within normal range. Sequential ECGs ruled out myocardial infarction.
The patient showed no signs of a tumor lysis syndrome, such as increase of serum potassium, phosphorus, or uric acid level, and sudden decrease in renal function, which was observed in previous clinical trials using CAR gene therapy.11,26 Her renal function rapidly deteriorated only shortly before death.
Immunological analyses
Blood counts of the patient showed a normal reconstitution pattern after lymphodepleting chemotherapy. At the moment of ICU admission, the patient was still lymphopenic and thrombopenic.
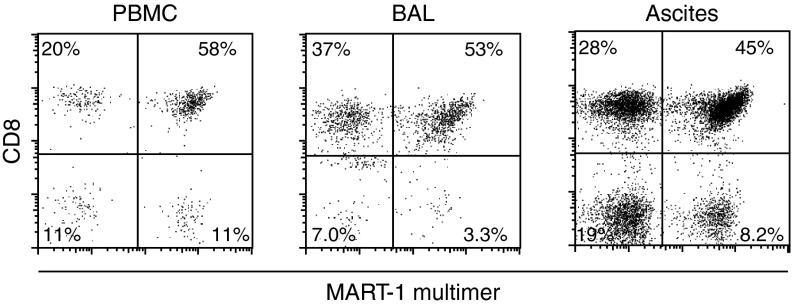
To test for the presence of the TCR transduced T cells, blood, BAL, and ascites samples were screened at day 7 postinfusion. Flow cytometric analyses demonstrated the presence of transduced T cells in the circulation (Figure 4). While the CD8+/CD4+ ratio was comparable to that seen in the infusion product, the percentage of MHC multimer+ cells appeared increased in the CD8+ T-cell population (from 62 to 74% in peripheral blood mononuclear cells (PBMCs)), while somewhat decreased in the CD4+ population (from 56 to 49% in the PBMCs), consistent with antigen-driven proliferation of 1D3HMCys-expressing CD8+ T cells.
Figure 4.
Flow cytometric analysis of PBMCs, broncho-alveolar lavage (BAL), and ascites samples withdrawn from the patient at day 7 upon T-cell infusion, gated on propidium iodide negative, CD3-positive lymphocytes. PBMC, peripheral blood mononuclear cell.
Patient-derived transduced T cells obtained from the blood at day 7, were able to produce high levels of IFN-γ, IL-2, and TNF-α upon incubation with the HLA-A2+, MART-1+ melanoma cell line mel624. The same cells incubated without the presence of target cells did not show any expression of these inflammatory cytokines (see Supplementary Figure S3).
The absolute number of transduced T cells present in blood was calculated to be 5.5 × 106, based on 5L of blood. The absolute amount of transduced T cells in ascites was calculated to be 2.2 × 106, based on 10L ascites. These numbers are very low, if compared with the cell number infused. This might suggest that a major part of the T cells are located in other compartments (like the spleen, liver, or tumor).
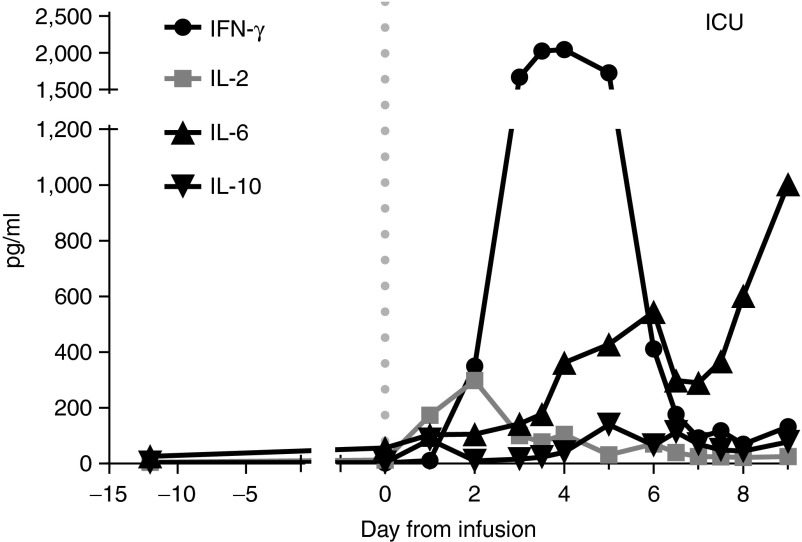
Analysis of cytokine levels in retrieved serum samples by cytometric bead array showed a peak in IL-2 (298 pg/ml) and in particular IFN-γ (2,040 pg/ml), on day 2 and 4 after T-cell infusion (Figure 5). IL-6 levels were steadily increasing upon T-cell infusion, up to 1,000 pg/ml (Figure 5). IL-10 levels were also elevated in serum (peaked at 140 pg/ml on day 5, Figure 5), but IL-4 and TNF-α were not detectable. Cytokine levels were also measured in BAL and ascites at day 7. These samples showed an identical cytokine profile to the serum samples obtained the same day, with elevated levels of IL-6, IFN-γ, and IL-10.
