Abstract
Increased precipitation and temperature variability as well as extreme events related to climate change are predicted to affect the availability and quality of water globally. Already heavily burdened with diarrheal diseases due to poor access to water, sanitation and hygiene facilities, communities throughout the developing world lack the adaptive capacity to sufficiently respond to the additional adversity caused by climate change. Studies suggest that diarrhea rates are positively correlated with increased temperature, and show a complex relationship with precipitation. Although climate change will likely increase rates of diarrheal diseases on average, there is a poor mechanistic understanding of the underlying disease transmission processes and substantial uncertainty surrounding current estimates. This makes it difficult to recommend appropriate adaptation strategies. We review the relevant climate-related mechanisms behind transmission of diarrheal disease pathogens and argue that systems-based mechanistic approaches incorporating human, engineered and environmental components are urgently needed. We then review successful systems-based approaches used in other environmental health fields and detail one modeling framework to predict climate change impacts on diarrheal diseases and design adaptation strategies.
Keywords: climate change, water, health, diarrhea, complex systems, coupled systems
1. Introduction
Enteric infections caused by inadequate drinking water supplies, sanitation and hand hygiene currently kill an estimated 842,000 children worldwide annually with the greatest number of deaths occurring in Sub-Saharan Africa and South-East Asia (Prüss-Ustün et al., 2014). They also frequently lead to stunted growth, impaired cognitive development and malnutrition - the effects of which can last into adulthood (Guerrant et al., 2013). It has been estimated that anthropogenic climate change will increase the relative risk of diarrhea by 22–29% by the end of the century (Kolstad & Johansson, 2011) possibly negating recent progress in reducing the global burden of diarrhea (Walker et al., 2012). The approximately 748 million people worldwide without an improved water supply (Bartram et al., 2014) and the additional 1.8 billion without consistent access to water free of microbial contamination (Bain et al., 2014) may bear the heaviest share of this additional disease burden. Their reliance on microbially contaminated or unreliable water supplies reduces their adaptive capacity to sufficiently respond to regional climate impacts. Furthermore, the nearly 2.5 billion who lack access to improved sanitation (Bartram et al., 2014) or who have other risk factors such as poor nutrition, hygiene or access to healthcare - are also at risk of increased incidence of diarrhea due to climate change impacts.
Predicting diarrhea incidence is complicated by the number of relevant risk factors and layers of complexity even when not accounting for climate change impacts (Mellor et al., 2012). This complexity includes factors such as individual genetics and physiology, personal behaviors, engineered infrastructure, local disease etiology and environmental factors as well as geographical, political, socioeconomic and cultural elements (Rehfuess & Bartram, 2014). In particular, the effects of environmental conditions such as temperature and precipitation might be modified by different factors, such as underlying social conditions, available infrastructure, and antecedent environmental conditions.
Epidemiological analyses have helped build our understanding of the relationships between weather-related drivers of diarrheal diseases. However, given the imminent threat of climate change, there is now a need for alternative approaches to augment our understanding of the risk of increased incidence of diarrhea, and to work towards designing effective interventions to improve adaptation strategies.
We propose that mechanistic, transdisciplinary systems-based methods that couple empirical field-based measurements with computational approaches incorporating human, engineered and environmental components are needed to better quantify this disease risk under diverse climate scenarios for disparate regions. These types of methods will enable engineers, scientists and policy-makers to design “no regrets” adaptation strategies that reduce both current and future risk against a backdrop of generally improving economic conditions in many developing countries. Such an approach can help improve resiliency which is the tendency to maintain integrity when subject to disturbance. Here, we first review the epidemiological literature on weather and climatic drivers of diarrheal diseases. We then assess the relevant weather and climate-affected transmission mechanisms that should be considered in improved approaches. Finally, we discuss examples of successful systems-based approaches and propose a way forward.
2. Discussion
2.1. Epidemiological Literature on Climate Drivers of Diarrheal Diseases
A number of epidemiological studies correlate all-cause diarrhea incidence to ambient temperature differences. In particular, studies have shown increases in diarrhea incidence of 3–11%, per degree Celsius ambient temperature increase in Fiji (Singh et al., 2001), Bangladesh (Hashizume et al., 2007), Peru (Checkley et al., 2000; Lama et al., 2004) and Japan (Onozuka et al., 2010). These regional results are corroborated by a recent global meta-analysis indicating a 7% increase in all-cause diarrhea per degree Celsius temperature increase based on the 10 studies that met the inclusion criteria (Carlton et al., 2015). While some of this research has shown a linear relationship between temperature and diarrhea rates (Hashizume et al., 2007), these associations can be non-linear and depend on local climatic conditions and pathogens. For example, in Peru, Checkley et al. (2000) found that a 5°C temperature increase in the winter was associated with a 77% increase in diarrhea-related hospital admissions, while the same temperature increase led to only a 21% increase in the summer (Checkley et al., 2000). Research in Botswana found that hospital admissions for diarrhea were associated with minimum temperature but not average or maximum temperature. Furthermore, increases in minimum temperatures corresponded with higher rates of diarrhea in the dry season, but lower rates in the wet season (Alexander et al., 2013).
Rainfall has likewise been shown to be an important driver of diarrheal diseases across diverse climatic regions. Research in Botswana found a bimodal cyclical pattern with peaks in the incidence of diarrhea in the wet and dry seasons. However, diarrhea incidence was only correlated to rainfall during the wet season (Alexander et al., 2013). Studies in Fiji (Singh et al., 2001) and Bangladesh (Hashizume et al., 2007) have shown that diarrhea incidence can increase above or below threshold rainfall amounts. Carlton et al. (2014) found that heavy rainfall events following dry periods increased the risk of diarrhea by 39%, while the risk decreased by 26% when the heavy rainfall event followed a wet period (Carlton et al., 2014). Furthermore, the exacerbation of diarrhea rates has been associated with severe flooding in Bangladesh (Hashizume et al., 2008) which can affect vulnerable regions (Christenson et al., 2014).
Studies have also investigated whether social and environmental conditions such as socioeconomic status or improved water and sanitation facilities modify the effect of weather or climate on diarrheal diseases. Research in Bangladesh indicated that effects of temperature on diarrhea rates were greater for those with lower educational attainment, households without a concrete roof and unsanitary toilets (Hashizume et al., 2007). The risk of diarrhea during severe flooding in Bangladesh was increased for individuals with lower education levels and non-concrete roofs, those drinking from a tube-well as opposed to tap water, and those with a distant water source or unsanitary toilets (Hashizume et al., 2008). Researchers in Ecuador found that unimproved sanitation increased the risk of diarrhea after one day of rainfall, but the effect was insignificant after five days of rainfall. Moreover, having an unimproved water source increased the risk of diarrhea, but only after several days of rainfall (Bhavnani et al., 2014). Despite the importance of such factors in modifying risk in these studies, other researchers have reported that sanitation, social cohesion and hygiene did not modify the relationship between heavy rainfall and diarrhea rates in Ecuador (Carlton et al., 2014). A study in the Pacific Islands found that diarrhea rates increased with the average annual temperature of the 17 islands studied, but there was no clear correlation between gross national product and diarrhea reports (Singh et al., 2001). The aforementioned recent meta-analysis found that the relationship between temperature and all-cause diarrhea was not modified by either geographical climate category or national income group. However, for bacterial diarrhea, there was a stronger effect size between temperature and diarrhea for low and middle income countries and in tropical climates (Carlton et al., 2015).
