Abstract
Gene transfer into hCD34+ hematopoietic stem/progenitor cells (HSCs) using human immunodeficiency virus type 1 (HIV-1)-based lentiviral vectors (LVs) has several promising therapeutic applications. Yet, efficiency, safety, and cost of LV gene therapy could be ameliorated by enhancing target cell transduction levels and reducing the amount of LV used on the cells. Several transduction enhancers already exist such as fibronectin fragments and cationic compounds, but all present limitations. In this study, we describe a new transduction enhancer called Vectofusin-1, which is a short cationic peptide, active on several LV pseudotypes. Vectofusin-1 is used as a soluble additive to safely increase the frequency of transduced HSCs and to augment the level of transduction to one or two copies of vector per cell in a vector dose-dependent manner. Vectofusin-1 acts at the entry step by promoting the adhesion and the fusion between viral and cellular membranes. Vectofusin-1 is therefore a promising additive that could significantly ameliorate hCD34+ cell-based gene therapy.
Keywords: cationic peptide, gene therapy, hematopoietic stem cell, lentiviral vector
Introduction
Human immunodeficiency virus type 1 (HIV-1)-based lentiviral vectors (LVs) are attractive delivery tools for gene therapy applications. These vectors are currently used in clinical applications to treat various diseases such as immune deficiencies, neurological diseases, cancers, anemias, and HIV infection.1,2
Numerous LV applications rely on ex vivo transduction of hematopoietic stem/progenitor cells (HSCs) expressing the hCD34 marker. Transduction of HSCs by LVs is initiated by binding of the viral envelope glycoprotein (GP) to cell surface receptors. Among the first and still most widely used GPs for pseudotyping LVs is vesicular stomatitis virus-G GP (VSV-G), which has broad tropism and generates stable particles that can be purified and cryopreserved.3,4 LVs can also be efficiently pseudotyped with other GPs harboring efficient hematopoietic tropism, such as amphotropic murine leukemia virus (MLV) GP, modified feline endogenous retrovirus RD114 GP (RD114TR), and modified gibbon ape leukemia virus GP (GALVTR).5 The capacity of pseudotyped LVs to interact with HSCs is thought to be a limiting factor that depends on the envelope GP used and the relative paucity of viral receptors. One strategy to optimize the binding and entry steps of LVs is the addition of cofactors during the transduction procedure. For laboratory research purposes, various culture additives can be used, such as cationic polymers (e.g., polybrene, refs. 6,7 and DEAE-Dextran, ref. 8), cationic lipids (e.g., lipofectin, lipofectamine, refs. 9,10), and cationic peptides (e.g., protamine sulfate, ref. 6 and human semen enhancer of viral infection (SEVI), ref. 11). The mechanism of action of these cationic additives is mainly based on their abilities to neutralize membrane charges and to promote virus aggregation.12,13 For clinical applications, transduction protocols include the fibronectin fragment FN CH-296 (also called Retronectin).14,15,16 Retronectin enhances transduction by facilitating the colocalization of viruses and cells. This peptide is essential to promote the infectivity of GALVTR-LV and RD114TR-LV, particularly with hCD34+ cells,5 but is less efficient with VSV-G-LV.5,17 Despite the limited effect of Retronectin to enhance infection of hCD34+ cells with VSV-G-LVs, this reagent continues to be the lead compound additive that is used in clinical gene therapy protocols. However, use of Retronectin is surface-based which is cumbersome both practically and for precise dosage of the additive relative to the vector and cell product-specific activity. Therefore, identification of new, soluble, easy to manipulate additives capable of enhancing the infectivity of a broad spectrum of LV pseudotypes including VSV-G-LVs is needed to provide an alternative to existing additives such as Retronectin.
Because the cationic property appears crucial for many enhancers of retroviral infectivity, we focused our attention on Vectofusin-1, a new histidine-rich cationic amphipathic peptide derived from the LAH4 peptide family.18,19 LAH4 peptides were previously used as an antimicrobial agent and also as efficient transfection agents for DNA and small-interfering RNA.20,21,22,23,24 LAH4 derivatives have been proposed to optimize nonviral gene transfer approaches,25 but have never been tested in the context of gene transfer strategies relying on enveloped viruses.
For the first time, we show that Vectofusin-1 was able to promote HSC transduction with every lentiviral pseudotype tested, including highly purified VSV-G-LVs. There were no deleterious effects of Vectofusin-1 on HSCs as tested by human immune system (HIS) reconstitution in the BALB-Rag/γC–immunodeficient mouse model. Finally, the adaptation of the widely used HIV-1 fusion assay26,27 to study the entry of LVs into target cells revealed that the enhancement of HSC transduction with LVs in the presence of Vectofusin-1 resulted from an increase in the adhesion and the fusion steps of LVs with the cellular membranes of HSCs.
Results
Enhanced lentiviral transduction of HSC in the presence of the Vectofusin-1 peptide
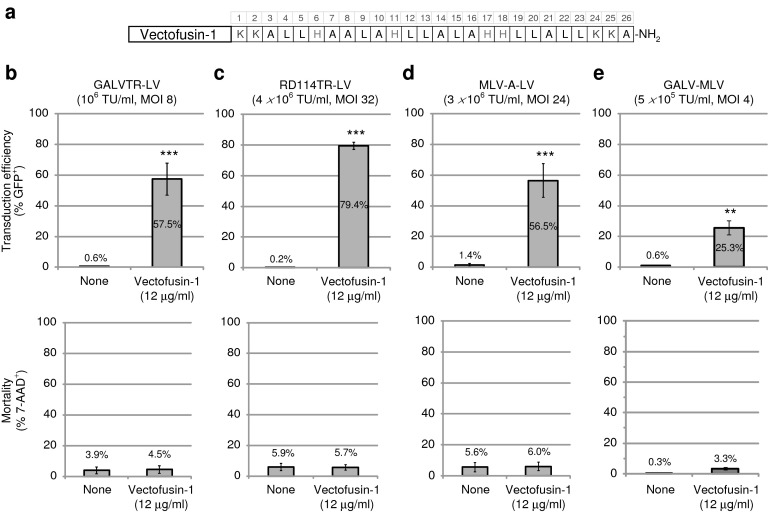
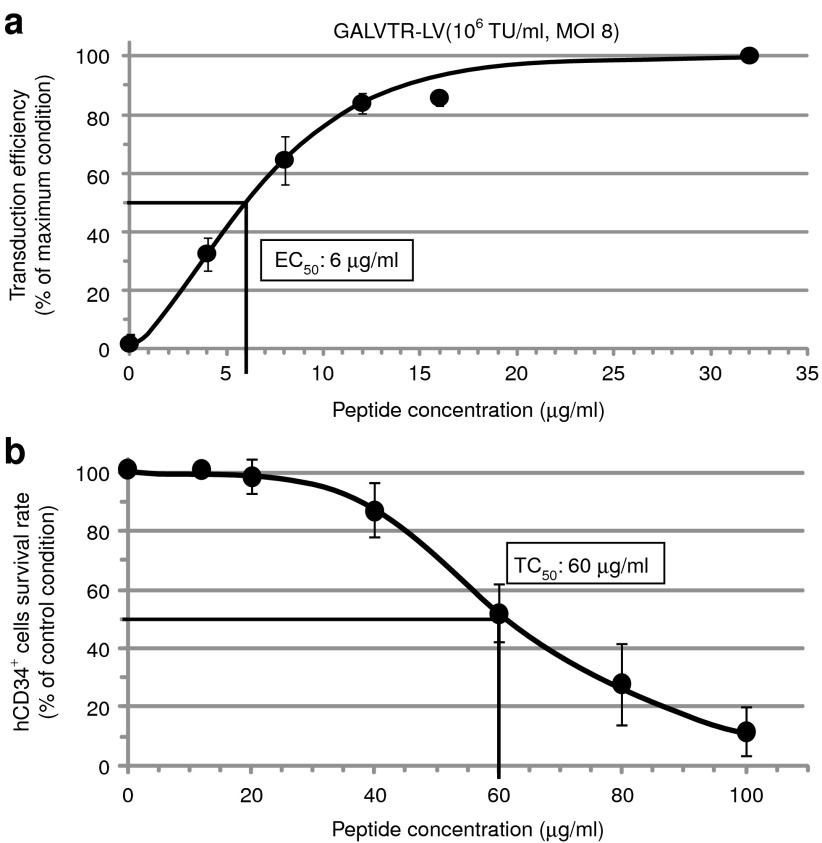