Figure 5.
Cytokine profile in serum samples of patient 1. Only the data point at day -13 was measured in plasma. A previous experiment showed that plasma and serum from the same patient gave the same result. Dotted line indicates time of T-cell infusion.
Analysis of off-target reactivity
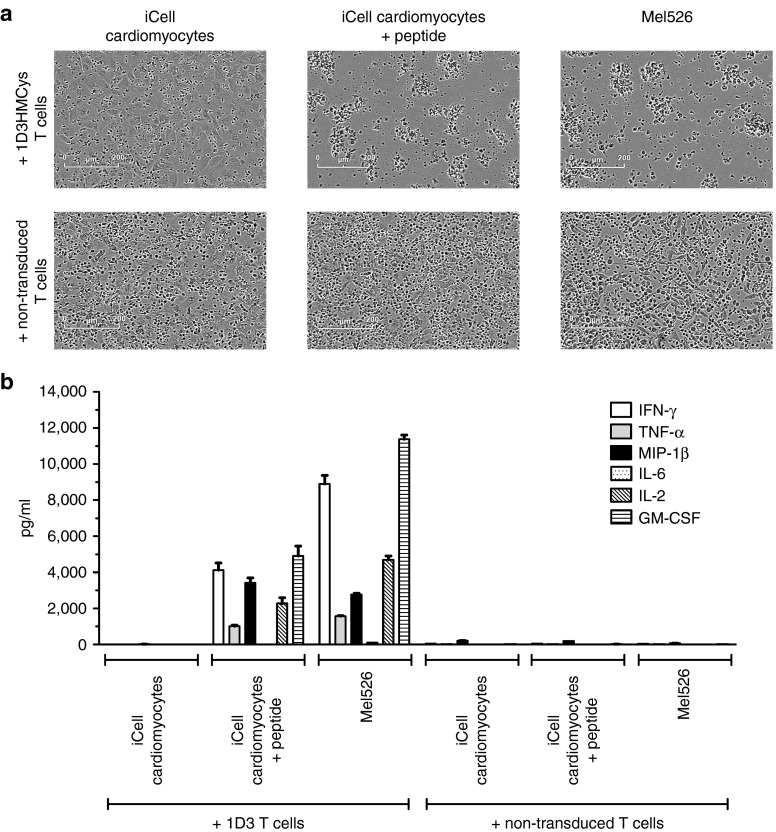
Severe cardiotoxicity was recently observed in a clinical trial testing an affinity enhanced MAGE-A3 specific TCR, and this was subsequently attributed to cross-reactivity of this TCR with a myocardial protein.9,10 To evaluate whether the 1D3HMCys TCR transduced T-cell product used here showed a similar cross reactivity, we performed cocultures of the infusion product with HLA-A*0201-positive (beating) cardiomyocytes.9 Phase contrast microscopy and cytokine analysis in the supernatant showed no recognition of cardiomyocytes by the 1D3HMCys TCR transduced T cells. In contrast, both MART-1 peptide pulsed cardiomyocyte cultures and the MART-1-positive mel526 melanoma cell line were recognized by 1D3HMCys TCR transduced cells, as measured by cell lysis and cytokine release (Figure 6).
Figure 6.
Lack of reactivity of the T-cell infusion against HLA-A2+ induced-pluripotent stem-cell (iPSC)–derived cardiomyocyte (iPSC-CM) cells, as observed by phase contrast microscopy (a) and cytokine analysis (b).
Discussion
This case report describes for the first time an unexpected fatal SAE in a stage IV melanoma patient following treatment with MART-1 TCR transduced T cells. In previous trials with TCR-modified T cells that used the same conditioning regimen, including trials that utilized TCRs directed against the same epitope,2,3,5 no such SAE have been observed.