As a further complicating factor, diarrhea is not a single disease. Rather, it is a symptom that can be caused by infection with a number of different pathogens (Walker et al., 2010; Guerrant et al., 1990) whose relative importance varies regionally (Kotloff et al., 2013). Diarrhea incidence directly attributable to these various pathogens shows substantial seasonal variations (Albert et al., 1999), which might change under future climates. For instance, rotavirus is positively associated with cooler and drier weather in the tropics (Levy et al., 2009a; Jagai et al., 2012), norovirus incidence peaks in the winter months in temperate climates (Ahmed et al., 2013), cryptosporidiosis is positively associated with warmer and wetter weather (Jagai et al., 2009), campylobacter incidence peaks in the springtime in temperate climates and is not correlated to rainfall (Kovats et al., 2005) and Shigella is associated with warmer weather (Gao et al., 2014). Indeed, a recent meta-analysis of the influence of temperature on diarrhea incidence found that pathogen taxa was a significant source of heterogeneity across the 26 studies they analyzed (Carlton et al., 2015). It is important to note that these pathogen-temperature associations are correlational relationships and may not be directly causally linked. An understanding of the relative importance of these pathogens under future climates and their sensitivity to climatic conditions is needed to fully quantify future diarrhea risk and design adaptation strategies. However, in order to predict how disease rates associated with each of these diarrheal disease agents might change under future climatic conditions, it will be important to incorporate other critical factors in addition to expected meteorological variables, such as projected demographic changes, development of infrastructure, and behavioral factors. Systems-based approaches can facilitate this more complex predictive modeling of pathogen sources and transmission pathways
Collectively, epidemiological studies have highlighted the relevance of temperature and precipitation to diarrhea incidence and therefore to the potential for diarrhea risk to change under future climates. However, these studies likewise highlight the geographical and climatic specificity of this risk, the frequently non-linear responses to temperature and rainfall, and the potential for risk to be modified with socioeconomic variables and local disease etiology. This makes it difficult to accurately predict impacts or design intervention strategies to improve adaptation under future climates. Despite this complexity, due to the lack of high quality empirical data, the most comprehensive study to date relying on the epidemiological literature to predict diarrhea rates under future climates, assumes a linear response to temperature and was not able to include empirical data from Africa or stratify by pathogen taxa. The authors were likewise unable to include rainfall, extreme weather events or economic growth effects (Kolstad & Johansson, 2011). This uncertainty and lack of a mechanistic understanding was highlighted in the recent IPCC Fifth Assessment Report (Barros & Field, 2014).
Mechanistic, systems-based methods can build upon the understanding that epidemiological studies have established about weather and climate drivers of diarrheal diseases by more fully incorporating the relevant human, engineered and environmental components to explain the variability in observed epidemiological results. Such methods can also integrate climate models to project their findings into the future for diverse economic and social scenarios.
2.2. Climate-Affected Diarrhea Transmission Mechanisms
Diarrhea-causing pathogens are transmitted from source to human ingestion through mainly water, food, surface, insect or hand-based pathways (Eisenberg et al., 2007). These pathways are likely to be differentially affected under future climates due to their underlying transmission mechanisms.
2.2.1. Water Quality
Water-borne transmission of enteric pathogens is likely to be affected by predicted mean global precipitation increases coupled with the likelihood that short, intense precipitation events will become more frequent and weaker storms will become less common (Collins et al., 2013). These changes in precipitation patterns are likely to affect microbial water quality. How this will occur will be impacted by the complex interplay of the natural and anthropogenic factors and will depend on local conditions, climatic and environmental processes (Barros & Field, 2014). In developing world communities with inadequate sanitation, there is an ample supply of fecal pollution from human and animal sources that can be transported into water supplies (Escamilla et al., 2013; Howard et al., 2003) making the contamination more transport than supply limited (Burns, 2005). Results from a study in coastal Ecuador were consistent with three major hydrological drivers of microbial contamination that are relevant for understanding how climate change might impact water quality at a local scale (Levy et al., 2009b). The “runoff effect” occurs when rainfall causes microbial contamination to be flushed into the environment. The “dilution effect” happens when high water flows dilute microbial concentrations. Lastly, the “concentration effect” occurs when dry conditions allow for the accumulation of microorganisms in the environment.
On balance, regions with increased precipitation variability could see higher levels of contamination after short, intense rainfall events that follow dry periods due to a combination of the concentration and runoff effects. Since microbial contamination tends to be worse during the rainy season in most places (Kostyla et al., 2015; Levy et al., 2009b; Cronin et al., 2006; Kumpel & Nelson, 2013), mean rainfall increases may degrade mean water quality. Despite these general mechanisms, drinking water quality from either untreated surface (Levy et al., 2009b) or nominally treated piped (Kumpel & Nelson, 2014) systems can vary substantially over very short timescales. Water quality also depends on the proximity of pollution sources (Escamilla et al., 2013) and the exact timing and intensity of rainfall events will ultimately control pathogen loading to water sources (Howard et al., 2003).
Zoonotic pathogens can also impair water quality and spread fecal contamination, especially in areas with high concentrations of domesticated animals (Cotruvo et al., 2004). Dry conditions and drought may lead to increased sharing of water sources between animals and humans, leading to outbreaks of new pathogens, as occurred in Swaziland in 1993 (Effer et al., 2001). In a systematic review of the literature, Zambrano et al. (2014) found consistent evidence of a positive association between exposure to domestic food-producing animals and diarrheal illness across a range of animal exposures and enteric pathogens (Zambrano et al., 2014). Factors such as the increased demand for animal protein, increased population densities and water scarcity may increase the public health risk of zoonotic enteric pathogen transmission, especially under climate change. Identifying these sources and transmission routes will be an important consideration for community adaptation strategies.
The survival of microbial pathogens in waterways is also likely to be affected directly by ambient temperatures. Diarrhea-causing pathogens differ in their response to environmental temperature increases but many, including Campylobacter (Thomas et al., 1999), Escherichia coli (Wang & Doyle, 1998; van Elsas et al., 2010), rotavirus (Hurst et al., 1989), norovirus (Ngazoa et al., 2008) and Cryptosporidium (King et al., 2005) show decreased survival in warmer waters. This could mean that the predicted warmer temperatures by the end of the century could lead to decreased pathogen survival in many regions. On the contrary, other pathogens, such as Vibrio cholerae, exhibit greater survival in warmer waters (Huq et al., 1984). However, these effects are non-linear and some pathogens might evolve to adapt to warmer climates (Koelle et al., 2005). Some of these results are inconsistent with the epidemiological evidence for diseases like cryptosporidiosis that shows higher incidence at warmer temperatures (Jagai et al., 2009). This contradiction could be explained by threshold effects, zoonotic transmission, nutrient availability, food spoilage, higher expression of virulence genes at warmer temperatures or behavioral factors. More research is need to understand these issues.
2.2.2. Water Quantity
In addition to water quality, the quantity of water available to communities is an important factor in helping communities maintain good health (Howard & Bartram, 2003). Poor access can limit the availability of water needed to maintain adequate hygiene thereby increasing the odds of hand and surface-based pathogen transmission.
While the impacts of climate change on both surface and groundwater resources are still debated (Barros & Field, 2014; De Wit & Stankiewicz, 2006; Taylor et al., 2013a, b; Fry et al., 2012), there is potential for changes to water availability at the community scale in many regions through both increased frequency of extreme events and longer term climatic changes. However, these changes will depend on regional hydrological and precipitation characteristics (De Wit & Stankiewicz, 2006; Taylor et al., 2013a).
At the community scale, the reduced access to water that may occur due to climate-induced hydrologic variability will likely have significant adverse impacts. For instance, if water supplies become limited due to drought, families will likely need to travel further to secure sufficient water. Evidence from Ethiopia suggests that water usage for hygiene (but not for drinking and cooking) drops significantly in the dry season when collection times rise and there is increased demand for wage labor. This effect was particularly pronounced for poorer households (Tucker et al., 2014). Long collection distances are associated with increased risk of diarrhea, child growth stunting and death amongst under five year old children in Africa (Pickering & Davis, 2012) due in part to post-collection contamination (Wright et al., 2004). Furthermore, research has shown that the long storage times associated with unreliable water sources are an important factor associated with diarrhea risk in South Africa (Mellor et al., 2012).