Vectofusin-1 is a 26-mer cationic amphipathic peptide identified in our laboratory and derived from the LAH4 peptide family18,19 (Figure 1a). Vectofusin-1 enhances the transduction of hCD34+ HSCs obtained from umbilical cord blood (UCB) as shown with several LV pseudotypes (Figure 1b–d). Vectofusin-1 enhances transduction with a green fluorescent protein (GFP)-encoding LV pseudotyped with the GALVTR, RD114TR or amphotropic MLV GPs, known to promote receptor-mediated entry into human hematopoietic cells.5,28,29 With unprocessed bulk harvest supernatant titering as low as 106 transducing units/ml, the transduction levels reached beyond 50% when Vectofusin-1 was added to the transduction medium, whereas transduction was nearly undetectable in the absence of this additive (Figure 1b–d). Notably, Vectofusin-1 reproducibly increased transduction levels with cells from several UCB donors and with no apparent cytotoxicity. The enhancing effects of Vectofusin-1 were also observed with Moloney MLV γ-retroviral vectors pseudotyped with GALV (GALV-MLV), showing that Vectofusin-1 activity is not restricted to the lentiviral genus (Figure 1e). Vectofusin-1 promotes GALVTR-LV infectivity of cord blood-derived CD34+ cells, with a half maximal efficient concentration (EC50) of ~6 µg/ml (Figure 2a) and showed a half maximal toxic concentration (TC50) of ~60 µg/ml (Figure 2b). Here, the TC50 of Vectofusin-1 was evaluated after overnight incubations with hCD34+ cells rather than 6 hours, for a more stringent assessment of toxicity. Hence, the Vectofusin-1 TC50/EC50 ratio represents a one log safety index which permits a safe and practical use of this reagent to transduce human CD34+ cells with various retroviral pseudotypes.
Figure 1.
Vectofusin-1 enhances CD34+ hematopoietic stem/progenitor cells transduction with various lentiviral or retroviral pseudotypes. (a) Schematic representation of the primary sequence of Vectofusin-1 peptide composed of four different types of residue: lysine (K), histidine (H), leucine (L), and alanine (A); carboxy-terminal amidation (-NH2). (b–e) A variety of green fluorescent protein (GFP)-encoding vectors (GALVTR-LVs (n = 5), RD114TR-LVs (n = 3), MLV-A-LVs (n = 3), GALV-MLV (n = 3)) were used to transduce hCD34+ cells during 6 hours in the absence (none) or presence of Vectofusin-1 (12 µg/ml). Data are shown as the average percentage of GFP+ or 7-AAD+ cells ± SD from number of umbilical cord blood samples treated in duplicate (**P < 0.01; ***P < 0.001, Student's t-test). 7-AAD, 7-aminoactinomycin D; GALV, gibbon ape leukemia virus; LV, lentiviral vector; MLV-A, amphotropic murine leukemia virus; MOI, multiplicity of infection; TU, transducing unit.
Figure 2.
Determination of the half maximal efficient (EC50) and toxic (TC50) concentrations of Vectofusin-1. (a) hCD34+ cells were infected with GALVTR-LVs in the absence or presence of various concentrations of Vectofusin-1. Transduction efficiencies (percentage of GFP+ cells) were obtained 5 days post-transduction (n = 4). Data are normalized to the maximum effect observed ± SD (average maximal value of transduction was 67%). (b) Evaluation of the TC50 of Vectofusin-1. The hCD34+ cells were incubated overnight with the indicated amounts of Vectofusin-1 (n = 6). The survival rate was estimated by counting the number of living cells using the Trypan blue exclusion method under light microscopy. Data are normalized to the control condition ± SD (average value of survival rate in the absence of peptides was 98.9%). GALV, gibbon ape leukemia virus; LV, lentiviral vector; MOI, multiplicity of infection; TU, transducing unit.
Vectofusin-1 augments CD34+ HSC transduction with highly purified VSV-G-LV
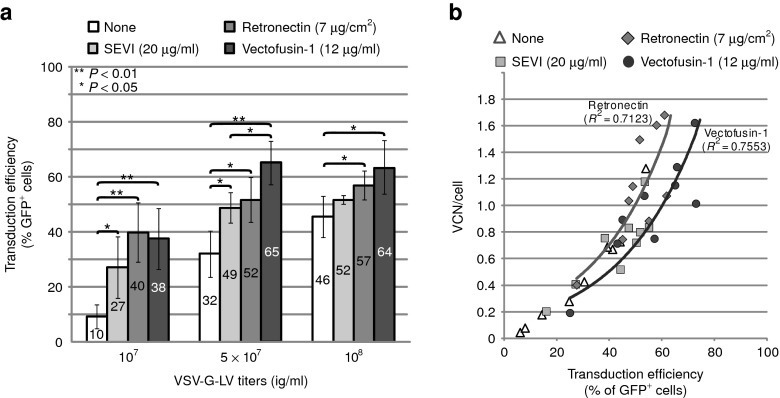
Most of the current clinical cell and gene therapy applications involving CD34+ HSCs use the pantropic VSV-G-LV pseudotype which enables robust virus purification protocols.4 However, the titer and infectivity of chromatographically purified vectors for HSCs can be low, requiring the help of transduction additives. Accordingly, the effects of Vectofusin-1 were compared with those of widely used additives: Retronectin, and also SEVI, a natural cationic peptide expressed in human semen and characterized as a strong enhancer of viral and vector infection.11,30 A purified GFP-expressing VSV-G-LV was obtained following a series of membrane and chromatography steps as described for the production of large-scale clinical grade LVs4 used to infect hCD34+ cells. As shown in Figure 3a, the different culture additives all enhanced transduction levels at each dose of vector tested. The enhancing effects were most prominent when suboptimal concentrations of vector were used, but became less evident as vector concentration increased because basal levels of transduction without additive also increased. Vectofusin-1 was more efficient than SEVI at the same optimal molar concentration (4.3 µmol/l) in enhancing the effects of high concentrations of vector. Vectofusin-1 was as efficient as Retronectin in promoting the transduction level of hCD34+ cells, and gave more reproducible results (as shown by a lower P value comparing effects versus “none” with 5 × 107 infectious genome (ig)/ml vector). Most importantly, the average vector copy number (VCN) per cell obtained in the presence of Vectofusin-1 ranged only between 1 and 2 for transduction levels reaching 60–80% of cells (Figure 3b), whereas the same range of VCN was observed when 45–65% of hCD34+ cells were transduced in the presence of Retronectin. These results illustrate the ability of Vectofusin-1 to transduce a high percentage of target cells in the bulk population, with a safe level of VCN (Figure 3b). Similarly, with other lentiviral pseudotypes, transduction efficiencies of hCD34+ cells in presence of Vectofusin-1 were able to reach 80% with VCN below 2.5 (Supplementary Figure S1). Thus, Vectofusin-1 safely enhanced the frequency of HSC transduction consistent with its predicted VCN value. In addition, we have shown that the effect of Vectofusin-1 is not restricted to hCD34+ cells isolated from UCBs. Indeed, using hCD34+ cells isolated from granulocyte colony-stimulating factor–mobilized peripheral blood (MPB), we observed a twofold increase of the transduction level in presence of Vectofusin-1 (Supplementary Figure S2). The slightly lower transduction efficiency that we observed for MPB-derived HSCs compared with UCB was previously described in the presence of Retronectin.31
Figure 3.