In the MART-1-DMF5 trial, reactivity against MART-1 expressing cells in skin, eye and ear was observed, starting at 5–7 days after cell infusion. The patient described here however, showed no signs of MART-1 directed toxicity such as hearing loss, renal function and severe skin rash (although a mild skin rash was observed on the trunk). In view of the young age of the patient and the absence of a history of cardiac disease, we assume that the observed deterioration and death was treatment related. Several possible explanations can be considered for the unexpected clinical development. Below we describe the potential causes and the evidence in favor and/or against them.
Septic shock due to bacterial infection (mucositis induced by chemotherapy)
Cardiovascular dysfunction is commonly observed during septic shock and a recent SAE in a CAR gene therapy trial was attributed to a septic shock syndrome.26 Consistent with this explanation is the observation that the patient had multiple ESBL-positive E. coli cultures from feces and blood in the days before and after T-cell infusion. The observed myocarditis is consistent with the known infiltration of immune cells into the heart upon severe sepsis.27 The high levels of the inflammatory markers, procalcitonin and IL-6 and the rise in CRP in this patient are also consistent with sepsis,28 although these increases may also be explained otherwise (see below). An argument against septic shock is the observation that the levels of CRP were high during the full treatment course of this patient and may be reflective of the large disease burden rather than an ongoing bacterial infection. In addition, blood, ascites, BAL, and liquor from day 6 were negative for bacteria, virus, and fungi, and the patient was broadly covered with antibiotics from day 1 on. Therefore, we consider septic shock at day 6 a less plausible explanation for the patient's deterioration and death.
High-dose cyclophosphamide induced cardiotoxicity
High doses of cyclophosphamide, such as used in this study, have been associated with acute heart failure, although this is a very rare event.29,30 So far, the same nonmyeloablative chemotherapy has been administered to 500–700 melanoma patients in adoptive T-cell therapies, to our knowledge without any reported cardiotoxicity.31 Cyclophosphamide toxicity leads to cardiac necrosis and/or fibrinous pericarditis, none of which were revealed at postmortem examination. As such, we consider cyclophosphamide induced cardiotoxicity an unlikely explanation.
Cardiac arrest induced by semi-acute heart failure
The levels of NT-proBNP, IL-6, and CRP were already slightly elevated shortly after chemotherapy. In the week following T-cell infusion, NT-proBNP and IL-6 rose to high levels. Although IL-6, NT-proBNP, and CRP levels can be elevated during infection32 or cancer progression, these biomarkers (in particular, NT-proBNP) can also be associated with diminished cardiac contractile function.33 The days following T-cell infusion, the patient's clinical condition was still relatively stable, although she had developed severe peripheral edema and ascites, resulting in a 10 kg increase in body weight (from the day of T-cell infusion). This fluid accumulation can be caused by IL-2 and her peritoneal metastases, but could also be the result of a semi-acute heart failure. The high heart rate, enlarged heart, and extremely high levels of NT-proBNP support this hypothesis.
It is possible that the acute hemorrhage in the brain metastasis caused the observed epileptic seizure,34 which was followed by an arrhythmia and a cardiac arrest.35 Although there was clear clinical evidence for a cardiac arrest (cyanosis and absence of a palpable radial pulse), this could not be confirmed, as ECG recording were unavailable during this episode. In conclusion, semi-acute heart failure occurred, possibly secondary to myocarditis and/or inflammatory cytokines (caused by bacteremia or tumor recognition of the T cells, see below) and may have increased the susceptibility to fatal arrhythmias which have caused the cardiac arrest.
T-cell-mediated toxicity
Cross-reactivity of the MART-1-specific TCR-transduced T cells with cardiac tissue. The 1D3HMCys TCR was obtained from an HLA-A2+ individual and the TCR-pMHC binding surface was left unaltered. Because of this, cross reactivity of the 1D3HMCys TCR with an HLA-A2-restricted epitope present in cardiac tissue would appear unlikely. In line with this, no reactivity of TCR-transduced T cells was observed against an HLA-A2+ beating heart culture. Cross-reactivity of the 1D3HMCys TCR with an allogeneic MHC-peptide complex forms another explanation for the observed cardiac arrest. However, this would then have to involve a (heart) tissue-specific antigen, as no fratricide among the TCR transduced T cells was observed and no T-cell activation was noted in the absence of target cells (Figure 1). Within the TCR gene therapy trials performed thus far, toxicity due to alloreactivity has not been observed. In addition, in preclinical work, reactivity of 1D3HMCys TCR transduced T cells against a panel of cell lines expressing common human leukocyte antigen alleles was evaluated, and no evidence for alloreactivity of the TCR transduced T cells was observed.18 The observation that T cells were present within post-mortem cardiac tissue does not provide clear evidence in favor of this explanation, as the patient was resuscitated, leading to heart damage, and as T cells were also found in other organs. In addition, no cardiac myonecrosis was observed. In summary, at this point, cross-reactivity with an allogeneic MHC-peptide complex remains a possibility. However, there is no direct evidence in favor of this explanation.