In some cases, increased water availability can also be detrimental in communities both with and without improved water sources due to behavioral changes. For example, there are large seasonal variations in water collection practices and the increased use of informal (and likely contaminated) water sources during the rainy seasons in Burkina Faso and Cambodia (Schweitzer et al., 2013; Shaheed et al., 2014). If climate change increases the duration of the rainy season in these types of areas, residents might then rely more heavily on informal sources because of their convenience and availability. However, even those with improved water sources may be affected if those water sources are compromised or damaged by extreme rainfall events especially in communities that rely on a single water source. This is a particular concern since contamination spikes to water supplies increase diarrhea incidence (Enger et al., 2012). Soap usage for hand washing is another behavior that can change seasonally. For example, in Mali, soap use dropped by 29% during the wet season when farmers spend more time in the field (Naughton, 2013).
2.2.3. Other Pathways
Insects- and food-borne transmission are two other important pathways by which diarrhea-causing pathogens can be transported (Collinet-Adler et al., 2015). However, little research has been pursued to understand the relationships between climate change and these pathways. Alexander et al. (2013) suggest increases in fly abundance as an explanation for observed increases in diarrhea in Botswana in the dry season. Evidence from the United Kingdom indicates that fly populations might increase by up to 244% by 2080 as a result of climate change (Goulson et al., 2005). Furthermore, other evidence from Australia suggests positive associations between ambient temperature and food borne disease (D’Souza et al., 2004). These results could indicate that insect-and food-based diarrhea incidence might increase in the future, though the evidence is limited.
2.2.4. Engineered Solutions
With climate change likely to affect many diarrheal disease transmission pathways, there is an urgent need to implement technologies that reduce diarrhea incidence under present as well as future climates by designing “no regret” adaptation strategies. Such strategies provide benefits under both current and potential future climate conditions. They can increase resilience to predicted impacts of climate change while providing immediate economic, environmental or social benefits (EPA, 2013).
Historically, engineered solutions have had a notable role in protecting populations from diarrheal diseases through protection of drinking water intakes, centralized drinking water treatment and wastewater treatment. Drinking water engineering was largely responsible for the epidemiological transition of the United States. It accounted for over half the reduction in total mortality and up to three-quarters of the reduction in infant mortality during the late nineteenth and early twentieth centuries (Cutler & Miller, 2005). More recent research conducted in low and middle-income countries suggests that drinking water solutions such as point-of-use treatment (Clasen et al., 2015) and high-quality piped water and sewer connections (Wolf et al., 2014) can lead to significant reductions in diarrhea rates. Likewise, studies suggest that improved sanitation and sewage reduce diarrhea incidence (Fewtrell et al., 2005; Heijnen et al., 2014; Barreto et al., 2011).
Global efforts including the WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation provide insights into the various water and sanitation technologies used around the world (Bartram et al., 2014). These reports indicate that coverage rates and technology types vary across countries and between urban and rural areas within countries. They also indicate substantial increases in access to improved water supplies and higher levels of service (e.g., water piped to home) in recent decades. However, even the presence of, for instance, an improved water supply, does not always fully address water quality (Onda et al., 2012; Bain et al., 2014) or reliability.
Various water infrastructure and treatment types typical in resource-limited settings are likely to be differently affected by climate extremes (Charles et al., 2010). For example, protected springs can become contaminated following rainfall events (Howard et al., 2003), while fluctuating water levels may cause problems for water infrastructure (Charles et al., 2010). Additionally, seasonal water quality changes can affect water treatment plant processes (Santana et al., 2014). In India, researchers found that while source water quality remained unchanged between monsoon and non-monsoon periods, post household-water-treatment drinking water had higher concentrations of indicator bacteria during the monsoon period (MacDonald et al., 2015). This was due to the longer household storage periods during the rainy periods, which is associated with growth of indicator bacteria (Mellor et al., 2013). They also found that chemical disinfection with sodium dichloroisocyanurate (NaDCC) tablets was less effective during that period (MacDonald et al., 2015). However, exactly how climate change will impact various water infrastructures and treatment technologies in diverse climates is still an open question. More research is needed to understand how to best improve the resiliency of water systems in such settings. That research should include both technical and social factors.
Similarly, sanitation infrastructure is subject to inefficiencies and failure under climate extremes. Poorly constructed or inadequately designed or maintained sanitation systems can lead to water source contamination or the spreading of fecal matter (Escamilla et al., 2013). Even when properly constructed, sanitation systems frequently rely on significant water resources (Fry et al., 2008), which can further strain water-stressed regions. This could lead some water-stressed communities to revert to open defecation.
Lastly, climate change is likely to increase the intensity and frequency of extreme weather events in much of the world. Given the correlation between extreme events like flooding and diarrhea prevalence (Hashizume et al., 2008), municipal governments can use systems approaches to design adequate flood control and prevent the spread of infectious diseases like diarrhea. These plans should not only prevent flood water from entering populated areas, but also include adequate disaster response plans.
Through a better understanding of the impact that climate change will have on water resources and diarrhea rates, we will be able to design “no regrets” water and sanitation infrastructure that will improve human security for both present and future climates. As such, more research is urgently needed to study how these engineered systems will be affected by climate variability. However, it is also clear that there are unlikely to be solutions that are effective in all settings. Engineered solutions need to be culturally appropriate, available, acceptable, monitored and evaluated to ensure ultimate success.
2.3. Systems-Based Approaches
Although prior epidemiological studies have shed light on the importance of the relationship between temperature, precipitation and diarrhea incidence, they are insufficient to predict climate change impacts or design adaptation strategies. This is because the causes and intervention strategies to prevent such environmentally-linked diseases are comprised of multiple layers of complexity (Rehfuess & Bartram, 2014) which can include social, economic, biological, hydrological and engineered factors as discussed above. These factors are difficult to fully integrate using traditional epidemiological means. In contrast to more localized environmental health hazards, climate change impact studies require a consideration of global-scale risk factors over time spans that last decades (McMichael, 1997) and epidemiological studies have limited ability to understand ecological effects on disease rates or effect mechanisms (Luke & Stamatakis, 2012).
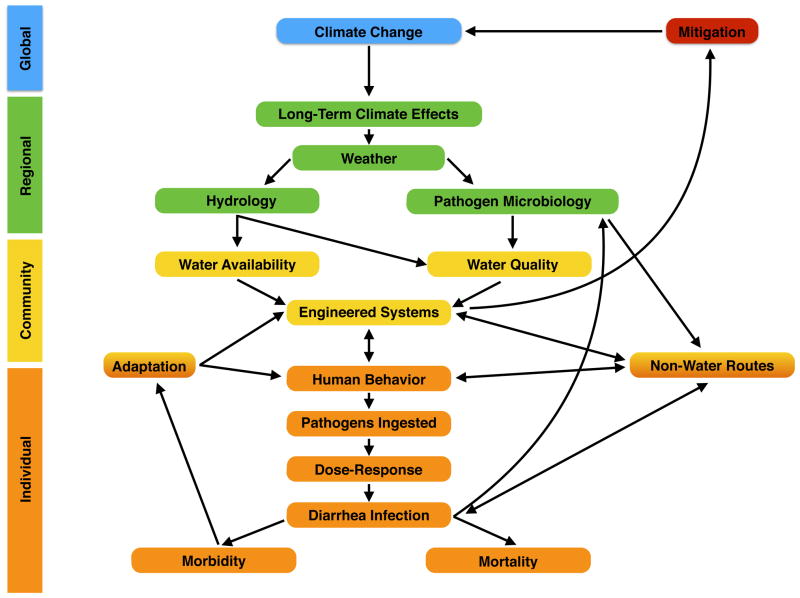
We therefore argue that mechanistic, systems approaches to study the effects of climate change on diarrheal diseases should incorporate the mechanisms described in this review and include human, engineered and environmental components. These mechanisms are summarized in Figure 1. This conceptual model is based on a qualitative analysis of the risk factors associated with diarrheal diseases as summarized in this review.