Comparison of Vectofusin-1 with other culture additives to promote transduction of hCD34+ cells with purified vesicular stomatitis virus-G-lentiviral vectors (VSV-G-LVs). hCD34+ cells (three umbilical cord blood donors tested in three independent experiments) were infected with increasing concentrations of purified VSV-G-LVs (107, 5 × 107, and 108 ig/ml corresponding to multiplicity of infection (MOI) 80, 400, and 800, respectively) in the absence (none) or presence of indicated transduction enhancers. (a) Transduction was measured in the bulk of cultured cells after 5 days by following the percentage of GFP+ cells ± SD using flow cytometry (**P < 0.01; *P < 0.05, Student's t-test) and (b) average vector copy number (VCN) of the cell population by quantitative PCR. GFP, green fluorescent protein; ig, infectious genome; SEVI, semen enhancer of viral infection.
Lack of hematopoietic toxicity of Vectofusin-1
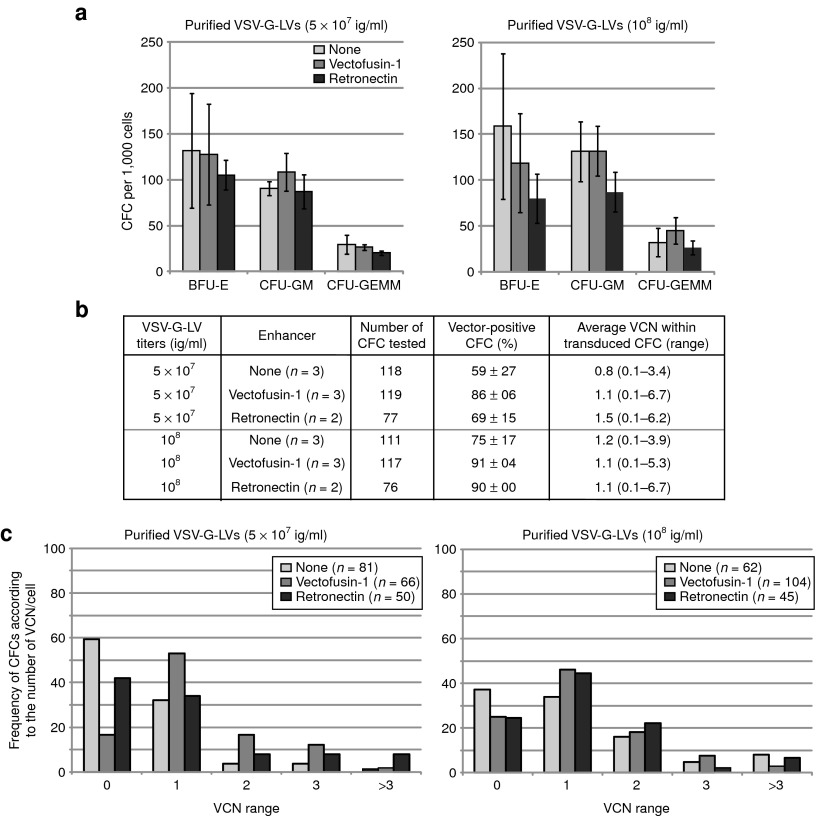
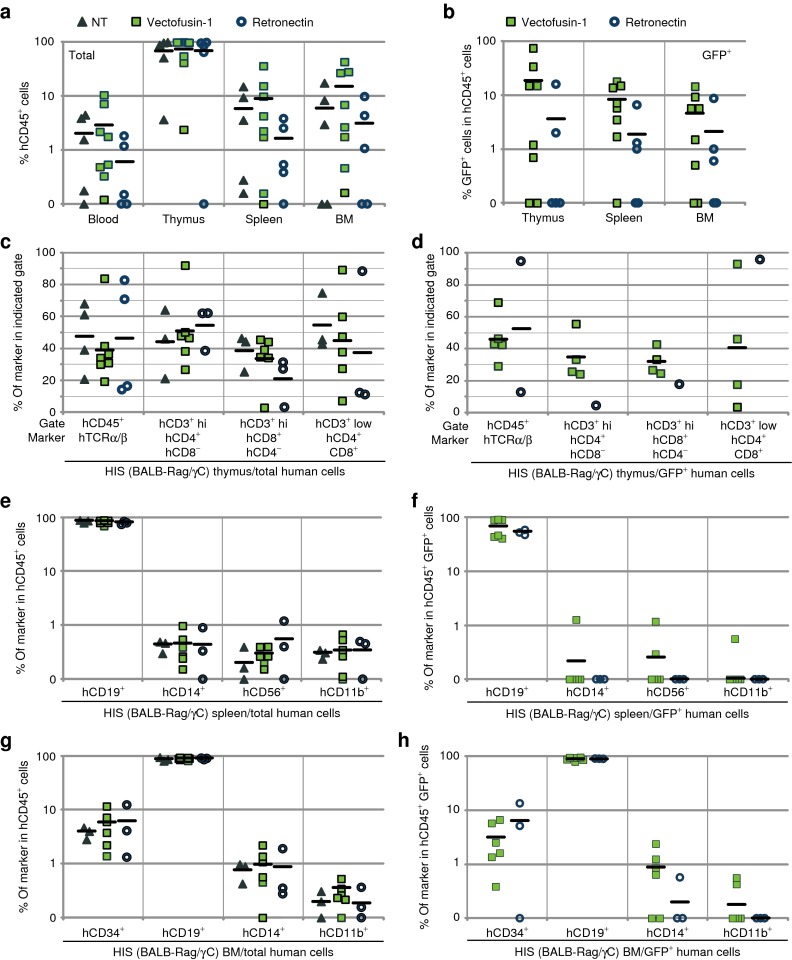
Hematopoietic toxicity was first evaluated in vitro by analyzing colony-forming cell (CFC) generated from CD34+ cells exposed to optimal concentrations of Vectofusin-1 during transduction. For these experiments, we used highly purified VSV-G-LV to transduce CD34+ cells because these vectors exhibit no measurable hematopoietic toxicity in this assay.4 There was no evidence of toxicity from Vectofusin-1 either. Exposure of CD34+ cells to Vectofusin-1 did not affect their subsequent growth as CFC and myeloerythroid differentiation (Figure 4a). Also, we did not observe any differences in the type and appearance of the CFCs transduced in the presence of Vectofusin-1 (data not shown). Genomic DNA extracted from single colonies was tested by Taqman real-time PCR (quantitative PCR) to evaluate transduction and VCNs at the clonal level.32 Up to 91% of individual CFCs were transduced in the presence of Vectofusin-1 (Figure 4b). Average VCN per CFC, and distribution of the number of VCN per CFC were comparable with the use of Vectofusin-1 or Retronectin to enhance the transduction of the initial hCD34+ cell population (Figure 4c). To further investigate the potential of Vectofusin-1 in the perspective of clinical applications, we evaluated whether this compound has hematopoietic toxicity in vivo by measuring the engraftment of Vectofusin-1–treated CD34+ cells in BALB-Rag/γC–immunodeficient mice. The effects of Vectofusin-1 were compared with those of the leading clinical additive Retronectin. To facilitate this comparison, UCB hCD34+ cells were transduced with GALVTR-LVs in the presence of optimal concentrations of Vectofusin-1 (12 µg/ml) or Retronectin (20 µg/cm2) and the transduced cells were injected into the liver of irradiated BALB-Rag/γC newborn mice to obtain a human immune lymphomyeloid system (HIS model) in the mouse as described previously.33 Twelve weeks after injection, equivalent numbers of hCD45+ cells were detected in PB, thymus, spleen, and bone marrow (BM) of mice, whether or not the cells had been transduced in presence of Vectofusin-1, Retronectin, or not transduced (Figure 5a). The engraftment of transduced GFP+ hCD45+ cells was at least as good when Vectofusin-1 was used compared with Retronectin (Figure 5b), and we confirmed that transduction levels of the bulk hCD34+ cell population were comparable (Supplementary Figure S3a). All observed mice exhibited active human thymopoiesis as evidenced by high percentages of immature CD3-low double-positive hCD4/hCD8 cells in the thymus and with an expected distribution of mature CD3-high single-positive hCD4 and hCD8 T cells (Figure 5c,d and Supplementary Figure S4a). These populations were efficiently transduced in the presence of Vectofusin-1 (Figure 5d). The spleen contained a large population of hCD19+ cells, indicating an active human B lymphoid development which was also transduced (Figure 5e,f and Supplementary Figure S4b). Human HSCs (hCD34+ hCD45+ cells) were also detectable in the BM and transduced in the presence of Vectofusin-1, as well as myeloid cells such as monocytes (hCD14+ cells) and granulocytes (hCD11b+ cells) (Figure 5g,h and Supplementary Figure S4c). We observed comparable potency of Vectofusin-1 and Retronectin in the capacity to promote the transduction of hCD34+ HSC cells engrafted in the BM, even though this conclusion is based on low numbers of transduced cells, around 2% of the engrafted CD34+ cells, in each condition tested (Supplementary Figure S3b). Such low level of transduction can be explained by suboptimal experimental conditions with the use of a single hit (6 hours) of unconcentrated GALVTR-LV in a murine model best known for efficient human thymic reconstitution than human HSC repopulation in BM (Figure 5a). In summary, Vectofusin-1 had no deleterious effects on the transduction, viability, differentiation or sustained engraftment of HSCs and HIS reconstitution. The encouraging safety profile of Vectofusin-1 indicates that it could potentially be used to enhance the ex vivo transduction of HSCs in clinical gene therapy applications.