Mixed dimer-dependent Graft-versus-host disease. In preclinical work, formation of mixed TCR dimers consisting of endogenous and exogenous chains has been shown to result in profound toxicity. However, several arguments make this explanation unlikely. First, two strategies to prevent mixed dimer formation were included in the design of the 1D3HMCys TCR. Furthermore, mixed dimer-dependent toxicity has not been reported to date in clinical TCR gene therapy trials, including trials that utilized fully human TCRα and TCRβ chains in which the risk of mixed dimer-dependent toxicity will be higher.36 In addition, the kinetics of the patient's deterioration were faster than was observed in mice developing mixed dimer dependent GvHD,8 and the observed symptomatology does not match GvHD. For these reasons, we believe that the formation of mixed dimers is unlikely to be the cause of the observed cardiac arrest.
Multiple organ failure due to on-target cytokine release. The cytokine profile of this patient showed a distinct pattern, including a strong peak in IFN-γ and IL-2 release shortly after T-cell infusion, which was already declining during deterioration, and a constant increase in IL-6 and IL-10 levels upon T-cell infusion. The strong peak in IFN-γ levels around day 4 post-T-cell infusion was similar to what was observed in the MART-1-DMF5 trial. However, in the patient described here, peak IFN-γ levels were markedly higher (2,000 pg/ml compared to 250–300 pg/ml in the MART-1-DMF5 trial, using similar cell numbers).3 In addition, a peak in IL-2 was observed. Since IFN-γ and IL-2 are produced by activated lymphocytes, and the patient was lymphopenic, the observed increase in IL-2 and IFN-γ levels is most likely due to the activity of the infused T cells. Nevertheless, it is remarkable that no TNF-α was observed in the circulation, since this cytokine is normally abundantly produced by transduced T cells upon tumor recognition (see Figure 1). Nevertheless, it could be that TNF-α has been internalized or is engaged to its receptor in vivo, and is therefore not detectable in the blood.
One of the most obvious difference between the MART-1-DMF5 trial and the current M11TCR trial is that a different TCR has been used (although with a similar affinity and reactivity). Furthermore, cells in the current trial are activated and isolated by anti-CD3/anti-CD28 microbeads and cultured in IL-7/IL-15 containing medium, whereas in the MART-1-DMF5 trial soluble anti-CD3 mAb and high dose IL-2 were used. These alterations may have led to a more active T-cell product,23 possibly explaining the higher peak levels of IFN-γ in our patient. In addition, the high tumor burden of our patient could have led to more pronounced T-cell activation and thereby higher IFN-γ levels.
A recent case report described a SAE upon infusion of anti-ErbB2-CAR transduced T cells. In this patient, a massive release of multiple cytokines (such as IFN-γ, TNF-α, GM-CSF, IL-6, and IL-10) was observed shortly after infusion (cytokine storm), attributed to recognition of low levels of ErbB2 on lung epithelial cells, and leading to multiorgan failure.37 In the two cases in which off-target recognition of heart cells by MAGE-A3 TCR-transduced T cells was observed, a similar ascending release profile of cytokines was observed.10 In contrast, in our patient, only the levels of IL-6 and IL-10 were increasing at the time of deterioration while the other measured cytokines (IFN-γ and IL-2) were already decreasing, which is more indicative for a cytokine release syndrome.