Figure 1.
In our definition, a mechanistic, systems approach would explicitly link in a modeling framework global climate change and its effects on local weather and hydrology; pathogen diversity, survival and transport; water quality and quantity available to communities and individuals; the impact of engineered infrastructure; as well as individual behaviors and changing socioeconomic factors. While similar approaches are more common for other environmental health diseases impacted by climate, to the best of our knowledge, very few studies have been conducted to date that model how climate change will impact diarrheal diseases using a systems-based mechanistic approach.
By using a mechanistic description that explicitly accounts for spatial and temporal dynamic processes, a systems approach can improve our understanding of how changes to climate will affect the spread of infectious diseases. Systems approaches have other benefits, including explicit assumptions that can be tested systematically. Such methods can also incorporate diverse data including climate predictions, pathogen biology, engineered infrastructure, predicted development and behavioral adaptation. This approach is also useful for identifying important variables to inform resource- and time-intensive field work. These methods can therefore not only predict outcomes, but also evaluate the influence of various assumptions, which helps to identify key transmission pathways to inform intervention strategies.
Mechanistic, systems based approaches can also account for economic development and social factors. This can occur by, for example, studying how periodic improvements of water delivery systems will impact enteric pathogen transmission pathways and water accessibility as economies grow. Methods such as agent-based computer modeling (An, 2009) can then be used to study how such improvements impact diarrheal disease rates. This can be be done by modeling both direct water-borne exposure and improvements to hygiene that can occur through increased water accessibility (Mellor et al., 2012). Such studies can motivate smarter investments by governments or other stakeholders.
While many have called for the use of systems-based methods to study the climate effects on environmental health diseases (McMichael, 1999; Altizer et al., 2013), many studies conducted to date are not explicitly mechanistic in nature. However, such non-mechanistic systems-based studies can use climate change scenarios to predict the impact of climate change based on epidemiological information and socioeconomic scenarios. For example, one study looked at the present-day global distribution of malaria and population density, which was used to establish climatic constraints on malaria distribution. The researchers integrated global climate model (GCM) results to project the changing distribution of malaria under different climate change scenarios (Rogers & Randolph, 2000). A recent study in China used estimates of the temperature sensitivity of diarrheal diseases, temperature projections from GCMs, WASH infrastructure development scenarios and projected demographic changes. That study showed that climate change is likely to delay China’s rapid progress towards reducing WASH-attributable disease burden by 8–85 months by 2030 (Hodges et al., 2014). Although such studies are important steps forward to predict the impact of climate change, they have limited ability to inform the engineering design of adaptation technologies.
To study the seasonality of enteric infections, time series analysis has been used to analyze cohort studies of the seasonality of rotavirus and the impact of temperature, humidity and other confounding variables in India (Sarkar et al., 2013). It has also been used to study the seasonal timing and intensity of six enterically transmitted diseases in Massachusetts, USA (Naumova et al., 2007). The results of such analyses could be coupled with GCM projections to predict impact under different climate scenarios. However, such a technique would be less suitable than mechanistic studies to help design interventions.
Many studies have taken a more fully system-based mechanistic approach to study how climate can impact a variety of environmental health diseases. Theses studies can motivate and inform the design of diarrhea modeling efforts. Researchers have studied the effects of climate or weather on schistosomiasis (Zhou et al., 2008; Perez-Saez et al., 2015), dengue fever (Patz et al., 1998), chikungunya (Ruiz-Moreno et al., 2012), cholera (Rinaldo et al., 2012) and malaria (Van Lieshout et al., 2004; Martens et al., 1995) among others. In these studies, researchers combined empirical data with climate models. For instance, to study schistosomiasis, Zhou et al (2008) experimentally measured the environmental determinants of snail life cycles. They then integrated these results into GIS maps and used projected temperature increases to predict disease risk by mid-century (Zhou et al., 2008). Similarly, to study malaria, van Lieshout et al (2004) included data about mosquito biting rates, mosquito survival, malaria parasite incubation periods, rainfall and temperature threshold values, mosquito distributions, GCMs, population scenarios and adaptive capacity (Van Lieshout et al., 2004). Another mechanistic systems-based study used a compartment model of both human and mosquito susceptibility, exposure and infection along with mosquito lifecycle information to study the geographic sensitivity of epidemic potential of the chikungunya virus in the United States (Ruiz-Moreno et al., 2012). Rinaldo et al (2012) studied the cholera epidemic in Haiti by incorporating hydrological transport, rainfall and human mobility among other factors to accurately reproduce historic cholera outbreaks (Rinaldo et al., 2012).
Such mechanistic system-based studies enable scientists to explicitly understand the underlying pathogen transmission pathways and mechanisms and how they are differentially affected by climate variables. This enables them to design targeted interventions to reduce transmission and improve health outcomes. These are the types of studies we are advocating for in this paper.
The case of diarrheal diseases is complicated by the multiple etiological agents and transmission pathways involved, as discussed in this review. Despite this challenge, several studies have taken systems approaches to model the environmental processes that mediate this transmission through air, water and fomite pathways (Li et al., 2009). Agent-based modeling has been used to focus on the mechanisms of pathogen transmission, growth and death through water pathways and the risk factors and intervention strategies that govern this transmission (Mellor et al., 2012, 2014). Another novel approach looked at social networks in Ecuador and found that disease risk was lower in remote communities (Eisenberg et al., 2006). Another study in Ecuador used a spatiotemporal Markov chain model to dynamically assess how the level of disease in a region dynamically affects the transmission dynamics in a local community (Goldstick et al., 2014).
Specific tools and frameworks of interest would depend on the nature of the specific research question. One sample modeling approach incorporating several of these tools is given in the Example Modeling Approach section. Such methods could be verified, validated and calibrated using new and existing, locally-based datasets. Parameter estimates can be drawn from prior laboratory, observational and intervention studies as appropriate. They can be supplemented by focused field-based studies designed to fill key knowledge gaps, allowing for lean, focused field-based study designs. Data about local and regional hydrology, rates and etiologies of diarrheal diseases, performance of engineered infrastructure, water-related practices and adaptive behaviors, as well as water quality measurements should be integrated. These data are partially available through existing datasets, but more comprehensive and integrated datasets are urgently needed over longer time periods in diverse regions. They should also rely on downscaled climate models or other regional climate change predictions to project change in the long term. Proven methods and models could then be scaled up to regional, continental or even global-scale models using larger datasets such as those of the Joint-Monitoring Programme (Bartram et al., 2014) or national records of disease rates and water quality.
The inclusion of many of the aforementioned mechanisms in a systems-based framework can provide additional insight that is absent in more traditional approaches. For instance, the fact that rotavirus is associated with cooler and drier temperatures suggests that rotavirus incidence might decline as regions of the earth get warmer and wetter. Likewise, the association of cryptosporidiosis and Shigella with warmer and wetter weather could increase diarrhea incidence attributable to those pathogens. While other, non-climatic factors such as demography and behavior might also affect future incidence of diarrhea attributable to each of these pathogens, the differential response of different pathogen taxa to weather has implications for adaptation strategies. For example, water treatment technologies such as ceramic water filters, which are effective against bacteria (Brown & Sobsey, 2010) and cryptosporidium (Bielefeldt et al., 2010) but less so against viruses might be preferred over chlorination, which is ineffective against protozoan. Mechanistic systems-based approaches would allow researchers to predict the microbial effectiveness, filter prevalence and compliance rates needed to mitigate current (Mellor et al., 2014) and future disease transmission, incorporating meteorological as well as social and demographic data. Similar mechanistic studies could allow researchers to optimize water treatment plant operations for cities in developing countries with the aim of improving microbial water quality and ensuring reliable water supplies. They can also be used to predict the level of system resiliency needed in the future and aid in the design of infrastructure systems to withstand the anticipated impact.