Figure 4.
Lack of in vitro hematopoietic toxicity of Vectofusin-1. (a) Differentiation of transduced hCD34+ cells in colony-forming cell (CFC) assays. Results represent the average number of different types of colonies obtained for 1,000 cells plated after transduction with 5 × 107 and 108 ig/ml (conditions of Figure 3). (b) Transduction was measured in individual CFCs obtained after 2 weeks of culture in methylcellulose. Transduction of CFCs was measured by determining the percentage of GFP+ CFCs using epifluorescence microscopy (“vector-positive CFC (%)”), and the vector copy number (VCN) in each CFC by quantitative PCR. The average VCN per vector-positive CFC and range are listed (right column). (c) Bars represent the percentage of CFCs in each category (VCN range) over the total number of CFCs analyzed. The number of CFCs analyzed is indicated between brackets for each group. BFU-E, burst-forming unit, erythroid; CFU-GEMM, colony-forming unit, granulocyte, erythrocyte, macrophage, megakaryocyte; CFU-GM, colony-forming unit, granulocyte-monocyte; GFP, green fluorescent protein; ig, infectious genome; VSV-G-LV, vesicular stomatitis virus-G-lentiviral vector.
Figure 5.
Evaluation of the safety of Vectofusin-1 in the immunodeficient BALB-Rag/γC mouse model. hCD34+ cells were transduced for 6 hours with GALVTR-LVs either in the presence of 12 µg/ml of Vectofusin-1 (squares) or 20 µg/cm2 of Retronectin (circle) and injected into the liver of newborn mice. Controls (NT) included non-infected cells not exposed to any transduction enhancer (triangle). (a,b) Eleven to thirteen weeks post-injection, the engraftment of human transduced cells into HIS (BALB-Rag/γC) mice was measured by flow cytometry in the peripheral blood, the thymus, the spleen, and the bone marrow (BM) using anti-hCD45 antibodies and green fluorescent protein (GFP) expression levels. (c,d) Human T lymphopoiesis was measured in the thymus by monitoring human TCRα/β in the hCD45+ gate, and CD4 and CD8 marker expression in CD3+ low or high (hi) gates. Human B lymphoid development, monocytes, natural killer cells, granulocytes, and hematopoietic progenitors were determined in the (e,f) spleen or the (g,h) BM by monitoring, respectively, the human CD19, CD14, CD56, CD11b, and CD34 surface markers in the hCD45+ gate. GALV, gibbon ape leukemia virus; HIS, human immune system; LV, lentiviral vector; NT, not transduced; TCRα/β, T-cell receptor α/β.
Vectofusin-1 promotes both the adhesion and the fusion steps of LVs with the plasma membrane of CD34+ cells
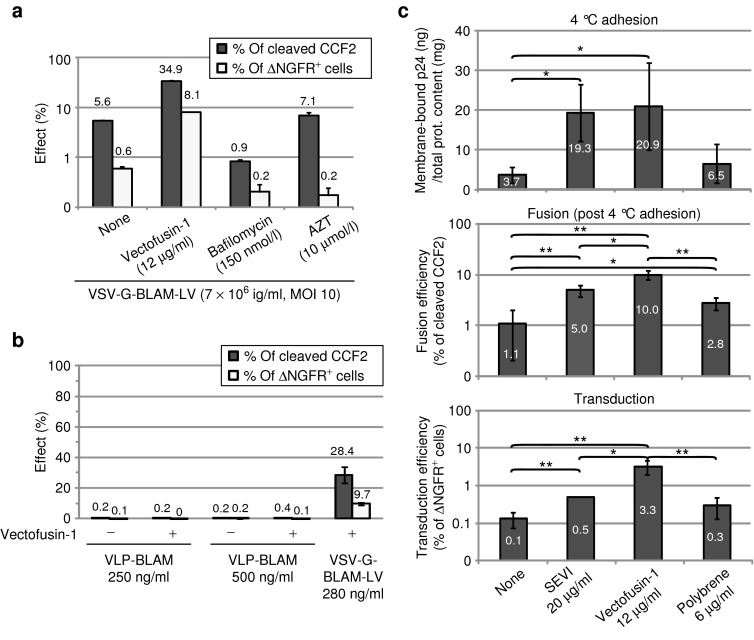
At a pH above 7, peptides that belong to the LAH4 family adopt a transmembrane orientation in lipid bilayers.34 The transduction of CD34+ cells is performed in buffered culture medium with a neutral pH. Therefore, we hypothesized that during transduction, Vectofusin-1 is able to insert itself in the viral and/or cellular membranes, leading to a modulation of the adhesion and the fusion steps of LVs with the membrane of CD34+ cells. To investigate this possibility, we adapted a fluorescence resonance energy transfer-based fusion assay, initially designed for replicative HIV-1 virions,26,27 to study nonreplicative LVs. The assay utilized recombinant LVs containing β-lactamase (BLAM)-Vpr chimeric proteins (BLAM-LVs) to measure fusion with target cells via viral incorporation and delivery of BLAM-Vpr into the cytoplasm.26 Concomitantly, the transduction efficiency was monitored by following ΔNGFR marker expression. As shown in Figure 6a, Vectofusin-1 strongly enhanced VSV-G-LV fusion with target cell membranes and consequently enhanced transduction efficiency. VSV-G-LVs fusion is specifically blocked using the endosome acidification inhibitor bafilomycin A1, whereas the azidothymidine, a reverse transcription inhibitor, is acting only on post-fusion events as expected. BLAM-VLPs, corresponding to BLAM-LVs produced in the absence of any envelope GP were unable to fuse with the plasma membrane, even in the presence of Vectofusin-1 (Figure 6b). These results suggest that Vectofusin-1 enhanced a receptor-mediated entry pathway rather than a simple induction of liposome fusion with the plasma membrane.