IL-6 can result in major organ toxicity. In a trial with recombinant IL-6, hepatotoxicity and cardiac arrhythmia were observed in two out of five patients treated with subcutaneous IL-6 injections of 30 µg/kg/day. The serum concentrations at this dose of recombinant IL-6 were comparable with the concentrations of IL-6 found in the serum of our patient,38 confirming that the IL-6 concentration reached possible toxic concentrations. Increased levels of IL-6 have been previously observed in patients treated in the MART-1/DC vaccination trial. Two patients that received an adoptive transfer of F5 TCR engineered T cells in this trial experienced respiratory distress that was attributed to elevated IL-6 levels (2,484–6,615-fold increase compared to baseline, our patient had a 300-fold increase).5 In addition, elevated IL-6 levels have been observed in patients treated with anti-CD19 CAR-transduced cells. This IL-6 induced toxicity, consisting of high fever, chills and hypotension may be counteracted by the infusion of anti-IL6R antibody (tocilizumab).15 While the source of the IL-6 in our patient is unknown, we have observed that coculture of the infused T cells of this patient with an HLA-A2+ MART-1+ melanoma cell line results in the release of IL-6. qPCR analysis showed that in this setting, the majority of IL-6 is derived from the melanoma cells (69-fold higher in absolute amount than produced by T cells, data not shown). These data suggest that the high levels of IL-6 observed in this patient could in part be released by the tumor upon T-cell recognition, but IL-6 release from other sources may also have contributed.
In conclusion, the cause of the unexpected death of our patient after infusion of MART-1 reactive T cells is probably multifactorial. Cross-reactivity with an (allogeneic) MHC-peptide complex remains a remote possibility, however, there is no direct evidence for this. We consider a cytokine release syndrome as a result of the activation of infused transduced T cells as the most likely cause of death. In addition, a major role for the previous bacteremia and/or the semi-acute heart failure in combination with epileptic seizure in the rapid deterioration of this patient appears plausible. Based on this analysis, we have amended our clinical protocol as follows:
1. Patients with a very high disease burden (>2× ULN in LDH), especially when involving pleural or peritoneal metastases giving rise to pleural effusion or ascites will not be eligible for this trial.
2. Patients with brain metastasis will not be eligible.
3. All patients (not only patients > 50 years of age) will undergo cardiac evaluation, and patients with pre-existing cardiac dysfunction will not be eligible.
4. A dose escalation design is incorporated, in which 5 × 107 TCR transduced T cells (100-fold lower than the amount infused in Patient 1) will be infused in the first cohort (n = 3), with escalation to 5 × 108 and 5 × 109 TCR transduced T cells in subsequent cohorts.
5. Serum IL-6 levels following T-cell infusion will be monitored in all patients, and infusion of anti-IL-6R antibody (tocilizumab) will be considered when IL-6 levels are above 200 pg/ml and/ or the patient shows signs of clinical deterioration.
6. In case of severe toxicity, high doses of corticosteroids in conjunction with anti-CD52 antibody will be used to eliminate T cells.
The introduction of a suicide gene was considered as an alternative way to eliminate T cells, but this would require the complete reconstruction of the retroviral vector.
The proposed amendments have been approved by the regulatory agency (CCMO) and following this medical ethical approval, two new patients have recently been treated in this trial without SAE. These patients received a dose of 5 × 107 transduced cells. In both patients, gene modified cells could be found back in the circulation for up to two months. Levels of IL-6 were monitored on a daily basis during hospitalization of these patients, and serum concentrations never exceeded 100 pg/ml. No clinical benefit was observed, although on-target toxicity against healthy, MART-1 expressing, melanocytes in the skin occurred, which led to a transient macular skin rash several days after cell infusion, later followed by the development of vitiligo in both two patients. No other toxicity was observed in these two patients.
Materials and Methods
Inclusion and clinical protocol. Patients are included in this study upon diagnosis of AJCC high-risk, inoperable stage IIIc or stage IV cutaneous melanoma, progressing after at least two lines of systemic therapy. Patients enrolled in this study have to be HLA-A*0201+ and the tumor must express MART-1. Patients undergo apheresis for retrieval of T cells, and once transduction of the obtained T cells with the 1D3HMCys TCR vector has succeeded (>10% transduction efficiency), patients undergo nonmyeloablative conditioning (cyclophosphamide: 60 mg/kg/day × 2 days i.v.; fludarabine: 25 mg/m2/day i.v. × 5 days). Following infusion of autologous transduced T cells, patients are treated with low-dose interleukin-2 (2 × 106 IU/once daily up to 2 weeks). The study was conducted according to national regulations and The Declaration of Helsinki. This study was registered under the number NL.37327.000.11.