Such approaches are complicated by the large uncertainties associated with human demographic behavior, non-linear and interactive relations of humans to natural systems and the sensitivity of health outcomes to multiple environmental stressors (McMichael, 1997). Furthermore, the level of spatial and temporal resolution is an important factor that warrants consideration. For instance, what resolution of climate models is needed to accurately predict climate impacts in heterogeneous landscapes? Or, at what rate will diarrhea pathogens evolve and societies adapt under future climates? These knowledge gaps mean that researchers must perform rigorous sensitivity analyses in order to fully characterize uncertainties.
These uncertainties are compounded by the fact that globally, diarrhea death rates have declined rapidly since 2004 which some have suggested is due to improved access to water and sanitation interventions and improved treatment of diarrheal diseases (WHO, 2004; Prüss-Ustün et al., 2014). It is likely that population adaptive responses and economic growth may likewise lessen this disease burden in the long term. Therefore, modeling efforts will need to incorporate any trends towards improvement, as has been done for malaria (Gething et al., 2010) and can be done using systems based approaches. By understanding such trends, we can learn what is working and build upon it.
2.4. Example Modeling Approach
One modeling approach to study climate impacts on diarrhea involves agent-based computer modeling (Mellor & Zimmerman, 2014). Agent-based modeling (An, 2009) is an effective and proven platform that can integrate many of the disparate mechanisms needed to quantify the effects of climate change on diarrheal disease rates and to design “no-regrets” adaptation strategies. For regional studies, such a platform could utilize a stochastic weather generator to downscale GCMs and generate simulated daily weather predictions for different climate change scenarios (Semenov & Stratonovitch, 2010). That simulated weather can then be used to determine water availability and quality for the human agents to consume. This can be done using a stand-alone or integrated watershed model appropriate for local conditions (Jamieson et al., 2004). Such water quality models can simulate the fate and transport of pathogens through natural and built systems. The water quality and quantity then available to the human agents on a given day can determine the propensity of those agents to get diarrhea. Quantitative microbial risk assessment (Haas et al., 1999) based on local disease etiology and pathogen abundance measurements can be used to quantify dose-response relationships and the relative risk associated with water availability can also be integrated (Fry et al., 2010). Other important factors like hand-washing, water source choice and other contamination vectors can also be included (Mellor et al., 2012). Then, different interventions such as point-of-use water treatment devices (Sobsey et al., 2008) or more reliable water supplies (Fry et al., 2010) can be included to test the ability of those interventions to reduce diarrhea incidence and help communities adapt to climate change. In addition to prioritizing adaptation strategies, such a method can quantify the links between climate model scenarios and diarrhea incidence over long time periods. It can also be used to explore the complex system dynamics, rank risk factor importance, identify key data gaps, find tipping points and test a broad range of counter-factual scenarios. It is a modular and adaptable technique that can simulate scenarios in silico which has obvious cost and ethical benefits. Bringing such a technique to a continental or global scale is challenging due to the computational limitations, however recent advances have made global-scale agent-based models feasible (Parker & Epstein, 2011).
3. Conclusions
New methods are needed to fully quantify the risk that climate change poses to rates of diarrheal diseases across diverse climates and to design “no-regrets” adaptation strategies to improve the resilience of developing world communities. Mechanistic, systems approaches and the coupling of the human, engineered and environmental systems have the potential to provide these benefits. Although common in other environmental health fields, such methods are not yet widely used to study climate impacts on diarrheal diseases.
In countries with the most adaptive capacity, global climate change impacts will range from crop failures and heat waves to sea level rise and the spread of serious infectious diseases. The costs of inaction will be high. In addition to those impacts, those populations with the least capacity and resilience in low income countries will be heavily impacted in terms of access to safe, reliable drinking water and basic sanitation (Barros & Field, 2014). A mechanistic understanding of these systems will support the development of robust adaptation strategies, advancing improved resiliency of developing world communities in the face of the added burdens of climate change impacts on water quantity, quality, and availability, and subsequently health.
References
- Ahmed SM, Lopman BA, Levy K. A systematic review and meta-analysis of the global seasonality of norovirus. PloS one. 2013;8:e75922. doi: 10.1371/journal.pone.0075922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MJ, Faruque A, Faruque S, Sack R, Mahalanabis D. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. Journal of Clinical Microbiology. 1999;37:3458–3464. doi: 10.1128/jcm.37.11.3458-3464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KA, Carzolio M, Goodin D, Vance E. Climate change is likely to worsen the public health threat of diarrheal disease in Botswana. International Journal of Environmental Research and Public Health. 2013;10:1202–1230. doi: 10.3390/ijerph10041202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer S, Ostfeld RS, Johnson PT, Kutz S, Harvell CD. Climate change and infectious diseases: from evidence to a predictive framework. science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- An G. Systems Biology. Springer; 2009. Dynamic knowledge representation using agent-based modeling: ontology instantiation and verification of conceptual models; pp. 445–468. [DOI] [PubMed] [Google Scholar]
- Bain R, Cronk R, Hossain R, Bonjour S, Onda K, Wright J, Yang H, Slaymaker T, Hunter P, Pruss-Ustn A, Bartram J. Global assessment of exposure to faecal contamination through drinking water based on a systematic review. Tropical Medicine & International Health. 2014;19:917–927. doi: 10.1111/tmi.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto ML, Genser B, Strina A, Teixeira MG, Assis A, Rego RF, Teles CA, Prado MS, Matos S, Alcântara-Neves NM, et al. Impact of a City-Wide Sanitation Programme in Northeast Brazil on Intestinal Parasites Infection in Young Children. Environmental Health Perspectives. 2011;118:1637–42. doi: 10.1289/ehp.1002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros V, Field C. Climate Change 2014: Impacts, Adaptation, and Vulnerability: Working Group II Contribution to the IPCC 5th Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press; 2014. [Google Scholar]
- Bartram J, Brocklehurst C, Fisher MB, Luyendijk R, Hossain R, Wardlaw T, Gordon B. Global monitoring of water supply and sanitation: History, methods and future challenges. International Journal of Environmental Research and Public Health. 2014;11:8137–8165. doi: 10.3390/ijerph110808137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavnani D, Goldstick JE, Cevallos W, Trueba G, Eisenberg JN. Impact of rainfall on diarrheal disease risk associated with unimproved water and sanitation. The American Journal of Tropical Medicine and Hygiene. 2014;90:705–711. doi: 10.4269/ajtmh.13-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt AR, Kowalski K, Schilling C, Schreier S, Kohler A, Scott Summers R. Removal of virus to protozoan sized particles in point-of-use ceramic water filters. Water Research. 2010;44:1482–1488. doi: 10.1016/j.watres.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Brown J, Sobsey M. Microbiological effectiveness of locally produced ceramic filters for drinking water treatment in Cambodia. Journal of Water and Health. 2010;8:1–10. doi: 10.2166/wh.2009.007. [DOI] [PubMed] [Google Scholar]