Figure 6.
Influence of Vectofusin-1 and other culture additives on the adhesion and fusion step of vesicular stomatitis virus-G-lentiviral vectors (VSV-G-LVs). (a) Viral fusion (percentage of cleaved CCF2) and level of transduction after 9 days (percentage of ΔNGFR+ cells) are represented as the average of duplicates ± SD. Data are representative of two different experiments. (b) Fusion and transduction assay on hCD34+ cells in the presence of BLAM-VLPs (250 or 500 ng/ml) or the positive control VSV-G-BLAM-LVs (280 ng/ml) in the absence or presence of Vectofusin-1 (12 µg/ml). Data are expressed as the average of two independent experiments performed in duplicate ± SD. (c) Adhesion, fusion, and transduction assays of hCD34+ cells in the presence of VSV-G-BLAM-LVs. Pre-cooled hCD34+ cells were incubated for 2.5–3 hours at 4 °C with the pre-cooled vector/peptide mix. Cells were then washed and half of the cells were lysed to evaluate the total membrane-bound p24 (top panel). The remaining cells were further cultured for 2–2.5 hours at 37 °C to induce viral fusion. Next, an aliquot of cells was used for the BLAM fusion assay (middle panel), whereas the remaining cells were further cultured. After 9 days, the transduction efficiency was evaluated as in a by monitoring ΔNGFR expression (lower panel). Data are expressed as the average of three independent experiments ± SD (**P < 0.01; *P < 0.05, Student's t-test). AZT, azidothymidine; BLAM, β-lactamase; MOI, multiplicity of infection; SEVI, semen enhancer of viral infection; VLP, virus-like particle.
To determine whether Vectofusin-1 acts on the adhesion step or exclusively on the fusion step, we implemented an entry assay capable of concomitantly evaluating the adhesion, fusion, and transduction steps. Briefly, hCD34+ cells were incubated with VSV-G-BLAM-LVs at 4 °C to allow viral adhesion, in the absence or presence of Vectofusin-1, SEVI, or polybrene. Unbound VSV-G-LVs were removed by washing and the cells were either lysed to determine the amount of membrane-bound p24, or cultured at 37 °C to allow the fusion process to occur. An aliquot of the hCD34+ cell suspension was used for the fusion assay, whereas the remaining cells were further cultured. After 9 days, the transduction efficiency was monitored by following ΔNGFR expression.35 As shown in Figure 6c, Vectofusin-1 was as efficient as SEVI, a known aggregating additive, to potentiate the adhesion step. In addition, the difference between SEVI and Vectofusin-1 was clearly shown in the fusion step. After an extensive wash of unbound VSV-G-LVs, we observed that viral particles bound to the cell surface are more prone to fusion and subsequent transduction in the presence of Vectofusin-1 (10%) than in the presence of SEVI (5%) or polybrene (2.8%) (Figure 6c). These results show that Vectofusin-1 has a major role on the cellular entry of LVs, acting both on the adhesion step and the fusion step.
Discussion
The Vectofusin-1 peptide is a new culture additive that potently enhances the transduction of HSCs with retroviral or lentiviral pseudotypes including highly purified VSV-G-LVs. The absence of hematopoietic toxicity of Vectofusin-1 has been demonstrated in vitro with CFC assays and in vivo in a surrogate murine model of HSC/progenitors engraftment, indicating that Vectofusin-1 is a nontoxic, effective, and versatile culture additive to be used as a transduction enhancer. Most importantly, Vectofusin-1 acts at the level of viral entry, by enhancing both the adhesion and the fusion of viral particles with the cellular plasma membrane. We have shown that these mechanisms are partially different from those of other transduction enhancers, either cationic polymers (polybrene) or peptides (SEVI). The effect on fusion is rather unique in this context.
It has been shown previously that peptides of the LAH4 family, to which Vectofusin-1 belongs, interact with membrane lipids, adopt a transmembrane orientation at neutral pH, and are able to disturb the plasma membrane.21 Because lipid mixing between viral and plasma membrane seems to precede viral pore formation,36,37,38,39 it is tempting to speculate that Vectofusin-1 may act in a two-step manner. The initial step could involve use of its cationic property to neutralize anionic lipids and heparan sulfates, leading to an increase of adhesion. In the second step, local destabilization of the membrane lipid leaflet could occur, leading to optimization of the lipid-mixing step and subsequent fusion that is independent of the envelope used to pseudotype LVs. This proposed mechanism of action will require further investigation to determine whether Vectofusin-1 acts on both the target cell and viral particle membranes. Structure function studies of Vectofusin-1 are also necessary to determine which amino acid residues are critical in promoting lentiviral transduction. Finally, it will be necessary to study these effects in various cell types that may have distinct membrane compositions and also to study the effects on other lentiviral pseudotypes harboring new viral GP (e.g., measle GP, refs. 40,41 and Tupaïa GP, ref. 42) or chimeric GP (e.g., measle hemagglutinin single-chain antibody, refs. 43,44 and designed ankyrin repeat proteins, ref. 45).
Vectofusin-1 significantly increased the entry of LVs. Major improvements in vector transduction have been obtained using Retronectin in clinical protocols, particularly with γ-retroviral gene therapy applications, but Vectofusin-1 has some advantages compared with Retronectin: (i) Vectofusin-1 is easier to use as it can be added directly into the transduction medium, whereas Retronectin requires a pre-coating step, which is difficult to standardize, leads to imprecise amounts of additive in relation to cells, and increases the steps of the viral transduction procedure; (ii) binding of CD34+ HSCs to Retronectin-coated surfaces may reduce the yields of cells obtained after transduction in culture bags,46 a side effect that is not expected with soluble factors like Vectofusin-1; (iii) the cell adhesion induced by Retronectin-coated surfaces may accelerate the differentiation of a subset of stem cells and this step may be better controlled in suspension cultures;47 (iv) Vectofusin-1 is a relatively small synthetic peptide of 26 amino acids, which is water soluble and can be easily synthesized and purified according to good manufacturing practices for ex vivo clinical applications. Interestingly, preliminary experiments suggest that Retronectin and Vectofusin-1 could be combined together to enhance the transduction of hCD34+ cells with LVs, but further experiments are needed to define optimal protocol conditions (data not shown).
In conclusion, Vectofusin-1 represents a new transduction enhancer which has numerous advantages over conventional additives now in widespread use. There are several potential applications of Vectofusin-1 in technological platforms, preclinical studies or clinical gene therapy applications. Vectofusin-1 could potentially ameliorate protocols for clinical transduction of CD34+ cells using retroviral vectors or LVs.48 Vectofusin-1 could be used to augment the levels of transduction or the VCN in cells. Alternatively, by enhancing the effects of suboptimal concentrations of vector, Vectofusin-1 could allow the use of lower amounts of vector on the cells thereby reducing cost and potentially toxicity. Based upon these results, we believe that there is strong rationale for future testing of this additive in clinical applications.