Production of the 1D3HMCys retroviral vector. The retrovirus used to transduce peripheral T cells in this study was produced by Eufets AG (Idar-Oberstein, Germany). Sequence integrity of the viral genes was confirmed by sequence analysis of both the Master Cell Bank and the final infusion product. Presence of replication competent retrovirus was tested at several moments during production (primary seed bank, master cell bank, retroviral supernatant, and end of production cell line). No replication competent retroviruses were detected in the clinical batch 1D3 HMCys retroviral supernatant, or in the final production cell line used to produce the clinical batch retroviral supernatant.
Production of TCR transduced T cells. The 1D3HMCys TCR transduced T-cell infusion product was generated according to a validated protocol under Good Manufacturing Practice conditions. In brief, at day 0, CD3+ cells were selected from an autologous apheresis product using anti-CD3/CD28 beads (Invitrogen, Carlsbad, CA). Subsequently, CD3+ cells were resuspended in medium (50%/50% Roswell Park Memorial Institute/AIM-V medium (Lonza, Verviers, Belgium), 5% HS (Sanquin Blood Supply, Amsterdam, The Netherlands)), containing IL-7 and IL-15 (5 ng/ml each, CellGenix, Freiburg, Germany) for 48 hours. At days 2 and 3, activated CD3+ cells were transduced with the 1D3HMCys retroviral vector (Eufets, Idar-Oberstein, Germany) using Retronectin (Takara, Tokyo, Japan) coated plates. After the second transduction, cells were collected from the plates and transferred to LifeCell culture bags containing fresh medium with IL-7 and IL-15 to allow expansion, and cells were split regularly. At day 13, aCD3/aCD28 beads were removed by magnetic separation and cells were washed and concentrated for reinfusion.25
Patient pathologic analysis and immunohistochemistry. Formalin-fixed paraffin-embedded tissues from the brain biopsy and the autopsy were sectioned at 4 µm and stained with hematoxylin and eosin. Immunohistochemistry was performed on serial sections using a Ventana automated immunostainer, according to the manufacturer's protocol (Ventana Medical Systems, Tuscon, AZ), using a panel of antibodies directed against CD3 (clone SP7, Neomarkers, Fremont, CA), CD4 (clone SP35, Cell Marque, Rocklin, CA), CD8 (clone C8/144B, Dako, Heverlee, Belgium), granzyme B (clone GrB-7, Monosan, Uden, The Netherlands), PD-1 (clone NAT, Abcam, Cambridge, MA) and MelanA/anti-MART1 (clone A103, Dako). Anti-TCR Vβ 14 (clone 1557, Immunotech, Woerden, The Netherlands) was used for immunohistochemistry on frozen sections.
Cytokine measurements. Serum samples were stored at −80 °C before analysis. Part of the samples were stored at 4 °C before transfer to −80 °C for logistic reasons, but this did not influence the measured cytokine levels, as evaluated in a direct comparison (data not shown). Levels of IL-2/IL-4/IL-6/IL-10/IFN-γ, and TNF-α were measured with the Cytometric Bead Array Human Th1/Th2 Cytokine Kit II (BD Biosciences, San Jose, CA), according to the manufacturer's protocol.
Flow cytometric analysis. Transduced T cells were stained with MART-1(26–35 A>L) HLA-A2 tetramers,39 anti-CD3 (clone SK7) and anti-CD8 (clone SK1) conjugated antibodies (BD Biosciences). For phenotypic analysis, cells were stained with antibodies directed against CD8 (clone SK1), CD45RA (clone HI100), CD45RO (clone UCHL1), CD27 (clone L128), CD28 (clone CD28.2), CD62L (clone DREG-56), and CCR7 (clone 3D12) (all BD Biosciences), Cells were analyzed on a FACSCalibur or Fortessa.
Intracellular cytokine release assay. 2 × 105 Mel624 cells and 2 × 105 transduced T cells were cultured in the presence of 1 μl/ml Golgiplug (BD Biosciences). After 15 hours of incubation, cells were washed and stained with antibodies directed against mouse TCR β chain (clone H57-597), CD3 (clone SK7), IL-2 (clone MQ1-17H12), IFN-γ (clone B27), and TNF-α (clone Mab 11) (all BD Biosciences) and analyzed on a FACSCalibur or Fortessa.