- Burns D. What do hydrologists mean when they use the term flushing? Hydrological Processes. 2005;19:1325–1327. [Google Scholar]
- Carlton EJ, Eisenberg JN, Goldstick J, Cevallos W, Trostle J, Levy K. Heavy rainfall events and diarrhea incidence: The role of social and environmental factors. American Journal of Epidemiology. 2014;179:344–352. doi: 10.1093/aje/kwt279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton EJ, Woster AP, DeWitt P, Goldstein RS, Levy K. A systematic review and meta-analysis of ambient temperature and diarrhoeal diseases. International Journal of Epidemiology. 2015 doi: 10.1093/ije/dyv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles K, Pond K, Pedley S. Vision 2030: The resilience of water supply and sanitation in the face of climate change. World Health Organization; 2010. [Google Scholar]
- Checkley W, Epstein LD, Gilman RH, Figueroa D, Cama RI, Patz JA, Black RE. Effects of El Niño and ambient temperature on hospital admissions for diarrhoeal diseases in Peruvian children. The Lancet. 2000;355:442–450. doi: 10.1016/s0140-6736(00)82010-3. [DOI] [PubMed] [Google Scholar]
- Christenson E, Elliott M, Banerjee O, Hamrick L, Bartram J. Climate-related hazards: A method for global assessment of urban and rural population exposure to cyclones, droughts, and floods. International Journal of Environmental Research and Public Health. 2014;11:2169–2192. doi: 10.3390/ijerph110202169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen T, Alexander K, Sinclair D, Boisson S, Peletz R, Chang H, Majorin F, Cairncross S. Interventions to improve water quality for preventing diarrhoea. Cochrane Database of Systematic Reviews. 2015 doi: 10.1002/14651858. CD004794.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinet-Adler S, Babji S, Francis M, Kattula D, Premkumar PS, Sarkar R, Mohan VR, Ward H, Kang G, Balraj V, et al. Environmental factors associated with high fly densities and diarrhea in Vellore, India. Applied and environmental microbiology. 2015;81:6053–6058. doi: 10.1128/AEM.01236-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M, Knutti R, Arblaster J, Dufresne J-L, Fichefet T, Friedlingstein P, Gao X, Gutowski W, Johns T, Krinner G, Shongwe M, Tebaldi C, Weaver A, MW Long-term Climate Change: Projections, Commitments and Irreversibility. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change.2013. [Google Scholar]
- Cotruvo J, Dufour A, Rees G, Bartram J, Carr R, Cliver D, Craun G, Fayer R, Gannon V. Waterborne zoonoses. Published on behalf of the World Health Organization by IWA Publishing; London, UK: 2004. pp. 242–255. [Google Scholar]
- Cronin A, Pedley S, Breslin N, Gibson J. Monitoring source and domestic water quality in parallel with sanitary risk identification in Northern Mozambique to prioritise protection interventions. J Water Health. 2006;4:333–345. doi: 10.2166/wh.2006.029. [DOI] [PubMed] [Google Scholar]
- Cutler D, Miller G. The role of public health improvements in health advances: The twentieth-century united states. Demography. 2005;42:1–22. doi: 10.1353/dem.2005.0002. [DOI] [PubMed] [Google Scholar]
- De Wit M, Stankiewicz J. Changes in surface water supply across Africa with predicted climate change. Science. 2006;311:1917–1921. doi: 10.1126/science.1119929. [DOI] [PubMed] [Google Scholar]
- D’Souza RM, Becker NG, Hall G, Moodie KB. Does ambient temperature affect foodborne disease? Epidemiology. 2004;15:86–92. doi: 10.1097/01.ede.0000101021.03453.3e. [DOI] [PubMed] [Google Scholar]
- Effler E, Isaäcson M, Arntzen L, Heenan R, Canter P, Barrett T, Lee L, Mambo C, Levine W, Zaidi A, et al. Factors contributing to the emergence of Escherichia coli O157 in Africa. Emerging Infectious Diseases. 2001;7:812. doi: 10.3201/eid0705.017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg J, Scott J, Porco T. Integrating disease control strategies: balancing water sanitation and hygiene interventions to reduce diarrheal disease burden. American Journal of Public Health. 2007;97:846. doi: 10.2105/AJPH.2006.086207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg JN, Cevallos W, Ponce K, Levy K, Bates SJ, Scott JC, Hubbard A, Vieira N, Endara P, Espinel M, et al. Environmental change and infectious disease: how new roads affect the transmission of diarrheal pathogens in rural Ecuador. Proceedings of the National Academy of Sciences. 2006;103:19460–19465. doi: 10.1073/pnas.0609431104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elsas JD, Semenov AV, Costa R, Trevors JT. Survival of escherichia coli in the environment: fundamental and public health aspects. The ISME journal. 2010;5:173–183. doi: 10.1038/ismej.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enger K, Nelson K, Rose J, Eisenberg J. The joint effects of efficacy and compliance: a study of household water treatment effectiveness against childhood diarrhea. Water Research. 2012;47:1181–1190. doi: 10.1016/j.watres.2012.11.034. [DOI] [PubMed] [Google Scholar]
- EPA. Climate Ready Water Utilities Adaptation Strategies Guide for Water Utilities. The Environmental Protection Agency; 2013. [Google Scholar]
- Escamilla V, Knappett PS, Yunus M, Streatfield P, Emch M. Influence of latrine proximity and type on tubewell water quality and diarrheal disease in Bangladesh. Annals of the Association of American Geographers. 2013;103:299–308. [Google Scholar]
- Fewtrell L, Kaufmann R, Kay D, Enanoria W, Haller L, Colford J. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- Fry LM, Cowden JR, Watkins DW, Jr, Clasen T, Mihelcic JR. Quantifying health improvements from water quantity enhancement: An engineering perspective applied to rainwater harvesting in West Africa. Environmental Science & Technology. 2010;44:9535–9541. doi: 10.1021/es100798j. [DOI] [PubMed] [Google Scholar]
- Fry LM, Mihelcic JR, Watkins DW. Water and nonwater-related challenges of achieving global sanitation coverage. Environmental Science & Technology. 2008;42:4298–4304. doi: 10.1021/es7025856. [DOI] [PubMed] [Google Scholar]
- Fry LM, Watkins DW, Reents N, Rowe MD, Mihelcic JR. Climate change and development impacts on the sustainability of spring-fed water supply systems in the Alto Beni region of Bolivia. Journal of Hydrology. 2012;468:120–129. [Google Scholar]
- Gao L, Zhang Y, Ding G, Liu Q, Zhou M, Li X, Jiang B. Meteorological Variables and Bacillary Dysentery Cases in Changsha City, China. The American Journal of Tropical Medicine and Hygiene. 2014;90:697–704. doi: 10.4269/ajtmh.13-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething PW, Smith DL, Patil AP, Tatem AJ, Snow RW, Hay SI. Climate change and the global malaria recession. Nature. 2010;465:342–345. doi: 10.1038/nature09098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstick JE, Trostle J, Eisenberg JNS. Ask When - Not Just Whether - It’s a Risk: How Regional Context Influences Local Causes of Diarrheal Disease. American Journal of Epidemiology. 2014 doi: 10.1093/aje/kwu034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson D, Derwent LC, Hanley ME, Dunn DW, Abolins SR. Predicting calyptrate fly populations from the weather, and probable consequences of climate change. Journal of Applied Ecology. 2005;42:795–804. [Google Scholar]
- Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. The impoverished gut? A triple burden of diarrhoea, stunting and chronic disease. Nature Reviews Gastroenterology and Hepatology. 2013;10:220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant RL, Hughes JM, Lima NL, Crane J. Diarrhea in developed and developing countries: magnitude, special settings, and etiologies. Review of Infectious Diseases. 1990;12:S41–S50. doi: 10.1093/clinids/12.Supplement_1.S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas CN, Rose JB, Gerba CP. Quantitative microbial risk assessment. John Wiley & Sons; 1999. [Google Scholar]
- Hashizume M, Armstrong B, Hajat S, Wagatsuma Y, Faruque AS, Hayashi T, Sack DA. Association between climate variability and hospital visits for non-cholera diarrhoea in Bangladesh: effects and vulnerable groups. International Journal of Epidemiology. 2007;36:1030–1037. doi: 10.1093/ije/dym148. [DOI] [PubMed] [Google Scholar]