Materials and Methods
Peptides and reagents. The Vectofusin-1 and SEVI peptides were produced by standard Fmoc solid-phase peptide synthesis, purified by preparative reverse phase high-performance liquid chromatography, and analyzed by high-performance liquid chromatography and mass spectrometry (Genecust, Dudelange, Luxembourg). Peptide purity was >99%. To promote full fibril formation, the SEVI peptide solution (5 mg/ml in phosphate-buffered saline) was agitated for 4 days at 37 °C at 1,400 rpm with an Eppendorf Thermomixer (Eppendorf, Le Pecq, France). Hexadimethrine bromide (Polybrene), Azidothymidine, Bafilomycin A1, 7-aminoactinomycin D (7-AAD), Trypan Blue, and Triton X-100 were obtained from Sigma-Aldrich (St-Quentin-Fallavier, France). Retronectin was from Ozyme (St-Quentin-en-Yvelines, France).
Cell line culture. HCT116 cells derived from a human colorectal carcinoma (CCL-247; ATCC, Manassas, VA) and 293T cells4 were cultured at 37 °C, 5% CO2 in Dulbecco's modified Eagle's medium + glutamax supplemented with 10% heat-inactivated fetal calf serum (Life Technologies, St-Aubin, France).
Viral vector production and vector tittering. HIV-1–derived LVs were generated by transient calcium phosphate transfection of 293T cells with four plasmids as described,4 consisting of the transfer vector plasmid expressing GFP (pCCLsin-cPPT-hPGK-eGFP-WPRE), the plasmid encoding HIV-1 Rev (pK.Rev), the plasmid encoding HIV-1 gagpol (pKLgagpol), and the appropriate envelope GP construct: pMDG (VSV-G) to generate VSV-G-LVs; pHCMV-RD114TR (modified feline endogenous retrovirus GP) to generate RD114TR-LVs;5 pBA-Ampho (amphotropic MLV GP) to generate MLV-A-LVs and pBA-GALV ampho-Kana (modified GALV GP) to generate GALVTR-LVs.5 Unless indicated otherwise, the viral supernatants were collected 48 hours post-transfection, filtered (0.45 µm), aliquoted, and stored at −80 °C before use. Physical particle titers were determined by measuring HIV-1 p24 capsid contents using a commercial ELISA kit (Perkin Elmer, Courtaboeuf, France). Infectious titers were determined on HCT116 cells using either the detection of GFP by flow cytometry (FACSCalibur; BD Biosciences, Le Pont de Claix, France), with titers expressed as transducing units per milliliter,49 or using quantitative PCR with titers expressed as ig/ml.4 LVs containing BLAM-Vpr (BLAM-LVs) and encoding the surface marker ΔNGFR are generated as described above but with a transfer plasmid encoding ΔNGFR (pRRLsin-cPPT-hPGK-ΔNGFR-WPRE, Généthon) and the addition of the BLAM-Vpr expression vector (pCMV4-BlaM-Vpr, plasmid 21950; Addgene, Cambridge, MA)26 and pAdVAntage (Promega, Charbonnières-les-Bains, France). Viral supernatants of MLV retroviral vector pseudotyped with the GALV envelope GP were obtained from the producer cell line PG13-MFG-GFP.50
Human CD34+ cells culture and transduction. UCB samples and human granulocyte colony-stimulating factor–MPB samples were obtained in accordance with international ethical principles and French national law under declaration N° DC-201-1655 to the French Ministry of Research and Higher Studies. UCB samples were collected from the Centre Hospitalier Sud-Francilien (CHSF) in Evry after uncomplicated births. MPB from adult donors were obtained from the French blood establishment (EFS, Evry, France) or from the Gustave Roussy Institute (IGR, Villejuif, France) following institutional agreements. The hCD34+ progenitors were isolated from UCB or MPB using immunomagnetic selection (Miltenyi Biotec, Paris, France) from the mononuclear cell fraction. The transduction of hCD34+ cells was performed first, by pre-activating the cells overnight in X-vivo 20 medium (Lonza, Levallois-Perret, France) supplemented with cytokines as described previously.32 Next, pre-activated cells were plated in 48-well plates (2.5 × 104 cells/100 µl). Transduction was initiated by adding 100 µl of LV supernatants mixed with transduction enhancers. Retronectin was used in 48-well plates and was pre-coated overnight (7–20 µg/cm2) and loaded with viral supernatants (dynamic pre-loading as previously described)28 before adding the cell suspension (2.5 × 104 cells/per well). At 6 hours post-transduction, viruses and additives were diluted by adding 1 ml of differentiation medium in each well. After 5–6 days, cellular mortality and transduction efficiency were evaluated respectively by 7-AAD labeling and measurement of GFP expression or ΔNGFR expression (anti-CD271 (NGFR) antibody conjugated to APC; Miltenyi Biotec) using flow cytometry (LSRII; BD Biosciences).
CFC assays and VCN determination. CFC assays were performed in duplicate by plating 500 transduced or untransduced cells per milliliter of Methocult (H4434), a complete methylcellulose medium supplemented with human cytokines (Stem Cell Technologies, Grenoble, France), according to the manufacturer's instructions. After 2 weeks of culture, colony-forming unit, erythroid/burst-forming unit, erythroid; colony-forming unit, granulocyte, monocyte; and colony-forming unit, granulocyte, erythrocyte, macrophage, megakaryocyte were counted using an inverted microscope with standard visual criteria. The protocols to isolate CFCs, to evaluate the GFP expression, and to determine VCN in each CFC have been described previously.32
BLAM-LVs adhesion, fusion, and transduction assay. LV-cell fusion was measured using the BLAM fusion assay.26 To study concomitantly the adhesion step, the fusion step, and the transduction efficiency of LVs, the BLAM fusion assay was adapted. Briefly, pre-activated hCD34+ cells (1.5 × 105 cells/well) were pre-cooled for 20 minutes at 4 °C. At the same time, VSV-G-BLAM-LVs were incubated for 15 minutes at 4 °C in the absence or in the presence of either SEVI (20 µg/ml, 4.3 µmol/l), Vectofusin-1 (12 µg/ml, 4.3 µmol/l) or polybrene (6 µg/ml). Cells were then incubated with BLAM-LVs (1.5 × 107 ig/ml, multiplicity of infection 20) for 2.5–3 hours at 4 °C. Next, cells were washed twice with cold phosphate-buffered saline 1x. For each condition, half of the cells were lysed with 100 µl of phosphate-buffered saline 1x containing 1% Triton X-100 and a cocktail of protease inhibitors (Complete; Roche Diagnostics, Meylan, France). In cell lysates, p24 contents were evaluated with an anti-p24 ELISA kit and normalized to total protein contents with the BCA protein assay kit (Thermo Fisher Scientific, Villebon-sur-Yvette, France). The remaining cells were resuspended in 200 µl of pre-activation medium and cultured for 2–2.5 hours at 37 °C, 5% CO2. Next an aliquot of the cells was processed for the fusion assay as described previously.26 Nine days post-transduction, cells were labeled with anti-human ΔNGFR and analyzed using flow cytometry.