Beating cardiac myocyte iPSC-CM assay. Induced-pluripotent stem-cell (iPSC)–derived cardiomyocyte (iPSC-CM) cells were thawed from liquid nitrogen storage and treated as per the manufacturer's instructions. The cells were HLA-A*0201 positive. iPSC-CM and control target cells were plated at 5 × 104 per well of a 96-well plate and incubated for 24 hours with nontransduced or 1D3HMcys-transduced T cells at a ratio of 1:1. Phase contrast images were captured using an IncuCyte FLR (Essen BioScience, Ann Arbor, MI). Quantification of cytokines (IFN-γ, IL-2, TNF-α, MIP-1β, GM-CSF, and IL-6) release in cell supernatants was performed by Luminex bead array technology (Life Technologies, Carlsbad, CA). Samples were processed in triplicate, and assays were performed as per the manufacturer's instructions.
SUPPLEMENTARY MATERIAL Figure S1. Intracellular cytokine production of TCR 1D3HMCys-transduced T-cells using two different ativation/stimulation protocols. Figure S2. Transduced T cells have a wide TCR repertoire and are polyclonal. Figure S3. Cytokine production of patient derived TCR modified T cells.
Acknowledgments
We are grateful to T. de Jong and C. Bierman for their assistance with immunohistochemistry and to H. Niessen (Department of Pathology, VUMC, Amsterdam) for consultation with regard to myocard pathology.
Supplementary Material
References
- Rosenberg, SA, Yang, JC, Sherry, RM, Kammula, US, Hughes, MS, Phan, GQ et al. (2011). Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17: 4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, RA, Dudley, ME, Wunderlich, JR, Hughes, MS, Yang, JC, Sherry, RM et al. (2006). Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314: 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, LA, Morgan, RA, Dudley, ME, Cassard, L, Yang, JC, Hughes, MS et al. (2009). Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, PF, Morgan, RA, Feldman, SA, Yang, JC, Sherry, RM, Dudley, ME et al. (2011). Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 29: 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodon, T, Comin-Anduix, B, Chmielowski, B, Koya, RC, Wu, Z, Auerbach, M et al. (2014). Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin Cancer Res 20: 2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst, MR, Yang, JC, Langan, RC, Dudley, ME, Nathan, DA, Feldman, SA et al. (2011). T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 19: 620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, RA, Chinnasamy, N, Abate-Daga, D, Gros, A, Robbins, PF, Zheng, Z et al. (2013). Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 36: 133–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendle, GM, Linnemann, C, Hooijkaas, AI, Bies, L, de Witte, MA, Jorritsma, A et al. (2010). Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med 16: 565–70, 1p following 570. [DOI] [PubMed] [Google Scholar]
- Cameron, BJ, Gerry, AB, Dukes, J, Harper, JV, Kannan, V, Bianchi, FC et al. (2013). Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med 5: 197ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linette, GP, Stadtmauer, EA, Maus, MV, Rapoport, AP, Levine, BL, Emery, L et al. (2013). Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122: 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, DL, Levine, BL, Kalos, M, Bagg, A and June, CH (2011). Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos, M, Levine, BL, Porter, DL, Katz, S, Grupp, SA, Bagg, A et al. (2011). T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 3: 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer, JN, Dudley, ME, Feldman, SA, Wilson, WH, Spaner, DE, Maric, I et al. (2012). B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119: 2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp, SA, Kalos, M, Barrett, D, Aplenc, R, Porter, DL, Rheingold, SR et al. (2013). Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila, ML, Riviere, I, Wang, X, Bartido, S, Park, J, Curran, K et al. (2014). Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6: 224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valmori, D, Dutoit, V, Schnuriger, V, Quiquerez, AL, Pittet, MJ, Guillaume, P et al. (2002). Vaccination with a Melan-A peptide selects an oligoclonal T cell population with increased functional avidity and tumor reactivity. J Immunol 168: 4231–4240. [DOI] [PubMed] [Google Scholar]
- Dietrich, PY, Le Gal, FA, Dutoit, V, Pittet, MJ, Trautman, L, Zippelius, A et al. (2003). Prevalent role of TCR alpha-chain in the selection of the preimmune repertoire specific for a human tumor-associated self-antigen. J Immunol 170: 5103–5109. [DOI] [PubMed] [Google Scholar]