- Hashizume M, Wagatsuma Y, Faruque A, Sack D, Hayashi T, Hunter P, Armstrong B. Factors determining vulnerability to diarrhoea during and after severe floods in Bangladesh. Journal of Water and Health. 2008;6:323–332. doi: 10.2166/wh.2008.062. [DOI] [PubMed] [Google Scholar]
- Heijnen M, Cumming O, Peletz R, Chan GKS, Brown J, Baker K, Clasen T. Shared sanitation versus individual household latrines: A systematic review of health outcomes. PloS one. 2014;9:e93300. doi: 10.1371/journal.pone.0093300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges M, Belle JH, Carlton EJ, Liang S, Li H, Luo W, Freeman MC, Liu Y, Gao Y, Hess JJ, Remais J. Delays in reducing waterborne and water-related infectious diseases in china under climate change. Nature Climate Change. 2014 doi: 10.1038/nclimate2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G, Bartram J. Domestic water quantity, service level, and health. World Health Organization; Geneva: 2003. [Google Scholar]
- Howard G, Pedley S, Barrett M, Nalubega M, Johal K. Risk factors contributing to microbiological contamination of shallow groundwater in Kampala, Uganda. Water Research. 2003;37:3421–3429. doi: 10.1016/S0043-1354(03)00235-5. [DOI] [PubMed] [Google Scholar]
- Huq A, West P, Small E, Huq M, Colwell R. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar 01 associated with live copepods in laboratory microcosms. Applied and Environmental Microbiology. 1984;48:420–424. doi: 10.1128/aem.48.2.420-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst CJ, Benton WH, McClellan KA. Thermal and water source effects upon the stability of enteroviruses in surface freshwaters. Canadian Journal of Microbiology. 1989;35:474–480. doi: 10.1139/m89-073. [DOI] [PubMed] [Google Scholar]
- Jagai JS, Castronovo DA, Monchak J, Naumova EN. Seasonality of cryptosporidiosis: A meta-analysis approach. Environmental Research. 2009;109:465–478. doi: 10.1016/j.envres.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagai JS, Sarkar R, Castronovo D, Kattula D, McEntee J, Ward H, Kang G, Naumova EN. Seasonality of rotavirus in South Asia: a meta-analysis approach assessing associations with temperature, precipitation, and vegetation index. PloS one. 2012;7:e38168. doi: 10.1371/journal.pone.0038168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson R, Gordon R, Joy D, Lee H. Assessing microbial pollution of rural surface waters: A review of current watershed scale modeling approaches. Agricultural Water Management. 2004;70:1–17. [Google Scholar]
- King BJ, Keegan AR, Monis PT, Saint CP. Environmental temperature controls cryptosporidium oocyst metabolic rate and associated retention of infectivity. Applied and Environmental Microbiology. 2005;71:3848–3857. doi: 10.1128/AEM.71.7.3848-3857.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle K, Pascual M, Yunus M. Pathogen adaptation to seasonal forcing and climate change. Proceedings of the Royal Society B: Biological Sciences. 2005;272:971–977. doi: 10.1098/rspb.2004.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolstad EW, Johansson KA. Uncertainties associated with quantifying climate change impacts on human health: a case study for diarrhea. Environmental Health Perspectives. 2011;119:299. doi: 10.1289/ehp.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyla C, Bain R, Cronk R, Bartram J. Seasonal variation of fecal contamination in drinking water sources in developing countries: A systematic review. Science of The Total Environment. 2015;514:333–343. doi: 10.1016/j.scitotenv.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. The Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- Kovats RS, Edwards SJ, Charron D, Cowden J, D?Souza RM, Ebi KL, Gauci C, Gerner-Smidt P, Hajat S, Hales S, et al. Climate variability and campylobacter infection: an international study. International Journal of Biometeorology. 2005;49:207–214. doi: 10.1007/s00484-004-0241-3. [DOI] [PubMed] [Google Scholar]
- Kumpel E, Nelson KL. Comparing microbial water quality in an intermittent and continuous piped water supply. Water Research. 2013;47:5176–5188. doi: 10.1016/j.watres.2013.05.058. [DOI] [PubMed] [Google Scholar]
- Kumpel E, Nelson KL. Mechanisms affecting water quality in an intermittent piped water supply. Environmental Science & Technology. 2014 doi: 10.1021/es405054u. [DOI] [PubMed] [Google Scholar]
- Lama JR, Seas CR, León-Barúa R, Gotuzzo E, Sack RB. Environmental temperature, cholera, and acute diarrhoea in adults in Lima, Peru. Journal of Health, Population and Nutrition. 2004:399–403. [PubMed] [Google Scholar]
- Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. International Journal of Epidemiology. 2009a;38:1487–1496. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy K, Hubbard AE, Nelson KL, Eisenberg JN. Drivers of water quality variability in northern coastal Ecuador. Environmental Science & Technology. 2009b;43:1788–1797. doi: 10.1021/es8022545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Eisenberg JN, Spicknall IH, Koopman JS. Dynamics and control of infections transmitted from person to person through the environment. American Journal of Epidemiology. 2009;170:257–265. doi: 10.1093/aje/kwp116. [DOI] [PubMed] [Google Scholar]
- Luke DA, Stamatakis KA. Systems science methods in public health: dynamics, networks, and agents. Annual Review of Public Health. 2012;33:357. doi: 10.1146/annurev-publhealth-031210-101222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald MC, Juran L, Jose J, Srinivasan S, Ali SI, Aronson KJ, Hall K. The impact of rainfall and seasonal variability on the removal of bacteria by a point-of-use drinking water treatment intervention in Chennai, India. International Journal of Environmental Health Research. 2015:1–14. doi: 10.1080/09603123.2015.1089532. [DOI] [PubMed] [Google Scholar]
- Martens W, Niessen LW, Rotmans J, Jetten TH, McMichael AJ. Potential impact of global climate change on malaria risk. Environmental health perspectives. 1995;103:458. doi: 10.1289/ehp.95103458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. Integrated assessment of potential health impact of global environmental change: prospects and limitations. Environmental Modeling & Assessment. 1997;2:129–137. [Google Scholar]
- McMichael AJ. Prisoners of the proximate: loosening the constraints on epidemiology in an age of change. American Journal of Epidemiology. 1999;149:887–897. doi: 10.1093/oxfordjournals.aje.a009732. [DOI] [PubMed] [Google Scholar]
- Mellor J, Abebe L, Ehdaie B, Dillingham R, Smith J. Modeling the sustainability of a ceramic water filter intervention. Water Research. 2014;49:286–299. doi: 10.1016/j.watres.2013.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor J, Zimmerman J. A Systems Approach to Climate, Water and Diarrhea in Hubli-Dharward, India. Abstract 13401 presented at 2014 AGU Fall Meeting; San Francisco, CA. 15–19 Dec; American Geophysical Union; 2014. [Google Scholar]
- Mellor JE, Smith JA, Learmonth GP, Netshandama VO, Dillingham RA. Modeling the complexities of water, hygiene, and health in Limpopo Province, South Africa. Environmental Science & Technology. 2012;46:13512–13520. doi: 10.1021/es3038966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor JE, Smith JA, Samie A, Dillingham RA. Coliform Sources and Mechanisms for Regrowth in Household Drinking Water in Limpopo, South Africa. Journal of Environmental Engineering. 2013;139:1152–1161. doi: 10.1061/(ASCE)EE.1943-7870.0000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton C. Master’s thesis. The University of South Florida; Tampa Florida: 2013. Assessing Appropriate Technology Handwashing Stations in Mali, West Africa. [Google Scholar]
- Naumova E, Jagai J, Matyas B, DeMaria A, MacNeill I, Griffiths J. Seasonality in six enterically transmitted diseases and ambient temperature. Epidemiology and Infection. 2007;135:281–292. doi: 10.1017/S0950268806006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngazoa E, Fliss I, Jean J. Quantitative study of persistence of human norovirus genome in water using taqman real-time rt-pcr. Journal of Applied Microbiology. 2008;104:707–715. doi: 10.1111/j.1365-2672.2007.03597.x. [DOI] [PubMed] [Google Scholar]