Production and monitoring of HIS (BALB-Rag/γC) mice. BALB-Rag/γC mice were housed under pathogen-free conditions at Généthon and treated in accordance with the guidelines of the Animal Ethical Committee under protocol CE11003 (approval dates 1 March 2011–1 March 2012). Briefly, transduced or untransduced hCD34+ cells (105 cells/mice) were injected intrahepatically into irradiated BALB-Rag/γC newborn pups. Eleven to thirteen weeks post-injection, HIS (BALB-Rag/γC) mice were euthanized and the engraftment level of human hematopoietic cells was monitored by flow cytometry in the PB, the thymus, the spleen, and the BM. The PB samples were treated with a RBC lysing solution (PharmLys; BD Biosciences). Next, WBCs and also thymus, spleen, and BM cells were resuspended in cold phosphate-buffered saline 1x/0.5% bovine serum albumin solution. In each well, the nonspecific binding was saturated with human γ-globulins. Next, cells were stained with fluorescently conjugated anti-human antibodies and dose-matched fluorescently labeled isotype antibodies according to the manufacturer's instruction. 7-AAD–positive, nonviable cells were excluded from the analysis. For engraftment analyses, we performed a nonparametric Mann–Whitney test.
SUPPLEMENTARY MATERIAL Figure S1. Determination of the VCN in hCD34+ cells transduced with various lentiviral pseudotypes. Figure S2. Transduction of hCD34+ cells isolated from G-CSF–mobilized peripheral blood (MPB) in presence of Vectofusin-1. Figure S3. hCD34+ transduction levels and engraftment efficiency of transduced cells in HIS (BALB-Rag/γC) mice. Figure S4. Monitoring of the engraftment of HIS (BALB-Rag/γC) mice.
Acknowledgments
This work was supported by the Association Française contre les Myopathies and the French ministry of research (PGT project: ANR-10-DPBS-01). We thank Généthon collaborators, in particular Armelle Viornery, Roseline Yao, the Flow Cytometry Core for technical assistance, the Bioexperimentation Department and Animal Care Facility for BALB-Rag/γC mice experiments, and the Standardized Production Department for lentiviral production of purified vesicular stomatitis virus-G glycoprotein-lentiviral vectors. We are very grateful to the mothers and staff of the Louise Michel hospital (Evry, France) for providing us with umbilical cord blood samples and to the Gustave Roussy Institute for providing us with mobilized peripheral blood samples. We also thank Fulvio Mavilio (Généthon) and Burkhard Bechinger (University of Strasbourg) for critical reading of the manuscript, Warner Greene (Gladstone Institute) for the pCMV4-BlaM-Vpr plasmid, and François-Loïc Cosset (University of Lyon) for helpful discussions and gift of GALVTR and RD114TR constructs. The authors declared no conflict of interest.
Supplementary material
Determination of the VCN in hCD34+ cells transduced with various lentiviral pseudotypes.
Transduction of hCD34+ cells isolated from G-CSF–mobilized peripheral blood (MPB) in presence of Vectofusin-1.
hCD34+ transduction levels and engraftment efficiency of transduced cells in HIS (BALB-Rag/γC) mice.
Monitoring of the engraftment of HIS (BALB-Rag/γC) mice.
References
- D'Costa, J, Mansfield, SG and Humeau, LM (2009). Lentiviral vectors in clinical trials: Current status. Curr Opin Mol Ther 11: 554–564. [PubMed] [Google Scholar]
- Naldini, L (2011). Ex vivo gene transfer and correction for cell-based therapies. Nat Rev Genet 12: 301–315. [DOI] [PubMed] [Google Scholar]
- Cronin, J, Zhang, XY and Reiser, J (2005). Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther 5: 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten, OW, Charrier, S, Laroudie, N, Fauchille, S, Dugué, C, Jenny, C et al. (2011). Large-scale manufacture and characterization of a lentiviral vector produced for clinical ex vivo gene therapy application. Hum Gene Ther 22: 343–356. [DOI] [PubMed] [Google Scholar]
- Sandrin, V, Boson, B, Salmon, P, Gay, W, Nègre, D, Le Grand, R et al. (2002). Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood 100: 823–832. [DOI] [PubMed] [Google Scholar]
- Cornetta, K and Anderson, WF (1989). Protamine sulfate as an effective alternative to polybrene in retroviral-mediated gene-transfer: implications for human gene therapy. J Virol Methods 23: 187–194. [DOI] [PubMed] [Google Scholar]
- Toyoshima, K and Vogt, PK (1969). Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology 38: 414–426. [DOI] [PubMed] [Google Scholar]
- Vogt, PK (1967). DEAE-dextran: enhancement of cellular transformation induced by avian sarcoma viruses. Virology 33: 175–177. [DOI] [PubMed] [Google Scholar]
- Innes, CL, Smith, PB, Langenbach, R, Tindall, KR and Boone, LR (1990). Cationic liposomes (Lipofectin) mediate retroviral infection in the absence of specific receptors. J Virol 64: 957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson, CP and Solaiman, F (1996). Virosomes: cationic liposomes enhance retroviral transduction. Nat Biotechnol 14: 339–342. [DOI] [PubMed] [Google Scholar]
- Wurm, M, Schambach, A, Lindemann, D, Hanenberg, H, Ständker, L, Forssmann, WG et al. (2010). The influence of semen-derived enhancer of virus infection on the efficiency of retroviral gene transfer. J Gene Med 12: 137–146. [DOI] [PubMed] [Google Scholar]
- Davis, HE, Rosinski, M, Morgan, JR and Yarmush, ML (2004). Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation. Biophys J 86: 1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roan, NR, Münch, J, Arhel, N, Mothes, W, Neidleman, J, Kobayashi, A et al. (2009). The cationic properties of SEVI underlie its ability to enhance human immunodeficiency virus infection. J Virol 83: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz, T, Patel, VP and Williams, DA (1994). Bone marrow extracellular matrix molecules improve gene transfer into human hematopoietic cells via retroviral vectors. J Clin Invest 93: 1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz, T, Dutt, P, Xiao, X, Carstanjen, D, Vik, T, Hanenberg, H et al. (1996). Fibronectin improves transduction of reconstituting hematopoietic stem cells by retroviral vectors: evidence of direct viral binding to chymotryptic carboxy-terminal fragments. Blood 88: 855–862. [PubMed] [Google Scholar]
- Hanenberg, H, Xiao, XL, Dilloo, D, Hashino, K, Kato, I and Williams, DA (1996). Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat Med 2: 876–882. [DOI] [PubMed] [Google Scholar]
- Haas, DL, Case, SS, Crooks, GM and Kohn, DB (2000). Critical factors influencing stable transduction of human CD34(+) cells with HIV-1-derived lentiviral vectors. Mol Ther 2: 71–80. [DOI] [PubMed] [Google Scholar]
- Kichler, A, Leborgne, C, März, J, Danos, O and Bechinger, B (2003). Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells. Proc Natl Acad Sci USA 100: 1564–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechinger, B, Vidovic, V, Bertani, P and Kichler, A (2011). A new family of peptide-nucleic acid nanostructures with potent transfection activities. J Pept Sci 17: 88–93. [DOI] [PubMed] [Google Scholar]