- Jorritsma, A, Gomez-Eerland, R, Dokter, M, van de Kasteele, W, Zoet, YM, Doxiadis, II et al. (2007). Selecting highly affine and well-expressed TCRs for gene therapy of melanoma. Blood 110: 3564–3572. [DOI] [PubMed] [Google Scholar]
- Cohen, CJ, Zhao, Y, Zheng, Z, Rosenberg, SA and Morgan, RA (2006). Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res 66: 8878–8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuball, J, Dossett, ML, Wolfl, M, Ho, WY, Voss, RH, Fowler, C et al. (2007). Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood 109: 2331–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, CJ, Li, YF, El-Gamil, M, Robbins, PF, Rosenberg, SA and Morgan, RA (2007). Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res 67: 3898–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak, AL, Workman, CJ, Wang, Y, Vignali, KM, Dilioglou, S, Vanin, EF et al. (2004). Correction of multi-gene deficiency in vivo using a single ‘self-cleaving' 2A peptide-based retroviral vector. Nat Biotechnol 22: 589–594. [DOI] [PubMed] [Google Scholar]
- Kaneko, S, Mastaglio, S, Bondanza, A, Ponzoni, M, Sanvito, F, Aldrighetti, L et al. (2009). IL-7 and IL-15 allow the generation of suicide gene-modified alloreactive self-renewing central memory human T lymphocytes. Blood 113: 1006–1015. [DOI] [PubMed] [Google Scholar]
- Kaiser, AD, Gadiot, J, Guislain, A and Blank, CU (2013). Mimicking homeostatic proliferation in vitro generates T cells with high anti-tumor function in non-lymphopenic hosts. Cancer Immunol Immunother 62: 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Eerland, R, Nuijen, B, Heemskerk, B, van Rooij, N, van den Berg, JH, Beijnen, JH et al. (2014). Manufacture of gene-modified human T-cells with a memory stem/central memory phenotype. Hum Gene Ther Methods 25: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens, R, Yeh, R, Bernal, Y, Riviere, I and Sadelain, M (2010). Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther 18: 666–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeding, L, Plötz, FB, Groeneveld, AB and Kneyber, MC (2012). Structural changes of the heart during severe sepsis or septic shock. Shock 37: 449–456. [DOI] [PubMed] [Google Scholar]
- Patil, VK, Morjaria, JB, De Villers, F and Babu, SK (2012). Associations between procalcitonin and markers of bacterial sepsis. Medicina (Kaunas) 48: 383–387. [PubMed] [Google Scholar]
- Morandi, P, Ruffini, PA, Benvenuto, GM, Raimondi, R and Fosser, V (2005). Cardiac toxicity of high-dose chemotherapy. Bone Marrow Transplant 35: 323–334. [DOI] [PubMed] [Google Scholar]
- de Jonge, ME, Huitema, AD, Rodenhuis, S and Beijnen, JH (2005). Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 44: 1135–1164. [DOI] [PubMed] [Google Scholar]
- Rosenberg, SA and Dudley, ME (2009). Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol 21: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López, L, Arai, K, Giménez, E, Jiménez, M, Pascuzo, C, Rodríguez-Bonfante, C et al. (2006). [C-reactive protein and interleukin-6 serum levels increase as Chagas disease progresses towards cardiac failure]. Rev Esp Cardiol 59: 50–56. [PubMed] [Google Scholar]
- Varpula, M, Pulkki, K, Karlsson, S, Ruokonen, E and Pettilä, V; FINNSEPSIS Study Group (2007). Predictive value of N-terminal pro-brain natriuretic peptide in severe sepsis and septic shock. Crit Care Med 35: 1277–1283. [DOI] [PubMed] [Google Scholar]
- De Herdt, V, Dumont, F, Hénon, H, Derambure, P, Vonck, K, Leys, D et al. (2011). Early seizures in intracerebral hemorrhage: incidence, associated factors, and outcome. Neurology 77: 1794–1800. [DOI] [PubMed] [Google Scholar]
- McLean, BN and Wimalaratna, S (2007). Sudden death in epilepsy recorded in ambulatory EEG. J Neurol Neurosurg Psychiatr 78: 1395–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, SA (2010). Of mice, not men: no evidence for graft-versus-host disease in humans receiving T-cell receptor-transduced autologous T cells. Mol Ther 18: 1744–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, RA, Yang, JC, Kitano, M, Dudley, ME, Laurencot, CM and Rosenberg, SA (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18: 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, J, Yang, JC, Topalian, SL, Parkinson, DR, Schwartzentruber, DS, Ettinghausen, SE et al. (1993). Phase I trial of subcutaneous interleukin-6 in patients with advanced malignancies. J Clin Oncol 11: 499–506. [DOI] [PubMed] [Google Scholar]
- Toebes, M, Coccoris, M, Bins, A, Rodenko, B, Gomez, R, Nieuwkoop, NJ et al. (2006). Design and use of conditional MHC class I ligands. Nat Med 12: 246–251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.