- Onda K, LoBuglio J, Bartram J. Global access to safe water: accounting for water quality and the resulting impact on MDG progress. International Journal of Environmental Research and Public Health. 2012;9:880–894. doi: 10.3390/ijerph9030880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onozuka D, Hashizume M, Hagihara A. Effects of weather variability on infectious gastroenteritis. Epidemiology and infection. 2010;138:236–243. doi: 10.1017/S0950268809990574. [DOI] [PubMed] [Google Scholar]
- Parker J, Epstein JM. A distributed platform for global-scale agent-based models of disease transmission. ACM Transactions on Modeling and Computer Simulation (TOMACS) 2011;22:2. doi: 10.1145/2043635.2043637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz JA, Martens W, Focks DA, Jetten TH. Dengue fever epidemic potential as projected by general circulation models of global climate change. Environmental Health Perspectives. 1998;106:147. doi: 10.1289/ehp.98106147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Saez J, Mari L, Bertuzzo E, Casagrandi R, Sokolow SH, De Leo GA, Mande T, Ceperley N, Froehlich JM, Sou M, Karambiri H, Yacouba H, Maiga A, Gatto M, Rinaldo A. A Theoretical Analysis of the Geography of Schistosomiasis in Burkina Faso Highlights the Roles of Human Mobility and Water Resources Development in Disease Transmission. PLoS Negl Trop Dis. 2015;9:e0004127. doi: 10.1371/journal.pntd.0004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering AJ, Davis J. Freshwater availability and water fetching distance affect child health in sub-Saharan Africa. Environmental Science & Technology. 2012;46:2391–2397. doi: 10.1021/es203177v. [DOI] [PubMed] [Google Scholar]
- Prüss-Ustün A, Bartram J, Clasen T, Colford JM, Cumming O, Curtis V, Bonjour S, Dangour AD, De France J, Fewtrell L, Freeman MC, Gordon B, Hunter PR, Johnston RB, Mathers C, Mausezahl D, Medlicott K, Neira M, Stocks M, Wolf J, Cairncross S. Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: a retrospective analysis of data from 145 countries. Tropical Medicine & International Health. 2014;19:894–905. doi: 10.1111/tmi.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfuess EA, Bartram J. Beyond direct impact: Evidence synthesis towards a better understanding of effectiveness of environmental health interventions. International Journal of Hygiene and Environmental Health. 2014;217:155–159. doi: 10.1016/j.ijheh.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Rinaldo A, Bertuzzo E, Mari L, Righetto L, Blokesch M, Gatto M, Casagrandi R, Murray M, Vesenbeckh SM, Rodriguez-Iturbe I. Reassessment of the 2010–2011 Haiti cholera outbreak and rainfall-driven multiseason projections. Proceedings of the National Academy of Sciences. 2012;109:6602–6607. doi: 10.1073/pnas.1203333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DJ, Randolph SE. The global spread of malaria in a future, warmer world. Science. 2000;289:1763–1766. doi: 10.1126/science.289.5485.1763. [DOI] [PubMed] [Google Scholar]
- Ruiz-Moreno D, Vargas IS, Olson KE, Harrington LC. Modeling dynamic introduction of chikungunya virus in the United States. PLoS Neglected Tropical Diseases. 2012;6 doi: 10.1371/journal.pntd.0001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana MVE, Zhang Q, Mihelcic JR. Influence of water quality on the embodied energy of drinking water treatment. Environmental Science & Technology. 2014;48:3084–3091. doi: 10.1021/es404300y. [DOI] [PubMed] [Google Scholar]
- Sarkar R, Kang G, Naumova EN. Rotavirus Seasonality and Age Effects in a Birth Cohort Study of Southern India. PloS one. 2013;8:e71616. doi: 10.1371/journal.pone.0071616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer R, Pezon C, Pinjari A, Fonseca C, Mihelcic JR. Household expenditure on water service: Financial and economic expenditures of rural and peri-urban households across socioeconomic classes and seasons in Burkina Faso 2013 [Google Scholar]
- Semenov MA, Stratonovitch P. Use of multi-model ensembles from global climate models for assessment of climate change impacts. Climate Research. 2010;41:1. [Google Scholar]
- Shaheed A, Orgill J, Ratana C, Montgomery M, Jeuland M, Brown J. Water quality risks of improved water sources: evidence from Cambodia. Tropical Medicine & International Health. 2014;19:186–194. doi: 10.1111/tmi.12229. [DOI] [PubMed] [Google Scholar]
- Singh RB, Hales S, de Wet N, Raj R, Hearnden M, Weinstein P. The influence of climate variation and change on diarrheal disease in the Pacific Islands. Environmental Health Perspectives. 2001;109:155. doi: 10.1289/ehp.01109155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M, Stauber C, Casanova L, Brown J, Elliott M. Point of use household drinking water filtration: a practical, effective solution for providing sustained access to safe drinking water in the developing world. Environmental Science & Technology. 2008;42:4261–4267. doi: 10.1021/es702746n. [DOI] [PubMed] [Google Scholar]
- Taylor RG, Scanlon B, Döll P, Rodell M, Van Beek R, Wada Y, Longuevergne L, Leblanc M, Famiglietti JS, Edmunds M, et al. Ground water and climate change. Nature Climate Change. 2013a;3:322–329. [Google Scholar]
- Taylor RG, Todd MC, Kongola L, Maurice L, Nahozya E, Sanga H, MacDonald AM. Evidence of the dependence of groundwater resources on extreme rainfall in East Africa. Nature Climate Change. 2013b;3:374–378. [Google Scholar]
- Thomas C, Hill D, Mabey M. Evaluation of the effect of temperature and nutrients on the survival of campylobacter spp. in water microcosms. Journal of Applied Microbiology. 1999;86:1024–1032. doi: 10.1046/j.1365-2672.1999.00789.x. [DOI] [PubMed] [Google Scholar]
- Tucker J, MacDonald A, Coulter L, Calow RC. Household water use, poverty and seasonality: Wealth effects, labour constraints, and minimal consumption in ethiopia. Water Resources and Rural Development. 2014;3:27–47. [Google Scholar]
- Van Lieshout M, Kovats R, Livermore M, Martens P. Climate change and malaria: analysis of the sres climate and socioeconomic scenarios. Global Environmental Change. 2004;14:87–99. [Google Scholar]
- Walker C, Perin J, Aryee M, Boschi-Pinto C, Black R. Diarrhea incidence in low-and middle-income countries in 1990 and 2010: a systematic review. BMC Public Health. 2012;12:220. doi: 10.1186/1471-2458-12-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CLF, Sack D, Black RE. Etiology of diarrhea in older children, adolescents and adults: a systematic review. PLoS Neglected Tropical Diseases. 2010;4:e768. doi: 10.1371/journal.pntd.0000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Doyle MP. Survival of enterohemorrhagic Escherichia coli O157: H7 in water. Journal of Food Protection. 1998;61:662–667. doi: 10.4315/0362-028x-61.6.662. [DOI] [PubMed] [Google Scholar]
- WHO. The global burden of disease: 2004 update. World Health Organization; 2004. [Google Scholar]
- Wolf J, Prüss-Ustün A, Cumming O, Bartram J, Bonjour S, Cairncross S, Clasen T, Colford JM, Curtis V, France J, et al. Assessing the impact of drinking water and sanitation on diarrhoeal disease in low-and middle-income settings: systematic review and meta-regression. Tropical Medicine & International Health. 2014 doi: 10.1111/tmi.12331. [DOI] [PubMed] [Google Scholar]
- Wright J, Gundry S, Conroy R. Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Tropical Medicine & International Health. 2004;9:106–117. doi: 10.1046/j.1365-3156.2003.01160.x. [DOI] [PubMed] [Google Scholar]
- Zambrano LD, Levy K, Menezes NP, Freeman MC. Human diarrhea infections associated with domestic animal husbandry: a systematic review and meta-analysis. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2014;108:313–325. doi: 10.1093/trstmh/tru056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XN, Yang GJ, Yang K, Wang XH, Hong QB, Sun LP, Malone JB, Kristensen TK, Bergquist NR, Utzinger J. Potential impact of climate change on schistosomiasis transmission in China. The American Journal of Tropical Medicine and Hygiene. 2008;78:188–194. [PubMed] [Google Scholar]