- Mason, AJ, Gasnier, C, Kichler, A, Prévost, G, Aunis, D, Metz-Boutigue, MH et al. (2006). Enhanced membrane disruption and antibiotic action against pathogenic bacteria by designed histidine-rich peptides at acidic pH. Antimicrob Agents Chemother 50: 3305–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, AJ, Martinez, A, Glaubitz, C, Danos, O, Kichler, A and Bechinger, B (2006). The antibiotic and DNA-transfecting peptide LAH4 selectively associates with, and disorders, anionic lipids in mixed membranes. FASEB J 20: 320–322. [DOI] [PubMed] [Google Scholar]
- Marquette, A, Mason, AJ and Bechinger, B (2008). Aggregation and membrane permeabilizing properties of designed histidine-containing cationic linear peptide antibiotics. J Pept Sci 14: 488–495. [DOI] [PubMed] [Google Scholar]
- Mason, AJ, Moussaoui, W, Abdelrahman, T, Boukhari, A, Bertani, P, Marquette, A et al. (2009). Structural determinants of antimicrobial and antiplasmodial activity and selectivity in histidine-rich amphipathic cationic peptides. J Biol Chem 284: 119–133. [DOI] [PubMed] [Google Scholar]
- Langlet-Bertin, B, Leborgne, C, Scherman, D, Bechinger, B, Mason, AJ and Kichler, A (2010). Design and evaluation of histidine-rich amphipathic peptides for siRNA delivery. Pharm Res 27: 1426–1436. [DOI] [PubMed] [Google Scholar]
- Ferrer-Miralles, N, Vázquez, E and Villaverde, A (2008). Membrane-active peptides for non-viral gene therapy: making the safest easier. Trends Biotechnol 26: 267–275. [DOI] [PubMed] [Google Scholar]
- Cavrois, M, De Noronha, C and Greene, WC (2002). A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat Biotechnol 20: 1151–1154. [DOI] [PubMed] [Google Scholar]
- Cavrois, M, Neidleman, J, Bigos, M and Greene, WC (2004). Fluorescence resonance energy transfer-based HIV-1 virion fusion assay. Methods Mol Biol 263: 333–344. [DOI] [PubMed] [Google Scholar]
- Jacome, A, Navarro, S, Río, P, Yañez, RM, González-Murillo, A, Lozano, ML et al. (2009). Lentiviral-mediated genetic correction of hematopoietic and mesenchymal progenitor cells from Fanconi anemia patients. Mol Ther 17: 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relander, T, Johansson, M, Olsson, K, Ikeda, Y, Takeuchi, Y, Collins, M et al. (2005). Gene transfer to repopulating human CD34+ cells using amphotropic-, GALV-, or RD114-pseudotyped HIV-1-based vectors from stable producer cells. Mol Ther 11: 452–459. [DOI] [PubMed] [Google Scholar]
- Münch, J, Rücker, E, Ständker, L, Adermann, K, Goffinet, C, Schindler, M et al. (2007). Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 131: 1059–1071. [DOI] [PubMed] [Google Scholar]
- Pollok, KE, van Der Loo, JC, Cooper, RJ, Hartwell, JR, Miles, KR, Breese, R et al. (2001). Differential transduction efficiency of SCID-repopulating cells derived from umbilical cord blood and granulocyte colony-stimulating factor-mobilized peripheral blood. Hum Gene Ther 12: 2095–2108. [DOI] [PubMed] [Google Scholar]
- Charrier, S, Ferrand, M, Zerbato, M, Précigout, G, Viornery, A, Bucher-Laurent, S et al. (2011). Quantification of lentiviral vector copy numbers in individual hematopoietic colony-forming cells shows vector dose-dependent effects on the frequency and level of transduction. Gene Ther 18: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand, N, Weijer, K and Spits, H (2008). Experimental model for the study of the human immune system: production and monitoring of “human immune system” Rag2-/-gamma c-/- mice. Methods Mol Biol 415: 65–82. [DOI] [PubMed] [Google Scholar]
- Georgescu, J, Munhoz, VH and Bechinger, B (2010). NMR structures of the histidine-rich peptide LAH4 in micellar environments: membrane insertion, pH-dependent mode of antimicrobial action, and DNA transfection. Biophys J 99: 2507–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini, C, Grez, M, Traversari, C, Ciceri, F, Marktel, S, Ferrari, G et al. (2003). Safety of retroviral gene marking with a truncated NGF receptor. Nat Med 9: 367–369. [DOI] [PubMed] [Google Scholar]
- de la Vega, M, Marin, M, Kondo, N, Miyauchi, K, Kim, Y, Epand, RF et al. (2011). Inhibition of HIV-1 endocytosis allows lipid mixing at the plasma membrane, but not complete fusion. Retrovirology 8: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha, NK, Latinovic, O, Martin, E, Novitskiy, G, Marin, M, Miyauchi, K et al. (2011). Imaging single retrovirus entry through alternative receptor isoforms and intermediates of virus-endosome fusion. PLoS Pathog 7: e1001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi, K, Kim, Y, Latinovic, O, Morozov, V and Melikyan, GB (2009). HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 137: 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien, MP, Lin, CH and Chang, DK (2009). Recruitment of HIV-1 envelope occurs subsequent to lipid mixing: a fluorescence microscopic evidence. Retrovirology 6: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frecha, C, Costa, C, Nègre, D, Gauthier, E, Russell, SJ, Cosset, FL et al. (2008). Stable transduction of quiescent T cells without induction of cycle progression by a novel lentiviral vector pseudotyped with measles virus glycoproteins. Blood 112: 4843–4852. [DOI] [PubMed] [Google Scholar]
- Funke, S, Schneider, IC, Glaser, S, Mühlebach, MD, Moritz, T, Cattaneo, R et al. (2009). Pseudotyping lentiviral vectors with the wild-type measles virus glycoproteins improves titer and selectivity. Gene Ther 16: 700–705. [DOI] [PubMed] [Google Scholar]
- Enkirch, T, Kneissl, S, Hoyler, B, Ungerechts, G, Stremmel, W, Buchholz, CJ et al. (2013). Targeted lentiviral vectors pseudotyped with the Tupaia paramyxovirus glycoproteins. Gene Ther 20: 16–23. [DOI] [PubMed] [Google Scholar]
- Funke, S, Maisner, A, Mühlebach, MD, Koehl, U, Grez, M, Cattaneo, R et al. (2008). Targeted cell entry of lentiviral vectors. Mol Ther 16: 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anliker, B, Abel, T, Kneissl, S, Hlavaty, J, Caputi, A, Brynza, J et al. (2010). Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors. Nat Methods 7: 929–935. [DOI] [PubMed] [Google Scholar]
- Münch, RC, Mühlebach, MD, Schaser, T, Kneissl, S, Jost, C, Plückthun, A et al. (2011). DARPins: an efficient targeting domain for lentiviral vectors. Mol Ther 19: 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers, CH, van Elzakker, P, van Steenbergen, SC, Sleijfer, S, Debets, R and Gratama, JW (2008). Retronectin-assisted retroviral transduction of primary human T lymphocytes under good manufacturing practice conditions: tissue culture bag critically determines cell yield. Cytotherapy 10: 406–416. [DOI] [PubMed] [Google Scholar]
- Guilak, F, Cohen, DM, Estes, BT, Gimble, JM, Liedtke, W and Chen, CS (2009). Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy, A and Thrasher, AJ (2011). Gene therapy for the Wiskott-Aldrich syndrome. Curr Opin Allergy Clin Immunol 11: 545–550. [DOI] [PubMed] [Google Scholar]
- Kutner, RH, Zhang, XY and Reiser, J (2009). Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc 4: 495–505. [DOI] [PubMed] [Google Scholar]
- Merten, OW (2004). State-of-the-art of the production of retroviral vectors. J Gene Med 6 (suppl. 1): S105–S124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Determination of the VCN in hCD34+ cells transduced with various lentiviral pseudotypes.
Transduction of hCD34+ cells isolated from G-CSF–mobilized peripheral blood (MPB) in presence of Vectofusin-1.
hCD34+ transduction levels and engraftment efficiency of transduced cells in HIS (BALB-Rag/γC) mice.
Monitoring of the engraftment of HIS (BALB-Rag/γC) mice.